N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES
|
|
|
- Gloria Hodge
- 5 years ago
- Views:
Transcription
1 N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES Zachery Matesich 24 February 2015
2 Roadmap 2 Introduction Synthetic Methods History of NHCs Properties of NHCs Nature of the carbene Structural properties Electronic properties Illustrative Examples Conclusion
3 N-Heterocyclic Carbene 3 Why? Highly nucleophilic What? Stable carbenes due to steric/electron effects Structurally versatile Neutral compound Divalent carbon atom with 6- electron valence shell Usually adjacent to electronegative atom Glorius, F. et al. Nature 2014, 510,
4 N-Heterocyclic Carbene: Precursor Synthesis 4 Illustration of syntheses for 4 general NHC cores
5 NHCs: Early Beginnings 5 Ofele (1968) Attempt to generate dihydro-complexes: When using imidazolium salts, noted a side reaction: Ofele, K. J. Organomet. Chem. 1968, P42-P43
6 NHCs: Early Beginnings 6 Wanzlick (1968) Isolation as a mercury dimer: 1 H NMR of product reveals downfield shift and lack of coupling DMSO -d 6 H(2) (ppm) H(4), H(5) (ppm) H(phenyl) (ppm) A (t) 1.47 (d) B (s) Wanzlick, H. W. et al ACIEE, 1968, 7,
7 NHCs: Early Beginnings 7 Wanzlick equilibrium Preferred product of carbene formation is dimer: Dimer can 170 ⁰C, about 50:50 mix Carbene can react with electrophiles whereas dimer is inactive Wanzlick, H. W. et al ACIEE, 1962, 1,
8 NHCs: First bottle-able carbene 8 Arduengo (1991) Deprotonation of a imidazolium salt X-ray and 1 H NMR analysis revealed interesting differences in A and B N 1 -C 2 -N 3 angle A ⁰ Avg C 2 - N 1(3) (pm) 1 H (4,5) (ppm) B ⁰ Arduengo, A. J. et al JACS, 1991, 113,
9 Stability of NHCs: Structural Properties 9 Synthesis of additional NHCs: 1992 A, C, D, & E stable as solids B stable in solution No rationale as to why Wanzlick was unable to isolate the same complexes Stability not solely the result of steric parameters or substituents on nitrogen Arduengo, A. J. et al JACS, 1992, 114,
10 Web of Science N-Heterocyclic Carbene 10 Number of publications per year since 1982 for NHC
11 Nature of the carbene: Overview 11 Hybridization of carbon breaks degeneracy of the p orbitals Ground state spin multiplicities for carbenes Singlet carbenes have filled & vacant orbital ambiphillic If singlet-triplet gap is >2 ev, singlet state predominant Both steric and electronic factors play a large role in influencing the orbital separation Bertrand, G. et al Chem. Rev. 2000, 100,
12 Nature of the carbene: Electronics 12 Inductive effects of σ-withdrawing substituents favor singlet state The σ nonbonding orbital has increased s character Smaller gap of σ-donating substituents favor triplet state Bertrand, G. et al Chem. Rev. 2000, 100,
13 Nature of the carbene: Electronics 13 Mesomeric effects involve π-donating/withdrawing substituents π-donating (X) include: -F, -Cl, -Br, -I, -NR 2, -PR 2, -SR π-withdrawing (Z) include: -COR, -CN, -CF 3, -BR 2, -SiR 3, -SR The energy of the vacant p π is increased with the combination of the lone pairs and as the σ orbital is unchanged, the σ- p π gap increases. Bertrand, G. et al Chem. Rev. 2000, 100,
14 Nature of the carbene: Sterics 14 If electronic effects are insignificant, sterics can dictate ground state Linear geometries favor triple state One p orbital is left perpendicular to the plane, unaffected, becomes p π Other p orbital is stabilized and acquires more s character, becomes σ With significant steric bulk, the carbene substituents broaden the bond angle Bertrand, G. et al Chem. Rev. 2000, 100,
15 Nature of the NH carbene: Stability 15 Nitrogen heteroatoms are σ-electron withdrawing and π-donating Therefore this breaks the degeneracy of the p x /p y energy level N-C-N bond is bent, forced by the structure of the ring Singlet state is made more favorable Steric bulk helps to kinetically stabilize Steric bulk is distal from carbene, which avoids making the bond more linear Bertrand, G. et al Chem. Rev. 2000, 100, Glorius, F. et al. Nature 2014, 510, Hoffman, R. et al. JACS 1968, 90,
16 Nature of the NH carbene: Stability 16 Frenking (1996): Effect of aromaticity on NHC stability via NBO The p π occupancy for 1 is 55.8% delocalized, for 2 is only 39.8% Heats of hydrogenation: 1 is kcal/mol and 2 is kcal/mol Shorter C-N bonds in 2 can be accounted for by steric factors of the backbone giving the bond more s character Frenking, G. et al. JACS 1996, 118,
17 Nature of the NHC-Metal bond 17 Tulloch (2001): Extent of π-bonding using Cu complexes Frenking (2004): Revealed that π-back bonding accounts for about 20% of a Ag-NHC product Tulloch, A. A. D et al Organometallics 2001, 20, Frenking, G. et al Organometallics 2004, 23,
18 Nature of the NHC-Metal bond 18 Hu (2004): Extent of π-back bonding using electron rich and deficient complexes Nolan (2007): Through 195 Pt NMR chemical shifts and 195 Pt- 13 C coupling, the influence of the carbene on the metal center was observed Larger coupling constants indicate more electron density in the σ-bond and upfield chemical shifts indicate more electron rich bonds Hu, X. et al Organometallics 2004, 23, Nolan, S. P. et al Organometallics 2007, 26,
19 Properties of NHCs: Sterics of Ligand 19 Due to shape of NHC (wedge-like), the buried volume (% V bur ) is used Defined as: percent of the total volume of a sphere occupied by a ligand Reveals that values are highly dependent on orientation of NHC-TM complex Furthermore, values are derived from X-ray structure, so not accurate representation in solution Nolan, S. P. et al Chem. Commun. 2001, 46, Glorius, F. et al. ACIEE 2010, 49,
20 Properties of NHCs: Sterics of Ligand 20 Cavallo (2010): Attempt for a dynamic model of the metal envionment The % V bur values obtained from geometry optimization are peak of a broad distribution Reveal that normal vibrations are not captured by static methods Unsaturated are slightly less bulky with shift to smaller values Cavallo, L. et al. JACS 2010, 132,
21 Properties of NHCs: pka s 21 Compiled pk a valued (DMSO) Significant rate difference between salts cores: Nolan, S. P. et al Chem. Soc. Rev. 2013, 42,
22 Properties of NHCs: 13 C NMR 22 Diagnostic 13 C NMR shift for carbene ( ppm) Saturation causes downfield shift due to a lower population of the carbene p π orbital population Correlation between N-C-N bond angle and 13 C shift Tapu, D. et al Chem. Rev. 2009, 109,
23 Properties of NHCs: Electronic Character 23 Tolman s Electronic Parameter Originally developed to describe phosphines Electron donating into metal caused CO ligand to weaken and can be examined by IR The lower the TEP, the more electron donating the NHC One caveat: As NHC-metal complexes involve multiple interactions with the NHCs, the values can be influenced Nolan, S. P. et al Chem. Soc. Rev. 2013, 42,
24 Properties of NHCs: in silico TEP 24 Use of DFT calculations to generate IR spectra of Ni(CO) 3 NHC complexes Values are in near agreement Solvent / source of IR data no longer a dissimilarity Can be used to predict structures not yet experimentally determined Gusev, D. G. Organometallics 2009, 28,
25 Properties of NHCs: NMR Measurements 25 Generation of carbene-phosphinidine adducts to measure the π-accepting properties of carbenes In resonance form B, free rotation should be observed in NMR Higher π-accepting NHC will involve more of A and the further 31 P shift will be downfield Complexes formed through: Bertrand, G. et al ACIEE. 2013, 52,
26 Properties of NHCs: NMR Measurements 26 Compounds with similar TEP values, but differing 31P shifts indicate differences in the π-accepting properties of the NHC 1 and 2 have same TEP, but 1 has more π-accepting character, therefore, 1 is more σ-donating Effect of annelation in 2 and 3 Comparison of 4/5 and 6/7 reveal more π-accepting in saturated, as there is less electron delocalization Bertrand, G. et al ACIEE. 2013, 52,
27 Stability of NHCs: Dimerization 27 Cavallo, L. et al Organometallics 2008, 27,
28 Group Problem: Cyclopentane Synthesis 28 Stereodivergence based upon use of HNC Each NHC essentially structurally identical with regards to sterics Bode, J. W. et al. Org. Lett. 2009, 11,
29 Group Problem: Cyclopentane Synthesis 29 1) Determine the mechanism for formation of each product 2) Rationalize the divergence in product formation based upon the NHC used Note: The counterion has no influence on the reaction outcome Bode, J. W. et al. Org. Lett. 2009, 11,
30 Group Problem: Answer 30 Divergence is result of ability for NHC to act as a leaving group More electron rich imidazolium HNC is less likely to leave Imiazolium prefers formation of more stable γ-lactone Bode, J. W. et al. Org. Lett. 2009, 11,
31 NHC Applications: Switching reactive pairs 31 Yang (2011): Acyloin Condensation Sterics guide the formation of the first intermediate More potential overlap when using triazolium catalyst with aryl aldehyde Yang, J. W. et al Org. Lett. 2011, 13,
32 Modifying the basic NHC structure 32 Bertrand (2012): Generating an electrophilic AND nucleophilic NHC Second nitrogen only acts as inductive electron withdrawing substituent C1-N2 bond is Å vs Å for C1-N1 revealing pyramidal N2 TEP value of 2047 cm -1 which puts it less electron donating than normal 6- membered NHC ligands (~ cm -1 ) Bertrand, G. et al ACIEE 2012, 51,
33 NHCs versus Phosphines 33 Monodentate, 2-electron ligands Synthesis for NHCs highly varied, with precursors being air-stable NHCs are typically irreversible binding ligands Steric/electronic properties in NHCs are typically separately alterable Larger trans-effect for NHCs (exploited in a later slide) TEP Values Phosphine typically cm -1 [P(tBu) 3 ] to cm -1 [PF 3 ] NHCs typically [IAd] to cm -1 [slide 31] stronger electron donor NHC more thermally and oxidatively stable Different modes of steric influence (NHC wedge, phosphine cone) Crabtree, R. H. J. Organmet. Chem. 2005, 690, Nolan, S. P. et al. Chem. Soc. Rev. 2013, 42,
34 NHCs versus Phosphines: Case-study 34 Grubbs II Ruthenium Olefin Metathesis Large rate difference reveals that binding to the alkene is 10 4 x more favorable than binding the phosphine ligand, leading to higher activity Glorius, F. et al. Nature 2014, 510, Grubbs, R. H. et al. JACS 2001, 123,
35 NHC Applications: Further catalyst improvements 35 Grubbs Z-selective Ruthenium Olefin Metathesis Inclusion of second bond between the metal and NHC ligand Steric clash of N-mesityl and alkylidene influences selectivity Grubbs, R. H. et al. JACS 2013, 135,
36 NHC Applications: Transition metals 36 Palladium cross-coupling reactions can be improved Aside from increased thermal stability of the catalyst, NHC can influence catalytic cycle More electron-rich Pd for OA Increased steric properties, as compared to phosphines aid reductive elimination Avoidance of Pd black through Pd(0) stabilization Glorius, F. et al. Nature 2014, 510,
37 NHC Applications: Palladium catalysis 37 Palladium catalyzed Heck reaction Pd-C bonds (1.990 Å) similar to other carbene complexes High thermal stability several days in boiling THF with O 2 No Pd decomposition observed during course of reaction Herrmann, W. A. et al. ACIEE 1995, 34,
38 Conclusion 38 Stabilization a combination of steric and electronics properties Methods for quantifying NHC properties are improving Some predictive models are being developed High versatility as ligands through synthesis Can be an improvement over use of phosphines High thermal and oxidative stability in NHC-metal complexes
39 References 39 Glorius, F. et al. Nature 2014, 510, Ofele, K. J. Organomet. Chem. 1968, P42-P43 Wanzlick, H. W. et al ACIEE, 1968, 7, Wanzlick, H. W. et al ACIEE, 1962, 1, Arduengo, A. J. et al JACS, 1991, 113, Arduengo, A. J. et al JACS, 1992, 114, Bertrand, G. et al Chem. Rev. 2000, 100, Hoffman, R. et al. JACS 1968, 90, Frenking, G. et al. JACS 1996, 118, Tulloch, A. A. D et al Organometallics 2001, 20, Frenking, G. et al Organometallics 2004, 23, Grubbs, R. H. et al. JACS 2013, 135, Hu, X. et al Organometallics 2004, 23, Nolan, S. P. et al Organometallics 2007, 26, Nolan, S. P. et al Chem. Commun. 2001, 46, Glorius, F. et al. ACIEE 2010, 49, Cavallo, L. et al. JACS 2010, 132, Nolan, S. P. et al Chem. Soc. Rev. 2013, 42, Tapu, D. et al Chem. Rev. 2009, 109, Gusev, D. G. Organometallics 2009, 28, Bertrand, G. et al ACIEE. 2013, 52, Cavallo, L. et al Organometallics 2008, 27, Bode, J. W. et al. Org. Lett. 2009, 11, Yang, J. W. et al Org. Lett. 2011, 13, Bertrand, G. et al ACIEE 2012, 51, Crabtree, R. H. J. Organmet. Chem. 2005, 690, Grubbs, R. H. et al. JACS 2001, 123, Herrmann, W. A. et al. ACIEE 1995, 34,
LIGAND DESIGN CARBENES. Fischer carbenes (B) have a heteroatom substituent on the alpha carbon atom.
 There are two main classes of carbene ligands Alkylidene (or Schrock carbene) ligands (A) have one or two alkyl or aryl substituents on the alpha carbon atom. Fischer carbenes (B) have a heteroatom substituent
There are two main classes of carbene ligands Alkylidene (or Schrock carbene) ligands (A) have one or two alkyl or aryl substituents on the alpha carbon atom. Fischer carbenes (B) have a heteroatom substituent
N-Heterocyclic Carbenes (NHCs)
 N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes
 N-Heterocyclic Carbenes References 1. "N-Heterocyclic Carbenes as Organocatalysts." Marion, N.; Diez- Gonzalez, S.; Nolan, S.P. Angew. Chem. Int. Ed. 2007, 26, 2988. (general review) 2. "Stable Carbenes."
N-Heterocyclic Carbenes References 1. "N-Heterocyclic Carbenes as Organocatalysts." Marion, N.; Diez- Gonzalez, S.; Nolan, S.P. Angew. Chem. Int. Ed. 2007, 26, 2988. (general review) 2. "Stable Carbenes."
N-Heterocyclic carbenes (NHCs): A comprehensive organocatalyst & reagent
 N-Heterocyclic carbenes (NHCs): A comprehensive organocatalyst & reagent Gowrisankar Parthasarathy Leibniz Universität Hannover, Institut für Organische Chemie AK Kalesse group seminar 1 Outline Introduction
N-Heterocyclic carbenes (NHCs): A comprehensive organocatalyst & reagent Gowrisankar Parthasarathy Leibniz Universität Hannover, Institut für Organische Chemie AK Kalesse group seminar 1 Outline Introduction
Metal Hydrides, Alkyls, Aryls, and their Reactions
 Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
N-Heterocyclic Carbenes (NHCs)
 N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito
 1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
deactivation or decomposition is therefore quantified using the turnover number.
 A catalyst may be defined by two important criteria related to its stability and efficiency. Name both of these criteria and describe how they are defined with respect to stability or efficiency. A catalyst
A catalyst may be defined by two important criteria related to its stability and efficiency. Name both of these criteria and describe how they are defined with respect to stability or efficiency. A catalyst
Oxidative Addition/Reductive Elimination 1. Oxidative Addition
 Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Organic Chemistry Laboratory Summer Lecture 6 Transition metal organometallic chemistry and catalysis July
 344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
O CH 3. Mn CH 3 OC C. 16eelimination
 igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
σ Bonded ligands: Transition Metal Alkyls and Hydrides
 σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
1. Which of the following compounds is the weakest base?
 I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
Organometallic Chemistry and Homogeneous Catalysis
 Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Nitrogen Centered Radical Ligands Nagashima Nozomu
 1 Nitrogen Centered Radical Ligands 2015. 7. 4. Nagashima Nozomu 1. Introduction 2 3 Aminyl radical 1) D. E. Wiliams, JACS, 1966, 88, 5665 2) Y. Teki et al. JOC, 2000, 65, 7889 Sterically protected aminyl
1 Nitrogen Centered Radical Ligands 2015. 7. 4. Nagashima Nozomu 1. Introduction 2 3 Aminyl radical 1) D. E. Wiliams, JACS, 1966, 88, 5665 2) Y. Teki et al. JOC, 2000, 65, 7889 Sterically protected aminyl
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Chapter 3 An Introduction to Organic Reactions: Acids and Bases
 There are 4 types of Organic Reactions Chapter 3 An Introduction to Organic Reactions: SUBSTITUTION: ADDITION: X Y + A X A + Y Example Example A B + X Y A B X Y ELIMINATION There are 4 Types of Organic
There are 4 types of Organic Reactions Chapter 3 An Introduction to Organic Reactions: SUBSTITUTION: ADDITION: X Y + A X A + Y Example Example A B + X Y A B X Y ELIMINATION There are 4 Types of Organic
Carbenes and Olefin Metathesis
 arbenes and Olefin etathesis Peter H.. Budzelaar etal-carbon multiple bonds any transition metals form not only - single bonds but also = and (more rare) even bonds. omplexes containing an = bond are called
arbenes and Olefin etathesis Peter H.. Budzelaar etal-carbon multiple bonds any transition metals form not only - single bonds but also = and (more rare) even bonds. omplexes containing an = bond are called
Oxidative Addition and Reductive Elimination
 xidative Addition and Reductive Elimination red elim coord 2 ox add ins Peter.. Budzelaar xidative Addition Basic reaction: n + X Y n X Y The new -X and -Y bonds are formed using: the electron pair of
xidative Addition and Reductive Elimination red elim coord 2 ox add ins Peter.. Budzelaar xidative Addition Basic reaction: n + X Y n X Y The new -X and -Y bonds are formed using: the electron pair of
Lecture Notes Chem 51B S. King I. Conjugation
 Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Organometallic Chemistry and Homogeneous Catalysis
 Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N6 Kashiwa Campus, November 27, 2009 Group VIB: Cr, Mo, W -Oxidation states from -2 to +6 -While +2 and +3 for Cr are quite
Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N6 Kashiwa Campus, November 27, 2009 Group VIB: Cr, Mo, W -Oxidation states from -2 to +6 -While +2 and +3 for Cr are quite
Insertion Reactions. 1) 1,1 insertion in which the metal and the X ligand end up bound to the same (1,1) atom
 Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Exam (6 pts) Show which starting materials are used to produce the following Diels-Alder products:
 Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
CHEMISTRY. Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para ipso attack, orientation in other ring systems.
 Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Reaction chemistry of complexes Three general forms: 1. Reactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b.
 eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
NOT TO BE REMOVED FROM THE EXAMINATION HALL
 A copy of the Level III (FHEQ Level 6) Equation and Data Sheet booklet is provided. The use of hand-held, battery-operated, electronic calculators will be permitted subject to the regulations governing
A copy of the Level III (FHEQ Level 6) Equation and Data Sheet booklet is provided. The use of hand-held, battery-operated, electronic calculators will be permitted subject to the regulations governing
Organometallic Chemistry and Homogeneous Catalysis
 rganometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N8 Kashiwa ampus, December 11, 2009 Types of reactions in the coordination sphere of T 3. Reductive elimination X-L n -Y L n +
rganometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N8 Kashiwa ampus, December 11, 2009 Types of reactions in the coordination sphere of T 3. Reductive elimination X-L n -Y L n +
A Summary of Organometallic Chemistry
 A Summary of Organometallic Chemistry Counting valence electrons (v.e.) with the ionic model 1. Look at the total charge of the complex Ph 3 P Cl Rh Ph 3 P PPh 3 OC CO 2 Fe OC CO Co + charge:0 charge:
A Summary of Organometallic Chemistry Counting valence electrons (v.e.) with the ionic model 1. Look at the total charge of the complex Ph 3 P Cl Rh Ph 3 P PPh 3 OC CO 2 Fe OC CO Co + charge:0 charge:
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
Chapter 8. Acidity, Basicity and pk a
 Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY
 4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
17.24 To name the compounds use the directions from Answer 17.3.
 Benzene and Aromatic Compounds 7 7 7.2 If benzene could be described by a single Kekulé structure, only one product would form in Reaction [], but there would be four (not three) dibromobenzenes (A ),
Benzene and Aromatic Compounds 7 7 7.2 If benzene could be described by a single Kekulé structure, only one product would form in Reaction [], but there would be four (not three) dibromobenzenes (A ),
Acids and Bases. Acids and Bases
 BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
Hydrides and Dihydrogen as Ligands: Lessons from Organometallic Chemistry. Lecture 9
 ydrides and Dihydrogen as Ligands: Lessons from Organometallic Chemistry Lecture 9 Inorganic Chemistry Chapter 1: Figure 10.1 2009 W.. Freeman Synthesis of Organometallic Complex ydrides Reaction of MCO
ydrides and Dihydrogen as Ligands: Lessons from Organometallic Chemistry Lecture 9 Inorganic Chemistry Chapter 1: Figure 10.1 2009 W.. Freeman Synthesis of Organometallic Complex ydrides Reaction of MCO
Amines and Heterocycles. McMurry: Chapter 24
 Amines and Heterocycles McMurry: Chapter 24 Introduction to Amines and Heterocycles Amines and heterocycles (cyclic amines) are ammonia derivatives, many of whichare found widely in livingorganisms: 2
Amines and Heterocycles McMurry: Chapter 24 Introduction to Amines and Heterocycles Amines and heterocycles (cyclic amines) are ammonia derivatives, many of whichare found widely in livingorganisms: 2
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
11/30/ Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Course 201N 1 st Semester Inorganic Chemistry Instructor: Jitendra K. Bera
 andout-9 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. rganometallic hemistry xidative Addition, Reductive Elimination, Migratory Insertion, Elimination
andout-9 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. rganometallic hemistry xidative Addition, Reductive Elimination, Migratory Insertion, Elimination
Synthesis Using Aromatic Materials
 Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Answers to Assignment #5
 Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
Chap. 5 Reactive intermediates
 Energy surface hap. 5 Reactive intermediates The plot of energy (potential and kinetic) as a function of 3N- 6 coordinates of the chemical system (reactants, intermediates, products) Reaction oordinate
Energy surface hap. 5 Reactive intermediates The plot of energy (potential and kinetic) as a function of 3N- 6 coordinates of the chemical system (reactants, intermediates, products) Reaction oordinate
SIX MEMBERED AROMATIC HETEROCYCLES
 SIX MEMBERED AROMATIC HETEROCYCLES Ṇ. Pyridine Pyridine is aromatic as there are six delocalized electrons in the ring. Six-membered heterocycles are more closely related to benzene as they are aromatic
SIX MEMBERED AROMATIC HETEROCYCLES Ṇ. Pyridine Pyridine is aromatic as there are six delocalized electrons in the ring. Six-membered heterocycles are more closely related to benzene as they are aromatic
There are two main electronic effects that substituents can exert:
 Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Paper No and Title Paper 5: Organic Chemistry-2 (Reaction mechanism- 1) Module No and Title 9, Reactive intermediates: Carbenes and Nitrenes
 Subject Chemistry Paper No and Title Paper 5: Organic Chemistry-2 (Reaction mechanism- 1) Module No and Title 9, Reactive intermediates: Carbenes and Module Tag CHE_P5_M9 TABLE OF CONTENTS 1. Learning
Subject Chemistry Paper No and Title Paper 5: Organic Chemistry-2 (Reaction mechanism- 1) Module No and Title 9, Reactive intermediates: Carbenes and Module Tag CHE_P5_M9 TABLE OF CONTENTS 1. Learning
Amines. Chapter 24 Organic Chemistry, 8th Edition. John McMurry
 Amines Chapter 24 Organic Chemistry, 8th Edition John McMurry 1 Introduction Amines are stronger bases and better nucleophiles than other neutral organic compounds. 2 Nomenclature 1 Amines are named using
Amines Chapter 24 Organic Chemistry, 8th Edition John McMurry 1 Introduction Amines are stronger bases and better nucleophiles than other neutral organic compounds. 2 Nomenclature 1 Amines are named using
Hydrides and Dihydrogen as Ligands: Hydrogenation Catalysis
 Hydrides and Dihydrogen as Ligands: Hydrogenation Catalysis Synthesis of Organometallic Complex Hydrides Reaction of MCO with OH -, H -, or CH 2 CHR 2 M(CO) n + OH - = M(CO) n-1 (COOH) - = HM(CO) n-1 -
Hydrides and Dihydrogen as Ligands: Hydrogenation Catalysis Synthesis of Organometallic Complex Hydrides Reaction of MCO with OH -, H -, or CH 2 CHR 2 M(CO) n + OH - = M(CO) n-1 (COOH) - = HM(CO) n-1 -
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
Chapter 3 Acids and Bases"
 Chapter 3 Acids and Bases BrØnsted-Lowry Acids and Bases A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton Acids and Bases Reactions of BrØnsted-Lowry Acids
Chapter 3 Acids and Bases BrØnsted-Lowry Acids and Bases A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton Acids and Bases Reactions of BrØnsted-Lowry Acids
Chem 634. Introduction to Transition Metal Catalysis. Reading: Heg Ch 1 2 CS-B 7.1, , 11.3 Grossman Ch 6
 Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
NUCLEOPHILICITY/BASICITY AND CAFFEINE- DERIVED N-HETEROCYCLIC CARBENE COMPLEXES. KOH SIOK BEE (B.Sc. (Hons.), NUS)
 13 C NMR SPECTROSCOPIC EVALUATION OF NUCLEOPHILICITY/BASICITY AND CAFFEINE- DERIVED N-HETEROCYCLIC CARBENE COMPLEXES KOH SIOK BEE (B.Sc. (Hons.), NUS) A THESIS SUBMITTED FOR THE DEGREE OF MASTER OF SCIENCE
13 C NMR SPECTROSCOPIC EVALUATION OF NUCLEOPHILICITY/BASICITY AND CAFFEINE- DERIVED N-HETEROCYCLIC CARBENE COMPLEXES KOH SIOK BEE (B.Sc. (Hons.), NUS) A THESIS SUBMITTED FOR THE DEGREE OF MASTER OF SCIENCE
Recapping where we are so far
 Recapping where we are so far Valence bond constructions, valence, valence electron counting, formal charges, etc Equivalent neutral classification and MLX plots Basic concepts for mechanism and kinetics
Recapping where we are so far Valence bond constructions, valence, valence electron counting, formal charges, etc Equivalent neutral classification and MLX plots Basic concepts for mechanism and kinetics
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo
 Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or
 Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or nonaromatic? 1 2 Classify cyclononatetrene and it s various ions
Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or nonaromatic? 1 2 Classify cyclononatetrene and it s various ions
Inorganic Chemistry Year 3
 Inorganic Chemistry Year 3 Transition Metal Catalysis Eighteen Electron Rule 1.Get the number of the group that the metal is in (this will be the number of d electrons) 2.Add to this the charge 1.Negative
Inorganic Chemistry Year 3 Transition Metal Catalysis Eighteen Electron Rule 1.Get the number of the group that the metal is in (this will be the number of d electrons) 2.Add to this the charge 1.Negative
Loudon Chapter 18 Review: Vinyl/Aryl Reactivity Jacquie Richardson, CU Boulder Last updated 2/21/2016
 Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Nucleophilic Heterocyclic Carbene Catalysis. Nathan Werner Denmark Group Meeting September 22 th, 2009
 Nucleophilic Heterocyclic Carbene Catalysis Nathan Werner Denmark Group Meeting September 22 th, 2009 Thiamine Thiamine Vitamin B 1 The first water-soluble vitamin described Is naturally synthesized by
Nucleophilic Heterocyclic Carbene Catalysis Nathan Werner Denmark Group Meeting September 22 th, 2009 Thiamine Thiamine Vitamin B 1 The first water-soluble vitamin described Is naturally synthesized by
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Organometallics: Hard to define usefully and completely at the same time, but generally: Compounds containing metal-carbon bond(s).
 eady; Catalysis rganometallics: Definitions rganometallics: ard to define usefully and completely at the same time, but generally: Compounds containing metal-carbon bond(s). C C C C C C K 3 o question:????
eady; Catalysis rganometallics: Definitions rganometallics: ard to define usefully and completely at the same time, but generally: Compounds containing metal-carbon bond(s). C C C C C C K 3 o question:????
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 1
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 12, 2015; 7-9 PM This is a 2-hour test, marked out
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 12, 2015; 7-9 PM This is a 2-hour test, marked out
Chapter 4: Aromatic Compounds. Bitter almonds are the source of the aromatic compound benzaldehyde
 Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts)
 CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting Timothy Chang
 Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting 01-15-2008 Timothy Chang Outlines - Fundamental considerations, C-H versus C-C activation - Orbital interactions -
Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting 01-15-2008 Timothy Chang Outlines - Fundamental considerations, C-H versus C-C activation - Orbital interactions -
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Deactivation Pathways in Transition Metal Catalysis
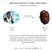 Deactivation Pathways in Transition tal Catalysis Why Study Catalyst Decomposition? decomposition active for catalysis inactive for catalysis "One of the reasons for [the] limited understanding [of catalyst
Deactivation Pathways in Transition tal Catalysis Why Study Catalyst Decomposition? decomposition active for catalysis inactive for catalysis "One of the reasons for [the] limited understanding [of catalyst
https://cuvillier.de/de/shop/publications/766
 Jelena Jenter (Autor) Nitrogen Donor Ligands in the Coordination Chemistry of the are Earth and Alkaline Earth Metals Synthesis - Structures - Catalysis https://cuvillier.de/de/shop/publications/766 Copyright:
Jelena Jenter (Autor) Nitrogen Donor Ligands in the Coordination Chemistry of the are Earth and Alkaline Earth Metals Synthesis - Structures - Catalysis https://cuvillier.de/de/shop/publications/766 Copyright:
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
EPIC LIGAND SURVEY: METAL ALKYLS - I
 EPIC LIGAND SURVEY: METAL ALKYLS - I With this post we finally reach the defining ligands of organometallic chemistry, alkyls. Metal alkyls feature a metal-carbon σ bond and are usually actor ligands,
EPIC LIGAND SURVEY: METAL ALKYLS - I With this post we finally reach the defining ligands of organometallic chemistry, alkyls. Metal alkyls feature a metal-carbon σ bond and are usually actor ligands,
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
p Bonds as Electrophiles
 Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
Electrophilic Aromatic Substitution. Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi
 Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
FRUSTRATED LEWIS PAIRS: structure and synthetic applications
 FRUSTRATED LEWIS PAIRS: structure and synthetic applications Zachery Matesich 17 November 2015 Gilbert Newton Lewis 2 Born: 1875 PhD: 1899 Harvard Berkley Professor: 1912-1946 Valence and the Structure
FRUSTRATED LEWIS PAIRS: structure and synthetic applications Zachery Matesich 17 November 2015 Gilbert Newton Lewis 2 Born: 1875 PhD: 1899 Harvard Berkley Professor: 1912-1946 Valence and the Structure
Organometallic Rections 1: Reactions at the Metal
 E Organometallic Rections 1: Reactions at the Metal Three major classes of reactions: 1 Ligand Substitution associative (cf. S N 2) dissociative (cf. S N 1) interchange (not dealt with in this course)
E Organometallic Rections 1: Reactions at the Metal Three major classes of reactions: 1 Ligand Substitution associative (cf. S N 2) dissociative (cf. S N 1) interchange (not dealt with in this course)
Reaction mechanisms offer us insights into how reactions work / how molecules react with one another.
 Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Chapter 14: Conjugated Dienes
 Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Chapter 15: Reactions of Substituted Benzenes
 Learning Objectives: Chapter 15: Reactions of Substituted Benzenes 1. Be able to recognize and utilize the oxidative and reductive reactions involving the substituents on benzene. 2. Recognize whether
Learning Objectives: Chapter 15: Reactions of Substituted Benzenes 1. Be able to recognize and utilize the oxidative and reductive reactions involving the substituents on benzene. 2. Recognize whether
Organic Chemistry Peer Tutoring Department University of California, Irvine
 Organic Chemistry Peer Tutoring Department University of California, Irvine Arash Khangholi (akhangho@uci.edu) Cassandra Amezquita (camezqu1@uci.edu) Jiana Machhor (jmachhor@uci.edu) OCHEM 51A Professor
Organic Chemistry Peer Tutoring Department University of California, Irvine Arash Khangholi (akhangho@uci.edu) Cassandra Amezquita (camezqu1@uci.edu) Jiana Machhor (jmachhor@uci.edu) OCHEM 51A Professor
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION METALS
 THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION METALS Second Edition ROBERT H. CRABTREE Yale University New Haven, Connecticut A Wiley-Interscience Publication JOHN WILEY & SONS New York / Chichester /
THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION METALS Second Edition ROBERT H. CRABTREE Yale University New Haven, Connecticut A Wiley-Interscience Publication JOHN WILEY & SONS New York / Chichester /
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
