Topic Review Group meeting
|
|
|
- Hector Parks
- 6 years ago
- Views:
Transcription
1 Topic Review Group meeting Memory of chirality Christian Gloor Based on: Zhao, H.; Hsu, D. C.; Carlier, P.R. Synthesis 2005,1, 1.
2 Table of content > Definitions of memory of chirality > Types of chirality > Requirements for memory of chirality > Memory of chirality in enolate chemistry > Memory of chirality in radical chemistry > Memory of chirality involving carbocation intermediates > Conclusion 25. October
3 Definitions of memory of chirality > Central chirality at a carbon alpha to a carbonyl group is preserved as transient axial chirality of the intermediate enolate and is then regenerated as central chirality in the reaction product (memory of chirality). 1 > The chirality of the starting ti material is preserved in a reactive intermediate t for a limited time. 2 > The chirality of a starting material having a chiral sp 3 -carbon is preserved in the reaction product even though h the reaction proceeds at the chiral carbon as a reaction center through reactive intermediates such as carbanion, singlet monoradicals, biradicals, or carbenium ions. 3 1 Kawabata, T.; Yahiro, K.; Fuji, K. J. Am. Chem. Soc. 1991,113, Fuji, K.; Kawabata, T. Chem. Eur. J. 1998, 4, Wanyoike, G. N.; Onomura, O.; Maki, T.; Matsumura, Y. Org. Lett. 2002, 4, October
4 Types of Chirality > Dynamic chirality is not a sufficient condition for MOC since the intermediate t has to be formed enantioselectively. l 1 Fuji, K.; Kawabata, T. Chem. Eur. J. 1998, 4, October
5 Requirements for memory of chirality > The three requirements for memory of chirality are: 1 1 Zhao, H.; Hsu, D.C.; Carlier, P.R. Synthesis 2005, 1, October
6 Memory of chirality in enolate chemistry > Seebach, D.; Wasmuth, D. Angew. Chem. 1981, 93, > Zhao, H.; Hsu, D. C.; Carlier, PR P.R. Synthesis 2005, 1, October
7 Memory of chirality in enolate chemistry > Kawabata, T.; Yahiro, K.; Fuji, K. J. Am. Chem. Soc. 1991, 113, October
8 Memory of chirality in enolate chemistry > Kawabata, T.; Yahiro, K.; Fuji, K. J. Am. Chem. Soc. 1991, 113, October
9 Memory of chirality in enolate chemistry > Kawabata, T.; Yahiro, K.; Fuji, K. J. Am. Chem. Soc. 1991, 113, October
10 Memory of chirality in enolate chemistry > Kawabata, T.; Suzuki, H.; Nagae, Y; Fuji, K. Angew. Chem. Int. Ed. 2000, 39, October
11 Memory of chirality in enolate chemistry > Kawabata, T.; Suzuki, H.; Nagae, Y; Fuji, K. Angew. Chem. Int. Ed. 2000, 39, October
12 Memory of chirality in enolate chemistry > Kawabata, T.; Kawakami, S.; Fuji, K. Tetrahedron Lett. 2002, 43, October
13 Memory of chirality in enolate chemistry > Kawabata, T.; Kawakami, S.; Majumdar, S. J. Am. Chem. Soc. 2003,125, October
14 Memory of chirality in enolate chemistry > Kawabata, T.; Kawakami, S.; Majumdar, S. J. Am. Chem. Soc. 2003,125, October
15 Memory of chirality in enolate chemistry > Zhao, H.; Hsu, D. C.; Carlier, P.R. Synthesis 2005,1, 1. > Vohra, S. et al. J. Chem. Soc. Perkin Trans , October
16 Memory of chirality in enolate chemistry > Zhao, H.; Hsu, D. C.; Carlier, P.R. Synthesis 2005,1, 1. > Vohra, S. et al. Chem. Commun. 1998, October
17 Memory of chirality in enolate chemistry > Bonache, M a, A. Et al. Tetrahedron Asymmetry 2003, 14, October
18 Memory of chirality in enolate chemistry > Bonache, M a, A. et al. Tetrahedron Asymmetry 2003, 14, October
19 Memory of chirality in enolate chemistry > Carlier, P. R. et al. JACS 2003, 125, October
20 Memory of chirality in complex chemistry > Schmalz, H.-G.; Koning, C.B.S.; Bernicke, D.; Siegel, S.; Pfletschinger, A. Angew. Chem. Int. Ed. 1999, 38, October
21 Retentive radical trapping controlled by a slow ring inversion > Buckmelter, A.J.; Powers, J.P.; Rychnovsky, S.D. J. Am. Chem. Soc. 1998, 120, October
22 Retentive radical trapping controlled by a slow ring inversion > Buckmelter, A.J.; Powers, J.P.; Rychnovsky, S.D. J. Am. Chem. Soc. 1998, 120, October
23 Retentive radical trapping controlled by a slow ring inversion > Buckmelter, A.J.; Kim, A.I.; Rychnovsky, S.D.J. Am. Chem. Soc. 2000, 122, October
24 Retentive radical trapping controlled by a slow ring inversion > Buckmelter, A.J.; Kim, A.I.; Rychnovsky, S.D.J. Am. Chem. Soc. 2000, 122, October
25 Memory of chirality in radical cyclisation > Dalgard, J.E.; Rychnovsky, S.D. Org. Lett. 2004, 6, October
26 Memory of chirality in radical cyclisation > Dalgard, J.E.; Rychnovsky, S.D. Org. Lett. 2004, 6, October
27 Memory of chirality in radical cyclisation > Curran, D.P.; Liu, W.; Chen, C.H.-T. J. Am. Soc. 1999, 121, October
28 Memory of chirality in radical cyclisation > Curran, D.P.; Liu, W.; Chen, C.H.-T. J. Am. Soc. 1999, 121, October
29 Memory of chirality in radical cyclisation > Curran, D.P.; Liu, W.; Chen, C.H.-T. J. Am. Soc. 1999,, 121,, October
30 Memory of chirality in radical cyclisation > Curran, D.P.; Chen, C.H.-T.; Geib, S.J.; Lapierre, A.J.B. Tetrahedron 1999, 60, October
31 Memory of chirality in radical cyclisation > Curran, D.P.; Chen, C.H.-T.; Geib, S.J.; Lapierre, A.J.B. Tetrahedron 1999, 60, October
32 Memory of chirality in radical cyclisation > Giese, B. et al. Angew. Chem. Int. Ed. 1999, 38, October
33 Memory of chirality involving carbocation intermediates > Wanyoike, G. N.; Onomura, O.; Maki, T.; Matsumura, Y. Org. Lett. 2002, 4, October
34 Memory of chirality involving carbocation intermediates > Wanyoike, G. N.; Onomura, O.; Maki, T.; Matsumura, Y. Org. Lett. 2002, 4, October
35 Memory of chirality involving carbocation intermediates > Wanyoike, G. N.; Onomura, O.; Maki, T.; Matsumura, Y. Org. Lett. 2002, 4, October
36 Memory of chirality involving carbocation intermediates > Wanyoike, G. N.; Onomura, O.; Maki, T.; Matsumura, Y. Org. Lett. 2002, 4, October
37 Conclusion > Memory of chirality is an emerging strategy for enantioselective synthesis > To date, most MOC experiments were carried out in enolate chemistry, but MOC is also performed in radical chemistry and with involvement of carbocations as reactive intermediates. > Three conditions have to be fulfilled for MOC: > The chiral starting material must be transformed in a conformationally chiral intermediate > The intermediate must not racemize during the timescale of the reaction > The reaction from the reactive intermediate has to work with a high ee. 25. October
Memory of Chirality: A Strategy for Asymmetric Synthesis
 Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
Memory of chirality in reactions involving monoradicals
 Free Radical Research ISSN: 1071-5762 (Print) 1029-2470 (Online) Journal homepage: http://www.tandfonline.com/loi/ifra20 Memory of chirality in reactions involving monoradicals Christian Simon Gloor, Fabrice
Free Radical Research ISSN: 1071-5762 (Print) 1029-2470 (Online) Journal homepage: http://www.tandfonline.com/loi/ifra20 Memory of chirality in reactions involving monoradicals Christian Simon Gloor, Fabrice
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito
 1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
Total Synthesis of (+)-Gelsemine. Xuan Dong group seminar 12/19/2012
 Total Synthesis of (+)-Gelsemine Xuan Dong group seminar 12/19/2012 Historical Background of Gelsemine First detected the presence of alk aloids in extracts of G. sempervir ens in 1870 by Wormley. 1876,
Total Synthesis of (+)-Gelsemine Xuan Dong group seminar 12/19/2012 Historical Background of Gelsemine First detected the presence of alk aloids in extracts of G. sempervir ens in 1870 by Wormley. 1876,
N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations
 Angew. Chem. Int. Ed. 2017, 10.1002. 1 N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations Reporter: En Li Supervisor: Prof. Yong
Angew. Chem. Int. Ed. 2017, 10.1002. 1 N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations Reporter: En Li Supervisor: Prof. Yong
Measuring enzyme (enantio)selectivity
 Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Corey-Bakshi. Bakshi-Shibata Reduction. Name Reaction Nilanjana Majumdar
 Corey-Bakshi Bakshi-Shibata Reduction Name Reaction Nilanjana Majumdar 02.27.09 utline Introduction Background CBS Reaction Application to Synthesis Introduction Born: 12 th July, 1928 in Methuen, Massachusetts,
Corey-Bakshi Bakshi-Shibata Reduction Name Reaction Nilanjana Majumdar 02.27.09 utline Introduction Background CBS Reaction Application to Synthesis Introduction Born: 12 th July, 1928 in Methuen, Massachusetts,
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Planar-Chiral Phosphine-Olefin Ligands Exploiting a (Cyclopentadienyl)manganese(I) Scaffold to Achieve High Robustness and High Enantioselectivity
 Planar-Chiral Phosphine-Olefin Ligands Exploiting a (Cyclopentadienyl)manganese(I) Scaffold to Achieve High Robustness and High Enantioselectivity Reporter: Cong Liu Checker: Hong-Qiang Shen Date: 2017/02/27
Planar-Chiral Phosphine-Olefin Ligands Exploiting a (Cyclopentadienyl)manganese(I) Scaffold to Achieve High Robustness and High Enantioselectivity Reporter: Cong Liu Checker: Hong-Qiang Shen Date: 2017/02/27
Chromium Arene Complexes
 Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
Povarov Reaction. Zain Yousaf 10/22/2013 University of Illinois at Urbana-Champaign
 Povarov Reaction Zain Yousaf 10/22/2013 University of Illinois at Urbana-Champaign Contents Introduction Chiral Lewis Acids Bronsted Acid Lanthanide complexes L-ramipril acid derived Sulfonamide Urea Derivative
Povarov Reaction Zain Yousaf 10/22/2013 University of Illinois at Urbana-Champaign Contents Introduction Chiral Lewis Acids Bronsted Acid Lanthanide complexes L-ramipril acid derived Sulfonamide Urea Derivative
I. Introduction. II. Retrosynthetic Analysis. Andrew Baggett. Liu lab
 Total Synthesis of Limonin Shuji Yamashita,* Akito Naruko, Yuki Nakazawa, Le Zhao, Yujiro Hayashi, Masahiro Hirama Tohoku University, Department of Chemistry, Aramaki-aza aoba, Aoba-ku, Sendai 980-8578
Total Synthesis of Limonin Shuji Yamashita,* Akito Naruko, Yuki Nakazawa, Le Zhao, Yujiro Hayashi, Masahiro Hirama Tohoku University, Department of Chemistry, Aramaki-aza aoba, Aoba-ku, Sendai 980-8578
The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction. Eric Gillis 4/19/07
 The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction Eric Gillis 4/19/07 Quaternary stereocenters in complex small molecules Retrosynthetic
The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction Eric Gillis 4/19/07 Quaternary stereocenters in complex small molecules Retrosynthetic
Synthesis, Mechanism, and Properties of Cyclopenta-fused Polycyclic Aromatic Hydrocarbons. Chaolumen. Introduction
 March 22, 2018 Synthesis, Mechanism, and Properties of Cyclopenta-fused Polycyclic Aromatic Hydrocarbons Reaxys Prize Club Symposium in Japan 2018 Chaolumen Institute for Chemical Research, Kyoto University
March 22, 2018 Synthesis, Mechanism, and Properties of Cyclopenta-fused Polycyclic Aromatic Hydrocarbons Reaxys Prize Club Symposium in Japan 2018 Chaolumen Institute for Chemical Research, Kyoto University
Recent Developments in Alkynylation
 --New approaches to introduce an alkynyl group Reporter: Zhao-feng Wang Supervisor: Yong Huang 2013-03-27 Contents 1. Introduction of Acetylene Chemistry 2. Nucleophilic alkynylation : Classic text book
--New approaches to introduce an alkynyl group Reporter: Zhao-feng Wang Supervisor: Yong Huang 2013-03-27 Contents 1. Introduction of Acetylene Chemistry 2. Nucleophilic alkynylation : Classic text book
Literature Report. Catalytic Enantioselective Synthesis of Isoindolinones through a Biomimetic Approach. : Zhong Yan : Ji Zhou :
 Literature Report Catalytic Enantioselective Synthesis of Isoindolinones through a Biomimetic Approach Reporter Checker Date : Zhong Yan : Ji Zhou : 2017-12-22 Min, C.; Lin, Y.; Seidel, D. Angew. Chem.
Literature Report Catalytic Enantioselective Synthesis of Isoindolinones through a Biomimetic Approach Reporter Checker Date : Zhong Yan : Ji Zhou : 2017-12-22 Min, C.; Lin, Y.; Seidel, D. Angew. Chem.
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo
 Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
C H Activated Trifluoromethylation
 Literature report C H Activated Trifluoromethylation Reporter:Yan Fang Superior:Prof. Yong Huang Jun. 17 th 2013 Contents Background Trifluoromethylation of sp-hybridized C-H Bonds Trifluoromethylation
Literature report C H Activated Trifluoromethylation Reporter:Yan Fang Superior:Prof. Yong Huang Jun. 17 th 2013 Contents Background Trifluoromethylation of sp-hybridized C-H Bonds Trifluoromethylation
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Name: CHEM 633: Advanced Organic Chemistry: Physical Final Exam. Please answer the following questions clearly and concisely.
 Name: 1 CEM 633: Advanced rganic Chemistry: Physical Final Exam Please answer the following questions clearly and concisely. You may write your answers in the space provided and/or on additional pages.
Name: 1 CEM 633: Advanced rganic Chemistry: Physical Final Exam Please answer the following questions clearly and concisely. You may write your answers in the space provided and/or on additional pages.
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid
 Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
Dr. P. Wipf Chem /26/2007
 I. Basic Principles I-L. Radicals & Carbenes Features of Radical Reactions Review: Curran, D. P. In Comprehensive Organic Synthesis; B. M. Trost and I. Fleming, Ed.; Pergamon Press: Oxford, 1991; Vol.
I. Basic Principles I-L. Radicals & Carbenes Features of Radical Reactions Review: Curran, D. P. In Comprehensive Organic Synthesis; B. M. Trost and I. Fleming, Ed.; Pergamon Press: Oxford, 1991; Vol.
C H activation of aliphatic amines without unnecessary mask M2 Takaya Togo
 C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
Morita Baylis Hillman Reaction. Aaron C. Smith 11/10/04
 Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
The Vinylogous Aldol Reaction
 The Vinylogous Aldol Reaction Reporter: Sixuan Meng Supervisor: Prof. Huang 2013-09-09 Zanardi, F. et al. Chem. Rev. 2000, 100, 1929 Zanardi, F. et al.. Chem. Rev. 2011, 111, 3076 Introduction 2 3 Regiochemical
The Vinylogous Aldol Reaction Reporter: Sixuan Meng Supervisor: Prof. Huang 2013-09-09 Zanardi, F. et al. Chem. Rev. 2000, 100, 1929 Zanardi, F. et al.. Chem. Rev. 2011, 111, 3076 Introduction 2 3 Regiochemical
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr.
 April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Chiral Supramolecular Catalyst for Asymmetric Reaction
 Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Construction of Chiral Tetrahydro-β-Carbolines: Asymmetric Pictet Spengler Reaction of Indolyl Dihydropyridines
 Literature Report V Construction of Chiral Tetrahydro-β-Carbolines: Asymmetric Pictet Spengler Reaction of Indolyl Dihydropyridines Reporter Checker Date : Xiao-Yong Zhai : Xin-Wei Wang : 2018-04-02 You,
Literature Report V Construction of Chiral Tetrahydro-β-Carbolines: Asymmetric Pictet Spengler Reaction of Indolyl Dihydropyridines Reporter Checker Date : Xiao-Yong Zhai : Xin-Wei Wang : 2018-04-02 You,
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates
 Pure & Appl. Chern., Vol. 70, No. 5, pp. 11 17-1 122, 1998. Printed in Great Britain. (0 1998 IUPAC Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates Mogens
Pure & Appl. Chern., Vol. 70, No. 5, pp. 11 17-1 122, 1998. Printed in Great Britain. (0 1998 IUPAC Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates Mogens
CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008
 First Three Letters of Last Name NAME Network ID CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed,
First Three Letters of Last Name NAME Network ID CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed,
The Career of Tristan H. Lambert
 The Career of Tristan H. Lambert Jian Rong( 荣健 ) Hu Group Meeting Apr 11, 2016 Tristan H. Lambert: Biographical Notes Professional experience 2011-present: Associate Professor, Columbia University 2006-2011:
The Career of Tristan H. Lambert Jian Rong( 荣健 ) Hu Group Meeting Apr 11, 2016 Tristan H. Lambert: Biographical Notes Professional experience 2011-present: Associate Professor, Columbia University 2006-2011:
Stereoselective Synthesis of Configurationally Stable Functionalized Ethano-Bridged Tröger Bases
 Stereoselective Synthesis of Configurationally Stable Functionalized Ethano-Bridged Tröger Bases Michon, C.; Sharma, A.; Bernardinelli, G.; Francotte, E.; Lacour, J. Chem. Commun., 2010, 46, 2206-2208
Stereoselective Synthesis of Configurationally Stable Functionalized Ethano-Bridged Tröger Bases Michon, C.; Sharma, A.; Bernardinelli, G.; Francotte, E.; Lacour, J. Chem. Commun., 2010, 46, 2206-2208
Dual enantioselective control by heterocycles of (S)-indoline derivatives*
 Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Hypervalent Iodine(III): Property and Reactivity. Penghao Chen g Dong Group Seminar October, 21 st, 2015
 Hypervalent Iodine(III): Property and Reactivity Penghao Chen g Dong Group Seminar October, 21 st, 2015 Structure and Nomenclature Aryl λ 3 iodanes: L I L L : hypervalent bond with a pure 5p orbital Ar
Hypervalent Iodine(III): Property and Reactivity Penghao Chen g Dong Group Seminar October, 21 st, 2015 Structure and Nomenclature Aryl λ 3 iodanes: L I L L : hypervalent bond with a pure 5p orbital Ar
A Simple Introduction of the Mizoroki-Heck Reaction
 A Simple Introduction of the Mizoroki-Heck Reaction Reporter: Supervisor: Zhe Niu Prof. Yang Prof. Chen Prof. Tang 2016/2/3 Content Introduction Intermolecular Mizoroki-Heck Reaction Intramolecular Mizoroki-Heck
A Simple Introduction of the Mizoroki-Heck Reaction Reporter: Supervisor: Zhe Niu Prof. Yang Prof. Chen Prof. Tang 2016/2/3 Content Introduction Intermolecular Mizoroki-Heck Reaction Intramolecular Mizoroki-Heck
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
Enantioselective photochemistry. Zhe Dong
 Enantioselective photochemistry Zhe Dong 2014-01-15 Introduction of Circular Polarization Light Left-CPL- S light Chiral light -> Chiral Product? Right-CPL- R light Introduction of Non-Linear Effects 20%ee
Enantioselective photochemistry Zhe Dong 2014-01-15 Introduction of Circular Polarization Light Left-CPL- S light Chiral light -> Chiral Product? Right-CPL- R light Introduction of Non-Linear Effects 20%ee
Asymmetric Palladium Catalyzed Cross-Coupling Reactions. Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1
 Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
Chiral Ionic Liquids (CILs) in Asymmetric Synthesis: The story so far.
 Chiral Ionic Liquids (CILs) in Asymmetric Synthesis: The story so far. Literature Presentation Aman Desai 06.16.06 1. Angew. Chem. Int. Ed. 2006, 45, 3689 2. Angew. Chem. Int. Ed. 2006, 45, 3093 3. Tetrahedron:
Chiral Ionic Liquids (CILs) in Asymmetric Synthesis: The story so far. Literature Presentation Aman Desai 06.16.06 1. Angew. Chem. Int. Ed. 2006, 45, 3689 2. Angew. Chem. Int. Ed. 2006, 45, 3093 3. Tetrahedron:
Enantioselective Borylations. David Kornfilt Denmark Group Meeting Sept. 14 th 2010
 Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
sp 3 C-H Alkylation with Olefins Yan Xu Dec. 3, 2014
 sp 3 C-H Alkylation with Olefins, Yan Xu Dec. 3, 2014 1) sp 3 C-H Alkylation via Directed C-H activation 2) Hydroaminoalkylation (still via C-H activation) 3) Hydrohydroxyalkylation (via radical chemistry)
sp 3 C-H Alkylation with Olefins, Yan Xu Dec. 3, 2014 1) sp 3 C-H Alkylation via Directed C-H activation 2) Hydroaminoalkylation (still via C-H activation) 3) Hydrohydroxyalkylation (via radical chemistry)
Department of Chemistry, University of Saskatchewan Saskatoon SK S7N 4C9, Canada. Wipf Group. Tyler E. Benedum Current Literature February 26, 2005
 Ward, D.E; Jheengut, V.; Akinnusi, O.T. Enantioselective Direct Intermolecular Aldol Reactions with Enantiotopic Group Selectivity and Dynamic Kinetic Resolution, Organic Letters 2005, ASAP. Department
Ward, D.E; Jheengut, V.; Akinnusi, O.T. Enantioselective Direct Intermolecular Aldol Reactions with Enantiotopic Group Selectivity and Dynamic Kinetic Resolution, Organic Letters 2005, ASAP. Department
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts. Larry Wolf SED Group Meeting
 The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts Larry Wolf SED Group Meeting 04-10-07 Outline Brief historical account and Utility Mechanism Different methods for asymmetric
The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts Larry Wolf SED Group Meeting 04-10-07 Outline Brief historical account and Utility Mechanism Different methods for asymmetric
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
I. Introduction. Peng Zhao. Liu lab
 Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Epoxidation with Peroxy Acids
 Epoxidation with Peroxy Acids RC 3 R C more reactive more likely Freccero, M.; Gandolfi, R.; Sarzi-Amadè, M.; Rastelli, A. J. rg. Chem. 2000, 65, 2030. Singleton, D. A.; Merrigan, S. R.; Liu, J.; ouk,
Epoxidation with Peroxy Acids RC 3 R C more reactive more likely Freccero, M.; Gandolfi, R.; Sarzi-Amadè, M.; Rastelli, A. J. rg. Chem. 2000, 65, 2030. Singleton, D. A.; Merrigan, S. R.; Liu, J.; ouk,
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Rhodium Carbenoids and C-H Insertion
 hodium Carbenoids and C- Insertion Literature Talk Uttam K. Tambar March 1, 2004 8pm, oyes 147 h h h h h h irreversible reversible carbenoid 2 h2l4 1 h2l4 or h2l4 2 utline I. What is a Carbene? II. What
hodium Carbenoids and C- Insertion Literature Talk Uttam K. Tambar March 1, 2004 8pm, oyes 147 h h h h h h irreversible reversible carbenoid 2 h2l4 1 h2l4 or h2l4 2 utline I. What is a Carbene? II. What
Palladium-catalyzed sp 3 C H activation. Yan Xu Dong Group Meeting Apr. 2, 2014
 Palladium-catalyzed sp 3 C H activation, Yan Xu Dong Group Meeting Apr. 2, 2014 Content 1 Allylic C H activation 2 Benzylic C H activation Palladiumcatalyzed sp 3 C H activation 3 4 Common sp 3 C H activation:
Palladium-catalyzed sp 3 C H activation, Yan Xu Dong Group Meeting Apr. 2, 2014 Content 1 Allylic C H activation 2 Benzylic C H activation Palladiumcatalyzed sp 3 C H activation 3 4 Common sp 3 C H activation:
Modern Synthetic Methods
 Modern Synthetic Methods Dr. Dorian Didier dodich@cup.uni-muenchen.de Functionnalized Organometallic Reagents C-N, C-O and C-S Bond Formation Introduction to Organoboron Chemistry Introduction to Organosilicon
Modern Synthetic Methods Dr. Dorian Didier dodich@cup.uni-muenchen.de Functionnalized Organometallic Reagents C-N, C-O and C-S Bond Formation Introduction to Organoboron Chemistry Introduction to Organosilicon
Rhodium Catalyzed Alkyl C-H Insertion Reactions
 Rhodium Catalyzed Alkyl C-H Insertion Reactions Rh Rh Jeff Kallemeyn 5/17/05 1. Cyclopropanation The Versatile and Reactive Rhodium Carbene R + Et Rh 2 (Ac) 4 R C 2 Et N 2 2. [2,3] sigmatropic rearrangement
Rhodium Catalyzed Alkyl C-H Insertion Reactions Rh Rh Jeff Kallemeyn 5/17/05 1. Cyclopropanation The Versatile and Reactive Rhodium Carbene R + Et Rh 2 (Ac) 4 R C 2 Et N 2 2. [2,3] sigmatropic rearrangement
Chiral Amplification. Literature Talk Fabian Schneider Konstanz, Universität Konstanz
 Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine N-oxide
 Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Some questions and answers that we will get out of this example synthesis:
 UTLINE 535 LECTURE 3 (2004) Page 22 The Synthesis of Cubane I am showing you this synthesis because it is elegant and exemplifies many different concepts. We can use it to talk in context about the philosophical
UTLINE 535 LECTURE 3 (2004) Page 22 The Synthesis of Cubane I am showing you this synthesis because it is elegant and exemplifies many different concepts. We can use it to talk in context about the philosophical
Nilson, M. G.; Funk, R. L. Org. Lett. 2010, ASAP. Chad Hopkins Wipf Group Literature Presentation
 TtlS Total Synthesis of ( ) akadomarin kd A ilson, M. G.; Funk, R. L. rg. Lett. 2010, ASAP. Chad opkins Wipf Group Literature Presentation 10 23 10 Chad opkins @ Wipf Group Page 1 of 11 12/5/2010 Isolation
TtlS Total Synthesis of ( ) akadomarin kd A ilson, M. G.; Funk, R. L. rg. Lett. 2010, ASAP. Chad opkins Wipf Group Literature Presentation 10 23 10 Chad opkins @ Wipf Group Page 1 of 11 12/5/2010 Isolation
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY
 STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY Pierre Deslongchamps (version du 16 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTION 8 Stereoelectronic
STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY Pierre Deslongchamps (version du 16 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTION 8 Stereoelectronic
Organic Reactions catalyzed by rhenium carbonyl complexes
 Organic Reactions catalyzed by rhenium carbonyl complexes Fanyang Mo Dong group seminar Feb. 26, 2014 Ref: Kuninobu, Y.; Takai, K. Chem Rev. 2011, 111, 1938. 1 Accidentally found by Ogawa in 1908, and
Organic Reactions catalyzed by rhenium carbonyl complexes Fanyang Mo Dong group seminar Feb. 26, 2014 Ref: Kuninobu, Y.; Takai, K. Chem Rev. 2011, 111, 1938. 1 Accidentally found by Ogawa in 1908, and
Problem Set 2: Reactions of Carbocations - KEY
 Problem Set 2: eactions of Carbocations - KEY Due Tuesday, 10/11/16 1. a. Formation of 1 and 2 silyl ethers is always high-yielding, but selective silylation of a single hydroxyl group can be challenging.
Problem Set 2: eactions of Carbocations - KEY Due Tuesday, 10/11/16 1. a. Formation of 1 and 2 silyl ethers is always high-yielding, but selective silylation of a single hydroxyl group can be challenging.
Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C. Claude Spino et al., J. Org. Chem. 2013, 78,
 Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C Claude Spino et al., J. Org. Chem. 2013, 78, 12532 12544. Poison frog alkaloids Isolated from poison frog skin.
Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C Claude Spino et al., J. Org. Chem. 2013, 78, 12532 12544. Poison frog alkaloids Isolated from poison frog skin.
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Complex Interactions of Pillar[5]arene with Paraquats and. Bis(pyridinium) Derivatives
![Complex Interactions of Pillar[5]arene with Paraquats and. Bis(pyridinium) Derivatives Complex Interactions of Pillar[5]arene with Paraquats and. Bis(pyridinium) Derivatives](/thumbs/94/119880372.jpg) Electronic supplementary information Complex Interactions of Pillar[5]arene with Paraquats and Bis(pyridinium) Derivatives Chunju Li,* Qianqian Xu, Jian Li, Feina Yao and Xueshun Jia* Department of Chemistry,
Electronic supplementary information Complex Interactions of Pillar[5]arene with Paraquats and Bis(pyridinium) Derivatives Chunju Li,* Qianqian Xu, Jian Li, Feina Yao and Xueshun Jia* Department of Chemistry,
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Review. Recent Developments in Pd-catalyzed Carbonylation Reaction. Li Yuanhe. Supervisors: Prof. Yang Prof. Chen Prof. Tang
 Review Recent Developments in Pd-catalyzed Carbonylation Reaction Li Yuanhe Supervisors: Prof. Yang Prof. Chen Prof. Tang History 1962, E. Fischer Z. Nafurforsch. 1962, 17b, 484. 1963, R. Heck J. Org.
Review Recent Developments in Pd-catalyzed Carbonylation Reaction Li Yuanhe Supervisors: Prof. Yang Prof. Chen Prof. Tang History 1962, E. Fischer Z. Nafurforsch. 1962, 17b, 484. 1963, R. Heck J. Org.
Carbonyl Ylide Cycloadditions
 Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
Stereochemistry of Molecules in Crystals (part 1, 2)
 Stereochemistry of Molecules in Crystals (part 1, 2) umio Toda kayama University of Science, kayama, Japan key word: solid state, -guest complex part 1: statistic aspect part 2: dynamic aspect : X a: X
Stereochemistry of Molecules in Crystals (part 1, 2) umio Toda kayama University of Science, kayama, Japan key word: solid state, -guest complex part 1: statistic aspect part 2: dynamic aspect : X a: X
Literature Report 2. Divergent Asymmetric Total Synthesis of Mulinane Diterpenoids. Date :
 Literature Report 2 Divergent Asymmetric Total Synthesis of Mulinane Diterpenoids Reporter : Zhou-Hao Zhu Checker : Xiao-Yong Zhai Date : 2018-04-23 Liu, Y.-T.; Li, L.-P.; Xie, J.-H.*; Zhou, Q.-L. Angew.
Literature Report 2 Divergent Asymmetric Total Synthesis of Mulinane Diterpenoids Reporter : Zhou-Hao Zhu Checker : Xiao-Yong Zhai Date : 2018-04-23 Liu, Y.-T.; Li, L.-P.; Xie, J.-H.*; Zhou, Q.-L. Angew.
Iron Catalysed Coupling Reactions
 LONG LITERATURE REPORT Iron Catalysed Coupling Reactions Mingyu Liu 2017. 8. 31 1 Fe [Ar]3d 6 4s 2 The fourth most common element in the Earth s crust Relatively less understanding and manipulation of
LONG LITERATURE REPORT Iron Catalysed Coupling Reactions Mingyu Liu 2017. 8. 31 1 Fe [Ar]3d 6 4s 2 The fourth most common element in the Earth s crust Relatively less understanding and manipulation of
Discussion Addendum for: Synthesis of 4-, 5-, and 6-Methyl-2,2 -Bipyridine by a Negishi Cross-Coupling Strategy: 5-Methyl-2,2 -Bipyridine
 DO:10.15227/orgsyn.089.0076 Discussion Addendum for: Synthesis of 4-, 5-, and 6-Methyl-2,2 -Bipyridine by a egishi Cross-Coupling Strategy: 5-Methyl-2,2 -Bipyridine A. H 2 SO 4, H 2 O ao H 2 2 OH 1 B.
DO:10.15227/orgsyn.089.0076 Discussion Addendum for: Synthesis of 4-, 5-, and 6-Methyl-2,2 -Bipyridine by a egishi Cross-Coupling Strategy: 5-Methyl-2,2 -Bipyridine A. H 2 SO 4, H 2 O ao H 2 2 OH 1 B.
Radical Reactions. Radical Stability!!! bond dissociation energies X Y X + Y. bond BDE (kcal/mol) bond BDE (kcal/mol) CH 3 CH 3 CH 2 95 O H R 2 C H
 adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
Supporting Information
 Supporting Information Enantioselective Synthesis of 3-Alkynyl-3-Hydroxyindolin-2-ones by Copper-Catalyzed Asymmetric Addition of Terminal Alkynes to Isatins Ning Xu, Da-Wei Gu, Jing Zi, Xin-Yan Wu, and
Supporting Information Enantioselective Synthesis of 3-Alkynyl-3-Hydroxyindolin-2-ones by Copper-Catalyzed Asymmetric Addition of Terminal Alkynes to Isatins Ning Xu, Da-Wei Gu, Jing Zi, Xin-Yan Wu, and
Stereoselective reactions of the carbonyl group
 1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
Michelangelo Gruttadauria
 Michelangelo Gruttadauria Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, Università di Palermo, Viale delle Scienze - Edificio 17, 90128, Palermo 1 Main focus theme: Organic
Michelangelo Gruttadauria Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, Università di Palermo, Viale delle Scienze - Edificio 17, 90128, Palermo 1 Main focus theme: Organic
Bio-inspired C-H functionalization by metal-oxo complexes
 1 Literature Seminar Bio-inspired C-H functionalization by metal-oxo complexes 2016. 7. 23. Nagashima Nozomu 2 C-H functionalization by enzymes Enzymes enable aliphatic C-H functionalization 3 P450 oxidation
1 Literature Seminar Bio-inspired C-H functionalization by metal-oxo complexes 2016. 7. 23. Nagashima Nozomu 2 C-H functionalization by enzymes Enzymes enable aliphatic C-H functionalization 3 P450 oxidation
Parallel Kinetic Resolution. Group Meeting Timothy Chang
 Parallel Kinetic Resolution (PKR) Group Meeting 09 29 2009 Timothy Chang Vedejs, E.; Chen, X. J. Am. Chem. Soc. 1997, 119, 2584. Tanaka, K.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 8078. KR versus PKR The
Parallel Kinetic Resolution (PKR) Group Meeting 09 29 2009 Timothy Chang Vedejs, E.; Chen, X. J. Am. Chem. Soc. 1997, 119, 2584. Tanaka, K.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 8078. KR versus PKR The
Organocatalytic asymmetric biomimetic transamination of aromatic ketone to optically active amine
 Organocatalytic asymmetric biomimetic transamination of aromatic ketone to optically active amine Ying Xie, a Hongjie Pan, a Xiao Xiao, a Songlei Li a and Yian Shi* a,b a Beijing National Laboratory for
Organocatalytic asymmetric biomimetic transamination of aromatic ketone to optically active amine Ying Xie, a Hongjie Pan, a Xiao Xiao, a Songlei Li a and Yian Shi* a,b a Beijing National Laboratory for
Radical cascade reactions triggered by single electron transfer
 Radical cascade reactions triggered by single electron transfer Reporter: Leming Wang Supervisor: Prof. Yong Huang 2017.11.13 Mateusz P. Plesniak, Huan-Ming Huang and David J. Procter; Nature Reviews Chemistry
Radical cascade reactions triggered by single electron transfer Reporter: Leming Wang Supervisor: Prof. Yong Huang 2017.11.13 Mateusz P. Plesniak, Huan-Ming Huang and David J. Procter; Nature Reviews Chemistry
Few Selected Total Synthesis in Group Meeting June 1, Anil Kumar Gupta
 Few Selected Total Synthesis in 2007 Group Meeting June 1, 2007 Anil Kumar Gupta Total Synthesis without Protecting Groups Chemoselectivity!! Solution: Protecting group BUT..It adds. Cost Complexity of
Few Selected Total Synthesis in 2007 Group Meeting June 1, 2007 Anil Kumar Gupta Total Synthesis without Protecting Groups Chemoselectivity!! Solution: Protecting group BUT..It adds. Cost Complexity of
Selectivity and Natural Product Synthesis. Reporter: Liang Xin-ting Supervisors: Prof. Yang Prof. Chen Prof. Tang
 Selectivity and Natural Product Synthesis Reporter: Liang Xin-ting Supervisors: Prof. Yang Prof. Chen Prof. Tang Content Introduction Selectivity of oxidative coupling Chemoselectivity Regioselectivity
Selectivity and Natural Product Synthesis Reporter: Liang Xin-ting Supervisors: Prof. Yang Prof. Chen Prof. Tang Content Introduction Selectivity of oxidative coupling Chemoselectivity Regioselectivity
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHAPTER 23 HW: ENOLS + ENOLATES
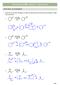 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Course Outline For: Organic Chemistry I (CHM 270) Credits: 5 Contact Hours: Lecture: 3 Lab: 4
 Course Outline For: Organic Chemistry I (CHM 270) Credits: 5 Contact Hours: Lecture: 3 Lab: 4 NOTE on Laboratory: Both Lecture and Laboratory must be taken simultaneously; separate grades will not be given
Course Outline For: Organic Chemistry I (CHM 270) Credits: 5 Contact Hours: Lecture: 3 Lab: 4 NOTE on Laboratory: Both Lecture and Laboratory must be taken simultaneously; separate grades will not be given
The Chemistry of Organozinc Reagent a long story. Reporter: Han Yixin Adivisors: Prof. Yang Prof. Chen Prof. Tang Prof. Luo Date: Nov. 22 nd.
 The Chemistry of Organozinc Reagent a long story Reporter: Han Yixin Adivisors: Prof. Yang Prof. Chen Prof. Tang Prof. Luo Date: Nov. 22 nd. 2013 2 Content Introduction Reactions of Organozinc reagent
The Chemistry of Organozinc Reagent a long story Reporter: Han Yixin Adivisors: Prof. Yang Prof. Chen Prof. Tang Prof. Luo Date: Nov. 22 nd. 2013 2 Content Introduction Reactions of Organozinc reagent
Asymmetric Catalysis by Chiral Hydrogen-Bond Donors
 Asymmetric Catalysis by Chiral Hydrogen-Bond Donors Angew. Chem. Int. Ed., 2006, 45, 1520~1543 Mark S. Taylor and Eric N. Jacobsen* Current Literature Presentation Zhenglai Fang Wipf s Group at Pitt Zhenglai
Asymmetric Catalysis by Chiral Hydrogen-Bond Donors Angew. Chem. Int. Ed., 2006, 45, 1520~1543 Mark S. Taylor and Eric N. Jacobsen* Current Literature Presentation Zhenglai Fang Wipf s Group at Pitt Zhenglai
Chiral Bronsted Acids as Catalysts
 Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S
![[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S [3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S](/thumbs/94/119475672.jpg) Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
One pot cascade reactions of glyoxylate
 Science One pot cascade reactions of glyoxylate By Shyam Sundar Samanta June 12 th 2012 One Pot Synthesis of Non-Proteinogenic Amino Acids and Elaborated Peptides One Pot synthesis. 1) The yield is very
Science One pot cascade reactions of glyoxylate By Shyam Sundar Samanta June 12 th 2012 One Pot Synthesis of Non-Proteinogenic Amino Acids and Elaborated Peptides One Pot synthesis. 1) The yield is very
