A Mathematical Model for Predicting Controlled Release of Bioactive Agents from Composite Fiber Structures
|
|
|
- Sherman Phelps
- 5 years ago
- Views:
Transcription
1 A Mathematical Model for Predicting Controlled Release of Bioactive Agents from Composite Fiber Structures Meital Zilberman, Moran Sofer Department of Biomedical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978, Israel Received 25 May 2006; revised 18 June 2006; accepted 12 July 2006 Published online 27 October 2006 in Wiley InterScience ( DOI: /jbm.a Abstract: A mathematical model for predicting bioactive agent release profiles from core/shell fiber structures was developed and studied. These new composite fibers, which combine good mechanical properties with desired protein release profiles, are designed for use in tissue regeneration and other biomedical applications. These fibers are composed of an inner dense polymeric core surrounded by a porous bioresorbable shell, which encapsulates the bioactive agent molecules. The model is based on Fick s second law of diffusion, and on two major assumptions: (a) firstorder degradation kinetics of the porous shell, and (b) a nonconstant diffusion coefficient for the bioactive agent, which increases with time because of degradation of the host polymer. Three factors are evaluated and included in this model: a porosity factor, a tortuosity factor, and a polymer concentration factor. Our study indicates that the model correlates well with in vitro release results, exhibiting a mean error of less than 2.2% for most studied cases. In this study, the model was used for predicting protein release profiles from fibers with shells of various initial molecular weights and for predicting the release of proteins with various molecular weights. This new model exhibits a potential for simulating fibrous systems for a wide variety of biomedical applications. Ó 2006 Wiley Periodicals, Inc. J Biomed Mater Res 80A: , 2007 Key words: protein-eluting fibers; bioresorbable polymers; porous scaffold; medical device; horseradish peroxidase INTRODUCTION Tissue regeneration involves the preparation of polymeric structures that serve as degradable scaffolds for bioactive agents or cells as well as the study of their structure and properties. 1 However, the key problem of how to incorporate bioactive agent molecules into thin delicate structures that construct devices and scaffolds remains unresolved, since they must be incorporated into dense polymeric structures without adversely affecting either the scaffold s properties or the agent s activity. Two types of drug-loaded fibers have been reported previously. 2 7 These are monolithic fibers in which the drug is dispersed throughout the fiber, and hollow reservoir fibers in which the drug is stored in the fiber s internal cavity. However, most such systems suffer from poor mechanical properties (due to drug incorporation) and/or require destructively high meltprocessing temperatures. Most drugs and all proteins cannot withstand high temperatures or endure many organic solvents. Correspondence to: M. Zilberman; meitalz@eng.tau. ac.il Contract grant sponsor: RAMOT (Horowitz) Foundation, Tel-Aviv University ' 2006 Wiley Periodicals, Inc. In one of our recent studies, we presented a new concept of core/shell fiber structures, which successfully meet these challenges. 8 These composite fibers combine a dense poly(l-lactic acid) (PLLA) core fiber and a drug/protein-loaded porous shell structure, i.e., the drug or protein is located in a separate compartment (a shell ) around a melt spun core fiber. A schematic representation of the composite fiber structure is presented in Figure 1. This results in good mechanical properties as well as in the desired drug release profile. Unlike conventional bulky multifiber structures that carry bioactive agent molecules in their pores, we incorporate these molecules directly into the shell (but not the core) of our composite fibers, rather than in the interstices between them. Better control over drug release can thus be achieved. In our initial studies, the shell was loaded with the model enzyme, horseradish peroxidase (HRP), which is very sensitive to both solvents and elevated temperatures and whose activity is a sensitive monitor of damage during processing. Our results indicated that HRP preserved % of its activity during the fiber preparation process. In addition to tissue regeneration applications, our new fibers are ideal for forming thin, delicate, biomedically important structures for various applications, such as fiber-based endovascular stents that mechanically support blood vessels while delivering drugs for preventing restenosis directly to the blood
2 680 ZILBERMAN AND SOFER Figure 1. A schematic representation of a core/shell fiber structure. [Color figure can be viewed in the online issue, which is available at vessel wall, or bioresorbable wound and burn dressings loaded with antimicrobial agents. Mathematical models for controlled release of bioactive agents from bioresorbable matrices are based on diffusion aspects, on the structural characteristics of the matrix polymer and its degradation and swelling, and on the micro-environmental ph changes inside polymer matrix pores that are due to the degradation products. Prediction of the drug release profile using a model is obviously very useful in the device s design phase, since the model enables fast evaluation and tuning of the various parameters for achieving an optimal release profile, while reducing laboratory tasks to a minimum. 9,10 Early drug delivery models described either systems based on nondegradable matrices or surface-eroding systems. 11 Several more recent models described bulkeroding systems, in which the drug is physically immobilized. For example, Siepman and Gopferich 12 quantified drug release from slab-shaped PLLA and poly(dl-lactic acid-co-glycolic acid) (PDLGA) matrices. Their model was based on Higuchi s classical pseudosteady-state equation for oversaturated, planar, nondegrading polymeric films, where the permeability of the drug within the polymer matrix was assumed to increase with time, due to cleavage of the polymer s bonds. Another possibility for simulating the effect of erosion on the diffusion process is to use a diffusion coefficient, which increases with time. Various theories therefore related the drug diffusion coefficient inside a degradable polymer directly to its molecular weight, since short chains offer less restriction to drug diffusion than do long chains. This was one of the main assumptions in the models of Charlier et al. 13 for predicting the release rate of Mifepristone from 50/50 PDLGA bulk-eroding films of various molecular weights, and Faisant et al. 14 who examined the release rate of 5-fluorouracil from 50/50 PDLGA microspheres. Each of them used an additional assumption regarding the polymer s first-order degradation kinetics, in order to calculate an appropriate time-dependent diffusion coefficient, which they used in Fick s laws of diffusion. Zhang et al. 15 addressed three mechanisms for drug release in a microspheric matrix, namely dissolution of the drug from the polymer matrix, diffusion of the dissolved drug, and erosion of the matrix. It should be noted that most drug delivery systems are either in the form of films or microspheres, and so are the above-described models. Sagiv et al., 16 however, developed a specific model for predicting protein release from monolithic PLLA fibers. Since PLLA degrades relatively slowly, they assumed that polymer erosion is negligible during the release time; thus allowing them to use a constant diffusion coefficient so as to simplify the model. This model is therefore obviously not applicable to our core/shell fiber structures. However, our promising results raised the need for a suitable model that could facilitate and shorten the design process of the core/shell fiber structures. This need was the driving force for the current research, in which a mathematical model was developed for predicting protein release profiles from our composite fiber structures. The hypotheses of our study are: (1) A model based on Fick s laws will be able to provide good prediction of protein release profile from our new core/shell fiber structures, and (2) the protein release profile from the fibers is affected by the molecular weights of the protein and the host polymer, and also by the emulsion s formulation parameters. CASE DEFINITION AND MODEL ASSUMPTIONS A mathematical model for predicting drug/protein release from our novel core/shell fiber structures was developed in this study, using Matlab 6.1. The release profiles that were predicted using this model were compared with that of the experimental results for certain fiber types. The experimental system About 8-cm long fibers, composed of an inner PLLA core fiber, were coated with a porous 75/25 PDLGA shell. The porous shell was fabricated using the freeze-drying technique, where an organic solution (containing PDLGA and chloroform as solvent) and an aqueous solution (containing distilled water and HRP) were mixed together in a test tube. Each PLLA fiber was then dipped in the resulting emulsion and immediately frozen in liquid nitrogen. The water and the solvent were removed by sublimation, leaving the HRP molecules mechanically trapped in the porous PDLGA shell surrounding the PLLA fibers. 8 The HRP load in the porous shell of all fibers was 5% (w/w; relative to the polymer weight). Various shell types were prepared and emulsions of organic:aqueous (o:a) phase ratios of 8:1 and 16:1 were obtained. Polymer contents
3 MATHEMATICAL MODEL FOR PREDICTING CONTROLLED RELEASE 681 TABLE I Fiber Types Used for Obtaining the Experimental Data Fiber Type of 13, 15, and 19% (w/v in the organic phase) were used for each o:a value. The types of fibers examined are presented in Table I. Assumptions used while deriving the model s equations i. The bulk-eroding PDLGA porous structure exhibits first-order degradation kinetics, i.e. the cleavage of its chains occurs at a constant rate ii. The protein s diffusion coefficient within the porous structure is only time-dependent and is assumed to increase with time proportional to the polymer s normalized molecular weight loss (M wl ). iii. The internal PLLA core exhibits minimal degradation, if any, within the considered protein release time-frame. This assumption is based on one of our previous studies. 20 iv. There is no protein flux towards the internal PLLA core. v. The external environment provides perfect sink conditions for the released proteins. When conducting the experiments the aqueous release medium was replaced at each sampling point. vi. The proteins within the porous PDLGA structure are released only when they find a continuous path to the surface, and their release occurs solely by diffusing through water. This assumption is based on other reports of drug delivery systems based on porous matrices. 10 MODEL EQUATIONS In this model, the protein concentration within the fiber obviously changes with time. Fick s second law of diffusion in cylindrical coordinates (r, y, z) 12 was therefore used, as ¼ 1 r Emulsion Organic: Aqueous Phase Ratio Polymer Content in the Organic Phase (%, w/v) C p t 1 8: : : : : D @z ð1þ where C is the HRP concentration and D is its diffusion coefficient within the porous PDLGA structure. Because of circular symmetry, the HRP concentration along y is constant, ¼ 0 ð2þ Furthermore, in the case of a fiber whose radius is significantly smaller than its length, the end effects are negligible, ¼ 0 ð3þ In conclusion, it may be stated that the diffusion of proteins from a fiber is limited to the radial axis, i.e. C ¼ f(r, t), thus simplifying equation (1) ¼ 1 rd ð4þ Assuming that the diffusion coefficient is only a function of time simplifies it even further, and the following equation can be ¼ 1 rd ¼ 1 r ¼ 1 r D C 2 D Thus, the final diffusion equation ¼ D 1 r D þ C 2 C 2 ð5þ ð6þ Appropriate initial and boundary conditions should be used in order to solve the diffusion equation (6). Assuming an initial uniform HRP concentration (C 0 ) leads to the following initial condition: C ¼ C 0 at t ¼ 0; r 1 < r < r 2 ð7þ where r 1 is the radius of the internal PLLA fiber and r 2 is the radius of the entire composite fiber (see Fig. 1). A no flux boundary condition was set on the internal wall of the porous structure, which is also the external wall of the inner PLLA fiber, (r ¼ r 1 ), indicating that the proteins within the porous structure are assumed to diffuse only toward the surface of the composite fiber and not toward its core: ¼ 0 at r ¼ r 1; t > 0 ð8þ The second boundary condition is a perfect sink at the external wall of the composite fiber, i.e. C ¼ 0 at r ¼ r 2 ; t > 0 ð9þ Using the above initial and boundary conditions, the differential equation (6) was resolved via the pdepe Matlab function, and yielded C(r, t), the HRP concentration function inside the fiber. Thus, the total mass of proteins still trapped within the porous structure
4 682 ZILBERMAN AND SOFER at any given moment could be estimated using the following equation: MðtÞ ¼ Z r2 r 1 Z r2 ACðr; tþdr ¼ Z r2 r 1 2prLCðr; tþdr ¼ 2pL rcðr; tþdr ð10þ r 1 Since the initial mass of proteins inside the fiber, (M(t ¼ 0)), is known, the evaluation of the released mass is given by M released ðtþ ¼ Mðt ¼ 0Þ MðtÞ ð11þ Estimation of the diffusion coefficient Previous reports on drug delivery systems based on porous matrices revealed that the drug is released much more slowly than that would be expected from the simplest consideration of aqueous diffusion. 10 The porous structure apparently slows the progress of the diffusing agents, since they go through a tortuous path on their way to the matrix surface. The length of this path is unknown, but it is greater than (r r 1 )byatortuosity factor t, which was initially proposed by Higuchi. 17,21,22 Thus, the HRP diffusion coefficient within the porous PDLGA structure is assumed to initially have a certain low effective value, D 0, which is determined by the microstructure of the matrix, i.e. tortuosity t and porosity e (the volume fraction accessible to the diffusing molecules). As degradation and finally erosion progress with time, the diffusion coefficient gradually increases, until it reaches HRP s molecular diffusion coefficient, i.e. its diffusion coefficient in water, D w. The above behavior can therefore be expressed using the following equations: e D 0 ¼ D w ð12þ t Polson s semiempirical equation 18,23,24 was used for the protein molecular diffusion coefficient, D w : D w ¼ A T ð14þ m M 1=3 wp where M wp is the protein s molecular weight, T is the absolute temperature, m is the fluid viscosity, and A is a constant that varies for each protein. Tortuosity, t, is an empirical parameter whose value was determined by us. The stereology sampling technique was used in order to estimate the matrix porosity. This technique enables estimating a sample s porosity using SEM (2D) images of sample cross-sections. 25 Point-counting estimation was therefore used, where a grid of points is placed on the fiber s cross-section image and the estimated porosity is the ratio between the number of points that fall on the pores themselves and the number of points that fall on the entire cross-section. Adding the polymer concentration effect As mentioned earlier, three polymer concentrations (w/v) were used in the organic phase while fabricating the different porous structures: 13, 15, and 19%. A higher polymer concentration results in a more viscous organic phase, thus creating a more stable emulsion. This higher viscosity, along with the obvious higher density, are expected to create the following hindering effects on the system: (1) slowing the matrix degradation rate due to a lower readiness to water penetration;(2)reducingthefreevolumeavailableforprotein diffusion, leading to a shorter initial burst effect in the release profile. An additional empirical parameter, C p, was therefore introduced in order to add the variant polymer concentration to the model, as follows. A function describing the decrease in molecular weight with time (degradation profile) for the relevant polymer (in this case the initial molecular weight ¼ 100 kda) had to be used. Data taken from a research by Wu and Wang 19 were used and interpolation was performed in order to obtain a good estimation for the degradation profile of 75/25 PDLGA with an initial molecular weight of 100 kda. The estimated degradation profiles of the 100 kda PDLGA and two other polymers, which will be used in this model, are presented in Figure 2. DðtÞ ¼ D 0 þðd w D 0 ÞM wl ðtþ ð13þ where M wl ðtþ ¼ M wðt ¼ 0Þ M w ðtþ M w ðt ¼ 0Þ Figure 2. Normalized degradation profile of 75/25 PDLGA with various initial polymer s molecular weights. The molecular weights are indicated. [Color figure can be viewed in the online issue, which is available at
5 MATHEMATICAL MODEL FOR PREDICTING CONTROLLED RELEASE 683 Figure 3. HRP release profile from various core/shell fiber structures containing 5% (w/w) HRP. The experimental results (red line) are compared with the predicted results (blue line): (a) o:a ¼ 8:1, 15% (w/v) polymer, (b) o:a ¼ 8:1, 19% (w/v) polymer, (c) o:a ¼ 16:1, 13% (w/v) polymer, (d) o:a ¼ 16:1, 15% (w/v) polymer, and (e) o:a ¼ 16:1, 19% (w/v) polymer. The mean error for each type is presented. [Color figure can be viewed in the online issue, which is available at The normalized molecular weight curves apparently fit a function of the type: M w (t)¼ exp( t/b). Since the polymer concentration was hypothesized to alter the degradation rate, it was added to the model as follows: M wl ðtþ ¼ 1 M w ðtþ ¼ 1 exp t B ¼ 1 exp C p B t ð15þ According to theory, the degradation of bulk-eroding polymers follows first-order kinetics, i.e. plotting the natural logarithm of the molecular weight vs. the hydrolyzing time yields a nearly linear curve The B coefficient was thus found using the least squares algorithm. RESULTS AND DISCUSSION As mentioned earlier, the experimental data used to validate this model were taken from one of our recent studies. 8 The chosen core/shell fiber types and their semiempirical C p and t values are presented in Table I. The predicted HRP release profile was compared with that of the experimental release profile for each type of composite fiber structures, and the results are presented in Figure 3. A very good fit was
6 684 ZILBERMAN AND SOFER Figure 4. The effect of the polymer s initial molecular weight on the predicted HRP release profile from various core/shell fiber structures containing 5% w/w HRP. Molecular weight: upper curve (red line) 40 kda, center curve (blue line) 100 kda, lower curve (green line) 160 kda; (a) o:a ¼ 8:1, 15% w/v polymer, (b) o:a ¼ 8:1, 19% w/v polymer, (c) o:a ¼ 16:1, 13% w/v polymer, (d) o:a ¼ 16:1, 15% w/v polymer, (e) o:a ¼ 16:1, 19% w/v polymer. [Color figure can be viewed in the online issue, which is available at obtained for all studied fibers, except for the fiber with an o:a of 8:1 and 15% (w/v) polymer, where the predicted HRP release during the first week is lower than the experimental HRP release (Fig. 2a). Hence, these results support our first hypothesis about good predictability of a model based on Fick s laws. As explained above, two main emulsion types were created by using a constant organic phase volume with two different aqueous phase volumes: o:a of 8:1 and 16:1. The fibers fabricated with a higher o:a (16:1) exhibit a more tortuous diffusion path, leading to higher values of the tortuosity factor (Table 1). Furthermore, the tortuosity factor within both 8:1 and 16:1 groups increases with the increase in polymer content. Therefore, either increasing the emulsion s o:a ratio (i.e., decreasing the aqueous phase volume) or increasing the polymer content, results in a decrease in the free space available for diffusion, leading to a higher tortuosity factor, which in turn leads to a lower release rate of the bioactive agent from the fiber s shell. These results are in agreement with our second hypothesis, saying that the emulsion formulation parameters affect the release profile. Since a higher polymer content leads to an emulsion with a more viscous and dense organic phase, we assumed that the resulting solid porous structure will tend to absorb less water, resulting in slower hydrolysis and hence degradation, leading to a shorter and
7 MATHEMATICAL MODEL FOR PREDICTING CONTROLLED RELEASE 685 more moderate burst effect. Following this hypothesis, C p was introduced into the model in a way that alters the porous structure s degradation rate. This is also supported by the experimental results, which demonstrate that as the polymer content increases, the matrix degradation decreases, leading to a smaller initial burst release. It is interesting to compare the C p and t values of different composite fiber types and elucidate the effect of processing conditions on these parameters (through microstructure). For example, the C p value of the 16:1 sample of fibers fabricated with the same polymer content of 15% (w/v) is 2.8 times higher than that of the 8:1 sample, and for 19% (w/v) polymer fibers the C p value of the 16:1 sample is 2.4 times higher than that of the 8:1 sample. A similar trend was obtained for t: thet value of the 16:1 sample of fibers fabricated with a polymer content of 15% (w/v) is 3.3 times higher than that of the 8:1 sample, and for 19% (w/v) polymer fibers the t value of the 16:1 sample is 3.0 times higher than that of the 8:1 sample (Table 1). This consistent behavior of both parameters as a function of the polymer concentration can simplify the model. Although it cannot be demonstrated without additional experiments, it seems that certain calibration curves and/or functions may be developed in order to simplify the model. The advantage of building a model for drug/protein delivery systems is the ability to elucidate the effect of the system s parameters on the release profile of the bioactive agent. In this regard, the effect of the molecular weight of both components, PDLGA and HRP, was studied. The effect of the PDLGA molecular weight on the release rate was examined using the degradation profiles of polymers with initial molecular weights of 40 and 160 kda, in addition to that of the standard 100 kda used in our experiments. These degradation profiles were obtained using interpolations based on the experimental results of Wu and Wang 19 and are presented in Figure 2. Our predicted HRP release profiles for the series of 75/25 PDLGA with three molecular weights are presented in Figure 4 for each of the studied fiber types. The decrease in initial molecular weight always resulted in an increased HRP release rate. This prediction is logical, since a lower initial molecular weight polymer will result in shorter polymer chains as degradation proceeds, giving rise to an enhanced drug release rate. It should be mentioned that the predicted release profiles are not accurate, and that the burst release values almost do not change with the initial molecular weight. This occurs mainly because the only parameter that was changed while making these predictions is the matrix degradation profile, leaving the same tortuosity factor that was calculated for the 100 kda fiber type. However, the tortuosity factor is supposed to increase with an increase in the molecular weight. Our future work will focus on an additional minimodel that predicts the effect of various parameters on the tortuosity of the polymer and therefore enables more accurate prediction of release profiles. The effect of HRP s molecular weight (i.e., size) on its release profile from the various fibers was also studied using our model. The results for fiber structures with a shell prepared from an emulsion of 5% (w/w) HRP and 19% (w/v) polymer are presented in Figure 5. The predicted profiles demonstrate that the HRP release rate decreases with the increase in its molecular weight, i.e. higher molecular weight proteins exhibit a lower diffusion coefficient, which results in lower mobility in water. Since protein release occurs by means of diffusion in water, this lower diffusion coefficient should result in a lower release rate. These results support our second hypothesis, saying that the release profile is affected by the sizes of the system s components, the bioactive agent, and the host polymer. We discovered that the effect of HRP s size on its Figure 5. TheeffectofHRP smolecularweightonitspredicted release profile from core/shell fiber structures containing5%w/vhrpand19%w/vpolymer.thehrp smolecular weights are indicated; (a) o:a ¼ 8:1, (b) o:a ¼ 16:1. [Color figure can be viewed in the online issue, which is available at
8 686 ZILBERMAN AND SOFER release profile is apparently higher than that of the host polymer s initial molecular weight. SUMMARY AND CONCLUSIONS The aim of this study was to develop a mathematical model for predicting protein release profiles from novel bioresorbable core/shell fiber structures designed to be used in tissue regeneration and other biomedical applications. These novel composite structures are composed of a dense polymeric core fiber coated with a porous protein-loaded PDLGA shell, and combine desired mechanical properties with versatile release profiles of the active agent. Use of the suggested model affords a good and rapid evaluation of the release profile, enabling further economical in vitro/in vivo release studies. The model is based on Fick s second law of diffusion and on two major assumptions: (a) first-order degradation kinetics of the porous shell, and (b) a time-dependent bioactive agent diffusion coefficient, which increases with time because of the degradation of the host polymer. The model also uses three empirical parameters that address the matrix microgeometry: porosity factor, tortuosity factor, and a polymer concentration factor that relates to the polymer concentration in the emulsion from which the matrix was fabricated. The model correlates well with in vitro release results, exhibiting a mean error of less than 2.2% for most studied cases. The behavior (values and tendencies) of the empirical tortuosity and polymer concentration factors in the model correlated well with theory. Furthermore, the model predicts an increased polymer concentration factor for higher polymer contents, resulting in a smaller burst release and a more moderate release profile. In this study, the model was used for predicting protein release profiles from fibers with shells of various initial molecular weights and for predicting the release of proteins with various molecular weights (sizes). This new model exhibits a potential for simulating fibrous systems for a wide variety of biomedical applications. References 1. Thomson RC, Shung AK, Yaszemski MJ, Mikos AG. Polymer scaffold processing. In: Lanza RP, Langer R, Vacanti J, editors. Principles of Tissue Engineering. New York:Academic Press; pp Su SH, Landau CL, Chao RY, Timmons RB, Meidell RS, Tang L, Eberhart RC. Expandable bioresorbable endovascular stent with anti-platelet and anti-inflammation treatments. Circulation 2001; 104:(Part II): Alikacem N, Yoshizawa T, Wilson C, Nelson KD. Quantitative MR imaging study of intravitreal sustained release of VEGF in rabbits. Invest Ophthalmol Vis Sci 2000;41: Dunn RL, English JP, Stoner WC, Potter AG, Perkins BH. Biodegradable fibers for the controlled release of tetracycline in treatment of peridontal disease. Proc Int Symp Controlled Release Bioact Mater 1987;14: Eenink MDJ, Feijen J, Oligslanger J, Albers JHM, Rieke JC, Greidonus PJ. Biodegradable hollow fibers for the controlled release of hormones. J Controlled Release 1987;6: Polacco G, Cascone MG, Lazzeri L, Ferrara S, Giusti P. Biodegradable hollow fibers containing drug-loaded nanoparticles as controlled release systems. Polym Int 2002;51: Lazzeri L, Cascone MG, Quiriconi S, Morabito L, Giusti P. Biodegradable hollow microfibers to produce bioactive scaffolds. Polym Int 2005;54: Levy Y, Zilberman M. Novel bioresorbable composite fiber structures loaded with proteins for tissue regeneration applications. J Biomed Mater Res A, in press. 9. Narasimhan B, Peppas A.The role of modeling studies in the development of future controlled-release devices. In: Park K, editor. Controlled Drug Delivery: Challenges and Strategies. Washington, DC: American Chemical Society; pp Tongwen X. Binglin H. Mechanism of sustained drug release in diffusion-controlled polymer matrix-application of percolation theory. Int J Pharm 1998;170: Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials 1996;17: Siepmann J, Gopferich A. Mathematical modeling of bioerodible polymeric drug delivery systems. Adv Drug Deliv Rev 2001;48: Charlier A, Leclerc B. Couarraze G. Release of mifepristone from biodegradable matrices: Experimental and theoretical evaluations. Int J Pharm 2000;200: Faisant N, Siepmann J, Benoit JP. PLGA-based microparticles: Education of mechanisms and a new, simple mathematical model quantifying drug release. Eur J Pharm Sci 2002;15: Zhang M, Yang Z, Chow L, Wang C. Simulation of drug release from biodegradable polymeric microspheres with bulk and surface erosions. J Pharm Sci 2003;92: Sagiv A, Parker N, Parkhi V, Nelson KD. Initial burst measures of release kinetics from fiber matrices. Ann Biomed Eng 2003;31: Gopferich A. Polymer bulk erosion. Macromolecules 1997;30: Saltzman WM. Drug Delivery: Engineering Principles for Drug Therapy. Oxford:Oxford University Press; Wu XS, Wang N. Synthesis, charachterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J Biomater Sci Polym Ed 2001;12: Zilberman M, Eberhart RC, Schwade ND. In vitro study of drug loaded bioresorbable films and support structures. J Biomater Sci Polym Ed 2002;13: Geankoplis CJ. Transport Process and Unit Operations, 2nd ed. Englewood Cliffs, NJ: Prentice Hall; Chapter Pismen LM. Diffusion in porous media of a random structure. Chem Eng Sci 1974;29: He L, Niemeyer B. A novel correlation for protein diffusion coefficients based on molecular weigt and radius of gyration. Biotechnol Prog 2003;19: Tyn MT, Gusek TW. Prediction of diffusion coefficients of proteins. Biotechnol Bioeng 1990;35: Saltzman WM, Pasternak SH, Langer R. Quantative image analysis for developing microstructural descriptions of heterogeneous materials. Chem Eng Sci 1987;42:
Controlled Release Theory
 Controlled Release Theory Last time: tailoring the structure of degradable polymers Fundamental concepts in controlled release Today: Theory of degradable polymer-based controlled release Reading: Charlier
Controlled Release Theory Last time: tailoring the structure of degradable polymers Fundamental concepts in controlled release Today: Theory of degradable polymer-based controlled release Reading: Charlier
Model based design of controlled release drug delivery systems
 Model based design of controlled release drug delivery systems Aditya Pareek, Praduman Arora, Venkataramana Runkana Copyright 2012 Tata Consultancy Services Limited 1 Why we need controlled drug delivery?
Model based design of controlled release drug delivery systems Aditya Pareek, Praduman Arora, Venkataramana Runkana Copyright 2012 Tata Consultancy Services Limited 1 Why we need controlled drug delivery?
Analysis of Case II drug transport with radial and axial release from cylinders
 International Journal of Pharmaceutics 254 (23) 183 188 Analysis of Case II drug transport with radial and axial release from cylinders Kosmas Kosmidis a, Eleni Rinaki b, Panos Argyrakis a, Panos Macheras
International Journal of Pharmaceutics 254 (23) 183 188 Analysis of Case II drug transport with radial and axial release from cylinders Kosmas Kosmidis a, Eleni Rinaki b, Panos Argyrakis a, Panos Macheras
Jacek Balcerzak, Maria Mucha
 ANALYSIS OF MODEL DRUG RELEASE KINETICS FROM COMPLEX MATRICES OF POLYLACTIDE-CHITOSAN Jacek Balcerzak, Maria Mucha Technical University of Lodz, Faculty of Process and Environmental Engineering Wolczanska
ANALYSIS OF MODEL DRUG RELEASE KINETICS FROM COMPLEX MATRICES OF POLYLACTIDE-CHITOSAN Jacek Balcerzak, Maria Mucha Technical University of Lodz, Faculty of Process and Environmental Engineering Wolczanska
Hydrogel Biomaterials: Structure and thermodynamics
 Hydrogel Biomaterials: Structure and thermodynamics Last Day: programmed/regulated/multifactor controlled release for drug delivery and tissue eng ineering Announcements: Today: Reading: Finish discussion
Hydrogel Biomaterials: Structure and thermodynamics Last Day: programmed/regulated/multifactor controlled release for drug delivery and tissue eng ineering Announcements: Today: Reading: Finish discussion
Biodegradable Solid Polymeric Materials (continued)
 Biodegradable Solid Polymeric Materials (continued) Last time: chemistry and physical chemistry of degrading polymeric solids for biomaterials Today: Factors controlling polymer degradation rates Theory
Biodegradable Solid Polymeric Materials (continued) Last time: chemistry and physical chemistry of degrading polymeric solids for biomaterials Today: Factors controlling polymer degradation rates Theory
A Novel Dissolution-Diffusion Model for Investigation of Drug Release from Polymeric Microspheres
 European Symposium on Computer Arded Aided Process Engineering 15. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. A Novel Dissolution-Diffusion Model for Investigation
European Symposium on Computer Arded Aided Process Engineering 15. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. A Novel Dissolution-Diffusion Model for Investigation
Issued: 02/07/06 Spring 2006 Due: 02/16/06 10 points/problem
 Problem Set 1 solutions 20.462J/3.962J Issued: 02/07/06 Spring 2006 Due: 02/16/06 10 points/problem 1. Listed in the table below are chemical and schematic structures of poly(l-lactide) and a number of
Problem Set 1 solutions 20.462J/3.962J Issued: 02/07/06 Spring 2006 Due: 02/16/06 10 points/problem 1. Listed in the table below are chemical and schematic structures of poly(l-lactide) and a number of
Mathematical Modeling and Simulation of Drug Release from Microparticles
 Mathematical Modeling and Simulation of Drug Release from Microparticles Qin H. 1 and Wang C.H. 2 Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive
Mathematical Modeling and Simulation of Drug Release from Microparticles Qin H. 1 and Wang C.H. 2 Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive
ELECTROSPRAY: NOVEL FABRICATION METHOD FOR BIODEGRADABLE POLYMERIC NANOPARTICLES FOR FURTHER APPLICATIONS IN DRUG DELIVERY SYSTEMS
 ELECTROSPRAY: NOVEL FABRICATION METHOD FOR BIODEGRADABLE POLYMERIC NANOPARTICLES FOR FURTHER APPLICATIONS IN DRUG DELIVERY SYSTEMS Ali Zarrabi a, Manouchehr Vossoughi b a Institute for Nanscience & Nanotechnology,
ELECTROSPRAY: NOVEL FABRICATION METHOD FOR BIODEGRADABLE POLYMERIC NANOPARTICLES FOR FURTHER APPLICATIONS IN DRUG DELIVERY SYSTEMS Ali Zarrabi a, Manouchehr Vossoughi b a Institute for Nanscience & Nanotechnology,
BENG 221 DRUG DIFFUSION THROUGH A COATED STENT
 BENG 221 DRUG DIFFUSION THROUGH A COATED STENT 12 TH NOVEMBER, 2010 1. INTRODUCTION: In this report, a bioengineering application of the one- dimensional diffusion equation, solved in both rectangular
BENG 221 DRUG DIFFUSION THROUGH A COATED STENT 12 TH NOVEMBER, 2010 1. INTRODUCTION: In this report, a bioengineering application of the one- dimensional diffusion equation, solved in both rectangular
IN VITRO DEGRADATION AND EROSION OF DEGRADABLE LACTATE SEGMENTED POLYURETHANES
 Journal of Optoelectronics and Advanced Materials Vol. 7, No. 6, December 2005, p. 2803-2808 IN VITRO DEGRADATION AND EROSION OF DEGRADABLE LACTATE SEGMENTED POLYURETHANES V. Melnig *, L. Obreja, A. Garlea
Journal of Optoelectronics and Advanced Materials Vol. 7, No. 6, December 2005, p. 2803-2808 IN VITRO DEGRADATION AND EROSION OF DEGRADABLE LACTATE SEGMENTED POLYURETHANES V. Melnig *, L. Obreja, A. Garlea
Biotransport: Principles
 Robert J. Roselli Kenneth R. Diller Biotransport: Principles and Applications 4 i Springer Contents Part I Fundamentals of How People Learn (HPL) 1 Introduction to HPL Methodology 3 1.1 Introduction 3
Robert J. Roselli Kenneth R. Diller Biotransport: Principles and Applications 4 i Springer Contents Part I Fundamentals of How People Learn (HPL) 1 Introduction to HPL Methodology 3 1.1 Introduction 3
CHAPTER 5 SURFACE TOPOGRAPHY STUDIES ON ELECTROPHORETICALLY DEPOSITED CHITOSAN ON POLYCAPROLACTONE MICRO FIBROUS SUBSTRATES
 89 CHAPTER 5 SURFACE TOPOGRAPHY STUDIES ON ELECTROPHORETICALLY DEPOSITED CHITOSAN ON POLYCAPROLACTONE MICRO FIBROUS SUBSTRATES 5.1 INTRODUCTION Synthetic bio polymers such as PCL, poly (glycolic acid)
89 CHAPTER 5 SURFACE TOPOGRAPHY STUDIES ON ELECTROPHORETICALLY DEPOSITED CHITOSAN ON POLYCAPROLACTONE MICRO FIBROUS SUBSTRATES 5.1 INTRODUCTION Synthetic bio polymers such as PCL, poly (glycolic acid)
NANO 243/CENG 207 Course Use Only
 L12: Drug Loading & Quantification May 15, 2018 1. Drug loading techniques 1.1 Physical approaches Nanprecipitation Single emulsion Double emulsion Encapsulation Remote loading 1.2 Chemical approaches
L12: Drug Loading & Quantification May 15, 2018 1. Drug loading techniques 1.1 Physical approaches Nanprecipitation Single emulsion Double emulsion Encapsulation Remote loading 1.2 Chemical approaches
Stable Encapsulation of Quantum Dot Barcodes with Silica Shells
 Stable Encapsulation of Quantum Dot Barcodes with Silica Shells Shang-Hsiu Hu and Xiaohu Gao Department of Bioengineering, University of Washington Seattle, WA 98195 (USA) Adv. Funct. Mater. 2010. ASAP
Stable Encapsulation of Quantum Dot Barcodes with Silica Shells Shang-Hsiu Hu and Xiaohu Gao Department of Bioengineering, University of Washington Seattle, WA 98195 (USA) Adv. Funct. Mater. 2010. ASAP
A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion
 A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion The MIT Faculty has made this article openly available. Please share how this access
A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion The MIT Faculty has made this article openly available. Please share how this access
Modelling of drug release from ensembles of aspirin microcapsules of certain particle size distribution
 Eichie & Okor, 23 Tropical Journal of Pharmaceutical Research, June 23; 2 (1): 137-145 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, Nigeria. All rights reserved. Available
Eichie & Okor, 23 Tropical Journal of Pharmaceutical Research, June 23; 2 (1): 137-145 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, Nigeria. All rights reserved. Available
Different Biodegradable Silica Structures In Drug Delivery. Mika Jokinen
 Different Biodegradable Silica Structures In Drug Delivery Mika Jokinen & Different Morphologies of Biodegradable Silica - Several levels of morphology ; different forms & structures - monoliths, fibers,
Different Biodegradable Silica Structures In Drug Delivery Mika Jokinen & Different Morphologies of Biodegradable Silica - Several levels of morphology ; different forms & structures - monoliths, fibers,
Diffusion and Adsorption in porous media. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Diffusion and Adsorption in porous media Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Devices used to Measure Diffusion in Porous Solids Modes of transport in
Diffusion and Adsorption in porous media Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Devices used to Measure Diffusion in Porous Solids Modes of transport in
Journal of Colloid and Interface Science
 Journal of Colloid and Interface Science 35 (2011) 78 82 Contents lists available at ScienceDirect Journal of Colloid and Interface Science www.elsevier.com/locate/jcis Diffusion through colloidosome shells
Journal of Colloid and Interface Science 35 (2011) 78 82 Contents lists available at ScienceDirect Journal of Colloid and Interface Science www.elsevier.com/locate/jcis Diffusion through colloidosome shells
Intrinsic Viscosity and Unperturbed Dimension of Poly(DL-lactic acid) Solution
 Macromolecular Research, Vol. 16, No. 7, pp 631-636 (2008) Intrinsic Viscosity and Unperturbed Dimension of Poly(DL-lactic acid) Solution Jae Sung Lee* and Sung Chul Kim Center for Advanced Functional
Macromolecular Research, Vol. 16, No. 7, pp 631-636 (2008) Intrinsic Viscosity and Unperturbed Dimension of Poly(DL-lactic acid) Solution Jae Sung Lee* and Sung Chul Kim Center for Advanced Functional
Model Solutions Spring 2003
 Exam I BE.462J/3.962J Model Solutions Spring 2003 (60 points total) 1. (5 points) Explain the following observation: autocatalysis generally has a smaller influence on the degradation rate of surface-eroding
Exam I BE.462J/3.962J Model Solutions Spring 2003 (60 points total) 1. (5 points) Explain the following observation: autocatalysis generally has a smaller influence on the degradation rate of surface-eroding
NANO 243/CENG 207 Course Use Only
 L8. Drug Dispersion and Diffusion in Biological Systems (2) April 26, 2018 2. Diffusion in Water (3) Diffusion of small molecule in water drug molecules/solvents are identical to solvent Assume molecules
L8. Drug Dispersion and Diffusion in Biological Systems (2) April 26, 2018 2. Diffusion in Water (3) Diffusion of small molecule in water drug molecules/solvents are identical to solvent Assume molecules
A theoretical approach to evaluate the release rate of. acetaminophen from erosive wax matrix dosage forms
 1 2 A theoretical approach to evaluate the release rate of acetaminophen from erosive wax matrix dosage forms 3 4 Yasuyoshi Agata, Yasunori Iwao, Kai Shiino, Atsuo Miyagishima, Shigeru Itai* 5 6 7 8 9
1 2 A theoretical approach to evaluate the release rate of acetaminophen from erosive wax matrix dosage forms 3 4 Yasuyoshi Agata, Yasunori Iwao, Kai Shiino, Atsuo Miyagishima, Shigeru Itai* 5 6 7 8 9
Lecture 10. Membrane Separation Materials and Modules
 ecture 10. Membrane Separation Materials and Modules Membrane Separation Types of Membrane Membrane Separation Operations - Microporous membrane - Dense membrane Membrane Materials Asymmetric Polymer Membrane
ecture 10. Membrane Separation Materials and Modules Membrane Separation Types of Membrane Membrane Separation Operations - Microporous membrane - Dense membrane Membrane Materials Asymmetric Polymer Membrane
Fast Deswelling of Microporous Cellulose Ether Gel Prepared by Freeze-drying
 Fast Deswelling of Microporous Cellulose Ether Gel Prepared by Freeze-drying N. Kato, 1 H. Suzuki, 1 Y. Sakai, 1 and S. H. Gehrke 2 1 Department of Applied Chemistry, Faculty of Engineering, Utsunomiya
Fast Deswelling of Microporous Cellulose Ether Gel Prepared by Freeze-drying N. Kato, 1 H. Suzuki, 1 Y. Sakai, 1 and S. H. Gehrke 2 1 Department of Applied Chemistry, Faculty of Engineering, Utsunomiya
Electrospinning of high-molecule PEO solution
 From the SelectedWorks of Ji-Huan He 2007 Electrospinning of high-molecule PEO solution Yu-Qin Wan Ji-Huan He, Donghua University Jian-Yong Yu Yue Wu Available at: https://works.bepress.com/ji_huan_he/20/
From the SelectedWorks of Ji-Huan He 2007 Electrospinning of high-molecule PEO solution Yu-Qin Wan Ji-Huan He, Donghua University Jian-Yong Yu Yue Wu Available at: https://works.bepress.com/ji_huan_he/20/
Gel Permeation Chromatography (GPC) or Size Exclusion Chromatography (SEC)
 Gel Permeation Chromatography (GPC) or Size Exclusion Chromatography (SEC) Size Exclusion Chromatography (SEC) is a non-interaction based separation mechanism in which compounds are retained for different
Gel Permeation Chromatography (GPC) or Size Exclusion Chromatography (SEC) Size Exclusion Chromatography (SEC) is a non-interaction based separation mechanism in which compounds are retained for different
Gelatine a physical gel
 Gelatine a physical gel W. Babel, Chemie in unserer Zeit, 86 (1996) binder in jogurts, aspic, capsules for medical drugs silver halogenide photography preparation from fibrous collagen (from skin and bones)
Gelatine a physical gel W. Babel, Chemie in unserer Zeit, 86 (1996) binder in jogurts, aspic, capsules for medical drugs silver halogenide photography preparation from fibrous collagen (from skin and bones)
Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith. Supporting Information
 Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith Ludivine van den Biggelaar 1, Patrice Soumillion 2 and Damien P. Debecker 1, * 1
Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith Ludivine van den Biggelaar 1, Patrice Soumillion 2 and Damien P. Debecker 1, * 1
International Journal of Pharma and Bio Sciences
 Research Article Bioinformatics International Journal of Pharma and Bio Sciences ISSN 0975-6299 MODEL DEPENDANT AND STATISTICAL APPROACHES TO STUDY RELEASE KINETICS OF MELOXICAM RELEASE MATRIX TABLETS
Research Article Bioinformatics International Journal of Pharma and Bio Sciences ISSN 0975-6299 MODEL DEPENDANT AND STATISTICAL APPROACHES TO STUDY RELEASE KINETICS OF MELOXICAM RELEASE MATRIX TABLETS
A comprehensive mathematical model describing drug release from collagen matrices
 Proceedings of the Fifth Workshop on Mathematical Modelling of Environmental and Life Sciences Problems Constanţa, Romania, September, 2006, pp. 27 34 A comprehensive mathematical model describing drug
Proceedings of the Fifth Workshop on Mathematical Modelling of Environmental and Life Sciences Problems Constanţa, Romania, September, 2006, pp. 27 34 A comprehensive mathematical model describing drug
NIH Public Access Author Manuscript J Control Release. Author manuscript; available in PMC 2014 January 10.
 NIH Public Access Author Manuscript Published in final edited form as: J Control Release. 2013 January 10; 165(1): 29 37. doi:10.1016/j.jconrel.2012.10.015. Mathematical Modeling of Drug Delivery from
NIH Public Access Author Manuscript Published in final edited form as: J Control Release. 2013 January 10; 165(1): 29 37. doi:10.1016/j.jconrel.2012.10.015. Mathematical Modeling of Drug Delivery from
Research Journal of Pharmaceutical, Biological and Chemical Sciences
 Research Journal of Pharmaceutical, Biological and Chemical Sciences Kinetics and Mechanisms of Drug Release from Swellable and Non Swellable Matrices: A Review Chime Salome A*, Onunkwo Godswill C and
Research Journal of Pharmaceutical, Biological and Chemical Sciences Kinetics and Mechanisms of Drug Release from Swellable and Non Swellable Matrices: A Review Chime Salome A*, Onunkwo Godswill C and
Encapsulation. Loughborough University Institutional Repository
 Loughborough University Institutional Repository Encapsulation This item was submitted to Loughborough University's Institutional Repository by the/an author. Citation: VLADISAVLJEVIC, G.T. and HOLDICH,
Loughborough University Institutional Repository Encapsulation This item was submitted to Loughborough University's Institutional Repository by the/an author. Citation: VLADISAVLJEVIC, G.T. and HOLDICH,
Technology White Papers nr. 10 Paul Holister Cristina Román Vas Tim Harper
 Technology White Papers nr. 10 Paul Holister Cristina Román Vas Tim Harper NANOCAPSULES Technology White Papers nr. 10 Release Date: Published by Científica Científica, Ltd. www.cientifica.com Científica
Technology White Papers nr. 10 Paul Holister Cristina Román Vas Tim Harper NANOCAPSULES Technology White Papers nr. 10 Release Date: Published by Científica Científica, Ltd. www.cientifica.com Científica
Numerical modeling of diffusion within composite media
 Numerical modeling of diffusion within composite media Miljan Milosevic To cite this version: Miljan Milosevic. Numerical modeling of diffusion within composite media. 2nd ECCOMAS Young Investigators Conference
Numerical modeling of diffusion within composite media Miljan Milosevic To cite this version: Miljan Milosevic. Numerical modeling of diffusion within composite media. 2nd ECCOMAS Young Investigators Conference
Quadratic ODE and PDE models of drug release kinetics from biodegradable polymers
 Quadratic ODE and PDE models of drug release kinetics from biodegradable polymers Michel C. Delfour and André Garon delfour@crm.umontreal.ca Andre.Garon@polymtl.ca Abstract. In order to achieve prescribed
Quadratic ODE and PDE models of drug release kinetics from biodegradable polymers Michel C. Delfour and André Garon delfour@crm.umontreal.ca Andre.Garon@polymtl.ca Abstract. In order to achieve prescribed
PRODUCTION OF DRUG NANOPARTICLES OF CONTROLLABLE SIZE USING SUPERCRITICAL FLUID ANTISOLVENT TECHNIQUE WITH ENHANCED MASS TRANSFER
 PRODUCTION OF DRUG NANOPARTICLES OF CONTROLLABLE SIZE USING SUPERCRITICAL FLUID ANTISOLVENT TECHNIQUE WITH ENHANCED MASS TRANSFER Gupta R.B 1, and Chattopadhyay P.* 2 1-Auburn University, 2-Ferro Corporation.
PRODUCTION OF DRUG NANOPARTICLES OF CONTROLLABLE SIZE USING SUPERCRITICAL FLUID ANTISOLVENT TECHNIQUE WITH ENHANCED MASS TRANSFER Gupta R.B 1, and Chattopadhyay P.* 2 1-Auburn University, 2-Ferro Corporation.
Contents. Foreword by Darrell H. Reneker
 Table of Foreword by Darrell H. Reneker Preface page xi xiii 1 Introduction 1 1.1 How big is a nanometer? 1 1.2 What is nanotechnology? 1 1.3 Historical development of nanotechnology 2 1.4 Classification
Table of Foreword by Darrell H. Reneker Preface page xi xiii 1 Introduction 1 1.1 How big is a nanometer? 1 1.2 What is nanotechnology? 1 1.3 Historical development of nanotechnology 2 1.4 Classification
CHAPTER 8 ACETONE + CARBON DIOXIDE AS TUNABLE MIXTURE SOLVENTS FOR. POLY (ε-caprolactone)
 CHAPTER 8 ACETONE + CARBON DIOXIDE AS TUNABLE MIXTURE SOLVENTS FOR POLY (ε-caprolactone) Poly (ε-caprolactone) is a semi-crystalline polymer that shows a high degree of miscibility with a number of different
CHAPTER 8 ACETONE + CARBON DIOXIDE AS TUNABLE MIXTURE SOLVENTS FOR POLY (ε-caprolactone) Poly (ε-caprolactone) is a semi-crystalline polymer that shows a high degree of miscibility with a number of different
A Thesis. entitled. Niloofar Alipourasiabi. Submitted to the Graduate Faculty as partial fulfillment of the requirements for
 A Thesis entitled Modeling of Controlled Drug Delivery from a Chitosan Microparticle by Niloofar Alipourasiabi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master
A Thesis entitled Modeling of Controlled Drug Delivery from a Chitosan Microparticle by Niloofar Alipourasiabi Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): The author reported an azobenzene-based MOF membrane, in which the permeation as well as selectivity can be remotely controlled by light irradiation.
Reviewers' comments: Reviewer #1 (Remarks to the Author): The author reported an azobenzene-based MOF membrane, in which the permeation as well as selectivity can be remotely controlled by light irradiation.
CHE 611 Advanced Chemical Reaction Engineering
 CHE 611 Advanced Chemical Reaction Engineering Dr. Muhammad Rashid Usman Institute of Chemical Engineering and Technology University of the Punjab, Lahore 54590 mrusman.icet@pu.edu.pk 1 Diffusion and reaction
CHE 611 Advanced Chemical Reaction Engineering Dr. Muhammad Rashid Usman Institute of Chemical Engineering and Technology University of the Punjab, Lahore 54590 mrusman.icet@pu.edu.pk 1 Diffusion and reaction
Continuous Microfluidic Synthesis of PLGA Microparticles by Droplet Method
 Microfluidic Synthesis PLGA Microparticles by Droplet Method - App. Note Continuous Microfluidic Synthesis of PLGA Microparticles by Droplet Method Dolomite s API encapsulation system for PLGA 20 µm to
Microfluidic Synthesis PLGA Microparticles by Droplet Method - App. Note Continuous Microfluidic Synthesis of PLGA Microparticles by Droplet Method Dolomite s API encapsulation system for PLGA 20 µm to
NUMERICAL APPROACH TO INTER-FIBER FLOW IN NON-WOVENS WITH SUPER ABSORBENT FIBERS
 THERMAL SCIENCE, Year 2017, Vol. 21, No. 4, pp. 1639-1644 1639 Introduction NUMERICAL APPROACH TO INTER-FIBER FLOW IN NON-WOVENS WITH SUPER ABSORBENT FIBERS by Zhi-Rong DING a*, Ying GUO a b, and Shan-Yuan
THERMAL SCIENCE, Year 2017, Vol. 21, No. 4, pp. 1639-1644 1639 Introduction NUMERICAL APPROACH TO INTER-FIBER FLOW IN NON-WOVENS WITH SUPER ABSORBENT FIBERS by Zhi-Rong DING a*, Ying GUO a b, and Shan-Yuan
The microparticle system has become an indispensable part of the controlled drug delivery fields for the past few decades since it can
 Microencapsulation Microencapsulation is a process or technique by which thin coatings can be applied reproducibly to small particles of solids, droplets of liquids, or dispersions, thus, forming microcapsules.
Microencapsulation Microencapsulation is a process or technique by which thin coatings can be applied reproducibly to small particles of solids, droplets of liquids, or dispersions, thus, forming microcapsules.
CARBON NANOTUBE-POLYMER COMPOSITES: AN OVERVIEW Brian Grady University of Oklahoma
 CARBON NANOTUBE-POLYMER COMPOSITES: AN OVERVIEW Brian Grady University of Oklahoma Abstract Carbon nanotubes are in many ways similar to polymers. Both molecules have contour lengths typically on the order
CARBON NANOTUBE-POLYMER COMPOSITES: AN OVERVIEW Brian Grady University of Oklahoma Abstract Carbon nanotubes are in many ways similar to polymers. Both molecules have contour lengths typically on the order
Biodegradable hollow fibres containing drug-loaded nanoparticles as controlled release systems
 Polymer International Polym Int 51:1464 1472 (2002) DOI: 10.1002/pi.1086 Biodegradable hollow fibres containing drug-loaded nanoparticles as controlled release systems Giovanni Polacco,* Maria Grazia Cascone,
Polymer International Polym Int 51:1464 1472 (2002) DOI: 10.1002/pi.1086 Biodegradable hollow fibres containing drug-loaded nanoparticles as controlled release systems Giovanni Polacco,* Maria Grazia Cascone,
Key words. moving boundary problem, boundary layer, exponentially small asymptotics, controlled drug release, nonlinear diffusion
 SIAM J. APPL. MATH. c 1998 Society for Industrial and Applied Mathematics Vol. 58, No. 4, pp. 1193 104, August 1998 010 CONTROLLED DRUG RELEASE ASYMPTOTICS DONALD S. COHEN AND THOMAS ERNEUX Abstract. The
SIAM J. APPL. MATH. c 1998 Society for Industrial and Applied Mathematics Vol. 58, No. 4, pp. 1193 104, August 1998 010 CONTROLLED DRUG RELEASE ASYMPTOTICS DONALD S. COHEN AND THOMAS ERNEUX Abstract. The
A Novel Self-aligned and Maskless Process for Formation of Highly Uniform Arrays of Nanoholes and Nanopillars
 Nanoscale Res Lett (2008) 3: 127 DOI 10.1007/s11671-008-9124-6 NANO EXPRESS A Novel Self-aligned and Maskless Process for Formation of Highly Uniform Arrays of Nanoholes and Nanopillars Wei Wu Æ Dibyendu
Nanoscale Res Lett (2008) 3: 127 DOI 10.1007/s11671-008-9124-6 NANO EXPRESS A Novel Self-aligned and Maskless Process for Formation of Highly Uniform Arrays of Nanoholes and Nanopillars Wei Wu Æ Dibyendu
file:///biology Exploring Life/BiologyExploringLife04/
 Objectives Describe the structure of a water molecule. List and describe water's unique properties. Distinguish between an acid and a base. Explain how Earth's conditions are fit for life. Key Terms polar
Objectives Describe the structure of a water molecule. List and describe water's unique properties. Distinguish between an acid and a base. Explain how Earth's conditions are fit for life. Key Terms polar
ANNOUNCEMENTS. HW5 is posted. It is due by 12:00 on 3/11.
 ANNOUNCEMENTS HW5 is posted. It is due by 12:00 on 3/11. Two Compartment Model for Drug Distribution from Blood to Peripheral Tissues Drug Injection Plasma/Blood Peripheral Tissues k 1 C 2 k e Mass Balance
ANNOUNCEMENTS HW5 is posted. It is due by 12:00 on 3/11. Two Compartment Model for Drug Distribution from Blood to Peripheral Tissues Drug Injection Plasma/Blood Peripheral Tissues k 1 C 2 k e Mass Balance
Chemical Engineering Seminar Series
 Effect of Reaction Conditions on Copolymer Properties Loretta Idowu Keywords: copolymer composition distribution; radical polymerization kinetics; semi-batch starved feed; hydroxyl-functionality Non-functional
Effect of Reaction Conditions on Copolymer Properties Loretta Idowu Keywords: copolymer composition distribution; radical polymerization kinetics; semi-batch starved feed; hydroxyl-functionality Non-functional
Preparation of porous polylactide microspheres by emulsion-solvent evaporation based on solution induced phase separation
 POLYMERS FOR ADVANCED TECHNOLOGIES Polym. Adv. Technol. 5; 16: 622 627 Published online 24 June 5 in Wiley InterScience (www.interscience.wiley.com). DOI: 1.12/pat.629 Preparation of porous polylactide
POLYMERS FOR ADVANCED TECHNOLOGIES Polym. Adv. Technol. 5; 16: 622 627 Published online 24 June 5 in Wiley InterScience (www.interscience.wiley.com). DOI: 1.12/pat.629 Preparation of porous polylactide
Potassium ion-recognizable responsive smart materials
 Potassium ion-recognizable responsive smart materials *Xiao-Jie Ju 1), Zhuang Liu 2), Hai-Rong Yu 2), Rui Xie 1), Wei Wang 3) and Liang-Yin Chu 4) 1), 2), 3), 4) School of Chemical Engineering, Sichuan
Potassium ion-recognizable responsive smart materials *Xiao-Jie Ju 1), Zhuang Liu 2), Hai-Rong Yu 2), Rui Xie 1), Wei Wang 3) and Liang-Yin Chu 4) 1), 2), 3), 4) School of Chemical Engineering, Sichuan
Encapsulation. Battelle Technology. Introduction
 Encapsulation Introduction The encapsulation methods reported in the literature 1-7 for the production of microcapsules are generally achieved using one of the following techniques: 1. Phase separation
Encapsulation Introduction The encapsulation methods reported in the literature 1-7 for the production of microcapsules are generally achieved using one of the following techniques: 1. Phase separation
Polyelectrolyte hydrogels
 Polyelectrolyte hydrogels Last Day: Physical hydrogels Structure and physical chemistry Today: polyelectrolyte hydrogels, complexes, and coacervates Polyelectrolyte multilayers theory of swelling in ionic
Polyelectrolyte hydrogels Last Day: Physical hydrogels Structure and physical chemistry Today: polyelectrolyte hydrogels, complexes, and coacervates Polyelectrolyte multilayers theory of swelling in ionic
,,, [ PEO2PPO2PEO ( EO) n ( PO) m ( EO) n ] , PEO2. (37 ), Lee [5 ] Pluronic F127 ( PEO 99 PPO 67 PEO 99 ) , F127 B 12
![,,, [ PEO2PPO2PEO ( EO) n ( PO) m ( EO) n ] , PEO2. (37 ), Lee [5 ] Pluronic F127 ( PEO 99 PPO 67 PEO 99 ) , F127 B 12 ,,, [ PEO2PPO2PEO ( EO) n ( PO) m ( EO) n ] , PEO2. (37 ), Lee [5 ] Pluronic F127 ( PEO 99 PPO 67 PEO 99 ) , F127 B 12](/thumbs/88/117118757.jpg) 34 2007 10 PEO2PPO2PEO 1,2, 3, 2 2 1,, 3 1, (1., 200433 ; 2., 201512) : 2 2, 2 2,, 2 2,, : 2 2 ; ; 2 2 [ PEO2PPO2PEO ( EO) n ( PO) m ( EO) n ],,PEO2PPO2PEO,,,, PEO2PPO2PEO, 1 PEO2PPO2PEO PEO2PPO2PEO,,
34 2007 10 PEO2PPO2PEO 1,2, 3, 2 2 1,, 3 1, (1., 200433 ; 2., 201512) : 2 2, 2 2,, 2 2,, : 2 2 ; ; 2 2 [ PEO2PPO2PEO ( EO) n ( PO) m ( EO) n ],,PEO2PPO2PEO,,,, PEO2PPO2PEO, 1 PEO2PPO2PEO PEO2PPO2PEO,,
Modeling Drug Release from Materials Based on Electrospun Nanofibers
 Modeling Drug Release from Materials Based on Electrospun Nanofibers Paweł Nakielski 1 *, Tomasz Kowalczyk 1,Tomasz A. Kowalewski 1 1 Institute of Fundamental Technological Research Polish Academy of Sciences
Modeling Drug Release from Materials Based on Electrospun Nanofibers Paweł Nakielski 1 *, Tomasz Kowalczyk 1,Tomasz A. Kowalewski 1 1 Institute of Fundamental Technological Research Polish Academy of Sciences
(ii) Compare the movement and arrangement of the molecules in solid nitrogen to those in nitrogen gas.
 1 Kinetic theory explains the properties of matter in terms of the arrangement and movement of particles. (a) Nitrogen is a gas at room temperature. Nitrogen molecules, N 2, are spread far apart and move
1 Kinetic theory explains the properties of matter in terms of the arrangement and movement of particles. (a) Nitrogen is a gas at room temperature. Nitrogen molecules, N 2, are spread far apart and move
POLYPYRROLE FILMS PREPARED BY CHEMICAL OXIDATION OF PYRROLE IN AQUEOUS FeCl 3 SOLUTION
 Journal of Science and Arts Year 10, No. 1 (12), pp. 53-58, 2010 POLYPYRROLE FILMS PREPARED BY CHEMICAL OXIDATION OF PYRROLE IN AQUEOUS FeCl 3 SOLUTION DRAGOŞ-VIOREL BREZOI Valahia University of Targoviste,130024,
Journal of Science and Arts Year 10, No. 1 (12), pp. 53-58, 2010 POLYPYRROLE FILMS PREPARED BY CHEMICAL OXIDATION OF PYRROLE IN AQUEOUS FeCl 3 SOLUTION DRAGOŞ-VIOREL BREZOI Valahia University of Targoviste,130024,
Steady-State Molecular Diffusion
 Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
PRODUCTION OF PEG SUBMICRON PARTICLES BY THE SOLUTION ENHANCED DISPERSION WITH ENHANCED MASS TRANSFER BY ULTRASOUND IN SUPERCRITICAL CO 2 (SEDS-EM)
 PRODUCTION OF PEG SUBMICRON PARTICLES BY THE SOLUTION ENHANCED DISPERSION WITH ENHANCED MASS TRANSFER BY ULTRASOUND IN SUPERCRITICAL CO 2 (SEDS-EM) Heyang Jin, Sining Li, Daode Hu and Yaping Zhao* Email
PRODUCTION OF PEG SUBMICRON PARTICLES BY THE SOLUTION ENHANCED DISPERSION WITH ENHANCED MASS TRANSFER BY ULTRASOUND IN SUPERCRITICAL CO 2 (SEDS-EM) Heyang Jin, Sining Li, Daode Hu and Yaping Zhao* Email
Magnetic Nano Particles for In-Vitro Diagnostics
 Magnetic Nano Particles for In-Vitro Diagnostics has utilized its many years of experience in creating a Magnetic Nano Particles Developer Kit for in-vitro biomedical applications. The kit offers a unique
Magnetic Nano Particles for In-Vitro Diagnostics has utilized its many years of experience in creating a Magnetic Nano Particles Developer Kit for in-vitro biomedical applications. The kit offers a unique
Elec Eng 3BA3: Structure of Biological Materials
 Elec Eng 3BA3: Structure of Biological Materials Page 1 of 12 Day Class Instructor: Dr. I. C. BRUCE Duration of Examination: 3 Hours McMaster University Final Examination December 5, 2008 This examination
Elec Eng 3BA3: Structure of Biological Materials Page 1 of 12 Day Class Instructor: Dr. I. C. BRUCE Duration of Examination: 3 Hours McMaster University Final Examination December 5, 2008 This examination
CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer
 CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer You are assigned to design a fallingcylinder viscometer to measure the viscosity of Newtonian liquids. A schematic
CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer You are assigned to design a fallingcylinder viscometer to measure the viscosity of Newtonian liquids. A schematic
Evaluation of diffusional resistances in the process of glucose isomerization to fructose by immobilized glucose isomerase
 Enzyme and Microbial Technology 28 (2001) 246 252 www.elsevier.com/locate/enzmictec Evaluation of diffusional resistances in the process of glucose isomerization to fructose by immobilized glucose isomerase
Enzyme and Microbial Technology 28 (2001) 246 252 www.elsevier.com/locate/enzmictec Evaluation of diffusional resistances in the process of glucose isomerization to fructose by immobilized glucose isomerase
An introduction to particle size characterisation by DCS:
 An introduction to particle size characterisation by DCS: Do you know the real size of your nano particles? By Dr Hiran Vegad, Analytik Ltd Introduction Differential centrifugal sedimentation (DCS) is
An introduction to particle size characterisation by DCS: Do you know the real size of your nano particles? By Dr Hiran Vegad, Analytik Ltd Introduction Differential centrifugal sedimentation (DCS) is
DISSOLUTION PROFILLING OF NIMESULIDE SOLID DISPERSIONS WITH POLYETHYLENE GLYCOL, TALC AND THEIR COMBINATIONS AS DISPERSION CARRIERS
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.2, No.1, pp 480-484, Jan-Mar 2010 DISSOLUTION PROFILLING OF NIMESULIDE SOLID DISPERSIONS WITH POLYETHYLENE GLYCOL, TALC
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.2, No.1, pp 480-484, Jan-Mar 2010 DISSOLUTION PROFILLING OF NIMESULIDE SOLID DISPERSIONS WITH POLYETHYLENE GLYCOL, TALC
CHEMICAL ENGINEERING (CHE)
 Chemical Engineering (CHE) 1 CHEMICAL ENGINEERING (CHE) CHE 2033 Introduction to Chemical Process Engineering Prerequisites: CHEM 1515 and ENSC 2213 Description: Concurrent enrollment in MATH 2233 or 3263,
Chemical Engineering (CHE) 1 CHEMICAL ENGINEERING (CHE) CHE 2033 Introduction to Chemical Process Engineering Prerequisites: CHEM 1515 and ENSC 2213 Description: Concurrent enrollment in MATH 2233 or 3263,
Supporting Information
 Supporting Information Fully acid-degradable biocompatible polyacetal microparticles for drug delivery Sergey E. Paramonov, Eric M. Bachelder, Tristan T. Beaudette, Stephany M. Standley, Cameron C. Lee,
Supporting Information Fully acid-degradable biocompatible polyacetal microparticles for drug delivery Sergey E. Paramonov, Eric M. Bachelder, Tristan T. Beaudette, Stephany M. Standley, Cameron C. Lee,
A Model for Non-Newtonian Flow in Porous Media at Different Flow Regimes
 A Model for Non-Newtonian Flow in Porous Media at Different Flow Regimes Quick introduction to polymer flooding Outline of talk Polymer behaviour in bulk versus porous medium Mathematical modeling of polymer
A Model for Non-Newtonian Flow in Porous Media at Different Flow Regimes Quick introduction to polymer flooding Outline of talk Polymer behaviour in bulk versus porous medium Mathematical modeling of polymer
Supplementary Figure 1. Cross-section SEM image of the polymer scaffold perovskite film using MAI:PbI 2 =1:1 in DMF solvent on the FTO/glass
 Supplementary Figure 1. Cross-section SEM image of the polymer scaffold perovskite film using MAI:PbI 2 =1:1 in DMF solvent on the FTO/glass substrate. Scale bar: 1 m. Supplementary Figure 2. Contact angle
Supplementary Figure 1. Cross-section SEM image of the polymer scaffold perovskite film using MAI:PbI 2 =1:1 in DMF solvent on the FTO/glass substrate. Scale bar: 1 m. Supplementary Figure 2. Contact angle
CFD Modeling of Hollow Fiber Membrane Contactor for Post-Combustion CO 2 Capture
 1 CFD Modeling of Hollow Fiber Membrane Contactor for Post-Combustion CO 2 Capture Muhammad Saeed, Liyuan Deng* Membrane Research Group (Memfo), Department of Chemical Engineering, Norwegian University
1 CFD Modeling of Hollow Fiber Membrane Contactor for Post-Combustion CO 2 Capture Muhammad Saeed, Liyuan Deng* Membrane Research Group (Memfo), Department of Chemical Engineering, Norwegian University
Carbon Dioxide-Philic Hybrid Polyhedral Oligomeric Silsesquioxanes
 Carbon Dioxide-Philic Hybrid Polyhedral Oligomeric Silsesquioxanes Basak KANYA, Novendra NOVENDRA, Cerag DILEK* Department of Chemical Engineering, Middle East Technical University, Ankara, 06800, Turkey
Carbon Dioxide-Philic Hybrid Polyhedral Oligomeric Silsesquioxanes Basak KANYA, Novendra NOVENDRA, Cerag DILEK* Department of Chemical Engineering, Middle East Technical University, Ankara, 06800, Turkey
Fundamentals of Heat Transfer Muhammad Rashid Usman
 Fundamentals of Heat Transfer Muhammad Rashid Usman Institute of Chemical Engineering and Technology University of the Punjab, Lahore. Figure taken from: http://heatexchanger-design.com/2011/10/06/heat-exchangers-6/
Fundamentals of Heat Transfer Muhammad Rashid Usman Institute of Chemical Engineering and Technology University of the Punjab, Lahore. Figure taken from: http://heatexchanger-design.com/2011/10/06/heat-exchangers-6/
Formulation and Ocular Bioavailability of Certain Drugs from Ophthalmic Preparations
 Formulation and Ocular Bioavailability of Certain Drugs from Ophthalmic Preparations Thesis presented by Ghada Saad Ahmed El-Moursy B. Pharm. Mansoura University (2007) Submitted in Partial Fulfillment
Formulation and Ocular Bioavailability of Certain Drugs from Ophthalmic Preparations Thesis presented by Ghada Saad Ahmed El-Moursy B. Pharm. Mansoura University (2007) Submitted in Partial Fulfillment
Medical Engineering and Physics
 Medical Engineering and Physics 45 (7) 5 6 Contents lists available at ScienceDirect Medical Engineering and Physics journal homepage: www.elsevier.com/locate/medengphy Mathematical modelling of variable
Medical Engineering and Physics 45 (7) 5 6 Contents lists available at ScienceDirect Medical Engineering and Physics journal homepage: www.elsevier.com/locate/medengphy Mathematical modelling of variable
Extraction Behaviour of Cu 2+ Ions with Used Cooking Oil-Based Organic Solvent
 International Proceedings of Chemical, Biological and Environmental Engineering, V0l. 96 (2016) DOI: 10.7763/IPCBEE. 2016. V96. 4 Extraction Behaviour of Cu 2+ Ions with Used Cooking Oil-Based Organic
International Proceedings of Chemical, Biological and Environmental Engineering, V0l. 96 (2016) DOI: 10.7763/IPCBEE. 2016. V96. 4 Extraction Behaviour of Cu 2+ Ions with Used Cooking Oil-Based Organic
Science Year 10 Unit 1 Biology
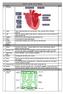 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Chapter Chemical Elements Matter solid, liquid, and gas elements atoms. atomic symbol protons, neutrons, electrons. atomic mass atomic number
 Chapter 2 2.1 Chemical Elements 1. Matter is defined as anything that takes up space and has mass. 2. Matter exists in three states: solid, liquid, and gas. A. Elements 1. All matter (both living and non-living)
Chapter 2 2.1 Chemical Elements 1. Matter is defined as anything that takes up space and has mass. 2. Matter exists in three states: solid, liquid, and gas. A. Elements 1. All matter (both living and non-living)
Model Solutions Spring 2003
 Exam 2 BE.462J/3.962J Model Solutions Spring 2003 (80 points total possible) 1. (10 points) Explain the phenomenon of phsensitive swelling in polyelectrolyte hydrogels. Why does the swelling depend on
Exam 2 BE.462J/3.962J Model Solutions Spring 2003 (80 points total possible) 1. (10 points) Explain the phenomenon of phsensitive swelling in polyelectrolyte hydrogels. Why does the swelling depend on
Drug Delivery with Alginate Dr. J. Vernengo and Dr. S. Farrell
 Objectives Drug Delivery with Alginate Dr. J. Vernengo and Dr. S. Farrell Define a hydrogel. Define the chemical structure and ionic crosslinking of alginate to form hydrogels. Discuss the role of hydrogels
Objectives Drug Delivery with Alginate Dr. J. Vernengo and Dr. S. Farrell Define a hydrogel. Define the chemical structure and ionic crosslinking of alginate to form hydrogels. Discuss the role of hydrogels
Convective Mass Transfer
 Convective Mass Transfer Definition of convective mass transfer: The transport of material between a boundary surface and a moving fluid or between two immiscible moving fluids separated by a mobile interface
Convective Mass Transfer Definition of convective mass transfer: The transport of material between a boundary surface and a moving fluid or between two immiscible moving fluids separated by a mobile interface
PRODUCTION OF L-PLA MICROPARTICLES BELOW AND ABOVE THE MIXTURE CRITICAL PRESSURE OF THE SYSTEM DCM-CO 2
 PRODUCTION OF L-PLA MICROPARTICLES BELOW AND ABOVE THE MIXTURE CRITICAL PRESSURE OF THE SYSTEM DCM-CO 2 Y. Pérez * (a), H. Pellikaan (b), F. E. Wubbolts (a), G. J. Witkamp (a), P. J. Jansens (a) (a) Laboratory
PRODUCTION OF L-PLA MICROPARTICLES BELOW AND ABOVE THE MIXTURE CRITICAL PRESSURE OF THE SYSTEM DCM-CO 2 Y. Pérez * (a), H. Pellikaan (b), F. E. Wubbolts (a), G. J. Witkamp (a), P. J. Jansens (a) (a) Laboratory
Recognition and Absorption of the Water-soluble X-ray Contrast Medium Iodixanol using Molecularly Imprinted Polymers for Biomedical Applications
 Recognition and Absorption of the Water-soluble X-ray Contrast Medium Iodixanol using Molecularly Imprinted Polymers for Biomedical Applications Zhan Liu 1, David G. Buckanll 1, and Mark G. Allen 2 1 School
Recognition and Absorption of the Water-soluble X-ray Contrast Medium Iodixanol using Molecularly Imprinted Polymers for Biomedical Applications Zhan Liu 1, David G. Buckanll 1, and Mark G. Allen 2 1 School
A mechanism on the drug release into a perfect sink from a coated planar matrix with a super-saturation loading in the core
 International Journal of Pharmaceutics 197 (2000) 23 34 www.elsevier.com/locate/ijpharm A mechanism on the drug release into a perfect sink from a coated planar matrix with a super-saturation loading in
International Journal of Pharmaceutics 197 (2000) 23 34 www.elsevier.com/locate/ijpharm A mechanism on the drug release into a perfect sink from a coated planar matrix with a super-saturation loading in
Supplementary Figure S1. Verifying the CH 3 NH 3 PbI 3-x Cl x sensitized TiO 2 coating UV-vis spectrum of the solution obtained by dissolving the
 Supplementary Figure S1. Verifying the CH 3 NH 3 PbI 3-x Cl x sensitized TiO 2 coating UV-vis spectrum of the solution obtained by dissolving the spiro-ometad from a perovskite-filled mesoporous TiO 2
Supplementary Figure S1. Verifying the CH 3 NH 3 PbI 3-x Cl x sensitized TiO 2 coating UV-vis spectrum of the solution obtained by dissolving the spiro-ometad from a perovskite-filled mesoporous TiO 2
2 THEORY OF TRANSPORT IN MEMBRANES
 2 THEORY OF TRANSPORT IN MEMBRANES 2.1 Driving forces for transport mechanisms A membrane process is a separation process that covers a broad range of problems from particles to molecules and a wide variety
2 THEORY OF TRANSPORT IN MEMBRANES 2.1 Driving forces for transport mechanisms A membrane process is a separation process that covers a broad range of problems from particles to molecules and a wide variety
Diffusion-Controlled Quasi-Stationary Mass. Transfer for an Isolated Spherical Particle in. an Unbounded Medium
 arxiv:1112.1861v2 [math-ph] 20 Sep 2012 Diffusion-Controlled Quasi-Stationary Mass Transfer for an Isolated Spherical Particle in an Unbounded Medium JAMES Q. FENG Boston Scientific Corporation, 3 Scimed
arxiv:1112.1861v2 [math-ph] 20 Sep 2012 Diffusion-Controlled Quasi-Stationary Mass Transfer for an Isolated Spherical Particle in an Unbounded Medium JAMES Q. FENG Boston Scientific Corporation, 3 Scimed
Prof. K. E. Healy 465 Evans Hall
 NAME: Prof. K. E. Healy 465 Evans Hall MSE/BioE Cl18 -Biological Performance of Materials Exam 1: October 18,2001 Closed Book Exam Please answer all of the questions clearly and box your final answer.
NAME: Prof. K. E. Healy 465 Evans Hall MSE/BioE Cl18 -Biological Performance of Materials Exam 1: October 18,2001 Closed Book Exam Please answer all of the questions clearly and box your final answer.
Effective diffusion coefficients measurement in polysaccharide based hydrogels.
 Effective diffusion coefficients measurement in polysaccharide based hydrogels. Aim of the work To estimate effective diffusion coefficients of substrate diffusion from limited volume of solution into
Effective diffusion coefficients measurement in polysaccharide based hydrogels. Aim of the work To estimate effective diffusion coefficients of substrate diffusion from limited volume of solution into
DRUG DELIVERY SYSTEM FOR ARTEMISININ
 DRUG DELIVERY SYSTEM FOR ARTEMISININ Timothy Dexter T. Tan, Anne Marianne Allison S. Tan, Drexel H. Camacho Chemistry Department, De La Salle University, 2401 Taft Avenue, Manila Abstract: Metal-organic
DRUG DELIVERY SYSTEM FOR ARTEMISININ Timothy Dexter T. Tan, Anne Marianne Allison S. Tan, Drexel H. Camacho Chemistry Department, De La Salle University, 2401 Taft Avenue, Manila Abstract: Metal-organic
Investigating the Relationship Between the Rheological Properties of Hyaluronic Acid and its Molecular Weight and Structure using Multidetector
 Investigating the Relationship Between the Rheological Properties of Hyaluronic Acid and its Molecular Weight and Structure using Multidetector SEC and SEC-MALS Presented by Bassem Sabagh, PhD Technical
Investigating the Relationship Between the Rheological Properties of Hyaluronic Acid and its Molecular Weight and Structure using Multidetector SEC and SEC-MALS Presented by Bassem Sabagh, PhD Technical
hydrogels Lecture 7 Spring
 hydrogels Lecture 7 Spring 2006 2 Thermodynamics of hydrogel swelling polymerize Move to a new, larger aqueous bath V r swelling V s Lecture 7 Spring 2006 3 Thermodynamics of hydrogel swelling Competing
hydrogels Lecture 7 Spring 2006 2 Thermodynamics of hydrogel swelling polymerize Move to a new, larger aqueous bath V r swelling V s Lecture 7 Spring 2006 3 Thermodynamics of hydrogel swelling Competing
Preparation and characterization of genipin-cross-linked chitosan microparticles by water-in-oil emulsion solvent diffusion method
 Vol.2, No.10, 101-105 (10) http://dx.doi.org/10.423/ns.10.210131 Natural Science Preparation and characterization of genipin-cross-linked chitosan microparticles by water-in-oil emulsion solvent diffusion
Vol.2, No.10, 101-105 (10) http://dx.doi.org/10.423/ns.10.210131 Natural Science Preparation and characterization of genipin-cross-linked chitosan microparticles by water-in-oil emulsion solvent diffusion
Water Soluble Polymers For Industrial Water Treatment Applications
 Water Soluble Polymers For Industrial Water Treatment Applications Presented By Technical Sales Jim Millard Course Objectives Explain what water soluble polymers are. Describe the 4 physical forms commonly
Water Soluble Polymers For Industrial Water Treatment Applications Presented By Technical Sales Jim Millard Course Objectives Explain what water soluble polymers are. Describe the 4 physical forms commonly
Studies on flow through and around a porous permeable sphere: II. Heat Transfer
 Studies on flow through and around a porous permeable sphere: II. Heat Transfer A. K. Jain and S. Basu 1 Department of Chemical Engineering Indian Institute of Technology Delhi New Delhi 110016, India
Studies on flow through and around a porous permeable sphere: II. Heat Transfer A. K. Jain and S. Basu 1 Department of Chemical Engineering Indian Institute of Technology Delhi New Delhi 110016, India
