Sampling for Gases and Vapors
|
|
|
- Buddy McCormick
- 6 years ago
- Views:
Transcription
1 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors: may exist in liquid state at STP e.g.: toluene, benzene, other solvents; mercury Distinction moot unless liquid is sprayed. 1
2 Methods of Sampling Grab sampling Evacuated flask: heavy walls, air removed. A valve is opened to allow workroom air to fill the flask. Analysis in laboratory, or with suitable field instruments. Wash bottle, carboy, or other rigid-walled container. Flush container with workroom air to obtain a sample. Flush the bottle with times the bottle volume. Water filled bottle is alternative. Methods of Sampling Plastic bags, made of Tedlar, polyester, polyvinylidine chloride, other plastics. Useful sizes 5-15 L. Bags must be tested for leaks before use; polymer selection will consider storage, permeability, and reaction with bag walls and memory effects. 2
3 Methods of Sampling Continuous (Integrated) sampling Absorbers: dissolving gas in a liquid. Methods of Sampling Continuous (Integrated sampling) Chemical reactions are often used to stabilize substance before it leaves the liquid. (reduce volatility, e.g.) 3
4 Methods of Sampling Samplers: gas washing bottles (impingers), fritted bubblers, spiral and helical absorbers, and glass-bead columns. Methanol, Butanol in water; Esters in alcohol examples of use for simple gas bubble (midget impinger). Reactive samplers also use the midget impinger TDI hydrolyzed to toluene diamine; ammonia neutralized by sulfuric acid. Cold traps use acetone in dry ice. Methods of Sampling Solid Adsorbents (gas onto a solid) Activated charcoal can be efficiently desorbed with carbon disulfide. Optimal for nonpolar solvents. Method Breakthrough 4
5 Methods of Sampling Silica gel Polar contaminants: amines, some inorganics Other sorbants include synthetic polymers like XAD and Tenax. Consistent in quality but more expensive. Useful for low concentrations and unusual chemicals. Calculations Calculations: to find concentration: m1 - m C = SE V C = m1+m2 m (DE V) blank blank ( impinger ) ( sorbent tube ) C corr 760 = C P T 298 5
6 Diffusive Samplers Depend on flow of pollutant across a quiescent layer of air, or a membrane. Synonymous with passive dosimeter. Uses no pump to draw air across adsorbent. Diffusion depends on well-established rules from physical chemistry, known as Fick's law. Two equations are used to predict solvent uptake Diffusive Samplers D( Ca - Co ) F = L M Ca - Co = (D)( ) At L D( A) Mass = t( Ca C L F is flux units mg/cm 2 /sec L is length if diffusion path, cm D is diffusion coefficient, cm 2 /sec o ) 6
7 Diffusive Samplers Equations allow prediction of how much mass will be collected by the sampler; sample rate and efficiency are dictated by design of the device. Diffusive Samplers Factors affecting performance: Independent of pressure, proportional to T ½. Humidity may reduce efficiency of charcoal samplers. Transients (peak exposures) do not affect performance, so long as sampling time is more than several minutes. Face velocity not too low or too high. 7
8 Calculation C = Diffusive Samplers m 1 + m2 - m DExV blank Real Time Monitoring Continuous monitors measure and report instantaneous observations of concentration. Supplemented by a means of recording data, a plot of air concentration vs. time can be generated. 8
9 Real Time Monitoring Technologic developments Digital meters Data logging Miniaturization Multipoint monitoring Stationary Monitoring Systems Uses Warning or alarm systems Monitor operation of controls Identify problem areas Generate a record of concentration Define exposure problems 9
10 Real Time Monitoring Description Multipoint monitor vs. single point Sensor(s) Response time Advantages, Disadvantages Real time data at several locations Capable of warning of leaks before personnel are injured Provide history of exposure data Expensive Complicated Area monitor (not personal) 10
11 Personal Monitoring Systems Personal dosimeters: data logging, computer interface, instantaneous response Personal alarm monitors: intended to warn if air concentrations get too high. Advantages, Disadvantages Portable Easy to use, stable and accurate Can be specific to vapors of concern Can monitor rapidly fluctuating concentrations Can provide detailed exposure profile for an individual worker Limited number of chemicals can be monitored this way. Increased maintenance (over pump/filter) 11
12 Direct-reading Instruments Characteristics Specific or nonspecific: advantages and disadvantages to each, depends on the use of instrument Direct-reading Instrument Characteristics Terms, accessories Remote probes Data storage, transfer Alarm (variable settings) Ease of operation (some devices are complex) Response time (lag time) Saturation Sensor (sensor life) Probe Active/passive collection 12
13 Direct-reading Instruments: Characteristics Hazardous atmospheres: instrument should be rated as intrinsically safe, if flammable air concentrations may be encountered. RF shielding may be needed. Direct-reading instruments Calibration: conduct before each use, preferably with same contaminant as being monitored, else a reliable standard. Use prepared standards, or generate your own. Calibrate flow of pump, if it is included. Calibration of aerosol monitors is more complex; may require factory calibration (periodic) 13
14 Recording Data Data loggers allow collection of digitized information. Data logger may be contained in monitor, or be a separate unit. Transfer to a PC via dedicated cable and software. Recording Data Dedicated printers: printers intended for use with specific pieces of equipment. Allows a simple method of generating hard copy, user needs little knowledge of how to connect devices together. 14
15 Principles of Detection Electrical Methods: Chemical and/or physical properties change electrical parameters in a gas or liquid. Amount of change is proportional to the concentration of the contaminant. Electrochemical cell 15
16 Principles of Detection Conductivity: nonspecific method; electrolytes in aqueous solution change solution's conductivity. Change is proportional to all ions present. Most useful when one contaminant predominates. Temperature regulation is required. Contaminants: H 2 S, SO 2, NH 3. Some devices can detect Cl 2, CO 2 Principles of Detection Potentiometry (measure potential difference): Gases react with chemicals in solution to change the ph of the solution. ph change is sensed electrically (ion flow.) Choice of reagents can improve specificity. Contaminants: Carbon monoxide, oxygen, carbon dioxide, many others. 16
17 Principles of Detection Voltammetry: polarized electrodes. Analyte moves to an electrode, gets oxidized, and resultant current is measured. Principles of Detection Coulometry: Maintain a current in a cell; chemicals react in solution. Contaminants: oxygen, ozone, H2S, nitrogen oxides, sulfur dioxide, HCN. 17
18 Principles of Detection Ionization: Generate ions in a chamber, measure the number of ions collected on an electrode. Method is nonspecific, in that any chemical species ionized will be measured. Two types: flame ionization detector (FID) photo ionization detector (PID). Contaminants: organic solvents, in a number of ranges (ppm to % LEL). Principles of Detection - FID 18
19 Principles of Detection - PID Principles of Detection Thermal methods: use thermal properties of a gas to measure concentration. Conductivity: Nonspecific method measuring thermal conductivity of a gas. Combustion: Nonspecific method that measures the heat released during combustion. Measure the change in electrical resistance across a heated filament. Instruments may be refined using particular filaments or oxidation catalysts. Very common detection technique. 19
20 Principles of Detection Spectroscopic and Photometric Methods Use of electromagnetic energy (UV, IR, visible) to measure levels of contaminants. Most common method is absorption, but fluorescence, scattering, acoustic techniques are being developed. Principles of Detection Methods depend on: molecular absorption emission scatter 20
21 Principles of Detection Infrared Photometry Nondispersive Infrared (NDIR): A band of IR light Lasers, detectors or filters tune to specific wavelength(s) CO 2 is often measured with NDIR. Infrared detector - NDIR 21
22 Principles of Detection Dispersive Infrared employ a grating or prism to separate broadband light into component wavelengths. Instruments can scan across IR region, to analyze for unknown gases. Infrared - IR 22
23 Principles of Detection Infrared Chemical compounds absorb energy at characteristic wavelengths, depending on chemical structure. IR absorption can be used to identify pure chemical compounds, or for air analysis. Principles of Detection Ultraviolet photometers: Absorption of UV radiation at particular wavelengths. Mercury absorbs UV at 254 nm. Reduction of UV energy is a measure of mercury concentration. 23
24 Principles of Detection Chemi-electromagnetic methods (chemical reaction followed by a measurement of electromagnetic radiation) Colorimetry: Gas is sampled in a liquid or solid matrix, and color change takes place. Choice of reactants can make monitor specific to vapor of concern. Analysis involves selection of a choice of sensing cell and wavelengths to measure. SO 2 NO x NH 3, Cl 2, TDI, formaldehyde, cyanide, others. Principles of Detection Chemiluminescent substances are induced to emit electromagnetic radiation Ozone reacts with Rhodamine B (a dye), and light at 585 nm is emitted. This emission can be detected by a photomultiplier tube, and is proportional to the mass of ozone in the detector. 24
25 Principles of Detection Ozone reacts with ethylene and nitrogen oxide; Each reaction results in specific light emission that can be measured proportionately to the mass of ozone or nitrogen oxide in the system. Principles of Detection Colorimetric indicators Development of detector tubes (SKC CD) Colorimetric indicators may use liquid reagents, chemically treated papers or solid reagents. Some simple laboratory methods have been packaged for field use; simple instructions can allow field analysis of reactive chemicals (e.g.: TDI, chromates, nitrogen oxides) 25
26 Principles of Detection Chemically treated papers may react with specific chemicals to give a single color change or a range of colors, indicating concentration in air. Sampling by diffusion or with use of a pump. Commercial instruments use a paper tape to indicate concentration. Principles of Detection Detector tubes contain specific chemicals in a small tube; reagents react with chemicals in the sampled air to provide a color change (most commonly length-of-stain indication) to define contaminant concentration. 26
27 Principles of Detection Applications: qualitative, quantitative evaluation of workroom air; air pollution studies subject to detection limitation; explosive atmosphere evaluation. Principles of Detection Because of limitations and sources of error, qualified persons should make interpretations of results; despite simplicity, unskilled users may derive misleading results. 27
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Real-Time Detection: From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953)
 Real-Time Detection: 1953 From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953) Sampling Gases and Vapors Gas: A state of matter characterized by very low density and
Real-Time Detection: 1953 From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953) Sampling Gases and Vapors Gas: A state of matter characterized by very low density and
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
Raw Material Finished product By product Exposure standards Length of shift Physical environment
 Measurement &Sampling Part 1 Introduction to Sampling What to sample? - Hazard Raw Material Finished product By product Exposure standards Length of shift Recognition Physical environment To Determine
Measurement &Sampling Part 1 Introduction to Sampling What to sample? - Hazard Raw Material Finished product By product Exposure standards Length of shift Recognition Physical environment To Determine
UV Hound Series. Dependable High Quality Air Monitoring. Accurate Readings Within Seconds. Portable UVDOAS Multi-gas Analyzers. Multi-Gas Capability
 UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
Analytical techniques: Environmental samples. Lecture 2 Universidade do Algarve
 Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
Chapter 3: Source Measurement Techniques
 Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j
 UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
Strategies For Selecting Air Sampling Methods AIHA-Florida Section Spring Conference
 Strategies For Selecting Air Sampling Methods 2011 AIHA-Florida Section Spring Conference Course Outline What are we going to sample for Sampling media Active sampling, diffusive samplers, direct read
Strategies For Selecting Air Sampling Methods 2011 AIHA-Florida Section Spring Conference Course Outline What are we going to sample for Sampling media Active sampling, diffusive samplers, direct read
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Questions, Myths and Misconceptions about Using Photoionization Detectors
 Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Spectroscopy. Page 1 of 8 L.Pillay (2012)
 Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
IV. DIRECT MEASUREMENT LABS. Introduction
 IV. DIRECT MEASUREMENT LABS VI-1 I. Purpose Introduction An industrial hygienist frequently needs to evaluate airborne gas and vapor concentrations "on the spot". In other cases, he needs to track down
IV. DIRECT MEASUREMENT LABS VI-1 I. Purpose Introduction An industrial hygienist frequently needs to evaluate airborne gas and vapor concentrations "on the spot". In other cases, he needs to track down
Chem 321 Name Answer Key D. Miller
 1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES
 THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES J Kouzaki 1*, S Sato 1, S Nakai 1, Y Shirasuna 2, K Hirano 2 1 Graduate School of Environmental and Information
THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES J Kouzaki 1*, S Sato 1, S Nakai 1, Y Shirasuna 2, K Hirano 2 1 Graduate School of Environmental and Information
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY. Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC
 PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
atomic absorption spectroscopy general can be portable and used in-situ preserves sample simpler and less expensive
 Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
TOUR OF SOIL CHEMISTRY LABS (09/22/07)
 Department of Crop and Soil Sciences Commemorating Our Centennial, 1907 2007 TOUR OF SOIL CHEMISTRY LABS (09/22/07) WELCOME TO THE TOUR OF SOIL CHEMISTRY LABS Instrument: Lachat QuikChem FIA + 8000 Analyzer
Department of Crop and Soil Sciences Commemorating Our Centennial, 1907 2007 TOUR OF SOIL CHEMISTRY LABS (09/22/07) WELCOME TO THE TOUR OF SOIL CHEMISTRY LABS Instrument: Lachat QuikChem FIA + 8000 Analyzer
METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD)
 683 METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and
683 METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and
CH0302 Process Instrumentation. Lecture 9 Composition Analysis
 CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
CH0302 Process Instrumentation Lecture 9 Composition Analysis Department of Chemical Engineering School of Bioengineering SRM University Kattankulathur 603203 4/3/15 Chemical Engineering 1 Industrial significance
AN INTRODUCTION TO ATOMIC SPECTROSCOPY
 AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
ANALYTICAL METHOD DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Air sampling and analysis
 DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry
 Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
High Pressure/Performance Liquid Chromatography (HPLC)
 High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
Introduction to Electromagnetic Radiation and Radiative Transfer
 Introduction to Electromagnetic Radiation and Radiative Transfer Temperature Dice Results Visible light, infrared (IR), ultraviolet (UV), X-rays, γ-rays, microwaves, and radio are all forms of electromagnetic
Introduction to Electromagnetic Radiation and Radiative Transfer Temperature Dice Results Visible light, infrared (IR), ultraviolet (UV), X-rays, γ-rays, microwaves, and radio are all forms of electromagnetic
MODULE 4.3 Atmospheric analysis of particulates
 MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
Methods of pollution control and waste management - laboratory. Adsorptive removal of volatile organic compounds from gases streams
 Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy
 Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
High-Speed Gas and Headspace Analysis for the Process-Line and Laboratory: SIFT- MS IFPAC 2017
 High-Speed Gas and Headspace Analysis for the Process-Line and Laboratory: SIFT- MS IFPAC 2017 Y.J. Mange D.B. Milligan V.S. Langford B.J. Prince M. Perkins C. Anderson T. Wilks Who is using Syft Technologies
High-Speed Gas and Headspace Analysis for the Process-Line and Laboratory: SIFT- MS IFPAC 2017 Y.J. Mange D.B. Milligan V.S. Langford B.J. Prince M. Perkins C. Anderson T. Wilks Who is using Syft Technologies
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases and vapours Date: January 15-17, 2016 Language: English
 Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
2101 Atomic Spectroscopy
 2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
Choosing the best detection technologies for measuring combustible gas and VOC vapors
 AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
Gaseous CEMS technology
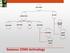 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Figure 1. Describe how the Law of Conservation of Energy applies to the chemical reaction that occurs in the hot pack
 NAME: TOTAL /25 1. A hot pack contains chemicals that can be activated to produce heat. A cold pack contains chemicals that feel cold when activated. Figure 1 Describe how the Law of Conservation of Energy
NAME: TOTAL /25 1. A hot pack contains chemicals that can be activated to produce heat. A cold pack contains chemicals that feel cold when activated. Figure 1 Describe how the Law of Conservation of Energy
Glossary of Common Laboratory Terms
 Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
AQA Chemistry (Combined Science) Specification Checklists. Name: Teacher:
 AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
Measurement Technique and its Application to Trace Components of Atmospheric Gas
 F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
F e a t u r e A r t i c l e Feature Article Measurement Technique and its Application to Trace Components of Atmospheric Gas Junji Kato From early on, HORIBA has been developing and adopting various measurement
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
CHLORINE THEORY & MEASUREMENT
 CHLORINE THEORY & MEASUREMENT Introduction Chlorine, dissolved in liquid, is one of the most effective and economical germ-killers for the treatment of water to make it potable or safe to drink. Chlorine's
CHLORINE THEORY & MEASUREMENT Introduction Chlorine, dissolved in liquid, is one of the most effective and economical germ-killers for the treatment of water to make it potable or safe to drink. Chlorine's
Understanding ammonia sensors and their applications
 : Understanding ammonia sensors and their applications Different types of ammonia sensors are optimized for use in specific applications. The key to success is understanding the monitoring environment,
: Understanding ammonia sensors and their applications Different types of ammonia sensors are optimized for use in specific applications. The key to success is understanding the monitoring environment,
Eppendorf UVette. Instructions for use
 Instructions for use Instructions Eppendorf see Fig. Tab. p. p. UVette for use Copyright 2014 Eppendorf AG, Hamburg, Germany. All rights reserved, including graphics and images. No part of this publication
Instructions for use Instructions Eppendorf see Fig. Tab. p. p. UVette for use Copyright 2014 Eppendorf AG, Hamburg, Germany. All rights reserved, including graphics and images. No part of this publication
Vapor Intrusion Sampling Options: Performance Data for Canisters, Badges, and Sorbent Tubes for VOCs
 Vapor Intrusion Sampling Options: Performance Data for s, Badges, and Sorbent Tubes for VOCs Linda S. Coyne SKC Inc., 863 Valley View Road, Eighty Four, PA 1533 George Havalias, Maria C. Echarte American
Vapor Intrusion Sampling Options: Performance Data for s, Badges, and Sorbent Tubes for VOCs Linda S. Coyne SKC Inc., 863 Valley View Road, Eighty Four, PA 1533 George Havalias, Maria C. Echarte American
Atomization. In Flame Emission
 FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
CHEM 103 Aqueous Solutions
 CHEM 103 Aqueous Solut Lecture Notes February 28, 2006 Prof. Sevian 1 Agenda What is a solution made of? Solubility of ionic compounds (salts), acids, and molecular compounds Exchange react that produce
CHEM 103 Aqueous Solut Lecture Notes February 28, 2006 Prof. Sevian 1 Agenda What is a solution made of? Solubility of ionic compounds (salts), acids, and molecular compounds Exchange react that produce
Monitoring Flammable Vapors and Gases in Industrial Processes
 Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
Introduction to Pharmaceutical Chemical Analysis
 Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Safety in the Chemistry Laboratory
 Safety in the Chemistry Laboratory CHAPTER1 Safety must be everyone s primary concern in the chemistry lab. Understanding and following all safety rules in the organic chemistry lab is critical to your
Safety in the Chemistry Laboratory CHAPTER1 Safety must be everyone s primary concern in the chemistry lab. Understanding and following all safety rules in the organic chemistry lab is critical to your
Experiment 1: Thin Layer Chromatography
 Experiment 1: Thin Layer Chromatography Part A: understanding R f values Part B: R f values & solvent polarity Part C: R f values & compound functionality Part D: identification of commercial food dye
Experiment 1: Thin Layer Chromatography Part A: understanding R f values Part B: R f values & solvent polarity Part C: R f values & compound functionality Part D: identification of commercial food dye
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
2001 Spectrometers. Instrument Machinery. Movies from this presentation can be access at
 2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
Chapter 15 Molecular Luminescence Spectrometry
 Chapter 15 Molecular Luminescence Spectrometry Two types of Luminescence methods are: 1) Photoluminescence, Light is directed onto a sample, where it is absorbed and imparts excess energy into the material
Chapter 15 Molecular Luminescence Spectrometry Two types of Luminescence methods are: 1) Photoluminescence, Light is directed onto a sample, where it is absorbed and imparts excess energy into the material
Chapter 3 Electrochemical methods of Analysis-Potentiometry
 Chapter 3 Electrochemical methods of Analysis-Potentiometry Electroanalytical chemistry Contents Introduction Galvanic and electrolytic cells Salt bridge Electrode potential and cell potential Indicator
Chapter 3 Electrochemical methods of Analysis-Potentiometry Electroanalytical chemistry Contents Introduction Galvanic and electrolytic cells Salt bridge Electrode potential and cell potential Indicator
Applications of Laser Spectroscopy to Highly Sensitive Analyses
 G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Applications of Laser Spectroscopy to Highly Sensitive Analyses Cavity Ring-Down Spectroscopy
G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Applications of Laser Spectroscopy to Highly Sensitive Analyses Cavity Ring-Down Spectroscopy
FAIMS Technology at a Glance
 FAIMS Technology at a Glance Field asymmetric ion mobility spectrometry (FAIMS), also known as differential mobility spectrometry (DMS), is a gas detection technology that separates and identifies chemical
FAIMS Technology at a Glance Field asymmetric ion mobility spectrometry (FAIMS), also known as differential mobility spectrometry (DMS), is a gas detection technology that separates and identifies chemical
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS)
 New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
Excimer Lasers Currently best UV laser sources Consist two atom types which repel each other eg nobel gas and halide or oxide which normally do not
 Excimer Lasers Currently best UV laser sources Consist two atom types which repel each other eg nobel gas and halide or oxide which normally do not bond But when excited/ionized these atoms attract Bound
Excimer Lasers Currently best UV laser sources Consist two atom types which repel each other eg nobel gas and halide or oxide which normally do not bond But when excited/ionized these atoms attract Bound
Chapter 9. Atomic emission and Atomic Fluorescence Spectrometry Emission spectrophotometric Techniques
 Chapter 9 Atomic emission and Atomic Fluorescence Spectrometry Emission spectrophotometric Techniques Emission Spectroscopy Flame and Plasma Emission Spectroscopy are based upon those particles that are
Chapter 9 Atomic emission and Atomic Fluorescence Spectrometry Emission spectrophotometric Techniques Emission Spectroscopy Flame and Plasma Emission Spectroscopy are based upon those particles that are
CHEMISTRY HIGHER LEVEL
 *P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
*P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
BRIEFING. Pharmacopeial Discussion Group Sign Off Document Attributes EP JP USP Definition Loss on drying Readily carbonizable substances
 BRIEFING Saccharin, NF 22 page 2825 and page 1711 of PF 29(5) [Sept. Oct. 2003]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
BRIEFING Saccharin, NF 22 page 2825 and page 1711 of PF 29(5) [Sept. Oct. 2003]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
AQA Chemistry Checklist
 Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Technology offer: Environmentally friendly holographic recording material
 Technology offer: Environmentally friendly holographic recording material Technology offer: Environmentally friendly holographic recording material SUMMARY Our research group has developed a new photopolymer
Technology offer: Environmentally friendly holographic recording material Technology offer: Environmentally friendly holographic recording material SUMMARY Our research group has developed a new photopolymer
https://www.chemicool.com/definition/chromatography.html
 CHROMATOGRAPHY 1 Chromatography - a physical method of mixture separation in which the components to be separated are distributed between two phases, one of which is stationary (stationary phase) while
CHROMATOGRAPHY 1 Chromatography - a physical method of mixture separation in which the components to be separated are distributed between two phases, one of which is stationary (stationary phase) while
NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25)
 Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Instrumental Chemical Analysis. Dr. Abdul Muttaleb Jaber Professor Faculty of Pharmacy Philadelphia University Fall 2012/2013
 0510212 Instrumental Chemical Analysis Dr. Abdul Muttaleb Jaber Professor Faculty of Pharmacy Philadelphia University Fall 2012/2013 Chapter 1 Electroanalytical Methods Electroanalytical Chemistry Electroanalytical
0510212 Instrumental Chemical Analysis Dr. Abdul Muttaleb Jaber Professor Faculty of Pharmacy Philadelphia University Fall 2012/2013 Chapter 1 Electroanalytical Methods Electroanalytical Chemistry Electroanalytical
Technical/Application Article 01 Version 1.2
 Technical/Application Article 01 Version 1.2 th 25 January 2017 WRH What is a PID? Introduction PID is the abbreviation for Photo-Ionization Detector. A PID is a hand-held, personal, or fixed wallmounted
Technical/Application Article 01 Version 1.2 th 25 January 2017 WRH What is a PID? Introduction PID is the abbreviation for Photo-Ionization Detector. A PID is a hand-held, personal, or fixed wallmounted
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB5009.
 Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
ISA QATAR : 90 th Seminar
 ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
"In Terms Of" 1. Explain, in terms of electron configuration, why arsenic and antimony are chemically similar.
 Name: Mrs. Vandergoot "In Terms Of" Regents Chemistry 1. Explain, in terms of electron configuration, why arsenic and antimony are chemically similar. 2. Base your answer to the following question on the
Name: Mrs. Vandergoot "In Terms Of" Regents Chemistry 1. Explain, in terms of electron configuration, why arsenic and antimony are chemically similar. 2. Base your answer to the following question on the
Exercise 9 - Petrochemicals and Climate
 113 Exercise 9 - Petrochemicals and Climate 1. The year of the first U.S. drilled oil well. c. 1859 2. Approximately, what percent of the world's remaining oil reserves are in the United States? a. 2%
113 Exercise 9 - Petrochemicals and Climate 1. The year of the first U.S. drilled oil well. c. 1859 2. Approximately, what percent of the world's remaining oil reserves are in the United States? a. 2%
Understanding catalytic LEL combustible gas sensor performance
 : Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
: Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
Pros and Cons of Water Analysis Methods
 Water Lens, LLC 4265 San Felipe, Suite 1100 Houston, Texas 77027 Office: (844) 987-5367 www.waterlensusa.com Pros and Cons of Water Analysis Methods Prepared by: Adam Garland, CTO Water Lens, LLC ICP-MS/OES
Water Lens, LLC 4265 San Felipe, Suite 1100 Houston, Texas 77027 Office: (844) 987-5367 www.waterlensusa.com Pros and Cons of Water Analysis Methods Prepared by: Adam Garland, CTO Water Lens, LLC ICP-MS/OES
Sodium Chloride - Analytical Standard
 Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
GENERAL PHARMACOPOEIA MONOGRAPH
 MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION GENERAL PHARMACOPOEIA MONOGRAPH Spectrophotometry in the ultraviolet GPM.1.2.1.1.0003.15 and visible spectral regions Replaces the SPRF X GPM, SPRF XI GPM,
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION GENERAL PHARMACOPOEIA MONOGRAPH Spectrophotometry in the ultraviolet GPM.1.2.1.1.0003.15 and visible spectral regions Replaces the SPRF X GPM, SPRF XI GPM,
Alphasense Ltd. Compliance Statements and Certificates. Contents. The Restriction of the Use of Certain Hazardous Substances (RoHS)...
 Alphasense Ltd Compliance Statements and Certificates Contents The Restriction of the Use of Certain Hazardous Substances (RoHS)... 2 Waste Electrical and Electronic Equipment (WEEE)... 3 Registration,
Alphasense Ltd Compliance Statements and Certificates Contents The Restriction of the Use of Certain Hazardous Substances (RoHS)... 2 Waste Electrical and Electronic Equipment (WEEE)... 3 Registration,
MEASURING H 2 S IN CRUDE OIL FOR QUALITY CONTROL & TRANSPORTATION SAFETY
 MEASURING IN CRUDE OIL FOR QUALITY CONTROL & TRANSPORTATION SAFETY COQA Meeting 2015 Marriott West Loop, Houston Wesley Kimbell Analytical Systems Keco www.h2sanalyzer.com Why measure in crude oil? Safety
MEASURING IN CRUDE OIL FOR QUALITY CONTROL & TRANSPORTATION SAFETY COQA Meeting 2015 Marriott West Loop, Houston Wesley Kimbell Analytical Systems Keco www.h2sanalyzer.com Why measure in crude oil? Safety
Steady-State Molecular Diffusion
 Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
CHEMISTRY. Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A.
 CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
Assessing Unknown Odors and Chemicals
 Treat yourself to better rental service. Thank You for Attending Today s Webinar: Assessing Unknown Odors and Chemicals Your Host Matt DeLacluyse Operations Mgr RAECO Rents mattd@raecorents.com Featured
Treat yourself to better rental service. Thank You for Attending Today s Webinar: Assessing Unknown Odors and Chemicals Your Host Matt DeLacluyse Operations Mgr RAECO Rents mattd@raecorents.com Featured
EXPERIMENT 7. Determination of Sodium by Flame Atomic-Emission Spectroscopy
 EXPERIMENT 7 Determination of Sodium by Flame Atomic-Emission Spectroscopy USE ONLY DEIONIZED WATER (NOT DISTILLED WATER!) THROUGHOUT THE ENTIRE EXPERIMENT Distilled water actually has too much sodium
EXPERIMENT 7 Determination of Sodium by Flame Atomic-Emission Spectroscopy USE ONLY DEIONIZED WATER (NOT DISTILLED WATER!) THROUGHOUT THE ENTIRE EXPERIMENT Distilled water actually has too much sodium
Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT?
 1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
2Fe 2 O 3 +3H 2 S FeS+FeS x +S+3H 2 O
 Elemental analysis of hydrocarbon streams using Dry colorimetry analyzers, a catalyst saviour Bushra Dawood, Application Coordinator C.I. Analytics www.cianalytics.com The Petrochemical industry has refined
Elemental analysis of hydrocarbon streams using Dry colorimetry analyzers, a catalyst saviour Bushra Dawood, Application Coordinator C.I. Analytics www.cianalytics.com The Petrochemical industry has refined
Catalytic bead sensors are used primarily to detect
 Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Atomic Emission Spectroscopy
 Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
Introduction to IH Analytical Chemistry
 Introduction to IH Analytical Chemistry Sampling and Analysis Manning, Eide, Van Etten (Apr 2013) Foreword Thank you for purchasing the Introduction to IH Analytical Chemistry self-study workbook. Course
Introduction to IH Analytical Chemistry Sampling and Analysis Manning, Eide, Van Etten (Apr 2013) Foreword Thank you for purchasing the Introduction to IH Analytical Chemistry self-study workbook. Course
Scope and application: For water, wastewater and seawater. Distillation is required for wastewater and seawater.
 Nitrogen, Ammonia DOC316.53.01078 USEPA 1 Nessler Method 2 Method 8038 0.02 to 2.50 mg/l NH 3 N Reagent Solution Scope and application: For water, wastewater and seawater. Distillation is required for
Nitrogen, Ammonia DOC316.53.01078 USEPA 1 Nessler Method 2 Method 8038 0.02 to 2.50 mg/l NH 3 N Reagent Solution Scope and application: For water, wastewater and seawater. Distillation is required for
NANDI CENTRAL DISTRICT JOINT MOCK 2013
 NAME:. SIGNATURE: INDEX NO:. DATE :.. 233/1 CHEMISTRY PAPER 1 THEORY JULY / AUGUST 2013 TIME: 2 HOURS NANDI CENTRAL DISTRICT JOINT MOCK 2013 Kenya Certificate of Secondary Education (K.C.S.E.) CHEMISTRY
NAME:. SIGNATURE: INDEX NO:. DATE :.. 233/1 CHEMISTRY PAPER 1 THEORY JULY / AUGUST 2013 TIME: 2 HOURS NANDI CENTRAL DISTRICT JOINT MOCK 2013 Kenya Certificate of Secondary Education (K.C.S.E.) CHEMISTRY
ELECTROCHEMICAL TECHNIQUES, OSMOMETRY AND THE PRINCIPLES OF RADIOACTIVITY
 ELECTROCHEMICAL TECHNIQUES, OSMOMETRY AND THE PRINCIPLES OF RADIOACTIVITY ELECTROCHEMISTY ELECTROCHEMISTRY IS THE STUDY OF CHEMICAL REACTIONS THAT RESULT IN THE FLOW OF ELECTRONS (CURRENT) OR THE DEVELOPMENT
ELECTROCHEMICAL TECHNIQUES, OSMOMETRY AND THE PRINCIPLES OF RADIOACTIVITY ELECTROCHEMISTY ELECTROCHEMISTRY IS THE STUDY OF CHEMICAL REACTIONS THAT RESULT IN THE FLOW OF ELECTRONS (CURRENT) OR THE DEVELOPMENT
Chromatographic Methods of Analysis Section 2: Planar Chromatography. Prof. Tarek A. Fayed
 Chromatographic Methods of Analysis Section 2: Planar Chromatography Prof. Tarek A. Fayed Planar chromatography includes two types: 1- Thin Layer Chromatography (TLC). 2- Paper Chromatography (PC). Thin
Chromatographic Methods of Analysis Section 2: Planar Chromatography Prof. Tarek A. Fayed Planar chromatography includes two types: 1- Thin Layer Chromatography (TLC). 2- Paper Chromatography (PC). Thin
THIN LAYER CHROMATOGRAPHY
 THIN LAYER CHROMATOGRAPHY OBJECTIVE In this laboratory you will separate spinach pigments using thin layer chromatography (TLC). INTRODUCTION Mixtures of compounds are very common in Organic Chemistry.
THIN LAYER CHROMATOGRAPHY OBJECTIVE In this laboratory you will separate spinach pigments using thin layer chromatography (TLC). INTRODUCTION Mixtures of compounds are very common in Organic Chemistry.
Atomic Absorption Spectrophotometry. Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon
 Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Determination of an Equilibrium Constant
 Last updated 1/29/2014 - GES Learning Objectives Students will be able to: Determine the numerical value of an equilibrium constant from measured concentrations of all reaction species. Use an absorption
Last updated 1/29/2014 - GES Learning Objectives Students will be able to: Determine the numerical value of an equilibrium constant from measured concentrations of all reaction species. Use an absorption
Chapter 5 Test. Directions: Write the correct letter on the blank before each question.
 Chapter 5 Test Name: Date: Directions: Write the correct letter on the blank before each question. Objective 1: Explain the science of fire as it relates to energy, forms of ignition, and modes of combustion.
Chapter 5 Test Name: Date: Directions: Write the correct letter on the blank before each question. Objective 1: Explain the science of fire as it relates to energy, forms of ignition, and modes of combustion.
