Bacterial Spores and Chemical Sporicidal Agents
|
|
|
- Charity James
- 5 years ago
- Views:
Transcription
1 CLINICAL MICROBIOLOGY REVIEWS, Apr. 1990, p Vol. 3, No /90/ $02.00/0 Copyright 1990, American Society for Microbiology Bacterial Spores and Chemical Sporicidal Agents A. D. RUSSELL Welsh School of Pharmacy, University of Wales College of Cardiff, Cardiff, CFJ 3XF, Wales INTRODUCTION THE BACTERIAL SPORE SPOROSTATIC AND SPORICIDAL ACTIVITY Group A: Sporostatic Compounds Phenols and cresols Organic acids and esters Alcohols QACs Biguanides Organomercury compounds Group B: Sporicidal Compounds Glutaraldehyde Formaldehyde Other aldehydes Chlorine-releasing agents Iodine and iodophors Peroxygens Ethylene oxide P-Propiolactone Other gases RECOVERY AND REVIVAL OF INJURED SPORES SPOROGENESIS, SUSCEPTIBILITY, AND RESISTANCE Sporulation Germination Outgrowth OVERCOMING SPORE RESISTANCE MECHANISMS OF SPORICIDAL ACTION MEDICAL AND OTHER USES OF CHEMICAL SPORICIDES Sporicidal Agents Inhibitors of Germination and Outgrowth CONCLUSIONS LITERATURE CITED INTRODUCTION Bacterial spores are highly resistant to chemical and physical agents (25, 88, 89, 91, 102, 139, 158, 166, , 178, 207, 208, 224, 225). Processes designed to achieve sterilization of food, pharmaceutical, medical, and other products have thus, of necessity, had to take this high level of resistance into account. Spores are also of importance in other contexts, notably, (i) as food-poisoning agents (Clostridium botulinum, C. perfringens, and Bacillus cereus), (ii) as etiological agents (C. perfringens and C. tetani) in some infections, and (iii) as sources of antibiotics, toxins, and insecticides. Add to these the complex and fascinating series of events that take place during sporulation, germination, and outgrowth and the stage is set for a comprehensive study encompassing many scientific and medical disciplines, several of which are outside the scope of the present paper. This paper will deal with chemical sporicidal agents of the disinfectant type. Such chemicals are comparatively few in number and their activity is often susceptible to environmental conditions, at least some of which can be readily controlled. Other agents that are bactericidal and sporostatic but not usually sporicidal will also be considered when relevant. More effective sporicidal action will only be achieved by 99 learning more about the ways in which sporicides act or spores resist their action, and due attention will be paid to these aspects. Finally, the clinical uses of sporicidal agents will be discussed. General aspects of disinfection and disinfectants are to be found in references 73, 101, and 183. These include details, when relevant, of sporicidal activity. Spore resistance is described by Russell et al. (177) and Gardner and Peel (72). In the United States, commercially available disinfectants are regulated by the Environmental Protection Agency and must be used according to the directions specified on their labels. Workers elsewhere should be familiar with regulations pertaining to their own country. THE BACTERIAL SPORE The most important sporeformers are members of the genera Bacillus and Clostridium. Certain other bacteria, e.g., Sporosarcinae, Desulfomaculum, and Sporolactobacillus spp. (52), can also form spores, but will not be considered here. True endospores are also produced by thermophilic actinomycetes. Thermoactinomyces vulgaris spores are highly refractile, do not take up simple stains, have a typical
2 100 RUSSELL CX FIG. 1. "Typical" bacterial spore. The exosporium is present in some, but not all, types of spores. EXO, Exosporium; OSC, outer spore coat; ISC, inner spore coat; CX, cortex-; GCW, germ cell wall; PM, plasma membrane. spore structure, contain dipicolinic acid, and are heat resistant (173). The structure of a so-called typical bacterial spore is depicted in Fig. 1. It is clear that the spore is a complex entity, being composed of several different layers, some of which are implicated in their greater resistance than vegetative cells to chemical or physical processes. The molecular structure of the bacterial spore is considered in detail by Ellar (57) and Warth (233). The germ cell (protoplast or core) and germ cell wall are surrounded by the cortex, external to which are the inner and denser outer spore coats. An exosporium is present in some spores, but may surround just one dense spore coat. In terms of its macromolecular constituents (Table 1), the protoplast is the location of RNA, DNA, dipicolinic acid, and most of the calcium, potassium, manganese, and phosphorus present in the spore. Also present is a substantial amount of low-molecular-weight basic proteins which are rapidly degraded during germination (187). The cortex consists largely of peptidoglycan, some 45 to 60% of the muramic acid residues not having either a peptide or an N-acetyl substituent but instead forming an internal amide, muramic lactam (233). Peptidoglycan is the site of action of lysozyme and of nitrous acid. A dense inner layer TABLE 1. Chemical composition of bacterial spores component Composition Comment Outer spore Mainly protein Alkali resistant; removed coat by disulfide bond-reducing agents Inner spore Mainly protein Alkali soluble coat Cortex Mainly peptidoglycan Differs from peptidoglycan of vegetative cell wall Core Protein, DNA, RNA, Unique spore proteins DPA,a divalent cat- associated with DNA ions a DPA, Dipicolinic acid. TABLE 2. CLIN. MICROBIOL. REV. Agents with bactericidal, sporostatic, and sporicidal activity Bactericidal agents Bactericidal agents Comment that are sporostatic that are sporicidal Group A Phenols None in group A Even high concentra- Organic acids and tions for prolonged estersa periods at ambient QACs temp are not spori- Biguanides cidal; may be Organomercurials sporicidal at ele- Alcohols vated temperatures Group B Glutaraldehyde All in group B Low concentrations Formaldehyde are sporostatic; Iodine compounds usually much higher concentra- Chlorine compounds tioner tons are needed Hydrogen peroxide for sporicidal effect Peroxy acids Ethylene oxide 13-Propiolactone a For example, the parabens [methyl, ethyl, propyl, and butyl esters of para-(4)-hydroxybenzoic acid]. (cortical membrane, germ cell wall, primordial cell wall) of the cortex develops into the cell wall of the emergent cell when the cortex is degraded during germination. Two membranes, the inner and outer forespore membranes, surround the forespore during germination. The inner forespore membrane eventually becomes the cytoplasmic membrane of the germinating spore, whereas the outer forespore membrane persists in the spore integuments. The spore coats make up a major portion of the spore (139), consisting mainly of protein with smaller amounts of complex carbohydrates and lipid and possibly large amounts of phosphorus. The outer spore coat contains the alkaliresistant protein fraction and is associated with the presence of disulfide-rich bonds. The alkali-soluble fraction is found in the inner spore coats and consists predominantly of acidic polypeptides which can be dissociated to their unit components by treatment with sodium dodecyl sulfate. From this brief consideration of the structure and composition of the bacterial spore, it is obvious that several sites exist for attack by biocides and equally obvious that the spore can possess barriers which limit biocide penetration. It is the purpose of this review not only to describe the activity, properties, and uses of sporicidal agents but also to consider their mechanism of action, insofar as this is known, how resistance may be presented by the spore, and how this may be overcome. SPOROSTATIC AND SPORICIDAL ACTIVITY Comparatively few antibacterial agents are actively sporicidal (101, 173, 180). Even quite powerful bactericides may be inhibitory to spore germination or outgrowth or both, i.e., sporostatic, rather than sporicidal. Examples include phenols and cresols, quaternary ammonium compounds (QACs), biguanides such as chlorhexidine, organic mercury compounds, and alcohols (group A in Table 2). Sporicidal activity may, however, be achieved at elevated temperatures. It is clear from Table 3 that concentrations effecting sporostasis are usually very close to those that inhibit vegetative cell growth.
3 VOL. 3, 1990 TABLE 3. Comparison of bacteriostatic and sporostatic concentrations Antibacterial Bacteriostatic concn Sporostatic concn agent (%, wt/vol) (%, wt vol) Benzalkonium chloride Cetylpyridinium chlo ride Chlorhexidine diace tate or gluconate Chlorocresol Cresol Phenol Phenylmercuric nitrate or acetate a Based on reference 173. It is also apparent from Table 4 that even chemicals that are considered to be sporicidal require much higher concentrations for this effect than for bactericidal activity. Also, a time factor must be considered since spores must invariably be exposed for longer periods. A recent example of this is described by Power and Russell (156), who demonstrate that 2% alkaline glutaraldehyde will sterilize an inoculum of ca. 108 CFU of Escherichia coli, Staphylococcus aureus, and vegetative cells of B. subtilis per ml within 10 min at 22C, whereas B. subtilis spores require several hours. Agents that are actively sporicidal (group B in Table 2) include aldehydes, halogens, peroxygens, and gaseous or vapor-phase disinfectants. The properties of chemical compounds in both groups A and B are considered below. Group A: Sporostatic Compounds Chemical compounds in group A are not sporicidal but inhibit germination or outgrowth at concentrations similar to bacteriostatic ones. Phenols and cresols. Even at high concentrations, phenolic disinfectants are poorly sporicidal (102, 180), 2.5 and 5% (wt/vol) having little effect on B. subtilis spores even after 100 h at 25 or 37TC. In contrast, concentrations as low as 0.2% (phenol), 0.08% (cresol), and 0.02% (chlorocresol) (wt/vol) are all effective inhibitors of germination (173). Furthermore, sporicidal activity is greatly accelerated when phenolics are used at elevated temperatures (33). Such a process, utilizing 0.2% (wt/vol) chlorocresol at 98 to 100'C, was for many years a pharmacopoeial process in the United Kingdom for sterilizing certain injectable and ophthalmic products, but is no longer official. TABLE 4. Comparison of bactericidal and sporicidal concentrations Antibacterial Bactericidal concn Sporicidal concn agent (%, wt/vol) (%, wt/vol) Chlorocresol 0.1 >0.4 Cresol 0.3 >0.5 Phenol 0.5 >5.0 Phenylmercuric nitrate >0.02 Chlorhexidine diacetate >0.05 Cetylpyridinium chloride >0.05b Glutaraldehyde < Formaldehyde <1 4-8 Hypochlorite 1-2 ppm 20 ppm a Kill may depend on ph and temperature and on period of treatment. b Not sporicidal at this concentration at ambient temperatures. SPORICIDAL AGENTS 101 Organic acids and esters. Organic acids such as benzoic and sorbic acids and esters (parabens) of para-(4)-hydroxybenzoic acid are widely used as preservatives (102, 126, 180, 196, 232). They are bactericidal but not sporicidal. The parabens inhibit the growth and toxin production of C. botulinum (165) and act at the germination stage. Their activity is only slightly affected over the ph range 4 to 8, whereas organic acids are most active in the undissociated form, at low ph values. Alcohols. Ethanol is rapidly lethal to nonsporing bacteria when the alcohol is used at appropriate concentrations, but has no sporicidal activity (171, 173). The addition of 1% sodium or potassium hydroxide, various acids, or 10% amylm-cresol to 70% alcohol is claimed to enhance sporicidal activity (208). Initial sporicidal activity of an ethanol-hypochlorite mixture is high but decreases on storage (40). Other alcohols, namely, methanol (methyl alcohol), propan-1-ol, propan-2-ol (isopropyl alcohol, isopropanol), phenethyl alcohol (phenylethanol), and octanol, also lack sporicidal activity (102, 180). It is of interest, however, to note that fresh mixtures of methanol (15%) and hypochlorite have a low sporicidal activity and that this activity increases as the mixture ages, in contrast to ethanol-hypochlorite or propan-2-ol plus hypochlorite, when the reverse applies (40, 51). Furthermore, increasing the methanol concentration to 25%, and especially to 50%, produces a rapid initial sporicidal action which can be maintained for at least 8 h after preparation (51). Alcohols have been used for the selective isolation of sporeforming bacteria (122). QACs. The QACs can be considered derivatives of ammonium salts (NH4X) in which the hydrogen atoms are replaced by alkyl groups (R1 to R4). The sum of the carbon atoms in the four R groups is >10, and at least one of the R groups must have a chain length in the range C8 to C18. As a group, the QACs are bactericidal in low concentrations to nonmycobacterial, nonsporeforming, gram-positive bacteria, are less active against gram-negative bacteria, and are not sporicidal (41, 181). Low concentrations are, however, sporostatic (Table 3), the QACs inhibiting outgrowth but not germination (172, 173). Activity is markedly reduced in the presence of organic matter and is greater at alkaline than at acid ph. Biguanides. The most important biguanide is chlorhexidine, which is used as the acetate (diacetate) and gluconate salts. It is an effective bacteriostatic and bactericidal agent towards many gram-positive and gram-negative bacteria, but is not mycobactericidal and is sporostatic rather than sporicidal (172, 173). Chlorhexidine is sporicidal at elevated temperatures (77, 190) and, like the QACs, it inhibits outgrowth rather than germination (189). Activity is greatly reduced in the presence of organic matter and is greater at alkaline than at acid ph. Organomercury compounds. In the last century, it was claimed that the inorganic mercury compound mercuric chloride was rapidly sporicidal towards B. anthracis. It was subsequently demonstrated that this incorrect conclusion was based on a failure to control adequately sporostasis in the subculture medium (reviewed in reference 174). Organomercury compounds such as phenylmercuric nitrate, phenylmercuric acid, and thiomersal (merthiolate) are important preservatives in many types of pharmaceutical products. These compounds are bacteriostatic and bactericidal and are effective sporostatic agents at low concentrations, but are only sporicidal when used at high temperatures (171, 173).
4 102 RUSSELL Group B: Sporicidal Compounds Chemical agents in group B are sporostatic at low concentrations and sporicidal at much higher levels. Glutaraldehyde. Glutaraldehyde [pentanedial; CHO (CH2)3 CHO] is a powerful antimicrobial agent and electron microscope fixative (84, 185). Its activity depends on ph, alkaline solutions being considerably more effective than acid ones (84, 185, 186). There is, however, a complex relationship among the parameters of concentration, temperature, and ph. The rate of bactericidal and sporicidal activity for aqueous acid solution is considerably lower than that for activated alkaline solution (26, 27, 84, 120, 137, 212). As temperature is increased, however, this difference between alkaline and acid solutions is reduced (84, 192, 213). One of the earliest indications of antimicrobial activity of glutaraldehyde arose from a survey of the sporicidal activity of saturated dialdehydes in a search for an efficient substitute for formaldehyde. This search revealed (150) that glutaraldehyde in alcoholic solution was superior as a sporicidal agent to both formaldehyde and glyoxal. Stonehill et al. (206) and Snyder and Cheatle (194) demonstrated that aqueous solutions of the dialdehyde were acidic and needed to be buffered ("activated") by suitable alkalinating agents to a ph of 7.5 to 8.5 for antimicrobial activity. A 2% (wt/vol) glutaraldehyde solution activated with 0.3% (wt/vol) sodium bicarbonate was advocated to provide the minimum concentration and conditions necessary for rapid sporicidal activity. At this in-use concentration, the dialdehyde was capable of killing spores of Bacillus and Clostridium spp. in 3 h (26, 203). Rubbo et al. (170) reported a 4-log (99.9%) kill of spores of B. anthracis and C. tetani in 15 and 30 min, respectively. Not all species are equally susceptible to glutaraldehyde, and B. subtilis (29) and B. pumilus (170) appear to be the most resistant. With B. subtilis spores in liquid suspension, a 3-h contact period with 2% alkaline glutaraldehyde produces ca. a 6-log drop in viable count (73, 116, 131, 192). Using the Association of Official Analytical Chemists sporicidal test (8) and vacuum-dried spores of B. subtilis, however, Boucher (29) found that 10 h was necessary to achieve a complete kill. Obviously, this long period cannot always be used in practice. The possible revival of glutaraldehydetreated spores will be considered later. Vegetative bacteria are more susceptible to the action of glutaraldehyde, a concentration as low as 0.02% alkaline aldehyde achieving an inactivation factor of 104 to 106 within 20 min at 20 C (164). At alkaline ph, glutaraldehyde solutions have a tendency to polymerize; polymerization in acid solution is very slow. Consequently, since monomeric glutaraldehyde is considered to be the active moiety (170) with interaction between amino groups in protein enhanced at alkaline ph (179), it is clear that acid solutions are more stable but less active and alkaline solutions are less stable but more active. These problems of stability and active life have prompted the development of novel formulations to overcome these drawbacks in use. Alkalination of glutaraldehyde produces a gradual decrease in aldehyde concentration (25, 155), the fall being temperature dependent (213). Some formulations utilize the benefits conferred by formulating in the lower alkaline range of ca. ph 7.5. One such product, a stabilized glutaraldehyde solution (131), also contains surfactants to promote rinsing of surfaces and is claimed to have the usual antimicrobial activity while also maintaining a stable glutaraldehyde concentration and ph over 28 days. In many instances, novel formulations have been produced which are CLIN. MICROBIOL. REV. based on acid rather than alkaline glutaraldehyde, thereby benefiting from the stability inherent in such solutions. The improved sporicidal activity claimed for these acidic solutions has often been obtained by the addition of agents that produce a potentiated or synergistic effect with the dialdehyde, e.g., nonionic surfactants (28-31) and anionic surfactants (81). Inorganic cation-anionic surfactant combinations greatly increase the antimicrobial activity at acid ph with a further increase in efficiency at higher temperatures (81). These solutions have a shelf life of years in terms of glutaraldehyde concentration, polymerization, and ph. Babb et al. (11) have examined different glutaraldehyde formulations and showed that acid dialdehyde preparations, although more stable than the alkaline ones, were less sporicidal and more corrosive. An enhanced activity is claimed for a combination (Sporicidin; The Sporicidin Co., Washington, D.C.) of glutaraldehyde with sodium phenate and phenol (106, 107). Studies from our laboratory (E. G. M. Power and A. D. Russell, J. Appl. Bacteriol., in press) agree to claims that the undiluted mixture is sporicidal but not that a 1:16 dilution is sporicidal. Inadequate neutralization of the high phenate concentration may be a contributory factor in reaching an erroneous conclusion. Formaldehyde. Formaldehyde (methanal, HCHO) is used in both the gaseous and liquid forms ( ). Formaldehyde solution (Formalin) is an aqueous solution containing ca. 34 to 38% (wt/wt) CH2O and methanol to delay polymerization. Formaldehyde is bactericidal and sporicidal, but at a slower rate than glutaraldehyde (170). It combines readily with proteins and is less effective in the presence of organic matter. Ortenzio et al. (146) claimed that formaldehyde solution was rapidly sporicidal to B. subtilis but not to C. sporogenes, which was not killed after 2 h of exposure. Borax-Formalin and formaldehyde-alcohol have been found to destroy B. anthracis, C. perfringens, and C. tetani (199), although some doubt must remain about the validity of these results since there could have been a failure to neutralize formaldehyde in subculture media. A lack of sporicidal activity of 8% formaldehyde has been noted by Pepper and Chandler (150), and this finding might take on an added significance when linked to proposals that spores apparently inactivated by formaldehyde may be revived by appropriate posttreatment procedures (200, 201). This aspect is considered in more detail later. Irrespective of whether aqueous or alcoholic solutions of formaldehyde are used, time-survivor curves of treated bacterial spores often show an initial shoulder (170), although this is by no means a universal finding (219). According to some workers (170), various alcohols (methanol, ethanol, and propan-2-ol) reduce the sporicidal activity of formaldehyde. The sporicidal activity of formaldehyde is influenced markedly by temperature, with extensive spore inactivation at temperatures of 40 C and above (219). Formaldehyde vapor may be obtained in various ways: (i) by evaporating appropriate dilutions of standardized batches of commercial Formalin (containing 10% methanol); (ii) by heating paraformaldehyde or the formaldehyde polymers, urea formaldehyde and melamine formaldehyde, under controlled conditions of time and temperature (221). Bacterial spores and nonsporulating bacteria are fairly readily killed by formaldehyde gas (207). A linear relationship exists between the formaldehyde concentration and killing rate. Nordgren (144) observed that the rate of disinfection of spores exposed to formaldehyde vapor increased as the
5 VOL. 3, 1990 TABLE 5. Comparative sporicidal activities of some aldehydesa Aldehyde Chemical formula Sporicidal activity Formaldehyde H * CHO Fair Glyoxal CHO * CHO Only at 10% (not 2%) Malonaldehyde CHO CH2 CHO Slight Succinaldehydec CHO (CH2)2 CHO Slight Glutaraldehyde CHO * (CH2)3 * CHO High Adipaldehyde CHO (CH2)4 CHO Slight a Comments on sporicidal activity of aldehydes based in part on Boucher (28-30). h Some aldehydes have been reexamined against B. subtilis NCTC 8236 spores (Power and Russell, in press). c Gigasept contains both succinaldehyde and formaldehyde and is more active (as a 10%o solution) than formaldehyde alone. SPORICIDAL AGENTS 103 temperature increased, that organic matter such as blood, sputum, or soil reduced the rate of spore inactivation, and that an increase in rate of kill occurred as the relative humidity was raised to 50% with little increase above this value. There is no universal agreement about the effect of relative humidity on activity. Russell (171, 172) reviewed the activity of formaldehyde and described work claiming that no bactericidal effect occurs unless the humidity is 70% or above. Confusingly, Hoffman (99) has reported that the aldehyde is quite effective even at humidity values of <50% and that formaldehyde generated from paraformaldehyde is more active than an equivalent amount generated from Formalin solution. Low concentrations of formaldehyde are sporostatic. Other aldehydes. The sporicidal activity of aldehydes other than glutaraldehyde and formaldehyde is equivocal (Table 5). Borick et al. (26) stated that glyoxal was sporicidal, and Boucher (28, 30) has considered the efficacy of various aldehydes. We have recently reexamined the effect of some aldehydes on B. subtilis NCTC 8236 spores (Power and Russell, in press). Over a 5-h period at 22 C, 10% butyraldehyde had no sporicidal action against the spores (109 CFU/ml at zero time), 10% glyoxal effected a 3-log reduction, and 8% formaldehyde produced a 4-log reduction. Gigasept (Sterling-Winthrop, Surrey, United Kingdom) (containing succinaldehyde [butan-1,4-dial], formaldehyde, and 2,5-dimethoxytetrahydrofuran, ph 6.5) achieved, at 5%, a 2-log reduction and, at 10%, a 5-log reduction. Malonaldehyde was inactive, and the greatest rates of kill were obtained with 2% alkaline glutaraldehyde, activated Cidex (Surgikos, Arlington, Tex.) and undiluted Sporicidin (glutaraldehyde, 2%; phenol, 7%; sodium, phenate, 1.2%; ph 7.4), all of which produced reductions of at least 8 logs. Sporicidin was marginally the most active. Chlorine-releasing agents. Essentially, chlorine compounds can be considered as being of three types: chlorine gas, which is too hazardous for normal use; sodium and calcium hypochlorites; and chlorine-releasing agents (55, 218). Of these, the agents of choice are sodium hypochlorite, which contains up to 15% (wt/vol) available chlorine, and sodium dichloroisocyanusate, which slowly releases hypochlorous acid (HOCl). The active species is undissociated hypochlorous acid, the hypochlorite ion (OCl-) being considerably less so, and disinfection by chlorination is optimal at around ph 6, at which dissociation of HOCI is minimal. Chlorine compounds are bactericidal and sporicidal, although spores are more resistant than vegetative cells (22, 23, 42, 55, 56, 147, 218). Activity of the hypochlorites is greatly reduced in the presence of organic matter. Organic N-chloro compounds, containing the =N-Cl- group, hydrolyze in water to produce an amino (=NH) group; their sporicidal activity is slower than that of the hypochlorites. Like the hypochlorites, activity of these compounds is greater at acid than at alkaline ph. Cousins and Allan (42) have demonstrated that sodium hypochlorite was the most effective of five halogens against B. cereus and that B. subtilis spores were more resistant to all sporicides tested. Generally, spores of Clostridium spp. are more susceptible to chlorine than are Bacillus spores (56). Freshly prepared hypochlorite solutions, buffered to about ph 7.6, have a very rapid sporicidal activity (11, 116). Mixtures of 1.5 to 4% sodium hydroxide with sodium hypochlorite (200 ppm [200 pg/g] available chlorine) are much more rapidly sporicidal than either sodium hydroxide or hypochlorite used singly (42). This could result from an effect of the alkali on the spore coat, thereby increasing hypochlorite penetration. Potentiation of sporicidal activity of hypochlorites is attained in the presence of methanol and other alcohols (40, 51, 116), and buffering to ph 7.6 to 8.1 of alcohol-hypochlorite solutions produces powerful sporicidal activity with optimum stability (51). Such solutions are still, however, inactivated by organic matter. Iodine and iodophors. Iodine and iodophors (iodophores; literally, iodine carriers) are considered to be effective bactericidal and sporicidal agents (207, 218). Iodine itself is sparingly soluble in cold, but more soluble in hot, water. Stronger solutions can be made in potassium iodide or in alcohol. Iodine is less reactive chemically than chlorine and is less affected by the presence of organic matter; nevertheless, these effects depend on iodine concentration. The activity of low, but not of high, concentrations of iodine is significantly reduced. The sporicidal efficacy of iodine is also ph dependent; at neutral and acid ph, diatomic iodine (12) is highly active and hypoiodous acid (HOI) also makes some contribution. At alkaline ph, activity is reduced, resulting from the formation of the hypoiodide (OF) ion, which has only a slight activity, and the inactive iodate (103), iodide (I-), and triiodide (13) ions. A major problem with iodine is that it is toxic and also stains fabric and tissues. The iodophors consist of a loose complex of elemental iodine solubilized by means of appropriate carriers which increase solubility while at the same time providing a sustained-release reservoir of iodine (85) and stabilized with phosphoric acid. Suitable carriers consist of neutral polymers such as nonionic surfactants and povidone (polyvinylpyrrolidone) and polyethylene glycols, which exhibit surface-active properties and which, therefore, improve wetting properties, thereby aiding in penetration into organic soil. The iodophors have been reviewed by several authors (85, , 207, 218). The concentration of free iodine in an iodophor is responsible for its bactericidal activity. In many iodophor preparations, the carrier is a nonionic surfactant in which the iodine is present as micellar aggregates. When the iodophor is diluted with water, the micelles disperse and most of the iodine is slowly liberated. Dilution below the critical micelle concentration of the surfactant results in iodine being in simple aqueous solution. Favero (60) has made the important point that iodophors formulated as antiseptics contain much less free iodine than those formulated as disinfectants, which should contain 30 to 50 mg of free iodine, or 70 to 150 mg of available iodine per liter. Iodophors at high concentrations may be sporicidal over a wide ph range, but are much less potent than glutaraldehyde; they do not stain and are nontoxic. Peroxygens. The two important peroxygens are hydrogen peroxide (H202) and peracetic acid (CH3COOOH).
6 104 RUSSELL The bactericidal and sporicidal properties of hydrogen peroxide have long been known (4-6, 16, 17, 121, 215, , ) and are influenced by a variety of factors. Of these, one of the most important is concentration, a low concentration (6%, wt/vol) being bactericidal, but only slowly sporicidal. However, at 25TC and levels of between 10 and 20% (wt/vol), the concentration exponent is about 1.5 (173). Survivor curves of spores exposed to low peroxide concentrations frequently exhibit a distinct shoulder. A "tailing" has also been observed (36), which has been attributed to the formation of spore clumps during treatment and the associated spore catalase, thereby destroying hydrogen peroxide in the immediate vicinity. Temperature exerts a marked effect on sporicidal activity; at ambient temperatures, peroxide is only slowly sporicidal, but the temperature coefficient (Q10) for each 10'C rise is about 2.5 (173). A factor that can influence activity of peroxide is its stability; it tends to be unstable and its decomposition is increased by metals, metallic salts, light, heat, and agitation, but it is comparatively stable in the presence of a slight excess of acid. Decomposition can be reduced by appropriate storage. Peracetic acid is a bactericidal and sporicidal agent (13, 14, 224). It decomposes ultimately to hydrogen peroxide, acetic acid, and oxygen, which at recommended in-use concentrations is toxologically safe. It is considered (13, 14) to be a more potent sporicide than hydrogen peroxide, and its activity is reduced only slightly in the presence of organic matter and is unaffected by the presence of catalase. Peracetic acid is more active at ph 5 than at neutral ph. Ethylene oxide. Ethylene oxide [EtO; (CH2)20] exists usually as a gas that is soluble in water, oils, rubber, and most organic solvents. A major problem with its use is that it is inflammable when in contact with air, but in practice this can be overcome by using mixtures of EtO with carbon dioxide or fluorocarbon compounds. EtO is freely diffusible and penetrates paper, cellophane, cardboard, fabrics, and some plastics but less readily through polyethylene. It is unable to penetrate crystalline materials. Early work ( , 151, 152) considered the chemical, physical, and microbiocidal properties of EtO. Later studies have fully supported these findings (34, 35, 39, 58, 59, 75, 99, 117, 129, 159, 162, , 180) and have shown that the activity of EtO depends on several factors. (i) Activity is concentration and time dependent. As would be expected, the higher the concentration of EtO, the more rapid its sporicidal activity. For example, Phillips (151) calculated values of 1/k (equivalent to the time in hours at 250C required [t90%i to kill 90% of B. subtilis var. globigii spores dried on cloth): values of 1/k were 7.2, 3.3, 1.6, 0.5, and 0.35 h for EtO concentrations of 22, 44, 88, 442, and 884 mg/liter, respectively. As the concentration of EtO increases, obviously 1/k or t90% decreases. These values demonstrate clearly that EtO is only slowly bactericidal, even a high concentration (884 mg/liter), taking 0.35 h to reduce the number of viable spores by 90%, i.e., 1 log cycle. This slow rate of kill is an obvious disadvantage of the gas and is a property that is taken into account in providing suitable conditions for sterilization. (ii) Activity is temperature dependent. Sporicidal activity of EtO is increased as the temperature is raised. Phillips (151) calculated that the temperature coefficient (010 or Q10) was 2.74 for each 10 C rise in temperature. The relationship among concentration, time, and temperature is, however, more complex than might be implied from this simple statement. Ernst (58, 59) has shown that the death rate is logarithmic and that the Q10 of 2.74 describes the tempera- CLIN. MICROBIOL. REV. ture effect for EtO concentrations of <880 mg/liter at temperatures of <350C. However, a critical temperature is reached for a particular concentration, after which an increase in concentration has no additional effect on the rate of kill of bacterial spores. At higher concentrations and temperatures about 32TC, the kinetics become zero order with respect to concentration, with a Q10 value of 1.9. (iii) Activity is water vapor dependent. Of all factors influencing EtO activity, moisture vapor is the most critical variable (34, 58). The area is a complex one; the fact that conflicting results were obtained by Phillips and Kaye ( , 151, 152) and Gilbert et al. (75), on the one hand, and Kereluk et al. (117) and Ernst (58, 59), on the other, is a reflection of the diverse test procedures used. The first group recommended the use of a relative humidity (RH) of between 30 and 40%, whereas the latter presented data to show that sporicidal efficacy increased with increasing RH. The lowlevel RH recommendations were based on work in which spores and their carrier materials were allowed to equilibrate with the RH of the test environment. The second group of workers were more interested in practical industrial applications, and the spore test pieces were below equilibrium for the moisture content of the load against the RH of the sterilizing environment. In a model theory (59) put forward to explain these conflicting data, spores are characterized with respect to their immediate environment and relative moisture content as compared with the gross environment surrounding them. The basis of this theory is that water molecules carry EtO to reactive sites; thus, in an environment with a relatively low moisture content with respect to the reactive site, the dynamic exchange must be directed outward, i.e., from the spore. The movement of EtO gas is thereby impeded, and the macromolecules of the cell are less amenable to alkylation. When the moisture content of the immediate environment increases, the equilibrium condition arises, which is intermediate in effectiveness. As the environmental water content rises further, the dynamic movement of water is directed towards the active site (the spore), the most ideal situation in practice. In the case of a relatively dry spore and low-rh environment, there is little exchange of moisture into and out of the spore, a situation very limiting for sterilization in practice. As the water content of the spore and of the environment increases, a relatively wet spore and high RH are obtained, thus resulting in a zone of high moisture which would have a diluting effect on EtO gas, reducing its availability to the spore (58, 59). This would be the situation in those experiments (75, , 151, 152) in which the RH is above 40%, with an intermediate RH thus representing the optimal RH of ca. 32%, designated by these workers. (iv) Activity depends on the type of organism. Contrary to the situation with most liquid biocides, to which spores are often several thousandfold more resistant than nonsporulating bacteria, spores are generally only some 2- to 10-fold more resistant to EtO gas than are vegetative organisms (35, 151, 152, 173). Spores of the thermophile B. stearothermophilus and of certain other organisms may, in fact, be less resistant to EtO than some vegetative bacteria such as S. aureus, Enterococcus faecalis, and Deinococcus radiodurans. IP-Propiolactone.,B-Propiolactone is not widely used as a sporicidal agent (39). It exists as a colorless liquid at room temperature, boils at 1630C, and may be vaporized in a special atomizer. P-Propiolactone is noninflammable, has low penetrating powers, and is claimed to be carcinogenic.
7 VOL. 3, 1990 SPORICIDAL AGENTS 105 TABLE 6. Neutralization of sporicidal and sporostatic activity Sporicidal agent Neutralizing agent Comment Glutaraldehyde Glycine ) Better then dilution or sodium bisulfite (could be toxic to germinating and outgrowing cells) Formaldehyde Glycine Hypochlorites Sodium thiosulfate Thiosulfate toxic to some streptococci; unlikely to be toxic Iodine and iodophors Sodium thiosulfate to germinating and outgrowing spores Hydrogen peroxide Catalase Very rapid effect of neutralizing agent Peracetic acid Dilution Ethylene oxide Dilution in recovery medium Specific neutralizer: guanine? Phenolsb and cresolsb Dilution or polysorbate Have high concn exponent; thus activity lost on dilution Organomercury compounds Sodium thioglycolate or cysteine' Thiglycolate might be toxic Chlorhexidine diacetate,b QACsb Lecithin + polysorbate Dilution inappropriate a Based on Russell et al. (176). b Sporostatic, but sporicidal at elevated temperatures. Its bactericidal and sporicidal activity is a direct function of the concentration and the time and temperature at which it is used (34, 35, 39, 99, ). The temperature coefficient, Q10, in the range of -10 to +25TC is 2 to 3 (99). As with EtO, however, the single most important factor determining its sporicidal potency is water vapor, and for optimum activity the RH should be kept above 70 to 75%. Again, however, it is not necessarily the atmospheric RH that is important, but the moisture content and location of water in the bacterial cell. B. subtilis var. globigii spores equilibrated to 98% RH are readily killed by P-propiolactone at an RH of 45%, an RH at which the spores are not usually susceptible. However, only a 2-log reduction (ca. 99% kill) is achieved when spores equilibrated to 75% RH are exposed to this agent at 45% RH, and a small percentage of spores preconditioned at 1% RH is thereafter very resistant to,-propiolactone at 75% RH (99). Other gases. Other gases with sporicidal activity include propylene oxide (reviewed in reference 173) and ozone. The former is bactericidal and sporicidal, but less so than EtO, and is allowed to be used in the food industry. As with EtO, its activity depends on concentration, on time and temperat'ure, and especially on RH. Ozone has bactericidal and sporicidal properties, but its instability and other undesirable properties were considered (99) to render it unsuitable for use as a gaseous disinfectant. However, more recent studies (63, 69) have demonstrated the sporicidal activity of the gas, especially under acidic ph conditions against spores (B. cereus, C. perfringens, and C. botulinum) of importance in food processing. RECOVERY AND REVIVAL OF INJURED SPORES When exposed to chemical or physical agents, microorganisms may be inhibited, sublethally injured, or irreversibly damaged, i.e., killed (7). In the laboratory, several types of tests are available for examining sporostatic and sporicidal activity. In essence, the sporostatic tests involve determining the MIC of a chemical agent, i.e., the lowest concentration preventing germination or outgrowth or both. Practical details can be obtained by consulting a forthcoming paper (A. D. Russell, B. N. Dancer, and E. G. M. Power, Soc. Appl. Bacteriol. Tech. Ser., in press). It must be noted that glutaraldehyde interacts strongly with nutrient media (80, 182) and that this may present an erroneous impression as to its apparently low sporostatic (or bacteriostatic) activity. Sporicidal evaluations may be of several types (8, 11, 36, 71, 77, 85, 161, 173, 190, 202, 203, 227; Russell et al., in press). Sporicidal activity can be tested against spores in liquid medium or suspended on appropriate carriers. Whatever method is adopted, and irrespective of whether quantitative (survival counts) or qualitative (extinction) assessments are made, an appropriate method must be used to determine spore survival (173). It cannot be emphasized too strongly that adequate neutralization (quenching) of the test chemical must be achieved to prevent sporostasis occurring in subculture media and, consequently, false-negatives (37, 78, 161, 173, 176). In brief, neutralization involves (i) diluting the biocide in the recovery medium to a level at which it ceases to have inhibitory activity, (ii) incorporating into the recovery medium a neutralizing agent (antidote) that specifically inactivates the biocide and is itself nontoxic to germination and outgrowth (177), or (iii) removing the biocide by means of a membrane filtration technique, followed by washing the membrane in situ (with, if necessary, an appropriate neutralizing agent, e.g., with QACs [38, 181]), and then placing it on the surface of a solid nutrient medium. Suitable neutralization procedures for specific sporicidal agents are summarized in Table 6. It must be added that sodium thioglycolate, widely used as an ingredient of anaerobic culture media, may in fact inhibit vegetative cell development from treated bacterial spores (50, 97, 136). When placed in nutrient media held at the desired temperature, normal (control, untreated) spores usually germinate very rapidly, a process complete within 30 to 60 min. Spores that have been damaged, however, invariably require long periods to repair this injury (7, 49, 50, 90, 159, 173, 200, 201, 228), and for this reason it is prudent to prolong the incubation period well beyond the usual period of, say, 48 h at (usually) 37 C; suboptimal incubation temperatures should also be examined, as should the effect of composition and ph of the recovery medium (134). The revival of chemically injured spores may also be
8 106 RUSSELL TABLE 7..poricie Revival of bacterial spores exposed to sporicidal agents Sporicide.Posttreatment procedures revival ence(s) Refer- Formaldehyde Heat activation (60-90'C), plating 201 Glutaraldehyde UDS ± sonication, incubation in 76 GML, plating in TSA Heat (50-90'C), dilution in GM or 76 GML, plating in TSA NaOH or KOH, plating 49, 154 Povidone-iodine Incubation in GML, plating in TSA 76 a UDS, Urea plus DTT plus sodium lauryl sulfate; GM, germination medium; GML, germination medium plus lysozyme; TSA, tryptose soy agar. achieved by specific procedures. It has been claimed that subjecting formaldehyde-treated spores of B. subtilis to a posttreatment heat shock at temperatures of 60 to 90'C enables most of the supposedly killed spores to revive (200, 201). A very small proportion of glutaraldehyde-exposed spores of various Bacillus spp. can be revived when, following neutralization of glutaraldehyde with glycine, the spores are treated with alkali (49, 153). The rate of NaOH-induced revival is ca (i.e., CFU per milliliter of glutaraldehydeexposed, NaOH-treated spores/unexposed, NaOH-treated spores), obviously a low value but one of potential importance (49). Experiments designed to distinguish between germination and outgrowth in the revival process have established that sodium hydroxide (range, 10 to 50 mm; optimum, 20 mm) added to glutaraldehyde-treated spores increased the potential for germination. In contrast, B. subtilis spores which are allowed to germinate before exposure to low concentrations of glutaraldehyde and then to sodium hydroxide are inhibited at the outgrowth phase to a much greater extent than germinated spores treated with the dialdehyde without subsequent alkali exposure (153). Sodium hydroxide can be replaced with potassium hydroxide or, to a lesser extent, sodium bicarbonate. The use of 2% (wt/vol) glycine (37) (Table 7) as an inactivator of glutaraldehyde is of paramount importance in these revival studies. Alkali-induced revival of spores exposed to another dialdehyde, glyoxal, has also been found with slight revival after exposure to Gigasept (containing succinaldehyde plus formaldehyde) but not to formaldehyde alone (E. G. M. Power, B. N. Dancer, and A. D. Russell, Lett. Apple. Microbiol., in press). This interesting phenomenon could be related to the far more damaging effects on the spores of glutaraldehyde than formaldehyde, with a consequent greater potential for revival of the former than the latter. Some revival of glutaraldehyde-treated spores can be achieved by means of a posttreatment heating (76, 154), but the extent of this revival (maximum, two- to threefold increase in viable count achieved at 57 C) is less than by alkali treatment and also markedly less than that reported with formaldehyde (201). Coat-removing agents fail to achieve any revival (154) despite reports that glutaraldehyde-treated spores damaged by one such treatment are capable of germination (76). Lysozyme, either used before plating or incorporated into recovery media, is likewise ineffective, and a combination of NaOH and lysozyme has a slight, but noticeable, deleterious effect on colony counts (154). To determine whether alkali induces protein release, CLIN. MICROBIOL. REV. B. subtilis NCTC 8236 spores have been treated with glutaraldehyde (which was then neutralized with glycine), washed with buffer, and exposed to 20 mm NaOH for 10 min; the release of protein was then determined chemically: only ca. 1 pfg was released by alkali treatment (49). It is, of course, possible that a small number of spores may possess a higher than average resistance to glutaraldehyde and become superdormant (86, 90) rather than damaged, so that they are able to germinate only under extreme conditions. Lysozyme may facilitate germination of damaged spores (90), although this phenomenon applies mainly to thermally injured spores (see later results with hypochlorites, however). Gorman et al. (76) reported that increased survivor counts of iodophortreated B. subtilis spores were obtained following exposure to lysozyme. Information on the revival of chemically damaged spores is still, on the whole, lacking. From an academic or theoretical point of view, considerable data may be generated about mechanisms of sporicidal action (64, 65), and thus studies on revival are always worthwhile. Likewise, any assessment of sterility must take into account conditions for repair of injured, but still viable and potentially harmful, bacterial spores. In the practical context, the studies from this laboratory with glutaraldehyde-damaged spores described above have used severe revival conditions that are unlikely to be encountered in practice; furthermore, an injured spore under such circumstances is effectively dead if it cannot germinate or outgrow. Nevertheless, there might be some small risk in overestimating the sporicidal efficacy of glutaraldehyde. Spores have been recovered after 24-h exposure to the dialdehyde, whereas the Association of Official Analytical Chemists test (8) recommends a 10-h exposure and much shorter exposure periods are commonly encountered, particularly in the hospital environment where time is often at a premium. An additional point is that in our studies (76, 153) only freshly activated glutaraldehyde solutions were used, whereas in practice older solutions are frequently used, with some deterioration occurring (155). Nevertheless, matters should be kept in perspective, and Babb et al. (11) have stated that a 3-h treatment with 2% alkaline glutaraldehyde should be sufficient for practical purposes to achieve a sporicidal effect, especially as bacterial spores are only infrequently found on clean medical equipment. The situation with formaldehyde is potentially more alarming. Although alkali treatment does not revive formaldehyde-treated spores (Power et al., in press), the studies of Spicher and Peters (200, 201) suggest that the sporicidal activity of this monoaldehyde might well have been overestimated. Confirmation or rebuttal of their findings is awaited with interest. Injured bacterial spores might be of concern in food microbiology. Hypochlorites are used as sanitizers and alter germination responses of C. botulinum spores (65). In addition, C. bifermentans spores are sensitized to lysozymeinduced germination following treatment with hypochlorite (237). Exposure of spores to EtO or H202 may alter requirements for growth (64). Cook and Pierson (41) have pointed out that conditions used to enumerate spores in foods might not be optimum for germination and outgrowth of all spores and that injured spores must be considered in this context. SPOROGENESIS, SUSCEPTIBILITY, AND RESISTANCE Sporulation, germination, and outgrowth are complex processes in the overall life cycle of Bacillus and Clostridium spp. Differing responses to biocides are shown at different
9 VOL. 3, 1990 stages, and these aspects will be considered, as some useful information can be obtained about the mechanisms of spore resistance. Sporulation Sporulation is a multiphase process leading to the development of a spore from a vegetative cell. The stages involved (57, 118, 119) can be summarized as follows. Stage 0 is the vegetative cell, stage I the presporulation phase (in which DNA is present as an axial filament), and stage II is the septation phase in which asymmetric cell formation occurs. Engulfment (encystment) of the forespore takes place in stage III and cortex formation between the inner and outer forespore membranes commences in stage IV, with synthesis of spore coats, dipicolinic acid, and uptake of Ca2+ in stage V. Spore maturation occurs in stage VI, with the coat material becoming more dense and refractility increasing. Lysis of the mother cell and liberation of the mature spore take place in stage VII. Clearly, there are several stages at which antibacterial agents could act or, conversely, when resistance to such agents could arise. Sporulation (Spo-) mutants which are unable to develop beyond a genetically determined point (98, 108, 109) are of considerable value in correlating structural changes, biochemical characteristics, and susceptibility or resistance to specific biocides. A practical consideration of these aspects is being published elsewhere (Russell et al., in press). Disinfectant-induced structural changes in fully developed spores have been described (123, 168, 189), but these have not been fully related to their biochemical effects on sporulating cells. Thus, the mechanism of action (see later section) of many sporicidal agents is still often poorly described. In contrast, the mechanisms of spore resistance to biocides are better understood, and these aspects will be considered here. Resistance of bacterial spores (209) can be examined by (i) comparing the response of wild-type and Spo- mutants; (ii) using other mutants, e.g., conditional cortexless mutants of B. sphaericus (103, 104); or (iii) comparing "normal" and coatless forms of a spore. Development of resistance to biocides and antibiotics during sporulation (82, 83, 130, 210) has been known for some time. Experiments designed specifically to associate changes in cell structure with altered responses to biocides can yield useful information in this area. There is a need, however, to correlate these structural changes more accurately with biochemical changes in the spore. Useful markers for monitoring the development of resistance are toluene (resistance to which is an early event), heat (intermediate event), and lysozyme (late event) ( ). In studies with a wild-type B. subtilis, strain 168, and its Spo- mutants, we have demonstrated (156a, 188) that resistance to chlorhexidine occurs later than that to toluene and at about the same time as heat resistance, whereas glutaraldehyde resistance is a very late event, occurring after the development of lysozyme resistance (Table 8). Some 12 or so polypeptides are found in the spore coat of B. subtilis ( ); these are synthesized at different times and are incorporated into the spore at stages V and VI. It has been suggested (108) that one polypeptide of molecular weight 36,000, which is formed very late in sporulation, may have a direct role in conferring resistance upon the spores. The development of glutaraldehyde resistance, however, is unlikely to result from the deposition of specific spore coat proteins because of the highly reactive nature of the dialdehyde molecule (Power TABLE 8. SPORICIDAL AGENTS 107 Onset of resistance to antibacterial agents during sporulationa Sporulation Sporulation Agent stage at which stage at resistance resistance which is Comment appears fully developed Toluene Late stage III Early stage IV Early event Chlorhexidine Stage IV Stage V Intermediate Heat Stage V Stage VI Intermediate Lysozyme Middle of Stage VI Late stage V Glutaraldehyde Late stage V Stage VI corm- Very late event pleted a Based on Power and Russell (in press) and Shaker et al. (188). and Russell, in press). Nevertheless, in general terms, the idea of attempting to correlate resistance with a specific components) of the spore coat is an attractive one and should be subjected to further experimentation. The increased resistance occurring during sporulation may thus be related to the stage of spore development (Table 8). In many instances, the chemicals studied, e.g., xylene, toluene, or benzene, have been organic solvents rather than preservatives or disinfectants. Resistance to chloroform and to phenol, however, develops late in the sporulation process (12, 130), and that to methanol and ethanol occurs at the same time as resistance to other alcohols, such as octanol and butanol. Alcohol-resistant sporulation mutants of B. subtilis can sporulate in the presence of alcohols at a frequency of 30 to 40% (24). In conditional spore cortexless mutants of B. sphaericus deficient in the synthesis of meso-diaminopimelic acid (Dap), the muramic lactam (and hence cortex) content increases with an increase in exogenous meso-diaminopimelic acid (103, 104). Characteristic spore properties have been found to be associated with different amounts of cortex; e.g., ca. 25% of maximum cortex content is necessary for the spore to present resistance to octanol but ca. 90% is necessary to show heat resistance. Such Dap- mutants might thus be useful in studying mechanisms of spore resistance to biocides, although as pointed out by Waites (224), changes other than variations in cortex development might occur elsewhere in the spore which must be considered before ascribing resistance solely to the cortex. Probably the most detailed approach to studying resistance of spores has involved the use of spore coatless forms (46, 62, 63, 69, 82, 88-91, 93, 124, 126, 132, 189, 194, 212, , 231, 237). Methods of removing one or both spore coats have been described in detail by Nishihara et al. ( ). Coats may be extracted by using 2-mercaptoethanol, sodium lauryl (dodecyl) sulfate, dithiothreitol (DTT), and urea. Treatments consist of urea plus DTT plus sodium lauryl sulfate, urea plus DTT, urea plus 2-mercaptoethanol, and sodium lauryl sulfate plus DTT. Of these treatments, urea plus DTT plus sodium lauryl sulfate is usually considered the most satisfactory. Both lysozyme and nitrous acid or sodium nitrite are effective against coatless, but not normal, spores, although pretreatment of the coatless spores with glutaraldehyde reduces the extent of this activity considerably (82). The role of the spore coat in resistance of spores to various antibacterial agents is summarized in Table 9. Hydrogen peroxide itself will remove coat protein from C. bifermentans (231); however, removal of coat protein by DTT before spore exposure to peroxide markedly increases its lethal effect, whereas B. cereus spores are much less
10 108 RUSSELL Alkali TABLE 9. Mechanisms or site of resistance of bacterial spores to chemical agents Antibacterial Spore Comment agent component Cortex Lysozyme Coat(s) 1 Hypochlorites Coat(s) UDS spores highly sensitive Glutaraldehyde Coat(s) Iodine Coat(s) Hydrogen peroxide Coat(s) Varies with strain Chlorhexidine Coat(s) UDS spores more sensitive than "normal" spores Ethylene oxide Coat(s) Exact relationship unclear Octanol Cortex Dap- mutants of B. sphaeri- Xylene Cortex J cus more sensitive a Dap, meso-diaminopimelic acid. affected (226). The spore coat is thus likely to confer a protective effect against peroxide to the former but not to the latter spores, thereby demonstrating that varying responses occur with different sporeformers and that the response may be associated with different composition and structure of the spore coat(s). Spores treated with urea plus DTT plus sodium lauryl sulfate are highly susceptible to glutaraldehyde, iodine, hydrogen peroxide, ozone, and chlorine (63, 76, 82, 174). Thus, even with agents that are known to be actively sporicidal, the coats play a role in limiting intracellular penetration. Nevertheless, the role of the spore coats in resistance to EtO is unclear. In B. subtilis, removal of the coats increases spore sensitivity (129). However, resistance to EtO of spores of B. cereus strain T pretreated with alkaline DTT remains unchanged (46). Furthermore, B. subtilis 4673 (a mutant of strain 4670) with defective coats and outer coat layers thinner and more diffuse than 4670 is more resistant to EtO than is Conversely, strain EV15 which overproduces coat material, thereby possessing an abnormally thick multilayered coat, has an exceptionally high resistance to EtO (46). On the other hand, these findings imply that the expected increased permeability to EtO in strain 4673 does not occur and, on the other, that increased resistance to EtO is associated with excessive coat production. EtO is a comparatively small molecule, but molecular size appears to be of little consequence where coat impermeability is concerned. For example, H202 (molecular weight, 34), ozone (03; molecular weight, 48), and chlorine dioxide (C102; molecular weight, 67.5) are all small molecules, yet spore coats are considered a primary protective barrier to their entry (63, 69, 70, 123). The spore coat appears to act as a permeability barrier to chlorine (123, 224, 226, 237), since coatless spores are rendered more permeable to hypochlorites. Chlorine will itself remove coat protein and allows lysozyme to initiate germination (237). Sodium hydroxide increases the permeability of bacterial spores to germinants, and the potentiation of hypochlorite action by sodium hydroxide (42) may be the result of the effect of the alkali on spore coats from which protein is removed (93), although the cortex is alkali resistant (123). Germination CLIN. MICROBIOL. REV. Activation is a treatment resulting in a spore which is poised for germination but which still retains most spore properties; activation is thus responsible for the breaking of dormancy in spores, but is reversible. In contrast, germination itself is an irreversible process and is defined as a change of an activated spore from a dormant to a metabolically active state within a short period of time. The first biochemical step in germination is the biological trigger reaction. This initiation process can be induced by metabolic or nonmetabolic means, although it is now generally believed that the trigger reaction is allosteric in nature rather than metabolic, because the inducer does not need to be metabolized to induce germination. Initiation of germination is followed rapidly by various degradative changes in the cell, leading within a short period of time to outgrowth. These changes include (87, 217) (i) a decrease in heat resistance accompanied by changes in staining properties, (ii) a decrease in refractility whereby phase-bright spores (Fig. 2a and b) become phase dark (Fig. 2b and c), (iii) a decrease in dry weight, and (iv) a decrease in optical density, a comparatively late event in germination (205), although it is a widely used method for measuring germination. Inhibition and control of spore germination are important considerations in many fields, including food preservation (74, 193), although dormancy may be a problem (86). Several antibacterial agents are known to inhibit germination. These include alcohols, aldehydes, phenols and cresols, parabens, sorbic acid, and mercuric chloride (2, 3, 47, 66, 68, 100, 128, 148, 149, 156, 166, 168, 171, 172, 181, 190, 193, 195, 198, 219, 220, 223, 234; B. M. Lund, Ph.D. dissertation, University of London, London, England, 1962). This inhibition (Table 10) occurs at concentrations that are closely related to those that inhibit the growth of vegetative bacteria. The effects of inhibitors of spore germination may be reversible. This is apparent from the results of studies with phenols (128, 148, 149, 181), formaldehyde (219), alcohols (220), and parabens (157, 234). These findings suggest a fairly loose binding of these agents to a site(s) on the spore surface since mere washing is often sufficient to dislodge the inhibitor. Mercuric chloride is a powerful inhibitor of the germination of spores of C. botulinum type A (2) and of Bacillus spp. (92, 100, 223). It appears to inhibit some reactions in germination before the loss of heat resistance but not the subsequent release of peptidoglycan (223). In contrast, an organomercurial compound, phenylmercuric nitrate, has been shown (148, 181) to have little effect on the germination of B. subtilis spores but a pronounced inhibitory effect on outgrowth. Germination (Ger) mutants of B. subtilis 168 deficient in the initiation of germination 135, 184), could be of value in studying the mechanism of action of antibacterial agents but do not, as yet, appear to have been studied in this context. Glutaraldehyde exerts an effect early in the germination process (153, 156). This belief is based on several experimental approaches, a very recent one (156a) involving the effect of the dialdehyde on the uptake of L-[14C]alanine to B. subtilis spores. This germinant is considered to act by binding to a specific receptor to the spore coat (184), and once spores are triggered to germinate, they are committed irreversibly to losing their dormant properties (205). The aldehyde could inhibit germination by (i) reducing L-alanine uptake as a consequence of competition for binding sites on
11 VOL. 3, 1990 SPORICIDAL AGENTS 109 (a) (b) (c) FIG. 2. Changes during spore germination examined by phase-contrast microscopy. (a) Mature, phase-bright spores; (b) development of phase-dark forms; (c) germination complete with full conversion to phase-dark cells. the spore, (ii) preventing passive diffusion of L-alanine into the spore, (iii) sealing the spore surface, or (iv) inhibiting the L-alanine-induced trigger reaction of germination by a later, as yet unexplained, mechanism (Fig. 3a to d, respectively; Table 11). In our experiments, despite earlier claims to the contrary (112, ), D-glucose has no significant effect on L-alanine uptake. These other workers, however, calculated the binding affinity of glucose solely on the loss of heat resistance and turbidity of germinated spores rather than by direct methods. Glutaraldehyde-treated spores retain their refractility, having the same appearance under the phasecontrast microscope as normal untreated spores (Fig. 2a), even after subsequent incubation in germination medium. The observation suggests that inhibition occurs very early in the germination process. At concentrations up to 0.1% (wt/vol), both acid and alkaline glutaraldehyde inhibit germination, but not L-[14C]alanine uptake, and therefore pre- TABLE 10. Inhibitors of germination and outgrowth Process Inhibitor Comment Germination Glutaraldehyde Probably inhibits trigger mechanism Sorbic acid Inhibitor of trigger mechanism? Formaldehyde, alcohols Diverse group, phenols, parabens probably different mercuric chloride J sites of action Sodium thioglycolate Caution needed with recovery media Outgrowth QACs, chlorexhidine No or little effect on EtO, organomercurials germination Hypochlorites I Glutaraldehyde Even more effective at these stages Sorbic acid Multiple sites of inhibition vent the trigger reaction by some unexplained means. At higher glutaraldehyde concentrations (01. to 1%, wt/vol), uptake of L-alanine is significantly reduced, presumably the result of a sealing effect by the aldehyde on the spore surface. Spores do not concentrate L-alanine and uptake proceeds rapidly without the necessity for an energy-dependent active transport system, demonstrating that the dormant spore is freely permeable to the amino acid, which enters by simple diffusion (53, 154). The effects of inhibitors of germination have been widely reported, but it is often not known at what stage of germination an inhibitor is active (193). Because of the nature of the germination process, the only types of antibacterial agents that are effective are those that inhibit the trigger reaction and those that prevent the degradative processes. Many antibacterial agents are known to affect the optical density changes that occur in germination. A decrease in L-AI SPORE Trigger for germ ination FIG. 3. Possible sites of action of an inhibitor of the trigger mechanism in spore germination.
12 110 RUSSELL TABLE 11. Process Low concn (<0.1%) inhibitory, con- siderably below sporicidal levels (2%) Decrease in optical density L-['4C]alanine binding to spores Effect of glutaraldehyde on the germination trigger mechanism Effect of glutaraldehyde Inhibition, but only at high aldehyde concn Phase darkening of Low concn (<0.1%) prevents (see spores Fig. 2) Even more inhibitory than vs germi- nation Outgrowth of previously germinated spores optical density is a late event in germination and is not suitable for studying the initial reactions (193). Thus, whereas many compounds are likely to prevent the degradative processes, it is unclear whether the trigger reaction is also affected. Even a procedure involving a short period of exposure to the inducer, e.g., L-alanine, followed by monitoring of the fall in optical density is considered to be inappropriate, as is one involving the release of 45Ca (205). Suitable methods are the direct one, described above, involving the uptake of labeled L-alanine, and one in which spores are exposed to L-alanine for a very short period of time. The reaction is then stopped by adding an excess of its competitive inhibitor, D-alanine. The commitment to germination is then measured by counting the conversion of phase-bright spores (Fig. 2a) to phase-dark spores (Fig. 2c). This technique has been used to study the effect of sorbate, an effective inhibitor of germination (198). Busta and his colleagues (21, 198) have concluded that sorbic acid does not compete with L-alanine for a common binding site on the bacterial spore, so that inhibition occurs after germinant binding (41). Alcohols inhibit the L-alanine-initiated germination of B. subtilis spores, suggesting that this inhibition results from an interaction of a hydrophobic region in or near the L-alanine receptor site on the spore with the hydrophobic group on the alcohol (242). Such interaction is presumably of a weak nature, because (as pointed out earlier) the inhibition of germination by alcohols is reversible. Unfortunately, only an optical density technique was used in these studies. The heat activation of C. perfringens spores at a temperature range of 70 to 80'C in water is enhanced in the presence of alcohols (43, 44). The concentration of a monohydric alcohol to produce optimum spore activation is inversely related to its hydrophobic character. Other inhibitors of germination include sodium bicarbonate (15, 45) and cyclic polypeptide antibiotics (96). Antibiotics are outside the scope of this paper, but the experimental approach involving morphological changes and inhibition of macromolecular syntheses has yet to be applied to many biocides. B. brevis Nagano wild type produces the antibiotic gramicidin S, which inhibits germinating spores (138). A particularly interesting property of this organism is that germination-initiated spores retain their resistance properties (48), and it is likely that this property could be studied further with a range of biocides. Outgrowth Outgrowth is defined as the development of a vegetative cell from a germinated spore and takes place in an orderly CLIN. MICROBIOL. REV. manner when germination is carried out in a medium that supports vegetative cell growth. After germination, germinated spores become swollen and shed their coats to allow the young vegetative cells to emerge, elongate, and divide. Of the macromolecular biosynthetic processes occurring after germination, RNA synthesis is the first, followed closely in Bacillus spp. by the onset of protein synthesis, with DNA synthesis occurring some time later. During outgrowth, all types of RNA are synthesized. Cell wall synthesis commences after RNA and protein but before DNA and coincides with swelling of the germinated spore. Several antibacterial agents act at the outgrowth rather than the germination stage (Table 10). These include QACs, organomercurials, chlorhexidine, and EtO (35, 148, 181, 190; Lund, Ph.D. dissertation), the first three of which are sporostatic agents, with EtO a sporicidal compound. QACs bind strongly to spores, and simple washing procedures will not remove them (38). QAC-treated spores which are membrane filtered are still prevented from undergoing outgrowth when transferred to an appropriate growth medium (38, 181), and a neutralizing medium must be used in conjunction with membrane filtration. The parabens and similar substances inhibit germination at sporostatic concentrations. Outgrowth is prevented at higher concentrations. High concentrations of hypochlorites are necessary to prevent spore germination, whereas moderate concentrations markedly retard outgrowth and low concentrations have only slight effects on either (237). Sublethal concentrations of EtO inhibit outgrowth but not germination (159), and resistance of spores to EtO does not decrease during germination (46, 47). Hydration of the spore core and alteration of spore coat layers do not therefore appear to be linked to an increased susceptibility. Even spores exposed to high EtO concentrations can germinate freely under a variety of conditions but will not outgrow (47), but asparagine acts as a germinant for untreated but not EtO-treated spores. A former food preservative, sodium nitrite, has been the subject of heated debate as to how it exerts its antimicrobial activity (173). It does not affect spore germination and in fact induces germination, but only at high concentrations (2, 3, 125). Nitrite inhibits postheating germination or outgrowth or both, heat-injured spores being rendered more susceptible to the salt (105). It is apparent from this section and the preceding one that most sporostatic compounds inhibit either germination or outgrowth. (An exception to this general statement is glutaraldehyde, low concentrations of which inhibit both processes [154]). What is not clear is why this should be so. There has been little basic research to explain why one compound should, for example, inhibit the degradative processes associated with germination, whereas another compound has no effect at this site but inhibits the later stage of outgrowth. Figure 4 summarizes the effects of antibacterial agents on germination and outgrowth, as well as detailing stages during sporulation at which specific resistances develop. OVERCOMING SPORE RESISTANCE Bacterial spores can pose a problem insofar as the activity of chemical agents is concerned. It is obviously essential to use appropriate concentrations at the optimum ph for a sufficient period of time to ensure a sporicidal effect. There are, however, means available for achieving the same, or an enhanced, response. These involve a combination of a chemical and a physical process or of two chemical agents (1).
13 VOL. 3, 1990 Glut, -SPORE İGlut? Lys. t Trigger.*-- SA? CHA- do Degradative.. Other germination Tol- processes inhibitors? CELL CULTURE GER INATION Several., V GlutO inhibitors? As Glt 0 OUTGROWTH FIG. 4. Summary of possible effects of some antibacterial agents on germination and outgrowth and of the development of resistance during sporulation. Glut, Glutaraldehyde; SA, sorbic acid; Tol, toluene; CHA, chlorhexidine diacetate; Lys, lysozyme; EtO, ethylene oxide. The bactericidal and sporicidal activity of a biocide increases with increasing temperature. For example, the temperature coefficient (0) per 1C rise in temperature for the phenolic agent chlorocresol is 1.1 (33). If the temperature is increased from 20 to 1000C, then assuming that 0 is the same over the entire temperature range, the activity increases by >2,000-fold ( = = 2,104). Use of this principle was, until 1988, made in the United Kingdom, where "heating with a bactericide" was one official (pharmacopoeial) method of sterilizing certain parenteral and ophthalmic products. Differences between acid and alkaline glutaraldehyde forms disappear at temperatures above about 400C (28-30, 213). A process that has been promising results is the use of saturated steam at subatmospheric pressure in the presence of formaldehyde (173). The complicated effect of heat and sodium nitrite has been described (105) and reviewed (173). Acid heat treatment is an important means of controlling spores in food, spores being more susceptible to heat at low ph (20). Spores can, in fact, exhibit a base exchange behavior which will reduce, restore, or enhance their thermal resistance. Sensitization involves converting them to the hydrogen (H) form, which can be transformed into the resistant calcium (Ca) form by treatment with calcium acetate at ph 11. H-form spores are also more susceptible to propylene oxide but less so than the Ca form is to glutaraldehyde (210, 214). Another physicochemical process is the use of glutaraldehyde with ultrasonics (28-31, 192). Although ultrasonic waves themselves possess little sporicidal activity (and have, in fact, been used to separate spores and vegetative cells), they have been claimed to potentiate the sporicidal activity of acid glutaraldehyde at 60'C and of hydrogen peroxide but not of iodophors. The QAC benzalkonium chloride still had a poor sporicidal effect (31). A synergistic effect of hydrogen peroxide used in conjunction with UV radiation has been shown to occur (17, 230). Combinations of chemical agents have also been studied. Examples have already been provided of hypochlorites and methanol (40, 51) and of glutaraldehyde with nonionic (28-31) or anionic (81) surfactants or with inorganic cationanionic surfactant combinations (81). In many instances, however, the underlying reasons for this potentiation remain obscure. Glutaraldehyde has also been combined with phenol plus phenate, the combination being claimed (106, 107) to have an enhanced sporicidal action. Alcohol is not sporicidal, and only high concentrations (9%) of hydrochloric acid have this property, whereas acid alcohol (1% HCl in 70% TABLE 12. Antibacterial agent Alkali Chlorine compounds Ethylene oxide Glutaraldehyde Hydrogen peroxide Lysozyme Nitrous acid SPORICIDAL AGENTS 111 Mechanisms of action of some chemical agents Site or mechanism of action Inner spore coat Cortex Alkylation of core protein and DNA Cortex Spore core? Cortex (1, 1--4 links) Cortex (at muramic acid residues) alcohol) kills spores in 4 h (208). Even combinations of local anesthetics and preservatives are claimed to be sporicidal (1) Ċlearly, no comprehensive studies have been undertaken to determine what factors are involved in designing a process with enhanced sporicidal activity. Also, reasons for any synergism are often totally inadequate. This aspect is one that could, with benefit, be addressed. MECHANISMS OF SPORICIDAL ACTION A considerable amount of information is available about the ways in which bactericidal agents affect nonsporing bacteria (175). In contrast, mechanisms of sporicidal activity are poorly understood (236). The major reason for this is undoubtedly the complex nature of the bacterial spore, to which may be added the possibility that an antibacterial compound might have more than one actual or potential site of action. While contributing to the overall lethal effect, this possibility can complicate still further the unraveling of the mechanism of sporicidal action. The spore does present several sites at which interaction with an antibacterial agent is possible, e.g., the inner and outer spore coats, cortex, spore membranes, and core (Table 12). Interaction with a particular site need not necessarily imply, however, that this is associated with death of the spore or that there is only one site or target in the spore that must be inactivated. Practical considerations have recently been described (Russell et al., in press). Data on uptake of an antibacterial compound to bacterial cells are often considered a useful starting point in examining the mode of action of the compound. Studies on the uptake of glutaraldehyde to different types of bacteria (156) have shown that E. coli, B. subtilis vegetative cells, and S. aureus bind more aldehyde than do resting B. subtilis spores. Uptake increases during spore germination and outgrowth but is less than to vegetative cells. The surface of bacterial spores is hydrophobic in nature (54). Low concentrations of both acid and alkaline glutaraldehyde increase this surface hydrophobicity (156), presumably as a consequence of the extensive interaction of the dialdehyde with outer layers of cells and spores (19, 84, 140, 160, 211). The greater sporicidal activity of the alkaline form is not reflected by the uptake patterns but it is likely that acid glutaraldehyde resides at the cell surfaces, whereas the alkalinating agent, sodium bicarbonate, assists in the increased penetration of the alkaline form into the spore (120, 137). The major initial effect of bicarbonate is believed to be on the outer layers of spores of bacterial cell walls (79-81, 84, 137), although it will also inhibit germination of Bacillus spores (15, 45). Glutaraldehyde is thus likely to seal the outer layers of spores, an action that would also be of importance in inhibiting spore germination (154), with penetration at alkaline ph into the spore. Glutaraldehyde combines with amino acids (94, 182,
14 112 RUSSELL 235) and has been found to interact strongly with the cortex and spore protoplast, the latter prepared by Fitz-James' technique (61). Interaction with the cortex might be responsible for the sporicidal action of the aldehyde. Penetration and reaction of glutaraldehyde with components of this layer may be assisted by the action of divalent cations (81, 84). Other antibacterial agents also interact with the outer spore layers. Chlorhexidine diacetate increases spore hydrophobicity (189) but is not sporicidal (188, 190) unless used at high concentrations at high temperatures (77). As with QACs, therefore (38), it is likely that this cationic agent combines strongly with spore coats, but is unable to penetrate into the spore (169). Hypochlorites solubilize the cell walls of nonsporing bacteria and the spore integuments of B. megaterium spores (167). Separation of spore coats from cortex, followed by sequential dissolution of spore layers, has been described (123). Bacillus and Clostridium spores exposed to hypochlorites leak dipicolinic acid (56, 62, 123), suggesting an increase in spore permeability that can also be achieved by heat alone (18). The spore coat appears to act to some extent as a permeability barrier to chlorine (123, , 237), since the removal of protein from spore coats renders spores more susceptible to hypochlorites. These chlorine compounds will themselves remove coat protein, thereby allowing accessibility of cortex to lysozyme, which initiates germination (67, 237). Pretreatment of spores with sublethal concentrations of chlorine renders the cells more susceptible to mild heating (56). This effect may result from an alteration of spore cortex (237) since the cortex is believed to control spore response to high temperatures (89-91). The cortex may therefore be the major site of chlorine action, particularly since the removal of spore coats does not affect spore viability. The mechanism of sporicidal action of hydrogen peroxide has also been widely studied (4-6, 13, 14, 16, 17, 93, 121, 166, 173, 174, 204, 215, , 229, 231). Hydrogen peroxide removes coat protein from C. bifermentans, but coat protein removal (by DTT) from spores prior to peroxide treatment markedly increases its effectiveness, although B. cereus is affected to a lesser extent. Sublethal levels of peroxide increase the germination rate in C. bifermentans. Exposure of peroxide-treated spores to monovalent cations or to increasing ph results in a complete loss of their refractility (231). H202 increases the lysis of spores of C. bifermentans in the presence of certain divalent cations such as Cu2+, but the effect with other spores is less marked (16, 17). Although C. bifermentans and B. subtilis var. niger spores take up Cu2+ at about the same rate, only the spore protoplasts of the former bind these cations. Activation of peroxide to hydroxyl radicals (-OH) is necessary for sporicidal action, which would explain the synergistic effect noted with the combined use of hydrogen peroxide and UV radiation (17, 228, 230). DTT-treated spores of C. perfringens are much more susceptible to peroxide-induced lysis in the presence of Cu2+ ions than are untreated spores (4), and, significantly, this lysis is reduced by -OH scavengers. Peroxide and Cu2+, alone, do not produce lysis of cortical fragments but in combination induce lysis. It has been suggested (4) that peroxide may react with Cu2+ bound to cortex peptidoglycan, thereby generating -OH radicals. These would be formed at the region near the germ cell wall and would be responsible for causing protoplast lysis. DTT-treated C. perfringens spores undergo germinationlike changes followed by lysis when exposed to peroxide-generating systems (5). CLIN. MICROBIOL. REV. Spores of C. bifermentans produced on different media react differently to hydrogen peroxide, the more resistant types having a thicker cortex and smaller protoplast (227, 229). Peroxide has a marked effect on spore structure (123, 231), the cortex becoming depleted or absent and the ribosomes becoming disordered. Taken as a whole, the above findings suggest that hydrogen peroxide has an effect on the spore coats in some organisms but that this in itself is insufficient to explain its sporicidal activity. Its major effects are undoubtedly on the cortex and core. The evidence to date implies tentatively that the core is the major site of action, but further studies are needed to substantiate this conclusion. Of the other sporicides, the mechanism of sporicidal action of only one (EtO) has been examined in detail. The mode of action of iodine has, surprisingly, been little studied (173). It is considered to bind to bacterial protein (207, 208), but this vague attribute does little to explain how it kills spores. Formaldehyde is considered (208) to be sporicidal because it can penetrate into the interior of the bacterial spore. This monoaldehyde is an extremely reactive chemical (173, 179), combining with protein, RNA, and DNA, but the reasons for its sporicidal action remain somewhat obscure. EtO is an alkylating agent believed to inactivate bacterial spores by combining with various groups in proteins and nucleic acids (34, 35, 173, 174). B. subtilis spores exposed to EtO release considerably greater quantities of DNA, RNA, protein, and dipicolinic acid than do untreated spores (129), but EtO is not mutagenic to bacteria or spores (75, 99), unlike liquid sulfur mustard, an alkylating agent which is also mutagenic. Exposure of spores to trichloroacetic acid alters their viability and germinability and their response to heat and alkali but not to lysozyme (191). Trichloroacetic acid could help provide useful data about mechanisms of action of other agents. MEDICAL AND OTHER USES OF CHEMICAL SPORICIDES Previous sections have dealt with the spectrum of activity and mechanisms of action and of bacterial resistance. This section will consider some uses of chemical sporicidal agents (see also references 73, 175, and 183). Sporicidal Agents Glutaraldehyde, one of the most potent sporicidal agents, is extensively used in the leather tanning industry and in tissue fixation for electron microscopy and has numerous biochemical applications (11, 84, 179). In the microbiological context, the dialdehyde has chiefly been used for the chemical sterilization of medical equipment which cannot be sterilized by physical methods (84, 163, 185, 186, 216, 222). The main advantages claimed for its use as a chemosterilizer are (i) its broad spectrum of activity, especially good sporicidal properties; (ii) its activity in the presence of organic matter; (iii) its rapid antimicrobial action, although spores are considerably less susceptible than nonsporing bacteria; (iv) its noncorrosive action towards metals, rubber, lenses, and most materials, although some formulations may not fulfil these criteria (9, 11); (v) its lack of harmful effects on cement or lenses of bronchoscopes, cystoscopes, or telescopes; and (vi) its ease of use. Nevertheless, its pungent and irritating odor to personnel over long periods of time is a distinct disadvantage (206). Rittenbury and Hench (164)
15 VOL. 3, 1990 and Haselhuhn et al. (95) recommended glutaraldehyde for the cold sterilization of hemostats, cystoscopes, food containers, and anesthesia equipment. The aldehyde has also been found to be completely satisfactory for the routine sterilization of urological instruments and endoscopes (133, 145) and has proved highly effective for the rapid and safe disinfection of gastrointestinal endoscopy equipment (216). Despite inadequate sterilization with glutaraldehyde, because of the short time periods often used in hospital practice, infection transmission appears to be rare, presumably because very few potentially pathogenic spores are to be found on cleaned endoscopes (9, 11). Bovallius and Anas (32) demonstrated the effectiveness of vapor-phase glutaraldehyde for surface disinfection against sporing and nonsporing bacteria. In spite of its low volatility, it was more effective than formaldehyde. Formaldehyde is used as a solution and in vapor-phase form. In the liquid phase, formaldehyde is used as a disinfectant and as a general farm disinfectant (180). Lowtemperature steam (without formaldehyde) for 10 min at 730C is probably the most suitable method for disinfecting cystoscopes between patients (11). Longer periods of use of low-temperature steam with formaldehyde are required for sterilizing laparoscopes and arthroscopes, but this damages most flexible fiber-optic endoscopes (11). Chlorine-based products are used as food sanitizers, many of which are designed to control bacterial spores (70). Hypochlorites are used as disinfectants in the dairy industry, for the disinfection of farm buildings (180), and as disinfectants in hospitals and food establishments (23). They have certain advantages over glutaraldehyde (11) in that they kill spores rapidly, so that instruments could be sterilized rather than disinfected between patients during busy endoscopy sessions. They can, however, cause instrument damage. The activity of hypochlorites is reduced drastically in the presence of organic matter, whereas organic chlorine compounds are less susceptible (23). Chlorine dioxide does not form chlorinated organic compounds and is an effective sporicide, and its activity is not significantly affected by ph (70). Concentrations required and conditions of use preclude the wide use of hydrogen peroxide as an effective sporicide. Nevertheless, peroxide has been used for sterilizing food contact surfaces, that is, in obtaining commercial sterility of the packaging material when rapid spore destruction is required. Its medical (e.g., for cleansing wounds and for ear drop formulations) and other uses do not usually rely on its sporicidal activity. EtO is mainly used as a chemical sterilizing agent (35, 173), but has also been used as a decontamination agent for articles handled by tuberculosis patients. In the United Kingdom, it is one of the pharmacopoeial methods described for sterilizing powders, and Russell (173) has listed equipment that has been sterilized by EtO, in each instance with spores used as an indicator for satisfactory sterilization. These materials included various ophthalmic instruments, anesthetic equipment, heart-lung machines, disposable syringes, and hospital blankets. A problem always associated with EtO is the possibility of toxic effects arising from residual EtO present in products (162). Although EtO gas diffuses rapidly in open air, porous materials adsorb the gas during the sterilization cycle in various amounts and then require various poststerilization periods for desorption of residual gas. The antibacterial agents with sporicidal activity described in this section, therefore, can be used as chemical sterilizing agents. Two points must be added, however: (i) they are SPORICIDAL AGENTS 113 much less potent than thermal sterilization methods, especially autoclaving, but often find a use in sterilizing thermolabile equipment; (ii) it is somewhat arbitrary to consider only their sporicidal activity, since many of the biocides are also used in other environments, e.g., as disinfectants, or as decontaminants in specific areas as with materials contaminated with mycobacteria, human immunodeficiency virus, or hepatitis B virus. Inhibitors of Germination and Outgrowth Antibacterial agents that are not sporicidal but instead inhibit germination or outgrowth or both have uses that are different from sporicides. Sporeforming bacteria are of particular concern in food products (74, 127), especially when they are capable of surviving food-processing treatments, of causing food spoilage, and of being foodborne pathogens, e.g., C. perfringens, C. botulinum, and B. cereus (41, 69). Because of changes in the food itself (palatability and nutritional aspects), it is often impossible to destroy all spores that might be present. Consequently, specific antimicrobial agents are often included to inhibit growth from spores. Sodium nitrite delays, but does not prevent, botulinal outgrowth (41, 105), but its potential toxicity to humans is now well known (196, 197). Methyl and propyl parabens are commonly used in the food industry as preservatives, with the propyl ester more effective in inhibiting C. botulinum growth and toxin production (171). Sorbic acid is a weak lipophilic acid widely used as a food preservative (127, 168); it inhibits botulinal spore germination (there being a loss of heat resistance), a property that is ph dependent and appears at ph values of <6 (165, 196, 197). Potassium sorbate delays C. botulinum growth and toxin production in cured meats (64) and, when added to acidic foods, is hydrolyzed to sorbic acid. The acid is effective in the undissociated form (pka, 4.75), and the maximum ph for activity is ca. 6 to 6.5. In addition to the effect on germination noted above, sorbate delays or inhibits the outgrowth of C. botulinum spores (20, 21). Sorbate plus nitrite is an effective combination also, but the need to eliminate the latter as a preservative does not make this a viable proposition. Antibacterial agents are also used as preservatives in pharmaceutical and cosmetic products. Here, however, specific sporeforming agents are not necessarily the major problem (172). CONCLUSIONS Comparatively few bactericidal agents are actively sporicidal. The most important chemical sporicides are glutaraldehyde, formaldehyde (liquid and vapor forms), chlorinereleasing agents, peroxygens, and ethylene oxide. Ozone may become an important addition in the near future. Even so, activity against spores is invariably considerably slower than against vegetative cells, and concentrations are higher for a sporicidal action to be achieved. Bactericidal and bacteriostatic chemicals that are not sporicidal are usually sporostatic, preventing spore germination or outgrowth or both. Exact mechanisms of sporicidal activity and of spore resistance have yet to be elucidated, and further studies are undoubtedly necessary. In the clinical context, a rational approach to disinfectants and sterilization, involving a consideration of both patient risk and the treatment of equipment and environment (9, 10, 60), is necessary. Moist heat is the preferred form of
16 114 RUSSELL sterilization (usually at temperatures of 121'C or above) or, as low-temperature steam (e.g., 730C), of disinfection. Other specific methods of sterilization, such as ionizing radiation, dry heat, filtration, and gaseous chemical agents (ethylene oxide and low-temperature steam with formaldehyde) are used when relevant. Chemical disinfectants (liquid chemical sterilants) should only be used when other methods of sterilization are inappropriate. Thus, glutaraldehyde is used for sterilization of medical equipment when heat cannot be used; for example, articles in a high-risk category may be thermolabile but require a process that is sporicidal. LITERATURE CITED 1. Abdelaziz, A. A., and M. A. El-Nakeeb Sporicidal activity of local anesthetics and their binary combinations with preservatives. J. Clin. Pharm. Ther. 13: Ando, Y Studies on germination of spores of clostridial strains capable of causing food poisoning. II. Effects of some chemicals on the germination of spores of Clostridium botulinum Type A (in Japanese). J. Food Hyg. Soc. Jpn. 14: Ando, Y Mechanism of nitrite-induced germination of Clostridium perfringens spores. J. Appl. Bacteriol. 49: Ando, Y., and T. Tsuzuki The effect of hydrogen peroxide on spores of Clostridium perfringens. Lett. Appl. Microbiol. 2: Ando, Y., and T. Tsuzuki Changes in decoated spores of Clostridium perfringens caused by treatment with some enzymatic and non-enzymatic systems. Lett. Appl. Microbiol. 3: Ando, Y., and T. Tsuzuki Hydrogen peroxide release from bacterial spores during germination. Lett. Appl. Microbiol. 4: Andrew, M. H. E., and A. D. Russell (ed.) The revival of injured microbes. Soc. Appl. Bacteriol. Symp. No Association of Official Analytical Chemists Official methods of analysis, 13th ed. Association of Official Analytical Chemists, Washington, D.C. 9. Ayliffe, G. A. J., D. Coates, and P. N. Hoffman Chemical disinfection in hospitals. Public Health Laboratory Service, London. 10. Ayliffe, G. A. J., and B. J. Collins Problems of disinfection in hospitals, p In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilisation. Blackwell Scientific Publications, Ltd., Oxford. 11. Babb, J. R., C. R. Bradley, and G. A. J. Ayliffe Sporicidal activity of glutaraldehyde and hypochlorites and some factors influencing their selection for the treatment of medical equipment. J. Hosp. Infect. 1: Balassa, G., P. Milhaud, E. Raulet, M. T. Silva, and J. C. F. Sousa A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J. Gen. Microbiol. 110: Baldry, M. G. C The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 54: Baldry, M. G. C., and J. A. L. Fraser Disinfection with peroxygens, p In K. R. Payne (ed.) Industrial biocides: critical reports on applied chemistry, vol. 23. John Wiley & Sons, Chichester, England. 15. Barker, A. N., and J. Wolf The inhibitory effect of bicarbonate on the germination of Bacillus spores, p In A. N. Barker, J. Wolf, D. J. Ellar, D. J. Dring, and G. W. Gould (ed.), Spore research Academic Press, Inc. (London), Ltd., London. 16. Bayliss, C. E., and W. M. Waites The effect of hydrogen peroxide on spores of Clostridium bifermentans. J. Gen. Microbiol. 96: Bayliss, C. E., and W. M. Waites The synergistic killing of spores of Bacillus subtilis by hydrogen peroxide and ultraviolet light irradiation. FEMS Microbiol. Lett. 5: Beaman, T. C., H. S. Pankratz, and P. Gerhardt Heat CLIN. MICROBIOL. REV. shock affects permeability and resistance of Bacillus stearothermophilus spores. Appl. Environ. Microbiol. 54: Beveridge, T. J., F. M. R. Williams, and J. J. Koral The effect of chemical fixatives on cell walls of Bacillus subtilis. Can. J. Microbiol. 24: Blocher, J. C., and F. F. Busta Bacterial spore resistance to acid. Food Technol. 37(11): Blocher, J. C., and F. F. Busta Multiple modes of inhibition of spore germination and outgrowth by reduced ph and sorbate. J. Appl. Bacteriol. 59: Bloomfield, S. F., and G. A. Miles The antibacterial properties of sodium dichloroisocyanurate and sodium hypochlorite formulations. J. Appl. Bacteriol. 46: Bloomfield, S. F., and E. E. Uso The antibacterial properties of sodium hypochlorite and sodium dichloroisocyanurate as hospital disinfectants. J. Hosp. Infect. 6: Bohin, J.-P., and B. Lubochinsky Alcohol-resistant sporulation mutants of Bacillus subtilis. J. Bacteriol. 150: Borick, P. M Chemical sterilizers (chemosterilizers). Adv. Appl. Microbiol. 10: Borick, P. M., F. H. Dondershine, and J. L. Chandler Alkalinized glutaraldehyde, a new antimicrobial agent. J. Pharm. Sci. 53: Borick, P. M., and R. E. Pepper The spore problem, p In M. A. Benarde (ed.), Disinfection. Marcel Dekker, Inc., New York. 28. Boucher, R. M. G Advances in sterilization techniques. Am. J. Hosp. Pharm. 29: Boucher, R. M. G Potentiated 1,5-pentanedial, a breakthrough in chemical sterilizing and disinfecting technology. Am. J. Hosp. Pharm. 31: Boucher, R. M. G On biocidal mechanisms in the aldehyde series. Can. J. Pharm. Sci. 10: Boucher, R. M. G Ultrasonics. A tool to improve biocidal efficacy of sterilants or disinfectants in hospital and dental practice. Can. J. Pharm. Sci. 12: Bovallius, A., and P. Anas Surface-decontaminating action of glutaraldehyde in the gas-aerosol phase. Appl. Environ. Microbiol. 34: Briggs, A., and Z. Z. Yazdany Resistance of Bacillus spores to combined sporicidal treatments. J. Apple. Bacteriol. 37: Bruch, C. W., and M. K. Bruch Gaseous disinfection, p In M. A. Benarde (ed.), Disinfection. Marcel Dekker, Inc., New York. 35. Caputo, R. A., and T. E. Odlaug Sterilization with ethylene oxide and other gases, p In S. S. Block (ed.), Disinfection, sterilization and preservation, 3rd ed. Lea & Febiger, Philadelphia. 36. Cerf, Tailing of survival curves of bacterial spores. J. Apple. Bacteriol. 42: Cheung, H.-Y., and M. R. W. Brown Glycine as an inactivator of glutaraldehyde. J. Pharm. Pharmacol. 34: Chiori, C. O., R. Hambleton, and G. J. Rigby The inhibition of Bacillus subtilis spores by cetrimide retained on washed membrane filters and on the washed spores. J. Appl. Bacteriol. 28: Christensen, E. A., and H. Kristensen Gaseous sterilisation, p In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilization. Blackwell Scientific Publications, Ltd., Oxford. 40. Coates, D., and J. E. Death Sporicidal activity of mixtures of alcohol and hypochlorites. J. Clin. Pathol. 31: Cook, F. M., and M. D. Pierson Inhibition of bacterial spores by antimicrobials. Food Technol. 37(11): Cousins, C. M., and C. D. Allan Sporicidal properties of some halogens. J. Appl. Bacteriol. 30: Craven, S. E Activation of Clostridium pefringens spores under conditions that disrupt hydrophobic interactions
17 VOL. 3, 1990 of biological macromolecules. Appl. Environ. Microbiol. 54: Craven, S. E., and L. C. Blankenship Activation and injury of Clostridium perfringens spores by alcohols. Appl. Environ. Microbiol. 50: Cross, G., J. Wolf, and A. N. Barker The effect of sodium bicarbonate on the germination of spores of some Bacills species, p In A. N. Barker, G. W. Gould, and J.Wolf (ed.), Spore research Academic Press, Inc. (London), Ltd., London. 46. Dadd, A. H., and G. M. Daley Role of the coat in resistance of bacterial spores to inactivation by ethylene oxide. J. Appl. Bacteriol. 53: Dadd, A. H., and J. E. Rumbelow Germination of spores of Bacillus subtilis var. niger following exposure to gaseous ethylene oxide. J. Appl. Bacteriol. 60: Daher, E., E. Rosenberg, and A. L. Demain Germination-initiated spores of Bacillus brevis Nagano retain their resistance properties. J. Bacteriol. 161: Dancer, B. N., E. G. M. Power, and A. D. Russell Alkaliinduced revival of Bacillus spores after inactivation by glutaraldehyde. FEMS Microbiol. Lett. 57: Davis, S. B., R. A. Carls, and J. R. Gillis Recovery of sublethal sterilization damaged Bacillus spores in various culture media. Dev. Ind. Microbiol. 20: Death, J. E., and D. Coates Effect of ph on sporicidal and microbicidal activity of buffered mixtures of alcohol and sodium hypochlorite. J. Clin. Pathol. 32: Doores, S Bacterial spore resistance-species of emerging importance. Food Technol. 37(11): Downing, R. G., and I. W. Dawes L-Alanine binding during initiation of germination in Bacillus subtilis, p In A. N. Barker, J. Wolf, D. J. Ellar, G. J. Dring, and G. W. Gould (ed.), Spore research Academic Press, Inc. (London), Ltd., London. 54. Doyle, R. J., F. Nedjat-Haiem, and J. S. Singh Hydrophobic characteristics of Bacillus spores. Curr. Microbiol. 10: Dychdala, G. R Chlorine and chlorine compounds, p In S. S. Block (ed.), Disinfection, sterilization and preservation, 3rd ed. Lea & Febiger, Philadelphia. 56. Dye, M., and G. C. Mead The effect of chlorine on the viability of clostridial spores. J. Food Technol. 7: Ellar, D. J Relations between structure and function in the prokaryotic cell. Symp. Soc. Gen. Microbiol. 28: Ernst, R. R Ethylene oxide sterilization kinetics. Biotechnol. Bioeng. Symp. 4: Ernst, R. R Sterilization by means of ethylene oxide. Acta Pharm. Suec. 12: Favero, M. S Sterilization, disinfection, and antisepsis in the hospital, p In E. H. Lennette, A. Balows, W. J. Hausler, Jr., and H. J. Shadomy (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, D.C. 61. Fitz-James, P. C Formation of protoplasts from resting spores. J. Bacteriol. 105: Foegeding, P. M Bacterial spore resistance to chlorine compounds. Food Technol. 37(11): Foegeding, P. M Ozone inactivation of Bacillus and Clostridium spores and the importance of the spore coat to resistance. Food Microbiol. 2: Foegeding, P. M., and F. F. Busta Bacterial spore injury-an update. J. Food Prot. 44: Foegeding, P. M., and F. F. Busta Hypochlorite injury of Clostridium botulinum spores alters germination responses. Appl. Environ. Microbiol. 45: Foegeding, P. M., and F. F. Busta Proposed role of lactate in germination of hypochlorite-treated Clostridium botulinum spores. Appl. Environ. Microbiol. 45: Foegeding, P. M., and F. F. Busta Proposed mechanism for sensitization by hypochlorite treatment of Clostridium botulinum spores. Appl. Environ. Microbiol. 45: Foegeding, P. M., and F. F. Busta Differing L-alanine SPORICIDAL AGENTS 115 germination requirements of hypochlorite-treated Clostridium botulinum spores from two crops. Appl. Environ. Microbiol. 45: Foegeding, P. M., and M. L. Fulp Comparison of coats and surface-dependent properties of Bacillus cereus T prepared in two sporulation environments. J. Apple. Bacteriol. 65: Foegeding, P. M., V. Hemstapat, and F. G. Giesbrecht Chlorine dioxide inactivation of Bacillus and Clostridium spores. J. Food Sci. 51: Forsyth, M. P A rate-of-kill test for measuring sporicidal properties of liquid sterilizers. Dev. Ind. Microbiol. 16: Gardner, J. F., and M. M. Peel Introduction to sterilization and disinfection. Churchill Livingstone, Ltd., Edinburgh. 73. Gardner, J. S., and M. S. Favero Guideline for handwashing and hospital infection control. Health and Human Services publication no Centers for Disease Control, Atlanta. 74. Genigeorgis, C. A Factors affecting the probability of growth of pathogenic microorganisms in foods. J. Am. Vet. Med. Assoc. 179: Gilbert, G. L., V. M. Gambill, D. R. Spiner, R. K. Hoffman, and C. R. Phillips Effect of moisture on ethylene oxide sterilization. Apple. Microbiol. 12: Gorman, S. P., E. P. Hutchinson, E. M. Scott, and L. M. McDermott Death, injury and revival of chemically treated Bacillus subtilis spores. J. Appl. Bacteriol. 54: Gorman, S. P., D. S. Jones, and A. M. Loftus The sporicidal activity and inactivation of chlorhexidine gluconate in aqueous and alcoholic solution. J. Appl. Bacteriol. 63: Gorman, S. P., and E. M. Scott Evaluation of potential inactivators of glutaraldehyde in disinfection studies with Escherichia coli. Microbios Lett. 1: Gorman, S. P., and E. M. Scott Effect of alkalination on the bacterial cell and glutaraldehyde molecules. Microbios Lett. 6: Gorman, S. P., and E. M. Scott Uptake and media reactivity of glutaraldehyde solutions related to structure and biocidal activity. Microbios Lett. 5: Gorman, S. P., and E. M. Scott Potentiation and stabilization of glutaraldehyde biocidal activity utilizing surfactant-divalent metal combinations. Int. J. Pharm. 4: Gorman, S. P., E. M. Scott, and E. P. Hutchinson Interaction of the Bacillus subtilis spore protoplast, cortex, ion-exchange and coatless forms with glutaraldehyde. J. Appl. Bacteriol. 56: Gorman, S. P., E. M. Scott, and E. P. Hutchinson Emergence and development of resistance to antimicrobial chemicals and heat in spores of Bacillus subtilis. J. Appl. Bacteriol. 57: Gorman, S. P., E. M. Scott, and A. D. Russell Antimicrobial activity, uses and mechanism of action of glutaraldehyde. J. Apple. Bacteriol. 48: Gottardi, W Iodine and iodine compounds, p In S. S. Block (ed.), Disinfection, sterilization and preservation, 3rd ed. Lea & Febiger, Philadelphia. 86. Gould, G. W Germination and the problem of dormancy. J. Apple. Bacteriol. 33: Gould, G. W Methods for studying bacterial spores, p In J. R. Norris and D. W. Ribbons (ed.), Methods in microbiology, vol. 6A. Academic Press, Inc., New York. 88. Gould, G. W Recent advances in the understanding of resistance and dormancy in bacterial spores. J. Appl. Bacteriol. 42: Gould, G. W Mechanisms of resistance and dormancy, p In A. Hurst and G. W. Gould (ed.), The bacterial spore, vol. 2. Academic Press, Inc., New York. 90. Gould, G. W The revival of injured microbes. Soc. Appl. Bacteriol. Symp. Ser. 12: Gould, G. W Modification of resistance and dormancy, p InG. J. Dring, D. J. Ellar, and G. W. Gould (ed.),
18 116 RUSSELL Fundamental and applied aspects of bacterial spores. Academic Press, Inc., New York. 92. Gould, G. W., and A. J. H. Salle Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60: Gould, G. W., J. M. Stubbs, and W. L. King Structure and composition of resistance layers in bacterial spore coats. J. Gen. Microbiol. 60: Hajdu, J., and P. Friedrich Reaction of glutaraldehyde with NH2 compounds. A spectrophotometric method for the determination of glutaraldehyde concentration. Anal. Biochem. 65: Haselhuhn, D. H., F. W. Brason, and P. M. Borick In use study of buffered glutaraldehyde for cold sterilization of anaesthesia equipment. Curr. Res. Anesth. Anaig. 46: Hayakawa, H., K. Tochikubo, and S. Kozuka Mutual relationship between antibiotics and resting spores of Bacillus subtilis: morphological changes and macromolecular synthesis after germination of spores treated with cyclic polypeptide and aminoglycoside antibiotics. Microbiol. Immunol. 25: Hibbert, H. R., and R. Spencer An investigation of the inhibitory properties of sodium thioglycollate in media for the recovery of bacterial spores. J. Hyg. 68: Hill, S. H. A spovh and spovj-new sporulation loci in Bacillus subtilis 168. J. Gen. Microbiol. 129: Hoffman, R. K Toxic gases, p In W. B. Hugo (ed.), Inhibition and destruction of the microbial cell. Academic Press, Inc., New York Hsich, L. K., and J. C. Vary Germination and peptidoglycan solubilization in Bacillus megaterium spores. J. Bacteriol. 123: Huber, W. G Antiseptics and disinfectants, p In N. H. Booth and L. E. McDonald (ed.), Veterinary pharmacology and therapeutics. Iowa State University Press, Ames Hugo, W. B., and A. D. Russell Types of antimicrobial agents, p In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilization. Blackwell Scientific Publications, Ltd., Oxford Imae, Y., and J. L. Strominger Relationship between cortex content and properties of Bacillus sphaericus spores. J. Bacteriol. 126: Imae, Y., and J. L. Strominger Conditional spore cortex-less mutants of Bacillus sphaericus J. Biol. Chem. 251: Ingram, M., and T. A. Roberts Application of the 'D-concept' to heat treatments involving curing salts. J. Food Technol. 6: Isenberg, H. D Clinical laboratory studies of disinfection with Sporicidin. J. Clin. Microbiol. 22: Isenberg, H. D., E. R. Giugliano, K. France, and P. Alperstein Evaluation of three disinfectants after in-use stress. J. Hosp. Infect. 11: Jenkinson, H. F Germination and resistance defects in spores of a Bacillus subtilis mutant lacking a coat polypeptide. J. Gen. Microbiol. 127: Jenkinson, H. F Altered arrangement of proteins in the spore coat of a germination mutant of Bacillus subtilis. J. Gen. Microbiol. 129: Jenkinson, H. F., D. Kay, and J. Mandelstam Temporal dissociation of late events in Bacillus subtilis sporulation from expression of genes that determine them. J. Bacteriol. 141: Jenkinson, H. F., W. D. Sawyer, and J. Mandelstam Synthesis and order of assembly of spore coat proteins in Bacillus subtilis. J. Gen. Microbiol. 123: Kanda, K., Y. Yasuda, and K. Tochikubo Germinationinitiating activities for Bacillus subtilis spores of analogues of L-alanine derived by modification at the amino or carboxyl group. J. Gen. Microbiol. 134: Kaye, S The sterilizing action of gaseous ethylene oxide. III. The effect of ethylene oxide and related compounds upon CLIN. MICROBIOL. REV. bacterial aerosols. Am. J. Hyg. 50: Kaye, S Use of ethylene oxide for the sterilization of hospital equipment. J. Lab. Clin. Med. 50: Kaye, S., and C. R. Phillips The sterilizing action of ethylene oxide. IV. The effect of moisture. Am. J. Hyg. 50: Kelsey, J. C., I. H. MacKinnon, and I. M. Maurer Sporicidal activity of hospital disinfectants. J. Clin. Pathol. 27: Kereluk, K., R. A. Gammon, and R. S. Lloyd Microbiological aspects of ethylene oxide sterilization. Apple. Microbiol. 19: Keynan, A., A. A. Berns, G. Dunn, M. Young, and J. Mandelstam Resporulation of outgrowing Bacillus subtilis spores. J. Bacteriol. 128: Keynan, A., and N. Sandler Spore research in historical perspective, p In A. Hurst and G. W. Gould (ed.), The bacterial spore, vol. 2. Academic Press, Inc., New York King, J. A., W. Woodside, and P. V. McGucken Relationship between ph and antibacterial activity of glutaraldehyde. J. Pharm. Sci. 63: King, W. L., and G. W. Gould Lysis of bacterial spores with peroxide. J. Apple. Bacteriol. 32: Koransky, J. R., S. D. Allen, and V. R. Dowell Use of ethanol for selective isolation of sporeforming microorganisms. Appl. Environ. Microbiol. 35: Kulikovsky, A., H. S. Pankratz, and H. L. Sadoff Ultrastructural and chemical changes in spores of Bacillus cereus after action of disinfectants. J. Appl. Bacteriol. 38: Kutima, P. M., and P. M. Foegeding Involvement of the spore coat in germination of Bacillus cereus T spores. Appl. Environ. Microbiol. 53: Labbe, R. G., and C. L. Duncan Growth from spores of Clostridium perfringens in the presence of sodium nitrite. Apple. Microbiol. 19: Labbe, R. G., R. R. Reich, and C. L. Duncan Alteration in ultrastructure and germination of Clostridium perfringens type A spores following extraction of spore coats. Can. J. Microbiol. 24: Leuck, E Antimicrobial food preservatives. Springer- Verlag, Berlin Lewis, J. C., and L. Jurd Sporostatic action of cynnamylphenols and related compounds on Bacillus megaterium, p In H. 0. Halvorson, R. Hanson, and L. L. Campbell (ed.), Spores V. American Society for Microbiology, Washington, D.C Marletta, J., and C. R. Stumbo Some effects of ethylene oxide on Bacillus subtilis. J. Food Sci. 35: Milhaud, P., and G. Balassa Biochemical genetics of bacterial sporulation. IV. Sequential development of resistances to chemical and physical agents during sporulation of Bacillus subtilis. Mol. Gen. Genet. 125: Miner, N. A., J. W. McDowell, G. W. Willcockson, N. I. Bruckner, R. L. Stark, and E. J. Whitmore Antimicrobial and other properties of a new stabilized alkaline glutaraldehyde disinfectant sterilizer. Am. J. Hosp. Pharm. 34: Mitani, T., and H. Kadota Chemical features of spore coat of Bacillus subtilis. J. Gen. Apple. Microbiol. 22: Mitchell, J. P., and V. G. Alder The disinfection of urological endoscopes. Br. J. Urol. 47: Mizuba, S., D. I. Zimmer, J. Woodrow, and F. E. Halleck Methods for exposing and recovering spores applicable for use in sterilization studies of complex mechanical equipment. Dev. Ind. Microbiol. 20: Moir, A., E. Lafferty, and D. A. Smith Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map locations. J. Gen. Microbiol. 111: Mossel, D. A. A., and H. Beerens Studies on the inhibitory properties of sodium thioglycollate on the germination of wet spores of clostridia. J. Hyg. 66:
19 VOL. 3, Munton, T. J., and A. D. Russell Aspects of the action of glutaraldehyde on Escherichia coli. J. Apple. Bacteriol. 33: Murray, T., I. Lazardis, and B. Seddon Germination of spores of Bacillus brevis and inhibition by gramicidin S: a stratagem for survival. Lett. Apple. Microbiol. 1: Murrell, W. G Chemical composition of spores and spore structures, p In G. W. Gould and A. Hurst (ed.) The bacterial spore. Academic Press, Inc., New York Navarro, J. M., and P. Monsan ttude du mechanism d'interaction du glutaraldehyde avec les micro-organisms. Ann. Microbiol. (Paris) 127B: Nishihara, T., Y. Takashi, T. Ichikawa, and M. Kondo Studies on the bacterial spore coat. 8. On the SDS-DTT extract from Bacillus megaterium spores. Microbiol. Immunol. 25: Nishihara, T., M. Tomita, N. Yamanaka, T. Ichikawa, and M. Kondo Studies on the bacterial spore coat. 7. Properties of alkali-soluble components from spore coat of Bacillus megaterium. Microbiol. Immunol. 24: Nishihara, T., I. Yoshimoto, and M. Kondo Studies on the bacterial spore coat. 9. The role of surface charge in germination of Bacillus megaterium spores. Microbiol. Immunol. 25: Nordgren, G Investigations on the sterilizing efficacy of gaseous formaldehyde. Acta Pathol. Microbiol. Scand. Suppl. 40: O'Brien, H. A., J. D. Mitchell, S. Haberman, D. F. Rowan, T. E. Winford, and J. Pellet The use of activated glutaraldehyde as a cold sterilizing agent for urological instruments. J. Urol. 95: Ortenzio, L. F., L. S. Stuart, and J. L. Friedl The resistance of bacterial spores to constant boiling hydrochloric acid. J. Assoc. Off. Agric. Chem. 36: Palin, A. T Water disinfection-chemical aspects and analytical control, p In J. D. Johnson (ed.), Disinfection: water and wastewater. Ann Arbor Science Publishers Inc., Ann Arbor, Mich Parker, M. S Some effects of preservatives on the development of bacterial spores. J. Appl. Bacteriol. 32: Parker, M. S., and T. J. Bradley A reversible inhibition of the germination of bacterial spores. Can. J. Microbiol. 14: Pepper, R. E., and V. L. Chandler Sporicidal activity of alkaline alcoholic saturated dialdehyde solutions. Appl. Microbiol. 11: Phillips, C. R The sterilizing action of gaseous ethylene oxide. II. Sterilization of contaminated objects with ethylene oxide and related compounds: time, concentration and temperature relationships. Am. J. Hyg. 50: Phillips, C. R., and S. Kaye The sterilizing action of ethylene oxide. I. Review. Am. J. Hyg. 50: Power, E. G. M., B. N. Dancer, and A. D. Russell Emergence of resistance to glutaraldehyde in spores of Bacillus subtilis 168. FEMS Microbiol. Lett. 50: Power, E. G. M., B. N. Dancer, and A. D. Russell Possible mechanisms for the revival of glutaraldehyde-treated spores of Bacillus subtilis NCTC J. Appl. Bacteriol. 67: Power, E. G. M., and A. D. Russell Assessment of 'Cold Sterilog Glutaraldehyde Monitor.' J. Hosp. Infect. 11: Power, E. G. M., and A. D. Russell Glutaraldehyde: its uptake by sporing and non-sporing bacteria, rubber, plastic and an endoscope. J. Apple. Bacteriol. 67: a.Power, E. G. M., and A. D. Russell Uptake of L- 14C-alanine to glutaraldehyde-treated and untreated spores of Bacillus subtilis. FEMS Microbiol. Lett. 66: Prasad, C Initiation of spore germination in Bacillus subtilis: relationship to inhibition of L-alanine metabolism. J. Bacteriol. 119: Quinn, P. J Evaluation of veterinary disinfectants and disinfection processes, p In A. H. Linton, W. B. SPORICIDAL AGENTS 117 Hugo, and A. D. Russell (ed.), Disinfection in veterinary and farm animal practice. Blackwell Scientific Publications, Ltd., Oxford Reich, R Effect of sublethal ethylene oxide exposure on Bacillus subtilis spores and biological indicator performance. J. Parent. Drug Assoc. 34: Relyveld, E. H Ittude du pouvoir bactericide du glutaraldehyde. Ann. Microbiol. (Paris) 128B: Reybrouck, G The evaluation of the antimicrobial activity of disinfectants, p In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilization. Blackwell Scientific Publications, Ltd., Oxford Richards, C., J. R. Furr, and A. D. Russell Inactivation of microorganisms by lethal gases, p In J. J. Kabara (ed.), Cosmetic and drug preservation: principles and practice. Marcel Dekker, Inc., New York Ridgway, G. L Decontamination of fibreoptic endoscopes. J. Hosp. Infect. 6: Rittenbury, M., and M. Hench Preliminary evaluation of an activated glutaraldehyde solution for cold disinfection. Ann. Surg. 161: Robach, M. C., and M. D. Pierson Influence of parahydroxybenzoic acid esters on the growth and toxin production of Clostridium botulinum 10755A. J. Food Sci. 43: Roberts, T. A., and A. D. Hitchins Resistance of spores, p In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, Inc., New York Rode, L. J., and M. G. Williams Utility of sodium hypochlorite for ultrastructure study of bacterial spore integuments. J. Bacteriol. 92: Ronning, I. E., and H. A. Frank Growth inhibition of putrefactive anaerobe 3679 caused by stringent-type response induced by protonophoric activity of sorbic acid. Appl. Environ. Microbiol. 53: Rosenberg, E., D. R. Brown, and A. L. Demain The influence of gramicidin S on hydrophobicity of germinating Bacillus brevis spores. Arch. Microbiol. 142: Rubbo, S. D., J. F. Gardner, and R. L. Webb Biocidal activities of glutaraldehyde and related compounds. J. Appl. Bacteriol. 30: Russell, A. D The destruction of bacterial spores, p In W. B. Hugo (ed.), Inhibition and destruction of the microbial cell. Academic Press, Inc., New York Russell, A. D Factors influencing the efficacy of antimicrobial agents, p In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilization. Blackwell Scientific Publications, Ltd., Oxford Russell, A. D The destruction of bacterial spores. Academic Press, Inc., New York Russell, A. D Mechanisms of action of chemical sporicidal and sporostatic agents. Int. J. Pharm. 16: Russell, A. D The effects of chemical and physical agents on microbes: disinfection and sterilization. In Topley & Wilson's principles of bacteriology, virology and immunity, 8th ed. Edward Arnold, London Russell, A. D., I. Ahonkhai, and D. T. Rogers Microbiological applications of the inactivation of antibiotics and other antimicrobial agents. J. Appl. Bacteriol. 46: Russell, A. D., B. N. Dancer, E. G. M. Power, and L. A. Shaker Mechanisms of bacterial spore resistance to disinfectants, p In Proceedings, 4th Conference on Progress in Chemical Disinfection Russell, A. D., S. A. Hammond, and J. R. Morgan Bacterial resistance to antiseptics and disinfectants. J. Hosp. Infect. 7: Russell, A. D., and D. Hopwood The biological uses and importance of glutaraldehyde. Prog. Med. Chem. 13: Russell, A. D., and W. B. Hugo Chemical disinfectants, p In A. H. Linton, W. B. Hugo, and A. D. Russell (ed.), Disinfection in veterinary and farm animal practice. Blackwell Scientific Publications, Ltd., Oxford.
20 118 RUSSELL 181. Russell, A. D., B. D. Jones, and P. Milburn Reversal of the inhibition of bacterial spore germination and outgrowth by antibacterial agents. Int. J. Pharm. 25: Russell, A. D., and T. J. Munton Bactericidal and bacteriostatic activity of glutaraldehyde and its interaction with lysine and proteins. Microbios 11: Rutala, W. A Disinfection, sterilization and waste disposal, p In R. P. Wenzel (ed.), Prevention and control of nosocomial infections. The Williams & Wilkins Co., Baltimore Sammons, R. L., A. Moir, and D. A. Smith Isolation and properties of spore germination mutants of Bacillus subtilis 168 deficient in the initiation of germination. J. Gen. Microbiol. 124: Scott, E. M., and S. P. Gorman Sterilization with glutaraldehyde, p In S. S. Block (ed.), Disinfection, sterilization and preservation, 3rd ed. Lea & Febiger, Philadelphia Scott, E. M., and E. P. Gorman Chemical disinfectants, antiseptics and preservatives, p In W. B. Hugo and A. D. Russell (ed.), Pharmaceutical microbiology, 4th ed. Blackwell Scientific Publications, Ltd., Oxford Setlow, P Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function and degradation. Annu. Rev. Microbiol. 42: Shaker, L. A., B. N. Dancer, A. D. Russell, and J. R. Furr Emergence and development of chlorhexidine resistance during sporulation of Bacillus subtilis 168. FEMS Microbiol. Lett. 51: Shaker, L. A., J. R. Furr, and A. D. Russell Mechanism of resistance of Bacillus subtilis spores to chlorhexidine. J. Appl. Bacteriol. 64: Shaker, L. A., A. D. Russell, and J. R. Furr Aspects of the action of chlorhexidine on bacterial spores. Int. J. Pharm. 34: Shibata, H., M. Uchida, H. Hayashi, and I. Tani Effect of trichloracetic acid treatment on certain properties of spores of Bacillus cereus T. Microbiol. Immunol. 23: Sierra, G., and R. M. G. Boucher Ultrasonic synergistic effects in liquid-phase chemical sterilization. Apple. Microbiol. 22: Smoot, L. A., and M. D. Pierson Inhibition and control of bacterial spore germination. J. Food Prot. 45: Snyder, R. W., and E. L. Cheatle Alkaline glutaraldehyde-an effective disinfectant. Am. J. Hosp. Pharm. 22: Sofos, J. N., and F. F. Busta Antimicrobial activity of sorbate. J. Food Prot. 44: Sofos, J. N., and F. F. Busta Chemical food preservatives, p In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilization. Blackwell Scientific Publications, Ltd., Oxford Sofos, J. N., F. F. Busta, and C. E. Allen Botulism control by nitrite and sorbate in cured meats: a review. J. Food Prot. 42: Sofos, J. N., M. D. Pierson, J. C. Blocher, and F. F. Busta Mode of action of sorbic acid on bacterial cells and spores. Int. J. Food Microbiol. 3: Spaulding, E. H Chemical sterilization of surgical instruments. Surg. Gynecol. Obstet. 69: Spicher, G., and J. Peters Microbial resistance to formaldehyde. I. Comparative quantitative studies in some selected species of vegetative bacteria, bacterial spores, fungi, bacteriophages and viruses. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. Reihe B 163: (In German.) 201. Spicher, G., and J. Peters Heat activation of bacterial spores after inactivation by formaldehyde. Dependence of heat activation on temperature and duration of action. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. Reihe B 173: Spooner, D. F., and G. Sykes Laboratory assessment of CLIN. MICROBIOL. REV. antibacterial activity, p In J. R. Norris and D. W. Ribbons (ed.), Methods in microbiology, vol. 7B. Academic Press, Inc., New York Starke, R. L., D. Ferguson, P. Garza, and N. A. Miner An evaluation of the Association of Official Analytical Chemists sporicidal test methods. Dev. Ind. Microbiol. 16: Stevenson, K. E., and B. D. Shafer Bacterial spore resistance to hydrogen peroxide. Food Technol. 37(11): Stewart, G. S. A. B., K. Johnstone, E. Hagelberg, and D. J. Ellar Commitment of bacterial spores to germinate. A measure of the trigger reaction. Biochem. J. 198: Stonehill, A. A., S. Krop, and P. M. Borick Buffered glutaraldehyde, a new chemical sterilizing solution. Am. J. Hosp. Pharm. 20: Sykes, G Disinfection and sterilization, 2nd ed. F. & N. Spon, London Sykes, G The sporicidal properties of chemical disinfectants. J. Apple. Bacteriol. 33: Takahashi, I., and L. W. MacKenzie Effects of inhibitory agents on sporulation of Bacillus subtilis. Can. J. Microbiol. 28: Tawasatani, T., M. Kakezawa, and I. Shibasaki Role of cellular calcium in the variation of propylene oxide sensitivity of bacterial spores. Hakko Kyokaishi 57: (In Japanese.) 211. Thomas, S Effect of high concentrations of glutaraldehyde upon bacterial spores. Microbios Lett. 4: Thomas, S., and A. D. Russell Studies on the mechanism of the sporicidal action of glutaraldehyde. J. Apple. Bacteriol. 37: Thomas, S., and A. D. Russell Temperature-induced changes in the sporicidal activity and chemical properties of glutaraldehyde. Apple. Microbiol. 28: Thomas, S., and A. D. Russell Sensitivity and resistance to glutaraldehyde of the hydrogen and calcium forms of Bacillus subtilis spores. J. Apple. Bacteriol. 38: Toledo, P. T., S. E. Escher, and J. C. Ayres Sporicidal properties of hydrogen peroxide against food-spoilage organisms. Apple. Microbiol. 26: Tolon, M., E. Thofern, and S. E. Miederer Disinfection procedures of fiberscopes in endoscopy departments. Endoscopy 8: Treadwell, P. E., G. J. Jann, and A. J. Salle Studies on factors affecting the rapid germination of spores of Clostridium botulinum. J. Bacteriol. 76: Trueman, J. R The halogens, p In W. B. Hugo (ed.), Inhibition and destruction of the microbial cell. Academic Press, Inc., New York Trujillo, P., and T. J. David Sporostatic and sporicidal properties of aqueous formaldehyde. Apple. Microbiol. 23: Trujillo, P., and N. Laible Reversible inhibition of spore germination by alcohols. Apple. Microbiol. 20: Tulis, J. J Formaldehyde gas as a sterilant, p In G. B. Phillips and W. S. Miller (ed.), Industrial sterilization. Duke University Press, Durham, N.C Varpela, E., S. Otterstrom, and R. Hackman Liberation of alkalinized glutaraldehyde by respirators after cold sterilization. Acta Anaesth. Scand. 15: Vinter, V Germination and outgrowth: effect of inhibitors. J. Apple. Bacteriol. 33: Waites, W. M Microbial resistance to non-antibiotic antimicrobial agents: resistance of bacterial spores, p In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilization. Blackwell Scientific Publications, Ltd., Oxford Waites, W. M Inactivation of spores with chemical agents, p In G. J. Dring, D. J. Ellar, and G. W. Gould (ed.), Fundamental and applied aspects of bacterial spores. Academic Press, Inc., New York Waites, W. M., and C. E. Bayliss The effect of changes in the spore coat on the destruction of Bacillus cereus spores
21 VOL. 3, 1990 by heat and chemical agents. J. Apple. Biochem. 1: Waites, W. M., and C. E. Bayliss Microbial growth and survival in extremes of environment. Soc. Apple. Bacteriol. Tech. Ser. 15: Waites, W. M., and C. E. Bayliss The revival of injured microbes. Soc. Apple. Bacteriol. Symp. Ser. 12: Waites, W. M., C. E. Bayliss, N. R. King, and A. M. C. Davies The effect of transition metal ions on the resistance of bacterial spores to hydrogen peroxide and to heat. J. Gen. Microbiol. 112: Waites, W. M., S. E. Harding, D. R. Fowler, S. H. Jones, D. Shaw, and M. Martin The destruction of spores of Bacillus subtilis by the combined effects of hydrogen perioxide and ultraviolet light. Lett. Apple. Microbiol. 7: Waites, W. M., L. R. Wyatt, N. R. King, and C. E. Bayliss Changes in spores of Clostridium bifermentans caused by treatment with hydrogen peroxide and cations. J. Gen. Microbiol. 93: WallhAuser, K. H Antimicrobial preservatives used by the cosmetic industry, p In J. J. Kabara (ed.), Cosmetic and drug preservation: principles and practice. Marcel Dekker, Inc., New York Warth, A. D Molecular structure of the bacterial spore. Adv. Microb. Physiol. 17: Watanabe, K., and S. Takesue Selective inhibition of the germination of Bacillus megaterium spores by alkyl p-hydroxybenzoates. Chem. Pharm. Bull. 24: SPORICIDAL AGENTS Whipple, E. B., and M. Ruta Structure of aqueous glutaraldehyde. J. Org. Chem. 39: Woods, D. R., and D. T. Jones Physiological responses of Bacteroides and Clostridium strains to environmental stress factors. Adv. Microb. Physiol. 28: Wyatt, L. R., and W. M. Waites The effect of chlorine on spores of Clostridium bifermentans, Bacillus subtilis and Bacillus cereus. J. Gen. Microbiol. 89: Yasuda, Y., and K. Tochikubo Relation between D- glucose and L- and D-alanine in the initiation of germination of Bacillus subtilis spore. Microbiol. Immunol. 28: Yasuda, Y., and K. Tochikubo Germination-initiation and inhibitory activities of L- and D-alanine analogues for B. subtilis spores. Modification of methyl group of L- and D- alanine. Microbiol. Immunol. 29: Yasuda, Y., and K. Tochikubo Disappearance of the cooperative effect of glucose on L-alanine binding during heat activation of germination of Bacillus subtilis spores. Microbiol. Immunol. 29: Yasuda, Y., K. Tochikubo, Y. Hachisuka, H. Tomida, and K. Ikeda Quantitative structure-inhibitory activity relationships of phenols and fatty acids for Bacillus subtilis spore germination. J. Med. Chem. 25: Yasuda-Yasaki, K., S. Namiki-Kanie, and Y. Hachisuka Inhibition of Bacillus subtilis spore germination by various hydrophobic compounds: demonstration of hydrophobic character of the L-alanine receptor site. J. Bacteriol. 136: Downloaded from on September 6, 2018 by guest
Sporicides and how to test them
 Live broadcast from Sporicides and how to test them Jean-Yves Maillard Cardiff School of Pharmacy and Pharmaceutical Sciences Cardiff University Live teleclass broadcast sponsored by www.clinell.com November
Live broadcast from Sporicides and how to test them Jean-Yves Maillard Cardiff School of Pharmacy and Pharmaceutical Sciences Cardiff University Live teleclass broadcast sponsored by www.clinell.com November
was prepared by the method of Beeby and Whitehouse and sodium hypochlorite were tested periodically; no changes were detected over the experimental
 Journal of Clinical Pathology, 1978, 31, 148-152 Sporicidal activity of mixtures of alcohol and hypochlorite D. COATES AND JANET E. DEATH From the Disinfection Reference Laboratory, Central Public Health
Journal of Clinical Pathology, 1978, 31, 148-152 Sporicidal activity of mixtures of alcohol and hypochlorite D. COATES AND JANET E. DEATH From the Disinfection Reference Laboratory, Central Public Health
Chemistry & Technology of Sanitizers
 Chemistry & Technology of Sanitizers Sterilize: Terms An Agent that will Destroys or Eliminates All Forms of Life, Including All Forms of Vegetative, or Actively Growing Bacteria, Bacterial Spores, Fungi
Chemistry & Technology of Sanitizers Sterilize: Terms An Agent that will Destroys or Eliminates All Forms of Life, Including All Forms of Vegetative, or Actively Growing Bacteria, Bacterial Spores, Fungi
Bacillus anthracis. Clostridium botulinum Clostridium perfringens and other, but never Gram-negative microbes
 SPORES (endospores) the spore is formed inside the parent vegetative cell hence the name endospores The spore is a dehydrated, multishelled structure that protects and allows the bacteria to exist in suspended
SPORES (endospores) the spore is formed inside the parent vegetative cell hence the name endospores The spore is a dehydrated, multishelled structure that protects and allows the bacteria to exist in suspended
Nursing college, Second stage Microbiology Dr.Nada Khazal K. Hendi L4: Sterilization & Disinfection
 1 L4: Sterilization & Disinfection Sterilization is the killing of all microorganisms, including bacterial spores, which are highly resistant. Sterilization is usually carried out by there are three methods:
1 L4: Sterilization & Disinfection Sterilization is the killing of all microorganisms, including bacterial spores, which are highly resistant. Sterilization is usually carried out by there are three methods:
Safety Services. Guidance on the Selection and Use of Disinfectants
 Safety Services Guidance on the Selection and Use of Disinfectants Guidance on the Selection and Use of Disinfectants Chemical disinfectants reduce the number of viable micro-organisms to a level below
Safety Services Guidance on the Selection and Use of Disinfectants Guidance on the Selection and Use of Disinfectants Chemical disinfectants reduce the number of viable micro-organisms to a level below
Sterilization. The complete killing of all forms of living organisms including bacterial spores.
 Sterilization The complete killing of all forms of living organisms including bacterial spores. Disinfection: The Killing of pathogenic microorganisms from objects. Disinfectant: A chemical agent used
Sterilization The complete killing of all forms of living organisms including bacterial spores. Disinfection: The Killing of pathogenic microorganisms from objects. Disinfectant: A chemical agent used
Chapter 002 The Chemistry of Biology
 Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
BIT TM Detailed Technical Discussion
 National Security Solutions BIT TM Detailed Technical Discussion Hydrogen peroxide is a strong oxidizer that is used for high-level disinfection and sterilization. It produces reactive hydroxyl free radicals
National Security Solutions BIT TM Detailed Technical Discussion Hydrogen peroxide is a strong oxidizer that is used for high-level disinfection and sterilization. It produces reactive hydroxyl free radicals
Disinfection. Disinfection is used to treat both domestic water and wastewater.
 Disinfection Disinfection is the selective destruction of disease causing organisms (viruses, bacteria, protozoans). It destroys most recognized pathogenic microorganisms, but not necessarily all microbial
Disinfection Disinfection is the selective destruction of disease causing organisms (viruses, bacteria, protozoans). It destroys most recognized pathogenic microorganisms, but not necessarily all microbial
Effect of ph on sporicidal and microbicidal activity of buffered mixtures of alcohol and sodium hypochlorite
 Journal of Clinical Pathology, 1979, 32, 148-153 Effect of on sporicidal and microbicidal activity of buffered mixtures of alcohol and sodium hypochlorite JANET E. DEATH AND D. COATES From the Disinfection
Journal of Clinical Pathology, 1979, 32, 148-153 Effect of on sporicidal and microbicidal activity of buffered mixtures of alcohol and sodium hypochlorite JANET E. DEATH AND D. COATES From the Disinfection
CE 370. Disinfection. Location in the Treatment Plant. After the water has been filtered, it is disinfected. Disinfection follows filtration.
 CE 70 Disinfection 1 Location in the Treatment Plant After the water has been filtered, it is disinfected. Disinfection follows filtration. 1 Overview of the Process The purpose of disinfecting drinking
CE 70 Disinfection 1 Location in the Treatment Plant After the water has been filtered, it is disinfected. Disinfection follows filtration. 1 Overview of the Process The purpose of disinfecting drinking
BASU. Healthcare. Knowledge brings the greatest benefit
 BASU Healthcare Knowledge brings the greatest benefit Knowledge brings the greatest benefit BASU is a privately owned company in Austria. We design and manufacture high quality products using simple reagents.
BASU Healthcare Knowledge brings the greatest benefit Knowledge brings the greatest benefit BASU is a privately owned company in Austria. We design and manufacture high quality products using simple reagents.
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1)
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
A Selective Medium for Bacillus anthracis
 56 R~ORRIS, E. J. (955). J. gen. Microbiol. 3, 566 A Selective Medium for Bacillus anthracis BY E. J. MORRIS Microbiological Research Department, Ministry of Supply, Porton, Wiltshire SUMMARY: A medium
56 R~ORRIS, E. J. (955). J. gen. Microbiol. 3, 566 A Selective Medium for Bacillus anthracis BY E. J. MORRIS Microbiological Research Department, Ministry of Supply, Porton, Wiltshire SUMMARY: A medium
of the work reported here was to define the point in the developmental process at which the curing salts act to prevent outgrowth.
 APPLIED MICROBIOLOGY, Feb. 1968, p. 406-411 Copyright 1968 American Society for Microbiology Vol. 16, No. 2 Printed in U.S.A. Effect of Sodium Nitrite, Sodium Chloride, and Sodium Nitrate on Germination
APPLIED MICROBIOLOGY, Feb. 1968, p. 406-411 Copyright 1968 American Society for Microbiology Vol. 16, No. 2 Printed in U.S.A. Effect of Sodium Nitrite, Sodium Chloride, and Sodium Nitrate on Germination
Microbial Genetics, Mutation and Repair. 2. State the function of Rec A proteins in homologous genetic recombination.
 Answer the following questions 1. Define genetic recombination. Microbial Genetics, Mutation and Repair 2. State the function of Rec A proteins in homologous genetic recombination. 3. List 3 types of bacterial
Answer the following questions 1. Define genetic recombination. Microbial Genetics, Mutation and Repair 2. State the function of Rec A proteins in homologous genetic recombination. 3. List 3 types of bacterial
LABORATORY 7 ENDOSPORE STAIN AND BACTERIAL MOTILITY
 LABORATORY 7 ENDOSPORE STAIN AND BACTERIAL MOTILITY A. Endospore Stain B. Bacterial Motility A. ENDOSPORE STAIN DISCUSSION A few genera of bacteria, such as Bacillus and Clostridium have the ability to
LABORATORY 7 ENDOSPORE STAIN AND BACTERIAL MOTILITY A. Endospore Stain B. Bacterial Motility A. ENDOSPORE STAIN DISCUSSION A few genera of bacteria, such as Bacillus and Clostridium have the ability to
Chapter Chemical Elements Matter solid, liquid, and gas elements atoms. atomic symbol protons, neutrons, electrons. atomic mass atomic number
 Chapter 2 2.1 Chemical Elements 1. Matter is defined as anything that takes up space and has mass. 2. Matter exists in three states: solid, liquid, and gas. A. Elements 1. All matter (both living and non-living)
Chapter 2 2.1 Chemical Elements 1. Matter is defined as anything that takes up space and has mass. 2. Matter exists in three states: solid, liquid, and gas. A. Elements 1. All matter (both living and non-living)
Mechanisms of Actions of Hypochlorous Acid as Cleaning and Disinfecting Agents in Relation to Injury to Bacteria
 Jpn. J. Food Microbiol., 26(2), 76 80, 2009 Symposium 1 Control of microorganisms in stress environment Mechanisms of Actions of Hypochlorous Acid as Cleaning and Disinfecting Agents in Relation to Injury
Jpn. J. Food Microbiol., 26(2), 76 80, 2009 Symposium 1 Control of microorganisms in stress environment Mechanisms of Actions of Hypochlorous Acid as Cleaning and Disinfecting Agents in Relation to Injury
Game plan Lecture Lab Prelabs
 Game plan Lecture Binary fission Growth curves Physical requirements for growth Chemical requirements for growth Lab Lab Exam Prelabs Growth Curve Bring books and APO-3 for next class Microbial growth
Game plan Lecture Binary fission Growth curves Physical requirements for growth Chemical requirements for growth Lab Lab Exam Prelabs Growth Curve Bring books and APO-3 for next class Microbial growth
CHLORINE THEORY & MEASUREMENT
 CHLORINE THEORY & MEASUREMENT Introduction Chlorine, dissolved in liquid, is one of the most effective and economical germ-killers for the treatment of water to make it potable or safe to drink. Chlorine's
CHLORINE THEORY & MEASUREMENT Introduction Chlorine, dissolved in liquid, is one of the most effective and economical germ-killers for the treatment of water to make it potable or safe to drink. Chlorine's
affected by the ph of the medium, the dependence of the bacteriostasis by dyes
 THE BACTERICIDAL AND BACTERIOSTATIC ACTION OF CRYSTAL VIOLET C. E. HOFFMANN AND OTTO RAHN Bacteriological Laboratory, New York State College of Agriculture, Cornell University, Ithaca, N. Y. Received for
THE BACTERICIDAL AND BACTERIOSTATIC ACTION OF CRYSTAL VIOLET C. E. HOFFMANN AND OTTO RAHN Bacteriological Laboratory, New York State College of Agriculture, Cornell University, Ithaca, N. Y. Received for
Chapter 02 Testbank. 1. Anything that occupies space and has mass is called. A. an electron. B. living. C. matter. D. energy. E. space.
 Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Chapter 02 Testbank. 1. Anything that occupies space and has mass is called. A. an electron. B. living. C. matter. D. energy. E. space.
 Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Safety Manual > Incompatible Chemicals Partial Listing
 Safety Manual > Incompatible Chemicals Partial Listing C. Incompatible Chemicals Partial Listing Chemical Incompatible Chemicals Acetic acid Chromic acid, nitric acid, permanganates, and peroxides Acetic
Safety Manual > Incompatible Chemicals Partial Listing C. Incompatible Chemicals Partial Listing Chemical Incompatible Chemicals Acetic acid Chromic acid, nitric acid, permanganates, and peroxides Acetic
Glove Selection Chart
 Glove Selection Chart The following guide is a general guide for glove selection in relation to chemicals handled. The information presented here is believed to be accurate; however, we cannot guarantee
Glove Selection Chart The following guide is a general guide for glove selection in relation to chemicals handled. The information presented here is believed to be accurate; however, we cannot guarantee
Denaturation and renaturation of proteins
 Denaturation and renaturation of proteins Higher levels of protein structure are formed without covalent bonds. Therefore, they are not as stable as peptide covalent bonds which make protein primary structure
Denaturation and renaturation of proteins Higher levels of protein structure are formed without covalent bonds. Therefore, they are not as stable as peptide covalent bonds which make protein primary structure
Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives
 Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives PHILIP NEL VP TECHNICAL AND R&D RADICAL WATERS CITREX CHILE A NEW ECO-SANITISER Electrochemically Activated
Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives PHILIP NEL VP TECHNICAL AND R&D RADICAL WATERS CITREX CHILE A NEW ECO-SANITISER Electrochemically Activated
Microbiology: An Introduction, 12e (Tortora) Chapter 2 Chemical Principles. 2.1 Multiple Choice Questions
 Microbiology An Introduction 12th Edition Tortora TEST BANK Full download at: https://testbankreal.com/download/microbiology-an-introduction-12thedition-tortora-test-bank/ Microbiology An Introduction
Microbiology An Introduction 12th Edition Tortora TEST BANK Full download at: https://testbankreal.com/download/microbiology-an-introduction-12thedition-tortora-test-bank/ Microbiology An Introduction
INTRODUCTION. Gram Stain
 INTRODUCTION In microbiology, organisms are so small that additional techniques are often required for proper viewing under the microscope. Cytological stains, or dyes that stain cells or cellular features,
INTRODUCTION In microbiology, organisms are so small that additional techniques are often required for proper viewing under the microscope. Cytological stains, or dyes that stain cells or cellular features,
Microbiology. Definition of a Microorganism. Microorganisms in the Lab. The Study of Microorganisms
 Microbiology The Study of Microorganisms Definition of a Microorganism Derived from the Greek: Mikros, «small» and Organismos, organism Microscopic organism which is single celled (unicellular) or a mass
Microbiology The Study of Microorganisms Definition of a Microorganism Derived from the Greek: Mikros, «small» and Organismos, organism Microscopic organism which is single celled (unicellular) or a mass
INTERNAL STRUCTURE Cytoplasmic membrane peripheral integral
 INTERNAL STRUCTURE Cytoplasmic membrane Under the cell wall and generally same structure with bacteria It consists of two layers On the surface of periplasmic space and cytoplasm, protein and phospholipid
INTERNAL STRUCTURE Cytoplasmic membrane Under the cell wall and generally same structure with bacteria It consists of two layers On the surface of periplasmic space and cytoplasm, protein and phospholipid
DISINFECTION IN A DAIRY MILKING PARLOUR USING ANOLYTE AS DISINFECTION
 DISINFECTION IN A DAIRY MILKING PARLOUR USING ANOLYTE AS DISINFECTION Prof T E Cloete and M S Thantsha, Department of Microbiology and Plant Pathology, University of Pretoria, South Africa INTRODUCTION
DISINFECTION IN A DAIRY MILKING PARLOUR USING ANOLYTE AS DISINFECTION Prof T E Cloete and M S Thantsha, Department of Microbiology and Plant Pathology, University of Pretoria, South Africa INTRODUCTION
CH 221 Chapter Four Part II Concept Guide
 CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
Solutions With Formaldehyde-Water Solutions
 APPLIED MICROBIOLOGY Vol., No., p. 9- May, 96 Copyright 96 American Society for Microbiology Printed in U.S.A. Comparison of Sterilizing Properties of Formaldehyde-Methanol Solutions With Formaldehyde-Water
APPLIED MICROBIOLOGY Vol., No., p. 9- May, 96 Copyright 96 American Society for Microbiology Printed in U.S.A. Comparison of Sterilizing Properties of Formaldehyde-Methanol Solutions With Formaldehyde-Water
Exercise 6-B STAINING OF MICROORGANISMS GRAM STAIN
 Exercise 6-B STAINING OF MICROORGANISMS GRAM STAIN Introduction The Gram stain, developed by Hans Christian Gram in 1884, is a staining technique allowing different types of microorganisms (usually bacteria)
Exercise 6-B STAINING OF MICROORGANISMS GRAM STAIN Introduction The Gram stain, developed by Hans Christian Gram in 1884, is a staining technique allowing different types of microorganisms (usually bacteria)
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. C is FALSE?
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about the atom 12 6 C is FALSE? 1) A) It has 12 neutrons
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about the atom 12 6 C is FALSE? 1) A) It has 12 neutrons
Sanitising wash water
 Sanitising wash water The issue Wash water sanitisers can prevent cross-contamination but they cannot reversecontamination and fresh produce cannot be entirely decontaminated. Listeria,salmonella and E.
Sanitising wash water The issue Wash water sanitisers can prevent cross-contamination but they cannot reversecontamination and fresh produce cannot be entirely decontaminated. Listeria,salmonella and E.
Growth from Spores of Clostridium perfringens
 APpuE MicRoBioLOGY, Feb. 1970, p. 353-359 Copyright 1970 American Society for Microbiology Vol. 19, No. 2 Printed in U.S.A. Growth from Spores of Clostridium perfringens in the Presence of Sodium Nitrite'
APpuE MicRoBioLOGY, Feb. 1970, p. 353-359 Copyright 1970 American Society for Microbiology Vol. 19, No. 2 Printed in U.S.A. Growth from Spores of Clostridium perfringens in the Presence of Sodium Nitrite'
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
 ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
Basic Chemistry. Chapter 2 BIOL1000 Dr. Mohamad H. Termos
 Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
CHMA2000 EXPT 7: The Physical and Chemical Properties of Alcohols
 CHMA2000 EXPT 7: The Physical and Chemical Properties of Alcohols Objectives: At the end of this experiment you should be able to: 1. Understand the physical and chemical properties of alcohols 2. Understand
CHMA2000 EXPT 7: The Physical and Chemical Properties of Alcohols Objectives: At the end of this experiment you should be able to: 1. Understand the physical and chemical properties of alcohols 2. Understand
Chemical Oxidation Oxidizing agents
 Chemical Oxidation CENG 4710 Environmental Control Chemical oxidation is used to detoxify waste by adding an oxidizing agent to chemically transform waste compounds. It is capable of destroying a wide
Chemical Oxidation CENG 4710 Environmental Control Chemical oxidation is used to detoxify waste by adding an oxidizing agent to chemically transform waste compounds. It is capable of destroying a wide
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1)
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
Unit 6 Solids, Liquids and Solutions
 Unit 6 Solids, Liquids and Solutions 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Unit 6 Solids, Liquids and Solutions 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
CH 2: SOLUTIONS
 1 CH 2: SOLUTIONS 2 SOLUTION, SOLVENT, SOLUTE Solutions are homogeneous mixtures of two or more than two components. i.e. composition and properties are uniform throughout the mixture. Eg: The component
1 CH 2: SOLUTIONS 2 SOLUTION, SOLVENT, SOLUTE Solutions are homogeneous mixtures of two or more than two components. i.e. composition and properties are uniform throughout the mixture. Eg: The component
PAPER 3: FINGERPRINTS AND OTHER IMPRESSIONS MODULE 16: Detection of Blood Fingerprints
 Subject FORENSIC SCIENCE Paper No and Title Module No and Title Module Tag Paper 3; Fingerprints and Other Impressions, Including Biometry Module 16; Detection of blood prints FSC_P3_M16 TABLE OF CONTENTS
Subject FORENSIC SCIENCE Paper No and Title Module No and Title Module Tag Paper 3; Fingerprints and Other Impressions, Including Biometry Module 16; Detection of blood prints FSC_P3_M16 TABLE OF CONTENTS
# Ans Workings / Remarks
 # Ans Workings / Remarks 1 B Atomic mass and temperature affects the rate of diffusion of gas. The lower the atomic mass, the lighter the substance. The higher the temperature, the higher the rate of collision
# Ans Workings / Remarks 1 B Atomic mass and temperature affects the rate of diffusion of gas. The lower the atomic mass, the lighter the substance. The higher the temperature, the higher the rate of collision
Evaluation of the efficiency of Mxxxx as a barrier against microrganisms crossing
 Evaluation of the efficiency of as a barrier against microrganisms crossing A) composition of filter The filter of has the following characteristics: 1. An outer layer, which is composed by a medical,
Evaluation of the efficiency of as a barrier against microrganisms crossing A) composition of filter The filter of has the following characteristics: 1. An outer layer, which is composed by a medical,
What Are Atoms? Chapter 2: Atoms, Molecules & Life
 Chapter 2: Atoms, Molecules & Life What Are Atoms? An atom are the smallest unit of matter. Atoms are composed of Electrons = negatively charged particles. Neutrons = particles with no charge (neutral).
Chapter 2: Atoms, Molecules & Life What Are Atoms? An atom are the smallest unit of matter. Atoms are composed of Electrons = negatively charged particles. Neutrons = particles with no charge (neutral).
THE REVERSE SELECTIVE BACTERIOSTATIC ACTION OF ACID FUCHSIN.
 THE REVERSE SELECTIVE BACTERIOSTATIC ACTION OF ACID FUCHSIN. BY JOHN W. CHURCHMAN, M.D. (From the Department of ygiene of Cornell University Medical College, New York.) PLATES 1 TO 3. (Received for publication,
THE REVERSE SELECTIVE BACTERIOSTATIC ACTION OF ACID FUCHSIN. BY JOHN W. CHURCHMAN, M.D. (From the Department of ygiene of Cornell University Medical College, New York.) PLATES 1 TO 3. (Received for publication,
Sporicidal activity of hospital disinfectants
 J. clin. Path., 1974, 27, 632-638 Sporicidal activity of hospital disinfectants J. C. KELSEY, I. H. MACKINNON, AND ISOBEL M. MAURER From the Disinfection Reference Laboratory, Central Public Health Laboratory,
J. clin. Path., 1974, 27, 632-638 Sporicidal activity of hospital disinfectants J. C. KELSEY, I. H. MACKINNON, AND ISOBEL M. MAURER From the Disinfection Reference Laboratory, Central Public Health Laboratory,
GCE O' LEVEL PURE CHEMISTRY (5073/02) Suggested Answers for 2016 O Level Pure Chemistry Paper 2
 Section A (50 M) Aa) trend The number of electron shell increases The number of valence electrons increases Proton number increases There is a change in character from metallic to non-metallic Only true
Section A (50 M) Aa) trend The number of electron shell increases The number of valence electrons increases Proton number increases There is a change in character from metallic to non-metallic Only true
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
General Chemistry A
 General Chemistry 1140 - A May 6, 2004 (6 Pages, 43 Parts) Name Each of the 40 multiple choice questions counts 2 point. Give the letter of the correct answer. 1. 2. Crystalline solids differ from amorphous
General Chemistry 1140 - A May 6, 2004 (6 Pages, 43 Parts) Name Each of the 40 multiple choice questions counts 2 point. Give the letter of the correct answer. 1. 2. Crystalline solids differ from amorphous
Chemical Storage According to Compatibility
 Chemical Storage According to Compatibility To lessen risk of exposure to hazardous chemicals, all chemicals should be separated and stored according to hazard category and compatibility. *Storage Groups
Chemical Storage According to Compatibility To lessen risk of exposure to hazardous chemicals, all chemicals should be separated and stored according to hazard category and compatibility. *Storage Groups
Essential Knowledge. 2.A.3 Organisms must exchange matter with the environment to grow, reproduce and maintain organization
 Ch3: Water Essential Knowledge 2.A.3 Organisms must exchange matter with the environment to grow, reproduce and maintain organization a. Molecules and atoms from the environment are necessary to build
Ch3: Water Essential Knowledge 2.A.3 Organisms must exchange matter with the environment to grow, reproduce and maintain organization a. Molecules and atoms from the environment are necessary to build
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
The Effect of Chlorine on Spores of Clostridium biyermentans, Bacillus subtilis and Bacillus cereus
 Journal of General Microbiology (1975), 89,337-344 Printed in Great Britain 337 The Effect of Chlorine on Spores of Clostridium biyermentans, Bacillus subtilis and Bacillus cereus By LINDA R. WYATT AND
Journal of General Microbiology (1975), 89,337-344 Printed in Great Britain 337 The Effect of Chlorine on Spores of Clostridium biyermentans, Bacillus subtilis and Bacillus cereus By LINDA R. WYATT AND
General concepts, history. Microscopy and staining. Review Questions-1
 Review Questions-1 General concepts, history What was the technique that Carl Woese used to identify another domain to classify m/o in? How did Pasteur help resolve the debate on spontaneous generation?
Review Questions-1 General concepts, history What was the technique that Carl Woese used to identify another domain to classify m/o in? How did Pasteur help resolve the debate on spontaneous generation?
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. Which salt is colorless? (A) KMn 4 (B) BaS 4 (C) Na 2 Cr 4 (D) CoCl 2 2. Which 0.10 M aqueous solution exhibits the lowest
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. Which salt is colorless? (A) KMn 4 (B) BaS 4 (C) Na 2 Cr 4 (D) CoCl 2 2. Which 0.10 M aqueous solution exhibits the lowest
Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT?
 1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
Chapter 12. Preview. Objectives Solutions Suspensions Colloids Solutes: Electrolytes Versus Nonelectrolytes
 Preview Objectives Solutions Suspensions Colloids Solutes: Electrolytes Versus Nonelectrolytes Section 1 Types of Mixtures Objectives Distinguish between electrolytes and nonelectrolytes. List three different
Preview Objectives Solutions Suspensions Colloids Solutes: Electrolytes Versus Nonelectrolytes Section 1 Types of Mixtures Objectives Distinguish between electrolytes and nonelectrolytes. List three different
Physical Pharmacy PHR 211. Lecture 1. Solubility and distribution phenomena.
 Physical Pharmacy PHR 211 Lecture 1 Solubility and distribution phenomena. Course coordinator Magda ELMassik, PhD Assoc. Prof. of Pharmaceutics 1 Objectives of the lecture After completion of thislecture,
Physical Pharmacy PHR 211 Lecture 1 Solubility and distribution phenomena. Course coordinator Magda ELMassik, PhD Assoc. Prof. of Pharmaceutics 1 Objectives of the lecture After completion of thislecture,
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
CHEM 261 Dec 4, 2017
 200 CEM 261 Dec 4, 2017 REVIEW: 1. N 3 2 S 4 1. 2 Al 3 N 2 2. 2 Al 3 2. N 3 2 S 4 N 2 I II For the left hand reaction, I - to create a meta positioned, the first molecule to be subsituted should be N 2,
200 CEM 261 Dec 4, 2017 REVIEW: 1. N 3 2 S 4 1. 2 Al 3 N 2 2. 2 Al 3 2. N 3 2 S 4 N 2 I II For the left hand reaction, I - to create a meta positioned, the first molecule to be subsituted should be N 2,
Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the study of physiology because
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
Catalytic bead sensors are used primarily to detect
 Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
The Influence of Magnesium on Cell Division
 480 WEBB, M. (1951). J. gen. Mimobiol. 5, 480-484. The Influence of Magnesium on Cell Division 4. The Specificity of Magnesium BY M. WEBB Chemistry Department, The University, Edgbaston, Birmingham 15,
480 WEBB, M. (1951). J. gen. Mimobiol. 5, 480-484. The Influence of Magnesium on Cell Division 4. The Specificity of Magnesium BY M. WEBB Chemistry Department, The University, Edgbaston, Birmingham 15,
Quat Absorption Onto Textiles
 Quat Absorption Onto Textiles Introduction Proper disinfection is a function of different variables, such as the concentration of disinfectant applied to surfaces, disinfectant interaction with wipes and
Quat Absorption Onto Textiles Introduction Proper disinfection is a function of different variables, such as the concentration of disinfectant applied to surfaces, disinfectant interaction with wipes and
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Gas Laws. Bonding. Solutions M= moles solute Mass %= mass solute x 100. Acids and Bases. Thermochemistry q = mc T
 Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
Name Period Teacher Practice Test: OTHS Academic Chemistry Spring Semester 2017 The exam will have 100 multiple choice questions (1 point each) Formula sheet (see below) and Periodic table will be provided
Compound. Math Focus. What are compounds? What is a chemical reaction? How are compounds used in everyday life?
 CHAPTER 3 2 Compounds SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What are compounds? What is a chemical reaction?
CHAPTER 3 2 Compounds SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What are compounds? What is a chemical reaction?
Chemistry 20 Lesson 17 Solubility
 Chemistry 20 Lesson 17 Solubility The ability of one compound to dissolve in another compound is called solubility. The term solubility can be used in two senses, qualitatively and quantitatively. Qualitatively,
Chemistry 20 Lesson 17 Solubility The ability of one compound to dissolve in another compound is called solubility. The term solubility can be used in two senses, qualitatively and quantitatively. Qualitatively,
8/24/2018. Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry. Chapter 2: Essential Chemistry for Biology
 1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
1. How many grams of gas are present in 1.50 L of hydrogen peroxide, H 2 O 2 (g), at STP?
 Chemistry 11 Fall 2010 Examination #2 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2010 Examination #2 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Physical pharmacy. The Gaseous State. dr basam al zayady. States of matter
 Physical pharmacy Lec 5 dr basam al zayady States of matter The Gaseous State Gas molecules have vigorous and rapid motion that result in collisions not only with one another but also with the walls of
Physical pharmacy Lec 5 dr basam al zayady States of matter The Gaseous State Gas molecules have vigorous and rapid motion that result in collisions not only with one another but also with the walls of
Chapter 1. Matter. 1.1 What is Chemistry. 1.2 The Scientific Method:
 Chapter 1. Matter 1.1 What is Chemistry CHEMISTRY The study of the structure, composition, properties and reactions of matter and the energy changes associated with matter. In other words the study of
Chapter 1. Matter 1.1 What is Chemistry CHEMISTRY The study of the structure, composition, properties and reactions of matter and the energy changes associated with matter. In other words the study of
Physical Science Lecture Notes Chapters 17, 18 & 19
 Physical Science Lecture Notes Chapters 17, 18 & 19 I. 17-1: Matter & Its Changes a. Changes in matter i. Physical Changes Alters form or appearance but doesn t change it into another substance ie. Water
Physical Science Lecture Notes Chapters 17, 18 & 19 I. 17-1: Matter & Its Changes a. Changes in matter i. Physical Changes Alters form or appearance but doesn t change it into another substance ie. Water
EXPERIMENT NINE Part I - The Standardization of Thiosulfate Solutions
 EXPERIMENT NINE Part I - The Standardization of Thiosulfate Solutions In general, thiosulfate solutions are standardized by indirect methods, Primary-standard oxidizing agents such as KIO 3, As 2 O 3,
EXPERIMENT NINE Part I - The Standardization of Thiosulfate Solutions In general, thiosulfate solutions are standardized by indirect methods, Primary-standard oxidizing agents such as KIO 3, As 2 O 3,
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS
 CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS CHEMICAL REACTIONS Occurs when matter combines or breaks apart to produce new kinds of matter with different properties with a change in energy. EVIDENCE FOR
CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS CHEMICAL REACTIONS Occurs when matter combines or breaks apart to produce new kinds of matter with different properties with a change in energy. EVIDENCE FOR
Differential Response of Various Spore Species to Sporicidal Disinfectants
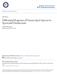 Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2007-08-13 Differential Response of Various Spore Species to Sporicidal Disinfectants Michael David Pratt Brigham Young University
Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2007-08-13 Differential Response of Various Spore Species to Sporicidal Disinfectants Michael David Pratt Brigham Young University
Alcohols, Phenols and Ethers
 SUBJECTIVE PROBLEMS: Alcohols, Phenols and Ethers Q1. An organic liquid (A), containing C, H and O with boiling point: 78 o C, and possessing a rather pleasant odour, on heating with concentrated sulphuric
SUBJECTIVE PROBLEMS: Alcohols, Phenols and Ethers Q1. An organic liquid (A), containing C, H and O with boiling point: 78 o C, and possessing a rather pleasant odour, on heating with concentrated sulphuric
may contain one or more neutrons
 Biology 115 Fall 2001 Campos/Saupe Atoms and Molecules I. Introduction - living things are composed of the same chemical elements as the nonliving world and obey the same physical and chemical laws - living
Biology 115 Fall 2001 Campos/Saupe Atoms and Molecules I. Introduction - living things are composed of the same chemical elements as the nonliving world and obey the same physical and chemical laws - living
Effect of Several Environmental Conditions on the "Thermal Death Rate" of Endospores of Aerobic, Thermophilic Bacteria
 APPLIED MICROBIOLOGY, Nov., 1965 Copyright 1965 American Society for Microbiology Vol. 13, No. 6 Printed in U.S.A. Effect of Several Environmental Conditions on the "Thermal Death Rate" of Endospores of
APPLIED MICROBIOLOGY, Nov., 1965 Copyright 1965 American Society for Microbiology Vol. 13, No. 6 Printed in U.S.A. Effect of Several Environmental Conditions on the "Thermal Death Rate" of Endospores of
Green Chemistry: A Greener Clean
 Green Chemistry: A Greener Clean Chicago ACS Chemistry Day Mary Kirchhoff Green Chemistry Institute What is Green Chemistry? Green Chemistry is the design of chemical products and processes that reduce
Green Chemistry: A Greener Clean Chicago ACS Chemistry Day Mary Kirchhoff Green Chemistry Institute What is Green Chemistry? Green Chemistry is the design of chemical products and processes that reduce
CHEMICAL OXIDATION. The use of oxidizing agents without the need of microorganisms for the reactions to proceed
 CHEMICAL OXIDATION The use of oxidizing agents without the need of microorganisms for the reactions to proceed oxidizing agents : O 3, H 2 O 2, Cl 2 or HOCl or O 2 etc catalysts : ph, transition metals,
CHEMICAL OXIDATION The use of oxidizing agents without the need of microorganisms for the reactions to proceed oxidizing agents : O 3, H 2 O 2, Cl 2 or HOCl or O 2 etc catalysts : ph, transition metals,
CHEMICAL COMPOUNDS. There are 92 different elements that occur naturally on the earth. The 3 most common elements in the Human Body are:
 BIOLOGY 12 CEMICAL COMPOUNDS NAME: CELL COMPOUNDS TE CEMICAL COMPONENTS OF MATTER To understand the nature of the substances found in cells, it is necessary to be familiar with the substances that make
BIOLOGY 12 CEMICAL COMPOUNDS NAME: CELL COMPOUNDS TE CEMICAL COMPONENTS OF MATTER To understand the nature of the substances found in cells, it is necessary to be familiar with the substances that make
Lecture 1 Solubility and Distribution Phenomena
 Physical Pharmacy Lecture 1 Solubility and Distribution Phenomena Assistant Lecturer in Pharmaceutics Overview Solubility Phenomena Introduction Solute-Solvent Interactions Solubility of gas in liquid
Physical Pharmacy Lecture 1 Solubility and Distribution Phenomena Assistant Lecturer in Pharmaceutics Overview Solubility Phenomena Introduction Solute-Solvent Interactions Solubility of gas in liquid
YEAR 10- Chemistry Term 1 plan
 YEAR 10- Chemistry Term 1 plan 2016-2017 Week Topic Learning outcomes 1 1. The particulate nature of matter State the distinguishing properties of solids, liquids and gases. Describe the structure of solids,
YEAR 10- Chemistry Term 1 plan 2016-2017 Week Topic Learning outcomes 1 1. The particulate nature of matter State the distinguishing properties of solids, liquids and gases. Describe the structure of solids,
Test Booklet. Subject: SC, Grade: HS CST High School Chemistry Part 2. Student name:
 Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
EFFECT OF ph AND AMMONIUM IONS ON THE PERMEABILITY
 EFFECT OF ph AND AMMONIUM IONS ON THE PERMEABILITY OF BACILLUS PASTEURII W. R. WILEY AND J. L. STOKES Department of Bacteriology and Public Health, Washington State University, Pullman, Washington ABSTRACT
EFFECT OF ph AND AMMONIUM IONS ON THE PERMEABILITY OF BACILLUS PASTEURII W. R. WILEY AND J. L. STOKES Department of Bacteriology and Public Health, Washington State University, Pullman, Washington ABSTRACT
PETE 203: Properties of oil
 PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
Biology 30 The Chemistry of Living Things
 Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
media), except those of aluminum and calcium
 1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
EDUCATIONAL COMMENTARY GRAM STAIN
 EDUCATIONAL COMMENTARY GRAM STAIN Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on the Continuing
EDUCATIONAL COMMENTARY GRAM STAIN Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on the Continuing
