A Theoretical Study for the Structure of Propranolol and Some Fluorinated Derivatives
|
|
|
- Daniella Sutton
- 5 years ago
- Views:
Transcription
1 Structural Chemistry, Vol. 6, Nos. 4/5, 1995 A Theoretical Study for the Structure of Propranolol and Some Fluorinated Derivatives Zarreen H. Farooqi 1 and Hassan Y. Aboul-Enein 2'3 Received September 6, 1994; revised January 5, 1995; accepted January 11, 1995 This paper reports on studies of the theoretical geometrical structure of propranolol and three of its fluorinated derivatives: l-(2,2,2-trifluoroethylamino)-3-(l-naphthyloxy)-2-propanol [trifluoroethyl-propranolol], l-(2,2,3,3,3-pentafluoropropylamino)-3-(1-naphthyloxy)-2-propanol [pentafluoropropyl-pmpranolol], and l-(2,2,3,3,4,4,4-heptafluorobutylamino)-3-(l-naphthyloxy)-2- propanol [heptafluorobutyl-propranolol]. The semiempirical method AM 1 was used to optimize the structures. In the minimum energy state the geometries of the naphthyl moiety and the nonfluorinated portions of the analogs are quite similar to that of the parent. Dipole moments, charge density distributions, and electrostatic potential distributions all point to the significance of the ether oxygen in all four compounds and the increasing contribution of the side-chain terminal to the activity of the molecule with increasing number of fluorines. KEY WORDS: Propranolol; fluorinated analogue; molecular models; semiempificaltechniques; fl-adrenergic blocke~. INTRODUCTION Propranolol, chemically known as 1-isopropylamino-3-(1-naphthyloxy)-2-propanol (see Fig. la) is the model parent drug for nonselective/~-blockers, a "pure" antagonist of catecholamines at the receptor sites. It has been used (i) to chronically lower blood pressure in (mild to moderate) hypertension, (ii) to prevent reflex tachycardia in severe hypertension, (iii) to reduce intraocular pressure in glaucomatous eyes, (iv) to reduce the frequency of anginal episodes and improve exercise tolerance in many patients with angina, (v) in the acute phase of a myocardial infarction to limit infarct size (a controversial use), (vi) in the treatment of both supraventricular and ventricular arrhythmias, (vii) to increase stroke i Department of Biomedical Statistics and Scientific Computing, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia. 2Department of Biological and Medical Research, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia. 3 Correspondence should be directed to Hassan Y. Aboul-Enein, BMR, MBC 03, King Faisal Specialist Hospital and Research Centre, Riyadh 11211, Kingdom of Saudi Arabia. volume in obstructive cardiomyopathy patients, (viii) to inhibit peripheral conversion of thyroxine to triiodothyronine (besides H-blockade), (ix) to reduce the frequency and intensity of migraine headaches, (x) to reduce somatic manifestations of anxiety and (xi) to treat alcohol withdrawal [1]. The principal toxicities of propranolol result from the blockade of cardiac, vascular, or bronchial /3-adrenoceptors. Most important predictable extensions are in patients with reduced myocardial reserve, asthma, peripheral vascular insufficiency, and diabetes. Some patients experience a withdrawal syndrome when discontinued after a long use. The manifestations of this are anxiety, tachycardia, increased intensity of angina, or increase in blood pressure [1]. The side effects of propranolol are not desired and a cardioselective H-blocker would be of great importance, not only in terms of specificity but also to reduce the dosage and thus toxicity. Aboul-Enein [2] recently reviewed the effect of introducing fluorine to medicinal agents and its influence in enhancing the pharmacological activity on these therapeutic agents. In search for compounds with better activity, some fluorinated derivatives of propranolol have been synthesized, the structures of three of which are given in Figs. lb and ld, namely, 1-(2,2,2-349 I040-04(0/95/I / Plenum Publishing Corporation
2 350 Com Dound Propranolol, la lb lc H ~ t e ~ - Propnma~, ld 19, '~2 ~1o9~ 2~2s 29'30 n "R R1 To C18 on the mdn stnctum 3S To C1tl on the n~ h n 33733~3(~ Toeleon~w~,~s To C1tl on the main structure F ToC16onthem~n~n~tum ~ 311 F 36 ~0 3~_r_ F42,f R2 4o H(34) H(34) H(34) Fig. 1. The chemical structure of: propranolol (la), trifiuoroethylpropranolol (lb), pentafluoropropyl-propranolol (lc), heptafluorobutyl-propranolol (ld). The schematic illustrates the common structure together the numbers of the atoms. The table below the schematic gives the side chains RI and R2 in the four molecules. trifluoroethylamino)-3-(1-naphthyloxy)-2-propanol (lb, known as trifluoroethyl-propranolol), 1-(2,2,3,3,3-pentafluoropropylamino)-3-(1-naphthyloxy)-2-propanol (lc, known as pentafluoropropyl-propranolol), and 1-(2,2, 3,3,4,4,4 - heptafl uorobutylamino) ( 1 - naphthyloxy) - 2-propanol (ld, known as heptafluorobutyl-propranolol). The synthesis of these fluorinated derivatives and the results of their pharmacological activity are presented elsewhere. This paper only describes the computed atomic structure of propranolol and these three fluorinated analogs. This is the first time that their structural geometry is being described. There have been publications describing the synthesis (for example, Ref. 3) and the pharmacological activity of drugs (for example, Ref. 4) that have side chains composed of the "(CF2)nCF3" (n >_ 0) group. There seems to be very little work done describing the electronic structure of organic compounds derived by using these side chains or an effect of fluorine on the molecular structure. What is known is that (i) replacing hydrogen by fluorine as in CF 3 (compared to CH3) can Farooqi and AbouI-Enein increase the lipid solubility and hence increase the rate of transport of biologically active compounds across lipid membranes [4]; and (ii) CF 2 is isopolar and isosteric with O and may be used to increase the stability of a drug by replacing an O at a biochemically labile position [4]. METHODS The four structures above were created using HyperChem for Windows [5] and optimized with a semiempirical technique, namely AM 1. The Polak-Ribiere conjugate gradient method was used for optimization. The minimum energy states (with minimum binding energy) that were achieved were as in Table I. RESULTS AND DISCUSSION Geometries The geometrical coordinates of propranolol together with the charges on each atom are presented in Table II. Similar information about the fluorinated derivatives is given in Tables III to V. All the coordinates and distances in this paper are in angstroms (.~,). Except where significant the particulars of the hydrogen atoms are not given here, and can be obtained from the authors. The naphthyl moiety in all the compounds is fiat. The bond angles of the naphthyl group are all approximately 120 ~ each with minimal torsion within the rings. All the other bond angles range between 105 ~ and 125 ~. In propranolol, the side chain zigzags around an axis in the plane of the naphthyl group (view a in Fig. 2). If the molecule is turned to view it from a side so that the naphthyl moiety becomes a straight line (view b in Fig. 2), the side chain is also more or less a straight line with its axis making an angle of approximately ~ with the plane of the naphthyl group. Keeping the rings fiat and viewing the molecule such that the side chain goes into the plane of the paper perpendicularly (view c in Fig. 2), the bond with O17 atom (oxygen of the hydroxyl group) makes an angle of about -63 ~ and the bond of the C34 atom an angle of about 121 ~ with the plane of the rings. In lb, the side chain zigzags in a similar way as in view a (Fig. 2) of the parent and its axis makes an angle of approximately ~ with the plane of the rings (in a view similar to view b of Fig. 2). While, when the side chain projects perpendicularly into the paper plane (like view c in Fig. 2) the bond with
3 i Computed Structure of Propranolol and Some Fluorinated Analogs 351 Table I. Optimized Energies of the Molecules. S.Nr Compound Ionization Heat of potential formation Min. binding (ev) kcal/mol) energy (kcal/mol) Gradient (kcal/mol/,~) Total energy (kcal/mol) Propranolol (la) Trifluoroethyl-propranolol (lb) Pentafluoropropyl-propranolol (lc) Heptafluorobutyl-propranolol (ld) O17 atom makes about 66 ~ the C36 atom an angle of about 45.5 ~ and the bond with C37 atom an angle of about ~ with the plane of the rings. In lc, zigzagging of the nonfluorinated portion of the side chain is similar to the parent (like view a in Fig. 2) and its axis makes an angle of approximately ~ with the plane of the rings (like in view b of Fig. 2). The --CF 3 group projects almost perpendicularly to the rest of the side chain (i.e., the nonfluorinated portion) in the direction opposite to that of the O17 atom. When viewed from the side with the side chain going perpendicularly into the paper plane (like in view c of Fig. 2) the bond with the O17 atom makes an angle of almost -118 ~ the bond of the C35 atom of about ~ and the bond of C37 atom of almost 115 ~ with the plane of the rings. In ld, the zigzagging of the nonfluorinated portion is similar to the parent (like in view a of Fig. 2) and the axis of the nonflourinated portion of the side chain makes an angle of about ~ with the plane of the rings (like Table 1I. Coordinates of Propranolol (la). # Atom (e-) (A,) (A) (A) 1 c c -0, c -0, C c C c c C o c C c c N C O C C in view b of Fig. 2), the first portion of the fluorinated region of the side chain (i.e., C33--C37 link) is at about 93 ~ to the nonfluorinated region of the side chain and the second region (i.e., C37--C39 link) is at about ~ to the first link (both of these in view b). In a view similar to view c of Fig. 2, the bond of the O17 atom makes an angle of about 67 ~ with the plane of the rings, the bond of C35 atom an angle of about 52 ~ and the bond of C37 atom of about -60 ~ In all four compounds the oxygen of the hydroxyl group (O17 atom) makes almost a fight angle to the side chain in the side view (-90 ~ in propranolol and lc and +90 ~ in lb and ld, respectively, in a view similar to view b of Fig. 2). There were mostly small changes in the geometry of the molecules with the introduction of the fluorine atoms, except near the side-chain terminus. The geom- Table IlL Coordinates of Trifluoroethyl-Propranolol (lb). # Atom (e-) (,~) (A,) (,~) 1 C C C C C C C C C C O C C C N C O C H F F F
4 352 Farooqi and Aboul-Enein Table IV. Coordinates of Pentafluoropropyl-Propranolol (lc). # Atom (e-) (A) (A) (A) 1 C C C C C C I C C C C O C C C N C O C H F F C F F F etries are illustrated in Fig. 3 with the molecules displayed along their longitudinal axes together with charges on individual atoms. The most important bond lengths, bond angles and the torsion angles of propranolol and the fluorinated analogs may be obtained from the supplementary material. Molecular Volumes The sizes of the four molecules are not very different. In fact, there is a reduction in size from the parent to the first derivative (--CF3). The dimensions of the molecule boxes together with the molecular volumes for the four molecules are as follows: propranolol A, 3, trifluoroethyl propranolol A, 3, pentafluoropropyl-propranolol ~3, and heptafluorobutyl propranolol A, 3. Energies The various energies (total energies, minimum binding energies, heats of formation, ionization potentials) relevant for the molecules are given in Table I. From the data presented above, it appears that the influence of the fluorine atoms is primarily at the terminal in Table V. Coordinates of Heptafluorobutyl-Propranolol (ld). # Atom (e-) (A) (A) (A) 1 C C C C C C C C C C O C C C N C O C H F F C F C F F F F ! Fig. 2. Propranolol viewed perpendicular to its (a) longitudinal (primary or 1") axis, (b) secondary (2 ~ axis, and (c) tertiary (3*) axis. 3
5 Computed Structure of Propranolol and Some Fluorinated Analogs 353 <tls7 - o.. s ~ -o.alw -o.in Ii ~ ;.,.- "*' la.~.i~ / The heat of formation of the CF 2 increment group may be estimated from the various compounds given in Table 1-1 of Ref. 6, and turns out to be in the range of kcal/mol to kcal/mol, with a mean of kcal/mol. From the same table [6] heat of formation attributable to CF 3 may be estimated to be approximately -160 kcal/mol. Given the heat of formation of propranolol (-58 kcal/mol), the estimated heats of formation for the three derivatives from these figures come out to be -218 kcal/mol, -321 kcal/mol and -424 kcal/mol which are not significantly different from the respective values obtained from the AM1 calculations (given in Table I) lb 127 ~.0.1S2 "%...,,.a~.o~.a.~a,..,,~" " ~/.a.f~... ~; I; ~.om.:oeo a,~ ~ yso "~ '~t ~*~ lc 9 0. fs7. O. 0 f ~ ~ f 3 7 ~a,1 'm.o.al~ Fig. 3. The atomic charges and the structure of (a) propranolol, (b) trifluoroethyl-propranolol, (c) pentafluoropmpyl-propranolol, and (d) heptafluorobutyl-propranolol. the geometry of the molecules. With each addition of the CF2 group the nonfluorinated portion of the side chain rotates about 180 ~ about its axis. In their stable configurations the molecules are in the following order with respect to the values of the energies: for binding energies: lb > la > lc > ld; for total energies: la > lb > le > ld; and for heat of formation: la > lb > le > ld. For the values of the energies see the tables. The ionization potentials of the four compounds discussed in this paper are almost the same (they differ in the second decimal place). As far as is known to the authors there does not seem to be a precedence of this in other series of polyfluoroalkyl amines. ld Dipole Moments The molecules do exhibit a sudden increase in the dipole moment from Debyes to Debyes when the two terminal methyl groups are replaced one by a hydrogen and the other by a --CF 3 group in lb. With further addition of fluorines there is a further increase in the dipole moment (to and then to Debyes), but not as dramatic. This simple measure indicates a significant redistribution of charge density. To quantify this change further, charge distributions and electrostatic potentials were studied and are discussed below. Charge Distributions The changes in the charges distribution in the four compounds involve the ether oxygen (O11) and all the terminal fluorines. In propranolol most of the charge is concentrated on the ether oxygen. In lb, the charge is mostly distributed around the same oxygen and the terminal fluorines and to a lesser extent around the nitrogen. In lc and ld, charge distribution is again mostly around the ether oxygen (O11) and the terminal fluorines and to a lesser extent around the hydroxyl oxygen (O17) and the nitrogen. The charge density distribution in the different molecules is shown in Fig. 4. Electrostatic Potential The sites of most negative electrostatic potential move toward the terminal of the molecules from the parent to the derivatives as the number of fluorines increase. In propranolol the site is near the ether oxygen, in lb it is more or less equally distributed between the ether oxygen and the fluorinated terminal. In lc the region of influence of the electrostatic potential progressively increases as it does again in ld. The volume
6 354 Farooqi and Aboul-Enein 'i i E L i f la i lb E! lc ~ ld Fig. 4. Distribution of charge densities in (a) propranolol, (b) tdfluoroethyl-propranolol, (c) pentafluoropropyl-propranolol, and (d) heptafluorobutyl-propranolol. For each molecule two views are presented, one perpendicular to the 2 ~ axis and the other perpendicular to the 3* axis. of this influence covers the region occupied by the nitrogen and the terminal fluorines in the three derivatives, but the increase in the number of fluorines make this region bigger. Thus the most likely site of protonation or electrophilic attack move from the ether oxygen in the parent through equally likely at this oxygen and the terminal to more likely at the fluorinated portion of the side-chain terminal particularly in ld. The regions are similar to those suggested by the charge density. As the number of fluorines increase in the molecule the corresponding region of influence also increases and reactivity would be expected to be stronger (i.e., stronger bonds are more likely to form). It is possible the binding to the receptor involves both the ether oxygen and the fluofines. CONCLUSION Optimized geometries (using the AM1 semiempirical technique) were discussed above. The molecular volumes and ionization potentials for the four compounds are almost equal. Significant changes in the molecules occur in the dipole moments, charge densities and the electrostatic potentials. Probably, the contribution from the changes in the charge density are sufficient to explain the changes in the biological activity of the four compounds as the number of fluorines on the molecules increases. Their activity increases (the volumic region of negative influence) with the increase in the number of fluorines. Maps of electrostatic potential distributions are given in Fig. 5.
7 Computed Structure of Propranolol and Some Fluorinated Analogs 355 ";2:. I ] G. "...~ '~ '.i f ld Fig. 5. Distribution of electrostatic potential in (a) propranolol, (b) trifluoroethyl-propranolol, (c) pentafluoropropylpropranolol, and (d) heptafluorobutyl-propranolol. This view is perpendicular to the 3 ~ axis of each molecule. Notice the changes in the dotted contours, which represent the negative values. SUPPLEMENTARY MATERIAL AVAILABLE A listing of the following is available from the authors upon request for all four compounds: bond lengths, bond angles, and torsion angles; dimensions of the molecule boxes and their volumes; and dipole moments of the molecules. ACKNOWLEDGMENTS The authors would like to thank the Administration of the King Faisal Specialist Hospital & Research Centre for their support of the Bioanalytical and Drug Development Research Program. REFERENCES 1. Katzung, B. G., Ed. Basic and Clinical Pharmacology, 5th ed.; Prentice-Hall: Englewood Cliffs, NJ, Aboul-Enein, H. Y. Toxicol. Environm. Chem. 1991, 29, Morken, P. A.; Bachand, P. C.; Swenson, D. C.; Burton, D. J. J. Am. Chem. Soc. 1993, 114, Bergstrom, D. E.; Swartling, D. J. In Molecular Structure and Energetics; Leibman, J. F.; Greenberg, A.; Dolbier, W. R. Jr., Eds.; VCH: New York, 1988; Vol HyperChem for Windows (version 3.0), Reference Manual for the Software; Autodesk, Inc.: Sausalito, CA, Smart, B. E., In Molecular Structure and Energetics; Liebman, J. F.; Greenberg, A., Eds.: VCH: New York, 1986; Vol. 3.
Saba Al Fayoumi. Tamer Barakat. Dr. Mamoun Ahram + Dr. Diala Abu-Hassan
 1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
Alcohols. Alcohol any organic compound containing a hydroxyl (R-OH) group. Alcohols are an extremely important organic source
 Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Introduction into Biochemistry. Dr. Mamoun Ahram Lecture 1
 Introduction into Biochemistry Dr. Mamoun Ahram Lecture 1 Course information Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 7 th edition Instructors Dr. Mamoun
Introduction into Biochemistry Dr. Mamoun Ahram Lecture 1 Course information Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 7 th edition Instructors Dr. Mamoun
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS
 CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
FACULTY OF PHARMACY. M. Pharmacy I Semester (Suppl.) Examination, November 2015 (Common To All) Subject: Pharmaceutical Analytical Techniques
 M. Pharmacy I Semester (Suppl.) Examination, November 2015 (Common To All) Subject: Pharmaceutical Analytical Techniques Code No. 6001 / S 1 a) Describe the instrumentation and applications of UV-visible
M. Pharmacy I Semester (Suppl.) Examination, November 2015 (Common To All) Subject: Pharmaceutical Analytical Techniques Code No. 6001 / S 1 a) Describe the instrumentation and applications of UV-visible
Organic Chemistry 6 th Edition Paula Yurkanis Bruice. Chapter 1. Electronic Structure and Bonding. Acids and Bases Pearson Education, Inc.
 Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Chapter 22. Organic and Biological Molecules
 Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
Chapter 8 Chemical Bonding
 Chapter 8 Chemical Bonding Types of Bonds Ionic Bonding Covalent Bonding Shapes of Molecules 8-1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Table 8.1 Two
Chapter 8 Chemical Bonding Types of Bonds Ionic Bonding Covalent Bonding Shapes of Molecules 8-1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Table 8.1 Two
1. (18) Multiple choice questions. Please place your answer on the line preceding each question.
 CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CHEM 261 Dec 4, 2017
 200 CEM 261 Dec 4, 2017 REVIEW: 1. N 3 2 S 4 1. 2 Al 3 N 2 2. 2 Al 3 2. N 3 2 S 4 N 2 I II For the left hand reaction, I - to create a meta positioned, the first molecule to be subsituted should be N 2,
200 CEM 261 Dec 4, 2017 REVIEW: 1. N 3 2 S 4 1. 2 Al 3 N 2 2. 2 Al 3 2. N 3 2 S 4 N 2 I II For the left hand reaction, I - to create a meta positioned, the first molecule to be subsituted should be N 2,
CHAPTER 2. Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules
 CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
Biochemistry I Fall 2015 Exam 1 Dr. Stone Name
 Biochemistry I Fall 2015 Exam 1 Dr. Stone Name Ka for acetic acid = 1.74 x 10-5 Ka for formic acid, CH 2 O 2 = 1.78 x 10-4 Ka for lactic acid, C 3 H 6 O 3 = 1.41 x 10-4 Ka = [H+][A-]/[HA] ph = pka + log
Biochemistry I Fall 2015 Exam 1 Dr. Stone Name Ka for acetic acid = 1.74 x 10-5 Ka for formic acid, CH 2 O 2 = 1.78 x 10-4 Ka for lactic acid, C 3 H 6 O 3 = 1.41 x 10-4 Ka = [H+][A-]/[HA] ph = pka + log
AMINES. 3. From your knowledge of the effects involved, predict or explain experimental results. Important areas include:
 AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
CHEM 263 Oct 11, Lecture Outline 3: Alcohols, Ethers, Stereochemistry, Ketones, and Aldehydes. Ethanol
 CEM 263 ct 11, 2016 Lecture utline 3: Alcohols, Ethers, Stereochemistry, Ketones, and Aldehydes Nomenclature of Alcohols Alcohols are compounds that have a hydroxyl group (-) bonded to a carbon atom (but
CEM 263 ct 11, 2016 Lecture utline 3: Alcohols, Ethers, Stereochemistry, Ketones, and Aldehydes Nomenclature of Alcohols Alcohols are compounds that have a hydroxyl group (-) bonded to a carbon atom (but
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Introductory Biochemistry
 Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Carbon Compounds. Chemical Bonding Part 2
 Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
BIOB111_CHBIO - Tutorial activity for Session 10. Conceptual multiple choice questions:
 BIOB111_CHBIO - Tutorial activity for Session 10 General Topics for Session 10 Week 5 Properties of the functional groups and examples. Amines, amides and Esters Physical properties and chemical reactions:
BIOB111_CHBIO - Tutorial activity for Session 10 General Topics for Session 10 Week 5 Properties of the functional groups and examples. Amines, amides and Esters Physical properties and chemical reactions:
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS
 COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR
![INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR](/thumbs/71/65562448.jpg) INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR Measom, N. D.; Down, K. D.; Hirst, D. J.; Jamieson, C.; Manas, E. S.; Patel, V. K.; and Somers, D. O. Celeste
INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR Measom, N. D.; Down, K. D.; Hirst, D. J.; Jamieson, C.; Manas, E. S.; Patel, V. K.; and Somers, D. O. Celeste
Chapter 14 Organic Compounds That Contain Oxygen, Halogen, or Sulfur
 Chapter 14 Organic Compounds That Contain Oxygen, Halogen, or Sulfur Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 14 Organic Compounds That Contain Oxygen, Halogen, or Sulfur Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 2: An Introduction to Organic Compounds
 Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
SAR. Structure - Activity Relationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) 2/28/2016
 Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Ethers can be symmetrical or not:
 Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
12.1 The Nature of Organic molecules
 12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
Abdullah Zreqat. Laith Abu Shekha. Mamoun Ahram
 2 Abdullah Zreqat Laith Abu Shekha Mamoun Ahram In this sheet we will talk about carbon, water, acid and bases. Carbon: Carbon is the only element that can form so many different compounds because each
2 Abdullah Zreqat Laith Abu Shekha Mamoun Ahram In this sheet we will talk about carbon, water, acid and bases. Carbon: Carbon is the only element that can form so many different compounds because each
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Lec.1 Chemistry Of Water
 Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Aside on Chapter 22, Organic Chemistry. Why is organic chemistry important:
 Aside on Chapter 22, Organic Chemistry Why is organic chemistry important: 1) Materials 2) Energy (oil & coal) 3) Human health a) diagnosis b) treatment (drugs) 4) A drug development logic progression
Aside on Chapter 22, Organic Chemistry Why is organic chemistry important: 1) Materials 2) Energy (oil & coal) 3) Human health a) diagnosis b) treatment (drugs) 4) A drug development logic progression
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Ping-Chiang Lyu. Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University.
 Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds
 Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols
Carbon and Molecular Diversity - 1
 Carbon and Molecular Diversity - 1 Although water is the most abundant compound of living organisms, and the "medium" for the existence of life, most of the molecules from which living organisms are composed
Carbon and Molecular Diversity - 1 Although water is the most abundant compound of living organisms, and the "medium" for the existence of life, most of the molecules from which living organisms are composed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models
 EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
Carbon and the Molecular Diversity of Life
 Chapter 4 Carbon and the Molecular Diversity of Life Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 4: 1. Organic chemistry is the study of carbon compounds 2. Carbon atoms
Chapter 4 Carbon and the Molecular Diversity of Life Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 4: 1. Organic chemistry is the study of carbon compounds 2. Carbon atoms
Chemistry Department, College of Science, University of Mutah, Karak, Jordan Reprint requests to Dr. H. S. M. Al-O.
 Semiempirical Method MNDO for the Evaluation of the Effect of Different Substituents at the Imine-Carbon Position on the Acetaldemine-Vinylamine Tauotomerization and Comparison to the Substitution at α-position
Semiempirical Method MNDO for the Evaluation of the Effect of Different Substituents at the Imine-Carbon Position on the Acetaldemine-Vinylamine Tauotomerization and Comparison to the Substitution at α-position
AMINES. 4. From your knowledge of the effects involved, predict or explain experimental results. Important areas include:
 AMINES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC or common name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole, pyridine, purine, pyrimidine,
AMINES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC or common name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole, pyridine, purine, pyrimidine,
Introduction to medicinal chemisry
 2017 1 st edition Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine
2017 1 st edition Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine
Organic Chemistry. Organic chemistry is the chemistry of compounds containing carbon.
 Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
L718Q mutant EGFR escapes covalent inhibition by stabilizing. a non-reactive conformation of the lung cancer drug. osimertinib
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) for L718Q mutant EGFR escapes covalent inhibition
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) for L718Q mutant EGFR escapes covalent inhibition
CHEMISTRY (CHE) CHE 104 General Descriptive Chemistry II 3
 Chemistry (CHE) 1 CHEMISTRY (CHE) CHE 101 Introductory Chemistry 3 Survey of fundamentals of measurement, molecular structure, reactivity, and organic chemistry; applications to textiles, environmental,
Chemistry (CHE) 1 CHEMISTRY (CHE) CHE 101 Introductory Chemistry 3 Survey of fundamentals of measurement, molecular structure, reactivity, and organic chemistry; applications to textiles, environmental,
Functional Groups SCH4C
 Functional Groups With the huge number of organic compounds in existence, it would be very difficult for you to memorize the properties of each compound separately. Fortunately the compounds fall into
Functional Groups With the huge number of organic compounds in existence, it would be very difficult for you to memorize the properties of each compound separately. Fortunately the compounds fall into
Carbon and the Molecular Diversity of Life
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 4 Carbon and the Molecular Diversity
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 4 Carbon and the Molecular Diversity
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION
 CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
REALLY, REALLY STRONG BASES. DO NOT FORGET THIS!!!!!
 CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR
 Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
Carbon and the Molecular Diversity of Life
 Chapter 4 Carbon and the Molecular Diversity of Life PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 4 Carbon and the Molecular Diversity of Life PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Full file at Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Carbon and the Molecular Diversity of Life CHAPTER 4
 Carbon and the Molecular Diversity of Life CHAPTER 4 1 Carbon: The Backbone of Life Although cells are 70 95% water, the rest consists mostly of carbon-based compounds Carbon is unparalleled in its ability
Carbon and the Molecular Diversity of Life CHAPTER 4 1 Carbon: The Backbone of Life Although cells are 70 95% water, the rest consists mostly of carbon-based compounds Carbon is unparalleled in its ability
2FAMILIES OF CARBON COMPOUNDS:
 P1: PBU/VY P2: PBU/VY Q: PBU/VY T1: PBU Printer: Bind Rite JWL338-02 JWL338-Solomons-v1 April 23, 2010 21:49 2AMILIES ARB MPUDS: UTIAL GRUPS, ITERMLEULAR RES, AD IRARED (IR) SPETRSPY SLUTIS T PRBLEMS 2.1
P1: PBU/VY P2: PBU/VY Q: PBU/VY T1: PBU Printer: Bind Rite JWL338-02 JWL338-Solomons-v1 April 23, 2010 21:49 2AMILIES ARB MPUDS: UTIAL GRUPS, ITERMLEULAR RES, AD IRARED (IR) SPETRSPY SLUTIS T PRBLEMS 2.1
Chemistry in Biology. Section 1. Atoms, Elements, and Compounds
 Section 1 Atoms, Elements, and Compounds Atoms! Chemistry is the study of matter.! Atoms are the building blocks of matter.! Neutrons and protons are located at the center of the atom.! Protons are positively
Section 1 Atoms, Elements, and Compounds Atoms! Chemistry is the study of matter.! Atoms are the building blocks of matter.! Neutrons and protons are located at the center of the atom.! Protons are positively
Outline. Organic Compounds. Overview: Carbon: The Backbone of Life. I. Organic compounds II. Bonding with Carbon III. Isomers IV.
 Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
file:///biology Exploring Life/BiologyExploringLife04/
 Objectives Identify carbon skeletons and functional groups in organic molecules. Relate monomers and polymers. Describe the processes of building and breaking polymers. Key Terms organic molecule inorganic
Objectives Identify carbon skeletons and functional groups in organic molecules. Relate monomers and polymers. Describe the processes of building and breaking polymers. Key Terms organic molecule inorganic
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
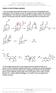 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Hydrogen Bonding & Molecular Design Peter
 Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Hydrogen Bonding & Molecular Design Peter Kenny(pwk.pub.2008@gmail.com) Hydrogen Bonding in Drug Discovery & Development Interactions between drug and water molecules (Solubility, distribution, permeability,
Chapter 8 Concepts of Chemical. Bonding
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 8 Concepts of John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 8 Concepts of John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice
Alcohols. Contents. Structure. structure
 Page 1 of 9 Alcohols Contents structure Physical Properties Classification of Alcohols Nomenclature of Alcohols Preparation of Alcohols Oxidation of Alcohols oxidation of aldehydes Structure Alcohols can
Page 1 of 9 Alcohols Contents structure Physical Properties Classification of Alcohols Nomenclature of Alcohols Preparation of Alcohols Oxidation of Alcohols oxidation of aldehydes Structure Alcohols can
3/30/2015. Third energy level. Second energy level. Energy absorbed. First energy level. Atomic nucleus. Energy released (as light)
 Chapter 2 An Introduction Chemistry Lecture 2: Energy Levels and Chemical Bonding Electrons are always moving Outside the nucleus in atomic orbitals Maybe usually Average distance from nucleus (size of
Chapter 2 An Introduction Chemistry Lecture 2: Energy Levels and Chemical Bonding Electrons are always moving Outside the nucleus in atomic orbitals Maybe usually Average distance from nucleus (size of
Biological Macromolecules
 Introduction for Chem 493 Chemistry of Biological Macromolecules Dr. L. Luyt January 2008 Dr. L. Luyt Chem 493-2008 1 Biological macromolecules are the molecules of life allow for organization serve a
Introduction for Chem 493 Chemistry of Biological Macromolecules Dr. L. Luyt January 2008 Dr. L. Luyt Chem 493-2008 1 Biological macromolecules are the molecules of life allow for organization serve a
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
HPLC Preparative Scaleup of Calcium Channel Blocker Pharmaceuticals Application
 HPLC Preparative Scaleup of Calcium Channel Blocker Pharmaceuticals Application Pharmaceuticals Author Cliff Woodward and Ronald Majors Agilent Technologies, Inc. 2850 Centerville Road Wilmington, DE 19808
HPLC Preparative Scaleup of Calcium Channel Blocker Pharmaceuticals Application Pharmaceuticals Author Cliff Woodward and Ronald Majors Agilent Technologies, Inc. 2850 Centerville Road Wilmington, DE 19808
Downloaded from
 I.I.T.Foundation - XI Chemistry MCQ #4 Time: 45 min Student's Name: Roll No.: Full Marks: 90 Chemical Bonding I. MCQ - Choose Appropriate Alternative 1. The energy required to break a chemical bond to
I.I.T.Foundation - XI Chemistry MCQ #4 Time: 45 min Student's Name: Roll No.: Full Marks: 90 Chemical Bonding I. MCQ - Choose Appropriate Alternative 1. The energy required to break a chemical bond to
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
4 Carbon and the Molecular Diversity of Life
 4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups
4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups
CHEM J-5 June 2010
 HEM1108 2010-J-5 June 2010 Hydrogen bond strength increases in the order H:::: < H:::: < F H::::F. Use this information and the data given in the table to explain the differences in boiling point of ammonia,
HEM1108 2010-J-5 June 2010 Hydrogen bond strength increases in the order H:::: < H:::: < F H::::F. Use this information and the data given in the table to explain the differences in boiling point of ammonia,
Organic and Biochemical Molecules. 1. Compounds composed of carbon and hydrogen are called hydrocarbons.
 Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Concepts of Chemical Bonding and Molecular Geometry Part 1: Ionic and Covalent Bonds. David A. Katz Pima Community College Tucson, AZ
 Concepts of Chemical Bonding and Molecular Geometry Part 1: Ionic and Covalent Bonds David A. Katz Pima Community College Tucson, AZ Chemical Bonds Three basic types of bonds: Ionic Electrostatic attraction
Concepts of Chemical Bonding and Molecular Geometry Part 1: Ionic and Covalent Bonds David A. Katz Pima Community College Tucson, AZ Chemical Bonds Three basic types of bonds: Ionic Electrostatic attraction
CHEM 109A Organic Chemistry
 CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
Research Article. Investigation of quantum-chemical properties of paracetamol
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(1):307-311 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Investigation of quantum-chemical properties of
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(1):307-311 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Investigation of quantum-chemical properties of
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
NAME. EXAM I I. / 36 September 25, 2000 Biochemistry I II. / 26 BICH421/621 III. / 38 TOTAL /100
 EXAM I I. / 6 September 25, 2000 Biochemistry I II. / 26 BIH421/621 III. / 8 TOTAL /100 I. MULTIPLE HOIE (6 points) hoose the BEST answer to the question by circling the appropriate letter. 1. An amino
EXAM I I. / 6 September 25, 2000 Biochemistry I II. / 26 BIH421/621 III. / 8 TOTAL /100 I. MULTIPLE HOIE (6 points) hoose the BEST answer to the question by circling the appropriate letter. 1. An amino
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
/15. Chem 202 Name KEY Exam 1 January 30, (3 pts total, 15 pts each) circle the best answer
 Chem 202 KEY Exam 1 January 30, 2006 1. (3 pts total, 15 pts each) circle the best answer Which of the following is (are) true? a. Charles Law assume V and T are constant b. Boyles law assumes P and V
Chem 202 KEY Exam 1 January 30, 2006 1. (3 pts total, 15 pts each) circle the best answer Which of the following is (are) true? a. Charles Law assume V and T are constant b. Boyles law assumes P and V
Technical Note. Introduction
 Technical Note Characterization of Eleven 2,5-Dimethoxy-N-(2-methoxybenzyl)- phenethylamine (NBOMe) Derivatives and Differentiation from their 3- and 4- Methoxybenzyl Analogues - Part II Patrick A. Hays*,
Technical Note Characterization of Eleven 2,5-Dimethoxy-N-(2-methoxybenzyl)- phenethylamine (NBOMe) Derivatives and Differentiation from their 3- and 4- Methoxybenzyl Analogues - Part II Patrick A. Hays*,
Electronic Structure. Hammett Correlations. Linear Free Energy Relationships. Quantitative Structure Activity Relationships (QSAR)
 Electronic Structure ammett Correlations Quantitative Structure Activity Relationships (QSAR) Quantitative Structure Activity Relationships (QSAR) We have already seen that by changing groups in a drug
Electronic Structure ammett Correlations Quantitative Structure Activity Relationships (QSAR) Quantitative Structure Activity Relationships (QSAR) We have already seen that by changing groups in a drug
Chapter 21. Organic Chemistry 4 th Edition Paula Yurkanis Bruice. More About Amines. Heterocyclic Compounds
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 21 More About Amines. Heterocyclic Compounds Irene Lee Case Western Reserve University Cleveland, OH 2004, Prentice Hall Amines An amine is
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 21 More About Amines. Heterocyclic Compounds Irene Lee Case Western Reserve University Cleveland, OH 2004, Prentice Hall Amines An amine is
The wavefunction that describes a bonding pair of electrons:
 4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS
 2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
1) Which type of compound does not contain a carbonyl group? A) ketone B) aldehyde C) amine D) ester E) carboxylic acid
 1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Ch 18 Ethers and Epoxides
 Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Fast Screening Methods for Beta Blockers by HPLC with Agilent Poroshell 120 Columns
 Fast Screening Methods for Beta Blockers by PLC with Agilent Poroshell Columns Application Note Pharma, Biopharma, and Clinical Author William Long Agilent Technologies, Inc. Introduction Beta blockers,
Fast Screening Methods for Beta Blockers by PLC with Agilent Poroshell Columns Application Note Pharma, Biopharma, and Clinical Author William Long Agilent Technologies, Inc. Introduction Beta blockers,
CHAPTER 3. Vibrational Characteristics of PTP-1B Inhibitors
 CHAPTER 3 Vibrational Characteristics of PTP-1B Inhibitors 3.1 Preface Theoretical Frequency calculations were performed on the molecules chosen in this study. Initially all the geometries were optimized
CHAPTER 3 Vibrational Characteristics of PTP-1B Inhibitors 3.1 Preface Theoretical Frequency calculations were performed on the molecules chosen in this study. Initially all the geometries were optimized
The Hückel Approximation Consider a conjugated molecule i.e. a molecule with alternating double and single bonds, as shown in Figure 1.
 The Hückel Approximation In this exercise you will use a program called Hückel to look at the p molecular orbitals in conjugated molecules. The program calculates the energies and shapes of p (pi) molecular
The Hückel Approximation In this exercise you will use a program called Hückel to look at the p molecular orbitals in conjugated molecules. The program calculates the energies and shapes of p (pi) molecular
Chemistry in Biology Section 1 Atoms, Elements, and Compounds
 Name Chemistry in Biology Section 1 Atoms, Elements, and Compounds Date Main Idea Details Scan the headings and boldfaced words in Section 1 of the chapter. Predict two things that you think might be discussed.
Name Chemistry in Biology Section 1 Atoms, Elements, and Compounds Date Main Idea Details Scan the headings and boldfaced words in Section 1 of the chapter. Predict two things that you think might be discussed.
