Are black carbon and soot the same?
|
|
|
- Allen Mason
- 6 years ago
- Views:
Transcription
1 Atmos. Chem. Phys. Discuss., 12, , 12 doi:.194/acpd Author(s) 12. CC Attribution 3.0 License. This discussion paper is/has been under review for the journal Atmospheric Chemistry and Physics (ACP). Please refer to the corresponding final paper in ACP if available. Are black carbon and soot the same? P. R. Buseck 1,2, K. Adachi 1,2,3, A. Gelencsér 4, É. Tompa 4, and M. Pósfai 4 Atmospheric Chemistry and Physics Discussions 1 School of Earth and Space Exploration, Arizona State University, Tempe, AZ 8282, USA 2 Department of Chemistry and Biochemistry, Arizona State University, Tempe, AZ 8282, USA 3 Atmospheric Environment and Applied Meteorology Research Department, Meteorological Research Institute, Tsukuba, Ibaraki, Japan 4 Department of Earth and Environmental Sciences, University of Pannonia, Veszprém, Hungary Received: 1 September 12 Accepted: 3 September 12 Published: 21 September 12 Correspondence to: P. R. Buseck (pbuseck@asu.edu) Published by Copernicus Publications on behalf of the European Geosciences Union Abstract The climate change and environmental literature, including that on aerosols, is replete with mention of black carbon (BC), but neither reliable samples nor standards exist. Thus, there is uncertainty about its exact nature. That confusion can be avoided if terms are defined and widely understood. Here we discuss an ambiguity between BC and soot and propose a more precise definition for soot as a specific material, which we call ns-soot, where ns refers to carbon nanospheres. We define ns-soot as particles that consist of nanospheres, typically with diameters < 0 nm, that possess distinct structures of concentrically wrapped, graphene-like layers of carbon and with grape-like (acinoform) morphologies. We additionally propose that, because of their importance for climate modeling and health issues, distinctions are made among bare, coated, and embedded ns-soot. BC, on the other hand, is not a well-defined material. We propose that the term should be restricted to light-absorbing refractory carbonaceous matter of uncertain character and that the uncertainty is stated explicitly. 1 Introduction Carbonaceous aerosol particles are important for climate, visibility, and health. They are major contributors to light absorption and thus radiative heating of the atmosphere. Many papers discuss the various types of such particles, but the terminology is confusing. Here we propose that a clearer definition of carbonaceous particle types and improved terminology can be established based on the results of transmission electron microscopy (TEM) analyses of individual aerosol particles. We previously identified spherical, amorphous, carbonaceous particles in the smoke of biofuel and biomass fires and termed them tar balls (Pósfai et al., 04). The identification and characterization of this widespread particle type led to subsequent studies that determined their compositions (Adachi and Buseck, 11; Tivanski et al., 07), hygroscopic properties (Semeniuk et al., 07), and complex refractive index 24822
2 (Alexander et al., 08; Chakrabarty et al., ), illustrating the utility of TEM-based categorization of particle types. In the present study, we focus on the major forms of light-absorbing carbonaceous aerosol particles, which are commonly called soot and black carbon (BC). They have uneven usage and different meanings (Andreae and Gelencsér, 06; Bond and Bergstrom, 06). IPCC (07), the definitive work on climate change, uses the definitions of soot and BC of Charlson and Heintzenberg (199), which are, respectively, Particles formed during the quenching of gases at the outer edge of flames of organic vapours, consisting predominantly of carbon, with lesser amounts of oxygen and hydrogen present as carboxyl and phenolic groups and exhibiting an imperfect graphitic structure and Operationally defined aerosol species based on measurement of light absorption and chemical reactivity and/or thermal stability; consists of soot, charcoal and/or possible light absorbing refractory organic matter. These definitions are based largely on origin and measurement type, respectively, rather than on material properties. More specifically, the definition of BC is unacceptably vague as a material description. In the section Changes in Atmospheric Constituents and Radiative Forcing, IPCC (07) uses BC instead of soot, e.g., Electron microscope images of BC particles show that they are emitted as complex chain structures (e.g., Pósfai et al., 03)..., even though the paper by Pósfai et al. (03) is mainly about soot and only mentions BC parenthetically and in passing. Thus, although soot and BC are used interchangeably by IPCC and others, we question the validity of that usage and conclude there is neither a clear consensus nor justification for they way they are used in much of the current literature. BC in the atmospheric sciences is the term used for materials that are strongly light absorbing, commonly determined by the attenuation of light by particles typically collected onto a filter and measured by an aethalometer (Hansen et al., 1984), although it is also measured using other methods (Table 1). A widely used approach for measuring BC is, contrary to its name, the Single Particle Soot Photometer (SP2) (Schwarz et al., 08). There are references in the SP2 literature to refractory BC or rbc, but even though the latter excludes non-refractory phases it is still not a clearly identifiable material. In the absence of an accepted BC standard, surrogate soot-like materials such as fullerene, graphite, and Aquadag are used as standards (e.g., Kondo et al., 11; Gysel et al., 11; Laborde et al., 12; Baumgardner et al., 12). Although BC brings to mind a material and is commonly used in the aerosol literature as if it were a specific material, laboratory samples of atmospheric BC that can be measured do not exist. Soot, on the other hand, is the solid, carbon-rich material that condenses from the vapor phase during combustion (Fig. 1) (Ishiguro et al., 1997; Richter and Howard, 00). Much aerosol usage has this as equivalent to BC (Jacobson, 01; Penner and Novakov, 1996), with the implicit assumption that the sole source of the optical absorption is soot, although a cause of confusion is that one term applies to origin and the other to optical measurements. No consensus exists about whether soot is a part of BC, BC is a part of soot, or BC is equivalent to soot. The definition used by IPCC indicates that soot is a part of BC. The usage of soot in electron microscopy studies commonly agrees with that of the IPCC (Adachi et al., ). In contrast, in other papers (e.g., Bond and Bergstrom, 06; Seinfeld and Pandis, 06; Salamanca et al., 12), soot consists of BC and other combustion-generated carbonaceous materials, and Ramanathan and Carmichael (08) wrote that BC refers to the absorbing components of soot, often defined using elemental carbon and some condensed organics. Elemental carbon (EC) is another term used in the aerosol community. It is essentially a high-t residue of thermal-optical measurements. EC is commonly measured together with organic carbon (OC), with the latter being the low-t fraction (Table 1). An additional complication is that these terms are used differently in various scientific disciplines. BC is widely used in the environmental communities dealing with sediments and soils for carbonaceous substances of pyrogenic origin that are resistant to thermal or chemical degradation. Such BC includes char, charcoal, and soot (International Steering Committee for Black Carbon Reference Materials; Preston and Schmidt, 06). This BC definition is unrelated to instrumental measurements of optical 24824
3 properties, i.e., the BC of atmospheric scientists and the BC of sedimentologists and soil scientists are not the same (Masiello, 04). The combustion community mainly uses soot instead of BC to describe carbonaceous combustion products that condense from aromatic hydrocarbons and have a characteristic microstructure and morphology (Maricq, 07; Ishiguro et al., 1997; Lapuerta et al., 12). Watson and Valberg (01) report that several morphologically distinct types of particulate carbon occur in soot, and Palotás et al. (1996) refer to different types of soots. Bond and Bergstrom (06) discussed the terminological issue and proposed grouping carbonaceous materials that strongly absorb light in the visible range as lightabsorbing carbon, LAC for short. LAC was also used by Andreae and Gelencsér (06) but with a different meaning, since their LAC includes all absorbing carbonaceous particles, even weakly absorbing brown carbon (BrC). We favor the use of LAC as intentionally being purely operational and thus neutral to both material type and origin. However, a material descriptor would be highly useful for atmospheric science in general, as well as uses such as source apportionment, assessment of particle health effects, and modeling radiative effects. Using transmission electron microscopy (TEM), we morphologically and structurally define nanosphere-soot (ns-soot), a major carbonaceous aerosol type. 2 Proposed ns-soot terminology As pointed out above, the term soot is used for both combustion products as a group and for a material with specific, defined properties. TEM has been widely used for studying this material. Although we have referred to soot extensively in our papers (Buseck and Pósfai, 1999; Pósfai et al., 1999, 03, 04; Li et al., 03; Buseck and Schwartz, 03; van Poppel et al., 0; Adachi et al., 07, ; Adachi and Buseck, 08; Pósfai and Buseck, ; Li et al., ), we believe it is appropriate to draw a clear distinction based on physical features rather than origin or optical properties for at least this one important type of aerosol particle. 248 We propose the term ns-soot for particles with grape-like (acinoform) morphologies that consist of nanospheres that possess distinct internal structures of concentrically wrapped, graphene-like layers of carbon. The nanosphere diameters are typically between and 0 nm (e.g., 44 nm for ambient ns-soot particles from Mexico City; Adachi and Buseck, 08). Regardless of their source, from fewer than to roughly 0 nanospheres typically occur in ns-soot particles in the atmosphere. Terms such as primary particles or monomers have been used for carbon nanospheres (e.g., Vander Wal et al., 1999; Liu et al., 08), but these terms depend on the aggregate form of the particles and do not indicate their chemical and structural features. Using the term carbon nanosphere, we can discuss the fundamental optical, chemical, and thermal properties of atmospheric ns-soot regardless of its aggregated shapes and coatings. Once such properties are known, the influence of aggregate morphology and internal mixing on optical and hygroscopic properties should be taken into account in order to model the atmospheric effects of ns-soot. High-resolution TEM images of carbon nanospheres show the poorly ordered, concentrically wrapped, wavy or curved graphene-like layers that resemble onion-like structures (Fig. 1c). The irregular stacking of these layers has been called turbostratic (Palotás et al., 1996), consistent with such nonuniform stacking in sheet silicates. Such carbon structures, and their electron energy-loss spectrometry (EELS) signals (Fig. 2a), indicate that carbon nanospheres consist largely of layered, π-bonded carbon that absorb visible light. The lack of distinct reflections in selected-area electron diffraction (SAED) patterns indicates the poorly ordered character of the carbon nanospheres. However, three blurred rings typically occur in SAED patterns as a result of short-range order within and between the graphene-like layers (Fig. 2b). The 0.36-nm lattice spacing that corresponds to the first ring can be matched to but is larger than the (002) crystal spacing in graphite, the distance between two neighboring graphene layers. First-neighbor atomic distances calculated from SAED patterns of carbon nanospheres indicate that 24826
4 the carbon atoms within the graphene-like layers are closer to one another than in graphite (Kis et al., 06). The basic structural features of carbon nanospheres appear to be independent of their sizes and are not affected by aggregation or mixing with other atmospheric constituents. Although both the interpretation of fine structure in EELS spectra and the quantitative evaluation of electron-diffraction patterns are perhaps beyond the scope of standard TEM users, SAED patterns and HRTEM images (e.g., Figs. 2b and 1, respectively) can be obtained routinely and provide enough information for the unambiguous identification of ns-soot. Tar balls, which are also spherical, lack distinctive diffraction features and the wavy, graphene-like layers that are characteristic of ns-soot. Thus, the two can be readily distinguished. 3 Associated materials and coatings The term soot has been used inconsistently in the atmospheric and combustion literature in the sense that in some instances it includes the embedding material (Salamanca et al., 12) and in other instances it does not (Virtanen et al., 04). Many ns-soot particles are coated with or embedded within other materials (e.g., organic matter, sulfate), forming internally mixed particles (Adachi et al., ) (Fig. 3). Consistent with our definition of ns-soot above, we propose that only the backbone of internally mixed particles, i.e., the aggregates of carbon nanospheres, should be termed ns-soot. We find this restrictive definition practical, since it does not depend on whether the mixing of ns-soot with organics, sulfate, or other materials occurred at the time of particle formation or during the aging of ns-soot in the atmosphere. Much ns-soot is either coated or embedded within other materials. The distinctions are somewhat arbitrary, but we propose that coated ns-soot has a thin (< 1 to nm) outer layer of a different material, whereas embedded ns-soot is thickly coated (Figs. 4, ). Outlines of embedded ns-soot tend to be smooth and round, whereas those of coated ns-soot tend to be irregular and similar to bare ns-soot TEM studies are typically performed under high-vacuum conditions that may result in the loss of volatile components. Thus, one could argue that ns-soot may be a definition for particles that do not even exist. However, atomic force microscopy (AFM) images obtained from vehicle emissions under ambient conditions clearly show that externally mixed, pure ns-soot particles do occur in the atmosphere, and both uncoated and coated ns-soot particles are emitted, depending on the combustion conditions (Fig. 6). 4 ns-soot vs. BC Although not practical for monitoring purposes, TEM can be used for rapid spot checks of morphologically distinct carbonaceous particles, as is done with asbestos measurements. We suggest that if one assumes that BC even exists as a distinct material, which we question (Buseck et al., 12), one should only speak of a BC particle if its material properties have been determined and it has been measured optically, and that combination has not yet been done. We further suggest that it is not acceptable to speak of BC particles unless the light absorption of the specific particles is known. In most instances, BC is applicable to an ensemble of carbonaceous materials that strongly absorb visible light (Fig. 4c). BC can contain ns-soot, commonly as a major constituent, but it can also include other light-absorbing carbonaceous materials. In contrast, all ns-soot is included within the BC (or rbc) category. Issues related to ns-soot Chemical analyses of combustion products are typically of bulk material, whether collected from flames or vehicle tailpipes, rather than the pure ns-soot considered here. Such analyses create a potential source of confusion. Although the dominant component of such bulk material is carbon, it also contains variable amounts of hydrogen, oxygen, and perhaps other elements. It is clear that ns-soot is a major component of 24828
5 this material, but it is less clear whether the elements other than carbon occur (a) within the structure of the ns-soot, (b) as coatings on its surfaces, or (c) in interstitial areas. There seems to be a broad consensus in the literature that hydrogen is a component of carbon nanospheres, and both spectroscopic methods and electron diffraction indicate the presence of aromatics (Salamanca et al., 12; Kis et al., 06). However, it is unclear how much of the hydrogen, and perhaps small amounts of other non-carbon elements, are essential parts of the nanospheres or simply adventitious. A related unresolved question is the origin of the curvature of the nanospheres. We assume that the fringes that are evident in high-resolution TEM images (Figs. 1c, b, 7a) are edge-on views of graphene-like layers containing mostly hexagonal rings of C. Pentagonal rings cause the curvature of fullerenes and similar materials and presumably occur in the carbon nanospheres in ns-soot. Chemical inhomogeneities can also contribute to the curvature. Hydrogen-bearing aromatics can disrupt the periodicity of graphene, resulting in layers that consist of non-periodically attached, misaligned islands of a few hexagonal rings of carbon (Fig. 7b). In the absence of a perfectly periodic crystalline structure, there is no constraint on the morphology, so that its layers can readily assume a spherical shape, resulting in the characteristic structure of carbon nanospheres. The refractive index of ns-soot is not well known. Reasons for the difficulty of measurements are (1) ns-soot commonly coexists with other materials and (2) aggregates of carbon nanospheres contain many voids and interstices (van Poppel et al., 0; Adachi et al., 07). Bond and Bergstrom (06) reviewed the issues related to the optical properties of strongly absorbing carbon (LAC) and showed a range of its refractive index. We believe that ns-soot is the main component of LAC, and therefore as a first approximation the refractive index determined for LAC can be used for ns-soot. The detailed morphology of aggregated carbon nanospheres, i.e., ns-soot, varies from source to source and changes upon aging during its transport (e.g., Abel et al., 03). Such aggregated shapes, which can be characterized using fractal dimensions, largely influence the radiative scattering of ns-soot in the atmosphere (Adachi et al., 07; Mackowski, 199). 6 Recommendations We propose that strongly absorbing carbonaceous species in atmospheric aerosols should be considered, and treated in models, as follows: 6.1 ns-soot We define ns-soot as particles that consist of nanospheres that possess distinct internal structures of concentrically wrapped, graphene-like layers of carbon and with grape-like (acinoform) morphologies. Until there are better data, we recommend using the current best estimates for the refractive index and mass absorption cross section of LAC, i.e., i and 7. ± 1.2 m 2 g 1 at 0 nm, respectively (Bond and Bergstrom, 06), for externally mixed ns-soot particles. The scattering cross sections of ns-soot depend strongly on morphology, type and extent of internal mixing, or embedding, all of which can be determined using TEM, with the limitation that volatile components remain undetected. Visual scanning is rapid and can be used to select a relatively smaller number that appear to be representative. We propose that for climate modeling and health issues distinctions be made between bare, coated, and embedded ns-soot. Each has distinct optical and chemical properties. Volume mixing of soot with other materials should be discarded from models, in agreement with suggestions of others (e.g., Jacobson, 01; Bond and Bergstrom, 06; Bond et al., 06). Ns-soot is never mixed with sulfate or organics as a homogeneous particle (Adachi and Buseck, ; Pósfai and Buseck, )
6 6.2 Black carbon (BC) Although a practical term for inferring atmospheric light absorption in atmospheric measurement and modeling, BC is not a well-defined material. The term should be restricted to light-absorbing refractory carbonaceous matter of uncertain character and should be used with a definition to explain what is meant, e.g., the total absorption resulting from ns-soot + organic carbon + other absorbing particle types. We recommend the use of BC equiv (equivalent black carbon) to indicate atmospheric light absorption by assuming BC consists of 0-nm monodisperse spheres with a refractive index of i. This convention would define optical properties for model calculations and clearly imply that BC equiv is a virtual rather than actual reference material. For SP2 measurements, the assumption that rbc = ns-soot should be made explicit, if that is indeed the assumption. 6.3 Elemental carbon (EC) The term elemental carbon should be restricted to describing the high-t component of thermal-optical analysis. Because EC does not exist in the atmosphere as such, it should never be used as a synonym of either ns-soot or BC. 6.4 Final thoughts To avoid ambiguity, we recommend using the term ns-soot if it is either known or there is reason to believe that the material consists of aggregated carbon nanospheres. BC should be restricted to mixtures of ns-soot plus additional material. Acknowledgement. The authors acknowledge the use of TEM facilities within the LeRoy Eyring Center for Solid State Science at Arizona State University. We are grateful to Andrew Metcalf and Rick Flagan for their helpful comments. K. Adachi acknowledges support from the global environment research fund of the Japanese Ministry of the Environment (A-11). M. Pósfai acknowledges support from an EU-Hungarian joint grant (TÁMOP 4.2.1/B-09/1/KONV ). This study was supported by NSF grants ATM and AGS References Abel, S. J., Haywood, J. M., Highwood, E. J., Li, J., and Buseck, P. R.: Evolution of biomass burning aerosol properties from an agricultural fire in Southern Africa, Geophys. Res. Lett., 30, 1783, doi:.29/03gl017342, 03. Adachi, K. and Buseck, P. R.: Internally mixed soot, sulfates, and organic matter in aerosol particles from Mexico City, Atmos. Chem. Phys., 8, , doi:.194/acp , 08. Adachi, K. and Buseck, P. R.: Hosted and free-floating metal-bearing atmospheric nanoparticles in Mexico City, Environ. Sci. Technol., 44, ,. Adachi, K. and Buseck, P. R.: Atmospheric tar balls from biomass burning in Mexico, J. Geophys. Res.-Atmos., 116, D04, doi:.29/jd02, 11. Adachi, K., Chung, S. H., Friedrich, H., and Buseck, P. R.: Fractal parameters of individual soot particles determined using electron tomography: implications for optical properties, J. Geophys. Res.-Atmos., 112, D142, doi:.29/06jd008296, 07. Adachi, K., Chung, S. H., and Buseck, P. R.: Shapes of soot aerosol particles and implications for their effects on climate, J. Geophys. Res.-Atmos., 1, D6, doi:.29/09jd012868,. Alexander, D. T. L., Crozier, P. A., and Anderson, J. R.: Brown carbon spheres in East Asian outflow and their direct radiative forcing properties, Science, 321, , 08. Andreae, M. O. and Gelencsér, A.: Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols, Atmos. Chem. Phys., 6, , doi:.194/acp , 06. Apicella, B., Alfe, M., Barbella, R., Tregrossi, A., and Ciajolo, A.: Aromatic structures of carbonaceous materials and soot inferred by spectroscopic analysis, Carbon, 42, 83 89, 04. Arnott, W. P., Hamasha, K., Moosmüller, H., Sheridan, P. J., and Ogren, J. A.: Towards aerosol light-absorption measurements with a 7-wavelength aethalometer: evaluation with a photoacoustic instrument and 3-wavelength nephelometer, Aerosol Sci. Tech., 39, 17 29, 0. Baumgardner, D., Popovicheva, O., Allan, J., Bernardoni, V., Cao, J., Cavalli, F., Cozic, J., Diapouli, E., Eleftheriadis, K., Genberg, P. J., Gonzalez, C., Gysel, M., John, A., Kirchstet- ter, T. W., Kuhlbusch, T. A. J., Laborde, M., Lack, D., Müller, T., Niessner, R., Petzold, A., Piazzalunga, A., Putaud, J. P., Schwarz, J., Sheridan, P., Subramanian, R., Swietlicki, E., Valli, G., 24832
7 30 Vecchi, R., and Viana, M.: Soot reference materials for instrument calibration and intercomparisons: a workshop summary with recommendations, Atmos. Meas. Tech.,, , doi:.194/amt , 12. Bond, T. C. and Bergstrom, R. W.: Light absorption by carbonaceous particles: an investigative review, Aerosol Sci. Tech., 40, 27 67, 06. Bond, T. C., Habib, G., and Bergstrom, R. W.: Limitations in the enhancement of visible light absorption due to mixing state, J. Geophys. Res.-Atmos., 111, D211, doi:.29/06jd0073, 06. Buseck, P. R. and Pósfai, M.: Airborne minerals and related aerosol particles: effects on climate and the environment, P. Natl. Acad. Sci. USA, 96, , doi:.73/pnas , Buseck, P. R. and Schwartz, S. E.: Tropospheric aerosols, in: Treatise on Geochemistry, Vol 4, edited by: Turekian, K. K. and Holland, H. D., Elsevier Science Ltd., San Diego, CA, , 03. Buseck, P. R., Adachi, K., and Pósfai, M.: Black carbon, aerosols, and the tooth fairy, AGU Fall Meeting, San Francisco, CA, 3 7 December 12, Abstract, submitted, 12. Cachier, H., Bremond, M. P., and Buat-Menard, P.: Determination of atmospheric soot carbon with a simple thermal method, Tellus B, 41B, , Chakrabarty, R. K., Moosmüller, H., Chen, L.-W. A., Lewis, K., Arnott, W. P., Mazzoleni, C., Dubey, M. K., Wold, C. E., Hao, W. M., and Kreidenweis, S. M.: Brown carbon in tar balls from smoldering biomass combustion, Atmos. Chem. Phys.,, , doi:.194/acp ,. Charlson, R. J. and Heintzenberg, J. (Eds.): Aerosol Forcing of Climate, John Wiley & Sons, New York, NY, 91 8, 199. Chow, J. C., Watson, J. G., Pritchett, L. C., Pierson, W. R., Frazier, C. A., and Purcell, R. G.: The DRI thermal-optical reflectance carbon analysis system description, evaluation and applications in United States air quality studies, Atmos. Environ., 27A, , Gysel, M., Laborde, M., Olfert, J. S., Subramanian, R., and Gröhn, A. J.: Effective density of Aquadag and fullerene soot black carbon reference materials used for SP2 calibration, Atmos. Meas. Tech., 4, , doi:.194/amt , 11. Hansen, A. D. A., Rosen, H., and Novakov, T.: The aethalometer an instrument for the realtime measurement of optical absorption by aerosol particles, Sci. Total Environ., 36, , Horvath, H.: Experimental calibration for aerosol light absorption measurements using the integrating plate method summary of the data, J. Aerosol Sci., 28, , Intergovernmental Panel on Climate Change (IPCC): Climate Change 07: The Physical Science Basis, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K. B., Tignor, M., and Miller, H. L., Cambridge University Press, Cambridge, UK, 996 pp., 07. Ishiguro, T., Takatori, Y., and Akihama, K.: Microstructure of diesel soot particles probed by electron microscopy: first observation of inner core and outer shell, Combust. Flame, 8, , Jacobson, M. Z.: Strong radiative heating due to the mixing state of black carbon in atmospheric aerosols, Nature, 409, , 01. Kis, V. K., Pósfai, M., and Lábár, J. L.: Nanostructure of atmospheric soot particles, Atmos. Environ., 40, 33 42, 06. Kondo, Y., Sahu, L., Moteki, N., Khan, F., Takegawa, N., Liu, X., Koike, M., and Miyakawa, T.: Consistency and traceability of black carbon measurements made by laser-induced incandescence, thermal-optical transmittance, and filter-based photo-absorption techniques, Aerosol Sci. Tech., 4, , 11. Laborde, M., Mertes, P., Zieger, P., Dommen, J., Baltensperger, U., and Gysel, M.: Sensitivity of the Single Particle Soot Photometer to different black carbon types, Atmos. Meas. Tech.,, 31 43, doi:.194/amt , 12. Lapuerta, M., Oliva, F., Agudelo, J. R., and Boehman, A. L.: Effect of fuel on the soot nanostructure and consequences on loading and regeneration of diesel particulate filters, Combust. Flame, 9, , 12. Li, J., Pósfai, M., Hobbs, P. V., and Buseck, P. R.: Individual aerosol particles from biomass burning in Southern Africa: 2. Compositions and aging of inorganic particles, J. Geophys. Res.-Atmos., 8, 8484, doi:.29/02jd0023, 03. Li, W. J., Shao, L. Y., and Buseck, P. R.: Haze types in Beijing and the influence of agricultural biomass burning, Atmos. Chem. Phys.,, , doi:.194/acp ,. Liu, L., Mishchenko, M. I., and Arnott, W. P.: A study of radiative properties of fractal soot aggregates using the superposition T-matrix method, J. Quant. Spectrosc. Ra., 9, ,
8 30 Mackowski, D. W.: Electrostatics analysis of radiative absorption by sphere clusters in the Rayleigh limit: application to soot particles, Appl. Optics, 34, 33 34, 199. Maricq, M. M.: Chemical characterization of particulate emissions from diesel engines: a review, J. Aerosol Sci., 38, , 07. Masiello, C. A.: New directions in black carbon organic geochemistry, Mar. Chem., 92, 1 213, 04. Mertes, S., Dippel, B., and Schwarzenböck, A.: Quantification of graphitic carbon in atmospheric aerosol particles by Raman spectroscopy and first application for the determination of mass absorption efficiencies, J. Aerosol Sci., 3, , 04. Moosmüller, H., Arnott, W. P., and Rogers, C. F.: Methods for real-time, in situ measurement of aerosol light absorption, J. Air Waste Manage. Assoc., 47, 7 166, Murphy, D. M., Cziczo, D. J., Froyd, K. D., Hudson, P. K., Matthew, B. M., Middlebrook, A. M., Peltier, R. E., Sullivan, A., Thomson, D. S., and Weber, R. J.: Single-particle mass spectrometry of tropospheric aerosol particles. J. Geophys. Res.-Atmos., 111, D23S32, doi:.29/06jd007340, 06. Novakov, T. and Corrigan, C. E.: Thermal characterization of biomass smoke particles, Microchim. Acta, 119, 7 166, 199. Palotás, Á. B., Rainey, L. C., Feldermann, C. J., Sarofim, A. F., and Vander Sande, J. B.: Soot morphology: an application of image analysis in high-resolution transmission electron microscopy, Microsc. Res. Techniq., 33, , Penner, J. E. and Novakov, T.: Carbonaceous particles in the atmosphere: a historical perspective to the Fifth International Conference on Carbonaceous Particles in the Atmosphere, J. Geophys. Res.-Atmos., 1, , Petzold, A. and Schönlinner, M.: Multi-angle absorption photometry a new method for the measurement of aerosol light absorption and atmospheric black carbon, J. Aerosol Sci., 3, , 04. Pósfai, M. and Buseck, P. R.: Nature and climate effects of individual tropospheric aerosol particles, Annu. Rev. Earth Pl. Sc., 38, 17 43, doi:.1146/annurev.earth ,. Pósfai, M., Anderson, J. R., Buseck, P. R., and Sievering, H.: Soot and sulfate aerosol particles in the remote marine troposphere, J. Geophys. Res.-Atmos., 4, , Pósfai, M., Simonics, R., Li, J., Hobbs, P. V., and Buseck, P. R.: Individual aerosol particles from biomass burning in southern Africa: 1. Compositions and size distributions of carbonaceous particles, J. Geophys. Res.-Atmos., 8, 8483, doi:.29/02jd002291, 03. Pósfai, M., Gelencsér, A., Simonics, R., Arató, K., Li, J., Hobbs, P. V., and Buseck, P. R.: Atmospheric tar balls: particles from biomass and biofuel burning, J. Geophys. Res.-Atmos., 9, D06213, doi:.29/03jd004169, 04. Preston, C. M. and Schmidt, M. W. I.: Black (pyrogenic) carbon: a synthesis of current knowledge and uncertainties with special consideration of boreal regions, Biogeosciences, 3, 397 4, doi:.194/bg , 06. Ramanathan, V. and Carmichael, G.: Global and regional climate changes due to black carbon, Nat. Geosci., 1, , doi:.38/ngeo6, 08. Richter, H. and Howard, J. B.: Formation of polycyclic aromatic hydrocarbons and their growth to soot a review of chemical reaction pathways, Prog. Energ. Combust., 26, 6 608, 00. Salamanca, M., Mondragón, F., Agudelo, J. R., Benjumea, P., and Santamaría, A.: Variations in the chemical composition and morphology of soot induced by the unsaturation degree of biodiesel and a biodiesel blend, Combust. Flame, 9, 10 18, 12. Schnaiter, M., Linke, C., Möhler, O., Naumann, K.-H., Saathoff, H., Wagner, R., Schurath, U., and Wehner, B.: Absorption amplification of black carbon internally mixed with secondary organic aerosol, J. Geophys. Res., 1, 1 11, 0. Schwarz, J. P., Gao, R. S., Spackman, J. R., Watts, L. A., Thomson, D. S., Fahey, D. W., Ryerson, T. B., Peischl, J., Holloway, J. S., Trainer, M., Frost, G. J., Baynard, T., Lack, D. A., de Gouw, J. A., Warneke, C., and Del Negro, L. A.: Measurement of the mixing state, mass, and optical size of individual black carbon particles in urban and biomass burning emissions, Geophys. Res. Lett., 3, L138, doi:.29/08gl033968, 08. Seinfeld, J. H. and Pandis, S. N.: Atmospheric Chemistry and Physics From Air Pollution to Climate Change, 2nd ed., John Wiley & Sons, New York, NY, 06. Semeniuk, T. A., Wise, M. E., Martin, S. T., Russell, L. M., and Buseck, P. R.: Hygroscopic behavior of aerosol particles from biomass fires using environmental transmission electron microscopy, J. Atmos. Chem., 6, 9 273, doi:.07/s , 07. Smith, D. M. and Chugtai, A. R.: The surface structure and reactivity of black carbon, Colloid Surface A,, 47 77,
9 Sullivan, R. C. and Prather, K. A.: Recent advances in our understanding of atmospheric chemistry and climate made possible by on-line aerosol analysis instrumentation, Anal. Chem., 77, , 0. Tivanski, A. V., Hopkins, R. J., Tyliszczak, T., and Gilles, M. K.: Oxygenated interface on biomass burn tar balls determined by single particle scanning transmission X-ray microscopy, J. Phys. Chem. A, 111, , 07. van Poppel, L. H., Friedrich, H., Spinsby, J., Chung, S. H., Seinfeld, J. H., and Buseck, P. R.: Electron tomography of nanoparticle clusters: implications for atmospheric lifetimes and radiative forcing of soot, Geophys. Res. Lett., 32, L24811, doi:.29/0gl024461, 0. Vander Wal, R. L, Ticich, T. M., and Stephens, A. B.: Can soot primary particle size be determined using laser-induced incandescence?, Combust. Flame, 116, , Virtanen A. K. K., Ristimäki, J. M., Vaaraslahti, K. M., and Keskinen, J.: Effect of engine load on diesel soot particles, Environ. Sci. Technol., 38, 1 6, 04. Watson, A. Y. and Valberg, P. A.: Carbon black and soot: two different substances, Am. Ind. Hyg. Assoc. J., 62, , 01. Wexler, A. S. and Johnston, M. V.: Real-time particle analysis by mass spectrometry, in: Aerosol Measurement: Principles, Techniques, and Applications, edited by: Kulkarni, P., Baron, P. A., and Willeke, K., John Wiley & Sons, New York, 233 4, Table 1. Methods for measuring the concentrations or properties of ns-soot and BC. Method (or instrument) Acronym Operating principle State of measured aerosol Microscopy Absorption measurements Transmission electron microscopy (coupled with electron diffraction, energy-dispersive X-ray spectroscopy, electron energy-loss spectroscopy and electron tomography) TEM (ED, EDS, EELS, ET) Interaction of electron beam with particle (signals for imaging, diffraction, and elemental composition are measured) Atomic force microscopy AFM Interaction between sample surface and a sharp tip mounted on a cantilever while scanning the surface Scanning transmission X-ray microscopy (coupled with near-edge X-ray absorption finestructure spectroscopy) Integrating plate (sphere, sandwich) Aethalometer Particle soot absorption photometer Multi-angle absorption photometer Photo-acoustic spectrometer Extinction cell + nephelometer ( difference method ) Single particle soot photometer STXM (NEXAFS) PSAP MAAP PAS Interaction of X-rays with particles single particle a Single particle b Single particle b What it purportedly measures Soot + OC (particle size, shape, crystal structure, elemental composition) Particle size, 3-D shape Composition and bonding character of C Problems, artifacts Labor-intensive, limited statistics, highly volatile species are lost Labor-intensive, no chemical information, ambiguous interpretation, scanning artifacts Labor-intensive, synchrotron radiation needed, no quantitative information Reference Pósfai and Buseck () Pósfai and Buseck () Tivanski et al. (07) Optical attenuation Bulk b BC Horvath (1997) Mass effect (multiple scattering), absorption enhancement by non-absorbing coat- Optical attenuation, measured Bulk b BC Hansen et al. continuously at several ings, uncertainty in parameters used for conversion be- (1984) wavelengths Optical attenuation, measured Bulk b BC tween absorption and BC Arnott et al. continuously at several wavelengths concentration, presence of (0) other absorbing particles (in- Optical attenuation, measured Bulk b BC cluding BrC), wavelength dependence of absorption Petzold and continuously at multiple angles Schönlinner (04) Energy from absorbed light converted to pressure Difference between extinction and scattering Bulk c BC Same as above except mass effect Bulk c BC Uses a small difference between two large numbers, needs large sample volumes, includes other absorbing species; affected by internal mixing SP2 Laser-induced incandescence Single rbc Uncertainty about nature of particle c coatings Moosmüller et al. (1997) Schnaiter et al. (0) Schwarz et al. (08)
10 Table 1. Continued. Method (or instrument) Acronym Operating principle State of measured aerosol Thermal analysis Single particle mass spectrometry Optical spectroscopy Evolved gas analysis EGA Conversion (volatilization) of C into CO 2 over a temperature range Chemical extraction + EGA Thermal-optical transmittance or reflectance analysis Aerosol mass spectrometry, particle analysis by laser mass spectrometry, aerosol time-of-flight mass spectrometry TOT, TOR AMS, PALMS, ATOFMS Extraction with water or organic solvent before volatilization to remove OC Transmittance or reflectance of filter monitored during heating and carbon volatilization Laser- or electron-impactinduced ionization of particles; fragments measured with a time-of-flight mass spectrometer Infrared spectroscopy IR Interaction of IR light with particles Raman spectroscopy RS Interaction of IR light with particles UV-visible spectroscopy UV-VIS Interaction of light with particles What it purportedly measures Problems, artifacts Bulk b TC OC = EC Interference with BrC and bioaerosol, interpretation of volatilization temperature ranges ambiguous, charring, filter effects Bulk b EC Same problems as above but somewhat reduced Bulk b EC Uncertainty about the cutpoint between OC and EC, charring within filter Single particle c Bulk b Bulk b EC + OC compounds Composition and bonding character of C Composition and bonding character of C a On substrate, in vacuum. b On substrate. c Airborne. d In suspension or solution. Bulk d Ionization efficiencies and matrix effects affect quantitative results, fragment recombination is needed to infer particle types Bulk method with relatively poor spatial resolution Reference Cachier et al. (1989) Novakov and Corrigan (199) Chow et al. (1993) Murphy et al. (06); Sullivan and Prather (0); Wexler and Johnston (11) Smith and Chugtai (199) Mertes et al. (04) Apicella et al. (04) Fig. 1. TEM and schematic images of soot and carbon nanospheres. (a) An ns-soot particle from NIST Standard Reference Material (SRM) 160b (diesel particulate matter). (b) Enlarged image of (a). (c) High-resolution image of ns-soot particle from (b). The lattice fringes of the ns-soot are evident. (d) Schematic image of carbonaceous nanospheres aggregated to form ns-soot
11 Fig. 2. (a) EELS of ns-soot from Mexico City showing peaks arising from carbon π- and σ- bonding. (b) Typical SAED pattern obtained from ns-soot from the emission of a diesel engine. The numbered rings correspond to lattice spacings of (1) 3.6, (2) 2.0, and (3) 1.2 Å Fig. 3. TEM images of internally mixed ns-soot particles from Mexico showing (a), (b) coating, and (c) embedding, although (b) shows that the distinction is gradational. The ribbon-like features are from the lacey carbon substrate. Samples (a) and (b) were collected from Mexico City and (c) was collected from biomass burning in Mexico. All samples were collected during the MILAGRO campaign (Adachi and Buseck, 08)
12 Fig. 4. Schematic images of (a) ns-soot, (b) internally mixed ns-soot particle, and (c) an ensemble of particles, the total absorption of which would be referred to as from BC when measured with an aethalometer. The aggregated, black layered spherules indicate ns-soot. The green and blue colors indicate organic matter and sulfate, respectively Fig.. High-resolution TEM images of (a) bare and (b) coated ns-soot particles from diesel emissions from buses. The arrows in (b) indicate an amorphous coating on carbon nanospheres
13 Fig. 6. Atomic force microscope images of (a) bare, (b) partly coated, and (c) embedded soot particles, all collected from the emissions of vehicles with internal combustion engines Fig. 7. (a) Part of Fig. b, with the contrast of graphene-like layers in ns-soot enhanced digitally. (b) A schematic model for three layers. They are graphene-like, but some C atoms are missing from the hexagonal nets. The missing atoms break the periodic order and possibly cause the misalignment of layer segments relative to one another, resulting in the layer curvature. H in ns-soot can be accommodated around the sites of missing C atoms, where some C atoms can be bonded to two C and one H
Cloud scavenging of anthropogenic refractory particles at a mountain site in North China Reply to Referee#1:
 Cloud scavenging of anthropogenic refractory particles at a mountain site in North China Lei Liu et al., We are grateful for referee#1 s comments that are helpful for improving the quality of our paper.
Cloud scavenging of anthropogenic refractory particles at a mountain site in North China Lei Liu et al., We are grateful for referee#1 s comments that are helpful for improving the quality of our paper.
Changes of ns-soot mixing states and shapes in an urban area during CalNex
 JOURNAL OF GEOPHYSICAL RESEARCH: ATMOSPHERES, VOL. 118, 3723 3730, doi:10.1002/jgrd.50321, 2013 Changes of ns-soot mixing states and shapes in an urban area during CalNex Kouji Adachi 1,2,3 and Peter R.
JOURNAL OF GEOPHYSICAL RESEARCH: ATMOSPHERES, VOL. 118, 3723 3730, doi:10.1002/jgrd.50321, 2013 Changes of ns-soot mixing states and shapes in an urban area during CalNex Kouji Adachi 1,2,3 and Peter R.
Shapes of soot aerosol particles and implications for their effects on climate
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2009jd012868, 2010 Shapes of soot aerosol particles and implications for their effects on climate Kouji Adachi, 1,2 Serena H. Chung, 3 and Peter
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2009jd012868, 2010 Shapes of soot aerosol particles and implications for their effects on climate Kouji Adachi, 1,2 Serena H. Chung, 3 and Peter
Supplement of Quantification of black carbon mixing state from traffic: implications for aerosol optical properties
 Supplement of Atmos. Chem. Phys., 16, 4693 4706, 2016 http://www.atmos-chem-phys.net/16/4693/2016/ doi:10.5194/acp-16-4693-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Quantification
Supplement of Atmos. Chem. Phys., 16, 4693 4706, 2016 http://www.atmos-chem-phys.net/16/4693/2016/ doi:10.5194/acp-16-4693-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Quantification
MULTI-WAVELENGTH OPTICAL CALIBRATION OF THERMAL/OPTICAL ANALYZER AND
 MULTI-WAVELENGTH OPTICAL CALIBRATION OF THERMAL/OPTICAL ANALYZER AND POTENTIAL APPLICATIONS John G. Watson, Judith C. Chow, L.-W. Antony Chen, Xiaoliang Wang, Ben Sumlin Division of Atmospheric Sciences,
MULTI-WAVELENGTH OPTICAL CALIBRATION OF THERMAL/OPTICAL ANALYZER AND POTENTIAL APPLICATIONS John G. Watson, Judith C. Chow, L.-W. Antony Chen, Xiaoliang Wang, Ben Sumlin Division of Atmospheric Sciences,
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
BC properties and its measurement techniques
 BC properties and its measurement techniques An overview 1 SAFER, SMARTER, GREENER Navigator 1. Introduction and overview some definitions 2. BC properties and how to measure them a. Light absorption b.
BC properties and its measurement techniques An overview 1 SAFER, SMARTER, GREENER Navigator 1. Introduction and overview some definitions 2. BC properties and how to measure them a. Light absorption b.
Response to Reviewer s comments
 Response to Reviewer s comments (MS Ref. No.: acp-2010-98): Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya by K. Ram
Response to Reviewer s comments (MS Ref. No.: acp-2010-98): Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya by K. Ram
Sensitivity of the Single Particle Soot Photometer to different black carbon types
 Atmos. Meas. Tech., 5, 131 143, 212 www.atmos-meas-tech.net/5/131/212/ doi:1.5194/amt-5-131-212 Author(s) 212. CC Attribution 3. License. Atmospheric Measurement Techniques Sensitivity of the Single Particle
Atmos. Meas. Tech., 5, 131 143, 212 www.atmos-meas-tech.net/5/131/212/ doi:1.5194/amt-5-131-212 Author(s) 212. CC Attribution 3. License. Atmospheric Measurement Techniques Sensitivity of the Single Particle
Constancy of soot refractive index absorption function: Implications for optical measurements of nanoparticles
 1. Introduction Constancy of soot refractive index absorption function: Implications for optical measurements of nanoparticles F. Migliorini*, K. A. Thomson, G. J. Smallwood National Research Council of
1. Introduction Constancy of soot refractive index absorption function: Implications for optical measurements of nanoparticles F. Migliorini*, K. A. Thomson, G. J. Smallwood National Research Council of
Indices of Refraction of Absorptive Aerosol Their Importance and Complexity
 Indices of Refraction of Absorptive Aerosol Their Importance and Complexity Steven T Massie NCAR Earth System Laboratory HITRAN Cambridge, Massachusetts June 16-18, 2010 NCAR is sponsored by the National
Indices of Refraction of Absorptive Aerosol Their Importance and Complexity Steven T Massie NCAR Earth System Laboratory HITRAN Cambridge, Massachusetts June 16-18, 2010 NCAR is sponsored by the National
Carbonaceous contaminants on support films for transmission electron microscopy
 PERGAMON Carbon 39 (2001) 909 913 Carbonaceous contaminants on support films for transmission electron microscopy P.J.F. Harris Department of Chemistry, University of Reading, Whiteknights, Reading RG6
PERGAMON Carbon 39 (2001) 909 913 Carbonaceous contaminants on support films for transmission electron microscopy P.J.F. Harris Department of Chemistry, University of Reading, Whiteknights, Reading RG6
The Absorption Ångström Exponent of black carbon: from numerical aspects
 The Absorption Ångström Exponent of black carbon: from numerical aspects Chao Liu 1,2,*, Chul Eddy Chung 3, Yan Yin 1,2 1 Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters,
The Absorption Ångström Exponent of black carbon: from numerical aspects Chao Liu 1,2,*, Chul Eddy Chung 3, Yan Yin 1,2 1 Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters,
Choosing the proper technique for measuring the particle light absorption
 Sino-German Symposium on Soot and its Climatic, Environmental and Health Impacts Choosing the proper technique for measuring the particle light absorption Development of an absorption reference Beijing,
Sino-German Symposium on Soot and its Climatic, Environmental and Health Impacts Choosing the proper technique for measuring the particle light absorption Development of an absorption reference Beijing,
Comparison of AERONET inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer
 Comparison of inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer Peter DeCarlo Remote Sensing Project April 28, 23 Introduction The comparison of direct in-situ
Comparison of inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer Peter DeCarlo Remote Sensing Project April 28, 23 Introduction The comparison of direct in-situ
ACTRIS Workshop on the Reference method for Multi-Wavelength Absorption
 ACTRIS Workshop on the Reference method for Multi-Wavelength Absorption During the reporting period a laboratory study for testing a reference system for determining the absorption coefficient at a single
ACTRIS Workshop on the Reference method for Multi-Wavelength Absorption During the reporting period a laboratory study for testing a reference system for determining the absorption coefficient at a single
European aerosol phenomenology-5: climatology of black carbon optical properties at 9 regional background sites across Europe
 European aerosol phenomenology-5: climatology of black carbon optical properties at 9 regional background sites across Europe M.Zanatta 1,2, F. Cavalli 3, M. Gysel 2, N. Bukowiecki 2, T. Müller 4, E. Weingartner
European aerosol phenomenology-5: climatology of black carbon optical properties at 9 regional background sites across Europe M.Zanatta 1,2, F. Cavalli 3, M. Gysel 2, N. Bukowiecki 2, T. Müller 4, E. Weingartner
Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol
 Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol P. S. Chhabra 1,N.L.Ng 2, M. R. Canagaratna 2, A. L. Corrigan 3, L. M. Russell 3, D. R. Worsnop 2, R. C. Flagan
Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol P. S. Chhabra 1,N.L.Ng 2, M. R. Canagaratna 2, A. L. Corrigan 3, L. M. Russell 3, D. R. Worsnop 2, R. C. Flagan
PUBLICATIONS. Journal of Geophysical Research: Atmospheres
 PUBLICATIONS Journal of Geophysical Research: Atmospheres RESEARCH ARTICLE Key Points: Occurrences of ns-soot/bc from TEM and SP2 data are generally consistent across the techniques Abundance and mixing
PUBLICATIONS Journal of Geophysical Research: Atmospheres RESEARCH ARTICLE Key Points: Occurrences of ns-soot/bc from TEM and SP2 data are generally consistent across the techniques Abundance and mixing
Melanie S. Hammer 1, Randall V. Martin 1,2, Aaron van Donkelaar 1, Virginie Buchard 3,4, Omar Torres 3, David A. Ridley 5, Robert J.D.
 Interpreting the Ultraviolet Aerosol Index Observed with the OMI Satellite Instrument to Understand Absorption by Organic Aerosols: Implications for Atmospheric Oxidation and Direct Radiative Effects Melanie
Interpreting the Ultraviolet Aerosol Index Observed with the OMI Satellite Instrument to Understand Absorption by Organic Aerosols: Implications for Atmospheric Oxidation and Direct Radiative Effects Melanie
Evolution of mixing state of black carbon in polluted air from Tokyo
 GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L16803, doi:10.1029/2007gl029819, 2007 Evolution of mixing state of black carbon in polluted air from Tokyo M. Shiraiwa, 1 Y. Kondo, 1 N. Moteki, 1 N. Takegawa, 1
GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L16803, doi:10.1029/2007gl029819, 2007 Evolution of mixing state of black carbon in polluted air from Tokyo M. Shiraiwa, 1 Y. Kondo, 1 N. Moteki, 1 N. Takegawa, 1
Supplementary Table 1. Geometry and flow rate of the individual components of the aerosol sampling system used in the A-FORCE 2013W campaign.
 Supplementary Table 1. Geometry and flow rate of the individual components of the aerosol sampling system used in the A-FORCE 2013W campaign. Component Shrouded solid diffuser inlet Inner diameter [mm]
Supplementary Table 1. Geometry and flow rate of the individual components of the aerosol sampling system used in the A-FORCE 2013W campaign. Component Shrouded solid diffuser inlet Inner diameter [mm]
Coatings and their enhancement of black carbon light absorption in the tropical atmosphere
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009042, 2008 Coatings and their enhancement of black carbon light absorption in the tropical atmosphere J. P. Schwarz,
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009042, 2008 Coatings and their enhancement of black carbon light absorption in the tropical atmosphere J. P. Schwarz,
Magee Scientific Aethalometer
 Magee Scientific Aethalometer Optical Transmission Analysis of NIST Reference Material 8785 and Neutral Density Filters www.mageescientific.com Berkeley, Ljubljana, July 2014 www.mageescientific.com Page
Magee Scientific Aethalometer Optical Transmission Analysis of NIST Reference Material 8785 and Neutral Density Filters www.mageescientific.com Berkeley, Ljubljana, July 2014 www.mageescientific.com Page
B C, a byproduct of combustion, has a unique role as a climate forcing agent. It absorbs the most solar light per
 Black carbon aerosol size in snow J. P. Schwarz 1,2, R. S. Gao 1, A. E. Perring 1,2, J. R. Spackman 3,4 & D. W. Fahey 1,2 SUBJECT AREAS: ATMOSPHERIC DYNAMICS CRYOSPHERIC SCIENCE 1 Chemical Sciences Division,
Black carbon aerosol size in snow J. P. Schwarz 1,2, R. S. Gao 1, A. E. Perring 1,2, J. R. Spackman 3,4 & D. W. Fahey 1,2 SUBJECT AREAS: ATMOSPHERIC DYNAMICS CRYOSPHERIC SCIENCE 1 Chemical Sciences Division,
Light Absorbing Carbonaceous Aerosols (BC and BrnC) and their Climate Impacts
 Light Absorbing Carbonaceous Aerosols (BC and BrnC) and their Climate Impacts J I U M E N G L I U E A S 8 8 0 2 : C L O U D S, A E R O S O L S A N D C L I M AT E M A R. 2 5, 2 0 1 3 Terminology Light absorbing
Light Absorbing Carbonaceous Aerosols (BC and BrnC) and their Climate Impacts J I U M E N G L I U E A S 8 8 0 2 : C L O U D S, A E R O S O L S A N D C L I M AT E M A R. 2 5, 2 0 1 3 Terminology Light absorbing
Standardisation of Particulate and Aerosol Measurements. Hanspeter Andres
 Standardisation of Particulate and Aerosol Measurements Hanspeter Andres Agenda 1. Particulates in Aerosols 2. Measures for Particulates in Aerosols 3. Traceability routes 4. International comparability
Standardisation of Particulate and Aerosol Measurements Hanspeter Andres Agenda 1. Particulates in Aerosols 2. Measures for Particulates in Aerosols 3. Traceability routes 4. International comparability
Chapter Eight: Conclusions and Future Work
 2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
Photoacoustic and filter measurements related to aerosol light absorption during. the Northern Front Range Air Quality Study (Colorado 1996/1997)
 1 Photoacoustic and filter measurements related to aerosol light absorption during the Northern Front Range Air Quality Study (Colorado 1996/1997) H. Moosmüller, W. P. Arnott, C. F. Rogers, J. C. Chow,
1 Photoacoustic and filter measurements related to aerosol light absorption during the Northern Front Range Air Quality Study (Colorado 1996/1997) H. Moosmüller, W. P. Arnott, C. F. Rogers, J. C. Chow,
SootParticle-AMS or LaserVaporizer-AMS. Aerodyne Research, Inc. et al.
 SootParticle-AMS or LaserVaporizer-AMS Aerodyne Research, Inc. et al. Outline SP-AMS instrument design Nomenclature Collection Efficiency Refractory carbon ion distributions SP-AMS Instruments in the Field
SootParticle-AMS or LaserVaporizer-AMS Aerodyne Research, Inc. et al. Outline SP-AMS instrument design Nomenclature Collection Efficiency Refractory carbon ion distributions SP-AMS Instruments in the Field
LASER MICROPROBE MASS SPECTROMETRY MICROANALYSIS OF POLYCYCLIC AROMATIC HYDROCARBONS IN FLAMES, IN DIESEL FUELS AND IN DIESEL EMISSIONS
 LASER MICROPROBE MASS SPECTROMETRY MICROANALYSIS OF POLYCYCLIC AROMATIC HYDROCARBONS IN FLAMES, IN DIESEL FUELS AND IN DIESEL EMISSIONS Robert A. Fletcher, 1 Richard A. Dobbins, 2 Bruce A. Benner, Jr.
LASER MICROPROBE MASS SPECTROMETRY MICROANALYSIS OF POLYCYCLIC AROMATIC HYDROCARBONS IN FLAMES, IN DIESEL FUELS AND IN DIESEL EMISSIONS Robert A. Fletcher, 1 Richard A. Dobbins, 2 Bruce A. Benner, Jr.
Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-offlight
 Supporting Information Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-offlight mass spectrometry- Part 1: Single particle atmospheric observations in Atlanta Lindsay
Supporting Information Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-offlight mass spectrometry- Part 1: Single particle atmospheric observations in Atlanta Lindsay
ATOC 3500/CHEM 3152 Week 9, March 8, 2016
 ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
Optical, physical, and chemical characterization of marine Black Carbon
 Optical, physical, and chemical characterization of marine Black Carbon Kevin Thomson Measurement Science for Emerging Technology September 16, 2015 2 nd ICCT BC Workshop, TNO, Utrecht, Netherlands Outline
Optical, physical, and chemical characterization of marine Black Carbon Kevin Thomson Measurement Science for Emerging Technology September 16, 2015 2 nd ICCT BC Workshop, TNO, Utrecht, Netherlands Outline
Supplementary Information: Twinning of cubic diamond explains reported nanodiamond polymorphs
 Supplementary Information: Twinning of cubic diamond explains reported nanodiamond polymorphs Péter Németh 1, Laurence A.J. Garvie 2,3, and Peter R. Buseck 3, 4 1 Institute of Materials and Environmental
Supplementary Information: Twinning of cubic diamond explains reported nanodiamond polymorphs Péter Németh 1, Laurence A.J. Garvie 2,3, and Peter R. Buseck 3, 4 1 Institute of Materials and Environmental
Characterization of dimers of soot and non-soot
 Characterization of dimers of soot and non-soot particles formed by charged coagulation Boston College and Aerodyne Leonid Nichman, Paola Massoli, Yue Zhang, Tim Onasch, Doug Worsnop, Paul Davidovits MTU
Characterization of dimers of soot and non-soot particles formed by charged coagulation Boston College and Aerodyne Leonid Nichman, Paola Massoli, Yue Zhang, Tim Onasch, Doug Worsnop, Paul Davidovits MTU
performance electrocatalytic or electrochemical devices. Nanocrystals grown on graphene could have
 Nanocrystal Growth on Graphene with Various Degrees of Oxidation Hailiang Wang, Joshua Tucker Robinson, Georgi Diankov, and Hongjie Dai * Department of Chemistry and Laboratory for Advanced Materials,
Nanocrystal Growth on Graphene with Various Degrees of Oxidation Hailiang Wang, Joshua Tucker Robinson, Georgi Diankov, and Hongjie Dai * Department of Chemistry and Laboratory for Advanced Materials,
Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility.
 Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility. Objectives: 1. Attenuation of atmospheric radiation by particulates. 2. Haze and Visibility. Readings:
Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility. Objectives: 1. Attenuation of atmospheric radiation by particulates. 2. Haze and Visibility. Readings:
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113, D16203, doi: /2007jd009699, 2008
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009699, 2008 Strong spectral variation of biomass smoke light absorption and single scattering albedo observed
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009699, 2008 Strong spectral variation of biomass smoke light absorption and single scattering albedo observed
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
Investigation of Optical Properties of Size-Selected Black Carbon Under Controlled Laboratory Conditions. Thesis
 Investigation of Optical Properties of Size-Selected Black Carbon Under Controlled Laboratory Conditions Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in
Investigation of Optical Properties of Size-Selected Black Carbon Under Controlled Laboratory Conditions Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
AMS Introduction Doug Worsnop. AMS Users Meeting
 AMS Introduction Doug Worsnop AMS Users Meeting Aerodyne Caltech Georgia Tech FZ - Juelich University of Minnesota Desert Research Institute October 21/22 October 24, 23 October 8, 24 25 August, 25 16
AMS Introduction Doug Worsnop AMS Users Meeting Aerodyne Caltech Georgia Tech FZ - Juelich University of Minnesota Desert Research Institute October 21/22 October 24, 23 October 8, 24 25 August, 25 16
Interactive comment on Reactive uptake of ammonia to secondary organic aerosols: kinetics of organonitrogen formation by Y. Liu et al.
 Atmos. Chem. Phys. Discuss., 15, C7412 C7424, 215 www.atmos-chem-phys-discuss.net/15/c7412/215/ Author(s) 215. This work is distributed under the Creative Commons Attribute 3. License. Atmospheric Chemistry
Atmos. Chem. Phys. Discuss., 15, C7412 C7424, 215 www.atmos-chem-phys-discuss.net/15/c7412/215/ Author(s) 215. This work is distributed under the Creative Commons Attribute 3. License. Atmospheric Chemistry
Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Chapter 12 Mass Spectrometry and Infrared Spectroscopy
 Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN
 KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to
 What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to 10µm Concentrations decrease exponentially with height N(z) = N(0)exp(-z/H) Long-lived
What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to 10µm Concentrations decrease exponentially with height N(z) = N(0)exp(-z/H) Long-lived
PMP Meeting. May 16th Presented on behalf of PEMS4NANO by Les Hill HORIBA Europe May
 PMP Meeting May 16th 2018 Presented on behalf of PEMS4NANO by Les Hill HORIBA Europe May 2018 www.pems4nano.eu WP3 Measurement integration into system development WP3 Aim & Objectives: 1. Particle characterization
PMP Meeting May 16th 2018 Presented on behalf of PEMS4NANO by Les Hill HORIBA Europe May 2018 www.pems4nano.eu WP3 Measurement integration into system development WP3 Aim & Objectives: 1. Particle characterization
Soot Property Reconstruction from Flame Emission Spectrometry
 Soot Property Reconstruction from Flame Emission Spectrometry Işıl AYRANCI KILINÇ Cambridge University Engineering Department ia262@cam.ac.uk Cambridge Particles Meeting 21 May 2010 I. Ayranci Soot Property
Soot Property Reconstruction from Flame Emission Spectrometry Işıl AYRANCI KILINÇ Cambridge University Engineering Department ia262@cam.ac.uk Cambridge Particles Meeting 21 May 2010 I. Ayranci Soot Property
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 ISSN
 156 Copper Nanoparticles: Green Synthesis Characterization Y.Suresh*1, S.Annapurna*2, G.Bhikshamaiah*3, A.K.Singh#4 Abstract Present work describes the synthesis nanoparticles using papaya extract as a
156 Copper Nanoparticles: Green Synthesis Characterization Y.Suresh*1, S.Annapurna*2, G.Bhikshamaiah*3, A.K.Singh#4 Abstract Present work describes the synthesis nanoparticles using papaya extract as a
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
7. Aerosols and Climate
 7. Aerosols and Climate I. Scattering 1. When radiation impinges on a medium of small particles, scattering of some of the radiation occurs in all directions. The portion scattered backward is called the
7. Aerosols and Climate I. Scattering 1. When radiation impinges on a medium of small particles, scattering of some of the radiation occurs in all directions. The portion scattered backward is called the
Infrared Spectroscopy
 Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Analysis of Flame-Formed Organic. Photoionization Measurements
 Analysis of Flame-Formed Organic Nanoparticles by UV Laser Photoionization Measurements Mario Commodo Istituto di Ricerche sulla Combustione, CNR, P.le Tecchio, 80, 80126, Napoli, Italy Patrizia Minutolo
Analysis of Flame-Formed Organic Nanoparticles by UV Laser Photoionization Measurements Mario Commodo Istituto di Ricerche sulla Combustione, CNR, P.le Tecchio, 80, 80126, Napoli, Italy Patrizia Minutolo
Journal of Aerosol Science
 Journal of Aerosol Science 42 (2011) 859 866 Contents lists available at ScienceDirect Journal of Aerosol Science journal homepage: www.elsevier.com/locate/jaerosci Inter-comparison of optical absorption
Journal of Aerosol Science 42 (2011) 859 866 Contents lists available at ScienceDirect Journal of Aerosol Science journal homepage: www.elsevier.com/locate/jaerosci Inter-comparison of optical absorption
Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer
 Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer A thesis submitted to the University of Manchester Institute of Science and Technology for the degree of Doctor of Philosophy
Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer A thesis submitted to the University of Manchester Institute of Science and Technology for the degree of Doctor of Philosophy
Single particle characterization using SP-AMS with light scattering module in urban environments
 Single particle characterization using SP-AMS with light scattering module in urban environments Alex K. Y. Lee 1, Megan D. Willis 1, Robert M. Healy 2,3, Tim Onasch 4 Jonathan Wang 3, Greg Evans 3, and
Single particle characterization using SP-AMS with light scattering module in urban environments Alex K. Y. Lee 1, Megan D. Willis 1, Robert M. Healy 2,3, Tim Onasch 4 Jonathan Wang 3, Greg Evans 3, and
Supplement of Evaluation of the performance of a particle concentrator for online instrumentation
 Supplement of Atmos. Meas. Tech., 7, 11 135, 1 http://www.atmos-meas-tech.net/7/11/1/ doi:1.519/amt-7-11-1-supplement Author(s) 1. CC Attribution 3. License. Supplement of Evaluation of the performance
Supplement of Atmos. Meas. Tech., 7, 11 135, 1 http://www.atmos-meas-tech.net/7/11/1/ doi:1.519/amt-7-11-1-supplement Author(s) 1. CC Attribution 3. License. Supplement of Evaluation of the performance
Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1
 Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1 ChiiDong Chen Institute of Physics, Academia Sinica chiidong@phys.sinica.edu.tw 02 27896766 Carbon contains 6 electrons: (1s) 2,
Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1 ChiiDong Chen Institute of Physics, Academia Sinica chiidong@phys.sinica.edu.tw 02 27896766 Carbon contains 6 electrons: (1s) 2,
Why is the sky blue?
 Why is the sky blue? Volcanic: June 12, 1991: Mt Pinatubo ejected 20 million tons of sulfur dioxide. Aerosols spread globally Haze lowered a drop of global temperature by 1F Size parameter: Rayleigh
Why is the sky blue? Volcanic: June 12, 1991: Mt Pinatubo ejected 20 million tons of sulfur dioxide. Aerosols spread globally Haze lowered a drop of global temperature by 1F Size parameter: Rayleigh
Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
 THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
Nanosphere Lithography
 Nanosphere Lithography Derec Ciafre 1, Lingyun Miao 2, and Keita Oka 1 1 Institute of Optics / 2 ECE Dept. University of Rochester Abstract Nanosphere Lithography is quickly emerging as an efficient, low
Nanosphere Lithography Derec Ciafre 1, Lingyun Miao 2, and Keita Oka 1 1 Institute of Optics / 2 ECE Dept. University of Rochester Abstract Nanosphere Lithography is quickly emerging as an efficient, low
Astrochemistry (2) Interstellar extinction. Measurement of the reddening
 Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects
 Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
Chemical mass closure of atmospheric aerosol collected over Athens, Greece.
 Chemical mass closure of atmospheric aerosol collected over Athens, Greece. Paraskevopoulou D. 1, 2, Liakakou E. 1, Theodosi C. 2, Zarmpas P. 2, Gerasopoulos E. 1 1, 2*, Mihalopoulos N. 1 Institute for
Chemical mass closure of atmospheric aerosol collected over Athens, Greece. Paraskevopoulou D. 1, 2, Liakakou E. 1, Theodosi C. 2, Zarmpas P. 2, Gerasopoulos E. 1 1, 2*, Mihalopoulos N. 1 Institute for
Soot Particle Aerosol Mass Spectrometer: Development, Validation, and Initial Application
 Aerosol Science and Technology, 46:804 817, 2012 Copyright C American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786826.2012.663948 Soot Particle Aerosol Mass
Aerosol Science and Technology, 46:804 817, 2012 Copyright C American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786826.2012.663948 Soot Particle Aerosol Mass
INTRODUCTION. Definition:-
 INTRODUCTION Definition:- Light scatteringis a form ofscatteringin whichlightis the form of propagating energy which is scattered. Light scattering can be thought of as the deflection of arayfrom a straight
INTRODUCTION Definition:- Light scatteringis a form ofscatteringin whichlightis the form of propagating energy which is scattered. Light scattering can be thought of as the deflection of arayfrom a straight
The Aerosol Quality Characterization System (AQCS) A Different Approach for Identifying Particle Properties
 The Aerosol Quality Characterization System (AQCS) A Different Approach for Identifying Particle Properties Darrel Baumgardner Centro de Ciencias de la Atmosfera Universidad Nacional Autonoma de Mexico
The Aerosol Quality Characterization System (AQCS) A Different Approach for Identifying Particle Properties Darrel Baumgardner Centro de Ciencias de la Atmosfera Universidad Nacional Autonoma de Mexico
Report on the Short Term Scientific Mission (STSM) conducted on the frame of the COST Action CM
 Report on the Short Term Scientific Mission (STSM) conducted on the frame of the COST Action CM - 1404 Chemical characterization of soot nanoparticles in nucleation flames Maurin Salamanca Universität
Report on the Short Term Scientific Mission (STSM) conducted on the frame of the COST Action CM - 1404 Chemical characterization of soot nanoparticles in nucleation flames Maurin Salamanca Universität
Correction for a measurement artifact of the Multi-Angle Absorption Photometer (MAAP) at high black carbon mass concentration levels
 doi:10.5194/amt-6-81-2013 Author(s) 2013. CC Attribution 3.0 License. Atmospheric Measurement Techniques Correction for a measurement artifact of the Multi-Angle Absorption Photometer (MAAP) at high black
doi:10.5194/amt-6-81-2013 Author(s) 2013. CC Attribution 3.0 License. Atmospheric Measurement Techniques Correction for a measurement artifact of the Multi-Angle Absorption Photometer (MAAP) at high black
Brown carbon absorption in the red and near infrared spectral region
 Brown carbon absorption in the red and near infrared spectral region A. Hoffer 1, A. Tóth 2, M. Pósfai 2, C.E. Chung 3, A. Gelencsér 1,2 [1] {MTA-PE Air Chemistry Research Group, Veszprém, P.O. Box 18,
Brown carbon absorption in the red and near infrared spectral region A. Hoffer 1, A. Tóth 2, M. Pósfai 2, C.E. Chung 3, A. Gelencsér 1,2 [1] {MTA-PE Air Chemistry Research Group, Veszprém, P.O. Box 18,
Supplement of Multi-wavelength optical measurement to enhance thermal/optical analysis for carbonaceous aerosol
 Supplement of Atmos. Meas. Tech., 8, 45 46, 25 http://www.atmos-meas-tech.net/8/45/25/ doi:.594/amt-8-45-25-supplement Author(s) 25. CC Attribution 3. License. Supplement of Multi-wavelength optical measurement
Supplement of Atmos. Meas. Tech., 8, 45 46, 25 http://www.atmos-meas-tech.net/8/45/25/ doi:.594/amt-8-45-25-supplement Author(s) 25. CC Attribution 3. License. Supplement of Multi-wavelength optical measurement
Ultraviolet-Visible and Infrared Spectrophotometry
 Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Chasing Aerosol Particles Down to Nano Sizes
 Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Vibrational Spectroscopies. C-874 University of Delaware
 Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
Aerosol Optical Properties
 ATM 507 Lecture 25 Text reading Chapter 15 Paper Due Dec. 9 Review Session Dec. 9 Final Dec. 12 (10:30 AM-12:30 PM) Today s topic Aerosol Optical Properties 1 Aerosol Optical Properties There are a number
ATM 507 Lecture 25 Text reading Chapter 15 Paper Due Dec. 9 Review Session Dec. 9 Final Dec. 12 (10:30 AM-12:30 PM) Today s topic Aerosol Optical Properties 1 Aerosol Optical Properties There are a number
Application of IR Raman Spectroscopy
 Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
CHARACTERIZATION of NANOMATERIALS KHP
 CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
IR LASER-INDUCED CARBOTHERMAL REDUCTION OF TITANIUM MONOXIDE: CARBON- PHASE SHIELD TO NANOSIZED TiO OXIDATION
 IR LASER-INDUCED CARBOTHERMAL REDUCTION OF TITANIUM MONOXIDE: CARBON- PHASE SHIELD TO NANOSIZED TiO OXIDATION Věra JANDOVÁ a, Zdeněk BASTL b, Jan ŠUBRT c, Josef POLA a a Institute of Chemical Process Fundamentals,
IR LASER-INDUCED CARBOTHERMAL REDUCTION OF TITANIUM MONOXIDE: CARBON- PHASE SHIELD TO NANOSIZED TiO OXIDATION Věra JANDOVÁ a, Zdeněk BASTL b, Jan ŠUBRT c, Josef POLA a a Institute of Chemical Process Fundamentals,
Biomass burning in Siberia and Kazakhstan as an important source for haze over the Alaskan Arctic in April 2008
 GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L02813, doi:10.1029/2008gl036194, 2009 Biomass burning in Siberia and Kazakhstan as an important source for haze over the Alaskan Arctic in April 2008 C. Warneke,
GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L02813, doi:10.1029/2008gl036194, 2009 Biomass burning in Siberia and Kazakhstan as an important source for haze over the Alaskan Arctic in April 2008 C. Warneke,
Evidence that Nitric Acid Increases Relative Humidity in Low-Temperature Cirrus
 Supporting Online Material for: Evidence that Nitric Acid Increases Relative Humidity in Low-Temperature Cirrus Clouds R. S. Gao, P. J. Popp, D. W. Fahey, T. P. Marcy, R. L. Herman, E. M. Weinstock, D.
Supporting Online Material for: Evidence that Nitric Acid Increases Relative Humidity in Low-Temperature Cirrus Clouds R. S. Gao, P. J. Popp, D. W. Fahey, T. P. Marcy, R. L. Herman, E. M. Weinstock, D.
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
FLAME-GENERATED CARBON PARTICLES: NEW INSIGHTS ON PARTICLE INCEPTION AND CHARACTERIZATION
 FLAME-GENERATED CARBON PARTICLES: NEW INSIGHTS ON PARTICLE INCEPTION AND CHARACTERIZATION G. De Falco*, M. Commodo**, L. Sgro**, P. Minutolo**, A. D Anna* p.minutolo@irc.cnr.it * Dipartimento di Ingegneria
FLAME-GENERATED CARBON PARTICLES: NEW INSIGHTS ON PARTICLE INCEPTION AND CHARACTERIZATION G. De Falco*, M. Commodo**, L. Sgro**, P. Minutolo**, A. D Anna* p.minutolo@irc.cnr.it * Dipartimento di Ingegneria
Aerosol Composition and Radiative Properties
 Aerosol Composition and Radiative Properties Urs Baltensperger Laboratory of Atmospheric Chemistry Paul Scherrer Institut, 5232 Villigen PSI, Switzerland WMO-BIPM Workshop Geneva, 30 March 1 April 2010
Aerosol Composition and Radiative Properties Urs Baltensperger Laboratory of Atmospheric Chemistry Paul Scherrer Institut, 5232 Villigen PSI, Switzerland WMO-BIPM Workshop Geneva, 30 March 1 April 2010
Advanced Spectroscopy Laboratory
 Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
Surface Analysis - The Principal Techniques
 Surface Analysis - The Principal Techniques Edited by John C. Vickerman Surface Analysis Research Centre, Department of Chemistry UMIST, Manchester, UK JOHN WILEY & SONS Chichester New York Weinheim Brisbane
Surface Analysis - The Principal Techniques Edited by John C. Vickerman Surface Analysis Research Centre, Department of Chemistry UMIST, Manchester, UK JOHN WILEY & SONS Chichester New York Weinheim Brisbane
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
CHEM*3440. Photon Energy Units. Spectrum of Electromagnetic Radiation. Chemical Instrumentation. Spectroscopic Experimental Concept.
 Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Multiple scattering of light by water cloud droplets with external and internal mixing of black carbon aerosols
 Chin. Phys. B Vol. 21, No. 5 (212) 5424 Multiple scattering of light by water cloud droplets with external and internal mixing of black carbon aerosols Wang Hai-Hua( 王海华 ) and Sun Xian-Ming( 孙贤明 ) School
Chin. Phys. B Vol. 21, No. 5 (212) 5424 Multiple scattering of light by water cloud droplets with external and internal mixing of black carbon aerosols Wang Hai-Hua( 王海华 ) and Sun Xian-Ming( 孙贤明 ) School
Monique Teich et al. Correspondence to: Hartmut Herrmann
 Supplement of Atmos. Chem. Phys., 7, 5 7, 7 http://www.atmos-chem-phys.net/7/5/7/ doi:.59/acp-7-5-7-supplement Author(s) 7. CC Attribution. License. Supplement of Contributions of nitrated aromatic compounds
Supplement of Atmos. Chem. Phys., 7, 5 7, 7 http://www.atmos-chem-phys.net/7/5/7/ doi:.59/acp-7-5-7-supplement Author(s) 7. CC Attribution. License. Supplement of Contributions of nitrated aromatic compounds
Consequences for Climate Feedback Interpretations
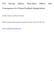 CO 2 Forcing Induces Semi-direct Effects with Consequences for Climate Feedback Interpretations Timothy Andrews and Piers M. Forster School of Earth and Environment, University of Leeds, Leeds, LS2 9JT,
CO 2 Forcing Induces Semi-direct Effects with Consequences for Climate Feedback Interpretations Timothy Andrews and Piers M. Forster School of Earth and Environment, University of Leeds, Leeds, LS2 9JT,
Crystalline Surfaces for Laser Metrology
 Crystalline Surfaces for Laser Metrology A.V. Latyshev, Institute of Semiconductor Physics SB RAS, Novosibirsk, Russia Abstract: The number of methodological recommendations has been pronounced to describe
Crystalline Surfaces for Laser Metrology A.V. Latyshev, Institute of Semiconductor Physics SB RAS, Novosibirsk, Russia Abstract: The number of methodological recommendations has been pronounced to describe
Optical Remote Sensing Techniques Characterize the Properties of Atmospheric Aerosols
 Optical Remote Sensing Techniques Characterize the Properties of Atmospheric Aerosols Russell Philbrick a,b,c, Hans Hallen a, Andrea Wyant c, Tim Wright b, and Michelle Snyder a a Physics Department, and
Optical Remote Sensing Techniques Characterize the Properties of Atmospheric Aerosols Russell Philbrick a,b,c, Hans Hallen a, Andrea Wyant c, Tim Wright b, and Michelle Snyder a a Physics Department, and
Direct and semi-direct radiative effects of absorbing aerosols in Europe: Results from a regional model
 GEOPHYSICAL SEARCH LETTERS, VOL. 39,, doi:10.1029/2012gl050994, 2012 Direct and semi-direct radiative effects of absorbing aerosols in Europe: Results from a regional model J. Meier, 1 I. Tegen, 1 B. Heinold,
GEOPHYSICAL SEARCH LETTERS, VOL. 39,, doi:10.1029/2012gl050994, 2012 Direct and semi-direct radiative effects of absorbing aerosols in Europe: Results from a regional model J. Meier, 1 I. Tegen, 1 B. Heinold,
Modeling of Mature Soot Dynamics and Optical Properties
 Modeling of Mature Soot Dynamics and Optical Properties Georgios A. Kelesidis, Sotiris E. Pratsinis Particle Technology Laboratory, ETH Zurich, Zurich, Switzerland Aleksandar Duric, Martin Allemann Siemens
Modeling of Mature Soot Dynamics and Optical Properties Georgios A. Kelesidis, Sotiris E. Pratsinis Particle Technology Laboratory, ETH Zurich, Zurich, Switzerland Aleksandar Duric, Martin Allemann Siemens
Ultraviolet-Visible and Infrared Spectrophotometry
 Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
