Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions
|
|
|
- Allen Tate
- 5 years ago
- Views:
Transcription
1 Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions Laura L. Kinner and James W. Peeler Emission Monitoring Inc. Raleigh, NC Daniel A. Willis Blue Circle Cement Marietta, GA ABSTRACT The measurement of gaseous hydrogen chloride (HCl) in Portland cement and lime kiln effluent poses great challenges because of the reactive nature of both the HCl and the entrained dust in the effluent. It will become necessary for these calcining facilities to measure HCl for the purpose of National Emission Standards for Hazardous Air Pollutant (NESHAP) or area source determinations, demonstrating compliance with state/local regulations, and/or establishing emissions inventories for air permits. Currently, Fourier transform infrared-based EPA Methods 320/321 are the only methods in the EPA promulgated Portland cement NESHAP Rule allowed to determine the 10 ton/year threshold for HCl MACT standard applicability at Portland cement plants 1. Because this method can be an economic disadvantage for many companies, the Portland Cement Association and the National Lime Association sponsored a laboratory investigation to compare directly FTIR and an improved impinger-based method similar to EPA Method The purpose of the laboratory study was to: 1) improve understanding of the complex HCl measurement issues, 2) solve the immediate problem associated with measurement of HCl by EPA Method 26 relative to infrared-based analyzers, and 3) to derive an improved impinger based measurement method that is acceptable to industry, that is more cost effective than IR-based methods, and that provides facilities a choice of HCl measurement methods. An American Society for Testing and Materials (ASTM) is presently drafting a method to measure gaseous chlorides from mineral calcining industries based on this effort. It is expected that the ASTM Method will be completed within the next year pending the results of field testing applications. This paper presents the results of the comparative laboratory study, and the improvements to Method 26 that will be incorporated into the ASTM test method. 1
2 INTRODUCTION In the past, numerous studies regarding HCl measurement in various effluent matrices have been conducted by EPA and by industry. Much of this work has fueled speculations regarding how and why these measurement methods failed in the various applications, and has evolved sometimes-mythological explanations about the reported results. Many issues remain misunderstood about the measurement of reactive condensable gases such as HCl, and much of the applicable knowledge from successful emissions tests has not been disseminated through the regulatory and measurement community. Work sponsored by the Portland Cement Association in 1996 resulted in developing Fourier transform infrared (FTIR) and gas filter correlation (GFCIR) based measurement methods that were validated by the cement industry using EPA Method 301. Some of this work involved the concurrent measurement of HCl using EPA Method 26. Because of sampling system discrepancies between the methods, results for Method 26 were low relative to GFCIR measurements. The EPA subsequently indicated in its proposal of the Portland cement MACT Standard 3 that validation of Method 26 was required on a kiln by kiln basis using an infrared-based analyzer as the reference method. Successful validation would then allow use of the impinger method at that facility only. In the summer of 1998, GFCIR work was performed by EPA contractors in gathering data for a future proposed MACT standard in the Lime industry. The poor results from these tests formed the basis for the EPA disallowing use of the GFCIR test method in their promulgation of the Portland cement MACT Standard 4. This EPA rulemaking decision reduced the HCl test method options to that of FTIR only. Because of these circumstances, the Portland Cement Association (PCA) and the National Lime Association (NLA) funded a program to demonstrate that simple modifications to Method 26 can produce data of known accuracy and precision, and can provide results comparable to instrumental infrared analyzers. Demonstration of method equivalency would allow member companies to choose which measurement technique best fits the technical and economical requirements of the particular test situation. Emission Monitoring was retained to develop an improved impinger method starting first with a laboratory investigation. The approach was intended to solve the immediate measurement problem with respect to Method 26 and the IR-based methods in the most cost-effective manner. It was not a comprehensive study, nor was it designed to determine the specific chemical reactions/mechanisms that cause the discrepancies between the impinger and the IR-based methods. The laboratory study was divided into iterative experiments to determine the adsorptive nature of glassware and two types of filter media as a function of sampling system temperature. The first series of experiments were conducted using HCl calibration gases in dry nitrogen. These experiments were performed both with and without cement kiln dust (CKD) loaded onto the two types of filters. 2
3 After deducing the optimal sampling system temperature, and filtration media, experiments were then performed using simulated effluent (i.e., SO 2, ammonia, and moisture). Simulated effluent and HCl calibration gas was used in the presence of dust samples to determine how these parameters effected quantifying HCl. Finally, as a test of the improved impinger method, comparative studies were conducted between an FTIR and the modified version of Method 26. LABORATORY STUDIES The laboratory study was conducted from July 21 through 28, 1999 at Clean Air Engineering's headquarters located in Palatine, Illinois. Clean Air provided the laboratory facilities and the FTIR instrumentation, and also conducted the ion chromatographic analyses of the impinger solutions. This was a well-suited facility from which to conduct these studies because of the proximity to the PCA Campus. Representatives from PCA and member companies were on-site to observe some of the laboratory work. The laboratory study was divided into simple and complex experiments. These experiments investigated the adsorptive nature of glassware and two common types of filter media as a function of sampling system temperature and degree of sampling system conditioning (i.e., exposure to HCl in simulated cement and lime kiln stack gas). Adsorption experiments were conducted using dry HCl calibration gases only, and then HCl calibration gas in the presence of simulated kiln gas and either cement or lime kiln dust (CKD or LKD). Comparative experiments were then performed between the FTIR and the improved impinger method to determine; 1) the degree to which these methods agreed with each other, and 2) the degree to which they could quantify accurately HCl at concentration levels of concern to industry (typically from 5-25 PPM). The following table presents a summary of the laboratory experiments. Table 1 Experiment Description and Simulated Effluent Matrix for Laboratory Studies Experimental Condition % H 2 O HCl PPM SO 2 PPM % - O 2 NH 3 - PPM Vapor PART I Temperature and Filtration media studies 7% % Quartz Vs Teflon Filters PART II HCl Evolution Studies 10% 109 5% 1.0 g of CKD and LKD on UHP quartz filters at 350 F 3
4 Table 1 (cont.) PART II HCl Adsorption Studies 0.05 g CKD # g CKD # g LKD (50:50 blend) 7% 7% 7% /475/0 0/245/475 0/245/475 15% 18% 18% O/10/40 PART III Improved Impinger Method /FTIR Comparisons 0.05 g CKD # g CKD # g CKD # g LKD (50:50 blend) 0.05 g CKD # g CKD # g CKD # g LKD (50:50 blend) 6% 6% 6% 6% 12% 12% 12% 12% % 15.5% 15.5% 15.5% 14.5% 14.5% 13.5% 15% PART I Effect of Measurement System Temperature and Filtration Media The purpose of these experiments was to investigate the effects on HCl quantification due to; 1) conditioning the front half of the impinger glassware (i.e., probe, filter holder and filter), 2) temperature, and 3) filtration media. An FTIR was used to measure the upscale and downscale response time for dry HCl calibration gas under these varying experimental parameters. Figure 1. presents a schematic of the experimental apparatus. Dilutions of a manufacturer s certified 165-PPM standard (±5% accuracy) were performed to generate the different HCl concentrations used during this study. The dilution system consisted of a series of mass flow meters calibrated specifically for this test program using a digital flow meter with a NIST traceable standard. (see Figure 1) Fresh, unconditioned Method 26 front half probes and filter holders were assembled and the time required to achieve a stable 99% upscale response for a 10 ppm standard of dry HCl calibration gas was measured at temperatures of 250 F and 350 F. This experiment was repeated to determine the effect on response time for conditioned glassware. Teflon and ultra high purity quartz filters manufactured by Pallflex (0.3µ) were evaluated side by side to determine the degree of HCl adsorption versus time at 350 F. 4
5 Part I - Results The time required to achieve a stable 99% upscale and downscale (zero) measurement system response was greater than 50 minutes for 10 ppm of HCl at 250 F and a 2 liters per minute flowrate using the "fresh" (off the shelf) front half glassware. Stable response times for conditioned glassware at the same temperature and flowrate was greater than 40 minutes. The time to achieve a stable upscale and downscale response at 350 F for the same 10 ppm HCl standard was reduced to about 25 minutes for unconditioned glassware, and 20 minutes for conditioned glassware. A stable HCl response time for both the ultra high purity quartz and Teflon coated filters was achieved in virtually the same amount of time (about 20 minutes), indicating that these filters have little affect on HCl adsorption. The following Table summarizes the results Table 2. Front-Half Glassware Measurement Temperature Response Time Unconditioned 250 F 50 minutes Conditioned 250 F 40 minutes Unconditioned 350 F 25 minutes Conditioned 350 F 20 minutes 5
6 Part I Discussions Using a 350 F measurement system temperature reduced the measurement system response time by a factor of 2. This suggests that many of the past noted discrepancies between Method 26 and the infrared methods were based on the large temperature difference between the methods. (350 F for the IR methods versus 250 F for Method 26) The measurement system response time for conditioned glassware was slightly less than that for fresh glassware. It is expected that the time to condition the glassware is a function of the relative surface area of the glass. These experiments used a 3 glass lined probe and 3 diameter filter holders and filters. This suggests that some of the past noted discrepancies between Method 26 and the infrared method results were due to lack of glassware equilibration between the effluent and the front half of the M26 trains. Together, the combined effect of temperature discrepancies between the methods and the conduct of the methods (lack of sampling system equilibration in M26) can account for the negative biases observed in past comparative efforts. The ultra high purity quartz and Teflon coated filtration media gave similar responses to the HCl in simulated effluent. PART II HCl Evolution and Adsorption Studies The purpose of these studies was to understand more fully the effects of CKD and LKD on quantifying HCl. Two sets of experiments were conducted to determine; 1) whether positive biases can arise from HCl evolving from CKD and LKD under sampling system conditions, and 2) the adsorptive capacity of the CDK and LKD for gaseous HCl. Different types of CKD and LKD samples were investigated. Figure 2 presents a schematic of the Part II experimental apparatus, and Table 3. presents the chemical analysis results of the various dust samples used in these experiments. 6
7 Table 3. CKD/LKD Analysis (all Numbers Expressed in Percentage) Compounds CKD#1 CKD#2 CKD#3* LKD#1** LKD#2** Process Type Long Wet Kiln Long Wet Alkali Long Dry Straight Rotary Straight Rotary By-pass Free Lime Not Done Not Done Cl Not Done Not Done SiO Al2O Fe2O CaO MgO SO Na2O K2O TiO P2O Mn2O SrO % Calcination LOI
8 * Used only during the M26/FTIR comparison studies (Part III) ** A 50:50 blend of these dusts were used in all experiments (kilns had common baghouse) A. HCl Evolution Studies Samples of cement kiln dusts 1 &2 and the lime kiln dust were used. These dusts represented varying degrees of calcination, percent free lime and chloride content. The dust samples were loaded onto quartz filters. Simulated effluent was directed through each of the 1.0-g samples at 350 F. No tests were conducted at the 250 F temperature since it was proven that this temperature could be the cause of negative bias. An FTIR was used to determine whether HCl could be evolved from the dust at measurement system temperatures. B. HCl Adsorption Studies on CKD and LKD with Simulated Effluent Samples of the same two cement kiln dusts and one lime kiln dust were loaded onto individual quartz filters and simulated effluent was directed through each of the 0.05-g samples to determine the effect of kiln dust on quantifying HCl. The concentration of water vapor, oxygen and HCl was held constant while the concentration of SO 2 was varied as each experiment progressed. During the LKD experiment ammonia was added also to determine the effect of quantifying HCl. Part II - Results A. HCl Evolution Studies - Gaseous HCl was not evolved from the CKD or LKD samples under the experimental conditions used during this study. This was true even with CKD #1 which had a chloride content of approximately 0.8%. In the latter case, a 2 lpm and a 1.0-g. of CKD sample could theoretically release 44 PPM of HCl. B. HCl Dust Adsorption Studies All CKD and LKD samples demonstrated some adsorptive capacity for HCl. The presence and amount of SO 2 in the simulated effluent greatly affects the amount of HCl that is adsorbed by the dust. An example of these phenomena is depicted in Figure 3. This figure shows that the HCl concentration drops as the simulated effluent is directed through the dust sample, and HCl adsorption on the dust decreases with increasing SO 2 concentrations in the simulated effluent. As expected, the addition of ammonia (NH 3 ) to the simulated effluent resulted in n immediate decreased the observed concentration of HCl as measured by the FTIR. Part II Discussions HCl is not evolved at 350 F from CKD or LKD in the presence of simulated effluent. This eliminates one source of suspected positive bias. All of the dust samples adsorbed HCl. This suggests that an effective HCl measurement system should minimize the collection of particulate matter during sampling. 8
9 The adsorption of HCl by the CKD and LKD samples is affected by the relative concentration of SO 2 in the effluent. The dust samples preferentially adsorb SO 2 over HCl. This suggests that effluent having a higher relative SO 2 concentration at the inlet to a baghouse will allow more HCl to pass through the filter cake collected on the bags. (An ESP likely will not exhibit as great of an effect due to the lack of filter cake through which the effluent passes.) The reaction of gaseous HCl with ammonia (NH 3 ) to form solid ammonium chloride (NH 4 Cl) is well known. At stack temperatures common to baghouse and ESP controlled kilns (300 F to 450 F), an equilibration between the gaseous HCl/NH 3 and the condensed NH 4 Cl certainly exists. It is impossible to know the exact partition ratio between the gas and particulate phases of these compounds. Furthermore, it is very difficult to control the effects of these partitioning reactions within various sampling system components. The only means to measure the gaseous HCl with any accuracy and precision in the presence of this mixture is to maintain the sampling system components as close to the stack temperature as possible within the practical constraints of the measurement system. Even with these precautions, the presence of NH 4 Cl in kiln effluent will probably result in some high bias in ion chromatographic analysis of impinger solutions. 9
10 HCl Conc. Changes with SO2 Addition - San Antonio By-Pass Dust FTIR Equilibration Switch to Dust 16 HCl - PPM Addition of ~ 400 ppm SO2 4 Addition of ~ 200 ppm SO Figure 3. CKD#3 Alkali by-pass Adsorptivity Study Time - Min. 10
11 PART III - FTIR/Modified M26 Comparison Studies The purpose of these studies was to determine whether the results from the previous experiments could be used to make simple modifications to EPA M26 so that the results of the ion chromatographic impinger analyses are compare to those provided by the FTIR. Samples of all three cement kiln dusts and one lime kiln dust (50:50 mixture of the two samples provided) were loaded onto separate quartz filters. Two sets of filters containing 0.05g samples were assembled for each experiment; one for the FTIR and one for the improved impinger method. The impinger method was modified from that prescribed by M26 by using conditioned glassware (glassware previously passivated by HCl and simulated effluent), and by operating the front half of the train at 350 F temperatures rather than 250 F. Figure 4 presents a schematic of the experimental apparatus used in these studies. Simulated effluent was directed simultaneously through the FTIR and the impinger train to compare the HCl concentration results. These experiments were conducted at two water vapor concentrations and at three HCl concentrations. The improved impinger method and the FTIR run were exactly 60 minutes in duration. The impinger train collected approximately 120 liters of gas sample (2 lpm for 60 minutes.) A blank run using no dust was conducted to compare directly the FTIR and impinger results in the absence of dust. The HCl certified gas standard also was analyzed directly by both methods. Figures 5 and 6 presents graphical representations of the FTIR response with time during two of these experiments. These graphs are annotated to contain information regarding the percent water vapor concentration, the expected results, and the results from the M26 ion chromatographic analysis of the impinger solutions. Figure 6 presents a bargraph (corresponding to Figures 5 and 6) that directly compares the FTIR and impinger results to each other and to the expected value(s). The CKD#1 results are presented in these figures for continuity. The expected values depicted in the bargraphs were calculated three separate ways; 1) the expected value based on the manufacturer certified analysis and application of dilution factors, 2) the expected value based on direct cylinder analysis by the FTIR and application of dilution factors, and 3) the expected value based on direct analysis of the cylinder by the impinger train and application of dilution factors. For each case, the error of the measurement is a combined effect of the calibration gas uncertainty (±5% or 5 ppm), the error of the calibrated dilution system, and the error of the analytical methodology used (in this case the FTIR quantification algorithm and the ion chromatographic analysis of the impinger solutions). 11
12 Part III - Results Overall, the FTIR measurement results were generally higher than expected and the improved impinger method results were generally lower than expected at concentration levels from 5-20 PPM. At the 5 PPM HCl concentration level, the FTIR results were approximately 2 PPM (40%) higher, and the impinger results were approximately 1 PPM (20%) lower than the expected value based on the certified tag value. At the 10-PPM HCl concentration level, the FTIR results were approximately 3-6 PPM (30-60%) higher than the value expected based on the certified tag value. The modified impinger results were approximately 0.8 to 3 PPM (8-30%) lower than the value expected based on the certified cylinder tag value. At the 25 PPM HCl concentration level, the FTIR results were approximately 11 PPM (45%) higher, and the impinger results were approximately 1 PPM (3%) lower than the expected value based on the certified tag value. From time to time the water injection system had to be refilled during the course of these experiments. This led to the discovery of an unexpected phenomenon. The HCl concentration varied proportionately with effluent water vapor content. 12
13 FTIR Continuous Data During M26 Comparison Run - CKD# Switch to dust - start M26 Run 14 Observed FTIR Run Average 11.2 PPM 12 HCl - PPM 10 8 FTIR Equilibration Expected FTIR Run Average 8.7 PPM Moisture Perturbation Moisture Concentration - Percent 0 Time - Min Figure 5. FTIR Trend with Time During Comparison Study - CKD#1 13
14 FTIR Continuous Data During M26 Run Comparison - CKD#1 18 FTIR Equilibration 16 Switch to dust - start M26 Run 14 Observed FTIR Run Average 11.3 PPM 12 HCl - PPM 10 8 Moisture Concentration - Percent Expected FTIR Run Average 8.7 PPM Moisture Perturbation 0 Time - Min Figure 6. FTIR Trend with Time During Comparison Study - CKD#1 14
15 14 12 FTIR Results M 26/FTIR Comparison results - CKD#1 FTIR Results HCl - PPM M 26 Results Exp. Value Tag Exp. Value FTIR Exp. Value M 26 M 26 Results Exp. Value Tag Exp. Value FTIR Exp. Value M % H 2 O 12% H 2 O Figure 7. FTIR/Modified M26 Comparison Results 15
16 Part III Discusions Operating the FTIR and the improved impinger measurement system at the same temperature with the same filtration media produces results that are similar. This is perhaps the most important aspect in obtaining comparable results for highly reactive gases in difficult to measure effluent. The experiments conducted during this study were operated at the 350 F. It is recommended that this temperature is used after baghouse and ESP controlled kilns where effluent temperatures typically exceed 350 F. For cooler stacks, the measurement system temperature should be at least 20 C higher than the effluent temperature to prevent condensation of the gas stream, but not so high as to vaporize material condensed on the particulate matter. In most cases, the FTIR results were higher and the improved impinger based results were lower than the value expected from the certified tag value. The FTIR results are likely high due to a non-linearity effect, which can be corrected using software. (It is particularly important to include low level HCl calibration standards in the FTIR reference library when attempting to analyze accurately these low concentration levels.) The improved impinger results are within 20% of the value (1ppm) at the 5-PPM concentration level. This is likely the expected accuracy of the method. The direct correlation of HCl with percent water vapor is likely explained by an adsorption effect. When water vapor is present, it occupies sites in the measurement system at the molecular level. Perhaps by hydrogen bonding with the fluorinated groups in the Teflon sampling components. Removing water vapor frees the active sites so that HCl can be adsorbed. The rapid displacement of HCl by water vapor as the moisture level is again increased suggests that the water is preferentially adsorbed in the system over HCl. This has a direct impact on instrumental test methods where dry calibration gases are used to test the sampling system integrity before switching to source effluent. RECOMMENDATIONS Numerous comparative studies between impinger-based and IR-based HCl measurement methods were conducted in the past using greatly disparate temperatures, and filtration media. The impinger testing was performed using freshly cleaned glassware, while the instrumental IR-based methods employed sample line conditioning with effluent before starting the run. Impinger-based methods are also designed to collect particulate matter effectively even when single point non-isokinetic sampling is performed, while the instrumental IR-based methods often take measures to reduce the amount of particulate collected (by turning the nozzle backwards, etc.). An additional source of error between the methods is the primary calibration standards used, and the degree of infrared analyzer linearity in the measurement range. Together the combination of sampling system temperature differences, the degree of sample component conditioning, the amount of particulate matter collected, and the 16
17 differences between the reference standards (calibration gases versus liquid ion chromatographic standards) can more than account for differences encountered in past comparative measurement results. Simple modifications to Method 26 such as raising the temperature, conditioning the front half of the sampling train components, and reducing the amount of particulate matter collected can be made to minimize the differences between the methods. Perhaps the most important issue that became evident during the course of these experiments is that measuring HCl accurately at the 3-10 PPM level of concern is very challenging. Given a 20-30% level of accuracy for both methods (under the best circumstances) means that a relative error of from 0.5 to 3 PPM can be realized at any time. This makes it difficult if not impossible for a large facility having 3 or more kilns to determine major source status under the Clean Air Act Amendments. Further work in this area should focus on how to measure HCl more accurately at low concentrations. Design of a sampling probe configuration that rejects particulate matter is recommended, along with field testing of the modified method. Testing should be conducted using a paired train configuration so that the precision of the method can be determined. Additionally, a means of determining the accuracy of the method should be investigated. This would entail procedures such as challenging the impinger method with a known quantity of HCl (from a certified gas standard) through the entire sampling train, including the probe and filter assembly, at the end of a test series. ACKNOWLEDGMENTS The authors would like to acknowledge the Portland Cement Association and its member companies particularly Blue Circle Cement, Capitol Aggregates, and California Portland. Acknowledgments also go to the National Lime Association, Clean Air Engineering, and Construction Test Laboratories. REFERENCES 1. Code of Federal Regulations 40 CFR Part 63, Appendix A, U.S. GPO, Washington, DC Code of Federal Regulations 40 CFR Part 60, Appendix A, U.S. GPO, Washington, DC National Emissions Standards for Hazardous Air Pollutants; Portland Cement Manufacturing Industry; March 24, 1998 (63 FR 14182). 4. National Emissions Standards for Hazardous Air Pollutants; Portland Cement Manufacturing Industry; June 14, 1999 (64 FR 31897). KEY WORDS FTIR, Method 26, ASTM, HCl, Emissions Measurement, Portland Cement, MACT Standards. 17
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
FTIR CEM Performance Specification Modification Considerations
 FTIR CEM Performance Specification Modification Considerations EPA/ICAC Emissions Measurements Roundtable Meeting September 17, 2013 Peter G. Zemek MKS Instruments Marty Spartz Prism Analytical MultiGas
FTIR CEM Performance Specification Modification Considerations EPA/ICAC Emissions Measurements Roundtable Meeting September 17, 2013 Peter G. Zemek MKS Instruments Marty Spartz Prism Analytical MultiGas
Pilot-Plant Performance Testing to Support Development of a Dilution Extractive HCl Monitoring System
 Pilot-Plant Performance Testing to Support Development of a Dilution Extractive HCl Monitoring System Ty Smith Cemtek Group Inc. and Ken Greaves Cemtek Instruments Inc. Background Cemtek is researching
Pilot-Plant Performance Testing to Support Development of a Dilution Extractive HCl Monitoring System Ty Smith Cemtek Group Inc. and Ken Greaves Cemtek Instruments Inc. Background Cemtek is researching
Draft PS 18 APPENDIX A Dynamic Spiking Procedure ( ) 1.1 This appendix to Performance Specification 18
 Draft PS 18 APPENDIX A Dynamic Spiking Procedure (4-4-2013) A1. Scope and Application 1.1 This appendix to Performance Specification 18 describes the procedure and performance requirements for dynamic
Draft PS 18 APPENDIX A Dynamic Spiking Procedure (4-4-2013) A1. Scope and Application 1.1 This appendix to Performance Specification 18 describes the procedure and performance requirements for dynamic
Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor
 13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
Appendix A - Test Methods Method 301--Field Validation of Pollutant Measurement Methods from Various Waste Media
 Appendix A - Test Methods Method 301--Field Validation of Pollutant Measurement Methods from Various Waste Media 1. APPLICABILITY AND PRINCIPLE 1.1 Applicability. This method, as specified in the applicable
Appendix A - Test Methods Method 301--Field Validation of Pollutant Measurement Methods from Various Waste Media 1. APPLICABILITY AND PRINCIPLE 1.1 Applicability. This method, as specified in the applicable
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS)
 Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
Chlorine and Hydrogen Chloride Monitoring Utilizing Ion Mobility Spectroscopy (IMS) Tad Bacon Jim Taylor Kurt Webber VP Engineering Sr. Chemical Engineer VP Sales Molecular Analytics Molecular Analytics
Mercury Stack Monitor SM-4
 Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Gaseous CEMS technology
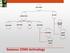 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION
 JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
Glossary of Common Laboratory Terms
 Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Scope and application: For water, wastewater and seawater. Distillation is required for wastewater and seawater.
 Nitrogen, Ammonia DOC316.53.01078 USEPA 1 Nessler Method 2 Method 8038 0.02 to 2.50 mg/l NH 3 N Reagent Solution Scope and application: For water, wastewater and seawater. Distillation is required for
Nitrogen, Ammonia DOC316.53.01078 USEPA 1 Nessler Method 2 Method 8038 0.02 to 2.50 mg/l NH 3 N Reagent Solution Scope and application: For water, wastewater and seawater. Distillation is required for
TO-15 Checklist Determination of VOCs in Air by GC-MS
 LAB ID: DATE: LAB NAME: ASSESSOR NAME: Method Number: TO-15 Checklist Determination of VOCs in Air by GC-MS SOP Number: Revision Number: SOP Date: Personnel records observed: Data records observed: Revision
LAB ID: DATE: LAB NAME: ASSESSOR NAME: Method Number: TO-15 Checklist Determination of VOCs in Air by GC-MS SOP Number: Revision Number: SOP Date: Personnel records observed: Data records observed: Revision
Standard Practice for Calculating Formulation Physical Constants of Paints and Coatings 1
 Designation: D 5201 05 Standard Practice for Calculating Formulation Physical Constants of Paints and Coatings 1 This standard is issued under the fixed designation D 5201; the number immediately following
Designation: D 5201 05 Standard Practice for Calculating Formulation Physical Constants of Paints and Coatings 1 This standard is issued under the fixed designation D 5201; the number immediately following
THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS
 International Gas Union Research Conference 14 THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS Main author Hironori IMANISHI Tokyo Gas Co., Ltd. JAPAN himanishi@tokyo-.co.jp
International Gas Union Research Conference 14 THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS Main author Hironori IMANISHI Tokyo Gas Co., Ltd. JAPAN himanishi@tokyo-.co.jp
Colorimetric Method Method to 0.70 mg/l Ag Powder Pillows
 Silver DOC316.53.01134 Colorimetric Method Method 8120 0.02 to 0.70 mg/l Ag Powder Pillows Scope and application: For water and wastewater. Test preparation Instrument-specific information Table 1 shows
Silver DOC316.53.01134 Colorimetric Method Method 8120 0.02 to 0.70 mg/l Ag Powder Pillows Scope and application: For water and wastewater. Test preparation Instrument-specific information Table 1 shows
SECTION D.2 AMMONIA NITROGEN
 SECTION D.2 AMMONIA NITROGEN CEDR Method Code: NH4F L01 a) Scope and Application i) This method describes the determination of low-level ammonia nitrogen concentrations in filtered samples taken from fresh
SECTION D.2 AMMONIA NITROGEN CEDR Method Code: NH4F L01 a) Scope and Application i) This method describes the determination of low-level ammonia nitrogen concentrations in filtered samples taken from fresh
Standard Operating Procedure for the Analysis of Dissolved Inorganic Carbon CCAL 21A.1
 Standard Operating Procedure for the Analysis of Dissolved Inorganic Carbon CCAL 21A.1 Cooperative Chemical Analytical Laboratory College of Forestry Oregon State University 321 Richardson Hall Corvallis,
Standard Operating Procedure for the Analysis of Dissolved Inorganic Carbon CCAL 21A.1 Cooperative Chemical Analytical Laboratory College of Forestry Oregon State University 321 Richardson Hall Corvallis,
Continuous measurement of airborne particles and gases
 Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
METHOD 13B - DETERMINATION OF TOTAL FLUORIDE EMISSIONS FROM STATIONARY SOURCES (SPECIFIC ION ELECTRODE METHOD)
 874 METHOD 13B - DETERMINATION OF TOTAL FLUORIDE EMISSIONS FROM STATIONARY SOURCES (SPECIFIC ION ELECTRODE METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and supplies)
874 METHOD 13B - DETERMINATION OF TOTAL FLUORIDE EMISSIONS FROM STATIONARY SOURCES (SPECIFIC ION ELECTRODE METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and supplies)
Hach Method Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis
 Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
Analyzing solubility of acid gas and light alkanes in triethylene glycol
 From the SelectedWorks of ali ali 208 Analyzing solubility of acid gas and light alkanes in triethylene glycol ali ali Available at: https://works.bepress.com/bahadori/8/ Journal of Natural Gas Chemistry
From the SelectedWorks of ali ali 208 Analyzing solubility of acid gas and light alkanes in triethylene glycol ali ali Available at: https://works.bepress.com/bahadori/8/ Journal of Natural Gas Chemistry
RADIOIODINE COLLECTION FILTER EFFICIENCY TESTING PROGRAM at F&J SPECIALTY PRODUCTS, INC. by FRANK M. GAVILA. Atlantic City, New Jersey June 2002
 F&J SPECIALTY PRODUCTS, INC. PO Box 2888 Ocala, Florida 34478-2888 Tel: (352) 680-1177 (352) 680-1178 Fax: (352) 680-1454 Email: fandj@fjspecialty.com Internet: www.fjspecialty.com The Nucleus of Quality
F&J SPECIALTY PRODUCTS, INC. PO Box 2888 Ocala, Florida 34478-2888 Tel: (352) 680-1177 (352) 680-1178 Fax: (352) 680-1454 Email: fandj@fjspecialty.com Internet: www.fjspecialty.com The Nucleus of Quality
Method to 0.50 mg/l NH 3 N Powder Pillows
 , 8155 Salicylate Method 1 Scope and Application: For water, wastewater and seawater 1 Adapted from Clin. Chim. Acta., 14, 403 (1966) DOC316.53.01077 Method 8155 0.01 to 0.50 mg/l NH 3 N Powder Pillows
, 8155 Salicylate Method 1 Scope and Application: For water, wastewater and seawater 1 Adapted from Clin. Chim. Acta., 14, 403 (1966) DOC316.53.01077 Method 8155 0.01 to 0.50 mg/l NH 3 N Powder Pillows
Certificate of Conformance
 Certificate of Conformance Product: Silver Impregnated Zeolite, Type Ag 400 G13 Mesh: 16 x 40 Mesh Part Number: AGX-4 Manufacturing Lot Number: 0507182-1 Purchase Order: Ref. Doc Number: Material Cure
Certificate of Conformance Product: Silver Impregnated Zeolite, Type Ag 400 G13 Mesh: 16 x 40 Mesh Part Number: AGX-4 Manufacturing Lot Number: 0507182-1 Purchase Order: Ref. Doc Number: Material Cure
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
METHOD 8033 ACETONITRILE BY GAS CHROMATOGRAPHY WITH NITROGEN-PHOSPHORUS DETECTION
 METHOD 80 ACETONITRILE BY GAS CHROMATOGRAPHY WITH NITROGEN-PHOSPHORUS DETECTION 1.0 SCOPE AND APPLICATION 1.1 Method 80 may be used to determine the concentration of acetonitrile (CAS No. 75-05-8) in aqueous
METHOD 80 ACETONITRILE BY GAS CHROMATOGRAPHY WITH NITROGEN-PHOSPHORUS DETECTION 1.0 SCOPE AND APPLICATION 1.1 Method 80 may be used to determine the concentration of acetonitrile (CAS No. 75-05-8) in aqueous
Worldwide Pollution Control Association
 Worldwide Pollution Control Association WPCA- Southern Company Mercury Seminar October 30-31, 2012 All presentations posted on this website are copyrighted by the Worldwide Pollution Control Association
Worldwide Pollution Control Association WPCA- Southern Company Mercury Seminar October 30-31, 2012 All presentations posted on this website are copyrighted by the Worldwide Pollution Control Association
Method to 0.50 mg/l NH 3 N Powder Pillows
 , 8155 Salicylate Method 1 Scope and Application: For water, wastewater and seawater 1 Adapted from Clin. Chim. Acta., 14, 403 (1966) DOC316.53.01077 Method 8155 0.01 to 0.50 mg/l NH 3 N Powder Pillows
, 8155 Salicylate Method 1 Scope and Application: For water, wastewater and seawater 1 Adapted from Clin. Chim. Acta., 14, 403 (1966) DOC316.53.01077 Method 8155 0.01 to 0.50 mg/l NH 3 N Powder Pillows
The Application of Method TO-15 to Naphthalene Measurements in Indoor Air
 The Application of Method TO-15 to Naphthalene Measurements in Indoor Air Extended Abstract #13 Heidi C. Hayes and Diane J. Benton Air Toxics Ltd. 180 Blue Ravine Rd. Ste. B Folsom, CA 95630 INTRODUCTION
The Application of Method TO-15 to Naphthalene Measurements in Indoor Air Extended Abstract #13 Heidi C. Hayes and Diane J. Benton Air Toxics Ltd. 180 Blue Ravine Rd. Ste. B Folsom, CA 95630 INTRODUCTION
Topsøe Catalysis Forum 2009
 Mercury Behaviour in Combustion Flue Gases Topsøe Catalysis Forum 9 Munkerupgaard 7 th -8 th of August 9 Dr. Harald Thorwarth Energie braucht Impulse Introduction clean gas Cr Co Ni Cd As Cu Pb Hg Input
Mercury Behaviour in Combustion Flue Gases Topsøe Catalysis Forum 9 Munkerupgaard 7 th -8 th of August 9 Dr. Harald Thorwarth Energie braucht Impulse Introduction clean gas Cr Co Ni Cd As Cu Pb Hg Input
AOAC Official Method 2016.xx. Determination of Total Sulfur in Fertilizers by High Temperature Combustion
 AOAC Official Method 2016.xx Determination of Total Sulfur in Fertilizers by High Temperature Combustion Proposed First Action 2015 (Applicable for measuring total sulfur concentration in solid and liquid
AOAC Official Method 2016.xx Determination of Total Sulfur in Fertilizers by High Temperature Combustion Proposed First Action 2015 (Applicable for measuring total sulfur concentration in solid and liquid
EXECUTIVE SUMMARY. especially in last 50 years. Industries, especially power industry, are the large anthropogenic
 EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Cyanide Analysis of Wastewater Samples from FCC and Hydrocracking Operations
 Cyanide Analysis of Wastewater Samples from FCC and Hydrocracking Operations Introduction Fluid catalytic cracking (FCC) is a major unit operation in refineries around the world. FCC is used to convert
Cyanide Analysis of Wastewater Samples from FCC and Hydrocracking Operations Introduction Fluid catalytic cracking (FCC) is a major unit operation in refineries around the world. FCC is used to convert
NITROGEN AND ITS COMPOUNDS Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure.
 NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Shirley E. Clark, Ph.D., P.E., D. WRE Penn State Harrisburg. Robert Pitt, Ph.D., P.E., BCEE, D. WRE University of Alabama
 Shirley E. Clark, Ph.D., P.E., D. WRE Penn State Harrisburg Robert Pitt, Ph.D., P.E., BCEE, D. WRE University of Alabama Site Stormwater Characteristics and Permit Limits Analytes on Permit 90 th percentile
Shirley E. Clark, Ph.D., P.E., D. WRE Penn State Harrisburg Robert Pitt, Ph.D., P.E., BCEE, D. WRE University of Alabama Site Stormwater Characteristics and Permit Limits Analytes on Permit 90 th percentile
Cyclones. Vane Axial Cyclone 10/30/2013. EVE 402 Air Pollution Generation and Control. Chapter #5 Lectures (Part 4) A mechanical gas cleaning device
 EVE 402 Air Pollution Generation and Control Chapter #5 Lectures (Part 4) Cyclones A mechanical gas cleaning device Gas is spun (centrifugal force) to separate particles Two types Vane axial A ring of
EVE 402 Air Pollution Generation and Control Chapter #5 Lectures (Part 4) Cyclones A mechanical gas cleaning device Gas is spun (centrifugal force) to separate particles Two types Vane axial A ring of
Laboratory Evaluation of EPA Methods 26 and 26A for Analysis of Halogens and Halides in Stack Gas
 Laboratory Evaluation of EPA Methods 26 and 26A for Analysis of Halogens and Halides in Stack Gas 3002003347 Laboratory Evaluation of EPA Methods 26 and 26A for Analysis of Halogens and Halides in Stack
Laboratory Evaluation of EPA Methods 26 and 26A for Analysis of Halogens and Halides in Stack Gas 3002003347 Laboratory Evaluation of EPA Methods 26 and 26A for Analysis of Halogens and Halides in Stack
CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature
 CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature Charles A. Lockert, Breen Energy Solution, 104 Broadway Street, Carnegie, PA 15106 Peter C. Hoeflich and Landis S. Smith, Progress Energy
CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature Charles A. Lockert, Breen Energy Solution, 104 Broadway Street, Carnegie, PA 15106 Peter C. Hoeflich and Landis S. Smith, Progress Energy
METHOD 8030A ACROLEIN AND ACRYLONITRILE BY GAS CHROMATOGRAPHY
 METHOD 8030A ACROLEIN AND ACRYLONITRILE BY GAS CHROMATOGRAPHY 1.0 SCOPE AND APPLICATION 1.1 Method 8030 is used to determine the concentration of the following volatile organic compounds: Compound Name
METHOD 8030A ACROLEIN AND ACRYLONITRILE BY GAS CHROMATOGRAPHY 1.0 SCOPE AND APPLICATION 1.1 Method 8030 is used to determine the concentration of the following volatile organic compounds: Compound Name
ASTM Designation: D Standard Test Method for Determination of Iodine Number of Activated Carbon
 ASTM Designation: D4607-94 Standard Test Method for Determination of Iodine Number of Activated Carbon 1. Scope 1.1 This test method covers the determination of the relative activation level of unused
ASTM Designation: D4607-94 Standard Test Method for Determination of Iodine Number of Activated Carbon 1. Scope 1.1 This test method covers the determination of the relative activation level of unused
Industrial Hygiene Report
 Industrial Hygiene Report Control of Hydrochloric Acid Vapors in a Lab Setting September, 24 2012 Sentry Air Systems 6999 W. Little York Rd, Suite P1 Houston, TX 77040 BACKGROUND: Hydrochloric acid, an
Industrial Hygiene Report Control of Hydrochloric Acid Vapors in a Lab Setting September, 24 2012 Sentry Air Systems 6999 W. Little York Rd, Suite P1 Houston, TX 77040 BACKGROUND: Hydrochloric acid, an
New good practices for emission measurements. and relevance of the SRM with respect to these requirements/practices CEM 2014
 New good practices for emission measurements and relevance of the SRM with respect to these requirements/practices CEM 2014 Jean Poulleau Cécile Raventos Evolution of French rules for emission measurements
New good practices for emission measurements and relevance of the SRM with respect to these requirements/practices CEM 2014 Jean Poulleau Cécile Raventos Evolution of French rules for emission measurements
Omega Chemicals Emissions Testing Report
 Omega Chemicals 18-Feb-2015 Doc No. 60336610_1.1_RPT Omega Chemicals Emissions Testing Report February 2015 NATA ACCREDITATION No. 2778 (14391) Accredited for compliance with ISO/IEC 17025 This document
Omega Chemicals 18-Feb-2015 Doc No. 60336610_1.1_RPT Omega Chemicals Emissions Testing Report February 2015 NATA ACCREDITATION No. 2778 (14391) Accredited for compliance with ISO/IEC 17025 This document
ANALYTICAL METHOD DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Air sampling and analysis
 DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
EFFECT OF CALIBRATION SPECIMEN PREPARATION TECHNIQUES ON NARROW RANGE X-RAY FLUORESCENCE CALIBRATION ACCURACY
 Copyright(c)JCPDS-International Centre for Diffraction Data 2000,Advances in X-ray Analysis,Vol.43 424 EFFECT OF CALIBRATION SPECIMEN PREPARATION TECHNIQUES ON NARROW RANGE X-RAY FLUORESCENCE CALIBRATION
Copyright(c)JCPDS-International Centre for Diffraction Data 2000,Advances in X-ray Analysis,Vol.43 424 EFFECT OF CALIBRATION SPECIMEN PREPARATION TECHNIQUES ON NARROW RANGE X-RAY FLUORESCENCE CALIBRATION
Fine Particles: Why We Care
 Fine Particles: Why We Care Visibility/Radiative Forcing Health Effects A function of chemical composition PM2.5 Mostly 1) Sulfate 2) Carbonaceous - Organic - Elemental (Soot) 3) Metals, minerals, Metals,
Fine Particles: Why We Care Visibility/Radiative Forcing Health Effects A function of chemical composition PM2.5 Mostly 1) Sulfate 2) Carbonaceous - Organic - Elemental (Soot) 3) Metals, minerals, Metals,
Point Level Capacitance Switch for Fly Ash Hopper Measurement
 Point Level Capacitance Switch for Fly Ash Hopper Measurement By: Bill Sholette Level Products Business Manager, Endress+Hauser Ravi Jethra Industry Manager Power/Renewables, Endress+Hauser If you re the
Point Level Capacitance Switch for Fly Ash Hopper Measurement By: Bill Sholette Level Products Business Manager, Endress+Hauser Ravi Jethra Industry Manager Power/Renewables, Endress+Hauser If you re the
Nitrogen, Total Inorganic
 Nitrogen, Total Inorganic DOC316.53.01090 Titanium Trichloride Reduction Method Method 10021 0.2 to 25.0 mg/l N Test N Tube Vials Scope and application: For water, wastewater and seawater. Test preparation
Nitrogen, Total Inorganic DOC316.53.01090 Titanium Trichloride Reduction Method Method 10021 0.2 to 25.0 mg/l N Test N Tube Vials Scope and application: For water, wastewater and seawater. Test preparation
Elements and Their Oxides
 Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles
 Air Pollution XIII 519 Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles K. D. Stewart & J. M. Andino Department of Environmental Engineering
Air Pollution XIII 519 Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles K. D. Stewart & J. M. Andino Department of Environmental Engineering
Analysis for Natural Gas and Similar Gaseous Mixtures by Gas Chromatography
 GPA Standard 2261-13 Analysis for Natural Gas and Similar Gaseous Mixtures by Gas Chromatography Adopted as Tentative Standard, 1961 Revised and Adopted as a Standard, 1964 Revised 1972, 1986, 1989, 1990,
GPA Standard 2261-13 Analysis for Natural Gas and Similar Gaseous Mixtures by Gas Chromatography Adopted as Tentative Standard, 1961 Revised and Adopted as a Standard, 1964 Revised 1972, 1986, 1989, 1990,
Hach Method Spectrophotometric Measurement of Free Chlorine (Cl 2 ) in Finished Drinking Water
 Hach Method 1041 Spectrophotometric Measurement of Free Chlorine (Cl ) in Finished Drinking Water Hach Company Method 1041 Revision 1. November 015 Spectrophotometric Measurement of Free Cl in Finished
Hach Method 1041 Spectrophotometric Measurement of Free Chlorine (Cl ) in Finished Drinking Water Hach Company Method 1041 Revision 1. November 015 Spectrophotometric Measurement of Free Cl in Finished
Application of Micro-Flow Imaging (MFI TM ) to The Analysis of Particles in Parenteral Fluids. October 2006 Ottawa, Canada
 Application of Micro-Flow Imaging (MFI TM ) to The Analysis of Particles in Parenteral Fluids October 26 Ottawa, Canada Summary The introduction of a growing number of targeted protein-based drug formulations
Application of Micro-Flow Imaging (MFI TM ) to The Analysis of Particles in Parenteral Fluids October 26 Ottawa, Canada Summary The introduction of a growing number of targeted protein-based drug formulations
Adsorption of Hg 0 on the Unburned Carbon with HF Acid Leaching
 Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.1, pp 41-51, 24 jmmce.org Printed in the USA. All rights reserved Adsorption of Hg on the Unburned Carbon with HF Acid Leaching
Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.1, pp 41-51, 24 jmmce.org Printed in the USA. All rights reserved Adsorption of Hg on the Unburned Carbon with HF Acid Leaching
CHEMISTRY. SCIENCE Paper 2
 CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
N10/4/CHEMI/SP2/ENG/TZ0/XX CHEMISTRY STANDARD LEVEL PAPER 2. Thursday 11 November 2010 (afternoon) Candidate session number.
 N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
Real Time On-Site Odor and VOC Emission Measurements Using a znose
 Real Time On-Site Odor and VOC Emission Measurements Using a znose Edward J. Staples, Electronic Sensor Technology, EST@ESTCAL.COM Remediation Site Description Remediation of contaminated soil from where
Real Time On-Site Odor and VOC Emission Measurements Using a znose Edward J. Staples, Electronic Sensor Technology, EST@ESTCAL.COM Remediation Site Description Remediation of contaminated soil from where
2Fe 2 O 3 +3H 2 S FeS+FeS x +S+3H 2 O
 Elemental analysis of hydrocarbon streams using Dry colorimetry analyzers, a catalyst saviour Bushra Dawood, Application Coordinator C.I. Analytics www.cianalytics.com The Petrochemical industry has refined
Elemental analysis of hydrocarbon streams using Dry colorimetry analyzers, a catalyst saviour Bushra Dawood, Application Coordinator C.I. Analytics www.cianalytics.com The Petrochemical industry has refined
The Theory of HPLC. Quantitative and Qualitative HPLC
 The Theory of HPLC Quantitative and Qualitative HPLC i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this reference
The Theory of HPLC Quantitative and Qualitative HPLC i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this reference
Persulfate Digestion Method Method to 25.0 mg/l N (LR) Test N Tube Vials
 Nitrogen, Total DOC316.53.01086 Persulfate Digestion Method Method 10071 0.5 to 25.0 mg/l N (LR) Test N Tube Vials Scope and application: For water and wastewater. Test preparation Instrument-specific
Nitrogen, Total DOC316.53.01086 Persulfate Digestion Method Method 10071 0.5 to 25.0 mg/l N (LR) Test N Tube Vials Scope and application: For water and wastewater. Test preparation Instrument-specific
Spectrophotometric Determination of Ferrocyanide in Effluents
 Spectrophotometric Determination of Ferrocyanide in Effluents ECN-0025-1 INTRODUCTION This method is used to determine the concentration of ferrocyanide ion in photoprocessing solution effluents. The ion
Spectrophotometric Determination of Ferrocyanide in Effluents ECN-0025-1 INTRODUCTION This method is used to determine the concentration of ferrocyanide ion in photoprocessing solution effluents. The ion
Persulfate Digestion Method Method to 150 mg/l N (HR) Test N Tube Vials
 Nitrogen, Total DOC316.53.01085 Persulfate Digestion Method Method 10072 2 to 150 mg/l N (HR) Test N Tube Vials Scope and application: For water and wastewater. Test preparation Instrument-specific information
Nitrogen, Total DOC316.53.01085 Persulfate Digestion Method Method 10072 2 to 150 mg/l N (HR) Test N Tube Vials Scope and application: For water and wastewater. Test preparation Instrument-specific information
VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing
 VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing Q. Xiang, M. Xanthos*, S. Mitra and S. H. Patel* Department of Chemical Engineering, Chemistry and Environmental Science
VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing Q. Xiang, M. Xanthos*, S. Mitra and S. H. Patel* Department of Chemical Engineering, Chemistry and Environmental Science
International General Certificate of Secondary Education UNIVERSITYOF CAMBRIDGELOCALEXAMINATIONSYNDICATE CHEMISTRY 0620/3
 Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITYOF CAMBRIDGELOCALEXAMINATIONSYNDICATE CHEMISTRY 0620/3 PAPER 3 Thursday 27 MAY 1999 Afternoon
Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITYOF CAMBRIDGELOCALEXAMINATIONSYNDICATE CHEMISTRY 0620/3 PAPER 3 Thursday 27 MAY 1999 Afternoon
It is the purpose of this paper to demonstrate that the detection limit is directly related to the sample size, for both TG and TG/FTIR.
 BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
A New On-line Cyanide Analyzer for Measurement of Cyanide in Hydrometallurgical Processing of Precious Metal Ores
 Application Note 37890312 A New On-line Cyanide Analyzer for Measurement of Cyanide in Hydrometallurgical Processing of Precious Metal Ores Keywords CNSolution 9310 On-line Cyanide Analyzer Cyanidation
Application Note 37890312 A New On-line Cyanide Analyzer for Measurement of Cyanide in Hydrometallurgical Processing of Precious Metal Ores Keywords CNSolution 9310 On-line Cyanide Analyzer Cyanidation
SULFUR DIOXIDE REMOVAL
 Report No. 63 A SULFUR DIOXIDE REMOVAL FROM STACK GASES Supplement A by EARL D. OLIVER August 1973 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE MENLO PARK, CALIFORNIA CONTENTS
Report No. 63 A SULFUR DIOXIDE REMOVAL FROM STACK GASES Supplement A by EARL D. OLIVER August 1973 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE MENLO PARK, CALIFORNIA CONTENTS
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE
 METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE 1.0 SCOPE AND APPLICATION 1.1 This method can be used for measuring total solubilized nitrate in drinking
METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE 1.0 SCOPE AND APPLICATION 1.1 This method can be used for measuring total solubilized nitrate in drinking
Cadmium Reduction Method Method 8171 MR (0.1 to 10.0 mg/l NO 3
 , MR, 8171 DOC316.53.01069 Cadmium Reduction Method Method 8171 MR (0.1 to 10.0 mg/l NO 3 N) Powder Pillows or AccuVac Ampuls Scope and Application: For water, wastewater and seawater Test preparation
, MR, 8171 DOC316.53.01069 Cadmium Reduction Method Method 8171 MR (0.1 to 10.0 mg/l NO 3 N) Powder Pillows or AccuVac Ampuls Scope and Application: For water, wastewater and seawater Test preparation
PA DEP SOUTHWEST REGIONAL OFFICE
 PA DEP SOUTHWEST REGIONAL OFFICE MEMO TO FROM Air Quality Permit File: OP-04-00043 / Thomas J. Joseph, P.E. Facilities Engineering Manager Air Quality Program THROUGH Mark R. Gorog, P.E. Environmental
PA DEP SOUTHWEST REGIONAL OFFICE MEMO TO FROM Air Quality Permit File: OP-04-00043 / Thomas J. Joseph, P.E. Facilities Engineering Manager Air Quality Program THROUGH Mark R. Gorog, P.E. Environmental
CHLORINE PROCESS ECONOMICS PROGRAM. Report No. 61A. Supplement A. by YEN CHEN YEN. May A private report by the STANFORD RESEARCH INSTITUTE
 Report No. 61A CHLORINE Supplement A by YEN CHEN YEN May 1074 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 2 3 INTRODUCTION... 1 SUMMARY...
Report No. 61A CHLORINE Supplement A by YEN CHEN YEN May 1074 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 2 3 INTRODUCTION... 1 SUMMARY...
(918) or (888)
 Corporate Headquarters 5634 S. 122nd E. Ave. Ste. F Tulsa, Oklahoma 74146 Las Vegas Office 5925 E. Lake Mead Blvd. Las Vegas, Nevada 89156 Philadelphia Office 8900 State Road Philadelphia, Pennsylvania
Corporate Headquarters 5634 S. 122nd E. Ave. Ste. F Tulsa, Oklahoma 74146 Las Vegas Office 5925 E. Lake Mead Blvd. Las Vegas, Nevada 89156 Philadelphia Office 8900 State Road Philadelphia, Pennsylvania
Gas Chromatography. Presented By Mr. Venkateswarlu Mpharm KTPC
 Gas Chromatography Gas Chromatography Presented By Mr. Venkateswarlu Mpharm KTPC What is Gas Chromatography? It is also known as Gas-Liquid Chromatography (GLC) GAS CHROMATOGRAPHY Separation of gaseous
Gas Chromatography Gas Chromatography Presented By Mr. Venkateswarlu Mpharm KTPC What is Gas Chromatography? It is also known as Gas-Liquid Chromatography (GLC) GAS CHROMATOGRAPHY Separation of gaseous
METHOD 3600C CLEANUP
 METHOD 3600C CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3600 provides general guidance on selection of cleanup methods that are appropriate for the target analytes of interest. Cleanup methods are applied
METHOD 3600C CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3600 provides general guidance on selection of cleanup methods that are appropriate for the target analytes of interest. Cleanup methods are applied
INVERSE PROBLEM AND CALIBRATION OF PARAMETERS
 INVERSE PROBLEM AND CALIBRATION OF PARAMETERS PART 1: An example of inverse problem: Quantification of the uncertainties of the physical models of the CATHARE code with the CIRCÉ method 1. Introduction
INVERSE PROBLEM AND CALIBRATION OF PARAMETERS PART 1: An example of inverse problem: Quantification of the uncertainties of the physical models of the CATHARE code with the CIRCÉ method 1. Introduction
Perfluorinated Alkyl Acids (PFAA) in Water by LC/MS/MS - PBM
 Organics Revision Date: July 19, 2017 Perfluorinated Alkyl Acids (PFAA) in Water by LC/MS/MS - PBM Parameter Perfluorinated Alkyl Acids (Perfluorobutane Sulphonate (PFBS), Perflourooctane Sulphonate (PFOS),
Organics Revision Date: July 19, 2017 Perfluorinated Alkyl Acids (PFAA) in Water by LC/MS/MS - PBM Parameter Perfluorinated Alkyl Acids (Perfluorobutane Sulphonate (PFBS), Perflourooctane Sulphonate (PFOS),
Simulator for Automotive Evaporative Emissions Restraint Systems
 Simulator for Automotive Evaporative Emissions Restraint Systems Presentation Dr.-Ing. Stefan Schlüter, Fraunhofer UMSICHT, Germany COMSOL Conference 2016 Munich Introduction Automotive Evaporative Emissions
Simulator for Automotive Evaporative Emissions Restraint Systems Presentation Dr.-Ing. Stefan Schlüter, Fraunhofer UMSICHT, Germany COMSOL Conference 2016 Munich Introduction Automotive Evaporative Emissions
Chemistry Instrumental Analysis Lecture 27. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 27 Gas Chromatography Introduction GC covers all chromatographic methods in which the mobile phase is gas. It may involve either a solid stationary phase (GSC)
Chemistry 4631 Instrumental Analysis Lecture 27 Gas Chromatography Introduction GC covers all chromatographic methods in which the mobile phase is gas. It may involve either a solid stationary phase (GSC)
Hydra-C Application Note: 1076
 Hydra-C Application Note: 1076 The Determination of Mercury in Samples by U.S. EPA SOW 846 Method 7473 using the Hydra-C Mercury Analyzer Introduction The accurate determination of mercury in various samples
Hydra-C Application Note: 1076 The Determination of Mercury in Samples by U.S. EPA SOW 846 Method 7473 using the Hydra-C Mercury Analyzer Introduction The accurate determination of mercury in various samples
SCR Catalyst Selection and Management for Improved Hg Oxidation Performance
 SCR Catalyst Selection and Management for Improved Hg Oxidation Performance Paper # 37 Presented at the Power Plant Pollutant Control MEGA Symposium August 19-21, 2014 Baltimore, MD Christopher T. Reeves,
SCR Catalyst Selection and Management for Improved Hg Oxidation Performance Paper # 37 Presented at the Power Plant Pollutant Control MEGA Symposium August 19-21, 2014 Baltimore, MD Christopher T. Reeves,
HYPHENATED TECHNOLOGY GUIDE
 HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
Cadmium Reduction Method Method to 30.0 mg/l NO 3 N (HR) Powder Pillows or AccuVac Ampuls
 Nitrate DOC316.53.01066 Cadmium Reduction Method Method 8039 0.3 to 30.0 mg/l NO 3 N (HR) Powder Pillows or AccuVac Ampuls Scope and application: For water, wastewater and seawater. Test preparation Instrument-specific
Nitrate DOC316.53.01066 Cadmium Reduction Method Method 8039 0.3 to 30.0 mg/l NO 3 N (HR) Powder Pillows or AccuVac Ampuls Scope and application: For water, wastewater and seawater. Test preparation Instrument-specific
INDIANA DEPARTMENT OF TRANSPORTATION OFFICE OF MATERIALS MANAGEMENT. VERIFYING THERMOMETERS ITM No T
 1.0 SCOPE. INDIANA DEPARTMENT OF TRANSPORTATION OFFICE OF MATERIALS MANAGEMENT VERIFYING THERMOMETERS ITM No. 909-08T 1.1 This test method covers the procedure for a verification of scale accuracy of liquid-in-glass
1.0 SCOPE. INDIANA DEPARTMENT OF TRANSPORTATION OFFICE OF MATERIALS MANAGEMENT VERIFYING THERMOMETERS ITM No. 909-08T 1.1 This test method covers the procedure for a verification of scale accuracy of liquid-in-glass
TECHNICAL DESCRIPTION SPECTRAFLOW ON LINE ANALYZER for BELT CONVEYOR APPLICATION
 TECHNICAL DESCRIPTION SPECTRAFLOW ON LINE ANALYZER for BELT CONVEYOR APPLICATION TECHNICAL SPECIFICATION SPECTRAFLOW ON LINE ANALYZER FOR BELT CONVEYOR CONTENTS 1. SpectraFlow Technical Description...
TECHNICAL DESCRIPTION SPECTRAFLOW ON LINE ANALYZER for BELT CONVEYOR APPLICATION TECHNICAL SPECIFICATION SPECTRAFLOW ON LINE ANALYZER FOR BELT CONVEYOR CONTENTS 1. SpectraFlow Technical Description...
METHOD 3520B CONTINUOUS LIQUID-LIQUID EXTRACTION
 METHOD 3520B CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
METHOD 3520B CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
Fusion UV/Persulfate TOC Analyzer
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +847.913.0777 for Refurbished & Certified Lab Equipment Fusion UV/Persulfate TOC Analyzer The Fusion Total Organic Carbon (TOC) Analyzer
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +847.913.0777 for Refurbished & Certified Lab Equipment Fusion UV/Persulfate TOC Analyzer The Fusion Total Organic Carbon (TOC) Analyzer
NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25)
 Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
High-Speed Environmental Analysis Using the Agilent 7500cx with Integrated Sample Introduction System Discrete Sampling (ISIS DS)
 High-Speed Environmental Analysis Using the Agilent 7500cx with Integrated Sample Introduction System Discrete Sampling (ISIS DS) Application Note Environmental Authors Steve Wilbur Agilent Technologies,
High-Speed Environmental Analysis Using the Agilent 7500cx with Integrated Sample Introduction System Discrete Sampling (ISIS DS) Application Note Environmental Authors Steve Wilbur Agilent Technologies,
Experiment 5: Analysis of Nutrients in Natural Waters CH3600 / ESP 5090: Environmental Chemistry, Plymouth State University
 Experiment 5: Analysis of Nutrients in Natural Waters CH3600 / ESP 5090: Environmental Chemistry, Plymouth State University Adapted from "Experiment 3: Analysis of Phosphate in Water," Laboratory Experiments
Experiment 5: Analysis of Nutrients in Natural Waters CH3600 / ESP 5090: Environmental Chemistry, Plymouth State University Adapted from "Experiment 3: Analysis of Phosphate in Water," Laboratory Experiments
Hydrated Lime for HCl Mitigation Benefits of High Reactivity Hydrate
 Hydrated Lime for HCl Mitigation Benefits of High Reactivity Hydrate July 18, 2013 Utility Focus - Support of Technical Advancement Support of key industry events WPCA member Sponsor of 2013 APC Conference
Hydrated Lime for HCl Mitigation Benefits of High Reactivity Hydrate July 18, 2013 Utility Focus - Support of Technical Advancement Support of key industry events WPCA member Sponsor of 2013 APC Conference
IMADUDDIN SCHOOL Second Term Examination 2017
 Index Register Class number number Name IMADUDDIN SCHOOL Second Term Examination 2017 GRADE 9 CHEMISTRY 5070/02 Paper 2 Theory Nov 2017 TIME 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Write your name,
Index Register Class number number Name IMADUDDIN SCHOOL Second Term Examination 2017 GRADE 9 CHEMISTRY 5070/02 Paper 2 Theory Nov 2017 TIME 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Write your name,
METHOD 3600B CLEANUP
 METHOD 3600B CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3600 provides general guidance on selection of cleanup methods that are appropriate for the target analytes of interest. Cleanup methods are applied
METHOD 3600B CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3600 provides general guidance on selection of cleanup methods that are appropriate for the target analytes of interest. Cleanup methods are applied
Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions Answers
 Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions 1980 2010 - Answers 1982 - #5 (a) From the standpoint of the kinetic-molecular theory, discuss briefly the properties of gas molecules
Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions 1980 2010 - Answers 1982 - #5 (a) From the standpoint of the kinetic-molecular theory, discuss briefly the properties of gas molecules
