CHM 15 EXAM #3 PRACTICE. Fill in the blanks or circle one answer as appropriate (2.5 points per question).
|
|
|
- Linette Watts
- 5 years ago
- Views:
Transcription
1 CHM 15 EXAM #3 PRACTICE Fill in the blanks or circle one answer as appropriate (2.5 points per question). 1. The equation which relates expansion work (w) done by a system to the change in the number of moles of gas in a reaction is: w = - n g RT 2. For a reaction which is endothermic, the final enthalpy of the system (Hf) is > the initial enthalpy (Hi) of the system. a. > b. < c. = d. e. 3. The sign of q (positive or negative) for heat lost by the system is negative. The sign of w (positive or negative) for work done on the system is positive 4. What is the change in the energy of a system that has 89 J of work done on it while losing 100 J of heat. a J b J c J d J e. none of the above 5. What is the formation reaction for C8H18 (l)? [C (s) is the stable form of carbon.] a. 8 C (s) + 9 H2 (g) C8H18 (g) b. 2 C4H8 (s) + H2 (g) C8H18 (l) c. 2 C4 (s) + 9 H2 (g) C8H18 (l) d. 8 C (g) + 9 H2 (g) C8H18 (l) e. none of the above 6. Under which of the following conditions will a gas behave least ideally?. a. low pressure and high temperature b. high pressure and high temperature c. low pressure and low temperature d. high pressure and low temperature 7. A gas is compressed to 1/3 of its initial volume while the temperature doubles. If moles of gas remains constant (& the gas behaves ideally), then the pressure will: a. increase by a factor of 2 b. increase by a factor of 3 c. increase by a factor of 6 d. decrease by a factor of 2 e. decrease by a factor of 3 f. decrease by a factor of 6 8. A gas is heated to four times its initial temperature in a sealed container. The average velocity of the gas will: a. decrease to ¼ of its initial value b. increase to 2 times its initial value c. decrease to ½ of its initial value d. increase to 4 times its initial value e. remain unchanged 167
2 Complete the following calculations (Show all of your work!) (12 points) gram sample of liquid octane, C8H18 (l), a component of gasoline, undergoes complete combustion in a bomb calorimeter that has a heat capacity of kj /ºC. If the initial temperature of the calorimeter is 20.25ºC and the final temperature of the calorimeter is 22.87ºC, what is the heat of combustion at constant volume (q v or E) of octane in the units kj /mol? [the molar mass of octane = g /mole] T = 22.87ºC 20.25ºC = +2.62ºC 0 = q cal + q comb... so... E = q comb = - q cal = - (11.93 kj /ºC)( +2.62ºC) = kj moles consumed = g /( gmol -1 ) = 5.733x10-3 mole E = kj / 5.733x10-3 mole = -5,45 2 kj/mole (15 points) 10. (a) Given the values for Hºf for the reactants and products, calculate Hºrxn for the following reaction in the units specified: 2 C8H18 (l) + 25 O2 (g) 16 CO2 (g) + 18 H2O (l) Hºrxn =?? kj /mole C8H18 Hºf [C8H18 (l)] = kj / mol Hºf [CO2 (g)] = kj / mol Hºf [H2O (l)] = kj / mol H = Σ m H f (prod) - Σ n H f (react) Simply divide the reaction two to find the energy change per mole of octane H = 8mole*( kj/mol) + 9mole*( kj/mol) 1mole*( kj/mol) H = -5,390.1 kj / mole octane Alternatively, do the calculation for the moles as shown and then divide by two. (b) Explain why the answers to questions 9 and 10(a) do not, and should not, match one another. The reaction at constant volume releases different energy per mole of octane due to the absence of the work term present at constant pressure. 168
3 (13 points) 11. Given the reactions below along with their enthalpy changes, use Hess Law to calculate the enthalpy of reaction for the following reaction in the units specified: 2 NOCl (g) 2 NO (g) + Cl2 (g) Hºrxn =?? kj /mol NO (a) 2 NOCl (g) + O2 (g) N2O4 (g) + Cl2 (g) Hºrxn = kj (b) 2 NO2 (g) 2 NO (g) + O2 (g) Hºrxn = kj (c) 2 NO2 (g) N2O4 (g) Hºrxn = kj rxn (a) 2 NOCl (g) + O2 (g) N2O4 (g) + Cl2 (g) Hºrxn = kj rev rxn (c) N2O4 (g) 2 NO2 (g) Hºrxn = kj rxn (b) 2 NO2 (g) 2 NO (g) + O2 (g) Hºrxn = kj 2 NOCl (g) 2 NO (g) + Cl2 (g) Hºrxn =?? kj /mol NO Hºrxn =?? kj /mol NO = (1/2)( )kJ = /2 = kj/mol NO (15 points) 12. Refer to the diagram below and answer the questions that follow. The valve between the two compartments is closed initially with the two gases isolated. When the valve is opened, the TOTAL pressure in the two connected compartments becomes 1.77 atm grams of unknown gas t at 300 K in L mole of He at 300 K in L (a) Calculate the pressure of He in the righthand compartment prior to opening the valve. P He = n He RT/V RT/V = atm/mole P He = 1.23 atm (b) When the valve is opened, calculate (in whatever order is easiest for you) the following: (i) the partial pressure of He in the two connected compartments, (ii) the partial pressure of the unknown gas in the two connected compartments, (iii) the moles of the unknown gas, and (iv) the molar mass of the unknown gas. (i) volume doubles so pressure is decreased to ½ initial value = 1.23 atm / 2 = atm (ii) P tot = P He + P unk, so P unk = P tot - P He = 1.77 atm atm = atm (iii) n = PV / RT = (1.154 atm)(30.00 L)/(R)(300 K) = mole (iv) MM = g/mole = g / mole = 16.0 g/mole 169
4 (25 points) 13. (a) Balance the following reaction (DO NOT add any other species to the reaction). 4 H 2 O (g) + 3 Fe (s) Fe 3 O 4 (s) + 4 H 2 (g) If g of Fe is reacted with 75.0 g of H 2 O: (b) What is the limiting reactant? (40.00 g Fe) / (55.85 g/mole) = mole Fe (75.0 g H 2 O) / (18.02 g/mole) = mole H 2 O Not even a close call / 3 < / 4... so Fe is limiting! (c) What is the theoretical yield of H 2 in BOTH moles AND in grams? mole H 2 = (4 mole H 2 / 3 mole Fe)*( mole Fe) = mole H 2 mass H 2 = (2.016 g H 2 / 1 mole)*( mole H 2 ) = g H 2 (d) What volume would the DRY H 2 occupy at 298 K and atm? V = nrt/p = ( mole H 2 )(R)(298 K)/(0.932 atm) V = L (e) If the actual amount of DRY H 2 collected at 298 K and atm is 3.01 L, what is the percent yield? % Yield = 100*(actual / theoretical) = 100*(3.01L/25.07 L) = 12.0 % (f) Is this reaction ENDOthermi or EXOthermic? Provide an answer and discuss why you believe you are correct. EXOthermic. Heat is required to convert iron ore (which is much like rust) into the metallic iron used to produce steel. Hydrogen gas can be used as a reducing agent in the process. Therefore, the REVERSE of the above reaction is ENDOthermic. As we learned in this chapter, if we reverse a reaction, the sign of the enthalpy change must also change. The actual process of making steel from iron ore is more involved than simply adding heat, but that needn t be addressed here. 170
5 BONUS!!!!!! (for up to 5 points complete the following). Avogadro s number is huge. To try to understand how big this number really is consider the following: A basketball has a diameter of 9.4 inches. The Earth has a surface area of 196,935,000 sq miles. How many basketballs are required to cover the Earth with a single layer of basketballs? (note: if your calculator does not have a key for π, try using 22 / 7 ) The projected area taken up by a sphere is equal to the cross-section of the sphere at its largest point. r = 9.4 inches / 2 = (1 mi / 1,760 yd)*(1 yd / 36 inch)*(9.4 in / 2) = 7.42x10-5 mi Area of that circle = πr 2 = π(7.42x10-5 mi) 2 = 1.73x10-8 mi 2 /BB # BB needed to cover the Earth = area of Earth/Area per BB = # BB = 196,935,000 sq miles / 1.73x10-8 mi 2 /BB = 1.14x10 16 BB [Note:] this is actually a larger # than we would need because we have not accounted for the less than 100% packing efficiency of the BB (i.e. there will be some empty spaces between parts of the BB) What amount in mole(s) does the number of basketballs represent? mole BB = (1 mole BB / 6.022x10 23 BB)*(1.14x10 16 BB) = 1.89x10-8 mole What mass of aluminum contains a number of aluminum atoms equal to the number of basketballs found above? mass Al = (27.0 g Al / 1 mole)*( 1.89x10-8 mole) = 5.11x10-7 g Al or µg Al WOW! Avogadro s number is REALLY enormous... and atoms are REALLY tiny!!!! 171
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Exam 4, Enthalpy and Gases
 CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
Name SUNY Chemistry Practice Test: Chapter 5
 Name SUNY Chemistry Practice Test: Chapter 5 Multiple Choice 1. 1... 3. 3. 4. 4. 5. 6. 7. 8. 9. 10. 11. 1. 13. 14. 15. 16. 17. 18. 19. 0. 1 1) Calculate the kinetic energy in joules of an automobile weighing
Name SUNY Chemistry Practice Test: Chapter 5 Multiple Choice 1. 1... 3. 3. 4. 4. 5. 6. 7. 8. 9. 10. 11. 1. 13. 14. 15. 16. 17. 18. 19. 0. 1 1) Calculate the kinetic energy in joules of an automobile weighing
Chem 105/107 Exam #3 Fall 2012
 November 12 th, 2012 Name: CLID: Score: Chem 105/107 Exam #3 Fall 2012 There are 17 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions,
November 12 th, 2012 Name: CLID: Score: Chem 105/107 Exam #3 Fall 2012 There are 17 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions,
General Chemistry 1 CHM201 Unit 3 Practice Test
 General Chemistry 1 CHM201 Unit 3 Practice Test 1. Heat is best defined as a. a substance that increases the temperature and causes water to boil. b. a form of potential energy. c. a form of work. d. the
General Chemistry 1 CHM201 Unit 3 Practice Test 1. Heat is best defined as a. a substance that increases the temperature and causes water to boil. b. a form of potential energy. c. a form of work. d. the
Enthalpy. Enthalpy. Enthalpy. Enthalpy. E = q + w. Internal Energy at Constant Volume SYSTEM. heat transfer in (endothermic), +q
 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = KE + PE ΔE = q + w Because most systems,
heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = KE + PE ΔE = q + w Because most systems,
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Chemical Reactions. Chapter 17
 Chemical Reactions Chapter 17 Chemical Equations C+O 2 CO 2 C (s) +O 2 (g) CO 2 (g) Reactants on left, products on right Each are balanced because same number of atoms of reactants as products Some equations
Chemical Reactions Chapter 17 Chemical Equations C+O 2 CO 2 C (s) +O 2 (g) CO 2 (g) Reactants on left, products on right Each are balanced because same number of atoms of reactants as products Some equations
Enthalpies of Reaction
 Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Chemistry. Chapter 17
 Chemistry Chapter 17 Chemical Equations C+O 2 CO 2 C (s) +O 2 (g) CO 2 (g) Reactants on left, products on right Each are balanced because same number of atoms of reactants as products Balancing Chemical
Chemistry Chapter 17 Chemical Equations C+O 2 CO 2 C (s) +O 2 (g) CO 2 (g) Reactants on left, products on right Each are balanced because same number of atoms of reactants as products Balancing Chemical
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Enthalpy Chapter 5.3-4,7
 Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Ch 9 Practice Problems
 Ch 9 Practice Problems 1. One mole of an ideal gas is expanded from a volume of 1.50 L to a volume of 10.18 L against a constant external pressure of 1.03 atm. Calculate the work. (1 L atm = 101.3 J) A)
Ch 9 Practice Problems 1. One mole of an ideal gas is expanded from a volume of 1.50 L to a volume of 10.18 L against a constant external pressure of 1.03 atm. Calculate the work. (1 L atm = 101.3 J) A)
Chapter 6. Thermochemistry
 Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Thermochemistry: energy considerations associated with chemical and physical change Energy: the capacity of a system to do work or produce heat potential energy energy of position;
Chapter 6: Thermochemistry Thermochemistry: energy considerations associated with chemical and physical change Energy: the capacity of a system to do work or produce heat potential energy energy of position;
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Energy Heat Work Heat Capacity Enthalpy
 Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Name: Thermochemistry. Practice Test C. General Chemistry Honors Chemistry
 Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions
 Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Chapter 6 Review. Part 1: Change in Internal Energy
 Chapter 6 Review This is my own personal review, this should not be the only thing used to study. You should also study using notes, PowerPoint, homework, ect. I have not seen the exam, so I cannot say
Chapter 6 Review This is my own personal review, this should not be the only thing used to study. You should also study using notes, PowerPoint, homework, ect. I have not seen the exam, so I cannot say
FACULTY OF SCIENCE MID-TERM EXAMINATION CHEMISTRY 120 GENERAL CHEMISTRY MIDTERM 1. Examiners: Prof. B. Siwick Prof. I. Butler Dr. A.
 FACULTY OF SCIENCE MID-TERM EXAMINATION CHEMISTRY 120 GENERAL CHEMISTRY MIDTERM 1 Examiners: Prof. B. Siwick Prof. I. Butler Dr. A. Fenster Name: INSTRUCTIONS 1. Enter your student number and name on the
FACULTY OF SCIENCE MID-TERM EXAMINATION CHEMISTRY 120 GENERAL CHEMISTRY MIDTERM 1 Examiners: Prof. B. Siwick Prof. I. Butler Dr. A. Fenster Name: INSTRUCTIONS 1. Enter your student number and name on the
Enthalpy. Slide 1. Slide 2 Reaction Energies. Slide 3 Enthalpy of Reactions. Calorimetry of Chemistry
 Slide 1 Enthalpy Calorimetry of Chemistry Slide 2 Reaction Energies In our earlier discussions of calorimetry, we used physical sources of heat (hot metal slug). It is also possible to use chemical sources
Slide 1 Enthalpy Calorimetry of Chemistry Slide 2 Reaction Energies In our earlier discussions of calorimetry, we used physical sources of heat (hot metal slug). It is also possible to use chemical sources
Exothermic process is any process that gives off heat transfers thermal energy from the system to the surroundings. H 2 O (l) + energy
 Exothermic process is any process that gives off heat transfers thermal energy from the system to the surroundings. H 2 O (g) H 2 O (l) + energy Endothermic process is any process in which heat has to
Exothermic process is any process that gives off heat transfers thermal energy from the system to the surroundings. H 2 O (g) H 2 O (l) + energy Endothermic process is any process in which heat has to
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
BCIT Fall Chem Exam #2
 BCIT Fall 2017 Chem 3310 Exam #2 Name: Attempt all questions in this exam. Read each question carefully and give a complete answer in the space provided. Part marks given for wrong answers with partially
BCIT Fall 2017 Chem 3310 Exam #2 Name: Attempt all questions in this exam. Read each question carefully and give a complete answer in the space provided. Part marks given for wrong answers with partially
Thermochemistry. Chapter 6. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Calorimetry. Chapter 5. Week 2 Unit 1. Calorimetry. Since we cannot know the of the reactants and products, we measure H through, the of.
 Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED
 CHM-201 General Chemistry and Laboratory I Unit #3 Take Home Test Due April 8, 2019 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
CHM-201 General Chemistry and Laboratory I Unit #3 Take Home Test Due April 8, 2019 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
Chemistry 200: General Chemistry I - Lecture
 Name Date Chemistry 200: General Chemistry I - Lecture Exam #3, 100 Points Total Form: A Read all directions carefully. Answers not conforming to the directions will be marked as incorrect! Section 1:
Name Date Chemistry 200: General Chemistry I - Lecture Exam #3, 100 Points Total Form: A Read all directions carefully. Answers not conforming to the directions will be marked as incorrect! Section 1:
The following gas laws describes an ideal gas, where
 Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Name Chem 161, Section: Group Number: ALE 27. Hess s Law. (Reference: Chapter 6 - Silberberg 5 th edition)
 Name Chem 161, Section: Group Number: ALE 27. Hess s Law (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a calculation you must show your work neatly using dimensional
Name Chem 161, Section: Group Number: ALE 27. Hess s Law (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a calculation you must show your work neatly using dimensional
Measuring and Expressing Enthalpy Changes. Copyright Pearson Prentice Hall. Measuring and Expressing Enthalpy Changes. Calorimetry
 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Useful Information to be provided on the exam: 1 atm = 760 mm Hg = 760 torr = lb/in 2 = 101,325 Pa = kpa. q = m C T. w = -P V.
 Chem 101A Study Questions, Chapters 5 & 6 Name: Review Tues 10/25/16 Due 10/27/16 (Exam 3 date) This is a homework assignment. Please show your work for full credit. If you do work on separate paper, attach
Chem 101A Study Questions, Chapters 5 & 6 Name: Review Tues 10/25/16 Due 10/27/16 (Exam 3 date) This is a homework assignment. Please show your work for full credit. If you do work on separate paper, attach
(E) half as fast as methane.
 Name AP Chem / / AP Chem Practice Exam #2 Part I: 40 Questions, 40 minutes, Multiple Choice, No Calculator Allowed Bubble the correct answer on the BLUE SIDE of your scantron for each of the following.
Name AP Chem / / AP Chem Practice Exam #2 Part I: 40 Questions, 40 minutes, Multiple Choice, No Calculator Allowed Bubble the correct answer on the BLUE SIDE of your scantron for each of the following.
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
47 people in recitation yesterday. Expect quizzes there and in class.
 Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
CHM 111 Dr. Kevin Moore
 CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
Thermodynamics - Energy Relationships in Chemical Reactions:
 Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Learning Check. How much heat, q, is required to raise the temperature of 1000 kg of iron and 1000 kg of water from 25 C to 75 C?
 Learning Check q = c * m * ΔT How much heat, q, is required to raise the temperature of 1000 kg of iron and 1000 kg of water from 25 C to 75 C? (c water =4.184 J/ C g, c iron =0.450 J/ C g) q Fe = 0.450
Learning Check q = c * m * ΔT How much heat, q, is required to raise the temperature of 1000 kg of iron and 1000 kg of water from 25 C to 75 C? (c water =4.184 J/ C g, c iron =0.450 J/ C g) q Fe = 0.450
Energy & Chemistry. Internal Energy (E) Energy and Chemistry. Potential Energy. Kinetic Energy. Energy and Chemical Reactions: Thermochemistry or
 Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
CHEMISTRY - TRO 4E CH.6 - THERMOCHEMISTRY.
 !! www.clutchprep.com CONCEPT: ENERGY CHANGES AND ENERGY CONSERVATION is the branch of physical science concerned with heat and its transformations to and from other forms of energy. is the branch of chemistry
!! www.clutchprep.com CONCEPT: ENERGY CHANGES AND ENERGY CONSERVATION is the branch of physical science concerned with heat and its transformations to and from other forms of energy. is the branch of chemistry
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
1. Entropy questions: PICK TWO (6 each)
 1. Entropy questions: PICK TWO (6 each) 1.00 mole of water freezes at 0.00ºC and 1 atm, releasing 6.01 kj of heat. Calculate the change in entropy and free energy for the process. Calculate the entropy
1. Entropy questions: PICK TWO (6 each) 1.00 mole of water freezes at 0.00ºC and 1 atm, releasing 6.01 kj of heat. Calculate the change in entropy and free energy for the process. Calculate the entropy
CHAPTER 16 REVIEW. Reaction Energy. SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 16 REVIEW Reaction Energy SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. For elements in their standard state, the value of H 0 f is 0. 2. The formation and decomposition
CHAPTER 16 REVIEW Reaction Energy SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. For elements in their standard state, the value of H 0 f is 0. 2. The formation and decomposition
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
_ + Units of Energy. Energy in Thermochemistry. Thermochemistry. Energy flow between system and surroundings. 100º C heat 50º C
 Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Chapter 5 Practice Multiple Choice & Free
 Name Response 1. A system has an increase in internal energy, E, of 40 kj. If 20 kj of work, w, is done on the system, what is the heat change, q? a) +60 kj d) -20 kj b) +40 kj e) -60 kj c) +20 kj 2. Which
Name Response 1. A system has an increase in internal energy, E, of 40 kj. If 20 kj of work, w, is done on the system, what is the heat change, q? a) +60 kj d) -20 kj b) +40 kj e) -60 kj c) +20 kj 2. Which
Exam 3, Ch 7, 19, 14 November 9, Points
 Chem 30 Name Exam 3, Ch 7, 9, 4 November 9, 206 00 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units
Chem 30 Name Exam 3, Ch 7, 9, 4 November 9, 206 00 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units
Chapter 8. Thermochemistry
 Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
Chemistry 212 Fall 2017 Exam III - A
 Chemistry 212 Fall 2017 Exam III - A Name MULTIPLE CHOICE. (1 point each) Choose the one alternative that best completes the statement or answers the question. 1) An important step in the synthesis of
Chemistry 212 Fall 2017 Exam III - A Name MULTIPLE CHOICE. (1 point each) Choose the one alternative that best completes the statement or answers the question. 1) An important step in the synthesis of
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
33. a. Heat is absorbed from the water (it gets colder) as KBr dissolves, so this is an endothermic process.
 31. This is an endothermic reaction so heat must be absorbed in order to convert reactants into products. The high temperature environment of internal combustion engines provides the heat. 33. a. Heat
31. This is an endothermic reaction so heat must be absorbed in order to convert reactants into products. The high temperature environment of internal combustion engines provides the heat. 33. a. Heat
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
mcdonald (pam78654) HW 6B: Thermodynamics laude (89560) 1 2. N 2 H 4 (g)+h 2 (g) 2NH 3 (g) 3. Na + (g)+cl (g) NaCl(s) 4. S 3 (g)+9f 2 (g) 3SF 6 (g)
 mcdonald (pam78654) HW 6B: Thermodynamics laude (89560) 1 This print-out should have 21 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001
mcdonald (pam78654) HW 6B: Thermodynamics laude (89560) 1 This print-out should have 21 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
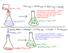 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Overview 4-8. Overview 4-8
 Overview 4-8 An unorthodox chemistry student decides to set out on a round-the-world trip using a balloon filled with hydrogen. Barely remembering his physics lessons, he figures out from his weight that
Overview 4-8 An unorthodox chemistry student decides to set out on a round-the-world trip using a balloon filled with hydrogen. Barely remembering his physics lessons, he figures out from his weight that
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Chapter 6. Energy Thermodynamics
 Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
CHEM 115 EXAM #1 Practice Fall 2007
 Name CHEM 115 EXAM #1 Practice Fall 007 Circle the correct answer or fill in the blanks. (numbers 1 -,.5 points each) 1. The term used to describe a substance composed of two or more elements combined
Name CHEM 115 EXAM #1 Practice Fall 007 Circle the correct answer or fill in the blanks. (numbers 1 -,.5 points each) 1. The term used to describe a substance composed of two or more elements combined
Chapter 6. Thermochemistry. Chapter 6. Chapter 6 Thermochemistry. Chapter 6 Thermochemistry Matter vs Energy 2/16/2016
 Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
CHEM 1423 Chapter 17 Homework Questions TEXTBOOK HOMEWORK
 CHEM 1423 Chapter 17 Homework Questions TEXTBOOK HOMEWORK 17.29 At 425 o C, Kp = 4.18x10-9 for the reaction 2HBr(g) H 2 (g) + Br 2 (g) In one experiment, 0.20 atm of HBr(g), 0.010 atm of H 2 (g), and 0.010
CHEM 1423 Chapter 17 Homework Questions TEXTBOOK HOMEWORK 17.29 At 425 o C, Kp = 4.18x10-9 for the reaction 2HBr(g) H 2 (g) + Br 2 (g) In one experiment, 0.20 atm of HBr(g), 0.010 atm of H 2 (g), and 0.010
Chemical Equations. Chemical Reaction: Interaction between substances that results in one or more new substances being produced
 Chemical Equations Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen water Reactants of a Reaction: Starting materials
Chemical Equations Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen water Reactants of a Reaction: Starting materials
Calculate the energy required to melt a 2.9 kg block of ice. Melting is a phase change - there is no change in temperature
 Calculation of Heat During a Phase Change or Reaction During a phase change, a physical process or chemical reaction, the temperature of the system does not change. Therefore the formula q = mc T would
Calculation of Heat During a Phase Change or Reaction During a phase change, a physical process or chemical reaction, the temperature of the system does not change. Therefore the formula q = mc T would
b. Free energy changes provide a good indication of which reactions are favorable and fast, as well as those that are unfavorable and slow.
 Chem 130 Name Exam 3, Ch 7, 19, 14 November 9, 2018 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct
Chem 130 Name Exam 3, Ch 7, 19, 14 November 9, 2018 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry: Part of Thermodynamics
 Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
AP Chapter 6: Thermochemistry Name
 AP Chapter 6: Thermochemistry Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. AP Chapter 6: Thermochemistry 2 Warm-Ups (Show your work for credit)
AP Chapter 6: Thermochemistry Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. AP Chapter 6: Thermochemistry 2 Warm-Ups (Show your work for credit)
Question 4. Calculate q when 0.10 g of ice is cooled from 10.ºC to -75ºC. (c ice = J/g ºC) A) -18 J B) -14 J C) -8.5 J D) + 14 J E) +18 J 5-4
 Question 1 A system conducts 1.07 kj of heat to the surroundings while delivering 1.79 kj of work. What is the change in internal energy of the system? A) +0.72 kj B) -0.72 kj C) +2.86 kj D) -2.86 kj 5-1
Question 1 A system conducts 1.07 kj of heat to the surroundings while delivering 1.79 kj of work. What is the change in internal energy of the system? A) +0.72 kj B) -0.72 kj C) +2.86 kj D) -2.86 kj 5-1
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
AP* Chapter 6. Thermochemistry
 AP* Chapter 6 Thermochemistry Section 6.1 The Nature of Energy Energy Capacity to do work or to produce heat. Law of conservation of energy energy can be converted from one form to another but can be neither
AP* Chapter 6 Thermochemistry Section 6.1 The Nature of Energy Energy Capacity to do work or to produce heat. Law of conservation of energy energy can be converted from one form to another but can be neither
AP Chemistry A. Allan Chapter Six Notes - Thermochemistry
 AP Chemistry A. Allan Chapter Six Notes - Thermochemistry 6.1 The Nature of Energy A. Definition 1. Energy is the capacity to do work (or to produce heat*) a. Work is a force acting over a distance (moving
AP Chemistry A. Allan Chapter Six Notes - Thermochemistry 6.1 The Nature of Energy A. Definition 1. Energy is the capacity to do work (or to produce heat*) a. Work is a force acting over a distance (moving
2. If the volume of a container holding a gas is reduced, what will happen to the presure within the container?
 1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
Chapter 6. Heat Flow
 Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
measure ΔT in water to get q = q surroundings and use q system = q surroundings
 example using water: Calculate the amount of energy required to heat 95.0 g of water from 22.5 C to 95.5 C. q = s m ΔT ( C (4.184 J g 1 C 1 ) (95.0 g) (73.0 = = 2.90 x 10 4 J or 29.0 kj Constant Pressure
example using water: Calculate the amount of energy required to heat 95.0 g of water from 22.5 C to 95.5 C. q = s m ΔT ( C (4.184 J g 1 C 1 ) (95.0 g) (73.0 = = 2.90 x 10 4 J or 29.0 kj Constant Pressure
2. When determining the ΔH rxn from ΔH f o, which of the following is not necessary in the calculation.
 Ch 6 and 7 Practice Problems - KEY The following problems are intended to provide you with additional practice in preparing for the exam. Questions come from the textbook, previous quizzes, previous exams,
Ch 6 and 7 Practice Problems - KEY The following problems are intended to provide you with additional practice in preparing for the exam. Questions come from the textbook, previous quizzes, previous exams,
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
