Chapter 6: Thermochemistry
|
|
|
- Emory Andrews
- 6 years ago
- Views:
Transcription
1 Chapter 6: Thermochemistry Thermochemistry: energy considerations associated with chemical and physical change Energy: the capacity of a system to do work or produce heat potential energy energy of position; stored energy kinetic energy energy of motion kinetic energy =! mυ 2 kinetic energy = 3 2 RT In a system, energy can be transferred in the form of heat or work: An Example of a System Configured for Energy Transferred as Work: heat, q energy transfer results in temperature change (ΔT); ΔT = Tfinal Tinitial work, w energy transfer during the act of moving an object against an opposing force overall: ΔU = q + w
2 The System, The Surroundings, The Universe & The First Law of Thermodynamics Units of Energy SI unit of energy: Joule, J 1 Joule is the amount of energy required to raise the temperature of g H2O by 1ºC. 1 kj = 103 J 1 MJ = 106 J thermochemical calorie, cal 1 calorie is the amount of energy required to raise the temperature of 1 g of H2O by 1ºC. 1 cal = J 1 kcal = 103 cal 1 nutritional calorie, 1 Cal = 1000 cal Endothermic Change: energy is absorbed by the system energy flow is from the surroundings into the system Esys increases Esurr decreases for the system: Efinal > Einitial if energy transferred in the form of heat, Tsys increases and Tsurr decreases E lost by surroundings = E gained by the system The System: what we re interested in The Surroundings: everything else The Universe: System + Surroundings = the Universe The 1st Law of Thermodynamics: The energy of the Universe is constant.
3 energy is released by the system energy flow is from the system into the surroundings Esys decreases Esurr increases Exothermic Change for the system: Efinal < Einitial if energy transferred in the form of heat, Tsys decreases and Tsurr increases E lost by system = E gained by the surroundings Changes in Thermchemical Quantities: importance of sign and magnitude if E is + Efinal > Einitial energy is absorbed by the system endothermic change ΔX = Xfinal Xinitial if ΔX is + : Xfinal > Xinitial if ΔX is! : Xfinal < Xinitial if E is! Efinal < Einitial energy is released by the system exothermic change if q is + qfinal > qinitial heat is absorbed by the system endothermic if q is! qfinal < qinitial heat is released by the system exothermic Calculate the change in internal energy of a system that releases 26.8 kj of heat as it does 68.7 kj of work on the surroundings. if w is + surroundings do work on the system endothermic if w is! system does work on the surroundings exothermic
4 PV Work expansion or compression of an ideal gas w =!P V notes: w and V are always opposite in sign; why? Calculate the quantity of work done (in kj) by an ideal gas as it expands from an initial volume of 2.50 L to a final volume of L against a constant external pressure of atm. 1 L atm = J Enthalpy, H H = E + PV We will be focussing on changes in enthalpy that accompany processes that occur at constant P. at constant P: H = qp H = Hfinal! Hinitial if H is + : heat is absorbed by the system endothermic process if H is! : heat is released by the system exothermic process Enthalpy Changes for Chemical Reactions: Thermochemical Equations Hrxn = Hfinal! Hinitial OR Hrxn = Hproducts! Hreactants Thermochemical Equation: balanced chemical equation + thermochemical data 2 Na (s) + 2 H2O (l)! 2 NaOH (aq) + H2 (g); H = kj notes: reaction is exothermic as written specifically, kj of energy is released when: 2 mol Na & 2 mol H2O are consumed 2 mol NaOH & 1 mol H2 are formed
5 An Enthalpy Diagram for This Reaction: Enthalpy Changes for Chemical Reactions: Thermochemical Equations interpretation of energy changes associated with a reaction must take into consideration the following: phases of reactants and products ex. 2 H2 (g) + O2 (g)! 2 H2O (g); H = kj vs. 2 H2 (g) + O2 (g)! 2 H2O (l); H = kj direction of the reaction: a reaction is endothermic in one direction and exothermic in the reverse direction ex. CH4 (g) + 4 Cl2 (g)! CCl4 (l) + 4 HCl (g); H = 433 kj vs. CCl4 (l) + 4 HCl (g)! CH4 (g) + 4 Cl2 (g); H = +433 kj Enthalpy Changes for Chemical Reactions: Thermochemical Equations stoichiometry of balanced equation energy released or absorbed is an extensive property (depends on size of sample) ex. combustion of propane C3H8 (g) + 5 O2 (g)! 3 CO2 (g) + 4 H2O (g); Hrxn = 2200 kj combustion of 100 g (2.27 mol) C3H8 generates ~5000 kj vs. combustion of 2.5 kg (56.7 mol) C3H8 generates ~125,000 kj N2 (g) + 3 H2 (g)! 2 NH3 (g); Determine the quantity of heat released (in kj) in the synthesis of 907 kg NH3. Stoichiometric Calculations: Using Hrxn as a Conversion Factor H = 91.8 kj 12.0 L N2 (g) at STP and 5.00 L H2 (g) at 30ºC and 3.02 atm are combined and allowed to react. What is the maximum amount of heat (in kj) that can be generated?
6 Calorimetry A laboratory technique in which a reaction proceeds in an insulated container (i.e. calorimeter) at constant P or constant V. adiabatic conditions for the system; no heat is lost to or gained from the surroundings record an observed T calculate H or E at constant P: H = qp at constant V: E = qv Heat Capacity (C) and Specific Heat (s) How does a substance respond to heating? Compare the observed temperature changes when 50.0 J energy are added to 10.0 g samples of diamond and tungsten: diamond m = 10.0 g q = 50.0 J T = 9.8ºC tungsten m = 10.0 g q = 50.0 J T = 37.3ºC Heat Capacity of a substance, C heat required to raise the temperature of a sample by 1º (C or K). heat supplied Heat Capacity = ; units J ºC or K T produced OR C = q T Specific Heat, s heat required to raise the temperature of 1g of substance by 1º (C or K) heat supplied Specific Heat = ; units J/g ºC or K ( T)(mass) OR s = q/(m T) note: use molar mass to convert between specific heat and molar heat capacity of a substance
7 Calculate the amount of heat required (in J) to raise the temperature of a 25.0 g block of nickel from 22ºC to 104ºC. For Ni, s = 0.44 J/g ºC g H2O at 280 K is mixed with 50.0 g H2O at 330 K. What will be the final temperature of the mixture? For H2O, s = J/g ºC. Constant Pressure Calorimetry to Determine H adiabatic conditions: no heat exchanged between system and surroundings Hsys = 0 if: Hsys = H1 + H2 then: H1 = H2 for an exothermic reaction: H1 = heat released by chemical reaction occurring in calorimeter H2 = heat absorbed by solution & calorimeter resulting in increase in T What do you measure in lab? amounts of reactants (mass, volume, mol) observed T order of determination (for the following examples): H2 H1 molar enthalpy of reaction +, units kj!, units kj!, units kj/mol Consider the neutralization reaction of hydrochloric acid and sodium hydroxide: HCl (aq) + NaOH (aq)! NaCl (aq) + H2O (l) Determine the molar enthalpy change (in kj/mol) for this reaction if, in a constant pressure calorimetry experiment ml of 1.20 M HCl (aq) is combined with 42.0 ml of 1.20 M NaOH (aq) resulting in an increase in temperature from an initial 25.00ºC to a final 31.80ºC. You can assume that, for the solutions, volume is additive; d = 1.00 g/ml; s = J/gºC.
8 When 23.6 g CaCl2 is dissolved in water in a constant pressure calorimeter, the temperature rose from 25.0ºC to 38.7ºC. If the heat capacity of the calorimeter and solution is 1258 J/ºC (i.e. Ccal = 1258 J/ºC), determine the heat released by the dissolution of 1.20 mol CaCl2. CaCl2 (s)! Ca 2+ (aq) + 2 Cl (aq) Constant Volume Calorimetry to Determine E adiabatic conditions: no heat exchanged between system and surroundings Esys = 0 if: Esys = E1 + E2 then: E1 = E2 for an exothermic reaction: E1 = heat released by chemical reaction occurring in calorimeter E2 = heat absorbed by solution & calorimeter resulting in increase in T The combustion of ethanol is studied in a constant volume (bomb) calorimeter with a calorimeter constant of 9.63 kj/ºc (i.e Ccal = 9.63 kj/ºc). The combustion of 2.84 g C2H5OH results in an increase in temperature from Ti = 25.00ºC to Tf = 33.73ºC. Determine the energy released in the combustion of 1 mol of C2H5OH. Determine the heat of combustion per gram of ethanol (in kj/g) Hess s Law of Heat Summation We can determine of H for one chemical reaction based on the H values for related reactions using Hess s Law: For a chemical reaction that can be written as the sum of 2 or more steps, H for the overall reaction is equal to the sum of H s for the individual steps. so: for a reaction that is a result of the sum of 3 individual steps: Hrxn = H1 + H2 + H3
9 Why does Hess s Law of Heat Summation work? Enthalpy is a state property: Hess s Law of Heat Summation dependent only on initial and final states independent of path Hess s Law of Heat Summation Determine H for the following reaction using equations 1 & 2 given below: 2 C (s) + O2 (g)! 2 CO (g) H =???? use: (1) 2 C (s) + 2 O2 (g)! 2 CO2 (g) H1 = 787 kj (2) 2 CO2 (g)! 2 CO (g) + O2 (g) H2 = +566 kj overall: 2 C (s) + O2 (g)! 2 CO (g) H = 221 kj An Enthalpy Diagram for This Process: To solve a Hess s Law of Heat Summation problem: consider the equations you have to work with identify how to manipulate them so that when you add them together, you end up with your target, overall equation your options are: multiply all stoichiometric coefficients in the equation by some factor, n " multiply H by the same factor, n write an equation in reverse (i.e. change direction) " change the sign on H
10 Determine H for: W (s) + C (s)! WC (s); H =???? using: (1) 2 W (s) + 3 O2 (g)! 2 WO3 (s) H = kj (2) C (s) + O2 (g)! CO2 (g) H = kj (3) 2 WC (s) + 5 O2 (g)! 2 WO3 (s) + 2 CO2 (g) H = kj solution: Determine H for: W (s) + C (s)! WC (s); H =???? using: (1) 2 W (s) + 3 O2 (g)! 2 WO3 (s) H = kj (2) C (s) + O2 (g)! CO2 (g) H = kj (3) 2 WC (s) + 5 O2 (g)! 2 WO3 (s) + 2 CO2 (g) H = kj actions to take: reverse equation (3) multiply equation (1) by factor of " multiply equation (3) by factor of " do nothing to equation (2) solution: Determine H for: W (s) + C (s)! WC (s); H =???? using: (1) W (s) + 3/2 O2 (g)! WO3 (s) H = kj (2) C (s) + O2 (g)! CO2 (g) H = kj (3) WO3 (s) + CO2 (g)! WC (s) + 5/2 O2 (g) H = kj overall: W (s) + C (s)! WC (s) H = 38.0 kj Standard Reaction Enthalpies Standard Reaction Enthalpies, Hº: reaction enthalpy corresponding to reactants in their standard states forming products in their standard states standard state: the physical state of a pure substance at P = 1 atm and a defined temperature
11 Standard Enthalpies of Formation, Hºf Hºf corresponds to the formation of 1 mol of a substance in its standard state from its elements in their standard states. ex: Na (s) + " Cl2 (g)! NaCl (s) 6 C (s) + 6 H2 (g) + 3 O2 (g)! C6H12O6 (s) Hºf of an element in its most stable form = 0 units of Hºf are kj/mol tabulated in Table 6.2 and Appendix C of text use Hºf s to calculate Hºrxn Using Standard Enthalpies of Formation, Hºf Hºrxn = n Hºf products n Hºf reactants Calcualte Hº (in kj) for the following reaction using the given Hºf values: 3 NO2 (g) + H2O (l)! 2 HNO3 (aq) + NO (g) Hºf: (in kj/mol) Determine Hºf (in kj/mol) for HCl (g) using the following data: CH4 (g) + 4 Cl2 (g)! CCl4 (l) + 4 HCl (g); Hº = kj Hºf : ??? (in kj/mol)
12 Determine Hºf (in kj/mol) for HCl (g) using the following data: CH4 (g) + 4 Cl2 (g)! CCl4 (l) + 4 HCl (g); Hº = kj Hºf : ??? (in kj/mol) solution: let x = Hºf of HCl (g) kj = [(1 mol CCl4)( 139 kj/mol) + (4 mol HCl)(x)]! [(1 mol CH4)( 74.9 kj/mol)] kj = 139 kj + (4 mol)(x) kj = 4 mol(x) so x = Hºf for HCl (g) = 92.3 kj/mol
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Topic 05 Energetics : Heat Change. IB Chemistry T05D01
 Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
All chemical reactions involve changes in energy. Typically this energy comes in the form of heat.
 Topic: Thermochemistry Essential Question: How does energy flow in chemical reactions? Name: Class: Date: / / Period: All chemical reactions involve changes in energy. Typically this energy comes in the
Topic: Thermochemistry Essential Question: How does energy flow in chemical reactions? Name: Class: Date: / / Period: All chemical reactions involve changes in energy. Typically this energy comes in the
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Enthalpy and Internal Energy
 Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 6: Thermochemistry
 Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Gilbert Kirss Foster. Chapter 9. Thermochemistry. Energy Changes in Chemical Reactions
 Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
5.1 Exothermic and endothermic reactions
 Topic 5: Energetics 5.1 Exothermic and endothermic reactions Chemical reactions involve the breaking and making of bonds. Breaking bonds requires energy,whereas energy is given out when new bonds are formed.
Topic 5: Energetics 5.1 Exothermic and endothermic reactions Chemical reactions involve the breaking and making of bonds. Breaking bonds requires energy,whereas energy is given out when new bonds are formed.
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY
 AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY Reaction Rate how fast a chemical reaction occurs Collision Theory In order for a chemical reaction to occur, the following conditions must
AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY Reaction Rate how fast a chemical reaction occurs Collision Theory In order for a chemical reaction to occur, the following conditions must
Chapter 6. Thermochemistry
 Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
The Nature of Energy Energy is the ability to do work or produce Heat, q or Q, is ; flows due to temperature differences (always to )
 CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Reaction Energy. Thermochemistry
 Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry. Chapter 6. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes
 Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Chapter 6. Energy Thermodynamics
 Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
I. Chemical Reactions that Involve Heat
 Unit 12 Energy I. Chemical Reactions that Involve Heat Thermochemistry: study of changes in heat in chemical reactions. Endothermic: absorbs heat; temp. goes down Exothermic: releases heat; temp. goes
Unit 12 Energy I. Chemical Reactions that Involve Heat Thermochemistry: study of changes in heat in chemical reactions. Endothermic: absorbs heat; temp. goes down Exothermic: releases heat; temp. goes
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
_ + Units of Energy. Energy in Thermochemistry. Thermochemistry. Energy flow between system and surroundings. 100º C heat 50º C
 Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Energy Transformations
 Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
5/14/14. How can you measure the amount of heat released when a match burns?
 CHEMISTRY & YOU Chapter 7 Thermochemistry How can you measure the amount of heat released when a match burns? 7. The Flow of Energy 7.3 Heat in Changes of State 7.4 Calculating Heats of Reaction Remember:
CHEMISTRY & YOU Chapter 7 Thermochemistry How can you measure the amount of heat released when a match burns? 7. The Flow of Energy 7.3 Heat in Changes of State 7.4 Calculating Heats of Reaction Remember:
Quiz I: Thermodynamics
 Quiz I: Thermodynamics SCH4U_2018-2019_V2 NAME: (Total Score: / 30) Multiple Choice (12) 1. What can be deduced from the following reaction profile? A. The reactants are less stable than the products and
Quiz I: Thermodynamics SCH4U_2018-2019_V2 NAME: (Total Score: / 30) Multiple Choice (12) 1. What can be deduced from the following reaction profile? A. The reactants are less stable than the products and
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
If neither volume nor pressure of the system changes, w = 0 and ΔE = q = ΔH. The change in internal energy is equal to the change in enthalpy.
 5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Heat (q) Two systems with different temperatures that are in thermal contact will exchange thermal energy, the quantity of which
AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Heat (q) Two systems with different temperatures that are in thermal contact will exchange thermal energy, the quantity of which
Lecture outline: Chapter 5
 Lecture outline: Chapter 5 1. The nature of energy Thermochemistryh 2. First law of thermodynamics 3. Enthalpies of reaction 4. Hess law 5. Enthalpies of formation 1 Chemical Reactivity (1) Does a chemical
Lecture outline: Chapter 5 1. The nature of energy Thermochemistryh 2. First law of thermodynamics 3. Enthalpies of reaction 4. Hess law 5. Enthalpies of formation 1 Chemical Reactivity (1) Does a chemical
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
6.5 Hess s Law of Heat Summation. 2 Chapter 6: First Law. Slide 6-2
 1/3/11 Thermochemistry: Energy Flow and Chemical Change Chapter 6 6.1 Forms of Energy and Their Interconversion 6.2 Enthalpy: Heats of Reaction and Chemical Change Thermochemistry: Energy Flow and Chemical
1/3/11 Thermochemistry: Energy Flow and Chemical Change Chapter 6 6.1 Forms of Energy and Their Interconversion 6.2 Enthalpy: Heats of Reaction and Chemical Change Thermochemistry: Energy Flow and Chemical
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
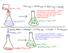 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Thermochemistry Ch. 8
 Definitions I. Energy (E): capacity to do work. II. Heat (q): transfer of energy from a body at a high temp. to a body at a low temp. III. Reaction perspectives: A. System: the focus. B. Surroundings:
Definitions I. Energy (E): capacity to do work. II. Heat (q): transfer of energy from a body at a high temp. to a body at a low temp. III. Reaction perspectives: A. System: the focus. B. Surroundings:
Energy Conversions. Energy. the ability to do work or produce heat. energy energy due to composition or position of an object
 Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
THERMODYNAMICS. Energy changes in reactions Text chapter 3, 4, 5, 6 & 7
 1 THERMODYNAMICS Energy changes in reactions Text chapter 3, 4, 5, 6 & 7 TERMINOLOGY: Thermodynamics: study of heat changes that occur during chemical reactions. Energy (J): Cannot be seen, touched, smelled,
1 THERMODYNAMICS Energy changes in reactions Text chapter 3, 4, 5, 6 & 7 TERMINOLOGY: Thermodynamics: study of heat changes that occur during chemical reactions. Energy (J): Cannot be seen, touched, smelled,
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Name: Thermochemistry. Practice Test C. General Chemistry Honors Chemistry
 Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chemical Thermodynamics
 Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Chapter 8. Thermochemistry 강의개요. 8.1 Principles of Heat Flow. 2) Magnitude of Heat Flow. 1) State Properties. Basic concepts : study of heat flow
 강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
Example 1: m = 100mL = 100g T i = 25 o C T f = 38 o C ΔT = 13 o C c = 4.18 J / (g o C) Q =??? Molar Heat of Dissolutions
 Molar Heat of Dissolutions It is observed that when 8.0g of Lithium chloride (LiCl) at 25 o C is dissolved in 100mL of water inside a calorimeter the final temperature of the water is 38 o C. Questions:
Molar Heat of Dissolutions It is observed that when 8.0g of Lithium chloride (LiCl) at 25 o C is dissolved in 100mL of water inside a calorimeter the final temperature of the water is 38 o C. Questions:
Name SUNY Chemistry Practice Test: Chapter 5
 Name SUNY Chemistry Practice Test: Chapter 5 Multiple Choice 1. 1... 3. 3. 4. 4. 5. 6. 7. 8. 9. 10. 11. 1. 13. 14. 15. 16. 17. 18. 19. 0. 1 1) Calculate the kinetic energy in joules of an automobile weighing
Name SUNY Chemistry Practice Test: Chapter 5 Multiple Choice 1. 1... 3. 3. 4. 4. 5. 6. 7. 8. 9. 10. 11. 1. 13. 14. 15. 16. 17. 18. 19. 0. 1 1) Calculate the kinetic energy in joules of an automobile weighing
Chapter 5: Thermochemistry. Molecular Kinetic Energy -Translational energy E k, translational = 1/2mv 2 -Rotational energy 5.
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
8.6 The Thermodynamic Standard State
 8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Name Date Class SECTION 16.1 PROPERTIES OF SOLUTIONS
 SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Thermochemistry: Part of Thermodynamics
 Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
AP CHEMISTRY. Unit 5 Thermochemistry. Jeff Venables Northwestern High School
 AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
Chapter 5: Thermochemistry
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Chapter 3. Thermochemistry: Energy Flow and Chemical Change. 5.1 Forms of Energy and Their Interconversion
 Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
17.2 Thermochemical Equations
 17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Law of conservation of energy: energy cannot be created or destroyed, only transferred One object to another One type of energy to another
 ch6blank Page 1 Chapter 6: Thermochemistry Thermochemistry: study of the relationships between chemistry and energy Energy: capacity to do work Work:result of a force acting over a certain distance, one
ch6blank Page 1 Chapter 6: Thermochemistry Thermochemistry: study of the relationships between chemistry and energy Energy: capacity to do work Work:result of a force acting over a certain distance, one
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Thermodynamics - Energy Relationships in Chemical Reactions:
 Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
3.2 Calorimetry and Enthalpy
 3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
Thermochemistry (chapter 5)
 Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
47 people in recitation yesterday. Expect quizzes there and in class.
 Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
THERMOCHEMISTRY -1. Dr. Sapna Gupta
 THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Table 1. Data for Heat Capacity Trial 1 Trial 2
 Thermochemistry: Measuring Enthalpy Change in Chemical Reactions Experiment created by the UMaine InterChemNet Team. Adapted with permission. Print this form and bring it with you to lab. You will complete
Thermochemistry: Measuring Enthalpy Change in Chemical Reactions Experiment created by the UMaine InterChemNet Team. Adapted with permission. Print this form and bring it with you to lab. You will complete
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
