The following examination contains 32 questions valued at 3.5 points/question, and some rather tasty opportunities for extra credit
|
|
|
- Beatrice Powers
- 5 years ago
- Views:
Transcription
1 Chem 131 Exam II Practice Spring 2018 The following examination contains 32 questions valued at 3.5 points/question, and some rather tasty opptunities f extra credit Name KEY 1. The cticosteroid prednisone suppresses the immune response and is useful in treating exposure to poison ivy and poison oak. Please list the functional groups present (HW 14.30) Prednisone Functional group 1: Alcohol (x2) Functional group 2: Ketone (x3) Functional group 3: Alkene (x2) While beyond the scope of this course, having both the alkene and alcohols at the carbon adjacent to the carbonyl (the carbon) influences their reactivities significantly. Make certain you do not confuse the terminal hydroxy ketone with a carboxylic acid, which connects oxygen directly to the carbonyl carbon Please name the following compounds. 1 EC point f crect alternative naming (I will use your best answer as the main answer, and if the 2 nd is a bit wobbly, I will use it as the extra credit) 2.
2 (in a pinch) 6. 7.
3 8. 9. If you have trouble seeing if different representations are the same molecule not, please feel free to bring ( beg f) model kits 10. Notice that even though the methyl group is indicated as directed towards the viewer, there are 2 of the same (ethylamino) groups; as such, (R) (S) isomers are not possible. Pure trickery! 11. F (preferred)
4 12. Please draw the structure of the following compounds directly below the name 13. butanedioic acid (succinic acid) 14. Dipotassium butanedioate (Dipotassium succinate) K + K Diethyl butanedioate (Diethyl succinate) 16. para-aminophenol
5 17. tho-chlo benzyl alcohol (2-chlophenylmethanol) 18. Triphenyl amine 19. Ethyl trans-2-hexenoate 20. Which of the following compounds would have the lowest boiling point? e.
6 Octane is non-polar. Only weak London fces hold a collection of octane molecules together. However the size (MM = 114 g/mol) does give a BP = o C. f comparison, heptanamide (MM = 129 g/mol) has a BP = 254 o C 21. Which of the following compounds would have the most limited water solubility? e. Like dissolves like and water is polar 22. Which of the following compounds would have the greatest solubility when mixed with a ph = 2 aqueous buffer? Acid will not react in low ph/acidic environment Amides are not basic e. N/A; All have about the same water solubility Weak base amine in acid bingo! Salt fms!
7 23. Which of the following compounds would have the greatest water solubility when mixed with a ph = 12 aqueous buffer? Acid in a basic environment bingo! Salt fms! Amides are not acidic either e. N/A; All have about the same water solubility Base will not react in a basic environment 24. Which of the following gives an acid when heated with aqueous HCl? e. N/A; all will give an acid when heated with aqueous HCl
8 Make certain you can name the acid fmed after hydrolysis in each case 25. Which of the following compounds will not dissolve completely in water? e. N/A; all of the above compounds dissolve completely in water Tough question, but recall esters are me limited in water solubility than one would expect based on carbon to oxygen ratio (pay close attention to page 5 of lecture 13 notes) In questions 25-30, please give the maj product f the following reactions. If there is no reaction, write N.R. 26. Room Temp Don t be fooled by the change in ientation, this is just a simple salt fmation between an acid and base 27.
9 28. Acid anhydride (similar to the acetic anhydride we used to make aspirin) allowing amide fmation between an amine and acid derivative, thus avoiding an acid-base reaction 1) KMnO4/OH - / NR No hydrogens available on the ketone f further oxidation 29. 1) NaBH4 2) H + Ketones resist further oxidation, but they can be readily reduced back to alcohols 30. H2O H + Recall Markovnikov s rule the most substituted alcohol is preferred 31.
10 H2O H + A bit tricky since the amine that initially went into making the cyclic amide (lactam) was part of the same molecule 32. Please synthesize pentanedioic acid from the -valerolactone (I) I Step 1: Aqueous acid and heat to generate 5-hydroxypentanoic acid Step 2: KMnO 4 in base with heat, Na 2 Cr 2 O 7 in H 2 SO 4 to oxidize alcohol to acid Bonus (3.5 EC): Please indicate a route by which styrene may be converted into acetophenone styrene acetophenone Step 1: Aqueous acid to generate 1-phenylethanol Step 2: KMnO 4 in base with heat, Na 2 Cr 2 O 7 in H 2 SO 4 to oxidize alcohol to ketone
The following examination contains 31 questions valued at 3.5 points/question. Please use a ½ sheet green Scantron for questions 1-23.
 Chemistry 106 Exam 1 Practice Spring 2018 The following examination contains 31 questions valued at 3.5 points/question. Please use a ½ sheet green Scantron for questions 1-23. Name 1. Which of the following
Chemistry 106 Exam 1 Practice Spring 2018 The following examination contains 31 questions valued at 3.5 points/question. Please use a ½ sheet green Scantron for questions 1-23. Name 1. Which of the following
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Alcohols, Phenols and Ethers
 SUBJECTIVE PROBLEMS: Alcohols, Phenols and Ethers Q1. An organic liquid (A), containing C, H and O with boiling point: 78 o C, and possessing a rather pleasant odour, on heating with concentrated sulphuric
SUBJECTIVE PROBLEMS: Alcohols, Phenols and Ethers Q1. An organic liquid (A), containing C, H and O with boiling point: 78 o C, and possessing a rather pleasant odour, on heating with concentrated sulphuric
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Naming Organic Halides. Properties of Organic Halides
 Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
HW #3: 14.26, 14.28, 14.30, 14.32, 14.36, 14.42, 14.46, 14.52, 14.56, Alcohols, Ethers and Thiols
 Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
Chemistry 2.5 AS WORKBOOK. Working to Excellence Working to Excellence
 Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:!
 CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
Organic Chemistry. Introduction to Organic Molecules and Functional Groups
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
Course Information. Instructor Information
 Jordan University of Science and Technology Department of Chemistry Course Syllabus Fall 2018/2019 Course Information Course Number: CHEM 108 Course Name: General and Organic Chemistry Credit Hours: 4
Jordan University of Science and Technology Department of Chemistry Course Syllabus Fall 2018/2019 Course Information Course Number: CHEM 108 Course Name: General and Organic Chemistry Credit Hours: 4
MSC. ISMAIL M.ALI DEPARTMENT OF CHEMICAL ENGINEEING COLLEGE OF ENGINEERING TIKRIT UNIVERSITY
 LECTURE 1 SYLLABUS FOR FIRST CLASS 2013-2014 MSC. ISMAIL M.ALI DEPARTMENT OF CHEMICAL ENGINEEING COLLEGE OF ENGINEERING TIKRIT UNIVERSITY MANDATORY CLASS: 1ST ORGANIC CHEMISTRY CH 122 Teaching scheme:
LECTURE 1 SYLLABUS FOR FIRST CLASS 2013-2014 MSC. ISMAIL M.ALI DEPARTMENT OF CHEMICAL ENGINEEING COLLEGE OF ENGINEERING TIKRIT UNIVERSITY MANDATORY CLASS: 1ST ORGANIC CHEMISTRY CH 122 Teaching scheme:
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Chem 3A - Practice Midterm I. Note: This is a slightly modified version of the first midterm exam from Chem 112A Fall 2012
 Chem 3A - Practice Midterm I Note: This is a slightly modified version of the first midterm exam from Chem 112A Fall 2012 Please provide all answers in the space provided. You are not allowed to use a
Chem 3A - Practice Midterm I Note: This is a slightly modified version of the first midterm exam from Chem 112A Fall 2012 Please provide all answers in the space provided. You are not allowed to use a
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
ORGANIC CHEMISTRY II
 ORGANIC EMISTRY II. CARBOXYLIC ACIDS Saturated mono carboxylic s are called fatty s. group is COOH which is made up of carbonyl and hydroxy groups. General molecular formula is CnH n O or C n H n+1 COOH.
ORGANIC EMISTRY II. CARBOXYLIC ACIDS Saturated mono carboxylic s are called fatty s. group is COOH which is made up of carbonyl and hydroxy groups. General molecular formula is CnH n O or C n H n+1 COOH.
(a) (b) CHAPTER 22. Practice exercises
 CAPTE 22 Practice exercises 22.1 ()-2,3-dihydroxypropanoic acid cis-butenedioic acid or (Z)-butenedioic acid (c) ()-3,5-dihydroxy-3-methylpenanoic acid 22.3 benzyl alcohol iodobenzene 22.5 pk a = 5.03
CAPTE 22 Practice exercises 22.1 ()-2,3-dihydroxypropanoic acid cis-butenedioic acid or (Z)-butenedioic acid (c) ()-3,5-dihydroxy-3-methylpenanoic acid 22.3 benzyl alcohol iodobenzene 22.5 pk a = 5.03
Chem 1120 Midterm points Dr. Luther Giddings
 Chem 1120 Midterm 1 100 points Dr. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Chem 1120 Midterm 1 100 points Dr. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Alkanes, Alkenes and Alkynes
 Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Module 4 revision guide: Compounds with C=O group
 opyright N Goalby Bancroft's School Module 4 revision guide: ompounds with = group arbonyls: Aldehydes and Ketones arbonyls are compounds with a = bond, they can be either aldehydes or ketones. 3 ethanal
opyright N Goalby Bancroft's School Module 4 revision guide: ompounds with = group arbonyls: Aldehydes and Ketones arbonyls are compounds with a = bond, they can be either aldehydes or ketones. 3 ethanal
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Assignment Isomers, Nomenclature, Polymers
 Assignment Isomers, Nomenclature, Polymers Anne-Marie Guirguis K /20 T /26 A /20 C /11 Total St. Francis Xavier SCH4U1 April 14 2017 Multiple Choice [Knowledge 20] Select the letter of the best answer
Assignment Isomers, Nomenclature, Polymers Anne-Marie Guirguis K /20 T /26 A /20 C /11 Total St. Francis Xavier SCH4U1 April 14 2017 Multiple Choice [Knowledge 20] Select the letter of the best answer
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Carbonyl groups react via nucleophilic addition, with the mechanism being represented as follows:
 Aldehydes and Ketones Introduction Aldehydes and ketones are two similar homologous groups both having the carbonyl group: The Carbon on the carbonyl group is slightly positive wince the Oxygen is pulling
Aldehydes and Ketones Introduction Aldehydes and ketones are two similar homologous groups both having the carbonyl group: The Carbon on the carbonyl group is slightly positive wince the Oxygen is pulling
ORGANIC - BROWN 8E CH CARBOXYLIC ACIDS.
 RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Carboxylic acid derivatives
 arboxylic acid derivatives Acyl hlorides and Acid Anhydrides N Goalby hemrevise.org arboxylic acid derivatives carboxylic acid (ethanoic acid) 3 l Acyl hloride (ethanoyl chloride) key 3 3 Acid Anhydride
arboxylic acid derivatives Acyl hlorides and Acid Anhydrides N Goalby hemrevise.org arboxylic acid derivatives carboxylic acid (ethanoic acid) 3 l Acyl hloride (ethanoyl chloride) key 3 3 Acid Anhydride
Ch08. Carbonyls. The carbonyl functional group. Exploring ketones, aldehydes and their reactions. version 1.0
 Ch08 Carbonyls The carbonyl functional group. Exploring ketones, aldehydes and their reactions. version 1.0 Nick DeMello, PhD. 2007-2015 Important Dates This Wednesday: - Lab Checkout (you must check out
Ch08 Carbonyls The carbonyl functional group. Exploring ketones, aldehydes and their reactions. version 1.0 Nick DeMello, PhD. 2007-2015 Important Dates This Wednesday: - Lab Checkout (you must check out
FIRST EXAMINATION. Name: CHM 332
 ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
CHEMISTRY 150. April 2012
 CHEMISTRY 150 Dr. B. MacLean April 2012 NAME: (please print) ID #: This is a three hour exam. SUGGESTION: Read over the entire exam before beginning, and begin by doing those questions which you find easiest.
CHEMISTRY 150 Dr. B. MacLean April 2012 NAME: (please print) ID #: This is a three hour exam. SUGGESTION: Read over the entire exam before beginning, and begin by doing those questions which you find easiest.
1) Which type of compound does not contain a carbonyl group? A) ketone B) aldehyde C) amine D) ester E) carboxylic acid
 1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
Hour Examination # 1
 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam # 1 Problem Booklet Page 1 of 11 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam Booklet No. Instructors: Paul Bracher & Erin Whitteck Hour Examination # 1 Wednesday,
CHEM 2410 Organic Chemistry 1 Fall 2017 Exam # 1 Problem Booklet Page 1 of 11 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam Booklet No. Instructors: Paul Bracher & Erin Whitteck Hour Examination # 1 Wednesday,
Full First and Last Name DO NOT WRITE YOUR NAME UNTIL TOLD TO START! CHEM 8B Organic Chemistry II EXAM 2, Winter 2017 (200 points)
 UCSC, Binder Exam 2, W17 Full First and Last Name D NT WRITE YUR NAME UNTIL TLD T START! CHEM 8B rganic Chemistry II EXAM 2, Winter 2017 (200 points) In each of the following problems, use your knowledge
UCSC, Binder Exam 2, W17 Full First and Last Name D NT WRITE YUR NAME UNTIL TLD T START! CHEM 8B rganic Chemistry II EXAM 2, Winter 2017 (200 points) In each of the following problems, use your knowledge
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
CHEM 60 Spring 2016 Exam 2 Ch 5-8, 100 points total.
 Name Exam No. F CHEM 60 Spring 2016 Exam 2 Ch 5-8, 100 points total. Multiple Choice. (20 questions, 3 points each = 60 points total) Mark the letter on the scantron form corresponding to the one best
Name Exam No. F CHEM 60 Spring 2016 Exam 2 Ch 5-8, 100 points total. Multiple Choice. (20 questions, 3 points each = 60 points total) Mark the letter on the scantron form corresponding to the one best
3) Oxidation of tertiary alcohol yields A) Aldehyde B) No reaction C) Ketone D) Carboxylic acid
 ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
ORGANIC - BRUICE 8E CH.3 - AN INTRODUCTION TO ORGANIC COMPOUNDS
 !! www.clutchprep.com CONCEPT: INDEX OF HYDROGEN DEFICIENCY (STRUCTURAL) A saturated molecule is any molecule that has the maximum number of hydrogens possible for its chemical structure. The rule that
!! www.clutchprep.com CONCEPT: INDEX OF HYDROGEN DEFICIENCY (STRUCTURAL) A saturated molecule is any molecule that has the maximum number of hydrogens possible for its chemical structure. The rule that
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
Chem 1220 Midterm points Dr. Luther Giddings
 Chem 1220 Midterm 1 100 points r. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Chem 1220 Midterm 1 100 points r. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Organic Chemistry. Organic chemistry is the chemistry of compounds containing carbon.
 Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Chemistry 263 Laboratory Experiment 5: Dibenzalacetone 5/10/18. Introduction
 Chemistry 263 Laboratory Experiment 5: Dibenzalacetone 5/10/18 Introduction We have been examining the electrophilic nature of the carbonyl of late. In particular, we have been trying to determine how
Chemistry 263 Laboratory Experiment 5: Dibenzalacetone 5/10/18 Introduction We have been examining the electrophilic nature of the carbonyl of late. In particular, we have been trying to determine how
Organic and Biochemical Molecules. 1. Compounds composed of carbon and hydrogen are called hydrocarbons.
 Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Part A - Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 SCH4U Unit Test Name: Date: Part A - Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. An amine is characterized by what functional group?
SCH4U Unit Test Name: Date: Part A - Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. An amine is characterized by what functional group?
The following exam contains 30 questions valued at 3 point/question and bonus opportunities. Name:
 Chemistry 261 Exam 3 all 2017 The following exam contains 30 questions valued at 3 point/question and bonus opportunities Name: ELECTROPILIC ADDITION REACTIONS BASIC REACTION OUTCOMES 1. Addition of hydrogen
Chemistry 261 Exam 3 all 2017 The following exam contains 30 questions valued at 3 point/question and bonus opportunities Name: ELECTROPILIC ADDITION REACTIONS BASIC REACTION OUTCOMES 1. Addition of hydrogen
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
CHEMISTRY 110 EXAM 2 Feb 25, 2013 FORM A
 EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
Aldehyde and Ketones with *Featured reactions
 * Aldehyde and Ketones with *Featured reactions *1.What is the general structure for an aldehyde? A ketone? *2.How are the common names of aldehydes and ketones determined? How are aldehydes and ketones
* Aldehyde and Ketones with *Featured reactions *1.What is the general structure for an aldehyde? A ketone? *2.How are the common names of aldehydes and ketones determined? How are aldehydes and ketones
HW #4: 15.28, 15.30, 15.32, 15.40, 15.42, 15.44, 15.50, 15.54, 15.56, 15.58, 15.62, AMINES
 Chemistry 131 Lecture 9: Amines - omenclature; Physical Properties; Heterocyclic Amines; Amine Reactivity, Amine Salts, Alkaloids Chapter 15 in McMurry, Ballantine, et. al. 7 th edition HW #4: 15.28, 15.30,
Chemistry 131 Lecture 9: Amines - omenclature; Physical Properties; Heterocyclic Amines; Amine Reactivity, Amine Salts, Alkaloids Chapter 15 in McMurry, Ballantine, et. al. 7 th edition HW #4: 15.28, 15.30,
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
CARBOXYLIC ACIDS AND THEIR DERIVATIVES. 3. Predict the relative acidity and basicity of compounds and ions. Important criteria include:
 CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
Chemical Reactions - Oxidation. Reactions Involving Aldehydes and Ketones. Learning Check. Learning Check. Chemical Reactions - Addition of Hydrogen
 Reactions Involving Aldehydes and Ketones Chemical Reactions - Oxidation When aldehydes are prepared by oxidizing primary alcohols with KMnO 4 or K 2 Cr 2 O 7, the reaction may continue and produce carboxylic
Reactions Involving Aldehydes and Ketones Chemical Reactions - Oxidation When aldehydes are prepared by oxidizing primary alcohols with KMnO 4 or K 2 Cr 2 O 7, the reaction may continue and produce carboxylic
CHAPTER 23 HW: ENOLS + ENOLATES
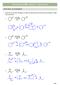 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions:
 1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Reducing Agents. Linda M. Sweeting 1998
 Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Naming for Chem 201 CH 4
 Naming for Chem 201 Functional groups are referred to as such because they function or react differently and give the molecule different properties. Here s a list of the groups you need to be able to name
Naming for Chem 201 Functional groups are referred to as such because they function or react differently and give the molecule different properties. Here s a list of the groups you need to be able to name
ORGANIC CHEMISTRY. Organic molecules are everywhere! The Alkanes (See pages 25-4 and 25-5) Naming Alkanes (See pages 25-7 to 25-10)
 RGANI EMISTRY hemistry 11 rganic molecules are everywhere! Some common examples: Sucrose (sugar) Methane (natural gas) Butane (lighter fluid) Plastic Acetic Acid (vinegar) Ethanol (fuel additive) What
RGANI EMISTRY hemistry 11 rganic molecules are everywhere! Some common examples: Sucrose (sugar) Methane (natural gas) Butane (lighter fluid) Plastic Acetic Acid (vinegar) Ethanol (fuel additive) What
Look for absorption bands in decreasing order of importance:
 1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
CARBOXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook
 CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Te Marau: Mātai Matū (Chemistry) Te Taumata: 2 Kaiako: Matua Matt Cassidy
 Te Marau: Mātai Matū (Chemistry) Te Taumata: 2 Kaiako: Matua Matt Cassidy Curriculum level: 7 Curriculum document: Medium of instruction: NZC English Tauira need to have gained 12 credits from T1 Pūtaiao
Te Marau: Mātai Matū (Chemistry) Te Taumata: 2 Kaiako: Matua Matt Cassidy Curriculum level: 7 Curriculum document: Medium of instruction: NZC English Tauira need to have gained 12 credits from T1 Pūtaiao
48% HBr Solvent 2. e. In order to determine the yield, the limiting reagent has to be identified first.
 1. a. The first reaction is a reduction of a carboxylic acid using lithium aluminum hydride leading to a benzylic alcohol, and not an aldehyde. The second reaction is a substitution reaction that affords
1. a. The first reaction is a reduction of a carboxylic acid using lithium aluminum hydride leading to a benzylic alcohol, and not an aldehyde. The second reaction is a substitution reaction that affords
Chemistry 11 Hydrocarbon Alkane Notes. In this unit, we will be primarily focusing on the chemistry of carbon compounds, also known as.
 1 Chemistry 11 Hydrocarbon Alkane Notes In this unit, we will be primarily focusing on the chemistry of carbon compounds, also known as. Why is organic chemistry so important? Many of the compounds that
1 Chemistry 11 Hydrocarbon Alkane Notes In this unit, we will be primarily focusing on the chemistry of carbon compounds, also known as. Why is organic chemistry so important? Many of the compounds that
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
TWO correct Names and / or structure. Definition correct OR Isomer correct
 NZIC 2015 Assessment Schedule for Chemistry 3.5 AS 91391: DEMONSTRATE UNDERSTANDING OF THE PROPERTIES OF SELECTED GANIC COMPOUNDS Question Evidence Achieved Merit Excellence 1(a) (2)-methylpropanoyl chloride
NZIC 2015 Assessment Schedule for Chemistry 3.5 AS 91391: DEMONSTRATE UNDERSTANDING OF THE PROPERTIES OF SELECTED GANIC COMPOUNDS Question Evidence Achieved Merit Excellence 1(a) (2)-methylpropanoyl chloride
tert-butyl alcohol n-butyl alcohol methyl propyl ether (c) Explain briefly why n-butyl alcohol has a much higher bp than methyl propyl ether.
 1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
CH310N Spring Anslyn. March 23, Exam 2
 C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
Drawing Hydrocarbons. Classifying Hydrocarbons. Four types of diagrams can be used to represent the structure of a hydrocarbon: e.g.
 Classifying Hydrocarbons alkanes- single C-C bonds, if all C s have H s attached, molecules are called hydrocarbons alkenes- have one or more C=C bonds alkynes- have one or more CΞC bonds alkenes & alkynes
Classifying Hydrocarbons alkanes- single C-C bonds, if all C s have H s attached, molecules are called hydrocarbons alkenes- have one or more C=C bonds alkynes- have one or more CΞC bonds alkenes & alkynes
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
Chemistry 12 Exam 1 September 27, 2000 Form A
 hemistry 12 Exam 1 September 27, 2000 Form A 1. The chemical formula for sodium phosphate is: A. S 3P 4 B. NaP 3. Na 3P 4 D. S 2P 3 E. Na 2P 4 2. ow many protons, neutrons and electrons does 201 g 2+ have?
hemistry 12 Exam 1 September 27, 2000 Form A 1. The chemical formula for sodium phosphate is: A. S 3P 4 B. NaP 3. Na 3P 4 D. S 2P 3 E. Na 2P 4 2. ow many protons, neutrons and electrons does 201 g 2+ have?
Lecture 2. The framework to build materials and understand properties
 Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
CHEM 203 Exam 2. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 2 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the IUPAC name of 5-ethyl-2,3-dimethyl-6-hexanol 2-ethyl-4-isopropyl-1-pentanol
CHEM 203 Exam 2 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the IUPAC name of 5-ethyl-2,3-dimethyl-6-hexanol 2-ethyl-4-isopropyl-1-pentanol
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Closed book exam, no books, notebooks, notes, etc. allowed. However, calculators, rulers, and molecular model sets are permitted.
 Massachusetts Institute of Technology Organic Chemistry 5.13 Friday, September 26, 2003 Prof. Timothy F. Jamison Hour Exam #1 Name (please both print and sign your name) Official Recitation Instructor
Massachusetts Institute of Technology Organic Chemistry 5.13 Friday, September 26, 2003 Prof. Timothy F. Jamison Hour Exam #1 Name (please both print and sign your name) Official Recitation Instructor
CHEM Chapter 21. Carboxylic Acid Derivatives_Nucleophilic Acyl Substitution Reactions (homework) W
 Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
CHAPTER 24 Organic Chemistry
 CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
Chapter 20: Carboxylic Acids
 1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
