COMPARISON OF WEEKLY AND DAILY WET DEPOSITION SAMPLES FROM AN EASTERN U.S. NATIONAL ATMOSPHERIC DEPOSITION PROGRAM SITE
|
|
|
- Briana Boyd
- 6 years ago
- Views:
Transcription
1 Illinois State Water Survey ATMOSPHERIC SCIENCES DIVISION SWS Contract Report 507 COMPARISON OF WEEKLY AND DAILY WET DEPOSITION SAMPLES FROM AN EASTERN U.S. NATIONAL ATMOSPHERIC DEPOSITION PROGRAM SITE by Elizabeth T. Prindiville and Van C. Bowersox Office of Precipitation Quality Prepared for the Baltimore Gas & Electric Company Baltimore, MD Champaign, Illinois December 1990 Illinois Department of Energy and Natural Resources
2 COMPARISON OF WEEKLY AND DAILY WET DEPOSITION SAMPLES FROM AN EASTERN U.S. NATIONAL ATMOSPHERIC DEPOSITION PROGRAM SITE by Elizabeth T. Prindiville and Van C. Bowersox Prepared for the Baltimore Gas & Electric Company Baltimore, MD Illinois State Water Survey 2204 Griffith Drive Champaign, Illinois December 1990
3 The body of this report was printed on recycled paper.
4 CONTENTS Introduction 1 Acknowledgments 1 Experimental Method 2 Results 8 Summary of Previously Published Studies 8 Test for Sample Evaporation 10 Test of Samples with Complete Measurement Sets 10 Sensitivity of Weekly Versus Daily Comparison to Size of Daily Set 15 Test of Samples with Complete Measurements Plus No-Volume Sample Sets 19 Assessing the Importance of the Unmeasured Mass from Low-Volume Samples 19 Test of All Valid Data Pairs 26 Test for Ion Balance Differences 28 Conclusion 31 References 32 TABLES 1. Test for Experimental Design Bias Concentration Analysis 6 2. Test for Experimental Design Bias Mass Analysis 7 3. Literature Review Summary 9 4. Summary of Data Collected Statistical Results for Samples with Complete Chemical Analyses Statistical Results for Samples with Complete Chemical Analyses Plus No-Volume Samples Regression Analysis Results Assessing the Importance of Unmeasured Mass from Low-Volume Samples Statistical Results for Samples with Complete Measurements Plus No-Volume Plus Low-Volume Samples Percent Differences in Mass between Weekly and Daily Sample Sets Statistical Results for Net Ion Balance Differences 29 Page FIGURES 1. Site location 3 2. Site layout 4 3. Sample volume comparison Notched box-and-whisker plot Analysis of variance Regression analysis Comparison of ph measurements 30
5 COMPARISON OF WEEKLY AND DAILY WET DEPOSITION SAMPLES FROM AN EASTERN U.S. NATIONAL ATMOSPHERIC DEPOSITION PROGRAM SITE by Elizabeth T. Prindiville and Van C. Bowersox INTRODUCTION Chemical constituents are continuously added to the atmosphere through natural processes and by human activities. Various monitoring networks, such as the National Atmospheric Deposition Program / National Trends Network (NADP/NTN), which has operated since 1978, have directed their efforts toward the quantification of the chemistry of wet-only deposition. Studies such as Sisterson et al. (1985) and Bowersox et al. (1990) have analyzed the sampling protocol employed in the various networks. In particular, the comparison between daily sampling (replace bucket after each event) and weekly sampling (replace bucket once a week) have been studied. The focus of these activities was to identify chemical differences resulting from temporal changes in sample collection. This report describes a controlled experiment to compare the chemical composition of daily and weekly samples. This experiment was conducted from January 1985 through February 1987 at an NADP site in central Maryland (designated MD03). Acknowledgments This work was sponsored by the Baltimore Gas & Electric Company of Baltimore, Maryland, through a cooperative agreement with the Illinois State Water Survey (University of Illinois at Urbana-Champaign). The authors would like to thank Bob Dalton for operation of the NADP site in central Maryland and Jack Lodge and Steve Farkas of the Baltimore Gas & Electric Company for their site management and input. Without the efforts of the analytical and data management groups at the NADP Central Analytical Laboratory of the Illinois State Water Survey, this work could not have been done. We would like to extend special thanks to Mark Peden, the laboratory manager. The sponsor of this work makes no warranty, express or implied, and assumes no liability with respect to the use of any information, apparatus, method, or process disclosed herein. This report was edited by Laurie Talkington. 1
6 EXPERIMENTAL METHOD This field study was designed to identify differences in the chemistry of NADP/NTN weekly and daily precipitation samples and to isolate the cause of the differences associated with sampling period length. Both weekly and daily samples were collected at the same site, both were collected with virtually identical wet deposition collectors, bom were handled using the same field collection and laboratory procedures, and both were analyzed at the same facility, the Central Analytical Laboratory (CAL) of the National Atmospheric Deposition Program at the Illinois State Water Survey (ISWS). Daily samples were collected each morning after precipitation occurred, and weekly samples were collected every Tuesday morning. To compare one or more daily samples unambiguously with a weekly sample, a daily sample was also collected every Tuesday, whether or not precipitation had occurred. NADP procedures were employed both in the field and at the CAL. Samples were sealed in the collection container, a 3.5-gallon polyethylene bucket, and sent by surface mail to the CAL. Upon arrival at the CAL, the precipitation was passed through a filter with 0.45-micron pores to remove insolubles from the sample and to stabilize the inorganic ions in solution. Sample filtration, the only sample preservation step taken, was completed within 72 hours of arrival at the CAL. This standard protocol has been used for NADP/NTN samples since Figure 1 shows the location where the study was conducted. Figure 2 is the site plan showing the specific locations of the NADP weekly and ISWS daily (or intercomparison) samplers. Location of the sampling equipment and separation of the equipment from surrounding obstructions met the NADP/NTN criteria for site installation. Both collectors were linked electronically, so that their open/close cycles would be nearly synchronized. Correct operation of the collectors was checked against the occurrence of precipitation by careful reviews of the precipitation gage record and the collector open/close record. Analog traces of these two records were captured on the precipitation gage chart, on which precipitation depth was resolved as a function of time. Results of this study were limited to uncontaminated, wet-only deposition samples. To test this experimental design for biases, weekly samples were compared to daily samples for which the sampling period was one week. Recall tiiat the daily sample was taken on 2
7 Figure 1. Site location 3
8 Figure 2. Site layout 4
9 the morning after precipitation occurred or on Tuesday morning, whichever came first During weeks with no precipitation or when precipitation did not occur until Monday, the daily sample was collected on Tuesday. In these cases, the sampling periods for daily and weekly samples were both one week. Because the sampling periods were virtually identical, the concentration measurements and sample volumes should have been the same. Daily samples that were complete one-week samples were compared to the corresponding weekly measurements. Table 1 lists the concentrations of the set of one-week daily and weekly samples that were compared. Both the mean, with its standard error (S.E.), and the median concentrations are tabulated. For the eight conservative cations and anions reported (SO 2-4, NO - 3, Cl -, NH + 4, Ca 2+, Mg 2+, Na +, and K + ), the coefficient of variation (COV) of the mean [=100 percent (standard error/mean)] ranged from 19 to 41 percent, except for daily K + measurements (282 percent). These large COVs indicate the highly variable nature of the concentrations in this small intercomparison data set. Further, tests of the null hypothesis (H o ) that the mean differences in the paired data set were zero showed that at the 5 percent significance level, this hypothesis could not be rejected for any measurement. Both parametric (t-test) and nonparametric (Wilcoxon) tests consistently showed that these eight samples were not significantly different. This suggests that there was no bias in the design of the experiment. In a final test for biases in the experimental design, the analyte masses and sample volumes in the one-week daily and weekly samples were compared. Analyte masses were calculated as the product of the measured concentration and the measured liquid volume of each sample. Results of the mean and its standard error and the median are presented in table 2. As for the concentration data, the COVs are large (25-56 percent), and the respective daily and weekly means and standard errors overlap for each variable tested. Furthermore, H o could not be rejected at the 5 percent level using either parametric or nonparametric tests. Tests of the experimental design showed no biases based on the subset of all precipitation samples in which the daily sampling period matched the weekly sampling period. 5
10 Table 1. Test for Experimental Design Bias Concentration Analysis Mean ± S.E. and median ph (ph units) Conductivity (micros/cm) Sulfate (mg/l) Nitrate (mg/l) Chloride (mg/l) Ammonium (mg/l) Calcium (mg/l) Magnesium (mg/l) Sodium (mg/l) Potassium (mg/l) Daily measurements 4.27 ± ± ± ± ± ± ± ± ± ± Weekly measurements 4.18 ± ± ± ± ± ± ± ± ± ±
11 Table 2. Test for Experimental Design Bias Mass Analysis Mean ± S.E. and median Volume (ml) Hydrogen (mg) Sulfate (mg) Nitrate (mg) Chloride (mg) Ammonium (mg) Calcium (mg) Magnesium (mg) Sodium (mg) Potassium (mg) Daily measurements ± ± ± ± ± ± ± ± ± ± Weekly measurements ± ± ± ± ± ± ± ± ± ±
12 RESULTS Summary of Previously Published Studies Results from published studies similar to this study were compared. The influence of sampling interval on chemical concentrations and depositions was found to be inconsistent. In general, the experimental designs of these studies lacked one or more of the controls included in the design of this study. Further, no explicit tests of design bias were reported in these accounts. That the results are inconsistent and difficult to compare is, perhaps, due to design biases, sampling protocols, or the approach used to summarize the results. Table 3 summarizes the key design features and results of previously published studies. The Sisterson et al. study (1985), which took place from April 1980 until March 1982, was most similar to this study. It is clear from the table that the weekly magnesium measurements were much higher than in tins study, while the daily ammonium measurements were higher by about me same percentage. Measurements for K +, Na +, and Cl - were not included in the analysis because of blank and analytical difficulties. The Topol et al. study (1986) compared daily and weekly sampling protocols at three sites in the Utility Acid Precipitation Study Program (UAPSP) network from October 1983 until October The samples containing 0.51 millimeter (mm) were discarded due to insufficient volume; however, the remaining daily samples were still compared to the weekly samples. The study reported here, however, addresses the problem of low-volume samples. The Madsen study (1982) lasted one year and was conducted in Florida. Results from both biweekly and daily sampling were compared to the weekly measurements, as seen on table 3. The normalized mass differences are similar to the results from the study reported here with daily NH + 4 masses much larger than the weekly values; however, these differences are statistically significant in this study, while they were not in the Madsen study. The depena analysis took place in central Pennsylvania from March 1981 until June Sample handling, storage, and analysis procedures followed the Multistate Atmospheric Power Production Pollution Study (MAP3S) program. The sample collectors were not set to open and close together, as they were in the other studies. Many data points were rejected due to low sample volume or to the application of the Chauvenet criterion, which removes outliers. The results of this study were different in direction from the other studies: daily measurements were 8
13 Table 3. Literature Review Summary Paper author S t a Normalized mass differences [(weekly - daily)/(daily)] 100% t e Ca 2+ Mg 2+ + NH 4 - NO 3 2- SO 4 Na + K + Cl - M o n t h s A e r o c h e m I n s y n c S a m e l a b Handling procedures Sisterson * * Y Y N weekly NADP ** Topol Topol Topol 1SWS Madsen depena Madsen Vet G a K s V t M d F 1 P a F 1 C a n * * 4.2 * 8.1 * Y N Y UAPSP Y N Y UAPSP * Y N Y UAPSP 25 Y Y Y NADP * 0.0 * Y N Y N N Y MAP3S Y N Y Biweekly 65.0 * N N N Monthly * Denotes significance at the 95th percentile. ** Flame photometry, atomic absorption, and liquid ion chromatography.
14 higher than weekly measurements for every analyte reported. In the present study, no data points were removed unless they had been contaminated or grossly mishandled. The Vet et al. study (1988) took place in Ontario, Canada, and accumulated data for 36 months. This study was conducted on a monthly basis and was concerned mainly with bulk versus wet-only sampling results. Comparisons of monthly to daily measurements were made, and the results showed that calcium and magnesium were more than 50 percent higher in monthly samples. Test for Sample Evaporation Evaporation of samples left in the field collector for one week could not be detected in this study. A comparison of daily composite and weekly sample volumes showed no biases. Figure 3 demonstrates the agreement between weekly and daily volumes. The line in the figure shows the one-to-one relationship. Because there is such excellent agreement between the daily and weekly volumes, all further comparisons are based on concentration alone. Test of Samples with Complete Measurement Sets To preclude any biases that may accrue from low-volume or no-volume samples, the initial comparison of daily and weekly samples was limited to those weekly or composite daily samples in which all analytes were measured/analyzed for all samples. Table 4 summarizes the data collected. None of the samples used for this initial comparison were low-volume "trace" samples or no-volume "blank" samples, nor were they coded for gross contamination or mishandling. In other words, each uncontaminated, one-week NADP precipitation sample with a complete set of analyses was compared with one or more uncontaminated daily precipitation samples with a complete set of analyses. These criteria were all satisfied in 40 cases (or weeks). Recall that 8 of these 40 cases were analyzed earlier to test for bias in the experimental design (see Experimental Method). For each set of daily samples in a case (i.e., one week), the weighted average concentration of each conservative ion was calculated, using the formula: 10
15 Figure 3. Sample volume comparison 11
16 Table 4. Summary of Data Collected Samples with complete analyses Low-volume samples out of total No-volume samples out of total Daily data 88 33/81 16/43 Weekly data 40 0/33 0/16 Total pairs
17 where the volume-weighted average concentration, C v, was calculated from the sample volumes, V j, and sample concentrations, C j. M was the number of daily precipitation samples in the week. Based on the volume-weighted average daily concentrations and corresponding weekly concentrations, the data set of 40 pairs of concentrations was created. The concentration distributions for the various ions were calculated for each of the 40 cases. Four statistical tests were applied to these distributions, three nonparametric and one parametric, to assess whether the daily composite data were biased relative to weekly measurements. A summary of the results of the statistical tests is presented in table 5. The rank correlation or Spearman test is a nonparametric test that uses data ranks, rather than data values. The volume-weighted average daily concentration and the weekly concentration for each variable are ranked separately. Then the differences between the ranks of paired observations are calculated to measure the disagreement between the pairs. The sum of squares of these differences is calculated and then scaled to fall between 1 (perfect agreement) and -1 (perfect disagreement). This test is sensitive to data extremes, and therefore it was the least preferred of the tests in this study. Data from this test suggest that the agreement between daily and weekly measurements is excellent, except for ammonium concentrations. The Wilcoxon signed rank test is designed for use with paired data, such as the concentration distributions from the daily and weekly sample sets. Each pair of values is compared by subtracting one from the other and converting the difference to a percentage. While the absolute magnitude of the differences is unimportant, the signs and ranks of the differences are important. These are used in the computation of the Z test statistic, which has a near-normal distribution. Values of 5 percent or less for the probability indicate that the null hypothesis (that is, the mean differences in the paired data set are zero) can be rejected at the 5 percent level of significance. Based on this test, all ions are accepted at the 95 percent level, except chloride. In the Kolmogorov-Smirnov two-sample test, the cumulative frequency distribution functions are determined for the two samples. Then an assessment is made as to whether the two functions can be independently drawn samples from the same population. Distances between the two distribution functions are calculated, and the significance levels of these deviations are shown in the table. A significance level of 5 percent or less indicates that the two samples (daily composite versus weekly) represent different phenomena. None of the values in the table are 13
18 Table 5. Statistical Results for Samples with Complete Chemical Analyses (percent) Rank correlation Wilcoxon signed rank Kolmogorov- Smirnov Student t-test Calcium Magnesium Potassium Ammonium Sodium Nitrate Chloride * Sulfate * Denotes significanc e at the 95th percenti] e. 14
19 statistically significant, although ammonium and potassium concentration distributions are below 80 percent. The student t-test is a parametric test that assumes that the data come from a normal distribution. All of the comparisons fall within the 95 percent confidence interval and therefore "pass" the student t-test. However, potassium and ammonium are closest to being rejected. While all the tests showed ion concentration distributions to be in agreement at a significance level of 5 percent, ammonium, chloride, and potassium showed consistently lower significance. Based on this weight of evidence, the concentrations of these three ions in these weekly and daily samples are most likely to be different. Sensitivity of Weekly Versus Daily Comparison to Size of Daily Set This study was designed to compare paired weekly and daily data sets by calculating a volume-weighted average concentration for the daily sample set for each week. This approach was used because it affords a direct and unambiguous comparison of samples collected in a controlled experiment in which paired observations are possible. Most other studies have not had the advantage of paired observations. Further, pairwise statistical tests yield more powerful and more insightful results. One question not yet addressed is whether contamination due to the number of samples in the daily set could bias the results. Each sample container is cleaned at the CAL according to NADP/NTN procedures; regular blind tests are performed to assess the effectiveness of these cleaning procedures. According to these tests, the contaminant masses are low enough to meet NADP/NTN standards, but they are not zero. As a result, each container has some background or "blank" contamination. Further contamination could result from sample handling when the container is installed and removed from the collector, when it is weighed and measured in the field laboratory, and when it is unpacked and processed at the CAL. Each handling step could contribute to the blank contaminant mass. While the importance of this contamination on comparisons of daily and weekly data has not been reported in the literature, the results of one previous study suggested a blank contamination problem (depena, et al., 1985). In that study, the ion concentrations in daily samples were higher than in weekly samples for every analyte reported. The low bias observed for every analyte in weekly samples was unique to that study. 15
20 To address the possible importance of blank contamination, an analysis of variance (ANOVA) was performed. The ANOVA is a test that compares the variance and the way the variance is partitioned in a data set to determine the relationship between the variance and some external variable. In this study, the ANOVA was applied to the ion concentration differences between the paired weekly and daily composite sets. The number of samples in the daily composite sample set was the prespecified external variable. ANOVA tests were performed for each conservative ion; the 56-pair data set was made up of the 40-pair set containing no lowvolume or no-volume samples plus the 16-pair set with one no-volume sample (see table 4). To display the results of these tests, "notched box-and-whisker" plots were used. Figure 4, an example of one of these plots, contains labels showing the meaning of the various marks. These plots display the 10th and 90th percentile values, the upper (75th percentile) and lower (25th percentile) quartile ranges, the median (50m percentile), and extreme values that lie outside of the "whiskers." The width of the box is proportional to the square root of the number of data points being displayed, and the notch surrounding the median provides a measure of the 95 percent confidence interval about the median. This confidence interval is based on the variability of the data set, and it is defined mathematically in the figure. Figure 5 shows the notched box-and-whisker plot for magnesium concentration differences as related to the number of samples in the daily composite set. Concentration differences were calculated as the weekly sample concentration minus the daily composite concentration. Negative differences indicated that the daily samples had more mass than the weekly samples. If the concentration differences decrease as the number of daily samples in the composite set increases, then the blank contaminant mass has a measurable effect on the results. Recall tiiat each sample adds its component of contamination to the set. For magnesium, the median concentration difference for all sample sizes is approximately zero, which implies tiiat the concentration difference is not affected by sample size. This is obvious from figure 5, where the median plus the confidence intervals are compared to one another. For each set, consisting of one to four samples per week, the confidence intervals overlap. The ANOVA results for each set of ion differences verify what is evident from the figure: concentration differences are not affected significandy by the number of samples in the daily composite set for magnesium. Results of the ANOVA tests for the other ions showed tiiat the number of samples in the daily composite set was not an important factor in concentration differences between weekly and 16
21 Figure 4. Notched box-and-whisker plot 17
22 Figure 5. Analysis of variance 18
23 daily samples; therefore, blank contamination was not an important factor in this study. From these results and the finding that the data were not sensitive to the number of samples collected, the 16 samples that contained a no-volume blank sample were included in the chemical comparisons of the two data sets. Test of Samples with Complete Measurements Plus No-Volume Sample Sets Table 6 presents the statistical summary of the comparisons of data sets of daily and weekly concentrations. The complete measurements of the 40-pair data set summarized in table 4 are combined with the 16-pair data set containing a no-volume blank sample. The statistical results in table 6 are stronger than in table 4 due to the larger data set. While the results are more obvious, all distributions are statistically insignificant at the 95 percent level. Notice that ammonium and chloride are now closer to having statistically significant differences in the daily and weekly distributions. Assessing the Importance of the Unmeasured Mass from Low-Volume Samples Of the 237 daily samples collected, 33 (or nearly 14 percent) were volumes of less than about 10 milliliters (ml). This is the operationally defined minimum volume needed to process a sample according to the CAL protocol. Samples of more than 10 ml are filtered and analyzed to obtain the full suite of cation and anion measurements, while samples of 10 ml or less are not filtered or measured for conservative cations or anions. If the volume of these samples is greater than 2 ml, a ph measurement is attempted. If the volume is greater than 5 ml, ph and conductance measurements are tried. Application of these standard procedures is contingent upon the quality of the sample and the success (or stability) of the attempted measurements. Measurements of samples with visible extrinsic contamination are avoided to preclude adsorption of contaminants to the glass ph electrode. Since chemical concentrations of ~17 percent of the samples are unknown, an approach was needed to assess the importance of the unmeasured mass of these samples. Some of the previous studies acknowledged the existence and possible importance of the unmeasured mass in low-volume samples, although no specific tests have been reported. In this study, data from the daily sample set with reported measurements were used to model or estimate the "missing mass" from samples with no measurements. The 115 daily samples, which 19
24 Table 6. Statistical Results for Samples with Complete Chemical Analyses Plus No-Volume Samples (percent) Rank correlation Wilcoxon signed rank Kolmogorov- Smirnov Student t-test Calcium Magnesium Potassium Ammonium Sodium Nitrate Chloride * Sulfate * Denotes significance at the 95th percentile. 20
25 comprised the 56 composite samples analyzed previously, were used to make mathematical estimates of the measurements for the 33 unmeasured low-volume samples. Based on scavenging theory, a relationship between concentration and volume is expected. For example, a soluble salt in the form of a monodispersed aerosol in the atmospheric boundary layer is captured by falling raindrops at a rate that approximates its concentration. That is, the removal of aerosol mass is proportional to the mass present This is referred to as a "first-order removal process," and the expected relationship of concentration to sample volume is logarithmic. None of the ions in precipitation samples strictly follow this simple conceptual model because none of the ions exist only in the boundary layer as monodispersed aerosols. Some fraction of the mass is scavenged well above the boundary layer during cloud formation. For some ions, a fraction of the mass is scavenged as a gas that is chemically transformed in the aqueous cloud media. For example, SO 2 dissolves in cloud water and is oxidized to sulfate by a number of mechanisms, most important of which is oxidation by H 2 O 2. In addition, aerosols in and above the boundary layer exist in a whole range of sizes, although sulfate aerosols tend to be submicron in size and calcium aerosols tend to be supermicron in size. These factors together result in a relationship between concentration and volume that is conceptually more complicated than a simple first-order removal process. As a result, the logarithmic model was only one of many that were attempted to fit a regressive relationship between concentration and volume. Figure 6 depicts the relationship of calcium concentrations versus sample volumes. In the figure, the "best fit" line of regression is represented by the solid line, and the two pairs of dashed lines represent the 95 percent confidence and prediction limits. The relationship is typical of most major inorganic ions in solution. Concentrations in low-volume samples exhibit a large variance, while in high volumes the variance is smaller. Concentrations in low volumes also tend to be higher than in high volumes; hence the tendency for an inverse relationship between concentration and volume. The large variance at low sample volumes reflects the extreme variability in atmospheric conditions during small precipitation events. Variations in air concentrations, evaporation of cloud droplets and precipitation, and sample contamination from collection and handling are all important factors in this variability. For the calcium concentrations in figure 6, the "best fit model" was a multiplicative model of the form concentration=(exp a ) (volume b ), (2) 21
26 Figure 6. Regression analysis 22
27 where a is the intercept, a constant; and b is the slope, also a constant. Values of a and b were determined from the minimum variance regression model. For calcium, the regression model was This model explained percent of the total variance of the calcium concentrations (hence R 2 = 53.01). Table 7 lists the best fit models for each ion. For all ions, the best fit model was a multiplicative regression of concentration to volume. These models had an R 2 ranging from 5.37 percent for sodium to percent for calcium. Given the large variability in precipitation chemistry data, models that account for half or more of the variance are very successful. These models were used to estimate the concentrations in low-volume trace samples having no measured concentration data. Thirty-three sets of daily samples contained a trace sample with no measured concentrations. Having estimated concentrations for these trace samples, volume-weighted average concentrations for the 33 sets were calculated. Composite concentrations for the 33 sets were calculated in an analogous fashion. As a basis for comparison, volume-weighted average concentrations for these 33 sets also were calculated assuming zero concentration and mass. This approach was used in some previously published studies. These two cases, one with a modeled or estimated mass and one with zero mass, were then compared with the full 56-pair data set. Since the 56-pair data set included no samples with missing measurements, the hypothesis was that its data distribution would emerge from the same population as the 33-pair data set with the modeled masses. Consequently, the 33-pair data set with zero-mass trace samples would have to be from a different population. Table 8 lists the results obtained from comparing the 56 composite daily concentrations to both the modeled trace samples and the trace samples with the missing concentration set at zero. Two statistical tests were used to compare the distributions, the Kolmogorov-Smirnov test and the student t-test. The first is a nonparametric test that exerts much less severe constraints on the actual distribution of data points. The student t-test is a parametric analysis; but data sets in this study contain significant outliers that could bias the results. In every case except nitrate, a better correlation was obtained between the 56 data points and the calculated trace values than the trace values left as zero. While none of the results were significant at the 95 percent level, 23
28 Table 7. Regression Analysis Results Correlation (R 2 ) (percent) Regression equation Calcium e (2.28) *X (-.714) Magnesium e (.145) *X (-.576) Potassium e (-.16) *X (-.439) Ammonium e (1.373) *X (-.463) Sodium 5.37 e (-1.15) *X (-.226) Nitrate e (3.202) *X (-.417) Chloride e (.208) *X (-.268) Sulfate e (2.614) *X (-.252) 24
29 Table 8. Assessing the Importance of Unmeasured Mass from Low-Volume Samples Trace sample concentration Kolmogorov - Smirnov (percent) Student t-test (percent) Calcium Magnesium Potassium Ammonium Sodium Nitrate Chloride Sulfate Zero Calculated Zero Calculated Zero Calculated Zero Calculated Zero Calculated Zero Calculated Zero Calculated Zero Calculated
30 potassium, ammonium, and sulfate were close. At a significance level of 85 percent, the distribution of approximately half of the ions was significantly different. This analysis suggests better agreement using calculated concentrations for trace samples. Thus these low-volume samples contain significant concentrations mat should not be ignored, that is, assumed to be zero. If actual chemical analysis is impossible on such small collected volumes, then some mathematical procedure must be used to estimate the missing mass. Test of AH Valid Data Pairs The calculated trace concentrations were used to obtain a composite daily sample for the 33 weeks in which trace samples were reported. These 33 points were combined with the 56 data points previously analyzed to form a data set composed of all valid pairs. A statistical analysis was employed once again to compare the daily and weekly concentration distributions. The results appear in table 9. Once again, table 9 can be compared to tables 5 and 6. Finally, to obtain the best estimate of the differences that could be observed in deposition from daily versus weekly sampling protocols, mass differences in the two data sets were calculated. The entire 89-point data set was used; for sample sets with trace samples, the mass was calculated using the respective algorithms in table 7. For each of the sample pairs an absolute mass difference between the weekly and daily values was calculated, and this difference was normalized by the daily mass and converted to a percentage. Table 10 lists the average normalized mass differences along with the respective standard errors. Statistically significant differences were found between the weekly and daily data sets for ammonium, sodium, nitrate, and sulfate at the 95th percentile level using the student t-test. The student t-test was used in this case because the mean of the distribution being compared to zero comes from a normalized data set; hence the use of a parametric test was appropriate. These results suggest significant and important losses of ammonium from weekly samples: about 12 percent It has been hypothesized (Sisterson et al., 1985) that ammonium degrades within the sample due to volatilization or biological conversion of the NH + 4 into NO - 3. This latter mechanism was not supported by this study because the nitrate was higher in the daily than in the weekly samples. The nitrate differences were only -7 percent, although at the 95 percent confidence level this average difference was significant. Daily sulfate masses were also higher by -5.5 percent, which was statistically significant but generally within analytical uncertainty. 26
31 Table 9. Statistical Results for Samples with Complete Measurements Plus No-Volume Plus Low-Volume Samples (percent) Rank correlation Wilcoxon signed rank Kolmogorov- Smirnov Student t-test Calcium Magnesium Potassium Ammonium Sodium Nitrate Chloride * Sulfate * Denotes statistical significance at the 95th percentile. Table 10. Percent Differences in Mass between Weekly and Daily Sample Sets Averaged normalized mass differences 100% [(weekly-daily)/daily] Calcium 4.28 ± 4.82 Magnesium 5.36 ± 5.76 Potassium ± 9.90 Ammonium ± 5.77 * * Denotes significance at the 95th percentile. Sodium ± 5.57 * Nitrate ± 2.25 * Chloride 3.96 ± 3.54 Sulfate ±2.13 * 27
32 Finally, it is important to note that all of the alkali and alkaline earth metals were higher in the weekly samples, while only the average sodium difference was statistically significant. At ~11 percent this difference exceeds analytical uncertainties and is important Previous studies, in particular Peden and Skowron (1978), have pointed to the slow solubilization of these metals from an exchangeable soil particle matrix as the possible cause for higher metal loadings in samples of longer duration. Test for Ion Balance Differences While daily and weekly ion concentrations differ, the effect of these differences on the sample acidity remains to be evaluated. To do so requires a calculation of the net ion balance of weekly and paired daily samples and then a comparison of the two distributions. The net ion balance for a sample is the difference of the anion and cation concentration sums in microequivalence units. For weekly samples, this is a straightforward calculation based on the actual measurements. For the daily composite samples, the volume-weighted average concentrations are first determined, and then used to calculate the net ion balance concentrations. To avoid the biases that may accrue from the modeled mass in the trace samples, this analysis was limited to the 56-pair data set previously analyzed. The results of the net ion balance calculations are reported in table 11. Daily values were subtracted from the weekly values, and the differences were then averaged. The table lists the average percent of normalized differences. To mitigate the influence of outliers, median differences are also shown. The averages of both distributions were compared to zero using the student t-test. Both distributions "passed" the test at the 95 percent level, which means that there is no significant difference in the weekly and daily ion balance results. Indeed, the average difference of approximately 2 microequivalents per liter (µeq/l) is virtually undetectable. Most ph measurement systems are not sufficiently sensitive to detect changes of 2µeq/L in free acidity in the ph 4 range of the typical samples from central Maryland. As a result, the net effect of anion concentration differences is on average offset by the cation concentration differences, which leaves the ph unchanged. Examination of the ph measurements for the daily and weekly sample sets confirms that the small anion and cation differences offset one another and the ph is unchanged. Figure 7 shows the cumulative frequency distributions of the daily and weekly ph measurements made at the CAL. For the daily samples, the ph values were not calculated from a weighted average 28
33 Table 11. Statistical Results for Net Ion Balance Differences (percent) Average of differences Average of normalized differences ± ± Median Student t-test
34 Figure 7. Comparison of ph measurements 30
35 of the daily samples for a week; rather they were the actual values for each sample. Hydrogen ion concentrations are conserved in such calculations, but the actual stoichiometric concentration of hydrogen ion is not conserved when samples are composited (Stensland and Bowersox, 1984). This occurs because precipitation is collected in a physically open system exposed to atmospheric trace gases, in particular CO 2, which chemically buffers the system. The distributions in figure 7 show that the curves are virtually identical, except for the extremes. The lowest and highest ph values in the daily data set are more extreme than for the weekly set. These differences manifest the weak buffering that occurs when precipitation events of different free acidities are composited, as they are during weekly sampling. The larger range of ph measurements in the daily set, however, apparently only affects the distribution below the 5th and above the 75th percentile. A statistical test of the two distributions suggests that they are from the same population, which is consistent with the net ion balance calculations listed in table 11. CONCLUSION Tests of the experimental design of this study showed no statistically significant biases, based on the subset of precipitation samples for which the daily sampling period matched the weekly sampling period. The weight of evidence from various tests suggests that low-volume samples are important and can significantly bias the comparison of weekly and daily sampling. The number of samples in the daily composite set did not significantly affect concentration differences between weekly and daily samples, so there was no evidence that bucket blank contamination was an important bias in this experiment. Evaporation of samples left in the field for one week could not be detected in this study. The normalized differences in mass expressed in percentages are listed in table 10. Values that are statistically significant from zero are shown with an asterisk. Ammonium in weekly samples appears to have an important low bias and sodium an important high bias. These differences are consistent with earlier studies by Sisterson et al. (1985) and Peden and Skowron (1978). Cumulative frequency distributions for daily and weekly ph measurements are not significantly different, although daily samples have both lower and higher values than weekly samples. This observation is consistent with the fact that the differences in total cation and anion concentrations typically offset one another. 31
36 REFERENCES Bowersox, V.C., D.L. Sisterson, and A.R. Olsen Acid rain, a world-wide phenomenon: a perspective from the United States. Intern. J. Environmental Studies 36: depena, R.G., K.C. Walker, L. Lebowitz, and J.G. Micka Wet deposition monitoring: effect of sampling period. Atmos. Environ. 19: Madsen, B.C An evaluation of sampling interval length on the chemical composition of wet-only deposition. Atmos. Environ. 16: Peden, M.E., and L.M. Skowron Ionic stability of precipitation samples. Atmos. Environ. 12: Sisterson, D.L., B.E. Wurfel, and B.M. Lesht Chemical differences between event and weekly precipitation samples in northeastern Illinois. Atmos. Environ. 19: Stensland, G.J., and V.C.Bowersox A comparison of methods of computing precipitation ph averages, In: Proceedings 77 th Annual APCA Meeting (paper ). Air Pollution Control Association, Pittsburgh, PA., 16pp. Topol, L.E., M. Lev-On, A.K. Pollack, and T.J. Permutt Comparison of Weekly and Daily Wet Deposition Sampling Results. UAPSP Report 113, Utility Acid Precipitation Study Program, Washington, DC, 123pp. Vet, R.J., A. Sirois, D.S. Jeffries, R.G. Semkin, N.W. Foster, P. Hazlett, and C.H. Chan Comparison of bulk, wet-only, and wet-plus-dry deposition measurements at the Turkey Lakes Watershed. Can. J. Fish. Aquat. Sci. 45:
The National Atmospheric Deposition Program (NADP)
 The National Atmospheric Deposition Program (NADP) Christopher Lehmann Director, Central Analytical Laboratory National Atmospheric Deposition Program Illinois State Water Survey - Prairie Research Institute
The National Atmospheric Deposition Program (NADP) Christopher Lehmann Director, Central Analytical Laboratory National Atmospheric Deposition Program Illinois State Water Survey - Prairie Research Institute
National Atmospheric Deposition Program 2002 Annual Summary
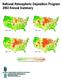 National Atmospheric Deposition Program 00 Annual Summary 5-7 0-5 - 7 000-00 0. 0.0 0.0 0.0 Average Ammonium Ion Concentration as NH (mg/l) 0 5 + 00 Highlights In 00, scientists, students, educators, and
National Atmospheric Deposition Program 00 Annual Summary 5-7 0-5 - 7 000-00 0. 0.0 0.0 0.0 Average Ammonium Ion Concentration as NH (mg/l) 0 5 + 00 Highlights In 00, scientists, students, educators, and
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING
 QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1987 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1987 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
Discontinuation of Support for Field Chemistry Measurements in the National Atmospheric Deposition Program National Trends Network (NADP/NTN)
 Discontinuation of Support for Field Chemistry Measurements in the National Atmospheric Deposition Program National Trends Network (NADP/NTN) Christopher Lehmann, NADP Program Office, Illinois State Water
Discontinuation of Support for Field Chemistry Measurements in the National Atmospheric Deposition Program National Trends Network (NADP/NTN) Christopher Lehmann, NADP Program Office, Illinois State Water
QUALITY ASSURANCE REPORT NATIONAL ATMOSPHERIC DEPOSITION PROGRAM,, 1996 and 1997
 Miscellaneous Publication 188 QUALITY ASSURANCE REPORT NATIONAL ATMOSPHERIC DEPOSITION PROGRAM,, 1996 and 1997 Laboratory Operations Central Analytical Laboratoryy NATIONAL ATMOSPHERIC DEPOSITION PROGRAM
Miscellaneous Publication 188 QUALITY ASSURANCE REPORT NATIONAL ATMOSPHERIC DEPOSITION PROGRAM,, 1996 and 1997 Laboratory Operations Central Analytical Laboratoryy NATIONAL ATMOSPHERIC DEPOSITION PROGRAM
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING
 QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1991 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1991 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
National Atmospheric Deposition Program
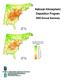 National Atmospheric Deposition Program 00 Annual ummary NAtChem 0 - Average non-sea-salt ulfate Wet Deposition NAtChem - - - - - - - - - > NADP and NAtChem In, the National Atmospheric Deposition Program
National Atmospheric Deposition Program 00 Annual ummary NAtChem 0 - Average non-sea-salt ulfate Wet Deposition NAtChem - - - - - - - - - > NADP and NAtChem In, the National Atmospheric Deposition Program
National Atmospheric Deposition Program 2004 Annual Summary
 National Atmospheric Deposition Program 00 Annual Summary ID MT WY Class I Areas UT CO Average Annual Wet Deposition of Inorganic Nitrogen (kg/ha), 000-00...... 00 Highlights In 00, scientists, students,
National Atmospheric Deposition Program 00 Annual Summary ID MT WY Class I Areas UT CO Average Annual Wet Deposition of Inorganic Nitrogen (kg/ha), 000-00...... 00 Highlights In 00, scientists, students,
Flushing Out the Moles in Lab: The Reaction of Calcium Chloride with Carbonate Salts
 Flushing Out the Moles in Lab: The Reaction of Calcium Chloride with Carbonate Salts Pre-lab Assignment: Reading: 1. Chapter sections 3.3, 3.4, 3.7 and 4.2 in your course text. 2. This lab handout. Questions:
Flushing Out the Moles in Lab: The Reaction of Calcium Chloride with Carbonate Salts Pre-lab Assignment: Reading: 1. Chapter sections 3.3, 3.4, 3.7 and 4.2 in your course text. 2. This lab handout. Questions:
During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction:
 Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING
 QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1994 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1994 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING
 QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1988 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1988 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
NITROGEN AND ITS COMPOUNDS Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure.
 NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
(a) Complete Figure 9 by placing one tick in each row to show whether the salt is soluble or insoluble. salt soluble insoluble.
 1 The method used to prepare a salt depends on its solubility in water. (a) Complete Figure 9 by placing one tick in each row to show whether the salt is soluble or insoluble. ammonium chloride salt soluble
1 The method used to prepare a salt depends on its solubility in water. (a) Complete Figure 9 by placing one tick in each row to show whether the salt is soluble or insoluble. ammonium chloride salt soluble
Chapter 6. Chemical Reactions. Sodium reacts violently with bromine to form sodium bromide.
 Chapter 6 Chemical Reactions Sodium reacts violently with bromine to form sodium bromide. Evidence of Chemical Reactions Chemical Equations Reactants Products Reactant(s): Substance(s) present before the
Chapter 6 Chemical Reactions Sodium reacts violently with bromine to form sodium bromide. Evidence of Chemical Reactions Chemical Equations Reactants Products Reactant(s): Substance(s) present before the
METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE
 METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE 1.0 SCOPE AND APPLICATION 1.1 This method can be used for measuring total solubilized nitrate in drinking
METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE 1.0 SCOPE AND APPLICATION 1.1 This method can be used for measuring total solubilized nitrate in drinking
Glossary of Common Laboratory Terms
 Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
REMEDIATION OF SALT IMPACTED GROUNDWATER WITH ELECTROKINETICS. Paper by: Sean Kelly, Rick Churko, Sean Frisky, Anjum Mullick, Stuart Torr.
 REMEDIATION OF SALT IMPACTED GROUNDWATER WITH ELECTROKINETICS. Paper by: Sean Kelly, Rick Churko, Sean Frisky, Anjum Mullick, Stuart Torr. Alberta Transportation is supporting leading research in the use
REMEDIATION OF SALT IMPACTED GROUNDWATER WITH ELECTROKINETICS. Paper by: Sean Kelly, Rick Churko, Sean Frisky, Anjum Mullick, Stuart Torr. Alberta Transportation is supporting leading research in the use
Part A Answer all questions in this part.
 Part A Directions (1-24): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
Part A Directions (1-24): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow
 1. Application Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow Parameters and Codes: Nitrogen, ammonia, dissolved, I-2522-90 (mg/l as N): 00608 Nitrogen, ammonia, total-in-bottom-material,
1. Application Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow Parameters and Codes: Nitrogen, ammonia, dissolved, I-2522-90 (mg/l as N): 00608 Nitrogen, ammonia, total-in-bottom-material,
Naming salts. Metal Acid Salt. Sodium hydroxide reacts with Hydrochloric acid to make Sodium chloride
 Naming salts A salt is any compound formed by the neutralisation of an acid by a base. The name of a salt has two parts. The first part comes from the metal, metal oxide or metal carbonate. The second
Naming salts A salt is any compound formed by the neutralisation of an acid by a base. The name of a salt has two parts. The first part comes from the metal, metal oxide or metal carbonate. The second
Part 01 - Notes: Reactions & Classification
 Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Glossary. The ISI glossary of statistical terms provides definitions in a number of different languages:
 Glossary The ISI glossary of statistical terms provides definitions in a number of different languages: http://isi.cbs.nl/glossary/index.htm Adjusted r 2 Adjusted R squared measures the proportion of the
Glossary The ISI glossary of statistical terms provides definitions in a number of different languages: http://isi.cbs.nl/glossary/index.htm Adjusted r 2 Adjusted R squared measures the proportion of the
Report of the Inter-laboratory Comparison Project 2004 on Wet Deposition
 The Network Center for the Acid Deposition Monitoring Network in East Asia Report of the Inter-laboratory Comparison Project 2004 on Wet Deposition 7th Attempt November 2005 Acid Deposition and Oxidant
The Network Center for the Acid Deposition Monitoring Network in East Asia Report of the Inter-laboratory Comparison Project 2004 on Wet Deposition 7th Attempt November 2005 Acid Deposition and Oxidant
IMADUDDIN SCHOOL Second Term Examination 2017
 Index Register Class number number Name IMADUDDIN SCHOOL Second Term Examination 2017 GRADE 9 CHEMISTRY 5070/02 Paper 2 Theory Nov 2017 TIME 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Write your name,
Index Register Class number number Name IMADUDDIN SCHOOL Second Term Examination 2017 GRADE 9 CHEMISTRY 5070/02 Paper 2 Theory Nov 2017 TIME 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Write your name,
Bases = Anti-Acids. The process is called neutralization (neither acidic nor basic) O H 3 2H 2
 Bases = Anti-Acids Example: HCl(aq) + H 2 (l) à H 3 + (aq) + Cl - (aq) NaH(aq) à Na + (aq) + H - (aq) H 3 + (aq) + H - (aq) à 2H 2 (l) Net: HCl(aq) + NaH(aq) à Na + (aq) + Cl - (aq) + H 2 (l) The process
Bases = Anti-Acids Example: HCl(aq) + H 2 (l) à H 3 + (aq) + Cl - (aq) NaH(aq) à Na + (aq) + H - (aq) H 3 + (aq) + H - (aq) à 2H 2 (l) Net: HCl(aq) + NaH(aq) à Na + (aq) + Cl - (aq) + H 2 (l) The process
GRAVIMETRIC ANALYSIS OF A CHLORIDE SALT. REFERENCES: Nelson, J., Chemistry: The Central Science, 3 rd edition, Prentice-Hall, 1985
 1 GRAVIMETRIC ANALYSIS OF A CHLORIDE SALT REFERENCES: Nelson, J., Chemistry: The Central Science, 3 rd edition, Prentice-Hall, 1985 Typical techniques used in gravimetric analyses by quantitatively determining
1 GRAVIMETRIC ANALYSIS OF A CHLORIDE SALT REFERENCES: Nelson, J., Chemistry: The Central Science, 3 rd edition, Prentice-Hall, 1985 Typical techniques used in gravimetric analyses by quantitatively determining
Draw one line from each solution to the ph value of the solution. Solution ph value of the solution
 1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
Review of the IMPROVE Equation for Estimating Ambient Light Extinction
 Review of the IMPROVE Equation for Estimating Ambient Light Extinction Jenny Hand 1 Bill Malm 2 1 CIRA, Colorado State University 2 National Park Service OUTLINE Introduction Sampling Biases Chemical forms
Review of the IMPROVE Equation for Estimating Ambient Light Extinction Jenny Hand 1 Bill Malm 2 1 CIRA, Colorado State University 2 National Park Service OUTLINE Introduction Sampling Biases Chemical forms
CH 221 Chapter Four Part II Concept Guide
 CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
Title: Gravimetric verification of chloride concentration from a precipitate of silver nitrate.
 Title: Gravimetric verification of chloride concentration from a precipitate of silver nitrate. Introduction: Gravimetric analysis is a method of quantitative chemical analysis used to determine the concentration
Title: Gravimetric verification of chloride concentration from a precipitate of silver nitrate. Introduction: Gravimetric analysis is a method of quantitative chemical analysis used to determine the concentration
CCR RULE GROUNDWATER STATISTICAL METHOD SELECTION CERTIFICATION
 This document summarizes the statistical methods which will be utilized for evaluating groundwater analytical results associated with the s (hereafter referred to as the Hatfield CCR unit ) CCR groundwater
This document summarizes the statistical methods which will be utilized for evaluating groundwater analytical results associated with the s (hereafter referred to as the Hatfield CCR unit ) CCR groundwater
"No matter what costume you wear, when you start eating Halloween candy, you will be a goblin. - Unknown
 CHEMISTRY 101 Hour Exam II October 31, 2017 Andino/McCarren Name Signature Section "No matter what costume you wear, when you start eating Halloween candy, you will be a goblin. - Unknown This exam contains
CHEMISTRY 101 Hour Exam II October 31, 2017 Andino/McCarren Name Signature Section "No matter what costume you wear, when you start eating Halloween candy, you will be a goblin. - Unknown This exam contains
CHEMISTRY 101 Hour Exam II. Dr. D. DeCoste T.A (30 pts.) 16 (15 pts.) 17 (15 pts.) Total (60 pts)
 CHEMISTRY 101 Hour Exam II March 16, 2017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 5 numbered pages. Check now to make sure you have a complete exam. You have one hour and
CHEMISTRY 101 Hour Exam II March 16, 2017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 5 numbered pages. Check now to make sure you have a complete exam. You have one hour and
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING
 QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1993 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
QUALITY ASSURANCE REPORT NADP/NTN DEPOSITION MONITORING Laboratory Operations Central Analytical Laboratory 1993 NATIONAL ATMOSPHERIC DEPOSITION PROGRAM A Cooperative Research Program of the State Agricultural
Solutions & Solubility: Net Ionic Equations (9.1 in MHR Chemistry 11)
 Solutions & Solubility: Net Ionic Equations (9.1 in MHR Chemistry 11) 1 Solubility vs. Temperature 2 Solubility Table Anions SOLUBILITY Table 8.3 page 363 in MHR Cl Br I S OH SO CO 3 PO 3 SO 3 C 2 H 3
Solutions & Solubility: Net Ionic Equations (9.1 in MHR Chemistry 11) 1 Solubility vs. Temperature 2 Solubility Table Anions SOLUBILITY Table 8.3 page 363 in MHR Cl Br I S OH SO CO 3 PO 3 SO 3 C 2 H 3
Shirley E. Clark, Ph.D., P.E., D. WRE Penn State Harrisburg. Robert Pitt, Ph.D., P.E., BCEE, D. WRE University of Alabama
 Shirley E. Clark, Ph.D., P.E., D. WRE Penn State Harrisburg Robert Pitt, Ph.D., P.E., BCEE, D. WRE University of Alabama Site Stormwater Characteristics and Permit Limits Analytes on Permit 90 th percentile
Shirley E. Clark, Ph.D., P.E., D. WRE Penn State Harrisburg Robert Pitt, Ph.D., P.E., BCEE, D. WRE University of Alabama Site Stormwater Characteristics and Permit Limits Analytes on Permit 90 th percentile
Understanding and Interpreting Soil and Plant Tissue Lab Reports
 Understanding and Interpreting Soil and Plant Tissue Lab Reports Dirk Holstege Director, UC Davis Analytical Laboratory dmholstege@ucdavis.edu 530-752-0148 224 Hoagland Hall UC Davis Anlab.ucdavis.edu
Understanding and Interpreting Soil and Plant Tissue Lab Reports Dirk Holstege Director, UC Davis Analytical Laboratory dmholstege@ucdavis.edu 530-752-0148 224 Hoagland Hall UC Davis Anlab.ucdavis.edu
insoluble partial very soluble (< 0.1 g/100ml) solubility (> 1 g/100ml) Factors Affecting Solubility in Water
 Aqueous Solutions Solubility is a relative term since all solutes will have some solubility in water. Insoluble substances simply have extremely low solubility. The solubility rules are a general set of
Aqueous Solutions Solubility is a relative term since all solutes will have some solubility in water. Insoluble substances simply have extremely low solubility. The solubility rules are a general set of
Solubility Rules and Net Ionic Equations
 Solubility Rules and Net Ionic Equations Why? Solubility of a salt depends upon the type of ions in the salt. Some salts are soluble in water and others are not. When two soluble salts are mixed together
Solubility Rules and Net Ionic Equations Why? Solubility of a salt depends upon the type of ions in the salt. Some salts are soluble in water and others are not. When two soluble salts are mixed together
CSUS Department of Chemistry Experiment 3 Chem.1A
 Experiment 3: Reactions in Aqueous Solutions: Pre lab Name: 10 points Due at the beginning of lab. Section: 1. Precipitation Reactions a. On the reverse side of this page or on a separate piece of paper,
Experiment 3: Reactions in Aqueous Solutions: Pre lab Name: 10 points Due at the beginning of lab. Section: 1. Precipitation Reactions a. On the reverse side of this page or on a separate piece of paper,
CHEMISTRY 102A Spring 2012 Hour Exam II. 1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with:
 . My answers for this Chemistry 0 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E. A sample of LSD (D-lysergic acid diethylamide, C 4 H 30
. My answers for this Chemistry 0 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E. A sample of LSD (D-lysergic acid diethylamide, C 4 H 30
JOHN BURKE HIGH SCHOOL
 JOHN BURKE HIGH SCHOOL Chemistry 2202 Midterm Examination January, 2013 Instructions: Part I: Multiple Choice: Choose the best answer to each item. Place all answers on the Answer Sheet provided. 40 marks
JOHN BURKE HIGH SCHOOL Chemistry 2202 Midterm Examination January, 2013 Instructions: Part I: Multiple Choice: Choose the best answer to each item. Place all answers on the Answer Sheet provided. 40 marks
Statistical Analysis of Chemical Data Chapter 4
 Statistical Analysis of Chemical Data Chapter 4 Random errors arise from limitations on our ability to make physical measurements and on natural fluctuations Random errors arise from limitations on our
Statistical Analysis of Chemical Data Chapter 4 Random errors arise from limitations on our ability to make physical measurements and on natural fluctuations Random errors arise from limitations on our
Solubility Rules See also Table 4.1 in text and Appendix G in Lab Manual
 Ch 4 Chemical Reactions Ionic Theory of Solutions - Ionic substances produce freely moving ions when dissolved in water, and the ions carry electric current. (S. Arrhenius, 1884) - An electrolyte is a
Ch 4 Chemical Reactions Ionic Theory of Solutions - Ionic substances produce freely moving ions when dissolved in water, and the ions carry electric current. (S. Arrhenius, 1884) - An electrolyte is a
Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions
 Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are two
Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are two
SECTION D.2 AMMONIA NITROGEN
 SECTION D.2 AMMONIA NITROGEN CEDR Method Code: NH4F L01 a) Scope and Application i) This method describes the determination of low-level ammonia nitrogen concentrations in filtered samples taken from fresh
SECTION D.2 AMMONIA NITROGEN CEDR Method Code: NH4F L01 a) Scope and Application i) This method describes the determination of low-level ammonia nitrogen concentrations in filtered samples taken from fresh
1. Current acid rain data and trends.
 Lecture 24. Acid rain. Part3. Controlling acid rain. Objectives: 1. Current acid rain data and trends. 2. Acid rain control. Readings: Turco: p. 284-287; 1. Current acid rain data and trends. Monitoring
Lecture 24. Acid rain. Part3. Controlling acid rain. Objectives: 1. Current acid rain data and trends. 2. Acid rain control. Readings: Turco: p. 284-287; 1. Current acid rain data and trends. Monitoring
Experiment 8 - Double Displacement Reactions
 Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
AIMALYTICAL CHEMISTRY
 Fundamentals of AIMALYTICAL CHEMISTRY Seventh Edition Douglas A. Skoog Stanford University Donald M. West San Jose State University F. James Holler University ;of Kentucky W r SAUNDERS COLLEGE PUBLISHING
Fundamentals of AIMALYTICAL CHEMISTRY Seventh Edition Douglas A. Skoog Stanford University Donald M. West San Jose State University F. James Holler University ;of Kentucky W r SAUNDERS COLLEGE PUBLISHING
"We are what we repeatedly do. Excellence, therefore, is not an act but a habit." --Aristotle--
 CHEMISTRY 101 Hour Exam II October 25, 2016 Adams/Huynh Name Signature Section "We are what we repeatedly do. Excellence, therefore, is not an act but a habit." --Aristotle-- This exam contains 17 questions
CHEMISTRY 101 Hour Exam II October 25, 2016 Adams/Huynh Name Signature Section "We are what we repeatedly do. Excellence, therefore, is not an act but a habit." --Aristotle-- This exam contains 17 questions
Lect. 2: Chemical Water Quality
 The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
Chem!stry. Assignment on Acids, Bases and Salts #
 Chem!stry Name: ( ) Class: Date: / / Assignment on Acids, Bases and Salts #5 Write your answers in the spaces below: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 1. Which of the
Chem!stry Name: ( ) Class: Date: / / Assignment on Acids, Bases and Salts #5 Write your answers in the spaces below: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 1. Which of the
Scientific Observations and Reaction Stoichiometry: The Qualitative Analysis and Chemical Reactivity of Five White Powders
 Scientific Observations and Reaction Stoichiometry: The Qualitative Analysis and Chemical Reactivity of Five White Powders Objectives Part 1: To determine the limiting reagent and percent yield of CuCO
Scientific Observations and Reaction Stoichiometry: The Qualitative Analysis and Chemical Reactivity of Five White Powders Objectives Part 1: To determine the limiting reagent and percent yield of CuCO
Studies of a Precipitation Reaction
 Studies of a Precipitation Reaction Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. Answer the following 6 questions in your
Studies of a Precipitation Reaction Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. Answer the following 6 questions in your
Identification of Ions and Gases
 Identification of Ions and Gases Question Paper 1 Level IGSE Subject hemistry (0620/0971) Exam oard ambridge International Examinations (IE) Topic cids, bases and salts Sub-Topic Identification of ions
Identification of Ions and Gases Question Paper 1 Level IGSE Subject hemistry (0620/0971) Exam oard ambridge International Examinations (IE) Topic cids, bases and salts Sub-Topic Identification of ions
METHOD 9035 SULFATE (COLORIMETRIC, AUTOMATED, CHLORANILATE)
 METHOD 9035 SULFATE (COLORIMETRIC, AUTOMATED, CHLORANILATE) 1.0 SCOPE AND APPLICATION 1.1 This automated method is applicable to ground water, drinking and surface waters, and domestic and industrial wastes
METHOD 9035 SULFATE (COLORIMETRIC, AUTOMATED, CHLORANILATE) 1.0 SCOPE AND APPLICATION 1.1 This automated method is applicable to ground water, drinking and surface waters, and domestic and industrial wastes
Personalised Learning Checklists Edexcel Combined: Chemistry Paper 1
 Edexcel (combined) Chemistry Topics (1SC0) from 2016 - Paper 1 (Topic 1 parts a&b) Topic Student Checklist R A G Describe how the Dalton model of an atom has changed over time because of the discovery
Edexcel (combined) Chemistry Topics (1SC0) from 2016 - Paper 1 (Topic 1 parts a&b) Topic Student Checklist R A G Describe how the Dalton model of an atom has changed over time because of the discovery
Unit 4: Chemical Changes (Higher Content)
 Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
CHEMISTRY 101 Hour Exam II. Dr. D. DeCoste T.A (30 pts.) 16 (15 pts.) 17 (15 pts.) Total (60 pts)
 CHEMISTRY 1 Hour Exam II March 16, 017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 5 numbered pages. Check now to make sure you have a complete exam. You have one hour and thirty
CHEMISTRY 1 Hour Exam II March 16, 017 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 5 numbered pages. Check now to make sure you have a complete exam. You have one hour and thirty
Spectrophotometric Determination of Ferrocyanide in Effluents
 Spectrophotometric Determination of Ferrocyanide in Effluents ECN-0025-1 INTRODUCTION This method is used to determine the concentration of ferrocyanide ion in photoprocessing solution effluents. The ion
Spectrophotometric Determination of Ferrocyanide in Effluents ECN-0025-1 INTRODUCTION This method is used to determine the concentration of ferrocyanide ion in photoprocessing solution effluents. The ion
-However, this definition can be expanded to include: biology (biometrics), environmental science (environmetrics), economics (econometrics).
 Chemometrics Application of mathematical, statistical, graphical or symbolic methods to maximize chemical information. -However, this definition can be expanded to include: biology (biometrics), environmental
Chemometrics Application of mathematical, statistical, graphical or symbolic methods to maximize chemical information. -However, this definition can be expanded to include: biology (biometrics), environmental
CCR RULE GROUNDWATER STATISTICAL METHOD SELECTION CERTIFICATION
 This document summarizes the statistical methods which will be utilized for evaluating groundwater analytical results associated with the s (hereafter referred to as the Harrison CCR unit ) CCR groundwater
This document summarizes the statistical methods which will be utilized for evaluating groundwater analytical results associated with the s (hereafter referred to as the Harrison CCR unit ) CCR groundwater
Physical Changes and Chemical Reactions
 Physical Changes and Chemical Reactions Gezahegn Chaka, Ph.D., and Sudha Madhugiri, Ph.D., Collin College Department of Chemistry Objectives Introduction To observe physical and chemical changes. To identify
Physical Changes and Chemical Reactions Gezahegn Chaka, Ph.D., and Sudha Madhugiri, Ph.D., Collin College Department of Chemistry Objectives Introduction To observe physical and chemical changes. To identify
Gas Laws. Bonding. Solutions M= moles solute Mass %= mass solute x 100. Acids and Bases. Thermochemistry q = mc T
 Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
CCR RULE GROUNDWATER STATISTICAL METHOD SELECTION CERTIFICATION
 This document summarizes the statistical methods which will be utilized for evaluating groundwater analytical results associated with the s CCR groundwater monitoring program. There are two neighboring
This document summarizes the statistical methods which will be utilized for evaluating groundwater analytical results associated with the s CCR groundwater monitoring program. There are two neighboring
A Global Assessment of Precipitation Chemistry and Deposition
 A Global Assessment of Precipitation Chemistry and Deposition A project of the World Meteorological Organization s Global Atmosphere Watch Scientific Advisory Group for Precipitation Chemistry Robert Vet
A Global Assessment of Precipitation Chemistry and Deposition A project of the World Meteorological Organization s Global Atmosphere Watch Scientific Advisory Group for Precipitation Chemistry Robert Vet
Nineteenth Progress Report Contract Number DE - AC02-76EV May Authors:
 COO-1199-63 SWS Contract Report 252 STUDY OF ATMOSPHERIC POLLUTION SCAVENGING Nineteenth Progress Report Contract Number DE - AC02-76EV01199 May 1981 Authors: Richard G. Semonin Van C. Bowersox Donald
COO-1199-63 SWS Contract Report 252 STUDY OF ATMOSPHERIC POLLUTION SCAVENGING Nineteenth Progress Report Contract Number DE - AC02-76EV01199 May 1981 Authors: Richard G. Semonin Van C. Bowersox Donald
Course Review. Kin 304W Week 14: April 9, 2013
 Course Review Kin 304W Week 14: April 9, 2013 1 Today s Outline Format of Kin 304W Final Exam Course Review Hand back marked Project Part II 2 Kin 304W Final Exam Saturday, Thursday, April 18, 3:30-6:30
Course Review Kin 304W Week 14: April 9, 2013 1 Today s Outline Format of Kin 304W Final Exam Course Review Hand back marked Project Part II 2 Kin 304W Final Exam Saturday, Thursday, April 18, 3:30-6:30
Chapter 4 Reactions in Aqueous Solutions. Copyright McGraw-Hill
 Chapter 4 Reactions in Aqueous Solutions Copyright McGraw-Hill 2009 1 4.1 General Properties of Aqueous Solutions Solution - a homogeneous mixture Solute: the component that is dissolved Solvent: the component
Chapter 4 Reactions in Aqueous Solutions Copyright McGraw-Hill 2009 1 4.1 General Properties of Aqueous Solutions Solution - a homogeneous mixture Solute: the component that is dissolved Solvent: the component
SOLUTIONS. Solutions - page
 SOLUTIONS For gases in a liquid, as the temperature goes up the solubility goes. For gases in a liquid, as the pressure goes up the solubility goes. Example: What is the molarity of a solution with 2.0
SOLUTIONS For gases in a liquid, as the temperature goes up the solubility goes. For gases in a liquid, as the pressure goes up the solubility goes. Example: What is the molarity of a solution with 2.0
2 EQUILIBRIUM 2.1 WHAT IS EQUILIBRIUM? 2.2 WHEN IS A SYSTEM AT EQUILIBRIUM? 2.3 THE EQUILIBRIUM CONSTANT
 2 EQUILIBRIUM 2.1 WHAT IS EQUILIBRIUM? In general terms equilibrium implies a situation that is unchanging or steady. This is generally achieved through a balance of opposing forces. In chemistry equilibrium
2 EQUILIBRIUM 2.1 WHAT IS EQUILIBRIUM? In general terms equilibrium implies a situation that is unchanging or steady. This is generally achieved through a balance of opposing forces. In chemistry equilibrium
4.4.1 Reactivity of metals Metal oxides The reactivity series. Key opportunities for skills development.
 4.4 Chemical changes Understanding of chemical changes began when people began experimenting with chemical reactions in a systematic way and organising their results logically. Knowing about these different
4.4 Chemical changes Understanding of chemical changes began when people began experimenting with chemical reactions in a systematic way and organising their results logically. Knowing about these different
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions
 Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
Chapter 6: Chemical Bonding
 Chapter 6: Chemical Bonding Learning Objectives Describe the formation of ions by electron loss/gain to obtain the electronic configuration of a noble gas. Describe the formation of ionic bonds between
Chapter 6: Chemical Bonding Learning Objectives Describe the formation of ions by electron loss/gain to obtain the electronic configuration of a noble gas. Describe the formation of ionic bonds between
What Do You Think? Investigate GOALS
 Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
Analysis of cations and anions by Ion- Selective Electrodes (ISEs)
 Analysis of cations and anions by Ion- Selective Electrodes (ISEs) Purpose: The purpose of this assignment is to introduce potentiometric measurements of ionic species by ion selective electrodes (ISEs)
Analysis of cations and anions by Ion- Selective Electrodes (ISEs) Purpose: The purpose of this assignment is to introduce potentiometric measurements of ionic species by ion selective electrodes (ISEs)
Section Four Structured questions
 Section Four Structured questions 1 For each of the following experiments, state ONE observable change and write a chemical equation for the reaction involved. a) Magnesium strip is added to dilute hydrochloric
Section Four Structured questions 1 For each of the following experiments, state ONE observable change and write a chemical equation for the reaction involved. a) Magnesium strip is added to dilute hydrochloric
Lab 6 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 6 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 6: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 6 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 6: This experiment will involve determining
Kenya Certificate of Secondary Education (K.C.S.E.)
 Name: School:. Date:... 233/1 CHEMISTRY PAPER 1 JULY /AUGUST 2011 TIME: 2 HOURS Index No. Candidate s Sign.... Kenya Certificate of Secondary Education (K.C.S.E.) Chemistry Paper 1 INSTRUCTIONS TO THE
Name: School:. Date:... 233/1 CHEMISTRY PAPER 1 JULY /AUGUST 2011 TIME: 2 HOURS Index No. Candidate s Sign.... Kenya Certificate of Secondary Education (K.C.S.E.) Chemistry Paper 1 INSTRUCTIONS TO THE
11.3 Reactions in Aqueous Solution. Chapter 11 Chemical Reactions Reactions in Aqueous Solution
 Chapter 11 Chemical Reactions 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in Aqueous Solution 1 CHEMISTRY & YOU How did soda straws get into limestone caves? These
Chapter 11 Chemical Reactions 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in Aqueous Solution 1 CHEMISTRY & YOU How did soda straws get into limestone caves? These
Comparison of Particulate Monitoring Methods at Fort Air Partnership Monitoring Stations
 Comparison of Particulate Monitoring Methods at Fort Air Partnership Monitoring Stations Melanie Larsen Harry Benders RS Environmental (Tom Dann) March 13, 2014 Executive Summary Historically FAP has acquired
Comparison of Particulate Monitoring Methods at Fort Air Partnership Monitoring Stations Melanie Larsen Harry Benders RS Environmental (Tom Dann) March 13, 2014 Executive Summary Historically FAP has acquired
Chemistry. Exam Choice. Student Number PRELIMINARY COURSE EXAMINATION. Total marks 75. General Instructions
 Student Number Exam Choice 2008 PRELIMINARY COURSE EXAMINATION Chemistry Total marks 75 General Instructions Reading time 5 minutes Working time 2 hours Write using black or blue pen Draw diagrams using
Student Number Exam Choice 2008 PRELIMINARY COURSE EXAMINATION Chemistry Total marks 75 General Instructions Reading time 5 minutes Working time 2 hours Write using black or blue pen Draw diagrams using
Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions)
 Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions) Metallic (electrostatic attraction between + metal ions and delocalised electrons) Group 1 ions 1+
Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions) Metallic (electrostatic attraction between + metal ions and delocalised electrons) Group 1 ions 1+
Who is polluting the Columbia River Gorge?
 Who is polluting the Columbia River Gorge? Final report to the Yakima Nation Prepared by: Dan Jaffe, Ph.D Northwest Air Quality, Inc. 7746 Ravenna Avenue NE Seattle WA 98115 NW_airquality@hotmail.com December
Who is polluting the Columbia River Gorge? Final report to the Yakima Nation Prepared by: Dan Jaffe, Ph.D Northwest Air Quality, Inc. 7746 Ravenna Avenue NE Seattle WA 98115 NW_airquality@hotmail.com December
ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION
 ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION Conventional Centrifugal Methods Centrifugal sedimentation of particles suspended in a fluid is a well known method (1, 2)
ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION Conventional Centrifugal Methods Centrifugal sedimentation of particles suspended in a fluid is a well known method (1, 2)
Supporting information
 Supporting information Chemistry of urban grime: Inorganic ion composition of grime vs. particles in Leipzig, Germany Alyson M. Baergen, 1 Sarah A. Styler, Dominik van Pinxteren, Konrad Müller, Hartmut
Supporting information Chemistry of urban grime: Inorganic ion composition of grime vs. particles in Leipzig, Germany Alyson M. Baergen, 1 Sarah A. Styler, Dominik van Pinxteren, Konrad Müller, Hartmut
METHOD 9252A CHLORIDE (TITRIMETRIC, MERCURIC NITRATE)
 METHOD 9252A CHLORIDE (TITRIMETRIC, MERCURIC NITRATE) 1.0 SCOPE AND APPLICATION 1.1 This method is applicable to ground water, drinking, surface, and saline waters, and domestic and industrial wastes.
METHOD 9252A CHLORIDE (TITRIMETRIC, MERCURIC NITRATE) 1.0 SCOPE AND APPLICATION 1.1 This method is applicable to ground water, drinking, surface, and saline waters, and domestic and industrial wastes.
AP Chemistry. 9. Which of the following species CANNOT function as an oxidizing agent? (A) Cr 2 O 72 (B) MnO 4 (C) NO 3 (D) S (E) I
 Name AP Chemistry AP Chemistry Exam Part I: 40 Questions, 40 minutes, Multiple Choice, No Calculator Allowed Bubble the correct answer on your scantron for each of the following. Use the following answers
Name AP Chemistry AP Chemistry Exam Part I: 40 Questions, 40 minutes, Multiple Choice, No Calculator Allowed Bubble the correct answer on your scantron for each of the following. Use the following answers
Determination of Cations and Amines in Hydrogen Peroxide by Ion Chromatography Using a RFIC (Reagent-Free) System
 Application Update 55 Determination of Cations and Amines in Hydrogen Peroxide by Ion Chromatography Using a RFIC (Reagent-Free System Introduction Hydrogen peroxide is an essential chemical in the fabrication
Application Update 55 Determination of Cations and Amines in Hydrogen Peroxide by Ion Chromatography Using a RFIC (Reagent-Free System Introduction Hydrogen peroxide is an essential chemical in the fabrication
Lecture Slides. Elementary Statistics. by Mario F. Triola. and the Triola Statistics Series
 Lecture Slides Elementary Statistics Tenth Edition and the Triola Statistics Series by Mario F. Triola Slide 1 Chapter 13 Nonparametric Statistics 13-1 Overview 13-2 Sign Test 13-3 Wilcoxon Signed-Ranks
Lecture Slides Elementary Statistics Tenth Edition and the Triola Statistics Series by Mario F. Triola Slide 1 Chapter 13 Nonparametric Statistics 13-1 Overview 13-2 Sign Test 13-3 Wilcoxon Signed-Ranks
Midwestern Climate Center Office of Applied Climatology Illinois State Water Survey 2204 Griffith Drive Champaign, Illinois (217)
 SWS Miscellaneous Publication 136 by Wayne M. Wendland (IL), Kenneth E. Kunkel, Glen Conner (KY), Wayne L. Decker (MO), Harry Hillaker (IA), Pant Naber-Knox (WI), Fred V. Nurnberger (MI), Jeffrey Rogers
SWS Miscellaneous Publication 136 by Wayne M. Wendland (IL), Kenneth E. Kunkel, Glen Conner (KY), Wayne L. Decker (MO), Harry Hillaker (IA), Pant Naber-Knox (WI), Fred V. Nurnberger (MI), Jeffrey Rogers
5.4 Chemical changes Reactivity of metals Metal oxides The reactivity series. Key opportunities for skills development
 5.4 Chemical changes Understanding of chemical changes began when people began experimenting with chemical reactions in a systematic way and organising their results logically. Knowing about these different
5.4 Chemical changes Understanding of chemical changes began when people began experimenting with chemical reactions in a systematic way and organising their results logically. Knowing about these different
Lecture Slides. Section 13-1 Overview. Elementary Statistics Tenth Edition. Chapter 13 Nonparametric Statistics. by Mario F.
 Lecture Slides Elementary Statistics Tenth Edition and the Triola Statistics Series by Mario F. Triola Slide 1 Chapter 13 Nonparametric Statistics 13-1 Overview 13-2 Sign Test 13-3 Wilcoxon Signed-Ranks
Lecture Slides Elementary Statistics Tenth Edition and the Triola Statistics Series by Mario F. Triola Slide 1 Chapter 13 Nonparametric Statistics 13-1 Overview 13-2 Sign Test 13-3 Wilcoxon Signed-Ranks
Chem 101 Review. Fall 2012
 Chem 101 Review Fall 2012 Elements, Atoms, Ions Elements in nature symbols Constant composition chemical formula Dalton s atomic theory Atomic structure what makes up the atom ions isotopes Periodic table
Chem 101 Review Fall 2012 Elements, Atoms, Ions Elements in nature symbols Constant composition chemical formula Dalton s atomic theory Atomic structure what makes up the atom ions isotopes Periodic table
CHAPTER 7 AEROSOL ACIDITY
 CHAPTER 7 AEROSOL ACIDITY It has been established by a number of investigators, that especially during the summer, aerosols along the coast of Washington are commonly acidic. 1-2 Although the measurements
CHAPTER 7 AEROSOL ACIDITY It has been established by a number of investigators, that especially during the summer, aerosols along the coast of Washington are commonly acidic. 1-2 Although the measurements
