Spectroscopic study of electrons emitted in Ar q 8 q 16 on Ar at 2.3q kev collision energy
|
|
|
- Angelina Lorena Stevenson
- 5 years ago
- Views:
Transcription
1 PHYSICAL REVIEW A VOLUME 53, NUMBER 4 APRIL 1996 Spectroscopic study of electrons emitted in Ar q 8 q 16 on Ar at 2.3q kev collision energy J. Vancura, P. J. Mucha, and V. O. Kostroun Nuclear Science and Engineering Program, Ward Laboratory, Cornell University, Ithaca, New York Received 27 September 1995 The spectra of electrons emitted in Ar q on Ar (8 q 16) collisions at 2.3q kev were measured in the eV energy range. Ar q ions were produced by the Cornell superconducting solenoid, cryogenic electron-beam ion source, and the emitted electrons analyzed by a /2 cylindrical electrostatic analyzer at 90 to the ion beam. The observed spectral features are interpreted in terms of energy differences between total electronic energies of final states of the collision system Ar q electron configuration Ar r and the Ar q Ar ground-state total electronic energy. The measured spectra appear to have a common interpretation. PACS number s : Fa, q, e, Fi I. INTRODUCTION Spectroscopic investigations of electron emissions in lowenergy, highly charged ion-atom collisions under singlecollision conditions were reported by Bordenave- Montesquieu et al. 1, who investigated electrons emitted in the decay of autoionizing states N 5 (nln 1 )(n 2,3,4 and n n) formed in collisions of N 7 with He and H 2 at 4.9 kev/amu. The number of systems that has been investigated since is quite large, with emphasis placed on bare and H- and He-like ions of C, N, and Ne incident on H 2 and He 2 8. Double-electron capture in 10q kev Ar 8,9 collisions with He atoms was investigated by electron spectroscopy by Hutton et al. 9. Multiple-electron capture in low-energy, highly charged Ar-ion collisions with Ne and Ar was investigated with electron spectroscopy by Posthumus and Morgenstern 10,11. Unlike double-electron capture from H 2 and He, which results in electron emission from only a few autoionizing states of the projectile formed in capture, multiple-electron capture from many-electron targets gives rise to electron spectra that are much more difficult to interpret because of the large number of excited projectile capture states possible. Furthermore, in a purely spectroscopic measurement, one cannot distinguish in an unambiguous manner between autoionization from states populated directly in capture and nonradiative decays from states populated in the deexcitation of higher-energy, multiply excited states. In collisions of low-energy, highly charged ions with many-electron targets, most of the projectiles undergo collisions in which the charge state q, observed long after the collision, decreases by one unit only. For example, for Ar q on Ar, collisions involving projectile charge-state changes by two units are less frequent, and by more than two units are quite rare 12. At the same time, the recoil target ion charge state distribution contains ions whose charge can range from one to eight 13. Since singly charged recoil ions account only for about one half of the ions observed 12, a significant fraction of the collisions has to be accompanied by electron emissions. By looking at the number and energy distribution of the electrons emitted, one might expect to learn something about collisions between low-energy, highly charged ions and many-electron target atoms. Unfortunately, the large number of target electrons which can be captured by the projectile complicates the interpretation of the observed electron spectra. There are at least two ways of approaching this problem of interpretation. One approach, taken by Posthumus and Morgenstern 10, is to measure energy-analyzed electrons in coincidence with target recoil ions. In this manner, it is possible to obtain partial electron energy spectra, each spectrum corresponding to a specific charge state of the target ion, and hence to the number of electrons initially captured assuming that in capture the target is not ionized or excited to a state that decays nonradiatively. In this paper we report on a somewhat different method of attack on the problem of interpretation. The method is based on a systematic study of the electron spectra emitted in Ar q on Ar (8 q 16) collisions at 2.3q kev. As far as we have been able to determine, this is the first investigation of electron emissions in low-energy, ion-atom collisions involving projectile charges q greater than ten. II. EXPERIMENT In the present experiment, Ar q (8 q 16) ions were produced by the Cornell superconducting solenoid, cryogenic electron-beam ion source CEBIS 14, and extracted at 2.3 kv. The pertinent experimental arrangement is shown in Fig. 1. Magnetically selected Ar charge states passed through a 10-cm-long gas cell which contained argon at mbar pressure. The gas cell was similar in design to that used in an earlier experiment to measure absolute total, and one- and two-electron transfer cross sections in Ar q on H 2 and He collisions 15. The ion beam transmitted through the gas cell could be charge state analyzed by a / 2 electrostatic analyzer. Inside the gas cell, the ion beam was surrounded by a cylinder made from stainless-steel mesh of 80% transparency. The mesh was insulated from ground, and its potential with respect to the grounded gas cell could be varied to accelerate or decelerate the electrons emitted in Ar q on Ar collisions. Electrons emitted from a 1.5-cm-long portion of the gasion beam were analyzed at 90 to the incident-beam direction by a / 2 cylindrical electrostatic analyzer with its en /96/53 4 / /$ The American Physical Society
2 2380 J. VANCURA, P. J. MUCHA, AND V. O. KOSTROUN 53 FIG. 2. Auger L 2,3 M 2,3 M 2,3 electron spectrum excited by 3-keV electron impact and recorded using the apparatus shown in Fig. 1. FIG. 1. Experimental arrangement used to measure electron spectra emitted in Ar q (8 q 16) on Ar collisions at 2.3q kev. trance slit parallel to the beam. The analyzer was made from aluminum which was copper and then gold plated. The outer and inner radii of the analyzer are 6.03 and 5.02 cm, respectively. The electrons were detected by a 1-cm-diameter cone channeltron detector. The gas cell and cylindrical electrostatic analyzer were mounted on a 20-cm-diameter stainlesssteel vacuum flange attached to a 15-cm six-way cross vacuum chamber. The system was pumped on by a Varian Cryostack 8 cryopump, and the background pressure, with no gas in the cell, was mbar. The analyzer was shielded by a thin -metal cylinder, 12 cm in diameter and 40 cm long. The electron spectrometer was located near the focus of the 90 bending magnet used to charge select the ion beam extracted from CEBIS. At this position, the measured ionbeam diameter was 1.5 mm. The beam diameter, together with a 1.0-mm-wide analyzer entrance slit, the analyzer and gas cell dimensions, and the 10-mm-diameter channeltron detector placed directly behind the exit slit, set the energy resolution E/E at The operation of the / 2 cylindrical electrostatic analyzer was verified by recording argon L 2,3 MM Augerelectron spectra excited by 3-keV electron impact, Fig. 2. The 3-keV electron beam was generated by an electron gun from a Westinghouse Electric Corp cm WX-30810P cathode ray tube, mounted at the entrance to the apparatus where the ion beam normally entered. The electron beam transmitted through the chamber was observed on a fluorescent screen. From the measured screen beam size diameter less than 1 mm, and the physical dimensions of the apparatus, the width of the unresolved L 2 M 2,3 M 2,3 peaks is consistent with the calculated resolution of the system, 5 ev at 205 ev. The most intense peaks in the spectrum are the L 2 M 2,3 M 2,3 ( 1 D) peak at ev and the ( 3 P) peak at ev 16,17. To record the electron spectra emitted in Ar q on Ar collisions, the potentials V/2 on the inner and outer electrodes of the electron analyzer were set to transmit 45-eV electrons, and the emitted electrons were either accelerated or decelerated to this energy by varying the potential applied between the cylindrical mesh surrounding the ion beam and the grounded gas cell. The fixed potential difference between the plates of the electrostatic analyzer ensured constant transmission through the analyzer. The geometry used to analyze the emitted electrons was chosen to maximize the detection solid angle subtended by a line source parallel to the entrance slit of a cylindrical electrostatic analyzer. In this case, the angular divergence in the analyzing plane is determined by the length of the line source, the source to detector distance, and the detector diameter. The disadvantage of this geometry is that at 90 observation angle, the Doppler shift variation of electron energy with angle is the greatest. However, at a projectile velocity of less than 0.2 a.u., corresponding to a 36.8-keV Ar 16 ion beam, the Doppler broadening of eV electrons due to a 3.5 acceptance angle around 90 is less than 0.4 ev. III. RESULTS Electrons emitted in Ar q -Ar collisions in the eV energy range were recorded for projectile charge states from 8 to 16 inclusive. The results are shown in Figs. 3 a 3 i.in the figure, the electron spectra were normalized to the same projectile beam intensity in order to show how the electron intensity increases with projectile charge state. A small but constant background due to electrons produced in other parts of the apparatus and scattered into the detector was subtracted from each of the spectra. For each incident projectile charge state, the ion beam transmitted through the cell was charge state analyzed to ensure that the transmitted beam contained mostly one and two electron transfer peaks. Each spectrum shown is a series of several runs recorded over a period of 1 6 h.
3 SPECTROSCOPIC STUDY OF ELECTRONS EMITTED IN Ar q... FIG. 3. a i spectra of electrons emitted in Ar q (8 q 16) on Ar collisions at 2.3q kev. The spectra were normalized to the same number of incident ions, and a small constant background was subtracted from each. Note the marked similarities between b and c, and among d f and g i. Figure 4 compares our electron spectrum for Ar 9 on Ar at 2.3q kev collision energy with that obtained by Posthumus and Morgenstern at 12.0q kev 10. Clearly the spectra are quite similar. Our spectrum is a little bit more smeared out than that of Posthumus and Morgenstern. We do not know whether or not this is an instrumental effect, their resolution being better than ours, or an effect due to the lower collision velocity in our experiment. To bring the main features into alignment on the same energy scale, the spectra had to be shifted by 8 ev relative to one another.
4 2382 J. VANCURA, P. J. MUCHA, AND V. O. KOSTROUN 53 FIG. 4. Comparison between our measured electron spectrum emitted in 2.3q kev Ar 9 on Ar collisions and that of Posthumus and Morgenstern 10 emitted at 12.0q kev inset. To bring the major peaks into alignment, the spectra had to be shifted by 8 ev relative to one another. IV. DISCUSSION The total electronic ground-state energies of the Ar q on Ar collision system before and after the collision determine the net change in the internal energy of this system. This change in internal energy appears as i a change in the total kinetic energy of the nuclei, and ii as the energy of photons and the kinetic energy of electrons emitted during and after the collisions. If r electrons are captured from the target by the projectile in the collision, Ar q Ar Ar q r * Ar r. In turn, the excited projectile and target deexcite, so that and Ar q r * Ar q s r s e photons Ar r * Ar r t te photons. 1 2a 2b In what follows, we neglect target excitation that deexcites by nonradiative transitions, and set t 0 in 2b. Let E(Ar q ) E Ar be the total electronic energy of the ground state of the system at infinite separation before the collision, and E(Ar (q s) ) E(Ar r ) the total electronic ground-state energy of the system at infinite separation after the collision. Ultimately, the energy difference E Ar q E Ar E Ar q s E Ar r has to be shared by the nuclei and the electrons and photons emitted during, or long after, the collision. From Eq. 3 we can calculate the maximum recoil charge that will be observed in the collision. For example, for one electron transfer, unaccompanied by target excitation, i.e., for the capture of r electrons in the collision, of which only one is permanently retained by the projectile, we require that E Ar r E Ar q E Ar E Ar q 1. With the help of the general Hartree-Fock program HF86 18, we obtain the following values for the total relativistic 3 4 electronic energies: E( Ar 8 ) a.u., E(Ar 7 ) a.u., and E(Ar) a.u. From Eq. 4 we obtain that E( Ar r ) a.u. Since E(Ar 4 ) , the maximum recoil charge state that is energetically allowed with Ar 7 is 4. If we consider twoelectron transfer, E(Ar (q 1) ) in Eq. 4 has to be replaced by E(Ar (q 2) ), and since E(Ar (q 1) ) E(Ar (q 2) ), the maximum recoil charge r observed in this case will be greater. For E(Ar 6 ) a.u., E(Ar r ) a.u., and since E(Ar 5 ) , the maximum recoil charge state energetically allowed with Ar 6 is 5. This is consistent with the measured recoil ion charge-state distribution for Ar 8 on Ar collisions 13, Ar 1, ; Ar 2, ; Ar 3, ; and Ar 4, Furthermore, since the fraction of Ar 7, Ar 6, and Ar 5 final charge projectiles observed is , , and , respectively 12, the maximum recoil charge state observed is accounted for. From the total electronic energies of excited atoms or ions, we can calculate Q values for the capture of r electrons during the collision. The Q value is the change in the total kinetic energy of the nuclear translational motion of the collision system that accompanies capture. The Q value for single-electron capture into a state nl of the projectile is Q nl E Ar q E Ar E Ar q nl E Ar 1, 5a that for the capture of two electrons into the state (nl) (n l )is Q nl n l E Ar q E Ar E Ar q nl n l E Ar 2, 5b and so on. If one assumes that the electrons transferred from the target to the projectile during the collision form an excited state that deexcites some time after the collision partners have separated, then we can use the energies of separated atoms and ions to fill in the energy levels between the ground states of the collision system long before and after the collision. For nonradiative transition rates of the order of 10 3 a.u. or less, 0.15-a.u. collision velocity, and 20-a.u. internuclear separation, t 0.133, and exp( t ) In this case very few decays take place while the collision partners are interacting strongly, and the above assumption for calculating Auger energies is reasonable. In what follows, it is convenient to measure the total electronic energies at infinite separation of singly, doubly, etc. excited states of ions accompanied by recoils of charge r, with respect to the initial ground-state total electronic energy of the Ar q Ar system. That is, let E q,r, nl E Ar q nl E Ar r E Ar q E Ar, 6a
5 SPECTROSCOPIC STUDY OF ELECTRONS EMITTED IN Ar q... FIG. 5. Schematic energy-level diagram of a hypothetical X q X collision system at infinite separation. The energies plotted, E q,r,config.)] E X q ( config.) E(X r E(X q ) E(X), are the total electronic energies of the collision system referenced to the X q X ground-state energy. The collision Q value for the capture of four electrons and two possible decay paths together with electron and photon energies are shown. Path 1 leads to the X (q 1) X 4, and path 2 to the X (q 2) X 4 ground state. E q,r, nl n l E Ar q nl n l E Ar r E Ar q E Ar, 6b etc. Note that the energies E q,1(nl)(n l ), E q,2, (nl)(n l )],... associated with recoil ions of charge 1, 2,... are all different, even though the Ar q projectile captured electron configuration (nl)(n l ) is the same in all cases. In this notation, the energy of an Auger electron emitted in the deexcitation of the state of energy E q,r, (nl)(n l )(n l )] to that of energy E q,r,(nl)(n l ) is E q,r,(nl)(n l )(n l ) E q,r,(nl)(n l ). The energy of a photon emitted in the transition from the state of energy E q,r,(n l ) to that of energy E q,r,(nl) is E q,r,(n l ) E q,r,(nl). Figure 5 illustrates this energy-level scheme and possible transitions for a hypothetical X q X system which decays to the ground state of the X (q 1) X 4 system. The decay scheme is what we believe to be typical of that observed for the capture of a larger number of electrons of which only one is retained by the projectile. For a given Q associated with the capture of four electrons, path 1 involves the emission of two low-energy electrons e 11 and e 12, plus an energetic electron e 13. The final transition to the ground state of X (q 1) X 4 emits a photon of energy h 11. Path 2 involves the emission of two energetic Auger electrons e 21 and e 22, to a doubly excited state of the X (q 2) X 4 system which lies just below the ground state of the X (q 1) X 4 system. This excited state and all other X q (nl)(n l ) X 4 states lying below it have to deexcite by photon emission, of which only the first, h 21, is shown. Path 2 leads to the X (q 2) X 4 ground state. In an attempt to understand the observed electron energy spectra in Figs. 3 a 3 i, we have calculated the energies of a number of excited states E q,r, electron configuration for the collision systems Ar q (8 q 16) Ar and r 1 4. The energies were calculated using the general Hartree-Fock code HF In order to keep the calculations tractable we considered only s and p states, since for the highly excited states of interest, the higher angular momentum states lie closer in energy to the p state than the s- p separation. In the same vein, we have only considered configurations, and not the possible terms. The very large number of energy states calculated presents a problem as to how best display the results. We chose the form shown in Figs. 6 a and 6 b through 8 a and 8 b. Energy levels convey information better than tables; they can be used to explain the overall features of the observed electron spectra, and, at the same time, provide a guide for more detailed calculations of excited states using any one of the readily available atomic structure computational packages. From our measured zero-degree energy-gain spectra for Ar q (8 q 14) on Ar 19, we know that most captures occur with Q values in the eV energy range. In this region of Q values, many of the calculated singly, doubly, triply, etc. excited capture states can be quite close in energy, and it may not be possible to identify unambiguously the features in the translational energy gain spectra or Q value distributions with specific single, double, etc. capture states on the basis of energetics alone. By capture we mean removal of electrons from the target during the collision. Figure 6 a shows a schematic energy-level diagram at infinite separation for the Ar 8 Ar system that goes over to the Ar 7 Ar 1 or Ar 7 Ar 2 ground state. The measured distribution of Q values for Ar 8 on Ar at 79.7q ev, is shown in Fig. 9 a 20. Single-electron capture occurs for Q values around 12 and 19 ev 21,22. The lower Q value is associated with capture to 6s and 6p and the higher value with capture to 5p, 5d, and 5 f. These excited states can decay by radiative transitions only. For two electron capture, the possible Q values range from about 25 to 45 ev, Fig. 9 a. This would suggest capture to states bracketed by (4p)(7s) to (5s)(6p) in Table I a. Given that the Ar 7 Ar 2 ground state is about 100 ev below the initial Ar 8 Ar ground state, for the Q in the eV energy range, we would expect Auger electrons in the eV range. Table I a lists nonradiative transition energies for selected E 8,2,(nl)(n l ) to E 8,2,(nl) transitions. The energies range from 20 to 80 ev. Although there are only three final states (3s), (3p), and (3d), there is a large number of initial states which leads to a considerable overlap of transition energies to these states. Figure 6 b shows the highly schematic energy-level diagram for the Ar 8 Ar system that goes to Ar 7 Ar 3. In this case we show triple capture to only a few states, (5s)(5p) 2,(5s) 2 (5p), and (5p) 3, that have Q values around 23 ev. These capture states can au-
6 2384 J. VANCURA, P. J. MUCHA, AND V. O. KOSTROUN 53 FIG. 6. Total electronic energies of selected states of the Ar 8 Ar collision system at infinite separation after the collision. The energies are plotted with respect to the total electronic energy of the ground state of the Ar 8 Ar system at infinite separation. a shows some of the energetically allowed states of Ar 8 (nl)] Ar, Ar 8 (nl) Ar 2, Ar 8 (nl) n l Ar 2. The ground states of the Ar 8 Ar, Ar 7 Ar, and Ar 7 Ar 2 systems are shown with thick lines. b shows some of the energetically allowed states of the Ar 8 (nl)] Ar 3, Ar 8 (nl)(n l)] Ar 3, and Ar 8 (nl) 2 (n l )] Ar 3 systems. FIG. 7. Total electronic energies of selected final states of the Ar 9 Ar collision system at infinite separation. For more details, see the caption for Fig. 6. toionize to levels of the doubly excited system just above the energy of the Ar 7 Ar 3 ground state. In this case the resulting Auger electrons have energies below 25 ev. If the capture states autoionize to levels below the Ar 7 Ar 3 ground state, e.g., to the (3p)(6s,6p) doubly excited states, or to the states below these, Fig. 6 b, then the E 8,3, (nl)(n l )] states can decay only by a series of radiative
7 SPECTROSCOPIC STUDY OF ELECTRONS EMITTED IN Ar q... TABLE I. a Nonradiative transition energies in ev for selected Ar 8 (nl)(n l )] Ar 2 to Ar 8 (nl)] Ar 2 transitions. b Nonradiative transition energies in ev for selected Ar 8 (nl) 2 (n l )] Ar 3 to Ar 8 (nl)(n l )] Ar 3 transitions. Q Initial state Final state energy Final state energy Final state energy a 10.2 (6s)(6p) to (3d) 49.3 to (3p) 73.0 to (3s) (5p)(7p) (5p)(7s) (5s)(7p) (5s)(7s) (5p)(6s) (5s)(6p) (5p) (5s)(5p) (5s) (4p)(7p) (4p)(7s) Q Initial State Final state energy b (5p) 2 (5p) to (3d)(5p) 30.6 (3p)(5p) 54.5 (3s)(5p) (5s)(5p) 2 (3d)(5p) 27.0 (3p)(5p) 50.8 (3s)(5p) (5s) 2 (5p) (3d)(5s) 27.2 (3p)(5s) 50.9 (3s)(5s) 68.6 transitions to the Ar 6 Ar 3 ground state (E 8,3,(3s) 2 ). Since the Q values associated with triple-electron capture are more likely to be around 40 ev or more, the triple-capture states will Auger decay to Ar 8 (nl)(n l )] Ar 3 states below the Ar 7 Ar 3 state. These states can only decay by radiative transitions to the Ar 6 Ar 3 ground state, and we would therefore expect Ar 6 scattered projectiles rather than Ar 7 projectiles in three-electron capture from the argon target. The capture of four electrons leading to the Ar 7 Ar 4 ground state would be accompanied by even lower-energy Auger electrons. From Fig. 3 a, we see that the maximum observed electron energy is below 90 ev, and there is a pronounced peak around 50 ev. Table I a shows that this peak is most likely associated with two-electron capture followed by Auger transitions to the (3d) and (3p) states in Ar 8 (nl)] Ar 2. The striking difference between the spectra observed for Ar 8 on Ar, Fig. 3 a and Ar 9 on Ar, Fig. 3 b, can be understood from Figs. 7 a and 7 b which show the energylevel diagram for the Ar 9 Ar system which eventually goes to the Ar 8 Ar 1, Ar 8 Ar 2, or Ar 8 Ar 3 ground state. The peak in the energy-gain spectrum of Ar 9 on Ar 19 corresponds to a Q value around 15 ev. For single-electron capture with this Q value, one would expect capture into 6p, 6d, or 6f states 21, which in turn can decay only by radiative transitions to the Ar 8 Ar 1 ground state. The transfer ionization peak in the energy-gain spectrum 19,21, which corresponds to Q values in the eV range, is associated with two-electron capture. From Table II a, we would expect capture to (61)(61 ), 51 (71 ), and (51)(61 ) states of the projectile. These excited states will in turn decay by nonradiative transitions to the 31 states of the Ar 9 (nl)] Ar 2 system. The possible electron energies range from 47 to 107 ev, Table II a. The largest transition energies occur to the Ar 9 (3s)] Ar 2 state. However, from Fig. 3 a, for the Ar 8 on Ar electron spectrum, there is very little intensity above 70 ev, which would rule out transitions to the Ar 8 (3s)] Ar 2 state, Table I a. If a similar situation holds for Ar 9, one would not expect electrons with energies greater than 110 ev in the decay of the doubly excited states. This can be readily seen from Fig. 7 a for Q 25 ev. A higher Q value for double capture would result in electrons of even lower energies. This interpretation is consistent with the partial electron energy spectra in coincidence with doubly charged recoil ions measured by Posthumus and Morgenstern 10.
8 2386 J. VANCURA, P. J. MUCHA, AND V. O. KOSTROUN 53 FIG. 9. Distribution of Q values vs Q for a Ar 8 on Ar, and b Ar 10 on Ar at 79.7q and 92.8q ev, respectively. The distribution, taken from Ref. 19, is integrated over all scattering angles. FIG. 8. Total electronic energies of selected final sates of the Ar 16 Ar collision system at infinite separation. For more details, see the caption for Fig. 6. Figure 7 b shows energy levels associated with the decay of triply excited states, bracketed by (5s) 2 (5p) and (6p) 3, formed in three-electron capture. Table II b lists nonradiative transition energies for selected Ar 9 (nl) 2 (n l )] Ar 3 to Ar 9 (nl)(n l )] Ar 3 transitions where (nl) is(5s), (5p), (6s), or (6p), since the (nl) 2 (n l ) cannot decay to an arbitrary (n l )(n l ). One set of quantum numbers in both initial and final states has to be the same neglecting higher order Auger processes, which is reasonable. The maximum electron energy in this case is 107 ev, but the bulk of energies is below 90 ev. Table II c lists nonradiative transition energies for selected Ar 9 (nl)(n l )] Ar 3 to Ar 9 (nl)] Ar 3 transition energies. If we take the final state (nl)tobe(2p), and consider only (n l )(n l ) states with (n l ) or(n l ) the same as in the triply excited capture states, the transition energies range from 186 to 273 ev. Table II d lists nonradiative transition energies for selected Ar 9 (nl)(n l )] Ar 4 to Ar 9 (nl)] Ar 4 transitions. These eV electrons would be observed in the decay of excited states formed in four-electron capture. Figure 3 b shows that there are fewer electrons in the eV range, and that the bulk of electrons falls in the energy ranges discussed above. The partial electron energy spectra in coincidence with the Ar 3 recoil show that there are indeed very few electron peaks in the eV electron range, and most of the electrons that appear in the spectrum have energies either below 80 ev or between 150 and 260 ev 10. From Fig. 7 b we see that it is possible to have transitions from the triply excited capture states Ar 9 (nl) 2 (n l )] Ar 3 to doubly excited states Ar 9 (nl)(n l )] Ar 3 that lie below the Ar 8 Ar 3 ground state. These states would then decay by radiative transitions to the Ar 7 Ar 3 ground state. However, because of the 300-eV or more transition energy, the Auger rate is small, and it is more likely that the Ar 7 two-electron transfer projectiles are produced in collisions in which four or more electrons are captured. The calculated Ar 10 on Ar energy-level schemes are very similar to those for Ar 9 on Ar, Figs. 7 a and 7 b.
9 SPECTROSCOPIC STUDY OF ELECTRONS EMITTED IN Ar q... TABLE II. a Nonradiative transition energies in ev for selected Ar 9 (nl)(n l )] Ar 2 to Ar 9 (nl)] Ar 2 transitions. b Nonradiative transition energies in ev for selected Ar 9 (nl) 2 (n l )] Ar 3 to Ar 9 (nl)(n l )] Ar 3 transitions. c Nonradiative transition energies in ev for selected Ar 9 (nl)(n l )] Ar 3 to Ar 9 (nl)] Ar 3 transitions. d Nonradiative transition energies in ev for selected Ar 9 (nl)(n l )] Ar 4 to Ar 9 (nl)] Ar 4 transitions. Q Initial state Final state energy Final state energy Final state energy a 13.5 (6p)(7s) to (3d) 72.9 to (3p) 96.5 to (3s) (6s)(7p) (6p) (6s)(6p) (6s) (5p)(7p) (5p)(7s) (5s)(7p) (5s)(7s) (5p)(6s) (5s)(6p) Q Initial state Final state energy b 8.84 (6s) 2 (6p) (4s)(6s) 24.6 (3d)(6p) 63.8 (3p)(6p) 87.4 (3p)(6s) 89.1 (3s)(6p) 105 (3s)(6s) (5s)(5p) 2 (3d)(5p) 35.4 (3d)(5s) 38.5 (3p)(5p) 59.2 (3p)(5s) 61.5 (3s)(5p) 77.0 (3s)(5s) 80.3 Initial state Final state energy c (4s)(6s) (2p) 273 (4p)(5s) 272 (4s)(5p) 272 (3d)(7p) 266 (3d)(7s) 264 (3d)(6p) 259 (3d)(6s) 250 (3d)(5p) 249 (3p)(7p) 242 (3d)(5s) 240 (3p)(7s) 232 (3p)(6p) 231 (3p)(6s) 228 (3s)(7p) 226 (3s)(7s) 226 (3p)(5p) 224 (3p)(5s) 220 (3s)(6p) 211 (3s)(6s) 211 (3d)(4s) 207 (3p)(4d) 196 (3s)(5p) 193 (3s)(5s) 186
10 2388 J. VANCURA, P. J. MUCHA, AND V. O. KOSTROUN 53 TABLE II. Continued. Initial state Final state energy d (3p)(4s) 196 (3s)(4d) 193 (3d) (3s)(4p) 184 (3s)(4s) 178 (3p)(3d) 162 (3s)(3d) 144 (3p) (3s)(3p) 119 (3s) Accordingly, one would expect the spectrum of electrons emitted in Ar 10 -on-ar collisions to be similar to that of Ar 9 on Ar, and Fig. 3 c shows that this is indeed so. The measured distributions of Q values for Ar 10 on Ar at 92.8q ev is shown in Fig. 9 b 20. The distribution is similar to that for Ar 8 on Ar. For single-electron capture around Q 14 ev, one would expect capture into 71 states, and for Q 20 ev into 61 states 21. For the other Ar q -on-ar charge states, one obtains basically the same energy-level structure, and hence the general analysis for Ar 8 and Ar 9 on Ar should also hold for these charge states. Thus in Fig. 3 f for Ar 13 on Ar, the composite peak between 50 and 80 ev, and the two broad peaks between 150 and 230 ev, can arise from two-electron capture into states with Q values around ev, which then autoionize to the Ar 13 (41)] Ar 2, ev, and Ar 13 (31)] Ar 2 states, ev. Triply excited states Ar 13 (7s or 7p) 2 (7s or 7p)] Ar 3 formed in three-electron capture with Q values around 50 ev can decay by nonradiative transitions to Ar 13 (3s or 3p)(7s or 7p)] Ar 3 states, which then decay by nonradiative transitions to Ar 13 (2p)] Ar 3. The electron energies involved are in the eV range. The broad peak extending from eV electrons could also be due to nonradiative transitions from Ar 13 (nl)(n l )] Ar 4 states to the Ar 13 (31)] Ar 4 state. These transitions are at the tail end of the cascade in the decay of quadruply excited states formed in four-electron capture. Figures 8 a and 8 b show the energy levels for the Ar 16 Ar system which goes to Ar 16 Ar 1, Ar 16 Ar 2,orAr 16 Ar 3. The general considerations applied to the previous collision systems should apply here also, and we would expect more or less the same situation as for the Ar 13 -on-ar system, except for an increase in transition energies. One observes that in Figs. 3 d 3 i there is a general trend of the features they shift to higher energies. The energy levels shown in Figs. 6 a and 6 b through 8 a and 8 b are for the collision system at infinite separation. To calculate these energies at different internuclear separations as the colliding partners approach one another, we used the ab initio self-consistent field quantum chemistry program GAMESS 23. Figure 10 a shows the calculated total electronic energies as a function of internuclear separation for the Ar 8 Ar initial state, and the arbitrarily chosen Ar 8 (5s)(6s)] Ar 2 final state. The calculations were performed using standard basis sets built into GAMESS, and our own basis set consisting of 11 (s) and eight (p) FIG. 10. a Plot of the total electronic energy of the initial Ar 8 Ar and final Ar 8 (5s)(6s)] Ar 2 collision systems as a function of internuclear separation R calculated using GAMESS 23. The solid squares are the difference between the total electronic energy of the Ar 8 (5s)(6s)] Ar 2 system at infinite separation and the Coulomb repulsion 18 2 /R between nuclei. b Total energies of the initial Ar 8 Ar and final Ar 8 (5s)(6s)] Ar 2 collision systems as a function of internuclear separation calculated using GAMESS. The solid dots show the total electronic energy at infinite nuclear separation for the Ar 8 (5s)(6s)] Ar 2 collision systems plus the ion-ion repulsion 6 2/R. In both a and b, all energies are measured with respect to the initial Ar 8 Ar total electronic ground-state energy.
11 SPECTROSCOPIC STUDY OF ELECTRONS EMITTED IN Ar q... uncontracted Gaussians fitted to the atomic radial wave functions obtained from Froese-Fischer s code HF We also used uncontracted Gaussians supplemented with a Binning-Curtiss Kr set centered between the Ar nuclei 23. In all three cases, the results were very similar. The calculations shown in Fig. 10 were done with our basis. In Fig. 10 a we show the electronic energies, and in Fig. 10 b the total energies, commonly used to calculate the nuclear motion in the Born-Oppenheimer approximation. The electronic energies are more useful when discussing nonradiative and radiative transitions, as these transitions are easier to visualize in this picture. If we take the total electronic energy of a particular final state of the collision system at infinite separation, and subtract from it the nuclear repulsion Z 2 /R at each internuclear separation R, where Z is the nuclear charge on the centers, we obtain excellent agreement with the energy calculated by GAMESS. For example, using HF86, the calculated total relativistic energies of Ar 8 (5s)(6s)] Ar 2 and Ar 8 Ar at infinite separation are and a.u., respectively. Subtracting 18 2 /R a.u. from 0.94 a.u., the energy of the (5s)(6s) state of the collision system with respect to the initial ground-state energy, gives the curve, solid squares, shown in Fig. 10 a. The agreement is actually better than that shown, since the GAMESS calculations at internuclear separations greater than 20 Å were done at only two points, one of which was at infinity. Similarly, by adding the repulsive term q 1 q 2 /R to the final total electronic energy of the collision system at infinite separation also gives a curve that agrees quite well with the GAMESS calculation, Fig. 10 b, solid squares. q 1 and q 2 are the net charges on the projectile and target in the final state of the collision system. In Fig. 10 b they are 6 and 2, respectively. Potential curves calculated in this manner are generally referred to as schematic in the literature. However, as the above calculation shows, such curves are very good down to the internuclear separations of interest, and, if anything, slightly overestimate crossing radii. At large internuclear separations, the change in the electronic energy is determined by the Coulomb interaction among the electrons localized on each center, and the ionic charge of the other partner. At smaller internuclear separations, the energies of the single-particle levels are Stark shifted by the electric field produced by the other partner, but this effect is small compared to the above-mentioned Coulomb interaction. As the internuclear separation is reduced further, mixing of the electronic orbitals occurs. When this mixing becomes important, the present calculation breaks down in that the orbitals tend to converge to electronic states other than those of the asymptotic system that one started out with. Interestingly, this occurs at internuclear distances corresponding to those extracted from total charge-transfer cross sections, tot, i.e., distances tot /. Finally, since the total electronic energies of the collision system at different internuclear separations R differ from those at infinite separation only by the nuclear repulsion energy Z 2 /R, one would expect the energies of Auger electrons emitted in the decay of excited states formed in the collision to be the same as those for an isolated system. At smaller internuclear distances, where the mixing of orbitals is strong, this should no longer be the case. V. CONCLUSIONS The spectra of electrons emitted in low energy, Ar q (9 q 16) on Ar collisions are qualitatively similar. This suggests that they might have a common interpretation. If one assumes that electrons transferred from the target to the projectile during the collision form an excited state that deexcites some time after the collision partners have separated, then the energies of the electrons emitted can be obtained from the total electronic energies of the excited projectile plus recoil ion of charge r referenced with respect to the initial total electronic energy of the projectile-target system. The total electronic energy of the excited projectiles is calculated for configurations that consist of the initial Ar q core plus one, two, etc. electrons in high-lying unoccupied orbitals. In this work, the total electronic energies of recoil ions of charge r were calculated for the ground state, although excited states of the recoils can easily be obtained. In all cases, the total electronic energies are for the projectile-target system at infinite separation. It should be noted that nonradiative transitions from a given initial to final projectile electron configuration will have different transition energies depending upon the charge state of the recoil ion accompanying the projectile. With the help of calculated average total electronic energies for different electronic configurations of excited ions plus ground-state recoil ions, the main features observed in Ar q -on-ar electron spectra were associated with specific transitions. For Ar 9 -on-ar collisions, an analysis of the electron spectra based on energetics is in qualitative agreement with the electron spectra observed in coincidence with recoil ions of charge 2 and The total electronic and total energies of the collision system at different internuclear separations were calculated using the ab initio self-consistent field quantum chemistry program GAMESS 23. It was noted that if one takes the total electronic energy of a particular state of the collision system at infinite separation, and subtracts from it the nuclear repulsion Z 2 /R at each internuclear separation R, one obtains excellent agreement with the energy calculated by GAMESS. Similarly, if one adds the repulsive term q 1 q 2 /R to the total electronic energy of the collision system at infinite separation, the potential-energy curves for different states of the collision system again agree very well with those calculated by GAMESS. The total electronic energies and potentialenergy curves so obtained for initial and arbitrary final sates of the collision system agree with the GAMESS calculations down to internuclear separations where appreciable mixing of orbitals takes place. ACKNOWLEDGMENTS The help of James Perotti in constructing much of the apparatus used in these experiments is gratefully acknowledged. This work was supported in part by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences.
12 2390 J. VANCURA, P. J. MUCHA, AND V. O. KOSTROUN 53 1 A. Bordenave-Montesquieu, P. Benoit-Cattin, A. Gleizes, A. I. Marrakchi, S. Dusson, and D. Hitz, J. Phys. B 17, L ; 17, L N. Stolterfoht, C. C. Havener, R. A. Phaneuf, J. K. Swenson, S. M. Shafroth, and F. W. Meyer, Phys. Rev. Lett. 57, M. Mack, J. H. Nijland, P. Van der Stratten, A. Niehaus, and R. Morgenstern, Phys. Rev. A 39, H. A. Sakaue, Y. Kanai, K. Ohta, M. Kushima, T. Inaba, S. Ohtani, K. Wakiya, H. Suzuki, T. Takayanagi, T. Kambara, A. Danjo, M. Yoshino, and Y. Awaya, J. Phys. B 23, L J. Posthumus and R. Morgenstern, J. Phys. B 23, M. Boudjema, M. Cornille, J. Dubau, P. Moretto-Capelle, A. Bordenave-Montesquieu, P. Benoit-Cattin, and A. Gleizes, J. Phys. B 24, ; 24, , and references therein. 7 F. Fremont, K. Sommer, D. Leclerc, S. Hicham, P. Boduch, X. Husson, and N. Stolterfoht, Phys. Rev. A 46, J. H. Posthumus, P. Lukey, and R. Morgenstern, J. Phys. B 25, R. Hutton, M. H. Prior, S. Chantrenne, Mau Hsiung Chen, and D. Schneider, Phys. Rev. A 39, J. H. Posthumus and R. Morgenstern, Phys. Rev. Lett. 68, J. H. Posthumus and R. Morgenstern, J. Phys. B 25, J. Vancura and V. O. Kostroun, Phys. Rev. A 49, J. Vancura, V. Marchetti, and V. O. Kostroun, in VIth International Conference on the Physics of Highly Charged Ions, edited by P. Richard, M. Stockli, C. L. Cocke, and C. D. Lin, AIP Conf. Proc. No. 274 AIP, New York, 1993, p V. O. Kostroun, in International Symposium on Electron Beam Ion Sources and their Applications, edited by A. Hershcovitch, AIP Conf. Proc. No. 188 AIP, New York, 1989, pp J. Vancura, V. J. Marchetti, J. J. Perotti, and V. O. Kostroun, Phys. Rev. A 47, D. J. Volz and M. E. Rudd, Phys. Rev. A 2, L. O. Werme, T. Bergmark, and K. Siegbahn, Phys. Scr. 8, C. Froese-Fischer, Comput. Phys. Commun. 43, J. Vancura and V. O. Kostroun unpublished. 20 M. Sadilek, J. Vancura, and V. O. Kostroun, J. Phys. Chem. 99, E. H. Nielsen, L. H. Andersen, A. Barany, H. Cederquist, J. Heinemeier, P. Hvelplund, H. Knudsen, K. B. MacAdam, and J. Sorensen, J. Phys. B 18, J. P. Giese, C. L. Cocke, W. Waggoner, L. Tunnell, and S. L. Varghese, Phys. Rev. A 34, General Atomic and Molecular Electronic Structure System GAMESS, by M. Dupuis, D. Spangler, and J. J. Wendoloski, National Resource for Computations in Chemistry Software Catalog University of California, Berkeley, CA, 1980, Program QG01. The version of GAMESS used is described by M. W. Schmidt, K. K. Baldridge, J. A. Boatz, J. H. Jensen, S. Kosski, M. S. Gordon, K. A. Nguyen, T. L. Windus, and S. T. Elbert in Quantum Chem. Program Exchange Bull. 10,
Advanced Lab Course. X-Ray Photoelectron Spectroscopy 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT Qualitative analysis Chemical Shifts 7
 Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Studies of charge exchange in symmetric ion ion collisions
 INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 34 (2001) 469 475 www.iop.org/journals/jb PII: S0953-4075(01)17355-4 Studies
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 34 (2001) 469 475 www.iop.org/journals/jb PII: S0953-4075(01)17355-4 Studies
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~pchemlab ; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~pchemlab ; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-RAY SPECTRA. Theory:
 12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
Experimental details. Sample preparation. Ln14MC5 complexes were prepared as described in 1. Magnetic Compton Scattering measurements
 Supporting information for Understanding Spin Structure in Metallacrown Single Molecule Magnets Aniruddha Deb, Thaddeus T. Boron, III, M Masayoshi Itou, Yoshiharu Sakurai, Talal Mallah, Vincent L. Pecoraro,
Supporting information for Understanding Spin Structure in Metallacrown Single Molecule Magnets Aniruddha Deb, Thaddeus T. Boron, III, M Masayoshi Itou, Yoshiharu Sakurai, Talal Mallah, Vincent L. Pecoraro,
Rb, which had been compressed to a density of 1013
 Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
5.8 Auger Electron Spectroscopy (AES)
 5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
Fully differential cross sections for transfer ionization a sensitive probe of high level correlation effects in atoms
 INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 37 (2004) L201 L208 PII: S0953-4075(04)75475-9 LETTER TO THE EDITOR Fully differential
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 37 (2004) L201 L208 PII: S0953-4075(04)75475-9 LETTER TO THE EDITOR Fully differential
Lecture 5. X-ray Photoemission Spectroscopy (XPS)
 Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
CHARGED PARTICLE INTERACTIONS
 CHARGED PARTICLE INTERACTIONS Background Charged Particles Heavy charged particles Charged particles with Mass > m e α, proton, deuteron, heavy ion (e.g., C +, Fe + ), fission fragment, muon, etc. α is
CHARGED PARTICLE INTERACTIONS Background Charged Particles Heavy charged particles Charged particles with Mass > m e α, proton, deuteron, heavy ion (e.g., C +, Fe + ), fission fragment, muon, etc. α is
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
EXPERIMENT 2-6. e/m OF THE ELECTRON GENERAL DISCUSSION
 Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Ma5: Auger- and Electron Energy Loss Spectroscopy
 Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Total and state-selective electron capture cross sections for C 3+ + H collisions
 J. Phys. B: At. Mol. Opt. Phys. 32 (1999) 5271 5278. Printed in the UK PII: S0953-4075(99)06231-8 Total and state-selective electron capture cross sections for C 3+ + H collisions H C Tseng and C D Lin
J. Phys. B: At. Mol. Opt. Phys. 32 (1999) 5271 5278. Printed in the UK PII: S0953-4075(99)06231-8 Total and state-selective electron capture cross sections for C 3+ + H collisions H C Tseng and C D Lin
1 The Cathode Rays experiment is associated. with: Millikan A B. Thomson. Townsend. Plank Compton
 1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
Kaonic nitrogen and hydrogen from DEAR
 Kaonic nitrogen and hydrogen from DEAR C. Curceanu (Petrascu) LNF INFN, Via. E. Fermi 40, 00044 Frascati (Roma), Italy On behalf on the DEAR Collaboration 1. DEAR scientific program DEAR (DAΦNE Exotic
Kaonic nitrogen and hydrogen from DEAR C. Curceanu (Petrascu) LNF INFN, Via. E. Fermi 40, 00044 Frascati (Roma), Italy On behalf on the DEAR Collaboration 1. DEAR scientific program DEAR (DAΦNE Exotic
Atomic structure and dynamics
 Atomic structure and dynamics -- need and requirements for accurate atomic calculations Analysis and interpretation of optical and x-ray spectra (astro physics) Isotope shifts and hyperfine structures
Atomic structure and dynamics -- need and requirements for accurate atomic calculations Analysis and interpretation of optical and x-ray spectra (astro physics) Isotope shifts and hyperfine structures
A simple electric thruster based on ion charge exchange
 A simple electric thruster based on ion charge exchange IEPC-2007-35 Presented at the 30 th International Electric Propulsion Conference, Florence, Italy Joe Khachan and Lachlan Blackhall University of
A simple electric thruster based on ion charge exchange IEPC-2007-35 Presented at the 30 th International Electric Propulsion Conference, Florence, Italy Joe Khachan and Lachlan Blackhall University of
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu
 Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
THE NATURE OF THE ATOM. alpha particle source
 chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
EUV spectra from the NIST EBIT
 EUV spectra from the NIST EBIT D. Kilbane and G. O Sullivan Atomic and Molecular Plasma Physics group, UCD, Ireland J. D. Gillaspy, Yu. Ralchenko and J. Reader National Institute of Standards and Technology,
EUV spectra from the NIST EBIT D. Kilbane and G. O Sullivan Atomic and Molecular Plasma Physics group, UCD, Ireland J. D. Gillaspy, Yu. Ralchenko and J. Reader National Institute of Standards and Technology,
Other electrons. ε 2s < ε 2p ε 3s < ε 3p < ε 3d
 Other electrons Consider effect of electrons in closed shells for neutral Na large distances: nuclear charge screened to 1 close to the nucleus: electron sees all 11 protons approximately:!!&! " # $ %
Other electrons Consider effect of electrons in closed shells for neutral Na large distances: nuclear charge screened to 1 close to the nucleus: electron sees all 11 protons approximately:!!&! " # $ %
Effect of angular electron correlation in He: Second-order calculations for transfer ionization
 PHYSICAL REVIEW A 78, 274 8 Effect of angular electron correlation in He: Second-order calculations for transfer ionization A. L. Godunov, Colm T. Whelan, and H. R. J. Walters 2 Department of Physics,
PHYSICAL REVIEW A 78, 274 8 Effect of angular electron correlation in He: Second-order calculations for transfer ionization A. L. Godunov, Colm T. Whelan, and H. R. J. Walters 2 Department of Physics,
Preliminary Quantum Questions
 Preliminary Quantum Questions Thomas Ouldridge October 01 1. Certain quantities that appear in the theory of hydrogen have wider application in atomic physics: the Bohr radius a 0, the Rydberg constant
Preliminary Quantum Questions Thomas Ouldridge October 01 1. Certain quantities that appear in the theory of hydrogen have wider application in atomic physics: the Bohr radius a 0, the Rydberg constant
ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY
![ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY](/thumbs/71/65949100.jpg) ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY E. Erturk, 1 L. Spielberger, 1 M. Achler, 1 L. Schmidt, 1 R. Dorner, 1 Th. Weber, 1
ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY E. Erturk, 1 L. Spielberger, 1 M. Achler, 1 L. Schmidt, 1 R. Dorner, 1 Th. Weber, 1
Joint ICTP-IAEA Workshop on Fusion Plasma Modelling using Atomic and Molecular Data January 2012
 2327-4 Joint ICTP- Workshop on Fusion Plasma Modelling using Atomic and Molecular Data 23-27 January 2012 Atomic Processes Modeling in Plasmas Modeling Spectroscopic Observables from Plasmas Hyun-Kyung
2327-4 Joint ICTP- Workshop on Fusion Plasma Modelling using Atomic and Molecular Data 23-27 January 2012 Atomic Processes Modeling in Plasmas Modeling Spectroscopic Observables from Plasmas Hyun-Kyung
The Bohr Model of Hydrogen
 The Bohr Model of Hydrogen Suppose you wanted to identify and measure the energy high energy photons. One way to do this is to make a calorimeter. The CMS experiment s electromagnetic calorimeter is made
The Bohr Model of Hydrogen Suppose you wanted to identify and measure the energy high energy photons. One way to do this is to make a calorimeter. The CMS experiment s electromagnetic calorimeter is made
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
Production of HCI with an electron beam ion trap
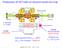 Production of HCI with an electron beam ion trap I=450 ma E= 5 kev axially: electrodes radially: electron beam space charge total trap potential U trap 200 V (U trap ion charge) 10000 ev 15000 A/cm 2 n
Production of HCI with an electron beam ion trap I=450 ma E= 5 kev axially: electrodes radially: electron beam space charge total trap potential U trap 200 V (U trap ion charge) 10000 ev 15000 A/cm 2 n
Chapter 9. Atomic structure and atomic spectra
 Chapter 9. Atomic structure and atomic spectra -The structure and spectra of hydrogenic atom -The structures of many e - atom -The spectra of complex atoms The structure and spectra of hydrogenic atom
Chapter 9. Atomic structure and atomic spectra -The structure and spectra of hydrogenic atom -The structures of many e - atom -The spectra of complex atoms The structure and spectra of hydrogenic atom
1.4 The Tools of the Trade!
 1.4 The Tools of the Trade! Two things are required for material analysis: excitation mechanism for originating characteristic signature (radiation) radiation detection and identification system (spectroscopy)
1.4 The Tools of the Trade! Two things are required for material analysis: excitation mechanism for originating characteristic signature (radiation) radiation detection and identification system (spectroscopy)
Nuclear Decays. Alpha Decay
 Nuclear Decays The first evidence of radioactivity was a photographic plate, wrapped in black paper and placed under a piece of uranium salt by Henri Becquerel on February 26, 1896. Like many events in
Nuclear Decays The first evidence of radioactivity was a photographic plate, wrapped in black paper and placed under a piece of uranium salt by Henri Becquerel on February 26, 1896. Like many events in
Electron momentum spectroscopy of metals
 Electron momentum spectroscopy of metals M. Vos, A.S. Kheifets and E. Weigold Atomic and Molecular Physics Laboratories, Research School of Physical Sciences and Engineering, Australian, National University
Electron momentum spectroscopy of metals M. Vos, A.S. Kheifets and E. Weigold Atomic and Molecular Physics Laboratories, Research School of Physical Sciences and Engineering, Australian, National University
Emphasis on what happens to emitted particle (if no nuclear reaction and MEDIUM (i.e., atomic effects)
 LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
Electron spectroscopy of doubly excited states in He produced by slow collisions of He 2 ions with Ba atoms
 PHYSICAL REVIEW A, VOLUME 64, 6279 Electron spectroscopy of doubly excited states in He produced by slow collisions of He 2 ions with Ba atoms K. Iemura, 1 S. Ohtani, 1 H. Suzuki, 1 J. Takeda, 2 S. Machida,
PHYSICAL REVIEW A, VOLUME 64, 6279 Electron spectroscopy of doubly excited states in He produced by slow collisions of He 2 ions with Ba atoms K. Iemura, 1 S. Ohtani, 1 H. Suzuki, 1 J. Takeda, 2 S. Machida,
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom
 Chapter 37 Early Quantum Theory and Models of the Atom Units of Chapter 37 37-7 Wave Nature of Matter 37-8 Electron Microscopes 37-9 Early Models of the Atom 37-10 Atomic Spectra: Key to the Structure
Chapter 37 Early Quantum Theory and Models of the Atom Units of Chapter 37 37-7 Wave Nature of Matter 37-8 Electron Microscopes 37-9 Early Models of the Atom 37-10 Atomic Spectra: Key to the Structure
MANIPAL INSTITUTE OF TECHNOLOGY
 SCHEME OF EVAUATION MANIPA INSTITUTE OF TECHNOOGY MANIPA UNIVERSITY, MANIPA SECOND SEMESTER B.Tech. END-SEMESTER EXAMINATION - MAY SUBJECT: ENGINEERING PHYSICS (PHY/) Time: 3 Hrs. Max. Marks: 5 Note: Answer
SCHEME OF EVAUATION MANIPA INSTITUTE OF TECHNOOGY MANIPA UNIVERSITY, MANIPA SECOND SEMESTER B.Tech. END-SEMESTER EXAMINATION - MAY SUBJECT: ENGINEERING PHYSICS (PHY/) Time: 3 Hrs. Max. Marks: 5 Note: Answer
Shells Orthogonality. Wave functions
 Shells Orthogonality Wave functions Effect of other electrons in neutral atoms Consider effect of electrons in closed shells for neutral Na large distances: nuclear charge screened to 1 close to the nucleus:
Shells Orthogonality Wave functions Effect of other electrons in neutral atoms Consider effect of electrons in closed shells for neutral Na large distances: nuclear charge screened to 1 close to the nucleus:
INTERACTIONS OF HIGHLY CHARGED IONS WITH C AND SURFACES
 Chapter 4 INTERACTIONS OF HIGHLY CHARGED IONS WITH C AND SURFACES U. Thumm J. R. Macdonald Laboratory, Department of Physics, Kansas State University Manhattan, Kansas 66506 2604, USA Uwe.Thumm@phys.ksu.edu
Chapter 4 INTERACTIONS OF HIGHLY CHARGED IONS WITH C AND SURFACES U. Thumm J. R. Macdonald Laboratory, Department of Physics, Kansas State University Manhattan, Kansas 66506 2604, USA Uwe.Thumm@phys.ksu.edu
Auger decay of excited Ar projectiles emerging from carbon foils
 J. Phys. B: Atom. Molec. Phys., Vol. 9, No. 15, 1976. Printed in Great Britain. @ 1976 LETTER TO THE EDITOR Auger decay of excited Ar projectiles emerging from carbon foils R A Baragiola?, P Ziem and N
J. Phys. B: Atom. Molec. Phys., Vol. 9, No. 15, 1976. Printed in Great Britain. @ 1976 LETTER TO THE EDITOR Auger decay of excited Ar projectiles emerging from carbon foils R A Baragiola?, P Ziem and N
X-Ray transitions to low lying empty states
 X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
X-RAY PRODUCTION. Prepared by:- EN KAMARUL AMIN BIN ABDULLAH
 X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
Differential Cross Section Measurements in Ion-molecule Collisions
 Differential Cross Section Measurements in Ion-molecule Collisions Song Cheng Department of Physics and Astronomy, University of Toledo, Toledo, Ohio 43606 A 14 m long beam line dedicated to study very
Differential Cross Section Measurements in Ion-molecule Collisions Song Cheng Department of Physics and Astronomy, University of Toledo, Toledo, Ohio 43606 A 14 m long beam line dedicated to study very
THE MODERN VIEW OF ATOMIC STRUCTURE
 44 CHAPTER 2 Atoms, Molecules, and Ions GO FIGURE What is the charge on the particles that form the beam? Experiment Interpretation Incoming a particles Beam of a particles Source of a particles Nucleus
44 CHAPTER 2 Atoms, Molecules, and Ions GO FIGURE What is the charge on the particles that form the beam? Experiment Interpretation Incoming a particles Beam of a particles Source of a particles Nucleus
Electron Spectroscopy
 Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
Nuclear Physics and Astrophysics
 Nuclear Physics and Astrophysics PHY-30 Dr. E. Rizvi Lecture 4 - Detectors Binding Energy Nuclear mass MN less than sum of nucleon masses Shows nucleus is a bound (lower energy) state for this configuration
Nuclear Physics and Astrophysics PHY-30 Dr. E. Rizvi Lecture 4 - Detectors Binding Energy Nuclear mass MN less than sum of nucleon masses Shows nucleus is a bound (lower energy) state for this configuration
Attosecond-correlated dynamics of two electrons in argon
 PRAMANA c Indian Academy of Sciences Vol. 82, No. 1 journal of January 2014 physics pp. 79 85 Attosecond-correlated dynamics of two electrons in argon V SHARMA 1,,NCAMUS 2, B FISCHER 2, M KREMER 2, A RUDENKO
PRAMANA c Indian Academy of Sciences Vol. 82, No. 1 journal of January 2014 physics pp. 79 85 Attosecond-correlated dynamics of two electrons in argon V SHARMA 1,,NCAMUS 2, B FISCHER 2, M KREMER 2, A RUDENKO
Scanning Auger Microprobe
 Scanning Auger Microprobe This enables images of the elements in the near surface layer of samples to be acquired. SAM a combination of the techniques of SEM and AES. An electron beam is scanned over the
Scanning Auger Microprobe This enables images of the elements in the near surface layer of samples to be acquired. SAM a combination of the techniques of SEM and AES. An electron beam is scanned over the
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
LECTURE 23 SPECTROSCOPY AND ATOMIC MODELS. Instructor: Kazumi Tolich
 LECTURE 23 SPECTROSCOPY AND ATOMIC MODELS Instructor: Kazumi Tolich Lecture 23 2 29.1 Spectroscopy 29.2 Atoms The first nuclear physics experiment Using the nuclear model 29.3 Bohr s model of atomic quantization
LECTURE 23 SPECTROSCOPY AND ATOMIC MODELS Instructor: Kazumi Tolich Lecture 23 2 29.1 Spectroscopy 29.2 Atoms The first nuclear physics experiment Using the nuclear model 29.3 Bohr s model of atomic quantization
Alpha Decay. Decay alpha particles are monoenergetic. Nuclides with A>150 are unstable against alpha decay. E α = Q (1-4/A)
 Alpha Decay Because the binding energy of the alpha particle is so large (28.3 MeV), it is often energetically favorable for a heavy nucleus to emit an alpha particle Nuclides with A>150 are unstable against
Alpha Decay Because the binding energy of the alpha particle is so large (28.3 MeV), it is often energetically favorable for a heavy nucleus to emit an alpha particle Nuclides with A>150 are unstable against
ACTIVITY 3 Introducing Energy Diagrams for Atoms
 Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 3 Introducing Energy Diagrams for Atoms Goal Now that we have explored spectral properties of LEDs, incandescent lamps, and gas lamps, we will
Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 3 Introducing Energy Diagrams for Atoms Goal Now that we have explored spectral properties of LEDs, incandescent lamps, and gas lamps, we will
PHYSICAL REVIEW A VOLUME 58, NUMBER 5
 PHYSICAL REVIEW A VOLUME 58, NUMBER 5 NOVEMBER 1998 Ionization dynamics in fast ion-atom collisions. II. Final-state momentum distributions of the ionization products in collisions of He with bare carbon
PHYSICAL REVIEW A VOLUME 58, NUMBER 5 NOVEMBER 1998 Ionization dynamics in fast ion-atom collisions. II. Final-state momentum distributions of the ionization products in collisions of He with bare carbon
Transverse momentum of ionized atoms and diatomic molecules acquired in collisions with fast highly-charged heavy ion. V. Horvat and R. L.
 Transverse momentum of ionized atoms and diatomic molecules acquired in collisions with fast highly-charged heavy ion V. Horvat and R. L. Watson The momenta of ions and electrons emerging from collisions
Transverse momentum of ionized atoms and diatomic molecules acquired in collisions with fast highly-charged heavy ion V. Horvat and R. L. Watson The momenta of ions and electrons emerging from collisions
Introduction to X-ray Photoelectron Spectroscopy (XPS) XPS which makes use of the photoelectric effect, was developed in the mid-1960
 Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Within the vast field of atomic physics, collisions of heavy ions with atoms define
 Chapter 1 Introduction Within the vast field of atomic physics, collisions of heavy ions with atoms define one of the most active areas of research. In the last decades, the design and construction of
Chapter 1 Introduction Within the vast field of atomic physics, collisions of heavy ions with atoms define one of the most active areas of research. In the last decades, the design and construction of
Sparks in Gases: Line Spectra
 Lecture 11 February 4, Chapter 3 The Particlelike Properties of Electromagnetic Radiation Sparks in Gases: Line Spectra This is one of the oldest tools available for the investigation of atoms and radiation.
Lecture 11 February 4, Chapter 3 The Particlelike Properties of Electromagnetic Radiation Sparks in Gases: Line Spectra This is one of the oldest tools available for the investigation of atoms and radiation.
Cold-target recoil-ion-momentum spectroscopy study of single electron capture from He by slow Ar 8 ions
 PHYSICAL REVIEW A VOLUME 57, NUMBER 6 JUNE 1998 Cold-target recoil-ion-momentum spectroscopy study of single electron capture from He by slow Ar 8 ions M. A. Abdallah, W. Wolff,* H. E. Wolf,* E. Sidky,
PHYSICAL REVIEW A VOLUME 57, NUMBER 6 JUNE 1998 Cold-target recoil-ion-momentum spectroscopy study of single electron capture from He by slow Ar 8 ions M. A. Abdallah, W. Wolff,* H. E. Wolf,* E. Sidky,
Magnetic sublevel population in 1s 2p excitation of helium by fast electrons and protons
 INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 34 (1) 2575 2580 www.iop.org/journals/jb PII: S0953-4075(01)21564-8 Magnetic
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 34 (1) 2575 2580 www.iop.org/journals/jb PII: S0953-4075(01)21564-8 Magnetic
The interaction of radiation with matter
 Basic Detection Techniques 2009-2010 http://www.astro.rug.nl/~peletier/detectiontechniques.html Detection of energetic particles and gamma rays The interaction of radiation with matter Peter Dendooven
Basic Detection Techniques 2009-2010 http://www.astro.rug.nl/~peletier/detectiontechniques.html Detection of energetic particles and gamma rays The interaction of radiation with matter Peter Dendooven
Introduction. X-Ray Production and Quality. Fluorescence Yield. Fluorescence X-Rays. Initiating event. Initiating event 3/18/2011
 X-Ray Production and Quality Chapter 9 F.A. Attix, Introduction to Radiological Physics and Radiation Dosimetry Introduction Physics of x-ray generation Fluorescence x-rays Bremsstrahlung x-rays Beam quality
X-Ray Production and Quality Chapter 9 F.A. Attix, Introduction to Radiological Physics and Radiation Dosimetry Introduction Physics of x-ray generation Fluorescence x-rays Bremsstrahlung x-rays Beam quality
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
arxiv:physics/ v1 3 Aug 2006
 Gamma Ray Spectroscopy with Scintillation Light in Liquid Xenon arxiv:physics/6834 v1 3 Aug 26 K. Ni, E. Aprile, K.L. Giboni, P. Majewski, M. Yamashita Physics Department and Columbia Astrophysics Laboratory
Gamma Ray Spectroscopy with Scintillation Light in Liquid Xenon arxiv:physics/6834 v1 3 Aug 26 K. Ni, E. Aprile, K.L. Giboni, P. Majewski, M. Yamashita Physics Department and Columbia Astrophysics Laboratory
Nuclear Physics. (PHY-231) Dr C. M. Cormack. Nuclear Physics This Lecture
 Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
ATOMIC WORLD P.1. ejected photoelectrons. current amplifier. photomultiplier tube (PMT)
 ATOMIC WORLD P. HKAL PAPER I 0 8 The metal Caesium has a work function of.08 ev. Given: Planck constant h = 6.63 0 34 J s, charge of an electron e =.60 0 9 C (a) (i) Calculate the longest wavelength of
ATOMIC WORLD P. HKAL PAPER I 0 8 The metal Caesium has a work function of.08 ev. Given: Planck constant h = 6.63 0 34 J s, charge of an electron e =.60 0 9 C (a) (i) Calculate the longest wavelength of
Radiation Detection for the Beta- Delayed Alpha and Gamma Decay of 20 Na. Ellen Simmons
 Radiation Detection for the Beta- Delayed Alpha and Gamma Decay of 20 Na Ellen Simmons 1 Contents Introduction Review of the Types of Radiation Charged Particle Radiation Detection Review of Semiconductor
Radiation Detection for the Beta- Delayed Alpha and Gamma Decay of 20 Na Ellen Simmons 1 Contents Introduction Review of the Types of Radiation Charged Particle Radiation Detection Review of Semiconductor
IB Physics SL Y2 Option B (Quantum and Nuclear Physics) Exam Study Guide Practice Problem Solutions
 IB Physics SL Y2 Option B (Quantum and Nuclear Physics) Exam Study Guide Practice Problem Solutions Objectives: 1. Describe the photoelectric effect. (B.1.1) 2. Describe the concept of the photon and use
IB Physics SL Y2 Option B (Quantum and Nuclear Physics) Exam Study Guide Practice Problem Solutions Objectives: 1. Describe the photoelectric effect. (B.1.1) 2. Describe the concept of the photon and use
Photoelectron spectroscopy Instrumentation. Nanomaterials characterization 2
 Photoelectron spectroscopy Instrumentation Nanomaterials characterization 2 RNDr. Věra V Vodičkov ková,, PhD. Photoelectron Spectroscopy general scheme Impact of X-ray emitted from source to the sample
Photoelectron spectroscopy Instrumentation Nanomaterials characterization 2 RNDr. Věra V Vodičkov ková,, PhD. Photoelectron Spectroscopy general scheme Impact of X-ray emitted from source to the sample
Atomic Structure. Chapter 8
 Atomic Structure Chapter 8 Overview To understand atomic structure requires understanding a special aspect of the electron - spin and its related magnetism - and properties of a collection of identical
Atomic Structure Chapter 8 Overview To understand atomic structure requires understanding a special aspect of the electron - spin and its related magnetism - and properties of a collection of identical
Physics of heavy multiply-charged ions: Studies on the borderile of atomic and nuclear physics
 Physics of heavy multiply-charged ions: Studies on the borderile of atomic and nuclear physics Andrey Surzhykov Technische Universität Braunschweig Physikalisch-Technische Bundesanstalt (PTB) Lecture 1
Physics of heavy multiply-charged ions: Studies on the borderile of atomic and nuclear physics Andrey Surzhykov Technische Universität Braunschweig Physikalisch-Technische Bundesanstalt (PTB) Lecture 1
Chapters 31 Atomic Physics
 Chapters 31 Atomic Physics 1 Overview of Chapter 31 Early Models of the Atom The Spectrum of Atomic Hydrogen Bohr s Model of the Hydrogen Atom de Broglie Waves and the Bohr Model The Quantum Mechanical
Chapters 31 Atomic Physics 1 Overview of Chapter 31 Early Models of the Atom The Spectrum of Atomic Hydrogen Bohr s Model of the Hydrogen Atom de Broglie Waves and the Bohr Model The Quantum Mechanical
A) m B) m C) m D) m E) m. 5. Which one of the following circuits has the largest resistance?
 Use the following to answer question 1. Two point charges, A and B, lie along a line separated by a distance L. The point x is the midpoint of their separation. 1. Which combination of charges would yield
Use the following to answer question 1. Two point charges, A and B, lie along a line separated by a distance L. The point x is the midpoint of their separation. 1. Which combination of charges would yield
EQUIPMENT Beta spectrometer, vacuum pump, Cs-137 source, Geiger-Muller (G-M) tube, scalar
 Modern Physics Laboratory Beta Spectroscopy Experiment In this experiment, electrons emitted as a result of the radioactive beta decay of Cs-137 are measured as a function of their momentum by deflecting
Modern Physics Laboratory Beta Spectroscopy Experiment In this experiment, electrons emitted as a result of the radioactive beta decay of Cs-137 are measured as a function of their momentum by deflecting
Resonant enhanced electron impact dissociation of molecules
 Journal of Physics: Conference Series Resonant enhanced electron impact dissociation of molecules Recent citations - An R-matrix study of singlet and triplet continuum states of N 2 Duncan A Little and
Journal of Physics: Conference Series Resonant enhanced electron impact dissociation of molecules Recent citations - An R-matrix study of singlet and triplet continuum states of N 2 Duncan A Little and
High-Resolution. Transmission. Electron Microscopy
 Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Modern Physics Laboratory Beta Spectroscopy Experiment
 Modern Physics Laboratory Beta Spectroscopy Experiment Josh Diamond and John Cummings Fall 2009 Abstract In this experiment, electrons emitted as a result of the radioactive beta decay of 137 55 Cs are
Modern Physics Laboratory Beta Spectroscopy Experiment Josh Diamond and John Cummings Fall 2009 Abstract In this experiment, electrons emitted as a result of the radioactive beta decay of 137 55 Cs are
Pt L X-RAYS PRODUCTION CROSS SECTIONS BY 12 C, 16 O, 32 S AND 48 Ti ION-BEAMS IN THE MeV/u ENERGY RANGE *
 Pt L X-RAYS PRODUCTION CROSS SECTIONS BY 12 C, 16 O, 32 S AND 48 Ti ION-BEAMS IN THE MeV/u ENERGY RANGE * M.M. GUGIU, C. CIORTEA, D.E. DUMITRIU, D. FLUERAŞU, A. ENULESCU, I. PITICU, A.C. SCAFEŞ, M.D. PENA
Pt L X-RAYS PRODUCTION CROSS SECTIONS BY 12 C, 16 O, 32 S AND 48 Ti ION-BEAMS IN THE MeV/u ENERGY RANGE * M.M. GUGIU, C. CIORTEA, D.E. DUMITRIU, D. FLUERAŞU, A. ENULESCU, I. PITICU, A.C. SCAFEŞ, M.D. PENA
Secondary Ion Mass Spectroscopy (SIMS)
 Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
G. Gantefdr and W. Eberhardt Institut fiir Festkiirperforschung, Forschungszentrum Jiilich, 5170 Jiilich, Germany
 Shell structure and s-p hybridization in small aluminum clusters G. Gantefdr and W. Eberhardt Institut fiir Festkiirperforschung, Forschungszentrum Jiilich, 5170 Jiilich, Germany Photoelectron spectra
Shell structure and s-p hybridization in small aluminum clusters G. Gantefdr and W. Eberhardt Institut fiir Festkiirperforschung, Forschungszentrum Jiilich, 5170 Jiilich, Germany Photoelectron spectra
IV. Surface analysis for chemical state, chemical composition
 IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
DEVELOPMENT OF A NEW POSITRON LIFETIME SPECTROSCOPY TECHNIQUE FOR DEFECT CHARACTERIZATION IN THICK MATERIALS
 Copyright JCPDS - International Centre for Diffraction Data 2004, Advances in X-ray Analysis, Volume 47. 59 DEVELOPMENT OF A NEW POSITRON LIFETIME SPECTROSCOPY TECHNIQUE FOR DEFECT CHARACTERIZATION IN
Copyright JCPDS - International Centre for Diffraction Data 2004, Advances in X-ray Analysis, Volume 47. 59 DEVELOPMENT OF A NEW POSITRON LIFETIME SPECTROSCOPY TECHNIQUE FOR DEFECT CHARACTERIZATION IN
Chapter 2 Problem Solutions
 Chapter Problem Solutions 1. If Planck's constant were smaller than it is, would quantum phenomena be more or less conspicuous than they are now? Planck s constant gives a measure of the energy at which
Chapter Problem Solutions 1. If Planck's constant were smaller than it is, would quantum phenomena be more or less conspicuous than they are now? Planck s constant gives a measure of the energy at which
Conversion Electron Spectroscopy in Transfermium Nuclei
 Conversion Electron Spectroscopy in Transfermium Nuclei R.-D. Herzberg University of iverpool, iverpool, 69 7ZE, UK Abstract Conversion electron spectroscopy is an essential tool for the spectroscopy of
Conversion Electron Spectroscopy in Transfermium Nuclei R.-D. Herzberg University of iverpool, iverpool, 69 7ZE, UK Abstract Conversion electron spectroscopy is an essential tool for the spectroscopy of
Evidence for K mixing in 178 Hf
 Hyperfine Interactions 107 (1997) 141^147 141 Evidence for K mixing in 178 Hf C.B. Collins The University of Texas at Dallas, Center for Quantum Electronics, PO Box 830688, Richardson, TX 75083-0688, USA
Hyperfine Interactions 107 (1997) 141^147 141 Evidence for K mixing in 178 Hf C.B. Collins The University of Texas at Dallas, Center for Quantum Electronics, PO Box 830688, Richardson, TX 75083-0688, USA
Energy Spectroscopy. Excitation by means of a probe
 Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Energy Spectroscopy. Ex.: Fe/MgO
 Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Some Beta-Decay Basics. Michael Edmison. Bluffton University
 Some Beta-Decay Basics Michael Edmiston Bluffton University The figure at bottom of the page is known as a Segrè Chart. The Segrè Chart is one way to arrange and observe information about nuclei. A Segrè
Some Beta-Decay Basics Michael Edmiston Bluffton University The figure at bottom of the page is known as a Segrè Chart. The Segrè Chart is one way to arrange and observe information about nuclei. A Segrè
The Vacuum Case for KATRIN
 The Vacuum Case for KATRIN Institute of Nuclear Physics, Forschungszentrum Karlsruhe,Germany, for the KATRIN Collaboration Lutz.Bornschein@ik.fzk.de The vacuum requirements of the KATRIN experiment have
The Vacuum Case for KATRIN Institute of Nuclear Physics, Forschungszentrum Karlsruhe,Germany, for the KATRIN Collaboration Lutz.Bornschein@ik.fzk.de The vacuum requirements of the KATRIN experiment have
A Comparison between Channel Selections in Heavy Ion Reactions
 Brazilian Journal of Physics, vol. 39, no. 1, March, 2009 55 A Comparison between Channel Selections in Heavy Ion Reactions S. Mohammadi Physics Department, Payame Noor University, Mashad 91735, IRAN (Received
Brazilian Journal of Physics, vol. 39, no. 1, March, 2009 55 A Comparison between Channel Selections in Heavy Ion Reactions S. Mohammadi Physics Department, Payame Noor University, Mashad 91735, IRAN (Received
Lecture 22 Ion Beam Techniques
 Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Total probability for reaction Yield
 Total probability for reaction Yield If target has thickness d, and target material has # nuclei/volume: n 0 [part./cm 3 ] Y=σ n 0 d The yield gives the intensity of the characteristic signal from the
Total probability for reaction Yield If target has thickness d, and target material has # nuclei/volume: n 0 [part./cm 3 ] Y=σ n 0 d The yield gives the intensity of the characteristic signal from the
( ) x10 8 m. The energy in a mole of 400 nm photons is calculated by: ' & sec( ) ( & % ) 6.022x10 23 photons' E = h! = hc & 6.
 Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
