Onofrio Annunziata, Luigi Paduano,, Arne J. Pearlstein, Donald G. Miller,, and John G. Albright*, 5916 J. Am. Chem. Soc. 2000, 122,
|
|
|
- Dwight Martin
- 5 years ago
- Views:
Transcription
1 5916 J. Am. Chem. Soc. 2000, 122, Extraction of Thermodynamic Data from Ternary Diffusion Coefficients. Use of Precision Diffusion Measurements for Aqueous Lysozyme Chloride-NaCl at 25 C To Determine the Change of Lysozyme Chloride Chemical Potential with Increasing NaCl Concentration Well into the Supersaturated Region Onofrio Annunziata, Luigi Paduano,, Arne J. Pearlstein, Donald G. Miller,, and John G. Albright*, Contribution from the Department of Chemistry, Texas Christian UniVersity, Fort Worth, Texas 76129, Geosciences and EnVironmental Technologies, Lawrence LiVermore National Laboratory, P.O. Box 808, LiVermore, California 94551, Department of Mechanical and Industrial Engineering, UniVersity of Illinois at Urbana-Champaign, 1206 West Green Street, Urbana, Illinois 61801, and Dipartimento di Chimica, UniVersità di Napoli, Via Mezzocannone 4, Naples, Italy ReceiVed NoVember 1, 1999 Abstract: For ternary systems, we present a method for using measured values of the four ternary diffusion coefficients and the Onsager reciprocal relations to extract derivatives of solute chemical potentials with respect to solute molar concentrations. The method is applicable to systems in which the molar concentration of one solute is very small compared to that of the other, and also small enough that an inverse concentration dependence dominates certain activity coefficient derivatives. These conditions apply to a large number of aqueous systems involving macromolecules of biological interest. Unlike other techniques, the present method can be used to study undersaturated and supersaturated solutions. The approach is illustrated for the lysozyme chloride- NaCl-H 2 O system at 25 C, using data reported here for ph 6.0 at 0.60 mm (8.6 mg/ml) lysozyme chloride and 0.25, 0.50, 0.65, 0.90, and 1.30 M (1.4, 2.8, 3.7, 5.1, and 7.2 wt %) NaCl concentrations, and our earlier data for ph 4.5 at the same concentrations. We use these solute chemical potential derivatives to compute the protein cation charge approximately, and to construct a function approximating the derivative of the lysozyme chloride chemical potential with respect to NaCl concentration, which we integrate over a range of NaCl concentrations. This provides the change of the lysozyme chloride chemical potential with NaCl concentration well into the supersaturated region, and hence provides the driving force for nucleation and crystal growth of lysozyme chloride as a function of the extent of supersaturation. We also compute the diffusion Onsager coefficients (L ij ) 0 for each composition at ph 4.5 and 6.0. Binary diffusion coefficients of aqueous lysozyme chloride at 0.89 mm (12.7 mg/ml) for ph values from 4.0 to 6.0, and at ph 6.0 for concentrations from 0.25 to 1.95 mm ( mg/ml) are also reported. Introduction Thermodynamic properties are typically determined from measurements in equilibrium or quasi-equilibrium experiments. However, that need not be the case. For example, binary diffusion coefficient measurements in dilute solutions have long been used to compute activity coefficients. 1 The use of ternary diffusion coefficients to determine binding coefficients and other thermodynamic data is also well established. 2-4 Portions of this work were presented at the Annual Meeting of the American Crystallographic Association in Buffalo, N.Y., May 22-27, * To whom correspondence should be addressed. Phone: (817) Fax: (817) albright@gamma.is.tcu.edu. Texas Christian University. Università di Napoli. University of Illinois at Urbana-Champaign. Lawrence Livermore National Laboratory. (1) Harned, H. S. Proc. Natl. Acad. Sci. U.S.A. 1954, 40, (2) Paduano, L.; Sartorio, R.; Vitagliano, V.; Albright, J. G.; Miller, D. G.; Mitchell, J. J. Phys. Chem. 1990, 94, (3) Paduano, L.; Sartorio, R.; Vitagliano, V.; Costantino, L. Ber. Bunsen- Ges. Phys. Chem. 1990, 94, (4) Kim, H. J. Solution Chem. 1974, 3, Here, we consider a class of ternary systems in which the molar concentration of one solute is very small compared to that of the other, and also small enough that an inverse concentration dependence dominates certain activity coefficient derivatives. For such systems, we show how the Onsager reciprocal relations (ORR), along with precision measurements of ternary diffusion coefficients, can be used to determine concentration derivatives of the chemical potentials of two solutes with respect to solute molar concentrations. The approach is illustrated for lysozyme chloride in aqueous NaCl. Lysozyme chloride-h 2 O and lysozyme chloride-nacl-h 2 O systems serve as models in a wide range of protein studies (e.g., crystal growth) involving kinetic, transport, and equilibrium processes, as discussed in ref 5. Multicomponent diffusion is important in many protein phenomena, including modeling of diffusion to the surface of a growing crystal. Diffusive transport is especially important under microgravity conditions, where buoyancy-driven convective transport is greatly reduced. Other (5) Albright, J. G.; Annunziata, O.; Miller, D. G.; Paduano, L.; Pearlstein, A. J. J. Am. Chem. Soc. 1999, 121, /ja993871l CCC: $ American Chemical Society Published on Web 06/08/2000
2 Thermodynamic Data from Diffusion Coefficients J. Am. Chem. Soc., Vol. 122, No. 25, than the work of Leaist and Hao 6-8 in dilute solution and our recent work 5 at higher concentrations relevant to crystal growth, we are unaware of reliable measurements of multicomponent diffusion coefficients in protein systems. 9 However, virtually all studies of protein diffusion are conducted in systems with two or more solutes, and pseudobinary diffusion coefficients for the protein are insufficient to describe the actual protein transport. This lack of measured multicomponent diffusion coefficients for protein systems motivates our systematic program to measure, interpret, and ultimately predict these important transport properties. Such data are critical to modeling of protein crystal growth. 5 In an earlier paper, 5 we reported precision interferometric measurements of binary diffusion coefficients for low to moderate concentrations of aqueous lysozyme chloride at 25 C and ph 4.5, as well as the four elements of the diffusion coefficient matrix for 0.60 mm (8.6 mg/ml) solutions of lysozyme chloride in M ( wt %) aqueous NaCl. At the higher NaCl concentrations, compositions extended well into the region in which the solution is supersaturated with respect to lysozyme chloride. In this paper, we report similar results for this system at ph 6.0. We also report binary diffusion coefficients of aqueous lysozyme chloride at 0.89 mm (12.7 mg/ml) as a function of ph. We now outline how our earlier data 5 and these additional data at ph 6.0, all at one fixed lysozyme chloride concentration and covering a range of NaCl concentrations, can be used to obtain the chemical potential derivatives, µ ij µ i / C j (i * j), from diffusion measurements. (A detailed analysis appears below under the heading Evaluation of the µ ij.) Here, lysozyme chloride and NaCl are denoted as solutes 1 and 2, respectively; C i is the molarity of solute i; and we have adopted a standard notation used in diffusion studies for the concentration derivatives of the chemical potentials. 10,11 Our procedure is limited to the case where one solute is very dilute in molar terms compared to the other. However, our system and many others of biological interest satisfy this restriction. In our ternary experiments, the molarity of lysozyme chloride is small compared to that of NaCl. Thus, as we discuss below, the self-derivative for lysozyme chloride, µ 11, is dominated by its concentration term, with smaller contributions from the charge and activity coefficient derivative terms. The selfderivative for NaCl, µ 22, is essentially that of the binary with minor corrections. As shown below, two additional equations for obtaining the molarity cross-derivatives (µ 12 and µ 21, which are unequal 11 ) are (a) equality of the molality cross-derivatives 12 µ 1 m 2 ) µ 2 m 1 (1) where m i is the molality of solute i, and (b) the ORR equation (6) Leaist, D. G. J. Phys. Chem. 1986, 90, (7) Leaist, D. G. J. Phys. Chem. 1989, 93, (8) Leaist, D. G.; Hao, L. J. Chem. Soc., Faraday Trans. 1993, 89, (9) In an earlier interferometric study of multicomponent diffusion in protein systems (Ram Mohan, G. Ph.D. Dissertation, Weizmann Institute of Science, Rehovot, Israel, 1965), apparatus quality was poor and data analysis was unsatisfactory. A 1969 conference paper by the same author (Ram Mohan, G. In Diffusion Processes: Proceedings of the Thomas Graham Memorial Symposium; Sherwood, J. N., Ed.; Gordon and Breach: London, 1971; pp ) contains no useful information. (10) Miller, D. G.; Vitagliano, V.; Sartorio, R. J. Phys. Chem. 1986, 90, (11) Miller, D. G. J. Phys. Chem. 1959, 63, (12) Lewis, G. N.; Randall, M. Thermodynamics, 2nd ed.; McGraw- Hill: New York, µ 11 (D 12 ) 0 - µ 12 (D 11 ) 0 ) µ 22 (D 21 ) 0 - µ 21 (D 22 ) 0 (2) relating the four molarity derivatives and the ternary diffusion coefficients in a solvent-fixed reference frame (D ij ) 0. 13,14 This approach yields some extremely useful results, including an estimate of the lysozyme charge and a functional approximation to the change of the chemical potential of lysozyme chloride with NaCl concentration. The latter provides the driving force for nucleation and crystal growth of lysozyme chloride as a function of the degree of supersaturation. This, together with the diffusion coefficients, will permit the modeling of protein crystallization processes. For isothermal diffusion, experiments have thoroughly verified the ORR in dilute 15 and nondilute 11,13,14,16-18 systems. The ORR have also been experimentally verified for linear processes other than isothermal diffusion For isothermal diffusion, activity coefficient derivatives and measured ternary diffusion coefficients are required to verify the ORR. Conversely, we take the ORR to be valid, and use the measured diffusion coefficients to extract the derivatives of the chemical potentials with respect to the concentrations. The significance of this approach is that it allows certain aspects of equilibrium behavior to be predicted using measured transport data and binary thermodynamic data, without resort to approximate theoretical techniques. 11,13,14,23,24 To the best of our knowledge, the only previous use of the ORR to compute a thermodynamic quantity is the onedimensional nonequilibrium molecular dynamics calculation of Hafskjold and Ikeshoji 25 for a thermodynamically ideal binary system in which the mass ratio was 10, and the two species had identical molecular diameters and Lennard-Jones intermolecular potential depths. The computed thermal diffusion coefficient and the ORR were then used to evaluate the derivative of the chemical potential of one species with respect to its own mass fraction. Also, to the best of our knowledge, the only previous use of the ORR to extract additional information from experimental data is the work of Hanot et al., 26 in which the ORR, activity coefficient data, and a measurement of the (Soret) separation ratio were used to compute the off-diagonal diffusion coefficients D 12 and D 21 for the ternary system H 2 O + ethanol + 2-propanol. This work differs fundamentally from earlier work in which thermodynamic properties and off-diagonal diffusion coefficients have been related by approximations, rather than the exact ORR. Havenga and Leaist 27 performed ternary diffusion measurements in 50:50 (w/w) water + dioxane (1) + electrolyte (2) solutions for twelve inorganic salts and one organic salt, and used vapor (13) Dunlop, P. J.; Gosting, L. J. J. Phys. Chem. 1959, 63, (14) Woolf, L. A.; Miller, D. G.; Gosting, L. J. J. Am. Chem. Soc. 1962, 84, (15) Leaist, D. G.; Lyons, P. A. Aust. J. Chem. 1980, 33, (16) Miller, D. G. J. Phys. Chem. 1965, 69, (17) Spera, F. J.; Trial, A. F. Science 1993, 259, (18) Käshammer, S.; Weingärtner, H.; Hertz, H. G. Z. Phys. Chem. 1994, 187, (19) Miller, D. G. Chem. ReV. 1960, 60, (20) Miller, D. G. In Transport Phenomena in Fluids; Hanley, H. J. M., Ed.; Marcel Dekker: New York, 1969; pp (21) Miller, D. G. In Foundations of Continuum Thermodynamics; Delgado Domingos, J. J., Nina, M. N. R., Whitelaw, J. H., Eds.; Wiley: New York, 1973; pp (22) Rowley, R. L.; Horne, F. H. J. Chem. Phys. 1978, 68, (23) Miller, D. G. J. Phys. Chem. 1958, 62, 767. (24) Fujita, H.; Gosting, L. J. J. Phys. Chem. 1960, 64, (25) Hafskjold, B.; Ikeshoji, T. Fluid Phase Equilib. 1995, 104, (26) Hanot, V. P.; Platten, J. K.; Chavepeyer, G.; Larre, J. P. Entropie 1998, 34 (214), (27) Havenga, E.; Leaist, D. G. J. Chem. Soc., Faraday Trans. 1998, 94,
3 5918 J. Am. Chem. Soc., Vol. 122, No. 25, 2000 Annunziata et al. pressure and activity data to assess the approximation µ 1 / C 2 (D 12 /D 11 ) µ 1 / C 1. That effort produced only qualitative agreement for each of the two values of µ 1 / C 1 obtained by differentiation of possibly inaccurate thermodynamic data. The present work is also significant in that it provides direct access to chemical potential variations in ternary protein systems ranging from undersaturated to significantly supersaturated. Most equilibrium techniques are limited to salt-protein saturation conditions. On the isothermal protein-salt saturation curve, measurements are complicated by long equilibration times and subjective judgments of what constitutes equilibrium. Most classical equilibrium techniques (isopiestic, vapor pressure, freezing-point depression) are insufficiently accurate to provide useful results, especially when the solute of interest is dilute. Electromotive force measurements require reversible solute-specific electrodes, which for proteins are typically unavailable and which could induce crystallization in supersaturated solution. Membrane osmometry is capable of dealing with dilute protein solutions, but lacks accuracy, and requires additional assumptions or generally unavailable data for complete determination of activity coefficients. Equilibrium dialysis and its variants also lack accuracy. Moreover, with either of the two latter techniques, the membrane poses precipitation problems for supersaturated solutions. Sedimentation equilibrium by ultracentrifugation requires long-duration experiments and is unsuitable for supersaturated solutions. Static light scattering, which can in principle be used for undersaturated and supersaturated systems obtains, at best, only the second virial coefficient, and has the disadvantage that additional assumptions or a generally unavailable thermodynamic derivative 28,29 ( ln γ 1 / m 2 in our notation) is required to obtain the true concentration variation of the chemical potential. In contrast, our technique requires only that the protein component be dilute with respect to the salt on a molar basis, and that binary thermodynamic data for the aqueous salt solution be available or measurable. Furthermore, our technique can be used to study supersaturated solutions as long as the experiment ends before the onset of precipitation. (28) Scatchard, G. J. Am. Chem. Soc. 1946, 68, (29) Tanford, C. Physical Chemistry of Macromolecules; Wiley: New York, (30) Gosting, L. J.; Kim, H.; Loewenstein, M. A.; Reinfelds, G.; Revzin, A. ReV. Sci. Instrum. 1973, 44, (31) Miller, D. G.; Albright, J. G. In Measurement of the Transport Properties of Fluids: Experimental Thermodynamics; Wakeham, W. A., Nagashima, A., Sengers, J. V., Eds.; Blackwell Scientific Publications: Oxford, 1991; pp Experimental Section The materials, solution preparation procedures, apparatus, and density measurement procedures were described earlier. 5 Seikagaku six times recrystallized hen egg-white lysozyme chloride from one lot (E96301) was used to prepare solutions for all binary experiments reported here. Solutions used in binary experiment LC8 were prepared with lysozyme chloride from the lot E96301 bottle which, as described earlier, 5 appears to be slightly drier than lysozyme chloride from other bottles of the same lot. For the ternary experiments, the lot numbers used in each experiment were the same as for the corresponding NaCl concentrations of our earlier paper. The molecular masses of neutral lysozyme, NaCl, and H 2O were taken to be , , and g mol -1, 5 respectively. As before, all mutual diffusion coefficients were measured with the Gosting optical interferometric diffusiometer operating in its automated Rayleigh mode. 5,30 Separations of Creeth pairs were used to analyze fringe patterns as described elsewhere. 31,32 All runs had approximately 50 fringes. The procedures for measuring binary and ternary diffusion coefficients were described earlier. 5 For each pair of mean concentrations in the ternary case, two measurements were performed with R 1 near 0 and two with R 1 near 1, except at C 2 ) 1.30 M (7.2 wt %) where we used R 1 ) 0.8 instead of 1.0 to avoid precipitation. Here, R i R i C i/(r 1 C 1 + R 2 C 2) is the refractive index fraction due to the ith solute. 5 Data analysis of the free-diffusion experiments 32 is based on the assumption that the concentration differences of the solutes across the initial boundary are small enough that the diffusion coefficients are constant and that Fick s second law C n i 2 C j t ) D ij 1 e i e n (3) j)1 x 2 can be applied, where n is the number of solutes. Since our concentration differences across the initial free-diffusion boundary were small, the volume changes on mixing were also negligible. Thus, all the measured diffusion coefficients are given relative to the volume-fixed frame of reference defined by n J i Vh i ) 0 (4) i)0 where J i and Vh i are the molar flux and partial molar volume of the ith component, respectively, and the subscript 0 denotes the solvent. For ph 6.0, we again observed that lysozyme chloride precipitated from the supersaturated 1.30 M NaCl solutions, typically after 2 days. Results We report results for two series of binary experiments and one ternary series. In the first binary series, we measure the concentration dependence of the binary D v for aqueous lysozyme chloride at ph 6.0 and compare it to the results at ph 4.5 reported earlier. 5 In the second binary series, we present the ph dependence of the binary D v at 0.89 mm. The ternary diffusion coefficient measurements are used with eq 1 and the ORR to extract the derivatives of chemical potential with respect to composition at ph 4.5 and 6.0. They are also an important part of our program to develop a complete understanding of diffusive transport in the aqueous lysozyme chloride-nacl system over the range of conditions relevant to crystal growth. Binary D v as a Function of Aqueous Lysozyme Chloride at ph 6.0. In this set of experiments, the mean lysozyme chloride concentration Ch 1 ranged from 0.25 to 1.95 mm ( mg/ml). Binary diffusion coefficients are shown in Figure 1, and are tabulated with the following supporting data in Table 1: Ch 1, the mean lysozyme chloride concentration; C 1, the lysozyme chloride concentration difference across the initial boundary; ph values for the bottom and top solutions; d bot and d top, the densities of the bottom and top solutions; t, a time offset for the inevitable deviation from exact step-function behavior of the solute distribution at the start of the clock; J, the total number of fringes; Vh 0 and Vh 1, the partial molar volumes of H 2 O and lysozyme chloride at the mean concentration for each experiment, determined from the densities and concentrations of the reported solution pair; 5 R 1 ) J/ C 1, the refractive index increment with respect to J 33,34 at the mean concentration; and D v, the mutual diffusion coefficient of lysozyme chloride in the volume-fixed reference frame. Because the HCl concentrations are much lower at ph 6.0 than at ph 4.5, there was no need to correct D v for ternary behavior associated with the increased relative importance of HCl as the second solute at (32) Miller, D. G.; Albright, J. G.; Mathew, R.; Lee, C. M.; Rard, J. A.; Eppstein, L. B. J. Phys. Chem. 1993, 97, (33) Miller, D. G.; Paduano, L.; Sartorio, R.; Albright, J. G. J. Phys. Chem. 1994, 98, 13,745-13,754.
4 Thermodynamic Data from Diffusion Coefficients J. Am. Chem. Soc., Vol. 122, No. 25, Figure 1. Diffusion coefficients of lysozyme chloride in H 2Oat25 C versus lysozyme chloride concentration: b, ph 4.5; 9, ph 6.0. Least-squares curves are described in the text. Table 1. Binary Experimental and Derived Data at 25 C, ph 6.0 (Series LC6-9) expt LC6 LC7 LC8 LC9 Ch 1 (mm) C 1 (mm) ph bottom ph top d bot (g cm -3 ) d top (g cm -3 ) t (s) J measd Vh 1 (10 3 cm 3 mol -1 ) Vh 0 (cm 3 mol -1 ) R 1 (10 5 dm 3 mol -1 ) D v (measd) (10-9 m 2 s -1 ) low lysozyme chloride concentrations. 5 The binary D v values reported are internally consistent within the series to better than 0.1%. The accuracy is significantly better than 1%, but ultimately depends on lysozyme purity. Binary D v at 0.89 mm Lysozyme Chloride as a Function of ph. Binary diffusion coefficients for a series of experiments performed at a mean lysozyme chloride concentration of 0.89 mm in the ph range are shown in Figure 2, and tabulated with supporting data in Table 2. The diagnostic Ω values 5,35 are small, as previously observed for solutions prepared from the same lysozyme chloride lots. This reconfirms that the diffusion is essentially binary, that the concentration dependence of the diffusion coefficient is unimportant, and that the lysozyme was of good purity. 5 Ternary (D ij ) v at ph 6.0 and a Lysozyme Chloride Concentration of 0.60 mm. Four ternary experiments were performed at each of five different mean compositions. Each experiment in a set of four had the same mean NaCl and 0.6 mm lysozyme chloride concentrations, but different concentration differences of the solutes across the diffusion boundary. The mean lysozyme chloride concentration and the five mean (34) The conventional refractive index increment, with respect to the fringe number J, isr i. The more fundamental quantity, R i / ) (a/λ)r i, can be computed from the tabulated quantities, where λ is the optical wavelength and a is the internal cell dimension along the optical axis, which in our case are nm and cm, respectively. Since extraction of diffusion coefficients depends only on the ratio R 1/R 2 ) R 1 / /R 2 /, either increment can be used. The refractive index increment with respect to J, which might also be called the fringe number increment, has the advantage that the cell size a is not required. (35) Creeth, J. M.; Gosting, L. J. J. Phys. Chem. 1958, 62, Figure 2. Diffusion coefficients of 0.89 mm lysozyme chloride in H 2Oat25 C versus ph. The fitted curve is piecewise quadratic. Table 2. Binary Experimental and Derived Data at 25 C, Ch 1 ) 0.89 M (Series LC10-12) expt LC10 LC2 a LC11 LC8 LC12 Ch 1 (mm) C 1 (mm) ph bottom ph top d bot (g cm -3 ) d top (g cm -3 ) t (s) J measd Vh 1 (10 3 cm 3 mol -1 ) Vh 0 (cm 3 mol -1 ) R 1 (10 5 dm 3 mol -1 ) D v (measd) (10-9 m 2 s -1 ) ph av a Data from this experiment were reported in ref 5. Table 3. Ternary Experimental Data at 25 C, [NaCl] ) 0.25 M (Series LNC6) expt LNC61 LNC62 LNC63 LNC64 Ch 1 (mm) Ch 2 (M) C 1 (mm) C 2 (M) ph bottom ph top d bot (g cm -3 ) d top (g cm -3 ) t (s) J measd J calcd D A (measd) (10-9 m 2 s -1 ) D A (calcd) (10-9 m 2 s -1 ) NaCl concentrations, 0.25, 0.50, 0.65, 0.90, and 1.30 M (1.4, 2.8, 3.7, 5.1, and 7.2 wt %), were identical to those previously investigated at ph Data from each experiment are presented in Tables 3-7. Besides the symbols introduced in Table 1, the data shown are the following: Ch 2, the mean NaCl concentration; C 2, the NaCl concentration difference across the free-diffusion starting boundary; J measd, the total number of fringes observed; J calcd, obtained from the C i and refractive index increments with respect to J R i defined below; and D A, the reduced-height-area ratio, 36 obtained as described in ref 5. (36) Dunlop, P. J.; Gosting, L. J. J. Am. Chem. Soc. 1955, 77,
5 5920 J. Am. Chem. Soc., Vol. 122, No. 25, 2000 Annunziata et al. Table 4. Ternary Experimental Data at 25 C, [NaCl] ) 0.50 M (Series LNC8) expt LNC81 LNC82 LNC83 LNC84 Ch 1 (mm) Ch 2 (M) C 1 (mm) C 2 (M) ph bottom ph top d bot (g cm -3 ) d top (g cm -3 ) t (s) J measd J calcd D A (measd) (10-9 m 2 s -1 ) D A (calcd) (10-9 m 2 s -1 ) Table 5. Ternary Experimental Data at 25 C, [NaCl] ) 0.65 M (Series LNC9) expt LNC91 LNC93 LNC94 LNC96c Ch 1 (mm) Ch 2 (M) C 1 (mm) C 2 (M) ph bottom ph top d bot (g cm -3 ) d top (g cm -3 ) t (s) J measd J calcd D A (meas) (10-9 m 2 s -1 ) D A (calc) (10-9 m 2 s -1 ) Table 6. Ternary Experimental Data at 25 C, [NaCl] ) 0.90 M (Series LNC7) expt LNC71 LNC72b LNC73 LNC74b Ch 1 (mm) Ch 2 (M) C 1 (mm) C 2 (M) ph bottom ph top d bot (g cm -3 ) d top (g cm -3 ) t (s) J measd J calcd D A (meas) (10-9 m 2 s -1 ) D A (calc) (10-9 m 2 s -1 ) Table 8 shows data derived from these five sets of experiments, including Ch i, the average concentration of solute i in all eight solutions (four bottom solutions and four top solutions) for each set-mean composition; dh and H i d/ C i (see eq 3 in ref 5), obtained as functions of C 1 and C 2 by a linear regression of the density data from all solution pairs shown in Tables 3-7, as well as solutions used in a few unsuccessful diffusion experiments; the partial molar volumes Vh 1 and Vh 2 of the solutes (calculated using eq A-7 of ref 13) at the mean concentration in each set of experiments; Vh 0, the partial molar volume of H 2 O, 37 implicit in n C i Vh i ) 1 (5) i)0 (37) Rard, J. A.; Albright, J. G.; Miller, D. G.; Zeidler, M. E. J. Chem. Soc., Faraday Trans. 1996, 92, Table 7. Ternary Experimental Data at 25 C, [NaCl] ) 1.30 M (Series LNC10) expt LNC101e LNC102 LNC103 LNC104 Ch 1 (mm) Ch 2 (M) C 1 (mm) C 2 (M) ph bottom ph top d bot (g cm -3 ) d top (g cm -3 ) t (s) J measd J calcd D A (measd) (10-9 m 2 s -1 ) D A (calcd) (10-9 m 2 s -1 ) and R i ) J/ C i, the refractive index increments with respect to J, 33,34 obtained to good approximation by linear regression of the J values versus C i for all four experiments of each set, and used to determine J calc. Also included are the ratio S A /I A, 32,38 a diagnostic which if smaller than about 0.2 implies strong sensitivity of the diffusion coefficients to errors in the computations by which they are extracted from the fringe data; the eigenvalues of the diffusion coefficient matrix, λ 1 and λ 2 (λ 1 < λ 2 ), associated with lysozyme chloride and NaCl, respectively; and (D ij ) v, the measured diffusion coefficients in the volume-fixed frame. The errors shown for (D ij ) v are approximately 4 times the standard error of the coefficients as determined from the propagation-of-error equations using the full covariance matrix of the least-squares parameters. 32,33,39 Equations to relate the volume-fixed frame to the solventfixed frame 10,13,14 require the Vh i of all components. Parts A and B of Table 9 give the values of (D ij ) 0 in the solvent-fixed frame (defined by J 0 ) 0) 10,40 for ph 4.5 and 6.0, respectively, with the ph 4.5 values calculated from the volume-fixed (D ij ) v reported earlier. 5 Discussion of Diffusion Coefficients Binary D v of Aqueous Lysozyme Chloride. Figure 1 shows plots of D v versus C 1 for ph 4.5 data previously reported 5 and for ph 6.0 data reported in Table 1. The plots are nearly linear. The equation D v ) ( C C 1 ) was fitted to the ph 6.0 data, where D v and C 1 have units of 10-9 m 2 s -1 and M, respectively. Extrapolating to zero protein concentration, we find that D v lies about 2% below the infinite-dilution value at ph Both values exceed, by about 10%, the value extrapolated to zero protein concentration by Cadman, Fleming, and Guy. 41 Those authors performed Goüy interferometric measurements at 25 C over a range of lysozyme chloride concentrations, and fitted a linear relation to the measured diffusion coefficients as a function of the mass fraction of lysozyme chloride in solution. The solutions were in the ph range , and no correction was made for the presence of HCl. 5 As in our earlier work, 5 we can compute the charge of the lysozyme cation. We take the limiting tracer diffusion coefficient of the aqueous lysozyme cation to be m 2 s -1,on the basis of the data shown in Figure 3 of ref 5. At ph 6.0, this gives a value of z P ) for the charge of the lysozyme cation. (38) Fujita, H.; Gosting, L. J. J. Am. Chem. Soc. 1956, 78, (39) Miller, D. G.; Sartorio, R.; Paduano, L.; Rard, J. A.; Albright, J. G. J. Solution Chem. 1996, 25, (40) Kirkwood, J. G.; Baldwin, R. L.; Dunlop, P. J.; Gosting, L. J.; Kegeles, G. J. Chem. Phys. 1960, 33, (41) Cadman, A. D.; Fleming, R.; Guy, R. H. Biophys. J. 1981, 37,
6 Thermodynamic Data from Diffusion Coefficients J. Am. Chem. Soc., Vol. 122, No. 25, Table 8. Derived Ternary Diffusion Data at 25 C, ph ) 6.0 series LNC6 LNC8 LNC9 LNC7 LNC10 Ch 1 (mm) Ch 2 (M) dh (g cm -3 ) H 1 (10 3 g mol -1 ) H 2 (10 3 g mol -1 ) Vh 1 (cm 3 mol -1 ) Vh 2 (cm 3 mol -1 ) Vh 0 (cm 3 mol -1 ) R 1 (10 2 dm 3 mol -1 ) R 2 (10 2 dm 3 mol -1 ) S A/I A λ 1 (10-9 m 2 s -1 ) λ 2 (10-9 m 2 s -1 ) (D 11) v (10-9 m 2 s -1 ) ( ( ( ( ( (D 12) v (10-9 m 2 s -1 ) ( ( ( ( ( (D 21) v (10-9 m 2 s -1 ) 9.0 ( ( ( ( ( 0.2 (D 22) v (10-9 m 2 s -1 ) ( ( ( ( ( Table 9 A. Solvent-Fixed Ternary Diffusion Coefficients for ph 4.5, C 1 ) 0.6 mm C 2 ) 0.25 M C 2 ) 0.50 M C 2 ) 0.65 M C 2 ) 0.90 M C 2 ) 1.30 M (D 11) 0 (10-9 m 2 s -1 ) (D 12) 0 (10-9 m 2 s -1 ) (D 21) 0 (10-9 m 2 s -1 ) (D 22) 0 (10-9 m 2 s -1 ) B. Solvent-Fixed Ternary Diffusion Coefficients for ph 6.0, C 1 ) 0.6 mm C 2 ) 0.25 M C 2 ) 0.50 M C 2 ) 0.65 M C 2 ) 0.90 M C 2 ) 1.30 M (D 11) 0 (10-9 m 2 s -1 ) (D 12) 0 (10-9 m 2 s -1 ) (D 21) 0 (10-9 m 2 s -1 ) (D 22) 0 (10-9 m 2 s -1 ) With this charge, the binary-system Nernst-Hartley equation gives the limiting D v. The chloride ion diffusion coefficient was taken to be m 2 s On the other hand, we can use a Harned-type analysis 1 of the concentration dependence of the diffusion coefficients, where in our application the protein charge z P is adjusted to match the diffusion data. This assumes that the slope of D v versus C 1 is due to the concentration dependence of the activity coefficient, and that the concentration dependence of the diffusion coefficient is given by the Debye-Hückel limiting law for dilute solutions. The resulting charge of the protein cation is z P ) However, this value should be interpreted with care, since the charge is high, and the lysozyme cation is not spherical with a central charge, as assumed for the Debye-Hückel limiting law. Comparison of z P values computed for ph 4.5 (z P ) 6. 7 and 3. 9 from the limiting diffusion coefficient and the Debye- Hückel limiting law, respectively) 5 to those at ph 6.0 shows the charge at ph 6.0 to be less, as expected. The slope of D v versus C 1 is only slightly greater at ph 6.0 than at ph 4.5, implying a marginally greater effective charge. Again, interpretation is complicated by the high charge and asphericity of the lysozyme cation. Figure 2 shows D v versus ph for 0.89 mm lysozyme chloride solutions. Interpretation of these data, which exhibit an apparent maximum at low ph, must consider several factors. These include the actual charge, the consequent dragging of lysozyme cations by more mobile chloride anions, and the concentration dependence of the lysozyme chloride activity coefficient as a function of ph. As the ph increases toward lysozyme s (42) Mills, R. J. Phys. Chem. 1957, 61, isoelectric value of 11, 43 the protein charge decreases. Along with a corresponding decrease in the number of associated chloride anions and the cations of lysozyme they drag, this contributes to the expected decrease in D v. At the lowest ph values, emergence of HCl as a third component and of hydrogen ions as charge carriers will reduce the flux of lysozyme cations dragged by chloride anions. Since the available data do not permit a definitive assignment of the ph contribution to the variation in D v, we have made no attempt to correct for the ph-dependent contribution of HCl. Ternary (D ij ) v at ph 6.0 and Lysozyme Chloride Concentration of 0.60 mm. The dependence of the ternary diffusion coefficients D ij on NaCl concentration at ph 4.5 and 6.0 is shown in Figure 3 for 0.25 M e C 2 e 1.30 M. The values of D 11 (Figure 3a), corresponding to diffusion of lysozyme chloride due to its own gradient, decrease with increasing NaCl concentration at both ph values. The increase of aqueous NaCl viscosity with increasing C 2 over the same C 2 range 44 accounts for most, but not all, of the decrease in D 11. At a given NaCl concentration, the D 11 values are smaller at the higher ph. Because the high NaCl concentration greatly reduces the dragging of lysozyme cations by chloride anions, the residual effects of those chlorides associated with lysozyme should be lower at the higher ph. Other effects might also be important, including differences in the degree of hydration of the protein as a function of ph. The values of D 12 (Figure 3b) are very small and, when expressed in terms of thermodynamic transport coefficients (D 12 (43) Tanford, C.; Wagner, M. L. J. Am. Chem. Soc. 1954, 76, (44) Jones, G.; Christian, S. M. J. Am. Chem. Soc. 1937, 59,
7 5922 J. Am. Chem. Soc., Vol. 122, No. 25, 2000 Annunziata et al. Figure 3. Elements of the matrix of diffusion coefficients for the ternary system lysozyme chloride + NaCl + H 2O at ph 6.0 and 25 C: b, ph 4.5; 9, ph 6.0. (a, top left) D 11, least-squares curves are cubic in C 2 1/2 ; (b, top right) D 12, least-squares curves are of the form a 0C 2-1/2 + a 1 + a 2C 2 1/2 ; (c, bottom left) D 21, least-squares curves are quadratic in C 2; (d, bottom right) D 22 and D v for binary diffusion of NaCl in H 2O (shown by [), least-squares curves are cubic in C 2 1/2. ) L 11 µ 12 + L 12 µ 22 ), result from near-cancellation of two terms with opposite signs and similar magnitudes. The D 12 values at lower concentration for ph 6.0 are larger than those at ph 4.5, but cross over around C 2 ) 0.6 M. The data are believed to be precise enough that this effect is probably real. Values of D 21 (Figure 3c) are large and increase rapidly with increasing C 2 for both ph values, but at each NaCl concentration are slightly lower at ph 6.0. The difference between D 21 values at ph 4.5 and 6.0 remains nearly constant as C 2 varies, as discussed below in terms of the decreased charge of the lysozyme cation as the isoelectric ph is approached. The ratio D 21 /D 11 indicates that each mole of lysozyme chloride cotransports 257 ( 2 mol of NaCl in a uniform 1.30 M NaCl solution at ph 6.0. This is identical, within experimental error, to the value of 260 ( 2 at ph The ratio D 21 /D 11 increases sharply and nearly linearly with NaCl concentration. The values at ph 6.0 exceed those at ph 4.5 by 1-8%. The dependence of D 21 on NaCl concentration can be interpreted in terms of excluded volume or salting-out effects. The relative merits of those interpretations, in light of precision ternary diffusion data to be published for lysozyme chloride in aqueous solutions of sodium, potassium, magnesium, calcium, and ammonium chlorides, will be thoroughly explored elsewhere. The values of D 22 (Figure 3d) are nearly the same for both ph values. Since this is the coefficient for NaCl diffusion due to its own gradient, no ph dependence is expected. Figure 4. Square root of the determinant of the diffusion coefficient matrix for the ternary system lysozyme chloride + NaCl + H 2OatpH 6.0 and 25 C: b, ph 4.5; 9, ph 6.0. Least-squares curves are cubic in C 2 1/2. Examination of the Determinant. Figure 4 shows the square root of the diffusion coefficient matrix, D (D 11 ) v (D 22 ) v - (D 12 ) v (D 21 ) v, as a function of C 2 for both ph values. At each NaCl concentration, the ph 6.0 values of D are consistently lower than those for ph 4.5. For each ph, D decreases by about 10% as C 2 increases from 0.25 M into the supersaturated
8 Thermodynamic Data from Diffusion Coefficients J. Am. Chem. Soc., Vol. 122, No. 25, region at 1.30 M. The plots suggest that if the determinant vanishes for 0.60 mm lysozyme chloride, it does so well above 1.30 M. Consequently, the data indicate that if the spinodal curve intersects the C 1 ) 0.6 mm line in the C 1 - C 2 plane, it does so at an NaCl concentration well in excess of 1.30 M. 5 Partial Molar Volumes. The partial molar volume Vh 1 of lysozyme chloride is independent of C 2 within experimental error, and apparently does not depend significantly on ph either, although experimental error may hide a small effect. In contrast, the partial molar volume Vh 2 of NaCl increases with increasing C 2. This behavior is very similar to that in binary NaCl + H 2 O. However, the values in the ternary system are slightly higher, consistent with an excluded volume effect. Again, there does not seem to be a significant ph dependence. Other Measured or Calculated Quantities. The parameters H 1 and H 2, defined in eq 3 of ref 5 and corresponding approximately to the derivatives of solution density with respect to C 1 and C 2, respectively, are independent of C 2 and ph within experimental error. The R 1 and R 2 values relating J to C 1 and C 2 are nearly the same for both ph values at a given mean concentration. Within experimental error, R 1 is apparently independent of concentration, whereas R 2 decreases slowly with increasing C 2, as it also does in binary aqueous NaCl. The eigenvalue λ 1 (associated with lysozyme chloride) at ph 4.5 is approximately 3% higher than at ph 6.0 at each concentration, and decreases with increasing NaCl concentration at both ph values. At ph 4.5 and 6.0, the λ 2 values (associated with NaCl) are approximately equal, as expected, and nearly independent of C 2. At ph 6.0, the values of λ 2 agree even more closely with measured values of D v in aqueous NaCl 46 than at ph 4.5, being within 1% of and always lower than D v. Use of Irreversible Thermodynamics and Diffusion Data To Calculate Chemical Potential Derivatives Our lysozyme chloride-nacl-h 2 O system has an interesting and useful attribute characteristic of many biological systems: the molar concentrations of a macromolecule and the supporting electrolyte are small and large, respectively. In this special case, it is possible to estimate the chemical potential derivatives µ 11 and µ 22, as will become clear below. The molality cross-derivative relation, eq 1, comes from classical thermodynamics. From eq 1, an expression can be derived relating the four molarity partial derivatives µ ij. 11 A second equation, the ternary ORR of irreversible thermodynamics, relates the four (D ij ) 0 values and the four µ ij values. 13,14 Therefore, we can get the two off-diagonal derivatives µ ij (i * j) from these two relations, provided that suitably accurate approximations are available for the self-derivatives of the lysozyme chloride and sodium chloride chemical potentials, µ 11 and µ 22, respectively. The ORR are of considerable theoretical interest, and for this reason have been rigorously tested. 11,13-22 However, particularly for bulk diffusion, they have had little practical application. Now, in an important application, we demonstrate use of the ORR to extract from our data thermodynamic properties that would otherwise be inaccessible. From binary thermodynamic data for the more concentrated solute and diffusion coefficients (transport data), it is possible to use the ORR to get the thermodynamic derivatives µ ij even for supersaturated solutions. We will illustrate this result for the system lysozyme chloride- NaCl-H 2 O, since it fulfills the required conditions. In addition, we can calculate the protein charge by least-squares fitting to an appropriate functional form, the thermodynamic derivative, µ 12, over the range of NaCl concentrations at constant lysozyme concentration. We now review the thermodynamic equations which lead to our results. Fundamental Equations. Our analysis is in terms of quantities referred to a solvent-fixed reference frame, identified by a subscript 0, with the diffusion Onsager coefficients denoted by (L ij ) 0. As noted in the Results, the solvent-fixed (D ij ) 0 values shown in Table 8 are obtained from experimental volume-fixed (D ij ) v by standard equations involving the Vh i. 10,13,14 It has been shown 10,13,14 that the (L ij ) 0 and (D ij ) 0 are related (in matrix form) by [ (D 11 ) 0 (D 12 ) 0 (D 21 ) 0 (D 22 ) 0 ] ) [ (L 11 ) 0 µ 11 + (L ) 12 0 µ 21 (L 11 ) 0 µ 12 + (L 12 ) 0 µ 22 (L 21 ) 0 µ 11 + (L 22 ) 0 µ 21 (L 21 ) 0 µ 12 + (L 22 ) 0 µ 22 ] The inverse relation is Since the ORR, (L 12 ) 0 ) (L 21 ) 0, 13,14 apply to the solvent-fixed frame, eq 7 yields eq 2. From the cross-derivative expression eq 1 and the relations between C i and m i, we can show that 11 (45) Vitagliano, V.; Sartorio, R. J. Phys. Chem. 1970, 74, (46) Rard, J. A.; Miller, D. G. J. Solution Chem. 1979, 8, (47) Miller, D. G. J. Phys. Chem. 1967, 71, (6) [ (L 11 ) 0 (L 12 ) 0 (L 21 ) 0 (L 22 ) 0] ) 1 µ 11 µ 22 - µ 12 µ [ 21 µ 22 (D 11 ) 0 - µ (D ) 0 µ 11 (D 12 ) 0 - µ 12 (D 11 ) 0 µ 22 (D 21 ) 0 - µ 21 (D 22 ) 0 µ 11 (D 22 ) 0 - µ 12 (D 21 ) 0] (7) 1 C 0 M 0 µ 1 m 2 ) µ 12 (1 - C 2 Vh 2 ) - µ 11 C 1 Vh 2 ) µ 21 (1 - C 1 Vh 1 ) - µ 22 C 2 Vh 1 ) 1 C 0 M 0 µ 2 m 1 (8) where M 0 is the molecular mass of H 2 O. The general thermodynamic expressions for µ ij in terms of volume concentrations and the corresponding mean ionic activity coefficients y i for volume concentrations have been derived. 11 Let z P, z Na, and z Cl be the absolute values of the charges on the protein cation, sodium cation, and chloride ion common to the salt and protein, respectively. Furthermore, Na + and Cl - are univalent, i.e., z Na ) z Cl ) 1. We have also assumed that lysozyme chloride has stoichiometry LyCl zp. Consequently, the cation stoichiometric coefficients 47 r 1c of LyCl zp and r 2c of NaCl are both unity. With these conditions, the µ ij for our particular case can be written in matrix form as [ µ 11 µ 12 µ 21 µ 22 ] ) RT [ 2 1 z P + + (z C 1 z P C 1 + C P + 1) ln y z 1 P + (z 2 C 1 z P C 1 + C P + 1) ln y 1 2 C 2 ] z P + 2 ln y ln y 2 z P C 1 + C 2 C 1 C 2 z P C 1 + C 2 C 2 (9)
9 5924 J. Am. Chem. Soc., Vol. 122, No. 25, 2000 Annunziata et al. Table 10 A. Chemical Potentials and Derivatives for ph 4.5, C 1 ) 0.6 mm C 2 ) 0.25 M C 2 ) 0.50 M C 2 ) 0.65 M C 2 ) 0.90 M C 2 ) 1.30 M µ 11/RT (M -1 ) µ 22/RT (M -1 ) µ 12/RT (M -1 ) µ 21/RT (M -1 ) (µ 1 - µ / 1 )/RT B. Chemical Potentials and Derivatives for ph 6.0, C 1 ) 0.6 mm C 2 ) 0.25 M C 2 ) 0.50 M C 2 ) 0.65 M C 2 ) 0.90 M C 2 ) 1.30 M µ 11/RT (M -1 ) µ 22/RT (M -1 ) µ 12/RT (M -1 ) µ 21/RT (M -1 ) (µ 1 - µ / 1 )/RT Note that the quantity z P C 1 + C 2 is equivalent to the total normality N of our ternary solution. Evaluation of the µ ij. With the above equations, we now show how to compute the partial derivatives of the chemical potentials, µ ij, for the case in which the molarity of at least one component is very low, a common situation in multicomponent protein solutions. (i) Calculation of µ 11. From eq 9, we can rewrite µ 11 as µ 11 ) RT C 1 [ 1 + z 2 PC 1 ln y 1 + (z z P C 1 + C P + 1)C 1 2 C 1 ] (10) from which we can see that the first term is dominant for small C 1. Values of µ 11 calculated by retaining the first two terms are given in Table 10 for ph 4.5 and 6.0 for all NaCl concentrations. Consideration of light-scattering measurements of the second virial coefficient of lysozyme chloride by Guo et al. 48 and formulas that relate µ 11 to the second virial coefficient 28 suggests that the absolute error associated with dropping the third term in eq 10 does not exceed 10%. For the application below, the accuracy required of µ 11 need not be high, so that the values calculated from eq 10 are satisfactory. (ii) Calculation of µ 22. Since C 2. C 1, we observe, not surprisingly, that the measured D 22 values are close to the values of the binary diffusion coefficients at corresponding NaCl concentrations. Similarly, it is expected that the chemical potential derivative µ 22 for the ternary case will be close to its corresponding binary value. Consequently, the expression for µ 22 can be written to a good approximation as µ 22 ) RT C 2 [ 1 + C 2 z P C 1 + C 2 + 2C 2( ln y 2 C 2 )binary] (11) The value of µ 22 is evaluated using the derivative of the activity coefficient 49 for the binary salt solution at the salt concentration of the ternary system. The activity coefficient correction in eq 11 is not very sensitive to the binary concentration chosen for its evaluation. Thus, eq 11 should yield an accurate value of µ 22. (iii) Calculation of µ 12 and µ 21. Solving eqs 2 and 8 for the cross-derivatives of the chemical potential in terms of µ 11, µ 22, and the four (D ij ) 0, we obtain µ 12 ) {µ 11 [C 1 Vh 2 (D 22 ) 0 - (1 - C 1 Vh 1 )(D 12 ) 0 ] - µ 22 [C 2 Vh 1 (D 22 ) 0 - (1 - C 1 Vh 1 )(D 21 ) 0 ]}/ [(1 - C 2 Vh 2 )(D 22 ) 0 - (1 - C 1 Vh 1 )(D 11 ) 0 ] (12a) µ 21 ) {µ 11 [C 1 Vh 2 (D 11 ) 0 - (1 - C 2 Vh 2 )(D 12 ) 0 ] - µ 22 [C 2 Vh 1 (D 11 ) 0 - (1 - C 2 Vh 2 )(D 21 ) 0 ]}/ [(1 - C 2 Vh 2 )(D 22 ) 0 - (1 - C 1 Vh 1 )(D 11 ) 0 ] (12b) We then calculate µ 12 and µ 21 from the estimated values of µ 11 and µ 22, and the four measured (D ij ) 0 values. Numerical examination of the various terms shows that µ 12 and µ 21 depend mostly on µ 22 and (D 21 ) 0. Values of all four µ ij values are given in Table 10 as µ ij /RT. If we linearly interpolate our ph 4.5 values of µ ij /RT at 0.90 and 1.30 M NaCl to 1.0 M NaCl, we get µ 11 ) 248 kcal mol -1 M -1 and µ 12 ) 6.74 kcal mol -1 M -1. Substituting these values into eq 8, we get µ 1 / m 2 ) 6.31 kcal mol -1 (mol kg -1 ) -1,in excellent agreement with the molality derivative µ 1 / m 2 ) (6.8 ( 1.7) kcal mol -1 (mol kg -1 ) -1 obtained densimetrically in equilibrium dialysis-like experiments by Arakawa and Timasheff 50 for lysozyme in buffered 1.0 M NaCl at ph 4.5 and 20 C. Our approach to obtaining the µ ij in the ternary case can also be used for four or more components, providing that the molar concentrations of all but one solute are (a) sufficiently low that terms inversely proportional to concentration dominate the generalization of eq 9 and (b) low compared to that of the remaining solute (e.g., the supporting electrolyte). For example, consider a four-component system. In that case, the six offdiagonal µ ij values are determined by three molality crossderivatives such as eq 1, three Onsager relations similar to but more complex than eq 2, and the three main-term µ ii values estimated as above. The analysis above has been applied to the case in which one component (the protein) has a molar concentration much lower than that of the other. However, the approach can be extended to ternary systems with solutes of comparable size by extrapolating data to the limit in which one solute is infinitely dilute. Taking the limit C 1 f 0 in eqs 12a and 12b yields eqs A-1 and A-2 of the Appendix. Here, D 11, D 21, and the limiting slope β ) lim C1 f0 (D 12 /C 1 ) (13) (48) Guo, H.; Kao, S.; McDonald, H.; Asanov, A.; Combs, L. L.; Wilson, are accessible by extrapolation, and D 22 and µ 22 are the binary W. W. J. Cryst. Growth 1999, 196, (49) Miller, D. G. J. Phys. Chem. 1966, 70, (50) Arakawa, T.; Timasheff, S. N. Biochemistry 1984, 23,
The Effect of Salt on Protein Chemical Potential Determined by Ternary Diffusion in Aqueous Solutions
 J. Phys. Chem. B 2006, 110, 1405-1415 1405 The Effect of Salt on Protein Chemical Potential Determined by Ternary Diffusion in Aqueous Solutions Onofrio Annunziata,*, Luigi Paduano,, Arne J. Pearlstein,
J. Phys. Chem. B 2006, 110, 1405-1415 1405 The Effect of Salt on Protein Chemical Potential Determined by Ternary Diffusion in Aqueous Solutions Onofrio Annunziata,*, Luigi Paduano,, Arne J. Pearlstein,
On the Role of Solute Solvation and Excluded-Volume Interactions in Coupled Diffusion
 11968 J. Phys. Chem. B 2008, 112, 11968 11975 On the Role of Solute Solvation and Excluded-Volume Interactions in Coupled Diffusion Onofrio Annunziata* Department of Chemistry, Texas Christian UniVersity,
11968 J. Phys. Chem. B 2008, 112, 11968 11975 On the Role of Solute Solvation and Excluded-Volume Interactions in Coupled Diffusion Onofrio Annunziata* Department of Chemistry, Texas Christian UniVersity,
Macromolecular hydration compared with preferential hydration and their role on macromolecule-osmolyte coupled diffusionwz
 PAPER www.rsc.org/pccp Physical Chemistry Chemical Physics Macromolecular hydration compared with preferential hydration and their role on macromolecule-osmolyte coupled diffusionwz Huixiang Zhang ab and
PAPER www.rsc.org/pccp Physical Chemistry Chemical Physics Macromolecular hydration compared with preferential hydration and their role on macromolecule-osmolyte coupled diffusionwz Huixiang Zhang ab and
Supporting Text Z = 2Γ 2+ + Γ + Γ [1]
![Supporting Text Z = 2Γ 2+ + Γ + Γ [1] Supporting Text Z = 2Γ 2+ + Γ + Γ [1]](/thumbs/91/105230015.jpg) Supporting Text RNA folding experiments are typically carried out in a solution containing a mixture of monovalent and divalent ions, usually MgCl 2 and NaCl or KCl. All three species of ions, Mg, M +
Supporting Text RNA folding experiments are typically carried out in a solution containing a mixture of monovalent and divalent ions, usually MgCl 2 and NaCl or KCl. All three species of ions, Mg, M +
Mathematical Model for Diffusion of a Protein and a Precipitant about a Growing Protein Crystal in Microgravity
 Mathematical Model for iffusion of a Protein and a Precipitant about a Growing Protein Crystal in Microgravity ONOFRIO ANNUNZIATA a,b AN JOHN G. ALBRIGHT a a epartment of Chemistry, Texas Christian University,
Mathematical Model for iffusion of a Protein and a Precipitant about a Growing Protein Crystal in Microgravity ONOFRIO ANNUNZIATA a,b AN JOHN G. ALBRIGHT a a epartment of Chemistry, Texas Christian University,
Modeling Viscosity of Multicomponent Electrolyte Solutions 1
 International Journal of Thermophysics, Vol. 19, No. 2, 1998 Modeling Viscosity of Multicomponent Electrolyte Solutions 1 M. M. Lencka, 2 A. Anderko, 2,3 S. J. Sanders, 2 and R. D. Young 2 A comprehensive
International Journal of Thermophysics, Vol. 19, No. 2, 1998 Modeling Viscosity of Multicomponent Electrolyte Solutions 1 M. M. Lencka, 2 A. Anderko, 2,3 S. J. Sanders, 2 and R. D. Young 2 A comprehensive
Lecture 6. NONELECTROLYTE SOLUTONS
 Lecture 6. NONELECTROLYTE SOLUTONS NONELECTROLYTE SOLUTIONS SOLUTIONS single phase homogeneous mixture of two or more components NONELECTROLYTES do not contain ionic species. CONCENTRATION UNITS percent
Lecture 6. NONELECTROLYTE SOLUTONS NONELECTROLYTE SOLUTIONS SOLUTIONS single phase homogeneous mixture of two or more components NONELECTROLYTES do not contain ionic species. CONCENTRATION UNITS percent
Recenltly,l'2 the determination of diffusion coefficients of electrolytes in dilute
 THE DIFFUSION COEFFICIENTS OF THE ALKALI METAL CHLORIDES AND POTASSIUM AND SILVER NITRATES IN DILUTE AQUEOUS SOLUTIONS AT 250* BY HERBERT S. HARNED DEPARTMENT OF CHEMISTRY, YALE UNIVERSITY Communicated
THE DIFFUSION COEFFICIENTS OF THE ALKALI METAL CHLORIDES AND POTASSIUM AND SILVER NITRATES IN DILUTE AQUEOUS SOLUTIONS AT 250* BY HERBERT S. HARNED DEPARTMENT OF CHEMISTRY, YALE UNIVERSITY Communicated
Chem 321 Lecture 17 - Potentiometry 10/24/13
 Student Learning Objectives Chem 321 Lecture 17 - Potentiometry 10/24/13 Electrodes The cell described in the potentiometric chloride titration (see 10/22/13 posting) consists of a Ag/AgCl reference electrode
Student Learning Objectives Chem 321 Lecture 17 - Potentiometry 10/24/13 Electrodes The cell described in the potentiometric chloride titration (see 10/22/13 posting) consists of a Ag/AgCl reference electrode
6, Physical Chemistry -II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules)
 Subject Paper No and Title Module No and Title Module Tag 6, Physical -II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) 25, Activity and Mean Activity coefficient
Subject Paper No and Title Module No and Title Module Tag 6, Physical -II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) 25, Activity and Mean Activity coefficient
Properties of Aqueous Mixtures of Pure Salts. Thermodynamics of the Ternary System: Water-Calcium Chloride-Magnesium Chloride at 25 C
 JOURNAL OF RESEARCH of the National bureau of Standards A. Physics and Chemistry Vol. 70A, No. 4, July-August 9 Properties of Aqueous Mixtures of Pure Salts. Thermodynamics of the Ternary System: Water-Calcium
JOURNAL OF RESEARCH of the National bureau of Standards A. Physics and Chemistry Vol. 70A, No. 4, July-August 9 Properties of Aqueous Mixtures of Pure Salts. Thermodynamics of the Ternary System: Water-Calcium
SITARAM K. CHAVAN * and MADHURI N. HEMADE ABSTRACT INTRODUCTION
 Int. J. Chem. Sci.: 11(1), 013, 619-67 ISSN 097-768X www.sadgurupublications.com DENSITIES, VISCOSITIES AND EXCESS THERMODYNAMIC PROPERTIES OF MONOMETHYL AMMONIUM CHLORIDE IN TETRAHYDROFURAN AND WATER
Int. J. Chem. Sci.: 11(1), 013, 619-67 ISSN 097-768X www.sadgurupublications.com DENSITIES, VISCOSITIES AND EXCESS THERMODYNAMIC PROPERTIES OF MONOMETHYL AMMONIUM CHLORIDE IN TETRAHYDROFURAN AND WATER
Initial position, x p (0)/L
 .4 ) xp().2 ) ( 2L 2 xp Dc ( Displacement, /L.2.4.5.5 Initial position, x p ()/L Supplementary Figure Computed displacements of (red) positively- and (blue) negatively-charged particles at several CO 2
.4 ) xp().2 ) ( 2L 2 xp Dc ( Displacement, /L.2.4.5.5 Initial position, x p ()/L Supplementary Figure Computed displacements of (red) positively- and (blue) negatively-charged particles at several CO 2
Some phenomenological and thermodynamic aspects of diffusion in multicomponent systems
 Pure &App/. Chern., Vol. 63, No. 10, pp. 1441-1448,1991, Printed in Great Britain. @ 1991 IUPAC Some phenomenological and thermodynamic aspects of diffusion in multicomponent systems Vincenzo Vitagliano
Pure &App/. Chern., Vol. 63, No. 10, pp. 1441-1448,1991, Printed in Great Britain. @ 1991 IUPAC Some phenomenological and thermodynamic aspects of diffusion in multicomponent systems Vincenzo Vitagliano
Analysis of cations and anions by Ion- Selective Electrodes (ISEs)
 Analysis of cations and anions by Ion- Selective Electrodes (ISEs) Purpose: The purpose of this assignment is to introduce potentiometric measurements of ionic species by ion selective electrodes (ISEs)
Analysis of cations and anions by Ion- Selective Electrodes (ISEs) Purpose: The purpose of this assignment is to introduce potentiometric measurements of ionic species by ion selective electrodes (ISEs)
ADSORPTION IN MICROPOROUS MATERIALS: ANALYTICAL EQUATIONS FOR TYPE I ISOTHERMS AT HIGH PRESSURE
 ADSORPTION IN MICROPOROUS MATERIALS: ANALYTICAL EQUATIONS FOR TYPE I ISOTHERMS AT HIGH PRESSURE A. L. MYERS Department of Chemical and Biomolecular Engineering University of Pennsylvania, Philadelphia
ADSORPTION IN MICROPOROUS MATERIALS: ANALYTICAL EQUATIONS FOR TYPE I ISOTHERMS AT HIGH PRESSURE A. L. MYERS Department of Chemical and Biomolecular Engineering University of Pennsylvania, Philadelphia
ON THE DETERMINATION OF THE SPINODAL CURVE FOR THE SYSTEM WATER + CHLOROFORM + ACETIC ACID FROM THE MUTUAL DIFFUSION COEFFICIENTS
 CONENSE MATTER ON THE ETERMINATION OF THE SPINOAL CURVE FOR THE SYSTEM WATER + CHLOROFORM + ACETIC ACI FROM THE MUTUAL IFFUSION COEFFICIENTS ANIELA BUZATU 1, FLORIN. BUZATU 2,3, RAU P. LUNGU 4, LUIGI PAUANO
CONENSE MATTER ON THE ETERMINATION OF THE SPINOAL CURVE FOR THE SYSTEM WATER + CHLOROFORM + ACETIC ACI FROM THE MUTUAL IFFUSION COEFFICIENTS ANIELA BUZATU 1, FLORIN. BUZATU 2,3, RAU P. LUNGU 4, LUIGI PAUANO
C deposits (63.5/2) g of copper; the quantity passed is therefore
 7. SOLUTIONS OF ELECTROLYTES n Faraday s Laws, Molar Conductivity, and Weak Electrolytes 7.1. 96 500 C deposits (63.5/2) g of copper; the quantity passed is therefore 96 500 0.04 2 63.5 C The current was
7. SOLUTIONS OF ELECTROLYTES n Faraday s Laws, Molar Conductivity, and Weak Electrolytes 7.1. 96 500 C deposits (63.5/2) g of copper; the quantity passed is therefore 96 500 0.04 2 63.5 C The current was
Mutual diffusion in the ternary mixture of water + methanol + ethanol: Experiments and Molecular Simulation
 - 1 - Mutual diffusion in the ternary mixture of water + methanol + ethanol: Experiments and Molecular Simulation Tatjana Janzen, Gabriela Guevara-Carrión, Jadran Vrabec University of Paderborn, Germany
- 1 - Mutual diffusion in the ternary mixture of water + methanol + ethanol: Experiments and Molecular Simulation Tatjana Janzen, Gabriela Guevara-Carrión, Jadran Vrabec University of Paderborn, Germany
Partial molar volumes at infinite dilution in aqueous solutions of NaCl, LiCl, NaBr, and CsBr at temperatures from 550 K to 725 K
 J. Chem. Thermodynamics 1998, 3, 312 Partial molar volumes at infinite dilution in aqueous solutions of NaCl, LiCl, NaBr, and CsBr at temperatures from 55 K to 725 K Josef Sedlbauer, Department of Chemistry,
J. Chem. Thermodynamics 1998, 3, 312 Partial molar volumes at infinite dilution in aqueous solutions of NaCl, LiCl, NaBr, and CsBr at temperatures from 55 K to 725 K Josef Sedlbauer, Department of Chemistry,
Physical Properties of Solutions
 Physical Properties of Solutions Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 12.1- Types of solutions A solution is a homogenous mixture of 2 or
Physical Properties of Solutions Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 12.1- Types of solutions A solution is a homogenous mixture of 2 or
Electrolyte Solutions
 Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
Lecture 6: Irreversible Processes
 Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP4, Thermodynamics and Phase Diagrams, H. K. D. H. Bhadeshia Lecture 6: Irreversible Processes Thermodynamics generally
Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP4, Thermodynamics and Phase Diagrams, H. K. D. H. Bhadeshia Lecture 6: Irreversible Processes Thermodynamics generally
Equation Writing for a Neutralization Reaction
 Equation Writing for a Neutralization Reaction An Acid-Base reaction is also called a Neutralization reaction because the acid (generates H + or H 3 O + ) and base (generates OH ) properties of the reactants
Equation Writing for a Neutralization Reaction An Acid-Base reaction is also called a Neutralization reaction because the acid (generates H + or H 3 O + ) and base (generates OH ) properties of the reactants
Phase Behavior of Aqueous Na K Mg Ca Cl NO 3 Mixtures: Isopiestic Measurements and Thermodynamic Modeling
 J Solution Chem (2007) 36: 723 765 DOI 10.1007/s10953-007-9145-2 ORIGINAL PAPER Phase Behavior of Aqueous Na K Mg Ca Cl NO 3 Mixtures: Isopiestic Measurements and Thermodynamic Modeling Mirosław S. Gruszkiewicz
J Solution Chem (2007) 36: 723 765 DOI 10.1007/s10953-007-9145-2 ORIGINAL PAPER Phase Behavior of Aqueous Na K Mg Ca Cl NO 3 Mixtures: Isopiestic Measurements and Thermodynamic Modeling Mirosław S. Gruszkiewicz
70 Example: If a solution is m citric acid, what is the molar concentration (M) of the solution? The density of the solution is 1.
 70 Example: If a solution is 0.688 m citric acid, what is the molar concentration (M) of the solution? The density of the solution is 1.049 g/ml molality definition molarity definition To solve the problem,
70 Example: If a solution is 0.688 m citric acid, what is the molar concentration (M) of the solution? The density of the solution is 1.049 g/ml molality definition molarity definition To solve the problem,
Diffusion of an Ionic Drug in Micellar Aqueous Solutions
 Langmuir 2009, 25, 3425-3434 3425 Diffusion of an Ionic Drug in Micellar Aqueous Solutions Huixiang Zhang, and Onofrio Annunziata*, Department of Chemistry, Texas Christian UniVersity, Fort Worth, Texas
Langmuir 2009, 25, 3425-3434 3425 Diffusion of an Ionic Drug in Micellar Aqueous Solutions Huixiang Zhang, and Onofrio Annunziata*, Department of Chemistry, Texas Christian UniVersity, Fort Worth, Texas
Observable Electric Potential and Electrostatic Potential in Electrochemical Systems
 658 J. Phys. Chem. B 2000, 104, 658-662 Observable Electric Potential and Electrostatic Potential in Electrochemical Systems Javier Garrido* and José A. Manzanares Departamento de Termodinámica, UniVersitat
658 J. Phys. Chem. B 2000, 104, 658-662 Observable Electric Potential and Electrostatic Potential in Electrochemical Systems Javier Garrido* and José A. Manzanares Departamento de Termodinámica, UniVersitat
Viscosities of oxalic acid and its salts in water and binary aqueous mixtures of tetrahydrofuran at different temperatures
 J. Chem. Sci., Vol. 117, No. 4, July 2005, pp. 351 357. Indian Academy of Sciences. Viscosities of oxalic acid and its salts in water and binary aqueous mixtures of tetrahydrofuran at different temperatures
J. Chem. Sci., Vol. 117, No. 4, July 2005, pp. 351 357. Indian Academy of Sciences. Viscosities of oxalic acid and its salts in water and binary aqueous mixtures of tetrahydrofuran at different temperatures
Salinity Gradients for Sustainable Energy: Primer, Progress, and Prospects
 Supporting Information Salinity Gradients for Sustainable Energy: Primer, Progress, and Prospects Ngai Yin Yip *,, Doriano Brogioli, Hubertus V. M. Hamelers, and Kitty Nijmeijer Department of Earth and
Supporting Information Salinity Gradients for Sustainable Energy: Primer, Progress, and Prospects Ngai Yin Yip *,, Doriano Brogioli, Hubertus V. M. Hamelers, and Kitty Nijmeijer Department of Earth and
Name Chemistry Pre-AP. Notes: Solutions
 Name Chemistry Pre-AP Notes: Solutions Period I. Intermolecular Forces (IMFs) A. Attractions Between Molecules Attractions between molecules are called and are very important in determining the properties
Name Chemistry Pre-AP Notes: Solutions Period I. Intermolecular Forces (IMFs) A. Attractions Between Molecules Attractions between molecules are called and are very important in determining the properties
PAPER No.6: PHYSICAL CHEMISTRY-II (Statistical
 Subject PHYSICAL Paper No and Title Module No and Title Module Tag 6, PHYSICAL -II (Statistical 34, Method for determining molar mass - I CHE_P6_M34 Table of Contents 1. Learning Outcomes 2. Introduction
Subject PHYSICAL Paper No and Title Module No and Title Module Tag 6, PHYSICAL -II (Statistical 34, Method for determining molar mass - I CHE_P6_M34 Table of Contents 1. Learning Outcomes 2. Introduction
An aqueous solution is 8.50% ammonium chloride by mass. The density of the solution is g/ml Find: molality, mole fraction, molarity.
 66 An aqueous solution is 8.50% ammonium chloride by mass. The density of the solution is 1.024 g/ml Find: molality, mole fraction, molarity. Find molality: mass percent molality Assuming 100 g solution,
66 An aqueous solution is 8.50% ammonium chloride by mass. The density of the solution is 1.024 g/ml Find: molality, mole fraction, molarity. Find molality: mass percent molality Assuming 100 g solution,
CHEM Exam 3 - March 31, 2017
 CHEM 3530 - Exam 3 - March 31, 2017 Constants and Conversion Factors NA = 6.02x10 23 mol -1 R = 8.31 J/mol-K = 8.31 kpa-l/mol-k 1 bar = 100 kpa = 750 torr 1 kpa = 7.50 torr 1 J = 1 kpa-l 1 kcal = 4.18
CHEM 3530 - Exam 3 - March 31, 2017 Constants and Conversion Factors NA = 6.02x10 23 mol -1 R = 8.31 J/mol-K = 8.31 kpa-l/mol-k 1 bar = 100 kpa = 750 torr 1 kpa = 7.50 torr 1 J = 1 kpa-l 1 kcal = 4.18
AR-7781 (Physical Chemistry)
 Model Answer: B.Sc-VI th Semester-CBT-602 AR-7781 (Physical Chemistry) One Mark Questions: 1. Write a nuclear reaction for following Bethe s notation? 35 Cl(n, p) 35 S Answer: 35 17Cl + 1 1H + 35 16S 2.
Model Answer: B.Sc-VI th Semester-CBT-602 AR-7781 (Physical Chemistry) One Mark Questions: 1. Write a nuclear reaction for following Bethe s notation? 35 Cl(n, p) 35 S Answer: 35 17Cl + 1 1H + 35 16S 2.
ELECTROCHEMICAL SYSTEMS
 ELECTROCHEMICAL SYSTEMS Third Edition JOHN NEWMAN and KAREN E. THOMAS-ALYEA University of California, Berkeley ELECTROCHEMICAL SOCIETY SERIES WILEY- INTERSCIENCE A JOHN WILEY & SONS, INC PUBLICATION PREFACE
ELECTROCHEMICAL SYSTEMS Third Edition JOHN NEWMAN and KAREN E. THOMAS-ALYEA University of California, Berkeley ELECTROCHEMICAL SOCIETY SERIES WILEY- INTERSCIENCE A JOHN WILEY & SONS, INC PUBLICATION PREFACE
LESSON 11. Glossary: Solutions. Boiling-point elevation
 LESSON 11 Glossary: Solutions Boiling-point elevation Colligative properties Freezing-point depression Molality Molarity (M) Mole (mol) Mole fraction Saturated solution a colligative property of a solution
LESSON 11 Glossary: Solutions Boiling-point elevation Colligative properties Freezing-point depression Molality Molarity (M) Mole (mol) Mole fraction Saturated solution a colligative property of a solution
Chapter 12. Properties of Solutions
 Chapter 12. Properties of Solutions What we will learn: Types of solutions Solution process Interactions in solution Types of concentration Concentration units Solubility and temperature Solubility and
Chapter 12. Properties of Solutions What we will learn: Types of solutions Solution process Interactions in solution Types of concentration Concentration units Solubility and temperature Solubility and
Chapter 17: Additional Aspects of Aqueous equilibria. Common-ion effect
 Chapter 17: Additional Aspects of Aqueous equilibria Learning goals and key skills: Describe the common ion effect. Explain how a buffer functions. Calculate the ph of a buffer solution. Calculate the
Chapter 17: Additional Aspects of Aqueous equilibria Learning goals and key skills: Describe the common ion effect. Explain how a buffer functions. Calculate the ph of a buffer solution. Calculate the
Chapter 11. Intermolecular Forces and Liquids & Solids
 Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
- Let's look at how things dissolve into water, since aqueous solutions are quite common. sucrose (table sugar)
 68 HOW THINGS DISSOLVE - Let's look at how things dissolve into water, since aqueous solutions are quite common. sucrose (table sugar)... what happens? - Water molecules pull the sugar molecules out of
68 HOW THINGS DISSOLVE - Let's look at how things dissolve into water, since aqueous solutions are quite common. sucrose (table sugar)... what happens? - Water molecules pull the sugar molecules out of
Properties of Solutions. Overview of factors affecting solubility Ways of expressing concentration Physical properties of solutions
 Properties of Solutions Overview of factors affecting solubility Ways of expressing concentration Physical properties of solutions Learning objectives Define terms solute, solvent and solution Distinguish
Properties of Solutions Overview of factors affecting solubility Ways of expressing concentration Physical properties of solutions Learning objectives Define terms solute, solvent and solution Distinguish
Chapter 12 & 13 Test Review. Bond, Ionic Bond
 Chapter 12 & 13 Test Review A solid solute dissolved in a solid solvent is an Alloy What is happening in a solution at equilibrium? The Ionic rate of Bond dissolving is equal to the rate of crystallization.
Chapter 12 & 13 Test Review A solid solute dissolved in a solid solvent is an Alloy What is happening in a solution at equilibrium? The Ionic rate of Bond dissolving is equal to the rate of crystallization.
Advanced Analytical Chemistry Lecture 12. Chem 4631
 Advanced Analytical Chemistry Lecture 12 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Advanced Analytical Chemistry Lecture 12 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Part I.
 Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
8. ELECTROCHEMICAL CELLS. n Electrode Reactions and Electrode Potentials a. H 2 2H + + 2e. Cl 2 + 2e 2Cl. H 2 + Cl 2 2H + + 2Cl ; z = 2
 8. ELECTROCHEMICAL CELLS n Electrode Reactions and Electrode Potentials 8.1. a. H H + + e Cl + e Cl H + Cl H + + Cl ; z = E = E RT F ln ( a H +a Cl ) b. Hg(l)+ Cl Hg Cl + e H + + e H Hg + H + + Cl Hg Cl
8. ELECTROCHEMICAL CELLS n Electrode Reactions and Electrode Potentials 8.1. a. H H + + e Cl + e Cl H + Cl H + + Cl ; z = E = E RT F ln ( a H +a Cl ) b. Hg(l)+ Cl Hg Cl + e H + + e H Hg + H + + Cl Hg Cl
Chemistry FINAL: CONTENT Review Packet
 Chemistry FINAL: CONTENT Review Packet Name: Period: Date: Classification of Matter & Chemical/ Physical Changes 1. are substances that are made up of two or more elements which are chemically combined
Chemistry FINAL: CONTENT Review Packet Name: Period: Date: Classification of Matter & Chemical/ Physical Changes 1. are substances that are made up of two or more elements which are chemically combined
Warm UP. between carbonate and lithium. following elements have? 3) Name these compounds: 1) Write the neutral compound that forms
 Warm UP 1) Write the neutral compound that forms between carbonate and lithium 2) How many valence electrons do the following elements have? a) Chlorine b) Neon c) Potassium 3) Name these compounds: a)
Warm UP 1) Write the neutral compound that forms between carbonate and lithium 2) How many valence electrons do the following elements have? a) Chlorine b) Neon c) Potassium 3) Name these compounds: a)
768 Lecture #11 of 18
 Lecture #11 of 18 768 769 Q: What s in this set of lectures? A: B&F Chapter 2 main concepts: Section 2.1 : Section 2.3: Salt; Activity; Underpotential deposition Transference numbers; Liquid junction potentials
Lecture #11 of 18 768 769 Q: What s in this set of lectures? A: B&F Chapter 2 main concepts: Section 2.1 : Section 2.3: Salt; Activity; Underpotential deposition Transference numbers; Liquid junction potentials
Chapter 13. Characteristics of a Solution. Example of A Homogenous Mixtures. Solutions
 Chapter 13 Solutions Characteristics of a Solution A solution is a homogeneous mixture A solution is composed of a: Solute: the substance in lesser amount Solvent: the substance in greater amount Two liquid
Chapter 13 Solutions Characteristics of a Solution A solution is a homogeneous mixture A solution is composed of a: Solute: the substance in lesser amount Solvent: the substance in greater amount Two liquid
Chem 321 Lecture 11 - Chemical Activities 10/3/13
 Student Learning Objectives Chem 321 Lecture 11 - Chemical Activities 10/3/13 One of the assumptions that has been made in equilibrium calculations thus far has been to equate K to a ratio of concentrations.
Student Learning Objectives Chem 321 Lecture 11 - Chemical Activities 10/3/13 One of the assumptions that has been made in equilibrium calculations thus far has been to equate K to a ratio of concentrations.
EXPERIMENT 3 THE IODINE CLOCK
 EXPERIMENT 3 THE IODINE CLOCK Introduction The Rates of Chemical Reactions Broadly defined, chemical kinetics is the study of the rates at which chemical reactions proceed. Oftentimes, reaction rate data
EXPERIMENT 3 THE IODINE CLOCK Introduction The Rates of Chemical Reactions Broadly defined, chemical kinetics is the study of the rates at which chemical reactions proceed. Oftentimes, reaction rate data
HONORS CHEMISTRY Putting It All Together II
 NAME: SECTION: HONORS CHEMISTRY Putting It All Together II Calculations in Chemistry It s time to pull out your calculators! In the first review sheet, you were able to write formulas of compounds when
NAME: SECTION: HONORS CHEMISTRY Putting It All Together II Calculations in Chemistry It s time to pull out your calculators! In the first review sheet, you were able to write formulas of compounds when
HYDRODYNAMIC AND THERMODYNAMIC ASPECTS OF DIFFUSION COEFFICIENTS IN THE TERNARY SYSTEM WATER-CHLOROFORM-ACETIC ACID AT 25 C
 U.P.B. Sci. Bull., Series A, Vol. 69, No. 3, 27 ISSN 223-727 HYDRODYNAMIC AND THERMODYNAMIC ASPECTS OF DIFFUSION COEFFICIENTS IN THE TERNARY SYSTEM WATER-CHLOROFORM-ACETIC ACID AT 25 C Daniela BUZATU,
U.P.B. Sci. Bull., Series A, Vol. 69, No. 3, 27 ISSN 223-727 HYDRODYNAMIC AND THERMODYNAMIC ASPECTS OF DIFFUSION COEFFICIENTS IN THE TERNARY SYSTEM WATER-CHLOROFORM-ACETIC ACID AT 25 C Daniela BUZATU,
READING A. INTRODUCTION CHE425L POTENTIOMETRY WITH K + ION-SELECTIVE ELECTRODE. Skoog, Holler and Crouch: Chapter 23 and Appendix 3.
 CHE425L POTENTIOMETRY WITH K + ION-SELECTIVE ELECTRODE READING Skoog, Holler and Crouch: Chapter 23 and Appendix 3. A. INTRODUCTION Potentiometry is a static electroanalytical method in which the potential
CHE425L POTENTIOMETRY WITH K + ION-SELECTIVE ELECTRODE READING Skoog, Holler and Crouch: Chapter 23 and Appendix 3. A. INTRODUCTION Potentiometry is a static electroanalytical method in which the potential
CHAPTER 7: Solutions & Colloids 7.2 SOLUBILITY. Degrees of Solution. Page PHYSICAL STATES of SOLUTIONS SOLUTION
 CHAPTER 7: Solutions & Colloids Predict the relative solubility of materials on the basis of polarity Describe solution formation in terms of solutesolvent interactions Calculate solution concentrations
CHAPTER 7: Solutions & Colloids Predict the relative solubility of materials on the basis of polarity Describe solution formation in terms of solutesolvent interactions Calculate solution concentrations
Honors Chemistry Unit 4 Exam Study Guide Solutions, Equilibrium & Reaction Rates
 Honors Chemistry Unit 4 Exam Study Guide Solutions, Equilibrium & Reaction Rates Define the following vocabulary terms. Solute Solvent Solution Molarity Molality Colligative property Electrolyte Non-electrolyte
Honors Chemistry Unit 4 Exam Study Guide Solutions, Equilibrium & Reaction Rates Define the following vocabulary terms. Solute Solvent Solution Molarity Molality Colligative property Electrolyte Non-electrolyte
Thermodynamics IV - Free Energy and Chemical Equilibria Chemical Potential (Partial Molar Gibbs Free Energy)
 Thermodynamics IV - Free Energy and Chemical Equilibria Chemical Potential (Partial Molar Gibbs Free Energy) increase in the Gibbs free energy of the system when 1 mole of i is added to a large amount
Thermodynamics IV - Free Energy and Chemical Equilibria Chemical Potential (Partial Molar Gibbs Free Energy) increase in the Gibbs free energy of the system when 1 mole of i is added to a large amount
Chapter 17. Additional Aspects of Aqueous Equilibria 蘇正寬 Pearson Education, Inc.
 Chapter 17 Additional Aspects of Aqueous Equilibria 蘇正寬 chengkuan@mail.ntou.edu.tw Additional Aspects of Aqueous Equilibria 17.1 The Common-Ion Effect 17.2 Buffers 17.3 Acid Base Titrations 17.4 Solubility
Chapter 17 Additional Aspects of Aqueous Equilibria 蘇正寬 chengkuan@mail.ntou.edu.tw Additional Aspects of Aqueous Equilibria 17.1 The Common-Ion Effect 17.2 Buffers 17.3 Acid Base Titrations 17.4 Solubility
Transport (kinetic) phenomena: diffusion, electric conductivity, viscosity, heat conduction...
 Transport phenomena 1/16 Transport (kinetic) phenomena: diffusion, electric conductivity, viscosity, heat conduction... Flux of mass, charge, momentum, heat,...... J = amount (of quantity) transported
Transport phenomena 1/16 Transport (kinetic) phenomena: diffusion, electric conductivity, viscosity, heat conduction... Flux of mass, charge, momentum, heat,...... J = amount (of quantity) transported
Electrochemistry SYBSc 2017
 Electrochemistry SYBSc 2017 Definition It is a branch in chemistry which deals with the qualitative and quantitative studies of chemical changes brought about by the passage of electricity. It is also
Electrochemistry SYBSc 2017 Definition It is a branch in chemistry which deals with the qualitative and quantitative studies of chemical changes brought about by the passage of electricity. It is also
Brass, a solid solution of Zn and Cu, is used to make musical instruments and many other objects.
 Brass, a solid solution of Zn and Cu, is used to make musical instruments and many other objects. 14.1 General Properties of Solutions 14.2 Solubility 14.3 Rate of Dissolving Solids 14.4 Concentration
Brass, a solid solution of Zn and Cu, is used to make musical instruments and many other objects. 14.1 General Properties of Solutions 14.2 Solubility 14.3 Rate of Dissolving Solids 14.4 Concentration
9.01 Solutions. The Chemistry of Matter in Water. Dr. Fred Omega Garces. Chemistry 100, Miramar College. 1 Solutions. Aug 17
 9.01 Solutions The Chemistry of Matter in Water Dr. Fred Omega Garces Chemistry 100, Miramar College 1 Solutions 8.01 Solutions How water Dissolves Salts 2 Solutions Components of Solution Homogeneous
9.01 Solutions The Chemistry of Matter in Water Dr. Fred Omega Garces Chemistry 100, Miramar College 1 Solutions 8.01 Solutions How water Dissolves Salts 2 Solutions Components of Solution Homogeneous
Chapter 12. Preview. Objectives Solutions Suspensions Colloids Solutes: Electrolytes Versus Nonelectrolytes
 Preview Objectives Solutions Suspensions Colloids Solutes: Electrolytes Versus Nonelectrolytes Section 1 Types of Mixtures Objectives Distinguish between electrolytes and nonelectrolytes. List three different
Preview Objectives Solutions Suspensions Colloids Solutes: Electrolytes Versus Nonelectrolytes Section 1 Types of Mixtures Objectives Distinguish between electrolytes and nonelectrolytes. List three different
Cell membrane resistance and capacitance
 Cell membrane resistance and capacitance 1 Two properties of a cell membrane gives rise to two passive electrical properties: Resistance: Leakage pathways allow inorganic ions to cross the membrane. Capacitance:
Cell membrane resistance and capacitance 1 Two properties of a cell membrane gives rise to two passive electrical properties: Resistance: Leakage pathways allow inorganic ions to cross the membrane. Capacitance:
Effects of concentration and temperature on the coupled heat and mass transport in liquid mixtures
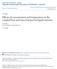 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Thermal Mechanics Chemical and Biomolecular Engineering Research and Publications 3-26-2001 Effects of concentration
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Thermal Mechanics Chemical and Biomolecular Engineering Research and Publications 3-26-2001 Effects of concentration
MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS
 MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS The following questions illustrate the range of the test in terms of the abilities measured, the disciplines covered, and the difficulty of the questions
MAJOR FIELD TEST IN CHEMISTRY SAMPLE QUESTIONS The following questions illustrate the range of the test in terms of the abilities measured, the disciplines covered, and the difficulty of the questions
Ions in Solution. Solvent and Solute
 Adapted from Peer-led Team Learning Begin at the beginning and go on till you come to the end: then stop." Early ideas of atoms and compounds, developed primarily through the reactions of solids and gases,
Adapted from Peer-led Team Learning Begin at the beginning and go on till you come to the end: then stop." Early ideas of atoms and compounds, developed primarily through the reactions of solids and gases,
DERIVATION OF PRACTICAL KEDEM - KATCHALSKY EQUATIONS FOR MEMBRANE SUBSTANCE TRANSPORT
 DERIVATION OF PRACTICAL KEDEM - KATCHALSKY EQUATIONS FOR MEMBRANE SUBSTANCE TRANSPORT M. Jarzyńska Technical High School of Environment Developing, Piotrków Trybunalski, Broniewskiego 16, Poland, e-mail:
DERIVATION OF PRACTICAL KEDEM - KATCHALSKY EQUATIONS FOR MEMBRANE SUBSTANCE TRANSPORT M. Jarzyńska Technical High School of Environment Developing, Piotrków Trybunalski, Broniewskiego 16, Poland, e-mail:
LIMITING IONIC PARTIAL MOLAR VOLUMES OF R 4 N + AND I IN AQUEOUS METHANOL AT K
 Int. J. Chem. Sci.: 11(1), 2013, 321-330 ISSN 0972-768X www.sadgurupublications.com LIMITING IONIC PARTIAL MOLAR VOLUMES OF R 4 N + AND I IN AQUEOUS METHANOL AT 298.15 K N. P. NIKAM * and S. V. PATIL a
Int. J. Chem. Sci.: 11(1), 2013, 321-330 ISSN 0972-768X www.sadgurupublications.com LIMITING IONIC PARTIAL MOLAR VOLUMES OF R 4 N + AND I IN AQUEOUS METHANOL AT 298.15 K N. P. NIKAM * and S. V. PATIL a
Learning Outcomes: At the end of this assignment, students will be able to:
 Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Sem /2007. Fisika Polimer Ariadne L. Juwono
 Chapter 8. Measurement of molecular weight and size 8.. End-group analysis 8.. Colligative property measurement 8.3. Osmometry 8.4. Gel-permeation chromatography 8.5. Ultracentrifugation 8.6. Light-scattering
Chapter 8. Measurement of molecular weight and size 8.. End-group analysis 8.. Colligative property measurement 8.3. Osmometry 8.4. Gel-permeation chromatography 8.5. Ultracentrifugation 8.6. Light-scattering
Slide 1. Slide 2. Slide 3. Colligative Properties. Compounds in Aqueous Solution. Rules for Net Ionic Equations. Rule
 Slide 1 Colligative Properties Slide 2 Compounds in Aqueous Solution Dissociation - The separation of ions that occurs when an ionic compound dissolves Precipitation Reactions - A chemical reaction in
Slide 1 Colligative Properties Slide 2 Compounds in Aqueous Solution Dissociation - The separation of ions that occurs when an ionic compound dissolves Precipitation Reactions - A chemical reaction in
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory
 Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title THE ACTIVITY COEFFICIENT OF AQUEOUS NaHCO3 Permalink https://escholarship.org/uc/item/4wv0n58j Author Pitzer, Kenneth S.
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title THE ACTIVITY COEFFICIENT OF AQUEOUS NaHCO3 Permalink https://escholarship.org/uc/item/4wv0n58j Author Pitzer, Kenneth S.
KCl in water at supercritical temperatures,3 made use of an expression in which
 A REPRESENTATION OF ISOTHERMAL ION-ION-PAIR-SOLVENT EQUILIBRIA INDEPENDENT OF CHANGES IN DIELECTRIC CONSTANT* By WILLIAM L. MARSHALL AND ARVIN S. QUIST REACTOR CHEMISTRY DIVISION, OAK RIDGE NATIONAL LABORATORY,
A REPRESENTATION OF ISOTHERMAL ION-ION-PAIR-SOLVENT EQUILIBRIA INDEPENDENT OF CHANGES IN DIELECTRIC CONSTANT* By WILLIAM L. MARSHALL AND ARVIN S. QUIST REACTOR CHEMISTRY DIVISION, OAK RIDGE NATIONAL LABORATORY,
Ajaya Bhattarai * and Bijan Das. Department of Chemistry, North Bengal University, Darjeeling, , India.
 J. Nepal Chem. Soc., Vol. 23, 28/29 Effects of Concentration, Temperature and Solvent Composition on the Partial Molar Volumes of Sodium Polystyrenesulphonate in Methanol -Water Solvent Media Ajaya Bhattarai
J. Nepal Chem. Soc., Vol. 23, 28/29 Effects of Concentration, Temperature and Solvent Composition on the Partial Molar Volumes of Sodium Polystyrenesulphonate in Methanol -Water Solvent Media Ajaya Bhattarai
SOLUTION CONCENTRATIONS
 SOLUTION CONCENTRATIONS The amount of solute in a solution (concentration) is an important property of the solution. A dilute solution contains small quantities of solute relative to the solvent, while
SOLUTION CONCENTRATIONS The amount of solute in a solution (concentration) is an important property of the solution. A dilute solution contains small quantities of solute relative to the solvent, while
Name Date Class PROPERTIES OF SOLUTIONS
 16.1 PROPERTIES OF SOLUTIONS Section Review Objectives Identify the factors that determine the rate at which a solute dissolves Identify the units usually used to express the solubility of a solute Calculate
16.1 PROPERTIES OF SOLUTIONS Section Review Objectives Identify the factors that determine the rate at which a solute dissolves Identify the units usually used to express the solubility of a solute Calculate
Soluble: A solute that dissolves in a specific solvent. Insoluble: A solute that will not dissolve in a specific solvent. "Like Dissolves Like"
 Solutions Homogeneous Mixtures Solutions: Mixtures that contain two or more substances called the solute and the solvent where the solute dissolves in the solvent so the solute and solvent are not distinguishable
Solutions Homogeneous Mixtures Solutions: Mixtures that contain two or more substances called the solute and the solvent where the solute dissolves in the solvent so the solute and solvent are not distinguishable
Quick Review. - Chemical equations - Types of chemical reactions - Balancing chemical equations - Stoichiometry - Limiting reactant/reagent
 Quick Review - Chemical equations - Types of chemical reactions - Balancing chemical equations - Stoichiometry - Limiting reactant/reagent Water H 2 O Is water an ionic or a covalent compound? Covalent,
Quick Review - Chemical equations - Types of chemical reactions - Balancing chemical equations - Stoichiometry - Limiting reactant/reagent Water H 2 O Is water an ionic or a covalent compound? Covalent,
HEATS OF MIXING OF MIXED SOLVENT SOLUTIONS OF ALKALI METAL SALTS OF SUBSTITUTED BENZENESULFONIC ACIDS
 Journal of Thermal Analysis and Calorimetry, Vol. 64 (2001) 707 712 HEATS OF MIXING OF MIXED SOLVENT SOLUTIONS OF ALKALI METAL SALTS OF SUBSTITUTED BENZENESULFONIC ACIDS C. Pohar, K. Arh and K. Škufca
Journal of Thermal Analysis and Calorimetry, Vol. 64 (2001) 707 712 HEATS OF MIXING OF MIXED SOLVENT SOLUTIONS OF ALKALI METAL SALTS OF SUBSTITUTED BENZENESULFONIC ACIDS C. Pohar, K. Arh and K. Škufca
Properties of Solutions. Chapter 13
 Properties of Solutions Chapter 13 Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. Saturated solution: contains the maximum amount of a
Properties of Solutions Chapter 13 Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. Saturated solution: contains the maximum amount of a
Water & Solutions Chapter 17 & 18 Assignment & Problem Set
 Water & Solutions Chapter 17 & 18 Assignment & Problem Set Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Water & Solutions 2 Vocabulary (know
Water & Solutions Chapter 17 & 18 Assignment & Problem Set Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Water & Solutions 2 Vocabulary (know
Thermodynamic study of the Na-Cu-Cl-SO 4 -H 2 O system at the temperature K
 J. Chem. Thermodynamics 2, 32, 285 295 doi:1.16/jcht.1999.564 Available online at http://www.idealibrary.com on Thermodynamic study of the Na-Cu-Cl-SO 4 -H 2 O system at the temperature 298.15 K Christomir
J. Chem. Thermodynamics 2, 32, 285 295 doi:1.16/jcht.1999.564 Available online at http://www.idealibrary.com on Thermodynamic study of the Na-Cu-Cl-SO 4 -H 2 O system at the temperature 298.15 K Christomir
Chapter 13. Ions in aqueous Solutions And Colligative Properties
 Chapter 13 Ions in aqueous Solutions And Colligative Properties Compounds in Aqueous Solution Dissociation The separation of ions that occurs when an ionic compound dissolves H2O NaCl (s) Na+ (aq) + Cl-
Chapter 13 Ions in aqueous Solutions And Colligative Properties Compounds in Aqueous Solution Dissociation The separation of ions that occurs when an ionic compound dissolves H2O NaCl (s) Na+ (aq) + Cl-
957 Lecture #13 of 18
 Lecture #13 of 18 957 958 Q: What was in this set of lectures? A: B&F Chapter 2 main concepts: Section 2.1 : Section 2.3: Salt; Activity; Underpotential deposition Transference numbers; Liquid junction
Lecture #13 of 18 957 958 Q: What was in this set of lectures? A: B&F Chapter 2 main concepts: Section 2.1 : Section 2.3: Salt; Activity; Underpotential deposition Transference numbers; Liquid junction
Evaluation of apparent and partial molar volume of potassium ferro- and ferricyanides in aqueous alcohol solutions at different temperatures
 Indian Journal of Chemical Technology ol. 11, September 4, pp. 714-718 Evaluation of apparent and partial molar volume of potassium ferro- and ferricyanides in aqueous alcohol solutions at different temperatures
Indian Journal of Chemical Technology ol. 11, September 4, pp. 714-718 Evaluation of apparent and partial molar volume of potassium ferro- and ferricyanides in aqueous alcohol solutions at different temperatures
Liquids and Solutions
 Liquids and Solutions Introduction This course examines the properties of liquids and solutions at both the thermodynamic and the molecular level. The main topics are: Liquids, Ideal and Regular Solutions,
Liquids and Solutions Introduction This course examines the properties of liquids and solutions at both the thermodynamic and the molecular level. The main topics are: Liquids, Ideal and Regular Solutions,
Temperature Effects on Electrophoresis. Supporting Information
 Temperature Effects on Electrophoresis Supporting Information Anita Rogacs, Juan G. Santiago* Department of Mechanical Engineering, Stanford University, Stanford, California, 94305 *To whom correspondence
Temperature Effects on Electrophoresis Supporting Information Anita Rogacs, Juan G. Santiago* Department of Mechanical Engineering, Stanford University, Stanford, California, 94305 *To whom correspondence
Part A Answer all questions in this part.
 Part A Directions (1-24): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
Part A Directions (1-24): For each statement or question, record on your separate answer sheet the number of the word or expression that, of those given, best completes the statement or answers the question.
Chemistry. Approximate Timeline. Students are expected to keep up with class work when absent.
 Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 15 SOLUTIONS Day Plans for the day Assignment(s) for the day 1 Begin Chapter 15
Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 15 SOLUTIONS Day Plans for the day Assignment(s) for the day 1 Begin Chapter 15
75 A solution of 2.500g of unknown dissolved in g of benzene has a freezing point of C. What is the molecular weight of the unknown?
 75 A solution of 2.500g of unknown dissolved in 100.0 g of benzene has a freezing point of 4.880 C. What is the molecular weight of the unknown? Solving for Cm (molality) will allow us to calculate how
75 A solution of 2.500g of unknown dissolved in 100.0 g of benzene has a freezing point of 4.880 C. What is the molecular weight of the unknown? Solving for Cm (molality) will allow us to calculate how
Unit 10 Solution Chemistry 1. Solutions & Molarity 2. Dissolving 3. Dilution 4. Calculation Ion Concentrations in Solution 5. Precipitation 6.
 Unit 10 Solution Chemistry 1. Solutions & Molarity 2. Dissolving 3. Dilution 4. Calculation Ion Concentrations in Solution 5. Precipitation 6. Formula, Complete, Net Ionic Equations 7. Qualitative Analysis
Unit 10 Solution Chemistry 1. Solutions & Molarity 2. Dissolving 3. Dilution 4. Calculation Ion Concentrations in Solution 5. Precipitation 6. Formula, Complete, Net Ionic Equations 7. Qualitative Analysis
We CAN have molecular solutions (ex. sugar in water) but we will be only working with ionic solutions for this unit.
 Solubility Equilibrium The Basics (should be mostly review) Solubility is defined as the maximum amount of a substance which can be dissolved in a given solute at a given temperature. The solubility of
Solubility Equilibrium The Basics (should be mostly review) Solubility is defined as the maximum amount of a substance which can be dissolved in a given solute at a given temperature. The solubility of
Topic 3200 Water; Hydrogen Ions Chemists are often faced with the situation where on adding salt MX to water (l)
 Topic 200 Water; Hydrogen Ions Chemists are often faced with the situation where on adding salt M to water (l) experimental evidence shows that the cation exists as a hydrate M 2 ) n. For example, adding
Topic 200 Water; Hydrogen Ions Chemists are often faced with the situation where on adding salt M to water (l) experimental evidence shows that the cation exists as a hydrate M 2 ) n. For example, adding
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Topic 2060 Gibbs Energies; Salt Solutions; Aqueous Mixtures The solubilities of chemical substance j in two liquids l
 Topic 6 Gibbs Energies; Salt Solutions; Aqueous Mixtures The solubilities of chemical substance in two liquids l and l (at the same T and p) offers a method for comparing the reference chemical potentials,
Topic 6 Gibbs Energies; Salt Solutions; Aqueous Mixtures The solubilities of chemical substance in two liquids l and l (at the same T and p) offers a method for comparing the reference chemical potentials,
Solutions and Their Properties
 Chapter 11 Solutions and Their Properties Solutions: Definitions A solution is a homogeneous mixture. A solution is composed of a solute dissolved in a solvent. When two compounds make a solution, the
Chapter 11 Solutions and Their Properties Solutions: Definitions A solution is a homogeneous mixture. A solution is composed of a solute dissolved in a solvent. When two compounds make a solution, the
Friction Coefficient Analysis of Multicomponent Solute Transport Through Polymer Membranes
 Friction Coefficient Analysis of Multicomponent Solute Transport Through Polymer Membranes NARASIMHAN SUNDARAM NIKOLAOS A. PEPPAS* School of Chemical Engineering, Purdue University, West Lafayette, Indiana
Friction Coefficient Analysis of Multicomponent Solute Transport Through Polymer Membranes NARASIMHAN SUNDARAM NIKOLAOS A. PEPPAS* School of Chemical Engineering, Purdue University, West Lafayette, Indiana
Florin-Dorian Buzatu [ISI] Articles:
![Florin-Dorian Buzatu [ISI] Articles: Florin-Dorian Buzatu [ISI] Articles:](/thumbs/86/93822039.jpg) List of Publications Florin-Dorian Buzatu [ISI] Articles: 1. F. D. Buzatu, Stud. Cerc. Fiz. 36 (1984) 163-182. Soliton solutions and the quantization of the sine-gordon theory 2. A. Corciovei and F. D.
List of Publications Florin-Dorian Buzatu [ISI] Articles: 1. F. D. Buzatu, Stud. Cerc. Fiz. 36 (1984) 163-182. Soliton solutions and the quantization of the sine-gordon theory 2. A. Corciovei and F. D.
