Prediction of the Antagonistic Activity of Aryl Benzyl Ethers against LTD4 by Using 3D-CoMFA Model Developed with Pranlukast Analogues
|
|
|
- Warren Thornton
- 5 years ago
- Views:
Transcription
1 3D-CoMFA Study of LTD4 Antagonist Bull. Korean Chem. Soc. 2006, Vol. 27, No Prediction of the Antagonistic Activity of Aryl Benzyl Ethers against LTD4 by Using 3D-CoMFA Model Developed with Pranlukast Analogues Jinyoung Kim, 1 Miryung Lee, 2 Seock-yong Kang, 2 Jina Park, 1 Yoongho Lim, 1,3 Dongsoo Koh, 4 Kwan Ha Park, 5 and Youhoon Chong 1,2,* 1 Division of Bioscience and Biotechnology, 2 Department of Molecular Biotechnology 3 Bio/Molecular Informatics Center, Konkuk University, Seoul , Korea. * chongy@konkuk.ac.kr 4 Department of Applied Chemistry, Dongduk Women's University, Seoul , Korea 5 Department of Marine Biomedical Sciences, Kunsan National University, Chonbuk , Korea Received May 16, 2006 A 3D-CoMFA model with pranlukast analogues was constructed, which could be applied to predict the antagonistic activity of aryl benzyl ether analogues against LTD4. Molecular modeling and 3D-CoMFA studies were performed on 78 pranlukast analogues and 14 aryl benzyl ethers to evaluate the antagonistic behavior of aryl benzyl ethers and provide information for further modification of this kind of compounds. The aryl benzyl ether core was found to be in excellent three dimensional match with the central planar moiety of pranlukast analogues, and the pranlukast 3D-CoMFA model could be successfully applied to predict the biological activity of aryl benzyl ether analogues. Key Words : 3D-CoMFA, QSAR, Pranlukast, Aryl benzyl ether Introduction In spite of the availability of effective and relatively cheap treatments, approximately 5% of asthmatic patients remain poorly controlled. Treatment with combination inhalers, which contain a corticosteroid and long-acting adrenoceptor agoinst, is the most effective current therapy, but the poor compliance has yet to be improved with oral therapy given once daily. However, the problem of systemic side effects conferred by oral therapy necessitates the development of oral drugs to specifically treat asthma that do not have effects on normal physiological mechanisms. There is therefore a search for new therapies, particularly safe and effective oral treatments and those that are more efficacious in severe asthma. Antileukotrienes (montelukast, pranlukast and zafirlukast, Fig. 1) were the first new class of anti-asthma treatment to be introduced in 30 years. 1 Owing to their anti-inflammatory properties, antileukotrienes have been the primary therapeutics in asthma management for several years. Although antileukotrienes have had some clinical success in asthma, they are considerably less effective and more expensive than inhaled corticosteroids. 2 In previous studies, 3 as a continuation of our efforts to Figure 1. Structures of pranlukast, montelukast, zafirlukast, and FPL The central planar triene-like moiety is shown bold.
2 1026 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 7 Jinyoung Kim et al. Figure 3. Superposition of pranlukast (dark) and aryl benzyl ether (3, light). Figure 2. Structures of aryl benzyl ethers. develop new classes of therapeutic agents for asthma, we demonstrated that aryl benzyl ethers (Fig. 2) show interesting biological activity as leukotriene D4 (LTD4) antagonists. The promising initial assay result warrants extensive structure-activity relationship study of the aryl benzyl ethers with various substituents at the aromatic rings. However, the structure-activity relationship study has been hampered due to the large number of possible combinations of substituents on both aromatic rings. Thus, in order to make the synthetic work simple and focused, a model system which can be used to predict the effects of the aromatic substituents on the activity of aryl benzyl ethers was required. In this study, we constructed a 3D-CoMFA model with pranlukast analogues, which could be applied to predict the antagonistic activity of aryl benzyl ether analogues against LTD4. The aim of CoMFA is to derive a correlation between the biological activity of a set of molecules and their 3D shape, electrostatic and hydrogen bonding characteristics. This correlation is derived from a series of superimposed conformations, one for each molecule in the set. These conformations are presumed to be the biologically active structures, overlaid in their common binding mode. Each conformation is taken in turn, and the molecular fields around it are calculated. Thus, a good alignment is the single most important part of doing a CoMFA analysis. The common substructure should have the same conformation in all molecules, and other parts should be superimposed as much as possible by adjusting internal torsional angles. Even though the three existing classes of antileukotriene drugs (montelukast, 4 pranlukast 5 and zafirlukast 6 ) have originated from different sources, they have been eventually modified by incorporating structural components present in FPL and/or leukotriene (Fig. 1). Therefore, it is of no surprise that antileukotrienes have a planar triene-like moiety with flanking hydrophobic chain and acidic head (Fig. 1). In order to find the similar triene-like moiety in aryl benzyl ether, its lowest energy conformation was investigated by Grid search around the three rotatory bonds bridging two aromatic rings at the center of the molecule. Interestingly, the energy minimized conformation of aryl benzyl ether showed perfect match with the antileukotrienes around the central planar region. In particular, pranlukast and aryl benzyl ether could be superimposed atom by atom, which allowed three-dimensional structure-activity relationship study with these two series of compounds (Fig. 3). At first, 78 pranlukast analogues were collected from the literature (15-92, Fig. 4). 4a They were then randomly divided into two groups: 69 compounds as training set and the other 9 compounds as test set (Table 1). The training set was used to build 3D-CoMFA models with CoMFA (comparative molecular field analysis) 7 methods, while the test set was used to validate the 3D-CoMFA model. The model was further validated using an external test set of 14 aryl benzyl ethers (Fig. 2). Finally, the contour plots of CoMFA were analyzed to provide helpful information on how to improve the biological activity of aryl benzyl ether derivatives by structural modifications. Materials and Methods All calculations were carried out on a linux enterprise operating system using molecular modeling software package SYBYL v 7.2. All compounds were constructed by the Sketch module in SYBYL base, protonated and assigned with MMFF94s charges. For more flexible compounds such as aryl benzyl ethers, systematic searches were performed with an interval of 10 o on every rotatory bonds to ensure their lowest energy conformations. Finally, they were minimized with MMFF94s force field. The most crucial step in performing CoMFA is to determine the bioactive conformations of the compounds so that all compounds could be aligned together. As discussed above, the central planar regions of the pranlukast analogues and aryl benzyl ethers were used as the common substructure for alignment. The most active compound among the series, pranlukast (41) was used as a template for structural alignment from the alignment facility in SYBYL, and 69 training set molecules, 9 test set molecules and 14 external aryl benzyl ether test set molecules were all aligned together (Fig. 5). As usual, PLS (partial least squares) method was used to establish and validate CoMFA. The IC 50 values were converted into pic 50 (-logic 50 ) values to describe the biological activities. CoMFA was set at standard values, with a sp 3
3 3D-CoMFA Study of LTD4 Antagonist Bull. Korean Chem. Soc. 2006, Vol. 27, No Figure 4. Structures of pranlukast analogues. a para-substituted unless otherwise mentioned. carbon atom with one positive charge used to probe steric and electrostatic fields. The standard cutoff value was set to 30 kcal/mol. LOO (leave-one-out) cross-validation method was used to evaluate the initial model. The cross-validated coefficient q 2 was calculated using the following equation: q 2 = 1.0 γ γ pred γ ( γ ) 2 actual ( γ γ actual mean) 2 Figure 5. Superposition of 69 training set molecules, 9 test set molecules, and 14 external test set molecules (aryl benzyl ethers). where γ pred, γ actual, and γ mean are predicted, actual, and mean values of the target property (pic 50 ), respectively, and PRESS = Σ γ (γ pred γ actual ) 2 is the sum of predictive sum of squares. The optimum number of components was then
4 1028 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 7 Jinyoung Kim et al. Table 1. CoMFA analysis on pranlukast analogues PRESS q 2 N r 2 SEE F SEEbs q 2 bs Pred. r a given, and CoMFA model was finally derived corresponding to the optimum number. The parameters of confidence intervals were further estimated by bootstrap in 10 runs. The column filtering box was kept unchecked during all operations (Table 1). In addition to LOO method to validate the CoMFA model, two test sets made up of 9 and 14 molecules, respectively, was used for model validation. Similar to cross-validated q 2 values of LOO method, the predictive performance of models on the test set was estimated by predictive r 2 value (Table 1). Results and Discussion b q 2 -leave one out (LOO) cross-validated correlation coefficient, N- optimum number of components, r 2 -noncross-validated correlation coefficient, SEE-standard error of estimate, F-F-test value, SEEbsstandard error of estimate by boot strapping analysis, q 2 bs-mean r 2 by boot strapping analysis (in 10 runs), Pred. r 2 -CoMFA predictive q 2 values on the test set: a test set composed of 9 pranlukast analogues, b external test set composed of 14 aryl benzyl ethers. The best prediction was obtained with CoMFA standard model (PRESS =3.91, q 2 = 0.845, N = 5), and its predictive performance on the test was r 2 = 0.971, which indicated that the built 3D-CoMFA model was reliable and able to predict biological activity of new derivatives accurately (Table 1). The relative contribution of steric and electrostatic field to the overall CoMFA field is and 0.358, respectively, which indicates dominant contribution by the steric factor. Also, the predictive r 2 values for both test sets were greater than 0.5, which indicates significant predictive power of the model. The conventional fit values on training set and prediction values on the test set made by the CoMFA model is shown in Table 2. The relationship curve between observed values versus conventional fit values (prediction values) on the training set and two test sets are also displayed in Fig. 6. CoMFA result is visualized by stdev*coeff contours, which shows that there is a large green contour around the phenyl ring at the end of the hydrophobic alkyl chain (Fig. 7a). Interestingly, the alkyl chain has a discrete length for optimum activity, and thus, even a slightly longer chain results in unfavorable steric interaction (yellow contour next to green one). Also, the small yellow contours around the alkyl chain indicate that branched alkyl chains would have unfavorable steric interaction. On the other hand, around the tetrazole moiety electrostatic interaction is favored (blue contour, Fig. 7b). The fused rings are sandwiched between two red contours, which show that the fused rings should remain planar between these two contours. The small blue contour around the aromatic alkyl substituent indicates that substitution of the alkyl chain with charged heteroatoms Table 2. Actual versus predicted activity of CoMFA (standard) model on the training set and test set Compds pic a 50 pic 50 Res Compds pic 50 pic 50 (act) b (pred) c (act) b (pred) c (a) Training set Res (b) Test set d (c) External test set e a pic 50 = logic 50; b actual activity; c predicted activity; d test set of 9 pranlukast analogues; e test set of 14 aryl benzyl ethers
5 3D-CoMFA Study of LTD4 Antagonist Bull. Korean Chem. Soc. 2006, Vol. 27, No Figure 6. Plot of observed pic 50 versus conventional fit predictions (predicted activity) of training set (a) and test set (b). Gray dot show conventional fit (prediction) of aryl benzyl ethers. Figure 7. CoMFA (standard model) Contour plots: pranlukast (41) is depicted as a reference molecule: (a) light contours predict bulky group enhance activity, whereas dark contours predict less bulky group is favored for activity; (b) light contours predict negative charge enhance activity, whereas dark contours predict positive charge enhance activity; (c) pranlukast (41) and 1 are shown together inside the combined map of steric and electrostatic contours; (d) aryl benzyl ether with pranlukast-like substitution is located inside the contour map. such as oxygen, or nitrogen would favor electrostatic interaction. Aryl benzyl ether 1 is located inside the combined map of steric and electrostatic contours with pranlukast (Fig. 7c), which provides insights into the structural modification of the aryl benzyl ether to improve its activity. As an example, a compound with simple introduction of the aromatic substituents of pranlukast (41) into the aryl benzyl ether core (Fig. 7d) snuggly fits into the contour map, and the QSAR program expects this compound to be even more active than pranlukast (predicted pic 50 for this compound is 10.83). Based on this information, various alkyl chains with optimum chain length and fused aromatic rings with polar substituents are being designed and installed onto the aryl benzyl ether core structure. The extensive structure-activity relationship study of aryl benzyl ethers as a novel class of antileukotriene would be reported in due course. Conclusions In summary, even though leukotriene modifiers have been used for asthma treatment for several years, problems associated with the current therapy such as the lack of clinical efficacy for severe asthma and the lack of specific LTB4 antagonists 8 have continuously prompted the discovery of novel leukotriene modifiers. Due to the promising biological activity as leukotriene D4 (LTD4) antagonists, 3,8 extensive structure-activity relationship study of the aryl benzyl ethers with various substituents at the aromatic rings are underway. In this study, in order to design an efficient structure-activity relationship study of aryl benzyl ethers, a 3D-CoMFA model was constructed by using the pranlukast analogues. The aryl benzyl ether core was found to be in excellent three dimensional match with the central planar moiety of pranlukast analogues, and the pranlukast 3D-
6 1030 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 7 Jinyoung Kim et al. CoMFA model could be successfully applied to predict the biological activity of aryl benzyl ether analogues. The 3D- CoMFA study also shows that hydrophobic alkyl chain with optimum length substituted at the benzylic aromatic ring as well as highly charged fused aromatic moiety at the other side of aryl benzyl ether core would greatly enhance the biological activity of aryl benzyl ether analogues. Acknowledgments. This work was supported by a grant Biogreen 21 (Korea Ministry of Agriculture and Forestry) and the second Brain Korea 21 (Korea Ministry of Education). Jinyoung Kim is supported by the second Brain Korea 21. References 1. Drazen, J. M.; Israel, E.; O Byme, P. M. N. Engl. J. Med. 1999, 340, Barnes, P. J. Curr. Opin. Pharmacol. 2003, 3, (a) Koh, D.; Park, K. H.; Lee, H.; Jung, J.; Cho, S. K.; Lim, Y. Agric. Chem. Biotechnol. 2000, 43, 277. (b) Koh, D.; Park, K. H.; Lee, H.; Jung, J.; Kim, K.; Cho, S. K.; Lim, Y. Agric. Chem. Biotechnol. 2000, 43, 281. (c) Koh, D.; Park, K. H.; Lim, Y. Agric. Chem. Biotechnol. 2001, 44, 35. (d) Koh, D.; Park, K. H.; Lee, H.; Jung, J.; Lim, Y. Agric. Chem. Biotechnol. 2001, 44, (a) Nakai, H.; Konno, M.; Kosuge, S.; Sakuyama, S.; Toda, M.; Arai, Y.; Obata, T.; Katusbe, N.; Miyamoto, T.; Okegawa, T.; Kawasaki, A. J. Med. Chem. 1988, 31, 84. (b) Taniguchi, Y.; Tamura, G.; Honma, M.; Aizawa, T.; Maruyama, N.; Shirato, K.; Takishima, T. J. Allergy Clin. Immunol. 1993, 92, (a) Bernstein, P. R.; Bird, T. G.; Brewster, A. G. In Burger s Medicinal Chemistry and Drug Discovery; Wolff, M. E., Ed.; Wiley: New York, 1997; Vol. 5, pp (b) Jones, T. R.; Labelle, M.; Belley, M.; Champion, E.; Charette, L.; Evans, J.; Ford-Hutchinson, A. W.; Gauthier, J.-Y.; Lord, A.; Masson, P.; McAuliffe, M.; McFarlane, C. S.; Metters, K. M.; Pickett, C.; Piechuta, H.; Rochette, C.; Rodger, I. W.; Sawyer, N.; Young, R. N.; Zamboni, R.; Abraham, W. M. Can. J. Physiol. Pharmacol. 1995, 73, (a) Brown, F. J.; Yee, Y. K.; Cronk, L. A.; Hebbel, K. C.; Krell, R. D.; Snyder, D. W. J. Med. Chem. 1990, 33, (b) Yee, Y. K.; Brown, F. J.; Hebbel, K. C.; Cronk, L. A.; Snyder, D. W.; Krell, R. D. Ann. N. Y. Acad. Sci. 1988, 524, (a) Cramer, R. D., III; Patterson, D. E.; Bunce, J. D. J. Am. Chem. Soc. 1988, 110, (b) Lee, D. Y.; Hyun, K. H.; Park, H. Y.; Lee, K. A.; Lee, B.-S.; Kim, C. K. Bull. Korean Chem. Soc. 2006, 27, 273. (c) Myung, P.-K.; Park, K.-Y.; Sung, N.-D. Bull. Korean Chem. Soc. 2005, 26, Ando, K.; Tsuji, E.; Ando, Y.; Kunitomo, J.-I.; Kobayashi, R.; Yokomizo, T.; Shimizu, T.; Yamashita, M.; Ohta, S.; Nabe, T.; Kohno, S.; Ohishi, Y. Org. Biomol. Chem. 2005, 3, 2129.
Ligand-based QSAR Studies on the Indolinones Derivatives Bull. Korean Chem. Soc. 2004, Vol. 25, No
 Ligand-based QSAR Studies on the Indolinones Derivatives Bull. Korean Chem. Soc. 2004, Vol. 25, No. 12 1801 Ligand-based QSAR Studies on the Indolinones Derivatives as Inhibitors of the Protein Tyrosine
Ligand-based QSAR Studies on the Indolinones Derivatives Bull. Korean Chem. Soc. 2004, Vol. 25, No. 12 1801 Ligand-based QSAR Studies on the Indolinones Derivatives as Inhibitors of the Protein Tyrosine
3D-QSAR Studies on Angiotensin-Converting Enzyme (ACE) Inhibitors: a Molecular Design in Hypertensive Agents
 952 Bull. Korean Chem. Soc. 2005, Vol. 26, No. 6 Amor A. San Juan and Seung Joo Cho 3D-QSAR Studies on Angiotensin-Converting Enzyme (ACE) Inhibitors: a Molecular Design in Hypertensive Agents Amor A.
952 Bull. Korean Chem. Soc. 2005, Vol. 26, No. 6 Amor A. San Juan and Seung Joo Cho 3D-QSAR Studies on Angiotensin-Converting Enzyme (ACE) Inhibitors: a Molecular Design in Hypertensive Agents Amor A.
T. J. Hou, Z. M. Li, Z. Li, J. Liu, and X. J. Xu*,
 1002 J. Chem. Inf. Comput. Sci. 2000, 40, 1002-1009 Three-Dimensional Quantitative Structure-Activity Relationship Analysis of the New Potent Sulfonylureas Using Comparative Molecular Similarity Indices
1002 J. Chem. Inf. Comput. Sci. 2000, 40, 1002-1009 Three-Dimensional Quantitative Structure-Activity Relationship Analysis of the New Potent Sulfonylureas Using Comparative Molecular Similarity Indices
Self-Organizing Molecular Field Analysis on a New Series of COX-2 Selective Inhibitors: 1,5-Diarylimidazoles
 Int. J. Mol. Sci. 2006, 7, 220-229 International Journal of Molecular Sciences ISSN 1422-0067 2006 by MDPI www.mdpi.org/ijms/ Self-Organizing Molecular Field Analysis on a New Series of COX-2 Selective
Int. J. Mol. Sci. 2006, 7, 220-229 International Journal of Molecular Sciences ISSN 1422-0067 2006 by MDPI www.mdpi.org/ijms/ Self-Organizing Molecular Field Analysis on a New Series of COX-2 Selective
5.1. Hardwares, Softwares and Web server used in Molecular modeling
 5. EXPERIMENTAL The tools, techniques and procedures/methods used for carrying out research work reported in this thesis have been described as follows: 5.1. Hardwares, Softwares and Web server used in
5. EXPERIMENTAL The tools, techniques and procedures/methods used for carrying out research work reported in this thesis have been described as follows: 5.1. Hardwares, Softwares and Web server used in
3D QSAR analysis of quinolone based s- triazines as antimicrobial agent
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.4, No.3, pp 1096-1100, July-Sept 2012 3D QSAR analysis of quinolone based s- triazines as antimicrobial agent Ramesh
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.4, No.3, pp 1096-1100, July-Sept 2012 3D QSAR analysis of quinolone based s- triazines as antimicrobial agent Ramesh
ISSN: ; CODEN ECJHAO E-Journal of Chemistry 2009, 6(3),
 ISS: 0973-4945; CODE ECJHAO E- Chemistry http://www.e-journals.net 2009, 6(3), 651-658 Comparative Molecular Field Analysis (CoMFA) for Thiotetrazole Alkynylacetanilides, a on-ucleoside Inhibitor of HIV-1
ISS: 0973-4945; CODE ECJHAO E- Chemistry http://www.e-journals.net 2009, 6(3), 651-658 Comparative Molecular Field Analysis (CoMFA) for Thiotetrazole Alkynylacetanilides, a on-ucleoside Inhibitor of HIV-1
Keywords: anti-coagulants, factor Xa, QSAR, Thrombosis. Introduction
 PostDoc Journal Vol. 2, No. 3, March 2014 Journal of Postdoctoral Research www.postdocjournal.com QSAR Study of Thiophene-Anthranilamides Based Factor Xa Direct Inhibitors Preetpal S. Sidhu Department
PostDoc Journal Vol. 2, No. 3, March 2014 Journal of Postdoctoral Research www.postdocjournal.com QSAR Study of Thiophene-Anthranilamides Based Factor Xa Direct Inhibitors Preetpal S. Sidhu Department
Nonlinear QSAR and 3D QSAR
 onlinear QSAR and 3D QSAR Hugo Kubinyi Germany E-Mail kubinyi@t-online.de HomePage www.kubinyi.de onlinear Lipophilicity-Activity Relationships drug receptor Possible Reasons for onlinear Lipophilicity-Activity
onlinear QSAR and 3D QSAR Hugo Kubinyi Germany E-Mail kubinyi@t-online.de HomePage www.kubinyi.de onlinear Lipophilicity-Activity Relationships drug receptor Possible Reasons for onlinear Lipophilicity-Activity
A 3D-QSAR Study of Celebrex-based PDK1 Inhibitors Using CoMFA Method
 Journal of the Chinese Chemical Society, 2009, 56, 59-64 59 A 3D-QSAR Study of Celebrex-based PDK1 Inhibitors Using CoMFA Method Wen-Hung Wang a ( ),N.R.Jena a ( ), Yi-Ching Wang b ( ) and Ying-Chieh Sun
Journal of the Chinese Chemical Society, 2009, 56, 59-64 59 A 3D-QSAR Study of Celebrex-based PDK1 Inhibitors Using CoMFA Method Wen-Hung Wang a ( ),N.R.Jena a ( ), Yi-Ching Wang b ( ) and Ying-Chieh Sun
Structural biology and drug design: An overview
 Structural biology and drug design: An overview livier Taboureau Assitant professor Chemoinformatics group-cbs-dtu otab@cbs.dtu.dk Drug discovery Drug and drug design A drug is a key molecule involved
Structural biology and drug design: An overview livier Taboureau Assitant professor Chemoinformatics group-cbs-dtu otab@cbs.dtu.dk Drug discovery Drug and drug design A drug is a key molecule involved
Drug Design 2. Oliver Kohlbacher. Winter 2009/ QSAR Part 4: Selected Chapters
 Drug Design 2 Oliver Kohlbacher Winter 2009/2010 11. QSAR Part 4: Selected Chapters Abt. Simulation biologischer Systeme WSI/ZBIT, Eberhard-Karls-Universität Tübingen Overview GRIND GRid-INDependent Descriptors
Drug Design 2 Oliver Kohlbacher Winter 2009/2010 11. QSAR Part 4: Selected Chapters Abt. Simulation biologischer Systeme WSI/ZBIT, Eberhard-Karls-Universität Tübingen Overview GRIND GRid-INDependent Descriptors
Molecular Modeling Study of Some Anthelmintic 2-phenyl Benzimidazole-1- Acetamides as β-tubulin Inhibitor
 Sawant et al : Molecular Modeling Study of Some Anthelmintic 2-phenyl Benzimidazole-1-Acetamides as -tubulin Inhibitor 1269 International Journal of Drug Design and Discovery Volume 5 Issue 1 January March
Sawant et al : Molecular Modeling Study of Some Anthelmintic 2-phenyl Benzimidazole-1-Acetamides as -tubulin Inhibitor 1269 International Journal of Drug Design and Discovery Volume 5 Issue 1 January March
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability
 Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
Using Bayesian Statistics to Predict Water Affinity and Behavior in Protein Binding Sites. J. Andrew Surface
 Using Bayesian Statistics to Predict Water Affinity and Behavior in Protein Binding Sites Introduction J. Andrew Surface Hampden-Sydney College / Virginia Commonwealth University In the past several decades
Using Bayesian Statistics to Predict Water Affinity and Behavior in Protein Binding Sites Introduction J. Andrew Surface Hampden-Sydney College / Virginia Commonwealth University In the past several decades
Ping-Chiang Lyu. Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University.
 Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Statistical concepts in QSAR.
 Statistical concepts in QSAR. Computational chemistry represents molecular structures as a numerical models and simulates their behavior with the equations of quantum and classical physics. Available programs
Statistical concepts in QSAR. Computational chemistry represents molecular structures as a numerical models and simulates their behavior with the equations of quantum and classical physics. Available programs
Description of Molecules with Molecular Interaction Fields (MIF)
 Description of Molecules with Molecular Interaction Fields (MIF) Introduction to Ligand-Based Drug Design Chimica Farmaceutica 2 Reduction of Dimensionality into Few New Highly Informative Entities -----
Description of Molecules with Molecular Interaction Fields (MIF) Introduction to Ligand-Based Drug Design Chimica Farmaceutica 2 Reduction of Dimensionality into Few New Highly Informative Entities -----
Quinazolinone Derivatives as Growth Hormone Secretagogue Receptor Inhibitors: 3D-QSAR study
 International Journal of ChemTech Research CODE (USA): IJCRGG, ISS: 0974-4290, ISS(Online):2455-9555 Vol.9, o.05 pp 896-903, 2016 Quinazolinone Derivatives as Growth Hormone Secretagogue Receptor Inhibitors:
International Journal of ChemTech Research CODE (USA): IJCRGG, ISS: 0974-4290, ISS(Online):2455-9555 Vol.9, o.05 pp 896-903, 2016 Quinazolinone Derivatives as Growth Hormone Secretagogue Receptor Inhibitors:
Mohmed et al., IJPSR, 2016; Vol. 7(3): E-ISSN: ; P-ISSN:
 IJPSR (2016), Vol. 7, Issue 3 (Research Article) Received on 23 September, 2015; received in revised form, 05 November, 2015; accepted, 17 December, 2015; published 01 March, 2016 3D-QSAR STUDY OF BENZOTHIAZOLE
IJPSR (2016), Vol. 7, Issue 3 (Research Article) Received on 23 September, 2015; received in revised form, 05 November, 2015; accepted, 17 December, 2015; published 01 March, 2016 3D-QSAR STUDY OF BENZOTHIAZOLE
Molecular docking, 3D-QSAR studies of indole hydrazone as Staphylococcus aureus pyruvate kinase inhibitor
 World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
Characterization of Binding Mode for Human Coagulation Factor XI (FXI) Inhibitors
 1212 Bull. Korean Chem. Soc. 2013, Vol. 34, No. 4 Jae Eun Cho et al. http://dx.doi.org/10.5012/bkcs.2013.34.4.1212 Characterization of Binding Mode for Human Coagulation Factor XI (FXI) Inhibitors Jae
1212 Bull. Korean Chem. Soc. 2013, Vol. 34, No. 4 Jae Eun Cho et al. http://dx.doi.org/10.5012/bkcs.2013.34.4.1212 Characterization of Binding Mode for Human Coagulation Factor XI (FXI) Inhibitors Jae
* Author to whom correspondence should be addressed; Tel.: ; Fax:
 Int. J. Mol. Sci. 2011, 12, 946-970; doi:10.3390/ijms12020946 OPEN ACCESS Article International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Structural Determination of Three
Int. J. Mol. Sci. 2011, 12, 946-970; doi:10.3390/ijms12020946 OPEN ACCESS Article International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Structural Determination of Three
International Journal of Research and Development in Pharmacy and Life Sciences. Review Article
 International Journal of Research and Development in Pharmacy and Life Sciences Available online at http//www.ijrdpl.com October - November, 2012, Vol. 1, No.4, pp 167-175 ISSN: 2278-0238 Review Article
International Journal of Research and Development in Pharmacy and Life Sciences Available online at http//www.ijrdpl.com October - November, 2012, Vol. 1, No.4, pp 167-175 ISSN: 2278-0238 Review Article
Applying Bioisosteric Transformations to Predict Novel, High Quality Compounds
 Applying Bioisosteric Transformations to Predict Novel, High Quality Compounds Dr James Chisholm,* Dr John Barnard, Dr Julian Hayward, Dr Matthew Segall*, Mr Edmund Champness*, Dr Chris Leeding,* Mr Hector
Applying Bioisosteric Transformations to Predict Novel, High Quality Compounds Dr James Chisholm,* Dr John Barnard, Dr Julian Hayward, Dr Matthew Segall*, Mr Edmund Champness*, Dr Chris Leeding,* Mr Hector
Hologram and Receptor-Guided 3D QSAR Analysis of Anilinobipyridine JNK3 Inhibitors
 3D QSAR Analysis of Anilinobipyridine JK3 Inhibitors Bull. Korean Chem. Soc. 2009, Vol. 30, o. 11 2739 Hologram and Receptor-Guided 3D QSAR Analysis of Anilinobipyridine JK3 Inhibitors Jae Yoon Chung,,
3D QSAR Analysis of Anilinobipyridine JK3 Inhibitors Bull. Korean Chem. Soc. 2009, Vol. 30, o. 11 2739 Hologram and Receptor-Guided 3D QSAR Analysis of Anilinobipyridine JK3 Inhibitors Jae Yoon Chung,,
Receptor Based Drug Design (1)
 Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
1. (18) Multiple choice questions. Please place your answer on the line preceding each question.
 CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
Organic Chemistry. Second Edition. Chapter 18 Aromatic Compounds. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 18 Aromatic Compounds Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 18.1 Introduction to Aromatic Compounds
Organic Chemistry Second Edition David Klein Chapter 18 Aromatic Compounds Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 18.1 Introduction to Aromatic Compounds
3D QSAR studies of Substituted Benzamides as Nonacidic Antiinflammatory Agents by knn MFA Approach
 INTERNATIONAL JOURNAL OF ADVANCES IN PARMACY, BIOLOGY AND CEMISTRY Research Article 3D QSAR studies of Substituted Benzamides as Nonacidic Antiinflammatory Agents by knn MFA Approach Anupama A. Parate*
INTERNATIONAL JOURNAL OF ADVANCES IN PARMACY, BIOLOGY AND CEMISTRY Research Article 3D QSAR studies of Substituted Benzamides as Nonacidic Antiinflammatory Agents by knn MFA Approach Anupama A. Parate*
Analogue and Structure Based Drug Designing of Prenylated Flavonoid Derivatives as PKB/Akt1 Inhibitors
 Available online at www.ijpcr.com International Journal of Pharmaceutical and Clinical esearch 2016; 8(8): 1205-1211 esearch Article ISS- 0975 1556 Analogue and Structure Based Drug Designing of Prenylated
Available online at www.ijpcr.com International Journal of Pharmaceutical and Clinical esearch 2016; 8(8): 1205-1211 esearch Article ISS- 0975 1556 Analogue and Structure Based Drug Designing of Prenylated
In silico pharmacology for drug discovery
 In silico pharmacology for drug discovery In silico drug design In silico methods can contribute to drug targets identification through application of bionformatics tools. Currently, the application of
In silico pharmacology for drug discovery In silico drug design In silico methods can contribute to drug targets identification through application of bionformatics tools. Currently, the application of
Overview. Descriptors. Definition. Descriptors. Overview 2D-QSAR. Number Vector Function. Physicochemical property (log P) Atom
 verview D-QSAR Definition Examples Features counts Topological indices D fingerprints and fragment counts R-group descriptors ow good are D descriptors in practice? Summary Peter Gedeck ovartis Institutes
verview D-QSAR Definition Examples Features counts Topological indices D fingerprints and fragment counts R-group descriptors ow good are D descriptors in practice? Summary Peter Gedeck ovartis Institutes
Identification of Active Ligands. Identification of Suitable Descriptors (molecular fingerprint)
 Introduction to Ligand-Based Drug Design Chimica Farmaceutica Identification of Active Ligands Identification of Suitable Descriptors (molecular fingerprint) Establish Mathematical Expression Relating
Introduction to Ligand-Based Drug Design Chimica Farmaceutica Identification of Active Ligands Identification of Suitable Descriptors (molecular fingerprint) Establish Mathematical Expression Relating
Characteristic Effects of 4,5-Disubstituted Pyridazin-3-one Derivatives with Various Functional Groups: Ab initio Study
 Structural and Electronic Effects Bull. Korean Chem. Soc. 2007, Vol. 28, No. 8 1363 Characteristic Effects of 4,5-Disubstituted Pyridazin-3-one Derivatives with Various Functional Groups: Ab initio Study
Structural and Electronic Effects Bull. Korean Chem. Soc. 2007, Vol. 28, No. 8 1363 Characteristic Effects of 4,5-Disubstituted Pyridazin-3-one Derivatives with Various Functional Groups: Ab initio Study
Molecular Graphics. Molecular Graphics Expt. 1 1
 Molecular Graphics Expt. 1 1 Molecular Graphics The study of organic chemistry has for more than a century and a half focussed on the relationship between the structure of an organic molecule (its three-dimensional
Molecular Graphics Expt. 1 1 Molecular Graphics The study of organic chemistry has for more than a century and a half focussed on the relationship between the structure of an organic molecule (its three-dimensional
CHAPTER 3. Vibrational Characteristics of PTP-1B Inhibitors
 CHAPTER 3 Vibrational Characteristics of PTP-1B Inhibitors 3.1 Preface Theoretical Frequency calculations were performed on the molecules chosen in this study. Initially all the geometries were optimized
CHAPTER 3 Vibrational Characteristics of PTP-1B Inhibitors 3.1 Preface Theoretical Frequency calculations were performed on the molecules chosen in this study. Initially all the geometries were optimized
Notes of Dr. Anil Mishra at 1
 Introduction Quantitative Structure-Activity Relationships QSPR Quantitative Structure-Property Relationships What is? is a mathematical relationship between a biological activity of a molecular system
Introduction Quantitative Structure-Activity Relationships QSPR Quantitative Structure-Property Relationships What is? is a mathematical relationship between a biological activity of a molecular system
Bioorganic & Medicinal Chemistry Letters
 Bioorganic & Medicinal Chemistry Letters 23 (2013) 5155 5164 Contents lists available at ciencedirect Bioorganic & Medicinal Chemistry Letters journal homepage: www.elsevier.com/locate/bmcl The discovery
Bioorganic & Medicinal Chemistry Letters 23 (2013) 5155 5164 Contents lists available at ciencedirect Bioorganic & Medicinal Chemistry Letters journal homepage: www.elsevier.com/locate/bmcl The discovery
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
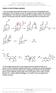 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
3D QSAR and Pharmacophore Modelling of Selected Benzimidazole Derivatives as Factor IXa Inhibitors
 Research Paper 3D QSAR and Pharmacophore Modelling of Selected Benzimidazole Derivatives as Factor IXa Inhibitors S. S. KUMBHAR*, P. B. CHOUDHARI AD M. S. BHATIA Drug Design and Development Group, Department
Research Paper 3D QSAR and Pharmacophore Modelling of Selected Benzimidazole Derivatives as Factor IXa Inhibitors S. S. KUMBHAR*, P. B. CHOUDHARI AD M. S. BHATIA Drug Design and Development Group, Department
Assignment 2: Conformation Searching (50 points)
 Chemistry 380.37 Fall 2015 Dr. Jean M. Standard September 16, 2015 Assignment 2: Conformation Searching (50 points) In this assignment, you will use the Spartan software package to investigate some conformation
Chemistry 380.37 Fall 2015 Dr. Jean M. Standard September 16, 2015 Assignment 2: Conformation Searching (50 points) In this assignment, you will use the Spartan software package to investigate some conformation
Studies of New Fused Benzazepine as Selective Dopamine D3 Receptor Antagonists Using 3D-QSAR, Molecular Docking and Molecular Dynamics
 Int. J. Mol. Sci. 2011, 12, 1196-1221; doi:10.3390/ijms12021196 OPEN ACCESS Article International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Studies of New Fused Benzazepine
Int. J. Mol. Sci. 2011, 12, 1196-1221; doi:10.3390/ijms12021196 OPEN ACCESS Article International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Studies of New Fused Benzazepine
Plan. Day 2: Exercise on MHC molecules.
 Plan Day 1: What is Chemoinformatics and Drug Design? Methods and Algorithms used in Chemoinformatics including SVM. Cross validation and sequence encoding Example and exercise with herg potassium channel:
Plan Day 1: What is Chemoinformatics and Drug Design? Methods and Algorithms used in Chemoinformatics including SVM. Cross validation and sequence encoding Example and exercise with herg potassium channel:
C. Correct! The abbreviation Ar stands for an aromatic ring, sometimes called an aryl ring.
 Organic Chemistry - Problem Drill 05: Drawing Organic Structures No. 1 of 10 1. What does the abbreviation Ar stand for? (A) Acetyl group (B) Benzyl group (C) Aromatic or Aryl group (D) Benzoyl group (E)
Organic Chemistry - Problem Drill 05: Drawing Organic Structures No. 1 of 10 1. What does the abbreviation Ar stand for? (A) Acetyl group (B) Benzyl group (C) Aromatic or Aryl group (D) Benzoyl group (E)
Interplay of hydrogen bonding and aryl-perfluoroaryl interactions in construction of supramolecular aggregates
 Interplay of hydrogen bonding and aryl-perfluoroaryl interactions in construction of supramolecular aggregates Katarzyna EICHSTAEDT Keywords: supramolecular chemistry, crystalengineering, Hydrogen bonding,
Interplay of hydrogen bonding and aryl-perfluoroaryl interactions in construction of supramolecular aggregates Katarzyna EICHSTAEDT Keywords: supramolecular chemistry, crystalengineering, Hydrogen bonding,
240 Chem. Aromatic Compounds. Chapter 6
 240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
Structure-Activity Modeling - QSAR. Uwe Koch
 Structure-Activity Modeling - QSAR Uwe Koch QSAR Assumption: QSAR attempts to quantify the relationship between activity and molecular strcucture by correlating descriptors with properties Biological activity
Structure-Activity Modeling - QSAR Uwe Koch QSAR Assumption: QSAR attempts to quantify the relationship between activity and molecular strcucture by correlating descriptors with properties Biological activity
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 01
 Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Exploring symmetry related bias in conformational data from the Cambridge Structural Database: A rare phenomenon?
 Exploring symmetry related bias in conformational data from the Cambridge Structural Database: A rare phenomenon? Aim To explore some well known cases where symmetry effects bias the distribution of conformational
Exploring symmetry related bias in conformational data from the Cambridge Structural Database: A rare phenomenon? Aim To explore some well known cases where symmetry effects bias the distribution of conformational
Hydrogen Bonding & Molecular Design Peter
 Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Coefficient Symbol Equation Limits
 1 Coefficient Symbol Equation Limits Squared Correlation Coefficient R 2 or r 2 0 r 2 N 1 2 ( Yexp, i Ycalc, i ) 2 ( Yexp, i Y ) i= 1 2 Cross-Validated R 2 q 2 r 2 or Q 2 or q 2 N 2 ( Yexp, i Ypred, i
1 Coefficient Symbol Equation Limits Squared Correlation Coefficient R 2 or r 2 0 r 2 N 1 2 ( Yexp, i Ycalc, i ) 2 ( Yexp, i Y ) i= 1 2 Cross-Validated R 2 q 2 r 2 or Q 2 or q 2 N 2 ( Yexp, i Ypred, i
Assignment 1: Molecular Mechanics (PART 1 25 points)
 Chemistry 380.37 Fall 2015 Dr. Jean M. Standard August 19, 2015 Assignment 1: Molecular Mechanics (PART 1 25 points) In this assignment, you will perform some molecular mechanics calculations using the
Chemistry 380.37 Fall 2015 Dr. Jean M. Standard August 19, 2015 Assignment 1: Molecular Mechanics (PART 1 25 points) In this assignment, you will perform some molecular mechanics calculations using the
Full Papers. 1 Introduction. Min Yang a, Lu Zhou a *, Zhili Zuo b *, Ricardo Mancera c, Hang Song a, Xiangyang Tang d, Xiang Ma a
 Docking Study and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Analyses of Min Yang a, Lu Zhou a *, Zhili Zuo b *, Ricardo Mancera c, Hang Song a, Xiangyang Tang d, Xiang Ma
Docking Study and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Analyses of Min Yang a, Lu Zhou a *, Zhili Zuo b *, Ricardo Mancera c, Hang Song a, Xiangyang Tang d, Xiang Ma
Assignment 1: Molecular Mechanics (PART 2 25 points)
 Chemistry 380.37 Fall 2015 Dr. Jean M. Standard September 2, 2015 Assignment 1: Molecular Mechanics (PART 2 25 points) In this assignment, you will perform some additional molecular mechanics calculations
Chemistry 380.37 Fall 2015 Dr. Jean M. Standard September 2, 2015 Assignment 1: Molecular Mechanics (PART 2 25 points) In this assignment, you will perform some additional molecular mechanics calculations
18.1 Intro to Aromatic Compounds
 18.1 Intro to Aromatic Compounds AROMATIC compounds or ARENES include benzene and benzene derivatives. Aromatic compounds are quite common. Many aromatic compounds were originally isolated from fragrant
18.1 Intro to Aromatic Compounds AROMATIC compounds or ARENES include benzene and benzene derivatives. Aromatic compounds are quite common. Many aromatic compounds were originally isolated from fragrant
Quiz QSAR QSAR. The Hammett Equation. Hammett s Standard Reference Reaction. Substituent Effects on Equilibria
 Quiz Select a method you are using for your project and write ~1/2 page discussing the method. Address: What does it do? How does it work? What assumptions are made? Are there particular situations in
Quiz Select a method you are using for your project and write ~1/2 page discussing the method. Address: What does it do? How does it work? What assumptions are made? Are there particular situations in
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
CHAPTER 6 QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIP (QSAR) ANALYSIS
 159 CHAPTER 6 QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIP (QSAR) ANALYSIS 6.1 INTRODUCTION The purpose of this study is to gain on insight into structural features related the anticancer, antioxidant
159 CHAPTER 6 QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIP (QSAR) ANALYSIS 6.1 INTRODUCTION The purpose of this study is to gain on insight into structural features related the anticancer, antioxidant
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
3D QSAR Analyses of Novel Tyrosine Kinase Inhibitors Based on Pharmacophore Alignment
 1032 J. Chem. Inf. Comput. Sci. 2001, 41, 1032-1040 3D QSAR Analyses of Novel Tyrosine Kinase Inhibitors Based on Pharmacophore Alignment L. L. Zhu, T. J. Hou, L. R. Chen, and X. J. Xu*, College of Chemistry
1032 J. Chem. Inf. Comput. Sci. 2001, 41, 1032-1040 3D QSAR Analyses of Novel Tyrosine Kinase Inhibitors Based on Pharmacophore Alignment L. L. Zhu, T. J. Hou, L. R. Chen, and X. J. Xu*, College of Chemistry
SAR. Structure - Activity Relationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) 2/28/2016
 Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Medicinal Chemistry and Chemical Biology
 Medicinal Chemistry and Chemical Biology Activities Drug Discovery Imaging Chemical Biology Computational Chemistry Natural Product Synthesis Current Staff Mike Waring Professor of Medicinal Chemistry
Medicinal Chemistry and Chemical Biology Activities Drug Discovery Imaging Chemical Biology Computational Chemistry Natural Product Synthesis Current Staff Mike Waring Professor of Medicinal Chemistry
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
3D-QSAR study on heterocyclic topoisomerase II inhibitors using CoMSIAy
 SAR and QSAR in Environmental Research Vol. 17, No. 2, April 2006, 121 132 3D-QSAR study on heterocyclic topoisomerase II inhibitors using CoMSIAy B. TEKINER-GULBAS, O. TEMIZ-ARPACI, I. YILDIZ*, E. AKI-SENER
SAR and QSAR in Environmental Research Vol. 17, No. 2, April 2006, 121 132 3D-QSAR study on heterocyclic topoisomerase II inhibitors using CoMSIAy B. TEKINER-GULBAS, O. TEMIZ-ARPACI, I. YILDIZ*, E. AKI-SENER
N-Arylhexahydropyrimidines. Electron impact mass spectrometry
 -rylhexahydropyrimidines. Electron impact mass spectrometry M. Beatriz García, Isabel. Perillo, and Liliana R. Orelli* Departamento de Química Orgánica, Facultad de Farmacia y Bioquímica. Junín 956, (1113)
-rylhexahydropyrimidines. Electron impact mass spectrometry M. Beatriz García, Isabel. Perillo, and Liliana R. Orelli* Departamento de Química Orgánica, Facultad de Farmacia y Bioquímica. Junín 956, (1113)
Substituent Effects on the Binding Energies of Benzyl Alcohol-H 2 O Clusters: Ab initio Study
 262 Bull. Korean Chem. Soc. 2002, Vol. 23, No. 2 Doo-Sik Ahn and Sungyul Lee Substituent Effects on the Binding Energies of Benzyl Alcohol-H 2 O Clusters: Ab initio Study Doo-Sik Ahn and Sungyul Lee *
262 Bull. Korean Chem. Soc. 2002, Vol. 23, No. 2 Doo-Sik Ahn and Sungyul Lee Substituent Effects on the Binding Energies of Benzyl Alcohol-H 2 O Clusters: Ab initio Study Doo-Sik Ahn and Sungyul Lee *
Marvin 5.4 A new generation of structure indexing at Elsevier. Dr. Michael Maier, Dr. Heike Nau, Elsevier
 Marvin 5.4 A new generation of structure indexing at Elsevier Dr. Michael Maier, Dr. Heike Nau, Elsevier Agenda Elsevier: Reaxys database Compound classes Structure requirements Marvin 5.4 Decision process
Marvin 5.4 A new generation of structure indexing at Elsevier Dr. Michael Maier, Dr. Heike Nau, Elsevier Agenda Elsevier: Reaxys database Compound classes Structure requirements Marvin 5.4 Decision process
Chapter 15. Free Radical Reactions
 Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
MEDICINAL CHEMISTRY I EXAM #1
 MEDICIAL CEMISTRY I EXAM #1 1 September 30, 2005 ame SECTI A. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 50 points
MEDICIAL CEMISTRY I EXAM #1 1 September 30, 2005 ame SECTI A. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 50 points
Synthesis and Reactivity of Vinyl Iodonium Salts
 Chemistry Honors Final Report Mackenzie Liebl Synthesis and Reactivity of Vinyl odonium Salts Background n Dr. Zhdankin s research lab, one of our main focuses is on the chemistry of iodine. odine is the
Chemistry Honors Final Report Mackenzie Liebl Synthesis and Reactivity of Vinyl odonium Salts Background n Dr. Zhdankin s research lab, one of our main focuses is on the chemistry of iodine. odine is the
Saba Al Fayoumi. Tamer Barakat. Dr. Mamoun Ahram + Dr. Diala Abu-Hassan
 1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Executive Summary. IP Owner Summary. Personal Description of Researcher. Market Feasibility. Key Technology Highlights. Trend & Partnership
 POSTECH Executive Summary Dr. Kimoon Kim, a professor of Postech, has researched supramolecular chemistry using cucurbituril and developed cucurbit[n]uril (CB[n], n=5-10) and functionalized CB[n] with
POSTECH Executive Summary Dr. Kimoon Kim, a professor of Postech, has researched supramolecular chemistry using cucurbituril and developed cucurbit[n]uril (CB[n], n=5-10) and functionalized CB[n] with
Analysis of a Large Structure/Biological Activity. Data Set Using Recursive Partitioning and. Simulated Annealing
 Analysis of a Large Structure/Biological Activity Data Set Using Recursive Partitioning and Simulated Annealing Student: Ke Zhang MBMA Committee: Dr. Charles E. Smith (Chair) Dr. Jacqueline M. Hughes-Oliver
Analysis of a Large Structure/Biological Activity Data Set Using Recursive Partitioning and Simulated Annealing Student: Ke Zhang MBMA Committee: Dr. Charles E. Smith (Chair) Dr. Jacqueline M. Hughes-Oliver
Spacer conformation in biologically active molecules*
 Pure Appl. Chem., Vol. 76, No. 5, pp. 959 964, 2004. 2004 IUPAC Spacer conformation in biologically active molecules* J. Karolak-Wojciechowska and A. Fruziński Institute of General and Ecological Chemistry,
Pure Appl. Chem., Vol. 76, No. 5, pp. 959 964, 2004. 2004 IUPAC Spacer conformation in biologically active molecules* J. Karolak-Wojciechowska and A. Fruziński Institute of General and Ecological Chemistry,
* Author to whom correspondence should be addressed; Tel.: ; Fax:
 Int. J. Mol. Sci. 2011, 12, 1807-1835; doi:10.3390/ijms12031807 OPEN ACCESS International Journal of Molecular Sciences Article ISSN 1422-0067 www.mdpi.com/journal/ijms Combined 3D-QSAR, Molecular Docking
Int. J. Mol. Sci. 2011, 12, 1807-1835; doi:10.3390/ijms12031807 OPEN ACCESS International Journal of Molecular Sciences Article ISSN 1422-0067 www.mdpi.com/journal/ijms Combined 3D-QSAR, Molecular Docking
Chapter 2: An Introduction to Organic Compounds
 Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Benzene a remarkable compound. Chapter 14 Aromatic Compounds. Some proposed structures for C 6 H 6. Dimethyl substituted benzenes are called xylenes
 Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Medicinal Chemistry/ CHEM 458/658 Chapter 4- Computer-Aided Drug Design
 Medicinal Chemistry/ CHEM 458/658 Chapter 4- Computer-Aided Drug Design Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Computer Aided Drug Design - Introduction Development
Medicinal Chemistry/ CHEM 458/658 Chapter 4- Computer-Aided Drug Design Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Computer Aided Drug Design - Introduction Development
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS
 COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
Aromatic Compounds I
 2302272 Org Chem II Part I Lecture 1 Aromatic Compounds I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 16 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 1 Aromatic Compounds I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 16 in Organic Chemistry, 8 th Edition, L.
Conformation Searching Applications
 Chemistry 380.37 Fall 2015 Dr. Jean M. Standard September 23, 2015 Conformation Searching Applications Cycloheptadecane Redux So what would a conformation search on cycloheptadecane using Spartan find?
Chemistry 380.37 Fall 2015 Dr. Jean M. Standard September 23, 2015 Conformation Searching Applications Cycloheptadecane Redux So what would a conformation search on cycloheptadecane using Spartan find?
Alkanes. Introduction
 Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Towards Physics-based Models for ADME/Tox. Tyler Day
 Towards Physics-based Models for ADME/Tox Tyler Day Overview Motivation Application: P450 Site of Metabolism Application: Membrane Permeability Future Directions and Applications Motivation Advantages
Towards Physics-based Models for ADME/Tox Tyler Day Overview Motivation Application: P450 Site of Metabolism Application: Membrane Permeability Future Directions and Applications Motivation Advantages
Exam. Name. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts)
 CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
3D QSAR and pharmacophore modeling studies on C-2 and C-4 substituted Quinazoline series as Anti-tubercular agents.
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (20):57-62 (http://scholarsresearchlibrary.com/archive.html) ISS 0975-5071 USA CODE: DPLEB4 3D
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (20):57-62 (http://scholarsresearchlibrary.com/archive.html) ISS 0975-5071 USA CODE: DPLEB4 3D
Molecular Interactions F14NMI. Lecture 4: worked answers to practice questions
 Molecular Interactions F14NMI Lecture 4: worked answers to practice questions http://comp.chem.nottingham.ac.uk/teaching/f14nmi jonathan.hirst@nottingham.ac.uk (1) (a) Describe the Monte Carlo algorithm
Molecular Interactions F14NMI Lecture 4: worked answers to practice questions http://comp.chem.nottingham.ac.uk/teaching/f14nmi jonathan.hirst@nottingham.ac.uk (1) (a) Describe the Monte Carlo algorithm
Structure-Based Drug Discovery An Overview
 Structure-Based Drug Discovery An Overview Edited by Roderick E. Hubbard University of York, Heslington, York, UK and Vernalis (R&D) Ltd, Abington, Cambridge, UK RSC Publishing Contents Chapter 1 3D Structure
Structure-Based Drug Discovery An Overview Edited by Roderick E. Hubbard University of York, Heslington, York, UK and Vernalis (R&D) Ltd, Abington, Cambridge, UK RSC Publishing Contents Chapter 1 3D Structure
Literature Review: Heterocyclic compounds are of particular interest in medicinal chemistry. The chemistry of heterocyclic compounds is one of the
 Literature Review: Heterocyclic compounds are of particular interest in medicinal chemistry. The chemistry of heterocyclic compounds is one of the most complex branches of chemistry. It is equally interesting
Literature Review: Heterocyclic compounds are of particular interest in medicinal chemistry. The chemistry of heterocyclic compounds is one of the most complex branches of chemistry. It is equally interesting
Saika Siddiqui, Bruce Gustafson, and Ramachandra, S. Hosmane*
 Third International Electronic Conference on Synthetic Organic Chemistry (ECSOC-3), www.mdpi.org/ecsoc-3.htm, September 1-30, 1999 [A0029] A New Synthetic Approach toward Ring-Expanded ("Fat") Purine Nucleobases:
Third International Electronic Conference on Synthetic Organic Chemistry (ECSOC-3), www.mdpi.org/ecsoc-3.htm, September 1-30, 1999 [A0029] A New Synthetic Approach toward Ring-Expanded ("Fat") Purine Nucleobases:
A Computational Screening Method in Deriving New Promising Explosive Molecules: ADD Method-1 and MS-HEMs
 A Computational Screening Method in Deriving New Promising Explosive Molecules: ADD Method-1 and MS-HEMs Soo Gyeong Cho, Eun Mee Goh, (ADD), Daejeon, South Korea 1 Research Purpose: Develop Better Warhead/Ammunition
A Computational Screening Method in Deriving New Promising Explosive Molecules: ADD Method-1 and MS-HEMs Soo Gyeong Cho, Eun Mee Goh, (ADD), Daejeon, South Korea 1 Research Purpose: Develop Better Warhead/Ammunition
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com Der Chemica Sinica, 2015, 6(11):1-6 ISSN: 0976-8505 CODEN (USA) CSHIA5 Computational study on the geometry optimization and excited-state properties triamterene
Available online at www.pelagiaresearchlibrary.com Der Chemica Sinica, 2015, 6(11):1-6 ISSN: 0976-8505 CODEN (USA) CSHIA5 Computational study on the geometry optimization and excited-state properties triamterene
CHCl (vinyl chloride, part of new car smell )
 ame Key 1) (20 points) Draw a Lewis dot structure for each of the following molecules; show all lone pairs. Indicate the hybridization at all atoms except hydrogen, chlorine, and fluorine. (a) 2 (b) C
ame Key 1) (20 points) Draw a Lewis dot structure for each of the following molecules; show all lone pairs. Indicate the hybridization at all atoms except hydrogen, chlorine, and fluorine. (a) 2 (b) C
Three Dimensional Quantitative Structure-Activity Relationship of 5H-Pyrido[4,3-b]indol-4-carboxamide JAK2 Inhibitors
![Three Dimensional Quantitative Structure-Activity Relationship of 5H-Pyrido[4,3-b]indol-4-carboxamide JAK2 Inhibitors Three Dimensional Quantitative Structure-Activity Relationship of 5H-Pyrido[4,3-b]indol-4-carboxamide JAK2 Inhibitors](/thumbs/85/91697299.jpg) Int. J. Mol. Sci. 2013, 14, 12037-12053; doi:10.3390/ijms140612037 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Three Dimensional Quantitative
Int. J. Mol. Sci. 2013, 14, 12037-12053; doi:10.3390/ijms140612037 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Three Dimensional Quantitative
Project I. Heterocyclic and medicinal chemistry
 Thesis projecten 2018-2019 onderzoeksgroep LOSA professor Wim Dehaen Project I. Heterocyclic and medicinal chemistry - Novel products with interesting biological properties In this line of research, novel
Thesis projecten 2018-2019 onderzoeksgroep LOSA professor Wim Dehaen Project I. Heterocyclic and medicinal chemistry - Novel products with interesting biological properties In this line of research, novel
SCULPT 3.0. Using SCULPT to Gain Competitive Insights. Brings 3D Visualization to the Lab Bench SPECIAL REPORT. 4 Molecular Connection Fall 1999
 SPECIAL REPORT SCULPT 3.0 Brings 3D Visualization to the Lab Bench ith the acquisition of Interactive Simulations, Inc., last April, MDL added to its arsenal of discovery informatics tools a powerful analysis
SPECIAL REPORT SCULPT 3.0 Brings 3D Visualization to the Lab Bench ith the acquisition of Interactive Simulations, Inc., last April, MDL added to its arsenal of discovery informatics tools a powerful analysis
