Stereocontrolled organocatalytic synthesis of prostaglandin PGF 2 in seven steps
|
|
|
- Sydney Hunt
- 5 years ago
- Views:
Transcription
1 Stereocontrolled organocatalytic synthesis of prostaglandin PGF 2 in seven steps Graeme Coulthard, William Erb, Varinder K. Aggarwal Nature. August 15, DI: /nature11411 N C 2 then [Bn 2 N 2 ][CF 3 C 2 ] C 2 Christopher Rosenker Wipf Group - Current Literature August 25, 2012
2 Biosynthetic Pathway to Prostaglandins Tyr cyclooxygenase archidonic acid 2 C 2 C 2 C peroxidase Tyr- C 2 C 2 C 2 C 2 C 2 PG 2 Rouzer, C. A.; Marnet, L. J. Chem. Rev. 2003, 103, 2239.
3 Biosynthetic Pathway to Prostaglandins PGJ 2 6-keto-PGF α C 2 PGD 2 Δ deoxy-PGJ 2 PGI 2 PGF 2 C 2 C 2 PGE 2 archidonic acid PG 2 TXA 2 PGC 2 PGA 2 Das, S.; Chandrasekhar, S.; Yadav, J. S.; Grée, R. Chem. Rev. 2007, 107, PGB 2
4 Biological Properties of Prostaglandins Implicated in a variety of biological processes: Sleep, pain, fever, inflammation, menstruation, birth, blood vessel constriction, and blood clotting Synthesized immediately in response to stimuli Lantanoprost is used for treatment of glaucoma $1.75 billion sales in step synthesis based on Corey s original synthetic strategy C 2 C 2 i-pr C 5 11 Ph Lantanoprost Coulthard, G.; Erb, W.; Aggarwal, V. K. Nature DI: /nature11411
5 Prostoglandin Synthesis and Biological Actions Funk, C. D.; Science 2001, 294, 1871.
6 Previous Prostaglandin Syntheses Wittig C 2 iodolactonization WE C 5 11 Ac C Me Ac Corey lactone Me Me Me Me Cl C Baeyer-Villiger oxidation Diels-Alder Corey: 17 steps; ~24% yield; >90% per step Corey, E. J.; Weinshenker, N. M.; Schaaf, T. K.; uber, W. J. Am. Chem. Soc. 1969, 91, Nicolaou, K. C.; Sorensen, E. J. Classics in Total Synthesis; VC: Weinheim, 1996, p
7 Previous Prostaglandin Syntheses Stork: 30 steps; 0.002% yield C-C bond formation C 5 11 C 2 Ts NC EE EE C 5 11 TBDPS lactonization C-C bond formation TBDPS C 2 Me Johnson ortho ester Claisen rearrangement Me α-d-glucose Stork, G.; Takahashi, T.; Kawamoto, I.; Suzuki, T. J. Am. Chem. Soc. 1978, 100, Nicolaou, K. C.; Sorensen, E. J. Classics in Total Synthesis; VC: Weinheim, 1996, p
8 Previous Prostaglandin Syntheses Stork: 11 steps; 15% yield; 84% per step Conjugate addition Alkylation C 2 C 5 11 C 5 11 Ph BM Conjugate addition Ph Noyori: 8 steps Alkylation C 2 C 2 Me C 5 11 C 5 11 TBS TBS TBS Conjugate addition Stork, G.; Isobe, M. J. Am. Chem. Soc.1975, 97, Stork G.; Isobe, M. J. Am. Chem. Soc. 1975, 97, Suzuiki, M.; Yanagisawa, A.; Noyori, R. J. Am. Chem. Soc. 1988, 110, 4718.
9 Previous Prostaglandin Syntheses Danishefsky: 8 steps Wittig Michael addition C 2 TBS C 2 Et Aldol TBS C 5 11 C 5 11 C 5 11 Ac Ac Ac Allylic transposition Danishefsky, S. J.; Cabal, M. P.; Chow, K. J. Am. Chem. Soc. 1989, 111, 3456.
10 Retrosynthesis of PGF 2 Wittig C 2 Conjugate addition FGI Aldol reaction EWG Coulthard, G.; Erb, W.; Aggarwal, V. K. Nature DI: /nature11411
11 Proline Catalyzed Aldol Dimerization ligomer aldol aldol - 2 N C 2 intramolecular aldol reaction and A A B B dehydration aldol aldol ligomer Coulthard, G.; Erb, W.; Aggarwal, V. K. Nature DI: /nature11411
12 Test Reactions for Aldol Reaction Me N C 2 (10 mol%) TF, rt Me 61%, 3.6:1 dr Me 3, Me catalyst (20 mol%) -78 C, Me 2 S TF, rt, 14 h Catalyst A: (S)-Proline - 5% Catalyst B: [Bn 2 N 2 ][C 2 CF 3 ] - 51% Coulthard, G.; Erb, W.; Aggarwal, V. K. Nature DI: /nature11411
13 Me Me Synthesis of PGF 2 2, 75 C (S)-proline (2 mol%) 4 h; then 115 C TF, rt, 20 h; 69% N Me then [Bn 2 N 2 ][C 2 CF 3 ] Me, amberlyst 15 MgS 4, C 2 Cl 2 (2 mol%), rt, 14 h 14 h, rt 65% per bond formation/ breaking step 14% (over two steps) 99:1 er; 15 g prepared from 110 g aldehyde Coulthard, G.; Erb, W.; Aggarwal, V. K. Nature DI: /nature11411
14 Synthesis of PGF 2 S Me Li 2 (NC)Cu C 5 11 TBS Me 3, C 2 Cl 2 :Me (3:1) Me TF; then TMSCl, Et 3 N TMS C 5 11 TBS -78 C, then NaB 4 (3 equiv.) -78 C to rt C 5 11 TBS 60% (over two steps) g 49% (over two steps) g 1.5 % aq. Cl:TF (3:2) BrPh 3 P C 2 C 2 rt, 16 h C 5 11 t-buk, TF, 0 C to rt 57% (over two steps) mg 47% (over two steps) g Coulthard, G.; Erb, W.; Aggarwal, V. K. Nature DI: /nature11411
15 Conclusion Seven step synthesis of prostaglandin from 2,5- dimethoxytetraydrofuran Total yield % (58-61% per step) Synthesis is shorter than the state of the art. Key organocatalytic aldol dimerization reaction in high enantiomeric purity (99:1 er) 4 bond forming/breaking reactions during process (~65% per rxn) Can be performed on +100 g scale Bicyclic enal is a useful intermediate for exploring prostaglandin derivatives. Me 4 steps R 1 R 2
Strategies for Stereocontrolled Synthesis
 Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Total Synthesis of the Sesquiterpenoid Periconianone A Based on a Postulated Biogenesis
 Total Synthesis of the Sesquiterpenoid Periconianone A Based on a Postulated Biogenesis C 3 C3 Liffert, R., Linden, A., Gademann, K. J. Am. Chem. Soc. 2017, 139, 16096 Presented by: Brendyn Smith CEM 852
Total Synthesis of the Sesquiterpenoid Periconianone A Based on a Postulated Biogenesis C 3 C3 Liffert, R., Linden, A., Gademann, K. J. Am. Chem. Soc. 2017, 139, 16096 Presented by: Brendyn Smith CEM 852
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
VINBLASTINE. H MeO 2 C MeO. OAc. CO 2 Me. Me H
 VIBLATIE 2 C 1 C 2 Ac a 3: catharanthine C 2 Ac C 2 2: ( )-vindoline xidation 5' 2 C 3' 16' 20' Ac C 2 1: (+)-vinblastine b 4 C 2 TFAA, -50 C Polonovski fragmentation 6' 5' 16' C 2 5 TFA 4' 3' 15' 16'
VIBLATIE 2 C 1 C 2 Ac a 3: catharanthine C 2 Ac C 2 2: ( )-vindoline xidation 5' 2 C 3' 16' 20' Ac C 2 1: (+)-vinblastine b 4 C 2 TFAA, -50 C Polonovski fragmentation 6' 5' 16' C 2 5 TFA 4' 3' 15' 16'
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Chiral Diol Promoted Boronates Addi3on Reac3ons. Lu Yan Morken Group Boston College
 Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang!
 1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
Synthesis of the Stenine Ring System from Pyrrole
 Current Literature Presentation gor psenica 06/18/2011 Synthesis of the Stenine Ring System from Pyrrole Bates, R. W.; Sridhar, S. J. rg. Chem., 2011, 76, 5026 5035 gor psenica @ Wipf Group Page 1 of 16
Current Literature Presentation gor psenica 06/18/2011 Synthesis of the Stenine Ring System from Pyrrole Bates, R. W.; Sridhar, S. J. rg. Chem., 2011, 76, 5026 5035 gor psenica @ Wipf Group Page 1 of 16
Back to Sugars: Enzymatic Synthesis
 Back to Sugars: Enzymatic Synthesis Zhensheng Ding ov. 04 orthrup, A. B.; M acm illan, D. W. C. Science 2004, 305, 1752 orthrup, A. B. and MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 6798-6799 orthrup,
Back to Sugars: Enzymatic Synthesis Zhensheng Ding ov. 04 orthrup, A. B.; M acm illan, D. W. C. Science 2004, 305, 1752 orthrup, A. B. and MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 6798-6799 orthrup,
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C-H Functionalization
 Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Lewis Base Activation of Lewis Acids: Development of a Lewis Base Catalyzed Selenolactonization
 Lewis Base Activation of Lewis Acids: Development of a Lewis Base Catalyzed Selenolactonization Denmark, S.E. and Collins, W.R. rg. Lett. 2007, 9, 3801-3804. C 2 H + Se Lewis Base CH 2 Cl 2 Se Presented
Lewis Base Activation of Lewis Acids: Development of a Lewis Base Catalyzed Selenolactonization Denmark, S.E. and Collins, W.R. rg. Lett. 2007, 9, 3801-3804. C 2 H + Se Lewis Base CH 2 Cl 2 Se Presented
Organocatalytic Umpolung via N- Heterocyclic Carbenes. Qinghe Liu Hu Group Meeting August 20 th 2015
 rganocatalytic Umpolung via N- Heterocyclic Carbenes Qinghe Liu Hu Group Meeting August 20 th 2015 Contents Part 1: Introduction Part 2: N-Heterocyclic carbene-catalyzed umpolung: classical umpolung, conjugated
rganocatalytic Umpolung via N- Heterocyclic Carbenes Qinghe Liu Hu Group Meeting August 20 th 2015 Contents Part 1: Introduction Part 2: N-Heterocyclic carbene-catalyzed umpolung: classical umpolung, conjugated
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Chemistry 432 Lecture Notes Updated: Spring Nucleophiles and Electrophiles: The Basis of Organic Chemistry
 Chemistry 432 Lecture Notes Updated: Spring 2016 Course Organization: Things You Need to Know 1. Named Reactions and Reagents 2. Vocabulary 3. Concepts 4. HOW TO DO SYNTHESIS Nucleophiles and Electrophiles:
Chemistry 432 Lecture Notes Updated: Spring 2016 Course Organization: Things You Need to Know 1. Named Reactions and Reagents 2. Vocabulary 3. Concepts 4. HOW TO DO SYNTHESIS Nucleophiles and Electrophiles:
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Large-Scale Synthesis of the Anti-Cancer Marine Natural Product (+)-Discodermolide
 61.7 g prepared in 39 steps 43 chemists worked on the project which lasted 20 months Chris Kendall @ Wipf Group 1 3/27/04 8 22 1 5 24 carbon linear polypropionate chain containing stereocenters (6 hydroxyl
61.7 g prepared in 39 steps 43 chemists worked on the project which lasted 20 months Chris Kendall @ Wipf Group 1 3/27/04 8 22 1 5 24 carbon linear polypropionate chain containing stereocenters (6 hydroxyl
(5) a. b. (6) N H CH 3 N H O H O (7) CH 3 O (8) OCH 3 2. explanation:
 ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
University of Bristol - Explore Bristol Research. Peer reviewed version. Link to published version (if available): /acs.orglett.
 Baars, H., Classen, M. J., & Aggarwal, V. K. (2017). Synthesis of Alfaprostol and PGF2 through 1,4-Addition of an Alkyne to an Enal Intermediate as the Key Step. Organic Letters, 19(21), 6008-6011. https://doi.org/10.1021/acs.orglett.7b03057
Baars, H., Classen, M. J., & Aggarwal, V. K. (2017). Synthesis of Alfaprostol and PGF2 through 1,4-Addition of an Alkyne to an Enal Intermediate as the Key Step. Organic Letters, 19(21), 6008-6011. https://doi.org/10.1021/acs.orglett.7b03057
Total Synthesis of the Proposed Structure of Briarellin J
 Total Synthesis of the Proposed Structure of Briarellin J Michael T. Crimmins, Mark C. Mans, and Abimael D. Rodríguez. rg. Lett. 2010, ASAP Ac Ac originally proposed structure revised structure Eric E.
Total Synthesis of the Proposed Structure of Briarellin J Michael T. Crimmins, Mark C. Mans, and Abimael D. Rodríguez. rg. Lett. 2010, ASAP Ac Ac originally proposed structure revised structure Eric E.
Classics in Total Synthesis
 Classics in Total Synthesis K. C. Nicolaou and E. J. Sorensen Targets, Strategies, Methods With a Foreword by E. J. Corey VCH Weinheim New York Basel Cambridge Tokyo Chapter 1 Introduction: Constructing
Classics in Total Synthesis K. C. Nicolaou and E. J. Sorensen Targets, Strategies, Methods With a Foreword by E. J. Corey VCH Weinheim New York Basel Cambridge Tokyo Chapter 1 Introduction: Constructing
Total Synthesis of (+)-Gelsemine. Xuan Dong group seminar 12/19/2012
 Total Synthesis of (+)-Gelsemine Xuan Dong group seminar 12/19/2012 Historical Background of Gelsemine First detected the presence of alk aloids in extracts of G. sempervir ens in 1870 by Wormley. 1876,
Total Synthesis of (+)-Gelsemine Xuan Dong group seminar 12/19/2012 Historical Background of Gelsemine First detected the presence of alk aloids in extracts of G. sempervir ens in 1870 by Wormley. 1876,
Total Synthesis of Maoecrystal V: Early-Stage of C-H Functionalization and Lactone
 Literature Report Total Synthesis of Maoecrystal V: arly-stage of C- Functionalization and Lactone Assembly by Radical Cyclization Reporter: Ji Yue Checker: Chen Muwang Date: 2013/12/02 Zakarian, A. et
Literature Report Total Synthesis of Maoecrystal V: arly-stage of C- Functionalization and Lactone Assembly by Radical Cyclization Reporter: Ji Yue Checker: Chen Muwang Date: 2013/12/02 Zakarian, A. et
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry.
 Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry. 1] Development of Asymmetric Catalysis 2] Total Synthesis of Natural products 3] Development of Novel Protecting-Group-Free
Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry. 1] Development of Asymmetric Catalysis 2] Total Synthesis of Natural products 3] Development of Novel Protecting-Group-Free
Rhenium-Catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage
 henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
Rhodium Catalyzed Hydroacylation
 Literature Report Changbin Yu 2012-12-04 检查 : 蔡先锋 Rhodium Catalyzed ydroacylation Vy M. Dong* Education h.d. California Institute of Technology, 2004 M.S. University of California at Berkeley, 2000 BS
Literature Report Changbin Yu 2012-12-04 检查 : 蔡先锋 Rhodium Catalyzed ydroacylation Vy M. Dong* Education h.d. California Institute of Technology, 2004 M.S. University of California at Berkeley, 2000 BS
Total Synthesis of ( )-Himandrine
 Total Synthesis of ( )-imandrine M. Movassaghi,* M. Tjandra and J. Qi J. Am. hem. Soc. 2009, 131, 9648-9650 Bz Adam T. oye urrent Literature ctober 3, 2009 Adam oye @ Wipf Group Page 1 of 22 10/3/2009
Total Synthesis of ( )-imandrine M. Movassaghi,* M. Tjandra and J. Qi J. Am. hem. Soc. 2009, 131, 9648-9650 Bz Adam T. oye urrent Literature ctober 3, 2009 Adam oye @ Wipf Group Page 1 of 22 10/3/2009
Literature Report. A 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed Dehydro Diels-Alder Reaction
 Literature Report 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed ehydro iels-lder Reaction Reporter: ong-qiang Shen Checker: Cong Liu ate: 2017/06/19 McGee, P.; Bétournay, G.; Barabé,
Literature Report 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed ehydro iels-lder Reaction Reporter: ong-qiang Shen Checker: Cong Liu ate: 2017/06/19 McGee, P.; Bétournay, G.; Barabé,
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
September [KV 804] Sub. Code: 3804
![September [KV 804] Sub. Code: 3804 September [KV 804] Sub. Code: 3804](/thumbs/89/97855699.jpg) September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Enantioselective Synthesis of Pactamycin, a Complex Antitumor Antibiotic
 Journal Club (3) Tomoya akamura Enantioselective Synthesis of Pactamycin, a Complex Antitumor Antibiotic Justin T. Malinowski, Robert J. Sharpe, Jeffrey S. Johnson Science 03, 30, 80 8.. Introduction -.
Journal Club (3) Tomoya akamura Enantioselective Synthesis of Pactamycin, a Complex Antitumor Antibiotic Justin T. Malinowski, Robert J. Sharpe, Jeffrey S. Johnson Science 03, 30, 80 8.. Introduction -.
CHEM 203. Final Exam December 18, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
A Highly Efficient Organocatalyst for Direct Aldol Reactions of Ketones and Aldehydes
 A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
Chemistry 3720, Spring 2004 Exam 3 Name:
 Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
CHAPTER 24 HW: CARBONYL CONDENSATIONS
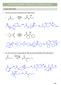 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
Total Synthesis of ( )-Virginiamycin M2
 Total Synthesis of ( )-Virginiamycin M2 Jie Wu and James S. Panek, Angewandte Chemie International Edition, 2010, 49, 6165-6168 btained from the CDC Public ealth Image Library. Image credit: CDC/Dr. David
Total Synthesis of ( )-Virginiamycin M2 Jie Wu and James S. Panek, Angewandte Chemie International Edition, 2010, 49, 6165-6168 btained from the CDC Public ealth Image Library. Image credit: CDC/Dr. David
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
[3,3]-Sigmatropic rearrangements
![[3,3]-Sigmatropic rearrangements [3,3]-Sigmatropic rearrangements](/thumbs/75/71504906.jpg) 1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon
 11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon Seth B. erzon, Liang Lu, Christina M. Woo, and Shivajirao L. Gholap J. Am. Chem. Soc. ASAP DI 10.1021/ja200034b Melissa Sprachman Current
11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon Seth B. erzon, Liang Lu, Christina M. Woo, and Shivajirao L. Gholap J. Am. Chem. Soc. ASAP DI 10.1021/ja200034b Melissa Sprachman Current
Palladium-Catalyzed Oxygenation of Unactivated sp 3 C-H Bonds
 Palladium-Catalyzed xygenation of Unactivated sp 3 C- Bonds Pd(Ac) 2 5 mol% PhI(Ac) 2 1.1 eq. Pd 2 Ac Desai, L. P.; ull, K. L.; Sanford *, M. S. University of Michigan J. Am. Chem. Soc. 2004, 126, ASAP
Palladium-Catalyzed xygenation of Unactivated sp 3 C- Bonds Pd(Ac) 2 5 mol% PhI(Ac) 2 1.1 eq. Pd 2 Ac Desai, L. P.; ull, K. L.; Sanford *, M. S. University of Michigan J. Am. Chem. Soc. 2004, 126, ASAP
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Nine-Step Enantioselective Total Synthesis of (+)-Minfiensine
 ine-step nantioselective Total Synthesis of (+)-Minfiensine Jones, S. B.; Simmons, B.; MacMillan, D. W. C.* J. Am. Chem. Soc. 2009, ASAP. DI: 10.1021/ja906472m Kara George Wipf Group - Current Literature
ine-step nantioselective Total Synthesis of (+)-Minfiensine Jones, S. B.; Simmons, B.; MacMillan, D. W. C.* J. Am. Chem. Soc. 2009, ASAP. DI: 10.1021/ja906472m Kara George Wipf Group - Current Literature
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes
 Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Hennoxazole A. Philip Williams Group Meeting December 12, OMe. OMe 1 6 O H
 ennoxazole A 1 6 11 17 24 Philip Williams Group eting December 12, 2007 Discovered Discovered by Paul cheuer at the University of awaii in 1991. Isolated 480mg ennoxazole A from 4.5kg from the sponge Polyfibrospongia
ennoxazole A 1 6 11 17 24 Philip Williams Group eting December 12, 2007 Discovered Discovered by Paul cheuer at the University of awaii in 1991. Isolated 480mg ennoxazole A from 4.5kg from the sponge Polyfibrospongia
Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates
 Pure & Appl. Chern., Vol. 70, No. 5, pp. 11 17-1 122, 1998. Printed in Great Britain. (0 1998 IUPAC Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates Mogens
Pure & Appl. Chern., Vol. 70, No. 5, pp. 11 17-1 122, 1998. Printed in Great Britain. (0 1998 IUPAC Metal-catalyzed asymmetric hetero-diels-alder reactions of unactivated dienes with glyoxylates Mogens
When something goes wrong. Goya: Mother showing her derformed child to two women Louvre, Paris
 1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
Organic Chemistry Curriculum Content Outline
 Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
CHO. OMe. endo. xylene, 140 o C, 2 h 70% 1. CH 2 (OMe) 2, MeOH TsOH, rt 2. Bu 2 O, 1,2-dichloroethane 140 o C, 2 h 3. 6 M HCl, THF, rt 44%
 VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
Concise Total Synthesis of Variocolortides A and B through an Unusual Hetero-Diels- Alder Reaction!
 Concise Total Synthesis of Variocolortides A and B through an Unusual etero-diels- Alder Reaction! Christian A. Kuttruff, endrik Zipse, and Dirk Trauner! ACIE Early View, DI: 10.1002/anie.201006154! Marija
Concise Total Synthesis of Variocolortides A and B through an Unusual etero-diels- Alder Reaction! Christian A. Kuttruff, endrik Zipse, and Dirk Trauner! ACIE Early View, DI: 10.1002/anie.201006154! Marija
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Convergent Route to ent-kaurane Diterpenoids: Total Synthesis of Lungshengenin D and 1α6α- Diacetoxy-ent-kaura-9(11),16-dien- 12,15-dione
 Convergent Route to ent-kaurane Diterpenoids: Total Synthesis of Lungshengenin D and 1α6α- Diacetoxy-ent-kaura-9(11),16-dien- 12,15-dione Xiangbo Zhao, Wu Li, Junjie Wang, and Dawei Ma* Shanghai Institute
Convergent Route to ent-kaurane Diterpenoids: Total Synthesis of Lungshengenin D and 1α6α- Diacetoxy-ent-kaura-9(11),16-dien- 12,15-dione Xiangbo Zhao, Wu Li, Junjie Wang, and Dawei Ma* Shanghai Institute
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction Mechanisms. Group Meeting Aaron Bailey 12 May 2009
 Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Diels Alder cycloaddition
 I p1.1.1 The Diels Alder Cycloaddition Reaction in the Context of Domino Processes J. G. West and E. J. Sorensen The Diels Alder cycloaddition has been a key component in innumerable, creative domino transformations
I p1.1.1 The Diels Alder Cycloaddition Reaction in the Context of Domino Processes J. G. West and E. J. Sorensen The Diels Alder cycloaddition has been a key component in innumerable, creative domino transformations
Branched-Regioselective Hydroformylation with Catalytic Amounts of a Reversibly Bound Directing Group
 1/12 Branched-egioselective ydroformylation with Catalytic Amounts of a eversibly Bound Directing Group h(i)/me C/ 2 MS 4A branched major by Christian U. Grünanger and Bernhard Breit Angew. Chem. Int.
1/12 Branched-egioselective ydroformylation with Catalytic Amounts of a eversibly Bound Directing Group h(i)/me C/ 2 MS 4A branched major by Christian U. Grünanger and Bernhard Breit Angew. Chem. Int.
Asymmetric Lewis Base Strategies for Heterocycle Synthesis
 Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Nickel-Catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides
 ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
A New Strategy for Efficient Synthesis of Medium and Large Ring Lactones without High Dilution or Slow Addition
 A ew Strategy for Efficient Synthesis of Medium and Large ing Lactones without High Dilution or Slow Addition BF 3 Et 2 TIPS 2,4,6-collidine n + n+2 ' ' Zhao, W.; Li, Z; Sun, J. J. Am. Chem. Soc. 2013
A ew Strategy for Efficient Synthesis of Medium and Large ing Lactones without High Dilution or Slow Addition BF 3 Et 2 TIPS 2,4,6-collidine n + n+2 ' ' Zhao, W.; Li, Z; Sun, J. J. Am. Chem. Soc. 2013
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
No Title. Li HUANG August 07, 2008
 o Title Li UAG August 07, 2008 utline Bioisostere and Isostere Report on the possibility of thioureas catalyzed Claisen rearrangement Isosteres are DefiniIon of isostere molecules or ions with the same
o Title Li UAG August 07, 2008 utline Bioisostere and Isostere Report on the possibility of thioureas catalyzed Claisen rearrangement Isosteres are DefiniIon of isostere molecules or ions with the same
Highly Cytotoxic, Structure Similar Polyke>des CO 2 H
 Current Literature Anguinomycins and Deriva2ves: Total Syntheses, Modeling, and Biological Evalua2on of the Inhibi2on of Nucleocytoplasmic Transport liv Eidam, Ulrike Kutay, Karl Gadermann, et al, JACS,
Current Literature Anguinomycins and Deriva2ves: Total Syntheses, Modeling, and Biological Evalua2on of the Inhibi2on of Nucleocytoplasmic Transport liv Eidam, Ulrike Kutay, Karl Gadermann, et al, JACS,
I. Liu Lab. Ka<e Boknevitz 1
 A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
TMSCl imidazole DMF. Ph Ph OTMS. Michael reaction. Michael reaction Ph R 3. epoxidation O R
 eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
Hirsutene and Δ 9(12) Capnellene. D. P. Curran (1986) Presenter: Mehdi Moemeni
 irsutene and Δ 9(12) Capnellene D. P. Curran (1986) Presenter: Mehdi Moemeni Back Ground of Radical Structure In a modern context the first organic free radical identified was triphenylmethyl radical.
irsutene and Δ 9(12) Capnellene D. P. Curran (1986) Presenter: Mehdi Moemeni Back Ground of Radical Structure In a modern context the first organic free radical identified was triphenylmethyl radical.
Nucleophilic Heterocyclic Carbene Catalysis. Nathan Werner Denmark Group Meeting September 22 th, 2009
 Nucleophilic Heterocyclic Carbene Catalysis Nathan Werner Denmark Group Meeting September 22 th, 2009 Thiamine Thiamine Vitamin B 1 The first water-soluble vitamin described Is naturally synthesized by
Nucleophilic Heterocyclic Carbene Catalysis Nathan Werner Denmark Group Meeting September 22 th, 2009 Thiamine Thiamine Vitamin B 1 The first water-soluble vitamin described Is naturally synthesized by
Enolates, Enols, and Enamines Part 3
 Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
ISOCHRYSOHERMIDIN. MeO 2 C. MeO. MeO. MeO 2 C OH. Me 1
 ISCRYSERMIDI 2 C 2 C 1 ormal demand Diels-Alder (M diene - LUM dienophile ) eutral Diels-Alder Inverse demand Diels-Alder (LUM diene - M dienophile ) EDG EWG EWG EDG LUM LUM LUM M E 1 E 2 E 3 M M E Scheme
ISCRYSERMIDI 2 C 2 C 1 ormal demand Diels-Alder (M diene - LUM dienophile ) eutral Diels-Alder Inverse demand Diels-Alder (LUM diene - M dienophile ) EDG EWG EWG EDG LUM LUM LUM M E 1 E 2 E 3 M M E Scheme
Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α-amino Acid Derivatives
 Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Suggested solutions for Chapter 34
 s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Spiro Monophosphite and Monophosphoramidite Ligand Kit
 Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides
 Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
OC IV: Organic Photochemistry Exercise 1 Exercise class Page 1 of 11. Exercise 1: Fundamentals, H-Abstraction reactions
 C IV: rganic otochemistry Exercise 1 Exercise class Page 1 of 11 Exercise 1: Fundamentals, -Abstraction reactions 1. Draw the energy potential curve of the ground state and the first two excited states
C IV: rganic otochemistry Exercise 1 Exercise class Page 1 of 11 Exercise 1: Fundamentals, -Abstraction reactions 1. Draw the energy potential curve of the ground state and the first two excited states
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Total Synthesis of Oxazolomycin A
 Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Short Literature 8/9/10
 Short Literature 8/9/10 Reiter, M.; Torssell, S.; Lee, S.; MacMillan, D. W. C. The organocatalytic three-step total synthesis of ()-frondosin B Chem. Sci. 2010, 37-42. Spangler, J. E.; Carson, C. A.; Sorensen,
Short Literature 8/9/10 Reiter, M.; Torssell, S.; Lee, S.; MacMillan, D. W. C. The organocatalytic three-step total synthesis of ()-frondosin B Chem. Sci. 2010, 37-42. Spangler, J. E.; Carson, C. A.; Sorensen,
Overview of Synthesizing Merrilactone A
 verview of Synthesizing Merrilactone A = Contents = I. Beginning II. Danishefsky's Route III. irama & Inoue's Route IV. Frontier's Route V. Conclusion 6th / Feb./ 2008 Literature Seminar ~ B4 part ~ Takafumi
verview of Synthesizing Merrilactone A = Contents = I. Beginning II. Danishefsky's Route III. irama & Inoue's Route IV. Frontier's Route V. Conclusion 6th / Feb./ 2008 Literature Seminar ~ B4 part ~ Takafumi
CHEM 330. Final Exam December 8, 2010 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
Direct Oxidative Heck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans
 Direct xidative eck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans by Zhang,.; Ferreira, E. M.; Stoltz, B. M. Angewandte
Direct xidative eck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans by Zhang,.; Ferreira, E. M.; Stoltz, B. M. Angewandte
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
David W.C. MacMillan: Career-in-Review. Yan Xu Dong Group Meeting Jan. 2, 2014
 David W.C. MacMillan: Career-in-Review Yan Xu Dong Group Meeting Jan. 2, 2014 David W.C. MacMillan: A Brief Introduction Career 1968 Born in Bellshill, Scotland. 1987-1991 Undergraduate degree in chemistry
David W.C. MacMillan: Career-in-Review Yan Xu Dong Group Meeting Jan. 2, 2014 David W.C. MacMillan: A Brief Introduction Career 1968 Born in Bellshill, Scotland. 1987-1991 Undergraduate degree in chemistry
Synthetic Methodology. Using Tertiary Phosphines. as Nucleophilic Catalysts
 Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
