Rotational Alignment of NO from Pt(ll1)
|
|
|
- Jessie Green
- 5 years ago
- Views:
Transcription
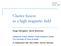
1 View Article Online / Journal Homepage / Table of Contents for this issue J. Chem. SOC., Faraday Trans. 2, 1989, 85(8), Rotational Alignment of NO from Pt(ll1) Inelastic Scattering and Molecular Desorption Dennis C. Jacobs,*? Kurt W. Kolasinski, Robert J. Madix and Richard N. Zare Department of Chemistry, Stanford University, Stanford, California 94305, U.S.A. The rotational alignment distribution of NO has been measured subsequent to the molecule s interaction with a well characterized Pt( 11 1) surface. Internal state distributions have been probed using resonance-enhanced multiphoton ionization (REMPI) spectroscopy in which lines of the NO A X+-X n (0,O) band constitute the resonant transition. NO/Pt( 11 1) scattering has been studied in two distinct regimes: inelastic scattering and trapping/desorption. In both cases, there is relatively no preferential alignment of rotation for J < However, molecules with higher rotational angular momentum show a marked increase in alignment. Inelastically scattered molecules prefer to rotate in a plane normal to the surface ( cartwheel motion), whereas desorbing molecules prefer to rotate in a plane parallel to the surface ( helicopter motion). These measurements provide new insight into momentum transfer at surfaces and the nature of the transition state which leads to molecular desorption. Rotational dynamics at the gas-surface interface reveal unique information about the molecule-surface potential. A molecule passing from the constrained two-dimensional environment of a solid surface lattice into a three-dimensional vacuum has the potential for exhibiting strong rotational polarization. Here, we investigate the rotational alignment of NO after it interacts with a Pt( 111) surface. We examine experimentally two distinct dynamical regimes, inelastic scattering and trapping/desorption. The first refers to a collision of short duration (one or a few bounces) between the molecule and the surface. This scattering event leaves the molecule with an energy distribution characteristic of both the molecule s initial conditions and the nature of the surface. The second involves a relatively long surface residence time in which the molecule completely loses memory of its initial conditions. In this latter case, the desorbed molecule s energy distribution is characterized solely by the conditions of the surface and the molecule-surface potential. There have been only two prior studies in which rotational alignment has been measured for gas-surface scattering: NO/Ag( 111)1-3 and N,/Ag( 11 l).4-6 These experiments both probed the regime of direct inelastic scattering by impinging an energetic supersonic molecular beam (10-90 kj mol- ) on a relatively cold Ag( 111) surface. Kleyn and co-worker~~-~ measured a preference for NO to scatter from Ag(ll1) with its rotational motion resembling that of a cartwheel (fig. 1). This behaviour is reconciled with a simple hard-cube model which permits forces only along the surface normal. These forces deliver an anisotropic torque on the molecule, causing an observed preference for cartwheel motion. Zare and co-~orkers~-~ found a similar result for N,/Ag( 111). Additionally, they measured the rotational orientation resulting from surface scattering. They observed a strong correlation between rotational orientation, rotational quantum number and scattering exit angle. Electric-field deflection techniques have probed surface scattering dynamics through the observation/ preparation of molecular orientation. Novakoski and McClelland7 i Present address: Department of Chemistry, University of Notre Dame, Notre Dame, IN 46556, U.S.A. 1325
2 1326 Rotational Alignment of NO from Pt( 11 1) View Article Online Fig. 1. Rotational alignment relative to the surface plane. The helicopter motion (a) is preferred for Ah2 > 0; the cartwheel motion (b) is favoured for AL < 0. measured a preferential molecular orientation for the desorption of CHF3 from Ag( 11 l), i.e. CHF3 tends to desorb with its hydrogen atom orientated toward the surface. Additionally, they found that scattering leaves the molecule with a weak orientation in the opposite sense. More recently, Kuipers et a1. observed the scattering angular distribution of NO from Ag(lll), where the incident NO was orientated in predominantly two opposite directions relative to the surface. There appeared to be a greater loss in normal translational energy when the oxygen atom, rather than the nitrogen atom, was directed toward the surface. The present study represents the first observation of rotational alignment in desorption. Additionally, it reports the rotational alignment of scattered NO in a previously unexplored regime, one in which the molecule s final rotational energy exceeds the initial energy of the incident molecular beam. Here, surface vibration-rotational energy transfer is the operative mechanism. The experiment utilizes REMPI through the NO A 2X+-X 211 (0,O) band to measure rotational alignment distributions in a statespecific manner. The quantitative reduction of REMPI spectra to alignment moments requires inclusion of both saturation in the resonant transition as well as incorporation of the symmetry character of the ionization transition. We present a brief summary of the methodology employed for the extraction of the quadrupole moment of the rotational alignment distribution from REMPI spectral intensities. Experimental The experiment utilizes an ultra-high vacuum (u.h.v.) scattering chamber, a tunable U.V. laser source and a variety of support electronics, all of which are described in detail elsewhere. A schematic diagram of the apparatus is shown in fig. 2. In short, the u.h.v. chamber is equipped with a sample manipulator (to facilitate sample translation rotation, surface heating and cooling), LEED and Auger surface diagnostics (to monitor surface order and cleanliness), a pulsed molecular beam source, a port for entry of a laser beam and ion time-of-flight tube. The main chamber is pumped by a 220 dm3 s- ion pump, a titanium sublimation pump, a liquid-nitrogen cryopanel and a 1500 dm3 s- turbomolecular pump (base pressure 2 x lo- * TorrS-). A differentially pumped invaginated source chamber houses the pulsed valve/skimmer assembly. The supersonic beam (50 psi$ neat expansion through a 0.1 mm nozzle orifice) is collimated with a 0.3 mm skimmer so that the beam strikes only a 5 mm t 1 Torr = /760 Pa. $ 1 psi = x 10 Pa.
3 View Article Online D. C. Jacobs et al Fig. 2. Experimental apparatus. A differentially pumped pulsed valve doses the R( 11 1) crystal. Laser radiation is focussed 2mm above the surface. Ions produced in the REMPI process are accelerated through the time-of-flight tube and collected by the CEMA charged particle multiplier. The crystal can be rotated in order to face LEED and Auger diagnostics. Not shown are Helmholtz coils which cancel the earth's magnetic field and leave a residual magnetic field in the direction of the surface normal. diameter region of the surface. The 10 cm nozzle-surface distance allows instantaneous pulsed molecular beam fluxes as high as 1017 molecule cm-* s-'. The rotational temperature of the neat beam is measured at 40 K and the beam translational energy is estimated to be 9 kj mol-l.lo A 330 dm3 s-' turbomomecular pump establishes a base pressure of 1 x lo-' Torr in the source chamber. The generation of tunable ultraviolet laser radiation relies on a commercial 10 Hz Quantel Nd:YAG system. The output of the dye laser (0.08 cm-' bandwidth) is frequency-doubled and Raman shifted (second-order anti-stokes) in HZ. This system creates 6 ns pulses at 224 nm with ca. 200 pj per pulse. A portion of the resulting laser beam is loosely focussed into the chamber (10 mj cm-* fluence). The direction of linear polarization is selected, on a shot-to-shot basis, by a photoelastic modulator (PEM) interfaced to a DEC PDP 11/23 computer. The energy of each laser pulse is measured by a pyroelectric detector and stored in the computer. The laser light runs parallel to and passes 2 mm above the surface. A fraction of the molecules leaving the surface pass through the laser interaction region and become ionized by the laser radiation via the REMPI process. The resulting ions are accelerated through a time-of-flight tube and collected by a charged particle multiplier (CEMA
4 1328 Rotational Alignment of NO from Pt( 11 1) multichannel plates). The ion signal is digitized by a CAMAC charge-sensitive gated integrator and passed to the computer for storage. Owing to the *II symmetry of the NO ground state, stray magnetic fields can scramble the rotational alignment of the product distribution during the molecule's 1 ps flight time from the surface to the laser ionization region. In order to minimize stray magnetic fields," Helmholtz coils are installed around the exterior of the main chamber. The regulated currents flowing through these coils are adjusted so that a small field, pointing along the surface normal, remains in the ionization region.? In addition, the filament current used to heat the surface is turned off for 10 ms during the laser pulse. The dynamical regimes of direct inelastic scattering and trapping/ desorption are differentiated according to their characteristic surface residence times. The residence time for direct inelastic scattering is infinitesimal on the microsecond timescale of the molecular-beam pulse. However, the trapped state will exhibit a half-life on the surface which is inversely proportional to the desorption rate constant. For our temperature regime, the half-life is estimated to be ms. Synchronizing the laser to fire during the nozzle pulse discriminates in favour of those molecules which have inelastically scattered. Conversely, firing the laser 200 ps after the 90ps nozzle pulse precludes inelastic scattering contributions and permits only detection of those molecules which have trapped and subsequently desorbed. The surface coverage at which these experiments are performed is less than 3% of a monolayer. Analysis View Article Online The accurate analysis of 1+1 REMPI spectra of NO through the A2C+-X211 (0,O) band requires characterization of both the extent of saturation in the resonant step as well as the M,-dependence of the ionization step. There are two stages in which the 1+1 REMPI data are treated. First, the data are power-normalized to account for variations in the laser power arising from shot-to-shot fluctuations and changes in the laser gain across the dye curve. In real time, the computer corrects the ion signal for variations in the laser energy through a least-squares fitting routine described elsewhere.i2 Secondly, the normalized data are reduced to population and alignment distributions while considering saturation of the resonant step and variations in the ionization probability with M, (the projection of J on the laser polarization direction).12 Our findings indicate that for our required sensitivity range, partial saturation is unavoidable. Complete saturation, however, eliminates all chances of measuring rotational alignment. Therefore we choose to work in a regime of moderately weak saturation and take great care in the reduction of population and alignment moments to remove the detrimental effects of saturation. The second photon absorbed in a REMPI scheme excites a continuum transition in the molecule. The dipole moment of ths transition points in some direction relative to the internuclear axis. The limiting direction of the transition dipole moment is either parallel or perpendicular to the internuclear axis. The projection of the dipole transition moment onto the internuclear axis directly determines the M,-dependent transition probability for the ionization step. This is required because different branch excitations in the resonant step create different alignment distributions in the intermediate level. The probability for ionization from the intermediate level is a function of both the intermediate-state alignment created by resonant excitation and the fraction of parallel versus perpendicular character in the ionization transition. Fortunately, rotational populations extracted from different branch excitations reveal redundant information. Additionally, different branches saturate at different laser power t A magnetic field along the cylindrical symmetry axis will not affect the observed alignment. Instead, it will ensure that stray transient fields will be insufficient to alter the net field direction.
5 View Article Online D. C. Jacobs et al. 1329,ex per imental -J ab initio calculation I +J Fig. 3. The M,-dependence of the ionization step in REMPI of NO through the A EC+-X II (0,O) band. The solid curve and shaded region represent the best fit and corresponding uncertainty in the experimental measurement. The vertical lines are calculated theoretically by ab initio methods. levels because of variations in the rotational line strength with branch excitation. Calibration spectra of NO in a room-temperature bulb are utilized to determine both the extent of saturation and the M,-dependent ionization probability in the REMPI process. This information is then used to analyse nascent distributions arising from NO molecules which have been scattered and desorbed from the Pt( 11 1) surface. The experimentally measured MJ -dependent ionization probability is shown in fig. 3,12 which reveals a relatively slight variation in the ionization transition probability with the quantum number, MJ. This has the consequence of limiting the amount of rotational alignment information which can be extracted from 1+1 REMPI of NO. Rotational alignment is conveniently described by the even moments of the rotational angular distribution. In the case of cylindrical symmetry, REMPI through the NO A-X(0,O) band will only be sensitive to the population and the quadrupole moment, Ah2) Because of the ionization dynamics, the AY moment cannot be extracted with any sensitivity. The value of the quadrupole moment ranges from +2 to -l,i3 where these limits correspond to the molecule rotating in a plane parallel to the surface ( helicopter motion) or in a plane perpendicular to the surface ( cartwheel motion), respectively (see fig. 1). A quadrupole moment of zero suggests no preferential alignment of rotation, ie., an isotropic distribution. Extraction of the population and quadrupole alignment moments requires that the ion signal be recorded for only two independent planes of linearly polarized light. In the laboratory, we record spectra under conditions where the polarization direction of the laser is either parallel or perpendicular to the surface normal. This is performed by
6 1330 Rotational AZignment of NO from Pt( 11 1) View Article Online Q2,+ R, 900 CI 5 h $ 600 c) C 0 2 2d / / / & 10 + C 3 s 5 / / Q,+ p21 R R2, : : :7 1 1 wavelength/nm I 226: 198 Fig. 4. Spectrum of NO scattering from a R( 11 1) surface at 400 K. The inset provides an expanded view of the high-l region. The rotational transitions of the resonant excitation step are assigned. alternating the polarization between these two directions throughout an entire wavelength scan. For a given rotational transition out of a rotational state J, we identify Ill and I, as the integrated ion intensities for which the laser polarization was parallel or perpendicular to the surface normal (the cylindrical symmetry axis), respectively. The quadrupole alignment moment AL* (J) is related to these measured intensities by where M, is the quantum number representing the projection of J on the cylindrical symmetry axis, ko,( M,) is the M,-dependent transition probability for the resonant photon step, ki2( M,) is the M,-dependent transition probability for the ionization step, IAt is the measured laser fluence and Fsa, is a function which calculates the overall REMPI ionization probability while including the effects of saturation and intermediate-state alignment. The analytical form of FSat, as well as ko,(m,) and k12(mj), have been worked out Inelastic Scattering Results The dynamical regime of inelastic scattering is selectively explored by firing the laser synchronously with the rising edge of the nozzle gas pulse.? A typical spectrum of NO scattered at normal incidence from a 400 K Pt(ll1) surface is shown in fig. 4. This t Normal incidence/normal detection prohibits angular discrimination against the incident molecular beam. Temporal discrimination is also impossible because of the slow falling edge on the molecular-beam pulse relative to the flight time of the molecules (0.5 mm ps-i). However, owing to the low rotational temperature of the beam, interference from the beam will affect only the states J < 7.5.
7 D. C. Jacobs et al. View Article Online I 1 Ah : -1.C 7 T T I I I Fig. 5. Quadrupole moment alignment distribution as a function of the rotational quantum number J plotted for the case of inelastic scattering of NO from Pt( 11 1) at 400 K. portion of the spectrum contains predominantly branches originating from the 211 ground spin-orbit state. A section of the figure is expanded to illustrate the high signal-to-background ratio that is routinely realized with REMPI. The spectra, recorded at orthogonal laser polarizations, are reduced to population and alignment distributions. The population distributions show considerable rotational excitation and are reported elsewhere. Fig. 5 shows the quadrupole alignment moment for the scattered molecules versus their rotational quantum number J. For J < 12.5 there is no appreciable alignment. Of course, interference by the incident beam will dilute the amount of observable alignment in this region. Beyond J = 12.5 there is a steady rise in the rotational alignment until it peaks near J=25.5. The maximum alignment observed corresponds to a quadrupole moment of Recall that Ar) has a limiting value of -1, which occurs when all the molecules rotate with a cartwheel-type motion. For J > 25.5, the alignment appears to decrease slightly. For clarity, fig. 5 includes only the quadrupole moment values measured for the 2111/2(A ) state. Here, the A notation refers to the symmetry of the ground-state A-do~b1et.l~ Measurement of the rotational alignment in the A state of the level is difficult because of congestion in one branch and branch-mixing in the other. However, the two A-doublets of opposite symmetry in the 2113/2 state exhibit similar rotational alignment distributions. J Trapping/Desorption The desorption regime is isolated by firing the laser 200 ps after the completion of the nozzle pulse,? thus eliminating contributions from inelastic scattering channels. The Pt( 11 1) sample is held at 553 K, while the molecular beam delivers pulses of NO to the t The nozzle pulse is characterized by a fast rising edge and a relatively slow tailing edge. Care is taken to ensure that the laser fires well after the visible tail of the pulse.
8 1332 Rotational Alignment of NO from Pt( 11 1) View Article Online 0.20 I "*) I Fig. 6. Quadrupole moment alignment distribution for NO desorption plotted against rotational quantum number J. The Pt(11 1) surface temperature is 553 K. 0, R2, branch member; 0, Q, + Pz, branch member. surface at 10 Hz. Because of the low signal intensity in this operating regime, accurate alignment moments cannot be extracted during the course of a wavelength scan. Therefore, the laser is tuned to a specific rotational transition and ions are collected for laser shots at alternating laser polarization directions. The measured intensities are analysed using eqn (1). Fig. 6 shows the quadrupole moment, Ag'(J), as calculated from two different rotational branches of the same 2111,2 A-doublet state.'' There is little to no rotational alignment for J < 12.5, while higher rotational levels show increasing values of the quadrupole moment. A positive quadrupole moment indicates a preference for rotational motion similar to that of a helicopter (see fig. 1). As in the case of direct inelastic scattering, the A-doublet states in both spin-orbit levels exhibit similar degrees of alignment. I, Discussion The technique of REMPI spectroscopy successfully probed rotational alignment in NO, subsequent to its interaction with a Pt( 11 1) surface. The extraction of alignment information from REMPI spectra requires a methodology which incorporates the effects of saturation in the resonant step and intermediate state alignment in the ionization step. As a check, the quadrupole moment was reduced from the polarization dependence of both RZ1 and Q1+P21 branch members. While the polarization ratios of these two branches show opposite preferences, they reduce to the same values of the quadrupole moment for each rotational level J. These experiments cover new ground in the field of gas-surface dynamics. They represent the first observation of rotational alignment in molecular desorption. Additionally, this marks the first study of inelastic scattering in which rotational levels up
9 View Article Online D. C. Jacobs et al to three times more energetic than the incident-beam energy are examined for rotational alignment. Although significant surface to rotational energy transfer is responsible for populating states with rotational energy exceeding the beam energy, rotational alignment is created in these states and is preserved in the final distribution. The observation of opposite preferences for rotational alignment in desorption and inelastic scattering further exemplifies the interesting dynamics at work in this chemical system. Both inelastic scattering and trapping/desorption regimes yield little rotational alignment for the levels, J < 12.5, while higher rotational levels show a monotonic increase in the magnitude of the quadrupole moment. For the case of desorption the alignment distribution implies a preference for helicopter motion. In contrast, the inelastic scattering process produces an alignment distribution which is indicative of cartwheel motion. Although both types of gas-surface interactions involve the same molecule-surface potential, their dynamics differ because of the way the molecule enters and exits the potential well. The following interpretations are supported by classical trajectory calculations which were performed to simulate the dynamics of the NO/Pt( 111) interaction. l6 In the case of inelastic scattering, the quadrupole moment shows a similar preference toward cartwheel motion as that seen for the scattering of NO -3 and N24-6 from Ag( 111). The preference for cartwheel motion in inelastic scattering arises from the predominantly out-of-plane forces that the molecule experiences during the impulsive surface collision. These forces torque the molecule such that it rotates in a plane normal to the surface. For NO/ Pt( 11 l), the observed quadrupole moment peaks at a value of There are four possible reasons why the quadrupole moment might not reach its limiting value of - 1. First, corrugation in the surface potential introduces tangential forces that are not present on a flat surface. Hence, on a corrugated surface the molecule can receive torques which induce rotational motion in a plane other than the pure cartwheel limit predicted for a flat surface. Secondly, multiple bounces on the surface will scramble alignment, as demonstrated by the trajectory model presented elsewhere.16 This effect potentially differentiates direct inelastic scattering from indirect inelastic scattering. Thirdly, the low populations in the highest rotational levels are susceptible to a weak but non-negligible contribution from trapping/desorption. This contribution would most likely have the opposite sense of alignment (as seen in the desorption results reported here) and thus affect the observed alignment more dramatically. Fourthly, breaking cylindrical symmetry in the system introduces new non-vanishing moments to the measured alignment distribution. The consequence of this effect is that measurement is made of the apparent quadrupole moment rather than the true quadrupole moment. However, we believe that deviations from cylindrical symmetry are minimal for this system. The quadrupole moment distribution measured for the case of molecular desorption is quite surprising. For rotational states with J > 12.5 a positive quadrupole moment is observed. The maximum measured quadrupole moment (+0.15) implies that T times as many molecules desorb with their plane of rotation resembling that of a helicopter as those resembling a cartwheel motion. The observed alignment cannot be reconciled with the simple picture of a direct transition from the known low-temperature equilibrium position (NO bound normal to the surface) to the gas-phase free rotor without the existence of an intermediate state occupied immediately prior to desorption. Our measurements are sensitive to the last few interactions the molecule makes with the surface before being desorbed, i.e. the nature of the molecule-surface potential near t The limiting values of this range assume that the rotational alignment distribution is best described by either an ellipsoid or a function which contains a linear combination of cos 8 and sin 8, respectively. The trajectory calculations suggest that the latter function provides a more accurate description of the distribution. In any case, these two functional forms probably represent the limiting cases for the alignment distribution.
10 View Article Online 1334 Rotational Alignment of NO from Pt( 111) L P Fig. 7. Proposed mechanism for desorption. Frame 1 shows the low-temperature equilibrium geometry for NO on Pt( 11 1). Rotation on the surface is less hindered in the plane parallel to the surface. Frames 2 and 3 show a rotationally excited species on the surface. Cleavage of the chemisorption bond (frame 4) leads to a preference for helicopter motion in the escaping NO molecule. the transition state. Rotation in the plane of the surface is less hindered than rotation in a plane normal to the surface because the former motion never forces the repulsive 0 end of the molecule closer to the surface than the attractive N end. This implies that there exists a higher density of rotational states associated with helicopter motion on the surface than those associated with a cartwheel motion. Thermal population of these surface hindered-rotor states followed by cleavage of the molecule-surface bond will leave the desorbed molecule with a preference toward helicopter motion (fig. 7). The observed rotational alignment distribution for molecular desorption is not inconsistent with the NO/?( 1 11) measurements of Ertl and co-worker~ ~ and Mantell et a1.20 The first group measured no detectable change in the populations extracted from Q-branch excitations relative to those of P and R branches. This is a relatively insensitive measure of rotational alignment and the magnitude of their error bars precludes a quantitative determination of the quadrupole moment. Additionally, the maximum rotational quantum state which they were able to observe was J = The latter study of Mantell et al. imposed the following limit on the quadrupole moment: AL* (J) < for J =4.5 and J = This agrees well with our reported value of AA2 (J) = 0.0 f 0.04 for these two quantum levels. Additionally, the experiments of Mantell et al2 were performed at a much higher coverage and lower temperature than those reported here. At higher coverages, lateral interactions become important and these may inhibit rotational motion in the plane parallel to the surface.
11 View Article Online D. C. Jacobs et al In conclusion, the ability to make quantitative measurements of the polarization dependence of 1 + REMPI allows us to determine rotational alignment. Such rotational alignment measurements provide new insight into the highly anisotropic processes of inelastic scattering and trapping/ desorption of molecules on surfaces. This work was supported by the Office of Naval Research (N K-00265). We gratefully acknowledge the assistance of Stacey F. Shane. References 1 A. Luntz, A. Kleyn and D. Auerbach, Phys. Rev. B, 1982, 25, A. Kleyn, A. Luntz and D. Auerbach, Surf: Sci., 1983, 117, A. Kleyn, A. Luntz and D. Auerbach, Surf: Sci., 1985, 152, G. 0. Sitz, A. C. Kummel and R. N. Zare, J. Vac. Sci. Technol. A, 1987, 5, G. 0. Sitz, A. C. Kummel and R. N. Zare, J. Chem. Phys., 1987, 87, G. 0. Sitz, A. C. Kummel and R. N. Zare, J. Chem. Phys., 1988, 89, L. V. Novakoski and G. M. McClelland, Phys. Rev. Lett., 1987, 59, E. W. Kuipers, M. G. Tenner, A. W. Kleyn and S. Stolte, Nature (London), 1988, 334, D. C. Jacobs, K. W. Kolasinski, S. F. Shane and R. N. Zare, J. Chem. Phys., submitted. 10 J. A. Serri, M. J. Cardillo and G. E. Becker, J. Chem. Phys., 1982, 77, D. C. Jacobs and R. N. Zare, J. Chem. Phys., 1986, 85, D. C. Jacobs, R. J. Madix and R. N. Zare, J. Chem. Phys., 1986,85, C. H. Greene and R. N. Zare, J. Chem. Phys., 1983, 78, M. Alexander, P. Andreson, R. Bacis, R. Bersohn, F. Comes, P. Dagdigian, R. Dixon, R. Field, G. Flynn, K. Gerecke, E. Grant, B. Howard, J. Huber, D. King, J. Kinsey, K. Kleinermanns, K. Kuchitsu, A. Luntz, A. McCafferty, B. Pouilly, H. Reisler, S. Rosenwaks, E. Rothe, M. Shapiro, J. Simons, R. Vasudev, J. Wiesenfeld, C. Wittig and R. Zare, J. Chem. Phys., 1988, 89, D. C. Jacobs, K. W. Kolasinski, R. J. Madix and R. N. Zare, J. Chem. Phys., 1987, 87, D. C. Jacobs and R. N. Zare, J. Chem. Phys., submitted. 17 A. C. Kummel, G. 0. Sitz and R. N. Zare, J. Chem. Phys., 1988, 88, Hindered rotation of chemisorbed molecules has been directly observed. See for example, M. D. Alvey, J. T. Yates Jr and K. J. Uram, J. Chem. Phys., 1987, 87, J. Segner, H. Robota, W. Vielhaber, G. Ertl, F. Frenkel, J. Hager, W. Krieger and H. Walther, Surf: Sci., 1983, 131, D. A. Mantell, R. R. Cavanagh and D. S. King, J. Chem. Phys., 1986, 84, S. N. Dixit, D. L. Lynch and W. M. Huo, Phys. Rev. A, 1985,32, Paper 8/03398F; Received 15th August, 1988
Lecture 9. Detailed Balance, and Nonreactive Scattering of Molecules
 Lecture 9 Detailed Balance, and Nonreactive Scattering of Molecules 2014 D.J. Auerbach All rights reserved Reading List 1. Cardillo, M.J., M. Balooch, and R.E. Stickney, Detailed Balancing and Quasi- Equilibrium
Lecture 9 Detailed Balance, and Nonreactive Scattering of Molecules 2014 D.J. Auerbach All rights reserved Reading List 1. Cardillo, M.J., M. Balooch, and R.E. Stickney, Detailed Balancing and Quasi- Equilibrium
Molecular Beam Scattering as a Probe of Surface Chemical Dynamics: part 1
 Molecular Beam Scattering as a Probe of Surface Chemical Dynamics: part 1 D. J. Auerbach Hitachi Global Storage Technologies San Jose, CA PIRE-ECCI Summer School Santa Barbara California August 21, 2006
Molecular Beam Scattering as a Probe of Surface Chemical Dynamics: part 1 D. J. Auerbach Hitachi Global Storage Technologies San Jose, CA PIRE-ECCI Summer School Santa Barbara California August 21, 2006
24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability ellipsoid. Selection rules.
 Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability
Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
Photoelectron Spectroscopy using High Order Harmonic Generation
 Photoelectron Spectroscopy using High Order Harmonic Generation Alana Ogata Yamanouchi Lab, University of Tokyo ABSTRACT The analysis of photochemical processes has been previously limited by the short
Photoelectron Spectroscopy using High Order Harmonic Generation Alana Ogata Yamanouchi Lab, University of Tokyo ABSTRACT The analysis of photochemical processes has been previously limited by the short
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Angular Correlation Experiments
 Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Answers to questions on exam in laser-based combustion diagnostics on March 10, 2006
 Answers to questions on exam in laser-based combustion diagnostics on March 10, 2006 1. Examples of advantages and disadvantages with laser-based combustion diagnostic techniques: + Nonintrusive + High
Answers to questions on exam in laser-based combustion diagnostics on March 10, 2006 1. Examples of advantages and disadvantages with laser-based combustion diagnostic techniques: + Nonintrusive + High
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
Building Blocks for Quantum Computing Part IV. Design and Construction of the Trapped Ion Quantum Computer (TIQC)
 Building Blocks for Quantum Computing Part IV Design and Construction of the Trapped Ion Quantum Computer (TIQC) CSC801 Seminar on Quantum Computing Spring 2018 1 Goal Is To Understand The Principles And
Building Blocks for Quantum Computing Part IV Design and Construction of the Trapped Ion Quantum Computer (TIQC) CSC801 Seminar on Quantum Computing Spring 2018 1 Goal Is To Understand The Principles And
Richard Miles and Arthur Dogariu. Mechanical and Aerospace Engineering Princeton University, Princeton, NJ 08540, USA
 Richard Miles and Arthur Dogariu Mechanical and Aerospace Engineering Princeton University, Princeton, NJ 08540, USA Workshop on Oxygen Plasma Kinetics Sept 20, 2016 Financial support: ONR and MetroLaser
Richard Miles and Arthur Dogariu Mechanical and Aerospace Engineering Princeton University, Princeton, NJ 08540, USA Workshop on Oxygen Plasma Kinetics Sept 20, 2016 Financial support: ONR and MetroLaser
Module 4 : Third order nonlinear optical processes. Lecture 28 : Inelastic Scattering Processes. Objectives
 Module 4 : Third order nonlinear optical processes Lecture 28 : Inelastic Scattering Processes Objectives In this lecture you will learn the following Light scattering- elastic and inelastic-processes,
Module 4 : Third order nonlinear optical processes Lecture 28 : Inelastic Scattering Processes Objectives In this lecture you will learn the following Light scattering- elastic and inelastic-processes,
Vibrational Spectroscopy of Molecules on Surfaces
 Vibrational Spectroscopy of Molecules on Surfaces Edited by John T. Yates, Jr. University of Pittsburgh Pittsburgh, Pennsylvania and Theodore E. Madey National Bureau of Standards Gaithersburg, Maryland
Vibrational Spectroscopy of Molecules on Surfaces Edited by John T. Yates, Jr. University of Pittsburgh Pittsburgh, Pennsylvania and Theodore E. Madey National Bureau of Standards Gaithersburg, Maryland
Direct inelastic scattering of N2 from Ag(111). I. Rotational populations and alignment
 Direct inelastic scattering of N2 from Ag(111).. Rotational populations and alignment Greg O. Sitz Andrew C. Kummel and Richard N. Zare Department of Chemistry Stanford University Stanford California 9435
Direct inelastic scattering of N2 from Ag(111).. Rotational populations and alignment Greg O. Sitz Andrew C. Kummel and Richard N. Zare Department of Chemistry Stanford University Stanford California 9435
Mass spectrometric determination of the surface compositions of ethanol water mixtures
 International Journal of Mass Spectrometry 212 (2001) 267 271 www.elsevier.com/locate/ijms Cluster/kinetic method Mass spectrometric determination of the surface compositions of ethanol water mixtures
International Journal of Mass Spectrometry 212 (2001) 267 271 www.elsevier.com/locate/ijms Cluster/kinetic method Mass spectrometric determination of the surface compositions of ethanol water mixtures
Atomic Structure. Chapter 8
 Atomic Structure Chapter 8 Overview To understand atomic structure requires understanding a special aspect of the electron - spin and its related magnetism - and properties of a collection of identical
Atomic Structure Chapter 8 Overview To understand atomic structure requires understanding a special aspect of the electron - spin and its related magnetism - and properties of a collection of identical
Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
 THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
Raman and stimulated Raman spectroscopy of chlorinated hydrocarbons
 Department of Chemistry Physical Chemistry Göteborg University KEN140 Spektroskopi Raman and stimulated Raman spectroscopy of chlorinated hydrocarbons WARNING! The laser gives a pulsed very energetic and
Department of Chemistry Physical Chemistry Göteborg University KEN140 Spektroskopi Raman and stimulated Raman spectroscopy of chlorinated hydrocarbons WARNING! The laser gives a pulsed very energetic and
requency generation spectroscopy Rahul N
 requency generation spectroscopy Rahul N 2-11-2013 Sum frequency generation spectroscopy Sum frequency generation spectroscopy (SFG) is a technique used to analyze surfaces and interfaces. SFG was first
requency generation spectroscopy Rahul N 2-11-2013 Sum frequency generation spectroscopy Sum frequency generation spectroscopy (SFG) is a technique used to analyze surfaces and interfaces. SFG was first
Spatial Intensity Nonuniformities of an OMEGA Beam due to Nonlinear Beam Propagation
 Spatial Intensity Nonuniformities of an OMEGA Beam due to Nonlinear Beam Propagation Several applications require that a laser beam maintain a high degree of wavefront quality after propagating a long
Spatial Intensity Nonuniformities of an OMEGA Beam due to Nonlinear Beam Propagation Several applications require that a laser beam maintain a high degree of wavefront quality after propagating a long
RYDBERG STATES (6s, 6s ) OF METHYL AND
 Laser Chem., Vol. 13, pp. 151-157 Reprints available directly from the Publisher Photocopying permitted by license only (C) 1993 Harwood Academic Publishers GmbH Printed in Malaysia 2+1 (2+2) REMPI-TOF
Laser Chem., Vol. 13, pp. 151-157 Reprints available directly from the Publisher Photocopying permitted by license only (C) 1993 Harwood Academic Publishers GmbH Printed in Malaysia 2+1 (2+2) REMPI-TOF
Energy Spectroscopy. Ex.: Fe/MgO
 Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Ma5: Auger- and Electron Energy Loss Spectroscopy
 Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Abstract... I. Acknowledgements... III. Table of Content... V. List of Tables... VIII. List of Figures... IX
 Abstract... I Acknowledgements... III Table of Content... V List of Tables... VIII List of Figures... IX Chapter One IR-VUV Photoionization Spectroscopy 1.1 Introduction... 1 1.2 Vacuum-Ultraviolet-Ionization
Abstract... I Acknowledgements... III Table of Content... V List of Tables... VIII List of Figures... IX Chapter One IR-VUV Photoionization Spectroscopy 1.1 Introduction... 1 1.2 Vacuum-Ultraviolet-Ionization
Photofragment angular momentum distributions in the molecular frame: Determination and interpretation
 JOURNAL O CHEMICAL PHYSICS VOLUME 110, NUMBER 7 15 EBRUARY 1999 Photofragment angular momentum distributions in the molecular frame: Determination and interpretation T. Peter Rakitzis and Richard N. Zare
JOURNAL O CHEMICAL PHYSICS VOLUME 110, NUMBER 7 15 EBRUARY 1999 Photofragment angular momentum distributions in the molecular frame: Determination and interpretation T. Peter Rakitzis and Richard N. Zare
IR Spectrography - Absorption. Raman Spectrography - Scattering. n 0 n M - Raman n 0 - Rayleigh
 RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
Requirements for scaleable QIP
 p. 1/25 Requirements for scaleable QIP These requirements were presented in a very influential paper by David Divincenzo, and are widely used to determine if a particular physical system could potentially
p. 1/25 Requirements for scaleable QIP These requirements were presented in a very influential paper by David Divincenzo, and are widely used to determine if a particular physical system could potentially
Comments to Atkins: Physical chemistry, 7th edition.
 Comments to Atkins: Physical chemistry, 7th edition. Chapter 16: p. 483, Eq. (16.1). The definition that the wave number is the inverse of the wave length should be used. That is much smarter. p. 483-484.
Comments to Atkins: Physical chemistry, 7th edition. Chapter 16: p. 483, Eq. (16.1). The definition that the wave number is the inverse of the wave length should be used. That is much smarter. p. 483-484.
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997
 The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
Molecular spectroscopy
 Molecular spectroscopy Origin of spectral lines = absorption, emission and scattering of a photon when the energy of a molecule changes: rad( ) M M * rad( ' ) ' v' 0 0 absorption( ) emission ( ) scattering
Molecular spectroscopy Origin of spectral lines = absorption, emission and scattering of a photon when the energy of a molecule changes: rad( ) M M * rad( ' ) ' v' 0 0 absorption( ) emission ( ) scattering
Photodissociation of 1-bromo-2-butene, 4-bromo-1-butene, and cyclopropylmethyl bromide at 234 nm studied using velocity map imaging
 THE JOURNAL OF CHEMICAL PHYSICS 125, 144312 2006 Photodissociation of 1-bromo-2-butene, 4-bromo-1-butene, and cyclopropylmethyl bromide at 234 nm studied using velocity map imaging Kai-Chung Lau, Yi Liu,
THE JOURNAL OF CHEMICAL PHYSICS 125, 144312 2006 Photodissociation of 1-bromo-2-butene, 4-bromo-1-butene, and cyclopropylmethyl bromide at 234 nm studied using velocity map imaging Kai-Chung Lau, Yi Liu,
Attosecond-correlated dynamics of two electrons in argon
 PRAMANA c Indian Academy of Sciences Vol. 82, No. 1 journal of January 2014 physics pp. 79 85 Attosecond-correlated dynamics of two electrons in argon V SHARMA 1,,NCAMUS 2, B FISCHER 2, M KREMER 2, A RUDENKO
PRAMANA c Indian Academy of Sciences Vol. 82, No. 1 journal of January 2014 physics pp. 79 85 Attosecond-correlated dynamics of two electrons in argon V SHARMA 1,,NCAMUS 2, B FISCHER 2, M KREMER 2, A RUDENKO
Study of Distributed Ion-Pumps in CESR 1
 Study of Distributed Ion-Pumps in CESR 1 Yulin Li, Roberto Kersevan, Nariman Mistry Laboratory of Nuclear Studies, Cornell University Ithaca, NY 153-001 Abstract It is desirable to reduce anode voltage
Study of Distributed Ion-Pumps in CESR 1 Yulin Li, Roberto Kersevan, Nariman Mistry Laboratory of Nuclear Studies, Cornell University Ithaca, NY 153-001 Abstract It is desirable to reduce anode voltage
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
EXPERIMENT 5. The Franck-Hertz Experiment (Critical Potentials) Introduction
 EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Table 1: Residence time (τ) in seconds for adsorbed molecules
 1 Surfaces We got our first hint of the importance of surface processes in the mass spectrum of a high vacuum environment. The spectrum was dominated by water and carbon monoxide, species that represent
1 Surfaces We got our first hint of the importance of surface processes in the mass spectrum of a high vacuum environment. The spectrum was dominated by water and carbon monoxide, species that represent
Throwing Light on Reaction Dynamics: H + HBr
 Throwing Light on Reaction Dynamics: H + HBr The thermal reaction of hydrogen gas (H 2 ) and bromine gas (Br 2 ) to form hydrogen bromide vapor (HBr) is a classic reaction: 22+2rHBrHBææÆ Energetics (thermodynamics)
Throwing Light on Reaction Dynamics: H + HBr The thermal reaction of hydrogen gas (H 2 ) and bromine gas (Br 2 ) to form hydrogen bromide vapor (HBr) is a classic reaction: 22+2rHBrHBææÆ Energetics (thermodynamics)
Mass Analyzers. Principles of the three most common types magnetic sector, quadrupole and time of flight - will be discussed herein.
 Mass Analyzers After the production of ions in ion sources, the next critical step in mass spectrometry is to separate these gas phase ions according to their mass-to-charge ratio (m/z). Ions are extracted
Mass Analyzers After the production of ions in ion sources, the next critical step in mass spectrometry is to separate these gas phase ions according to their mass-to-charge ratio (m/z). Ions are extracted
Rb, which had been compressed to a density of 1013
 Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Surface Defects on Natural MoS 2
 Supporting Information: Surface Defects on Natural MoS 2 Rafik Addou 1*, Luigi Colombo 2, and Robert M. Wallace 1* 1 Department of Materials Science and Engineering, The University of Texas at Dallas,
Supporting Information: Surface Defects on Natural MoS 2 Rafik Addou 1*, Luigi Colombo 2, and Robert M. Wallace 1* 1 Department of Materials Science and Engineering, The University of Texas at Dallas,
EXPERIMENT 2-6. e/m OF THE ELECTRON GENERAL DISCUSSION
 Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Fundamentals of Spectroscopy for Optical Remote Sensing. Course Outline 2009
 Fundamentals of Spectroscopy for Optical Remote Sensing Course Outline 2009 Part I. Fundamentals of Quantum Mechanics Chapter 1. Concepts of Quantum and Experimental Facts 1.1. Blackbody Radiation and
Fundamentals of Spectroscopy for Optical Remote Sensing Course Outline 2009 Part I. Fundamentals of Quantum Mechanics Chapter 1. Concepts of Quantum and Experimental Facts 1.1. Blackbody Radiation and
Precision VUV spectroscopy of Ar I at 105 nm
 J. Phys. B: At. Mol. Opt. Phys. 32 (999) L5 L56. Printed in the UK PII: S0953-4075(99)05625-4 LETTER TO THE EDITOR Precision VUV spectroscopy of Ar I at 05 nm I Velchev, W Hogervorst and W Ubachs Vrije
J. Phys. B: At. Mol. Opt. Phys. 32 (999) L5 L56. Printed in the UK PII: S0953-4075(99)05625-4 LETTER TO THE EDITOR Precision VUV spectroscopy of Ar I at 05 nm I Velchev, W Hogervorst and W Ubachs Vrije
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/329/5991/553/dc1 Supporting Online Material for Steric Effects in the Chemisorption of Vibrationally Excited Methane on Ni(1) This PDF file includes: Bruce L. Yoder,
www.sciencemag.org/cgi/content/full/329/5991/553/dc1 Supporting Online Material for Steric Effects in the Chemisorption of Vibrationally Excited Methane on Ni(1) This PDF file includes: Bruce L. Yoder,
2.B Laser Ionization of Noble Gases by Coulomb Barrier Suppression
 ADVANCED TECHNOLOGY DEVELOPMENTS 2.B Laser Ionization of Noble Gases by Coulomb Barrier Suppression Introduction The ionization of atoms and ions in high-intensity laser fields allows a study of atomic
ADVANCED TECHNOLOGY DEVELOPMENTS 2.B Laser Ionization of Noble Gases by Coulomb Barrier Suppression Introduction The ionization of atoms and ions in high-intensity laser fields allows a study of atomic
What happens when light falls on a material? Transmission Reflection Absorption Luminescence. Elastic Scattering Inelastic Scattering
 Raman Spectroscopy What happens when light falls on a material? Transmission Reflection Absorption Luminescence Elastic Scattering Inelastic Scattering Raman, Fluorescence and IR Scattering Absorption
Raman Spectroscopy What happens when light falls on a material? Transmission Reflection Absorption Luminescence Elastic Scattering Inelastic Scattering Raman, Fluorescence and IR Scattering Absorption
7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text , , 12.10)
 2009, Department of Chemistry, The University of Western Ontario 7a.1 7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text 11.1 11.5, 12.1 12.5, 12.10) A. Electromagnetic Radiation Energy is
2009, Department of Chemistry, The University of Western Ontario 7a.1 7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text 11.1 11.5, 12.1 12.5, 12.10) A. Electromagnetic Radiation Energy is
Vibrational Autoionization in Polyatomic molecules
 Vibrational Autoionization in Polyatomic molecules S.T. Pratt Annu. Rev. Phys. Chem. 2005. 56:281-308 2006. 12. 4. Choi, Sunyoung 1 Schedule 12/4 (Mon) - Introduction - Theoretical background 12/6 (Wed)
Vibrational Autoionization in Polyatomic molecules S.T. Pratt Annu. Rev. Phys. Chem. 2005. 56:281-308 2006. 12. 4. Choi, Sunyoung 1 Schedule 12/4 (Mon) - Introduction - Theoretical background 12/6 (Wed)
Excitation of high angular momentum Rydberg states
 J. Phys. B: At. Mol. Phys. 19 (1986) L461-L465. Printed in Great Britain LE ITER TO THE EDITOR Excitation of high angular momentum Rydberg states W A Molanderi, C R Stroud Jr and John A Yeazell The Institute
J. Phys. B: At. Mol. Phys. 19 (1986) L461-L465. Printed in Great Britain LE ITER TO THE EDITOR Excitation of high angular momentum Rydberg states W A Molanderi, C R Stroud Jr and John A Yeazell The Institute
An axis-specific rotational rainbow in the direct scatter of formaldehyde from Au(111) and its influence on trapping probability
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Supplementary Information An axis-specific rotational rainbow in the direct scatter
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Supplementary Information An axis-specific rotational rainbow in the direct scatter
Revival Structures of Linear Molecules in a Field-Free Alignment Condition as Probed by High-Order Harmonic Generation
 Journal of the Korean Physical Society, Vol. 49, No. 1, July 2006, pp. 337 341 Revival Structures of Linear Molecules in a Field-Free Alignment Condition as Probed by High-Order Harmonic Generation G.
Journal of the Korean Physical Society, Vol. 49, No. 1, July 2006, pp. 337 341 Revival Structures of Linear Molecules in a Field-Free Alignment Condition as Probed by High-Order Harmonic Generation G.
4. Inelastic Scattering
 1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
NPTEL/IITM. Molecular Spectroscopy Lectures 1 & 2. Prof.K. Mangala Sunder Page 1 of 15. Topics. Part I : Introductory concepts Topics
 Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Photoemission Spectroscopy
 FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
Harris: Quantitative Chemical Analysis, Eight Edition
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 21: MASS SPECTROMETRY CHAPTER 21: Opener 21.0 Mass Spectrometry Mass Spectrometry provides information about 1) The elemental composition of
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 21: MASS SPECTROMETRY CHAPTER 21: Opener 21.0 Mass Spectrometry Mass Spectrometry provides information about 1) The elemental composition of
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Hyperfine structure and isotope shift measurements on 4d 10 1 S 0 4d 9 5p J = 1 transitions in Pd I using deep-uv cw laser spectroscopy
 Eur. Phys. J. D 19, 25 29 (22) DOI: 1.114/epjd/e2251 THE EUROPEAN PHYSICAL JOURNAL D c EDP Sciences Società Italiana di Fisica Springer-Verlag 22 Hyperfine structure and isotope shift measurements on 4d
Eur. Phys. J. D 19, 25 29 (22) DOI: 1.114/epjd/e2251 THE EUROPEAN PHYSICAL JOURNAL D c EDP Sciences Società Italiana di Fisica Springer-Verlag 22 Hyperfine structure and isotope shift measurements on 4d
Supplementary Figure 1 Schematics of an optical pulse in a nonlinear medium. A Gaussian optical pulse propagates along z-axis in a nonlinear medium
 Supplementary Figure 1 Schematics of an optical pulse in a nonlinear medium. A Gaussian optical pulse propagates along z-axis in a nonlinear medium with thickness L. Supplementary Figure Measurement of
Supplementary Figure 1 Schematics of an optical pulse in a nonlinear medium. A Gaussian optical pulse propagates along z-axis in a nonlinear medium with thickness L. Supplementary Figure Measurement of
Surface Analysis - The Principal Techniques
 Surface Analysis - The Principal Techniques Edited by John C. Vickerman Surface Analysis Research Centre, Department of Chemistry UMIST, Manchester, UK JOHN WILEY & SONS Chichester New York Weinheim Brisbane
Surface Analysis - The Principal Techniques Edited by John C. Vickerman Surface Analysis Research Centre, Department of Chemistry UMIST, Manchester, UK JOHN WILEY & SONS Chichester New York Weinheim Brisbane
CHEM*3440. Photon Energy Units. Spectrum of Electromagnetic Radiation. Chemical Instrumentation. Spectroscopic Experimental Concept.
 Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
CMSC 33001: Novel Computing Architectures and Technologies. Lecture 06: Trapped Ion Quantum Computing. October 8, 2018
 CMSC 33001: Novel Computing Architectures and Technologies Lecturer: Kevin Gui Scribe: Kevin Gui Lecture 06: Trapped Ion Quantum Computing October 8, 2018 1 Introduction Trapped ion is one of the physical
CMSC 33001: Novel Computing Architectures and Technologies Lecturer: Kevin Gui Scribe: Kevin Gui Lecture 06: Trapped Ion Quantum Computing October 8, 2018 1 Introduction Trapped ion is one of the physical
Studies on the photodissociation and symmetry of SO 2 D
 JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 20 22 MAY 2003 Studies on the photodissociation and symmetry of SO 2 D Limin Zhang, a) Zhong Wang, Jiang Li, Feng Wang, Shilin Liu, Shuqin Yu, and Xingxiao
JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 20 22 MAY 2003 Studies on the photodissociation and symmetry of SO 2 D Limin Zhang, a) Zhong Wang, Jiang Li, Feng Wang, Shilin Liu, Shuqin Yu, and Xingxiao
State resolved inelastic scattering of N 2 from Ru 0001
 JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 24 22 JUNE 2003 State resolved inelastic scattering of N 2 from Ru 0001 H. Mortensen, a) E. Jensen, L. Diekhöner, b) A. Baurichter, A. C. Luntz, and V. V.
JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 24 22 JUNE 2003 State resolved inelastic scattering of N 2 from Ru 0001 H. Mortensen, a) E. Jensen, L. Diekhöner, b) A. Baurichter, A. C. Luntz, and V. V.
Vibrational Spectroscopies. C-874 University of Delaware
 Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
Chapter 7. Nuclear Magnetic Resonance Spectroscopy
 Chapter 7 Nuclear Magnetic Resonance Spectroscopy I. Introduction 1924, W. Pauli proposed that certain atomic nuclei have spin and magnetic moment and exposure to magnetic field would lead to energy level
Chapter 7 Nuclear Magnetic Resonance Spectroscopy I. Introduction 1924, W. Pauli proposed that certain atomic nuclei have spin and magnetic moment and exposure to magnetic field would lead to energy level
Academic and Research Staff. Dr. C.H. Becker Dr. R.A. Gottscho Dr. M.L. Zimmerman Dr. T.A. Brunner Dr. A. Morales-Mori Dr. W.H.
 V. ATOMIC RESONANCE AND SCATTERING Academic and Research Staff Prof. D. Kleppner Prof. D.E. Pritchard Dr. T.W. Ducas* Dr. M.B. Elbel Dr. J.R. Rubbmark Dr. J.A. Serri Dr. C.H. Becker Dr. R.A. Gottscho Dr.
V. ATOMIC RESONANCE AND SCATTERING Academic and Research Staff Prof. D. Kleppner Prof. D.E. Pritchard Dr. T.W. Ducas* Dr. M.B. Elbel Dr. J.R. Rubbmark Dr. J.A. Serri Dr. C.H. Becker Dr. R.A. Gottscho Dr.
Harmonic Generation for Photoionization Experiments Christian J. Kornelis Physics REU Kansas State University
 Harmonic Generation for Photoionization Experiments Christian J. Kornelis Physics REU Kansas State University The Basic Setup for the KLS Photoionization Experiment V. Kumarappan Femtosecond Pump-Probe
Harmonic Generation for Photoionization Experiments Christian J. Kornelis Physics REU Kansas State University The Basic Setup for the KLS Photoionization Experiment V. Kumarappan Femtosecond Pump-Probe
HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS
 HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS J. Liu, T. Tsong To cite this version: J. Liu, T. Tsong. HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS. Journal de
HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS J. Liu, T. Tsong To cite this version: J. Liu, T. Tsong. HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS. Journal de
Molecular alignment, wavepacket interference and Isotope separation
 Molecular alignment, wavepacket interference and Isotope separation Sharly Fleischer, Ilya Averbukh and Yehiam Prior Chemical Physics, Weizmann Institute Yehiam.prior@weizmann.ac.il Frisno-8, Ein Bokek,
Molecular alignment, wavepacket interference and Isotope separation Sharly Fleischer, Ilya Averbukh and Yehiam Prior Chemical Physics, Weizmann Institute Yehiam.prior@weizmann.ac.il Frisno-8, Ein Bokek,
Scattering of xenon from Ni 111 : Collision-induced corrugation and energy transfer dynamics
 JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 4 22 JANUARY 2000 Scattering of xenon from Ni 111 : Collision-induced corrugation and energy transfer dynamics Mark D. Ellison, a) Carl M. Matthews, and Richard
JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 4 22 JANUARY 2000 Scattering of xenon from Ni 111 : Collision-induced corrugation and energy transfer dynamics Mark D. Ellison, a) Carl M. Matthews, and Richard
two slits and 5 slits
 Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Electron Spectroscopy
 Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
Energy Spectroscopy. Excitation by means of a probe
 Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Autoresonant Ion Trap Mass Spectrometer The RGA Alternative
 Autoresonant Ion Trap Mass Spectrometer The GA Alternative 1 What is a Mass Spectrometer? Ionizer Mass Spectrometer Mass Separator Magnetic Sector, Quadrupole or Ion Trap Detector Partial Pressure Measurement
Autoresonant Ion Trap Mass Spectrometer The GA Alternative 1 What is a Mass Spectrometer? Ionizer Mass Spectrometer Mass Separator Magnetic Sector, Quadrupole or Ion Trap Detector Partial Pressure Measurement
Rotational states and rotational transitions of molecules. Microwave spectroscopic methods
 Rotational states and rotational transitions of molecules Microwave spectroscopic methods Consequences of the BO approximation Within the BO approximation, the Schrödinger equation can be solved using
Rotational states and rotational transitions of molecules Microwave spectroscopic methods Consequences of the BO approximation Within the BO approximation, the Schrödinger equation can be solved using
Liquid Crystals IAM-CHOON 1(1100 .,4 WILEY 2007 WILEY-INTERSCIENCE A JOHN WILEY & SONS, INC., PUBLICATION. 'i; Second Edition. n z
 Liquid Crystals Second Edition IAM-CHOON 1(1100.,4 z 'i; BICENTCNNIAL 1 8 0 7 WILEY 2007 DICENTENNIAL n z z r WILEY-INTERSCIENCE A JOHN WILEY & SONS, INC., PUBLICATION Contents Preface xiii Chapter 1.
Liquid Crystals Second Edition IAM-CHOON 1(1100.,4 z 'i; BICENTCNNIAL 1 8 0 7 WILEY 2007 DICENTENNIAL n z z r WILEY-INTERSCIENCE A JOHN WILEY & SONS, INC., PUBLICATION Contents Preface xiii Chapter 1.
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
Abstract Submitted for the DPP99 Meeting of The American Physical Society
 Abstract Submitted for the DPP99 Meeting of The American Physical Society Sorting Category: 5.1.1.2(Experimental) Calibration of a Three Path Thomson System at DIII- D 1 B.D. BRAY, C.L. HSIEH, T.N. CARLSTROM,
Abstract Submitted for the DPP99 Meeting of The American Physical Society Sorting Category: 5.1.1.2(Experimental) Calibration of a Three Path Thomson System at DIII- D 1 B.D. BRAY, C.L. HSIEH, T.N. CARLSTROM,
high temp ( K) Chapter 20: Atomic Spectroscopy
 high temp (2000-6000K) Chapter 20: Atomic Spectroscopy 20-1. An Overview Most compounds Atoms in gas phase high temp (2000-6000K) (AES) (AAS) (AFS) sample Mass-to-charge (ICP-MS) Atomic Absorption experiment
high temp (2000-6000K) Chapter 20: Atomic Spectroscopy 20-1. An Overview Most compounds Atoms in gas phase high temp (2000-6000K) (AES) (AAS) (AFS) sample Mass-to-charge (ICP-MS) Atomic Absorption experiment
Supplementary Material for In situ frequency gating and beam splitting of vacuum- and extreme-ultraviolet pulses
 Supplementary Material for In situ frequency gating and beam splitting of vacuum- and extreme-ultraviolet pulses Rajendran Rajeev, Johannes Hellwagner, Anne Schumacher, Inga Jordan, Martin Huppert, Andres
Supplementary Material for In situ frequency gating and beam splitting of vacuum- and extreme-ultraviolet pulses Rajendran Rajeev, Johannes Hellwagner, Anne Schumacher, Inga Jordan, Martin Huppert, Andres
Lecture 5. X-ray Photoemission Spectroscopy (XPS)
 Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Cluster fusion in a high magnetic field
 Santa Fe July 28, 2009 Cluster fusion in a high magnetic field Roger Bengtson, Boris Breizman Institute for Fusion Studies, Fusion Research Center The University of Texas at Austin In collaboration with:
Santa Fe July 28, 2009 Cluster fusion in a high magnetic field Roger Bengtson, Boris Breizman Institute for Fusion Studies, Fusion Research Center The University of Texas at Austin In collaboration with:
AMOR the time-of-flight neutron reflectometer at SINQ/PSI
 PRAMANA c Indian Academy of Sciences Vol. 63, No. 1 journal of July 2004 physics pp. 57 63 AMOR the time-of-flight neutron reflectometer at SINQ/PSI MUKUL GUPTA 1, T GUTBERLET 1, J STAHN 1, P KELLER 1
PRAMANA c Indian Academy of Sciences Vol. 63, No. 1 journal of July 2004 physics pp. 57 63 AMOR the time-of-flight neutron reflectometer at SINQ/PSI MUKUL GUPTA 1, T GUTBERLET 1, J STAHN 1, P KELLER 1
van Quantum tot Molecuul
 10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
PAPER No. : 8 (PHYSICAL SPECTROSCOPY) MODULE No. : 5 (TRANSITION PROBABILITIES AND TRANSITION DIPOLE MOMENT. OVERVIEW OF SELECTION RULES)
 Subject Chemistry Paper No and Title Module No and Title Module Tag 8 and Physical Spectroscopy 5 and Transition probabilities and transition dipole moment, Overview of selection rules CHE_P8_M5 TABLE
Subject Chemistry Paper No and Title Module No and Title Module Tag 8 and Physical Spectroscopy 5 and Transition probabilities and transition dipole moment, Overview of selection rules CHE_P8_M5 TABLE
ScienceDirect. Gas flow visualization using laser-induced fluorescence
 Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 106 (2015 ) 92 96 Dynamics and Vibroacoustics of Machines (DVM2014) Gas flow visualization using laser-induced fluorescence
Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 106 (2015 ) 92 96 Dynamics and Vibroacoustics of Machines (DVM2014) Gas flow visualization using laser-induced fluorescence
Spectroscopy in Inorganic Chemistry. Vibration and Rotation Spectroscopy
 Spectroscopy in Inorganic Chemistry Symmetry requirement for coupling combination bands and Fermi resonance 2 3 V 3 1505 cm -1 (R, IR) E' stretches v 1 888 cm -1 (R) A 1 ' stretch V 2 718 cm -1 (IR) A
Spectroscopy in Inorganic Chemistry Symmetry requirement for coupling combination bands and Fermi resonance 2 3 V 3 1505 cm -1 (R, IR) E' stretches v 1 888 cm -1 (R) A 1 ' stretch V 2 718 cm -1 (IR) A
Application of IR Raman Spectroscopy
 Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
( ) x10 8 m. The energy in a mole of 400 nm photons is calculated by: ' & sec( ) ( & % ) 6.022x10 23 photons' E = h! = hc & 6.
 Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
As a partial differential equation, the Helmholtz equation does not lend itself easily to analytical
 Aaron Rury Research Prospectus 21.6.2009 Introduction: The Helmhlotz equation, ( 2 +k 2 )u(r)=0 1, serves as the basis for much of optical physics. As a partial differential equation, the Helmholtz equation
Aaron Rury Research Prospectus 21.6.2009 Introduction: The Helmhlotz equation, ( 2 +k 2 )u(r)=0 1, serves as the basis for much of optical physics. As a partial differential equation, the Helmholtz equation
Lecture 3 Vacuum Science and Technology
 Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
- A spark is passed through the Argon in the presence of the RF field of the coil to initiate the plasma
 THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
THE PLASMA Inductively Coupled Plasma Mass Spectrometry (ICP-MS) What is a Plasma? - The magnetic field created by a RF (radio frequency) coil produces a current within a stream of Argon (Ar) gas, which
MOLECULAR SURFACE PUMPING: CRYOPUMPING
 51 MOLECULAR SURFACE PUMPING: CRYOPUMPING C. Benvenuti CERN, Geneva, Switzerland Abstract Weak van der Waals attractive forces may provide molecular gas pumping on surfaces cooled at sufficiently low temperatures.
51 MOLECULAR SURFACE PUMPING: CRYOPUMPING C. Benvenuti CERN, Geneva, Switzerland Abstract Weak van der Waals attractive forces may provide molecular gas pumping on surfaces cooled at sufficiently low temperatures.
Measurements of Cl-atom photofragment angular momentum distributions in the photodissociation of Cl 2 and ICl
 JOURNAL OF CHEMICAL PHYSICS VOLUME 110, NUMBER 7 15 FEBRUARY 1999 Measurements of Cl-atom photofragment angular momentum distributions in the photodissociation of Cl 2 and ICl T. Peter Rakitzis, S. Alex
JOURNAL OF CHEMICAL PHYSICS VOLUME 110, NUMBER 7 15 FEBRUARY 1999 Measurements of Cl-atom photofragment angular momentum distributions in the photodissociation of Cl 2 and ICl T. Peter Rakitzis, S. Alex
Femtosecond X-Ray Experiments
 Femtosecond X-Ray Experiments Christian Bressler FXE Hamburg, January 25, 2017 FXE Workshop Dec 2016: Users overall very happy with implemented components 2 Scientific Instrument FXE The FXE scientific
Femtosecond X-Ray Experiments Christian Bressler FXE Hamburg, January 25, 2017 FXE Workshop Dec 2016: Users overall very happy with implemented components 2 Scientific Instrument FXE The FXE scientific
Progress Report on Chamber Dynamics and Clearing
 Progress Report on Chamber Dynamics and Clearing Farrokh Najmabadi, Rene Raffray, Mark S. Tillack, John Pulsifer, Zoran Dragovlovic (UCSD) Ahmed Hassanein (ANL) Laser-IFE Program Workshop May31-June 1,
Progress Report on Chamber Dynamics and Clearing Farrokh Najmabadi, Rene Raffray, Mark S. Tillack, John Pulsifer, Zoran Dragovlovic (UCSD) Ahmed Hassanein (ANL) Laser-IFE Program Workshop May31-June 1,
CEE 772 Lecture #27 12/10/2014. CEE 772: Instrumental Methods in Environmental Analysis
 Updated: 10 December 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #21 Mass Spectrometry: Mass Filters & Spectrometers (Skoog, Chapt. 20, pp.511 524) (Harris, Chapt.
Updated: 10 December 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #21 Mass Spectrometry: Mass Filters & Spectrometers (Skoog, Chapt. 20, pp.511 524) (Harris, Chapt.
APPLICATION OF SPONTANEOUS RAMAN SCATTERING TO THE FLOWFIELD IN A SCRAMJET COMBUSTOR T. Sander and T. Sattelmayer Lehrstuhl für Thermodynamik,
 APPLICATION OF SPONTANEOUS RAMAN SCATTERING TO THE FLOWFIELD IN A SCRAMJET COMBUSTOR T. Sander and T. Sattelmayer Lehrstuhl für Thermodynamik, TU-München, D-85747, Garching, Germany Introduction The weight
APPLICATION OF SPONTANEOUS RAMAN SCATTERING TO THE FLOWFIELD IN A SCRAMJET COMBUSTOR T. Sander and T. Sattelmayer Lehrstuhl für Thermodynamik, TU-München, D-85747, Garching, Germany Introduction The weight
