Caging Molecules: Catch Me If You Can!
|
|
|
- Collin Webb
- 5 years ago
- Views:
Transcription
1 Caging Molecules: Catch Me If You Can! Xiaoyong Li Department of Chemistry Michigan State University ov. 2nd, 2005 Aida, T. et.al. Angew. Chem. Int. Ed. 2001, 40, 1857.
2 Three Questions To Be Answered What?are caging molecules? How?to cage molecules? Why? to cage molecules?
3 K + What are Caging Molecules? K + H 18-Crown-6-K + H H H H H H HH Guest H H H H H H H H H n CD α β γ n Cyclodextrin Rocuronium bromide euromuscular blocker Born, A.; Bradley, M.; Cameron, K.; Clark, J. K.; Egmond, J. van; Feilden, H.; Maclean, E. J.; Muir, A. W.; Palin, R.; Rees, D.C.; Zhang, M.-Q. Agnew. Chem., Int. Ed. 2002, 41, 265. H H - Br Ac +
4 K + What are Caging Molecules? H 18-Crown-6-K + H H H H H HH Guest H H H H H H H H H n CD α β γ n Cyclodextrin H Z = C 2 Me ~83% Fullenrene Iwamatsu, S.; Murata, S.; Andoh, Y.; Minoura, M.; Kobayashi, K.; Mizorogi,.; agase, S. J. rg. Chem. 2005, 70, 4820.
5 What are Caging Molecules? Host-guest chemistry CH H 3 C CH 3 3 CH H 3 H H H Host S S Guest S S H CH3 H H H CH CH 3 3 C 3 H Cacerand Encapsulation / imprisonment Cram, D. J.; Karbach, S.; Kim, Y. H.; Aczynskyj, L.; Kalleymeyn, G. W. J. Am. Chem. Soc. 1985, 107, 2575.
6 How to Cage Molecules Building a host cage Synthesis by covalent bonding Self-assembly by noncovalent interactions Caging guest molecules van der Waals force Donor-acceptor interaction π - π stacking / CH - π interaction Electrostatic attraction
7 Why to Cage Molecules? Molecular storage - stabilize reactive species / dyes / drugs - selective extraction Acquire insights for enzymatic process - conduct chemical transformations in cages
8 Molecular storage Stabilize reactive species / dyes / drugs EtH + Et CEt rhodamine 6G Ultrastable Extended fluorescence life Unchanged fluorescent activity K a > M -1 Cucurbit[7]uril au, W. M.; Mohanty J. Angew. Chem. Int. Ed. 2005, 44, 3750.
9 Why to Cage Molecules? Molecular storage - stabilize reactive species / dyes / drugs - selective extraction
10 Molecular Extraction of Fullerenes - Aida s Story Carbon nanotube Superconductor HIV protease inhibitor C 60 Fullerenes C 60 (98%) C 60 / C 70 (9:1) C 70 (98%) C 84 (98%) Unite 1g 500mg 50mg 5mg Price $ 246 $ 141 $ 153 $ 697 Aldrich Catalog , 937
11 Caging Fullerenes by Dimeric Dimeric Metalloporphyrin Metalloporphyrins Host Hex Hex Hex Hex (CH 2 ) 6 (CH2 ) 6 Hex Hex Hex Hex Guest C 60 Bpy K a =6.7X10 5 M -1 << 3.2X10 8 M -1 C 60 Host Host-C 60 Tashiro, K.; Aida, T.; Zheng, J.-Y.; Kinbara, K.; Saigo, K.; Sakamoto, S.; Yamaguchi, K. J. Am. Chem. Soc. 1999, 121, 9477.
12 Caging C 60 by Dimeric Metalloporphyrins C 60 Free host -C23=2.918 Å -C24=2.765 Å UV titration of host with C 60 in benzene Proof from MR C 60 as acceptor Zheng, J.-Y.;Tashiro, K.; Hirabayashi, Y.; Kinbara, K.; Saigo, K.; Aida, T.; Sakamoto,S.; Yamaguchi, K.; Aida, T. Angew. Chem. Int. Ed. 2001, 40, 1857.
13 Caging Different Fullerenes Hex Hex Hex Hex (CH 2 ) 6 (CH2 ) 6 Hex Hex Hex Hex Log K association C 60 C 70 C 96 Guest C 12 H 25 C 12 H 25 (CH 2 ) 6 (CH 2 ) 6 C 12 H 25 C 12 H 25 Shoji, Y.; Tashiro, K.; Aida, T. J. Am. Chem. Soc. 2004, 126, 6570.
14 Selective Extraction of Higher Fullerenes C 12 H 25 C 12 H 25 Mixture of fullerenes host (CH 2 ) 6 (CH 2 ) 6 C 12 H 25 C 12 H 25 Host-C >70 Enriched C 96 host Host-C >100 Enriched C 102 ~C 110 Shoji, Y.; Tashiro, K.; Aida, T. J. Am. Chem. Soc. 2004, 126, 6570.
15 Selective Extraction of Higher Fullerenes 20% C 70 in C 60 Et Et Et Et Et Et Et Et (CH 2 ) 6 (CH2 ) 6 Et Et Et Et Et Et Et Et 91% C 70 in C 60 Porphyrincolumn Zheng, J.-Y.;Tashiro, K.; Hirabayashi, Y.; Kinbara, K.; Saigo, K.; Aida, T.; Sakamoto,S.; Yamaguchi, K.; Aida, T. Angew. Chem. Int. Ed. 2001, 40, 1857.
16 Why to Cage Molecules? Molecular storage - stabilize reactive species / dyes / drugs - selective extraction Acquire insights for enzymatic process - conduct chemical transformations in cages
17 A Comparison between atural Enzyme & Supermolecular Cage atural enzyme: selective substrate binding induced proximity hydrophobic interior complex superstructure hard to make Supermolecular cage: selective caging by size, shape, etc space-restricted environment hydrophobic interior well-defined and tunable structure
18 Approach to Supermolecular Cage Synthesis by covalent bonding Self-assembly by noncovalent interactions
19 Self-assembly Even a common, ordinary brick wants to be something more than it is. --Louis Kahn The Encyclopedia of Twentieth-Century Architecture (Ed.: R.Stephen Sennott), Fitzroy Dearborn, Chicago, 2002.
20 Self-assembly by oncovanlent Interactions - Hydrogen bonding fast equilibration / reversible solvent competition for H-bonding - Metal-ligand coordination greater strength more rigidity stable in aqueous solution
21 H-bonding Based Supermolecular Cage H R R H H H H H H R R H R= 4-n-heptylphenyl Volume = 320 Å 3 Softball Kang, J.; Rebek, J., Jr. ature 1997, 385, 50. Kang, J.; Santamarı a, J.; Hilmersson,G.; Rebek, J., Jr. J. Am. Chem. Soc. 1998, 120, 7389.
22 Functions of Metal-ligand Based Supermolecular Cages Fujita s story - molecular triangles Hupp s story molecular squares
23 Self-Assembly of Molecular Triangle -Fujita s Story n
24 M 6 L 4 Host Assembly-Molecular Paneling Pd Pd 12+ Pd Pd Pd Pd H 2 H 2 Pd 2 2 tube , water soluble Hydrophobic cavity bowl Pd = cage H 2 Pd H 2
25 [2+2] Photodimerization of lefins within anocages 2 hv + syn Poor yield and selectivity in solution phase Improved reactions need to be conducted in crystalline state The cage effect restrictions from lattice anti 2 hv + D 2 syn 2 mm, 0.5h > 98% yield anti Yoshizawa, M.; Takeyama, Y.; Kusukawa, T.; Fujita, M. Angew. Chem. Int. Ed. 2002, 41, 1347.
26 [2+2] Photodimerization of lefins within anocages 2 hv X benzene HT-syn HH-syn HT-anti HH-anti 150mM Yoshizawa, M.; Takeyama, Y.; Kusukawa, T.; Fujita, M. Angew. Chem. Int. Ed. 2002, 41, 1347.
27 [2+2] Photodimerization of lefins within anocages 2 hv D mM, 3h HT-syn HH-syn HT-anti HH-anti CDCl 3 HT-syn > 98% yield Yoshizawa, M.; Takeyama, Y.; Kusukawa, T.; Fujita, M. Angew. Chem. Int. Ed. 2002, 41, 1347.
28 [2+2] Cross-Photodimerization A + B hv A'-B' + A'-A' + B'-B' syn / anti syn / anti syn / anti + caging A+ B A B + A A + B B hv A'-B' + A'-A' + B'-B' syn syn syn
29 [2+2] Cross-Photodimerization of lefins within anocages + 1 : 1 Cage (1eq.) 80 o C, 10min D inert to hv D 2 hv, 3h size compatibility CDCl 3 97% yield Yoshizawa, M. Takeyama,Y.; kano,t.; Fujita M. J. Am. Chem. Soc. 2003, 125, 3243.
30 Photochemical xidation of Alkanes H H hv 2 + CH 3 C Air, Lewis Acid Can we do it in a greener way? Pd = Pd Shul pin, G. B.; izova, G. V.; Kozlov, Y.. ew. J. Chem. 1996, 20, Yoshizawa, M.; Miyagi, S.; Kawano, M.; Ishiguro, K.; Fujita, M. J. Am. Chem. Soc. 2004, 126, 9172.
31 Alkane xidation via Photochemical Excitation of Molecular Cage H H 4 hv H 2, r.t., 30min + 1 : Pd = Pd Yoshizawa, M.; Miyagi, S.; Kawano, M.; Ishiguro, K.; Fujita, M. J. Am. Chem. Soc. 2004, 126, 9172.
32 Alkane xidation via Photochemical Excitation of Molecular Cage H 4 hv H 2, r.t., 30min Ar hv 12+ Ar Substrate? Solvent? Metal? H 2 CH 3 C Solid Pd 2+ Pt 2+ Pd = Pd Anion? Ligand? 3 - PF 6 - Yoshizawa, M.; Miyagi, S.; Kawano, M.; Ishiguro, K.; Fujita, M. J. Am. Chem. Soc. 2004, 126, 9172.
33 Mechanism of Alkane xidation in Photo Reactor hv G G G' H H' H 12+ Generate radical in triazine ligand by hv e transfer from alkane to L generating R. & L Trap R. by H 2 or 2 releasing product Yoshizawa, M.; Miyagi, S.; Kawano, M.; Ishiguro, K.; Fujita, M. J. Am. Chem. Soc. 2004, 126, 9172.
34 Alkane xidation via Photochemical Excitation of Molecular Cage 2.6 Å Close contact is crucial! o oxidation with nanobowl Yoshizawa, M.; Miyagi, S.; Kawano, M.; Ishiguro, K.; Fujita, M. J. Am. Chem. Soc. 2004, 126, 9172.
35 Metal-ligand Based Supermolecular Cages Fujita s story - molecular triangles Hupp s story molecular squares
36 A Paradigm of Artificial Enzyme -Hupp s Story Fe Ⅱ Mn Ⅲ Cl - R 1 R 4 + xidant Catalyst R 1 R 4 R 2 R 3 R 2 R 3 Cytochrome P450 T = 50 t 1/2 = 10 min Merlau, M. L.; Mejia, M. del P.; guyen, S. T.; Hupp, J. T. Angew. Chem. Int. Ed. 2001, 40, 4239.
37 Mechanism for Metalloporphyrin Catalyzed Epoxidation R 1 Mn Ⅲ Cl [] R 2 R 1 R 1 R 2 Mn Ⅴ Mn Ⅴ Mn Ⅲ + Cl Cl Cl R 2 Mn Ⅲ Cl + Mn Ⅴ Mn Ⅳ Mn Ⅳ Cl oxo-dimer 2 Cl - Collman, J. P.; Kodadek, T.; Raybuck, S. A.; Brauman, J. I. J. Am. Chem. Soc. 1985, 107, Collman, J. P.; Brauman, J. I.; Meunier, B.; Hayashi, T.; Kodadek, T.; Raybuck, S. A. J. Am.. Chem. Soc. 1985, 107, 2000.
38 Artificial Enzyme from Directed Selfassembly of Molecular Squares Design a cage 18 Å Cl(C) 3 Re Re(C) 3 Cl 18 Å Mn Ⅲ 14 Å Cl(C) 3 Re Re(C) 3 Cl A perfect cavity! Merlau, M. L.; Mejia, M. del P.; guyen, S. T.; Hupp, J. T. Angew. Chem. Int. Ed. 2001, 40, 4239.
39 Artificial Enzyme from Directed Selfassembly of Molecular Squares 9 Å Cl(C) 3 Re Re(C) 3 Cl Host + Mn Ⅲ K a =10 6 M -1 Mn Ⅲ 18 Å Cl(C) 3 Re Re(C) 3 Cl + PHI Ph Catalyst CH 2 Cl 2, r.t. Ph 92% T = 500 t 1/2 = 3h Merlau, M. L.; Mejia, M. del P.; guyen, S. T.; Hupp, J. T. Angew. Chem. Int. Ed. 2001, 40, 4239.
40 Improve the Catalytic Activity of Artificial Enzyme by Enhance the Caging Cl(C) 3 Re Re(C) 3 Cl Mn Ⅲ Mn Ⅲ T= 65 Cl(C) 3 Re Re(C) 3 Cl Catalyst + PHI Ph CH 2 Cl 2, r.t. Ph 92% K a =10 7 M -1 T=1500 Merlau, M. L.; Mejia, M. del P.; guyen, S. T.; Hupp, J. T. Angew. Chem. Int. Ed. 2001, 40, 4239.
41 Summary Caging molecules has been extensively investigated as an important tool for molecule storage, catalysis and mimicking nature Fabricating supermolecular cages was achieved by many methods especially by self-assembly via noncovalent interactions as H-bonding & metal-ligand coordination Understanding the nature of caging molecules promotes the development of molecular reactors and artificial enzymes It s all about the space --Julius Rebek Jr.
42 Louis Kahn Yale University Art Gallery 1953, ew Haven, Connecticut
43 Louis Kahn Salk Institute for Biologic Studies 1965, La Jolla, California
44 Louis Kahn ational Assembly Building 1963, Dhaka, Bangladesh
45
46 Cl(C) 3 Re Re(C) 3 Cl Mn Ⅲ Cl(C) 3 Re Re(C) 3 Cl
47 Acknowledgement Dr. Borhan Dr. Wagner Chrysoula, Dan, Jennifer, Jun, Marina, Shang, Sing, Stewart, Tao, Xiaofei Yana
Reactivity within Confined Nano-spaces
 Reactivity within Confined Nano-spaces Larry Wolf Group Meeting 11-17-09 Encapsulating Cyclobutadiene hemicarcerand Anslyn, E. V; Dougherty, D. A. Modern Physical Organic Chemistry Cram. D. J. et. al.
Reactivity within Confined Nano-spaces Larry Wolf Group Meeting 11-17-09 Encapsulating Cyclobutadiene hemicarcerand Anslyn, E. V; Dougherty, D. A. Modern Physical Organic Chemistry Cram. D. J. et. al.
Supramolecular catalysis
 Supramolecular catalysis Catalyst: a chemical species that accelerates a chemical reactions without being consumed rganometallic catalyst: soluble metal complex with organic ligands that accelerates the
Supramolecular catalysis Catalyst: a chemical species that accelerates a chemical reactions without being consumed rganometallic catalyst: soluble metal complex with organic ligands that accelerates the
Paper No. 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry)
 Subject Chemistry Paper No and Title Paper 1: ORGANIC - I (Nature of Bonding Module No and Title Module Tag CHE_P1_M10 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Non-Covalent Interactions
Subject Chemistry Paper No and Title Paper 1: ORGANIC - I (Nature of Bonding Module No and Title Module Tag CHE_P1_M10 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Non-Covalent Interactions
Metalloporphyrin. ~as efficient Lewis acid catalysts with a unique reaction-field~ and. ~Synthetic study toward complex metalloporphyrins~
 Metalloporphyrin ~as efficient Lewis acid catalysts with a unique reaction-field~ and ~Synthetic study toward complex metalloporphyrins~ Literature Seminar Kenta Saito (D1) 1 Topics Chapter 1 ~as efficient
Metalloporphyrin ~as efficient Lewis acid catalysts with a unique reaction-field~ and ~Synthetic study toward complex metalloporphyrins~ Literature Seminar Kenta Saito (D1) 1 Topics Chapter 1 ~as efficient
Supporting Information
 Supporting Information Cyclodextrin Supramolecular Complex as Water Soluble Ratiometric Sensor for ferric Ion Sensing Meiyun Xu, Shuizhu Wu,* Fang Zeng, Changmin Yu College of Materials Science & Engineering,
Supporting Information Cyclodextrin Supramolecular Complex as Water Soluble Ratiometric Sensor for ferric Ion Sensing Meiyun Xu, Shuizhu Wu,* Fang Zeng, Changmin Yu College of Materials Science & Engineering,
Bio-inspired C-H functionalization by metal-oxo complexes
 1 Literature Seminar Bio-inspired C-H functionalization by metal-oxo complexes 2016. 7. 23. Nagashima Nozomu 2 C-H functionalization by enzymes Enzymes enable aliphatic C-H functionalization 3 P450 oxidation
1 Literature Seminar Bio-inspired C-H functionalization by metal-oxo complexes 2016. 7. 23. Nagashima Nozomu 2 C-H functionalization by enzymes Enzymes enable aliphatic C-H functionalization 3 P450 oxidation
Spectroscopy and laser characterization of synthesized supramolecular host cucurbit[7]uril using aqueous Rhodamine B dye
![Spectroscopy and laser characterization of synthesized supramolecular host cucurbit[7]uril using aqueous Rhodamine B dye Spectroscopy and laser characterization of synthesized supramolecular host cucurbit[7]uril using aqueous Rhodamine B dye](/thumbs/83/88339580.jpg) PRAMAA c Indian Academy of Sciences Vol. 82, o. 2 journal of February 2014 physics pp. 271 275 Spectroscopy and laser characterization of synthesized supramolecular host cucurbit[7]uril using aqueous Rhodamine
PRAMAA c Indian Academy of Sciences Vol. 82, o. 2 journal of February 2014 physics pp. 271 275 Spectroscopy and laser characterization of synthesized supramolecular host cucurbit[7]uril using aqueous Rhodamine
BAE 820 Physical Principles of Environmental Systems
 BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
Ali Ahmadpour. Fullerenes. Ali Ahmadpour. Department of Chemical Engineering Faculty of Engineering Ferdowsi University of Mashhad
 Ali Ahmadpour Fullerenes Ali Ahmadpour Department of Chemical Engineering Faculty of Engineering Ferdowsi University of Mashhad 2014 World of Carbon Materials 2 Fullerenes 1985 Robert F. Curl Jr. Richard
Ali Ahmadpour Fullerenes Ali Ahmadpour Department of Chemical Engineering Faculty of Engineering Ferdowsi University of Mashhad 2014 World of Carbon Materials 2 Fullerenes 1985 Robert F. Curl Jr. Richard
Solvent Scales. ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute
 Solvent Scales ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute Water 78 1.17 0.47 DMS 47 0.00 0.76 DM 37 0.00 0.76 Methanol 33 0.93 0.66 MPA 29 0.00 1.05 Acetone 21 0.08 0.43 Methylene
Solvent Scales ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute Water 78 1.17 0.47 DMS 47 0.00 0.76 DM 37 0.00 0.76 Methanol 33 0.93 0.66 MPA 29 0.00 1.05 Acetone 21 0.08 0.43 Methylene
Chem G8316_10 Supramolecular Organic Chemistry
 Chem G8316_10 Supramolecular Organic Chemistry Lecture 5, Wednesday, February 3, 2010 Photophysics of aromatic hydrocarbons Supramolecular effects on the photophysics of aromatic hydrocarbons 1 Course
Chem G8316_10 Supramolecular Organic Chemistry Lecture 5, Wednesday, February 3, 2010 Photophysics of aromatic hydrocarbons Supramolecular effects on the photophysics of aromatic hydrocarbons 1 Course
Enzyme function: the transition state. Enzymes & Kinetics V: Mechanisms. Catalytic Reactions. Margaret A. Daugherty A B. Lecture 16: Fall 2003
 Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 Enzyme function: the transition state Catalytic Reactions A B Catalysts (e.g. enzymes) act by lowering the transition state
Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 Enzyme function: the transition state Catalytic Reactions A B Catalysts (e.g. enzymes) act by lowering the transition state
Catalytic Reactions. Intermediate State in Catalysis. Lecture 16: Catalyzed reaction. Uncatalyzed reaction. Enzymes & Kinetics V: Mechanisms
 Enzyme function: the transition state Catalytic Reactions Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 A B Catalysts (e.g. enzymes) act by lowering the transition state
Enzyme function: the transition state Catalytic Reactions Lecture 16: Enzymes & Kinetics V: Mechanisms Margaret A. Daugherty Fall 2003 A B Catalysts (e.g. enzymes) act by lowering the transition state
Metal-organic frameworks in heterogeneous catalysis
 WIR SCHAFFE WISSE HEUTE FÜR MRGE Dr. Marco Ranocchiari :: Syncat Group Leader - LSK :: Paul Scherrer Institut Metal-organic frameworks in heterogeneous catalysis Catalysis Lecture 2017 ETH Zurich Catalysis:
WIR SCHAFFE WISSE HEUTE FÜR MRGE Dr. Marco Ranocchiari :: Syncat Group Leader - LSK :: Paul Scherrer Institut Metal-organic frameworks in heterogeneous catalysis Catalysis Lecture 2017 ETH Zurich Catalysis:
Chapter 16: Ethers, Epoxides, and Sulfides
 Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
Handout IRTG, May Lectures overview. Ligands for metals
 andout IRTG, ay 2009 Jan Reedijk. Leiden Institute of Chemistry Slide copies, stored as pdf; allowed for private use only. Bifunctionality in ligands and coordination compounds: application in design of
andout IRTG, ay 2009 Jan Reedijk. Leiden Institute of Chemistry Slide copies, stored as pdf; allowed for private use only. Bifunctionality in ligands and coordination compounds: application in design of
A. Loupy, B.Tchoubar. Salt Effects in Organic and Organometallic Chemistry
 A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
Palladium-Catalyzed Oxygenation of Unactivated sp 3 C-H Bonds
 Palladium-Catalyzed xygenation of Unactivated sp 3 C- Bonds Pd(Ac) 2 5 mol% PhI(Ac) 2 1.1 eq. Pd 2 Ac Desai, L. P.; ull, K. L.; Sanford *, M. S. University of Michigan J. Am. Chem. Soc. 2004, 126, ASAP
Palladium-Catalyzed xygenation of Unactivated sp 3 C- Bonds Pd(Ac) 2 5 mol% PhI(Ac) 2 1.1 eq. Pd 2 Ac Desai, L. P.; ull, K. L.; Sanford *, M. S. University of Michigan J. Am. Chem. Soc. 2004, 126, ASAP
Chapter 15: Enyzmatic Catalysis
 Chapter 15: Enyzmatic Catalysis Voet & Voet: Pages 496-508 Slide 1 Catalytic Mechanisms Catalysis is a process that increases the rate at which a reaction approaches equilibrium Rate enhancement depends
Chapter 15: Enyzmatic Catalysis Voet & Voet: Pages 496-508 Slide 1 Catalytic Mechanisms Catalysis is a process that increases the rate at which a reaction approaches equilibrium Rate enhancement depends
schematic diagram; EGF binding, dimerization, phosphorylation, Grb2 binding, etc.
 Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Anglo-Chinese School (Independent) International Baccalaureate Diploma Programme Scheme Of Work Year 5 Chemistry HL
 Topic 1 Quantitative Chemistry Topic 11 Measurement and Data Processing Topic 9 Redox equation 1.1 The mole concept and Avogadro s constant a) Determine the number of particles and the amount of substance
Topic 1 Quantitative Chemistry Topic 11 Measurement and Data Processing Topic 9 Redox equation 1.1 The mole concept and Avogadro s constant a) Determine the number of particles and the amount of substance
C a h p a t p e t r e r 6 E z n y z m y e m s
 Chapter 6 Enzymes 4. Examples of enzymatic reactions acid-base catalysis: give and take protons covalent catalysis: a transient covalent bond is formed between the enzyme and the substrate metal ion catalysis:
Chapter 6 Enzymes 4. Examples of enzymatic reactions acid-base catalysis: give and take protons covalent catalysis: a transient covalent bond is formed between the enzyme and the substrate metal ion catalysis:
Chem 1102 Semester 1, 2011
 Chem 1102 Semester 1, 2011 1 Lecture 29-30: Calculations involving K sp and Q (sat solutions of sparingly soluble electrolytes) Common Ion, T, p, solvent effects Enthalpy-entropy interplay Solubility curves
Chem 1102 Semester 1, 2011 1 Lecture 29-30: Calculations involving K sp and Q (sat solutions of sparingly soluble electrolytes) Common Ion, T, p, solvent effects Enthalpy-entropy interplay Solubility curves
The following molecules are related:
 Isolobal Analogy Inclusion of the ligand η-c 5 H 5 - which, as a donor of 3 π-electron pairs formally occupies 3 coordination sites, yields the analogies: The following molecules are related: 1 Isolobal
Isolobal Analogy Inclusion of the ligand η-c 5 H 5 - which, as a donor of 3 π-electron pairs formally occupies 3 coordination sites, yields the analogies: The following molecules are related: 1 Isolobal
Reversible Interaction between Substrate and Ligand
 Reversible Interaction between Substrate and Ligand 2010.6.9.YoheiShimizu(D3) is is is Ser 271 P Lys 229-2 3 P Zn 2+ Tyr P 3 2- + 3 Lys 107 P 3 2- class 1 aldolase class 2 aldolase Glu 185 Asp 211 C 2
Reversible Interaction between Substrate and Ligand 2010.6.9.YoheiShimizu(D3) is is is Ser 271 P Lys 229-2 3 P Zn 2+ Tyr P 3 2- + 3 Lys 107 P 3 2- class 1 aldolase class 2 aldolase Glu 185 Asp 211 C 2
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): Review for Nature Communications manuscript NCOMMS-17-03154-T "Polyaromatic Molecular Peanuts" by Yoshizawa and co-workers reports the synthesis
Reviewers' comments: Reviewer #1 (Remarks to the Author): Review for Nature Communications manuscript NCOMMS-17-03154-T "Polyaromatic Molecular Peanuts" by Yoshizawa and co-workers reports the synthesis
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Halogen Bond Applications in Organic Synthesis. Literature Seminar 2018/7/14 M1 Katsuya Maruyama
 Halogen Bond Applications in Organic Synthesis Literature Seminar 2018/7/14 M1 Katsuya Maruyama 1 Contents 1. Introduction 2. Property of Halogen Bond 3. Application to Organic Synthesis 2 1. Introduction
Halogen Bond Applications in Organic Synthesis Literature Seminar 2018/7/14 M1 Katsuya Maruyama 1 Contents 1. Introduction 2. Property of Halogen Bond 3. Application to Organic Synthesis 2 1. Introduction
Supramolecular Organic Photochemistry: The Control of Organic Photochemistry and Photophysics through Intermolecular Interactions
 CAPTER 13 Supramolecular rganic Photochemistry: The Control of rganic Photochemistry and Photophysics through Intermolecular Interactions 13.1 The Current and Emerging Paradigm of Supramolecular rganic
CAPTER 13 Supramolecular rganic Photochemistry: The Control of rganic Photochemistry and Photophysics through Intermolecular Interactions 13.1 The Current and Emerging Paradigm of Supramolecular rganic
14: Organic Synthesis: Disconnection Approach. 17: Principles of molecular associations and organizations: Non-covalent synthesis
 Subject Chemistry Paper No and Title Module No and Title Module Tag 14: Organic Synthesis: Disconnection Approach 17: Principles of molecular associations and organizations: Non-covalent synthesis CHE_P14_M17
Subject Chemistry Paper No and Title Module No and Title Module Tag 14: Organic Synthesis: Disconnection Approach 17: Principles of molecular associations and organizations: Non-covalent synthesis CHE_P14_M17
Biochemistry. Lecture 8 Enzyme Kinetics
 Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Supplementary Information
 Catalytically Efficient Palladium anoparticles Stabilized by Click rrocenyl Dendrimers Cátia rnelas, Lionel Salmon, Jaime Ruiz Aranzaes, Didier Astruc Supplementary Information Cyclic Voltammetry (CV),
Catalytically Efficient Palladium anoparticles Stabilized by Click rrocenyl Dendrimers Cátia rnelas, Lionel Salmon, Jaime Ruiz Aranzaes, Didier Astruc Supplementary Information Cyclic Voltammetry (CV),
!n[a] =!n[a] o. " kt. Half lives. Half Life of a First Order Reaction! Pressure of methyl isonitrile as a function of time!
![!n[a] =!n[a] o. kt. Half lives. Half Life of a First Order Reaction! Pressure of methyl isonitrile as a function of time! !n[a] =!n[a] o. kt. Half lives. Half Life of a First Order Reaction! Pressure of methyl isonitrile as a function of time!](/thumbs/77/76481346.jpg) Half lives Half life: t 1/2 t 1/2 is the time it takes for the concentration of a reactant to drop to half of its initial value. For the reaction A! products Half Life of a First Order Reaction! Pressure
Half lives Half life: t 1/2 t 1/2 is the time it takes for the concentration of a reactant to drop to half of its initial value. For the reaction A! products Half Life of a First Order Reaction! Pressure
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Chem 634. Introduction to Transition Metal Catalysis. Reading: Heg Ch 1 2 CS-B 7.1, , 11.3 Grossman Ch 6
 Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
Lecture 14 (10/18/17) Lecture 14 (10/18/17)
 Lecture 14 (10/18/17) Reading: Ch6; 190-191, 194-195, 197-198 Problems: Ch6 (text); 7, 24 Ch6 (study guide-facts); 4, 13 NEXT Reading: Ch6; 198-203 Ch6; Box 6-1 Problems: Ch6 (text); 8, 9, 10, 11, 12,
Lecture 14 (10/18/17) Reading: Ch6; 190-191, 194-195, 197-198 Problems: Ch6 (text); 7, 24 Ch6 (study guide-facts); 4, 13 NEXT Reading: Ch6; 198-203 Ch6; Box 6-1 Problems: Ch6 (text); 8, 9, 10, 11, 12,
Organic Electron Donors
 rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
Chemistry Unit: Chemical Bonding (chapter 7 and 8) Notes
 Name: Period: Due Date: 1-18-2019 / 100 Formative pts. Chemistry Unit: Chemical Bonding (chapter 7 and 8) Notes Topic-1: Review: 1. Valence electrons: The electrons in the outermost of an atom Valence
Name: Period: Due Date: 1-18-2019 / 100 Formative pts. Chemistry Unit: Chemical Bonding (chapter 7 and 8) Notes Topic-1: Review: 1. Valence electrons: The electrons in the outermost of an atom Valence
Minnesota Density Functionals
 Main Group Thermochemistry Minnesota Density Functionals D e 2 oncovalent Interactions M06-ass Functionals Long-range Charge Transfer Thermochemical Kinetics Transition Metal Chemistry We have developed
Main Group Thermochemistry Minnesota Density Functionals D e 2 oncovalent Interactions M06-ass Functionals Long-range Charge Transfer Thermochemical Kinetics Transition Metal Chemistry We have developed
Direct Oxidative Heck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans
 Direct xidative eck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans by Zhang,.; Ferreira, E. M.; Stoltz, B. M. Angewandte
Direct xidative eck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans by Zhang,.; Ferreira, E. M.; Stoltz, B. M. Angewandte
Recommended Reading: 23, 29 (3rd edition); 22, 29 (4th edition) Ch 102 Problem Set 7 Due: Thursday, June 1 Before Class. Problem 1 (1 points) Part A
 Recommended Reading: 23, 29 (3rd edition); 22, 29 (4th edition) Ch 102 Problem Set 7 Due: Thursday, June 1 Before Class Problem 1 (1 points) Part A Kinetics experiments studying the above reaction determined
Recommended Reading: 23, 29 (3rd edition); 22, 29 (4th edition) Ch 102 Problem Set 7 Due: Thursday, June 1 Before Class Problem 1 (1 points) Part A Kinetics experiments studying the above reaction determined
Scission of Dinitrogen by a Molybdenum(III) Xylidene Complex. CHM 5.33 Fall 2005
 Scission of Dinitrogen by a Molybdenum(III) Xylidene Complex CHM 5.33 Fall 2005 Introduction The experiment is based on research performed in the laboratory of Professor Cummins during the early 90 s.
Scission of Dinitrogen by a Molybdenum(III) Xylidene Complex CHM 5.33 Fall 2005 Introduction The experiment is based on research performed in the laboratory of Professor Cummins during the early 90 s.
Deactivation Pathways in Transition Metal Catalysis
 Deactivation Pathways in Transition tal Catalysis Why Study Catalyst Decomposition? decomposition active for catalysis inactive for catalysis "One of the reasons for [the] limited understanding [of catalyst
Deactivation Pathways in Transition tal Catalysis Why Study Catalyst Decomposition? decomposition active for catalysis inactive for catalysis "One of the reasons for [the] limited understanding [of catalyst
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Story Behind the Well-Developed Chiral Lewis Acid in Asymmetric Diels-Alder reaction
 Story Behind the Well-Developed Chiral Lewis Acid in Asymmetric Diels-Alder reaction Reporter: Zhang Sulei Supervisors: Prof. Yang Zhen Prof. Chen Jiahua Prof. Tang Yefeng 2015-10-05 1 Contents Background
Story Behind the Well-Developed Chiral Lewis Acid in Asymmetric Diels-Alder reaction Reporter: Zhang Sulei Supervisors: Prof. Yang Zhen Prof. Chen Jiahua Prof. Tang Yefeng 2015-10-05 1 Contents Background
Solving Zeolite Jigsaw:
 Solving Zeolite Jigsaw: A Synthetic Chemist's Approach R. Murugavel Dept. of Chemistry, IIT-Bombay rmv@chem.iitb.ac.in muruks@iitb.ac.in Tel. 7163 ur major research efforts: Solving the Zeolite Jigsaw
Solving Zeolite Jigsaw: A Synthetic Chemist's Approach R. Murugavel Dept. of Chemistry, IIT-Bombay rmv@chem.iitb.ac.in muruks@iitb.ac.in Tel. 7163 ur major research efforts: Solving the Zeolite Jigsaw
Q.1 Predict what will happen when SiCl 4 is added to water.
 Transition etals F325 1 The aqueous chemistry of cations Hydrolysis when salts dissolve in water the ions are stabilised by polar water molecules hydrolysis can occur and the resulting solution can become
Transition etals F325 1 The aqueous chemistry of cations Hydrolysis when salts dissolve in water the ions are stabilised by polar water molecules hydrolysis can occur and the resulting solution can become
Lecture 15: Enzymes & Kinetics. Mechanisms ROLE OF THE TRANSITION STATE. H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl. Margaret A. Daugherty.
 Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Q.1 Predict what will happen when SiCl 4 is added to water.
 Transition etals 1 The aqueous chemistry of cations Hydrolysis when salts dissolve in water the ions are stabilised by polar water molecules hydrolysis can occur and the resulting solution can become acidic
Transition etals 1 The aqueous chemistry of cations Hydrolysis when salts dissolve in water the ions are stabilised by polar water molecules hydrolysis can occur and the resulting solution can become acidic
Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage
 Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage William Cullen, a M. Cristina Misuraca, b Christopher A. Hunter,*,b Nicholas H. Williams*,a and Michael D.
Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage William Cullen, a M. Cristina Misuraca, b Christopher A. Hunter,*,b Nicholas H. Williams*,a and Michael D.
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Green Oxidations with Tungsten Catalysts. by Mike Kuszpit Michigan State University
 Green xidations with Tungsten Catalysts by Mike Kuszpit Michigan State University xidations in rganic Chemistry [] [] R 1 R 1 R 1 [] R 1 R 2 R 1 R 2 [] R 1 R 2 R 1 R 2 R 1 R 2 [] R 1 R 2 Essential as building
Green xidations with Tungsten Catalysts by Mike Kuszpit Michigan State University xidations in rganic Chemistry [] [] R 1 R 1 R 1 [] R 1 R 2 R 1 R 2 [] R 1 R 2 R 1 R 2 R 1 R 2 [] R 1 R 2 Essential as building
Bifunctional Asymmetric Catalysts: Design and Applications. Junqi Li CHEM Sep 2010
 Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
Ligand Substitution Reactivity of Coordinated Ligands
 Reactivity of Coordinated Ligands 2 C 2 H 4 (0) + H + + + 2 2 e (Cu 2 Cu) H CH 3 CH H "βh elim" ins βh elim H Peter H.M. Budzelaar Why care about substitution? Basic premise about metalcatalyzed reactions:
Reactivity of Coordinated Ligands 2 C 2 H 4 (0) + H + + + 2 2 e (Cu 2 Cu) H CH 3 CH H "βh elim" ins βh elim H Peter H.M. Budzelaar Why care about substitution? Basic premise about metalcatalyzed reactions:
Electronic Supplementary Information Effective lead optimization targeted for displacing bridging water molecule
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Electronic Supplementary Information Effective lead optimization targeted for displacing
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Electronic Supplementary Information Effective lead optimization targeted for displacing
Free Energy. because H is negative doesn't mean that G will be negative and just because S is positive doesn't mean that G will be negative.
 Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Organic Chemistry Laboratory Summer Lecture 6 Transition metal organometallic chemistry and catalysis July
 344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
Chapter 3: Elements and Compounds. 3.1 Elements
 Chapter 3: Elements and Compounds 3.1 Elements An element is a fundamental substance that cannot be broken down by chemical or physical methods to simpler substances. The 118 known elements are nature
Chapter 3: Elements and Compounds 3.1 Elements An element is a fundamental substance that cannot be broken down by chemical or physical methods to simpler substances. The 118 known elements are nature
Study on the Complexation of Macromolecule Cucurbituril with Metals and Acetamide
 International Journal of Chemistry and Applications. ISSN 0974-3111 Volume 4, Number 3 (2012), pp. 219-226 International Research Publication House http://www.irphouse.com Study on the Complexation of
International Journal of Chemistry and Applications. ISSN 0974-3111 Volume 4, Number 3 (2012), pp. 219-226 International Research Publication House http://www.irphouse.com Study on the Complexation of
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Molecularly imprinted polymers
 Molecularly imprinted polymers Presentation in Sensors, Arrays, Screening Lennart Niehues, Jan Philip Meyer 1 Overview Introduction Advantages Disadvantages Theory of MIP Requirements for the optimal MIP
Molecularly imprinted polymers Presentation in Sensors, Arrays, Screening Lennart Niehues, Jan Philip Meyer 1 Overview Introduction Advantages Disadvantages Theory of MIP Requirements for the optimal MIP
Chemistry Instrumental Analysis Lecture 11. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 11 Molar Absorptivities Range 0 to 10 5 Magnitude of e depends on capture cross section of the species and probability of the energy-absorbing transition. e
Chemistry 4631 Instrumental Analysis Lecture 11 Molar Absorptivities Range 0 to 10 5 Magnitude of e depends on capture cross section of the species and probability of the energy-absorbing transition. e
PHOTOCATALYSIS: FORMATIONS OF RINGS
 PHOTOCATALYSIS: FORMATIONS OF RINGS Zachery Matesich 15 April 2014 Roadmap 2 Photoredox Catalysis Cyclizations Reductive Oxidative Redox-neutral Electron Transfer Conclusion http://www.meta-synthesis.com/webbook/11_five/five.html
PHOTOCATALYSIS: FORMATIONS OF RINGS Zachery Matesich 15 April 2014 Roadmap 2 Photoredox Catalysis Cyclizations Reductive Oxidative Redox-neutral Electron Transfer Conclusion http://www.meta-synthesis.com/webbook/11_five/five.html
Unraveling the Mechanism of Water Oxidation by Ruthenium-Oxo Complexes
 Unraveling the Mechanism of Water xidation by thenium-xo Complexes Casseday Richers Literature Seminar ovember 20, 2007 The oxidation of water to dioxygen and protons represents one half the watersplitting
Unraveling the Mechanism of Water xidation by thenium-xo Complexes Casseday Richers Literature Seminar ovember 20, 2007 The oxidation of water to dioxygen and protons represents one half the watersplitting
Solvent & geometric effects on non-covalent interactions
 Solvent & geometric effects on non-covalent interactions Scott L. Cockroft PhysChem Forum 10, Syngenta, Jealott s Hill, 23 rd March 11 QSAR & Physical Organic Chemistry Quantifiable Physicochemical Properties
Solvent & geometric effects on non-covalent interactions Scott L. Cockroft PhysChem Forum 10, Syngenta, Jealott s Hill, 23 rd March 11 QSAR & Physical Organic Chemistry Quantifiable Physicochemical Properties
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction Mechanisms. Group Meeting Aaron Bailey 12 May 2009
 Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Organic Molecules, Photoredox, and. Catalysis
 Organic Molecules, Photoredox, and Catalysis 1 What is Photoredox Catalysis 2 Transition Metal vs Organic Photoredox Transition Metal Catalysts Organic Catalyst Reprinted (2017) with permission from (Wangelin,
Organic Molecules, Photoredox, and Catalysis 1 What is Photoredox Catalysis 2 Transition Metal vs Organic Photoredox Transition Metal Catalysts Organic Catalyst Reprinted (2017) with permission from (Wangelin,
Figure 1. Oxidation by iron-oxo complex. supported by porous solid
 Oxidation of Ethane to Ethanol by N 2 O in a Metal-Organic Framework with Coordinatively Unsaturated Iron(II) Sites Long, J.R, et al., Nat. Chem. 2014, 6, 590. Mechanism of Oxidation of Ethane to Ethanol
Oxidation of Ethane to Ethanol by N 2 O in a Metal-Organic Framework with Coordinatively Unsaturated Iron(II) Sites Long, J.R, et al., Nat. Chem. 2014, 6, 590. Mechanism of Oxidation of Ethane to Ethanol
PART I FUNDAMENTALS OF SUPRAMOLECULAR POLYMERS COPYRIGHTED MATERIAL
 PART I FUNDAMENTALS OF SUPRAMOLECULAR POLYMERS COPYRIGHTED MATERIAL CHAPTER 1 A BRIEF INTRODUCTION TO SUPRAMOLECULAR CHEMISTRY IN A POLYMER CONTEXT RAYMOND J. THIBAULT and VINCENT M. ROTELLO 1.1. INTRODUCTION
PART I FUNDAMENTALS OF SUPRAMOLECULAR POLYMERS COPYRIGHTED MATERIAL CHAPTER 1 A BRIEF INTRODUCTION TO SUPRAMOLECULAR CHEMISTRY IN A POLYMER CONTEXT RAYMOND J. THIBAULT and VINCENT M. ROTELLO 1.1. INTRODUCTION
Supporting information
 Supporting information Design and applications of an efficient amphiphilic click Cu I catalyst in water Changlong Wang, a Dong Wang, a Shilin Yu, a Thomas Cornilleau, a Jaime Ruiz, a Lionel Salmon, b and
Supporting information Design and applications of an efficient amphiphilic click Cu I catalyst in water Changlong Wang, a Dong Wang, a Shilin Yu, a Thomas Cornilleau, a Jaime Ruiz, a Lionel Salmon, b and
Transition Metal Chemistry
 Transition Metal Chemistry 2 2011.12.2 Ⅰ Fundamental Organometallic Reactions Following four reactions are important formal reaction patterns in organotransition metal complexes, which would conveniently
Transition Metal Chemistry 2 2011.12.2 Ⅰ Fundamental Organometallic Reactions Following four reactions are important formal reaction patterns in organotransition metal complexes, which would conveniently
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit. Lecture 2 (9/11/17)
 16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
18 th IRTG Joint Symposium
 18 th IRTG Joint Symposium University of Münster and Nagoya University Thursday, November 27 th and Saturday, November 29 th 2014 Westfälische Wilhelms-Universität Münster, Hörsaalgebäude der chemischen
18 th IRTG Joint Symposium University of Münster and Nagoya University Thursday, November 27 th and Saturday, November 29 th 2014 Westfälische Wilhelms-Universität Münster, Hörsaalgebäude der chemischen
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chiral Supramolecular Catalyst for Asymmetric Reaction
 Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Recent applications of chiral binaphtholderived phosphoric acid in catalytic asymmetric reactions
 Recent applications of chiral binaphtholderived phosphoric acid in catalytic asymmetric reactions 1. Seayad, J.; Seayad, A. M.; List, B. J. Am. Chem. Soc. 2006, ASAP. 2. Storer, R. L.; Carrera, D. E.;
Recent applications of chiral binaphtholderived phosphoric acid in catalytic asymmetric reactions 1. Seayad, J.; Seayad, A. M.; List, B. J. Am. Chem. Soc. 2006, ASAP. 2. Storer, R. L.; Carrera, D. E.;
Atomic and molecular interaction forces in biology
 Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
Synthesis of New Carbamate Materials from Primary Amines and CO 2
 Synthesis of ew Carbamate Materials from Primary Amines and C 2 Stephen Chiang University of Texas at Arlington 2006 Abstract The synthesis and characterization of novel linear polymer carbamates are reported.
Synthesis of ew Carbamate Materials from Primary Amines and C 2 Stephen Chiang University of Texas at Arlington 2006 Abstract The synthesis and characterization of novel linear polymer carbamates are reported.
What is an enzyme? Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics. Margaret A. Daugherty Fall General Properties
 Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2003 ENZYMES: Why, what, when, where, how? All but the who! What: proteins that exert kinetic control over
Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2003 ENZYMES: Why, what, when, where, how? All but the who! What: proteins that exert kinetic control over
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
The concept of the organic molecule as
 Perspective Supramolecular organic photochemistry: Control of covalent bond formation through noncovalent supramolecular interactions and magnetic effects Nicholas J. Turro* Department of Chemistry, Columbia
Perspective Supramolecular organic photochemistry: Control of covalent bond formation through noncovalent supramolecular interactions and magnetic effects Nicholas J. Turro* Department of Chemistry, Columbia
CONTAINER MOLECULES AS CATALYSTS IN ORGANIC CHEMISTRY. Reported by Ronald A. Smaldone February 21, 2005
 CTAIE MLECULES AS CATALYSTS I GAIC CEMISTY eported by onald A. Smaldone February 21, 2005 ITDUCTI Since Linus Pauling first postulated that enzymes preferentially bind the transition state to catalyze
CTAIE MLECULES AS CATALYSTS I GAIC CEMISTY eported by onald A. Smaldone February 21, 2005 ITDUCTI Since Linus Pauling first postulated that enzymes preferentially bind the transition state to catalyze
Green Chemistry Experiment: A Template-Directed [2+2] Photodimerization Conducted in the Solid State
![Green Chemistry Experiment: A Template-Directed [2+2] Photodimerization Conducted in the Solid State Green Chemistry Experiment: A Template-Directed [2+2] Photodimerization Conducted in the Solid State](/thumbs/77/75020075.jpg) Green Chemistry Experiment: A Template-Directed [2+2] Photodimerization Conducted in the Solid State Introduction A misconception often encountered in organic chemistry is that all organic reactions that
Green Chemistry Experiment: A Template-Directed [2+2] Photodimerization Conducted in the Solid State Introduction A misconception often encountered in organic chemistry is that all organic reactions that
Surface Modification of Biomaterials
 Lecture 9: Surface Modification of Biomaterials Supporting notes 3.051J/20.340J Materials for Biomedical Applications, Spring 2006 1 Purpose: Alter surface properties to enhance performance in biological
Lecture 9: Surface Modification of Biomaterials Supporting notes 3.051J/20.340J Materials for Biomedical Applications, Spring 2006 1 Purpose: Alter surface properties to enhance performance in biological
Recent Advances of Alkyne Metathesis. Group Meeting Timothy Chang
 Recent Advances of Alkyne Metathesis Group Meeting Timothy Chang 11-09-10 Fischer Carbyne and Schrock Alkylidyne Fischer Doublet LX type 4e Schrock Quartet X 3 type 6e -1-3 lone pair covalent p-back bonding
Recent Advances of Alkyne Metathesis Group Meeting Timothy Chang 11-09-10 Fischer Carbyne and Schrock Alkylidyne Fischer Doublet LX type 4e Schrock Quartet X 3 type 6e -1-3 lone pair covalent p-back bonding
METAL-ORGANIC FRAMEWORKS
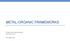 METAL-ORGANIC FRAMEWORKS Engle Lab Group Meeting Aug 10 th, 2017 Rei Matsuura A rapidly growing field Articles on "Metal-Organic Frameworks" 160000 140000 120000 100000 80000 60000 40000 20000 0 What are
METAL-ORGANIC FRAMEWORKS Engle Lab Group Meeting Aug 10 th, 2017 Rei Matsuura A rapidly growing field Articles on "Metal-Organic Frameworks" 160000 140000 120000 100000 80000 60000 40000 20000 0 What are
DOWNLOAD PDF MOLECULAR AND SUPRAMOLECULAR BIOINORGANIC CHEMISTRY
 Chapter 1 : From bio-inorganic chemistry to metal supramolecular chemistry DÃ partement de chimie For students of chemistry and medical sciences, this book will help shed some light on, for instance, the
Chapter 1 : From bio-inorganic chemistry to metal supramolecular chemistry DÃ partement de chimie For students of chemistry and medical sciences, this book will help shed some light on, for instance, the
Macroscopic Self-Assembly and Self-Healing through Molecular Recognition
 December 12, 2012, Den Haag Macroscopic Self-Assembly and Self-Healing through Molecular Recognition Osaka University Akira Harada Molecular Recognition O O Host-Guest Chemistry O M + O O O O O Crown Ethers,
December 12, 2012, Den Haag Macroscopic Self-Assembly and Self-Healing through Molecular Recognition Osaka University Akira Harada Molecular Recognition O O Host-Guest Chemistry O M + O O O O O Crown Ethers,
All chemical bonding is based on the following relationships of electrostatics: 2. Each period on the periodic table
 UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
Photoinduced Water Oxidation at the Aqueous. GaN Interface: Deprotonation Kinetics of. the First Proton-Coupled Electron-Transfer Step
 Supporting Information Photoinduced Water Oxidation at the Aqueous Interface: Deprotonation Kinetics of the First Proton-Coupled Electron-Transfer Step Mehmed Z. Ertem,,,* eerav Kharche,,* Victor S. Batista,
Supporting Information Photoinduced Water Oxidation at the Aqueous Interface: Deprotonation Kinetics of the First Proton-Coupled Electron-Transfer Step Mehmed Z. Ertem,,,* eerav Kharche,,* Victor S. Batista,
Hydrides and Dihydrogen as Ligands: Hydrogenation Catalysis
 Hydrides and Dihydrogen as Ligands: Hydrogenation Catalysis Synthesis of Organometallic Complex Hydrides Reaction of MCO with OH -, H -, or CH 2 CHR 2 M(CO) n + OH - = M(CO) n-1 (COOH) - = HM(CO) n-1 -
Hydrides and Dihydrogen as Ligands: Hydrogenation Catalysis Synthesis of Organometallic Complex Hydrides Reaction of MCO with OH -, H -, or CH 2 CHR 2 M(CO) n + OH - = M(CO) n-1 (COOH) - = HM(CO) n-1 -
Nucleophilic Fluorination. Souvik Rakshit Burke group Literature Seminar July 13, 2013
 Nucleophilic Fluorination Souvik Rakshit Burke group Literature Seminar July 13, 2013 Relevance 20% of pharmaceuticals contain fluorine 5-fluorouracil Antineoplastic agent, 1957 Lipitor (Atorvastatin)
Nucleophilic Fluorination Souvik Rakshit Burke group Literature Seminar July 13, 2013 Relevance 20% of pharmaceuticals contain fluorine 5-fluorouracil Antineoplastic agent, 1957 Lipitor (Atorvastatin)
Copper-Catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)-
 Literature Report Copper-Catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)- aseseazines A and B Reporter: Mu-Wang Chen Checker: Zhang-Pei Chen Date: 2013-05-28 Reisman,
Literature Report Copper-Catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)- aseseazines A and B Reporter: Mu-Wang Chen Checker: Zhang-Pei Chen Date: 2013-05-28 Reisman,
Personalised Learning Checklists AQA Chemistry Paper 1
 AQA Chemistry (8462) from 2016 Topics C4.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State that elements
AQA Chemistry (8462) from 2016 Topics C4.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State that elements
CHEMISTRY XL-14A CHEMICAL BONDS
 CHEMISTRY XL-14A CHEMICAL BONDS July 16, 2011 Robert Iafe Office Hours 2 July 18-July 22 Monday: 2:00pm in Room MS-B 3114 Tuesday-Thursday: 3:00pm in Room MS-B 3114 Chapter 2 Overview 3 Ionic Bonds Covalent
CHEMISTRY XL-14A CHEMICAL BONDS July 16, 2011 Robert Iafe Office Hours 2 July 18-July 22 Monday: 2:00pm in Room MS-B 3114 Tuesday-Thursday: 3:00pm in Room MS-B 3114 Chapter 2 Overview 3 Ionic Bonds Covalent
It s the amino acids!
 Catalytic Mechanisms HOW do enzymes do their job? Reducing activation energy sure, but HOW does an enzyme catalysis reduce the energy barrier ΔG? Remember: The rate of a chemical reaction of substrate
Catalytic Mechanisms HOW do enzymes do their job? Reducing activation energy sure, but HOW does an enzyme catalysis reduce the energy barrier ΔG? Remember: The rate of a chemical reaction of substrate
CHEM 4170 Problem Set #1
 CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
Dana Alsulaibi. Jaleel G.Sweis. Mamoon Ahram
 15 Dana Alsulaibi Jaleel G.Sweis Mamoon Ahram Revision of last lectures: Proteins have four levels of structures. Primary,secondary, tertiary and quaternary. Primary structure is the order of amino acids
15 Dana Alsulaibi Jaleel G.Sweis Mamoon Ahram Revision of last lectures: Proteins have four levels of structures. Primary,secondary, tertiary and quaternary. Primary structure is the order of amino acids
Coupling Reactions Using Excited State Organonickel Complex _LS_Daiki_Kamakura
 Coupling eactions Using Excited State rganonickel Complex 171118_LS_Daiki_Kamakura i Catalysis in Coupling eactions 9 10 11 Co 28 i Cu h Pd Ag Ir Pt Au Features of i d electrons: 8 relatively inexpensive
Coupling eactions Using Excited State rganonickel Complex 171118_LS_Daiki_Kamakura i Catalysis in Coupling eactions 9 10 11 Co 28 i Cu h Pd Ag Ir Pt Au Features of i d electrons: 8 relatively inexpensive
