Swarming is defined as flagellum-driven bacterial group motility
|
|
|
- Calvin Bryant
- 6 years ago
- Views:
Transcription
1 MINIREVIEW Swarming: Flexible Roaming Plans Jonathan D. Partridge, Rasika M. Harshey Section of Molecular Genetics and Microbiology and Institute of Cellular and Molecular Biology, University of Texas at Austin, Austin, Texas, USA Movement over an agar surface via swarming motility is subject to formidable challenges not encountered during swimming. Bacteria display a great deal of flexibility in coping with these challenges, which include attracting water to the surface, overcoming frictional forces, and reducing surface tension. Bacteria that swarm on hard agar surfaces (robust swarmers) display a hyperflagellated and hyperelongated morphology. Bacteria requiring a softer agar surface (temperate swarmers) do not exhibit such a dramatic morphology. For polarly flagellated robust swarmers, there is good evidence that restriction of flagellar rotation somehow signals the induction of a large number of lateral flagella, but this scenario is apparently not relevant to temperate swarmers. Swarming bacteria can be further subdivided by their requirement for multiple stators (Mot proteins) or a statorassociated protein (FliL), secretion of essential polysaccharides, cell density-dependent gene regulation including surfactant synthesis, a functional chemotaxis signaling pathway, appropriate cyclic (c)-di-gmp levels, induction of virulence determinants, and various nutritional requirements such as iron limitation or nitrate availability. Swarming strategies are as diverse as the bacteria that utilize them. The strength of these numerous designs stems from the vantage point they offer for understanding mechanisms for effective colonization of surface niches, acquisition of pathogenic potential, and identification of environmental signals that regulate swarming. The signature swirling and streaming motion within a swarm is an interesting phenomenon in and of itself, an emergent behavior with properties similar to flocking behavior in diverse systems, including birds and fish, providing a convenient new avenue for modeling such behavior. Swarming is defined as flagellum-driven bacterial group motility on a surface (1). Beyond this common definition, the strategies for effective movement are as varied as the bacteria themselves (2 6). Swarming was originally observed on the surface of media solidified with agar in petri dishes, and the agar surface remains the surface of choice for studying this form of motility. The remarkably similar appearances of moving bacterial colonies on an agar surface, where groups of cells (referred to also as rafts or packs) move together side by side with individual groups continuously forming and reforming and with cohesion of the group being important for motility of individuals within the group, suggest that common attributes, such as cell density, cell shape, and flagellar mechanics in a water-restricted surface environment, rather than cellcell signaling, likely dictate the emergent group dynamics seen in all swarming bacteria (see Movies S1 to S4 in the supplemental material; swarming movies are also available at /faculty/weibel/lab/gallery/movies.aspx). However, under compatible laboratory conditions, the requirements for initiating movement in different bacteria vary, likely reflecting specific adaptations for a surface niche that the bacteria naturally inhabit. Understanding these varied requirements is important for anticipating the sorts of challenges a solid surface might present for bacterial movement, how bacteria cope with these challenges, and how they integrate environmental signals to assess whether to swarm. Given that many swarmers are adapted for a pathogenic state, this knowledge is relevant not only for host invasion but also for other surface-specific behaviors, such as biofilm formation, and will eventually find applications in controlling the successful establishment and spread of bacterial surface communities. WHAT IS DIFFERENT BETWEEN SWIMMING AND SWARMING HABITATS? Swimming motility has been best studied in Escherichia coli, a bacterium that is 1 mby2 m in size when grown in aqueous medium. When individual bacteria swim in bulk liquid, they move by rotating several helical flagellar filaments, each driven at its base by a rotary motor (7 9). The rotating helical filament creates a viscous slip down the long axis of the helix, displacing the water. Forward motion is attained when the motors turn counterclockwise (CCW), sweeping the individual filaments into a bundle that exerts enough thrust to propel the cell to run at speeds averaging 30 m/s in water. The cell changes course when one or more motors turn clockwise (CW), often generating a tumble. Swimming cells can perform chemotaxis by moving up or down chemical gradients, using an elaborate signaling system that modulates the CCW/CW bias of the motors (10). Swarming motility is flagellum driven and must therefore generate forward motion in the same manner as swimming, with helical filaments pushing against the surrounding water (11, 12). Water is therefore the most critical element for swarming, but it is trapped within the agar gel; there is no free water at the surface of the gel (13, 14). Therefore, the foremost challenge for bacteria is to attract sufficient water to the surface to fully immerse the cells whose height, as they lie lengthwise on the surface, is 1 m. The sensitivity of swarming to moist conditions is explained by this requirement for water (2, 6). A second obstacle the bacteria will encounter as they attempt to move is surface friction, defined as an electrostatic force between two surfaces sliding past each other. The magnitude of this force depends on the material properties of Published ahead of print 21 December 2012 Address correspondence to Rasika M. Harshey, rasika@uts.cc.utexas.edu. Supplemental material for this article may be found at /JB Copyright 2013, American Society for Microbiology. All Rights Reserved. doi: /jb March 2013 Volume 195 Number 5 Journal of Bacteriology p jb.asm.org 909
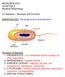
2 FIG 1 Multiple swarming strategies. See the text for details. the surfaces and whether they are dry or coated in viscous or in lubricated fluid (15). Once bacteria are bathed in water/fluid, their movement will create a viscous drag, which will also offer frictional resistance to movement (16). There are no measurements currently available for the magnitude of these forces in swarming bacteria. To overcome frictional resistance, the bacteria must lubricate the cell-surface interface, reduce charge, and/or generate more thrust by increasing flagellar motor power. This is the second critical requirement for swarming. Once bacteria overcome the first two obstacles and initiate movement, the moving fluid will encounter surface tension due to the tendency of water to adhere to itself, impeding wetting of the virgin (uncolonized) territory ahead of the advancing colony (17). This third substantial impediment is overcome either by bacterial production of surfactants or by a substrate with an inherently low surface tension. Swarming is therefore a daunting proposition compared to swimming. The challenges for surface navigation are summarized in Fig. 1. The fact that so many bacterial species display this form of motility in the laboratory, and therefore possess mechanisms to override surface impediments, argues that swarming must be an important means of invading more territory in the bacteria s natural habitats. BACTERIAL MECHANISMS FOR ENABLING SURFACE NAVIGATION Bacterial adaptations/mechanisms for overcoming surface challenges are summarized in Fig. 1. The three main challenges shown are derived from conditions present on an agar surface, the only substrate on which swarming has been studied. The reader should be mindful of the possibility that in native swarming habitats, the rankings reported below may change in importance or be subsumed into each other if swarming occurs on submerged solidliquid interfaces or surfaces where frictional forces are minimal. (i) Attracting water to the surface. When bacteria grown in liquid media are transferred to the surface of swarm media, they all exhibit a lag before swarming begins. Reasons for the lag have been deduced to include expression of swarming-specific functions and/or attainment of a confluent and dense arrangement of cells (for specific references, see reference 6 and references cited therein). The former might include accumulation of osmotic agents to help extract water from the agar beneath. The extracellular matrix (ECM) of Proteus mirabilis shows the presence of polysaccharides and the osmolyte glycine betaine, both of which have been linked to hydration (18). The essential swarming function of an acidic polysaccharide Cmf (colony migration factor) secreted by P. mirabilis is also hypothesized to aid colony hydration (19 21). The gene controlling Cmf production is related to sugar transferases required for lipopolysaccharide (LPS) core modification and is located near genes involved in the synthesis of LPS O side chains, enterobacterial common antigen (ECA), and other outer polysaccharides (19). The LPS O antigen and ECA are surmised to serve similar functions during swarming by E. coli and Salmonella (22, 23). (See alternate explanations for the role of these molecules in the next section.) Transcriptome analysis of Salmonella found that irrespective of whether bacteria were propagated on hard (nonswarming) or soft (swarming) agar, a large number of genes showed surface-specific upregulation, including those for LPS synthesis (24). Thus, the altered metabolome on a surface includes increased synthesis of LPS, which the bacteria might exploit to promote swarming (25). Transcriptome studies of Proteus and of several other bacteria have not revealed a swarm- 910 jb.asm.org Journal of Bacteriology
3 ing-specific alteration in synthesis of these molecules (26 28). We note, however, that swarmers upregulate the synthesis of the osmoprotectants glutamate and proline in Pseudomonas aeruginosa (26), potassium uptake in Bacillus cereus (29), and a sodium/solute symporter in Vibrio parahaemolyticus (30). It is possible that these or other osmotic agents are also secreted by posttranscriptional signaling mechanisms that sense the surface, or as part of a general bacterial metabolic program. In the latter scenario, the lag period spent in increasing cell density would concomitantly be spent increasing the external accumulation of osmolytes (22). Observations that are apparently contrary to the proposed involvement of the lag period in attracting water include reports showing continued swarming of a P. mirabilis colony when replica printed on fresh medium (31) or shortening/abolition of the lag by artificial upregulation of the flagellar master regulators (32 34). It is possible that in the former case, the ECM is transferred along with the bacteria to the fresh plate, and in the latter, upregulation of a flagellar master regulator known to control the expression of other regulators (35, 36) induced sugar transport or other pathways that increase colony hydration (37 39). In summary, Gram-negative bacteria appear to use osmolytes, polysaccharides, LPS, and ECA to draw water toward the cells. The synthesis of some of these molecules is increased during surface growth in organisms in which such comparisons have been made. Solute transport systems show swarming-specific upregulation in some bacteria, and it is likely that other as-yet-unidentified osmolytes play an important role in both Gram-negative and Grampositive bacteria. New technologies using mass spectral techniques for detecting metabolites directly on the surface of bacterial colonies should aid in their identification (40). (ii) Overcoming frictional forces. Interaction of surfaces in relative motion is influenced by their design, friction, adhesion, lubrication, and wear; the study of this interaction is a specialized branch of engineering called tribology, with bio-tribology being a large subfield (41, 42). Examples of moving surfaces in biology range in scale from bacterial flagellar motors rotating in the membrane, to diatoms sliding past each other, to bone/cartilage joints. Swarming bacteria are another example of moving surfaces whose tribology has not yet been explored. The frictional forces they experience may arise from charge interactions between the agar and bacterial surfaces and/or from viscous drag of the fluid between the agar and the moving bacteria (16). The agar surface is not completely hydrophilic, as judged by the faster spreading of a drop of water placed on its surface if the drop includes surfactants (13, 14), suggesting that charge interactions may contribute to friction. In this context, the nonswarming phenotypes of LPS and Cmf mutants discussed above could also be due to alterations of cell surface charge. Nanoparticle tribology suggests that lubrication of surfaces reduces frictional forces (15). Biosurfactants are lubricants, as their amphipathic properties allow them to straddle hydrophobic and hydrophilic surfaces. Molecules with surfactant properties include LPS in Gram-negative bacteria (43), rhamnolipids and their derivatives in Pseudomonas (44), and lipopeptides in both Gram-negative (Serratia) and Gram-positive (Bacillus) bacterial species (44). These molecules are either attached to the bacterial surface or secreted into the medium, and so it is safe to assume that they will alter the interactive surface between the bacteria and the agar and perhaps contribute to lowering friction during swarming. Frictional resistance may also be overcome by increasing force (45). Flagellar motors generate the force that moves a bacterium through water. Moving on a surface is likely to require more force to overcome the frictional resistance, but there are no studies measuring the magnitude of this resistance. However, we can infer that swarming bacteria encounter frictional resistance during swarming because they require more flagella (hence more motors), a special set of stators that increase power to the motor, or a specific stator-associated protein (FliL) that likely provides more power to existing motors. In order to appreciate the contribution of flagellar propulsive power to surface motility, we divide swarming bacteria into two categories: robust swarmers, which can navigate across a hard agar surface (1.5% agar and above), and temperate swarmers, which can swarm only on a softer agar surface (0.5 to 0.8% agar). Robust swarmers include polarly flagellated bacteria (which induce peritrichous flagellation upon surface contact), such as Azospirillum, Rhodospirillum, and Vibrio species, as well as the peritrichously flagellated Proteus species. Temperate swarmers include E. coli and Bacillus, Pseudomonas, Rhizobium, Salmonella, Serratia, and Yersinia species. Robust swarmers synthesize many more flagella than temperate swarmers. Increasing flagellar numbers in temperate swarmers allows them to swarm on harder agar (see our accompanying article [33]), and increasing these numbers in robust swarmers allows them to swim through more viscous medium (46). Viscosity represents liquid friction (45). Thus, one may infer that frictional forces are greater on harder agar and that at least one function of higher flagellar numbers in robust swarmers is to provide more thrust for moving on this surface. Robust swarmers also undergo a substantial increase in cell length ( 30 m); this adaptation could provide greater surface area to accommodate the increased numbers of flagella, increase stride (i.e., generate longer steps), minimize drag, or promote lengthwise alignment of cells into rafts (12). Temperate swarmers do not generally overproduce flagella and show only modest increases in cell length (33, 36). Those that do increase flagellar numbers (e.g., B. subtilis) do not increase them to the extent seen with robust swarmers (36). Swarmers that do not show increased flagellar numbers display a requirement for special stators or stator-associated proteins. For example, P. aeruginosa has five stator proteins (MotAB, MotCD, and MotY), which likely associate into two sets of stators, whereas only two such proteins (MotAB) drive swimming motility in E. coli and Salmonella. While swimming is enabled by either set of stators in P. aeruginosa, swarming requires a combination of these (47, 48). The stator set required for swarming in P. aeruginosa also enables swimming in a highly viscous solution (47). Therefore, the stators that permit swarming are the ones that can work more effectively against friction and must transmit more power to the motor. This does not mean that the swarming fluid is more viscous (to date, there have been no measurements of fluid viscosity within the swarm). In fact, the similar swarming and swimming speeds of E. coli would suggest that the swarming fluid is not more viscous for this bacterium (2, 49). E. coli and Salmonella, which have only one stator set (MotAB) that enables swimming, absolutely require an additional protein called FliL for swarming, deduced to allow the flagellar rod to withstand higher torque on swarm agar (50). FliL appears to be associated with the MotAB stators in Borrelia burgdorferi, as determined by cryo-electron tomography (51). When overexpressed together with the stators in Salmonella, FliL allows swarming on harder agar surfaces in the absence of an increase in flagellar/motor num- March 2013 Volume 195 Number 5 jb.asm.org 911
4 bers and is also more effective for swimming against fluids with increased viscosity (33). This stator relationship is seen in swimming as well, with loss of FliL causing an 20% reduction in swim speeds (50). Thus, in addition to providing structural support, FliL is likely engaged with the MotAB stators to increase their power output during swarming. This was exemplified recently in Rhodobacter sphaeroides, in which absence of FliL halted swimming entirely, a defect that was overcome by suppressor mutations in the stator component MotB that are known to increase the proton motive force (PMF) (52, 53). It is possible that the increased torque needed and generated under swarming conditions makes structural reinforcement of the flagella a necessity, underscoring the critical nature of FliL to this mode of motility. In summary, swarming is enhanced by amphipathic molecules, such as LPS and surfactants, which may contribute to lowering friction, as well as by increased force on the moving cells through increased flagellar propulsive power. Multiple solutions work to increase thrust: increased flagellar motor numbers (robust swarmers), different stators that transmit more power to the motor (Pseudomonas), and increased current through the motors and perhaps a flagellar structure more able to withstand the increased physical demands of moving on a surface (FliL function). (iii) Reducing surface tension. The phenomenon of surface tension is due to the cohesive forces between liquid molecules, i.e., molecules interact more strongly with each other than with those associated with them on a surface. Breaking through this tension is difficult for a moving object. It stands to reason therefore that swarming would benefit from a lowering of surface tension. Agents that lower surface tension have amphipathic properties, which allow them to interact with both the liquid and the surface. The term wetting agent is often used in the literature to describe such agents, which include surfactants, emulsifiers, and detergents. The term wettability is used to describe the ability of a liquid to spread or to reduce its contact angle with a surface (17). Confusingly, osmolytes such as salts or sugars, which attract water, are also referred to as wetting agents. As the discussion below will reveal, both play important but separate roles in swarming. We therefore recommend avoidance of the term wetting agents to describe these molecules in the context of swarming. The requirement for surfactants for swarming varies in the different bacterial species studied. There are no reports of specific surfactants in most robust swarmers, so it is assumed that LPS may serve this function, although glycolipids have been found in the ECM of P. mirabilis (18). On the other hand, swarming is greatly enhanced by lipopeptide and rhamnolipid surfactants produced by the temperate swarmers Serratia, Bacillus, Pseudomonas, and Rhizobium species (2, 6, 54). However, even these generous surfactant producers show striking differences in swarming proficiency in the absence of surfactant. For example, mutations that abolish surfactant production inhibit swarming completely in B. subtilis (55). In Serratia marcescens, however, such mutants swarm in place, i.e., show movement only within the zone of inoculation and do not move outward (56, 57). The contrasting behavior of surfactant-deficient mutants of B. subtilis and S. marcescens is noteworthy because their surfactants (called surfactin and serrawettin, respectively) have a related cyclic lipopeptide structure (6) and display surfactant activity that is stronger in B. subtilis (14). Yet, S. marcescens is able to initiate swarming motility in the absence of serrawettin, while B. subtilis is completely nonmotile on swarming agar in the absence of surfactin. The S. marcescens data show that serrawettin does not extract water from the agar, i.e., is not an osmolyte: the bacteria are clearly able to hydrate the colony independently of serrawettin, as evidenced by their robust motility within the zone of inoculation; they are simply unable to spread this water in the absence of serrawettin. (We sound a cautionary note in this regard when assuming that surfactants extract liquid from the substrate [58, 59].) In B. subtilis, the complete lack of motility in the absence of surfactin could stem from an additional signaling function for surfactin, which includes the production of more flagella, as judged by poor flagellation of surfactantdeficient mutants (60). Surfactant-deficient mutants of P. aeruginosa show more complex behaviors, likely because this bacterium makes multiple surfactant molecules with different properties (reference 6 and references cited therein). Salmonella and E. coli display little, if any, secreted surfactant activity in their supernatants (14, 61). These bacteria, as well as surfactant-deficient mutants of S. marcescens, will swarm on a special agar surmised to have a lower surface tension (Eiken agar) or on agar supplemented with other surfactants (22, 57, 62, 63). In summary, secreted surfactants are more prevalent in temperate than in robust swarmers. There is no evidence that surfactants serve as osmolytes. Rather, surfactants enhance the rate at which a swarming colony advances after it is hydrated. In B. subtilis, the surfactant may play an additional role in regulating flagellar numbers. IS THERE A COMMON SURFACE-SENSING MECHANISM? To answer the question of whether there is a common surfacesensing mechanism, one must first ask if there is a program, genetic or otherwise, specific for swarming. If so, one would expect to see either a transcriptional upregulation of components essential for swarming, such as osmolytes, surfactants, and flagella, or posttranscriptional mechanisms that enhance the amounts or function of these components. Let us consider the genetic program first. The transcriptomes and proteomes of several swarming bacteria have now been analyzed, although not all of these studies made a distinction between swarming and nonswarming agar, i.e., transcriptome changes in response to surface-related alterations in metabolism that were independent of swarming (24 29, 64). Nonetheless, a general picture that emerges is that a large number of genes show differential regulation on a surface compared to that in broth (swimming conditions) but increased flagellar synthesis is observed in a swarm agar-specific manner only with P. mirabilis, V. parahaemolyticus, B. subtilis, and B. cereus, consistent with their altered swarmer cell morphologies. Gram-negative temperate swarmers do not show an increase in flagellar gene expression, consistent with the absence of increased flagella in these bacteria. In bacteria that showed dramatic changes in cell length, genes controlling cell division (mincd) were upregulated in P. mirabilis (27) but not in V. parahaemolyticus (28). Surfactant synthesis is cell density specific rather than swarming specific (see below), although nitrates, which are essential for swarming in P. aeruginosa, upregulate rhamnolipid synthesis independent of cell density sensing on a surface (65). A study with Pseudomonas syringae showed that synthesis of the surfactant HAA ( -hydroxydecanoyl- -hydroxydecanoate) is under the control of the flagellar regulon and that absence of HAA specifically affected swarming motility (66). No specific genes could be linked to osmolyte secretion in any organism, as discussed above, although a spike in LPS synthesis was 912 jb.asm.org Journal of Bacteriology
5 observed in Salmonella on swarm agar (24), and upregulation of osmoprotectant or sodium/solute symporter systems was seen in several bacteria (26, 29, 30). Many virulence genes were upregulated either in a surface-specific manner in Salmonella (24)or in a swarming agar-specific manner in P. aeruginosa, P. mirabilis, and V. parahaemolyticus (26 29, 64), supporting the idea that surfaceadapted cells are more pathogenic (67, 68). Is there evidence for nongenetic or posttranscriptional mechanisms as part of the swarming program? Although there is no clear evidence for such mechanisms, one can infer that they likely exist. For example, transcriptome studies did not uncover a mechanism that would explain how cell division is inhibited to generate the hyperelongated cell morphology of robust swarmers (27, 28). Similarly, increased biosynthesis of the Cmf polysaccharide essential for Proteus swarming was not detected in the swarming transcriptome (27). Such adaptations could well be controlled by nongenetic just-in-time surface-sensing mechanisms. In summary, all swarmers generally increase virulence gene expression, in either a swarming-specific or a surface-specific manner. There is evidence for a genetic program for increasing flagellar numbers in some but not all swarming bacteria. There are likely to be posttranscriptional mechanisms for enhancing osmolyte secretion and suppressing cell division in all swarming bacteria; however, these mechanisms have yet to be identified. The flagellar motor as a sensor. Several bacteria, including Aeromonas, Azospirillum, Rhodospirillum, and Vibrio species, possess dual flagellar systems where a single polar flagellum is employed for swimming but numerous lateral flagella are induced upon contact with a surface (3). Frictional forces are surmised to slow polar flagellar rotation (69, 70). A clear-cut demonstration of slow rotation speed being a trigger for induction of gene expression was provided by experiments conducted in broth (i.e., swim conditions) with V. parahaemolyticus: addition of a sodium ion channel inhibitor decreased the rotation rate of the sodiumdriven polar flagellum motor in a dose-dependent manner, with a concomitant induction of lateral flagellar (laf) gene transcription (71). The relationship between the average swimming speed and laf induction was similar to that observed when viscosity (liquid friction) was changed. Despite the clarity of these experiments, we are still in the dark about how motor speed or ion current might be sensed and transduced to activate laf expression. The observation that absence of the polar flagellum, where the motor is expected to rotate at top speeds, also induces lateral flagellar synthesis in several of these bacteria (70, 72) suggests that laf regulation via the flagellar motor is likely to be complex, with an additional nutritional input of iron limitation required (73). There is no evidence for a similar mechanism(s) in other swarming bacteria. Perturbations of the stator-associated FliL protein have been reported to affect induction of swarming-related proteins in P. mirabilis, implicating the C-terminal periplasmic segment of FliL in a sensory role (74, 75). However, it is not known if this sensory role is related to motor speed monitoring or to some other surface-sensing pathway (see below). The cell envelope as a sensor. In P. mirabilis, four Umo (upregulator of the master operon flhdc) proteins associated with the cell envelope are thought to participate in sensing the surface, based on the observations that these proteins activated flhdc expression and were themselves upregulated during swarming and that their loss either abolished or reduced the flhdc transcript (27, 75, 76). Control of the flhdc operon (albeit negative) by a phosphorylated response regulator in the Rcs signaling pathway, which senses cell envelope stress (77), has been offered as a model for how Umo proteins might similarly communicate surface information via a response regulator to activate flhdc (36, 78). In one scenario, the periplasmic region of FliL, implicated to have a sensory role (75), could perhaps communicate with the Umo signaling pathway. An analogous pathway might be envisioned for B. subtilis, where the response regulator DegU controls flagellar gene expression during swarming (36, 79). The upstream signals that activate DegU are unknown. In summary, in bacteria that regulate gene expression in response to a surface, no universal surface-sensing mechanism has emerged. The cell envelope and flagellar motor have been implicated in the sensory process in different bacteria, but details of the transduction pathway are still unknown. SIGNALING PATHWAYS THAT CONTROL THE DECISION TO MOVE Three signaling pathways influence swarming, but with different outcomes in different bacteria. (i) Chemotaxis. The movement of swimming bacteria in nutrient gradients is controlled by a two-component system in which a sensor kinase mediates the phosphorylation of response regulators, which control the direction of flagellar rotation as well as adaptation to the sensory signal (10). Chemotaxis is required for outward migration of the swarming colony in V. parahaemolyticus and Vibrio alginolyticus (80, 81), but not for any of the other bacteria examined to date. In Rhodospirillum centenum, B. subtilis, and P. aeruginosa, nonchemotactic mutants whose flagellar motors are CCW biased (expected to form flagellar bundles) can swarm, while those that are CW biased do not (82 84). Aberrant and nonswarming patterns have been reported for nonchemotactic mutants of P. mirabilis (85), but chemotaxis is apparently not required for swarming (86). The chemotaxis pathway does not influence swarming-related gene expression in any of these organisms. In E. coli and Salmonella, the chemotaxis (che) pathway was originally thought to control flagellar gene expression in swarmer cells, because che mutants had shorter flagella when propagated on swarm agar (62). Transcriptome analysis showed that only the last (class 3) stage of flagellar transcription is inhibited in these mutants under swarming conditions, and this inhibition was traced to inhibition of FlgM export, a negative regulator that binds 28 and inhibits class 3 gene expression (35, 87). che mutants have a second phenotype, which is that they are unable to hydrate their swarm colonies (87). External hydration of the colony, or conditions that abrogate the extreme CCW or CW bias of che mutants, was observed to restore swarming in these mutants (87, 88). These data can be explained by the finding that the rate of secretion of flagellin subunits through the flagellum is monitored by a rheostatic mechanism by which feedback regulates various promoter classes in the flagellar regulon (89). This makes the flagellum a drought sensor, turning down class 3 gene expression in response to dry conditions outside. Thus, the chemotaxis signaling pathway does not control gene expression directly, but rather its control of motor bias influences the ability of the colony to attract water in an unknown manner, perhaps by controlling osmolyte production. Like E. coli and Salmonella, che mutants of S. marcescens are nonswarming despite unimpeded copious surfactant production March 2013 Volume 195 Number 5 jb.asm.org 913
6 (56), and they can be rescued for swarming by hydrating the colony (R. M. Harshey, unpublished data), demonstrating again that the surfactant is not an osmolyte (see Reducing surface tension above). A related observation was made for B. subtilis, where a CW-biased che mutant colony was observed to be poorly hydrated, as measured by the diffusion of MgO beads on the colony surface (14). MgO beads on a surfactin-producing nonflagellate mutant were completely immobile, not only showing that surfactin itself is insufficient to extract water from the agar but also suggesting the involvement of flagella in this effort. Rotating flagellar bundles have also been proposed to aid in hydration through capillary action (33). In summary, chemotaxis is not required for swarming, although there are exceptions to this rule. Nonswarming phenotypes in some bacteria are specific to CW-biased mutants, which cannot readily form flagellar bundles. The most intriguing phenotype of nonchemotactic mutants of some bacteria is their inability to hydrate the swarm colony, implicating flagellar switching/rotation in this function. (ii) Quorum sensing. Bacteria produce and release small molecules, termed autoinducers, whose external concentration rises with increasing cell density. A minimal threshold stimulatory concentration of these autoinducers is detected either directly or via sensor kinases to alter gene expression, a phenomenon generally called quorum sensing (90), although alternative nomenclature for this regulation has been suggested (91, 92). Surfactant synthesis is under quorum-sensing control in Serratia, P. aeruginosa and B. subtilis and facilitates swarming as described above (83, 93 95), although nitrates upregulate rhamnolipid synthesis independent of quorum sensing in P. aeruginosa (65). Surfactant-deficient mutants of these bacteria have swarming defects but are otherwise motile, also as described above. Interestingly, the autoinducer in Rhizobium etli doubles as a surfactant (54), showing that surfactants play roles in addition to surface tension/friction reduction (see also references 60 and 96). Quorum-sensing pathways control expression of many other genes as well, which could also account for the nonswarming phenotypes of either surfactant or quorumsensing mutants of B. subtilis and Pseudomonas (94, 97). In V. parahaemolyticus, the canonical quorum-sensing pathway normally inhibits swarming, but this pathway is silenced in most strains studied (30, 98). Quorum sensing by a novel autoinducer signal stimulates swarming-specific gene expression in V. parahaemolyticus by influencing levels of a second signaling molecule, cyclic (c)-di-gmp (99). In Salmonella, there is no evidence that quorum sensing is essential for swarming (100). In summary, the major pathway known to be influenced by quorum sensing during swarming is surfactant production. Surfactants can themselves be autoinducers and control a large network of gene expression. Novel quorum-sensing mechanisms influence swarming-related gene expression in V. parahaemolyticus. Quorum sensing does not appear to have a role during E. coli or Salmonella swarming. (iii) c-di-gmp signaling. c-di-gmp is an important signaling molecule in the transition between motile and sessile forms of bacterial life (101, 102). High levels of c-di-gmp inhibit motility and promote biofilm formation, whereas low levels favor motile behaviors. Bacteria can possess multiple proteins that synthesize or degrade c-di-gmp via guanyl cyclases and phosphodiesterases, respectively. These proteins are associated with diverse input and output domains, suggesting that they receive a variety of signals and respond through varied mechanisms. c-di-gmp inhibits flagellar motility at the level of either gene expression, flagellar assembly, or function (103, 104). In V. parahaemolyticus, the scrabc operon encodes both a quorum-sensing and a c-di-gmp-regulating system, which increases phosphodiesterase activity in response to growth on a surface, decreasing c-di-gmp levels and thereby increasing swarming-related gene expression (28, 105). This system is not required for the flagellummediated pathway of surface sensing in V. parahaemolyticus (106). In Salmonella, the phosphodiesterase YhjH and the c-di-gmp receptor protein YcgR are members of the class 3 flagellar regulon ( ). It is not known what keeps c-di-gmp levels low during swarming, and despite the existence of multiple phosphodiesterases, mutation of this specific phosphodiesterase (YhjH) inhibits swarming in both E. coli and Salmonella. The YcgR::c-di-GMP complex binds directly to flagellar motor components in these bacteria to induce a CCW motor bias, i.e., inhibit chemotaxis, and slow or brake the motor ( ). A related mechanism to inhibit swarming motility appears to be operating in B. subtilis (113). In P. aeruginosa, c-di-gmp levels are additionally regulated by the type IV pilus assembly machinery (114), with high levels inhibiting swarming also by inhibiting chemotaxis, but likely by a different mechanism acting upstream of the motor ( ). In summary, maintaining low c-di-gmp levels is crucial for flagellar motility. A quorum-sensing mechanism for keeping these levels low during swarming exists in V. parahaemolyticus, where c-di-gmp influences swarming-specific gene expression. In E. coli, Salmonella, and B. subtilis, c-di-gmp complexed with a receptor protein interacts directly with the flagellar motor to induce a CCW bias and inhibit chemotaxis and/or to put a brake on the motor. Environmental signals that increase c-di-gmp levels to inhibit swarming are not known. GROUP DYNAMICS Swarming is a well-known behavior in many species, from schooling fish to flocking birds to marching locusts. Coordinated patterns that emerge as a result of a multiplicity of simple interactions are referred to as emergent behavior (see references 49 and 118 and references therein). The movement of swarming bacteria displays spatiotemporal features of collective motion similar to those seen in these other swarming systems and is being increasingly used to study the general principles of emergent behavior (49, 118). Features being modeled include colony expansion, pattern formation, and behavior of individuals within the swarm (11, 12, 58, ). Fluorescent labeling of flagellar filaments of E. coli showed that flagellar mechanics during swarming are similar to those during swimming (11, 12), except that CW flagellar rotations reverse the direction of travel rather than promote a tumble (12). The different outcomes of motor reversals on bacterial trajectories have been attributed to differences in polymorphic transformations of filaments during swimming in bulk fluid versus swarming in confined spaces (12). Neighboring cells were not observed to form shared flagellar bundles, as was suggested based on scanning electron micrographs of P. mirabilis, where the flagella appeared to be interwoven in phase to form helical connections between adjacent swarmer cells (125); however, the methods used to visualize the E. coli flagella necessitated observation of cells that were not as dense as normally seen on swarm agar (11, 12). Cells that stalled at the 914 jb.asm.org Journal of Bacteriology
7 edge of the colony extended their flagella outward, likely pumping fluid outward and promoting swarm expansion (11, 12). The advancing front of most swarms is monolayered, with the cell density increasing toward the center of the colony. Direct observation of an E. coli swarm in the monolayered area led to many interesting observations (49): mean swarming and swimming speeds of the bacteria were about the same, but variations in speed were much larger within a swarm. The normal run-tumble behavior seen in swimming was largely suppressed. Cells attempted to move straight ahead but were continuously jostled either from true collisions with neighbors or from forces induced by the moving fluid. Collisions tended to align cell bodies along their length, and the groups/packs/rafts so created transported this alignment from place to place, resulting in the swirling motion typical of swarms (see Movies S1 to S4 in the supplemental material). The longer length of swarmer cells may be expected to facilitate alignment into rafts. Packs did not swim any faster, but they were deflected less easily, favoring a forward trajectory. They changed directions randomly as a result of the collisions rather than due to flagellar reversal. Qualitatively similar observations have been reported previously for other bacteria (2, 126). A minimal model of swarm expansion, requiring only cell growth and hydrated surfaces, has been proposed for E. coli (127). Cells travel in a group, likely because they collectively trap a larger pool of water (123), because they entrain each other (12), and/or because the larger torque produced by a group of cells might be more effective in breaking the surface tension of liquid for forward motion. These models may explain why accidentally stranded individual cells remain immotile until they are swept up by a passing raft (see Movie S2). In summary, detailed observations of individual cells within a swarm are just beginning to be made. The pack behaviors of E. coli and B. subtilis, both temperate swarmers, appear to be similar despite differences in surfactant production by their swarm colonies. Flagellar mechanics have been visualized only in E. coli. It remains to be seen if these observations can be extended to robust swarmers. ADVANTAGES OF COLLECTIVE MOTION The upper surface of swarms of S. marcescens and B. subtilis, which secrete powerful surfactants, show superdiffusive behavior as monitored by the motion of MgO particles deposited on the swarm colony surface (14). Superdiffusive behavior was not observed on the surface of a drop of bacterial culture, on bacteriumfree culture supernatant, or on nonswarming surfactant-producing colonies, suggesting that superdiffusion is an emergent property resulting from the interaction of the collective motion of the bacteria within the swarm with the surfactant layer above. Such behavior may confer a means of long-range communication, such as circulation of nutrients encountered by the advancing edge of the swarm to inner or older regions of the colony, transport of signaling molecules, more efficient acquisition of oxygen from the atmosphere to reach bacteria under a multilayered pile, and better regulation of temperature. E. coli swarms display little or no surfactant activity (14). In these swarms, a rapid chiral flow of 10 m/s was observed along the edge (128). The speed of the streaming fluid was about three times faster than the swarm expansion and would also be expected to circulate nutrients to distant parts of the colony. Thus, there are multiple ways to effect long-range communication within a swarm. These and as-yetundiscovered mechanisms may account for the increased resistance of swarms to antimicrobials (100, 129, 130). The pathogenic potential of a surface-adapted colony, whose virulence pathways are upregulated on a surface, makes the moving group an imposing force. CONCLUDING REMARKS Swarming has been observed (and studied) almost exclusively on agar surfaces in laboratory settings. The large number of bacteria that swarm on agar suggests that swarming is an important means of surface colonization in natural habitats. We speculate that open surfaces of animal tissues or ripe fruits that exhibit agar-like gelatinous properties may present a fertile ground for swarming. In the wild, solid-liquid interfaces on organic or inorganic substrates could conceivably also support swarming. Given that chemotaxis is generally not required for outward migration, the endgame of swarming appears to be acquiring more territory and increasing population size. As this review highlights, the challenges en route are many. Bacteria overcome these obstacles by banding together in large numbers, exhibiting a plethora of mechanisms to attract water, to increase flagellar motor power, to lubricate the cell-surface interface, and to reduce surface tension. Mechanisms specific to each species must reflect adaptations to specific niches. Some bacteria induce a swarming-specific gene expression program, and others induce a surface-specific metabolic program. The polar flagellum is a surface sensor in some bacteria, and the cell envelope is implicated in others, but no single mechanism accounts for surface-related gene expression, which invariably includes upregulating virulence factors. Thus, swarming plans include pathogenesis. Nongenetic mechanisms for increasing osmolyte production and cell length are likely to be operative, but have not as yet been defined. A variety of adaptations increase flagellar propulsive force. Although bacteria suppress flagellar reversals and move predominantly forward during swarming, control of motor bias is important. A CCW bias allowing flagellar bundling is sufficient in some bacteria for forward motion, but the ability to switch directions is important for initiating swarming in others. Flagellar rotation/bias appears to be important for colony hydration. The flagellum therefore plays varied roles during swarming, but the mechanisms underlying these functions remain to be elucidated. c-di-gmp levels, controlled by quorum sensing in some but not all bacteria, influence stay or swarm decisions via different mechanisms. Although a variety of biochemical programs are deployed for promoting motility, once movement is under way, the common physics of rod-shaped cells packed shoulder to shoulder lengthwise, attempting to navigate two-dimensional space in a thin film of water, likely create a perfect storm for the emergent collective motion seen in all swarming bacteria. ACKNOWLEDGMENTS We thank Avraham Be er for helpful comments. Swarming research in our lab is supported by a grant from the NIH (GM 57400). REFERENCES 1. Henrichsen J Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36: Harshey RM Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57: McCarter LL Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol. 7: March 2013 Volume 195 Number 5 jb.asm.org 915
8 4. Rather PN Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 7: Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, Vermant J, Michiels J Living on a surface: swarming and biofilm formation. Trends Microbiol. 16: Kearns DB A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 8: Berg HC The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72: Berg, HC E. coli in motion. Springer, New York, NY. 9. Brown MT, Delalez NJ, Armitage JP Protein dynamics and mechanisms controlling the rotational behaviour of the bacterial flagellar motor. Curr. Opin. Microbiol. 14: Hazelbauer GL, Falke JJ, Parkinson JS Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem. Sci. 33: Copeland MF, Flickinger ST, Tuson HH, Weibel DB Studying the dynamics of flagella in multicellular communities of Escherichia coli by using biarsenical dyes. Appl. Environ. Microbiol. 76: Turner L, Zhang R, Darnton NC, Berg HC Visualization of flagella during bacterial swarming. J. Bacteriol. 192: Banaha M, Daerr A, Limat L Spreading of liquid drops on agar gels. Eur. Phys. J. Spec. Top. 166: Be er A, Harshey RM Collective motion of surfactant-producing bacteria imparts superdiffusivity to their upper surface. Biophys. J. 101: Akbulut M, Belman N, Golan Y, Israelachvili J Frictional properties of confined nanorods. Adv. Mater. 18: Kralchevsky PA, Nagayama K Capillary interactions between particles bound to interfaces, liquid films and biomembranes. Adv. Colloid Interface Sci. 85: de Gennes PG Wetting: statics and dynamics. Rev. Mod. Phys. 57: Lahaye E, Aubry T, Fleury V, Sire O Does water activity rule P. mirabilis periodic swarming? II. Viscoelasticity and water balance during swarming. Biomacromolecules 8: Gygi D, Rahman MM, Lai HC, Carlson R, Guard-Petter J, Hughes C A cell-surface polysaccharide that facilitates rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol. Microbiol. 17: Stahl SJ, Stewart KR, Williams FD Extracellular slime associated with Proteus mirabilis during swarming. J. Bacteriol. 154: Rauprich O, Matsushita M, Weijer CJ, Siegert F, Esipov SE, Shapiro JA Periodic phenomena in Proteus mirabilis swarm colony development. J. Bacteriol. 178: Toguchi A, Siano M, Burkart M, Harshey RM Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysaccharide. J. Bacteriol. 182: Inoue T, Shingaki R, Hirose S, Waki K, Mori H, Fukui K Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J. Bacteriol. 189: Wang Q, Frye JG, McClelland M, Harshey RM Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol. Microbiol. 52: Kim W, Surette MG Metabolic differentiation in actively swarming Salmonella. Mol. Microbiol. 54: Tremblay J, Deziel E Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genomics 11:587. doi: / Pearson MM, Rasko DA, Smith SN, Mobley HL Transcriptome of swarming Proteus mirabilis. Infect. Immun. 78: Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 79: Salvetti S, Faegri K, Ghelardi E, Kolsto AB, Senesi S Global gene expression profile for swarming Bacillus cereus bacteria. Appl. Environ. Microbiol. 77: Gode-Potratz CJ, McCarter LL Quorum sensing and silencing in Vibrio parahaemolyticus. J. Bacteriol. 193: Matsuyama T, Takagi Y, Nakagawa Y, Itoh H, Wakita J, Matsushita M Dynamic aspects of the structured cell population in a swarming colony of Proteus mirabilis. J. Bacteriol. 182: Furness RB, Fraser GM, Hay NA, Hughes C Negative feedback from a Proteus class II flagellum export defect to the flhdc master operon controlling cell division and flagellum assembly. J. Bacteriol. 179: Partridge JD, Harshey RM More than motility: Salmonella flagella contribute to overriding friction and facilitating colony hydration during swarming. J. Bacteriol. 195: Kearns DB, Losick R Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 19: Chevance FF, Hughes KT Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 6: Patrick JE, Kearns DB Swarming motility and the control of master regulators of flagellar biosynthesis. Mol. Microbiol. 83: Pruss BM, Liu X, Hendrickson W, Matsumura P FlhD/FlhCregulated promoters analyzed by gene array and lacz gene fusions. FEMS Microbiol. Lett. 197: Pruss BM, Matsumura P A regulator of the flagellar regulon of Escherichia coli, flhd, also affects cell division. J. Bacteriol. 178: Chubiz JE, Golubeva YA, Lin D, Miller LD, Slauch JM FliZ regulates expression of the Salmonella pathogenicity island 1 invasion locus by controlling HilD protein activity in Salmonella enterica serovar Typhimurium. J. Bacteriol. 192: Watrous J, Roach P, Alexandrov T, Heath BS, Yang JY, Kersten RD, van der Voort M, Pogliano K, Gross H, Raaijmakers JM, Moore BS, Laskin J, Bandeira N, Dorrestein PC Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. U. S. A. 109:E1743 E Dowson D Bio-tribology. Faraday Discuss. 156: Gebeshuber IC, Stachelberger H, Drack M Diatom bionanotribology: biological surfaces in relative motion. Their design, friction, adhesion, lubrication and wear. J. Nanosci. Nanotechnol. 5: Raetz CR, Whitfield C Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71: Desai JD, Banat IM Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 61: Walker JS Physics: an introduction, 2nd ed. Prentice-Hall Inc., Cranbury, NJ. 46. Tuson HH, Copeland MF, Carey S, Sacotte R, Weibel DB Flagellum density regulates Proteus mirabilis swarmer cell motility in viscous environments. J. Bacteriol. 195: Toutain CM, Zegans ME, O Toole GA Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. J. Bacteriol. 187: Doyle TB, Hawkins AC, McCarter LL The complex flagellar torque generator of Pseudomonas aeruginosa. J. Bacteriol. 186: Darnton NC, Turner L, Rojevsky S, Berg HC Dynamics of bacterial swarming. Biophys. J. 98: Attmannspacher U, Scharf BE, Harshey RM FliL is essential for swarming: motor rotation in absence of FliL fractures the flagellar rod in swarmer cells of Salmonella enterica. Mol. Microbiol. 68: Motaleb MA, Pitzer JE, Sultan SZ, Liu J A novel gene inactivation system reveals altered periplasmic flagellar orientation in a Borrelia burgdorferi flil mutant. J. Bacteriol. 193: Suaste-Olmos F, Domenzain C, Mireles-Rodriguez JC, Poggio S, Osorio A, Dreyfus G, Camarena L The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides. J. Bacteriol. 192: Hosking ER, Vogt C, Bakker EP, Manson MD The Escherichia coli MotAB proton channel unplugged. J. Mol. Biol. 364: Daniels R, Reynaert S, Hoekstra H, Verreth C, Janssens J, Braeken K, Fauvart M, Beullens S, Heusdens C, Lambrichts I, De Vos DE, Vanderleyden J, Vermant J, Michiels J Quorum signal molecules as biosurfactants affecting swarming in Rhizobium etli. Proc. Natl. Acad. Sci. U. S. A. 103: Patrick JE, Kearns DB Laboratory strains of Bacillus subtilis do not exhibit swarming motility. J. Bacteriol. 191: O Rear J, Alberti L, Harshey RM Mutations that impair swarming motility in Serratia marcescens 274 include but are not limited to those affecting chemotaxis or flagellar function. J. Bacteriol. 174: jb.asm.org Journal of Bacteriology
Bacterial Chemotaxis
 Bacterial Chemotaxis Bacteria can be attracted/repelled by chemicals Mechanism? Chemoreceptors in bacteria. attractant Adler, 1969 Science READ! This is sensing, not metabolism Based on genetic approach!!!
Bacterial Chemotaxis Bacteria can be attracted/repelled by chemicals Mechanism? Chemoreceptors in bacteria. attractant Adler, 1969 Science READ! This is sensing, not metabolism Based on genetic approach!!!
The flagellar motor of Caulobacter crescentus generates more torque when a cell swims backwards
 The flagellar motor of Caulobacter crescentus generates more torque when a cell swims backwards Pushkar P. Lele a1, Thibault Roland a, Abhishek Shrivastava a, Yihao Chen and Howard C. Berg a Affiliations:
The flagellar motor of Caulobacter crescentus generates more torque when a cell swims backwards Pushkar P. Lele a1, Thibault Roland a, Abhishek Shrivastava a, Yihao Chen and Howard C. Berg a Affiliations:
Soft Matter. Self-assembly. Volume 5 Number 6 21 March 2009 Pages
 Soft Matter www.softmatter.org Volume 5 Number 6 21 March 2009 Pages1093 1296 ISSN 1744-683X REVIEW Matthew F. Copeland and Douglas B. Weibel Bacterial swarming: a model system for studying dynamic self-assembly
Soft Matter www.softmatter.org Volume 5 Number 6 21 March 2009 Pages1093 1296 ISSN 1744-683X REVIEW Matthew F. Copeland and Douglas B. Weibel Bacterial swarming: a model system for studying dynamic self-assembly
Salmonella enterica Serovar Typhimurium Swarming Mutants with Altered Biofilm-Forming Abilities: Surfactin Inhibits Biofilm Formation
 JOURNAL OF BACTERIOLOGY, Oct. 2001, p. 5848 5854 Vol. 183, No. 20 0021-9193/01/$04.00 0 DOI: 10.1128/JB.183.20.5848 5854.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Salmonella
JOURNAL OF BACTERIOLOGY, Oct. 2001, p. 5848 5854 Vol. 183, No. 20 0021-9193/01/$04.00 0 DOI: 10.1128/JB.183.20.5848 5854.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Salmonella
Collective Motion of Surfactant-Producing Bacteria Imparts Superdiffusivity to Their Upper Surface
 iophysical Journal Volume September 8 Collective Motion of Surfactant-Producing acteria Imparts Superdiffusivity to Their Upper Surface vraham e er * and Rasika M. Harshey * Center for Nonlinear Dynamics
iophysical Journal Volume September 8 Collective Motion of Surfactant-Producing acteria Imparts Superdiffusivity to Their Upper Surface vraham e er * and Rasika M. Harshey * Center for Nonlinear Dynamics
New Insights into the Dynamics of Swarming Bacteria: A Theoretical Study
 New Insights into the Dynamics of Swarming Bacteria: A Theoretical Study David Hansmann 1,2*, Guido Fier 2, Rubén C. Buceta 1,2 1 Departamento de Física, Universidad Nacional de Mar del Plata, Funes 3350,
New Insights into the Dynamics of Swarming Bacteria: A Theoretical Study David Hansmann 1,2*, Guido Fier 2, Rubén C. Buceta 1,2 1 Departamento de Física, Universidad Nacional de Mar del Plata, Funes 3350,
56:198:582 Biological Networks Lecture 10
 56:198:582 Biological Networks Lecture 10 Temporal Programs and the Global Structure The single-input module (SIM) network motif The network motifs we have studied so far all had a defined number of nodes.
56:198:582 Biological Networks Lecture 10 Temporal Programs and the Global Structure The single-input module (SIM) network motif The network motifs we have studied so far all had a defined number of nodes.
Bio Microbiology - Spring 2014 Learning Guide 04.
 Bio 230 - Microbiology - Spring 2014 Learning Guide 04 http://pessimistcomic.blogspot.com/ Cell division is a part of a replication cycle that takes place throughout the life of the bacterium A septum
Bio 230 - Microbiology - Spring 2014 Learning Guide 04 http://pessimistcomic.blogspot.com/ Cell division is a part of a replication cycle that takes place throughout the life of the bacterium A septum
56:198:582 Biological Networks Lecture 11
 56:198:582 Biological Networks Lecture 11 Network Motifs in Signal Transduction Networks Signal transduction networks Signal transduction networks are composed of interactions between signaling proteins.
56:198:582 Biological Networks Lecture 11 Network Motifs in Signal Transduction Networks Signal transduction networks Signal transduction networks are composed of interactions between signaling proteins.
MICROBIOLOGIA GENERALE. Structure and function of prokaryotic cells 3
 MICROBIOLOGIA GENERALE Structure and function of prokaryotic cells 3 Structure and function of prokaryotic cells: in the cytosol The bacterial chromosome is typically one large circular molecule of DNA
MICROBIOLOGIA GENERALE Structure and function of prokaryotic cells 3 Structure and function of prokaryotic cells: in the cytosol The bacterial chromosome is typically one large circular molecule of DNA
Deposited on: 23 August 2010
 Bees, M.A., Andresen, P., Mosekilde, E. and Givskov, M. (2002) Quantitative effects of medium hardness and nutrient availability on the swarming motility of Serratia liquefaciens. Bulletin of Mathematical
Bees, M.A., Andresen, P., Mosekilde, E. and Givskov, M. (2002) Quantitative effects of medium hardness and nutrient availability on the swarming motility of Serratia liquefaciens. Bulletin of Mathematical
Lecture 8: Temporal programs and the global structure of transcription networks. Chap 5 of Alon. 5.1 Introduction
 Lecture 8: Temporal programs and the global structure of transcription networks Chap 5 of Alon 5. Introduction We will see in this chapter that sensory transcription networks are largely made of just four
Lecture 8: Temporal programs and the global structure of transcription networks Chap 5 of Alon 5. Introduction We will see in this chapter that sensory transcription networks are largely made of just four
Hybrid Quorum sensing in Vibrio harveyi- two component signalling
 Hybrid Quorum sensing in Vibrio harveyi- two component signalling Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of
Hybrid Quorum sensing in Vibrio harveyi- two component signalling Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of
Bio Microbiology - Spring 2012 Learning Guide 04.
 Bio 230 - Microbiology - Spring 2012 Learning Guide 04 http://pessimistcomic.blogspot.com/ A septum assembles at the center of the cell. This molecular "purse string" is linked to the inner surface of
Bio 230 - Microbiology - Spring 2012 Learning Guide 04 http://pessimistcomic.blogspot.com/ A septum assembles at the center of the cell. This molecular "purse string" is linked to the inner surface of
return in class, or Rm B
 Last lectures: Genetic Switches and Oscillators PS #2 due today bf before 3PM return in class, or Rm. 68 371B Naturally occurring: lambda lysis-lysogeny decision lactose operon in E. coli Engineered: genetic
Last lectures: Genetic Switches and Oscillators PS #2 due today bf before 3PM return in class, or Rm. 68 371B Naturally occurring: lambda lysis-lysogeny decision lactose operon in E. coli Engineered: genetic
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Light controlled motility in E.coli bacteria: from individual response to population dynamics
 Light controlled motility in E.coli bacteria: from individual response to population dynamics Ph.D. Candidate: Supervisor: GIACOMO FRANGIPANE Dr. ROBERTO DI LEONARDO Escherichia Coli A model organism for
Light controlled motility in E.coli bacteria: from individual response to population dynamics Ph.D. Candidate: Supervisor: GIACOMO FRANGIPANE Dr. ROBERTO DI LEONARDO Escherichia Coli A model organism for
Bacterial Morphology and Structure م.م رنا مشعل
 Bacterial Morphology and Structure م.م رنا مشعل SIZE OF BACTERIA Unit for measurement : Micron or micrometer, μm: 1μm=10-3 mm Size: Varies with kinds of bacteria, and also related to their age and external
Bacterial Morphology and Structure م.م رنا مشعل SIZE OF BACTERIA Unit for measurement : Micron or micrometer, μm: 1μm=10-3 mm Size: Varies with kinds of bacteria, and also related to their age and external
Creative Genomic Webs -Kapil Rajaraman PHY 498BIO, HW 4
 Creative Genomic Webs -Kapil Rajaraman (rajaramn@uiuc.edu) PHY 498BIO, HW 4 Evolutionary progress is generally considered a result of successful accumulation of mistakes in replication of the genetic code.
Creative Genomic Webs -Kapil Rajaraman (rajaramn@uiuc.edu) PHY 498BIO, HW 4 Evolutionary progress is generally considered a result of successful accumulation of mistakes in replication of the genetic code.
Regulation and signaling. Overview. Control of gene expression. Cells need to regulate the amounts of different proteins they express, depending on
 Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Introduction to the mathematical modeling of multi-scale phenomena
 Introduction to the mathematical modeling of multi-scale phenomena Diffusion Brownian motion Brownian motion (named after botanist Robert Brown) refers to the random motion of particles suspended in a
Introduction to the mathematical modeling of multi-scale phenomena Diffusion Brownian motion Brownian motion (named after botanist Robert Brown) refers to the random motion of particles suspended in a
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
MINIREVIEW. Get the Message Out: Cyclic-Di-GMP Regulates Multiple Levels of Flagellum-Based Motility. Alan J. Wolfe and Karen L.
 JOURNAL OF BACTERIOLOGY, Jan. 2008, p. 463 475 Vol. 190, No. 2 0021-9193/08/$08.00 0 doi:10.1128/jb.01418-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. MINIREVIEW Get the Message
JOURNAL OF BACTERIOLOGY, Jan. 2008, p. 463 475 Vol. 190, No. 2 0021-9193/08/$08.00 0 doi:10.1128/jb.01418-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. MINIREVIEW Get the Message
Outline. Collective behavior in bacteria. Know your horsemen. Importance. Cooperation and disease. Medical applications?
 Collective behavior in bacteria Will Driscoll April 30 th, 2008 Outline Importance Our macrobial bias Quorum sensing Biofilms Physiology Development Prokaryotic stab at multicellularity? Discussion But
Collective behavior in bacteria Will Driscoll April 30 th, 2008 Outline Importance Our macrobial bias Quorum sensing Biofilms Physiology Development Prokaryotic stab at multicellularity? Discussion But
ADVANCES IN BACTERIA MOTILITY MODELLING VIA DIFFUSION ADAPTATION
 ADVANCES IN BACTERIA MOTILITY MODELLING VIA DIFFUSION ADAPTATION Sadaf Monajemi a, Saeid Sanei b, and Sim-Heng Ong c,d a NUS Graduate School for Integrative Sciences and Engineering, National University
ADVANCES IN BACTERIA MOTILITY MODELLING VIA DIFFUSION ADAPTATION Sadaf Monajemi a, Saeid Sanei b, and Sim-Heng Ong c,d a NUS Graduate School for Integrative Sciences and Engineering, National University
Microbiology Helmut Pospiech
 Microbiology 20.03.2018 Helmut Pospiech The control of what gets in Passive transport along a concentration gradient often inefficient Active transport Requires energy consumption and what gets out ABC
Microbiology 20.03.2018 Helmut Pospiech The control of what gets in Passive transport along a concentration gradient often inefficient Active transport Requires energy consumption and what gets out ABC
Introduction to Microbiology BIOL 220 Summer Session I, 1996 Exam # 1
 Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220 Summer Session I, 1996 Exam # 1 B 1. Which is possessed by eukaryotes but not by prokaryotes? A. Cell wall B. Distinct nucleus
Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220 Summer Session I, 1996 Exam # 1 B 1. Which is possessed by eukaryotes but not by prokaryotes? A. Cell wall B. Distinct nucleus
Asymmetry in the Clockwise and Counter-Clockwise Rotation of the Bacterial Flagellar Motor
 Asymmetry in the Clockwise and Counter-Clockwise Rotation of the Bacterial Flagellar Motor The Harvard community has made this article openly available. Please share how this access benefits you. Your
Asymmetry in the Clockwise and Counter-Clockwise Rotation of the Bacterial Flagellar Motor The Harvard community has made this article openly available. Please share how this access benefits you. Your
Paenibacillus dendritiformis bacterial colony growth depends on surfactant but not on bacterial motion
 JB Accepts, published online ahead of print on July J. Bacteriol. doi:./jb.- Copyright, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. Paenibacillus dendritiformis
JB Accepts, published online ahead of print on July J. Bacteriol. doi:./jb.- Copyright, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. Paenibacillus dendritiformis
CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON
 PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
BACTERIA AND ARCHAEA 10/15/2012
 BACTERIA AND ARCHAEA Chapter 27 KEY CONCEPTS: Structural and functional adaptations contribute to prokaryotic success Rapid reproduction, mutation, and genetic recombination promote genetic diversity in
BACTERIA AND ARCHAEA Chapter 27 KEY CONCEPTS: Structural and functional adaptations contribute to prokaryotic success Rapid reproduction, mutation, and genetic recombination promote genetic diversity in
Paenibacillus dendritiformis Bacterial Colony Growth Depends on Surfactant but Not on Bacterial Motion
 JOURNAL OF BACTERIOLOGY, Sept. 2009, p. 5758 5764 Vol. 191, No. 18 0021-9193/09/$08.00 0 doi:10.1128/jb.00660-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Paenibacillus dendritiformis
JOURNAL OF BACTERIOLOGY, Sept. 2009, p. 5758 5764 Vol. 191, No. 18 0021-9193/09/$08.00 0 doi:10.1128/jb.00660-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Paenibacillus dendritiformis
BACTERIAL PHYSIOLOGY SMALL GROUP. Monday, August 25, :00pm. Faculty: Adam Driks, Ph.D. Alan Wolfe, Ph.D.
 BACTERIAL PHYSIOLOGY SMALL GROUP Monday, August 25, 2014 1:00pm Faculty: Adam Driks, Ph.D. Alan Wolfe, Ph.D. Learning Goal To understand how bacterial physiology applies to the diagnosis and treatment
BACTERIAL PHYSIOLOGY SMALL GROUP Monday, August 25, 2014 1:00pm Faculty: Adam Driks, Ph.D. Alan Wolfe, Ph.D. Learning Goal To understand how bacterial physiology applies to the diagnosis and treatment
Dynamics of Bacterial Swarming
 Dynamics of Bacterial Swarming The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version Accessed Citable Link
Dynamics of Bacterial Swarming The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version Accessed Citable Link
Using Evolutionary Approaches To Study Biological Pathways. Pathways Have Evolved
 Pathways Have Evolved Using Evolutionary Approaches To Study Biological Pathways Orkun S. Soyer The Microsoft Research - University of Trento Centre for Computational and Systems Biology Protein-protein
Pathways Have Evolved Using Evolutionary Approaches To Study Biological Pathways Orkun S. Soyer The Microsoft Research - University of Trento Centre for Computational and Systems Biology Protein-protein
Trail-following bacteria: from single particle dynamics to collective behaviour
 Trail-following bacteria: from single particle dynamics to collective behaviour K. Zhao, C.K. Lee & G.C.L. Wong (UCLA) A. Gelimson, W.T. Kranz & Ramin Golestanian (Oxford) Biofilms: Strongly Correlated
Trail-following bacteria: from single particle dynamics to collective behaviour K. Zhao, C.K. Lee & G.C.L. Wong (UCLA) A. Gelimson, W.T. Kranz & Ramin Golestanian (Oxford) Biofilms: Strongly Correlated
Chapter 16. Cellular Movement: Motility and Contractility. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
How bacteria in colonies can survive by killing their brothers and sisters
 How bacteria in colonies can survive by killing their brothers and sisters Harry Swinney University of Texas at Austin bacterial colony 6 cm at U. Texas: Avraham Be er Hepeng Zhang Shelley Payne E.L. Florin
How bacteria in colonies can survive by killing their brothers and sisters Harry Swinney University of Texas at Austin bacterial colony 6 cm at U. Texas: Avraham Be er Hepeng Zhang Shelley Payne E.L. Florin
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS
 2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
AGITATION AND AERATION
 AGITATION AND AERATION Although in many aerobic cultures, gas sparging provides the method for both mixing and aeration - it is important that these two aspects of fermenter design be considered separately.
AGITATION AND AERATION Although in many aerobic cultures, gas sparging provides the method for both mixing and aeration - it is important that these two aspects of fermenter design be considered separately.
TER 26. Preview for 2/6/02 Dr. Kopeny. Bacteria and Archaea: The Prokaryotic Domains. Nitrogen cycle
 Preview for 2/6/02 Dr. Kopeny Bacteria and Archaea: The Prokaryotic Domains TER 26 Nitrogen cycle Mycobacterium tuberculosis Color-enhanced images shows rod-shaped bacterium responsible for tuberculosis
Preview for 2/6/02 Dr. Kopeny Bacteria and Archaea: The Prokaryotic Domains TER 26 Nitrogen cycle Mycobacterium tuberculosis Color-enhanced images shows rod-shaped bacterium responsible for tuberculosis
Tineke Jones Agriculture and Agri-Food Canada Lacombe Research Centre Lacombe, Alberta
 Growth of Escherichia Coli at Chiller Temperatures Tineke Jones Agriculture and Agri-Food Canada Lacombe Research Centre Lacombe, Alberta \ Introduction The responses of mesophilic microorganisms to chiller
Growth of Escherichia Coli at Chiller Temperatures Tineke Jones Agriculture and Agri-Food Canada Lacombe Research Centre Lacombe, Alberta \ Introduction The responses of mesophilic microorganisms to chiller
MORPHOLOGY: the study of form and structure
 MICROBIOLOGY CHAPTER 3 Bacteria Morphology 3:1 Bacteria Structure and Function MORPHOLOGY: the study of form and structure Structure of Bacteria 1. PROKARYOTIC no membrane bound nucleus nor other organelles
MICROBIOLOGY CHAPTER 3 Bacteria Morphology 3:1 Bacteria Structure and Function MORPHOLOGY: the study of form and structure Structure of Bacteria 1. PROKARYOTIC no membrane bound nucleus nor other organelles
Vicente Fernandez. Group of Prof. Roman Stocker
 Microbial motility in complex fluid environments Vicente Fernandez Group of Prof. Roman Stocker Microbial motility in ecological context 5 70% of bacteria in the ocean are motile Hotspots dominate the
Microbial motility in complex fluid environments Vicente Fernandez Group of Prof. Roman Stocker Microbial motility in ecological context 5 70% of bacteria in the ocean are motile Hotspots dominate the
Organisms: We will need to have some examples in mind for our spherical cows.
 Lecture 4: Structure and Composition (Sept. 15) 4.1 Reading Assignment for Lectures 3-4: Phillips, Kondev, Theriot (PKT), Chapter 2 Problem Set 1 (due Sept. 24) now posted on the website. Cellular materials:
Lecture 4: Structure and Composition (Sept. 15) 4.1 Reading Assignment for Lectures 3-4: Phillips, Kondev, Theriot (PKT), Chapter 2 Problem Set 1 (due Sept. 24) now posted on the website. Cellular materials:
LABORATORY 7 ENDOSPORE STAIN AND BACTERIAL MOTILITY
 LABORATORY 7 ENDOSPORE STAIN AND BACTERIAL MOTILITY A. Endospore Stain B. Bacterial Motility A. ENDOSPORE STAIN DISCUSSION A few genera of bacteria, such as Bacillus and Clostridium have the ability to
LABORATORY 7 ENDOSPORE STAIN AND BACTERIAL MOTILITY A. Endospore Stain B. Bacterial Motility A. ENDOSPORE STAIN DISCUSSION A few genera of bacteria, such as Bacillus and Clostridium have the ability to
SPECIES OF ARCHAEA ARE MORE CLOSELY RELATED TO EUKARYOTES THAN ARE SPECIES OF PROKARYOTES.
 THE TERMS RUN AND TUMBLE ARE GENERALLY ASSOCIATED WITH A) cell wall fluidity. B) cell membrane structures. C) taxic movements of the cell. D) clustering properties of certain rod-shaped bacteria. A MAJOR
THE TERMS RUN AND TUMBLE ARE GENERALLY ASSOCIATED WITH A) cell wall fluidity. B) cell membrane structures. C) taxic movements of the cell. D) clustering properties of certain rod-shaped bacteria. A MAJOR
Multi-Scale Modeling and Simulation of the Growth of Bacterial Colony with Cell-Cell Mechanical Interactions
 Multi-Scale Modeling and Simulation of the Growth of Bacterial Colony with Cell-Cell Mechanical Interactions Hui Sun Department of Mathematics and Statistics California State University Long Beach SIAM
Multi-Scale Modeling and Simulation of the Growth of Bacterial Colony with Cell-Cell Mechanical Interactions Hui Sun Department of Mathematics and Statistics California State University Long Beach SIAM
SUPPLEMENTARY FIGURE 1. Force dependence of the unbinding rate: (a) Force-dependence
 (b) BSA-coated beads double exponential low force exponential high force exponential 1 unbinding time tb [sec] (a) unbinding time tb [sec] SUPPLEMENTARY FIGURES BSA-coated beads without BSA.2.1 5 1 load
(b) BSA-coated beads double exponential low force exponential high force exponential 1 unbinding time tb [sec] (a) unbinding time tb [sec] SUPPLEMENTARY FIGURES BSA-coated beads without BSA.2.1 5 1 load
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Contains ribosomes attached to the endoplasmic reticulum. Genetic material consists of linear chromosomes. Diameter of the cell is 1 m
 1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
Quorum sensing in plantassociated. S. Brook Peterson Parsek Lab UW Microbiology
 Quorum sensing in plantassociated bacteria S. Brook Peterson Parsek Lab UW Microbiology Outline What is quorum sensing? QS in plant associated bacteria What traits are regulated by QS? What benefits does
Quorum sensing in plantassociated bacteria S. Brook Peterson Parsek Lab UW Microbiology Outline What is quorum sensing? QS in plant associated bacteria What traits are regulated by QS? What benefits does
Coupling between switching regulation and torque
 Coupling between switching regulation and torque generation in bacterial flagellar motor Fan Bai 1, Tohru Minamino 1, Zhanghan Wu 2, Keiichi Namba 1*, Jianhua Xing 2* 1.Graduate School of Frontier Biosciences,
Coupling between switching regulation and torque generation in bacterial flagellar motor Fan Bai 1, Tohru Minamino 1, Zhanghan Wu 2, Keiichi Namba 1*, Jianhua Xing 2* 1.Graduate School of Frontier Biosciences,
Evolution in Action: The Power of Mutation in E. coli
 Evolution in Action: The Power of Mutation in E. coli by Merle K. Heidemann, College of Natural Science, Emeritus Peter J. T. White, Lyman Briggs College James J. Smith, Lyman Briggs College Michigan State
Evolution in Action: The Power of Mutation in E. coli by Merle K. Heidemann, College of Natural Science, Emeritus Peter J. T. White, Lyman Briggs College James J. Smith, Lyman Briggs College Michigan State
Sensing wetness: a new role for the bacterial flagellum
 The EMBO Journal (2005) 24, 2034 2042 & 2005 European Molecular Biology Organization All Rights Reserved 0261-4189/05 www.embojournal.org Sensing wetness: a new role for the bacterial flagellum THE EMBO
The EMBO Journal (2005) 24, 2034 2042 & 2005 European Molecular Biology Organization All Rights Reserved 0261-4189/05 www.embojournal.org Sensing wetness: a new role for the bacterial flagellum THE EMBO
Genetic Variation: The genetic substrate for natural selection. Horizontal Gene Transfer. General Principles 10/2/17.
 Genetic Variation: The genetic substrate for natural selection What about organisms that do not have sexual reproduction? Horizontal Gene Transfer Dr. Carol E. Lee, University of Wisconsin In prokaryotes:
Genetic Variation: The genetic substrate for natural selection What about organisms that do not have sexual reproduction? Horizontal Gene Transfer Dr. Carol E. Lee, University of Wisconsin In prokaryotes:
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Tuesday, December 27, 16
 Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Radial and spiral stream formation in Proteus mirabilis colonies Chuan Xue, Elena Budrene, and Hans G. Othmer
 Radial and spiral stream formation in Proteus mirabilis colonies Chuan Xue, Elena Budrene, and Hans G. Othmer Mathematical Biosciences Institute, the Ohio State University, Columbus, OH, USA, Department
Radial and spiral stream formation in Proteus mirabilis colonies Chuan Xue, Elena Budrene, and Hans G. Othmer Mathematical Biosciences Institute, the Ohio State University, Columbus, OH, USA, Department
arxiv:physics/ v2 [physics.bio-ph] 24 Aug 1999
![arxiv:physics/ v2 [physics.bio-ph] 24 Aug 1999 arxiv:physics/ v2 [physics.bio-ph] 24 Aug 1999](/thumbs/83/88149091.jpg) Adaptive Ising Model of Bacterial Chemotactic Receptor Network Yu Shi arxiv:physics/9901053v2 [physics.bio-ph] 24 Aug 1999 Cavendish Laboratory, University of Cambridge, Cambridge CB3 0HE, United Kingdom
Adaptive Ising Model of Bacterial Chemotactic Receptor Network Yu Shi arxiv:physics/9901053v2 [physics.bio-ph] 24 Aug 1999 Cavendish Laboratory, University of Cambridge, Cambridge CB3 0HE, United Kingdom
Principles of Cellular Biology
 Principles of Cellular Biology آشنایی با مبانی اولیه سلول Biologists are interested in objects ranging in size from small molecules to the tallest trees: Cell Basic building blocks of life Understanding
Principles of Cellular Biology آشنایی با مبانی اولیه سلول Biologists are interested in objects ranging in size from small molecules to the tallest trees: Cell Basic building blocks of life Understanding
LIFE CYCLE OF DICTYOSTELIUM DISCOIDEUM
 LIFE CYCLE OF DICTYOSTELIUM DISCOIDEUM l 1. HISTORICAL Cellular slime molds were first discovered by Brefeld in 1869 and the uniqueness of their asexual life cycle was first recognized by the french mycologist
LIFE CYCLE OF DICTYOSTELIUM DISCOIDEUM l 1. HISTORICAL Cellular slime molds were first discovered by Brefeld in 1869 and the uniqueness of their asexual life cycle was first recognized by the french mycologist
Advanced Higher Biology. Unit 1- Cells and Proteins 2c) Membrane Proteins
 Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
The outer membrane of Borrelia 7/3/2014. LDA Conference Richard Bingham 1. The Outer Membrane of Borrelia; The Interface Between Them and Us
 The Outer Membrane of Borrelia; The Interface Between Them and Us Richard Bingham The University of Huddersfield Lecture Outline I will give an overview of the outer membrane of Borrelia I will present
The Outer Membrane of Borrelia; The Interface Between Them and Us Richard Bingham The University of Huddersfield Lecture Outline I will give an overview of the outer membrane of Borrelia I will present
The kinky propulsion of Spiroplasma
 The kinky propulsion of Spiroplasma Anna Tuttle Microbiology Journal Club October 13, 2008 How do bacteria move? Flagellar helical propellor driven by rotary motor Some, like Spirochetes, have internal
The kinky propulsion of Spiroplasma Anna Tuttle Microbiology Journal Club October 13, 2008 How do bacteria move? Flagellar helical propellor driven by rotary motor Some, like Spirochetes, have internal
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Supporting Information
 Supporting Information López et al. 10.1073/pnas.0810940106 1. Ivey DM, et al. (1993) Cloning and characterization of a putative Ca2 /H antiporter gene from Escherichia coli upon functional complementation
Supporting Information López et al. 10.1073/pnas.0810940106 1. Ivey DM, et al. (1993) Cloning and characterization of a putative Ca2 /H antiporter gene from Escherichia coli upon functional complementation
Under the Radar Screen: How Bugs Trick Our Immune Defenses
 Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 2: Phagocytosis Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research Salmonella Gram negative bacteria
Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 2: Phagocytosis Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research Salmonella Gram negative bacteria
Microbial Interactions: Essential Part of Below-Ground Biocontrol Wietse de Boer
 Microbial Interactions: Essential Part of Below-Ground Biocontrol Wietse de Boer NIOO-KNAW (Microbial Ecology) WUR (Soil Quality) Wageningen Email: w.deboer@nioo.knaw.nl Rhizosphere: Hotspot of Microbial
Microbial Interactions: Essential Part of Below-Ground Biocontrol Wietse de Boer NIOO-KNAW (Microbial Ecology) WUR (Soil Quality) Wageningen Email: w.deboer@nioo.knaw.nl Rhizosphere: Hotspot of Microbial
ATP hydrolysis 1 1 1
 ATP hydrolysis 1 1 1 ATP hydrolysis 2 2 2 The binding zipper 1 3 3 ATP hydrolysis/synthesis is coupled to a torque Yasuda, R., et al (1998). Cell 93:1117 1124. Abrahams, et al (1994). Nature 370:621-628.
ATP hydrolysis 1 1 1 ATP hydrolysis 2 2 2 The binding zipper 1 3 3 ATP hydrolysis/synthesis is coupled to a torque Yasuda, R., et al (1998). Cell 93:1117 1124. Abrahams, et al (1994). Nature 370:621-628.
NOTE: Questions are written on both sides of the sheets of paper making up this exam booklet!
 Biology 1010 Section A Midterm 1 January 30, 2008 (print): ANSWER KEY Name (signature): Student I.D. #: Time: 50 minutes Read the following instructions: 1. Do not open the examination until you are instructed
Biology 1010 Section A Midterm 1 January 30, 2008 (print): ANSWER KEY Name (signature): Student I.D. #: Time: 50 minutes Read the following instructions: 1. Do not open the examination until you are instructed
Molecular Mechanisms of self-assembly and motion of the bacterial flagellum
 Molecular Mechanisms of self-assembly and motion of the bacterial flagellum Mexico-Japan Workshop on Pharmacobiology and Nanobiology Universidad Nacional Autonoma de México (UNAM) Mexico City, Mexico 2009.02.25-26
Molecular Mechanisms of self-assembly and motion of the bacterial flagellum Mexico-Japan Workshop on Pharmacobiology and Nanobiology Universidad Nacional Autonoma de México (UNAM) Mexico City, Mexico 2009.02.25-26
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement
 1 Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement In the last lecture, we saw that a repeating alternation between chemical (ATP hydrolysis) and vectorial
1 Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement In the last lecture, we saw that a repeating alternation between chemical (ATP hydrolysis) and vectorial
CE 421/521 Environmental Biotechnology. The Cell: The common denominator of all living things Chapter 4 in Vaccari et al. Tim Ellis August 24, 2006
 CE 421/521 Environmental Biotechnology The Cell: The common denominator of all living things Chapter 4 in Vaccari et al. Tim Ellis August 24, 2006 Introduction Cells were discovered around the same time
CE 421/521 Environmental Biotechnology The Cell: The common denominator of all living things Chapter 4 in Vaccari et al. Tim Ellis August 24, 2006 Introduction Cells were discovered around the same time
Biomaterial Scaffolds
 Biomaterial Scaffolds Biomaterial Properties Surface properties Bulk properties Biological properties Types of Biomaterials Biological materials Synthetic materials Surface Properties The body reads the
Biomaterial Scaffolds Biomaterial Properties Surface properties Bulk properties Biological properties Types of Biomaterials Biological materials Synthetic materials Surface Properties The body reads the
IMECE TRACKING BACTERIA IN A MICROFLUIDIC CHEMOTAXIS ASSAY
 Proceedings of IMECE 2008 2008 ASME International Mechanical Engineering Congress and Exposition October 31 November 6, 2008, Boston, Massachusetts, USA IMECE2008-66436 TRACKING BACTERIA IN A MICROFLUIDIC
Proceedings of IMECE 2008 2008 ASME International Mechanical Engineering Congress and Exposition October 31 November 6, 2008, Boston, Massachusetts, USA IMECE2008-66436 TRACKING BACTERIA IN A MICROFLUIDIC
Investigation of the E. coli Flagellar Motor using Optical Tweezers
 FOPRA 2017/2018 Physik-Department Lehrstuhl für Biophysik E22 Technische Universität München Investigation of the E. coli Flagellar Motor using Optical Tweezers Alfredo Sciortino (alfredo.sciortino@tum.de)
FOPRA 2017/2018 Physik-Department Lehrstuhl für Biophysik E22 Technische Universität München Investigation of the E. coli Flagellar Motor using Optical Tweezers Alfredo Sciortino (alfredo.sciortino@tum.de)
Ch 3. Bacteria and Archaea
 Ch 3 Bacteria and Archaea SLOs for Culturing of Microorganisms Compare and contrast the overall cell structure of prokaryotes and eukaryotes. List structures all bacteria possess. Describe three basic
Ch 3 Bacteria and Archaea SLOs for Culturing of Microorganisms Compare and contrast the overall cell structure of prokaryotes and eukaryotes. List structures all bacteria possess. Describe three basic
Authors Nicholas W Frankel, William Pontius, Yann S Dufour, Junjiajia Long, Luis Hernandez- Nunez, Thierry Emonet*
 Title Adaptability of non-genetic diversity in bacterial chemotaxis Authors Nicholas W Frankel, William Pontius, Yann S Dufour, Junjiajia Long, Luis Hernandez- Nunez, Thierry Emonet* Department of Molecular
Title Adaptability of non-genetic diversity in bacterial chemotaxis Authors Nicholas W Frankel, William Pontius, Yann S Dufour, Junjiajia Long, Luis Hernandez- Nunez, Thierry Emonet* Department of Molecular
ENTEROBACTER AEROGENES UNKNOWN BACTERIA FLOW CHART UNKNOWN LAB REPORT, MICROBIOLOGY ENTEROBACTER AEROGENES
 ENTEROBACTER AEROGENES UNKNOWN BACTERIA PDF UNKNOWN LAB REPORT, MICROBIOLOGY ENTEROBACTER AEROGENES IDENTIFICATION OF AN UNKNOWN BACTERIAL SPECIES OF 1 / 5 2 / 5 3 / 5 enterobacter aerogenes unknown bacteria
ENTEROBACTER AEROGENES UNKNOWN BACTERIA PDF UNKNOWN LAB REPORT, MICROBIOLOGY ENTEROBACTER AEROGENES IDENTIFICATION OF AN UNKNOWN BACTERIAL SPECIES OF 1 / 5 2 / 5 3 / 5 enterobacter aerogenes unknown bacteria
Membranes 2: Transportation
 Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Biology: Life on Earth
 Teresa Audesirk Gerald Audesirk Bruce E. Byers Biology: Life on Earth Eighth Edition Lecture for Chapter 4 Cell Structure and Function Copyright 2008 Pearson Prentice Hall, Inc. Chapter 4 Outline 4.1 What
Teresa Audesirk Gerald Audesirk Bruce E. Byers Biology: Life on Earth Eighth Edition Lecture for Chapter 4 Cell Structure and Function Copyright 2008 Pearson Prentice Hall, Inc. Chapter 4 Outline 4.1 What
Heme-based NO sensors. HNOX: Heme-nitric oxide/oxygen binding domain/protein Bacterial
 Heme-based sensors HX: Heme-nitric oxide/oxygen binding domain/protein Bacterial 1 J. Inorg. Biochem. 99, 892 (2005). Soluble guanylate cyclase (sgc) is a nitric oxide () sensing hemoprotein that has been
Heme-based sensors HX: Heme-nitric oxide/oxygen binding domain/protein Bacterial 1 J. Inorg. Biochem. 99, 892 (2005). Soluble guanylate cyclase (sgc) is a nitric oxide () sensing hemoprotein that has been
Radial and Spiral Stream Formation in Proteus mirabilis Colonies
 in Proteus mirabilis Colonies Chuan Xue 1 *, Elena O. Budrene 2, Hans G. Othmer 3 1 Mathematical Biosciences Institute, the Ohio State University, Columbus, Ohio, United States of America, 2 Department
in Proteus mirabilis Colonies Chuan Xue 1 *, Elena O. Budrene 2, Hans G. Othmer 3 1 Mathematical Biosciences Institute, the Ohio State University, Columbus, Ohio, United States of America, 2 Department
Shape, Arrangement, and Size. Cocci (s., coccus) bacillus (pl., bacilli) 9/21/2013
 Shape, Arrangement, and Size Cocci (s., coccus) are roughly spherical cells. The other common shape is that of a rod, sometimes called a bacillus (pl., bacilli). Spiral-shaped procaryotes can be either
Shape, Arrangement, and Size Cocci (s., coccus) are roughly spherical cells. The other common shape is that of a rod, sometimes called a bacillus (pl., bacilli). Spiral-shaped procaryotes can be either
The Prokaryotic World
 The Prokaryotic World A. An overview of prokaryotic life There is no doubt that prokaryotes are everywhere. By everywhere, I mean living in every geographic region, in extremes of environmental conditions,
The Prokaryotic World A. An overview of prokaryotic life There is no doubt that prokaryotes are everywhere. By everywhere, I mean living in every geographic region, in extremes of environmental conditions,
The type IV pilus - WWU Münster Kalvi Institute,
 The type IV pilus - a multifunctional bacterial motor Berenike Maier WWU Münster Kalvi Institute, 1.2.2011 1 Type IV pili are ubiquitous multifunctional polymers 2 Type IV pili are ubiquitous multifunctional
The type IV pilus - a multifunctional bacterial motor Berenike Maier WWU Münster Kalvi Institute, 1.2.2011 1 Type IV pili are ubiquitous multifunctional polymers 2 Type IV pili are ubiquitous multifunctional
Molecular Motors. Structural and Mechanistic Overview! Kimberly Nguyen - December 6, 2013! MOLECULAR MOTORS - KIMBERLY NGUYEN
 Molecular Motors Structural and Mechanistic Overview!! Kimberly Nguyen - December 6, 2013!! 1 Molecular Motors: A Structure and Mechanism Overview! Introduction! Molecular motors are fundamental agents
Molecular Motors Structural and Mechanistic Overview!! Kimberly Nguyen - December 6, 2013!! 1 Molecular Motors: A Structure and Mechanism Overview! Introduction! Molecular motors are fundamental agents
Analysis of Escherichia coli amino acid transporters
 Ph.D thesis Analysis of Escherichia coli amino acid transporters Presented by Attila Szvetnik Supervisor: Dr. Miklós Kálmán Biology Ph.D School University of Szeged Bay Zoltán Foundation for Applied Research
Ph.D thesis Analysis of Escherichia coli amino acid transporters Presented by Attila Szvetnik Supervisor: Dr. Miklós Kálmán Biology Ph.D School University of Szeged Bay Zoltán Foundation for Applied Research
Optical Tweezers. The Useful Micro-Manipulation Tool in Research
 Optical Tweezers The Useful Micro-Manipulation Tool in Research Student: Nikki Barron Class: Modern Physics/ Biophysics laboratory Advisor: Grant Allen Instructor: David Kleinfeld Date: June 15, 2012 Introduction
Optical Tweezers The Useful Micro-Manipulation Tool in Research Student: Nikki Barron Class: Modern Physics/ Biophysics laboratory Advisor: Grant Allen Instructor: David Kleinfeld Date: June 15, 2012 Introduction
Horizontal transfer and pathogenicity
 Horizontal transfer and pathogenicity Victoria Moiseeva Genomics, Master on Advanced Genetics UAB, Barcelona, 2014 INDEX Horizontal Transfer Horizontal gene transfer mechanisms Detection methods of HGT
Horizontal transfer and pathogenicity Victoria Moiseeva Genomics, Master on Advanced Genetics UAB, Barcelona, 2014 INDEX Horizontal Transfer Horizontal gene transfer mechanisms Detection methods of HGT
THE THIRD GENERAL TRANSPORT SYSTEM BRANCHED-CHAIN AMINO ACIDS IN SALMONELLA T YPHIMURI UM KEIKO MATSUBARA, KUNIHARU OHNISHI, AND KAZUYOSHI KIRITANI
 J. Gen. Appl. Microbiol., 34, 183-189 (1988) THE THIRD GENERAL TRANSPORT SYSTEM BRANCHED-CHAIN AMINO ACIDS IN SALMONELLA T YPHIMURI UM FOR KEIKO MATSUBARA, KUNIHARU OHNISHI, AND KAZUYOSHI KIRITANI Department
J. Gen. Appl. Microbiol., 34, 183-189 (1988) THE THIRD GENERAL TRANSPORT SYSTEM BRANCHED-CHAIN AMINO ACIDS IN SALMONELLA T YPHIMURI UM FOR KEIKO MATSUBARA, KUNIHARU OHNISHI, AND KAZUYOSHI KIRITANI Department
Zool 3200: Cell Biology Exam 5 4/27/15
 Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
Name: Trask Zool 3200: Cell Biology Exam 5 4/27/15 Answer each of the following short answer questions in the space provided, giving explanations when asked to do so. Circle the correct answer or answers
Adventures in Multicellularity
 Adventures in Multicellularity The social amoeba (a.k.a. slime molds) Dictyostelium discoideum Dictyostelium discoideum the most studied of the social amoebae / cellular slime molds predatory soil amoeba
Adventures in Multicellularity The social amoeba (a.k.a. slime molds) Dictyostelium discoideum Dictyostelium discoideum the most studied of the social amoebae / cellular slime molds predatory soil amoeba
Assist. Prof. Martina Šeruga Musić acad. year 2016/17
 Assist. Prof. Martina Šeruga Musić acad. year 2016/17 PHYTOPATHOGENIC BACTERIA there are more than 100 species of known phytopathogenic bacteria genera Agrobacterium, Erwinia, Ralstonia, Pseudomonas, Xanthomonas,
Assist. Prof. Martina Šeruga Musić acad. year 2016/17 PHYTOPATHOGENIC BACTERIA there are more than 100 species of known phytopathogenic bacteria genera Agrobacterium, Erwinia, Ralstonia, Pseudomonas, Xanthomonas,
Lecture 4: viscoelasticity and cell mechanics
 Teaser movie: flexible robots! R. Shepherd, Whitesides group, Harvard 1 Lecture 4: viscoelasticity and cell mechanics S-RSI Physics Lectures: Soft Condensed Matter Physics Jacinta C. Conrad University
Teaser movie: flexible robots! R. Shepherd, Whitesides group, Harvard 1 Lecture 4: viscoelasticity and cell mechanics S-RSI Physics Lectures: Soft Condensed Matter Physics Jacinta C. Conrad University
SYLLABUS. Meeting Basic of competence Topic Strategy Reference
 SYLLABUS Faculty : Mathematics and science Study Program : Biology education Lecture/Code : Microbiology/BIO 236 Credits : 2 unit of semester credit Semester : 5 Prerequisites lecture : Biochemistry, Cell
SYLLABUS Faculty : Mathematics and science Study Program : Biology education Lecture/Code : Microbiology/BIO 236 Credits : 2 unit of semester credit Semester : 5 Prerequisites lecture : Biochemistry, Cell
Gene expression in prokaryotic and eukaryotic cells, Plasmids: types, maintenance and functions. Mitesh Shrestha
 Gene expression in prokaryotic and eukaryotic cells, Plasmids: types, maintenance and functions. Mitesh Shrestha Plasmids 1. Extrachromosomal DNA, usually circular-parasite 2. Usually encode ancillary
Gene expression in prokaryotic and eukaryotic cells, Plasmids: types, maintenance and functions. Mitesh Shrestha Plasmids 1. Extrachromosomal DNA, usually circular-parasite 2. Usually encode ancillary
Mechanical analysis of phase transition experiments of the bacterial flagellar filament
 Acta Mech Sin (2010) 26:777 785 DOI 10.1007/s10409-010-0364-1 RESEARCH PAPER Mechanical analysis of phase transition experiments of the bacterial flagellar filament Xiao-Ling Wang Qing-Ping Sun Received:
Acta Mech Sin (2010) 26:777 785 DOI 10.1007/s10409-010-0364-1 RESEARCH PAPER Mechanical analysis of phase transition experiments of the bacterial flagellar filament Xiao-Ling Wang Qing-Ping Sun Received:
Model Solutions Spring 2003
 Exam 2 BE.462J/3.962J Model Solutions Spring 2003 (80 points total possible) 1. (10 points) Explain the phenomenon of phsensitive swelling in polyelectrolyte hydrogels. Why does the swelling depend on
Exam 2 BE.462J/3.962J Model Solutions Spring 2003 (80 points total possible) 1. (10 points) Explain the phenomenon of phsensitive swelling in polyelectrolyte hydrogels. Why does the swelling depend on
