SUPERVISORS. Professor Jo Klaveness School of Pharmacy University of Oslo. Associate professor Pål Rongved School of Pharmacy University of Oslo
|
|
|
- Daniella Jones
- 6 years ago
- Views:
Transcription
1 SUPERVISRS Professor Jo Klaveness School of Pharmacy University of slo Associate professor Pål Rongved School of Pharmacy University of slo 1
2 TABLE F CTETS TABLE F CTETS 1 ABBREVIATIS ABSTRACT ITRDUCTI Acetylcholine- a neurotransmitter Synthesis, release and inactivation Structure of acetylcholinesterase Anticholinesterases interfere with acetylcholine activity Effects of anticholinesterases Different groups of anticholinesterases Short- acting anticholinesterases Medium- duration anticholinesterases Irreversible anticholinesterases erve agents: Irreversible anticholinesterases The dawn of a deadly weapon Biochemistry Ageing half- life Acetylcholinesterase reactivators xime deprotonation xime reactivation mechanism of organophosphate- inhibited acetylcholinesterase Stability of oximes in solution Reactivation efficacy Therapeutic efficacy Clinical recommendations Reaching and maintaining a therapeutic oxime blood concentration xime transport across the blood-brain-barrier; the use of prodrugs Synthesis of a pralidoxime prodrug Problems associated with the pralidoxime prodrug AIM F THE STUDY
3 TABLE F CTETS 5 RESULTS AD DISCUSSI Synthesis Pralidoxime/ 2- PAM in acetone PAM in absolute ethanol Reduction of 2- PAM to 1- methyl- 1, 2, 3, 6- tetrahydropyridine- 2- carbaldoxime with abh pyridinealdoxime--methyl iodide/ 4-PAM Reduction of 4-PAM to 1- methyl- 1, 2, 3, 6- tetrahydropyridine- 4- carbaldoxime with abh TMB pyridinealdoxime- - propyl bromide TMB- 4 from 4- pyridinealdoxime and 4- pyridinealdoxime- - propyl bromide. Synthesis via a two- step route Attempted synthesis pyridinealdoxime- - propyl bromide in ethanol pyridinealdoxime- - propyl bromide in acetone trimetylenebis- 2, 2- (hydroxyiminomethyl)- pyridine bromide trimetylenebis- 2, 4- (hydroxyiminomethyl)- pyridine bromide Reduction of TMB- 4 with abh Reduction of TMB-4 with LiBH 4 in diethyl ether Conclusion EXPERIMETAL Materials and methods Reagents, solvents and solution Methods Synthesis Pralidoxime/2- PAM in acetone Pralidoxime/2- PAM in absolute ethanol Reduction of 2- PAM to 1-methyl- 1, 2, 3, 6- tetrahydropyridine- 2- carbaldoxime with abh pyridinealdoxime- - methyl iodide/ 4- PAM Reduction of 4-PAM to 1- methyl-1, 2, 3, 6- tetrahydropyridine-4- carbaldoxime with abh trimetylenebis- 4- (hydroxyiminomethyl)- pyridine bromide/tmb pyridinealdoxime- - propyl bromide TMB- 4 from 4- pyridinealdoxime and 4- pyridinealdoxime- - propyl bromide. Synthesis via a two- step route
4 TABLE F CTETS 6.3 Attempted synthesis pyridinealdoxime- - propyl bromide in ethanol pyridinealdoxime- - propyl bromide in acetone trimetylenebis- 2, 2- (hydroxyiminomethyl)- pyridine bromide trimetylenebis- 2, 4- (hydroxyiminomethyl)- pyridine bromide Reduction of TMB-4 with abh Reduction of TMB-4 with LiBH 4 in diethyl ether REFERECES
5 ABBREVIATS 1 ABBREVIATIS Acetyl CoA- acetyl coenzyme A ACh- acetylcholine AChE- acetylcholinesterase BBB- blood brain barrier CAT- choline acetyltransferase CS- central nervous system DMS- dimethyl sulfoxide EtH- ethanol Li BH 4 - lithium borohydride, reduction agent MeH- methanol MS- mass spectroscopy MS- EI- mass spectroscopy method, electron ionization (EI) MS- ES- mass spectroscopy method, electrospray (ES) abh 4 - sodium borohydride, reduction agent MR- nuclear magnetic resonance P- organophosphate PC- organophosphorous compound PS- peripheral nervous system Pro- PAM- pralidoxime prodrug 2- PAM- pralidoxime TLC- thin layer chromatography TMB- 4- trimedoxime, an oxime 5
6 ABSTRACT 2 ABSTRACT Acetylcholinesterase (AChE) is an enzyme crucial to normal nerve signaling because of its rapid hydrolysis of acetylcholine, a common neurotransmitter. Inhibition of this enzyme may cause bradycardia, hypotension and difficulty in breathing, and the outcome might be deadly. erve agents are a group of lipophilic and volatile organophosphorous AChE inhibitors. Because of their ability to penetrate unbroken skin and pass into the central nervous system (CS), they are ideal for chemical warfare. A group of mono-and bispyridinium compounds called oximes are able to reactivate inhibited AChE, and there is great interest in synthesizing better and more effective oximes. To be able to reactivate inhibited AChE the pyridine nitrogen has to be positively charged, but due this positive charge the oximes have problems passing the blood- brain- barrier (BBB) and enter the CS. As a consequence of this, nerve agent intoxication is difficult to treat. The solution to this problem may be to synthesize an oxime prodrug; in other words a compound that can freely pass the BBB (e. g has lipophilic properties) and enter the CS, and once inside the CS regain its active form. When a polar compound like an oxime has successfully entered the CS, it is in reality trapped. Little progress has been made in regard to synthesizing an effective oxime prodrug since the pralidoxime prodrug was synthesized in ew oximes have been introduced the last decade, but none of these have properties that allow a passing of the BBB to a satisfactory extent. In this master thesis I have synthesized some known mono- and bispyridinium oximes according to literature protocol. Pralidoxime, a monopyridinium oxime and TMB- 4, a bispyridinium oxime, were successfully synthesized in high yields. TMB- 4 was synthesized via two different protocols, a direct route and a new, indirect route of synthesis. Potential oxime prodrugs were synthesized by reduction of mono- and bispyridinium oximes with abh 4. ximes that resisted reduction by abh 4 were later attempted reduced using LiBH 4. ther oximes were synthesized using new synthetic methods developed by the author. A new monopyridinium oxime was synthesized and isolated, whereas synthesis of bispyridinium derivatives of TMB- 4 proved difficult. 6
7 ITRDUCTI 3 ITRDUCTI 3.1 Acetylcholine- a neurotransmitter Synthesis, release and inactivation Acetylcholine (ACh) is synthesized in the nerve terminus from choline, which is taken up into the nerve terminal by an active transport system. Free choline is acetylated by a cytosolic enzyme, choline acetyltransferase, (CAT), which transfers the acetyl group from acetyl coenzyme A (acetyl CoA). Most of the synthesized ACh is packed into vesicles with a concentration of approximately 100 mmol/l. Release happens through exocytosis, which is triggered by a calcium influx to the nerve terminus. After release, ACh diffuse across the synaptic cleft to reach receptors on the surface of the postsynaptic cell. In fast cholinergic synapses, for example neuromuscular and ganglionic synapses, ACh is hydrolyzed very fast (within 1 ms) to inactive choline and acetate by acetylcholinesterase (AChE). AChE is an enzyme bound to the basal membrane of the nerve terminal. The basal membrane is located between the pre- and postsynaptic membranes. Some of the released ACh is actually hydrolyzed before being able to interact with postsynaptic receptors. AChmolecules that reach a receptor remains bound to the receptor for, on average, 2 ms, and are quickly hydrolyzed after dissociation, preventing them from combining with a second receptor. The result is that transmitter action is very rapid and very brief, which is important for the initiation of fast muscular responses in synapses than may have to transmit high frequency signals. 7
8 ITRDUCTI Structure of acetylcholinesterase Each molecule of AChE consists of six active sites, where each site has a central esteratic site and a peripheral anionic site (air, Hunter 2004). The esteratic site is composed of three amino acid residues (Ser, His and Glu). These residues are referred to as a catalytic triad. Together these residues perform the catalytic functions of the enzyme. The catalytic triad enables nucleophilic attack on ACh and subsequent hydrolysis. The other component of the active site is the anionic subsite. This term is in fact misleading since the site is actually uncharged and lipophilic (Harel et al. 1993; Sussman et al. 1991). The anionic site binds ACh, enabling the ester linkage of ACh to interact with the esteratic site of AChE. ACh is hydrolyzed and the acetyl group is transferred to the serine residue (air, Hunter 2004). A free choline molecule is released. The acetylated enzyme is hydrolyzed rapidly, forming free enzyme and acetic acid. Approximately molecules of ACh are hydrolyzed pr second in each of the six active sites on the enzyme (air, Hunter 2004). The structure of the active site with ACh attached is shown in Fig.1. Fig.1: Active site of acetylcholine esterase with its substrate acetylcholine (air, Hunter 2004). 8
9 ITRDUCTI 3.2 Anticholinesterases interfere with acetylcholine activity Anticholinesterases inhibit AChE by binding reversibly or irreversibly to the esteratic site. With the esteratic site occupied, the enzyme is no longer able to hydrolyze its substrate ACh, and ACh is not inactivated after being released from the postsynaptic receptor. This means that one molecule of ACh can activate the same receptor several times, or activate several receptors. The consequence of AChE inhibition is a constant activation of postsynaptic receptors, which may lead to a depolarization block, a decrease in the postsynaptic cell s electrical excitability. The main reason for this loss of excitability is the inactivation of voltage- sensitive sodium channels, which are rendered unable to open in response to brief depolarizing stimuli. A depolarization block is associated with accumulation of ACh in plasma and other tissue fluids. This can cause life threatening physiological effects unless the inhibited AChE regains its normal function. A group of organophosphate anticholinesterases inhibit AChE by phosphorylating the esteratic site. Spontaneous hydrolysis of phosphorylated AChE is extremely slow, about 10 7 to fold slower than deacetylation (Johnson et al. 2000). This causes exposure to anticholinesterases to be potentially life threatening. 3.3 Effects of anticholinesterases Effects of anticholinesterases on autonomic cholinergic synapses mainly reflect increased ACh- activity at parasympathetic postganglionic synapses (Rang et al. 1999). This means increased secretions from salivary, lachrymal, bronchial and gastrointestinal glands, increased peristaltic activity, bronco constriction, bradycardia and hypotension, pupillary constriction and fall in oculary pressure. Large doses of anticholinesterases can stimulate and later block autonomic ganglia and cause complex autonomic effects. (Depolarization block, see chapter 3.2). Poisoning from contact with anticholinesterases may cause difficulty in breathing. An additional depolarization block may lead to a fatal outcome for the individual or animal in question. Effects on neuromuscular junction can be seen as initial muscle cramps and later paralysis due to depolarization block (Rang et al. 1999). Tertiary anticholinesterases compounds such as physostigmine and non-polar organophoshates can penetrate the bloodbrain- barrier (BBB) freely and affect the CS. Initially anticholinesterases cause an excitation of the CS, often seen as convulsions. The excitation is followed by a subsequent depression which may lead to unconsciousness and respiratory failure (Rang et al. 1999), due to an inhibition of the respiratory centre in the medulla oblongata (Johnson et al. 2000). These 9
10 ITRDUCTI central effects are mainly the result of activation of muscarinic receptors, and are antagonized by atropine (Rang et al. 1999). 3.4 Different groups of anticholinesterases Peripherally acting anticholinesterases fall into three main categories according to their way of interaction with the active site on the AChE- enzyme, which also determines the duration of action (Rang et al. 1999): Short- acting anticholinesterases Medium- duration anticholinesterases Irreversible anticholinesterases Short- acting anticholinesterases Edrophonium is a quaternary ammonium compound that binds to the anionic site of the enzyme only. The ionic bond is readily reversible, and the drug action is very brief. Edrophonium is too short- acting for therapeutic use, and its main use is diagnosis of myasthenia gravis (Rang et al. 1999). In myasthenia gravis transmission fails because there are not enough ACh receptors, and anticholinesterases improve transmission because they increase the number of free ACh molecules. The chemical structure of edrophonium is shown in Fig. 2. H Fig. 2: Structure of edrophonium, a short- acting anticholinesterase (Rang et al. 1999). 10
11 ITRDUCTI Medium- duration anticholinesterases Medium- duration anticholinesterases include neostigmine (used in combination with atropine to reverse non- depolarizing neuromuscular block), pyridostigmine (treatment of myasthenia gravis), and rivastigmine (treatment of dementia caused by Alzheimer s disease) (Felleskatalogen 2004). They are quaternary compounds, in contrast to physostigmine, a tertiary amine anticholinesterase found in the Calabar bean (used in combination with pilocarpine for treatment of glaucoma) (Samuelsson 1999). The medium- duration anticholinesterases all have strongly basic groups which bind to the anionic site of the AChE. They are however carbamyl esters, whereas ACh is an acetate ester. The carbamyl group is transferred to the serine H of the esteratic site in similar fashion to acetyl from ACh, but the carbamylated enzyme is much slower to hydrolyze. The hydrolysis can take several minutes, compared to microseconds for an acetylated enzyme (Rang et al. 1999). The slow recovery of the carbamylated enzyme means these drugs are quite long- lasting; AChE inhibited by rivastigmine regains its normal activity approximately 10 hours after administration of the drug, and pyridostigmine has a time of duration of 4-6 hours (Felleskatalogen 2004). Structures of the medium duration anticholinesterases mentioned above are found in Fig. 3. H a b c d 11
12 ITRDUCTI Fig. 3: Structure of the most important medium duration anticholinesterases a) neostigmine b) physostigmine c) rivastigmine d) pyridostigmine (Rang et al. 1999; Goodman&Gilman s 2001) Irreversible anticholinesterases rganophosphorous compounds (PCs) are extremely potent irreversible inhibitors of AChE. They have properties that make them useful in different areas. rganophosphates are used as insecticides, softening agents and additives to lubricants, and as nerve agents (Kassa 2002). Most of these compounds interact only with the esteratic site of AChE and have no cationic group (Rang et al. 1999). The general structure is shown in Fig.4. Irreversible anticholinesterases are pentavalent compounds containing a labile group such as fluoride or an organic group. After bonding to the esteratic site, the labile group is released (Rang et al. 1999). The residue of the molecule remains covalently attached to the enzyme s serine Hgroup, effectively blocking the esteratic site and hence inhibiting the normal function of the enzyme. The inactive phosphorylated enzyme is usually very stable. With some anticholinesterases virtually no hydrolysis occurs, and recovery of enzymatic activity depends on the synthesis of new enzyme molecules. This is a process that may take weeks (Rang et al. 1999). R 1 P X R 2 Fig. 4: General structure of organophosphorous compounds R1 and R2 are normally simple alkyl- and aryl groups which both may be linked directly to the P atom (in phosphinates) or linked via -- or -S- (in phosphates), or R1 may be bonded directly and R2 may be bonded via one of the groups mentioned above (in phosphonates). In phosphoroamidates, C is bonded to P through an amino group. The X- group is a leaving group (LG) and may be a substituted or branched aliphatic, aromatic or heterocyclic group, linked to P -, - or S-. The atom linked to P via a double bond may be S or, and related compounds are called phosphorothioates and phosphates respectively. The P=- analogue of a thioate ester is called an oxon, and this term is often incorporated in the trivial name, like parathion and paraoxon. (Johnson et al. 2000). 12
13 ITRDUCTI 3.5 erve agents: Irreversible anticholinesterases erve agents are related chemically to organophosphorous insecticides and have a similar mechanism of toxicity, but a much higher mammalian acute toxicity, particularly via the dermal route (Vale 2004). They are non- polar substances with high lipid solubility, and they are rapidly absorbed through mucous membranes, and even unbroken skin (Rang et al. 1999). The discovery of these properties lead to their use as chemical warfare agents, and they are referred to as nerve agents. The nerve agents acquired their name because they affect the transmission of nerve impulses in the nervous system (Vale 2004). The outdated term nerve gas is misleading, since all nerve agents in their pure state are colorless liquids. Their volatility varies widely; VX on one extreme is oil- like, whereas sarin, being the most volatile nerve agent, is approximately as volatile as water ( Holstege, Dobmeier 2005). The nerve agent, either as a gas, aerosol or liquid, enters the body through inhalation or through the skin. Systemic poisoning may follow inhalation, ingestion or dermal exposure, though the onset of systemic toxicity is slower by the latter route (Vale 2004). The route for entering the body is of importance for the period of time required for the nerve agent to start having effect. The nerve agents have the quickest effect when absorbed through the respiratory system. This is due to the rapid diffusion into the blood circulation, and subsequent transport to target organs ( Exposure to high doses of a nerve agent will cause muscle paralysis of the respiratory muscles, and in addition the respiratory center of the CS is affected (Johnson et al. 2000). The combination of these two is the direct cause of death. Consequently, death caused by a nerve agent is death by suffocation. 13
14 ITRDUCTI The dawn of a deadly weapon Prior to and during World War 2, efforts were directed towards the development of chemical warfare agents. During his work with developing pesticides, a German chemist, Gerhard Schrader, discovered tabun by chance in 1936 (Holstege, Dobmeier 2005). Schrader firsthandedly observed the effects of nerve agents on human beings in January 1937, when a drop of tabun spilled onto a lab bench. Within minutes Schrader and his laboratory assistant began to experience miosis, dizziness, and severe shortness of breath. It took them three weeks to fully recover ( Tabun was the first of the substances later referred to as nerve agents. The nerve agents are stable and easily dispersed, they have rapid effects both when absorbed through the skin and via respiration, and they are extremely toxic. Because of these properties, the nerve agents are considered among the most dangerous chemical warfare agents in the world. They are in fact classified as weapons of mass destruction by the United ations according to U Resolution 687 ( The structures of the most important nerve agents are given in Fig.5. Up to the end of the war, Schrader and his co-workers synthesized about new organophosphorous compounds. Sarin was developed in 1938, and was named after the men who participated in synthesizing it: Schrader, Ambrose, Rudringer, and Vander Linde. Soman was the last nerve agent to be developed by the Germans in 1944 by Dr. Richard Kuhn (Holstege, Dobmeier 2005). The G agents, or German agents, tabun, sarin and soman are volatile, and both dermal and respiratory hazards (Vale 2004). A British scientist synthesized an extremely toxic compound in 1952 while searching for a new pesticide to replace DDT (Holstege, Dobmeier 2005). This substance was forwarded to the US for production and was later coded VX. VX belong to a family of nerve agents called the V agents. The V agents are less volatile than the G-agents, and are primarily percutaneous contact hazards unless aerosolized (Vale 2004). In the morning rush hour on March 20, 1995, a group of terrorists placed containers of sarin in carriages on three underground railway lines in open plastic bags, enabling the agent, which is liquid under temperate conditions, to evaporate (J. A. Vale 2004). ver 5,000 sought medical 14
15 ITRDUCTI attention, 984 of these were moderately poisoned and 54 were severely poisoned. 12 people died as a direct cause of the exposure to sarin (kumura et al.1996, Sidell 1996). Two years later, The Chemical Weapons Convention forbid development, production, stockpiling and the use of chemical war agents (Johnson et al. 2002). There is however little reason to believe nerve agents won t be used again in future, being a very effective weapon for terrorists and in warfare. P C CH P F P F a b c P S P F d e Fig. 5: The most common nerve agents are the following: a) Tabun, -ethyl dimethylamidophosphorylcyanide (GA) is the easiest to manufacture. Consequently, it is more likely that developing countries start their chemical warfare arsenal with this nerve agent whereas industrialized countries consider tabun to be out-of-date and of limited use. b) Sarin, isopropyl methylphosphonofluoridate, (GB) is a volatile substance mainly taken up through inhalation. c) Soman, pinacoyl methylphosphonofluoridate, (GD) and it is a moderately volatile substance which can be taken up by inhalation or skin contact. d) VX, -ethyl S-diisopropylaminomethyl methylphosphonothiolate, is a persistent substance which can remain on material, equipment and terrain for long periods of time. Uptake is mainly through the skin but also through inhalation of the substance as a gas or aerosol. e) Cyclosarin /GF agent, cyclohexyl methylphosphonofluoridate, is a substance with low volatility which is taken up through skin contact and inhalation of the substance either as a gas or aerosol. 15
16 ITRDUCTI Biochemistry The interaction between organophosphorous compounds (PCs) and AChE all take place in the same manner, but the rate of each step depend on the structure of the PC. In every case, the initial step of the inhibition occur when the PC is in the oxon (P=)-form. Pure thioates (P=S- compounds) are not significant inhibitors in their original form, but are metabolically activated to oxons in vivo (Johnson et al. 2000). The interaction between P- oxons and AChE include four stages (Johnson et al. 2000): 1. A serine group on AChE is phosphorylated. 2. A LG X leaves the complex. This leads to the formation of a relatively stabile covalent bond between the PC and the enzyme, with a consequent inhibition of catalytic activity. After inhibition, two reactions are possible: 3. Reactivation may happen spontaneously but slowly at a rate determined by the properties of the attached PC and the enzyme. The rate might be changed by adding nucleophilic reagents such as oximes, which may accelerate the reactivation rate and thereby act as antidotes. 4. The AChE- PC complex may go through an ageing. Ageing is a time dependent loss of the phosphorylated enzyme s ability to be reactivated. The ageing involves cleavage of one or more bonds in the R- - P- chain with a subsequent loss of R, and formation of a charged monosubstituted phosphoric acid still attached to the enzyme. The reaction is called an ageing because it is usually a slow process, and the product, a covalent PC- AChE complex, can no longer be reactivated by nucleophilic reactivating agents. Therapeutic oximes cannot break such a bond. The inhibition mechanism of AChE is shown in Fig
17 ITRDUCTI Enzyme-H + X P R 1 1 R 2 Enzyme X H Michaelis complex P R 2 R 1 2 Enzyme 3 P R 1 R 2 1. Reversible formation of the Michaelis complex 2. Phosphorylation of the enzyme 3. Reactivation (dephosphorylation) 4. Ageing 4 Enzyme P R 1 - Fig. 6: AChE inhibition mechanism Inhibition of AChE starts with the formation of the Michaelis complex in step 1. In step 2 the enzyme is phosphorylated and inactivated. The enzyme- PC- complex may now spontaneously hydrolyze (step 3), rendering the enzyme reactivated, or the complex may go through an ageing (step 4). nce aged, reactivation is impossible (Johnson et al. 2000) Ageing half- life The ageing half- life of the nerve agent- AChE complex is an important factor when it comes to outcome of oxime treatment. If treatment is initiated too late, the oxime will not be able to reactivate inhibited AChE, and the result may be fatal. The ageing half- life for three of the most dangerous nerve agents are (presko et al. 1998): Tabun-AChE: 46 hours Sarin- AChE: 5 hours Soman- AChE: 1.3 minutes 17
18 ITRDUCTI 3.6 Acetylcholinesterase reactivators Compounds containing an oxime group (RCH= H) attached to a pyridine ring with a quaternary nitrogen are able to reactivate PC- inhibited AChE by dephosphorylating the enzyme s active sites. The quaternary nitrogen atom is bonded to the anionic site, thereby placing the oxime group in the vicinity of the esteratic site. Several oximes are potentially effective in reactivating PC- inhibited AChE. The basic structure for these compounds differs only by the number of pyridine rings (mono or bis) and by the position of the oxime group on the pyridine ring. The structure of the most important oximes can be found in Fig. 7. The more potent antimuscarinic bisquartarnary pyridine derivatives are those containing a hydrophobic substituent at position 3 or 4 in the pyridine ring (Kloog et al. 1986). 18
19 ITRDUCTI CH H CH H 2Cl CH 3 CH Cl H CH 2 CH 2 a b HC H 2Cl HC H HC H HC H 2Br C H 2 H 2 H 2 C C CH 2 c d H 2 H CH C H 2 2Cl 2Cl H 2 C CH 2 C H H H C H H 2 C CH 2 e f Fig. 7: The structures of the most common oximes a) Pralidoxime chloride, 2-pyridinealdoxime--methyl chloride b) bidoxime chloride, bis-4-pyridinealdoxime--methylether dichloride c) Methoxime chloride,, -methylen-4-pyridinealdoxime dichloride d) Trimedoxime bromide/ TMB-4, 1, 1 - trimethylenebis- 4-(hydroxyiminomethyl) pyridine bromide e) HI-6 chloride, 4- aminocarbonylpyridine- 1- methylenoxy- 2 -(hydroxyiminomethyl)- 1 -methylpyridine dichloride monohydrate f) HLø-7 chloride, 1-[[[4-(aminocarbonyl) pyridine] methoksy] methyl] -2, 4-bis [(hydroxyimino) methyl] pyridine dichloride 19
20 ITRDUCTI xime deprotonation To be pharmacologically active, oximes need to undergo a prior deprotonation. This deprotonation is shown in Fig. 8 (Brüggeman 1998). CH 3 H 2-PAM CH 3 Active species Fig. 8: xime deprotonation (Brüggeman 1998) xime deprotonation is an essential step to render the oxime active and capable of reactivating PC- inhibited AChE. The deprotonation causes a loss of a proton from the oxime H- group, leaving the oxygen negatively charged xime reactivation mechanism of organophosphate- inhibited acetylcholinesterase ximes reactivate PC- inhibited AChE through a nucleophilic attack on phosphorous. An oxime- phosphonate leaves the active site, and the regenerated esteratic site is subsequentially able to return to normal function, and bind and split its substrate ACh. The reactivation mechanism is shown in Fig. 9 below (Brüggeman 1998). 20
21 ITRDUCTI Serine moiety of AChE C H H C C CH 2 P R 1 R 2 H CH 3 H P R 1 C H H C C CH 3 R 1 CH 2 H 2 Reactivated serine moiety in the active site of AChE H H H P R 1 CH 3 R 2 Fig. 9: Reactivation of PC- inhibited AChE The oxime group attacks phosphorous, and subsequentially removes the PC from the active site of AChE. The active site (e.g. the serine moiety) is reactivated and the enzyme can return to normal function. The oxime- PC complex goes through hydrolysis and is split in oxime, now inactive, and PC (Brüggeman 1998). 21
22 ITRDUCTI Stability of oximes in solution The stability of the oxime in aqueous solution is an important chemical property because of the necessity of storage in advance of possible clinical use. Pralidoxime, obidoxime and methoxime are all relatively stabile in water and can be stored in solution. Stability studies on obidoxime show that obidoxime ampoules can be stored for over 30 years at room temperature before the content decreases by 10% (Rubnov et al. 1999). HI-6 and HLø-7 on the other hand are unstable in aqueous solution and must be stored as lyophilized powder (Eyer et al. 1986). When these compounds are in solution, they decompose quickly under ambient or physiological conditions (ph 7.4 and 37º C). The degradation half- time of both these oximes in 1 mm solution have been found to be about 12 hours (Eyer et al. 1986; Eyer et al. 1989). This short degradation half- life is definitely one of the biggest problems associated with the use of these oximes. Therefore, dry/wet autoinjectors have been developed. For practical reasons, they contain both atropine and oxime, comprising the oxime as a powder which is dissolved in the atropine-containing solvent immediately before intramuscular injection. The autoinjectors are activated by breaking a membrane followed by shaking the device. The commercially available autoinjectors (Astra Tech AB, Sweden, and STI International, UK) are specified to deliver a dose of some 500 mg HI 6 dichloride and 2 mg atropine sulphate after a shaking time of 5 seconds (Thiermann et al. 1998) Reactivation efficacy Generally speaking, the reactivating efficiency of an oxime is determined by the nucleophilic strength of the oxime and its affinity for the PC- inhibited enzyme. ximes may differ in the position of the oxime group on the pyridine ring, but their reactivity is comparable because of the similar basic structure (Kassa 2002). The oximes affinity for the intact enzyme is characterized by the dissociation constant K dis of the enzyme- oxime complex. A high value of K dis means a decreased affinity of the oxime for the intact enzyme. The affinity of the PC- inhibited enzyme is characterized by the dissociation constant of the inhibited enzymeoxime complex K R. An increase in K R means a decrease in the affinity of the oxime for the PC- inhibited enzyme. The affinity is determined by a number of physiochemical factors such as steric compatibility, electrostatic effects, hydrophobic interactions and the general shape and size of the reactivator, in addition to functional groups (Kassa 2002). Pralidoxime and obidoxime have relatively low affinity for both active and phosphonylated AChE, while the affinity of the H- oximes is quite high. This corresponds to the reactivating 22
23 ITRDUCTI potency in vitro; pralidoxime and obidoxime are poor reactivators and the H- oximes are relatively good reactivators (Kassa, Cabal 1999 A; Kassa, Cabal 1999 B; Kassa, Cabal 1999 C). Treatment of soman intoxication Soman is one of the most treatment resistant war gases due to the presence of as slowly distributed soman depot in the poisoned individuals (Kassa 2002) and the extremely rapid ageing of soman- phosphonylated AChE (presko et al. 1998). In pyridostigmine and control rabbits intoxicated with soman and treated with oxime and atropine, HI- 6 was found to be three to five times more effective than pralidoxime (Koplovitz, Steward 1995). Pralidoxime, obidoxime, and methoxime were found to be virtually ineffective in reactivating somaninhibited AChE in the peripheral nervous system (PS) and in the CS. bidoxime may actually worsen the AChE- inhibition by soman (Kassa, Cabal 1999). The H-oximes have been found to be significantly more effective in reactivation of soman- inhibited AChE than the monopyridinium oximes, although the percentage reactivation within the CS is unsatisfactory due to the H- oximes poor ability to penetrate the BBB (Kassa 2002). The research data on the H- oximes are contradictory; J. Kassa have shown that the H- oximes cannot pass the BBB in sufficient concentration and exhibit their action within the CS to a satisfactory extent (Kassa 2002), while other studies have shown that the H- oximes can in fact pass the BBB in sufficient concentration to produce biochemical and physiological effects in soman poisoning (Eyer et al.1992; Clement 1992; Kassa 1998; Koplovitz, Steward 1995). Treatment of tabun intoxication Bispyridinium oximes show different ability to reactivate tabun- inhibited AChE in vitro, whereas no monopyridinium oxime including pralidoxime is able to reactivate tabuninhibited AChE (Cabal et al. 2004). bidoxime, methoxime and HI-6 are poor AChEreactivators; the percentage of AChE reactivation is lower than 10%. Pralidoxime has been found to be more effective against tabun poisoning than HI- 6, but the efficacy of HI- 6 increased three- fold when rabbits were pretreated with pyridostigmine before being treated with oxime and atropine (Koplovitz, Steward 1995). Trimedoxime (TMB-4) seems to be the most effective reactivator of tabun inhibited AChE; it has been found to be able to reactivate more than 40% of tabun-inhibited AChE at 1 mmol/l concentration in vitro (Cabal et al. 2004). The extremely poor effect by most oximes on tabun intoxicated individuals is caused 23
24 ITRDUCTI by a free electron pair located on the amidic nitrogen, making a nucleophilic attack by the oximes almost impossible (Dawson 1994; Koplovitz et al. 1995; Eto 1976; Cabal, Bajgar 1999). Treatment of VX intoxication VX has been reported to respond to all oxime treatment (Kassa 2002), and VX intoxication is therefore easy to treat compared to intoxication with the other nerve agents mentioned. Treatment of sarin intoxication The ability of pralidoxime, obidoxime and methoxime to reactivate sarin- inhibited AChE is relatively low. The H- oximes have proven to be very effective both peripherally and centrally, the latter in spite of the quaternary structure that should, at least to a certain extent, limit penetration of the BBB (Kassa 2002) Therapeutic efficacy Therapeutic efficacy of the oximes is normally measured by the protective ratio (PR) of the LD 50 of the particular PC in an animal being treated with an oxime, compared to the LD 50 in unprotected animals. In most published experiments, a combination of atropine and oxime is used as an antidotal treatment since this is the treatment most likely to be used in for example combat. Therapeutic efficacy can also be measured by comparing the median effective dose (ED 50 ) that prevents death of rats after exposure to supralethal doses of tested PCs, when the oximes are combined with the same dose of atropine. ED 50 data published show that pralidoxime in recommended doses alone is not able to prevent mortality in rats exposed to supralethal doses of any nerve agent tested (Kassa, Cabal 1999 A; Kassa, Cabal 1999 B, Kassa, Cabal 1999 C). Data show that obidoxime must be administered in doses higher than the recommended dose (approximately 2% of LD 50 ) to be effective, which gives a low safety ratio. The required dose of obidoxime is not sufficiently safe and may cause dangerous side effects. HI-6 and HLø-7 are effective in protecting rats poisoned with supralethal doses nerve agent, like soman, in doses comparable to recommended human therapeutic doses. The safety ratio of required doses of the H- oximes is very high, and HI-6 and HLø-7 are considered to be safe in use (Kassa 2002). 24
25 ITRDUCTI The therapeutic efficacy of the oximes seems to depend on many different factors, one of them being the particular nerve agent involved. Especially intoxications by soman and tabun are difficult to treat, whereas VX yields readily to common antidotal treatment. ther factors seem to be the administration route for both nerve agent and antidote, and timing. Treatment need to be initiated as soon as possible. one of the oximes, including the H- oximes, can be regarded as a universally suitable reactivator (Worek et al.1997). Generally speaking, the H- oximes are promising antidotes because they are able to protect experimental animals from toxic effects, and improve the survival rate in animals intoxicated with lethal doses. The H- oximes are more effective in nerve agent intoxication than pralidoxime and obidoxime, especially when it comes to soman poisoning (Kassa 2002). bidoxime has been found to be inferior to HI 6 against soman, sarin, cyclosarin and VX, and pralidoxime is generally less potent than the other oximes (Worek ET al.1997). However, pralidoxime and especially obidoxime are sufficiently effective in the treatment of P- insecticide poisoning, since these insecticides are considerably less toxic than the nerve agents (Kassa, Bajgar 1996; Worek et al. 1996). bidoxime has turned out to be the most potent and most efficacious oxime in reactivating AChE inhibited by various classes of P insecticides and tabun (Worek et al.1997). 25
26 ITRDUCTI 3.7 Clinical recommendations It is important with immediate treatment after exposure to nerve agents. The first clinical signs of intoxication may not appear until about 50% of AChE is inhibited and some molecules of AChE will remain uninhibited for a long time (Johnson et al. 2000). The body has a huge surplus of AChE compared to what is needed to maintain normal body function, a kind of biochemical safety buffer. This means that initiation of treatment at the first sign of symptoms may be too late. It is important to notice that clinical signs of PC intoxication become severe before all AChE is inhibited, perhaps at a 75-90% inhibition (Johnson et al. 2000). At this point of enzymatic inhibition, the body responds by increasing ACh levels, and the ACh will compete with the P-oxons for the remaining uninhibited active sites on AChE (Johnson et al. 2000). When all AChE is inhibited, the patient will be dead. There is limited experience with nerve agent intoxication in human, but it is generally accepted that clinically relevant amounts of nerve agent can be found in blood for a shorter period of time than the P insecticides (Kassa 2002). The time of action for the nerve agents is in other words shorter than for the insecticides. Despite of the rapid disappearance from the blood, the toxic effects of the nerve agents may be prolonged because of the rapid ageing of the PC- AChE- complex, especially regarding soman (Kassa 2002; presko et al. 1998). In absence of a clinical response after administration of an oxime, it is unlikely that treatment in excess of hours will lead to further reactivation of AChE (Kassa 2002). Preferred treatment is a combination treatment of diazepam, oxime and atropine. These drugs should be administered as soon as possible after intoxication. In mild cases of intoxication, drying of bronchial secretions by administering atropine may be adequate therapy. This may be sufficient treatment until spontaneous reactivation of inhibited AChE or natural synthesis of new enzyme occurs (Johnson et al. 2000). Serious cases are treated with atropine, diazepam and oxime in combination with supportive ventilation, since AChE- inhibition affects the respiratory centre in the medulla oblongata (Johnson et al. 2000). In the case of a massive overdose, high concentrations of PC may remain in the blood for days, causing a depot that slowly releases inhibiting oxon (Moretto, Johnson 1987). ximes are cleared from the blood quite rapidly (Johnson 1975), and even if some inactivation has been achieved, another inhibition cycle and possible ageing of the inhibited AChE may follow. It is therefore important to achieve and maintain a sufficient blood concentration of the oxime, and not abort 26
27 ITRDUCTI treatment at an early point despite of immediate treatment response. It is recommended that the oxime should be administered for as long as atropine is indicated. For the majority of individuals this will be for less than 48 hours; the exception would be individuals exposed dermally to VX where a depot of VX might result in prolonged intoxication (Marrs 2004). Diazepam Diazepam is used as an anticonvulsant. Treatment with diazepam may be a valuable adjunct antidote in serious cases, even though the reasons for this are not clear. The structure is shown in Fig. 10. Diazepam appears to be more effective than other anticonvulsants such as the barbiturates, even though results from comparative studies have not been published. Diazepam has anti- GABA-ergic properties, which may cause diazepam to act as a specific antagonist in secondary GABA- ergic central pathways activated by ACh (Johnson et al. 2000). Diazepam may also counteract the unwanted CS side effects of atropine (Kusic et al. 1991). If convulsions are not controlled by diazepam, severe rhabdomyolysis may be the result (Johnson et al. 2000). CH 3 Cl Fig. 10: Structure of diazepam, used as an anticonvulsant in the treatment of anticholinesterase poisoning. ximes It has been found that bisquaternary pyridinium oximes inhibit K + - evoked ACh release from rat brain stem slices (Kloog et al. 1986). Bisquaternary pyridinium oximes appear to behave as agonists at presynaptic muscarinic autoreceptors in the brain, thereby mimicking ACh s negative feedback on its own release. These oximes have also shown to possess antimuscarinic activity at postsynaptic receptors of guinea pig ileum. This suggests that bispyridinium oximes may act simultaneously as presynaptic agonists and postsynaptic 27
28 ITRDUCTI antagonists at cholinergic synapses (Kloog et al. 1986). Three oximes are available in autoinjectors for self- and buddy- administration: Pralidoxime as the methanesulphonate, methyl sulphate and chloride salts, obidoxime dichloride and HI-6 dichloride. Patients still showing symptoms following use of the auto injector need additional doses of oxime and atropine to reactivate inhibited enzyme and reduce cholinergic symptoms (Kassa 2002). Atropine Atropine is a physiological antidote and completely blocks the action of ACh at muscarinic receptors (Johnson et al. 2000).The structure of atropine is shown in Fig. 11. Atropine also reverses the parasympathetic overstimulation resulting from the inhibition of AChE. At the dose levels normally needed, atropine is also an antagonist at muscarinic receptors in the CS, and may prevent convulsions and inhibition of the respiratory centre (Johnson et al. 2000). Atropine should be administered until the patient is fully atropinized; this will manifest itself with dry skin and sinus tachycardia (Vale 2004). CH 3 CH 2 H C CH Fig.11: Structure of atropine, a physiological antidote that blocks the action of ACh. Atropine reverses the neuronal overstimulation caused by inhibition of AChE 28
29 ITRDUCTI Reaching and maintaining a therapeutic oxime blood concentration There is still an ongoing debate as to what is the best way of reaching and maintaining a therapeutic oxime blood concentration. Bolus- dosing, and continuous intravenous infusion may both be an option. Bolus dosing Bolus-dosing schedules of the oximes currently available are based on data derived from human cases of P insecticide poisoning. Under extreme conditions, for instance in battle, single bolus- doses given intramuscularly is probably the only practical solution. Bolusdosing of the oxime by intravenous injection is by far more effective, but depending on the circumstances, intravenous bolus- dosing may not be possible. Continuous intravenous infusion An alternative method for administering therapeutic doses of an oxime is by continuous intravenous infusion. The threshold plasma concentration of oxime to counteract nerve agent poisoning in humans is presumed to be 4µg/ml (Sundwall 1961). Clinical studies indicate that both pralidoxime and obidoxime preferably should be given as continuous infusions after an initial bolus dose (Willems et al. 1992; Thiermann et al. 1997; Medicis et al. 1996). There is no reason to believe that these results cannot be extrapolated to include the newer H-oximes such as HI- 6 and HLø- 7. When considering nerve agent intoxication in for instance battle, the patient would most likely be given an intramuscular bolus- dose initially, and not receive intravenous infusion until medical personnel arrived to the scene or he or she was moved to a hospital or a similar facility. 29
30 ITRDUCTI 3.8 xime transport across the blood-brain-barrier; the use of prodrugs Treatment of anticholinesterase poisoning with oximes is highly effective in the PS, but fails to restore activity to a large fraction of the inhibited brain AChE (Ellyn, Wills 1968). The oximes are polar substances, a feature necessary for them to act as competitive agonists at the esteratic site of AChE. Unfortunately the oximes cannot readily pass the BBB because of their polar nature. PCs in general and nerve agents in particular are small, lipophilic compounds that can freely pass the BBB. Since anticholinesterase intoxication causes a wide array of physiological effects due to inhibitory action within the CS, it is desirable that the oxime is able to cross the BBB and reach the CS. A solution to this problem may be to formulate a prodrug of the oxime. A prodrug is a compound that can freely pass the BBB and enter the CS, and once inside the CS regain its active form. A polar compound once inside the CS is in reality trapped and unable to escape. Such a prodrug will in this context be lipophilic Synthesis of a pralidoxime prodrug A reduced, lipophilic pralidoxime analogue, able to being oxidized to active pralidoxime in vivo, was synthesized in 1975 (Bodor et al. 1975). This compound is called pro- PAM (1-methyl-1, 6-dihydropyridine-2- carbaldoxime hydrochloride). The structure of pro- PAM is shown in Fig. 12. Pro- PAM is rapidly oxidized to pralidoxime in vivo. Pro- PAM is a tertiary amine at physiological ph and is therefore in principle able to cross the BBB and enter the CS (Bodor et al. 1975). When inside the CS, pro- PAM can be oxidized to pralidoxime, and once on its active form it can reactivate inhibited AChE in the CS. CH 3 Cl H Fig.12: The pralidoxime prodrug pro- PAM. At physiological ph this compound exists as its free base, and it is significantly more lipophilic than pralidoxime. It can be quantitatively oxidized to pralidoxime, making it a pralidoxime prodrug (Bodor et al. 1975). 30
31 ITRDUCTI Problems associated with the pralidoxime prodrug Despite of pro- PAM s lipophilic character, investigations have shown that pro- PAM does not have a better effect in the treatment of anticholinesterase intoxication than pralidoxime (Boscovic et al. 1980; Clement 1979; Heffron, Hobbiger 1980). Research shows that pro- PAM has limited effect when given subcutaneously. This is probably related to the short halflives for formation (t ½ = 1 min) and elimination (t ½ 20 min) of the dihydropyridine prodrug in combination with slow transport from the site of injection to the brain (Kinley et al. 1982). Intravenous injection of an oxime is an effective administration route under certain clinical circumstances, but for emergency first- aid the intramuscular and subcutaneous routes is more practical. Under these conditions, it is unlikely that pro- PAM will be more effective in reactivating inhibited AChE in the CS than pralidoxime, because of minor differences in the degree of reactivation of PC- inhibited brain AChE (Kinley et al. 1982). 31
32 AIM F THE STUDY 4 AIM F THE STUDY Little has happened the last decade in regard to synthesizing therapeutically active prodrugs of known pyridine oximes, and this is definitely an area of great interest in the future. In my master thesis I have been interested in oximes, and potential prodrugs of these oximes. In this master thesis, the following goals were set: Achieve an understanding of the biochemistry of anticholinesterases, anticholinesterase poisoning, oximes, and oxime facilitated AChE reactivation Synthesize a few well known mono- and bispyridinium oximes Synthesis of potential oxime prodrugs based on reduction of the pyridine ring system Development of improved synthetic methods for synthesis of mono- and bispyridinium oximes If any compounds were to exhibit prodrug potential, these were to be tested against anticholinesterase poisoning by the orwegian Defense Research Establishment (FFI) 32
33 RESULTS AD DISCUSSI 5 RESULTS AD DISCUSSI 5.1 Synthesis Pralidoxime/ 2- PAM in acetone The synthesis of 2- PAM iodide was performed as described by Shek et al with some modifications of the protocol. The procedure was originally meant for synthesis of [ 14 C] methyl labeled 2- PAM iodide, and the methyl iodide added to the solution was a mixture of 14 C methyl iodide and regular methyl iodide. In this synthesis the temperature was kept at approximately 50 ºC under normal pressure and the reaction mixture was allowed to react for 24 hours, thereby deviating from the original protocol where a pressure bottle was used, and the temperature was kept at 95ºC for 6 hours. Two separate experiments were completed. The yields were 44.2% and 54%, respectively. The difference in yield, though considerable, is probably due to the operator, since the molar ratio in the two experiments was equal. The structure was confirmed by 1 H- MR- and MS- data for both experiments, and the structures of the two reaction product were identical. The high temperature in the reference article seems not to be essential for formation of the reaction product (2-PAM), at least not when the reaction time is increased. The reaction mechanism for the synthesis of 2- PAM is shown in Fig. 13 below. H CH 3 I H I CH 3 Fig. 13: Reaction mechanism of 2- PAM synthesis 33
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Chemistry Module Quiz for Science and Technology for NPTS Spring 2017
 Name Chemistry Module Quiz for Science and Technology for NPTS Spring 2017 1. Write the term in the blank that corresponds to the following definitions. (1 point each) a. Any chemical reactant that takes
Name Chemistry Module Quiz for Science and Technology for NPTS Spring 2017 1. Write the term in the blank that corresponds to the following definitions. (1 point each) a. Any chemical reactant that takes
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
Technical Inquiry Sarin/GB Overview
 Technical Inquiry Sarin/GB Overview Developed by: HDIAC 104 Union Valley Rd. Oak Ridge, TN 37830 HDIAC Contract Number: FA8075-13-D-0001 Technical Inquiry Summary: HDIAC received an inquiry to provide
Technical Inquiry Sarin/GB Overview Developed by: HDIAC 104 Union Valley Rd. Oak Ridge, TN 37830 HDIAC Contract Number: FA8075-13-D-0001 Technical Inquiry Summary: HDIAC received an inquiry to provide
Organization of the nervous system. Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2
 Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
The Nervous System. Nerve Impulses. Resting Membrane Potential. Overview. Nerve Impulses. Resting Membrane Potential
 The Nervous System Overview Nerve Impulses (completed12/03/04) (completed12/03/04) How do nerve impulses start? (completed 19/03/04) (completed 19/03/04) How Fast are Nerve Impulses? Nerve Impulses Nerve
The Nervous System Overview Nerve Impulses (completed12/03/04) (completed12/03/04) How do nerve impulses start? (completed 19/03/04) (completed 19/03/04) How Fast are Nerve Impulses? Nerve Impulses Nerve
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS
 COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Animal structure and function
 Animal structure and function The nervous system Parts of the nervous system 43C, 44B, 45D Brain structure and function Eyes Retina Neurons: How neurons communicate: Resting potential: The resting
Animal structure and function The nervous system Parts of the nervous system 43C, 44B, 45D Brain structure and function Eyes Retina Neurons: How neurons communicate: Resting potential: The resting
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Acetylcholine and An.cholinesterase Drugs. Nicholas J. Silvestri, M.D. Assistant Professor of Neurology
 Acetylcholine and An.cholinesterase Drugs Nicholas J. Silvestri, M.D. Assistant Professor of Neurology Acetylcholine Major neurotransmi?er Involved in both central, autonomic, and peripheral nervous system
Acetylcholine and An.cholinesterase Drugs Nicholas J. Silvestri, M.D. Assistant Professor of Neurology Acetylcholine Major neurotransmi?er Involved in both central, autonomic, and peripheral nervous system
R7.3 Receptor Kinetics
 Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Information processing. Divisions of nervous system. Neuron structure and function Synapse. Neurons, synapses, and signaling 11/3/2017
 Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Nervous System AP Biology
 Nervous System 2007-2008 Why do animals need a nervous system? What characteristics do animals need in a nervous system? fast accurate reset quickly Remember Poor think bunny! about the bunny signal direction
Nervous System 2007-2008 Why do animals need a nervous system? What characteristics do animals need in a nervous system? fast accurate reset quickly Remember Poor think bunny! about the bunny signal direction
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lectures for Biology, Eighth Edition Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp and Janette Lewis Copyright
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lectures for Biology, Eighth Edition Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp and Janette Lewis Copyright
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Nerve Signal Conduction. Resting Potential Action Potential Conduction of Action Potentials
 Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
ACETONE. PRODUCT IDENTIFICATION CAS NO EINECS NO MOL WT H.S. CODE Oral rat LD50: 5800 mg/kg
 ACETONE www.pawarchemicals.com PRODUCT IDENTIFICATION CAS NO 67-64-1 EINECS NO. 200-662-2 FORMULA (CH3)2C=O MOL WT. 58.08 H.S. CODE 2914.11 TOXICITY SYNONYMS Oral rat LD50: 5800 mg/kg Dimethyl ketone;
ACETONE www.pawarchemicals.com PRODUCT IDENTIFICATION CAS NO 67-64-1 EINECS NO. 200-662-2 FORMULA (CH3)2C=O MOL WT. 58.08 H.S. CODE 2914.11 TOXICITY SYNONYMS Oral rat LD50: 5800 mg/kg Dimethyl ketone;
Bio-elements. Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components.
 Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
BIOLOGY. 1. Overview of Neurons 11/3/2014. Neurons, Synapses, and Signaling. Communication in Neurons
 CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016
 PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS
 MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS The following questions illustrate the range of the test in terms of the abilities measured, the disciplines covered, and the difficulty of the questions
MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS The following questions illustrate the range of the test in terms of the abilities measured, the disciplines covered, and the difficulty of the questions
10/16/17 ACIDS AND BASES, DEFINED WATER IS AMPHOTERIC OUTLINE. 9.1 Properties of Acids and Bases. 9.2 ph. 9.3 Buffers
 ACIDS AND BASES, DEFINED A hydrogen atom contains a proton and an electron, thus a hydrogen ion (H + ) is a proton: Acids: Proton (H + ) transfer between molecules is the basis of acid/base chemistry Ø
ACIDS AND BASES, DEFINED A hydrogen atom contains a proton and an electron, thus a hydrogen ion (H + ) is a proton: Acids: Proton (H + ) transfer between molecules is the basis of acid/base chemistry Ø
Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
C a h p a t p e t r e r 6 E z n y z m y e m s
 Chapter 6 Enzymes 4. Examples of enzymatic reactions acid-base catalysis: give and take protons covalent catalysis: a transient covalent bond is formed between the enzyme and the substrate metal ion catalysis:
Chapter 6 Enzymes 4. Examples of enzymatic reactions acid-base catalysis: give and take protons covalent catalysis: a transient covalent bond is formed between the enzyme and the substrate metal ion catalysis:
CHEM 345 Problem Set 07 Key
 CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
NOTES: CH 48 Neurons, Synapses, and Signaling
 NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
An Introduction to Metabolism
 An Introduction to Metabolism I. All of an organism=s chemical reactions taken together is called metabolism. A. Metabolic pathways begin with a specific molecule, which is then altered in a series of
An Introduction to Metabolism I. All of an organism=s chemical reactions taken together is called metabolism. A. Metabolic pathways begin with a specific molecule, which is then altered in a series of
37 Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
12U Biochemistry Unit Test
 1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Exercise 9 - Petrochemicals and Climate
 113 Exercise 9 - Petrochemicals and Climate 1. The year of the first U.S. drilled oil well. c. 1859 2. Approximately, what percent of the world's remaining oil reserves are in the United States? a. 2%
113 Exercise 9 - Petrochemicals and Climate 1. The year of the first U.S. drilled oil well. c. 1859 2. Approximately, what percent of the world's remaining oil reserves are in the United States? a. 2%
Patrick, An Introduction to Medicinal Chemistry 5e Chapter 7 Enzymes as drug targets. 1) The structures of isoleucine and valine are as follows.
 Answers to end-of-chapter questions 1) The structures of isoleucine and valine are as follows. 2 C 2 2 C 2 3 C C 3 3 C C 3 Isoleucine Valine Isoleucine has a larger side chain than valine, and so there
Answers to end-of-chapter questions 1) The structures of isoleucine and valine are as follows. 2 C 2 2 C 2 3 C C 3 3 C C 3 Isoleucine Valine Isoleucine has a larger side chain than valine, and so there
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Introduction to Cellular Communication *
 OpenStax-CNX module: m53235 1 Introduction to Cellular Communication * Steven Telleen This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Why Cells Communicate
OpenStax-CNX module: m53235 1 Introduction to Cellular Communication * Steven Telleen This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Why Cells Communicate
Unit 2: Chemistry Test Review
 Name: Period: Unit 2: Chemistry Test Review 1. List the three states of matter. 2. Describe an atom in terms of its nucleus, valence,shell, electrons, protons, and neutrons. 3. Define the term element
Name: Period: Unit 2: Chemistry Test Review 1. List the three states of matter. 2. Describe an atom in terms of its nucleus, valence,shell, electrons, protons, and neutrons. 3. Define the term element
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
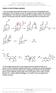 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Ch 33. The nervous system
 Ch 33 The nervous system AP bio schedule Tuesday Wed Thursday Friday Plant test Animal behavior lab Nervous system 25 Review Day (bring computer) 27 Review Day (bring computer) 28 Practice AP bio test
Ch 33 The nervous system AP bio schedule Tuesday Wed Thursday Friday Plant test Animal behavior lab Nervous system 25 Review Day (bring computer) 27 Review Day (bring computer) 28 Practice AP bio test
Dendrites - receives information from other neuron cells - input receivers.
 The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
Reminder: These notes are meant to supplement, not replace, the textbook and lab manual. Electrophilic Aromatic Substitution notes
 Reminder: These notes are meant to supplement, not replace, the textbook and lab manual. Electrophilic Aromatic Substitution notes History and Application: The rate of a reaction directly impacts the commercial
Reminder: These notes are meant to supplement, not replace, the textbook and lab manual. Electrophilic Aromatic Substitution notes History and Application: The rate of a reaction directly impacts the commercial
Biochemistry. Lecture 8 Enzyme Kinetics
 Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water
 Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Lec.1 Chemistry Of Water
 Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lab 3: Solubility of Organic Compounds
 Lab 3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Lab 3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Saba Al Fayoumi. Tamer Barakat. Dr. Mamoun Ahram + Dr. Diala Abu-Hassan
 1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
Synthesis and In Vitro Evaluation of 2-PAM Analogs as Potential Resurrecting Agents for. Aged Human Acetylcholinesterase
 Synthesis and In Vitro Evaluation of 2-AM Analogs as otential Resurrecting Agents for Aged uman Acetylcholinesterase S. ilanthi Yasapala Department of Chemistry University of Iowa 1 Introduction Acetylcholinesterase
Synthesis and In Vitro Evaluation of 2-AM Analogs as otential Resurrecting Agents for Aged uman Acetylcholinesterase S. ilanthi Yasapala Department of Chemistry University of Iowa 1 Introduction Acetylcholinesterase
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry
 Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
Acids, Bases, Salts, Buffers
 Acids, Bases, Salts, Buffers Acids, Bases, Salts, Buffers An acid is any solute that dissociates in a solution and releases hydrogen ions, thereby lowering ph Since a hydrogen ion consist solely of a proton,
Acids, Bases, Salts, Buffers Acids, Bases, Salts, Buffers An acid is any solute that dissociates in a solution and releases hydrogen ions, thereby lowering ph Since a hydrogen ion consist solely of a proton,
Chapter Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow,
 Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
MEMBRANE STRUCTURE. Lecture 9. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
A. Visceral and somatic divisions. B. Sympathetic and parasympathetic divisions. C. Central and peripheral divisions
 Ch 8: Neurons: Cellular and Network Properties, Part 1 Review of the Nervous System Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve cell
Ch 8: Neurons: Cellular and Network Properties, Part 1 Review of the Nervous System Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve cell
Chapter 15 Amines. Amines contain one or more organic groups bonded to nitrogen the compounds have these general formulas:
 Chapter 15 Amines Amines have single bonds of Carbon bound to Nitrogen. Many of the molecules that carry chemical messages (such as neurotransmitters) are amines. They are present in many kinds of essential
Chapter 15 Amines Amines have single bonds of Carbon bound to Nitrogen. Many of the molecules that carry chemical messages (such as neurotransmitters) are amines. They are present in many kinds of essential
Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions:
 Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Introduction Acetylcholinesterase (AChE) is one of the most important enzymes involved in nerve transmission. The enzyme is bound to cellular membrane
 Cell Technology PROTOCOL acella - AChE * Bioluminescence Assay for Monitoring Acetylcholinesterase Activity *Patent Pending Contact Information Address Cell Technology Inc 950 Rengstorff Ave Suite D Mountain
Cell Technology PROTOCOL acella - AChE * Bioluminescence Assay for Monitoring Acetylcholinesterase Activity *Patent Pending Contact Information Address Cell Technology Inc 950 Rengstorff Ave Suite D Mountain
Toxicological Targets. Russell L. Carr Department of Basic Sciences College of Veterinary Medicine
 Toxicological Targets Russell L. Carr Department of Basic Sciences College of Veterinary Medicine Toxicology Definitions = study of poisons Poison = any agent capable of producing a deleterious response
Toxicological Targets Russell L. Carr Department of Basic Sciences College of Veterinary Medicine Toxicology Definitions = study of poisons Poison = any agent capable of producing a deleterious response
Membranes 2: Transportation
 Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
thebiotutor.com A2 Biology Unit 5 Responses, Nervous System & Muscles
 thebiotutor.com A2 Biology Unit 5 Responses, Nervous System & Muscles 1 Response Mechanism tropism Definition A growth movement of part of plant in response to a directional stimulus examples Positive:
thebiotutor.com A2 Biology Unit 5 Responses, Nervous System & Muscles 1 Response Mechanism tropism Definition A growth movement of part of plant in response to a directional stimulus examples Positive:
media), except those of aluminum and calcium
 1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION
 CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity
 ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity Absorption/Acid-Base Case (p. 42) Question #1: Drug Cetirizine Clemastin e Functional groups
ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity Absorption/Acid-Base Case (p. 42) Question #1: Drug Cetirizine Clemastin e Functional groups
AMINES. 3. Secondary When two hydrogen atoms are replaced by two alkyl or aryl groups.
 AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
2013 W. H. Freeman and Company. 6 Enzymes
 2013 W. H. Freeman and Company 6 Enzymes CHAPTER 6 Enzymes Key topics about enzyme function: Physiological significance of enzymes Origin of catalytic power of enzymes Chemical mechanisms of catalysis
2013 W. H. Freeman and Company 6 Enzymes CHAPTER 6 Enzymes Key topics about enzyme function: Physiological significance of enzymes Origin of catalytic power of enzymes Chemical mechanisms of catalysis
Chapter 22 Amines. Nomenclature Amines are classified according to the degree of substitution at nitrogen.
 CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
BIOLOGY. Neurons, Synapses, and Signaling CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 48 Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Lines of Communication The
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 48 Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Lines of Communication The
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
H H O C C O H Carboxylic Acids and Derivatives C CH 2 C. N Goalby chemrevise.org. Strength of carboxylic acids.
 19 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending -oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
19 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending -oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
Acetylcholinesterase Assay Kit (Fluorometric - Red)
 ab138873 Acetylcholinesterase Assay Kit (Fluorometric - Red) Instructions for Use For the detection of Acetylcholinesterase activity in blood, cell extracts, and in other solutions. This product is for
ab138873 Acetylcholinesterase Assay Kit (Fluorometric - Red) Instructions for Use For the detection of Acetylcholinesterase activity in blood, cell extracts, and in other solutions. This product is for
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Enzymes are biological macromolecules that increase the rate of the reaction. Six major groups of enzymes (pgs. 94-95/98-99) Oxidoreductases:
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Enzymes are biological macromolecules that increase the rate of the reaction. Six major groups of enzymes (pgs. 94-95/98-99) Oxidoreductases:
G. GENERAL ACID-BASE CATALYSIS
 G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
Class XII: Chemistry Chapter 13: Amines Top concepts
 Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
OT Exam 1, August 9, 2002 Page 1 of 8. Occupational Therapy Physiology, Summer Examination 1. August 9, 2002
 Page 1 of 8 Occupational Therapy Physiology, Summer 2002 Examination 1 August 9, 2002 Dr. Heckman's section is questions 1-6 and each question is worth 5 points for a total of 30 points. Dr. Driska's section
Page 1 of 8 Occupational Therapy Physiology, Summer 2002 Examination 1 August 9, 2002 Dr. Heckman's section is questions 1-6 and each question is worth 5 points for a total of 30 points. Dr. Driska's section
Experiment : Reduction of Ethyl Acetoacetate
 Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes
 Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes 1. Lipids are good energy-storage molecules because a) the can absorb a large amount of energy while maintaining a constant temperature b)
Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes 1. Lipids are good energy-storage molecules because a) the can absorb a large amount of energy while maintaining a constant temperature b)
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems
 Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
