5. Draw the alkene that gives the product shown, and specify its stereochemistry. (2 points)
|
|
|
- Doris Owen
- 6 years ago
- Views:
Transcription
1 JSPERSE HEM 350 TEST 3 VERSIN 1 h. 7 Structure and Synthesis of lkenes h. 8 Reactions of lkenes 1 1. How many elements of unsaturation are in the formula 6 H 9 N 2? (3 points) a. 0 b. 1 c. 2 d. 3 e. 4 f. 5 g For the three structures shown, which of the statements is true? (3 points) N 2 N 2 D a.,, and D are Z; is E b. and are the only Z compounds c. is the only Z compound; is the only E compound d.,, and D are Z; is E e. is the only Z compound; is the only E compound 3. Rank the reactivity of the following toward H 2 S 4 /Î catalyzed dehydration. (3 points) a. is fastest; is slowest heat b. is fastest; is slowest c. is fastest; is slowest d. is fastest; is slowest e. is fastest; is slowest f. is fastest; is slowest H 4. Which of the following reactants would give exaactly the same products from both (E)- and (Z)-2-butene? (3 points) If two chiral centers are produced, then diastereomeric products are produced. a. 2 b. Ph 3 H c. 1) H 3 -THF 2) Na, H 2 2 d. s 4, H 2 2 e. D 2, Pt ut if only one (or zero) chiral centers are produced, then the two alkenes don't give different products. In this case, with H- being added, only the carbon to which is added ends up being chiral, so you get the same racemic mix of 2-butanol either way. 5. Draw the alkene that gives the product shown, and specify its stereochemistry. (2 points) The normal "E" alkene would have given the wrong product stereochemistry. If the cis/ trans sense of the addition and the cis/trans appearance of the product match, then "E" alkene would have worked. 1 s 4, H 2 2
2 2 6. Draw the major product for each of the following reactions or reaction sequences. You needn t bother to show side products or minor products. For chiral molecules that are racemic, you needn t draw both enantiomers. E REFUL T SHW THE RRET RIENTTIN, ND THE RRET STEREHEMISTRY IN SES WHERE STEREHEM IS FTR. (3 points each) 1. Hg(c) 2, H 2 2. NaH 4 NEt 3 H 2 S 4, heat H- H 3 3 H H 2 1. H- 2. Na E2 1. NaH 2 H 3 2. KMn 4, heat 2, H 2 H, peroxides 2
3 7. single unknown reacts with 3 /Me 2 S to give the following three products. What is the structure for the unknown? (3 points) Me 2 S H H Provide the name or structure for the following. (3 points each) l (racemic, don t do R/S stuff) or 9. Provide a possible structure for a compound with formula 5 H 8, given that it reacts with excess H 2 /Pt to give 5 H 10. (3 points) 10. Fill in the blanks for the following reaction sequence: (6 points) 2, hv NaH 3 H 3 Ph 3 H 11. onsider how the Se- bond would be polarized and predict the product which would result when H 3 Se adds to propene: (Selenium is located two rows directly below oxygen on the periodic table). (3 points) H 3 Se- Markovnikov's rule. Electronegativity/periodic table shows yxgen more electronegative than selenium, so the oxygen adds to the more substituted end of the alkene. 3
4 4 12. When the following isomeric alkenes are fully burned, rank the amount of heat produced in the combustions, from most heat produced (1) to least heat produced (4). (3 points) 13. Provide structures for starting material and reactions products and, given the formula of starting material and the stereochemical status of products and. (5 points) racemic mixture l 2 4 H 8 Ph 3 H meso compound With (E)-but-2-ene, l2 would give meso and Ph3H would have given chiral. ut-1-ene would have given chiral with both reactions. 14. Draw mechanisms for the following reactions, using formal arrow-pushing. Each intermediate along the mechanism pathway must be shown. (6 points, 3 points, 6 points) H 2 S 4 heat H H 2, H 2 (be sure your mech. is consistent with the observed stereochemistry) 4
5 15. Provide reagents for the following transformations. (5 points each) 5 I may or may not have had time to discuss and require the KMn4 reaction. H3 5
A. Provide reagents for the following transformations. T 6. Cl OH
 rganic Chemistry I est 3 Extra Synthesis Practice Problems Page 1: Synthesis Design Practice. Page 2+3: Predict the Product Practice (including some that involve stereochemistry). Page 4: Cis/trans Stereospecific
rganic Chemistry I est 3 Extra Synthesis Practice Problems Page 1: Synthesis Design Practice. Page 2+3: Predict the Product Practice (including some that involve stereochemistry). Page 4: Cis/trans Stereospecific
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
diene. If stereoisomeric mixtures form, indicate by drawing one and writing "+ enantiomer" and/or "+ diastereomer" in the box.
 I. (9 points) Page A. Consider how varying the conditions of a reaction can vastly influence the rate of a reaction as well as the product(s) formed. First draw the product(s) that form in the reference
I. (9 points) Page A. Consider how varying the conditions of a reaction can vastly influence the rate of a reaction as well as the product(s) formed. First draw the product(s) that form in the reference
2. Hydrohalogenation: Propylene reacts with HBr to form 2-bromopropane.
 Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. KH (1 equiv.) + KCl THF. + HBr.
 1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
Chemistry 201. MW 12:00pm 1:15pm Examination #2 August 15 th Bronco ID. Question Score Possible Points. 1 (12pts) 2 (24pts) 3 (25pts)
 Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
Chemistry 250B Final Exam Answer Key December 19, 2008
 Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
HO HO. 1) BH 3 HCl. + enantiomer either one may be drawn 4. + enantiomer either one may be drawn 4 1) O 3
 . (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
. (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
California State Polytechnic University, Pomona Nomenclature (one structure) 25
 alifornia State Polytechnic University, Pomona 1 hem 314 Final Exam Spring, 2005 Beauchamp ame Problem Points redit 1. omenclature (one structure) 25 2. 2D Lewis structure (large structure with 20 possible
alifornia State Polytechnic University, Pomona 1 hem 314 Final Exam Spring, 2005 Beauchamp ame Problem Points redit 1. omenclature (one structure) 25 2. 2D Lewis structure (large structure with 20 possible
If Compound 2 (below) was used in the above reaction, how might things change? Name Page 1. I. (23 points)
 I. (2 points) ame Page itrogen atoms in amines can undergo substitution reactions repeatedly under mildly basic conditions. Provide the structure of the starting amine and supply the missing reagent(s).
I. (2 points) ame Page itrogen atoms in amines can undergo substitution reactions repeatedly under mildly basic conditions. Provide the structure of the starting amine and supply the missing reagent(s).
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron sheet.
 Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Exam 4. Name CHEM 210. PBr 3. HBr. 1) NaH 2) Br H 2 SO 4. 1) NaOCH 3 2) dil. H 3 O + O CH 3 OH, H 2 SO 4. 1) LDA, -78 o C.
 Exam 4 Name CEM 210 1. (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where
Exam 4 Name CEM 210 1. (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where
California State Polytechnic University, Pomona Chem 315. Exam Points 1. Nomenclature (1) 30
 hem 315 Final Exam Fall, 2007 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 30 redit 2. Relative rder of Reactivity 20 3. Reactions Page, using reactions learned thus far (30) 30 4.
hem 315 Final Exam Fall, 2007 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 30 redit 2. Relative rder of Reactivity 20 3. Reactions Page, using reactions learned thus far (30) 30 4.
Chem ORGANIC CHEMISTRY I
 ORGANIC CHEMISTRY I CHEM 221 /2 01 Final Examination December 20, 2005 1400-1700 Dr. Cerrie ROGERS x x molecular model kits (without instructions) programmable calculators must be reset Chem 221 --- ORGANIC
ORGANIC CHEMISTRY I CHEM 221 /2 01 Final Examination December 20, 2005 1400-1700 Dr. Cerrie ROGERS x x molecular model kits (without instructions) programmable calculators must be reset Chem 221 --- ORGANIC
Homework Problem Set 8 Iverson CH320M/328M Due Friday, Nov. 9
 omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
CHAPTER 8 HW: ELIMINATIONS REACTIONS
 APTER 8 W: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult to
APTER 8 W: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult to
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
CHEM 231 (Davis) Organic Chemistry Exam III 19 April, YOUR NAME (Last, First, M.I.) DISCUSSION SECTION #53 (5 Points)
 EM 23 (avis) rganic hemistry Exam III 9 pril, 2006 YUR NME (Last, First, M.I.) ISUSSIN SETIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and on the next
EM 23 (avis) rganic hemistry Exam III 9 pril, 2006 YUR NME (Last, First, M.I.) ISUSSIN SETIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and on the next
Chem 350 Jasperse Ch. 8 Notes 1
 hem 350 Jasperse h. 8 Notes 1 Summary of Alkene Reactions, h. 8. Memorize Reaction, rientation where Appropriate, Stereochemistry where Appropriate, and Mechanism where Appropriate. -all are drawn using
hem 350 Jasperse h. 8 Notes 1 Summary of Alkene Reactions, h. 8. Memorize Reaction, rientation where Appropriate, Stereochemistry where Appropriate, and Mechanism where Appropriate. -all are drawn using
I. (32 points) Name. Page 1. (ii) its diastereomer its structural isomer its enantiomer. The product you have drawn will. H 3 CO Cl form with H 3 CO
 ame. ( points) Page Each of the reactions yields a PAR of main organic products, but you should only draw one. Then answer the other questions about the starting and the products formed. Check boxes are
ame. ( points) Page Each of the reactions yields a PAR of main organic products, but you should only draw one. Then answer the other questions about the starting and the products formed. Check boxes are
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
a) 1. O 3 2. (CH 3 ) 2 S
 Name: 1 Chemistry 250B Final Exam December 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) Complete the following reactions. Show the stereochemistry
Name: 1 Chemistry 250B Final Exam December 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) Complete the following reactions. Show the stereochemistry
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
CHEM 3311 Exam 2 ANSWER KEY
 CEM 3311 Exam 2 ANSWER KEY March 11, 2014 Time: 2 ours Please sign the onor Pledge h in this sheet with your scantron. I pledge that n my honor, as a University of Colorado-Boulder student, I have neither
CEM 3311 Exam 2 ANSWER KEY March 11, 2014 Time: 2 ours Please sign the onor Pledge h in this sheet with your scantron. I pledge that n my honor, as a University of Colorado-Boulder student, I have neither
350 Organic Chemistry I Winona State University
 350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
NAME: STUDENT NUMBER: Page 1 of 4
 NAME: STUDENT NUMBER: Page 1 of 4 Chemistry 2.339 Midterm Test Friday ctober 28, 2005 This test is worth 40 Marks. You must complete all work within the class period. Put all answers in the space provided.
NAME: STUDENT NUMBER: Page 1 of 4 Chemistry 2.339 Midterm Test Friday ctober 28, 2005 This test is worth 40 Marks. You must complete all work within the class period. Put all answers in the space provided.
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Homework Problem Set 9 Iverson CH320M/328M Due Friday, November 30
 omework Problem Set 9 Iverson C0M/8M Due Friday, November 0 NAME (Print): SIGNATURE: Chemistry 0M/8M Dr. ent Iverson 9th omework November 9, 08 Please print the first three letters of your last name in
omework Problem Set 9 Iverson C0M/8M Due Friday, November 0 NAME (Print): SIGNATURE: Chemistry 0M/8M Dr. ent Iverson 9th omework November 9, 08 Please print the first three letters of your last name in
Chemistry 143 Exam #2 November 15, Name: Student Number: Section Number: TA: INSTRUCTIONS:
 Chemistry 143 Exam #2 November 15, 2017 Name: Student Number: Section Number: TA: INSTRUCTINS: This exam consists of 32 questions on 9 pages. Please make certain that your exam is complete. Write your
Chemistry 143 Exam #2 November 15, 2017 Name: Student Number: Section Number: TA: INSTRUCTINS: This exam consists of 32 questions on 9 pages. Please make certain that your exam is complete. Write your
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Chemistry 233 Exam 3 (Green) The Periodic Table
 Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
CM 241 Organic Chemistry Fall 1999
 M 241 rganic hemistry Fall 1999 1 UR EXAM III KEY Date: 12/14/99 The marks for each question are given in italics. Write all answers in booklets provided. Question 1. (2+2+2+2) Give names for the following
M 241 rganic hemistry Fall 1999 1 UR EXAM III KEY Date: 12/14/99 The marks for each question are given in italics. Write all answers in booklets provided. Question 1. (2+2+2+2) Give names for the following
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
Chemistry 233 Exam 3. The Periodic Table
 Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Score: NAME (Print): Chemistry 320N Dr. Brent Iverson 3rd Homework February 3, 2017 SIGNATURE:
 omework Problem Set 3 Iverson 30N Due Friday February 10 NAME (Print): SIGNATURE: hemistry 30N Dr. ent Iverson 3rd omework February 3, 017 Please print the first three letters of your last name in the
omework Problem Set 3 Iverson 30N Due Friday February 10 NAME (Print): SIGNATURE: hemistry 30N Dr. ent Iverson 3rd omework February 3, 017 Please print the first three letters of your last name in the
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CHEM 232 Exam Two April 5, 2010
 CEM 232 Exam Two April 5, 2010 Name (-1 pt) TA Name (-1 pt) UIN (-1 pt) Question 1 [Qualitative anking, 40 points] A significant amount of time has been devoted to describing the physical and chemical
CEM 232 Exam Two April 5, 2010 Name (-1 pt) TA Name (-1 pt) UIN (-1 pt) Question 1 [Qualitative anking, 40 points] A significant amount of time has been devoted to describing the physical and chemical
NAME (Print): Chemistry 610A/618A Dr. Brent Iverson 2nd Exam Oct. 29, 2003 SIGNATURE:
 NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 FALL 2018 CEM 251: Organic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only
FALL 2018 CEM 251: Organic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only
CHEM Exam 2 ANSWER KEY
 CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
Time: 3 hours (180 minutes) Marking Scheme For The Exam
 hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
More Tutorial at
 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points)
 Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22
 Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
CHEM 2423 Unit 8 Homework Answers
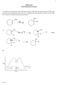 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
Knowing how many elements of unsaturation are present helps to classify, and helps in isomer problems. Theory # H's - Actual # H's 2 (2C + 2) - H
 hem 341 Jasperse h. 6 andouts 1 h. 6 Structure and Synthesis of lkenes Review ond Strength - σ ond 83 kcal/mol = π ond 63 kcal/mol π onds are much weaker π onds are thus more breakable and more reactive
hem 341 Jasperse h. 6 andouts 1 h. 6 Structure and Synthesis of lkenes Review ond Strength - σ ond 83 kcal/mol = π ond 63 kcal/mol π onds are much weaker π onds are thus more breakable and more reactive
+ HBr ΔH = OH CH CH 2. HgOAc
 radical substitution l hv + l 2 + l Energy radical addition RR + electrophilic addition + 3 3 Energy Energy + 2 acetone water + 2 2 + Energy Energy acid catalyzed hydration + 2 + Energy + + 2 + + 2 = 2
radical substitution l hv + l 2 + l Energy radical addition RR + electrophilic addition + 3 3 Energy Energy + 2 acetone water + 2 2 + Energy Energy acid catalyzed hydration + 2 + Energy + + 2 + + 2 = 2
Chem 341 Jasperse Ch. 9 Handouts 1
 Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
Special Topics for Final Exam: Topics that Frequently Appear on ACS or Standardized Tests, But That I Didn t Cover in Class This Year
 hem 360 Jasperse Final xam otes. Special Topics 3 Special Topics for Final xam: Topics that Frequently ppear on S or Standardized Tests, ut That I idn t over in ass This Year 1. poxides as lectrophiles
hem 360 Jasperse Final xam otes. Special Topics 3 Special Topics for Final xam: Topics that Frequently ppear on S or Standardized Tests, ut That I idn t over in ass This Year 1. poxides as lectrophiles
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 11, 2002 ANSWERS
 Name INSTRUTINS Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 11, 2002 ANSWERS This examination has two parts. The first part is in multiple choice format; the
Name INSTRUTINS Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 11, 2002 ANSWERS This examination has two parts. The first part is in multiple choice format; the
More tutorial at
 Answer all the questions. 1) (12 pts) Assign (R) or (S) to all the chiral centers in the following molecules and mark the priority of each group around a chiral center (just the top chiral carbon in molecule
Answer all the questions. 1) (12 pts) Assign (R) or (S) to all the chiral centers in the following molecules and mark the priority of each group around a chiral center (just the top chiral carbon in molecule
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
O N N. electrons in ring
 ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS
 NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Lecture Notes Chem 51B S. King I. Conjugation
 Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
I. (42 points) (a) Label the indicated sites (circles and oval box) with the appropriate stereochemical designation.
 I. ( points) ame Page A. etamethasone is a powerful anti-inflammatory drug that comes with unfortunate immunosupressive properties. It is often prescribed as a topical cream that can be used to alleviate
I. ( points) ame Page A. etamethasone is a powerful anti-inflammatory drug that comes with unfortunate immunosupressive properties. It is often prescribed as a topical cream that can be used to alleviate
Sometimes the DEPT function stops working or the undergraduate NMRs at your University don't have the DEPT option. This is the situation here.
 ) Calculate the degree of unsaturation for the formula (C9H2) ( pt) H-NMR NMR (Show structure fragment work in spectrum) (2 pts) 2H 8H 3 2 3 C-NMR (Show structure fragments work in spectrum) (2 pts). Sometimes
) Calculate the degree of unsaturation for the formula (C9H2) ( pt) H-NMR NMR (Show structure fragment work in spectrum) (2 pts) 2H 8H 3 2 3 C-NMR (Show structure fragments work in spectrum) (2 pts). Sometimes
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
CHEM 240: Survey of Organic Chemistry at North Dakota State University Midterm Exam 01 - Tue, 23 Sep 2014!! Name:! KEY!
 EM 240: Survey of rganic hemistry at North Dakota State University Midterm Exam 01 - Tue, 23 Sep 2014!! Name:! KEY! Please read through each question carefully and answer in the spaces provided. A good
EM 240: Survey of rganic hemistry at North Dakota State University Midterm Exam 01 - Tue, 23 Sep 2014!! Name:! KEY! Please read through each question carefully and answer in the spaces provided. A good
much more stable then the
 8.1 Give the structure and name of the product that would be obtained from the ionic addition of I to propene. I I I 8.3 Provide mechanistic explanations for the following observations:(a)the addition
8.1 Give the structure and name of the product that would be obtained from the ionic addition of I to propene. I I I 8.3 Provide mechanistic explanations for the following observations:(a)the addition
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Problem Points Credit. 1. Nomenclature (one structure) 30
 alifornia State Polytechnic University, Pomona 1 hem 2010 Midterm #2 Fall, 2018 Beauchamp ame KY (int your name legibly) oblem Points redit 1. omenclature (one structure) 30 2. xplain relative stabilities
alifornia State Polytechnic University, Pomona 1 hem 2010 Midterm #2 Fall, 2018 Beauchamp ame KY (int your name legibly) oblem Points redit 1. omenclature (one structure) 30 2. xplain relative stabilities
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
stereoselection view Product 1 with its enantiomer
 . ( points) A. Complete the following reactions as directed. (a) Cl C1 C i) major product DBU C C C C C Page 1 ii) Draw a ewman Projection for the conformation of the C1-C bond indicated that specifically
. ( points) A. Complete the following reactions as directed. (a) Cl C1 C i) major product DBU C C C C C Page 1 ii) Draw a ewman Projection for the conformation of the C1-C bond indicated that specifically
Solutions. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #2 - October 23, 2000
 Solutions Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 23, 2000 INSTRUTIONS --- This examination has two parts. The first part is in multiple choice format;
Solutions Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 23, 2000 INSTRUTIONS --- This examination has two parts. The first part is in multiple choice format;
- NH2 or NH 3 H 2 O or CH 3 COO - BF 3 or F - I Br. or Br
 CEMSTRY 313-02 MDTERM # 3 answer key November 09, 2004 Statistics: Average: 71 pts (71%); ighest: 90 pts (90%); Lowest: 49 pts (49%) Number of students perfming at above average: 22 (51%) 1. (8 pts) Mark
CEMSTRY 313-02 MDTERM # 3 answer key November 09, 2004 Statistics: Average: 71 pts (71%); ighest: 90 pts (90%); Lowest: 49 pts (49%) Number of students perfming at above average: 22 (51%) 1. (8 pts) Mark
5. Reactions of Alkenes (text )
 2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
Chem 112A: Final Exam
 Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
"Check" all descriptions that apply. is a meso compound has at least one chiral diastereomer has an enantiomer has at least one achiral diastereomer
 I. ( points) ame Page For each of the compounds shown below: () Identify any sources of stereochemistry by circling the site(s) and providing appropriate stereolabel(s) for each site. If no stereochemistry
I. ( points) ame Page For each of the compounds shown below: () Identify any sources of stereochemistry by circling the site(s) and providing appropriate stereolabel(s) for each site. If no stereochemistry
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Part C- section 1 p-bonds as nucleophiles
 Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Chemistry 210 Organic Chemistry I Winter Semester 2002 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I Winter Semester 2002 Dr. Rainer Glaser Examination #6 Reactions of Alkenes & Alkyne hemistry. Friday, March 26, 2002, 9 9:50 am Name: Answer Key Question 1. alogenation Reactions.
hemistry 210 rganic hemistry I Winter Semester 2002 Dr. Rainer Glaser Examination #6 Reactions of Alkenes & Alkyne hemistry. Friday, March 26, 2002, 9 9:50 am Name: Answer Key Question 1. alogenation Reactions.
CEM 351 2nd EXAM/Version A Friday, October 17, :50 2:40 p.m. Room 138, Chemistry
 Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
FALL 2018 CEM 251: Organic Chemistry I Sections Oct 23 rd, 2018
 FALL 2018 CEM 251: Organic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM Oct 23 rd, 2018 Name: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
FALL 2018 CEM 251: Organic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM Oct 23 rd, 2018 Name: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
CHEM 240: Survey of Organic Chemistry at North Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! Name:! KEY!
 CEM 240: Survey of rganic Chemistry at orth Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! ame:! KEY! Please read through each question carefully and answer in the spaces provided. A good
CEM 240: Survey of rganic Chemistry at orth Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! ame:! KEY! Please read through each question carefully and answer in the spaces provided. A good
Chem 121 Winter 2016: Section 03, Sample Problems. Alkenes and Alkynes
 Chem 121 Winter 2016: Section 03, Sample Problems Alkenes and Alkynes Problems adapted from Chemistry - The Central Science, 3 rd edition. 24.2 Consider the hydrocarbon drawn below. (a) What is the hybridisation
Chem 121 Winter 2016: Section 03, Sample Problems Alkenes and Alkynes Problems adapted from Chemistry - The Central Science, 3 rd edition. 24.2 Consider the hydrocarbon drawn below. (a) What is the hybridisation
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Chapter 6. Isomers and Stereochemistry
 Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Chem 3719 Example Exams. Chemistry 3719 Practice Exams
 Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chem 2061 Final Exam. Fall Andy Aspaas, Instructor. Thursday, December 15, Instructions: Please print: Last name: First name:
 Please print: Last name: First name: hem 2061 Final Exam Fall 2005 Andy Aspaas, Instructor Thursday, December 15, 2005 Instructions: You may start as soon as you arrive. The exam was designed to be finished
Please print: Last name: First name: hem 2061 Final Exam Fall 2005 Andy Aspaas, Instructor Thursday, December 15, 2005 Instructions: You may start as soon as you arrive. The exam was designed to be finished
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams.
 2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
STEREOGENIC CENTER (Chiral Center,Asymmetric Center)
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
H 3 C C C H H. Hydride Shift
 Exam II SN1, E1, SN2, E2 Reactions Markovnikov Addition Ant-Markovnikov Addition Reaction Mechanisms offman and Saytzeff Eliminations Enantiomers and Diastereomers SN1 Reactions: By-Products - Whenever
Exam II SN1, E1, SN2, E2 Reactions Markovnikov Addition Ant-Markovnikov Addition Reaction Mechanisms offman and Saytzeff Eliminations Enantiomers and Diastereomers SN1 Reactions: By-Products - Whenever
Stereochemistry. Based on McMurry s Organic Chemistry, 6 th edition
 Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
