On the aliphatic versus aromatic content of the carriers of the unidentified infrared emission features
|
|
|
- Moses Elliott
- 5 years ago
- Views:
Transcription
1 Advance Access publication 2016 July 20 doi: /mnras/stw1740 On the aliphatic versus aromatic content of the carriers of the unidentified infrared emission features X. J. Yang, 1,2 R. Glaser, 3 Aigen Li 2 and J. X. Zhong 1 1 Department of Physics, Xiangtan University, Xiangtan, Hunan Province, China 2 Department of Physics and Astronomy, University of Missouri, Columbia, MO 65211, USA 3 Department of Chemistry, University of Missouri, Columbia, MO 65211, USA Accepted 2016 July 15. Received 2016 July 9; in original form 2015 July 4 1 INTRODUCTION A series of strong and relatively broad infrared (IR) emission features at 3.3, 6.2, 7.7, 8.6, and 11.3 µm are ubiquitously seen in almost all astronomical objects with associated gas and dust, including protoplanetary nebulae, planetary nebulae, reflection nebulae, young stellar objects, H II regions, the Galactic IR cirrus, and external galaxies (see Tielens 2008). These features are a common characteristic of the interstellar medium (ISM) of the Milky Way and nearby galaxies as well as distant galaxies out to redshifts of z 4 (e.g. see Riechers et al. 2014). Since their first detection four decades ago in two planetary nebulae (NGC 7027 and BD+30 o 3639; Gillett, Forrest & Merrill 1973), the carriers of these IR emission features have remained unidentified. Because of this, they are collectively known as the unidentified infrared emission (UIE or UIR) bands. Nevertheless, it is now generally accepted that these features are characteristic of the stretching and bending vibrations of some sorts of aromatic hydrocarbon materials and, lia@missouri.edu ABSTRACT Although it is generally accepted that the unidentified infrared emission (UIE) features at 3.3, 6.2, 7.7, 8.6, and 11.3 µm are characteristic of the stretching and bending vibrations of aromatic hydrocarbon materials, the exact nature of their carriers remains unknown: whether they are free-flying, predominantly aromatic gas-phase molecules, or amorphous solids with a mixed aromatic/aliphatic composition are being debated. Recently, the 3.3 and 3.4 µm features which are commonly respectively attributed to aromatic and aliphatic C H stretches have been used to place an upper limit of 2 per cent on the aliphatic fraction of the UIE carriers (i.e. the number of C atoms in aliphatic chains to that in aromatic rings). Here we further explore the aliphatic versus aromatic content of the UIE carriers by examining the ratio of the observed intensity of the 6.2 µm aromatic C C feature (I 6.2 ) to that of the 6.85 µm aliphatic C H deformation feature (I 6.85 ). To derive the intrinsic oscillator strengths of the 6.2 µm stretch (A 6.2 ) and the 6.85 µm deformation (A 6.85 ), we employ density functional theory to compute the vibrational spectra of seven methylated polycyclic aromatic hydrocarbon molecules and their cations. By comparing I 6.85 /I 6.2 with A 6.85 /A 6.2, we derive the fraction of C atoms in methyl(ene) aliphatic form to be at most 10 per cent, confirming the earlier finding that the UIE emitters are predominantly aromatic. We have also computed the intrinsic strength of the 7.25 µm feature (A 7.25 ), another aliphatic C H deformation band. We find that A 6.85 appreciably exceeds A This explains why the 6.85 µm feature is more frequently detected in space than the 7.25 µm feature. Key words: dust, extinction ISM: lines and bands ISM: molecules infrared: ISM. therefore, the UIE features are sometimes also referred to as the aromatic infrared bands (AIB). A large number of candidate materials have been proposed as carriers of the UIE bands. All of these materials contain aromatic structures of fused benzene rings. The major debate lies in the exact structure of the UIE carriers: (1) are they free-flying, predominantly aromatic gas-phase macro-molecules like polycyclic aromatic hydrocarbon (PAH) molecules (Léger & Puget 1984; Allamandola, Tielens & Barker 1985, 1989), or (2) are they amorphous solids with a mixed aromatic/aliphatic composition like hydrogenated amorphous carbon (HAC; Jones, Duley & Williams 1990), quenched carbonaceous composites (QCC; Sakata et al. 1990), coal or kerogen (Papoular et al. 1989)? As originally suggested by Duley & Williams (1981), all of these solid materials share the basic molecular structure of PAHs by containing arenes. 1 They also contain 1 A benzene ring is C 6 H 6. If the H atoms are gone, then it is not really benzene anymore. It is an aromatic ring system which can be called arene. Arene is a hydrocarbon with alternating double and single bonds between carbon atoms forming rings. C 2016 The Authors Published by Oxford University Press on behalf of the Royal Astronomical Society
2 1552 X. J. Yang et al. aliphatic C H bonds as well as other molecular structures often with other elements besides C and H. The MAON model recently proposed by Kwok & Zhang (2011, 2013) also falls in this category, where MAON stands for mixed aromatic/aliphatic organic nanoparticle. Are the UIE carriers aromatic or aliphatic? A straightforward way to address this question is to examine the aliphatic fraction of the UIE carriers (i.e. the fraction of C atoms in aliphatic chains). Aliphatic hydrocarbons have a vibrational band at 3.4 µm due to the C H stretching mode (Pendleton & Allamandola 2002). In many interstellar and circumstellar environments the 3.3 µm emission feature is indeed often accompanied by a weaker feature at 3.4 µm(see Li & Draine 2012 and references therein). As demonstrated by Li & Draine (2012) and Yang et al. (2013), one can place an upper limit of 2 per cent on the aliphatic fraction of the emitters of the UIE features by assigning the 3.4 µm emission exclusively to aliphatic C H (also see Rouillé et al. 2012; Steglich et al. 2013). This is indeed an upper limit as the 3.4 µm emission feature could also be due to anharmonicity of the aromatic C H stretch (Barker, Allamandola & Tielens 1987) and superhydrogenated PAHs whose edges contain excess H atoms (Bernstein, Sandford & Allamandola 1996; Sandford, Bernstein & Materese 2013). In addition to the 3.4 µm C H stretching mode, aliphatic hydrocarbon materials also have two C H deformation bands at 6.85 and 7.25 µm. These two bands have been observed in weak absorption in the Galactic diffuse ISM (Chiar et al. 2000). They are also seen in emission, with the 6.85 µm feature detected both in the Milky Way and in the Large and Small Magellanic Clouds while the 7.25 µm feature so far mostly seen in the Magellanic Clouds (e.g. see Sloan et al. 2014). Their strengths (relative to the nearby 6.2 and 7.7 µm C C stretching bands) also allow an estimate of the aliphatic fraction of the UIE carrier. In this work, we further explore the aliphatic versus aromatic content of the UIE carriers by examining the ratio of the observed intensity of the 6.2 µm aromatic C C feature (I 6.2 ) 2 to that of the 6.85 µm aliphatic C H deformation features (I 6.85 ). At this moment we will not apply the 7.25 µm emission feature to constrain the aliphatic fraction of the UIE carriers since this feature appears much weaker than the 6.85 µm feature. 3 Nevertheless, to help understand why the 6.85 µm feature is more frequently detected than the 7.25 µm feature, we will also compute the intrinsic strength of the 7.25 µm feature and compare it with the 6.85 µm feature. To derive the intrinsic oscillator strengths of the 6.2 µm aromatic C C stretch (A 6.2 ) and the 6.85 µm aliphatic C H deformation (A 6.85 ), we employ density functional theory to compute the IR vibrational spectra of seven methylated PAH molecules and their cations. The methyl group is taken to represent the aliphatic component of the UIE carriers. PAH cations with aliphatic sidegroups are of particular interest since ionized PAHs are expected to be dominant or at least 2 The 7.7 µm C C stretching feature is often stronger than the 6.2 µm C C stretch for most of the interstellar UIE sources. However, the IR vibrational spectra of methylated PAHs calculated from density function theory are too complicated in the 7.7 µm wavelength range to allow us to reliably identify the C C stretching modes from which the 7.7 µm feature originates. In contrast, the C C stretching modes in the 6.2 µm wavelength range are much cleaner. 3 The detection of the 7.25 µm emission feature has so far only been reported in several protoplanetary nebulae and one planetary nebula in the Magellanic Clouds (see Sloan et al. 2014). This feature is not seen in the Milky Way except in some protoplanetary discs around young stars (e.g. see Sloan et al. 2005; Acke et al. 2010;Seok&Li2016). as abundant as neutrals in astronomical environments in which the 6.2, 7.7, and 8.6 µm bands are observed. In Section 2 we briefly describe the computational methods and the parent molecules based on which we derive the band strengths. We also present in Section 2 the computed band-strength ratios A 6.85 /A 6.2 and A 7.25 /A 6.2. We summarize in Section 3 the intensities of the 6.2 µm aromatic C C stretch and the 6.85 and 7.25 µm aliphatic C H deformations observed in the Milky Way and Magellanic Clouds. In Section 4 we estimate the aliphatic fractions of the UIE carriers from the observed intensity ratio (I 6.85 /I 6.2 ). We discuss and summarize our major results in Sections 5 and 6, respectively. 2 COMPUTING A 6.85 /A 6.2 AND A 7.25 /A 6.2 We usethe GAUSSIAN09software (Frischetal.2009) to calculate the IR vibrational spectra for a range of methylated aromatic molecules and their cations. We employ the hybrid density functional theoretical method (B3LYP) at the G level. 4 We adopt the vibrational frequency scale factor of for the B3LYP/6-311+G method (Borowski 2012). We have considered both neutral and singly-ionized benzene (C 6 H 6 ), naphthalene (C 10 H 8 ), anthracene (C 14 H 10 ), phenanthrene (C 14 H 10 ), pyrene (C 16 H 10 ), perylene (C 20 H 12 ), and coronene (C 24 H 12 ), as well as all of their methyl derivatives. We focus on methyl-substituted PAHs as PAHs with larger side chains are not as stable against photolytic dissociation as methyl-pahs and are therefore not expected to be present in the ISM in a large abundance. The molecules studied are shown in Fig. 1 together with the standard International Union of Pure and Applied Chemistry (IUPAC) numbering scheme. 5 We use the first four letters of the molecules to refer to them and attach the position number of the location of the methyl group. For example, 1-methylnaphthalene is referred to as Naph1. The methyl conformations are indicated in Fig. 1 and there are several possibilities. For their cation counterparts, we add a + sign (e.g. Naph1+ refers to singly ionized 1-methylnaphthalene). Depending on the symmetry of the neutral molecule, there are one or two stereoisomers in which one of the methyl-ch bonds lies in the plane of the arene. We differentiate between these stereoisomers by addition of a or b to the name of the structure isomer, and the in-plane C H bond points into the less (more) crowded hemisphere in the a-conformation (b-conformation). 6 In most cases, either the 4 Yang et al. (2013) have carried out computations with the B3LYP method in conjunction with a variety of basis sets: 6-31G,6-31+G,6-311+G, 6-311G,6-31+G,6-31++G, G, G,6-311+G(3df,3pd), and G(3df,3pd). It is found that the results computed with the basis sets G, G, G(3df,3pd), and G(3df,3pd) essentially reach the convergence limit. The B3LYP/6-311+G method presents an excellent compromise between accuracy and computational demand. Yang et al. (2013) have also examined the accuracy of the B3LYP method by employing second-order Møller Plesset perturbation theory (MP2) which is thought to be more accurate in computing band intensities than B3LYP (see Cramer 2004). It is found that the results computed from B3LYP/ G(3df,3pd) closely agree with that from MP2/ G(3df,3pd). Therefore, in this work we adopt the B3LYP/6-311+G method Take Naph1a and Naph1b as examples. In Naph1a, the in-plane methyl-h is four bonds away from the closest H-atom, H at C2. In Naph1b, the inplane methyl-h is five bonds away from the closest H-atom, H at C8. This leaves more space between the in-plane methyl-h and H(C2) in Naph1a than between in-plane methyl-h and H(C8) in Naph1b, and Naph1a is less crowded than Naph1b.
3 Aliphatic versus aromatic content of the carriers of the UIE features 1553 Figure 1. Structures of the mono-methyl ( CH 3 ) derivatives of seven aromatic molecules together with the standard IUPAC numbering: benzene (C 6 H 6 ), naphthalene (C 10 H 8 ), anthracene (C 14 H 10 ), phenanthrene (C 14 H 10 ), pyrene (C 16 H 10 ), perylene (C 20 H 12 ), and coronene (C 24 H 12 ). We use the first four letters of the parent molecules to refer to them and attach the position number of the location of the methyl group (e.g. Naph1 for 1-methylnaphthalene). The mono-methyl derivative of benzene is known as toluene (i.e. Tolu, C 7 H 8 ). Depending on where the methyl side-group is attached, a molecule will have several isomers (e.g. monomethyl-pyrene has three isomers in which the CH 3 group is attached to carbon 1, 2, or 4, respectively). We also indicate whether the structure is a minimum (M) or a transition state (TS) structure for the methyl rotation. a-conformation or the b-conformation corresponds to the minimum while the other conformation corresponds to the transition state structure for methyl rotation. 7 Note that the a-conformation can be the minimum (i.e. Naph1a) or the rotational transition state structure (i.e. Naph2a). 7 A structure on the potential energy surface is a stationary structure if the net inter-atomic forces on each atom are acceptably close to zero. A minimum is a stationary structure for which a small distortion along any internal coordinate increases the energy (all curvatures are positive). A transition state structure is a stationary structure for which a small distortion along one internal coordinate lowers the energy while distortions along any of the other internal coordinates increases the energy. The internal coordinate with the negative curvature is called the transition vector. For the rotational transition state structures, the transition vector describes a rotation of the methyl group about the H 3 C C bond and serves to scramble the H atoms in the associated minimum structures (i.e. Naph1a can be realized with any one of the three methyl-hs in the plane). The neutral molecules Tolu, Anth9 and Pyre2 are symmetric and the a-andb-conformations are identical. In these cases there exists an additional conformation type, the c-conformation, in which one of the methyl-ch bonds is almost perpendicular with respect to the arene plane. For Tolu and Pery2, the c-conformation is the minimum while the conformations with in-plane CH-bonds are the rotational transition state structures. In contrast, for Anth9 the c-conformation serves as the transition state for interconversion between the conformations with in-plane CH-bonds. We have also calculated all the minima of the cations of all the isomers of the methyl derivatives of the PAH molecules shown in Fig. 1. The structures and Mulliken charge distributions of these cations are shown in Fig. 2. The presence of a positive charge in the π-system causes polarization and results in complicated charge patterns which are characterized by positively charged centres and centres with partial negative charges. The centres of positive charges are always tertiary carbons (attached to three C atoms), while the centres of partial negative charges are secondary carbons (attached
4 1554 X. J. Yang et al. Figure 2. Charge distributions of the cations of the mono-methyl ( CH 3 ) derivatives of seven aromatic molecules: benzene, naphthalene, anthracene, phenanthrene, pyrene, perylene, and coronene. We adopt the same naming code as in Fig. 1, with a + sign specifying that the molecule is singly ionized. The bottom horizontal bar shows the colour coding for the charge distribution, with red being negatively charged and green being positively charged. to two C atoms) and primary carbons (attached to one C atom). The monocyclic aromatic hydrocarbon Tolu1c+ is atypical in that it is the only cation with only one tertiary carbon and thus this carbon is the centre of positive charges. All PAHs contain tertiary carbons at the bonds shared between rings and these carbons have the capacity to accept partial positive charges. Charge delocalization is highly effective in only a few of the cations (Anth1a+, Anth2b+, Pyre2c+). Most cations prefer a distribution of charges over two or more C atoms and concomitant bond polarizations, with Pery3a+ being an extreme example. The variety of charge distribution patterns has several consequences on the vibrational properties. First, the methyl group may be overall neutral (i.e. Pyre2c+); it may contain a partially negative methyl C (i.e. Naph1a+, Antha1a+, and especially Pery2c+); the methyl group may be attached to a cationic centre (i.e. Tolu1c+, Phen1a+) or to an essentially uncharged carbon (i.e. Anth2b+,
5 Aliphatic versus aromatic content of the carriers of the UIE features 1555 Table 1. IR intensity (km mol 1 ) of the 6.85 and 7.25 µm aliphatic C H deformation bands and the 6.2µm aromatic C C stretch band computed at the B3LYP/6-311+G level for all the neutral methyl PAHs shown in Fig. 1. Compound A 6.2 a A 6.85 b A 6.85 /A 6.2 A 7.25 c A 7.25 /A 6.2 Toluc Naph1a Naph2b Anth1a Anth2b Anth9a Phen1a Phen2b Phen3b Phen4a Phen9a Pyre1a Pyre2c Pyre4a Pery1c Pery2a Pery3a Coro1a Notes. a Intensity of the aromatic C C stretch at 6.2 µm in kmmol 1 (per C C bond). b Intensity of the aliphatic C H deformation at 6.85 µm in kmmol 1 (per C H bond). c Intensity of the aliphatic C H deformation at 7.25 µm in kmmol 1 (per C H bond). Pyre2c+). As a result, the force constants of the aliphatic C H modes will vary much more in the cations than in the neutral PAHs. Secondly, the charge distribution creates more diversity in the bond strengths of the aromatic C H and of the C C bonds and the group vibrations will cover broader spectral ranges. Thirdly, the presence of a charge and the resulted charge distribution pattern affect the dipole polarizabilities and cause significant differences in the intensities of the cations as compared to the neutrals. The vibrational frequencies and intensities for the 6.2 µm aromatic C C stretching mode and the 6.85 and 7.85 µm aliphatic C H deformation modes of all the isomers of the seven methylated PAH species and their cations are computed. The standard scaling is applied to the frequencies by employing a scale factor of The scaling will not be applied to the intensities since we are mostly interested in the intensity ratios A 6.85 /A 6.2 and A 7.25 /A 6.2 and the scaling will be cancelled out. We tabulate the computed band strengths in Tables 1 and 2, respectively, for neutral methyl PAHs and their cations. As shown in Fig. 3, both A 6.2 and A 6.85 vary appreciably for different neutral molecules and their isomers. For the 6.2 µm aromatic C C stretch, it has an average value (per aromatic C C bond) of A km mol 1, with a standard deviation of σ (A 6.2 ) 0.70 km mol 1. The 6.85 µm aliphatic C H deformation band is relatively less dependent on the nature of the molecule and the specific isomer. The average band strength (per aliphatic C H bond) is A km mol 1, and the standard deviation is σ (A 6.85 ) 1.93 km mol 1.TheA 6.85 /A 6.2 ratio, as shown in Fig. 3, varies considerably and depends significantly on the specific molecule and its specific isomers. Nevertheless, the A 6.85 /A 6.2 ratio for neutral molecules clearly shows a low-end value of 5 (i.e. A 6.85 /A 6.2 5). For these molecules, the intrinsic strength of the 7.25 µm aliphatic C H deformation band, A 7.25, is substan- Table 2. Same as Table 1 but for the cations of all the isomers of the methyl PAHs shown in Fig. 1. Compound A 6.2 A 6.85 A 6.85 /A 6.2 A 7.25 A 7.25 /A 6.2 Toluc Naph1a Naph2a Anth1a Anth2b Anth9b Phen1a Phen2b Phen3b Phen4a Phen9a Pyre1a Pyre2c Pyre4a Pery1c Pery2c Pery3a Coro1a tially weaker than the 6.85 µm band.asshowninfig.4, the strength (per aliphatic C H bond) of the 7.25 µm band is less than 2 km mol 1 for 72 per cent of the molecules and less than 1 km mol 1 for 61 per cent of the molecules. Similar to the 6.85 µm band, although the A 7.25 /A 6.2 ratio varies considerably for different molecules and their different isomers, the A 7.25 /A 6.2 ratio also clearly shows a low-end value of 1.0 (i.e. A 7.25 /A ; see Fig. 4b). For the cations of the methyl PAHs considered in this work, the computed band strengths (A 6.85, A 7.25 ) and their ratios (A 6.85 /A 6.2, A 7.25 /A 6.2 ) are tabulated in Table 2 and shown in Fig. 5. Compared to their neutral counterparts, the band-strength ratios of cations show even more substantial differences among different molecules. However, similar to neutral molecules, the band-strength ratios of cations also show low-end values, with A 6.85 /A and A 7.25 /A , respectively, for the 6.85 and 7.25 µm bands. 3 OBSERVATIONAL CONSTRAINTS Unlike the 3.4 µm aliphatic C H stretch, the detection of the 6.85 and 7.25 µm aliphatic C H deformation bands in the ISM of the Milky Way is much rarer. We are not aware of any Galactic sources in which the 7.25 µm feature is seen in emission except several protoplanetary discs (Seok & Li 2016). On the other hand, to the best of our knowledge, only seven Galactic objects exhibit a noticeable emission feature at 6.85 µm: NGC 7023, a reflection nebula (RN); NGC 7027, a planetary nebula (PN), the Orion bar, a photodissociated region (PDR); and four protoplanetary nebulae (PPNe; IRAS , IRAS , IRAS , and IRAS ). Except for the four Galactic PPNe, the observed 6.85 µm feature intensity is much weaker than that of the 6.2 µm feature. As shown in Table 3, for Galactic sources other than PPNe, I 6.85 /I For the four Galactic PPNe, the 6.85 µm feature is much stronger, with I 6.85 /I 6.2 > 1 for three of these four PPNe. However, these Galactic PPNe all exhibit unusual UIE properties compared to the typical interstellar UIE bands (e.g. these PPNe show a broad 8.2 µm feature rather than separately resolved 7.7/8.6 µm UIE features; moreover, the 11.3 and 12.7 µm C H outof-plane bending features seen in sources with normal -looking UIE features are blended in a flat-top broad-band at 11.8 µm; see
6 1556 X. J. Yang et al. Figure 3. Band strengths for the 6.85 µm aliphatic C H deformation (A 6.85 ) and for the ratio of A 6.85 to the 6.2 µm aromatic C C stretch (A 6.2 ) as determined with the B3LYP/6-311+G method for the mono-methyl derivatives of seven aromatic neutral molecules (benzene, naphthalene, anthracene, phenanthrene, pyrene, perylene, and coronene) and all of their isomers. Tokunaga 1997, Peeters et al. 2002). The origin of these unusual UIE features is unclear and their carriers are very likely different from that of the normal-looking interstellar UIE features. While the UIE carriers of PPNe are freshly synthesized in the outflows of asymptotic giant branch (AGB) and/or post-agb stars, their interstellar counterparts must have experienced various physical and chemical processes in the ISM. In this work we will restrict ourselves to the interstellar sources which show typical-looking UIE features for which I 6.85 /I It is interesting to note that the 6.85 and 7.25 µm emission features appear to be more frequently seen in the Magellanic Clouds than in the Galaxy. Sloan et al. (2014) systematically analysed the Spitzer/IRS spectra of a large number of carbon-rich sources in the Large and Small Magellanic Clouds. They derived the intensities of the 6.2, 6.85, 7.25, 7.7 and 8.6 µm emission features of all the Magellanic sources which exhibit the 6.85 and 7.25 µm emission features. We compile the observed intensity ratios I 6.85 /I 6.2 and I 7.25 /I 6.2 from Sloan et al. (2014) for all these sources and tabulate them in Table 3 and show them in Fig. 6. These sources are predominantly PPNe, with only three out of 18 not PPNe: SMP SMC 011 (PN), SMP SMC 020 (PN), and IRAS (AGB). While the Magellanic PPNe have a wide range of intensity ratios from I 6.85 /I toi 6.85 /I and from I 7.25 /I to I 7.25 /I , two of the non-ppn sources (SMP SMC 011, IRAS ) have I 6.85 /I and one non-ppn source (SMP SMC 020) has I 6.85 /I The 7.25 µm feature is not seen in SMP SMC 011 and IRAS Even in SMP SMC 020, the only non-ppn source in the Magellanic Clouds in which the detection of the 7.25 µm feature was reported, the intensity ratio has a large error bar (i.e. I 7.25 /I ± 0.11). Combining the intensity ratios observed for both Galactic and Magellanic sources, in the following we will take I 6.85 /I 6.2 = 0.1 to determine the aliphatic fractions of the UIE carriers. The 7.25 µm emission feature will not be used to estimate the aliphatic content of the UIE carriers. 4 CONSTRAINTS ON THE ALIPHATIC FRACTION OF THE UIE CARRIERS FROM THE 6.85 µm FEATURES Let N C, ali and N C, aro, respectively, be the numbers of aliphatic and aromatic C atoms in the emitters of the 6 8 µm UIE bands. Let B λ (T) = (2 hc 2 /λ 5 )/[exp (hc/λkt) 1] be the Planck function at
7 Aliphatic versus aromatic content of the carriers of the UIE features 1557 Figure 4. Same as Fig. 3 but for the 7.25 µm aliphatic C H deformation band (A 7.25 ). wavelength λ and temperature T (where h is the Planck constant, c is the speed of light, and k is the Boltzman constant). The aliphatic fraction can be estimated from N C,ali N C,aro I 6.85 I 6.2 A 6.2 A 6.85 B 6.2 B 6.85 ( ) 1.25, (1) 2.5 where the term (1.25/2.5) arises from that we assume that one aliphatic C atom corresponds to 2.5 aliphatic C H bonds (intermediate between methylene CH 2 and methyl CH 3 ) and one aromatic C atom corresponds to 1.25 aromatic C C bond (intermediate between benzene C 6 H 6 and coronene C 24 H 12 ). The major UIE features fall in the wavelength range of 3 12 µm, implying a temperature range of 200 T 800 K. 8 With I 6.85 /I and B 6.85 /B ± 0.24 for 200 T 800 K we obtain an upper limit of N C, ali /N C, aro 0.96 per cent for neutral methylated- PAHs with A 6.2 /A , and N C, ali /N C, aro 9.6 per cent for ionized methylated-pahs with A 6.2 /A We therefore conclude that the carriers of the 6 8 µm UIE bands are largely aromatic, with <10 per cent of the C atoms in aliphatic form. The above derivation of N C, ali /N C, aro is based on the assumption that the UIE carriers emit at a single temperature in the range of 200 T 800 K. However, the UIE carriers are nano-sized (or smaller) and will be transiently heated by single stellar photons (see Li2004). Illuminated by starlight, they will not attain an equilibrium temperature, instead, they will have a distribution of temperatures. Following Draine & Li (2001), we will calculate the temperature probability distribution functions and emission spectra of methyl PAHs of N C, aro aromatic C atoms and N C, ali aliphatic C atoms. For such molecules, we approximate their absorption cross-sections by adding three Drude functions to that of PAHs of N C, aro C atoms. These Drude functions represent the 3.4 µm aliphatic C H stretch, and the 6.85 and 7.25 µm aliphatic C H deformations. The absorption cross-section of a methyl PAH molecule of N C, aro aromatic C atoms and N C, ali aliphatic C atoms becomes C abs (N C,λ) = C PAH abs (N C,aro,λ) (2) 2 γ 3.4 λ 3.4 σ int,3.3 (A 3.4 /A 3.3 ) + N C,ali π (λ/λ 3.4 λ 3.4 /λ) 2 + γ3.4 2 (3) 8 Let C abs (λ) be the dust absorption cross-section at wavelength λ and j λ be the dust IR emissivity. For C abs (λ) λ β, λj λ peaks at λ p (hc/kt)/(4 + β) (see Li 2005). With β 2 for PAH-like UIE carriers, we have T 800 K for λ p 3 µm andt 200 K for λ p 12 µm. 2 γ 6.85 λ 6.85 σ int,6.2 (A 6.85 /A 6.2 ) + N C,ali π (λ/λ 6.85 λ 6.85 /λ) 2 + γ (4) 2 γ 7.25 λ 7.25 σ int,6.2 (A 7.25 /A 6.2 ) + N C,ali π (λ/λ 7.25 λ 7.25 /λ) 2 + γ7.25 2, (5)
8 1558 X. J. Yang et al. Figure 5. Band-strength ratios of the 6.85 and 7.25µm aliphatic C H deformation bands to the 6.2 µm aromatic C C stretch as determined with the B3LYP/6-311+G method for the mono-methyl derivatives of seven aromatic cations (benzene, naphthalene, anthracene, phenanthrene, pyrene, perylene, and coronene) and all of their isomers. The inset panels enlarge the band-strength ratios of those molecules with A 6.85 /A 6.2 < 4 (upper panel) and with A 7.25 /A 6.2 < 2 (bottom panel). where N C = N C, aro + N C, ali ; λ 3.4 = 3.4 µm, λ 6.85 = 6.85 µm, and λ 7.25 = 7.25 µm are, respectively, the peak wavelengths of the 3.4, 6.85 and 7.25 µm features; γ 3.4 λ 3.4, γ 6.85 λ 6.85,andγ 7.25 λ 7.25 are, respectively, the full width at half-maximum of the 3.4, 6.85 and 7.25 µm features (γ 3.4, γ 6.85,andγ 7.25 are dimentionless parameters; see Draine & Li 2007); and σ int, 3.3 and σ int, 6.2 are, respectively, the integrated strengths per (aromatic) C atom of the 3.3 µm aromatic C H stretch and 6.2 µm aromatic C C stretch (see Draine & Li 2007). We take A 3.4 /A 3.3 = 1.76 (see Yang et al. 2013). We take the lower limits of A 6.85 /A and A 7.25 /A for neutrals, A 6.85 /A and A 7.25 /A for cations as derived in Section 2. We note that, for a given observed intensity ratio I 6.85 /I 6.2, a lower limit on A 6.85 /A 6.2 leads to an upper limit on the aliphatic fraction N C, ali /N C, aro. Let dp be the probability that the temperature of the methyl molecule will be in [T, T + dt]. The emissivity of this molecule becomes j λ (N C ) = C abs (N C,λ)4πB λ (T ) dp dt. (6) dt Asshowninfigs6and7ofDraine&Li(2007), the 6 8 µm interstellar UIE emitters are in the size range of N C C atoms. For illustrative purpose, we therefore consider N C = 80. We adopt the thermal-discrete method of Draine & Li (2007) to compute the temperature probability distribution functions of both neutral and ionized methyl PAHs of N C, ali = 0, 1, 2,..., 8 aliphatic C atoms and (80 N C, ali ) aromatic C atoms. In Fig. 7 we show the IR emission spectra of both neutral and ionized methyl PAHs of N C, ali = 0, 1, 3, 5, 8 illuminated by the solar neighbourhood interstellar radiation field (ISRF) of Mathis, Mezger & Panagia (1983, hereafter MMP83). Fig. 7 shows that, while the 6.85 µm feature is clearly visible in the IR emission spectrum for N C, ali = 3, the 7.25 µm feature remains hardly noticeable even for N C, ali = 8. We will discuss this in more detail in Section 5. For a given N C, ali,wederive(i 6.85 /I 6.2 ) mod,the model intensity ratio of the 6.85 µm band to the 6.2 µm band, from ( ) I6.85 = 6.85 j λ(n C ) dλ I 6.2 mod 6.2 j λ(n C ) dλ, (7) where 6.2 j λ (N C ) dλ and 6.85 j λ (N C ) dλ are, respectively, the feature-integrated excess emission of the methyl PAH above the 6.2 and 6.85 µm features. In Fig. 8 we show the model intensity ratios (I 6.85 /I 6.2 ) mod as a function of N C, ali /N C, aro. For an observational upper limit of (I 6.85 /I 6.2 ) obs 0.1, Fig. 8 allows us to place an upper limit of N C, ali /N C, aro 1.8 per cent for neutral methyl PAHs and
9 Aliphatic versus aromatic content of the carriers of the UIE features 1559 Table 3. Observed intensity ratios of the 6.85 and 7.25 µm aliphatic C H deformation bands to the 6.2 µm aromatic C C stretch band (I 6.85 /I 6.2, I 7.25 /I 6.2 ). Object Type I 6.85 /I 6.2 I 7.25 /I 6.2 Reference NGC 7023 RN This work NGC 7027 PN Kwok & Zhang (2011) Orion Bar PDR Kwok & Zhang (2011) IRAS PPN Zhang, Kwok & Hrivnak (2010) IRAS PPN Zhang et al. (2010) IRAS PPN Zhang et al. (2010) IRAS PPN Tokunaga (1997) IRAS PPN 0.72 ± ± 0.24 Sloan et al. (2014) IRAS PPN 0.35 ± ± 0.04 Sloan et al. (2014) IRAS PPN 1.05 ± ± 0.04 Sloan et al. (2014) IRAS PPN 0.09 ± ± 0.01 Sloan et al. (2014) IRAS PPN 1.66 ± ± 0.02 Sloan et al. (2014) IRAS PPN 0.06 ± ± 0.01 Sloan et al. (2014) IRAS F PPN 0.23 ± ± 0.09 Sloan et al. (2014) IRAS Z PPN 0.33 ± ± 0.01 Sloan et al. (2014) IRAS AGB 0.05 ± ± 0.01 Sloan et al. (2014) IRAS PPN 0.27 ± ± 0.10 Sloan et al. (2014) IRAS PPN 0.14 ± ± 0.02 Sloan et al. (2014) IRAS PPN 1.05 ± ± 0.01 Sloan et al. (2014) 2MASS J PPN 1.03 ± ± 0.02 Sloan et al. (2014) 2MASS J PPN 0.09 ± ± 0.03 Sloan et al. (2014) 2MASS J PPN 0.42 ± ± 0.03 Sloan et al. (2014) NGC 1978 WBT 2665 PPN 1.69 ± ± 0.08 Sloan et al. (2014) SMP SMC 011 PN 0.06 ± ± 0.02 Sloan et al. (2014) SMP SMC 020 PN 0.20 ± ± 0.11 Sloan et al. (2014) Figure 6. Observed intensity ratios I 6.85 /I 6.2 versus I 7.25 /I 6.2 of the Magellanic Cloud sources compiled in Sloan et al. (2014). The right panel enlarges those sources shown in the left panel with I 6.85 /I 6.2 < 0.5 and I 7.25 /I 6.2 < 0.2. N C, ali /N C, aro 7.5 per cent for ionized methyl PAHs. We therefore can also conclude that the carriers of the 6 8 µm UIE bands are largely aromatic, with < 10 per cent of the C atoms in aliphatic form. 5 DISCUSSION In Section 4 we have shown that the aliphatic fractions derived from the simple single-temperature approach (N C, ali /N C, aro 0.96 per cent for neutrals and N C, ali /N C, aro 9.6 per cent for cations) are generally consistent with that derived from the more rigorous calculations of the model emission spectra (N C, ali /N C, aro 1.8 per cent for neutrals and N C, ali /N C, aro 7.5 per cent for cations). This can be understood in terms of the single-photon heating process. Upon absorption of a photon of energy hν (where ν is the photon frequency), a methyl PAH molecule will be heated to a maximum temperature T p determined by its specific heat C(T) and hν: T p 0 C(T ) dt = hν. The molecule will then rapidly cool down and radiate away most of the absorbed energy at temperature T p. Before it absorbs another photon, the molecule will spend most of the time at very low temperatures (Draine & Li 2001). Therefore, a single temperature of T p provides a reasonably good measure of the IR emission. The aliphatic fractions derived in Section 4 from the model intensity ratio (I 6.85 /I 6.2 ) mod are calculated for methyl PAHs excited by the MMP83ISRF. If the actual radiation intensity is U-times that of
10 1560 X. J. Yang et al. Figure 7. IR emission spectra of neutral (black lines) and ionized (red lines) methyl PAHs of N C, ali = 0, 1, 3, 5, 8 aliphatic C atoms and (80 N C, ali ) aromatic C atoms illuminated by the MMP83 ISRF. For clarity, the spectra for methyl PAHs with N C, ali = 1, 3, 5, 8 are vertically shifted. Figure 8. Model-calculated intensity ratios (I 6.85 /I 6.2 ) mod as a function of the aliphatic fraction N C, ali /N C, aro for neutral methyl PAHs and their cations. The molecules and their cations are illuminated by the MMP83 ISRF (squares), a solar-type star of T eff = 6000 K (triangles), and a B1.5V star of T eff = K (circles). The dashed horizontal line plots the observed upper limit of the intensity ratio (I 6.85 /I 6.2 ) obs 0.1. The lines passing through the origin are linear fits to the model-calculated intensity-ratio data points. the MMP83ISRF, the results will remain unchanged because the IR emission spectra of stochastically heated nano-sized grains or very large molecules, after scaled by U, are essentially independent of U (e.g. see fig. 13 of Li & Draine 2001, and fig. 1(f) of Draine & Li 2007). To examine whether and how the hardness of the exciting starlight affects the aliphatic fraction estimate, we consider methyl PAHs of N C, ali = 0, 1, 2,..., 8 aliphatic C atoms and (80 N C, ali ) aromatic C atoms excited by stars with an effective temperature of T eff = 6000 K like our Sun and by stars of T eff = K like the B1.5V star HD which illuminates the reflection nebula NGC The starlight intensity in the 912 Å 1 µm wavelength range is fixed at U = 1, with U defined as U 912 Å 1µm 4πJ (λ, T eff ) dλ, (8) 912 Å 1µm 4πJ ISRF(λ) dλ where J (λ, T eff ) is the intensity of starlight approximated by the Kurucz model atmospheric spectrum, and J ISRF (λ) isthemmp83 ISRF starlight intensity. As shown in Fig. 8,foragivenN C, ali /N C, aro, the T eff = 6000 K model results in a larger (I 6.85 /I 6.2 ) mod than that of the MMP83 ISRF model, while the T eff = K model results in a smaller (I 6.85 /I 6.2 ) mod than that of the MMP83 ISRF
11 Aliphatic versus aromatic content of the carriers of the UIE features 1561 (c) Figure 9. Effects of ionization on the intrinsic strengths of the 6.2, 6.85 and 7.25 µm bands. model. This is because a softer radiation field rises the methyl PAH molecule to a lower temperature and therefore it radiates relatively more at 6.82 µm than at 6.2 µm. Nevertheless, the effects on the derived N C, ali /N C, aro are small: the MMP83 ISRF model, the T eff = 6000 K model, and the T eff = K model, respectively, derive N C, ali /N C, aro 1.6 per cent, 1.8 per cent, 1.8 per cent for neutrals, and N C, ali /N C, aro 6.2 per cent, 7.5 per cent, 8.2 per cent for cations (see Fig. 8). To examine the effects of ionization of methyl PAHs on the intrinsic strengths of the 6.2, 6.85 and 7.25 µm bands, we show in Fig. 9 the intrinsic strengths of these bands of ionized methyl PAHs relative to that of their neutral counterparts. As is long known (Allamandola, Hudgins & Sandford 1999; Hudgins & Allamandola 2005), the 6.2 µm C C stretch is substantially enhanced upon ionization (see Fig. 9a). In contrast, the 6.85 µm aliphatic C H deformation is only moderately enhanced (see Fig. 9b), while the 7.25 µm aliphatic C H deformation is more like the 6.2 µm C Cstretchandshows considerable enhancement upon ionization (see Fig. 9c). As mentioned in Sections 3 and 4, the detection of the 7.25 µm emission feature in the Milky Way or the Magellanic Clouds is rarer than the 6.85 µm feature. This is because, as shown in Fig. 10, the intrinsic strength of the 7.25 µm feature is weaker than that of the 6.85 µm feature by a factor of 8 for neutral methyl PAHs and by a factor of 3 for their cations. In addition, the weak 7.25 µm feature could be hidden by the pronounced 7.7 µm C C stretch. The aliphatic fraction of the UIE carriers derived from the 6.85 µm aliphatic C H deformation is consistent with that derived from the 3.4 µm aliphatic C H stretch in the sense that the UIE carrier is predominantly aromatic and the aliphatic component is only a minor part of the UIE emitters. Based on the observed intensity ratios of the 3.4 µm feature to the 3.3 µm feature, Li & Draine (2012) placed an upper limit of 9 per cent on the aliphatic fraction of the UIE carriers in NGC 7027 and the Orion bar. Yang et al. (2013) further constrained the aliphatic fraction to be at most 2 per cent by examining a large sample of 35 UIE sources which exhibit both the 3.3 µm and 3.4 µm C H features. Unlike Li & Draine (2012) who adopted the 3.3 µm feature strength (A 3.3 ) of small neutral PAHs (Draine & Li 2007) and the 3.4 µm feature strength (A 3.4 ) obtained by averaging over three pure aliphatic molecules (i.e. ethane, hexane, and methyl-cyclo-hexane) and one aromatic molecule with an aliphatic sidegroup (i.e. ethyl-benzene; d Hendecourt & Allamandola 1986), Yang et al. (2013) employed density functional theory to compute A 3.4 /A 3.3 for a range of methyl-substituted PAHs. Li & Draine (2012) have also examined the 6.85 and 7.7 µm features of NGC 7027 and the Orion bar by comparing the observed intensity ratios of these two bands (I 6.85 /I 7.7 ) with their intrinsic band strengths (A 6.85, A 7.7 ). Adopting the 7.7 µm feature strength of charged aromatic molecules (Draine & Li 2007)forA 7.7 and the 6.85 µm feature strength obtained by averaging over that measured for methyl-cylco-hexane (d Hendecourt & Allamandola 1986) and for HAC (Dartois & Muñoz-Caro 2007) fora 6.85, they derived the aliphatic fraction of the UIE emitters to be <15 per cent. With the band-strength ratio A 6.85 /A 6.2 obtained over many methylated aromatic species (see Section 2), we derive an aliphatic fraction of <10 per cent from the observed intensity ratio I 6.85 /I 6.2 (see Section 4). Finally, we note that the aliphatic C atoms considered here are all in the form of methyl side groups. The aliphatic fraction derived in Section 4 is the ratio between the number of C atoms in the aromatic skeleton of PAHs and the number of C atoms in methyl side groups. This is not necessarily all of the aliphatic C atoms in astronomical PAHs: there may be some in linear bridges, and there may also be some that are in the regular PAH skeleton but at a superhydrogenated site. 9 These aliphatic C atoms are not yet included in this study. But Yang et al. (2016) have shown that the aliphatic fraction of the UIE carriers derived from the A 3.4 /A 3.3 ratios of PAHs with a wide range of sidegroups (e.g. ethyl, propyl, butyl, dimethyl) was close to that basedonthea 3.4 /A 3.3 ratios of mono-methyl PAHs. Also, the sample of regular and methylated PAHs considered here (see Fig. 1) may not be representative of what are actually present in astronomical environments (e.g. the actual astro-pah molecules may have N C 40 C atoms (Tielens 2008) while the largest molecule considered here is coronene of N C = 24). Future experimental and/or quantum-chemical computational studies of larger species would be very helpful to obtain more reliable estimates of the A 6.85 /A 6.2 ratio, and, therefore, of the aliphatic fraction of the UIE carriers. 6 SUMMARY We have examined the nature of the UIE emitters by comparing the observed intensity ratios of the 6.2 µm aromatic C C stretch and the 9 To bond to an additional H atom, a C atom has to switch from sp 2 to sp 3 orbitals, thereby by definition becoming aliphatic.
12 1562 X. J. Yang et al. Figure 10. Comparison of the intrinsic strength of the 7.25 µm aliphatic C H deformation with that of the 6.85 µm aliphatic C H deformation for neutral methyl PAHs and ionized methyl PAHs µm aliphatic C H deformation with the intrinsic band strengths of these two features computed from density functional theory for seven PAH molecules (and their cations) with a methyl side chain. We derive the fraction of C atoms in methyl(ene) aliphatic form to be at most 10 per cent, confirming the earlier finding that the UIE emitters are largely aromatic. ACKNOWLEDGEMENTS We thank T. Onaka, J.Y. Seok, G. Sloan, and the anonymous referee for very helpful suggestions. AL and XJY are supported in part by NSFC , NSF AST , NNX13AE63G, Hunan Provincial NSF 2015JJ3124, and the University of Missouri Research Board. RG is supported in part by NSF-PRISM grant Mathematics and Life Sciences ( ). Acknowledgment is also made to the donors of the American Chemical Society Petroleum Research Fund for partial support of this research (53415-ND4). Computations were performed using the high-performance computer resources of the University of Missouri Bioinformatics Consortium. REFERENCES Acke B., Bouwman J., Juhász A., Henning T., van den Ancker M. E., Meeus G., Tielens A. G. G. M., Waters L. B. F. M., 2010, ApJ, 718, 558 Allamandola L. J., Tielens A. G. G. M., Barker J. R., 1985, ApJ, 290, L25 Allamandola L. J., Tielens A. G. G. M., Barker J. R., 1989, ApJS, 71, 733 Allamandola L. J., Hudgins D. M., Sandford S. A., 1999, ApJ, 511, L115 Barker J. R., Allamandola L. J., Tielens A. G. G. M., 1987, ApJ, 315, L61 Bernstein M. P., Sandford S. A., Allamandola L. J., 1996, ApJ, 472, L127 Borowski P., 2012, J. Phys. Chem. A, 116, 3866 Cramer C. J., 2004, Essentials of Computational Chemistry: Theories and Models, 2nd edn. John Wiley & Sons, New York Chiar J. E., Tielens A. G. G. M., Whittet D. C. B., Schutte W. A., Boogert A. C. A., Lutz D., van Dishoeck E. F., Bernstein M. P., 2000, ApJ, 537, 749 Dartois E., Muñoz-Caro G. M., 2007, A&A, 476, 1235 Draine B. T., Li A., 2001, ApJ, 551, 807 Draine B. T., Li A., 2007, ApJ, 657, 810 Duley W. W., Williams D. A., 1981, MNRAS, 196, 269 d Hendecourt L. B., Allamandola L. J., 1986, A&AS, 64, 453 Frisch M. J. et al., 2009, Gaussian 09, Revision B01. Gaussian, Inc., Wallingford, CT Gillett F. C., Forrest W. J., Merrill K. M., 1973, ApJ, 183, 87 Hudgins D. M., Allamandola L. J., 2005, in Lis D. C., Blake G. A., Herbst E. eds, IAU Symp. 231, Astrochemistry: Recent Successes and Current Challenges. Cambridge Univ. Press, Cambridge, p. 443 Jones A. P., Duley W. W., Williams D. A., 1990, QJRAS, 31, 567 Kwok S., Zhang Y., 2011, Nature, 479, 80 Kwok S., Zhang Y., 2013, ApJ, 771, 5 Léger A., Puget J., 1984, A&A, 137, L5 Li A., 2004, in Witt A. N., Clayton G. C., Draine B. T., eds, ASP Conf. Ser. Vol. 309, Astrophysics of Dust. Astron. Soc. Pac., San Francisco, p. 417 Li A., 2005, in Popescu C. C., Tuffs R. J., eds, AIP Conf. Ser. Vol. 761, The Spectral Energy Distributions of Gas-rich Galaxies: Confronting Models with Data. Astron. Soc. Pac, San Francisco, p. 123 Li A., Draine B. T., 2001, ApJ, 554, 778 Li A., Draine B. T., 2012, ApJ, 760, L35 Mathis J. S., Mezger P. G., Panagia N., 1983, A&A, 128, 212 (MMP83) Papoular R., Conrad J., Giuliano M., Kister J., Mille G., 1989, A&A, 217, 204 Peeters E., Hony S., Van Kerckhoven C., Tielens A. G. G. M., Allamandola L. J., Hudgins D. M., Bauschlicher C. W., 2002, A&A, 390, 1089 Pendleton Y. J., Allamandola L. J., 2002, ApJS, 138, 75 Riechers D. A. et al., 2014, ApJ, 796, 84 Rouillé G. et al., 2012, ApJ, 752, 25 Sakata A., Wada S., Onaka T., Tokunaga A. T., 1990, ApJS, 353, 543 Sandford S. A., Bernstein M. P., Materese C. K., 2013, ApJS, 205, 8 Seok J. Y., Li A., 2016, ApJ, in press Sloan G. C. et al., 2005, ApJ, 632, 956 Sloan G. C. et al., 2014, ApJ, 791, 28 Steglich M., Jäger C., Huisken F., Friedrich M., Plass W., Räder H.-J., Müllen K., Henning Th., 2013, ApJS, 208, 26 Tielens A. G. G. M., 2008, ARA&A, 46, 289 Tokunaga A. T., 1997, in Okuda H., Matsumoto T., Roellig T., eds, ASP Conf. Ser. Vol. 124, Diffuse Infrared Radiation and the IRTS, Astron. Soc. Pac., San Francisco, p. 149 Yang X. J., Glaser R., Li A., Zhong J. X., 2013, ApJ, 776, 110 Yang X. J., Li A., Glaser R., Zhong J. X., 2016, ApJ, 825, 22 Zhang Y., Kwok S., Hrivnak B. J., 2010, ApJ, 725, 990 This paper has been typeset from a TEX/LATEX file prepared by the author.
Chemical Enrichment of the ISM by Stellar Ejecta
 Chemical Enrichment of the ISM by Stellar Ejecta Sun Kwok The University of Hong Kong IAU GA Beijing, Special Session 12, August 31, 2012 Molecular synthesis in the late stages of stellar evolution It
Chemical Enrichment of the ISM by Stellar Ejecta Sun Kwok The University of Hong Kong IAU GA Beijing, Special Session 12, August 31, 2012 Molecular synthesis in the late stages of stellar evolution It
ANALYZING ASTRONOMICAL OBSERVATIONS WITH THE NASA AMES PAH DATABASE
 PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 117-122 www.eas.org ANALYZING ASTRONOMICAL OBSERVATIONS WITH THE NASA AMES PAH DATABASE J. Cami 1, 2 Abstract.
PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 117-122 www.eas.org ANALYZING ASTRONOMICAL OBSERVATIONS WITH THE NASA AMES PAH DATABASE J. Cami 1, 2 Abstract.
Aromatic Features in M101 HII Regions and Starburst Galaxies
 **FULL TITLE** ASP Conference Series, Vol. **VOLUME**, **YEAR OF PUBLICATION** **NAMES OF EDITORS** Aromatic Features in M101 HII Regions and Starburst Galaxies Karl D. Gordon, Charles Engelbracht, J.D.T.
**FULL TITLE** ASP Conference Series, Vol. **VOLUME**, **YEAR OF PUBLICATION** **NAMES OF EDITORS** Aromatic Features in M101 HII Regions and Starburst Galaxies Karl D. Gordon, Charles Engelbracht, J.D.T.
8: Composition and Physical state of Interstellar Dust
 8: Composition and Physical state of Interstellar Dust James Graham UC, Berkeley 1 Reading Tielens, Interstellar Medium, Ch. 5 Mathis, J. S. 1990, AARA, 28, 37 Draine, B. T., 2003, AARA, 41, 241 2 Nature
8: Composition and Physical state of Interstellar Dust James Graham UC, Berkeley 1 Reading Tielens, Interstellar Medium, Ch. 5 Mathis, J. S. 1990, AARA, 28, 37 Draine, B. T., 2003, AARA, 41, 241 2 Nature
Interstellar Dust and Extinction
 University of Oxford, Astrophysics November 12, 2007 Outline Extinction Spectral Features Emission Scattering Polarization Grain Models & Evolution Conclusions What and Why? Dust covers a range of compound
University of Oxford, Astrophysics November 12, 2007 Outline Extinction Spectral Features Emission Scattering Polarization Grain Models & Evolution Conclusions What and Why? Dust covers a range of compound
Exploring ISM dust with IRSIS. Emmanuel DARTOIS IAS-CNRS
 Exploring ISM dust with IRSIS Emmanuel DARTOIS IAS-CNRS IRSIS meeting 05-12-2007 Overview Intestellar ice mantles Hydrocarbons in the galaxy and outside Polycyclic Aromatic Hydrocarbons (PAHs) Interstellar
Exploring ISM dust with IRSIS Emmanuel DARTOIS IAS-CNRS IRSIS meeting 05-12-2007 Overview Intestellar ice mantles Hydrocarbons in the galaxy and outside Polycyclic Aromatic Hydrocarbons (PAHs) Interstellar
Astrochemistry (2) Interstellar extinction. Measurement of the reddening
 Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
Astrochimistry Spring 2013 Lecture 4: Interstellar PAHs NGC HST
 Astrochimistry Spring 2013 Lecture 4: Interstellar PAHs NGC 7023 - HST Julien Montillaud 8th February 2013 Outline I. From Unidentified to Aromatic Infrared Bands (7 p.) I.1 Historical background I.2 Observational
Astrochimistry Spring 2013 Lecture 4: Interstellar PAHs NGC 7023 - HST Julien Montillaud 8th February 2013 Outline I. From Unidentified to Aromatic Infrared Bands (7 p.) I.1 Historical background I.2 Observational
Lecture 5. Interstellar Dust: Chemical & Thermal Properties
 Lecture 5. Interstellar Dust: Chemical & Thermal Properties!. Spectral Features 2. Grain populations and Models 3. Thermal Properties 4. Small Grains and Large Molecules -------------------------------------------------
Lecture 5. Interstellar Dust: Chemical & Thermal Properties!. Spectral Features 2. Grain populations and Models 3. Thermal Properties 4. Small Grains and Large Molecules -------------------------------------------------
Excited States Calculations for Protonated PAHs
 52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
Physics and Chemistry of the Interstellar Medium
 Physics and Chemistry of the Interstellar Medium Sun Kwok The University of Hong Kong UNIVERSITY SCIENCE BOOKS Sausalito, California * Preface xi The Interstellar Medium.1.1 States of Matter in the ISM
Physics and Chemistry of the Interstellar Medium Sun Kwok The University of Hong Kong UNIVERSITY SCIENCE BOOKS Sausalito, California * Preface xi The Interstellar Medium.1.1 States of Matter in the ISM
Lecture 18 - Photon Dominated Regions
 Lecture 18 - Photon Dominated Regions 1. What is a PDR? 2. Physical and Chemical Concepts 3. Molecules in Diffuse Clouds 4. Galactic and Extragalactic PDRs References Tielens, Ch. 9 Hollenbach & Tielens,
Lecture 18 - Photon Dominated Regions 1. What is a PDR? 2. Physical and Chemical Concepts 3. Molecules in Diffuse Clouds 4. Galactic and Extragalactic PDRs References Tielens, Ch. 9 Hollenbach & Tielens,
Physics 224 The Interstellar Medium
 Physics 224 The Interstellar Medium Lecture #11: Dust Composition, Photoelectric Heating, Neutral Gas Outline Part I: Dust Heating & Cooling continued Part III: Dust Emission & Photoelectric Heating Part
Physics 224 The Interstellar Medium Lecture #11: Dust Composition, Photoelectric Heating, Neutral Gas Outline Part I: Dust Heating & Cooling continued Part III: Dust Emission & Photoelectric Heating Part
Interstellar Chemistry
 4 Interstellar Chemistry Pascale Ehrenfreund Leiden Observatory, Leiden, The Netherlands The space between the stars, called the interstellar medium (ISM), is composed primarily of H and He gases incorporating
4 Interstellar Chemistry Pascale Ehrenfreund Leiden Observatory, Leiden, The Netherlands The space between the stars, called the interstellar medium (ISM), is composed primarily of H and He gases incorporating
Class 3. The PAH Spectrum, what does it tell us??
 Class 3 The PAH Spectrum, what does it tell us?? PAH Vibrations! CH str! CC str! CC str /CH ip! CH oop! 3! 4! 5! 6! 7! 8! 9! 10! 15! Wavelength (µm)! NASA Ames! Astrochemisty Lab! Vibration - S. Langhoff!
Class 3 The PAH Spectrum, what does it tell us?? PAH Vibrations! CH str! CC str! CC str /CH ip! CH oop! 3! 4! 5! 6! 7! 8! 9! 10! 15! Wavelength (µm)! NASA Ames! Astrochemisty Lab! Vibration - S. Langhoff!
Interstellar Medium. Alain Abergel. IAS, Université Paris-Sud, Orsay Cedex, France
 Interstellar Medium Alain Abergel IAS, Université Paris-Sud, 91405 Orsay Cedex, France ISO Legacy Colloquium, Dec 13, 2006 Main Topics Elemental abundances in the ionised gas of HII regions Interstellar
Interstellar Medium Alain Abergel IAS, Université Paris-Sud, 91405 Orsay Cedex, France ISO Legacy Colloquium, Dec 13, 2006 Main Topics Elemental abundances in the ionised gas of HII regions Interstellar
Photodissociation Regions Radiative Transfer. Dr. Thomas G. Bisbas
 Photodissociation Regions Radiative Transfer Dr. Thomas G. Bisbas tbisbas@ufl.edu Interstellar Radiation Field In the solar neighbourhood, the ISRF is dominated by six components Schematic sketch of the
Photodissociation Regions Radiative Transfer Dr. Thomas G. Bisbas tbisbas@ufl.edu Interstellar Radiation Field In the solar neighbourhood, the ISRF is dominated by six components Schematic sketch of the
A SMALL FULLERENE (C 24 ) MAY BE THE CARRIER OF THE 11.2 MICRON UNIDENTIFIED INFRARED BAND
 accepted for publication in The Astrophysical Journal A SMALL FULLERENE (C 24 ) MAY BE THE CARRIER OF THE 11.2 MICRON UNIDENTIFIED INFRARED BAND L. S. Bernstein 1, R. M. Shroll 1, D. K. Lynch 2, & F. O.
accepted for publication in The Astrophysical Journal A SMALL FULLERENE (C 24 ) MAY BE THE CARRIER OF THE 11.2 MICRON UNIDENTIFIED INFRARED BAND L. S. Bernstein 1, R. M. Shroll 1, D. K. Lynch 2, & F. O.
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
arxiv: v1 [astro-ph.ga] 5 Dec 2018
![arxiv: v1 [astro-ph.ga] 5 Dec 2018 arxiv: v1 [astro-ph.ga] 5 Dec 2018](/thumbs/91/107040438.jpg) Draft version December 7, 2018 Typeset using LATEX twocolumn style in AASTeX62 Examining the class B-to-A shift of the 7.7 µm PAH band with the NASA Ames PAH IR Spectroscopic Database Matthew J. Shannon
Draft version December 7, 2018 Typeset using LATEX twocolumn style in AASTeX62 Examining the class B-to-A shift of the 7.7 µm PAH band with the NASA Ames PAH IR Spectroscopic Database Matthew J. Shannon
The 22 micron emission feature in supernova remnants and massive star-forming regions
 The Evolving ISM in the Milky Way & Nearby Galaxies The 22 micron emission feature in supernova remnants and massive star-forming regions Takashi Onaka 1, Thomas. L. Roellig 2, Yoko Okada 3, and Kin-Wing
The Evolving ISM in the Milky Way & Nearby Galaxies The 22 micron emission feature in supernova remnants and massive star-forming regions Takashi Onaka 1, Thomas. L. Roellig 2, Yoko Okada 3, and Kin-Wing
Comparison between 30 micron sources in different galaxies
 Journal of Physics: Conference Series PAPER OPEN ACCESS Comparison between 30 micron sources in different galaxies To cite this article: Marcin Gadkowski et al 2016 J. Phys.: Conf. Ser. 728 062007 View
Journal of Physics: Conference Series PAPER OPEN ACCESS Comparison between 30 micron sources in different galaxies To cite this article: Marcin Gadkowski et al 2016 J. Phys.: Conf. Ser. 728 062007 View
1. The AGB dust budget in nearby galaxies
 **Volume Title** ASP Conference Series, Vol. **Volume Number** **Author** c **Copyright Year** Astronomical Society of the Pacific Identifying the chemistry of the dust around AGB stars in nearby galaxies
**Volume Title** ASP Conference Series, Vol. **Volume Number** **Author** c **Copyright Year** Astronomical Society of the Pacific Identifying the chemistry of the dust around AGB stars in nearby galaxies
Dust in the Diffuse Universe
 Dust in the Diffuse Universe Obscuring Effects Chemical Effects Thermal Effects Dynamical Effects Diagnostic Power Evidence for Grains: Chemical Effects Catalyzes molecular hydrogen formation. Depletion
Dust in the Diffuse Universe Obscuring Effects Chemical Effects Thermal Effects Dynamical Effects Diagnostic Power Evidence for Grains: Chemical Effects Catalyzes molecular hydrogen formation. Depletion
Astronomy 106, Fall September 2015
 Today in Astronomy 106: molecules to molecular clouds to stars Aromatic (benzene-ring) molecules in space Formation of molecules, on dust-grain surfaces and in the gas phase Interstellar molecular clouds
Today in Astronomy 106: molecules to molecular clouds to stars Aromatic (benzene-ring) molecules in space Formation of molecules, on dust-grain surfaces and in the gas phase Interstellar molecular clouds
Far- and mid-infrared spectroscopy of complex organic matter of astrochemical interest: coal, heavy petroleum fractions and asphaltenes
 MNRAS 429, 3025 3039 (2013) doi:10.1093/mnras/sts558 Far- and mid-infrared spectroscopy of complex organic matter of astrochemical interest: coal, heavy petroleum fractions and asphaltenes Franco Cataldo,
MNRAS 429, 3025 3039 (2013) doi:10.1093/mnras/sts558 Far- and mid-infrared spectroscopy of complex organic matter of astrochemical interest: coal, heavy petroleum fractions and asphaltenes Franco Cataldo,
Status of the Diffuse Interstellar Band Problem
 Status of the Diffuse Interstellar Band Problem Ben McCall Department of Chemistry and Department of Astronomy University of Illinois at Urbana-Champaign APO DIB Collaboration: Tom Fishman (Chicago), Scott
Status of the Diffuse Interstellar Band Problem Ben McCall Department of Chemistry and Department of Astronomy University of Illinois at Urbana-Champaign APO DIB Collaboration: Tom Fishman (Chicago), Scott
Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium
 1 Chapter 1 Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium 1.1 Interstellar Molecules Although hydrogen is the most abundant element in the universe, it is other elements that
1 Chapter 1 Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium 1.1 Interstellar Molecules Although hydrogen is the most abundant element in the universe, it is other elements that
Unscrambling the Egg. Yvonne Pendleton NASA Ames Research Center. JWST Workshop Nov. 14, 2017
 Unscrambling the Egg Yvonne Pendleton NASA Ames Research Center JWST Workshop Nov. 14, 2017 From interstellar dust to new stars and planets Comparisons between material forming new planetary systems and
Unscrambling the Egg Yvonne Pendleton NASA Ames Research Center JWST Workshop Nov. 14, 2017 From interstellar dust to new stars and planets Comparisons between material forming new planetary systems and
LABORATORY SPECTROSCOPY OF PROTONATED PAH MOLECULES RELEVANT FOR INTERSTELLAR CHEMISTRY
 PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 103-108 www.eas.org LABORATORY SPECTROSCOPY OF PROTONATED PAH MOLECULES RELEVANT FOR INTERSTELLAR CHEMISTRY
PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 103-108 www.eas.org LABORATORY SPECTROSCOPY OF PROTONATED PAH MOLECULES RELEVANT FOR INTERSTELLAR CHEMISTRY
arxiv: v1 [astro-ph.ga] 29 Jun 2011
![arxiv: v1 [astro-ph.ga] 29 Jun 2011 arxiv: v1 [astro-ph.ga] 29 Jun 2011](/thumbs/73/68434711.jpg) Astronomy & Astrophysics manuscript no. final c ESO 2 June 3, 2 arxiv:6.5899v [astro-ph.ga] 29 Jun 2 Coupled Blind Signal Separation and Spectroscopic Database Fitting of the Mid Infrared PAH Features
Astronomy & Astrophysics manuscript no. final c ESO 2 June 3, 2 arxiv:6.5899v [astro-ph.ga] 29 Jun 2 Coupled Blind Signal Separation and Spectroscopic Database Fitting of the Mid Infrared PAH Features
AKARI Near-Infrared Spectroscopy Towards Young Stellar Objects
 AKARI Near-Infrared Spectroscopy Towards Young Stellar Objects Aleksandra Ardaseva 1,TakashiOnaka 2 1 University of St Andrews, United Kingdom 2 Department of Astronomy, Graduate School of Science, University
AKARI Near-Infrared Spectroscopy Towards Young Stellar Objects Aleksandra Ardaseva 1,TakashiOnaka 2 1 University of St Andrews, United Kingdom 2 Department of Astronomy, Graduate School of Science, University
Chapter 10 The Interstellar Medium
 Chapter 10 The Interstellar Medium Guidepost You have begun your study of the sun and other stars, but now it is time to study the thin gas and dust that drifts through space between the stars. This chapter
Chapter 10 The Interstellar Medium Guidepost You have begun your study of the sun and other stars, but now it is time to study the thin gas and dust that drifts through space between the stars. This chapter
The Interstellar Medium in Galaxies: SOFIA Science
 The Interstellar Medium in Galaxies: SOFIA Science Margaret Meixner (STScI) Xander Tielens (NASA/Ames/Leiden Univ.), Jesse Dotson (NASA/ARC), Bruce Draine (Princeton), Mark Wolfire (U. Maryland), Jackie
The Interstellar Medium in Galaxies: SOFIA Science Margaret Meixner (STScI) Xander Tielens (NASA/Ames/Leiden Univ.), Jesse Dotson (NASA/ARC), Bruce Draine (Princeton), Mark Wolfire (U. Maryland), Jackie
arxiv: v1 [astro-ph.sr] 15 May 2015
![arxiv: v1 [astro-ph.sr] 15 May 2015 arxiv: v1 [astro-ph.sr] 15 May 2015](/thumbs/87/96798259.jpg) ON THE ORIGIN OF THE 11.3 MICRON UNIDENTIFIED INFRARED EMISSION FEATURE arxiv:1505.03971v1 [astro-ph.sr] 15 May 2015 SeyedAbdolreza Sadjadi, Yong Zhang ( 張泳 ), and Sun Kwok ( 郭新 ) Space Astronomy Laboratory,
ON THE ORIGIN OF THE 11.3 MICRON UNIDENTIFIED INFRARED EMISSION FEATURE arxiv:1505.03971v1 [astro-ph.sr] 15 May 2015 SeyedAbdolreza Sadjadi, Yong Zhang ( 張泳 ), and Sun Kwok ( 郭新 ) Space Astronomy Laboratory,
A. G. G. M. TIELENS SRON, Kapteyn Astronomical Institute, P.O. Box 800, 9700 AV Groningen, The Netherlands
 THE ASTROPHYSICAL JOURNAL, 560:261È271, 2001 October 10 ( 2001. The American Astronomical Society. All rights reserved. Printed in U.S.A. THEORETICAL MODELING OF INFRARED EMISSION FROM NEUTRAL AND CHARGED
THE ASTROPHYSICAL JOURNAL, 560:261È271, 2001 October 10 ( 2001. The American Astronomical Society. All rights reserved. Printed in U.S.A. THEORETICAL MODELING OF INFRARED EMISSION FROM NEUTRAL AND CHARGED
A Far-ultraviolet Fluorescent Molecular Hydrogen Emission Map of the Milky Way Galaxy
 A Far-ultraviolet Fluorescent Molecular Hydrogen Emission Map of the Milky Way Galaxy (The Astrophysical Journal Supplement Series, 231:21 (16pp), 2017 August) November 14, 2017 Young-Soo Jo Young-Soo
A Far-ultraviolet Fluorescent Molecular Hydrogen Emission Map of the Milky Way Galaxy (The Astrophysical Journal Supplement Series, 231:21 (16pp), 2017 August) November 14, 2017 Young-Soo Jo Young-Soo
arxiv: v1 [astro-ph] 7 Feb 2008
![arxiv: v1 [astro-ph] 7 Feb 2008 arxiv: v1 [astro-ph] 7 Feb 2008](/thumbs/83/88512079.jpg) The infrared spectra of very large, compact, highly symmetric, polycyclic aromatic hydrocarbons (PAHs) Charles W. Bauschlicher, Jr. 1, Els Peeters 2,3,4, Louis J. Allamandola 5 arxiv:0802.1071v1 [astro-ph]
The infrared spectra of very large, compact, highly symmetric, polycyclic aromatic hydrocarbons (PAHs) Charles W. Bauschlicher, Jr. 1, Els Peeters 2,3,4, Louis J. Allamandola 5 arxiv:0802.1071v1 [astro-ph]
Universe Now. 9. Interstellar matter and star clusters
 Universe Now 9. Interstellar matter and star clusters About interstellar matter Interstellar space is not completely empty: gas (atoms + molecules) and small dust particles. Over 10% of the mass of the
Universe Now 9. Interstellar matter and star clusters About interstellar matter Interstellar space is not completely empty: gas (atoms + molecules) and small dust particles. Over 10% of the mass of the
Dust: Grain Populations, Extinction Curves, and Emission Spectra Monday, January 31, 2011
 Dust: Grain Populations, Extinction Curves, and Emission Spectra Monday, January 31, 2011 CONTENTS: 1. Introduction 2. The Extinction Curve and Abundance Constraints A. Formalities B. Features 3. Infrared
Dust: Grain Populations, Extinction Curves, and Emission Spectra Monday, January 31, 2011 CONTENTS: 1. Introduction 2. The Extinction Curve and Abundance Constraints A. Formalities B. Features 3. Infrared
Simulating chemistry on interstellar dust grains in the laboratory
 From Stars to Life, April 3-6 2013, Gainesville FL Simulating chemistry on interstellar dust grains in the laboratory Nicolas Polfer University of Florida Department of Chemistry http://www.cosmotography.com/images/cosmic_nurseries.html
From Stars to Life, April 3-6 2013, Gainesville FL Simulating chemistry on interstellar dust grains in the laboratory Nicolas Polfer University of Florida Department of Chemistry http://www.cosmotography.com/images/cosmic_nurseries.html
The Dusty Universe. Joe Weingartner George Mason University Dept of Physics and Astronomy
 The Dusty Universe Joe Weingartner George Mason University Dept of Physics and Astronomy To astronomers, dust means: sub micron solid grains (1 micron = 1 m = 10 6 m = one millionth of a meter) Typical
The Dusty Universe Joe Weingartner George Mason University Dept of Physics and Astronomy To astronomers, dust means: sub micron solid grains (1 micron = 1 m = 10 6 m = one millionth of a meter) Typical
12/27/2010. Chapter 14 Aromatic Compounds
 Nomenclature of Benzene Derivatives Benzene is the parent name for some monosubstituted benzenes; the substituent name is added as a prefix Chapter 14 Aromatic Compounds For other monosubstituted benzenes,
Nomenclature of Benzene Derivatives Benzene is the parent name for some monosubstituted benzenes; the substituent name is added as a prefix Chapter 14 Aromatic Compounds For other monosubstituted benzenes,
FAR AND MID INFRARED SPECTROSCOPY OF COMPLEX ORGANIC MATTER OF ASTROCHEMICAL INTEREST: COAL, HEAVY PETROLEUM FRACTIONS, AND ASPHALTENES
 1 FAR AND MID INFRARED SPECTROSCOPY OF COMPLEX ORGANIC MATTER OF ASTROCHEMICAL INTEREST: COAL, HEAVY PETROLEUM FRACTIONS, AND ASPHALTENES Franco Cataldo 1,2, D. A. García Hernández 3,4, Arturo Manchado
1 FAR AND MID INFRARED SPECTROSCOPY OF COMPLEX ORGANIC MATTER OF ASTROCHEMICAL INTEREST: COAL, HEAVY PETROLEUM FRACTIONS, AND ASPHALTENES Franco Cataldo 1,2, D. A. García Hernández 3,4, Arturo Manchado
NIR Silicate features and Statistics from IRAS data
 NIR Silicate features and Statistics from IRAS data Ranjan Gupta Inter University Center for Astronomy and Astrophysics Pune-411007, India NIR Silicate features and Statistics from IRAS data p.1/46 Abstract
NIR Silicate features and Statistics from IRAS data Ranjan Gupta Inter University Center for Astronomy and Astrophysics Pune-411007, India NIR Silicate features and Statistics from IRAS data p.1/46 Abstract
Astr 2310 Thurs. March 23, 2017 Today s Topics
 Astr 2310 Thurs. March 23, 2017 Today s Topics Chapter 16: The Interstellar Medium and Star Formation Interstellar Dust and Dark Nebulae Interstellar Dust Dark Nebulae Interstellar Reddening Interstellar
Astr 2310 Thurs. March 23, 2017 Today s Topics Chapter 16: The Interstellar Medium and Star Formation Interstellar Dust and Dark Nebulae Interstellar Dust Dark Nebulae Interstellar Reddening Interstellar
arxiv: v1 [astro-ph.ga] 21 Nov 2018
![arxiv: v1 [astro-ph.ga] 21 Nov 2018 arxiv: v1 [astro-ph.ga] 21 Nov 2018](/thumbs/90/103887410.jpg) Article The Astrochemistry Implications of Quantum Chemical Normal Modes Vibrational Analysis SeyedAbdolreza Sadjadi ID & Quentin Andrew Parker ID Laboratory for Space Research and the Department of Physics,
Article The Astrochemistry Implications of Quantum Chemical Normal Modes Vibrational Analysis SeyedAbdolreza Sadjadi ID & Quentin Andrew Parker ID Laboratory for Space Research and the Department of Physics,
Polycyclic Aromatic Hydrocarbon from the Magellanic Clouds
 Polycyclic Aromatic Hydrocarbon from the Magellanic Clouds Hony and Spitzer/SAGE-SPEC team Galliano, Seok, Neelamkodan, Kemper, Madden, Indebetouw, Gordon, Sandstrom 11 Aug 2017 S. Hony (Heidelberg University)
Polycyclic Aromatic Hydrocarbon from the Magellanic Clouds Hony and Spitzer/SAGE-SPEC team Galliano, Seok, Neelamkodan, Kemper, Madden, Indebetouw, Gordon, Sandstrom 11 Aug 2017 S. Hony (Heidelberg University)
Do the Infrared Emission Features Need Ultraviolet Excitation?
 Draft 16 Sept 1997; to be submitted to ApJ Letters Do the Infrared Emission Features Need Ultraviolet Excitation? K.I. Uchida2 3, K. Sellgren2, and M. Werner 4 Received ) accepted lbased on observations
Draft 16 Sept 1997; to be submitted to ApJ Letters Do the Infrared Emission Features Need Ultraviolet Excitation? K.I. Uchida2 3, K. Sellgren2, and M. Werner 4 Received ) accepted lbased on observations
6. Interstellar Medium. Emission nebulae are diffuse patches of emission surrounding hot O and
 6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
Review from last class:
 Review from last class: Properties of photons Flux and luminosity, apparent magnitude and absolute magnitude, colors Spectroscopic observations. Doppler s effect and applications Distance measurements
Review from last class: Properties of photons Flux and luminosity, apparent magnitude and absolute magnitude, colors Spectroscopic observations. Doppler s effect and applications Distance measurements
Interstellar Medium by Eye
 Interstellar Medium by Eye Nebula Latin for cloud = cloud of interstellar gas & dust Wide angle: Milky Way Summer Triangle (right) α&β Centauri, Coal Sack Southern Cross (below) Dust-Found in the Plane
Interstellar Medium by Eye Nebula Latin for cloud = cloud of interstellar gas & dust Wide angle: Milky Way Summer Triangle (right) α&β Centauri, Coal Sack Southern Cross (below) Dust-Found in the Plane
Interstellar Medium and Star Birth
 Interstellar Medium and Star Birth Interstellar dust Lagoon nebula: dust + gas Interstellar Dust Extinction and scattering responsible for localized patches of darkness (dark clouds), as well as widespread
Interstellar Medium and Star Birth Interstellar dust Lagoon nebula: dust + gas Interstellar Dust Extinction and scattering responsible for localized patches of darkness (dark clouds), as well as widespread
A World of Dust. Bare-Eye Nebula: Orion. Interstellar Medium
 Interstellar Medium Physics 113 Goderya Chapter(s): 10 Learning Outcomes: A World of Dust The space between the stars is not completely empty, but filled with very dilute gas and dust, producing some of
Interstellar Medium Physics 113 Goderya Chapter(s): 10 Learning Outcomes: A World of Dust The space between the stars is not completely empty, but filled with very dilute gas and dust, producing some of
7. Dust Grains & Interstellar Extinction. James R. Graham University of California, Berkeley
 7. Dust Grains & Interstellar Extinction James R. Graham University of California, Berkeley Visual Extinction Presence of interstellar gas or nebulae has a long history Existence of absorbing interstellar
7. Dust Grains & Interstellar Extinction James R. Graham University of California, Berkeley Visual Extinction Presence of interstellar gas or nebulae has a long history Existence of absorbing interstellar
The Interstellar Medium
 The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
Beyond the Visible -- Exploring the Infrared Universe
 Beyond the Visible -- Exploring the Infrared Universe Prof. T. Jarrett (UCT) Infrared Window Telescopes ISM -- Galaxies Infrared Window Near-infrared: 1 to 5 µm Mid-infrared: 5 to 50 µm
Beyond the Visible -- Exploring the Infrared Universe Prof. T. Jarrett (UCT) Infrared Window Telescopes ISM -- Galaxies Infrared Window Near-infrared: 1 to 5 µm Mid-infrared: 5 to 50 µm
INDEX OF SUBJECTS 6, 14, 23, 50, 95, 191 4, 191, 234
 INDEX OF SUBJECTS Abundances, elemental Abundances, ionic AGB stars (see Stars, AGB) Age, nebulae Asymptotic Giant Branch (AGB) Be stars (see Stars, Be) Bipolar structure, nebulae Carbon stars Carbon stars,
INDEX OF SUBJECTS Abundances, elemental Abundances, ionic AGB stars (see Stars, AGB) Age, nebulae Asymptotic Giant Branch (AGB) Be stars (see Stars, Be) Bipolar structure, nebulae Carbon stars Carbon stars,
Ground State Calculations for Protonated PAHs
 15 Chapter 2 Ground State Calculations for Protonated PAHs 2.1 Introduction Ab initio ground electronic state calculations can be used to determine geometries and energies of all possible protonated PAH
15 Chapter 2 Ground State Calculations for Protonated PAHs 2.1 Introduction Ab initio ground electronic state calculations can be used to determine geometries and energies of all possible protonated PAH
Number of Stars: 100 billion (10 11 ) Mass : 5 x Solar masses. Size of Disk: 100,000 Light Years (30 kpc)
 THE MILKY WAY GALAXY Type: Spiral galaxy composed of a highly flattened disk and a central elliptical bulge. The disk is about 100,000 light years (30kpc) in diameter. The term spiral arises from the external
THE MILKY WAY GALAXY Type: Spiral galaxy composed of a highly flattened disk and a central elliptical bulge. The disk is about 100,000 light years (30kpc) in diameter. The term spiral arises from the external
Lecture 6 - spectroscopy
 Lecture 6 - spectroscopy 1 Light Electromagnetic radiation can be thought of as either a wave or as a particle (particle/wave duality). For scattering of light by particles, air, and surfaces, wave theory
Lecture 6 - spectroscopy 1 Light Electromagnetic radiation can be thought of as either a wave or as a particle (particle/wave duality). For scattering of light by particles, air, and surfaces, wave theory
Dust. The four letter word in astrophysics. Interstellar Emission
 Dust The four letter word in astrophysics Interstellar Emission Why Dust Dust attenuates and scatters UV/optical/NIR Amount of attenuation and spectral shape depends on dust properties (grain size/type)
Dust The four letter word in astrophysics Interstellar Emission Why Dust Dust attenuates and scatters UV/optical/NIR Amount of attenuation and spectral shape depends on dust properties (grain size/type)
THE MID-INFRARED LABORATORY SPECTRA OF NAPHTHALENE (C 10 H 8 ) IN SOLID H 2 O
 The Astrophysical Journal, 607:346 360, 2004 May 20 # 2004. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE MID-INFRARED LABORATORY SPECTRA OF NAPHTHALENE (C 10 H 8 ) IN SOLID
The Astrophysical Journal, 607:346 360, 2004 May 20 # 2004. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE MID-INFRARED LABORATORY SPECTRA OF NAPHTHALENE (C 10 H 8 ) IN SOLID
Fullerenes and PAHs: from laboratory to the detection in interstellar space
 Highlights of Spanish Astrophysics VII, Proceedings of the X Scientific Meeting of the Spanish Astronomical Society held on July 9-13, 2012, in Valencia, Spain. J. C. Guirado, L.M. Lara, V. Quilis, and
Highlights of Spanish Astrophysics VII, Proceedings of the X Scientific Meeting of the Spanish Astronomical Society held on July 9-13, 2012, in Valencia, Spain. J. C. Guirado, L.M. Lara, V. Quilis, and
Extended Red Emission: Photoluminescence by Interstellar Nanoparticles
 **TITLE** ASP Conference Series, Vol. **VOLUME***, **YEAR OF PUBLICATION** **NAMES OF EDITORS** Extended Red Emission: Photoluminescence by Interstellar Nanoparticles Adolf N. Witt Ritter Astrophysical
**TITLE** ASP Conference Series, Vol. **VOLUME***, **YEAR OF PUBLICATION** **NAMES OF EDITORS** Extended Red Emission: Photoluminescence by Interstellar Nanoparticles Adolf N. Witt Ritter Astrophysical
INVESTIGATION OF THE ULTRAVIOLET, VISIBLE, AND NEAR-INFRARED ABSORPTION SPECTRA OF HYDROGENATED POLYCYCLIC AROMATIC HYDROCARBONS AND THEIR CATIONS
 The Astrophysical Journal, 628:555 566, 2005 July 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. INVESTIGATION OF THE ULTRAVIOLET, VISIBLE, AND NEAR-INFRARED ABSORPTION
The Astrophysical Journal, 628:555 566, 2005 July 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. INVESTIGATION OF THE ULTRAVIOLET, VISIBLE, AND NEAR-INFRARED ABSORPTION
Alkanes. Introduction
 Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Benzene a remarkable compound. Chapter 14 Aromatic Compounds. Some proposed structures for C 6 H 6. Dimethyl substituted benzenes are called xylenes
 Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Midterm Results. The Milky Way in the Infrared. The Milk Way from Above (artist conception) 3/2/10
 Lecture 13 : The Interstellar Medium and Cosmic Recycling Midterm Results A2020 Prof. Tom Megeath The Milky Way in the Infrared View from the Earth: Edge On Infrared light penetrates the clouds and shows
Lecture 13 : The Interstellar Medium and Cosmic Recycling Midterm Results A2020 Prof. Tom Megeath The Milky Way in the Infrared View from the Earth: Edge On Infrared light penetrates the clouds and shows
Studies of diffuse UV radiation
 Bull. Astr. Soc. India (2007) 35, 295 300 Studies of diffuse UV radiation N. V. Sujatha and Jayant Murthy Indian Institute of Astrophysics, Bangalore 560 034, India Abstract. The upcoming TAUVEX mission
Bull. Astr. Soc. India (2007) 35, 295 300 Studies of diffuse UV radiation N. V. Sujatha and Jayant Murthy Indian Institute of Astrophysics, Bangalore 560 034, India Abstract. The upcoming TAUVEX mission
Structure Determination. How to determine what compound that you have? One way to determine compound is to get an elemental analysis
 Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Delivery of Complex Organic Compounds from Evolved Stars to the Solar System. Title
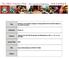 Title Delivery of Complex Organic Compounds from Evolved Stars to the Solar System Author(s) Kwok, S Citation Origins Of Life And Evolution Of Biospheres, 2011, v. 41 n. 6, p. 497-502 Issued Date 2011
Title Delivery of Complex Organic Compounds from Evolved Stars to the Solar System Author(s) Kwok, S Citation Origins Of Life And Evolution Of Biospheres, 2011, v. 41 n. 6, p. 497-502 Issued Date 2011
A STUDY OF THE 3.3 AND 3.4 m EMISSION FEATURES IN PROTOYPLANETARY NEBULAE 1
 The Astrophysical Journal, 662:1059Y1066, 2007 June 20 # 2007. The American Astronomical Society. All rights reserved. Printed in U.S.A. A STUDY OF THE 3.3 AND 3.4 m EMISSION FEATURES IN PROTOYPLANETARY
The Astrophysical Journal, 662:1059Y1066, 2007 June 20 # 2007. The American Astronomical Society. All rights reserved. Printed in U.S.A. A STUDY OF THE 3.3 AND 3.4 m EMISSION FEATURES IN PROTOYPLANETARY
Our Galaxy. We are located in the disk of our galaxy and this is why the disk appears as a band of stars across the sky.
 Our Galaxy Our Galaxy We are located in the disk of our galaxy and this is why the disk appears as a band of stars across the sky. Early attempts to locate our solar system produced erroneous results.
Our Galaxy Our Galaxy We are located in the disk of our galaxy and this is why the disk appears as a band of stars across the sky. Early attempts to locate our solar system produced erroneous results.
MID-INFRARED SPECTRA OF POLYCYCLIC AROMATIC HYDROCARBON EMISSION IN HERBIG Ae/Be STARS
 The Astrophysical Journal, 632:956 963, 2005 October 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. MID-INFRARED SPECTRA OF POLYCYCLIC AROMATIC HYDROCARBON EMISSION
The Astrophysical Journal, 632:956 963, 2005 October 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. MID-INFRARED SPECTRA OF POLYCYCLIC AROMATIC HYDROCARBON EMISSION
1. INTRODUCTION. Received 2003 January 28; accepted 2003 March 31
 The Astrophysical Journal, 592:947 952, 2003 August 1 # 2003. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE ROLE OF POLYCYCLIC AROMATIC HYDROCARBONS IN ULTRAVIOLET EXTINCTION.
The Astrophysical Journal, 592:947 952, 2003 August 1 # 2003. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE ROLE OF POLYCYCLIC AROMATIC HYDROCARBONS IN ULTRAVIOLET EXTINCTION.
ASTR2050 Spring Please turn in your homework now! In this class we will discuss the Interstellar Medium:
 ASTR2050 Spring 2005 Lecture 10am 29 March 2005 Please turn in your homework now! In this class we will discuss the Interstellar Medium: Introduction: Dust and Gas Extinction and Reddening Physics of Dust
ASTR2050 Spring 2005 Lecture 10am 29 March 2005 Please turn in your homework now! In this class we will discuss the Interstellar Medium: Introduction: Dust and Gas Extinction and Reddening Physics of Dust
Taking fingerprints of stars, galaxies, and interstellar gas clouds. Absorption and emission from atoms, ions, and molecules
 Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules 1 Periodic Table of Elements The universe is mostly hydrogen H and helium He
Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules 1 Periodic Table of Elements The universe is mostly hydrogen H and helium He
Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines!
 Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines Idealized 21cm spectra Example observed 21cm spectra HI densities
Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines Idealized 21cm spectra Example observed 21cm spectra HI densities
University of Groningen. Infrared emission features Boersma, Christiaan
 University of Groningen Infrared emission features Boersma, Christiaan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen Infrared emission features Boersma, Christiaan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
Astrochemistry the summary
 Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
Physics Homework Set 2 Sp 2015
 1) A large gas cloud in the interstellar medium that contains several type O and B stars would appear to us as 1) A) a reflection nebula. B) a dark patch against a bright background. C) a dark nebula.
1) A large gas cloud in the interstellar medium that contains several type O and B stars would appear to us as 1) A) a reflection nebula. B) a dark patch against a bright background. C) a dark nebula.
Taking fingerprints of stars, galaxies, and interstellar gas clouds
 - - Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules Periodic Table of Elements The universe is mostly hydrogen H and helium He
- - Taking fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules Periodic Table of Elements The universe is mostly hydrogen H and helium He
Astrochemistry. Lecture 10, Primordial chemistry. Jorma Harju. Department of Physics. Friday, April 5, 2013, 12:15-13:45, Lecture room D117
 Astrochemistry Lecture 10, Primordial chemistry Jorma Harju Department of Physics Friday, April 5, 2013, 12:15-13:45, Lecture room D117 The first atoms (1) SBBN (Standard Big Bang Nucleosynthesis): elements
Astrochemistry Lecture 10, Primordial chemistry Jorma Harju Department of Physics Friday, April 5, 2013, 12:15-13:45, Lecture room D117 The first atoms (1) SBBN (Standard Big Bang Nucleosynthesis): elements
18. Stellar Birth. Initiation of Star Formation. The Orion Nebula: A Close-Up View. Interstellar Gas & Dust in Our Galaxy
 18. Stellar Birth Star observations & theories aid understanding Interstellar gas & dust in our galaxy Protostars form in cold, dark nebulae Protostars evolve into main-sequence stars Protostars both gain
18. Stellar Birth Star observations & theories aid understanding Interstellar gas & dust in our galaxy Protostars form in cold, dark nebulae Protostars evolve into main-sequence stars Protostars both gain
PART 3 Galaxies. Gas, Stars and stellar motion in the Milky Way
 PART 3 Galaxies Gas, Stars and stellar motion in the Milky Way The Interstellar Medium The Sombrero Galaxy Space is far from empty! Clouds of cold gas Clouds of dust In a galaxy, gravity pulls the dust
PART 3 Galaxies Gas, Stars and stellar motion in the Milky Way The Interstellar Medium The Sombrero Galaxy Space is far from empty! Clouds of cold gas Clouds of dust In a galaxy, gravity pulls the dust
Taking Fingerprints of Stars, Galaxies, and Other Stuff. The Bohr Atom. The Bohr Atom Model of Hydrogen atom. Bohr Atom. Bohr Atom
 Periodic Table of Elements Taking Fingerprints of Stars, Galaxies, and Other Stuff Absorption and Emission from Atoms, Ions, and Molecules Universe is mostly (97%) Hydrogen and Helium (H and He) The ONLY
Periodic Table of Elements Taking Fingerprints of Stars, Galaxies, and Other Stuff Absorption and Emission from Atoms, Ions, and Molecules Universe is mostly (97%) Hydrogen and Helium (H and He) The ONLY
Stars, Galaxies & the Universe Lecture Outline
 Stars, Galaxies & the Universe Lecture Outline A galaxy is a collection of 100 billion stars! Our Milky Way Galaxy (1)Components - HII regions, Dust Nebulae, Atomic Gas (2) Shape & Size (3) Rotation of
Stars, Galaxies & the Universe Lecture Outline A galaxy is a collection of 100 billion stars! Our Milky Way Galaxy (1)Components - HII regions, Dust Nebulae, Atomic Gas (2) Shape & Size (3) Rotation of
CHM Physical Chemistry II Chapter 12 - Supplementary Material. 1. Einstein A and B coefficients
 CHM 3411 - Physical Chemistry II Chapter 12 - Supplementary Material 1. Einstein A and B coefficients Consider two singly degenerate states in an atom, molecule, or ion, with wavefunctions 1 (for the lower
CHM 3411 - Physical Chemistry II Chapter 12 - Supplementary Material 1. Einstein A and B coefficients Consider two singly degenerate states in an atom, molecule, or ion, with wavefunctions 1 (for the lower
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY
 EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
Outline. Continuum Phenomena Interstellar Dust Ionized Gas Synchrotron Radiation. Emission Line Phenomena HII Regions Photo-Dissociation Regions
 Characterizing the Interstellar Medium within Galaxies Daniel Dale University of Wyoming Outline Continuum Phenomena Interstellar Dust Ionized Gas Synchrotron Radiation Emission Line Phenomena HII Regions
Characterizing the Interstellar Medium within Galaxies Daniel Dale University of Wyoming Outline Continuum Phenomena Interstellar Dust Ionized Gas Synchrotron Radiation Emission Line Phenomena HII Regions
Hot Dust and Molecular Gas in Interacting Galaxies:
 Hot Dust and Molecular Gas in Interacting Galaxies: Vassilis Charmandaris Cornell University ISO/mid-IR : I.F. Mirabel (CEA), O.Laurent (MPE), & ISOCAM-GTO team (CEA) CO/mm: F. Combes (Obs. Paris), C.
Hot Dust and Molecular Gas in Interacting Galaxies: Vassilis Charmandaris Cornell University ISO/mid-IR : I.F. Mirabel (CEA), O.Laurent (MPE), & ISOCAM-GTO team (CEA) CO/mm: F. Combes (Obs. Paris), C.
5) What spectral type of star that is still around formed longest ago? 5) A) F B) A C) M D) K E) O
 HW2 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The polarization of light passing though the dust grains shows that: 1) A) the dust grains
HW2 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The polarization of light passing though the dust grains shows that: 1) A) the dust grains
THE INTERSTELLAR MEDIUM
 THE INTERSTELLAR MEDIUM An IR view of dust clouds In particular, light from polycyclic aromatic hydrocarbons (PAH s) Little bit of carbon out there, forms hydrocarbons like car exhaust Associated with
THE INTERSTELLAR MEDIUM An IR view of dust clouds In particular, light from polycyclic aromatic hydrocarbons (PAH s) Little bit of carbon out there, forms hydrocarbons like car exhaust Associated with
Lecture 5. Interstellar Dust: Optical Properties
 Lecture 5. Interstellar Dust: Optical Properties 1. Introduction 2. Extinction 3. Mie Scattering 4. Dust to Gas Ratio 5. Appendices References Spitzer Ch. 7, Osterbrock Ch. 7 DC Whittet, Dust in the Galactic
Lecture 5. Interstellar Dust: Optical Properties 1. Introduction 2. Extinction 3. Mie Scattering 4. Dust to Gas Ratio 5. Appendices References Spitzer Ch. 7, Osterbrock Ch. 7 DC Whittet, Dust in the Galactic
Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different types are classified by frequency or
 CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
Stellar Life Cycle in Giant Galactic Nebula NGC edited by David L. Alles Western Washington University
 Stellar Life Cycle in Giant Galactic Nebula NGC 3603 edited by David L. Alles Western Washington University e-mail: alles@biol.wwu.edu Introduction NGC 3603 is a giant HII region in the Carina spiral arm
Stellar Life Cycle in Giant Galactic Nebula NGC 3603 edited by David L. Alles Western Washington University e-mail: alles@biol.wwu.edu Introduction NGC 3603 is a giant HII region in the Carina spiral arm
THE UNUSUAL HYDROCARBON EMISSION FROM THE EARLY CARBON STAR HD : THE CONNECTION BETWEEN AROMATICS AND ALIPHATICS
 The Astrophysical Journal, 664:1144Y1153, 2007 August 1 # 2007. The American Astronomical Society. All rights reserved. Printed in U.S.A. A THE UNUSUAL HYDROCARBON EMISSION FROM THE EARLY CARBON STAR HD
The Astrophysical Journal, 664:1144Y1153, 2007 August 1 # 2007. The American Astronomical Society. All rights reserved. Printed in U.S.A. A THE UNUSUAL HYDROCARBON EMISSION FROM THE EARLY CARBON STAR HD
BUT, what happens when atoms, with electrons attached, are packed really close together? The electrons from the neighboring atoms can have a small
 Quiz #5 There are two stars, star A and star B. Star A is approaching the Earth at 100 km/s and Star B is moving away from the Earth at 200 km/s. Compare the Doppler shift for these two stars by explaining
Quiz #5 There are two stars, star A and star B. Star A is approaching the Earth at 100 km/s and Star B is moving away from the Earth at 200 km/s. Compare the Doppler shift for these two stars by explaining
Possible Extra Credit Option
 Possible Extra Credit Option Attend an advanced seminar on Astrophysics or Astronomy held by the Physics and Astronomy department. There are seminars held every 2:00 pm, Thursday, Room 190, Physics & Astronomy
Possible Extra Credit Option Attend an advanced seminar on Astrophysics or Astronomy held by the Physics and Astronomy department. There are seminars held every 2:00 pm, Thursday, Room 190, Physics & Astronomy
