Slide 1. Slide 2. Slide 3. Choosing and Using the Best Sensor Technologies for Marine Chemistry Applications
|
|
|
- Derek Morris
- 5 years ago
- Views:
Transcription
1 Slide 1 Choosing and Using the Best Sensor Technologies for Marine Chemistry Applications Bob Henderson, BS, MBA GFG Instrumentation Inc. Ann Arbor, MI Toll free: Mobile: bhenderson@gfg-inc.com GfG website: Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 1 Slide 2 GfG Instrumentation World-wide manufacturer of gas detection solutions Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 2 Slide 3 GfG Instrumentation Exceptional designs with best cost of ownership in the gas detection industry Aug September 5, , 2014 Best Sensor GfG Technologies Instrumentation for Marine company Chemistry overviewapplications Slide Slide 3 3
2 Slide 4 GfG, Inc. website Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 4 Slide 5 Technical support and downloads Manuals Software and software updates Data sheets Training materials and presentations Application Notes and Technical Notes: appnotes.htm Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 5 Slide 6 Multigas detectors GfG multi-gas instruments are compact, reliable and simple to operate. Loud audible and bright visual alarms give immediate warning of up to 6 gas hazards at the same time.
3 Slide 7 Available for use with a wide range of dependable, substance specific electrochemical sensors Optional range and resolution available for many sensors Plug-and-play sensor design makes changing or replacing sensors a snap MICRO IV Single Sensor Gas Detector Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 7 Slide 8 Interchangeable rechargeable (NiMH) or alkaline battery packs last over 20 hours per charge Top-mounted, three color, full graphics LCD Durable IP-67 water resistant design O 2 sensor rated for continuous use in 30 C temperatures G450 / G460 Multi-Gas Detector Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 8 Slide 9 Available sensors include: O 2 NO Catalytic LEL NO 2 Infrared LEL HCL Infrared CO 2 HF CO EtO H 2S O 3 COSH COCl 2 PID HBr SO 2 THT Cl 2 and more! ClO 2 NH 3 H 2 G460 Interchangeable Plug-and-Play Smart Sensors PH 3 HCN Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 9
4 Slide 10 Calibration adapters Sampling probes Leather holsters Calibration kits Other accessories Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 10 Slide 11 Measuring Oxygen (Deficiency and Enrichment) Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 11 Slide 12 Sensor generates electrical current proportional to the O 2 concentration Sensor used up over time (one to three years) Oxygen reduced to hydroxyl ions at cathode: O 2 + 2H 2O + 4e - 4OH - Hydroxyl ions oxidize lead (anode): 2Pb + 4OH - 2PbO + 2H 2O + 4e - Overall cell reaction: 2Pb + O 2 2PbO Fuel Cell Oxygen Sensors Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 12
5 Slide 13 Oxygen Sensor Major components of a fuel cell type oxygen sensor Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 13 Slide 14 Most O 2 sensors have a capillary pore used to allow sensor to self-stabilize at new pressure O 2 sensors with capillary pore are true percent by volume measurement devices Are able to self stabilize to changes in pressure due to Stabilization at new pressure is not instantaneous, may take 30 seconds or longer Rear vent allows faster stabilization, with less stress to internal components Rear vent 2015 AIHA PDC 415: Methods and Applications for Chemical Detection in Real Time Slide 14 Capillary pore (located under external moisture barrier filter) Slide 15 Response of G460 equipped with O2-A3 and 4OX-V sensors to changes in pressure Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 15
6 Slide 16 Oxygen Pump Detection Principle Oxygen passively diffuses into polymer (catalyst) substrate Power from instrument battery used to pump the oxygen back out Reactions: Oxidation / Reduction of target gas by catalyst Sensing: O 2 + 4H + + 4e - 2 H 2O Counter: 2 H 2O O 2 + 4H + + 4e - Oxygen generated on counter electrode Amount electricity required to remove reaction product and return sensor to ground state (by generating O 2 at counter electrode) proportional to concentration of oxygen present Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 16 Slide 17 Advantages: Oxygen Pump Sensor Advantages and disadvantages Non-consuming detection technique (sensor does not lose sensitivity or consume itself over time) Disadvantages / concerns: Detection reaction may be influenced by shifts in humidity and density Sensor is net consumer of electricity (drain on power supply) Important to ensure that reaction product (H 2O) is removed from sensor Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 17 Slide 18 G460 with 4OX-LL (lead free) O 2, O2-A3 (fuel cell) O 2 and IR CO 2 sensors exposed to 100% volume N 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 18
7 Slide 19 G460 with O2-A3 oxygen and carbon dioxide sensors exposed to 4% Vol CO 2, 18% Vol O 2, balance N 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 19 Slide 20 G460 with O2-A3 O 2 sensor response to 4.9% O 2, 18.3% N 2, 76.8% CO 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 20 Slide 21 G460 with O2-A3 (fuel cell) O 2 and IR CO 2 sensors exposed to 94% volume CO 2, balance (6% volume) O 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 21
8 Slide 22 G460 with O2-A3 (fuel cell) O 2 and IR CO 2 sensors exposed to 94% volume CO 2, balance (6% volume) O 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 22 Slide 23 G460 with 4OX-LL (lead free) O 2 exposed to 94% volume CO 2, balance (6% volume) O 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 23 Slide 24 G460 with 4OX-LL (lead free) O 2, O2-A3 (fuel cell) O 2 and IR CO 2 sensors exposed to 94% volume CO 2, balance (6% volume) O 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 24
9 Slide 25 O 2 in dry air = 20.95% O 2 at 20 C and 25% RH 20.8% O 2 at 40 C and 85% RH 19.7% O 2 at 40 C and 90% RH 19.5% O 2 at 40 C and 100% RH 19.4% What should you do if the instrument is used in high temp, high RH conditions? Use cylinder of Zero Air to fresh air adjust the O 2 sensor. O 2 concentration in fresh air as function of temperature and humidity Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 25 Slide 26 Measuring combustible gases and vapors Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 26 Slide 27 Flame proof sensors depend on physical barriers such as stainless steel housings and flame arrestors to limit the amount of energy that can ever be released by the sensor The flame arrestor can slow, reduce, or even prevent larger molecules from entering the sensor The larger the molecule, the slower it diffuses through the flame arrestor into the sensor Traditional LEL sensors are Flame proof devices Stainless steel housing Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 27 Flame arrestor (sinter)
10 Slide 28 Combustible Gas Sensor The catalyst in the LEL sensor bead can be harmed if it is exposed to certain substances Porous Platinum refractory The larger the molecule, the wire coil slower it diffuses into the bead, bead with the longer it takes to be oxidized, catalyst and the lower the relative response 0.1 mm Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 28 Slide 29 Conditions created by oxidation of large molecules affects diffusion of molecules into the sensor Oxidation occurs on step-by-step basis, and proceeds only when molecules are in physical contact with catalyst coated surfaces within the bead. The very hot reaction by-products create convective currents as they rapidly diffuse away from the catalyst surfaces in the bead. Water vapor produced by oxidation of larger molecules creates a significant net outward flux, impeding diffusion of new molecules into the bead. Oxidation of methane: CH 4 + 2O 2 CO 2 + 2H 2O To oxidize one molecule CH 4 three molecules enter bead, and three molecules produced as by-products. Oxidation of pentane: C 5H O 2 5CO 2 + 6H 2O To oxidize one molecule of pentane, nine molecules enter bead, and 11 molecules produced as by-products. Oxidation of nonane: C 9H O 2 9CO H 2O To oxidize one molecule of nonane, 15 molecules enter bead, but 19 need to leave the sensor. Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 29 Slide 30 Typical carbon number distribution in No. 2 Diesel Fuel (liquid) Less than 2% of molecules in diesel vapor are small enough to be measured by means of standard LEL sensor Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 30
11 Slide 31 Reading % LEL True LEL Concentration Catalytic pellistor combustible gas response curves Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 31 Slide 32 Typical catalytic percent LEL sensor response to 50% LEL methane (2.5% vol. CH 4 ) Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 32 Slide 33 Typical catalytic percent LEL sensor response to 50% LEL pentane (0.7% vol. C 5 H 12 ) Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 33
12 Slide 34 Catalytic combustible sensor exposed to various gases Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 34 Slide 35 Non-dispersive infrared (NDIR) sensors When infra-red radiation passes through a sensing chamber containing a specific contaminant, only those frequencies that match one of the vibration modes are absorbed The rest of the light is transmitted through the chamber without hindrance The presence of a particular chemical group within a molecule thus gives rise to characteristic absorption bands Non-dispersive IR sensors measure at a specific range of wavelengths associated with a particular gas or class of gases Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 35 Slide 36 I 1 =I 0 *e -αlc L Beer-Lambert Law I 0 is the intensity of the incident light I 1 is the intensity after passing through the material L is the distance that the light travels through the material (the path length) c is the concentration of absorbing species in the material α is the absorption coefficient or the molar Size (length) matters... absorptivity of the absorber Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 36
13 Slide 37 Must have a COVALENT CHEMICAL BOND O S Linear molecules: SO Energy Absorbed by Bond Stretching and Bending Vibration Symmetric Asymmetric Bend Stretch Stretch Nonlinear Molecules Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 37 Slide 38 Geometry and specific bonds in molecule give rise to IR spectrum Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 38 Slide 39 Lower quantum levels must be populated Dipole moment (degree of charge imbalance) must change with the vibrational motion CO 2 and CH 4 absorb IR Homonuclear diatomics such as hydrogen DO NOT absorb IR IR-transparent gases: H 2 N 2 O 2 F 2 Cl 2 Hg 2 Ar Requirements for IR Absorption Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 39
14 Slide 40 Wavelengths typically used for IR LEL measurement Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 40 Slide 41 LEL measurement at 3.33μm vs. 3.4μm Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 41 Slide 42 Limitations: Combustible gas NDIR sensor advantages and limitations Molecule must include chemical bonds that absorb at the wavelength(s) used for measurement Not all combustible gases can be detected! Advantages: Diatomic molecules like hydrogen (H 2) cannot be detected at all Gases with double and triple bonds (like acetylene) detect poorly or not at all at some measurement wavelengths NDIR sensors with short optical path-lengths may have limited ability to measure gases with lower relative responses Sensor cannot be poisoned Does not require oxygen to detect gas Can be used for high-range combustible gas measurement Responds well to large hydrocarbon molecules that cannot be measured by means of standard LEL sensor Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 42
15 Slide 43 Three detection wavelengths Entirely separate optical paths (and pathlengths) for combustible and and CO 2 measurement Light path through three wavelength GfG NDIR sensor Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 43 Slide 44 Be very cautious when using O 2 concentration to estimate concentration of some other displacing gas Every 5% of displacing gas introduced into a confined space reduces O 2 concentration by only about 1% Presence of displacing gas on oxygen concentration Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 44 Slide 45 Shape of raw NDIR CH 4 curve (at 3.33 μm) is less linear than other detectable gases CH 4 curve can be mathematically corrected (normalized) against the response curves of other gases of interest Response of NDIR LEL sensor (3.33 μm, 44 mm path) to various target gases Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 45
16 Slide 46 Linearized 3.33 μm NDIR combustible gas response curves Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 46 Slide 47 Relative response of pellistor and infrared sensors to n-pentane Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 47 Slide 48 Both sensors were calibrated to 50% LEL n-hexane Readings for both sensors are now very close to the true 50% LEL concentration Initial response of IR sensor is slightly quicker than the pellistor sensor However, the time to the final stable response (T100) is virtually identical for both sensors, (about 150 seconds) Response of calibrated pellistor and IR sensors to 50% LEL n-hexane Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 48
17 Slide 49 Substance-specific electrochemical sensors Gas diffusing into sensor reacts at surface of the sensing electrode Sensing electrode made to catalyze a specific reaction Use of selective external filters further limits cross sensitivity Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 49 Slide 50 Cross sensitivities of City Technology 4S Rev. 2 sensor at 20 C Relative responses of City Technology 4S Rev. 2 sulfur dioxide (Sh2) sensor at 20 C Gas Concentration used (ppm) Carbon monoxide (Ch) 300 < 1 Reading (ppm Sh2) Nitric oxide (Nh) 50 0 to 5.0 Nitrogen dioxide (Nh2) 6 < 10 Hydrogen sulfide (H2S) 25 < 0.1 Chlorine (Cl2) 5 < 2 Ammonia (NH3) 20 0 Hydrogen (H2) 400 < 1 Hydrogen cyanide (HCN) 10 < 5 Acetylene (C2H2) 10 < 30 Ethene (C2H4) 50 < 45 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 50 Slide 51 Effects of hydrogen on CO sensor readings Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 51
18 Slide 52 G460 with 4COSH H 2S, 4HS-LM H 2S and THT (C 4H 8S) sensors exposed to 20 ppm H 2S, balance air Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 52 Slide 53 G460 with 4COSH H 2S, 4HS-LM H 2S and THT (C 4H 8S) sensors exposed to 3.0 ppm tert-butylmercaptan, balance N 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 53 Slide 54 G460 with 4COSH H 2S, 4HS-LM H 2S and THT (C 4H 8S) sensors exposed to 3.0 ppm methylmercaptan, balance N 2 Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 54
19 Slide 55 Setting toxic sensor alarms Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 55 Slide 56 Exposure limits for H2S Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 56 Slide 57 Where should you set the H 2 S alarms? H 2S TLV only includes STEL and TWA limits; does not include a Ceiling or Peak limit Instruments typically have four user settable alarms for each toxic sensor (Low, High, STEL and TWA) Suggested alarms: NIOSH: Low: 10.0 ppm High: 15.0 ppm STEL: 15.0 ppm TWA: 10.0 ppm TLV : Low: 3.0 ppm High: 5.0 ppm STEL: 5.0 ppm TWA: 1.0 ppm Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 57
20 Slide 58 What should you do for extended work shifts? Most industrial hygienists use Brief and Scala model Corrects for increased exposure time and decreased recovery time Simple to use Very conservative Adjusted TLV = Where h = # of hours worked per day Model used for chemicals where the TLV is based on acute or chronic toxicity and not for chemicals that have a TLV based on irritation (i.e. Ammonia) For 12 hour shift according to this model the TWA TLV limit should be reduced to one-half the 8-hour value Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 58 Slide 59 Photoionization Detectors Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 59 Slide 60 Catalytic LEL and photoionization detectors are complementary detection techniques Catalytic LEL sensors excellent for measurement of methane, propane, and other common combustible gases NOT detectable by PID PIDs detect large VOC and hydrocarbon molecules that are undetectable by catalytic sensors Best approach to VOC measurement is to use multi-sensor instrument capable of measuring all atmospheric hazards that may be potentially present Catalytic (CC) LEL vs. PID Sensors Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 60
21 Slide 61 Combustible sensor limitations Contaminant LEL (Vol %) Flashpoint OSHA PEL NIOSH REL TLV 5% LEL in Temp (ºF) PPM Acetone 2.5% -4ºF 1,000 PPM 250 PPM TWA 500 PPM 1250 PPM (-20 ºC) TWA TWA; 750 PPM STEL Diesel (No.2) 0.6% 125ºF None Listed None Listed 15 PPM 300 PPM vapor (51.7ºC) Ethanol 3.3% 55ºF 1,000 PPM 1000 PPM 1000 PPM 1,650 PPM (12.8 ºC) TWA TWA TWA Gasoline 1.3% -50ºF None Listed None Listed 300 PPM 650 PPM (-45.6ºC) TWA; 500 PPM STEL n-hexane 1.1% -7ºF 500 PPM TWA 50 PPM 50 PPM TWA 550 PPM (-21.7 ºC) TWA Isopropyl 2.0% 53ºF 400 PPM 400 PPM 200 PPM 1000 PPM alcohol (11.7ºC) TWA TWA; 500 TWA; 400 PPM STEL PPM STEL Kerosene/ 0.7% ºF None Listed 100 mg/m3 200 mg/m3 350 PPM Jet Fuels ( ºC ) TWA (approx. TWA (approx PPM) 29 PPM) MEK 1.4% 16ºF 200 PPM 200 PPM 200 PPM 700 PPM (-8.9ºC) TWA TWA; 300 TWA; 300 PPM STEL PPM STEL Turpentine ºF 100 PPM 100 PPM 20 PPM TWA 400 PPM (35ºC) TWA TWA Xylenes (o, m % 81 90ºF 100 PPM 100 PPM 100 PPM & p Aug isomers) 5, 2015 Best Sensor (27.3 Technologies 32.3 ºC) for TWA Marine Chemistry TWA; Applications 150 TWA; Slide PPM PPM STEL STEL Slide 62 Condensation and contamination on lamp window and sensor surfaces can create surface conduction paths between sensing and counter electrodes Buildup of contamination provides nucleation points for condensation, leading to surface currents If present, surface currents cause false readings and / or add significant noise that masks intended measurement (sometimes called moisture leakage ) PID designs MAY require periodic cleaning of the lamp and detector to minimize the effects of contaminants and humidity condensation on PID readings Critical PID Performance Issues: Effects of Humidity and Contamination Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 62 Slide 63 Planar "3D" 2-electrode PID design Benefits: Rapid response and clearing times Limitations: Gap between window and electrodes increases "quenching" effect of water vapor on signal Potential for drawing particulate contaminants into sensor More ionic fragments left behind to be adsorbed onto electrodes and window Results: Increased sensitivity to water vapor and humidity Must clean lamp more frequently Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 63
22 Slide 64 3-electrode PID Design Benefits: Diffusion design includes "fence electrode" to provide mechanical short circuit between sensing and counter electrodes Electrodes housed in replaceable "stack" Diffusion of molecules into and out of glow zone means less ionic fragments or particulates left behind Limitations: Slightly slower response Operation at higher voltage increases vulnerability to EMI / RFI Results: Reduced "moisture leakage" response due to humidity Clean lamp less frequently Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 64 Slide 65 PID Components Detector assembly Electrodes: sensing, counter and (in some designs) fence Lamp: most commonly 10.6EV, 11.7eV or 9.8 ev Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 65 Slide 66 What are broad-range sensors? Broad-range sensors (like LEL and PID sensors) are non-specific Broad-range sensors provide aggregate measurements for all detectable molecules within a specified class, for instance: Molecules capable of oxidation by standard LEL sensor Molecules capable of ionization by standard PID with 10.6 ev lamp Cannot distinguish between different contaminants they are able to detect Provide single total reading for all detectable substances present PID and LEL readings always relative to gas used to calibrate detector Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 66
23 Slide 67 The Controlling Compound Every mixture of gases and vapors has a compound that is the most toxic and controls the setpoint for the whole mixture Determine that chemical and you can determine a conservative mixture setpoint If we are safe for the worst chemical we will be safe for all chemicals Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 67 PID Alarms: Varying Mixtures Slide 68 Chemical Name 10.6eV CF Ethanol appears to be the safest compound Turpentine appears to be the most toxic NIOSH REL Exposure Limit (8-hr. TWA) Ethanol Turpentine Acetone This table only provides half of the decision making equation Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 68 PID Alarms: Varying Mixtures Slide 69 Set the PID for the compound with the lowest Exposure Limit (EL) in equivalent units and you are safe for all of the chemicals in the mixture Divide the EL in chemical units by CF to get the EL in isobutylene EL Isobutylene = EL chemical CF chemical PID Alarms: Varying Mixtures Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 69
24 Slide 70 Chemical name CF iso (10.6eV) NIOSH REL (8 hr. TWA) EL ISO (PEL) TLV IF you are following the NIOSH REL then ethanol is the controlling compound when the exposure limits are expressed in equivalent Isobutylene Units The equivalent ELiso is a calculation that involves a manufacturer specific Correction Factor (CF) Similar calculations can be done for any PID brand that has a published CF list PID Alarms: Varying Mixtures (8hr. TWA) BE CAREFUL: If you are following the TLV the controlling chemical would be turpentine! Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 70 EL ISO (TLV) Ethanol Turpentine Acetone Slide 71 Choosing the best sensor configuration GfG multi-sensor instruments can include up to seven channels of realtime measurement Available sensors for combustible gas and VOC measurement: CC %LEL IR %LEL IR %Vol Electrochemical toxic PID Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 71 Slide 72 Selection matrix for Sensors for measurement of combustible gas and VOCs Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 72
25 Slide 73 Questions? Aug 5, 2015 Best Sensor Technologies for Marine Chemistry Applications Slide 73
Questions, Myths and Misconceptions about Using Photoionization Detectors
 Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Understanding catalytic LEL combustible gas sensor performance
 : Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
: Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
Choosing the best detection technologies for measuring combustible gas and VOC vapors
 AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
Catalytic bead sensors are used primarily to detect
 Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
UV Hound Series. Dependable High Quality Air Monitoring. Accurate Readings Within Seconds. Portable UVDOAS Multi-gas Analyzers. Multi-Gas Capability
 UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
Training Coarse ECOPROBE 5 CHEMISTRY RS DYNAMICS
 Training Coarse ECOPROBE 5 CHEMISTRY Contents Ecoprobe analytical principles Photoionization Infrared spectroscopy Oxygen measurement Pollutants Types and responses Head space New application of Ecoprobe
Training Coarse ECOPROBE 5 CHEMISTRY Contents Ecoprobe analytical principles Photoionization Infrared spectroscopy Oxygen measurement Pollutants Types and responses Head space New application of Ecoprobe
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases and vapours Date: January 15-17, 2016 Language: English
 Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Monitoring Flammable Vapors and Gases in Industrial Processes
 Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS)
 New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
Sampling for Gases and Vapors
 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Technical/Application Article 01 Version 1.2
 Technical/Application Article 01 Version 1.2 th 25 January 2017 WRH What is a PID? Introduction PID is the abbreviation for Photo-Ionization Detector. A PID is a hand-held, personal, or fixed wallmounted
Technical/Application Article 01 Version 1.2 th 25 January 2017 WRH What is a PID? Introduction PID is the abbreviation for Photo-Ionization Detector. A PID is a hand-held, personal, or fixed wallmounted
Alphasense Ltd. Compliance Statements and Certificates. Contents. The Restriction of the Use of Certain Hazardous Substances (RoHS)...
 Alphasense Ltd Compliance Statements and Certificates Contents The Restriction of the Use of Certain Hazardous Substances (RoHS)... 2 Waste Electrical and Electronic Equipment (WEEE)... 3 Registration,
Alphasense Ltd Compliance Statements and Certificates Contents The Restriction of the Use of Certain Hazardous Substances (RoHS)... 2 Waste Electrical and Electronic Equipment (WEEE)... 3 Registration,
Combustible Gas Catalytic Bead (0-100 %LEL) Part No FM Performance Certified 1,4 FM Performance Certified 1
 Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Using 11.7eV PID Lamps To Accurately Measure VOCs With Ionization Energy Above 10.6eV
 Using 11.7eV PID Lamps To Accurately Measure VOCs With Ionization Energy Above 10.6eV Summary Honeywell RAE Systems strongly recommends using 100 ppm propane as calibration gas for instruments with 11.7eV
Using 11.7eV PID Lamps To Accurately Measure VOCs With Ionization Energy Above 10.6eV Summary Honeywell RAE Systems strongly recommends using 100 ppm propane as calibration gas for instruments with 11.7eV
Cracking. 191 minutes. 186 marks. Page 1 of 27
 3.1.6.2 Cracking 191 minutes 186 marks Page 1 of 27 Q1. (a) Gas oil (diesel), kerosine (paraffin), mineral oil (lubricating oil) and petrol (gasoline) are four of the five fractions obtained by the fractional
3.1.6.2 Cracking 191 minutes 186 marks Page 1 of 27 Q1. (a) Gas oil (diesel), kerosine (paraffin), mineral oil (lubricating oil) and petrol (gasoline) are four of the five fractions obtained by the fractional
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
Assessing Unknown Odors and Chemicals
 Treat yourself to better rental service. Thank You for Attending Today s Webinar: Assessing Unknown Odors and Chemicals Your Host Matt DeLacluyse Operations Mgr RAECO Rents mattd@raecorents.com Featured
Treat yourself to better rental service. Thank You for Attending Today s Webinar: Assessing Unknown Odors and Chemicals Your Host Matt DeLacluyse Operations Mgr RAECO Rents mattd@raecorents.com Featured
Measurement Technique and its Application to Trace Components of Atmospheric Gas
 F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
Chapter 9 Oxidation-Reduction Reactions. An Introduction to Chemistry by Mark Bishop
 Chapter 9 Oxidation-Reduction Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Oxidation Historically, oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn
Chapter 9 Oxidation-Reduction Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Oxidation Historically, oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn
a) ion-ion attractions b) London dispersion forces c) hydrogen bonding forces d) dipole-dipole attractions
 Asgn #48: Intermolecular Forces Name Dec. 13, 2016 1. The intermolecular forces that are most significant in accounting for the high boiling point of liquid water relative to other substances of similar
Asgn #48: Intermolecular Forces Name Dec. 13, 2016 1. The intermolecular forces that are most significant in accounting for the high boiling point of liquid water relative to other substances of similar
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
National 5 Whole Course Revision Questions
 National 5 Whole Course Revision Questions Unit 1 Chemical changes 1. Describe how temperature, concentration and particle size affect the rate of a chemical reaction- mention collision theory in your
National 5 Whole Course Revision Questions Unit 1 Chemical changes 1. Describe how temperature, concentration and particle size affect the rate of a chemical reaction- mention collision theory in your
Understanding ammonia sensors and their applications
 : Understanding ammonia sensors and their applications Different types of ammonia sensors are optimized for use in specific applications. The key to success is understanding the monitoring environment,
: Understanding ammonia sensors and their applications Different types of ammonia sensors are optimized for use in specific applications. The key to success is understanding the monitoring environment,
Chapter 7. Oxidation-Reduction Reactions
 Chapter 7 Oxidation-Reduction Reactions Chapter Map Oxidation Historically oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn 2Zn 2+ + 4e O + 2e O 2 or O 2 + 4e 2O 2 Oxidation
Chapter 7 Oxidation-Reduction Reactions Chapter Map Oxidation Historically oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn 2Zn 2+ + 4e O + 2e O 2 or O 2 + 4e 2O 2 Oxidation
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine?
 Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
5 Energy from chemicals
 5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
1.11 Redox Equilibria
 1.11 Redox Equilibria Electrochemical cells Electron flow A cell has two half cells. The two half cells have to be connected with a salt bridge. Simple half cells will consist of a metal (acts an electrode)
1.11 Redox Equilibria Electrochemical cells Electron flow A cell has two half cells. The two half cells have to be connected with a salt bridge. Simple half cells will consist of a metal (acts an electrode)
Far UV Absorbance Detector
 Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Chem 321 Name Answer Key D. Miller
 1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
Name May 2, 2012 Physical Behavior of Matter and Bonding Review
 Name May 2, 2012 Physical Behavior of Matter and Bonding Review Base your answers to questions 1 through 3 on the information below. Starting as a gas at 206 C, a sample of a substance is allowed to cool
Name May 2, 2012 Physical Behavior of Matter and Bonding Review Base your answers to questions 1 through 3 on the information below. Starting as a gas at 206 C, a sample of a substance is allowed to cool
Chemistry A: States of Matter Packet Name: Hour: Page 1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
Elements and Their Oxides
 Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Page 2. The hydrocarbon but-1-ene (C 4H 8) is a member of the homologous series of alkenes. But-1-ene has structural isomers.
 Q1.(a) The hydrocarbon but-1-ene (C 4H 8) is a member of the homologous series of alkenes. But-1-ene has structural isomers. State the meaning of the term structural isomers. Give the IUPAC name of the
Q1.(a) The hydrocarbon but-1-ene (C 4H 8) is a member of the homologous series of alkenes. But-1-ene has structural isomers. State the meaning of the term structural isomers. Give the IUPAC name of the
Introduction to Chemical Reactions. Chapter 6
 Introduction to Chemical Reactions Chapter 6 Instructional Goals 1. Given the reactants and product in a chemical reaction, the student will be able to write and balance chemical equations. 2. Identify
Introduction to Chemical Reactions Chapter 6 Instructional Goals 1. Given the reactants and product in a chemical reaction, the student will be able to write and balance chemical equations. 2. Identify
Name May 2, 2012 Physical Behavior of Matter and Bonding Review
 Name May 2, 2012 Physical Behavior of Matter and Bonding Review Base your answers to questions 1 through 3 on the information below. Starting as a gas at 206 C, a sample of a substance is allowed to cool
Name May 2, 2012 Physical Behavior of Matter and Bonding Review Base your answers to questions 1 through 3 on the information below. Starting as a gas at 206 C, a sample of a substance is allowed to cool
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Strategies For Selecting Air Sampling Methods AIHA-Florida Section Spring Conference
 Strategies For Selecting Air Sampling Methods 2011 AIHA-Florida Section Spring Conference Course Outline What are we going to sample for Sampling media Active sampling, diffusive samplers, direct read
Strategies For Selecting Air Sampling Methods 2011 AIHA-Florida Section Spring Conference Course Outline What are we going to sample for Sampling media Active sampling, diffusive samplers, direct read
MASS and INFRA RED SPECTROSCOPY
 MASS and INFRA RED SPECTRSCPY Mass Spectroscopy The mass spectrometer was looked at in Unit 1. It was noted there that compounds produce fragmentation patterns when passes through a mass spectrometer.
MASS and INFRA RED SPECTRSCPY Mass Spectroscopy The mass spectrometer was looked at in Unit 1. It was noted there that compounds produce fragmentation patterns when passes through a mass spectrometer.
HOW ADVANCED PYROMETERS INCREASE THERMAL PROCESS REPEATABILITY AND PRODUCT QUALITY
 HOW ADVANCED PYROMETERS INCREASE THERMAL PROCESS REPEATABILITY AND PRODUCT QUALITY Accurate temperature measurement is key for controlling the stability and repeatability of many temperature-critical processes.
HOW ADVANCED PYROMETERS INCREASE THERMAL PROCESS REPEATABILITY AND PRODUCT QUALITY Accurate temperature measurement is key for controlling the stability and repeatability of many temperature-critical processes.
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
Assessment Schedule 2015 Chemistry: Demonstrate understanding of aspects of carbon chemistry (90932)
 NCEA Level 1 Chemistry (90932) 2015 page 1 of 6 Assessment Schedule 2015 Chemistry: Demonstrate understanding of aspects of carbon chemistry (90932) Evidence Statement Q Evidence Achievement Merit Excellence
NCEA Level 1 Chemistry (90932) 2015 page 1 of 6 Assessment Schedule 2015 Chemistry: Demonstrate understanding of aspects of carbon chemistry (90932) Evidence Statement Q Evidence Achievement Merit Excellence
ISA QATAR : 90 th Seminar
 ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ICSE Board Class X Chemistry Board Paper Time: 1½ hrs Total Marks: 80
 ICSE Board Class X Chemistry Board Paper 2011 Time: 1½ hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will NOT be allowed to
ICSE Board Class X Chemistry Board Paper 2011 Time: 1½ hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will NOT be allowed to
GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. Bonding. GCSE OCR Revision Chemistry
 Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
N Goalby chemrevise.org
 Redox Equilibria Electrochemical cells This type of cell can be called a Voltaic cell or Galvanic cell. Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
Redox Equilibria Electrochemical cells This type of cell can be called a Voltaic cell or Galvanic cell. Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
Period 11: Chemical Energy and Fossil Fuels
 Name Section Period 11: Chemical Energy and Fossil Fuels 11.1 What is the Composition of Matter? 1) Atoms a) What are the building blocks of an atom? b) Which nucleons make up an atomic nucleus? c) What
Name Section Period 11: Chemical Energy and Fossil Fuels 11.1 What is the Composition of Matter? 1) Atoms a) What are the building blocks of an atom? b) Which nucleons make up an atomic nucleus? c) What
Test Booklet. Subject: SC, Grade: HS 2009 End of Course Chemistry. Student name:
 Test Booklet Subject: SC, Grade: HS 2009 End of Course Chemistry Student name: Author: Virginia District: Virginia Released Tests Printed: Tuesday April 23, 2013 1 Which of these would be best to measure
Test Booklet Subject: SC, Grade: HS 2009 End of Course Chemistry Student name: Author: Virginia District: Virginia Released Tests Printed: Tuesday April 23, 2013 1 Which of these would be best to measure
Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT?
 1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
Organic Chemistry. Alkanes are hydrocarbons in which the carbon atoms are joined by single covalent bonds.
 Organic Chemistry Organic compounds: The branch of chemistry which deals with the study of carbon compounds is called organic chemistry. Catenation: The carbon atom has a property to undergo self linking
Organic Chemistry Organic compounds: The branch of chemistry which deals with the study of carbon compounds is called organic chemistry. Catenation: The carbon atom has a property to undergo self linking
Reaction Rates and Chemical Equilibrium
 Reaction Rates and Chemical Equilibrium 12-1 12.1 Reaction Rates a measure of how fast a reaction occurs. Some reactions are inherently fast and some are slow 12-2 12.2 Collision Theory In order for a
Reaction Rates and Chemical Equilibrium 12-1 12.1 Reaction Rates a measure of how fast a reaction occurs. Some reactions are inherently fast and some are slow 12-2 12.2 Collision Theory In order for a
Reaction Rates and Chemical Equilibrium
 Reaction Rates and Chemical Equilibrium : 12-1 12.1 Reaction Rates : a measure of how fast a reaction occurs. Some reactions are inherently fast and some are slow: 12-2 1 12.2 Collision Theory In order
Reaction Rates and Chemical Equilibrium : 12-1 12.1 Reaction Rates : a measure of how fast a reaction occurs. Some reactions are inherently fast and some are slow: 12-2 1 12.2 Collision Theory In order
Gaseous CEMS technology
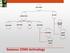 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Gas Sensors and Solar Water Splitting. Yang Xu
 Gas Sensors and Solar Water Splitting Yang Xu 11/16/14 Seite 1 Gas Sensor 11/16/14 Seite 2 What are sensors? American National Standards Institute (ANSI) Definition: a device which provides a usable output
Gas Sensors and Solar Water Splitting Yang Xu 11/16/14 Seite 1 Gas Sensor 11/16/14 Seite 2 What are sensors? American National Standards Institute (ANSI) Definition: a device which provides a usable output
OH, is an important feedstock for the chemical industry.
 1 Methanol, CH 3 OH, is an important feedstock for the chemical industry. In the manufacture of methanol, carbon dioxide and hydrogen are reacted together in the reversible reaction shown below. CO 2 (g)
1 Methanol, CH 3 OH, is an important feedstock for the chemical industry. In the manufacture of methanol, carbon dioxide and hydrogen are reacted together in the reversible reaction shown below. CO 2 (g)
Proportions to find moles Correctly organized Example. Problem
 Reactions continued And chemical review!! Steps to find grams in chemical Balance the equation to get molar ratios Find molar mass of the substances in question Find moles of the one given in grams Set
Reactions continued And chemical review!! Steps to find grams in chemical Balance the equation to get molar ratios Find molar mass of the substances in question Find moles of the one given in grams Set
Chemical Reactions (Chapter 13)
 Chemical Reactions (Chapter 13) coefficients reactants products https://www.youtube.com/watch?v=dummopdw By4 Student Learning Objectives Utilize chemical equations to determine the amounts of reactants,
Chemical Reactions (Chapter 13) coefficients reactants products https://www.youtube.com/watch?v=dummopdw By4 Student Learning Objectives Utilize chemical equations to determine the amounts of reactants,
Unit Five: Intermolecular Forces MC Question Practice April 14, 2017
 Unit Five: Intermolecular Forces Name MC Question Practice April 14, 2017 1. Which of the following should have the highest surface tension at a given temperature? 2. The triple point of compound X occurs
Unit Five: Intermolecular Forces Name MC Question Practice April 14, 2017 1. Which of the following should have the highest surface tension at a given temperature? 2. The triple point of compound X occurs
Chapter 19. Molecules and Compounds
 Chapter 19 Molecules and Compounds 1 Mini Quiz Which elements will react with water the same way that Na does? A. Ar B. B C. Cl D. K E. Mg 2 Another Which of the following has the highest ionization energy?
Chapter 19 Molecules and Compounds 1 Mini Quiz Which elements will react with water the same way that Na does? A. Ar B. B C. Cl D. K E. Mg 2 Another Which of the following has the highest ionization energy?
Chapter 8: Energy from Electron Transfer
 Chapter 8: Energy from Electron Transfer In his 2006 State of the Union address, President George W. Bush proclaimed... we are addicted to oil Are we doomed as a country to go on to the bitter end in terms
Chapter 8: Energy from Electron Transfer In his 2006 State of the Union address, President George W. Bush proclaimed... we are addicted to oil Are we doomed as a country to go on to the bitter end in terms
Use your knowledge of organic reaction mechanisms to complete the mechanism for this step by drawing two curly arrows on the following equation.
 Q1.The carboxylic acid 3-methylbutanoic acid is used to make esters for perfumes. The following scheme shows some of the reactions in the manufacture of this carboxylic acid. (a) One of the steps in the
Q1.The carboxylic acid 3-methylbutanoic acid is used to make esters for perfumes. The following scheme shows some of the reactions in the manufacture of this carboxylic acid. (a) One of the steps in the
Chemistry 40S Chemical Kinetics (This unit has been adapted from
 Chemistry 40S Chemical Kinetics (This unit has been adapted from https://bblearn.merlin.mb.ca) Name: 1 2 Lesson 1: Introduction to Kinetics Goals: Identify variables used to monitor reaction rate. Formulate
Chemistry 40S Chemical Kinetics (This unit has been adapted from https://bblearn.merlin.mb.ca) Name: 1 2 Lesson 1: Introduction to Kinetics Goals: Identify variables used to monitor reaction rate. Formulate
AASS, Örebro University
 Mobile Robotics Achim J. and Lilienthal Olfaction Lab, AASS, Örebro University 1 Contents 1. "Classical" Electronic Nose 2. Further Gas Sensing Technologies for Mobile Robots 3. (Signal Processing in Electronic
Mobile Robotics Achim J. and Lilienthal Olfaction Lab, AASS, Örebro University 1 Contents 1. "Classical" Electronic Nose 2. Further Gas Sensing Technologies for Mobile Robots 3. (Signal Processing in Electronic
Chemistry Standard level Paper 1
 M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
Rates of Reaction HL
 Name: Rates of Reaction Objectives 16. Rates of Reaction -define rate of reaction -define catalysis -monitor the rate of production of oxygen from hydrogen peroxide, using manganese dioxide as a catalyst
Name: Rates of Reaction Objectives 16. Rates of Reaction -define rate of reaction -define catalysis -monitor the rate of production of oxygen from hydrogen peroxide, using manganese dioxide as a catalyst
3.2 Alkanes. Refining crude oil. N Goalby chemrevise.org 40 C 110 C 180 C. 250 C fuel oil 300 C 340 C. Fractional Distillation: Industrially
 3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
Exampro GCSE Chemistry
 Exampro GCSE Chemistry C Chapter 4 Higher Name: Class: Author: Date: Time: 59 Marks: 59 Comments: Page of 0 Q. The picture shows a lump of phosphate rock. Rob Lavinsky, irocks.com CC-BY-SA-3.0 [CC-BY-SA-3.0],
Exampro GCSE Chemistry C Chapter 4 Higher Name: Class: Author: Date: Time: 59 Marks: 59 Comments: Page of 0 Q. The picture shows a lump of phosphate rock. Rob Lavinsky, irocks.com CC-BY-SA-3.0 [CC-BY-SA-3.0],
Edexcel Chemistry Checklist
 Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
CHEMISTRY HIGHER LEVEL
 *P15* Pre-Leaving Certificate Examination, 2013 Triailscrúdú na hardteistiméireachta, 2013 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
*P15* Pre-Leaving Certificate Examination, 2013 Triailscrúdú na hardteistiméireachta, 2013 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
Topics to Expect: Periodic Table: s, p, d, f blocks Metal, Metalloid, Non metal, etc. Periodic Trends, Family names Electron Configuration: Orbitals a
 Chemistry Final Exam Review and Practice Chapters Covered ESSENTIALLY CUMMULATIVE List of Chapters: Ch: 6, 7, 8, 9, 10, 13, 14, 15, 16, 19, 20 Topics to Expect: Periodic Table: s, p, d, f blocks Metal,
Chemistry Final Exam Review and Practice Chapters Covered ESSENTIALLY CUMMULATIVE List of Chapters: Ch: 6, 7, 8, 9, 10, 13, 14, 15, 16, 19, 20 Topics to Expect: Periodic Table: s, p, d, f blocks Metal,
A Level Chemistry. Ribston Hall High School. Pre Course Holiday Task. Name: School: ii) Maths:
 A Level Chemistry Ribston Hall High School Pre Course Holiday Task Name: School: GCSE Grades in i) Chemistry or Science: ii) Maths: 1 The following are a series of questions on topics you have covered
A Level Chemistry Ribston Hall High School Pre Course Holiday Task Name: School: GCSE Grades in i) Chemistry or Science: ii) Maths: 1 The following are a series of questions on topics you have covered
Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed
 Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed Gas Chromatography (GC) In gas chromatography, the sample is vaporized and injected onto the head of a chromatographic
Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed Gas Chromatography (GC) In gas chromatography, the sample is vaporized and injected onto the head of a chromatographic
MC 17 C SECTION - I (40 marks) Compulsory : Attempt all questions from this section.
 Question 1. (a) SECTION - I (40 marks) Compulsory : Attempt all questions from this section. Choose from the following list of substances, as to what matches the description from to given below : [Bronze,
Question 1. (a) SECTION - I (40 marks) Compulsory : Attempt all questions from this section. Choose from the following list of substances, as to what matches the description from to given below : [Bronze,
1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time
 Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Nomenclature. 133 minutes. 130 marks. Page 1 of 22
 3.1.5.1 Nomenclature 133 minutes 130 marks Page 1 of 22 Q1. (a) Write an equation for the formation of epoxyethane from ethene, showing the structure of the product. Explain why the epoxyethane molecule
3.1.5.1 Nomenclature 133 minutes 130 marks Page 1 of 22 Q1. (a) Write an equation for the formation of epoxyethane from ethene, showing the structure of the product. Explain why the epoxyethane molecule
H 8. ) is a member of the homologous series of alkenes. But-1-ene has structural isomers (2)... (1)...
 Q1. (a) The hydrocarbon but-1-ene (C 4 H 8 ) is a member of the homologous series of alkenes. But-1-ene has structural isomers. (i) State the meaning of the term structural isomers. (ii) Give the IUPAC
Q1. (a) The hydrocarbon but-1-ene (C 4 H 8 ) is a member of the homologous series of alkenes. But-1-ene has structural isomers. (i) State the meaning of the term structural isomers. (ii) Give the IUPAC
Volatile Organic Compounds (VOC) Probes
 Volatile Organic Compounds (VOC) Probes New Plug and Play accessory probes Compatible with the new 9565 VelociCalc and 7575 Qtrak Also compatible with the 9555 VelociCalc and 7565 Qtrak Firmware must be
Volatile Organic Compounds (VOC) Probes New Plug and Play accessory probes Compatible with the new 9565 VelociCalc and 7575 Qtrak Also compatible with the 9555 VelociCalc and 7565 Qtrak Firmware must be
Balancing chemical reaction equations (stoichiometry)
 Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
Introduction to electrochemistry
 Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Reactions continued. And chemical review!!
 Reactions continued And chemical review!! Steps to find grams in chemical reaction Balance the equation to get molar ratios Find molar mass of the substances in question Find moles of the one given in
Reactions continued And chemical review!! Steps to find grams in chemical reaction Balance the equation to get molar ratios Find molar mass of the substances in question Find moles of the one given in
PAPER 2 MAY/JUNE SESSION hour
 Centre Number Candidate Number Candidate Name CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level CHEMISTRY 9701/2 PAPER 2 MAY/JUNE SESSION
Centre Number Candidate Number Candidate Name CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level CHEMISTRY 9701/2 PAPER 2 MAY/JUNE SESSION
AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES
 JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
CHEMISTRY. SCIENCE Paper 2
 CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
ALE 1. Chemical Kinetics: Rates of Chemical Reactions
 Name Chem 163 Section: Team Number: ALE 1. Chemical Kinetics: Rates of Chemical Reactions (Reference: Sections 16.1 16.2 + parts of 16.5 16.6 Silberberg 5 th edition) How do the surface area, concentration
Name Chem 163 Section: Team Number: ALE 1. Chemical Kinetics: Rates of Chemical Reactions (Reference: Sections 16.1 16.2 + parts of 16.5 16.6 Silberberg 5 th edition) How do the surface area, concentration
Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet
 C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? 2. What is saturated? Unsaturated? Supersaturated? 3. How does
C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? 2. What is saturated? Unsaturated? Supersaturated? 3. How does
Test Booklet. Subject: SC, Grade: HS CST High School Chemistry Part 2. Student name:
 Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
IV. DIRECT MEASUREMENT LABS. Introduction
 IV. DIRECT MEASUREMENT LABS VI-1 I. Purpose Introduction An industrial hygienist frequently needs to evaluate airborne gas and vapor concentrations "on the spot". In other cases, he needs to track down
IV. DIRECT MEASUREMENT LABS VI-1 I. Purpose Introduction An industrial hygienist frequently needs to evaluate airborne gas and vapor concentrations "on the spot". In other cases, he needs to track down
# Ans Workings / Remarks
 # Ans Workings / Remarks 1 B Atomic mass and temperature affects the rate of diffusion of gas. The lower the atomic mass, the lighter the substance. The higher the temperature, the higher the rate of collision
# Ans Workings / Remarks 1 B Atomic mass and temperature affects the rate of diffusion of gas. The lower the atomic mass, the lighter the substance. The higher the temperature, the higher the rate of collision
High Pressure/Performance Liquid Chromatography (HPLC)
 High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
AP Chem Chapter 14 Study Questions
 Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
e - Galvanic Cell 1. Voltage Sources 1.1 Polymer Electrolyte Membrane (PEM) Fuel Cell
 Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
DM70 Hand-Held Dewpoint Meter for Spot-Checking Applications
 DM70 Hand-Held Dewpoint Meter for Spot-Checking Applications The Vaisala DRYCAP Hand-Held Dewpoint Meter DM70 offers accurate and fast measurement for industrial dewpoint applications, such as compressed
DM70 Hand-Held Dewpoint Meter for Spot-Checking Applications The Vaisala DRYCAP Hand-Held Dewpoint Meter DM70 offers accurate and fast measurement for industrial dewpoint applications, such as compressed
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Unit 6 Solids, Liquids and Solutions
 Unit 6 Solids, Liquids and Solutions 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Unit 6 Solids, Liquids and Solutions 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Page 2. Q1.Which of these substances does not contribute to the greenhouse effect? Unburned hydrocarbons. Carbon dioxide. Water vapour. Nitrogen.
 Q1.Which of these substances does not contribute to the greenhouse effect? A B C D Unburned hydrocarbons. Carbon dioxide. Water vapour. Nitrogen. (Total 1 mark) Q2.(a) The hydrocarbon but-1-ene (C 4H 8)
Q1.Which of these substances does not contribute to the greenhouse effect? A B C D Unburned hydrocarbons. Carbon dioxide. Water vapour. Nitrogen. (Total 1 mark) Q2.(a) The hydrocarbon but-1-ene (C 4H 8)
Gas Laws. Bonding. Solutions M= moles solute Mass %= mass solute x 100. Acids and Bases. Thermochemistry q = mc T
 Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
This place covers: Electrolytic production of inorganic compounds, of non-metals and of organic compounds
 C25B ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR (anodic or cathodic protection C23F 13/00; single-crystal growth C30B) Electrolytic production
C25B ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR (anodic or cathodic protection C23F 13/00; single-crystal growth C30B) Electrolytic production
