Supplementary Information. for. Mechanistic investigation of the iridium-catalysed alkylation of amines with alcohols
|
|
|
- Melvin Rich
- 5 years ago
- Views:
Transcription
1 Supplementary Information for Mechanistic investigation of the iridium-catalysed alkylation of amines with alcohols Peter Fristrup,* Matyas Tursky and Robert Madsen* Department of Chemistry, Building 21 Technical University of Denmark DK-28 Kgs. Lyngby, Denmark Table of Contents GC retention times... 2 Competition experiments... 4 Primary KIE determination... 2 Primary KIE determination with GC-FID and GC-MS measurements Racemisation experiment Validation of computational method using two available X-ray structures XYZ coordinates and energies from computational study Calculation of theoretical KIE IRC scan calculations of representative TS s Dihedral scan, cis-imine to trans-imine
2 GC retention times Injector temperature 3 C, detector temperature 35 C. Table A. Programmed oven temperature: start 1 C, ramp 2 C/min to 3 C, 5 3 C. Compound aniline benzyl alcohol 1,2,4,5-tetramethylbenzene naphthalene p-anisalcohol Retention Time 1.52 min 1.7 min 2.11 min 2.47 min 2.92 min Table B. Programmed oven temperature: start 1 C, ramp 2 C/min to 2 C, 5 2 C, ramp 2 C/min to 3 C, 5 3 C. Compound p-toluidine p-(trifluoromethyl)aniline p-(trifluoromethyl)benzyl alcohol p-methylbenzyl alcohol p-chloroaniline p-anisidine p-chlorobenzyl alcohol p-(dimethylamino)aniline p-(hydroxymethyl)benzonitrile p-aminobenzonitrile p-(dimethylamino)benzyl alcohol methyl p-(hydroxymethyl)benzoate methyl p-aminobenzoate p-nitrobenzyl alcohol Retention Time 1.84 min 1.85 min 1.91 min 2.14 min 2.43 min 2.51 min 2.64 min 3.31 min 3.52 min 3.76 min 3.98 min 4.8 min 4.18 min 4.21 min 2
3 Table C. Programmed oven temperature: start 1 C, ramp 5 C/min to 13 C, 2 C/min to 2 C, 5 2 C, ramp 2 C to 3 C, 5 3 C. Compound aniline benzyl alcohol 1,2,4,5-tetramethylbenzene naphthalene p-chloroaniline p-aminobenzonitrile Retention Time 1.73 min 2.5 min 2.85 min 3.53 min 3.39 min 6.72 min 3
4 OMe Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Competition experiment 1, p-methoxybenzyl alcohol vs. benzyl alcohol K 2 CO 3 (14 mg,.1 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) p-methoxybenzyl alcohol (125 μl, 1 mmol) naphthalene (129 mg, 1 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion Time PhNH 2 BnOH p-meo-bnoh Naphthalene PhNH 2 BnOH p-meo-bnoh - no cat cat min h h h h h h h y = x R 2 = H 4
5 Me Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Competition experiment 2, p-methylbenzyl alcohol vs. benzyl alcohol K 2 CO 3 (14 mg,.1 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) p-methylbenzyl alcohol (122 mg, 1 mmol) naphthalene (126 mg, 1 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion time PhNH2 BnOH p-me-bnoh Naphthalene PhNH2 BnOH p-me-bnoh - no cat cat min h h h h h h h h y = 1.669x R 2 = H 5
6 CF3 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Competition experiment 3, p-(trifluoromethyl)benzyl alcohol vs. benzyl alcohol K 2 CO 3 (15 mg,.11 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) p-(trifluoromethyl)benzyl alcohol (135 μl,.99 mmol) naphthalene (127 mg, 1 mmol) [Cp*IrCl 2 ] 2 (39 mg,.49 mmol) Areas Conversion time PhNH 2 BnOH p-cf 3-BnOH Naphthalene PhNH 2 BnOH p-cf 3-BnOH - no cat cat min h h h h h h y =.1952x R 2 = H 6
7 Cl Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Competition experiment 4, p-chlorobenzyl alcohol vs. benzyl alcohol K 2 CO 3 (14 mg,.1 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) p-chlorobenzyl alcohol (143 mg, 1 mmol) naphthalene (128 mg, 1 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion time PhNH2 BnOH p-cl-bnoh Naphthalene PhNH2 BnOH p-cl-bnoh - no cat cat min h h h h h h y =.768x R 2 = H 7
8 COOMe Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Competition experiment 5, methyl p-(hydroxymethyl)benzoate vs. benzyl alcohol K 2 CO 3 (15 mg,.11 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) methyl p-(hydroxymethyl)benzoate (165 mg,.99 mmol) naphthalene (128 mg, 1 mmol) [Cp*IrCl 2 ] 2 (39 mg,.49 mmol) Areas Conversion time PhNH2 BnOH p-coome-bnoh Naphthalene PhNH2 BnOH p-coome-bnoh - no cat cat min h h h h h h h h y =.4381x R 2 = H 8
9 CN Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Competition experiment 6, p-(hydroxymethyl)benzonitrile vs. benzyl alcohol K 2 CO 3 (14 mg,.1 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) p-(hydroxymethyl)benzonitrile (132 mg,.99 mmol) naphthalene (127 mg, 1 mmol) [Cp*IrCl 2 ] 2 (39 mg,.49 mmol) Areas Conversion time PhNH 2 BnOH p-cn-bnoh Naphthalene PhNH 2 BnOH p-cn-bnoh - no cat cat min h h h h h h h h y =.2682x R 2 = H 9
10 Me2N Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Competition experiment 7, p-(dimethylamino)benzyl alcohol vs. benzyl alcohol K 2 CO 3 (14 mg,.1 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) p-(dimethylamino)benzyl alcohol (156 mg, 1.3 mmol) naphthalene (126 mg, 1 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion time PhNH 2 BnOH p-nme 2-BnOH Naphthalene PhNH 2 BnOH p-nme 2-BnOH - no cat cat min h h h h h h h y = x R 2 = H 1
11 Competition experiment 8, p-nitrobenzyl alcohol vs. p-chlorobenzyl alcohol K 2 CO 3 (14 mg,.1 mmol) aniline (18 μl, 2 mmol) p-chlorobenzyl alcohol (144 mg, 1.1 mmol) p-nitrobenzyl alcohol (154 mg, 1.2 mmol) naphthalene (13 mg, 1.1 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion time aniline p-cl-bnoh p-no 2-BnOH Naphthalene PhNH 2 p-cl-bnoh p-no 2-BnOH - no cat cat min h h h h h h y =.2943x R 2 =
12 Competition experiment 9, p-toluidine vs. aniline K 2 CO 3 (15 mg,.11 mmol) aniline (9 μl, 1 mmol) p-toluidine (17 mg, 1. mmol) benzyl alcohol (21 μl, 2.3 mmol) naphthalene (126 mg,.98 mmol) [Cp*IrCl 2 ] 2 (41 mg,.51 mmol) Areas Conversion time BnOH p-h-aniline p-me-aniline Naphthalene BnOH p-h-aniline p-me-aniline - no cat cat min h h h h h h h y = x R 2 =
13 Competition experiment 1, p-anisidine vs. aniline K 2 CO 3 (14 mg,.1 mmol) aniline (9 μl, 1 mmol) p-anisidine (122 mg,.99 mmol) benzyl alcohol (21 μl, 2.3 mmol) naphthalene (126 mg,.98 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion time BnOH p-h-aniline p-meo-aniline Naphthalene BnOH p-h-aniline p-meo-aniline - no cat cat min h h h h h h h y = x R 2 =
14 Competition experiment 11, p-(trifluoromethyl)aniline vs. aniline K 2 CO 3 (14 mg,.1 mmol) aniline (9 μl, 1 mmol) p-(trifluoromethyl)aniline (125 μl, 1. mmol) benzyl alcohol (21 μl, 2.3 mmol) naphthalene (126 mg,.98 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion time BnOH p-h-aniline p-cf 3-aniline Naphthalene BnOH p-h-aniline p-cf 3-aniline - no cat cat min h h h h h h h y =.665x R 2 =
15 Competition experiment 12, p-chloroaniline vs. aniline K 2 CO 3 (14 mg,.1 mmol) aniline (9 μl, 1 mmol) p-chloroaniline (129 mg, 1.1 mmol) benzyl alcohol (21 μl, 2.3 mmol) 1,2,4,5-tetramethylbenzene (126 mg,.94 mmol) [Cp*IrCl 2 ] 2 (39 mg,.49 mmol) Areas Conversion time BnOH p-h-aniline p-cl-aniline Tetramethylbenzene BnOH p-h-aniline p-cl-aniline - no cat cat min h h h h h h h y =.4431x R 2 =
16 Competition experiment 13, methyl p-aminobenzoate vs. aniline K 2 CO 3 (15 mg,.11 mmol) aniline (9 μl, 1 mmol) methyl p-aminobenzoate (152 mg, 1. mmol) benzyl alcohol (21 μl, 2.3 mmol) naphthalene (125 mg,.98 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion time BnOH p-h-aniline p-coome-aniline Naphthalene BnOH p-h-aniline p-coome-aniline - no cat cat min h h h h h h h h y =.2245x R 2 =
17 Competition experiment 14, p-aminobenzonitrile vs. p-chloroaniline K 2 CO 3 (14 mg,.1 mmol) p-chloroaniline (124 mg,.97 mmol) p-aminobenzonitrile (119 mg, 1. mmol) benzyl alcohol (21 μl, 2.3 mmol) 1,2,4,5-tetramethylbenzene (132 mg,.98 mmol) [Cp*IrCl 2 ] 2 (39 mg,.49 mmol) Areas Conversion time BnOH p-cl-aniline p-cn-aniline Tetramethylbenzene BnOH p-cl-aniline p-cn-aniline - no cat cat min h h h h h h h h y =.128x R 2 =
18 Competition experiment 15, p-(dimethylamino)aniline vs. p-anisidine K 2 CO 3 (14 mg,.1 mmol) p-anisidine (122 mg,.99 mmol) p-(dimethylamino)aniline (135 mg,.99 mmol) benzyl alcohol (21 μl, 2.3 mmol) 1,2,4,5-tetramethylbenzene (132 mg,.98 mmol) [Cp*IrCl 2 ] 2 (39 mg,.49 mmol) Areas Conversion time BnOH p-meo-aniline p-nme 2-aniline Tetramethylbenzene BnOH p-meo-aniline p-nme 2-aniline - no cat cat min min min h min min min h min h h h h
19 2.5 2 y = 5.45x R 2 =
20 d2-bnoh Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry Primary KIE determination Compounds loads: K 2 CO 3 (15 mg,.11 mmol) aniline (18 μl, 2 mmol) benzyl alcohol (15 μl, 1 mmol) p-methoxybenzyl alcohol (125 μl, 1 mmol) naphthalene (125 mg,.98 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol).6.5 y =.273x R 2 = OMe k D /k OMe =.27 k OMe /k H =1.944 k H / k D =
21 Primary KIE determination with benzyl alcohol, -d 2 vs. p-chlorobenzyl alcohol K 2 CO 3 (14 mg,.1 mmol) aniline (186 mg, 2 mmol) benzyl alcohol, -d 2 (11 mg, 1 mmol) p-chlorobenzyl alcohol (142 mg, 1 mmol) naphthalene (129 mg, 1 mmol) [Cp*IrCl 2 ] 2 (4 mg,.5 mmol) Areas Conversion Time d2-bnoh p-cl-bnoh Naphthalene d2-bnoh p-cl-bnoh - no cat min h h h h h h y = 1.755x R² =
22 There seems to be a slight curvature which may arise from loss of deuterium from benzyl alcohol, -d 2. When only the first part of the curve is plotted the difference in reactivity is significantly higher (1.37)..7.6 y = x R² = Using these data one can again determine the kinetic isotope effect (KIE) as follows: k Cl /k D = 1.37 k Cl /k H =.71 k H / k D =
23 GC-MS data from primary KIE determination, benzyl alcohol, -d 2 vs. p-chlorobenzyl alcohol m/z=17 m/z=18 m/z=19 m/z=11 m/z= GC-MS data showing that the benzyl alcohol, -d 2 (m/z=11) loses its deuterium content during the reaction m/z=14 m/z=141 m/z=142 m/z=143 m/z=144 m/z= GC-MS data showing that the undeuterated p-chlorobenzyl alcohol (m/z=142) increases its deuterium content during the reaction (m/z=144). 23
24 m/z=181 m/z=182 m/z=183 m/z=184 m/z=185 m/z= GC-MS data showing the data for the unsubstituted product. Here, the deuterium content is high (m/z=184) and also remains relatively high during the reaction which illustrates that the final reduction to the amine is virtually irreversible m/z=216 m/z=217 m/z=218 m/z=219 m/z= GC-MS data for the chloro-substituted amine product. Here, the deuterium content is low in the beginning (m/z=217), but gradually increases (m/z=218). 24
25 To estimate the degree of reversibility of the initial alcohol oxidation we decided to plot the conversion of benzyl alcohol measured by GC-FID (i.e. total amount irrespective of deuterium content) versus the decrease of the m/z=11 signal benzyl alcohol (GC-FID) m/z= Since the experiment was run under competetion conditions one will not obtain absolute kinetics or reaction orders, but it is still clear that the disappearance of the benzyl alcohol is faster than the deuterium exchange of the unreacted benzyl alcohol, -d 2. The same type of analysis was carried out for p-chlorobenzyl alcohol m/z=142 p-cl-benzyl alcohol Also here the disappearance of the total amount of p-chlorobenzyl alcohol is significantly faster than the proton/deuterium exchange. 25
26 Racemisation experiment In an oven-dried Schlenk tube were placed K 2 CO 3 (4 mg,.3 mmol), (R)-1-deutero-1- phenylethanol (29 mg,.24 mmol, 81% ee), acetophenone (3 mg,.24 mmol) and dry toluene (.5 ml). The mixture was placed in an oil bath preheated to 11 C and a GC sample was taken out. Then, [Cp*IrCl 2 ] 2 (1 mg,.125 mmol) was added. After addition of the catalyst, a GC sample was taken out immediately. Then, GC samples were taken out after 3 min, 1h, 2h, 3h, 4h, 5h. At that time, GC analysis showed 8% ee (Column: Chrompack CP Chirasil-Dex CB.25 mm x 25 m column), the reaction mixture was cooled down to rt and subjected to column chromatography (hexane/et 2 O 4/1), which yielded 1 mg of 1-phenylethanol. The 1 H content was determined by NMR to be 6%. 26
27 Computational study The description of the computational method is included in the main text of the manuscript. Two available crystal structures (Refcode: DCPMIR, ARADAY1) 1 were used to access the accuracy of the applied computational method (B3LYP in combination with an effective core potential for iridium). Below is shown an overlay of the crystal structure (blue) with the calculated structure (red) (figure S1 and S2). The superimposition was performed for atoms heavier than hydrogen to avoid the otherwise dominating influence of the methyl rotation. For the DCPMIR structure the RMSD was.951 Å, indicating an acceptable overall structural similarity. The largest errors in the computational structure was found to be overly long Ir-Cl and Ir-Cpstar bonds, in both cases on the order of.4-.5å which we are confident will not influence the overall conclusions reached in this study. For ARADAY1 structure the RMSD was calculated to.1112 Å, slightly higher than for the DCPMIR structure, which to some degree can be assigned to the lack of symmetry in the crystal structure, resulting in three slightly different experimental Ir-Cl bond lengths. Figure S1 Overlay of X-ray structure (blue, DCPMIR) with a structure energy minimized using DFT/B3LYP (red). RMSD for non-hydrogen atoms were.951 Å. Figure S2 Overlay of X-ray structure (blue, ARADAY1) with a structure energy minimized using DFT/B3LYP (red). RMSD for non-hydrogen atoms were.1112 Å. (1) (a) DCPMIR: M. R. Churchill, S. A. Julis, Inorg. Chem., 1977, 16, ; (b) ARADAY1: M. Maekawa, Y. Suenaga, T. Kuroda-Sowa, M. Munakata, Anal. Sci., 24, 2, x11-x12. 27
28 XYZ Coordinates and energies for calculated complexes. Ir2_Cpstar2_Cl4_dimer E_scf (B3LYP/6-31G*) = a.u. E_scf (M6/6-31G*) = a.u. E_Gibbs = a.u. E_solv = a.u. ZPE = kcal/mol Ir Cl Cl C C C C C C C C C C Cl Ir Cl C C C C C C C C C C H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H
29 Ir_Cpstar_Cl2_monomer E_scf (B3LYP/6-31G*) = a.u. E_scf (M6/6-31G*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol Ir Cl Cl C C C C C C H H H C H H H C H H H C H H H C H H H Ir_Cpstar_PhNH2_PhCO_alcohol (1a) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol C... C C C C C H H H C H H H C H H H C H
30 H H C H H H H Ir C O H C C C C C C H H H H H N H H C C C C C C H H H H H Ir_Cpstar_PhNH2_PhCO_E_TS (1b) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol C... C C C C C H H H C H H H C H H H C H H
31 H C H H H H Ir C O H C C C C C C H H H H H N H H C C C C C C H H H H H Ir_Cpstar_PhNH2_PhCO_aldehyde (1c) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol C... C C C C C H H H C H H H C H H H C H H H
32 C H H H H Ir C O H C C C C C C H H H H H N H H C C C C C C H H H H H Ir_Cpstar_H_Hemiaminal_bidentate (1d) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol C O H H N C C C C C C H H H H H C C C C C
33 C H H H H H H Ir C C C C C C H H H C H H H C H H H C H H H C H H H H Ir_Cpstar_H_Hemiaminal_Hshift (1e) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol C O H H N C C C C C C H H H H H C C C C
34 C C H H H H H Ir C C C C C C H H H C H H H C H H H C H H H C H H H H H Ir_Cpstar_PhNH2_PhCNPh_imine (1f) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol C... C C C C C H H H C H H H C H H H C H H H
35 C H H H H Ir C N H C C C C C C H H H H H C C C C C C H H H H H N H H C C C C C C H H H H H Ir_Cpstar_PhNH2_PhCNPh_E_TS (1g) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol C... C C C C C H H H C
36 H H H C H H H C H H H C H H H H Ir C N H C C C C C C H H H H H C C C C C C H H H H H N H H C C C C C C H H H H H Ir_Cpstar_PhNH2_PhCNPh_amine (1h) E_scf (B3LYP/6-31G*) = a.u. E_scf (B3LYP/6-31G+*) = a.u. E_scf (M6/6-31G*) = a.u. E_scf (M6/6-31G+*) = a.u. E_solv = a.u. E_Gibbs = a.u. ZPE = kcal/mol 36
37 C... C C C C C H H H C H H H C H H H C H H H C H H H H Ir C N H C C C C C C H H H H H C C C C C C H H H H H N H H C C C C C C H H H H H
38 38
39 Calculation of theoretical KIE The calculation of the theoretical KIE was performed using the Hessian calculated in gas phase for the complexes 1a and 1b. To allow comparison with experimental results the Gibbs free energy was evaluated at T=383 K (=11 C, bp. of toluene). This gave the following results: Ir_Cpstar_PhNH2_PhCO_E_TS_freq_D_383K Ir_Cpstar_PhNH2_PhCO_E_TS_alcohol_min_full_freq_D_383K Ir_Cpstar_PhNH2_PhCO_E_TS_freq_H_383K Ir_Cpstar_PhNH2_PhCO_E_TS_alcohol_min_full_freq_H_383K a.u a.u a.u a.u. From this we find an activation energy of 37.7 kj/mol (proton) and 4.9 kj/mol (deuterium). This difference in energy of 3.2 kj/mol corresponds to a difference in reaction rate of 2.7 when using a Boltzmann expression evaluated at T=383K. Performing a similar analysis using differences in zero-point energies (ZPE) resulted in a calculated KIE of 2.73, thus documenting that the observed effect arises mainly from differences in bond strengths, as expected. 39
40 IRC scan calculations An IRC scan calculations was carried out for a representative beta-hydride elimination. Below are shown three structures, one close to the TS and one from either end of the IRC scan. Figure 1 IRC step close to TS (forward1) Figure 2 IRC step close to alcohol (reverse 8) 4
41 Figure 3 IRC step close to aldehyde (forward 1) Table 1 Data from the IRC scan of a representative beta-hydride abstraction Ir_Cpstar_PhNH2_PhCO_Z_TS_freq_IRC point # Rxn. Coord Energy (Hartree) Relative Energy (kj/mol) C-H distance (Å)
42 Figure 4 Relative energy vs. IRC coordinate for beta-hydride elimination Figure 5 Relative energy vs. C-H distance (Å) for the C-H bond which is being broken/formed. 42
43 An IRC scan calculations was also carried out for a representative imine reduction. Below are shown three structures, one close to the TS and one from either end of the IRC scan. Figure 6 IRC step close to TS (forward1) Figure 7 IRC step close to amine (reverse 1) 43
44 Figure 8 IRC step close to imine (forward 1) Table 2 Data from the IRC scan of a representative imine reduction. Ir_Cpstar_PhNH2_PhCNPh_E_TS_freq_IRC point # Rxn. Coord Energy Relative Energy (kj/mol) C-H distance (Å)
45 Figure 9 Relative energy vs. IRC coordinate for imine reduction. Figure 1 Relative energy vs. C-H distance (Å) for the C-H bond which is being broken/formed. 45
46 Dihedral scan, cis-imine to trans-imine To estimate the possibility of cis to trans isomerization of the intermediate imine, a dihedral scan calculation was carried out (B3LYP/6-31G*, gas phase). It is clear that the barrier is very high, which can be ascribed to the very strong electrostatic interaction between the hydride and the proton on the phenyl group of the imine. Figure 11 Energy profile for the dihedral scan. Table 3 The data obtained from the dihedral scan. dihedral angle Energy (Ha) Relative Energy Below are shown the structures obtained during the scan from cis-imine to trans-imine: 46
47 Figure 12 Structures obtained in the dihedral scan from cis-imine (top-left) to trans-imine (bottom right). 47
Supporting Information
 Supporting Information Efficient copper-catalyzed coupling of aryl chlorides, bromides and iodides with aqueous ammonia Hanhui Xu and Christian Wolf* Department of Chemistry, Georgetown University, Washington
Supporting Information Efficient copper-catalyzed coupling of aryl chlorides, bromides and iodides with aqueous ammonia Hanhui Xu and Christian Wolf* Department of Chemistry, Georgetown University, Washington
SUPPORTING INFORMATION. Elucidation of the role of betaine hydrochloride in glycerol esterification: towards bio-based ionic building blocks
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2017 SUPPORTING INFORMATION Elucidation of the role of betaine hydrochloride in glycerol esterification:
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2017 SUPPORTING INFORMATION Elucidation of the role of betaine hydrochloride in glycerol esterification:
Supporting Information
 Supporting Information A General thod for Two-Step Transamidation of Secondary Amides using Commercially Available, Airand Moisture-Stable Palladium/C (-eterocyclic Carbene) Complexes Guangrong ng, Peng
Supporting Information A General thod for Two-Step Transamidation of Secondary Amides using Commercially Available, Airand Moisture-Stable Palladium/C (-eterocyclic Carbene) Complexes Guangrong ng, Peng
Thiourea Derivatives as Brønsted Acid Organocatalysts
 Supporting Information Thiourea Derivatives as Brønsted Acid Organocatalysts Ádám Madarász, Zsolt Dósa, Szilárd Varga, * Tibor Soós, Antal Csámpai, Imre Pápai * Institute of Organic Chemistry, Research
Supporting Information Thiourea Derivatives as Brønsted Acid Organocatalysts Ádám Madarász, Zsolt Dósa, Szilárd Varga, * Tibor Soós, Antal Csámpai, Imre Pápai * Institute of Organic Chemistry, Research
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2014 Supporting Information Palladium-Catalyzed Construction of Spirooxindoles by Arylative Cyclization of 3-( -Disubstituted)allylidene-2-Oxindoles
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2014 Supporting Information Palladium-Catalyzed Construction of Spirooxindoles by Arylative Cyclization of 3-( -Disubstituted)allylidene-2-Oxindoles
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Pd-Catalyzed C-H Activation/xidative Cyclization of Acetanilide with orbornene:
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Pd-Catalyzed C-H Activation/xidative Cyclization of Acetanilide with orbornene:
QUATERNARY AMMONIUM SALTS AS ALKYLATING REAGENTS IN C-H ACTIVATION CHEMISTRY
 Electronic Supporting Information for QUATERNARY AMMONIUM SALTS AS ALKYLATING REAGENTS IN C-H ACTIVATION CHEMISTRY Manuel Spettel, Robert Pollice, and Michael Schnürch* Institute of Applied Synthetic Chemistry,
Electronic Supporting Information for QUATERNARY AMMONIUM SALTS AS ALKYLATING REAGENTS IN C-H ACTIVATION CHEMISTRY Manuel Spettel, Robert Pollice, and Michael Schnürch* Institute of Applied Synthetic Chemistry,
Figure 1: Transition State, Saddle Point, Reaction Pathway
 Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Potential Energy Surfaces
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Potential Energy Surfaces
Instructor Notes for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System
 Instructor otes for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison,
Instructor otes for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison,
Catalytic hydrogenation of liquid alkenes with a silica grafted hydride. pincer iridium(iii) complex: Support for a heterogeneous mechanism
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information for Catalysis Science & Technology Catalytic
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information for Catalysis Science & Technology Catalytic
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Catalytic performance of Keplerate polyoxomolybdates in green epoxidation of alkenes with hydrogen
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Catalytic performance of Keplerate polyoxomolybdates in green epoxidation of alkenes with hydrogen
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 07 Electronic Supplementary Information Hydroaminomethylation/hydrohydroxymethylation sequence
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 07 Electronic Supplementary Information Hydroaminomethylation/hydrohydroxymethylation sequence
The Mechanistic Studies of the Wacker Oxidation. Tyler W. Wilson SED Group Meeting
 The Mechanistic Studies of the Wacker xidation Tyler W. Wilson SE Group Meeting 11.27.2007 Introduction xidation of ethene by (II) chloride solutions (Phillips, 1894) -First used as a test for alkenes
The Mechanistic Studies of the Wacker xidation Tyler W. Wilson SE Group Meeting 11.27.2007 Introduction xidation of ethene by (II) chloride solutions (Phillips, 1894) -First used as a test for alkenes
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Content Synthesis of compounds 2a, 2b in Scheme
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Content Synthesis of compounds 2a, 2b in Scheme
A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones. Jin-Quan Yu, a and E. J.
 A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones Jin-Quan Yu, a and E. J. Corey b * a Department of Chemistry, Cambridge University, Cambridge CB2 1EW, United
A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones Jin-Quan Yu, a and E. J. Corey b * a Department of Chemistry, Cambridge University, Cambridge CB2 1EW, United
Supporting Information
 Supporting Information Activation of Ene-Diamido Samarium Methoxide with Hydrosilane for Selectively Catalytic Hydrosilylation of Alkenes and Polymerization of Styrene: an Experimental and Theoretical
Supporting Information Activation of Ene-Diamido Samarium Methoxide with Hydrosilane for Selectively Catalytic Hydrosilylation of Alkenes and Polymerization of Styrene: an Experimental and Theoretical
Iridium-catalyzed regioselective decarboxylative allylation of. β-ketoacids: efficient construction of γ, δ-unsaturated ketones
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Iridium-catalyzed regioselective decarboxylative allylation of β-ketoacids: efficient construction
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Iridium-catalyzed regioselective decarboxylative allylation of β-ketoacids: efficient construction
Supplementary Material
 10.1071/CH17506_AC CSIRO 2018 Australian Journal of Chemistry 2018, 71(5), 341-347 Supplementary Material Magnesium alkoxide complexes of (benzimidazolylmethyl)amino ligands: Synthesis and applications
10.1071/CH17506_AC CSIRO 2018 Australian Journal of Chemistry 2018, 71(5), 341-347 Supplementary Material Magnesium alkoxide complexes of (benzimidazolylmethyl)amino ligands: Synthesis and applications
Development of Safe and Scalable Continuous-Flow Methods for. Palladium-Catalyzed Aerobic Oxidation Reactions
 Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2019 Supporting Information Difluorocarbene-derived trifluoromethylselenolation of benzyl halides Xin-Lei
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2019 Supporting Information Difluorocarbene-derived trifluoromethylselenolation of benzyl halides Xin-Lei
Electronic Supplementary Information. for
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information for Two Chiral Catalysts in Action: Insights on Cooperativity
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information for Two Chiral Catalysts in Action: Insights on Cooperativity
Activation of a hydroamination gold catalyst by oxidation of a redox non-innocent chlorostibine Z-ligand
 Activation of a hydroamination gold catalyst by oxidation of a redox non-innocent chlorostibine Z-ligand Haifeng Yang and François P. Gabbaï Department of Chemistry, Texas A&M University, College Station,
Activation of a hydroamination gold catalyst by oxidation of a redox non-innocent chlorostibine Z-ligand Haifeng Yang and François P. Gabbaï Department of Chemistry, Texas A&M University, College Station,
Silver-Catalyzed Cascade Reaction of β-enaminones and Isocyanoacetates to Construct Functionalized Pyrroles
 Supporting Information for Silver-Catalyzed Cascade Reaction of β-enaminones and Isocyanoacetates to Construct Functionalized Pyrroles Guichun Fang, a, Jianquan Liu a,c, Junkai Fu,* a Qun Liu, a and Xihe
Supporting Information for Silver-Catalyzed Cascade Reaction of β-enaminones and Isocyanoacetates to Construct Functionalized Pyrroles Guichun Fang, a, Jianquan Liu a,c, Junkai Fu,* a Qun Liu, a and Xihe
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Merging visible-light photoredox and copper catalysis
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Merging visible-light photoredox and copper catalysis
Syntheses and Structures of Mono-, Di- and Tetranuclear Rhodium or Iridium Complexes of Thiacalix[4]arene Derivatives
![Syntheses and Structures of Mono-, Di- and Tetranuclear Rhodium or Iridium Complexes of Thiacalix[4]arene Derivatives Syntheses and Structures of Mono-, Di- and Tetranuclear Rhodium or Iridium Complexes of Thiacalix[4]arene Derivatives](/thumbs/88/115555933.jpg) Supplementary Information Syntheses and Structures of Mono-, Di- and Tetranuclear Rhodium or Iridium Complexes of Thiacalix[4]arene Derivatives Kenji Hirata, Toshiaki Suzuki, Ai Noya, Izuru Takei and Masanobu
Supplementary Information Syntheses and Structures of Mono-, Di- and Tetranuclear Rhodium or Iridium Complexes of Thiacalix[4]arene Derivatives Kenji Hirata, Toshiaki Suzuki, Ai Noya, Izuru Takei and Masanobu
SUPPORTING INFORMATION. A simple asymmetric organocatalytic approach to optically active cyclohexenones
 SUPPRTING INFRMATIN A simple asymmetric organocatalytic approach to optically active cyclohexenones Armando Carlone, Mauro Marigo, Chris North, Aitor Landa and Karl Anker Jørgensen* Danish National Research
SUPPRTING INFRMATIN A simple asymmetric organocatalytic approach to optically active cyclohexenones Armando Carlone, Mauro Marigo, Chris North, Aitor Landa and Karl Anker Jørgensen* Danish National Research
Supplementary Materials for
 www.sciencemag.org/content/350/6258/298/suppl/dc1 Supplementary Materials for Hydrogenation of carboxylic acids with a homogeneous cobalt catalyst Ties J. Korstanje, Jarl Ivar van der Vlugt, Cornelis J.
www.sciencemag.org/content/350/6258/298/suppl/dc1 Supplementary Materials for Hydrogenation of carboxylic acids with a homogeneous cobalt catalyst Ties J. Korstanje, Jarl Ivar van der Vlugt, Cornelis J.
Direct Alkylation of Amines with Alcohols Catalyzed by Base
 Supporting Information for Direct Alkylation of Amines with Alcohols Catalyzed by Base Qiang-Qiang Li, Zu-Feng Xiao, Chuan-Zhi Yao, Hong-Xing Zheng, and Yan-Biao Kang* Center of Advanced Nanocatalysis,
Supporting Information for Direct Alkylation of Amines with Alcohols Catalyzed by Base Qiang-Qiang Li, Zu-Feng Xiao, Chuan-Zhi Yao, Hong-Xing Zheng, and Yan-Biao Kang* Center of Advanced Nanocatalysis,
Supporting Information
 Supporting Information (Tetrahedron. Lett.) Cavitands with Inwardly and Outwardly Directed Functional Groups Mao Kanaura a, Kouhei Ito a, Michael P. Schramm b, Dariush Ajami c, and Tetsuo Iwasawa a * a
Supporting Information (Tetrahedron. Lett.) Cavitands with Inwardly and Outwardly Directed Functional Groups Mao Kanaura a, Kouhei Ito a, Michael P. Schramm b, Dariush Ajami c, and Tetsuo Iwasawa a * a
12A Lab Activity Notes
 12A Lab Activity Notes Lab Activity 12 In this experiment, RX reacts with a base. The methoxide ion (CH 3 O - ) is a small, strong base. The tert-butoxide ion ((CH 3 ) 3 CO - ) is a large, strong base.
12A Lab Activity Notes Lab Activity 12 In this experiment, RX reacts with a base. The methoxide ion (CH 3 O - ) is a small, strong base. The tert-butoxide ion ((CH 3 ) 3 CO - ) is a large, strong base.
Aerobic Alcohol Oxidation and Oxygen Atom Transfer Reactions Catalyzed by a Nonheme Iron(II)-α-Keto Acid Complex
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for Aerobic Alcohol Oxidation and Oxygen Atom Transfer
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for Aerobic Alcohol Oxidation and Oxygen Atom Transfer
Palladium-Catalyzed Alkylarylation of Acrylamides with
 Supporting Information Palladium-Catalyzed Alkylarylation of Acrylamides with Unactivated Alkyl Halides Hua Wang, Li-a Guo, and Xin-Hua Duan* Department of Chemistry, School of Science and ME Key Laboratory
Supporting Information Palladium-Catalyzed Alkylarylation of Acrylamides with Unactivated Alkyl Halides Hua Wang, Li-a Guo, and Xin-Hua Duan* Department of Chemistry, School of Science and ME Key Laboratory
Supporting information. A Nonradical Zinc-Barbier Reaction for Diastereoselective Synthesis of Vicinal Amino Alcohols
 Supporting information S1 A Nonradical Zinc-Barbier Reaction for Diastereoselective Synthesis of Vicinal Amino Alcohols Lise Keinicke, Peter Fristrup, Per-Ola Norrby,* and Robert Madsen* Center for Sustainable
Supporting information S1 A Nonradical Zinc-Barbier Reaction for Diastereoselective Synthesis of Vicinal Amino Alcohols Lise Keinicke, Peter Fristrup, Per-Ola Norrby,* and Robert Madsen* Center for Sustainable
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Catalyst-Free and Solvent-Free ydroboration of Aldehydes. Stachowiak,
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Catalyst-Free and Solvent-Free ydroboration of Aldehydes. Stachowiak,
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/10/eaas9319/dc1 Supplementary Materials for Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions
advances.sciencemag.org/cgi/content/full/4/10/eaas9319/dc1 Supplementary Materials for Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions
Supporting Information
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Palladium-Catalyzed Regio-selective xidative C-H
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Palladium-Catalyzed Regio-selective xidative C-H
Supporting Information
 Supporting Information Synthesis of H-Indazoles from Imidates and Nitrosobenzenes via Synergistic Rhodium/Copper Catalysis Qiang Wang and Xingwei Li* Dalian Institute of Chemical Physics, Chinese Academy
Supporting Information Synthesis of H-Indazoles from Imidates and Nitrosobenzenes via Synergistic Rhodium/Copper Catalysis Qiang Wang and Xingwei Li* Dalian Institute of Chemical Physics, Chinese Academy
Study on the Selective Hydrogenation of Nitroaromatics to N-aryl hydroxylamines using a Supported Pt nanoparticle Catalyst
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Supporting Information
 Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information Unmasking Representative Structures of TMP-Active Hauser and Turbo Hauser Bases Pablo García-Álvarez, David V. Graham,
Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information Unmasking Representative Structures of TMP-Active Hauser and Turbo Hauser Bases Pablo García-Álvarez, David V. Graham,
Qile Wang, and Nan Zheng* Department of Chemistry and Biochemistry, University of Arkansas. Fayetteville, Arkansas,
 Supporting Information A Photocatalyzed Synthesis of Naphthalenes by Using Aniline as a Traceless Directing Group in [4+2] Annulation of AminoBenzocyclobutenes with Alkynes Qile Wang, and Nan Zheng* Department
Supporting Information A Photocatalyzed Synthesis of Naphthalenes by Using Aniline as a Traceless Directing Group in [4+2] Annulation of AminoBenzocyclobutenes with Alkynes Qile Wang, and Nan Zheng* Department
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown.
 Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Supporting Information. DBU-Mediated Metal-Free Oxidative Cyanation of α-amino. Carbonyl Compounds: Using Molecular Oxygen as the Oxidant
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information DBU-Mediated Metal-Free Oxidative Cyanation of α-amino
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information DBU-Mediated Metal-Free Oxidative Cyanation of α-amino
Triazabicyclodecene: an Effective Isotope. Exchange Catalyst in CDCl 3
 Triazabicyclodecene: an Effective Isotope Exchange Catalyst in CDCl 3 Supporting Information Cyrille Sabot, Kanduluru Ananda Kumar, Cyril Antheaume, Charles Mioskowski*, Laboratoire de Synthèse Bio-rganique,
Triazabicyclodecene: an Effective Isotope Exchange Catalyst in CDCl 3 Supporting Information Cyrille Sabot, Kanduluru Ananda Kumar, Cyril Antheaume, Charles Mioskowski*, Laboratoire de Synthèse Bio-rganique,
List of contents: 1. Table S1 of The crystallographic and refinement data of 1, 2, and TableS2 of Bond lengths and bond angles of 1 3
 Transfer Hydrogenation (ph independent) of Ketones and Aldehydes in Water with Glycerol: Ru, Rh and Ir Catalysts with COOH Group Near Metal on (Phenylthio)methyl-2-Pyridine Scaffold Om Prakash, Hemant
Transfer Hydrogenation (ph independent) of Ketones and Aldehydes in Water with Glycerol: Ru, Rh and Ir Catalysts with COOH Group Near Metal on (Phenylthio)methyl-2-Pyridine Scaffold Om Prakash, Hemant
CHEM 344 Fall 2015 Final Exam (100 pts)
 CHEM 344 Fall 2015 Final Exam (100 pts) Name: TA Name: DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Have a swell winter break. Directions for drawing molecules, reactions, and electron-pushing mechanisms:
CHEM 344 Fall 2015 Final Exam (100 pts) Name: TA Name: DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Have a swell winter break. Directions for drawing molecules, reactions, and electron-pushing mechanisms:
Supporting Information for. an Equatorial Diadduct: Evidence for an Electrophilic Carbanion
 Supporting Information for Controlled Synthesis of C 70 Equatorial Multiadducts with Mixed Addends from an Equatorial Diadduct: Evidence for an Electrophilic Carbanion Shu-Hui Li, Zong-Jun Li,* Wei-Wei
Supporting Information for Controlled Synthesis of C 70 Equatorial Multiadducts with Mixed Addends from an Equatorial Diadduct: Evidence for an Electrophilic Carbanion Shu-Hui Li, Zong-Jun Li,* Wei-Wei
Aerobic Oxidation Using Air Catalyzed by [Cp*IrCl 2 ] 2
![Aerobic Oxidation Using Air Catalyzed by [Cp*IrCl 2 ] 2 Aerobic Oxidation Using Air Catalyzed by [Cp*IrCl 2 ] 2](/thumbs/86/94726523.jpg) Worcester Polytechnic Institute Department of Chemistry and Biochemistry Aerobic Oxidation Using Air Catalyzed by [Cp*IrCl 2 ] 2 A Major Qualifying Project Report presented by Wenbo Wu. Chemistry -2014-
Worcester Polytechnic Institute Department of Chemistry and Biochemistry Aerobic Oxidation Using Air Catalyzed by [Cp*IrCl 2 ] 2 A Major Qualifying Project Report presented by Wenbo Wu. Chemistry -2014-
Supporting Information. Ruthenium Catalyzed Oxidative Homocoupling of Arylboronic Acids in Water:
 Supporting Information Ruthenium Catalyzed Oxidative Homocoupling of Arylboronic Acids in Water: Ligand Tuned Reactivity and Mechanistic Study Deepika Tyagi, Chinky Binnani, Rohit K. Rai, Ambikesh D. Dwivedi,
Supporting Information Ruthenium Catalyzed Oxidative Homocoupling of Arylboronic Acids in Water: Ligand Tuned Reactivity and Mechanistic Study Deepika Tyagi, Chinky Binnani, Rohit K. Rai, Ambikesh D. Dwivedi,
F G C G H H C B B A A DD E E
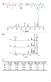 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
SUPPLEMENTARY INFORMATION
 A sustainable catalytic pyrrole synthesis Stefan Michlik, 1 Rhett Kempe* 1 1 Lehrstuhl Anorganische Chemie II, Universität Bayreuth, 95440 Bayreuth, Germany *To whom correspondence should be addressed.
A sustainable catalytic pyrrole synthesis Stefan Michlik, 1 Rhett Kempe* 1 1 Lehrstuhl Anorganische Chemie II, Universität Bayreuth, 95440 Bayreuth, Germany *To whom correspondence should be addressed.
Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril
![Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril](/thumbs/94/119194061.jpg) SUPPORTING INFORMATION Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril Lidia Strimbu Berbeci, Wei Wang and Angel E. Kaifer* Center for Supramolecular Science and
SUPPORTING INFORMATION Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril Lidia Strimbu Berbeci, Wei Wang and Angel E. Kaifer* Center for Supramolecular Science and
A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor
 A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor Andrew Bogdan 1 and D. Tyler McQuade 2, * Address: 1 Department of Chemistry and Chemical Biology, Cornell
A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor Andrew Bogdan 1 and D. Tyler McQuade 2, * Address: 1 Department of Chemistry and Chemical Biology, Cornell
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. DFT optimized structure of the [Ag III (L 1 )](ClO 4 ) 2 (1 ClO4 ) complex (CCDC code 978368). Hydrogen atoms and the two perchlorate anions have been omitted
Supplementary Figures Supplementary Figure 1. DFT optimized structure of the [Ag III (L 1 )](ClO 4 ) 2 (1 ClO4 ) complex (CCDC code 978368). Hydrogen atoms and the two perchlorate anions have been omitted
Supporting Information. Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6
![Supporting Information. Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6 Supporting Information. Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6](/thumbs/89/99017119.jpg) Supporting Information Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6 Nan Jiang and Arthur J. Ragauskas * Department of Chemistry, Georgia Institute
Supporting Information Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6 Nan Jiang and Arthur J. Ragauskas * Department of Chemistry, Georgia Institute
A General and Efficient Method for the Palladium Catalysed Conversion of Allylic Alcohols into their Corresponding Dienes
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 A General and Efficient Method for the Palladium Catalysed Conversion of
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 A General and Efficient Method for the Palladium Catalysed Conversion of
Electronic Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2012
 Ring Expansion of Alkynyl Cyclopropanes to Highly substituted Cyclobutenes via a N-Sulfonyl-1,2,3-Triazole Intermediate Renhe Liu, Min Zhang, Gabrielle Winston-Mcerson, and Weiping Tang* School of armacy,
Ring Expansion of Alkynyl Cyclopropanes to Highly substituted Cyclobutenes via a N-Sulfonyl-1,2,3-Triazole Intermediate Renhe Liu, Min Zhang, Gabrielle Winston-Mcerson, and Weiping Tang* School of armacy,
Bi-Aryl Rotation in Phenyl-dihydroimidazoquinoline. Catalysts for Kinetic Resolution of Arylalkyl Carbinols
 Bi-Aryl Rotation in Phenyl-dihydroimidazoquinoline Catalysts for Kinetic Resolution of Arylalkyl Carbinols Zheng Wang, a Jinjin Ye, a Rui Wu, a Yang-Zi Liu, a John S. Fossey, a,b Jiagao Cheng,* a and Wei-Ping
Bi-Aryl Rotation in Phenyl-dihydroimidazoquinoline Catalysts for Kinetic Resolution of Arylalkyl Carbinols Zheng Wang, a Jinjin Ye, a Rui Wu, a Yang-Zi Liu, a John S. Fossey, a,b Jiagao Cheng,* a and Wei-Ping
Supplementary information for:
 Supplementary information for: Solvent dispersible nanoplatinum-carbon nanotube hybrids for application in homogeneous catalysis Yuhong Chen, Xueyan Zhang and Somenath Mitra* Department of Chemistry and
Supplementary information for: Solvent dispersible nanoplatinum-carbon nanotube hybrids for application in homogeneous catalysis Yuhong Chen, Xueyan Zhang and Somenath Mitra* Department of Chemistry and
Reaction Progress Kinetics Analysis of 1,3-Disiloxanediols as Hydrogenbonding
 Supporting Information for: Reaction Progress Kinetics Analysis of 1,3-Disiloxanediols as Hydrogenbonding Catalysts Kayla M. Diemoz, Jason E. Hein, Sean O. Wilson, James C. Fettinger, Annaliese K. Franz*
Supporting Information for: Reaction Progress Kinetics Analysis of 1,3-Disiloxanediols as Hydrogenbonding Catalysts Kayla M. Diemoz, Jason E. Hein, Sean O. Wilson, James C. Fettinger, Annaliese K. Franz*
Supplementary Figure S1 Stable structures of I, I', II, and II' optimized at the
 H C Si 2.492 (2.348) 1.867 (2.005) Fe O 2.106 (2.077) 2.360 (2.497) N 1.126 (1.115) 2.105 (2.175) I quintet (triplet) 0.0 (+4.1) kcal/mol II triplet (quintet) 0.0 (+4.3) kcal/mol 1.857 (2.076) 2.536 (2.351)
H C Si 2.492 (2.348) 1.867 (2.005) Fe O 2.106 (2.077) 2.360 (2.497) N 1.126 (1.115) 2.105 (2.175) I quintet (triplet) 0.0 (+4.1) kcal/mol II triplet (quintet) 0.0 (+4.3) kcal/mol 1.857 (2.076) 2.536 (2.351)
Supplementary Information
 Supplementary Information C aryl -C alkyl bond formation from Cu(ClO 4 ) 2 -mediated oxidative cross coupling reaction between arenes and alkyllithium reagents through structurally well-defined Ar-Cu(III)
Supplementary Information C aryl -C alkyl bond formation from Cu(ClO 4 ) 2 -mediated oxidative cross coupling reaction between arenes and alkyllithium reagents through structurally well-defined Ar-Cu(III)
Structural Elucidation of Sumanene and Generation of its Benzylic Anions
 Structural Elucidation of Sumanene and Generation of its Benzylic Anions idehiro Sakurai, Taro Daiko, iroyuki Sakane, Toru Amaya, and Toshikazu irao Department of Applied Chemistry, Graduate School of
Structural Elucidation of Sumanene and Generation of its Benzylic Anions idehiro Sakurai, Taro Daiko, iroyuki Sakane, Toru Amaya, and Toshikazu irao Department of Applied Chemistry, Graduate School of
Enantioselective Organocatalytic Michael Addition of Malonate Esters to Nitro Olefins Using Bifunctional Cinchonine Derivatives
 Enantioselective rganocatalytic Michael Addition of Malonate Esters to itro lefins Using Bifunctional Cinchonine Derivatives Jinxing Ye, Darren J. Dixon * and Peter S. Hynes School of Chemistry, University
Enantioselective rganocatalytic Michael Addition of Malonate Esters to itro lefins Using Bifunctional Cinchonine Derivatives Jinxing Ye, Darren J. Dixon * and Peter S. Hynes School of Chemistry, University
Carbene) Catalyzed Alcohol Oxidation Using. Molecular Oxygen
 Supporting information for [Pd(HC)(PR 3 )] (HC = -Heterocyclic Carbene) Catalyzed Alcohol Oxidation Using Molecular Oxygen Václav Jurčík, Thibault E. Schmid, Quentin Dumont, Alexandra M. Z. Slawin and
Supporting information for [Pd(HC)(PR 3 )] (HC = -Heterocyclic Carbene) Catalyzed Alcohol Oxidation Using Molecular Oxygen Václav Jurčík, Thibault E. Schmid, Quentin Dumont, Alexandra M. Z. Slawin and
Straightforward Synthesis of Enantiopure (R)- and (S)-trifluoroalaninol
 S1 Supplementary Material (ESI) for Organic & Biomolecular Chemistry This journal is (c) The Royal Society of Chemistry 2010 Straightforward Synthesis of Enantiopure (R)- and (S)-trifluoroalaninol Julien
S1 Supplementary Material (ESI) for Organic & Biomolecular Chemistry This journal is (c) The Royal Society of Chemistry 2010 Straightforward Synthesis of Enantiopure (R)- and (S)-trifluoroalaninol Julien
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 ucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 ucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry Supporting Information General Remarks Most of chemicals were purchased from Sigma-Aldrich, Strem,
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry Supporting Information General Remarks Most of chemicals were purchased from Sigma-Aldrich, Strem,
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Tuning the Lewis Acidity of Boranes in rustrated Lewis Pair Chemistry: Implications for the Hydrogenation of Electron-Poor
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Tuning the Lewis Acidity of Boranes in rustrated Lewis Pair Chemistry: Implications for the Hydrogenation of Electron-Poor
Kinetics and Mechanism of the Selective Oxidation of Benzyl Alcohols by Acidified Dichromate in Aqueous Acetic Acid Medium
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (3): Pg. 1391-1396 Kinetics and Mechanism
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (3): Pg. 1391-1396 Kinetics and Mechanism
CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY]
![CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY] CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY]](/thumbs/83/88348834.jpg) CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY] 1 Introduction Outline Mass spectrometry (MS) 2 INTRODUCTION The analysis of the outcome of a reaction
CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY] 1 Introduction Outline Mass spectrometry (MS) 2 INTRODUCTION The analysis of the outcome of a reaction
1. Reagents: All commercial materials were used as received unless otherwise noted. The following solvents were obtained from a JC Meyer solvent dispe
 Supporting Information Pd-catalyzed Mono-selective ortho-c H Alkylation of N-Quinolyl Benzamides: Evidence for Stereo-retentive Coupling of Secondary Alkyl Iodides Shu-Yu Zhang, Qiong Li, Gang He, William
Supporting Information Pd-catalyzed Mono-selective ortho-c H Alkylation of N-Quinolyl Benzamides: Evidence for Stereo-retentive Coupling of Secondary Alkyl Iodides Shu-Yu Zhang, Qiong Li, Gang He, William
Supplementary Note 1 : Chemical synthesis of (E/Z)-4,8-dimethylnona-2,7-dien-4-ol (4)
 Supplementary Note 1 : Chemical synthesis of (E/Z)-4,8-dimethylnona-2,7-dien-4-ol (4) A solution of propenyl magnesium bromide in THF (17.5 mmol) under nitrogen atmosphere was cooled in an ice bath and
Supplementary Note 1 : Chemical synthesis of (E/Z)-4,8-dimethylnona-2,7-dien-4-ol (4) A solution of propenyl magnesium bromide in THF (17.5 mmol) under nitrogen atmosphere was cooled in an ice bath and
Chapter 04 Alcohols and Alkyl Halides part 01
 hapter 04 Alcohols and Alkyl alides part 01 EM 341: Spring 2012 Prof. Greg ook Functional Groups A functional group is a structural feature in a molecule that has characteristic reactivity. A functional
hapter 04 Alcohols and Alkyl alides part 01 EM 341: Spring 2012 Prof. Greg ook Functional Groups A functional group is a structural feature in a molecule that has characteristic reactivity. A functional
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
2,5-DIMETHOXY-4-IODOPHENETHYLAMINE Latest Revision: August 22, 2005
 2,5-DIMETHOXY-4-IODOPHENETHYLAMINE Latest Revision: August 22, 2005 1. SYNONYMS CFR: CAS #: Other Names: Not Available Base: Not Available Hydrochloride: 69587-11-7 4-Iodo-2,5-dimethoxyphenethylamine 4-Iodo-2,5-dimethoxybenzeneethanamine
2,5-DIMETHOXY-4-IODOPHENETHYLAMINE Latest Revision: August 22, 2005 1. SYNONYMS CFR: CAS #: Other Names: Not Available Base: Not Available Hydrochloride: 69587-11-7 4-Iodo-2,5-dimethoxyphenethylamine 4-Iodo-2,5-dimethoxybenzeneethanamine
Supporting Information:
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information: A metal free reduction of aryl-n-nitrosamines to corresponding hydrazines
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information: A metal free reduction of aryl-n-nitrosamines to corresponding hydrazines
Supporting Information
 Supporting Information Formation of Ruthenium Carbenes by gem-hydrogen Transfer to Internal Alkynes: Implications for Alkyne trans-hydrogenation Markus Leutzsch, Larry M. Wolf, Puneet Gupta, Michael Fuchs,
Supporting Information Formation of Ruthenium Carbenes by gem-hydrogen Transfer to Internal Alkynes: Implications for Alkyne trans-hydrogenation Markus Leutzsch, Larry M. Wolf, Puneet Gupta, Michael Fuchs,
Supporting Information for
 Supporting Information for Chelated Ruthenium Catalysts for Z-Selective Olefin Metathesis Koji Endo and Robert H. Grubbs* Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry
Supporting Information for Chelated Ruthenium Catalysts for Z-Selective Olefin Metathesis Koji Endo and Robert H. Grubbs* Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry
Supporting Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Sulfonato-imino copper(ii) complexes : fast and general Chan-
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Sulfonato-imino copper(ii) complexes : fast and general Chan-
Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Single-atom dispersed Co--C catalyst: structure identification and
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Single-atom dispersed Co--C catalyst: structure identification and
Nickel Phosphine Catalysts with Pendant Amines. for the Electrocatalytic Oxidation of Alcohols
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Nickel Phosphine Catalysts with Pendant Amines for the Electrocatalytic Oxidation of Alcohols Charles
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Nickel Phosphine Catalysts with Pendant Amines for the Electrocatalytic Oxidation of Alcohols Charles
The version of SI posted May 6, 2004 contained errors. The correct version was posted October 21, 2004.
 The version of SI posted May 6, 2004 contained errors. The correct version was posted October 21, 2004. Sterically Bulky Thioureas as Air and Moisture Stable Ligands for Pd-Catalyzed Heck Reactions of
The version of SI posted May 6, 2004 contained errors. The correct version was posted October 21, 2004. Sterically Bulky Thioureas as Air and Moisture Stable Ligands for Pd-Catalyzed Heck Reactions of
4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester
 NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
Supporting Text Synthesis of (2 S ,3 S )-2,3-bis(3-bromophenoxy)butane (3). Synthesis of (2 S ,3 S
 Supporting Text Synthesis of (2S,3S)-2,3-bis(3-bromophenoxy)butane (3). Under N 2 atmosphere and at room temperature, a mixture of 3-bromophenol (0.746 g, 4.3 mmol) and Cs 2 C 3 (2.81 g, 8.6 mmol) in DMS
Supporting Text Synthesis of (2S,3S)-2,3-bis(3-bromophenoxy)butane (3). Under N 2 atmosphere and at room temperature, a mixture of 3-bromophenol (0.746 g, 4.3 mmol) and Cs 2 C 3 (2.81 g, 8.6 mmol) in DMS
Supplementary Information
 3 Et 2 and TMSTf: A Synergistic Combination of Lewis Acids Eddie L. Myers a, Craig P. utts a and Varinder K. Aggarwal* a Contents Supplementary Information (a) Analysis of mixtures of TMSTf and 3 Et 2
3 Et 2 and TMSTf: A Synergistic Combination of Lewis Acids Eddie L. Myers a, Craig P. utts a and Varinder K. Aggarwal* a Contents Supplementary Information (a) Analysis of mixtures of TMSTf and 3 Et 2
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
Polymer Chemistry SUPPORTING INFORMATION
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Polymer Chemistry Thiol-Maleimide Click Chemistry: Evaluating the Influence of Solvent,
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Polymer Chemistry Thiol-Maleimide Click Chemistry: Evaluating the Influence of Solvent,
Hydroxylation Reactions of Substituted Cyclohexanes Using FeII. Complex-Triethylamine Oxide-Trifluoroacetic Acid
 Hydroxylation Reactions of Substituted Cyclohexanes Using FeII Complex-Triethylamine Oxide-Trifluoroacetic Acid (Received January 17, 1986) Substituted cyclohexanes, such as methyl-, ethyl-, and tert-butyl-cyclohexanes,
Hydroxylation Reactions of Substituted Cyclohexanes Using FeII Complex-Triethylamine Oxide-Trifluoroacetic Acid (Received January 17, 1986) Substituted cyclohexanes, such as methyl-, ethyl-, and tert-butyl-cyclohexanes,
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
Supplementary Methods
 Supplementary Methods General Information Unless stated otherwise, the following procedural techniques were used: Glassware was dried in an oven (140 C) overnight before use. All reactions were carried
Supplementary Methods General Information Unless stated otherwise, the following procedural techniques were used: Glassware was dried in an oven (140 C) overnight before use. All reactions were carried
Synergistic Cu/Ir Catalysis. Table of Contents
 Supporting Information for Stereodivergent Synthesis of, -Disubstituted -Amino Acids via Synergistic Cu/Ir Catalysis Liang Wei, 1 Qiao Zhu, 1 Shi-Ming Xu, 1 Xin Chang 1 and Chun-Jiang Wang* 1,2 1 College
Supporting Information for Stereodivergent Synthesis of, -Disubstituted -Amino Acids via Synergistic Cu/Ir Catalysis Liang Wei, 1 Qiao Zhu, 1 Shi-Ming Xu, 1 Xin Chang 1 and Chun-Jiang Wang* 1,2 1 College
Supporting information. A Brønsted Acid-Catalyzed Generation of Palladium Complexes: Efficient Head-to-Tail Dimerization of Alkynes.
 Supporting information A Brønsted Acid-Catalyzed Generation of Palladium Complexes: Efficient Head-to-Tail Dimerization of Alkynes Tieqiao Chen, a,b Cancheng Guo, a Midori Goto, b and Li-Biao Han* a,b
Supporting information A Brønsted Acid-Catalyzed Generation of Palladium Complexes: Efficient Head-to-Tail Dimerization of Alkynes Tieqiao Chen, a,b Cancheng Guo, a Midori Goto, b and Li-Biao Han* a,b
CHAPTER PRACTICE PROBLEMS CHEMISTRY
 APTER PRACTICE PRBLEMS EMISTRY Electrophilic Aromatic Substitution Name : Batch : Date : rientation influence of groups 1. Predict the characteristics of -NH + as a substituent. activating, o/p directing
APTER PRACTICE PRBLEMS EMISTRY Electrophilic Aromatic Substitution Name : Batch : Date : rientation influence of groups 1. Predict the characteristics of -NH + as a substituent. activating, o/p directing
O H Hydrogen bonding promotes H-atom transfer from C H bonds for C-alkylation of alcohols
 ydrogen bonding promotes -atom transfer from C bonds for C-alkylation of alcohols Jenna L. Jeffrey, Jack A. Terrett, David W. C. MacMillan Science 2015, 349, 1532-1536 Raffaele Colombo 9/26/2015 Raffaele
ydrogen bonding promotes -atom transfer from C bonds for C-alkylation of alcohols Jenna L. Jeffrey, Jack A. Terrett, David W. C. MacMillan Science 2015, 349, 1532-1536 Raffaele Colombo 9/26/2015 Raffaele
1G (bottom) with the phase-transition temperatures in C and associated enthalpy changes (in
 Supplementary Figure 1. Optical properties of 1 in various solvents. UV/Vis (left axis) and fluorescence spectra (right axis, ex = 420 nm) of 1 in hexane (blue lines), toluene (green lines), THF (yellow
Supplementary Figure 1. Optical properties of 1 in various solvents. UV/Vis (left axis) and fluorescence spectra (right axis, ex = 420 nm) of 1 in hexane (blue lines), toluene (green lines), THF (yellow
Catalyst-Free Reaction of Ethynyl-π-Extended Electron Acceptors with Amines
 Catalyst-Free Reaction of Ethynyl-π-Extended Electron Acceptors with Amines Atsuro Takai* and Masayuki Takeuchi* 2018 The Chemical Society of Japan Table of Contents S1. Synthesis and Characterization
Catalyst-Free Reaction of Ethynyl-π-Extended Electron Acceptors with Amines Atsuro Takai* and Masayuki Takeuchi* 2018 The Chemical Society of Japan Table of Contents S1. Synthesis and Characterization
Supporting Information for
 Supporting Information for Deuteration of boranes: catalysed versus non-catalysed processes David J. Nelson, Jonathan B. Egbert and Steven P. Nolan* EaStCHEM, School of Chemistry, University of St. Andrews,
Supporting Information for Deuteration of boranes: catalysed versus non-catalysed processes David J. Nelson, Jonathan B. Egbert and Steven P. Nolan* EaStCHEM, School of Chemistry, University of St. Andrews,
ACID-CATALYZED DEHYDRATION OF 2-METHYLCYCLOHEXANOL. Douglas G. Balmer. (T.A. Mike Hall) Dr. Dailey
 ACID-CATALYZED DEYDRATIN F 2-METYLCYCLEXANL Douglas G. Balmer (T.A. Mike all) Dr. Dailey Submitted 8 August 2007 Balmer 1 Introduction: The purpose of this experiment is to acid-catalyze the dehydration
ACID-CATALYZED DEYDRATIN F 2-METYLCYCLEXANL Douglas G. Balmer (T.A. Mike all) Dr. Dailey Submitted 8 August 2007 Balmer 1 Introduction: The purpose of this experiment is to acid-catalyze the dehydration
Supplementary Figure S1 X-ray crystallographic structure of (C)-(-)-6. (a) ORTEP drawing of (C)-(-)-6 at probability ellipsoids of 50%: tope view.
 Supplementary Figure S1 X-ray crystallographic structure of (C)-(-)-6. (a) ORTEP drawing of (C)-(-)-6 at probability ellipsoids of 50%: tope view. (b) Side view. All hydrogen atoms are omitted for clarity.
Supplementary Figure S1 X-ray crystallographic structure of (C)-(-)-6. (a) ORTEP drawing of (C)-(-)-6 at probability ellipsoids of 50%: tope view. (b) Side view. All hydrogen atoms are omitted for clarity.
Theoretical study of the BF 3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates
 Theoretical study of the BF 3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates Margarita M. Vallejos a* and Silvina C. Pellegrinet b* a Laboratorio de Química Orgánica, IQUIBA-NEA,
Theoretical study of the BF 3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates Margarita M. Vallejos a* and Silvina C. Pellegrinet b* a Laboratorio de Química Orgánica, IQUIBA-NEA,
