Figure 20.3 Molecular Models Representing an Enantiomeric Pair in a Chiral Molecule
|
|
|
- Gilbert Griffith
- 6 years ago
- Views:
Transcription
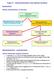
1 Topic 20 Additional igher Level 2. ptical Isomerism ptical isomerism occurs in molecules which have at least one carbon atom which is bonded to four different atoms or groups. A carbon bonded to four different atoms or groups is asmmetric. A molecule containing an asmmetric atom is also usuall (though not alwas) asmmetric. This means the molecule has neither a plane of smmetr nor a centre of smmetr. At least in open-chain compounds, an sp 3 hbridised atom is tetrahedrall coordinated. The four single bonds point towards to the corners of a tetrahedron. If we look more closel, we find there are two different was to organise four different atoms or groups around an asmmetric carbon atom. These two arrangements are mirror images of one another. If we take these two mirror images and tr and place them on top of one another to superimpose them we find the do not match. No matter how the two molecules are twisted or turned or rotated, the are not the same molecule; the are stereoisomers of each other. The two isomers are nonsuperimposable mirror images. These mirror-image stereoisomers are called enantiomers. The simplest eample of a pair of non-superimposable mirror images is our hands. ur left and right hand are mirror images of one another. Tr and put a left-handed glove on our right hand and a right-handed glove on our left hand and ou find that the don t fit. Imagine (but not too hard) that both of our hands were cut off at the wrists in a freak chemistr accident. The surgeon then makes the mistake of sewing our right hand on our left wrist. If ou wanted our thumb to face forward, the palm on our new left hand would face outwards. Figure 20.3 shows an asmmetric atom at the centre of a ball-and-stick molecular model. A pair of model molecules is constructed that are non-superimposable mirror images of one another. You would need to take one of the models apart and reconstruct it so that it had eactl the same spatial arrangement as the other. The models represent a pair of mirror-image molecules called an enantiomeric pair. The molecules cannot be converted into one another without breaking single covalent bonds. It is recommended that ou get a set of molecular models to reall understand the spatial difference between these two molecules. Figure 20.3 Molecular Models Representing an Enantiomeric Pair in a hiral Molecule Mirror 1. An asmmetric carbon atom sits at the centre of a tetrahedron bonded to four different atoms or 1. groups. Its mirror image is seen in the mirror. 2. A second molecular model is made that eactl represents the mirror image of the first. No matter how this mirror image molecule is rotated it cannot be superimposed on the other molecule. 3. The molecules eist as a pair of non-superimposable Asmmetric mirror images called an enantiomeric pair. atom bjects like our hands that can eist as a pair of non-superimposable mirror images are said to be chiral (pronounced kiral ). The same terminolog is used for molecules that have this propert (es, there is a lot of terminolog in this topic). The asmmetric carbon atom is called a chiral centre. hiral atoms are normall shown with an asteri when the structure of a chiral molecule is drawn. hiralit can occur in relativel simple molecules. onsider butan-2-ol, which is shown in two was on the right. The chiral (asmmetric) carbon atom is asteried. It is bonded to four different atoms or groups
2 Topic 20 rganic hemistr The best wa of showing the difference in spatial arrangement between a pair of enantiomers is to draw the chiral atom at the centre of a tetrahedron. The two enantiomers are separated b an imaginar mirror plane. Sometimes when ou draw the enantiomers, it is necessar to reverse the order of some of the groups attached to the chiral atom to make it clear which atom 3 3 is bonded to which. This is done with the group in the Mirror plane butan-2-ol isomer drawn to the right of the mirror plane. 5. Work out whether the following molecules are chiral. Identif an chiral centres and draw the mirror images in the wa shown for butan-2-ol above: (a) 2-hdropropanoic acid (lactic acid) 2-bromopropane (c) 2-bromobutane (d) 1-bromobutane (e) 2-aminopropanoic acid (alanine) (f) aminoethanoic acid (glcine) Solutions: (a) 3 3 lactic acid is chiral (c) (e) Br 3 2-bromobutane is chiral N 2 alanine is chiral The spatial origin of chiralit in molecules and some of its consequences are highlighted in the following video: So wh are these non-superimposable mirror image molecules called optical isomers? The answer lies in how pure samples of these isomers interact with plane-polarised light. We sa that these isomers are opticall active. Let s first eplain what plane-polarised light is and, before that, the nature of light itself. Light is a transverse wave. The wave s electrical field vibrates at right angles to the direction of travel. Not onl does the electrical field vibrate up and down and from side to side, it vibrates in all directions perpendicular to the direction of travel. Light becomes polarised when it is passed through a polarising filter, like a Polaroid film, or though certain tpes of prism. This polarising filter is called the polariser. Polaroid film can be thought of having aligned rod-like molecules. The molecules allow onl that portion of light with its electric field vibrating in one direction (one plane) to pass through; all the other light is absorbed (the Polaroid film makes things darker because less light is transmitted overall). This selectivel transmitted light is called plane-polarised light (Figure 20.4). The electrical field of plane-polarised light vibrates in onl one plane (in Figure 20.4 it vibrates along the -ais). 3 (d) (f) N 2 N 2 N
3 Topic 20 Additional igher Level Figure 20.4 rigin of Plane-Polarised Light This wave is blocked b the Polaroid. Polaroid The wave that is transmitted through the Polaroid vibrates in one plane onl. This is plane-polarised light. The electric field vibrates in all directions at right angles to the direction of travel of the light wave along the -ais. nl two of these waves are shown to keep the diagram clear. Figure 20.5 shows what happens if we shine the plane-polarised light onto a second Polaroid film. This second filter is called the analser. If the two Polaroid films (polariser and analser) have their polarising ais aligned, the maimum amount of plane-polarised light can pass through the analser (Figure 20.5 (a)). If the analser is now twisted through 90 o, no light can pass through (Figure 20.5 ). This is called etinction. (a) Figure 20.5 Light Passing Through Two Polarising Filters If the analser is aligned along the same ais as the polariser, then light will be transmitted. Polariser Plane polarised light Analser If the ais of polarisation of the analser is at right angles to the polariser, etinction occurs. No light is transmitted when we have crossed polars. You can see the same effect as that illustrated in Figure 20.5 if ou have some high qualit polarising sunglasses. The lenses in these sunglasses contain a thin polarising laer. Rotate our head when ou have the sunglasses on and ou will find that objects become lighter or darker. That s because a lot of the glare that ou get when sun reflects off a surface like water is polarised. Alternativel, ask our teacher if the have some polarising lenses ou can investigate. Rotate the two polarising lenses relative to each other and ou will find the go light then dark as the polarising aes are aligned then crossed. 22
4 Topic 20 rganic hemistr Now let s see what happens when an opticall active substance is included in the arrangement illustrated in Figure In Figure 20.6 (a), plane-polarised light is passed through water. The light that is transmitted is unchanged; it is still polarised in the same plane. Water is opticall inactive. Figure 20.6 shows what happens when plane-polarised light is passed through a pure solution of a single enantiomer. The plane in which the plane-polarised light is vibrating has been rotated. As we look back at the light source along the -ais, we see that the plane has been rotated to the right through an angle of about 20 o. Figure 20.6 Rotation of Plane-Polarised Light (a) Water When passed through an opticall inactive substance like water the plane of polarisation is unchanged. Plane polarised light Solution of single enantiomer The opticall active substance in the solution has rotated the plane of polarisation b about 20 o to the right, as seen b an observer looking along the ais toward the light source. 20 o A polarimeter can be used to measure the direction and etent b which the plane of the polarised light has been rotated b an opticall active substance. A polarimeter is essentiall nothing more than a small tube with two polarising filters at either end. At one end of the tube there is the polariser and at the other there is the analser. The sample is placed in the tube between the two filters. Monochromatic light (light of one wavelength) is shone along the tube through the polariser and analser. When an opticall inactive substance like water is placed in the polarimeter then the maimum amount of light will pass through the tube when the polarising ais of the polariser and analser are aligned (as in Figure 20.5 (a)). But when an opticall active solution is placed in the polarimeter, the analser must be twisted in order to transmit the maimum amount of light through the polarimeter. In our eample shown in Figure 20.6, the analser needs to be rotated clockwise, or to the right, through an angle of about 20 o. The angle through which the plane of the plane-polarised light is rotated depends on the molecule and also on other factors such as concentration, how far the light must pass through the sample, and the wavelength of the light. But the crucial thing to understand is that the plane of plane-polarised light is rotated b a pure sample of a single enantiomer, that is, a sample of one of the two mirror image molecules. ur eample isomer in Figure 20.6 rotates the plane of plane-polarised light to the right (or put another wa, we must turn the analser clockwise to have the maimum amount of light passing through the polarimeter). This isomer is called the 23
5 Topic 20 Additional igher Level detrororator isomer (+ or plus isomer). Shine light through a pure sample of the other enantiomer and the plane of plane-polarised light will be rotated in the opposite direction (in this case anticlockwise). The other isomer is called the laevorotator isomer ( or minus isomer). The nature of plane-polarised light and the use of a polarimeter in detecting optical rotation is summarised in the following animation: There are man opticall active molecules with asmmetric carbon atoms. Man but not all, as we will see are found in nature or made in the laborator as a miture of the two enantiomers. This miture is called a racemic miture (or racemate). Shine plane-polarised light through a solution of a racemate and the plane of plane-polarised light is unaffected. This is because the rotator effects of the two stereoisomers in the miture cancel out. Tring to separate one isomer from the other in a racemic miture (a process called resolving) is a trick business. But wh would we need to resolve a racemic miture anwa? The phsical properties of enantiomers are identical and, given that the have the same chemical groups, surel the chemical properties are identical too? Nature shows us that this is not alwas the case. We might epect that in nature all things are smmetrical. After all there are an equal number of left and right hands in the world. But when we look more carefull, we find that -amino acids that are coded for b DNA in a human bod. All ecept for the simplest one, glcine, are R' R N 2 The two optical isomers in a pair of enantiomers rotate the plane of plane-polarised light in opposite directions chiral. Amaingl, all the amino acids found in nature have the same spatial arrangement. In other words, onl one of the isomers of the enantiomeric pair is found naturall. What this means that peptides, proteins and enmes, all compounds made from linking amino acids together in a chain, have a fied handedness. arbohdrates are also chiral molecules and so are nucleic acids (have ou noticed that DNA twists one wa and not the other?). In short, our own bodies are chiral environments. ne consequence of this is that the receptors in our bodies often respond differentl to the two enantiomers of an asmmetric molecule. Take, for eample, the molecule aspartame. Aspartame is opticall active. ne of the enantiomers of aspartame tastes sweet and is used in artificial sweeteners like Nutrasweet; the other enantiomer tastes bitter. The analgesic morphine is also a chiral molecule. ne of the enantiomers of morphine is a ver powerful pain reliever and is non-addictive; the other enantiomer is highl addictive and not as effective as a pain-reliever. ne of the most heartbreaking eamples of the stereoselectivit of the human bod is the stor of thalidomide. The drug thalidomide was prescribed to thousands of pregnant women in the late 1950s and earl 1960s. Thalidomide is an effective drug for relieving morning sickness. Tragicall, man babies born to women who had taken thalidomide during the first trimester of pregnanc were born with terrible defects. Man so-called thalidomide babies were born with abnormall short legs and flipper-like arms. The drug thalidomide is chiral. The asmmetric carbon atom Thalidomide N -amino acids are chiral when R R (all ecept glcine when R=R =) N is asterisked in the structure. A line is used in this structure when we don t specif if the bond points up ( ), as in one enantiomer, or down ( ), as in the other enantiomer. ne of the enantiomers is an effective antiemetic (it relieves sickness); the other enantiomer is a powerful foetus deformer. The drug was withdrawn from the market but not before over 10,000 babies had been affected. An emotive but informative histor of Thalidomide can be seen at ne of the greatest advances in organic chemistr in the past decade or so has been the development of asmmetric snthesis. Asmmetric snthesis is the selective preparation of one of the enantiomers of an asmmetric molecule. Three pioneers of cataltic asmmetric snthesis, William S. Knowles, K. Barr Sharpless and Roji Noori, were jointl awarded the Nobel Prie for hemistr in This new technolog would not have prevented the thalidomide traged, even if it had been available at the time. This is because the two enantiomers of thalidomide 24
Chem!stry. Optical Isomerism
 hem!stry ame: ( ) lass: Date: / / ptical Isomerism ptical isomers are compounds that share the same molecular formula, but which rotate plane polarised light in opposite directions, either clockwise or
hem!stry ame: ( ) lass: Date: / / ptical Isomerism ptical isomers are compounds that share the same molecular formula, but which rotate plane polarised light in opposite directions, either clockwise or
Optical Isomerism. Types of isomerism. chemrevise.org 20/08/2013. N Goalby Chemrevise.org. Isomerism. Structural isomerism.
 ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
It is possible for organic molecules with the same molecular formula to have different structures
 Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
E30 ENANTIOMERS Chirality in organic chemistry
 E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
Amides, Amino acids and Chirality
 R hemistry A 432 Amides, Amino Acids & hirality Amides, Amino acids and hirality aming of Amides The amide functional group consists of a carbonyl group bonded to the nitrogen of an amine. Like amines,
R hemistry A 432 Amides, Amino Acids & hirality Amides, Amino acids and hirality aming of Amides The amide functional group consists of a carbonyl group bonded to the nitrogen of an amine. Like amines,
Topic 5 Stereochemistry and optical isomers Isomerism
 Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
OPTICAL ISOMERISM UNIT-1
 OPTICAL ISOMERISM UNIT-1 K.Anita priyadharshini, Lecturer, Dept.of Pharmaceutical Chemistry, SRM College of Pharmacy TYPES OF ISOMERISM CHAIN ISOMERISM STRUCTURAL ISOMERISM Same molecular formula but different
OPTICAL ISOMERISM UNIT-1 K.Anita priyadharshini, Lecturer, Dept.of Pharmaceutical Chemistry, SRM College of Pharmacy TYPES OF ISOMERISM CHAIN ISOMERISM STRUCTURAL ISOMERISM Same molecular formula but different
Lecture Topics: I. Stereochemistry Stereochemistry is the study of the three dimensional structure of molecules
 Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
Introduction to Organic Chemistry, Unit 3: Chirality at Carbon Centers Bring your model kits to class!
 Introduction to rganic hemistry, Unit 3: hirality at arbon enters Bring your model kits to class! rganic hemistry #3 1 bjectives: by the end of this unit, you should be able to... Identify the chiral centres
Introduction to rganic hemistry, Unit 3: hirality at arbon enters Bring your model kits to class! rganic hemistry #3 1 bjectives: by the end of this unit, you should be able to... Identify the chiral centres
Lab 9: Polarization Phy208 Spring 2008
 Lab 9: Polarization Ph208 Spring 2008 Name Section This sheet is the lab document our TA will use to score our lab. It is to be turned in at the end of lab. To receive full credit ou must use complete
Lab 9: Polarization Ph208 Spring 2008 Name Section This sheet is the lab document our TA will use to score our lab. It is to be turned in at the end of lab. To receive full credit ou must use complete
Chapter 6. Isomers and Stereochemistry
 hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
ISOMERISM - A general survey
 Isomerism 1 ISOMERISM - A general survey STRUTURAL ISOMERS have the same molecular formula but different structural formulae They occur due to variations in... the carbon skeleton AIN ISOMERISM 2 2 positions
Isomerism 1 ISOMERISM - A general survey STRUTURAL ISOMERS have the same molecular formula but different structural formulae They occur due to variations in... the carbon skeleton AIN ISOMERISM 2 2 positions
Chapter 3: Stereochemistry & Chirality
 Chapter 3: Stereochemistry & Chirality 1. Chiral & Achiral Compounds - Identifying Stereocenters 2. Assigning R & S configurations 3. Diastereomers - Molecules with two or more stereocenters 4. Properties
Chapter 3: Stereochemistry & Chirality 1. Chiral & Achiral Compounds - Identifying Stereocenters 2. Assigning R & S configurations 3. Diastereomers - Molecules with two or more stereocenters 4. Properties
x y plane is the plane in which the stresses act, yy xy xy Figure 3.5.1: non-zero stress components acting in the x y plane
 3.5 Plane Stress This section is concerned with a special two-dimensional state of stress called plane stress. It is important for two reasons: () it arises in real components (particularl in thin components
3.5 Plane Stress This section is concerned with a special two-dimensional state of stress called plane stress. It is important for two reasons: () it arises in real components (particularl in thin components
Lab 10: Polarization Phy248 Spring 2009
 Lab 10: Polarization Ph248 Spring 2009 Name Section This sheet is the lab document our TA will use to score our lab. It is to be turned in at the end of lab. To receive full credit ou must use complete
Lab 10: Polarization Ph248 Spring 2009 Name Section This sheet is the lab document our TA will use to score our lab. It is to be turned in at the end of lab. To receive full credit ou must use complete
Stereochemistry. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects.
 Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Chapter 6. Isomers and Stereochemistry
 Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Stereochemistry. Based on McMurry s Organic Chemistry, 6 th edition
 Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Chapter 5: Stereoisomerism
 hapter 5: Stereoisomerism [Sections: 5.1-5.9] 1. dentifying Types of somers Same MolecularFormula? A B compounds are not isomers Same onnectivity? D E constitutional isomers have different names (parent
hapter 5: Stereoisomerism [Sections: 5.1-5.9] 1. dentifying Types of somers Same MolecularFormula? A B compounds are not isomers Same onnectivity? D E constitutional isomers have different names (parent
Lesson 4. Molecular Geometry and Isomers II. Lesson 4 CH 3 HO H OH
 Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
This section will involve expanding your knowledge of different types of ISOMERS.
 This section will involve expanding your knowledge of different types of ISOMERS. Isomerism arises whenever there is more than one way to organise a given number of atoms. It is very common in organic
This section will involve expanding your knowledge of different types of ISOMERS. Isomerism arises whenever there is more than one way to organise a given number of atoms. It is very common in organic
Organic Chemistry. Chemical Bonding and Structure (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
02/07/2017. Isomerism. Structural isomerism. 1. Structural isomerism different linkages of atoms. Same molecular formula Different structural formulae
 hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
6 Properties of polarized light - polarimetry
 6 Properties of polarized light - polarimetr Supervisors : J.Geandrot, O.Frantz This practical work aims to stud some phenomena caused b the transversalit of light : dichroism, birefingence, rotating power.
6 Properties of polarized light - polarimetr Supervisors : J.Geandrot, O.Frantz This practical work aims to stud some phenomena caused b the transversalit of light : dichroism, birefingence, rotating power.
Chapter 5 Stereochemistry
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Chapter 5 Stereochemistry
 Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Chapter 5 Stereochemistry. Stereoisomers
 Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Stereochemistry & Polarimetry notes
 Reminder: These notes are meant to supplement, not replace, the laboratory manual. Stereochemistry & Polarimetry notes History Application: Approximately 25% of all drugs are marketed as either racemates
Reminder: These notes are meant to supplement, not replace, the laboratory manual. Stereochemistry & Polarimetry notes History Application: Approximately 25% of all drugs are marketed as either racemates
Stereochemistry CHAPTER SUMMARY
 2 7 2 7. Introduction APTER SUMMARY Isomers are compounds with identical molecular formulas but different structural formulas. Structural or constitutional isomers differ in the bonding arrangement of
2 7 2 7. Introduction APTER SUMMARY Isomers are compounds with identical molecular formulas but different structural formulas. Structural or constitutional isomers differ in the bonding arrangement of
CHAPTER 5. Stereoisomers
 CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
240 Chem. Stereochemistry. Chapter 5
 240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
Symmetry Arguments and the Role They Play in Using Gauss Law
 Smmetr Arguments and the Role The la in Using Gauss Law K. M. Westerberg (9/2005) Smmetr plas a ver important role in science in general, and phsics in particular. Arguments based on smmetr can often simplif
Smmetr Arguments and the Role The la in Using Gauss Law K. M. Westerberg (9/2005) Smmetr plas a ver important role in science in general, and phsics in particular. Arguments based on smmetr can often simplif
Molecules, Medicine and Life
 Molecules, Medicine and Life Ravi P. Singh Organic Chemistry Division NCL PUNE Exciting Science 24 th March 2013 Life and its basic elements What is life? What are we made of? What is our surrounding made
Molecules, Medicine and Life Ravi P. Singh Organic Chemistry Division NCL PUNE Exciting Science 24 th March 2013 Life and its basic elements What is life? What are we made of? What is our surrounding made
Chapter 4: Stereochemistry
 Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
C 4 H 10 O. butanol. diethyl ether. different carbon skeleton different functional group different position of FG
 hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
PRESENTATION ISOMERISM. Dr. Susmita Bajpai
 PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
geometric isomers (diastereomers)
 Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
Enantiomers. nonsuperimposable mirror image Both Configuration will be opposite. Both Configuration will be opposite
 Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
9. Stereochemistry. Stereochemistry
 9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
STEREOCHEMISTRY. 2. Define the following, and tell whether or not a given compound or structure fits the description or possesses the feature.
 A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
x find all of the symmetry operations/elements: o character table headings: E, 2C φ σ v, i, S φ C 2 φ
 Construct and annotate a valence molecular orbital diagram for the linear molecule Mg 2. Assume the 3pAOs lie deeper in energ than the Mg 3sAO and that the Mg and orbitals interact onl slightl. Mg is a
Construct and annotate a valence molecular orbital diagram for the linear molecule Mg 2. Assume the 3pAOs lie deeper in energ than the Mg 3sAO and that the Mg and orbitals interact onl slightl. Mg is a
Enantiomers 2:22 PM 1
 1 Definition (Revisited) Enantiomers are stereoisomers that are nonsuperimposable mirror images. Note that all the chiral centres in a pair of enantiomers are mirror images of each other. 2 Practice Questions
1 Definition (Revisited) Enantiomers are stereoisomers that are nonsuperimposable mirror images. Note that all the chiral centres in a pair of enantiomers are mirror images of each other. 2 Practice Questions
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
Unit One Part 8: stereochemistry
 Unit ne Part 8: stereochemistry 1 Describe the difference between stereoisomers & structural isomers omenclature used for double bonds (cis-trans or E-Z) Predict conformations of cyclohexanes Define chirality
Unit ne Part 8: stereochemistry 1 Describe the difference between stereoisomers & structural isomers omenclature used for double bonds (cis-trans or E-Z) Predict conformations of cyclohexanes Define chirality
Chapter 3 The Chemistry of Carbon
 Complex molecules assembled like TinkerToys Chapter 3 The Chemistry of Carbon Why study Carbon? All living things are made of cells Cells ~72% H 2 O ~3% salts (Na, Cl, K ) ~25% carbon compounds carbohydrates
Complex molecules assembled like TinkerToys Chapter 3 The Chemistry of Carbon Why study Carbon? All living things are made of cells Cells ~72% H 2 O ~3% salts (Na, Cl, K ) ~25% carbon compounds carbohydrates
220A Solutions. Assignment 5.
 4-40. Draw the free-bod diagram. 220A Solutions Assignment 5. - cos sin here are two stumbling points. he first is to resist the temptation to draw a force pointing down the inclined plane. he onl forces
4-40. Draw the free-bod diagram. 220A Solutions Assignment 5. - cos sin here are two stumbling points. he first is to resist the temptation to draw a force pointing down the inclined plane. he onl forces
CHAPTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS. ƒ C Cl ƒ
 EINS26-400-417.v1.qxd 11/9/07 1:13 PM Page 400 APTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS 1. A chiral carbon atom is one to which four different atoms or groups are attached and is a center
EINS26-400-417.v1.qxd 11/9/07 1:13 PM Page 400 APTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS 1. A chiral carbon atom is one to which four different atoms or groups are attached and is a center
LESSON #11 - FORMS OF A LINE COMMON CORE ALGEBRA II
 LESSON # - FORMS OF A LINE COMMON CORE ALGEBRA II Linear functions come in a variet of forms. The two shown below have been introduced in Common Core Algebra I and Common Core Geometr. TWO COMMON FORMS
LESSON # - FORMS OF A LINE COMMON CORE ALGEBRA II Linear functions come in a variet of forms. The two shown below have been introduced in Common Core Algebra I and Common Core Geometr. TWO COMMON FORMS
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers)
 Suggested Reading: Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers) Jones Section 4.9 Physical Properties of Diastereomers: Optical Resolution pages 176-178 Introduction
Suggested Reading: Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers) Jones Section 4.9 Physical Properties of Diastereomers: Optical Resolution pages 176-178 Introduction
Chapter 18 Quadratic Function 2
 Chapter 18 Quadratic Function Completed Square Form 1 Consider this special set of numbers - the square numbers or the set of perfect squares. 4 = = 9 = 3 = 16 = 4 = 5 = 5 = Numbers like 5, 11, 15 are
Chapter 18 Quadratic Function Completed Square Form 1 Consider this special set of numbers - the square numbers or the set of perfect squares. 4 = = 9 = 3 = 16 = 4 = 5 = 5 = Numbers like 5, 11, 15 are
Name. Optical Isomers
 Name KEY Lab Day Optical Isomers Introduction: Stereoisomers are compounds that have the same structural formulas, but differ in their spatial arrangements. Two major types of stereoisomers are geometric
Name KEY Lab Day Optical Isomers Introduction: Stereoisomers are compounds that have the same structural formulas, but differ in their spatial arrangements. Two major types of stereoisomers are geometric
11.4 Polar Coordinates
 11. Polar Coordinates 917 11. Polar Coordinates In Section 1.1, we introduced the Cartesian coordinates of a point in the plane as a means of assigning ordered pairs of numbers to points in the plane.
11. Polar Coordinates 917 11. Polar Coordinates In Section 1.1, we introduced the Cartesian coordinates of a point in the plane as a means of assigning ordered pairs of numbers to points in the plane.
LESSON #12 - FORMS OF A LINE COMMON CORE ALGEBRA II
 LESSON # - FORMS OF A LINE COMMON CORE ALGEBRA II Linear functions come in a variet of forms. The two shown below have been introduced in Common Core Algebra I and Common Core Geometr. TWO COMMON FORMS
LESSON # - FORMS OF A LINE COMMON CORE ALGEBRA II Linear functions come in a variet of forms. The two shown below have been introduced in Common Core Algebra I and Common Core Geometr. TWO COMMON FORMS
Stereochemistry. 3-dimensional Aspects of Tetrahedral Atoms
 Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
chapter 1 Chemical structure and bonding
 Sa Ph m ar ple m c ac on eu te tic nt al co P r p es rig s ht chapter 1 Chemical structure and bonding Overview After learning the material presented in this chapter ou should: understand how an atom is
Sa Ph m ar ple m c ac on eu te tic nt al co P r p es rig s ht chapter 1 Chemical structure and bonding Overview After learning the material presented in this chapter ou should: understand how an atom is
Mathematics 309 Conic sections and their applicationsn. Chapter 2. Quadric figures. ai,j x i x j + b i x i + c =0. 1. Coordinate changes
 Mathematics 309 Conic sections and their applicationsn Chapter 2. Quadric figures In this chapter want to outline quickl how to decide what figure associated in 2D and 3D to quadratic equations look like.
Mathematics 309 Conic sections and their applicationsn Chapter 2. Quadric figures In this chapter want to outline quickl how to decide what figure associated in 2D and 3D to quadratic equations look like.
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Organic: module 4 revision guide. Basic definitions to know. Drawing Displayed formulae
 opyright Goalby Bancroft's School rganic: module 4 revision guide ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds
opyright Goalby Bancroft's School rganic: module 4 revision guide ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds
10/4/2010. Sequence Rules for Specifying Configuration. Sequence Rules for Specifying Configuration. 5.5 Sequence Rules for Specifying.
 5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
10/4/2010. Chapter 5 Stereochemistry at Tetrahedral Centers. Handedness. 5.1 Enantiomers and the Tetrahedral Carbon
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 5 Stereochemistry at Tetrahedral Centers Richard Morrison University of Georgia, Athens Handedness Right and left hands are not identical
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 5 Stereochemistry at Tetrahedral Centers Richard Morrison University of Georgia, Athens Handedness Right and left hands are not identical
The Rotation of Polarized Light by Chiral Molecules E6-1
 Experiment 6 The Rotation of Polarized Light by hiral Molecules Smell of (S)-limonene Smell of (R)-limonene E6-1 E6-2 The Task In this experiment you will learn how a polarimeter works and use one to measure
Experiment 6 The Rotation of Polarized Light by hiral Molecules Smell of (S)-limonene Smell of (R)-limonene E6-1 E6-2 The Task In this experiment you will learn how a polarimeter works and use one to measure
STEREOCHEMISTRY CHIRALITY
 TERECEMITRY CIRALITY A A C C B C D D C B Chirality - tereoisomers 2 Chirality Enantiomers - molecules that are not superimposable on their mirror image. Molecules that can exist as enantiomers are called
TERECEMITRY CIRALITY A A C C B C D D C B Chirality - tereoisomers 2 Chirality Enantiomers - molecules that are not superimposable on their mirror image. Molecules that can exist as enantiomers are called
Chemistry of Carbon. Building Blocks of Life
 Chemistry of Carbon Building Blocks of Life 2007-2008 Why study Carbon? All of life is built on carbon Cells ~72% H2O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl,
Chemistry of Carbon Building Blocks of Life 2007-2008 Why study Carbon? All of life is built on carbon Cells ~72% H2O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl,
3.7 Organic naming and Isomerism continued
 3.7 rganic naming and Isomerism continued See chapter 3.1 for basic naming of organic molecules. This chapter extends the naming for functional groups met in next few chapters homologous series functional
3.7 rganic naming and Isomerism continued See chapter 3.1 for basic naming of organic molecules. This chapter extends the naming for functional groups met in next few chapters homologous series functional
15. Polarization. Linear, circular, and elliptical polarization. Mathematics of polarization. Uniaxial crystals. Birefringence.
 15. Polarization Linear, circular, and elliptical polarization Mathematics of polarization Uniaial crstals Birefringence Polarizers Notation: polarization near an interface Parallel ("p") polarization
15. Polarization Linear, circular, and elliptical polarization Mathematics of polarization Uniaial crstals Birefringence Polarizers Notation: polarization near an interface Parallel ("p") polarization
Lab 5 Forces Part 1. Physics 211 Lab. You will be using Newton s 2 nd Law to help you examine the nature of these forces.
 b Lab 5 Forces Part 1 Phsics 211 Lab Introduction This is the first week of a two part lab that deals with forces and related concepts. A force is a push or a pull on an object that can be caused b a variet
b Lab 5 Forces Part 1 Phsics 211 Lab Introduction This is the first week of a two part lab that deals with forces and related concepts. A force is a push or a pull on an object that can be caused b a variet
STEREOCHEMISTRY A STUDENT WHO HAS MASTERED THE MATERIAL IN THIS SECTION SHOULD BE ABLE TO:
 STEREOEMISTRY A STUDENT WO AS MASTERED TE MATERIAL IN TIS SETION SOULD BE ABLE TO: 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of
STEREOEMISTRY A STUDENT WO AS MASTERED TE MATERIAL IN TIS SETION SOULD BE ABLE TO: 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of
Ch 3 Alg 2 Note Sheet.doc 3.1 Graphing Systems of Equations
 Ch 3 Alg Note Sheet.doc 3.1 Graphing Sstems of Equations Sstems of Linear Equations A sstem of equations is a set of two or more equations that use the same variables. If the graph of each equation =.4
Ch 3 Alg Note Sheet.doc 3.1 Graphing Sstems of Equations Sstems of Linear Equations A sstem of equations is a set of two or more equations that use the same variables. If the graph of each equation =.4
Basic Stereochemical Considerations
 Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
Chapter 6 Principles of Stereochemistry
 6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
Experiment 6. Stereochemistry
 Experiment 6. Stereochemistry Introduction Organic molecules with the same molecular formula but different arrangements of atoms are called isomers. Structural isomers are different because the location
Experiment 6. Stereochemistry Introduction Organic molecules with the same molecular formula but different arrangements of atoms are called isomers. Structural isomers are different because the location
Chem 341 Jasperse Ch. 9 Handouts 1
 Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Carbohydrates - Introduction
 arbohydrates - Introduction arbohydrates - General Description A. Polyhydroxy Aldehydes or Ketones ARBN AIN B. Serve a variety of functions ARBN AIN ARBN AIN 1. Energy storage (Glucose, Glycogen, Starch)
arbohydrates - Introduction arbohydrates - General Description A. Polyhydroxy Aldehydes or Ketones ARBN AIN B. Serve a variety of functions ARBN AIN ARBN AIN 1. Energy storage (Glucose, Glycogen, Starch)
Stereochemistry. Conformers: Compounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds.
 Stereochemistry Terms onformers: ompounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds. onstitutional isomers (also called structural isomers):
Stereochemistry Terms onformers: ompounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds. onstitutional isomers (also called structural isomers):
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop
 Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
1.6 ELECTRONIC STRUCTURE OF THE HYDROGEN ATOM
 1.6 ELECTRONIC STRUCTURE OF THE HYDROGEN ATOM 23 How does this wave-particle dualit require us to alter our thinking about the electron? In our everda lives, we re accustomed to a deterministic world.
1.6 ELECTRONIC STRUCTURE OF THE HYDROGEN ATOM 23 How does this wave-particle dualit require us to alter our thinking about the electron? In our everda lives, we re accustomed to a deterministic world.
This is the most difficult part of stereochemistry; that is the visualization of molecules just by thought process in three-dimensional space.
 Course on Stereochemistry Professor Amit Basak Department of Chemistry Indian Institute of Technology Kharagpur Module No 01 Lecture 01: Constitution and Configuration (V2-note-version) Welcome to this
Course on Stereochemistry Professor Amit Basak Department of Chemistry Indian Institute of Technology Kharagpur Module No 01 Lecture 01: Constitution and Configuration (V2-note-version) Welcome to this
Lecture 4: 12.4 Isomerism
 Lecture 4: 12.4 Isomerism Learning Outcomes: At the end of the lesson the students should be able to : Define isomerism. Explain constitutional isomerism. chain isomers positional isomers functional group
Lecture 4: 12.4 Isomerism Learning Outcomes: At the end of the lesson the students should be able to : Define isomerism. Explain constitutional isomerism. chain isomers positional isomers functional group
9. Stereochemistry: Introduction to Using Molecular Models
 9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
2/25/2015. Chapter 4. Introduction to Organic Compounds. Outline. Lecture Presentation. 4.1 Alkanes: The Simplest Organic Compounds
 Lecture Presentation Outline Chapter 4 Introduction to Organic Compounds 4.2 Representing Structures of Organic Compounds Julie Klare Fortis College Smyrna, GA Alkanes are structurally simple organic compounds
Lecture Presentation Outline Chapter 4 Introduction to Organic Compounds 4.2 Representing Structures of Organic Compounds Julie Klare Fortis College Smyrna, GA Alkanes are structurally simple organic compounds
Carbon and the Molecular Diversity of Life
 The Star of The Show arbon and the Molecular Diversity of Life hapter 4 Pgs. 58-67 arbon is the Backbone of Life arbon enters the biosphere via plants It is the most vital atom in proteins, DNA, carbohydrates,
The Star of The Show arbon and the Molecular Diversity of Life hapter 4 Pgs. 58-67 arbon is the Backbone of Life arbon enters the biosphere via plants It is the most vital atom in proteins, DNA, carbohydrates,
Names. Chiral: A chiral object is not superimposable upon its mirror image. A chiral object contains the property of "handedness.
 CEM 241 IN-CLASS #3 MOLECULAR MODELS EXERCISE Names Stereoisomerism Construct a model containing a tetrahedral carbon (black ball) that is attached to four different atoms (use the green, orange, purple
CEM 241 IN-CLASS #3 MOLECULAR MODELS EXERCISE Names Stereoisomerism Construct a model containing a tetrahedral carbon (black ball) that is attached to four different atoms (use the green, orange, purple
Organic Chemistry Chapter 5 Stereoisomers H. D. Roth
 Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
3.7 InveRSe FUnCTIOnS
 CHAPTER functions learning ObjeCTIveS In this section, ou will: Verif inverse functions. Determine the domain and range of an inverse function, and restrict the domain of a function to make it one-to-one.
CHAPTER functions learning ObjeCTIveS In this section, ou will: Verif inverse functions. Determine the domain and range of an inverse function, and restrict the domain of a function to make it one-to-one.
Copyright 2009 James K Whitesell
 Copyright 2009 James K Whitesell 5-1 These two molecules, cyclopropylcyclopentane and cyclobutycyclobutane have the same number of carbon and hydrogen atoms and thus they are constitutional isomers. 5-2
Copyright 2009 James K Whitesell 5-1 These two molecules, cyclopropylcyclopentane and cyclobutycyclobutane have the same number of carbon and hydrogen atoms and thus they are constitutional isomers. 5-2
8. BOOLEAN ALGEBRAS x x
 8. BOOLEAN ALGEBRAS 8.1. Definition of a Boolean Algebra There are man sstems of interest to computing scientists that have a common underling structure. It makes sense to describe such a mathematical
8. BOOLEAN ALGEBRAS 8.1. Definition of a Boolean Algebra There are man sstems of interest to computing scientists that have a common underling structure. It makes sense to describe such a mathematical
Algebra II Notes Unit Six: Polynomials Syllabus Objectives: 6.2 The student will simplify polynomial expressions.
 Algebra II Notes Unit Si: Polnomials Sllabus Objectives: 6. The student will simplif polnomial epressions. Review: Properties of Eponents (Allow students to come up with these on their own.) Let a and
Algebra II Notes Unit Si: Polnomials Sllabus Objectives: 6. The student will simplif polnomial epressions. Review: Properties of Eponents (Allow students to come up with these on their own.) Let a and
CHEMISTRY PAPER No. : 7 MODULE No. : 23 (Optical Isomerism)
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 7 : Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 23
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 7 : Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 23
5. Zeros. We deduce that the graph crosses the x-axis at the points x = 0, 1, 2 and 4, and nowhere else. And that s exactly what we see in the graph.
 . Zeros Eample 1. At the right we have drawn the graph of the polnomial = ( 1) ( 2) ( 4). Argue that the form of the algebraic formula allows ou to see right awa where the graph is above the -ais, where
. Zeros Eample 1. At the right we have drawn the graph of the polnomial = ( 1) ( 2) ( 4). Argue that the form of the algebraic formula allows ou to see right awa where the graph is above the -ais, where
Atomic Properties of Carbon
 Organic Compounds Atomic Properties of Carbon Organic molecules have structural complexity and chemical diversity. Carbon can lose 4 electrons and have the same electronic configuration as He. OR Carbon
Organic Compounds Atomic Properties of Carbon Organic molecules have structural complexity and chemical diversity. Carbon can lose 4 electrons and have the same electronic configuration as He. OR Carbon
Why study Carbon? Chemistry of Life. Chemistry of Life. Hydrocarbons can grow. Hydrocarbons. Building Blocks. Combinations of C & H
 Chemistry of Life Building Blocks Why study Carbon? All of life is built on carbon Cells ~72% 2 O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl, K Chemistry of Life
Chemistry of Life Building Blocks Why study Carbon? All of life is built on carbon Cells ~72% 2 O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl, K Chemistry of Life
The Force Table Introduction: Theory:
 1 The Force Table Introduction: "The Force Table" is a simple tool for demonstrating Newton s First Law and the vector nature of forces. This tool is based on the principle of equilibrium. An object is
1 The Force Table Introduction: "The Force Table" is a simple tool for demonstrating Newton s First Law and the vector nature of forces. This tool is based on the principle of equilibrium. An object is
Methods for Advanced Mathematics (C3) Coursework Numerical Methods
 Woodhouse College 0 Page Introduction... 3 Terminolog... 3 Activit... 4 Wh use numerical methods?... Change of sign... Activit... 6 Interval Bisection... 7 Decimal Search... 8 Coursework Requirements on
Woodhouse College 0 Page Introduction... 3 Terminolog... 3 Activit... 4 Wh use numerical methods?... Change of sign... Activit... 6 Interval Bisection... 7 Decimal Search... 8 Coursework Requirements on
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
Topic 3 Notes Jeremy Orloff
 Topic 3 Notes Jerem Orloff 3 Line integrals and auch s theorem 3.1 Introduction The basic theme here is that comple line integrals will mirror much of what we ve seen for multivariable calculus line integrals.
Topic 3 Notes Jerem Orloff 3 Line integrals and auch s theorem 3.1 Introduction The basic theme here is that comple line integrals will mirror much of what we ve seen for multivariable calculus line integrals.
STEREOGENIC CENTER (Chiral Center,Asymmetric Center) Atom (usually carbon) to which 4 different groups are attached: W Z C X Y
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
ES.182A Topic 36 Notes Jeremy Orloff
 ES.82A Topic 36 Notes Jerem Orloff 36 Vector fields and line integrals in the plane 36. Vector analsis We now will begin our stud of the part of 8.2 called vector analsis. This is the stud of vector fields
ES.82A Topic 36 Notes Jerem Orloff 36 Vector fields and line integrals in the plane 36. Vector analsis We now will begin our stud of the part of 8.2 called vector analsis. This is the stud of vector fields
Isomerism. Introduction
 Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
