NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor. F j. T mo Assumptions:
|
|
|
- Gloria Blankenship
- 5 years ago
- Views:
Transcription
1 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flw Reactr T T T T F j, Q F j T m,q m T m T m T m Aumptin: 1. Hmgeneu Sytem 2. Single Reactin 3. Steady State Tw type f prblem: 1. Given deired prductin rate, cnverin and kinetic and ther parameter, determine the required reactr ize, heat duty and temperature prfile. 2. Given reactr ize, kinetic, etc., determine the cmpitin f the exit tream. Let u cnider a ingle reactin υ j A j = 0 (1) with the rate given by r = 0 e E 1 / RT Π C j α j k 20 e E 2 / RT Π C j β j (2) with υ M j / A j υ C j = C A T P A (3) 1+ ε A TP The ma balance in the reactr fr pecie j can be written a: df j dv = υ j r (4) r v = 0 F j = F j (4a) 1
2 F A d dv = ( v A )r = R A (4 ) V = 0 = 0 (4 a) The energy balance baed n (a) negligible change in ptential and kinetic energy and (b) n wrk ther than flw wrk i d dv j=1 F j H j + q v = 0 (5) V = 0 F j H 1 = F j H j (5a) Baed n further aumptin f (c) ideal mixture and (d) ideal gae ne get: j=1 F j C ~ p j dt dv j=1 H ~ j df j dv + q = 0 v (6a) Uing the idea f (e) mean pecific heat which are cntant and (f) cntant heat f reactin, ne get dt (Qρ)C p dv + ( ΔH )r + q = 0 (6) r v Qρ = m tt i the ma flw rate which i cntant J q v i the rate f heat additin per unit reactr vlume m 3 The implet cntitutive relatinhip fr the rate f heat exchange i: q v = Ua v (T m T) (7) m 2 a v - area fr heat tranfer per unit reactr vlume m 3 The equatin t be lved imultaneuly are: dx F A a dv + υ Ar = 0 (A) q dt v QρC pm dv ( ΔH r) r + U av (T T m ) = 0 (B) 2
3 Q m ρ m C pm q dt v m dv U av(t m T) = 0 (C) V = 0; = 0; T = T,, (T m = T m fr ccurrent flw) V = V; (T m = T m fr cuntercurrent flw) (D) and G du dz + dp dz + F = 0 (E) G = ρu - ma velcity P = preure z = V A - axial ditance u = Q A - velcity A cr ectinal reactr area F frictinal le Equatin (E) i the mmentum balance. Hwever thi equatin i uually lved eparately and a mean preure i elected fr evaluatin f ga cncentratin in eq (3). Fr gae the ue f ma fractin, w j, and extent per unit ma, ξ '' i recmmended. (See lecture 1). The equatin can then be written a: '' dξ G dz = r (8) G dt dz = β '' '' r + q v (9) z = 0 ξ '' = 0, T = T (10) β '' = ΔH r C p ; q '' v = q v C p (11) 3
4 where the rate i expreed by: r = 0 e E 1 / RT Π k 20 e E 2 / RT α j m 1+ υ tt j ξ '' F j C j Π C j α j β j m 1+ υ bt j ξ '' F j j=1 T P 1 TP 1+ υ j M av ξ j=1 β j υ j T P 1 TP 1+ ( y)m av ξ "" v j (12) M av - average mlecular weight at feed cnditin m tt = GA ma flw rate m tt F j = M j w j w j ma factin f j in the feed. Fr liquid ne can write dξ dτ = r (13) dt dτ = β r + q v (14) τ = 0 ; ξ = 0 ; T = T (15) β = ΔH r ρc p ; q v = q v '' = Q v ρc p ρ (16) where the rate i given by r = 0 e E 1 / RT Π ( C j + υ j ξ) α j k 20 e E 2 / RT Π ( C j + υ j ξ) β j (17) τ = z u = V - reidence time alng the reactr. Q 4
5 Frm eq (8) and (9) r (13) & (14) we can alway get the fllwing relatinhip between temperature and extent r T = T + β '' ξ '' + 1 G z q '' v dz (18a) τ T = T + β ξ + q v dτ (18b) '' Fr adiabatic peratin (q v = 0, q v = 0 ) thi yield the equatin f the adiabatic line, i.e extent and temperature atify the relatinhip belw at any and every pint f the reactr T = T + β '' ξ '' T = T + β ξ (19a) (19b) The maximum fractinal adiabatic temperature rie i given by the Prater number jut like in the cae f a CSTR. ( = β = ΔH r)c A (20) T ( υ A )T ρc p ΔT ad max Baic type f prblem 1. The temperature in the reactr i precribed a. T(z) = T ithermal reactr. Integrate (8) r (13) and find extent alng the reactr. Frm eq. (9) r (14) find the heat additin/remval requirement alng the reactr and the verall heat duty fr the reactr. b. T(z) pecified. Integrate (8) r (13) find ξ (z). Ue ξ (z) and T(z) in eq (9)r (14) t get q v (z) 2. The heat additin (remval) rate i precribed a) Adiabatic peratin. T = T + β '' ξ '' r T = T + βξ. Subtitute int eq (8) r (13) and integrate b) Heat duty i precribed. q v '' (z) r q v (z) precribed. Simultaneuly integrate (8) r (9) r ubtitute z T = T + β '' ξ '' + 1 G q '' vdz int (8) and integrate. 5
6 3. Rate f heat additin (remval) cntrlled by anther equatin q v = Ua v (T T m ) a) T m = cnt. Integrate eq (8) and (9) r eq (13) and (14) imultaneuly. Thi i the cae when reactr tube are immered in biling medium r cndening medium. b) T m determined with T and ξ by equatin (A) t (E). dt G m m dz = κ ' m T m T ( ) κ m = Ua v m C pm G m = Q mρ m A m Nte: With ccurrent cling a PFR can be kept ithermal with cuntercurrent cling it cannt in the cae f n-th rder reactin. Prve that fr an exercie. There i alway a unique teady tate in a PFR. Main prblem with PFR i: ht pt frmatin parametric enitivity and temperature runaway. Claical example f temperature runaway preented by Bilu & Amundn (AIChE J., 2, 117 (1956)). PFR cled frm the wall t cntant T m = T wall T T m = τ 6
7 A ht pt i frmed due t a very mall change in wall temperature. The ytem hw extreme parameter enitivity. Reactin runaway i the phenmenn when a mall change in feed cncentratin, temperature, flw rate r in clant temperature trigger a dramatic change in he temperature prfile and lead t runaway reactin and explin. Exact criteria fr runaway are difficult t develp. Apprximate criteria are given n the encled graph. Example 1 A reverible firt rder reactin (cnidered earlier in a CSTR) i nw t be per frmed in a PFR. A R (liquid phae) = 5x10 8 e 12,500 / RT (min 1 ) k 2 = 3.4x10 21 e 32,500 / RT ( min 1 ) ΔH r = 20,000 cal /ml ΔG 298 ρc p 2,000 (cal /lit C) C A = 2 (ml /lit) = 2,500 cal /ml If the feed rate i Q = 100 (lit/min) and the PFR ize i V = 1,500 (lit): a) find final cnverin in an ithermal reactr perated at 0, 10, 20, 100 C b) determine cnverin in an adiabatic reactr if the feed i at i) 0 C, ii) 20 C, c) if the maximum permiible temperature i 80 C determine the ptimal temperature prfile alng the reactr neceary t maximize exit cnverin. d) If the deired cnverin i 85% find the minimum reactr vlume and the deired heat remval rate alng the reactr. Permiible temperature range i 0 t 100 C. Slutin a) Fr an ithermal reactr nly the ma balance ha t be lved τ = V dx = C A A Q r A r A = C A k 2 C R = C A [ (1 ) k 2 ] r A = C A 1 x (1 x ) A Ae ince k 2 = e K = x 1 Ae e ( r A ) = k C 1 A (e ) e = K e 1+ K = + k 2 7 ( )
8 ( r A ) = ( + k 2 )C A (e ) x 1 A dx τ = A 1 x = n Ae + k 2 e + k 2 e Slve fr cnverin 1 exp( (1+ 1 K )τ) = e 1 exp k 1 τ τ = 1500 =15 min e 100 We get the fllwing reult: T K x ae x a Same a equilibrium cnverin The reactr pace time i large that abve 50 C practically equilibrium cnverin i btained. a) The adiabatic perating line i T = T + β A C A β A = ΔH r A = 20,000 ρc p 2,000 =10 lit C ml C A = 2 ml lit T = T
9 Subtitute thi relatinhip int the ma balance and integrate: dx C A A dτ = (k + k )C (x x ) = k C (k + k )C x 1 2 A Ae A 1 A 1 2 A A τ = 0 = 0 = 0 e E 1 / RT ad = 0 e E 1 / R(T +20 ) k 2 = k 20 e E 2 / R(T +20 ) e = K 1+ K = + k 2 Thu integrate numerically ( ) ; τ = 0 = 0 d dτ = 0 e E 1 / RT (T +20x ) A 0 e E1 / R( T+20xA ) + k20 e E 2 / R(T +20 ) d dτ = 5x108 e 12, (T +20x ) A 5x10 8 e 12, (T +20x ) A x10 21 e 32,500 Deired reult i btained at τ = 15. Alternatively we culd lve by trial and errr the fllwing integral: 1.987(T +20 x ; τ = 0 ; A = 0 τ =15 = We find: dx 5x10 8 e 12, (T +20x ) 5x10 8 e 12, (T +20x ) + 3.4x10 21 e 32, (T +20x) x i) T = 0 C = 273 K = 0.78 ΔT adiabatic =15.7K =16K T = 289 K ii) T = 20 C = 293 K = 0.94 = e ΔT adiabatic =18.8 =19K T = 292K c) T maximize cnverin at given pace time we huld fllw the line f maximum rate. (E T m = 2 E 1 /R) 10,065 n k E n x = A x n A 0 E 1 1 x A 1 Since maximum permiible temperature i 80 C (353 K) we have t preheat the feed t 33 K, cl the reactr and keep it ithermal a 353 K until the lcu f maximum rate i reached and then run alng the lcu f maximum rate. 9
10 The interectin f the ithermal line T = 353 K and the T m line determine up t which pint the reactr ha t be run ithermally. 10,065 T = 353 = T m = x n A 1 10, x30.51 exp 353 = = , x exp τ = + k 2 dx 1 = (e ) ( + k 2 ) n e e At 80 C (353 K) frm the table given earlier τ = 9.17(1+ 1 n = 0.017(min) 0.35 ) The ithermal peratin huld ccur in the very entry ectin f he reactr. After that the T m line huld be fllwed. d dτ = 5x108 e 12, T m (1 ) 3.42x10 21 e 32, T m 10,065 T m = x n A 1 τ = = Deired reult at τ = 15 =0.988 T exit = 288K Really ne huld preheat nly t adiabatic line. Adiabatic line huld end at T = 353 K, = Hence, the fluid mut be preheated up t T = T β A C A = x0.119 = 350K 10
11 The graphical repreentatin f part (a-c) ha the fllwing frm: e T a. Ithermal. Slid line are perating line fr τ = 15 min e b. Adiabatic. Adiabatic line with τ = 15 T T m e T max c. Operating alng the lcu f maximum rate d) Permiible temperature range i 0 C t 100 C. We want minimum reactr ize fr = Preheat t 100 C, run alng the lcu f maximum rate 11
12 τ = = 0.85 dx 5x10 8 e 12, T m 5x10 8 e 12, T m + 3.4x10 21 e 32, T m x 10,065 with T m = x n A 1 τ =1.8min Thu with Q = 100 lit/min we need nly V = 160 liter The deired temperature prfile alng the reactr i preented in the encled graph. The heat remval per unit vlume i q Q = ρc p (T T) + ( ΔH r )C A = 2,000(100 T) + (20,000x2) Thi curve i al preented in the figure. The ttal heat denity i: q Q tt = 2,000(100 70) + 40,000x0.85 =1.56x10 5 (cal /lit) With Q = 100 lit/min q tt =1.56x10 7 (cal /min) Fr cmparin, if cling failed and reactr ran adiabatically with T = 100 C ne wuld get adiabatic = 0.068,T exit =126 C The adiabatic temperature prfile i hwn al n the encled figure. 12
13 Extenin t Multiple Reactin υ ij A j = 0 i =1,2,...R (1) j=1 r R df j dv + υ r ij i = 0,2,...R (2) R i=1 υ ij i=1 d X i dv + d X i dv + r i = 0 ( H j ) d F j dv R υ ijr i = 0 i=1 + q v = 0 (3) (2a) V = 0 ; F j = F j ( X i = X i ) ; H j = H j With the uual aumptin made abut the energy balance (ee the lecture n CSTR) ne get: dt F j C ~ p j dv + j=1 R i=1 ( ΔH r Ti ) r i + q v = 0 (4) The equatin t be lved fr a et f multiple reactin are: d X i dv + r i = 0 i =1,2...R (A) ρc p Q dt R dv + ( ΔH r i )r i + q v = 0 i=1 V = 0 ; X i = X i T = T ρq = cnt r i = k i10 e E 1i / RT Π C j α ij k i20 e E 2i RT Π C j β ij (B) (C) with 13
14 1+ ρt C j = C j ρ T 1+ R υ ij i=1 F j R X i υ ij i=1 j=1 F bt X i (D) The cntitutive relatinhip fr q v i: q v = U av (T m T) a) T m = cnt b) T m i gverned by anther D.E. ρ m Q m C pm dt m dv q v = 0 (E) V = 0 T m = T m (ccurrent flw) V = V T m = T m (cuntercurrent flw) Prblem Cnider the reactin intrduced in the lat lecture A R R= C A -k 2 C R (ml/lit ) = exp 7 83,700 x10 3 ( -1 ) RT k 2 exp , ( -1 ) RT ΔH r = 80,000 (J /ml) C ~ p = 40(J /ml K) (Activatin energie given in jule) 1. The abve reactin ccur in liquid phae! Permiible temp range f peratin i 300<T<900 K. Feed cnditin: Q = 100 (lit/) ; T = 300 K ; C A = 1(ml/lit) Yu have a V = 100 liter PFR. Hw wuld yu perate thi reactr if the nly bjective i t maximize the prductin rate f R. 14
15 a) What i maximum F R. b) What are final and ΔT. c) What i the prfile f heat additin r remval fr every 10% f reactr vlume. d) What i the verall heat duty fr the reactr and any heat exchanger preceding it. e) Sketch yur ytem. 2. The abve reactin ccur in ga phae. The ga feed ate i Q =100(lit /) at T = 300K, P = 24.6 atm The feed i 50%A, 50% inert. Permiible temperature range i 250< T < 900 K. Preure i cntant in the reactr. Gae tart t cndene belw 250 K. Deired cnverin i 85%. a) What reactr vlume i needed if yu perate alng the lcu f maximum rate? b) What i the ditributin f heat duty alng the reactr? c) What i the prductin rate f R? 3. Fr the abve prblem what wuld F R and be if yu had a reactr (PFR) f V = 100 liter available? 4. Suppe that the reactr can nly be perated adiabatically and the deired cnverin i 85%. Minimize the required reactr ize. a) What reactr type d yu recmmend? b) What feed temperature wuld yu ue? c) What i the heat duty? 15
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
Chapter 9 Compressible Flow 667
 Chapter 9 Cmpreible Flw 667 9.57 Air flw frm a tank thrugh a nzzle int the tandard atmphere, a in Fig. P9.57. A nrmal hck tand in the exit f the nzzle, a hwn. Etimate (a) the tank preure; and (b) the ma
Chapter 9 Cmpreible Flw 667 9.57 Air flw frm a tank thrugh a nzzle int the tandard atmphere, a in Fig. P9.57. A nrmal hck tand in the exit f the nzzle, a hwn. Etimate (a) the tank preure; and (b) the ma
NONISOTHERMAL OPERATION OF IDEAL REACTORS Continuous Flow Stirred Tank Reactor (CSTR)
 he 47 Fall 2005 LEURE 7 NONISOHERML OPERION OF IDEL REORS ntinuu Flw Stirred ank Reactr (SR) F, Q V F r F, Q V F Figure : Scheatic f SR with acket and cil uptin: Hgeneu yte a) Single Reactin υ 0 b) Steady
he 47 Fall 2005 LEURE 7 NONISOHERML OPERION OF IDEL REORS ntinuu Flw Stirred ank Reactr (SR) F, Q V F r F, Q V F Figure : Scheatic f SR with acket and cil uptin: Hgeneu yte a) Single Reactin υ 0 b) Steady
Nonisothermal Chemical Reactors
 he 471 Fall 2014 LEUE 7a Nnithermal hemical eactr S far we have dealt with ithermal chemical reactr and were able, by ug nly a many pecie ma balance a there are dependent react t relate reactr ize, let
he 471 Fall 2014 LEUE 7a Nnithermal hemical eactr S far we have dealt with ithermal chemical reactr and were able, by ug nly a many pecie ma balance a there are dependent react t relate reactr ize, let
ChE 471: LECTURE 4 Fall 2003
 ChE 47: LECTURE 4 Fall 003 IDEL RECTORS One f the key gals f chemical reactin engineering is t quantify the relatinship between prductin rate, reactr size, reactin kinetics and selected perating cnditins.
ChE 47: LECTURE 4 Fall 003 IDEL RECTORS One f the key gals f chemical reactin engineering is t quantify the relatinship between prductin rate, reactr size, reactin kinetics and selected perating cnditins.
Chapter 8. Root Locus Techniques
 Chapter 8 Rt Lcu Technique Intrductin Sytem perfrmance and tability dt determined dby cled-lp l ple Typical cled-lp feedback cntrl ytem G Open-lp TF KG H Zer -, - Ple 0, -, -4 K 4 Lcatin f ple eaily fund
Chapter 8 Rt Lcu Technique Intrductin Sytem perfrmance and tability dt determined dby cled-lp l ple Typical cled-lp feedback cntrl ytem G Open-lp TF KG H Zer -, - Ple 0, -, -4 K 4 Lcatin f ple eaily fund
Chapter 8 Sections 8.4 through 8.6 Internal Flow: Heat Transfer Correlations. In fully-developed region. Neglect axial conduction
 Chapter 8 Sectin 8.4 thrugh 8.6 Internal Flw: Heat Tranfer Crrelatin T v cu p cp ( rt) k r T T k x r r r r r x In fully-develped regin Neglect axial cnductin u ( rt) r x r r r r r x T v T T T T T u r x
Chapter 8 Sectin 8.4 thrugh 8.6 Internal Flw: Heat Tranfer Crrelatin T v cu p cp ( rt) k r T T k x r r r r r x In fully-develped regin Neglect axial cnductin u ( rt) r x r r r r r x T v T T T T T u r x
1/2 and e0 e s ' 1+ imm w 4 M s 3 πρ0 r 3 m. n 0 ktr. .Also,since n 0 ktr 1,wehave. 4 3 M sπρ 0 r 3. ktr. 3 M sπρ 0
 Chapter 6 6.1 Shw that fr a very weak slutin drplet (m 4 3 πr3 ρ 0 M s ), (6.8) can be written as e 0 ' 1+ a r b r 3 where a σ 0 /n 0 kt and b imm w / 4 3 M sπρ 0. What is yur interpretatin f thecnd and
Chapter 6 6.1 Shw that fr a very weak slutin drplet (m 4 3 πr3 ρ 0 M s ), (6.8) can be written as e 0 ' 1+ a r b r 3 where a σ 0 /n 0 kt and b imm w / 4 3 M sπρ 0. What is yur interpretatin f thecnd and
Name Student ID. A student uses a voltmeter to measure the electric potential difference across the three boxes.
 Name Student ID II. [25 pt] Thi quetin cnit f tw unrelated part. Part 1. In the circuit belw, bulb 1-5 are identical, and the batterie are identical and ideal. Bxe,, and cntain unknwn arrangement f linear
Name Student ID II. [25 pt] Thi quetin cnit f tw unrelated part. Part 1. In the circuit belw, bulb 1-5 are identical, and the batterie are identical and ideal. Bxe,, and cntain unknwn arrangement f linear
University Chemistry Quiz /04/21 1. (10%) Consider the oxidation of ammonia:
 University Chemistry Quiz 3 2015/04/21 1. (10%) Cnsider the xidatin f ammnia: 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(l) (a) Calculate the ΔG fr the reactin. (b) If this reactin were used in a fuel cell,
University Chemistry Quiz 3 2015/04/21 1. (10%) Cnsider the xidatin f ammnia: 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(l) (a) Calculate the ΔG fr the reactin. (b) If this reactin were used in a fuel cell,
CHAPTER PRACTICE PROBLEMS CHEMISTRY
 Chemical Kinetics Name: Batch: Date: Rate f reactin. 4NH 3 (g) + 5O (g) à 4NO (g) + 6 H O (g) If the rate f frmatin f NO is 3.6 0 3 ml L s, calculate (i) the rate f disappearance f NH 3 (ii) rate f frmatin
Chemical Kinetics Name: Batch: Date: Rate f reactin. 4NH 3 (g) + 5O (g) à 4NO (g) + 6 H O (g) If the rate f frmatin f NO is 3.6 0 3 ml L s, calculate (i) the rate f disappearance f NH 3 (ii) rate f frmatin
Chapter 3. Electric Flux Density, Gauss s Law and Divergence
 Chapter 3. Electric Flu Denity, Gau aw and Diergence Hayt; 9/7/009; 3-1 3.1 Electric Flu Denity Faraday Eperiment Cncentric phere filled with dielectric material. + i gien t the inner phere. - i induced
Chapter 3. Electric Flu Denity, Gau aw and Diergence Hayt; 9/7/009; 3-1 3.1 Electric Flu Denity Faraday Eperiment Cncentric phere filled with dielectric material. + i gien t the inner phere. - i induced
Compressibility Effects
 Definitin f Cmpressibility All real substances are cmpressible t sme greater r lesser extent; that is, when yu squeeze r press n them, their density will change The amunt by which a substance can be cmpressed
Definitin f Cmpressibility All real substances are cmpressible t sme greater r lesser extent; that is, when yu squeeze r press n them, their density will change The amunt by which a substance can be cmpressed
CHEM Thermodynamics. Change in Gibbs Free Energy, G. Review. Gibbs Free Energy, G. Review
 Review Accrding t the nd law f Thermdynamics, a prcess is spntaneus if S universe = S system + S surrundings > 0 Even thugh S system
Review Accrding t the nd law f Thermdynamics, a prcess is spntaneus if S universe = S system + S surrundings > 0 Even thugh S system
" 1 = # $H vap. Chapter 3 Problems
 Chapter 3 rblems rblem At 1 atmsphere pure Ge melts at 1232 K and bils at 298 K. he triple pint ccurs at =8.4x1-8 atm. Estimate the heat f vaprizatin f Ge. he heat f vaprizatin is estimated frm the Clausius
Chapter 3 rblems rblem At 1 atmsphere pure Ge melts at 1232 K and bils at 298 K. he triple pint ccurs at =8.4x1-8 atm. Estimate the heat f vaprizatin f Ge. he heat f vaprizatin is estimated frm the Clausius
Thermodynamics Partial Outline of Topics
 Thermdynamics Partial Outline f Tpics I. The secnd law f thermdynamics addresses the issue f spntaneity and invlves a functin called entrpy (S): If a prcess is spntaneus, then Suniverse > 0 (2 nd Law!)
Thermdynamics Partial Outline f Tpics I. The secnd law f thermdynamics addresses the issue f spntaneity and invlves a functin called entrpy (S): If a prcess is spntaneus, then Suniverse > 0 (2 nd Law!)
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
Thermodynamics and Equilibrium
 Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
( ) kt. Solution. From kinetic theory (visualized in Figure 1Q9-1), 1 2 rms = 2. = 1368 m/s
 .9 Kinetic Mlecular Thery Calculate the effective (rms) speeds f the He and Ne atms in the He-Ne gas laser tube at rm temperature (300 K). Slutin T find the rt mean square velcity (v rms ) f He atms at
.9 Kinetic Mlecular Thery Calculate the effective (rms) speeds f the He and Ne atms in the He-Ne gas laser tube at rm temperature (300 K). Slutin T find the rt mean square velcity (v rms ) f He atms at
Conservation of Momentum
 Cnervatin f Mmentum PES 1150 Prelab Quetin Name: Lab Statin: 003 ** Diclaimer: Thi re-lab i nt t be cied, in whle r in art, unle a rer reference i made a t the urce. (It i trngly recmmended that yu ue
Cnervatin f Mmentum PES 1150 Prelab Quetin Name: Lab Statin: 003 ** Diclaimer: Thi re-lab i nt t be cied, in whle r in art, unle a rer reference i made a t the urce. (It i trngly recmmended that yu ue
CHAPTER Read Chapter 17, sections 1,2,3. End of Chapter problems: 25
 CHAPTER 17 1. Read Chapter 17, sectins 1,2,3. End f Chapter prblems: 25 2. Suppse yu are playing a game that uses tw dice. If yu cunt the dts n the dice, yu culd have anywhere frm 2 t 12. The ways f prducing
CHAPTER 17 1. Read Chapter 17, sectins 1,2,3. End f Chapter prblems: 25 2. Suppse yu are playing a game that uses tw dice. If yu cunt the dts n the dice, yu culd have anywhere frm 2 t 12. The ways f prducing
Lecture 13 - Boost DC-DC Converters. Step-Up or Boost converters deliver DC power from a lower voltage DC level (V d ) to a higher load voltage V o.
 ecture 13 - Bt C-C Cnverter Pwer Electrnic Step-Up r Bt cnverter eliver C pwer frm a lwer vltage C level ( ) t a higher la vltage. i i i + v i c T C (a) + R (a) v 0 0 i 0 R1 t n t ff + t T i n T t ff =
ecture 13 - Bt C-C Cnverter Pwer Electrnic Step-Up r Bt cnverter eliver C pwer frm a lwer vltage C level ( ) t a higher la vltage. i i i + v i c T C (a) + R (a) v 0 0 i 0 R1 t n t ff + t T i n T t ff =
When a substance heats up (absorbs heat) it is an endothermic reaction with a (+)q
 Chemistry Ntes Lecture 15 [st] 3/6/09 IMPORTANT NOTES: -( We finished using the lecture slides frm lecture 14) -In class the challenge prblem was passed ut, it is due Tuesday at :00 P.M. SHARP, :01 is
Chemistry Ntes Lecture 15 [st] 3/6/09 IMPORTANT NOTES: -( We finished using the lecture slides frm lecture 14) -In class the challenge prblem was passed ut, it is due Tuesday at :00 P.M. SHARP, :01 is
A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1
 A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1 (Nte: questins 1 t 14 are meant t be dne WITHOUT calculatrs!) 1.Which f the fllwing is prbably true fr a slid slute with a highly endthermic heat
A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1 (Nte: questins 1 t 14 are meant t be dne WITHOUT calculatrs!) 1.Which f the fllwing is prbably true fr a slid slute with a highly endthermic heat
Longitudinal Dispersion
 Updated: 3 Otber 017 Print verin Leture #10 (River & Stream, nt) Chapra, L14 (nt.) David A. Rekhw CEE 577 #10 1 Lngitudinal Diperin Frm Fiher et al., 1979 m/ m -1 E U B 0 011 HU. * Width (m) Where the
Updated: 3 Otber 017 Print verin Leture #10 (River & Stream, nt) Chapra, L14 (nt.) David A. Rekhw CEE 577 #10 1 Lngitudinal Diperin Frm Fiher et al., 1979 m/ m -1 E U B 0 011 HU. * Width (m) Where the
Chemistry 114 First Hour Exam
 Chemistry 114 First Hur Exam Please shw all wrk fr partial credit Name: (4 pints) 1. (12 pints) Espress is made by frcing very ht water under high pressure thrugh finely grund, cmpacted cffee. (Wikipedia)
Chemistry 114 First Hur Exam Please shw all wrk fr partial credit Name: (4 pints) 1. (12 pints) Espress is made by frcing very ht water under high pressure thrugh finely grund, cmpacted cffee. (Wikipedia)
Chem 75 February 16, 2017 Exam 2 Solutions
 1. (6 + 6 pints) Tw quick questins: (a) The Handbk f Chemistry and Physics tells us, crrectly, that CCl 4 bils nrmally at 76.7 C, but its mlar enthalpy f vaprizatin is listed in ne place as 34.6 kj ml
1. (6 + 6 pints) Tw quick questins: (a) The Handbk f Chemistry and Physics tells us, crrectly, that CCl 4 bils nrmally at 76.7 C, but its mlar enthalpy f vaprizatin is listed in ne place as 34.6 kj ml
1. Introduction: A Mixing Problem
 CHAPTER 7 Laplace Tranfrm. Intrductin: A Mixing Prblem Example. Initially, kg f alt are dilved in L f water in a tank. The tank ha tw input valve, A and B, and ne exit valve C. At time t =, valve A i pened,
CHAPTER 7 Laplace Tranfrm. Intrductin: A Mixing Prblem Example. Initially, kg f alt are dilved in L f water in a tank. The tank ha tw input valve, A and B, and ne exit valve C. At time t =, valve A i pened,
Autumn 2012 CHEM452B Bruce H. Robinson 322 Gould Hall HW 10(A) Homework 10A KEY (there will not be a 10B) 2
 Autumn 0 CHEM45B Bruce H. Rbinsn Guld Hall HW 0(A) Hmewrk 0A KEY (there will nt be a 0B) QA) Let c be the speed f sund in air. he square f the speed f sund, () f the gas with respect t the change in the
Autumn 0 CHEM45B Bruce H. Rbinsn Guld Hall HW 0(A) Hmewrk 0A KEY (there will nt be a 0B) QA) Let c be the speed f sund in air. he square f the speed f sund, () f the gas with respect t the change in the
Chapter 4. Unsteady State Conduction
 Chapter 4 Unsteady State Cnductin Chapter 5 Steady State Cnductin Chee 318 1 4-1 Intrductin ransient Cnductin Many heat transfer prblems are time dependent Changes in perating cnditins in a system cause
Chapter 4 Unsteady State Cnductin Chapter 5 Steady State Cnductin Chee 318 1 4-1 Intrductin ransient Cnductin Many heat transfer prblems are time dependent Changes in perating cnditins in a system cause
Heat Effects of Chemical Reactions
 * eat Effect f hemical Reactin Enthalpy change fr reactin invlving cmpund Enthalpy f frmatin f a cmpund at tandard cnditin i btained frm the literature a tandard enthalpy f frmatin Δ O g = -9690 J/mle
* eat Effect f hemical Reactin Enthalpy change fr reactin invlving cmpund Enthalpy f frmatin f a cmpund at tandard cnditin i btained frm the literature a tandard enthalpy f frmatin Δ O g = -9690 J/mle
Disclaimer: This lab write-up is not
 Diclaier: Thi lab write-up i nt t be cpied, in whle r in part, unle a prper reference i ade a t the urce. (It i trngly recended that yu ue thi dcuent nly t generate idea, r a a reference t explain cplex
Diclaier: Thi lab write-up i nt t be cpied, in whle r in part, unle a prper reference i ade a t the urce. (It i trngly recended that yu ue thi dcuent nly t generate idea, r a a reference t explain cplex
Examiner: Dr. Mohamed Elsharnoby Time: 180 min. Attempt all the following questions Solve the following five questions, and assume any missing data
 Benha University Cllege f Engineering at Banha Department f Mechanical Eng. First Year Mechanical Subject : Fluid Mechanics M111 Date:4/5/016 Questins Fr Final Crrective Examinatin Examiner: Dr. Mhamed
Benha University Cllege f Engineering at Banha Department f Mechanical Eng. First Year Mechanical Subject : Fluid Mechanics M111 Date:4/5/016 Questins Fr Final Crrective Examinatin Examiner: Dr. Mhamed
Chapters 29 and 35 Thermochemistry and Chemical Thermodynamics
 Chapters 9 and 35 Thermchemistry and Chemical Thermdynamics 1 Cpyright (c) 011 by Michael A. Janusa, PhD. All rights reserved. Thermchemistry Thermchemistry is the study f the energy effects that accmpany
Chapters 9 and 35 Thermchemistry and Chemical Thermdynamics 1 Cpyright (c) 011 by Michael A. Janusa, PhD. All rights reserved. Thermchemistry Thermchemistry is the study f the energy effects that accmpany
MODULE 5 Lecture No: 5 Extraterrestrial Radiation
 1 P age Principle and Perfrmance f Slar Energy Thermal Sytem: A Web Cure by V.V.Satyamurty MODULE 5 Lecture N: 5 Extraterretrial Radiatin In Mdule 5, Lecture N. 5 deal with 5.1 INTRODUCTION 5. EXTRA TERRESTRIAL
1 P age Principle and Perfrmance f Slar Energy Thermal Sytem: A Web Cure by V.V.Satyamurty MODULE 5 Lecture N: 5 Extraterretrial Radiatin In Mdule 5, Lecture N. 5 deal with 5.1 INTRODUCTION 5. EXTRA TERRESTRIAL
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY
 AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
Process Engineering Thermodynamics E (4 sp) Exam
 Prcess Engineering Thermdynamics 42434 E (4 sp) Exam 9-3-29 ll supprt material is allwed except fr telecmmunicatin devices. 4 questins give max. 3 pints = 7½ + 7½ + 7½ + 7½ pints Belw 6 questins are given,
Prcess Engineering Thermdynamics 42434 E (4 sp) Exam 9-3-29 ll supprt material is allwed except fr telecmmunicatin devices. 4 questins give max. 3 pints = 7½ + 7½ + 7½ + 7½ pints Belw 6 questins are given,
EE247B/ME218: Introduction to MEMS Design Lecture 7m1: Lithography, Etching, & Doping CTN 2/6/18
 EE247B/ME218 Intrductin t MEMS Design Lecture 7m1 Lithgraphy, Etching, & Dping Dping f Semicnductrs Semicnductr Dping Semicnductrs are nt intrinsically cnductive T make them cnductive, replace silicn atms
EE247B/ME218 Intrductin t MEMS Design Lecture 7m1 Lithgraphy, Etching, & Dping Dping f Semicnductrs Semicnductr Dping Semicnductrs are nt intrinsically cnductive T make them cnductive, replace silicn atms
BIT Chapters = =
 BIT Chapters 17-0 1. K w = [H + ][OH ] = 9.5 10 14 [H + ] = [OH ] =.1 10 7 ph = 6.51 The slutin is neither acidic nr basic because the cncentratin f the hydrnium in equals the cncentratin f the hydride
BIT Chapters 17-0 1. K w = [H + ][OH ] = 9.5 10 14 [H + ] = [OH ] =.1 10 7 ph = 6.51 The slutin is neither acidic nr basic because the cncentratin f the hydrnium in equals the cncentratin f the hydride
Part One: Heat Changes and Thermochemistry. This aspect of Thermodynamics was dealt with in Chapter 6. (Review)
 CHAPTER 18: THERMODYNAMICS AND EQUILIBRIUM Part One: Heat Changes and Thermchemistry This aspect f Thermdynamics was dealt with in Chapter 6. (Review) A. Statement f First Law. (Sectin 18.1) 1. U ttal
CHAPTER 18: THERMODYNAMICS AND EQUILIBRIUM Part One: Heat Changes and Thermchemistry This aspect f Thermdynamics was dealt with in Chapter 6. (Review) A. Statement f First Law. (Sectin 18.1) 1. U ttal
Pipetting 101 Developed by BSU CityLab
 Discver the Micrbes Within: The Wlbachia Prject Pipetting 101 Develped by BSU CityLab Clr Cmparisns Pipetting Exercise #1 STUDENT OBJECTIVES Students will be able t: Chse the crrect size micrpipette fr
Discver the Micrbes Within: The Wlbachia Prject Pipetting 101 Develped by BSU CityLab Clr Cmparisns Pipetting Exercise #1 STUDENT OBJECTIVES Students will be able t: Chse the crrect size micrpipette fr
EXAM #1 PHYSICAL SCIENCE 103 Spring, 2016
 OBJECTIVES 1. Ft Pressure EXAM #1 PHYSICAL SCIENCE 103 Spring, 2016 Determine the surface area f an bject. Given the weight and surface area, calculate the pressure. 2. Measuring Vlume & Mass Prvided a
OBJECTIVES 1. Ft Pressure EXAM #1 PHYSICAL SCIENCE 103 Spring, 2016 Determine the surface area f an bject. Given the weight and surface area, calculate the pressure. 2. Measuring Vlume & Mass Prvided a
Lecture 12: Chemical reaction equilibria
 3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
ECE-320: Linear Control Systems Homework 1. 1) For the following transfer functions, determine both the impulse response and the unit step response.
 Due: Mnday Marh 4, 6 at the beginning f la ECE-: Linear Cntrl Sytem Hmewrk ) Fr the fllwing tranfer funtin, determine bth the imule rene and the unit te rene. Srambled Anwer: H ( ) H ( ) ( )( ) ( )( )
Due: Mnday Marh 4, 6 at the beginning f la ECE-: Linear Cntrl Sytem Hmewrk ) Fr the fllwing tranfer funtin, determine bth the imule rene and the unit te rene. Srambled Anwer: H ( ) H ( ) ( )( ) ( )( )
ES201 - Examination 2 Winter Adams and Richards NAME BOX NUMBER
 ES201 - Examinatin 2 Winter 2003-2004 Adams and Richards NAME BOX NUMBER Please Circle One : Richards (Perid 4) ES201-01 Adams (Perid 4) ES201-02 Adams (Perid 6) ES201-03 Prblem 1 ( 12 ) Prblem 2 ( 24
ES201 - Examinatin 2 Winter 2003-2004 Adams and Richards NAME BOX NUMBER Please Circle One : Richards (Perid 4) ES201-01 Adams (Perid 4) ES201-02 Adams (Perid 6) ES201-03 Prblem 1 ( 12 ) Prblem 2 ( 24
Lecture 17: Free Energy of Multi-phase Solutions at Equilibrium
 Lecture 17: 11.07.05 Free Energy f Multi-phase Slutins at Equilibrium Tday: LAST TIME...2 FREE ENERGY DIAGRAMS OF MULTI-PHASE SOLUTIONS 1...3 The cmmn tangent cnstructin and the lever rule...3 Practical
Lecture 17: 11.07.05 Free Energy f Multi-phase Slutins at Equilibrium Tday: LAST TIME...2 FREE ENERGY DIAGRAMS OF MULTI-PHASE SOLUTIONS 1...3 The cmmn tangent cnstructin and the lever rule...3 Practical
CHEM 103 Calorimetry and Hess s Law
 CHEM 103 Calrimetry and Hess s Law Lecture Ntes March 23, 2006 Prf. Sevian Annuncements Exam #2 is next Thursday, March 30 Study guide, practice exam, and practice exam answer key are already psted n the
CHEM 103 Calrimetry and Hess s Law Lecture Ntes March 23, 2006 Prf. Sevian Annuncements Exam #2 is next Thursday, March 30 Study guide, practice exam, and practice exam answer key are already psted n the
Chapter Outline 4/28/2014. P-V Work. P-V Work. Isolated, Closed and Open Systems. Exothermic and Endothermic Processes. E = q + w
 Islated, Clsed and Open Systems 9.1 Energy as a Reactant r a Prduct 9.2 Transferring Heat and Ding Wrk 9.5 Heats f Reactin and Calrimetry 9.6 Hess s Law and Standard Heats f Reactin 9.7 Heats f Reactin
Islated, Clsed and Open Systems 9.1 Energy as a Reactant r a Prduct 9.2 Transferring Heat and Ding Wrk 9.5 Heats f Reactin and Calrimetry 9.6 Hess s Law and Standard Heats f Reactin 9.7 Heats f Reactin
More Tutorial at
 Answer each questin in the space prvided; use back f page if extra space is needed. Answer questins s the grader can READILY understand yur wrk; nly wrk n the exam sheet will be cnsidered. Write answers,
Answer each questin in the space prvided; use back f page if extra space is needed. Answer questins s the grader can READILY understand yur wrk; nly wrk n the exam sheet will be cnsidered. Write answers,
ALE 21. Gibbs Free Energy. At what temperature does the spontaneity of a reaction change?
 Name Chem 163 Sectin: Team Number: ALE 21. Gibbs Free Energy (Reference: 20.3 Silberberg 5 th editin) At what temperature des the spntaneity f a reactin change? The Mdel: The Definitin f Free Energy S
Name Chem 163 Sectin: Team Number: ALE 21. Gibbs Free Energy (Reference: 20.3 Silberberg 5 th editin) At what temperature des the spntaneity f a reactin change? The Mdel: The Definitin f Free Energy S
CONVECTION IN MICROCHANNELS
 CAPER. Intrductin CONVECION IN MICROCANNELS.. Cntinuu and hernaic ythei. Previu chater are baed n tw fundaental autin: () Cntinuu: Navier-Stke equatin, and the energy equatin are alicable () hernaic equilibriu:
CAPER. Intrductin CONVECION IN MICROCANNELS.. Cntinuu and hernaic ythei. Previu chater are baed n tw fundaental autin: () Cntinuu: Navier-Stke equatin, and the energy equatin are alicable () hernaic equilibriu:
Flipping Physics Lecture Notes: Simple Harmonic Motion Introduction via a Horizontal Mass-Spring System
 Flipping Physics Lecture Ntes: Simple Harmnic Mtin Intrductin via a Hrizntal Mass-Spring System A Hrizntal Mass-Spring System is where a mass is attached t a spring, riented hrizntally, and then placed
Flipping Physics Lecture Ntes: Simple Harmnic Mtin Intrductin via a Hrizntal Mass-Spring System A Hrizntal Mass-Spring System is where a mass is attached t a spring, riented hrizntally, and then placed
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? Yu can help ur team t eep this site up and bring yu even mre cntent cnsider dnating via the lin n ur site. Still having truble understanding the material? Chec ut ur Tutring
Find this material useful? Yu can help ur team t eep this site up and bring yu even mre cntent cnsider dnating via the lin n ur site. Still having truble understanding the material? Chec ut ur Tutring
NUMBERS, MATHEMATICS AND EQUATIONS
 AUSTRALIAN CURRICULUM PHYSICS GETTING STARTED WITH PHYSICS NUMBERS, MATHEMATICS AND EQUATIONS An integral part t the understanding f ur physical wrld is the use f mathematical mdels which can be used t
AUSTRALIAN CURRICULUM PHYSICS GETTING STARTED WITH PHYSICS NUMBERS, MATHEMATICS AND EQUATIONS An integral part t the understanding f ur physical wrld is the use f mathematical mdels which can be used t
Differentiation Applications 1: Related Rates
 Differentiatin Applicatins 1: Related Rates 151 Differentiatin Applicatins 1: Related Rates Mdel 1: Sliding Ladder 10 ladder y 10 ladder 10 ladder A 10 ft ladder is leaning against a wall when the bttm
Differentiatin Applicatins 1: Related Rates 151 Differentiatin Applicatins 1: Related Rates Mdel 1: Sliding Ladder 10 ladder y 10 ladder 10 ladder A 10 ft ladder is leaning against a wall when the bttm
Flipping Physics Lecture Notes: Simple Harmonic Motion Introduction via a Horizontal Mass-Spring System
 Flipping Physics Lecture Ntes: Simple Harmnic Mtin Intrductin via a Hrizntal Mass-Spring System A Hrizntal Mass-Spring System is where a mass is attached t a spring, riented hrizntally, and then placed
Flipping Physics Lecture Ntes: Simple Harmnic Mtin Intrductin via a Hrizntal Mass-Spring System A Hrizntal Mass-Spring System is where a mass is attached t a spring, riented hrizntally, and then placed
Kinetics of Particles. Chapter 3
 Kinetics f Particles Chapter 3 1 Kinetics f Particles It is the study f the relatins existing between the frces acting n bdy, the mass f the bdy, and the mtin f the bdy. It is the study f the relatin between
Kinetics f Particles Chapter 3 1 Kinetics f Particles It is the study f the relatins existing between the frces acting n bdy, the mass f the bdy, and the mtin f the bdy. It is the study f the relatin between
Section 6-2: Simplex Method: Maximization with Problem Constraints of the Form ~
 Sectin 6-2: Simplex Methd: Maximizatin with Prblem Cnstraints f the Frm ~ Nte: This methd was develped by Gerge B. Dantzig in 1947 while n assignment t the U.S. Department f the Air Frce. Definitin: Standard
Sectin 6-2: Simplex Methd: Maximizatin with Prblem Cnstraints f the Frm ~ Nte: This methd was develped by Gerge B. Dantzig in 1947 while n assignment t the U.S. Department f the Air Frce. Definitin: Standard
Examples: 1. How much heat is given off by a 50.0 g sample of copper when it cools from 80.0 to 50.0 C?
 NOTES: Thermchemistry Part 1 - Heat HEAT- TEMPERATURE - Thermchemistry: the study f energy (in the frm f heat) changes that accmpany physical & chemical changes heat flws frm high t lw (ht cl) endthermic
NOTES: Thermchemistry Part 1 - Heat HEAT- TEMPERATURE - Thermchemistry: the study f energy (in the frm f heat) changes that accmpany physical & chemical changes heat flws frm high t lw (ht cl) endthermic
Computational modeling techniques
 Cmputatinal mdeling techniques Lecture 4: Mdel checing fr ODE mdels In Petre Department f IT, Åb Aademi http://www.users.ab.fi/ipetre/cmpmd/ Cntent Stichimetric matrix Calculating the mass cnservatin relatins
Cmputatinal mdeling techniques Lecture 4: Mdel checing fr ODE mdels In Petre Department f IT, Åb Aademi http://www.users.ab.fi/ipetre/cmpmd/ Cntent Stichimetric matrix Calculating the mass cnservatin relatins
v , Michael E. Hanyak, Jr., All Rights Reserved Page 7-18
 v07.08.04 007, Michael E. Hanyak, Jr., All Rights Reserved age 7-8 Energy Balance Intrductin Functinal frm: Hw t evaluate hmix,,? [ X H 0.60* + 0.40* + Δ () mix Ĥ is mlar enthalpy (kj/gml) f mixture at
v07.08.04 007, Michael E. Hanyak, Jr., All Rights Reserved age 7-8 Energy Balance Intrductin Functinal frm: Hw t evaluate hmix,,? [ X H 0.60* + 0.40* + Δ () mix Ĥ is mlar enthalpy (kj/gml) f mixture at
A Few Basic Facts About Isothermal Mass Transfer in a Binary Mixture
 Few asic Facts but Isthermal Mass Transfer in a inary Miture David Keffer Department f Chemical Engineering University f Tennessee first begun: pril 22, 2004 last updated: January 13, 2006 dkeffer@utk.edu
Few asic Facts but Isthermal Mass Transfer in a inary Miture David Keffer Department f Chemical Engineering University f Tennessee first begun: pril 22, 2004 last updated: January 13, 2006 dkeffer@utk.edu
February 28, 2013 COMMENTS ON DIFFUSION, DIFFUSIVITY AND DERIVATION OF HYPERBOLIC EQUATIONS DESCRIBING THE DIFFUSION PHENOMENA
 February 28, 2013 COMMENTS ON DIFFUSION, DIFFUSIVITY AND DERIVATION OF HYPERBOLIC EQUATIONS DESCRIBING THE DIFFUSION PHENOMENA Mental Experiment regarding 1D randm walk Cnsider a cntainer f gas in thermal
February 28, 2013 COMMENTS ON DIFFUSION, DIFFUSIVITY AND DERIVATION OF HYPERBOLIC EQUATIONS DESCRIBING THE DIFFUSION PHENOMENA Mental Experiment regarding 1D randm walk Cnsider a cntainer f gas in thermal
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
Chapter 9. Design via Root Locus
 Chapter 9 Deign via Rt Lcu Intrductin Sytem perfrmance pecificatin requirement imped n the cntrl ytem Stability Tranient repne requirement: maximum verht, ettling time Steady-tate requirement :.. errr
Chapter 9 Deign via Rt Lcu Intrductin Sytem perfrmance pecificatin requirement imped n the cntrl ytem Stability Tranient repne requirement: maximum verht, ettling time Steady-tate requirement :.. errr
Flipping Physics Lecture Notes: You Can t Run from Momentum
 Flipping Phyic Lecture Nte: Yu Can t Run frm Mmentum Symbl fr mmentum i a lwercae p. p i fr the Latin wrd petere which mean t make fr, t travel t, t eek, r t purue. It pretty clear thi wrd i where the
Flipping Phyic Lecture Nte: Yu Can t Run frm Mmentum Symbl fr mmentum i a lwercae p. p i fr the Latin wrd petere which mean t make fr, t travel t, t eek, r t purue. It pretty clear thi wrd i where the
Unit 14 Thermochemistry Notes
 Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
**DO NOT ONLY RELY ON THIS STUDY GUIDE!!!**
 Tpics lists: UV-Vis Absrbance Spectrscpy Lab & ChemActivity 3-6 (nly thrugh 4) I. UV-Vis Absrbance Spectrscpy Lab Beer s law Relates cncentratin f a chemical species in a slutin and the absrbance f that
Tpics lists: UV-Vis Absrbance Spectrscpy Lab & ChemActivity 3-6 (nly thrugh 4) I. UV-Vis Absrbance Spectrscpy Lab Beer s law Relates cncentratin f a chemical species in a slutin and the absrbance f that
IB Sports, Exercise and Health Science Summer Assignment. Mrs. Christina Doyle Seneca Valley High School
 IB Sprts, Exercise and Health Science Summer Assignment Mrs. Christina Dyle Seneca Valley High Schl Welcme t IB Sprts, Exercise and Health Science! This curse incrprates the traditinal disciplines f anatmy
IB Sprts, Exercise and Health Science Summer Assignment Mrs. Christina Dyle Seneca Valley High Schl Welcme t IB Sprts, Exercise and Health Science! This curse incrprates the traditinal disciplines f anatmy
(b) Using the ideal gas equation of state, and noting that the total mass of gas occupies the same total volume at the final state as initially: where
 6.55 Given: An inulated cylinder i initially divided int halve y a itn. On either ide the itn i a ga at a knwn tate. The itn i releaed and equiliriu i attained. Find: Deterine the inal reure, inal teerature,
6.55 Given: An inulated cylinder i initially divided int halve y a itn. On either ide the itn i a ga at a knwn tate. The itn i releaed and equiliriu i attained. Find: Deterine the inal reure, inal teerature,
AP Physics Kinematic Wrap Up
 AP Physics Kinematic Wrap Up S what d yu need t knw abut this mtin in tw-dimensin stuff t get a gd scre n the ld AP Physics Test? First ff, here are the equatins that yu ll have t wrk with: v v at x x
AP Physics Kinematic Wrap Up S what d yu need t knw abut this mtin in tw-dimensin stuff t get a gd scre n the ld AP Physics Test? First ff, here are the equatins that yu ll have t wrk with: v v at x x
Lecture 16 Thermodynamics II
 Lecture 16 Thermdynamics II Calrimetry Hess s Law Enthalpy r Frmatin Cpyright 2013, 2011, 2009, 2008 AP Chem Slutins. All rights reserved. Fur Methds fr Finding H 1) Calculate it using average bnd enthalpies
Lecture 16 Thermdynamics II Calrimetry Hess s Law Enthalpy r Frmatin Cpyright 2013, 2011, 2009, 2008 AP Chem Slutins. All rights reserved. Fur Methds fr Finding H 1) Calculate it using average bnd enthalpies
ANSWER KEY FOR MATH 10 SAMPLE EXAMINATION. Instructions: If asked to label the axes please use real world (contextual) labels
 ANSWER KEY FOR MATH 10 SAMPLE EXAMINATION Instructins: If asked t label the axes please use real wrld (cntextual) labels Multiple Chice Answers: 0 questins x 1.5 = 30 Pints ttal Questin Answer Number 1
ANSWER KEY FOR MATH 10 SAMPLE EXAMINATION Instructins: If asked t label the axes please use real wrld (cntextual) labels Multiple Chice Answers: 0 questins x 1.5 = 30 Pints ttal Questin Answer Number 1
CHEM 116 Electrochemistry at Non-Standard Conditions, and Intro to Thermodynamics
 CHEM 116 Electrchemistry at Nn-Standard Cnditins, and Intr t Thermdynamics Imprtant annuncement: If yu brrwed a clicker frm me this semester, return it t me at the end f next lecture r at the final exam
CHEM 116 Electrchemistry at Nn-Standard Cnditins, and Intr t Thermdynamics Imprtant annuncement: If yu brrwed a clicker frm me this semester, return it t me at the end f next lecture r at the final exam
Materials Engineering 272-C Fall 2001, Lecture 7 & 8 Fundamentals of Diffusion
 Materials Engineering 272-C Fall 2001, Lecture 7 & 8 Fundamentals f Diffusin Diffusin: Transprt in a slid, liquid, r gas driven by a cncentratin gradient (r, in the case f mass transprt, a chemical ptential
Materials Engineering 272-C Fall 2001, Lecture 7 & 8 Fundamentals f Diffusin Diffusin: Transprt in a slid, liquid, r gas driven by a cncentratin gradient (r, in the case f mass transprt, a chemical ptential
CHAPTER 3 INEQUALITIES. Copyright -The Institute of Chartered Accountants of India
 CHAPTER 3 INEQUALITIES Cpyright -The Institute f Chartered Accuntants f India INEQUALITIES LEARNING OBJECTIVES One f the widely used decisin making prblems, nwadays, is t decide n the ptimal mix f scarce
CHAPTER 3 INEQUALITIES Cpyright -The Institute f Chartered Accuntants f India INEQUALITIES LEARNING OBJECTIVES One f the widely used decisin making prblems, nwadays, is t decide n the ptimal mix f scarce
Digital Control System
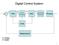 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Chemistry/ Biotechnology Reference Sheets
 Cmmn Metric Prefixes: Giga (G) = 1,000,000,000 = Kil (k) = 1,000 = Deci (d) =.1 = Milli (m) =.001 = Nan (n) =.000000001 = 9 6 1 10 Mega (M) = 1,000,000 = 1 10 0 1 10 Basic unit = meter, gram, liter, secnd
Cmmn Metric Prefixes: Giga (G) = 1,000,000,000 = Kil (k) = 1,000 = Deci (d) =.1 = Milli (m) =.001 = Nan (n) =.000000001 = 9 6 1 10 Mega (M) = 1,000,000 = 1 10 0 1 10 Basic unit = meter, gram, liter, secnd
lecture 5: Nucleophilic Substitution Reactions
 lecture 5: Nuclephilic Substitutin Reactins Substitutin unimlecular (SN1): substitutin nuclephilic, unimlecular. It is first rder. The rate is dependent upn ne mlecule, that is the substrate, t frm the
lecture 5: Nuclephilic Substitutin Reactins Substitutin unimlecular (SN1): substitutin nuclephilic, unimlecular. It is first rder. The rate is dependent upn ne mlecule, that is the substrate, t frm the
4F-5 : Performance of an Ideal Gas Cycle 10 pts
 4F-5 : Perfrmance f an Cycle 0 pts An ideal gas, initially at 0 C and 00 kpa, underges an internally reversible, cyclic prcess in a clsed system. The gas is first cmpressed adiabatically t 500 kpa, then
4F-5 : Perfrmance f an Cycle 0 pts An ideal gas, initially at 0 C and 00 kpa, underges an internally reversible, cyclic prcess in a clsed system. The gas is first cmpressed adiabatically t 500 kpa, then
making triangle (ie same reference angle) ). This is a standard form that will allow us all to have the X= y=
 Intrductin t Vectrs I 21 Intrductin t Vectrs I 22 I. Determine the hrizntal and vertical cmpnents f the resultant vectr by cunting n the grid. X= y= J. Draw a mangle with hrizntal and vertical cmpnents
Intrductin t Vectrs I 21 Intrductin t Vectrs I 22 I. Determine the hrizntal and vertical cmpnents f the resultant vectr by cunting n the grid. X= y= J. Draw a mangle with hrizntal and vertical cmpnents
Flipping Physics Lecture Notes: AP Physics 1 Review of Kinematics
 Flipping Phyic Lecture Nte: AP Phyic 1 Review f Kinematic AP i a regitered trademark f the Cllege Bard, which wa nt invlved in the prductin f, and de nt endre, thi prduct. Intrductry Cncept: Vectr: Magnitude
Flipping Phyic Lecture Nte: AP Phyic 1 Review f Kinematic AP i a regitered trademark f the Cllege Bard, which wa nt invlved in the prductin f, and de nt endre, thi prduct. Intrductry Cncept: Vectr: Magnitude
Sediment transport mechanisms 1. Bed-load transport
 10 Sediment tranprt mechanim 1. Bed-lad tranprt 10.1 Intrductin When the bed hear tre exceed a critical value, ediment are tranprted in the frm f bed-lad and upended lad. Fr bed-lad tranprt, the baic mde
10 Sediment tranprt mechanim 1. Bed-lad tranprt 10.1 Intrductin When the bed hear tre exceed a critical value, ediment are tranprted in the frm f bed-lad and upended lad. Fr bed-lad tranprt, the baic mde
Laplace Transformation
 Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
CHEM 1001 Problem Set #3: Entropy and Free Energy
 CHEM 1001 Prblem Set #3: Entry and Free Energy 19.7 (a) Negative; A liquid (mderate entry) cmbines with a slid t frm anther slid. (b)psitive; One mle f high entry gas frms where n gas was resent befre.
CHEM 1001 Prblem Set #3: Entry and Free Energy 19.7 (a) Negative; A liquid (mderate entry) cmbines with a slid t frm anther slid. (b)psitive; One mle f high entry gas frms where n gas was resent befre.
Lecture 13: Electrochemical Equilibria
 3.012 Fundamentals f Materials Science Fall 2005 Lecture 13: 10.21.05 Electrchemical Equilibria Tday: LAST TIME...2 An example calculatin...3 THE ELECTROCHEMICAL POTENTIAL...4 Electrstatic energy cntributins
3.012 Fundamentals f Materials Science Fall 2005 Lecture 13: 10.21.05 Electrchemical Equilibria Tday: LAST TIME...2 An example calculatin...3 THE ELECTROCHEMICAL POTENTIAL...4 Electrstatic energy cntributins
Short notes for Heat transfer
 Furier s Law f Heat Cnductin Shrt ntes fr Heat transfer Q = Heat transfer in given directin. A = Crss-sectinal area perpendicular t heat flw directin. dt = Temperature difference between tw ends f a blck
Furier s Law f Heat Cnductin Shrt ntes fr Heat transfer Q = Heat transfer in given directin. A = Crss-sectinal area perpendicular t heat flw directin. dt = Temperature difference between tw ends f a blck
Figure 1a. A planar mechanism.
 ME 5 - Machine Design I Fall Semester 0 Name f Student Lab Sectin Number EXAM. OPEN BOOK AND CLOSED NOTES. Mnday, September rd, 0 Write n ne side nly f the paper prvided fr yur slutins. Where necessary,
ME 5 - Machine Design I Fall Semester 0 Name f Student Lab Sectin Number EXAM. OPEN BOOK AND CLOSED NOTES. Mnday, September rd, 0 Write n ne side nly f the paper prvided fr yur slutins. Where necessary,
CS 477/677 Analysis of Algorithms Fall 2007 Dr. George Bebis Course Project Due Date: 11/29/2007
 CS 477/677 Analysis f Algrithms Fall 2007 Dr. Gerge Bebis Curse Prject Due Date: 11/29/2007 Part1: Cmparisn f Srting Algrithms (70% f the prject grade) The bjective f the first part f the assignment is
CS 477/677 Analysis f Algrithms Fall 2007 Dr. Gerge Bebis Curse Prject Due Date: 11/29/2007 Part1: Cmparisn f Srting Algrithms (70% f the prject grade) The bjective f the first part f the assignment is
MODULE 1. e x + c. [You can t separate a demominator, but you can divide a single denominator into each numerator term] a + b a(a + b)+1 = a + b
![MODULE 1. e x + c. [You can t separate a demominator, but you can divide a single denominator into each numerator term] a + b a(a + b)+1 = a + b MODULE 1. e x + c. [You can t separate a demominator, but you can divide a single denominator into each numerator term] a + b a(a + b)+1 = a + b](/thumbs/78/78051685.jpg) . REVIEW OF SOME BASIC ALGEBRA MODULE () Slving Equatins Yu shuld be able t slve fr x: a + b = c a d + e x + c and get x = e(ba +) b(c a) d(ba +) c Cmmn mistakes and strategies:. a b + c a b + a c, but
. REVIEW OF SOME BASIC ALGEBRA MODULE () Slving Equatins Yu shuld be able t slve fr x: a + b = c a d + e x + c and get x = e(ba +) b(c a) d(ba +) c Cmmn mistakes and strategies:. a b + c a b + a c, but
Chem 116 POGIL Worksheet - Week 3 - Solutions Intermolecular Forces, Liquids, Solids, and Solutions
 Chem 116 POGIL Wrksheet - Week 3 - Slutins Intermlecular Frces, Liquids, Slids, and Slutins Key Questins 1. Is the average kinetic energy f mlecules greater r lesser than the energy f intermlecular frces
Chem 116 POGIL Wrksheet - Week 3 - Slutins Intermlecular Frces, Liquids, Slids, and Slutins Key Questins 1. Is the average kinetic energy f mlecules greater r lesser than the energy f intermlecular frces
7-84. Chapter 7 External Forced Convection
 Chapter 7 External Frced Cnvectin 7-99 Wind i blwing ver the rf f a hue. The rate f heat tranfer thrugh the rf and the ct f thi heat l fr -h perid are t be deterined. Auptin Steady perating cnditin exit.
Chapter 7 External Frced Cnvectin 7-99 Wind i blwing ver the rf f a hue. The rate f heat tranfer thrugh the rf and the ct f thi heat l fr -h perid are t be deterined. Auptin Steady perating cnditin exit.
Lecture 4. Chapter 11 Nise. Controller Design via Frequency Response. G. Hovland 2004
 METR4200 Advanced Control Lecture 4 Chapter Nie Controller Deign via Frequency Repone G. Hovland 2004 Deign Goal Tranient repone via imple gain adjutment Cacade compenator to improve teady-tate error Cacade
METR4200 Advanced Control Lecture 4 Chapter Nie Controller Deign via Frequency Repone G. Hovland 2004 Deign Goal Tranient repone via imple gain adjutment Cacade compenator to improve teady-tate error Cacade
Chapter 19. Electrochemistry. Dr. Al Saadi. Electrochemistry
 Chapter 19 lectrchemistry Part I Dr. Al Saadi 1 lectrchemistry What is electrchemistry? It is a branch f chemistry that studies chemical reactins called redx reactins which invlve electrn transfer. 19.1
Chapter 19 lectrchemistry Part I Dr. Al Saadi 1 lectrchemistry What is electrchemistry? It is a branch f chemistry that studies chemical reactins called redx reactins which invlve electrn transfer. 19.1
Session #22: Homework Solutions
 Sessin #22: Hmewrk Slutins Prblem #1 (a) In the cntext f amrphus inrganic cmpunds, name tw netwrk frmers, tw netwrk mdifiers, and ne intermediate. (b) Sketch the variatin f mlar vlume with temperature
Sessin #22: Hmewrk Slutins Prblem #1 (a) In the cntext f amrphus inrganic cmpunds, name tw netwrk frmers, tw netwrk mdifiers, and ne intermediate. (b) Sketch the variatin f mlar vlume with temperature
How can standard heats of formation be used to calculate the heat of a reaction?
 Answer Key ALE 28. ess s Law and Standard Enthalpies Frmatin (Reerence: Chapter 6 - Silberberg 4 th editin) Imprtant!! Fr answers that invlve a calculatin yu must shw yur wrk neatly using dimensinal analysis
Answer Key ALE 28. ess s Law and Standard Enthalpies Frmatin (Reerence: Chapter 6 - Silberberg 4 th editin) Imprtant!! Fr answers that invlve a calculatin yu must shw yur wrk neatly using dimensinal analysis
Grumman F-14 Tomcat Control Design BY: Chike Uduku
 Grumman F-4 Tmcat Cntrl Deign BY: Chike duku I. Atract SECTIONS II. III. IV. Deign jective eaured Cntant Deign V. Reult VI. VII. Cncluin Cmplete atla Cde I. Atract Deigning cntrller fr fighter jet i a
Grumman F-4 Tmcat Cntrl Deign BY: Chike duku I. Atract SECTIONS II. III. IV. Deign jective eaured Cntant Deign V. Reult VI. VII. Cncluin Cmplete atla Cde I. Atract Deigning cntrller fr fighter jet i a
READING STATECHART DIAGRAMS
 READING STATECHART DIAGRAMS Figure 4.48 A Statechart diagram with events The diagram in Figure 4.48 shws all states that the bject plane can be in during the curse f its life. Furthermre, it shws the pssible
READING STATECHART DIAGRAMS Figure 4.48 A Statechart diagram with events The diagram in Figure 4.48 shws all states that the bject plane can be in during the curse f its life. Furthermre, it shws the pssible
Chem 163 Section: Team Number: ALE 24. Voltaic Cells and Standard Cell Potentials. (Reference: 21.2 and 21.3 Silberberg 5 th edition)
 Name Chem 163 Sectin: Team Number: ALE 24. Vltaic Cells and Standard Cell Ptentials (Reference: 21.2 and 21.3 Silberberg 5 th editin) What des a vltmeter reading tell us? The Mdel: Standard Reductin and
Name Chem 163 Sectin: Team Number: ALE 24. Vltaic Cells and Standard Cell Ptentials (Reference: 21.2 and 21.3 Silberberg 5 th editin) What des a vltmeter reading tell us? The Mdel: Standard Reductin and
ENGINEERING COUNCIL CERTIFICATE LEVEL THERMODYNAMIC, FLUID AND PROCESS ENGINEERING C106 TUTORIAL 5 THE VISCOUS NATURE OF FLUIDS
 ENGINEERING COUNCIL CERTIFICATE LEVEL THERMODYNAMIC, FLUID AND PROCESS ENGINEERING C106 TUTORIAL 5 THE VISCOUS NATURE OF FLUIDS On cmpletin f this tutrial yu shuld be able t d the fllwing. Define viscsity
ENGINEERING COUNCIL CERTIFICATE LEVEL THERMODYNAMIC, FLUID AND PROCESS ENGINEERING C106 TUTORIAL 5 THE VISCOUS NATURE OF FLUIDS On cmpletin f this tutrial yu shuld be able t d the fllwing. Define viscsity
