Monolayer and multilayer adsorption isotherm models for sorption from aqueous media
|
|
|
- Elwin Randall
- 5 years ago
- Views:
Transcription
1 Kora J. Chm. Eg., 32(5), (2015) DOI: /s REVIEW PAPER INVITED REVIEW PAPER pissn: ISSN: Moolayr ad multilayr adsorptio isothrm modls for sorptio from auous mdia Ryhah Saadi*,, Zahra Saadi*,, Rza Fazali*,, ad Nargs Elmi Fard** *Dpartmt of Chmical Egirig, Faculty of Egirig, South Thra Brach, Islamic Azad Uivrsity, Thra, Ira **Dpartmt of Chmistry, Faculty of Scic, East Thra Brach, Islamic Azad Uivrsity, Thra, Ira (Rcivd 21 Jauary 2015 accptd 10 March 2015) Abstract Idustrial wastwatr pollutd with various cotamiats, icludig havy mtals, dys, tc., dagrs huma halth ad th viromt. Various sparatio tchius hav b dvlopd for th rmoval of pollutats from auous solutios. Adsorptio procss has draw cosidrabl atttio du to its simplicity of dsig, high rmoval fficicy, v at dilut coctratio, ad coomical aspct. W rviwd th most commo two, thr, four, ad fiv paramtr adsorptio isothrm modls corrspodig to moolayr ad multilayr adsorptio o th basis of paramtrs that ca b usd for xplorig ovl adsorbts. Thrmodyamic assumptios of th modls giv iformatio about th surfac proprtis, capacity of th adsorbt ad adsorptio mchaism. Sv rror fuctios wr ivstigatd to valuat th fitss uality of isothrm modls with th xprimtal uilibrium data. Kywords: Adsorptio, Isothrm, Moolayr, Multilayr, Error INTRODUCTION Th rapid progrss of diffrt idustris ad tchologis has rsultd i a hug amout of wastwatr big producd from idustrial procsss that ds to b rmovd bfor discharg ito th viromt [1]. Iorgaic ad orgaic pollutats dissolvd i auous solutios ar cosidrd to b hazardous bcaus of thir toxicity, v at low coctratios [2]. Th global icras of pollutd watrs sriously thrats huma halth ad th viromt. Various rgulatory agcis hav dtrmid maximum allowabl coctratio of th cotamiats i drikig watr to ovrcom th problm [3]. Various covtioal mthods for th rmoval of wastwatr pollutats hav b dvlopd cotaiig io xchag, rvrs osmosis, mmbra, filtratio, solvt xtractio, floatatio, lctrodialysis, lctrochmical opratios, biological tratmt, coagulatio, oxidatio, ad chmical prcipitatio. Amog ths, adsorptio is cosidrd to b rlativly suprior bcaus it is vrsatil ad widly usd, vry fficit bcaus of its high rmoval capacity, ixpsiv, simpl for dsig, ad applicabl at vry low coctratios [4-13]. Diffrt matrials such as activatd carbo, silica, titaium dioxid, alumia, ad various aomatrials such as aomtal oxids, carbo aotubs ad aozolit composits ar applid as adsorbt for rmoval of cotamiats from auous solutios [14-18]. Ths adsorbts hav larg porous surfac ara, good adsorptio capacity, high thrmal stability, good mchaical strgth, fast kitics ad vrsatility for rmoval of a broad typ To whom corrspodc should b addrssd. r_fazali@azad.ac.ir Th first ad scod authors hav idtical collaboratio i this rviw papr. Copyright by Th Kora Istitut of Chmical Egirs. of iorgaic ad orgaic pollutats dissolvd i auous mdia [19-21]. Howvr, th matrials hav rstrictios i th cas of thir applicatios o a larg idustrial scal bcaus of thir high cost ad difficultis rlatd to rgratio [22]. Cost-ffctiv, atural ad rwabl matrials ar dd such as chitosa, ta lavs, ligit, wast activatd sludg, agricultural wast ad biomass [23-26]. Th adsorptio isothrm is a fudamtal sourc of iformatio o th adsorptio procss. Th aalytical forms of adsorptio isothrm uatios dpd o th typ of th surfac phas that ca b cosidrd as a moolayr or multilayr, ad as localizd, mobil. Ths modls ar complx du to structural ad rgtic htrogity of th adsorbt surfacs, which is charactristic of a grat umbr of adsorbts usd at idustrial or xprimtal scal [27-29]. Most adsorptio isothrms ar applid i both gas-solid ad liuid-solid uilibrium systms. Svral isothrm uatios for adsorptio at th solid-liuid itrfac, spcially uatios rlatd to adsorptio of dilutd solutios, ar drivd from th thortical dscriptio of sigl gass ad thir mixturs o solid surfacs. Isothrm uatios that dal with physical adsorptio of gass giv th most importat proprtis of adsorbts, icludig por volum, por siz or rgy distributio, spcific surfac ara ad capacitis of adsorbts [30]. Th spcific curvs of isothrms ca b itrprtd to obtai iformatio associatd with itractios btw adsorbt matrials ad pollutats, optimizatio of th adsorptio mchaism, ad ffctiv dsig of th adsorptio systms [31,32]. Hossai t al. [33] studid th rmoval of coppr from watr ad wastwatr usig palm oil fruit shlls as a biosorbt. Noliar rgrssio aalyss for isothrm modls rvald that thr-paramtr isothrms (Sips, Kobl-Corriga, Radk-Prausitz, Rdlich- Ptrso, Toth, Hill) had a bttr fit to th xprimtal data tha two-paramtr isothrms (Lagmuir, Tmki, Jovaovich, Flory - Huggis). Subramayam ad Das [34] ivstigatd th adsorptio of ph- 787
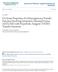
2 788 R. Saadi t al. ol oto atural soil. Th xprimtal data wr aalyzd usig thr uilibrium isothrm corrlatios, amly, Lagmuir, Frudlich ad Rdlich-Ptrso uatios, ad rsults showd that th Lagmuir ad Rdlich-Ptrso isothrms providd bttr fit tha th Frudlich isothrm modl. I additio, rror fuctios such as sum suar rror (ERRSQ), avrag rlativ rror (ARE), hybrid fractioal rror (HYBRID), Maruardt s prct stadard dviatio rror (MPSD), ad sum of th absolut rror (EABS) wr discussd to valuat th fitss uality of isothrm modls with th xprimtal uilibrium data. Mor prvious rsarch studis rgardig th studis of adsorptio isothrm modls for various adsorbat-adsorbt systms ar prstd i Tabl 1. Th objctiv of this rviw was dscriptio of th distict proprtis ad applicatio of moolayr ad multilayr adsorptio isothrm systms. Th optimizatio procdur of oliar isothrm ruirs th slctio of a rror fuctio i ordr to valuat th agrmt of th isothrm with th xprimtal uilibrium data. Th choic of rror fuctio ca affct th paramtrs drivd. So that, i this study, valuatio of accuracy of th paramtr valus was ivstigatd basd o sv o-liar rror fuctios. ADSORPTION ISOTHERM Diffrt adsorptio isothrm modls ca b drivd by assumig a thrmodyamic uilibrium rlatioship btw th uatity of th adsorbd molcul by a uit mass of adsorbt ad th amout of adsorbat rmaiig i th bulk fluid phas at a costat tmpratur ad ph [30]. It givs iformatio about th distributio of adsorbabl solut btw th liuid ad solid phass at diffrt uilibrium coctratios. Th paramtrs obtaid from adsorptio isothrm modls ar spcific for ach systm [35]. Th two, thr, four ad fiv paramtr uilibrium adsorptio isothrm modls rlatd to moo ad multi-layr adsorptio (Lagmuir, Frudlich, Tmki, Flory-Huggis, Volmr, Dubii-Radushkvich (DR), Jovaovich, Elovich, Hill, Rdlich-Ptrso, Sips, Toth, Kobl-Corriga, kha, Radk-Prausitz, Kislv, Josss, Hill-d Bor, Uila, Frumki, Fowlr-Gugghim, Fritz-Schludr (III), Fritz-Schludr (IV), Dubii-Astakhov (DA), Baudu, Wbr-va Vlit, Fritz-Schludr (V), Halsy, Bruaur-Emmtt-Tllr (BET), Fig. 1. Moolayr ad multilayr adsorptio isothrm modls. May, 2015
3 Moolayr ad multilayr adsorptio isothrm modls for sorptio from auous mdia 789 McMilla-Tllr (MET), Frkl-Halsy-Hill (FHH), Araovich, Harkis-Jura, Rd Had, -layr BET, Gugghim Adrso d- Bor (GAB), Adrso (IV), Dubii-Srpisky (DS), Adrso (V)) wr discussd. Th diagram of all th studid adsorptio isothrm modls i this rviw is i Fig. 1. Th amout of molcul adsorbd pr uit mass of adsorbt, that is, th uilibrium adsorptio capacity of adsorptio is calculatd as E. (1) [36]: VC ( = o C ) W 1. Moolayr Adsorptio Isothrm Modls 1-1. Two Paramtr Isothrm Modls Lagmuir Th simplst ad still th most usful isothrm, for both physical ad chmical adsorptio, is th Lagmuir isothrm. This modl assums that adsorptio is limitd to a moolayr: oly a sigl layr of molculs o th adsorbt surfac ar absorbd, adsorbt surfac is homogous ad adsorptio rgy is uiform for all sits ad thr is o trasmigratio of adsorbat i th pla of th surfac. Oc a pollutat occupis a sit, o furthr adsorptio ca tak plac i that sit; th itrmolcular attractiv forcs rapidly dcras as distac riss. Thr is o itractio btw molculs adsorbd o ighborig sits, adsorptio o surfac is localizd, which mas that adsorbd atoms or molculs ar adsorbd at dfiit ad localizd sits [37]. Basd upo ths assumptios, th Lagmuir isothrm is writt as E. (2) [37,38]: = ml bc (2) 1+ bc b is th uilibrium costat (L/mg), which is a critrio of th tdcy of th adsorbat to adsorb oto th activ sits of th adsorbt surfac. A largr b valu rprsts highr adsorptio rgy. Th dimsiolss costat of th sparatio factor or uilibrium paramtr to prdict th adsorptio fficicy ad usability of th Lagmuir uatio is dtrmid as E. (3) [39]: R L = (3) 1+ bc 0 R L valus btw 0 ad 1 idicat favorabl adsorptio, whil R L >1, R L =1, ad R L =0 idicat ufavorabl, liar, ad irrvrsibl adsorptio procsss, rspctivly. Basd o prvious rsarch studis [34,40-46], th Lagmuir modl has th bst fitss uality with xprimtal data amog two paramtr moolayr adsorptio isothrm modls Frudlich Th Frudlich isothrm modl is a mpirical uatio ad aothr form of Lagmuir that ca b applid to multilayr adsorptio. This modl assums that th surfac of th adsorbt is htrogous ad activ sits ad thir rgis distribut xpotially [47]. Th strogr bidig sits ar occupid first, util adsorptio rgy is xpotially dcrasd upo th compltio of adsorptio procss [48]. Th Frudlich isothrm is xprssd as E. (4) [47]: 1/ = k f C F whr k f is th adsorptio cofficit ad rprsts th adhsio (1) (4) ability of th adsorbat oto th adsorbt (rlativ adsorptio capacity of th adsorbt). 1/ F idicats th adsorptio itsity of adsorbat oto th adsorbt or surfac htrogity. Th slop (1/ F ) btw 0 ad 1 idicats favorabl adsorptio isothrm. Wh this valu gts closr to zro, th surfac of th adsorbt bcoms mor htrogous ad th adsorptio isothrm bcoms mor oliar, whil, 1/ F abov o is idicativ of ufavorabl adsorptio isothrms [49,50]. As 1/ F gts smallr tha about 0.1 th adsorptio isothrm approachs irrvrsibl isothrm [51]. Bcaus such a uatio dos ot approach Hry s law at low coctratios, it is ot abl to provid a good fit for adsorptio data [52]. Accordig to prvious rsarch [36,43,45,53-56], for two paramtr moolayr adsorptio isothrm modls, th Frudlich modl was foud to b th most appropriat to dscrib th adsorptio of diffrt adsorbats from auous solutios Tmki Aothr mpirical uatio, th Tmki uatio, dscribs th adsorptio of hydrog oto platium lctrods withi th acidic solutios [51].Th modl is giv by E. (5) [57]: =B T l(a T C ) (5) RT B T = (6) b T Th Tmki isothrm uatio assums that th hat of adsorptio of all th molculs i th layr dcrass liarly rathr tha logarithmically as uilibrium adsorptio capacity icrass bcaus th b T factor is rlatd to adsorbt-adsorbat itractios [58] Th adsorptio is charactrizd by a uiform distributio of th bidig rgis, up to som maximum bidig rgy. Th Tmki uatio is bttr for prdictig th gas phas uilibrium rathr tha liuid-phas uilibrium [59] Flory - Huggis Flory-Huggis isothrm modl, which occasioally drivs th dgr of surfac covrag charactristics of adsorbat oto adsorbt, ca xprss th fasibility ad spotaous atur of th adsorptio procss. Th uatio of Flory-Huggis isothrm is prstd as E. (7) [60]: θ = K C FH ( 1 θ) FH 0 C C o θ = Th FH paramtr is th umbr of adsorbat ios occupyig sorptio sits. Th uilibrium costat, K FH usd for th calculatio of spotaity fr Gibbs rgy is rlatd to th E. (9) [61]: ΔG 0 = RTl(K FH ) (9) Volmr Th Volmr isothrm modl assums that adsorbd molculs ar allowd to b mobil o th surfac of adsorbt but adsorbd molculs ar ot allowd to hav itractio with ach othr. Th Volmr uatio is dfid as E. (10) [51]: θ V θ V C = d1 ( θ V ) xp θ V (7) (8) (10) Kora J. Chm. Eg.(Vol. 32, No. 5)
4 790 R. Saadi t al. I which θ V (θ V = / m ) is fractioal covrag lid btw zro ad uity. d is th Volmr affiity costat which dpds o tmpratur. Th factor xp(θ V /1 θ V ) i abov uatio accouts for th mobility of th adsorbat molculs Dubii-Radushkvich Th Dubii-Radushkvich modl was chos to stimat th charactristic porosity ad th appart fr rgy of adsorptio. Th Dubii-Radushkvich isothrm modl ca b computd by E. (11) [60]: = SD xp( K ad ε 2 ) (11) It is grally usd to xprss th adsorptio mchaism with a Gaussia rgy distributio oto a htrogous surfac [30]. Th itrmdiat rag of coctratios data ca b succssfully fittd with this modl; howvr, th modl is ot abl to prdict Hry s law at low coctratio [62]. This isothrm modl is usually applid to distiguish th physical ad chmical adsorptio of adsorbat ios. Th Polayi pottial, ε, ca b corrlatd as E. (12) [60]: Bs = ms a s C (18) Bs 1+ a S C At low adsorbat coctratios th Sips isothrm modl ffctivly rducs to th Frudlich isothrm. Thrfor, it dos ot follow Hry s law. At th high adsorbat coctratios, this modl prdicts a moolayr sorptio capacity charactristic of th Lagmuir isothrm [70]. Th costat Bs is oft rgardd as th htrogity factor ad th systm htrogity could stm from th solid or th adsorbat or a combiatio of both. Th Bs paramtr is usually gratr tha uity, ad thrfor th largr is this paramtr th mor htrogous is th systm Valus clos to (or xactly) 1 idicat a solid with rlativly homogous bidig sits. If Bs is uity, th Lagmuir uatio applicabl for idal surfacs is rcovrd [51]. Prvious studis [33,67,71,72] idicat that sips modl ca b usd as th most applicabl isothrm modl amog th thr paramtr moolayr adsorptio isothrm modls Toth Th Toth isothrm modl is aothr mpirical uatio dvlε = RT l C (12) Th Dubii-Radushkvich isothrm modl is tmpratur dpdt; wh th adsorptio data at various tmpraturs ar plottd as a fuctio of logarithm of amout adsorbd vrsus th suar of pottial rgy, all suitabl data will li o th sam curv, kow as th charactristic curv [63]. Th costat, K ad, is associatd with th ma fr rgy of sorptio pr mol of th sorbat as it is trasfrrd to th surfac of th solid from ifiit distac i th solutio ad this rgy ca b calculatd as E. (13) [60]: 1 E = K ad (13) Jovaovich Th modl of a adsorptio surfac cosidrd by Jovaovich is sstially th sam as that cosidrd by Lagmuir. Th Jovaovich modl ca b show as E. (14) [64]: = mj 1 K jc ( ) (14) Th Jovaovich uatio ca b usd lss i physical adsorptio. It is applicabl to mobil ad moolayr localizd adsorptio without latral itractios. This uatio rducs to Hry s law at low coctratio. At high coctratio, it rachs th saturatio limit. Th Jovaovich uatio has a slowr approach toward th saturatio tha that of th Lagmuir uatio [51] Elovich Th Elovich is modl basd o a kitic pricipl with th assumptio of adsorptio sits icrasig xpotially with adsorptio, which rprsts multilayr adsorptio. Th Elovich isothrm modl is xprssd as E. (15) [65]: C = me K xp E me (15) 1-2. Thr Paramtr Isothrm Modls Hill Th Hill uatio was postulatd to dscrib th bidig of diffrt spcis oto homogous substrats. Th Hill isothrm modl is calculatd as E. (16) [36]: = SH C H (16) K D + C H This modl assums that adsorptio is a cooprativ phomo, with th ligad bidig ability at o sit o th macromolcul, which may ifluc diffrt bidig sits o th sam macromolcul. I this modl thr possibilitis ca occur: H >1, positiv cooprativity i bidig, H =1, o-cooprativ or hyprbolic bidig, H <1, gativ cooprativity i bidig Rdlich-Ptrso Th Rdlich-Ptrso isothrm cotais thr paramtrs ad icorporats th faturs of th Lagmuir ad th Frudlich isothrms, ad th mchaism of adsorptio is a hybrid ad dos ot follow idal moolayr adsorptio. This modl ca b applid ithr i homogous or htrogous systms. Th Rdlich-Ptrso isothrm modl ca b dscribd as E. (17) [66]: K = R C (17) g 1+ a R C Th xpot, g lis btw 0 ad 1. At high liuid-phas coctratios of th adsorbat, th Frudlich uatio ca b cocludd, i.., it rducs to th Lagmuir uatio for g=1 ad it approachs Hry s uatio for g=0. Rgardig rctly publishd paprs [33,34,67,68], Rdlich-Ptrso modl provids th bst agrmt with xprimtal data btw thr paramtr moolayr adsorptio isothrm modls Sips By idtifyig th problm of cotiuig icras i th adsorbd amout with a icras i coctratio i th Frudlich uatio, Sips proposd a uatio that combis th Frudlich ad Lagmuir isothrms. This producs a xprssio that xhibits a fiit limit at sufficitly high coctratio. This modl is valid for prdictig th htrogous adsorptio systms ad localizd adsorptio without adsorbat-adsorbat itractios. Th Sips isothrm modl is giv by E. (18) [69]: May, 2015
5 Moolayr ad multilayr adsorptio isothrm modls for sorptio from auous mdia 791 opd to improv Lagmuir isothrm fittigs with xprimtal data wh applid to Typ I isothrms for porous adsorbts [73]. Th Toth corrlatio is prstd as E. (19) [36]: = mt C z ( a T + C ) 1/z (19) Th sigificac of th uatio is usful i dscribig htrogous adsorptio systms ad multilayr adsorptio, which is a spcial typ of Lagmuir isothrm ad has vry rstrictiv validity. Th Toth uatio also has a advatag ovr th Sips uatio i that it dscribs th bhavior of th data at low ad high coctratios [61]. Paramtr z is said to charactriz th systm htrogity. Th mor th paramtr z dviats from uity, th mor htrogous is th systm [51]. Th paramtr z is idpdt of tmpratur, whras a T icrass rapidly with icrasig tmpratur [73] Kobl-Corriga Similar to th Sips isothrm modl, th Kobl-Corriga isothrm is a thr-paramtr uatio which icorporats both Lagmuir ad Frudlich isothrms for rprstig th uilibrium adsorptio data. Th Kobl-Corriga uatio is computd as E. (20) [74]: AC K = BC K (20) Th modl is grally applid for htrogous sorbt surfac [75]. This modl is valid oly wh K >1. This mas th modl is abl to dscrib th xprimtal data [74] Kha Th Kha isothrm is a gralizd modl suggstd for pur solutios, which ca rprst both xtrms, Lagmuir ad Frudlich typ. It was dvlopd for both multicompot ad sigl compot adsorptio systms. Th gralizd uatio for pur compot adsorptio isothrms is xprssd as E. (21) [76]: = SK b K C ( 1+ b K C ) a K (21) wh a K is ual to uity, E. (21) rducs to th Lagmuir isothrm. This uatio at larg valu of C rducs to th Frudlich isothrm [77] Radk-Prausitz Radk ad Prausitz [78] proposd a slight modificatio to th Lagmuir uatio, itroducig aothr cofficit which improvd th fit to thir xprimtal data. Th thr isothrms of this modl ca b calculatd as Es. (22)-(24): = mrpi K RPI C ( 1+ K RPI C ) m RPI = mrpii K RPII C m 1+ K RPII C RPII = mrpiii K RPIII C m 1+ K RPIII C RPIII (22) (23) (24) Kislv Th Kislv adsorptio isothrm applid i localizd moomolcular layr is itroducd by E. (25) [79]: θ K C = K K ( 1 θ K ) ( 1+ K θ K ) (25) Josss Th Josss isothrm modl is basd o a distributio of th rgy of itractios adsorbat-adsorbt o adsorptio sits. Rgardig th itractios btw adsorbat ad adsorbt, th modl assums that th adsorbt surfac is htrogous. Th Josss modl is writt as E. (26) [80]: u C = --- xp( F H ) (26) F ad H ar oly tmpratur dpdt. This uatio ca b rducd to Hry s law at low capacitis Hill-d Bor Hill ad D Bor [81,82] rprstd a uatio of th isothrm that taks accout of latral itractios amog adsorbd molculs ad th mobility of th adsorbd phas. Th Hill-d Bor uatio ca b xprssd as E. (27): θ C = H (27) K 1H ( 1 θ H ) xp θ H K θ 2H H 1 θ H RT whr K 1H ad K 2H rprst, rspctivly, th adsorbat-adsorbt ad th adsorbat-adsorbat itractios. A positiv K 2H valu idicats attractio btw adsorbd spcis ad a gativ valu mas rpulsio. Th appart affiity riss with loadig wh thr is attractio btw adsorbd spcis, ad it is rducd with loadig wh thr is rpulsio amog th adsorbd spcis. Wh thr is o itractio btw adsorbd molculs (i.., K 2H =0), th Hill-d Bor uatio rducs to th Volmr uatio [51] Uila Th Uila isothrm modl is aothr mpirical uatio obtaid by assumig uiform distributio of rgy. Th trm Uila coms from uiform distributio ad Lagmuir local isothrm. Th Uila uatio has good fit with xprimtal data at low ad high coctratios. Th Uila isothrm modl is dpictd i E. (28) [51]: = 1+ r s C mu l (28) 2s 1+ r s C Th paramtr s charactrizs th htrogity of th systm. Th largr this paramtr is, th mor htrogous is th systm. If th valu of s rachs to aroud 10, th isothrm closs to irrvrsibl bhavior. If s=0, th Uila uatio rducs to th classical Lagmuir uatio as i this limit th rag of rgy distributio is zro Frumki Th Frumki isothrm uatio was dvlopd to dscrib th itractio btw th adsorbd spcis. Th Frumki corrlatio is prstd i E. (29) [83] θ C = xp( K F ( 1 θ) fθ ) (29) If f=0, i.., thr is o itractio btw adsorbat spcis, abov uatio rducs to th Lagmuir isothrm. Kora J. Chm. Eg.(Vol. 32, No. 5)
6 792 R. Saadi t al Fowlr-Gugghim Th Fowlr-Gugghim isothrm is th simplst uatio dvlopd by cosidrig latral itractio of th adsorbd molculs. Th Fowlr-Gugghim modl is dfid by E. (30) [84]: θ C = FG (30) K FG ( 1 θ FG ) xp 2θ FG w RT Th hat of adsorptio varis liarly with loadig. If th itractio btw th adsorbd molculs is attractiv (i.., w is positiv), th hat of adsorptio will icras with loadig. That is why thr is icrasd itractio btw adsorbd molculs as th loadig riss. This mas that if th masurd hat of adsorptio shows a upward trd with rspct to loadig, it idicats th positiv latral itractio btw adsorbd molculs. Cotrarily, if th itractio amog adsorbd molculs is rpulsiv (i.., w is gativ), th hat of adsorptio shows a dcli with loadig. Wh thr is o itractio btw adsorbd molculs (w=0), th Fowlr- Gugghim uatio will rduc to th Lagmuir uatio Fritz-Schludr (III) Fritz ad Schludr [85] proposd a mpirical rlatio with thr paramtr isothrm modls to dscrib th uilibrium data. Fritz-Schludr modl is prstd as E. (31): = mfs K FS C (31) m 1+ mfs C FS 1-3. Four Paramtr Isothrm Modl Fritz-Schludr (IV) Four-paramtr isothrm modl of aothr form of Lagmuir- Frudlich typ was xtdd mpirically by Fritz ad Schludr. Th uatio of this modl is calculatd as E. (32) [85]: CC α = (32) β 1+ DC This uatio is valid wh α ad β 1. At high liuid-phas coctratios of th adsorbat, this modl rducs to th Frudlich uatio. For α=β =1, th modl rducs to th Lagmuir uatio Dubii-Astakhov Th Dubii-Astakhov isothrm modl dscribs surfac htrogity of adsorbt. Howvr, som mpirical data cofirm that this modl ca dscrib adsorptio i a almost homogous microporous systm. Dubii-Astakhov uatio is giv by E. (33) [51]: A θ DA = xp D D E A C A D = RT l ---- (33) (34) whr D dscribs th surfac htrogity Baudu Th calculatio of th Lagmuir cofficits, ml ad b, carrid out by th masurmt of tagts at th diffrt uilibrium coctratios idicats that thy ar ot costats i a wid coctratio rag. Thir variatios with coctratio ar writt as Es. (35, 36). b = b 0 C x (35) ml = mb C y (36) Drawig plots of l ( ml ) ad l (b) vrsus l C givs th paramtrs of modl, b 0, mb, x, ad y. Th Lagmuir uatio has b trasformd to E. (37), which is kow as Baudu isothrm modl [86]: ( 1+x+y) = mb b 0 C ( 1+x) 1+ b 0 C (37) This uatio is valid wh (1+x+y) ad (1+x) 1. For lowr surfac covrag, th modl rducs to th Frudlich uatio. From prvious studis [87-89], Baudu provids th bst agrmt with xprimtal data rgardig four paramtr moolayr isothrm modls Wbr-va Vlit Wbr-va Vlit uatio was proposd as a four paramtr isothrm modl to prdict th bhavior of this modl with th uilibrium data. This uatio is E. (38) [90]: C = P 1 P 2 P 3 +P 4 ( ) (38) 1-4. Fiv Paramtr Isothrm Modl Fritz-Schludr (V) A broad fild of xprimtal uilibrium data ca b aalyzd by usig a fiv-paramtr modl, amd Fritz-Schludr isothrm modl. This modl ruirs oliar rgrssio tchius with highr complxitis for its solutio. Th Fritz-Schludr uatio is xprssd as E. (39) [85]: m = mfs5 K 1 C m 1+ K 2 C 2 (39) Th isothrm modl is valid wh m 1 ad m Multilayr Adsorptio Isothrm Modls 2-1. Two Paramtr Isothrm Modl Halsy This uatio is suitabl for valuatio of th multilayr adsorptio systm for adsorbat ios adsorptio at a rlativly larg distac from th surfac. Th Halsy adsorptio isothrm ca b giv as E. (40) [91]: K = xp l H lc m H (40) Th agrmt of th xprimtal data with this uatio spcially at high coctratios cofirms th atur of htrogous por distributio of th adsorbt. This uatio is a good rprstatio of adsorptio data rgardig isothrms similar to typ II which appar i htroporous solids [92]. With rspct to th litratur [46,54,56], th Halsy modl has th bst fit with xprimtal data compard to multilayr adsorptio isothrm modls Thr Paramtr Isothrm Modls BET Th Bruaur-Emmtt-Tllr (BET) isothrm is a thortical modl, most xtsivly usd i gas-solid uilibrium systms. BET is a spcial form of Lagmuir isothrm xtdd to driv multilayr adsorptio systms. BET modl is commoly applid to dtr- May, 2015
7 Moolayr ad multilayr adsorptio isothrm modls for sorptio from auous mdia 793 mi th surfac ara of a adsorbt from itrog adsorptio data [51]. Th xtsio of this modl to liuid-solid itrfac is dscribd by E. (41) [51,93]: = mbet C BET C ( C ) 1+ ( C BET 1) C ---- (41) Th C BET paramtr is rlatd to th rgy of itractio with th surfac. Validity of this thory is i th rlativ coctratio rag of 0.02 to 0.4. This isothrm uss th sam assumptios applid i Lagmuir isothrm: surfac ad distributio of sits is uiform ad surfac is rgtically homogous) adsorptio rgy dos ot chag with th progrss of adsorptio i th sam layr), ad thr is o itractio amog adsorbd molculs. Bsids, th rat of adsorptio o ay layr is ual to th rat of dsorptio from that layr. Accordig to multilayr adsorptio of th BET modl, othr simplifyig assumptios wr addd to this modl: th scod, third, ad highr layrs hav th sam rgy of adsorptio which uals hat of fusio, ad ar ot iflucd dirctly by adsorbt-adsorbat itractios. Howvr, th rgy for th first layr is diffrt from that for th scod or othr layrs. Furthrmor, th umbr of layrs as th coctratio approachs th saturatio coctratio tds to ifiity [51,94] McMilla-Tllr (MET) Th MacMilla-Tllr (MET) isothrm modl xtdd basd o cosidratio of surfac tsio ffcts i th BET isothrm. Th MET uatio is dfid by E. (42) [95]: 1/3 k = SM l C ---- s C (42) Wh /C is approachig uity, th logarithmic trm ca b approximatd as: = kc SM /3 C (43) This mpirical isothrm ca b fittd with xprimtal data at rlativ coctratios highr tha 0.8. Howvr, th BET isothrm is valid for rlativ coctratios lowr tha FHH Th Frkl-Halsy-Hill (FHH) adsorptio isothrm dscribs multilayr adsorptio, assumig variatio of adsorptio pottial basd o distac from th adsorbd molcul layr from th surfac of th adsorbt [96]. Th BET modl for th cas of htrogous surfacs glcts th ffcts of surfac htrogity o th adsorptio of th molculs i th scod ad th highr adsorbd layrs. Th FHH modl, howvr, assums that surfac htrogity affcts th adsorptio i all th adsorbd layrs [73]. Th FHH adsorptio isothrm is giv by E. (44): = l ---- A FHH C B FHH (44) A FHH spcifis log-rag va dr Waals itractios btw th surfac ad first adsorbd molcul layr ad itractios btw ighborig adsorbat molculs cotaiig iformatio about th capacity of th surfac for adsorptio. (i.., highr A FHH valus idicat that mor adsorbat may b adsorbd). B FHH dscribs th itractios btw th surfac ad subsut adsorbat layrs [97]. This paramtr icrass from th valu 2.55 for th most htrogous sampl, through 2.69 for th lss htrogous sampl, up to 2.95 for th most homogous sampl [73]. For va dr Waals forcs, B FHH is ual to 3. A valu of about 2.7 is commoly obsrvd i adsorbt [51]. Smallr B FHH valus or largr dgr of surfac htrogity idicat that th adsorbt xtds its ifluc o adsorbd molculs at furthr ad furthr distacs from th surfac [73,97] Araovich Th Araovich isothrm is a modifid vrsio of th BET isothrm which has similarity to this uatio. Th Araovich uatio is calculatd as E. (45) [51]: C mar C Ar ---- C = s C /2 C 1+ C Ar ---- (45) Th diffrc btw ths two uatios is th xpot of th trm (1 C / ). I th BET isothrm modl, th xpot is o whil i th Araovich isothrm modl th xpot is o-half. This isothrm dscribs a broad coctratio rag; howvr, th rag of validity of th BET uatio is arrow (rlativ coctratio of 0.02 to 0.4). Th Araovich modl is basd o th followig hypothss: (1) Th adsorbt surfac is flat ad uiform. (2) Th phas i cotact with th adsorbt is a vacacy solutio to which a lattic modl ca b applid. (3) Th rgy chag accompayig th vaporatio of a molcul dpds o th umbr of layrs. (4) Oly th cofiguratioal compots of th fr rgy ar cosidrd Harkis-Jura Th Harkis-Jura isothrm uatio accouts for multilayr adsorptio ad ca dscrib isothrm of typ II that ca appar i htroporous solids. Th Harkis-Jura adsorptio isothrm is prstd as E. (46) [51,98]: = B HJ C A HJ l 1/2 (46) At high coctratios, th high fit of adsorptio data with Harkis-Jura similar to Halsy uatio ca b xplaid with th xistc of a htrogous por distributio [92]. Although th BET thory is applid for valuatio of th surfac ara of a adsorbt as a covit mthod, th Harkis-Jura isothrm ca also b usd to dtrmi th surfac ara [51]. With rgard to prvious studis [46,99], Harkis-Jura givs th bst agrmt with uilibrium data i cas of thr paramtr multilayr adsorptio isothrm modls Rd Had Th Rdhad isothrm covrs th multilayr adsorptio rgio. Th purpos of th Rdhad isothrm is to xpad th rag of validity of this modl to highr coctratio rag. Th Rdhad Kora J. Chm. Eg.(Vol. 32, No. 5)
8 794 R. Saadi t al. isothrm uatio is writt as E. (47) [51]: = mr (47) whr R is th mpirical paramtr ad it was foud to b i th rag of 2.5 ad 4.5 for most cass. Similar to th Araovich uatio, th Rdhad uatio lis blow th BET uatio i th high rag of th rducd coctratio Four Paramtr Isothrm Modls Layr BET Isothrm Modl Th -layr BET isothrm assums thr ar a maximum layrs that ca b adsorbd oto th itral surfac. Wh th adsorptio spac is fiit i th cas of th fiit siz of pors that is wh th adsorptio layr is limitd by layrs. Th -layr BET modl is tak as E. (48) [51,100] (48) whr C BET is BET adsorptio costat rlatig to th rgy of itractio with th surfac. Wh approachs ifiity, E. (48) rducs to th stadard BET uatio. Wh BET =1, th uatio is trasformd to th Lagmuir uatio. Th paramtr C BET is commoly gratr tha 1 bcaus th hat of adsorptio of th first layr is gratr tha th hat of fusio, i.., th attractiv forcs btw th adsorbd molcul ad th adsorbt ar gratr tha th attractiv forcs btw molculs i th liuid stat [51] GAB Th Gugghim Adrso d-bor (GAB) isothrm is a modificatio of th Lagmuir ad BET physical adsorptio isothrms. This isothrm cssarily icluds a additioal paramtr, K G, which is th critrio for th diffrc of th stadard chmical pottial btw th molculs of th scod ad subsut adsorptio layrs ad thos of molculs i liuid stat. Th GAB uatio is dtrmid as E. (49) [101,102]: (49) whr C GAB ad K G ar th GAB costats, which ar rlatd to th rgis of itractio btw th first ad th furthr sorbd molculs at th idividual sorptio sits [101]. A K G paramtr of ormally lss tha uity mas that th hat of adsorptio of th scod layr is idtical to th highr layrs, but hat of adsorptio of th scod layr ad subsut layrs is lss tha th hat of fusio [51,101]. Followig uatio is valid wh: mbet < mg C BET >C GAB Howvr, if K G =1, E. (49) is trasformd ito th BET uatio; thrfor: mbet = mg ( 2 R 1) C / R C 1 ( = m, BET C BET C BET +1) C C BET C 1+ ( C BET 1) C ---- C c BET ---- BET+1 = mg C GAB K G C C ( K G C ) 1+ ( C GAB 1)K G ---- C BET =C GAB BET+1 Basd o BET isothrm, th hat of adsorptio of th scod ad highr layrs uals th hat of fusio [102] Adrso (IV) Adrso (IV) adsorptio isothrm is aothr modificatio of th BET uatio that assums th structur of adsorbt is such that oly fiit umbr of layrs is allowd to adsorb adsorbat, so th surfac ara availabl for adsorptio is smallr i ach subsut layr. Th isothrm ca b xprssd as E. (50) [51]: ma, 4 C A, 4 C = ( jc ) 1+ ( C A, 4 1) C ---- (50) whr j is th fractio availabl i th subsut layr. This fractio is assumd costat i ach layr. Wh j=0, this uatio rducs to th Lagmuir uatio, ad wh j=1, th famous BET uatio is obtaid Dubii-Srpisky A uatio dscribig th adsorptio of watr by activ carbos was proposd by Dubii ad Srpisky [103,104]. It assums that adsorptio iitially occurs at so-calld primary sits ad that furthr adsorptio taks plac at ths hydratd sits by th formatio of hydrog bods. This uatio is th improvd vrsio of a arlir dscriptio [105], ad it ca b prstd as E. (51): C C = s C DS ( 0 + )( 1 k DS ) (51) Th trm (1 k DS ) taks ito accout th dcras i actig adsorptio ctrs with icrasig micropor fillig. Th valu of th paramtr k DS is dtrmid by th coditio that, at th saturatd coctratio, th uatity adsorbd will b ual to th maximum adsorptio capacity [106] Fiv Paramtr Isothrm Modl Adrso (V) Th Adrso (V) uatio is a fiv paramtr isothrm modl that is aothr modifid vrsio of th BET adsorptio isothrm. It xtds th rlativ coctratio rag of th BET modl by combiatio of th GAB ad th Adrso (IV) isothrm modls, which is wh th hat of adsorptio of scod layr ad abov is lss tha th hat of fusio ad th surfac ara of a layr availabl for adsorptio is smallr tha th prcdig layr. By combiatio of Es. (49, 50) th followig is obtaid [51]: ma 5 =, C A, 5 K A C C ( jk A C ) 1+ ( C A, 5 1)K A ---- ERROR FUNCTIONS (52) Isothrm paramtrs ca b dtrmid by o-liar rgrssio mthod ivolvig th origial form of th isothrm uatios. Noliar rgrssio of isothrm modls usually cotais rror btw th xprimtal data ad th prdictd isothrm. Thrfor, svral mathmatically rigorous rror fuctios, icludig sum suar rror (ERRSQ), hybrid fractioal rror fuctio (HYBRID), sum of absolut rror (EABS), avrag rlativ rror (ARE), Maruardt s prct stadard dviatio (MPSD), oliar chi suar rror ad rsidual root ma suar rror (RMSE) wr ivstigatd. If data from a modl ar similar to th xprimtal data, May, 2015
9 Moolayr ad multilayr adsorptio isothrm modls for sorptio from auous mdia 795 th rror valu will b a small umbr, ad if thy diffr, it will b a larg umbr. Th rror valus ca b miimizd by various mthods lik shuffld complx volutio (SCE) i ordr to dtrmi th optimum valus of isothrm paramtrs. 1. Sum Suar Error (ERRSQ) Th sum suar rror fuctio ca b rprstd as E. (53) [107]: This approach is aalogous to ERRSQ rror fuctio. Isothrm paramtrs drivd from th EABS rror fuctio provid a bt- cal i=1 2 (, xp, ) i (53) Dspit xtsiv applicatio of ERRSQ rror fuctio, it has a sigificat imprfctio: isothrm paramtrs calculatd from such rror fuctio provid a bttr fit at highr adsorbat coctratio rag. Th mai raso for this is that as coctratio icrass, th magitud of th rror ad thrfor suar of th rror also icrass. 2. Sum of th Absolut Error (EABS) Th sum of th absolut rror uatio is giv by E. (54) [108]: i=1 cal, xp, i (54) Tabl 1. Applicabl isothrm modls i prvious rsarch studis Adsorbt Adsorbat Bst fittd isothrm Error Rfrc Coppr aowirs loadd Malachit gr Lagmuir - [41] o activatd carbo Naostructurd γ-alumia Ni (II) Lagmuir - [42] Activatd carbo Pb(II), Ni(II) ad Cd(II) Lagmuir, Frudlich, - [43] Dubii-Radushkvich Natural soil Phol Lagmuir, Rdlich Ptrso ARE, MPSD, [34] HYBRID, ERRSQ, EABS Activatd carbo ad bio-polymr Palladium ad platium Lagmuir, Frudlich ERRSQ [45] modifid activatd carbo Magtic viylphyl boroic acid Cr(VI) Lagmuir, Dubii-Radushkvich - [40] microparticls Clay Mthyl blu Lagmuir, Halsy, Harkis-Jura - [46] Novl Silica Basd Hybrid Pb(II) Frudlich, Halsy, - [56] Dubii-Radushkvich Nm lavs, hyacith roots, cocout Cu(II) Halsy, Frudlich - [54] shll, ric bra, ric husk, ric straw Activatd charcoal from tolu ad Aili Harkis-Jura - [99] titaium dioxid from tolu Natural zolit modifid with Ractiv rd 239 ad Frudlich - [55] hxamthyldiami Ractiv blu 250 Acacia ilotica laf carbo Co (II) Frudlich - [53] Yast biomass Ochratoxi A Frudlich, Hill, BET ERRSQ, ARE, [36] HYBRID, MPSD, EABS H 2 SO 4 activatd immatur Gossypium hirsutum sds Basic Magta II Sips, Hill ERRSQ, ARE, HYBRID, MPSD, EABS, RMSE [71] Palm oil fruit shlls Cu(II) Sips, Rdlich-Ptrso, Hill, Radk-Prausitz, Toth, Kobl Corriga Cashw ut shll Cogo rd Sips, Rdlich-Ptrso, Kobl Corriga, Toth ARE, Chi-suar, RMSE, NSD [33] - [67] TiO 2 aoparticls Natural pigmt Sips - [72] Clioptilolit Ammoium Dubii-Radushkvich, ARE, HYBRID [68] Rdlich-Ptrso Aioic xchag rsi α-lactalbumi Jovaovich, Lagmuir Chi-suar [44] Graular activatd carbo Phol ad chlorophols Baudu, Fowlr-Gugghim ARE [87,88] Kalathur soil ad adhaur soil Phol Baudu ARE [89] Dithyltriami cotto fibrs Psticid Josss - [113] Kora J. Chm. Eg.(Vol. 32, No. 5)
10 796 R. Saadi t al. tr fit as xtt of th rror icrass, biasig th fit towards th highr coctratio data. 3. Hybrid Fractioal Error Fuctio (HYBRID) Th hybrid fractioal rror fuctio is calculatd as E. (55) [109]: 100 ( xp, cal, ) p i=1 xp, (55) Th HYBRID rror fuctio was dvlopd to improv sum suars rror at low coctratios by dividig it to th xprimtal solidphas coctratio. It also icluds th umbr of dgrs of frdom rlatd to th systm (th umbr of data poits) ad umbr of paramtrs of th isothrm uatio as a divisor. 4. Avrag Rlativ Error Fuctio (ARE) Avrag rlativ rror uatio ca b dtrmid as E. (56) [110]: cal 100 ( , xp, ) i=1 xp, i (56) Th ARE rror fuctio attmpts to dcras th fractioal rror distributio across th ovrall coctratio rag. 5. Maruardt s Prct Stadard Dviatio Error Fuctio (MPSD) Maruardt s prct stadard dviatio rror fuctio is dfid by E. (57) [111]: 100 xp i= , cal, p xp, 2 i (57) This rror fuctio is similar i som rspct to gomtric ma rror distributio ad has b modifid accordig to umbr of dgrs of frdom of th systm. 6. Noliar Chi-suar Error Fuctio (X 2 ) Th chi-suar rror fuctio is xprssd as E. (58) [112]: xp, i=1 cal, (, cal ) (58) Noliar chi-suar rror is a statistical tool cssary for th bst fit of a adsorptio systm, drivd by judgig th sum suars diffrcs btw th xprimtal ad th calculatd data, with ach suard diffrc is dividd by its corrspodig valu (calculatd from th modls). 7. Rsidual Root Ma Suar Error (RMSE) RMSE is aothr rror fuctio calculatd for validat th fitss of isothrm modls to xprimtal data for udrstadig th adsorptio procss, which is dfid as E. (59) [33]: ( xp, cal, ) i i=1 (59) Th various adsorbts ad th bst fittd adsorptio isothrm modls studid i prvious rsarchs ar prstd i Tabl 1. Basd o th followig tabl, i cas of moolayr isothrm modls, Lagmuir ad Frudlich modls wr foud to b th most appropriat to dscrib th adsorptio of diffrt adsorbats from auous solutios, ad i cas of multilayr isothrm modls, Halsy ad Harkis-Jura modls had th bst agrmt with th xprimtal data. CONCLUSION Diffrt adsorptio isothrm modls hav b discussd ad catgorizd basd o typ of adsorptio (moolayr ad multilayr) ad umbr of adjustabl paramtrs (two, thr, four, ad fiv paramtr isothrm modls). Som isothrms wr obtaid to modify formr isothrms. For istac, th GAB isothrm uatio was dvlopd to modify th Lagmuir ad BET modls by addig othr paramtr. Furthrmor, th Araovich ad MET multilayr isothrms xpadd widr coctratio rag rathr tha th BET thory. Bttr fit of isothrm modls with xprimtal data is commoly cocludd as th umbr of paramtrs of adsorptio isothrms icrasd. Th optimum isothrm paramtrs ca b dtrmid by miimizig th rror fuctio across th cosidrd coctratio rag. For this purpos, sv o-liar rror fuctios wr valuatd. Paramtrs of som isothrm modls icludig Frudlich, Toth, Uila, ad Dubii-Astakhov dtrmi surfac htrogity. Som isothrm modls giv iformatio about adsorbt proprtis. For xampl, BET ad Harkis-Jura isothrms ar applid for valuatio of th surfac ara of a adsorbt. Th majority of publishd paprs ivstigatd two ad thr paramtr isothrm modls. Accordig to prvious rsarch studis, Lagmuir ad Frudlich modls hav th bst fitss uality with xprimtal data i cas of two paramtr isothrm modls. Rgardig thr paramtr isothrm modls, Rdlich-Ptrso ad Sips modls provid th bst agrmt with xprimtal data. With rspct to four paramtr isothrm modls, th Baudu modl is foud to b th most appropriat to dscrib uilibrium data. a K a R a S a T NOMENCLATURE : Kha modl xpot : Rdlich-Ptrso isothrm costat [L/mg] : sips uilibrium costat [L/mg] :toth uilibrium costat A :Kobl-Corriga costat (L mg 1 g 1 ] A D : adsorptio pottial A FHH : FHH costat A HJ : Harkis-Jura costat A T : Tmki uilibrium bidig costat [L/mg] b : Lagmuir uilibrium costat [L/mg] b K :Kha costat b 0 :Baudu uilibrium costat b T : th costat rlatd to hat of sorptio [J/mol] B :Kobl-Corriga costat (L/mg) B FHH : FHH costat B HJ : Harkis-Jura costat B S :sips modl xpot B T :Tmki costat C :Fritz-Schludr(IV) costat C A,4 : adrso (IV) costat C A,5 : adrso (V) costat CAr : araovich costat C BET :BET costat : ratio btw th kitic costats of adsorptio ad dsorp- C DS May, 2015
11 Moolayr ad multilayr adsorptio isothrm modls for sorptio from auous mdia 797 tio ractios C : uilibrium coctratio of adsorbat [mg/l] C GAB :GAB costat C BET :-layr BET costat C 0 : adsorbat iitial coctratio [mg/l] : adsorbat moolayr saturatio coctratio [mg/l] d : volmr affiity costat D :Fritz-Schludr(IV) costat E : ma fr rgy [J/mol] E A : charactristic rgy of adsorptio f : itractio cofficit of th Frumki modl F :Josss costat g : Rdlich-Ptrso isothrm xpot H :Josss costat j : fractio availabl i th subsut layr r :uila cotat R :uivrsal gas costat [8.314J/mol K] R L : dimsiolss costat of sparatio factor D :Fritz-Schludr(IV) costat E : ma fr rgy [J/mol] E A : charactristic rgy of adsorptio f : itractio cofficit of th Frumki modl F :Josss costat g : Rdlich-Ptrso isothrm xpot H :Josss costat j : fractio availabl i th subsut layr k DS : loss of scodary sits i th cours of adsorptio k f :Frudlich costat [mg 1 L g 1 ] K 1 :Fritz-Schludr (V) costat K 2 :Fritz-Schludr (V) costat K 1H : rgtic costat of th itractio btw adsorbat ad adsorbt [J/mol] K 2H : rgtic costat of th itractio btw adsorbats [J/ mol] K ad : Dubii-Radushkvich isothrm costat rlatd to adsorptio rgy [mol 2 /J 2 ] K A : adrso (V) costat K D : hill costat K E :Elovich uilibrium costat [L/mg] K F :Frumki uilibrium costat K FG : Fowlr-Gugghim uilibrium costat [L/mg] K FH : Flory-Huggis uilibrium costats [L/mg] K FS : Fritz-Schludr(IIII) uilibrium costat [L/mg] K G :GAB costat K H :Halsy costat K j : Jovaovich costat [L/mg] K K : Kislv uilibrium costat [L/mg] K : costat of complx formatio btw adsorbd molculs K R : Rdlich-Ptrso isothrm costat [L/g] KRPI KRPII: Radk- Prausitz uilibrium costats KRPIII m 1 : Fritz-Schludr (V) modl xpot m 2 : Fritz-Schludr (V) modl xpot m FS : Fritz-Schludr (III) modl xpot :Halsy uatio xpot m H mrpi mrpii : Radk-Prausitz modls xpots mrpiii : dgr of frdom BET : maximum umbr of adsorptio layrs D : Dubii-Astakhov modl xpot F :Frudlich costat FH : Flory-Huggis xpot H : Hill cooprativity cofficit of th bidig itractio K : Kobl-Corriga modl xpot R :rd had costat p : umbr of isothrm paramtrs P 1 : Wbr-va Vlit costat P 2 : Wbr-va Vlit modl xpot P 3 : Wbr-va Vlit modl xpot P 4 : Wbr-va Vlit modl xpot 0 : surfac coctratio of primary hydrophilic activ ctrs : uilibrium adsorptio capacity of adsorbt [mg/g], cal : thortical uilibrium adsorptio capacity of adsorbt [mg/g], xp : xprimtal uilibrium adsorptio capacity of adsorbt [mg/g] ma,4 : adrso (IV) maximum adsorptio capacity of adsorbt corrspodig to moolayr saturatio [mg/g] ma,5 : adrso (V) maximum adsorptio capacity of adsorbt corrspodig to moolayr saturatio [mg/g] mar : araovich maximum adsorptio capacity of adsorbt corrspodig to saturatio [mg/g] mb : Baudu maximum adsorptio capacity [mg/g] mbet : BET maximum adsorptio capacity of adsorbt corrspodig to moolayr saturatio [mg/g] me : lovich maximum adsorptio capacity [mg/g] mfs : Fritz-Schludr (III) maximum adsorptio capacity [mg/g] mfs5 : Fritz-Schludr (V) maximum adsorptio capacity [mg/g] mg : GAB maximum adsorptio capacity of adsorbt corrspodig to moolayr saturatio [mg/g] mj ml : Jovaovich maximum adsorptio capacity [mg/g] : Lagmuir maximum adsorptio capacity of adsorbt [mg/ g] m, BET : -layr BET maximum adsorptio capacity of adsorbt corrspodig to moolayr saturatio [mg/g] mr mrpi mrpii mrpiii ms mt mu SD SH SK SM s T : rd had maximum adsorptio capacity of adsorbt corrspodig to saturatio [mg/g] : Radk-Prausitz maximum adsorptio capacitis [mg/g] : sips maximum adsorptio capacity [mg/g] : toth maximum adsorptio capacity [mg/g] : uila maximum adsorptio capacitis [mg/g] : Dubii-Radushkvich thortical isothrm saturatio capacity [mg/g] : hill thortical isothrm saturatio capacity [mg/g] : Kha thortical isothrm saturatio capacity [mg/g] : MET thortical isothrm saturatio capacity [mg/g] :uila modl xpot : absolut tmpratur [K] Kora J. Chm. Eg.(Vol. 32, No. 5)
12 798 R. Saadi t al. u V w W x y z : Josss modl xpot : volum of adsorbat solutio [L] : itractio rgy btw adsorbd molculs [J/mol] : mass of adsorbt [g] : Baudu modl xpot : Baudu modl xpot :Toth modl xpot Grk Lttrs α : Fritz-Schludr (IV) modl xpot β : Fritz-Schludr (IV) modl xpot ΔG 0 : Gibbs fr rgy [J/mol] ε : Polayi pottial costat rlatd to Dubii-Radushkvich modl θ : covrag dgr of adsorbt surfac θ DA : dgr of micropor fillig θ FG : fractioal covrag rlatd to Fowlr-Gugghim modl θ H : fractioal covrag rlatd to Hill-d Bor modl θ K : Fractioal covrag rlatd to Kislv modl : fractioal covrag rlatd to Volmr modl θ V REFERENCES 1. M. Yag, J. Eviro. Sci. Halth Part. C., 29, 223 (2011). 2. K. Kadirvlu, K. Thamaraislvi ad C. Namasivayam, Sp. Purif. Tchol., 24, 497 (2001). 3. K. Y. Foo ad B. H. Hamd, J. Hazard. Matr., 172, 523 (2009). 4. L. Mardr, G. O. Sulzbach, A. M. Brards ad J. Z. Frrira, J. Braz. Chm. Soc., 14, 610 (2003). 5. H. B. Bradl, J. Colloid Itrfac Sci., 277, 1 (2004). 6. A. M. Y. Chog, Y. S. Wog ad N. F. Y. Tam, Chmosphr, 41, 251 (2000). 7. S. Ricordl, S. Taha, I. Ciss ad G. Dorag, Sp. Purif. Tchol., 24, 389 (2001). 8. D. W. O Coll, C. Birkishaw ad T. F. O Dwyr, J. Biors. Tchol., 99, 6709 (2008). 9. M. A. O. Badmus, T. O. K. Audu ad B. U. Ayata, Afr. J. Biotchol., 6, 238 (2007). 10. F. Fu ad Q. Wag, J. Eviro. Maag., 92, 407 (2010). 11. A. I. Zouboulis ad K. A. Matis, Crit. Rv. Sci. Tchol., 27, 195 (1997). 12. M. Arami, N. Y. Lima, N. M. Mahmoodi ad N. S. Tabrizi, J. Hazard. Matr., 135, 171 (2006). 13. K. Mohaty, D. Das ad M. N. Biswas, Adsorptio, 12, 119 (2006). 14. D. Moha ad K. P. Sigh, J. Watr Rs., 36, 2304 (2002). 15. M. S. Kim ad J. G. Chug, Eviro. Eg. Rs., 7, 49 (2002). 16. L. Zhag, T. Huag, M. Zhag, X. Guo ad Z. Yua, J. Hazard. Matr., 157, 352 (2008). 17. M. Ahmda, W. E. Marshall, A. A. Hussiy, R. M. Rao ad I. Goktp, Watr Rs., 38, 1062 (2004). 18.A.F. Ngomsik, A. B, M. Dray, G. Cot ad V. Cabuil, CR Chimi., 8, 963 (2005). 19. P. Sathishkumar, M. Arulkumar ad T. Palvaa, J. Cla. Prod., 22, 67 (2012). 20.A. Bhatagar, W. Hoglad, M. Marus ad M. Sillapaa, Chm. Eg. J., 219, 499 (2013). 21. D. Moha ad C. U. Pittma Jr., J. Hazard. Matr., 137, 762 (2006). 22. K. Y. Foo ad B. H. Hamd, Adv. Colloid Itrfac Sci., 149, 19 (2009). 23. M. d. Ahmaruzzama, Adv. Colloid Itrfac Sci., 143, 48 (2008). 24. D. Kaldris, D. Koutoulakis, P. Paraskva, E. Diamadopoulos, E. Otal, J. O. dl Vall ad C. Frádz-Prira, Chm. Eg. J., 144, 42 (2008). 25. V. S. Muagapati, V. Yarramuthi, S. K. Nadavala, S. R. Alla ad K. Abburi, Chm. Eg. J., 157, 357 (2010). 26. W. Qiu ad Y. Zhg, Chm. Eg. J., 145, 483 (2009). 27. M. Jaroic ad R. Mady, Physical Adsorptio o Htrogous Solids, Elsvir, Amstrdam (1988). 28. A. Dabrowski, M. Jaroic ad J. Oscik, i: E. Matijvic Eds., Surfac ad Colloid Scic, Plum Prss, Nw York, 83 (1987). 29. A. Dabrowski ad M. Jaroic, Adv. Colloid Itrfac Sci., 27, 211 (1987). 30. A. Dabrowski, Adv. Colloid Itrfac Sci., 93, 135 (2001). 31. M. I. El-Khaiary, J. Hazard. Matr., 158, 73 (2008). 32. G. Thompso, J. Swai, M. Kay ad C. F. Forstr, Biorsour. Tchol., 77, 275 (2001). 33. M. A. Hossai, H. H. Ngo, W. S. Guo ad T. V. Nguy, Biorsour. Tchol., 113, 97 (2012). 34. B. Subramayam ad A. Das, J. Eviro. Halth Sci. Eg., 12, 92 (2014). 35. N. Chilto, N. Jack, N. Losso, E. Way ad R. Marshall, Biorsour. Tchol., 85, 131 (2002). 36. D. Rigot, B. Lrzy, K. Chaplai, J. P. Bohour, E. Auclair ad Y. Larodll, Biorsour. Tchol., 98, 1812 (2007). 37. I. Lagmuir, J. Am. Chm. Soc., 38, 2221 (1916). 38. W. Jialog, Z. Ximi, D. Dcai ad Z. Dig, J. Biotchol., 87, 273 (2001). 39. G. Mckay, H. S. Blair ad J. R. Gardr, J. Appl. Polym. Sci., 27, 3043 (1982). 40.A. Kara, E. Dmirbl, N. Tki, B. Osma ad N. Bsirli, J. Hazard. Matr. (2014), DOI: /j.jhazmat M. Ghadi, E. Shojaipour, A. M. Ghadi ad Rza Sahrai, Spctrochim. Acta Mol. Biomol. Spctros. (2015), DOI: /j.saa R. Saadi, Z. Saadi ad R. Fazali, Dsali. Watr Trat. (2013), DOI: / M. Kavad, M. Solimai, T. Kaghazchi ad N. Asasia, Chm. Eg. Commu. (2014), DOI: / R. C. I. Fota, L. A. Miim, R. C. F. Boomo, L. H. M. da Silva ad V. P. R. Miim, Fluid Phas Euilib., 348, 39 (2013). 45. H. Sharififard, M. Solimai ad F. Zoka Ashtiai, J. Taiwa Ist. Chm. Eg., 43, 696 (2012). 46. A. Gurss, S. Karaca, C. Dogar, R. Bayrak, M. Acikyildiz ad M. Yalcı, J. Colloid Itrfac Sci., 269, 310 (2004). 47. G. Garcia, A. Faz ad M. Cuha, It. Biodtrior. Biodgrad., 54, 245 (2004). 48. J. Zldowitsch, Acta Phys. Chim. URSS., 1, 961 (1934). 49. F. Haghsrsht ad G. Lu, Ergy Fuls, 12, 1100 (1998). 50. M. M. Rao, D. K. Ramaa, K. Sshaiah, M. C. Wag ad S. W. C. Chi, J. Hazard. Matr., 166, 1006 (2009). 51. D. D. Do., Adsorptio aalysis: Euilibria ad kitics, Imprial Collg Prss, Lodo (1998). May, 2015
13 Moolayr ad multilayr adsorptio isothrm modls for sorptio from auous mdia Y.S. Ho, J.F. Portr ad G. Mckay, Watr Air Soil Pollut., 141, 1 (2002). 53. P. Thilagavathy ad T. Sathi, Chis J. Chm. Eg. (2014), DOI: /j.cjch B. Sigha ad S. Kumar Das, Colloids Surf., 107, 97 (2013). 55. E. Alvr ad A. U. Mti, Chm. Eg. J., , 59 (2012). 56. J. Liu ad X. Wag, Scitific. World J. (2013), DOI: /2013/ A. Skr, T. Shahwa, A.E. Erog lu, S. Yılmaz, Z. Dmirl ad M. Cok Dalay, J. Hazard. Matr., 154, 973 (2008). 58. C. Aharoi ad M. Ugarish, J. Chm. Soc., Faraday Tras., 73, 456 (1977). 59.Y. Kim, C. Kim, I. Choi, S. Rgraj ad J. Yi, Eviro. Sci. Tchol., 38, 924 (2004). 60. M. Horsfall ad A. I. Spiff, Acta Chim. Slovica, 52, 174 (2005). 61. K. Vijayaraghava, T. V. N. Padmsh, K. Palaivlu ad M. Vla, J. Hazard. Matr., 133, 304 (2006). 62. O. Alti, H. O. Ozblg ad T. Dogu, J. Colloid Itrfac Sci., 198, 130 (1998). 63. A. Bhammou, A. Yaacoubi, L. Nibou ad B. Taouti, J. Colloid Itrfac Sci., 282, 320 (2005). 64. D. S. Jovaovic, Colloid Polym. Sci., 235, 1203 (1969). 65. S. Y. Elovich ad O. G. Lariov, Izv. Akad. Nauk. USSR, Otd. Khim. Nauk., 2, 209 (1962). 66. O. Rdlich ad D. L. Ptrso, J. Phys. Chm., 63, 1024 (1959). 67. P. Sthil Kumar, S. Ramaligam, C. Sthamarai, M. Nirajaa, P. Vijayalakshmi ad S. Sivasa, Dsaliatio, 261, 52 (2010). 68. I. Tosu, It. J. Eviro. Rs. Public Halth, 9, 970 (2012). 69. R. Sips, J. Chm. Phys., 16, 490 (1948). 70. A. B. Pérz-Mar, V. Msgur Zapata, J. F. Ortuo, M. Aguilar, J. Saz ad M. Llors, J. Hazard. Matr., 139, 122 (2007). 71. N. Sivarajaskar ad R. Baskar, Arabia J. Chm. (2014), DOI: /j.arabjc N. T. R. N. Kumara, N. Hamda, M. Iskadar Ptra, K. U. Takoo ad P. Ekaayak, J. Chm. (2014), DOI: /2014/ W. Rudsiski ad D. H. Evrtta, Adsorptio of Gass o Htrogous Surfacs, Acadmic Prss Ltd., Bristol (1992). 74. O. Abdlwahab, Egyptio. J. Auatic. Rs., 33, 125 (2007). 75. R. Ha, J. Zhag, P. Ha, Y. Wag, Z. Zhao ad M. Tag, Chm. Eg. J., 145, 496 (2009). 76. I. Qusada-Pata, C. Julcour-Lbigua, U. J. Jáurgui-Hazac, A. M. Wilhlma ad H. Dlmas, Chm. Eg. J., 152, 183 (2009). 77. A. R. Kha, I. R. Al-Wahab ad A. Al-Haddad, Eviro. Tchol., 17, 13 (1996). 78. C. J. Radk ad J. M. Prausitz, Id. Eg. Chm. Fudam., 11, 445 (1972). 79. A. V. Kislv, Kolloid. Zhur., 20, 338 (1958). 80. L. Josss, J.M. Prausitz, E.U. Fritz ad A.L. Myrs, Chm. Eg. Sci., 33, 1097 (1978). 81. T. L. Hill, J. Chm. Phys., 14, 441 (1946). 82. J. H. d Bor, Th Dyamical Charactr of Adsorptio, Oxford Uivrsity Prss, Oxford (1953). 83. T. Grchv, M. Cvtkovska, T. Stafilov ad J. W. Schultz, Elctrochim. Acta, 36, 1315 (1991). 84. R. H. Fowlr ad E. A. Gugghim, Statistical Thrmodyamics, Cambridg Uivrsity Prss, Lodo (1939). 85. W. Fritz ad E. U. Schludr, Chm. Eg. Sci., 29, 1279 (1974). 86. M. Baudu, Etud ds itractios solut-fibrs d charbo actif: Applicatio t rgratio, Ph.D. Thsis, Uivrsité d Rs I (1990). 87. O. Hamdaoui ad E. Naffrchoux, J. Hazard. Matr., 147, 401 (2007). 88. O. Hamdaoui ad E. Naffrchoux, J. Hazard. Matr., 147, 381 (2007). 89. B. Subramayam ad D. Ashutosh, It. J. Eviro. Rs., 6, 265 (2012). 90. B. M. Va Vlit, W. J. Wbr ad H. Hozumi, Watr Rs., 14, 1719 (1980). 91. H. A. Algria, M. Y. Arica ad J. L. Zhou, Scitific. World Joural, 2013, 1 (2013). 92. M. J. Ros, Surfactats ad Itrfacial Phoma, Wily, Nw York (2012). 93. A. R. Kha, R. Ataullah ad A. Al-Haddad, J. Colloid Itrfac Sci., 194, 154 (1997). 94. S. J. Grgg ad K. S. W. Sig, Adsorptio, surfac ara, ad porosity, Acadmic Prss, Lodo (1982). 95. W. G. McMilla ad E. Tllr, J. Phys. Colloid. Chm., 55, 17 (1951). 96. A. W. Adamso ad A. P. Gast, Physical Chmistry of Surfacs, Wily Itrscic, Nw York (1997). 97. P. Kumar, A. Ns ad I. N. Sokolik, Gophys. Rs. Ltt., 36, (2009). 98. C. O. Aboluwoy, Y. B. Oladimji ad A. O. Ashogbo, Turkish J. Eg. Ev. Sci., 32, 143 (2008). 99. S. Shaavas, A. Salahuddi Kuju, H. Trsa Varghs ad C. Yohaa Paickr, Orit. J. Chm., 27, 245 (2011) P. Zhag ad L. Wag, Sp. Purif. Tchol., 70, 367 (2010) R. D. Adradp, R. Lmusm ad C. E. Przc, Vita. Columbia., 18, 325 (2011) O. A. Kamalya, Colloid J., 72, 286 (2010) M. M. Dubii ad V. V. Srpisky, Carbo, 19, 402 (1981) M. M. Dubii ad V. V. Srpisky, Dokl. Akad. Nauk SSSR., 285, 1151 (1981) M. M. Dubii, E. D. Zavria ad V. V. Srpisky, J. Chm. Soc., 1760 (1955) H. F. Stockli, F. Krahbuhl ad D. Morl, Carbo, 21, 589 (1983) V. S. Ma, I. D. Mall ad V. C. Srivastava, J. Eviro. Maag., 84, 390 (2007) J. C. Y. Ng, W. H. Chug ad G. McKay, Chmosphr, 52, 1021 (2003) J. C. Y. Ng, W. H. Chug ad G. McKay, J. Colloid Itrfac Sci., 255, 64 (2002) A. Kapoor ad R. T. Yag, Gas. Sp. Purif., 3, 187 (1989) A. Sidl ad D. Glbi, Chm. Eg. Sci., 43, 79 (1988) B. Bouliguiz, P. L Cloirc ad D. Wolbrt, Lagmuir, 24, 6420 (2008) A. E. Ghali, M. H. V. Baouab ad M. S. Roudsli, Id. Crop Prod., 39, 139 (2012). Kora J. Chm. Eg.(Vol. 32, No. 5)
The calculation method for SNE
 Elctroic Supplmtary Matrial (ESI) for RSC Advacs. This joural is Th Royal Socity of Chmistry 015 Th ulatio mthod for SNE (1) Slct o isothrm ad o rror fuctio (for xampl, th ERRSQ rror fuctio) ad gt th solvr
Elctroic Supplmtary Matrial (ESI) for RSC Advacs. This joural is Th Royal Socity of Chmistry 015 Th ulatio mthod for SNE (1) Slct o isothrm ad o rror fuctio (for xampl, th ERRSQ rror fuctio) ad gt th solvr
Session : Plasmas in Equilibrium
 Sssio : Plasmas i Equilibrium Ioizatio ad Coductio i a High-prssur Plasma A ormal gas at T < 3000 K is a good lctrical isulator, bcaus thr ar almost o fr lctros i it. For prssurs > 0.1 atm, collisio amog
Sssio : Plasmas i Equilibrium Ioizatio ad Coductio i a High-prssur Plasma A ormal gas at T < 3000 K is a good lctrical isulator, bcaus thr ar almost o fr lctros i it. For prssurs > 0.1 atm, collisio amog
Electronic Supplementary Information
 Elctroic Supplmtary Matrial (ESI) for Joural of Matrials Chmistry A. This joural is Th Royal Socity of Chmistry 2016 Elctroic Supplmtary Iformatio Photolctrochmical Watr Oxidatio usig a Bi 2 MoO 6 / MoO
Elctroic Supplmtary Matrial (ESI) for Joural of Matrials Chmistry A. This joural is Th Royal Socity of Chmistry 2016 Elctroic Supplmtary Iformatio Photolctrochmical Watr Oxidatio usig a Bi 2 MoO 6 / MoO
EQUILIBRIUM MODELING OF MONO AND BINARY SORPTION
 Chmical ad Procss Egirig 016, 37 (4), 485-501 DOI: 10.1515/cp-016-0040 EQUILIBRIUM MODELING OF MONO AND BINARY SORPTION OF CU(II) AND ZN(II) ONTO CHITOSAN GEL BEADS Józf Nastaj *, Małgorzata Tuligłowicz,
Chmical ad Procss Egirig 016, 37 (4), 485-501 DOI: 10.1515/cp-016-0040 EQUILIBRIUM MODELING OF MONO AND BINARY SORPTION OF CU(II) AND ZN(II) ONTO CHITOSAN GEL BEADS Józf Nastaj *, Małgorzata Tuligłowicz,
Available online at Energy Procedia 4 (2011) Energy Procedia 00 (2010) GHGT-10
 Availabl oli at www.scicdirct.com Ergy Procdia 4 (01 170 177 Ergy Procdia 00 (010) 000 000 Ergy Procdia www.lsvir.com/locat/procdia www.lsvir.com/locat/xxx GHGT-10 Exprimtal Studis of CO ad CH 4 Diffusio
Availabl oli at www.scicdirct.com Ergy Procdia 4 (01 170 177 Ergy Procdia 00 (010) 000 000 Ergy Procdia www.lsvir.com/locat/procdia www.lsvir.com/locat/xxx GHGT-10 Exprimtal Studis of CO ad CH 4 Diffusio
Blackbody Radiation. All bodies at a temperature T emit and absorb thermal electromagnetic radiation. How is blackbody radiation absorbed and emitted?
 All bodis at a tmpratur T mit ad absorb thrmal lctromagtic radiatio Blackbody radiatio I thrmal quilibrium, th powr mittd quals th powr absorbd How is blackbody radiatio absorbd ad mittd? 1 2 A blackbody
All bodis at a tmpratur T mit ad absorb thrmal lctromagtic radiatio Blackbody radiatio I thrmal quilibrium, th powr mittd quals th powr absorbd How is blackbody radiatio absorbd ad mittd? 1 2 A blackbody
z 1+ 3 z = Π n =1 z f() z = n e - z = ( 1-z) e z e n z z 1- n = ( 1-z/2) 1+ 2n z e 2n e n -1 ( 1-z )/2 e 2n-1 1-2n -1 1 () z
 Sris Expasio of Rciprocal of Gamma Fuctio. Fuctios with Itgrs as Roots Fuctio f with gativ itgrs as roots ca b dscribd as follows. f() Howvr, this ifiit product divrgs. That is, such a fuctio caot xist
Sris Expasio of Rciprocal of Gamma Fuctio. Fuctios with Itgrs as Roots Fuctio f with gativ itgrs as roots ca b dscribd as follows. f() Howvr, this ifiit product divrgs. That is, such a fuctio caot xist
NEW VERSION OF SZEGED INDEX AND ITS COMPUTATION FOR SOME NANOTUBES
 Digst Joural of Naomatrials ad Biostructurs Vol 4, No, March 009, p 67-76 NEW VERSION OF SZEGED INDEX AND ITS COMPUTATION FOR SOME NANOTUBES A IRANMANESH a*, O KHORMALI b, I NAJAFI KHALILSARAEE c, B SOLEIMANI
Digst Joural of Naomatrials ad Biostructurs Vol 4, No, March 009, p 67-76 NEW VERSION OF SZEGED INDEX AND ITS COMPUTATION FOR SOME NANOTUBES A IRANMANESH a*, O KHORMALI b, I NAJAFI KHALILSARAEE c, B SOLEIMANI
APPENDIX: STATISTICAL TOOLS
 I. Nots o radom samplig Why do you d to sampl radomly? APPENDI: STATISTICAL TOOLS I ordr to masur som valu o a populatio of orgaisms, you usually caot masur all orgaisms, so you sampl a subst of th populatio.
I. Nots o radom samplig Why do you d to sampl radomly? APPENDI: STATISTICAL TOOLS I ordr to masur som valu o a populatio of orgaisms, you usually caot masur all orgaisms, so you sampl a subst of th populatio.
1985 AP Calculus BC: Section I
 985 AP Calculus BC: Sctio I 9 Miuts No Calculator Nots: () I this amiatio, l dots th atural logarithm of (that is, logarithm to th bas ). () Ulss othrwis spcifid, th domai of a fuctio f is assumd to b
985 AP Calculus BC: Sctio I 9 Miuts No Calculator Nots: () I this amiatio, l dots th atural logarithm of (that is, logarithm to th bas ). () Ulss othrwis spcifid, th domai of a fuctio f is assumd to b
H2 Mathematics Arithmetic & Geometric Series ( )
 H Mathmatics Arithmtic & Gomtric Sris (08 09) Basic Mastry Qustios Arithmtic Progrssio ad Sris. Th rth trm of a squc is 4r 7. (i) Stat th first four trms ad th 0th trm. (ii) Show that th squc is a arithmtic
H Mathmatics Arithmtic & Gomtric Sris (08 09) Basic Mastry Qustios Arithmtic Progrssio ad Sris. Th rth trm of a squc is 4r 7. (i) Stat th first four trms ad th 0th trm. (ii) Show that th squc is a arithmtic
LECTURE 13 Filling the bands. Occupancy of Available Energy Levels
 LUR 3 illig th bads Occupacy o Availabl rgy Lvls W hav dtrmid ad a dsity o stats. W also d a way o dtrmiig i a stat is illd or ot at a giv tmpratur. h distributio o th rgis o a larg umbr o particls ad
LUR 3 illig th bads Occupacy o Availabl rgy Lvls W hav dtrmid ad a dsity o stats. W also d a way o dtrmiig i a stat is illd or ot at a giv tmpratur. h distributio o th rgis o a larg umbr o particls ad
Ali Mehrizad 1 *, Parvin Gharbani 2. Pol. J. Environ. Stud. Vol. 23, No. 6 (2014), DOI: /pjoes/26779
 Pol. J. Eviro. Stud. Vol. 3, No. 6 (14), 111-116 DOI: 1.1544/pjos/6779 Origial Rsarch Dcotamiatio of 4-Chloro--Nitrophol from Auous Solutio by Graph Adsorptio: Euilibrium, Kitic, ad Thrmodyamic Studis
Pol. J. Eviro. Stud. Vol. 3, No. 6 (14), 111-116 DOI: 1.1544/pjos/6779 Origial Rsarch Dcotamiatio of 4-Chloro--Nitrophol from Auous Solutio by Graph Adsorptio: Euilibrium, Kitic, ad Thrmodyamic Studis
Washington State University
 he 3 Ktics ad Ractor Dsig Sprg, 00 Washgto Stat Uivrsity Dpartmt of hmical Egrg Richard L. Zollars Exam # You will hav o hour (60 muts) to complt this xam which cosists of four (4) problms. You may us
he 3 Ktics ad Ractor Dsig Sprg, 00 Washgto Stat Uivrsity Dpartmt of hmical Egrg Richard L. Zollars Exam # You will hav o hour (60 muts) to complt this xam which cosists of four (4) problms. You may us
Bipolar Junction Transistors
 ipolar Juctio Trasistors ipolar juctio trasistors (JT) ar activ 3-trmial dvics with aras of applicatios: amplifirs, switch tc. high-powr circuits high-spd logic circuits for high-spd computrs. JT structur:
ipolar Juctio Trasistors ipolar juctio trasistors (JT) ar activ 3-trmial dvics with aras of applicatios: amplifirs, switch tc. high-powr circuits high-spd logic circuits for high-spd computrs. JT structur:
Mixed Mode Oscillations as a Mechanism for Pseudo-Plateau Bursting
 Mixd Mod Oscillatios as a Mchaism for Psudo-Platau Burstig Richard Brtram Dpartmt of Mathmatics Florida Stat Uivrsity Tallahass, FL Collaborators ad Support Thodor Vo Marti Wchslbrgr Joël Tabak Uivrsity
Mixd Mod Oscillatios as a Mchaism for Psudo-Platau Burstig Richard Brtram Dpartmt of Mathmatics Florida Stat Uivrsity Tallahass, FL Collaborators ad Support Thodor Vo Marti Wchslbrgr Joël Tabak Uivrsity
DTFT Properties. Example - Determine the DTFT Y ( e ) of n. Let. We can therefore write. From Table 3.1, the DTFT of x[n] is given by 1
![DTFT Properties. Example - Determine the DTFT Y ( e ) of n. Let. We can therefore write. From Table 3.1, the DTFT of x[n] is given by 1 DTFT Properties. Example - Determine the DTFT Y ( e ) of n. Let. We can therefore write. From Table 3.1, the DTFT of x[n] is given by 1](/thumbs/79/79094742.jpg) DTFT Proprtis Exampl - Dtrmi th DTFT Y of y α µ, α < Lt x α µ, α < W ca thrfor writ y x x From Tabl 3., th DTFT of x is giv by ω X ω α ω Copyright, S. K. Mitra Copyright, S. K. Mitra DTFT Proprtis DTFT
DTFT Proprtis Exampl - Dtrmi th DTFT Y of y α µ, α < Lt x α µ, α < W ca thrfor writ y x x From Tabl 3., th DTFT of x is giv by ω X ω α ω Copyright, S. K. Mitra Copyright, S. K. Mitra DTFT Proprtis DTFT
The Interplay between l-max, l-min, p-max and p-min Stable Distributions
 DOI: 0.545/mjis.05.4006 Th Itrplay btw lma lmi pma ad pmi Stabl Distributios S Ravi ad TS Mavitha Dpartmt of Studis i Statistics Uivrsity of Mysor Maasagagotri Mysuru 570006 Idia. Email:ravi@statistics.uimysor.ac.i
DOI: 0.545/mjis.05.4006 Th Itrplay btw lma lmi pma ad pmi Stabl Distributios S Ravi ad TS Mavitha Dpartmt of Studis i Statistics Uivrsity of Mysor Maasagagotri Mysuru 570006 Idia. Email:ravi@statistics.uimysor.ac.i
INTRODUCTION TO SAMPLING DISTRIBUTIONS
 http://wiki.stat.ucla.du/socr/id.php/socr_courss_2008_thomso_econ261 INTRODUCTION TO SAMPLING DISTRIBUTIONS By Grac Thomso INTRODUCTION TO SAMPLING DISTRIBUTIONS Itro to Samplig 2 I this chaptr w will
http://wiki.stat.ucla.du/socr/id.php/socr_courss_2008_thomso_econ261 INTRODUCTION TO SAMPLING DISTRIBUTIONS By Grac Thomso INTRODUCTION TO SAMPLING DISTRIBUTIONS Itro to Samplig 2 I this chaptr w will
Partition Functions and Ideal Gases
 Partitio Fuctios ad Idal Gass PFIG- You v lard about partitio fuctios ad som uss ow w ll xplor tm i mor dpt usig idal moatomic diatomic ad polyatomic gass! for w start rmmbr: Q( N ( N! N Wat ar N ad? W
Partitio Fuctios ad Idal Gass PFIG- You v lard about partitio fuctios ad som uss ow w ll xplor tm i mor dpt usig idal moatomic diatomic ad polyatomic gass! for w start rmmbr: Q( N ( N! N Wat ar N ad? W
Journal of Modern Applied Statistical Methods
 Joural of Modr Applid Statistical Mthods Volum Issu Articl 6 --03 O Som Proprtis of a Htrogous Trasfr Fuctio Ivolvig Symmtric Saturatd Liar (SATLINS) with Hyprbolic Tagt (TANH) Trasfr Fuctios Christophr
Joural of Modr Applid Statistical Mthods Volum Issu Articl 6 --03 O Som Proprtis of a Htrogous Trasfr Fuctio Ivolvig Symmtric Saturatd Liar (SATLINS) with Hyprbolic Tagt (TANH) Trasfr Fuctios Christophr
(Reference: sections in Silberberg 5 th ed.)
 ALE. Atomic Structur Nam HEM K. Marr Tam No. Sctio What is a atom? What is th structur of a atom? Th Modl th structur of a atom (Rfrc: sctios.4 -. i Silbrbrg 5 th d.) Th subatomic articls that chmists
ALE. Atomic Structur Nam HEM K. Marr Tam No. Sctio What is a atom? What is th structur of a atom? Th Modl th structur of a atom (Rfrc: sctios.4 -. i Silbrbrg 5 th d.) Th subatomic articls that chmists
Ideal crystal : Regulary ordered point masses connected via harmonic springs
 Statistical thrmodyamics of crystals Mooatomic crystal Idal crystal : Rgulary ordrd poit masss coctd via harmoic sprigs Itratomic itractios Rprstd by th lattic forc-costat quivalt atom positios miima o
Statistical thrmodyamics of crystals Mooatomic crystal Idal crystal : Rgulary ordrd poit masss coctd via harmoic sprigs Itratomic itractios Rprstd by th lattic forc-costat quivalt atom positios miima o
Statistics 3858 : Likelihood Ratio for Exponential Distribution
 Statistics 3858 : Liklihood Ratio for Expotial Distributio I ths two xampl th rjctio rjctio rgio is of th form {x : 2 log (Λ(x)) > c} for a appropriat costat c. For a siz α tst, usig Thorm 9.5A w obtai
Statistics 3858 : Liklihood Ratio for Expotial Distributio I ths two xampl th rjctio rjctio rgio is of th form {x : 2 log (Λ(x)) > c} for a appropriat costat c. For a siz α tst, usig Thorm 9.5A w obtai
Chapter 2 Infinite Series Page 1 of 11. Chapter 2 : Infinite Series
 Chatr Ifiit Sris Pag of Sctio F Itgral Tst Chatr : Ifiit Sris By th d of this sctio you will b abl to valuat imror itgrals tst a sris for covrgc by alyig th itgral tst aly th itgral tst to rov th -sris
Chatr Ifiit Sris Pag of Sctio F Itgral Tst Chatr : Ifiit Sris By th d of this sctio you will b abl to valuat imror itgrals tst a sris for covrgc by alyig th itgral tst aly th itgral tst to rov th -sris
Chapter Taylor Theorem Revisited
 Captr 0.07 Taylor Torm Rvisitd Atr radig tis captr, you sould b abl to. udrstad t basics o Taylor s torm,. writ trascdtal ad trigoomtric uctios as Taylor s polyomial,. us Taylor s torm to id t valus o
Captr 0.07 Taylor Torm Rvisitd Atr radig tis captr, you sould b abl to. udrstad t basics o Taylor s torm,. writ trascdtal ad trigoomtric uctios as Taylor s polyomial,. us Taylor s torm to id t valus o
Chapter 11.00C Physical Problem for Fast Fourier Transform Civil Engineering
 haptr. Physical Problm for Fast Fourir Trasform ivil Egirig Itroductio I this chaptr, applicatios of FFT algorithms [-5] for solvig ral-lif problms such as computig th dyamical (displacmt rspos [6-7] of
haptr. Physical Problm for Fast Fourir Trasform ivil Egirig Itroductio I this chaptr, applicatios of FFT algorithms [-5] for solvig ral-lif problms such as computig th dyamical (displacmt rspos [6-7] of
ln x = n e = 20 (nearest integer)
 H JC Prlim Solutios 6 a + b y a + b / / dy a b 3/ d dy a b at, d Giv quatio of ormal at is y dy ad y wh. d a b () (,) is o th curv a+ b () y.9958 Qustio Solvig () ad (), w hav a, b. Qustio d.77 d d d.77
H JC Prlim Solutios 6 a + b y a + b / / dy a b 3/ d dy a b at, d Giv quatio of ormal at is y dy ad y wh. d a b () (,) is o th curv a+ b () y.9958 Qustio Solvig () ad (), w hav a, b. Qustio d.77 d d d.77
PURE MATHEMATICS A-LEVEL PAPER 1
 -AL P MATH PAPER HONG KONG EXAMINATIONS AUTHORITY HONG KONG ADVANCED LEVEL EXAMINATION PURE MATHEMATICS A-LEVEL PAPER 8 am am ( hours) This papr must b aswrd i Eglish This papr cosists of Sctio A ad Sctio
-AL P MATH PAPER HONG KONG EXAMINATIONS AUTHORITY HONG KONG ADVANCED LEVEL EXAMINATION PURE MATHEMATICS A-LEVEL PAPER 8 am am ( hours) This papr must b aswrd i Eglish This papr cosists of Sctio A ad Sctio
Figure 2-18 Thevenin Equivalent Circuit of a Noisy Resistor
 .8 NOISE.8. Th Nyquist Nois Thorm W ow wat to tur our atttio to ois. W will start with th basic dfiitio of ois as usd i radar thory ad th discuss ois figur. Th typ of ois of itrst i radar thory is trmd
.8 NOISE.8. Th Nyquist Nois Thorm W ow wat to tur our atttio to ois. W will start with th basic dfiitio of ois as usd i radar thory ad th discuss ois figur. Th typ of ois of itrst i radar thory is trmd
On the approximation of the constant of Napier
 Stud. Uiv. Babş-Bolyai Math. 560, No., 609 64 O th approximatio of th costat of Napir Adri Vrscu Abstract. Startig from som oldr idas of [] ad [6], w show w facts cocrig th approximatio of th costat of
Stud. Uiv. Babş-Bolyai Math. 560, No., 609 64 O th approximatio of th costat of Napir Adri Vrscu Abstract. Startig from som oldr idas of [] ad [6], w show w facts cocrig th approximatio of th costat of
Option 3. b) xe dx = and therefore the series is convergent. 12 a) Divergent b) Convergent Proof 15 For. p = 1 1so the series diverges.
 Optio Chaptr Ercis. Covrgs to Covrgs to Covrgs to Divrgs Covrgs to Covrgs to Divrgs 8 Divrgs Covrgs to Covrgs to Divrgs Covrgs to Covrgs to Covrgs to Covrgs to 8 Proof Covrgs to π l 8 l a b Divrgt π Divrgt
Optio Chaptr Ercis. Covrgs to Covrgs to Covrgs to Divrgs Covrgs to Covrgs to Divrgs 8 Divrgs Covrgs to Covrgs to Divrgs Covrgs to Covrgs to Covrgs to Covrgs to 8 Proof Covrgs to π l 8 l a b Divrgt π Divrgt
A Simple Proof that e is Irrational
 Two of th most bautiful ad sigificat umbrs i mathmatics ar π ad. π (approximatly qual to 3.459) rprsts th ratio of th circumfrc of a circl to its diamtr. (approximatly qual to.788) is th bas of th atural
Two of th most bautiful ad sigificat umbrs i mathmatics ar π ad. π (approximatly qual to 3.459) rprsts th ratio of th circumfrc of a circl to its diamtr. (approximatly qual to.788) is th bas of th atural
MONTGOMERY COLLEGE Department of Mathematics Rockville Campus. 6x dx a. b. cos 2x dx ( ) 7. arctan x dx e. cos 2x dx. 2 cos3x dx
 MONTGOMERY COLLEGE Dpartmt of Mathmatics Rockvill Campus MATH 8 - REVIEW PROBLEMS. Stat whthr ach of th followig ca b itgratd by partial fractios (PF), itgratio by parts (PI), u-substitutio (U), or o of
MONTGOMERY COLLEGE Dpartmt of Mathmatics Rockvill Campus MATH 8 - REVIEW PROBLEMS. Stat whthr ach of th followig ca b itgratd by partial fractios (PF), itgratio by parts (PI), u-substitutio (U), or o of
10. Joint Moments and Joint Characteristic Functions
 0 Joit Momts ad Joit Charactristic Fctios Followig sctio 6 i this sctio w shall itrodc varios paramtrs to compactly rprst th iformatio cotaid i th joit pdf of two rvs Giv two rvs ad ad a fctio g x y dfi
0 Joit Momts ad Joit Charactristic Fctios Followig sctio 6 i this sctio w shall itrodc varios paramtrs to compactly rprst th iformatio cotaid i th joit pdf of two rvs Giv two rvs ad ad a fctio g x y dfi
Digital Signal Processing, Fall 2006
 Digital Sigal Procssig, Fall 6 Lctur 9: Th Discrt Fourir Trasfor Zhg-Hua Ta Dpartt of Elctroic Systs Aalborg Uivrsity, Dar zt@o.aau.d Digital Sigal Procssig, I, Zhg-Hua Ta, 6 Cours at a glac MM Discrt-ti
Digital Sigal Procssig, Fall 6 Lctur 9: Th Discrt Fourir Trasfor Zhg-Hua Ta Dpartt of Elctroic Systs Aalborg Uivrsity, Dar zt@o.aau.d Digital Sigal Procssig, I, Zhg-Hua Ta, 6 Cours at a glac MM Discrt-ti
ECE 340 Lecture 38 : MOS Capacitor I Class Outline:
 ECE 34 Lctur 38 : MOS Capacitor I Class Outli: Idal MOS Capacitor higs you should ow wh you lav Ky Qustios What ar th diffrt ias rgios i MOS capacitors? What do th lctric fild ad lctrostatic pottial loo
ECE 34 Lctur 38 : MOS Capacitor I Class Outli: Idal MOS Capacitor higs you should ow wh you lav Ky Qustios What ar th diffrt ias rgios i MOS capacitors? What do th lctric fild ad lctrostatic pottial loo
Outline. Ionizing Radiation. Introduction. Ionizing radiation
 Outli Ioizig Radiatio Chaptr F.A. Attix, Itroductio to Radiological Physics ad Radiatio Dosimtry Radiological physics ad radiatio dosimtry Typs ad sourcs of ioizig radiatio Dscriptio of ioizig radiatio
Outli Ioizig Radiatio Chaptr F.A. Attix, Itroductio to Radiological Physics ad Radiatio Dosimtry Radiological physics ad radiatio dosimtry Typs ad sourcs of ioizig radiatio Dscriptio of ioizig radiatio
Solution to 1223 The Evil Warden.
 Solutio to 1 Th Evil Ward. This is o of thos vry rar PoWs (I caot thik of aothr cas) that o o solvd. About 10 of you submittd th basic approach, which givs a probability of 47%. I was shockd wh I foud
Solutio to 1 Th Evil Ward. This is o of thos vry rar PoWs (I caot thik of aothr cas) that o o solvd. About 10 of you submittd th basic approach, which givs a probability of 47%. I was shockd wh I foud
They must have different numbers of electrons orbiting their nuclei. They must have the same number of neutrons in their nuclei.
 37 1 How may utros ar i a uclus of th uclid l? 20 37 54 2 crtai lmt has svral isotops. Which statmt about ths isotops is corrct? Thy must hav diffrt umbrs of lctros orbitig thir ucli. Thy must hav th sam
37 1 How may utros ar i a uclus of th uclid l? 20 37 54 2 crtai lmt has svral isotops. Which statmt about ths isotops is corrct? Thy must hav diffrt umbrs of lctros orbitig thir ucli. Thy must hav th sam
Empirical Study in Finite Correlation Coefficient in Two Phase Estimation
 M. Khoshvisa Griffith Uivrsity Griffith Busiss School Australia F. Kaymarm Massachustts Istitut of Tchology Dpartmt of Mchaical girig USA H. P. Sigh R. Sigh Vikram Uivrsity Dpartmt of Mathmatics ad Statistics
M. Khoshvisa Griffith Uivrsity Griffith Busiss School Australia F. Kaymarm Massachustts Istitut of Tchology Dpartmt of Mchaical girig USA H. P. Sigh R. Sigh Vikram Uivrsity Dpartmt of Mathmatics ad Statistics
Discrete Fourier Transform (DFT)
 Discrt Fourir Trasorm DFT Major: All Egirig Majors Authors: Duc guy http://umricalmthods.g.us.du umrical Mthods or STEM udrgraduats 8/3/29 http://umricalmthods.g.us.du Discrt Fourir Trasorm Rcalld th xpotial
Discrt Fourir Trasorm DFT Major: All Egirig Majors Authors: Duc guy http://umricalmthods.g.us.du umrical Mthods or STEM udrgraduats 8/3/29 http://umricalmthods.g.us.du Discrt Fourir Trasorm Rcalld th xpotial
Worksheet: Taylor Series, Lagrange Error Bound ilearnmath.net
 Taylor s Thorm & Lagrag Error Bouds Actual Error This is th ral amout o rror, ot th rror boud (worst cas scario). It is th dirc btw th actual () ad th polyomial. Stps:. Plug -valu ito () to gt a valu.
Taylor s Thorm & Lagrag Error Bouds Actual Error This is th ral amout o rror, ot th rror boud (worst cas scario). It is th dirc btw th actual () ad th polyomial. Stps:. Plug -valu ito () to gt a valu.
Discrete Fourier Transform. Nuno Vasconcelos UCSD
 Discrt Fourir Trasform uo Vascoclos UCSD Liar Shift Ivariat (LSI) systms o of th most importat cocpts i liar systms thory is that of a LSI systm Dfiitio: a systm T that maps [ ito y[ is LSI if ad oly if
Discrt Fourir Trasform uo Vascoclos UCSD Liar Shift Ivariat (LSI) systms o of th most importat cocpts i liar systms thory is that of a LSI systm Dfiitio: a systm T that maps [ ito y[ is LSI if ad oly if
MILLIKAN OIL DROP EXPERIMENT
 11 Oct 18 Millika.1 MILLIKAN OIL DROP EXPERIMENT This xprimt is dsigd to show th quatizatio of lctric charg ad allow dtrmiatio of th lmtary charg,. As i Millika s origial xprimt, oil drops ar sprayd ito
11 Oct 18 Millika.1 MILLIKAN OIL DROP EXPERIMENT This xprimt is dsigd to show th quatizatio of lctric charg ad allow dtrmiatio of th lmtary charg,. As i Millika s origial xprimt, oil drops ar sprayd ito
Chapter (8) Estimation and Confedence Intervals Examples
 Chaptr (8) Estimatio ad Cofdc Itrvals Exampls Typs of stimatio: i. Poit stimatio: Exampl (1): Cosidr th sampl obsrvatios, 17,3,5,1,18,6,16,10 8 X i i1 17 3 5 118 6 16 10 116 X 14.5 8 8 8 14.5 is a poit
Chaptr (8) Estimatio ad Cofdc Itrvals Exampls Typs of stimatio: i. Poit stimatio: Exampl (1): Cosidr th sampl obsrvatios, 17,3,5,1,18,6,16,10 8 X i i1 17 3 5 118 6 16 10 116 X 14.5 8 8 8 14.5 is a poit
Chemical Physics II. More Stat. Thermo Kinetics Protein Folding...
 Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
ECE594I Notes set 6: Thermal Noise
 C594I ots, M. odwll, copyrightd C594I Nots st 6: Thrmal Nois Mark odwll Uivrsity of Califoria, ata Barbara rodwll@c.ucsb.du 805-893-344, 805-893-36 fax frcs ad Citatios: C594I ots, M. odwll, copyrightd
C594I ots, M. odwll, copyrightd C594I Nots st 6: Thrmal Nois Mark odwll Uivrsity of Califoria, ata Barbara rodwll@c.ucsb.du 805-893-344, 805-893-36 fax frcs ad Citatios: C594I ots, M. odwll, copyrightd
An Introduction to Asymptotic Expansions
 A Itroductio to Asmptotic Expasios R. Shaar Subramaia Asmptotic xpasios ar usd i aalsis to dscrib th bhavior of a fuctio i a limitig situatio. Wh a fuctio ( x, dpds o a small paramtr, ad th solutio of
A Itroductio to Asmptotic Expasios R. Shaar Subramaia Asmptotic xpasios ar usd i aalsis to dscrib th bhavior of a fuctio i a limitig situatio. Wh a fuctio ( x, dpds o a small paramtr, ad th solutio of
Reliability of time dependent stress-strength system for various distributions
 IOS Joural of Mathmatcs (IOS-JM ISSN: 78-578. Volum 3, Issu 6 (Sp-Oct., PP -7 www.osrjourals.org lablty of tm dpdt strss-strgth systm for varous dstrbutos N.Swath, T.S.Uma Mahswar,, Dpartmt of Mathmatcs,
IOS Joural of Mathmatcs (IOS-JM ISSN: 78-578. Volum 3, Issu 6 (Sp-Oct., PP -7 www.osrjourals.org lablty of tm dpdt strss-strgth systm for varous dstrbutos N.Swath, T.S.Uma Mahswar,, Dpartmt of Mathmatcs,
Ch. 24 Molecular Reaction Dynamics 1. Collision Theory
 Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Search sequence databases 3 10/25/2016
 Sarch squnc databass 3 10/25/2016 Etrm valu distribution Ø Suppos X is a random variabl with probability dnsity function p(, w sampl a larg numbr S of indpndnt valus of X from this distribution for an
Sarch squnc databass 3 10/25/2016 Etrm valu distribution Ø Suppos X is a random variabl with probability dnsity function p(, w sampl a larg numbr S of indpndnt valus of X from this distribution for an
2617 Mark Scheme June 2005 Mark Scheme 2617 June 2005
 Mark Schm 67 Ju 5 GENERAL INSTRUCTIONS Marks i th mark schm ar plicitly dsigatd as M, A, B, E or G. M marks ("mthod" ar for a attmpt to us a corrct mthod (ot mrly for statig th mthod. A marks ("accuracy"
Mark Schm 67 Ju 5 GENERAL INSTRUCTIONS Marks i th mark schm ar plicitly dsigatd as M, A, B, E or G. M marks ("mthod" ar for a attmpt to us a corrct mthod (ot mrly for statig th mthod. A marks ("accuracy"
Probability & Statistics,
 Probability & Statistics, BITS Pilai K K Birla Goa Campus Dr. Jajati Kshari Sahoo Dpartmt of Mathmatics BITS Pilai, K K Birla Goa Campus Poisso Distributio Poisso Distributio: A radom variabl X is said
Probability & Statistics, BITS Pilai K K Birla Goa Campus Dr. Jajati Kshari Sahoo Dpartmt of Mathmatics BITS Pilai, K K Birla Goa Campus Poisso Distributio Poisso Distributio: A radom variabl X is said
ROLE OF SAWDUST IN THE REMOVAL OF IRON FROM AQUEOUS SOLUTION
 AJSTD Vol. 23 Issu 3 pp. 223-229 (2006) ROLE OF SAWDUST IN THE REMOVAL OF IRON FROM AQUEOUS SOLUTION H.B. Snin *, O. Subhi, R. Rosliza, N. Kancono, M.S. Azhar, S. Hasiah, and W.B. Wan Nik Faculty of Scinc
AJSTD Vol. 23 Issu 3 pp. 223-229 (2006) ROLE OF SAWDUST IN THE REMOVAL OF IRON FROM AQUEOUS SOLUTION H.B. Snin *, O. Subhi, R. Rosliza, N. Kancono, M.S. Azhar, S. Hasiah, and W.B. Wan Nik Faculty of Scinc
ph People Grade Level: basic Duration: minutes Setting: classroom or field site
 ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
Numerov-Cooley Method : 1-D Schr. Eq. Last time: Rydberg, Klein, Rees Method and Long-Range Model G(v), B(v) rotation-vibration constants.
 Numrov-Cooly Mthod : 1-D Schr. Eq. Last tim: Rydbrg, Kli, Rs Mthod ad Log-Rag Modl G(v), B(v) rotatio-vibratio costats 9-1 V J (x) pottial rgy curv x = R R Ev,J, v,j, all cocivabl xprimts wp( x, t) = ai
Numrov-Cooly Mthod : 1-D Schr. Eq. Last tim: Rydbrg, Kli, Rs Mthod ad Log-Rag Modl G(v), B(v) rotatio-vibratio costats 9-1 V J (x) pottial rgy curv x = R R Ev,J, v,j, all cocivabl xprimts wp( x, t) = ai
Blackbody Radiation. All bodies at a temperature T emit and absorb thermal electromagnetic radiation. How is blackbody radiation absorbed and emitted?
 All bodis at a tmpratur T mit ad absorb thrmal lctromagtic radiatio Blackbody radiatio I thrmal quilibrium, th powr mittd quals th powr absorbd How is blackbody radiatio absorbd ad mittd? 1 2 A blackbody
All bodis at a tmpratur T mit ad absorb thrmal lctromagtic radiatio Blackbody radiatio I thrmal quilibrium, th powr mittd quals th powr absorbd How is blackbody radiatio absorbd ad mittd? 1 2 A blackbody
Chapter 4 - The Fourier Series
 M. J. Robrts - 8/8/4 Chaptr 4 - Th Fourir Sris Slctd Solutios (I this solutio maual, th symbol,, is usd for priodic covolutio bcaus th prfrrd symbol which appars i th txt is ot i th fot slctio of th word
M. J. Robrts - 8/8/4 Chaptr 4 - Th Fourir Sris Slctd Solutios (I this solutio maual, th symbol,, is usd for priodic covolutio bcaus th prfrrd symbol which appars i th txt is ot i th fot slctio of th word
t i Extreme value statistics Problems of extrapolating to values we have no data about unusually large or small ~100 years (data) ~500 years (design)
 Extrm valu statistics Problms of xtrapolatig to valus w hav o data about uusually larg or small t i ~00 yars (data h( t i { h( }? max t i wids v( t i ~500 yars (dsig Qustio: Ca this b do at all? How log
Extrm valu statistics Problms of xtrapolatig to valus w hav o data about uusually larg or small t i ~00 yars (data h( t i { h( }? max t i wids v( t i ~500 yars (dsig Qustio: Ca this b do at all? How log
Comparison of Simple Indicator Kriging, DMPE, Full MV Approach for Categorical Random Variable Simulation
 Papr 17, CCG Aual Rport 11, 29 ( 29) Compariso of Simpl Idicator rigig, DMPE, Full MV Approach for Catgorical Radom Variabl Simulatio Yupg Li ad Clayto V. Dutsch Ifrc of coditioal probabilitis at usampld
Papr 17, CCG Aual Rport 11, 29 ( 29) Compariso of Simpl Idicator rigig, DMPE, Full MV Approach for Catgorical Radom Variabl Simulatio Yupg Li ad Clayto V. Dutsch Ifrc of coditioal probabilitis at usampld
Principles of Humidity Dalton s law
 Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
International Journal of Advanced and Applied Sciences
 Itratioal Joural of Advacd ad Applid Scics x(x) xxxx Pags: xx xx Cotts lists availabl at Scic Gat Itratioal Joural of Advacd ad Applid Scics Joural hompag: http://wwwscic gatcom/ijaashtml Symmtric Fuctios
Itratioal Joural of Advacd ad Applid Scics x(x) xxxx Pags: xx xx Cotts lists availabl at Scic Gat Itratioal Joural of Advacd ad Applid Scics Joural hompag: http://wwwscic gatcom/ijaashtml Symmtric Fuctios
Homotopy perturbation technique
 Comput. Mthods Appl. Mch. Engrg. 178 (1999) 257±262 www.lsvir.com/locat/cma Homotopy prturbation tchniqu Ji-Huan H 1 Shanghai Univrsity, Shanghai Institut of Applid Mathmatics and Mchanics, Shanghai 272,
Comput. Mthods Appl. Mch. Engrg. 178 (1999) 257±262 www.lsvir.com/locat/cma Homotopy prturbation tchniqu Ji-Huan H 1 Shanghai Univrsity, Shanghai Institut of Applid Mathmatics and Mchanics, Shanghai 272,
Part B: Transform Methods. Professor E. Ambikairajah UNSW, Australia
 Part B: Trasform Mthods Chaptr 3: Discrt-Tim Fourir Trasform (DTFT) 3. Discrt Tim Fourir Trasform (DTFT) 3. Proprtis of DTFT 3.3 Discrt Fourir Trasform (DFT) 3.4 Paddig with Zros ad frqucy Rsolutio 3.5
Part B: Trasform Mthods Chaptr 3: Discrt-Tim Fourir Trasform (DTFT) 3. Discrt Tim Fourir Trasform (DTFT) 3. Proprtis of DTFT 3.3 Discrt Fourir Trasform (DFT) 3.4 Paddig with Zros ad frqucy Rsolutio 3.5
On the Reformulated Zagreb Indices of Certain Nanostructures
 lobal Joural o Pur ad Applid Mathmatics. ISSN 097-768 Volum, Numbr 07, pp. 87-87 Rsarch Idia Publicatios http://www.ripublicatio.com O th Rormulatd Zagrb Idics o Crtai Naostructurs Krthi. Mirajar ad Priyaa
lobal Joural o Pur ad Applid Mathmatics. ISSN 097-768 Volum, Numbr 07, pp. 87-87 Rsarch Idia Publicatios http://www.ripublicatio.com O th Rormulatd Zagrb Idics o Crtai Naostructurs Krthi. Mirajar ad Priyaa
ELECTRONIC APPENDIX TO: ELASTIC-PLASTIC CONTACT OF A ROUGH SURFACE WITH WEIERSTRASS PROFILE. Yan-Fei Gao and A. F. Bower
 ELECTRONIC APPENDIX TO: ELASTIC-PLASTIC CONTACT OF A ROUGH SURFACE WITH WEIERSTRASS PROFILE Ya-Fi Gao ad A. F. Bowr Divisio of Egirig, Brow Uivrsity, Providc, RI 9, USA Appdix A: Approximat xprssios for
ELECTRONIC APPENDIX TO: ELASTIC-PLASTIC CONTACT OF A ROUGH SURFACE WITH WEIERSTRASS PROFILE Ya-Fi Gao ad A. F. Bowr Divisio of Egirig, Brow Uivrsity, Providc, RI 9, USA Appdix A: Approximat xprssios for
A Strain-based Non-linear Elastic Model for Geomaterials
 A Strai-basd No-liar Elastic Modl for Gomatrials ANDREW HEATH Dpartmt of Architctur ad Civil Egirig Uivrsity of Bath Bath, BA2 7AY UNITED KINGDOM A.Hath@bath.ac.uk http://www.bath.ac.uk/ac Abstract: -
A Strai-basd No-liar Elastic Modl for Gomatrials ANDREW HEATH Dpartmt of Architctur ad Civil Egirig Uivrsity of Bath Bath, BA2 7AY UNITED KINGDOM A.Hath@bath.ac.uk http://www.bath.ac.uk/ac Abstract: -
Triple Play: From De Morgan to Stirling To Euler to Maclaurin to Stirling
 Tripl Play: From D Morga to Stirlig To Eulr to Maclauri to Stirlig Augustus D Morga (186-1871) was a sigificat Victoria Mathmaticia who mad cotributios to Mathmatics History, Mathmatical Rcratios, Mathmatical
Tripl Play: From D Morga to Stirlig To Eulr to Maclauri to Stirlig Augustus D Morga (186-1871) was a sigificat Victoria Mathmaticia who mad cotributios to Mathmatics History, Mathmatical Rcratios, Mathmatical
Narayana IIT Academy
 INDIA Sc: LT-IIT-SPARK Dat: 9--8 6_P Max.Mars: 86 KEY SHEET PHYSIS A 5 D 6 7 A,B 8 B,D 9 A,B A,,D A,B, A,B B, A,B 5 A 6 D 7 8 A HEMISTRY 9 A B D B B 5 A,B,,D 6 A,,D 7 B,,D 8 A,B,,D 9 A,B, A,B, A,B,,D A,B,
INDIA Sc: LT-IIT-SPARK Dat: 9--8 6_P Max.Mars: 86 KEY SHEET PHYSIS A 5 D 6 7 A,B 8 B,D 9 A,B A,,D A,B, A,B B, A,B 5 A 6 D 7 8 A HEMISTRY 9 A B D B B 5 A,B,,D 6 A,,D 7 B,,D 8 A,B,,D 9 A,B, A,B, A,B,,D A,B,
Estimation of apparent fraction defective: A mathematical approach
 Availabl onlin at www.plagiarsarchlibrary.com Plagia Rsarch Library Advancs in Applid Scinc Rsarch, 011, (): 84-89 ISSN: 0976-8610 CODEN (USA): AASRFC Estimation of apparnt fraction dfctiv: A mathmatical
Availabl onlin at www.plagiarsarchlibrary.com Plagia Rsarch Library Advancs in Applid Scinc Rsarch, 011, (): 84-89 ISSN: 0976-8610 CODEN (USA): AASRFC Estimation of apparnt fraction dfctiv: A mathmatical
A Novel Approach to Recovering Depth from Defocus
 Ssors & Trasducrs 03 by IFSA http://www.ssorsportal.com A Novl Approach to Rcovrig Dpth from Dfocus H Zhipa Liu Zhzhog Wu Qiufg ad Fu Lifag Collg of Egirig Northast Agricultural Uivrsity 50030 Harbi Chia
Ssors & Trasducrs 03 by IFSA http://www.ssorsportal.com A Novl Approach to Rcovrig Dpth from Dfocus H Zhipa Liu Zhzhog Wu Qiufg ad Fu Lifag Collg of Egirig Northast Agricultural Uivrsity 50030 Harbi Chia
Iterative Methods of Order Four for Solving Nonlinear Equations
 Itrativ Mods of Ordr Four for Solvig Noliar Equatios V.B. Kumar,Vatti, Shouri Domii ad Mouia,V Dpartmt of Egirig Mamatis, Formr Studt of Chmial Egirig Adhra Uivrsity Collg of Egirig A, Adhra Uivrsity Visakhapatam
Itrativ Mods of Ordr Four for Solvig Noliar Equatios V.B. Kumar,Vatti, Shouri Domii ad Mouia,V Dpartmt of Egirig Mamatis, Formr Studt of Chmial Egirig Adhra Uivrsity Collg of Egirig A, Adhra Uivrsity Visakhapatam
On a problem of J. de Graaf connected with algebras of unbounded operators de Bruijn, N.G.
 O a problm of J. d Graaf coctd with algbras of uboudd oprators d Bruij, N.G. Publishd: 01/01/1984 Documt Vrsio Publishr s PDF, also kow as Vrsio of Rcord (icluds fial pag, issu ad volum umbrs) Plas chck
O a problm of J. d Graaf coctd with algbras of uboudd oprators d Bruij, N.G. Publishd: 01/01/1984 Documt Vrsio Publishr s PDF, also kow as Vrsio of Rcord (icluds fial pag, issu ad volum umbrs) Plas chck
A Propagating Wave Packet Group Velocity Dispersion
 Lctur 8 Phys 375 A Propagating Wav Packt Group Vlocity Disprsion Ovrviw and Motivation: In th last lctur w lookd at a localizd solution t) to th 1D fr-particl Schrödingr quation (SE) that corrsponds to
Lctur 8 Phys 375 A Propagating Wav Packt Group Vlocity Disprsion Ovrviw and Motivation: In th last lctur w lookd at a localizd solution t) to th 1D fr-particl Schrödingr quation (SE) that corrsponds to
A Mathematical Study of Electro-Magneto- Thermo-Voigt Viscoelastic Surface Wave Propagation under Gravity Involving Time Rate of Change of Strain
 Thortical Mathmatics & Applicatios vol.3 o.3 3 87-6 ISSN: 79-9687 (prit) 79-979 (oli) Sciprss Ltd 3 A Mathmatical Study of Elctro-Magto- Thrmo-Voigt Viscolastic Surfac Wav Propagatio udr Gravity Ivolvig
Thortical Mathmatics & Applicatios vol.3 o.3 3 87-6 ISSN: 79-9687 (prit) 79-979 (oli) Sciprss Ltd 3 A Mathmatical Study of Elctro-Magto- Thrmo-Voigt Viscolastic Surfac Wav Propagatio udr Gravity Ivolvig
macro Road map to this lecture Objectives Aggregate Supply and the Phillips Curve Three models of aggregate supply W = ω P The sticky-wage model
 Road map to this lctur macro Aggrgat Supply ad th Phillips Curv W rlax th assumptio that th aggrgat supply curv is vrtical A vrsio of th aggrgat supply i trms of iflatio (rathr tha th pric lvl is calld
Road map to this lctur macro Aggrgat Supply ad th Phillips Curv W rlax th assumptio that th aggrgat supply curv is vrtical A vrsio of th aggrgat supply i trms of iflatio (rathr tha th pric lvl is calld
Restricted Factorial And A Remark On The Reduced Residue Classes
 Applid Mathmatics E-Nots, 162016, 244-250 c ISSN 1607-2510 Availabl fr at mirror sits of http://www.math.thu.du.tw/ am/ Rstrictd Factorial Ad A Rmark O Th Rducd Rsidu Classs Mhdi Hassai Rcivd 26 March
Applid Mathmatics E-Nots, 162016, 244-250 c ISSN 1607-2510 Availabl fr at mirror sits of http://www.math.thu.du.tw/ am/ Rstrictd Factorial Ad A Rmark O Th Rducd Rsidu Classs Mhdi Hassai Rcivd 26 March
Folding of Hyperbolic Manifolds
 It. J. Cotmp. Math. Scics, Vol. 7, 0, o. 6, 79-799 Foldig of Hyprbolic Maifolds H. I. Attiya Basic Scic Dpartmt, Collg of Idustrial Educatio BANE - SUEF Uivrsity, Egypt hala_attiya005@yahoo.com Abstract
It. J. Cotmp. Math. Scics, Vol. 7, 0, o. 6, 79-799 Foldig of Hyprbolic Maifolds H. I. Attiya Basic Scic Dpartmt, Collg of Idustrial Educatio BANE - SUEF Uivrsity, Egypt hala_attiya005@yahoo.com Abstract
Bohr type models of the atom give a totally incorrect picture of the atom and are of only historical significance.
 VISUAL PHYSICS ONLIN BOHR MODL OF TH ATOM Bhr typ mdls f th atm giv a ttally icrrct pictur f th atm ad ar f ly histrical sigificac. Fig.. Bhr s platary mdl f th atm. Hwvr, th Bhr mdls wr a imprtat stp
VISUAL PHYSICS ONLIN BOHR MODL OF TH ATOM Bhr typ mdls f th atm giv a ttally icrrct pictur f th atm ad ar f ly histrical sigificac. Fig.. Bhr s platary mdl f th atm. Hwvr, th Bhr mdls wr a imprtat stp
Electronic Supplementary Information (ESI)
 Elctronic Supplmntary Matrial (ESI) for RSC Advancs. This journal is Th Royal Socity of Chmistry 2015 Elctronic Supplmntary Information (ESI) Bouqut-lik calcium sulfat dihydrat: a highly fficint adsorbnt
Elctronic Supplmntary Matrial (ESI) for RSC Advancs. This journal is Th Royal Socity of Chmistry 2015 Elctronic Supplmntary Information (ESI) Bouqut-lik calcium sulfat dihydrat: a highly fficint adsorbnt
ANALYSIS OF UNSTEADY HEAT CONDUCTION THROUGH SHORT FIN WITH APPLICABILITY OF QUASI THEORY
 It. J. Mch. Eg. & Rob. Rs. 013 Tjpratap Sigh t al., 013 Rsarch Papr ISSN 78 0149 www.ijmrr.com Vol., No. 1, Jauary 013 013 IJMERR. All Rights Rsrvd ANALYSIS OF UNSTEADY HEAT CONDUCTION THROUGH SHORT FIN
It. J. Mch. Eg. & Rob. Rs. 013 Tjpratap Sigh t al., 013 Rsarch Papr ISSN 78 0149 www.ijmrr.com Vol., No. 1, Jauary 013 013 IJMERR. All Rights Rsrvd ANALYSIS OF UNSTEADY HEAT CONDUCTION THROUGH SHORT FIN
Physics 2D Lecture Slides Lecture 14: Feb 3 rd 2004
 Bria Wcht, th TA is back! Pl. giv all rgrad rqusts to him Quiz 4 is This Friday Physics D Lctur Slids Lctur 14: Fb 3 rd 004 Vivk Sharma UCSD Physics Whr ar th lctros isid th atom? Early Thought: Plum puddig
Bria Wcht, th TA is back! Pl. giv all rgrad rqusts to him Quiz 4 is This Friday Physics D Lctur Slids Lctur 14: Fb 3 rd 004 Vivk Sharma UCSD Physics Whr ar th lctros isid th atom? Early Thought: Plum puddig
coulombs or esu charge. It s mass is about 1/1837 times the mass of hydrogen atom. Thus mass of electron is
 1 ATOMIC STRUCTURE Fudamtal Particls: Mai Fudamtal Particl : (a) Elctro: It is a fudamtal particl of a atom which carris a uit gativ charg. It was discovrd by J.J. Thomso (1897) from th studis carrid out
1 ATOMIC STRUCTURE Fudamtal Particls: Mai Fudamtal Particl : (a) Elctro: It is a fudamtal particl of a atom which carris a uit gativ charg. It was discovrd by J.J. Thomso (1897) from th studis carrid out
Chapter 8: Electron Configurations and Periodicity
 Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Discrete Fourier Transform. Discrete Fourier Transform. Discrete Fourier Transform. Discrete Fourier Transform. Discrete Fourier Transform
 Discrt Fourir Trasform Dfiitio - T simplst rlatio btw a lt- squc x dfid for ω ad its DTFT X ( ) is ω obtaid by uiformly sampli X ( ) o t ω-axis btw ω < at ω From t dfiitio of t DTFT w tus av X X( ω ) ω
Discrt Fourir Trasform Dfiitio - T simplst rlatio btw a lt- squc x dfid for ω ad its DTFT X ( ) is ω obtaid by uiformly sampli X ( ) o t ω-axis btw ω < at ω From t dfiitio of t DTFT w tus av X X( ω ) ω
The pn junction: 2 Current vs Voltage (IV) characteristics
 Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
NET/JRF, GATE, IIT JAM, JEST, TIFR
 Istitut for NET/JRF, GATE, IIT JAM, JEST, TIFR ad GRE i PHYSICAL SCIENCES Mathmatical Physics JEST-6 Q. Giv th coditio φ, th solutio of th quatio ψ φ φ is giv by k. kφ kφ lφ kφ lφ (a) ψ (b) ψ kφ (c) ψ
Istitut for NET/JRF, GATE, IIT JAM, JEST, TIFR ad GRE i PHYSICAL SCIENCES Mathmatical Physics JEST-6 Q. Giv th coditio φ, th solutio of th quatio ψ φ φ is giv by k. kφ kφ lφ kφ lφ (a) ψ (b) ψ kφ (c) ψ
A Note on Quantile Coupling Inequalities and Their Applications
 A Not o Quatil Couplig Iqualitis ad Thir Applicatios Harriso H. Zhou Dpartmt of Statistics, Yal Uivrsity, Nw Hav, CT 06520, USA. E-mail:huibi.zhou@yal.du Ju 2, 2006 Abstract A rlatioship btw th larg dviatio
A Not o Quatil Couplig Iqualitis ad Thir Applicatios Harriso H. Zhou Dpartmt of Statistics, Yal Uivrsity, Nw Hav, CT 06520, USA. E-mail:huibi.zhou@yal.du Ju 2, 2006 Abstract A rlatioship btw th larg dviatio
Adsorption of Ni(II) on ion exchange resin: Kinetics, equilibrium and thermodynamic studies
 Kora J. Ch. Eg., 9(9), 13-138 (01) DOI: 10.1007/s11814-01-001-5 INVITED REVIEW PAPER Adsortio of Ni(II) o io xchag rsi: Kitics, quilibriu ad throdyaic studis Baybars Ali Fil, Rc Bocukcuoglu, Alr Erd Yilaz,
Kora J. Ch. Eg., 9(9), 13-138 (01) DOI: 10.1007/s11814-01-001-5 INVITED REVIEW PAPER Adsortio of Ni(II) o io xchag rsi: Kitics, quilibriu ad throdyaic studis Baybars Ali Fil, Rc Bocukcuoglu, Alr Erd Yilaz,
CDS 101: Lecture 5.1 Reachability and State Space Feedback
 CDS, Lctur 5. CDS : Lctur 5. Rachability ad Stat Spac Fdback Richard M. Murray ad Hido Mabuchi 5 Octobr 4 Goals: Di rachability o a cotrol systm Giv tsts or rachability o liar systms ad apply to ampls
CDS, Lctur 5. CDS : Lctur 5. Rachability ad Stat Spac Fdback Richard M. Murray ad Hido Mabuchi 5 Octobr 4 Goals: Di rachability o a cotrol systm Giv tsts or rachability o liar systms ad apply to ampls
Page 1. Before-After Control-Impact (BACI) Power Analysis For Several Related Populations (Variance Known) Richard A. Hinrichsen. September 24, 2010
 Pag for-aftr Cotrol-Impact (ACI) Powr Aalysis For Svral Rlatd Populatios (Variac Kow) Richard A. Hirichs Sptmbr 4, Cavat: This primtal dsig tool is a idalizd powr aalysis built upo svral simplifyig assumptios
Pag for-aftr Cotrol-Impact (ACI) Powr Aalysis For Svral Rlatd Populatios (Variac Kow) Richard A. Hirichs Sptmbr 4, Cavat: This primtal dsig tool is a idalizd powr aalysis built upo svral simplifyig assumptios
2.29 Numerical Fluid Mechanics Spring 2015 Lecture 12
 REVIEW Lctur 11: Numrical Fluid Mchaics Sprig 2015 Lctur 12 Fiit Diffrcs basd Polyomial approximatios Obtai polyomial (i gral u-qually spacd), th diffrtiat as dd Nwto s itrpolatig polyomial formulas Triagular
REVIEW Lctur 11: Numrical Fluid Mchaics Sprig 2015 Lctur 12 Fiit Diffrcs basd Polyomial approximatios Obtai polyomial (i gral u-qually spacd), th diffrtiat as dd Nwto s itrpolatig polyomial formulas Triagular
8(4 m0) ( θ ) ( ) Solutions for HW 8. Chapter 25. Conceptual Questions
 Solutios for HW 8 Captr 5 Cocptual Qustios 5.. θ dcrass. As t crystal is coprssd, t spacig d btw t plas of atos dcrass. For t first ordr diffractio =. T Bragg coditio is = d so as d dcrass, ust icras for
Solutios for HW 8 Captr 5 Cocptual Qustios 5.. θ dcrass. As t crystal is coprssd, t spacig d btw t plas of atos dcrass. For t first ordr diffractio =. T Bragg coditio is = d so as d dcrass, ust icras for
Elements of Statistical Thermodynamics
 24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
Lens Design II. Lecture 6: Chromatical correction I Herbert Gross. Winter term
 Ls Dsig II Lctur 6: Chromatical corrctio I 05--4 Hrbrt Gross Witr trm 05 www.iap.ui-a.d Prlimiary Schdul 0.0. Abrratios ad optimizatio Rptitio 7.0. Structural modificatios Zro oprads, ls splittig, ls additio,
Ls Dsig II Lctur 6: Chromatical corrctio I 05--4 Hrbrt Gross Witr trm 05 www.iap.ui-a.d Prlimiary Schdul 0.0. Abrratios ad optimizatio Rptitio 7.0. Structural modificatios Zro oprads, ls splittig, ls additio,
Normal Form for Systems with Linear Part N 3(n)
 Applid Mathmatics 64-647 http://dxdoiorg/46/am7 Publishd Oli ovmbr (http://wwwscirporg/joural/am) ormal Form or Systms with Liar Part () Grac Gachigua * David Maloza Johaa Sigy Dpartmt o Mathmatics Collg
Applid Mathmatics 64-647 http://dxdoiorg/46/am7 Publishd Oli ovmbr (http://wwwscirporg/joural/am) ormal Form or Systms with Liar Part () Grac Gachigua * David Maloza Johaa Sigy Dpartmt o Mathmatics Collg
Osmium doping of UAl 2. Department of Physics and Engineering Greenville, SC Department of Physics Gainesville, FL
 Osmium doping of UAl 2 T. D. Scott 1,2, D. J. Burntt 2, J. S. Kim 2, and G. R. Stwart 2 1 Bob Jons Univrsity Dpartmnt of Physics and Enginring Grnvill, SC 29614 2 Univrsity of Florida Dpartmnt of Physics
Osmium doping of UAl 2 T. D. Scott 1,2, D. J. Burntt 2, J. S. Kim 2, and G. R. Stwart 2 1 Bob Jons Univrsity Dpartmnt of Physics and Enginring Grnvill, SC 29614 2 Univrsity of Florida Dpartmnt of Physics
Thermodynamical insight on the role of additives in shifting the equilibrium between white and grey tin
 hrmodynamical insight on th rol of additivs in shifting th quilibrium btwn whit and gry tin Nikolay Dmntv Dpartmnt of Chmistry, mpl Univrsity, Philadlphia, PA 19122 Abstract In this study mthods of statistical
hrmodynamical insight on th rol of additivs in shifting th quilibrium btwn whit and gry tin Nikolay Dmntv Dpartmnt of Chmistry, mpl Univrsity, Philadlphia, PA 19122 Abstract In this study mthods of statistical
Bayesian Test for Lifetime Performance Index of Exponential Distribution under Symmetric Entropy Loss Function
 Mathmatics ttrs 08; 4(): 0-4 http://www.scicpublishiggroup.com/j/ml doi: 0.648/j.ml.08040.5 ISSN: 575-503X (Prit); ISSN: 575-5056 (Oli) aysia Tst for iftim Prformac Idx of Expotial Distributio udr Symmtric
Mathmatics ttrs 08; 4(): 0-4 http://www.scicpublishiggroup.com/j/ml doi: 0.648/j.ml.08040.5 ISSN: 575-503X (Prit); ISSN: 575-5056 (Oli) aysia Tst for iftim Prformac Idx of Expotial Distributio udr Symmtric
