Farr High School HIGHER PHYSICS. Unit 2 Particles and Waves. Exam Question Booklet
|
|
|
- Laurence Phelps
- 5 years ago
- Views:
Transcription
1 Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Exam Question Booklet 1
2 2
3 MULTIPLE CHOICE QUESTIONS
4
5
6
7
8
9
10
11
12 1. The diagram shows the apparatus used by Rutherford to investigate the scattering of alpha particles by a gold foil. From the observations made as the microscope and screen were moved from P to Q, Rutherford deduced that an atom has a nucleus which is: THE STANDARD MODEL (a) positively charged; (b) massive; (c) much smaller than the volume of the atom. Explain how the observations from the scattering experiment led to these three deductions. 3 (3) 2. About one hundred years ago Rutherford designed an experiment to investigate the structure of the atom. He used a radioactive source to fire alpha particles at a thin gold foil target. His two assistants, Geiger and Marsden, spent many hours taking readings from the detector as it was moved to different positions between X and Y. (a) How did the number of alpha particles detected at X compare with the number detected at Y? 1 (b) State three conclusions Rutherford deduced from the results. 3 (4) 12
13 3. Information on the properties of three elementary particles together with two types of quarks and their corresponding antiquarks is shown in the tables below. (a) Using information from the tables above, show that a proton consists of two up quarks and one down quark. 1 (b) State the combination of quarks that forms a pi-meson The following strong interaction has been observed. K + p n + X The K is a strange meson of quark composition ū s. The u quark has a charge of +2/3. The d quark has a charge of 1/3. (a) Determine the charge of the strange quark. 1 (b) Use the appropriate conservation law to determine whether particle X is positive, negative or neutral. 1 (c) State whether particle X is a baryon or a meson. Justify your answer. 2 (d) State the quark composition of X. Justify your answer. 2 (2) (6) 13
14 5. The equation for a decay can be written as: n p + + v For each of these four particles, state its name, and where appropriate, its quark composition. (3) 6. (a) A conversation is overheard between two young pupils who are discussing their science lessons. Pupil A We learned in science today that the nucleus of an atom is made of protons which are positively charged and neutrons which have no charge. Pupil B That s interesting because we learned in science that like charges repel. How come the protons in the nucleus don t fly apart? Pupil A I don t know. Write a paragraph that would explain to the pupils why the protons in a nucleus do not fly apart. 3 (b) Protons and neutrons each contain two different types of quark: the up quark which has an electric charge of + 2/3 and the down quark which has an electric charge of 1/3. Use this information to show: (i) the overall charge on the proton is +1; 1 (ii) the overall charge on the neutron is zero. 1 (c) The Large Hadron Collider (LHC) accelerates beams of protons in opposite directions. The protons are allowed to collide within a very small space, releasing a substantial amount of energy. By referring to the equation E = mc 2, explain how this produces a shower of particles. 1 (6) 14
15 FORCES ON CHARGED PARTICLES 1. The apparatus shown in the diagram is designed to accelerate alpha particles. An alpha particle travelling at a speed of m s 1 passes through a hole in plate A. The mass of an alpha particle is kg and its charge is C. (a) When the alpha particle reaches plate B, its kinetic energy has increased to J. Show that the work done on the alpha particle as it moves from plate A to plate B is J, 4 (b) Calculate the potential difference between plates A and B. 3 (c) The apparatus is now adapted to accelerate electrons from A to B through the same potential difference. How does the increase in kinetic energy of an electron compare with the increase in kinetic energy of the alpha particle in part (a)? Justify your answer. 2 (9) 2. The diagram shows an arrangement which is used to accelerate electrons. The potential difference between the cathode and the anode is 2 5 kv. Assuming that the electrons start from rest at the cathode, calculate the speed of an electron just as it reaches the anode. 4 (4) 15
16 3. A particle accelerator increases the speed of protons by accelerating them between a pair of metal plates, A and B, connected to a power supply as shown below. The potential difference between A and B is 25 kv. (a) Show that the kinetic energy gained by a proton between plates A and B is J. 3 (b) The kinetic energy of a proton at plate A is J. Calculate the velocity of the proton on reaching plate B. 3 (c) The plates are separated by a distance of 1 2 m. Calculate the force produced by the particle accelerator on a proton as it travels between plates A and B The diagram below shows a cathode ray tube used in an oscilloscope. (9) The electrons which are emitted from the cathode start from rest and reach the anode with a speed of m s 1. (a) (i) Calculate the kinetic energy of each electron just before it reaches the anode. 3 (ii) Calculate the p.d. between the anode and the cathode. 3 (b) Describe how the spot at the centre of the screen produced by the electron beam can be moved to position X. Your answer must make reference in the relative sizes and polarity (signs) of the voltages applied to plates P and Q. 2 (6) 16
17 5. The diagram below shows the basic features of a proton accelerator. It is enclosed in an evacuated container. Protons released from the proton source start from rest at P. A potential difference of 200 kv is maintained between P and Q. (a) What is meant by the term potential difference of 200 kv? 1 (b) Explain why protons released at P are accelerated towards Q. 1 (c) Calculate: (i) the work done on a proton as it accelerates from P and Q; 3 (ii) the speed of a proton as it reaches Q. 3 (d) The distance between P and Q is now halved. What effect, if any, does this change have on the speed of a proton as it reaches Q? Justify your answer Identification of elements in a semiconductor sample can be carried out using an electron scanner to release atoms from the surface of the sample for analysis. Electrons are accelerated from rest between a cathode and anode by a potential difference of 2 40 kv. A variable voltage supply connected to the deflection plates enables the beam to scan the sample between points A and B shown in the figure below. (10) (a) Calculate the speed of the electrons as they pass through the anode. 3 (b) Explain why the electron beam follows: (i) a curved path between the plates; 1 (ii) a straight path beyond the plates. 1 (c) The anode voltage is now increased. State what happens to the length of the sample scanned by the electron beam. You must justify your answer. 2 (7) 17
18 7. A cyclotron is a particle accelerator which consists of two D-shaped hollow structures, called dees, placed in a vacuum. The diagram below shows the cyclotron viewed from above. (a) Protons are released from rest at point A and accelerated across the gap between the dees by a voltage of 2 00 kv. Show that the speed of the protons as they first reach the right hand dee is m s 1. 2 (b) Inside the dees the electric field strength is zero but there is a uniform magnetic field. This forces the protons to move in semi-circular paths when inside the dees. State the direction of the magnetic field in the dees. 1 (c) While the protons are inside the dee, the polarity of the applied voltage is reversed so that the protons are again accelerated when they cross to the left hand dee. Calculate the speed of the protons as they first enter the left hand dee. 2 (5) 18
19 8. (a) A charged particle moves with a speed of m s 1 in a circular orbit in a uniform magnetic field directed into the page as shown below. State whether the charge on the particle is positive or negative. 1 (b) An electron enters a uniform magnetic field at an angle to the magnetic field lines as shown below. Explain the shape of the electron path in the magnetic field. 2 (c) Charged particles which enter the Earth s atmosphere near the North pole collide with air molecules. The light emitted in this process is called the Aurora Borealis. In the figure below, the Earth s magnetic field is indicated by continuous lines which show the magnetic field direction in the region surrounding the Earth. The extent of the Earth s atmosphere is also shown. Charged particles approach the Earth in the direction shown in the diagram. Explain why these particles do not cause an aurora above the Equator. 2 (5) 19
20 NUCLEAR REACTIONS 1. A smoke alarm contains a very small sample of the radioactive isotope Americium-241, represented by the symbol (a) How many neutrons are there in a nucleus of this isotope? 1 (b) This isotope decays by emitting alpha particles as shown in the following statement. (i) Determine the numbers represented by the letters r and s. 1 (ii) Use the data booklet to identify the element T Some power stations use nuclear fission reactions to provide energy for generating electricity. The following statement represents a fission reaction. (3) (a) Determine the numbers represented by the letters r and s in the above statement. 1 (b) Explain why a nuclear fission reaction releases energy. 1 (c) The masses of the particles involved in the reaction are shown in the table. Calculate the energy released in this reaction. 5 (7) 20
21 3. A nuclear fission reaction is represented by the following statement. (a) Is this a spontaneous or induced reaction? You must justify your answer. 1 (b) Determine the numbers represented by the letters r and s in the above reaction. 1 (c) Use the data booklet to identify the element represented by T. 1 (d) The masses of the nuclei and particles in the reaction are given below. Calculate the energy released in the reaction. 5 (8) 4. The following statement represents a nuclear reaction which may form the basis of a nuclear power station of the future. (a) State the name given to the above type of nuclear reaction. 1 (b) Explain, using E = mc 2, how this nuclear reaction results in the production of energy. 2 (c) Using the information given below, and any other data required from the Data Sheet, calculate the energy released in the above nuclear reaction. 5 (8) 21
22 5. A ship is powered by a nuclear reactor. One reaction that takes place in the core of the nuclear reactor is represented by the statement below (a) The symbol for the Uranium nucleus is U. What information about the nucleus is provided by the following numbers? (i) 92 1 (ii) (b) Describe how neutrons produced during the reaction can cause further nuclear reactions. 1 (c) The masses of the particles involved in the reaction are shown in the table. Calculate the energy released in the reaction. 5 (8) 22
23 6. Radium (Ra) decays to Radon (Rn) by the emission of an alpha particle. Some energy is also released by this decay. The decay is represented by the statement shown below. The masses of the nuclides involved are as follows. (a) What are the values of x and y for the nuclide Rn? 1 (b) Why is energy released by this decay? 1 (c) Calculate the energy released by one decay of this type. 5 (7) x y 7. Energy is released from stars as a result of nuclear reactions. One of these reactions is represented by the statement given below. (a) What type of nuclear reaction is described by this statement? 1 (b) Explain why this reaction results in the release of energy. You should make reference to an equation in your explanation. 2 (3) 23
24 WAVE PARTICLE DUALITY 1. Ultraviolet radiation from a lamp is incident on the surface of a metal. This causes the release of electrons from the surface of the metal. The energy of each photon of ultraviolet radiation is J. The work function of the metal is J. (a) Calculate: (i) the maximum kinetic energy of an electron released from this metal by this radiation; 3 (ii) the maximum speed of an emitted electron. 3 (b) The source of ultraviolet radiation is now moved further away from the surface of the metal. State the effect, if any, this has on the maximum speed of an emitted electron. Justify your answer. 2 (8) 2. To explain the photoelectric effect, light can be considered as consisting of tiny bundles of energy. These bundles of energy are called photons. (a) Sketch a graph to show the relationship between photon energy and frequency. 1 (b) Photons of frequency Hz are incident on the surface of a metal. This releases photoelectrons from the surface of the metal. The maximum kinetic energy of any of these photoelectrons is J. Calculate the work function of the metal. 3 (c) The irradiance due to these photons on the surface of the metal is now reduced. Explain why the maximum kinetic energy of each photoelectron is unchanged. 1 (5) 24
25 3. A metal plate emits electrons when certain wavelengths of electromagnetic radiation are incident on it. When light of wavelength 605 nm is incident on the metal plate, electrons are released with zero kinetic energy. (a) Show that the work function of this metal is J. 5 (b) The wavelength of the incident radiation is now altered. Photons of energy J are incident on the metal plate. (i) Calculate the maximum kinetic energy of the electrons just as they leave the metal plate. 1 (ii) The irradiance of this radiation on the metal plate is now decreased. State the effect this has on the ammeter reading. Justify your answer In 1902, P. Lenard set up an experiment similar to the one shown below. (8) There is a constant potential difference between the metal plate and the metal cylinder. Monochromatic radiation is directed onto the plate. Photoelectrons produced at the plate are collected by the cylinder. The frequency and the irradiance can be altered independently. The frequency of the radiation is set at a value above the threshold frequency. (a) The irradiance of the radiation is slowly increased. Sketch a graph of the current against irradiance of radiation. 1 (b) The metal of the plate has a work function of J. The wavelength of the radiation is 400 nm. (i) Calculate the maximum kinetic energy of a photoelectron. 4 (ii) The battery connections are now reversed. Explain why there could still be a reading on the ammeter. 1 (5) 25
26 5. An image intensifier is used to improve night vision. It does this by amplifying the light from an object. Light incident on a photocathode causes the emission of photoelectrons. These electrons are accelerated by an electric field and strike a phosphorescent screen causing it to emit light. This emitted light is of a greater intensity than the light that was incident on the photocathode. The voltage between the photocathode and the phosphorescent screen is V. The minimum frequency of the incident light that allows photoemission to take place is Hz. (a) What name is given to the minimum frequency of the light required for photoemission to take place? 1 (b) (i) Show that the work function of the photocathode material is J. 3 (ii) Light of frequency Hz is incident on the photocathode. Calculate the maximum kinetic energy of an electron emitted from the photocathode. 4 (iii) Calculate the kinetic energy gained by an electron as it is accelerated from the photocathode to the phosphorescent screen. 3 (10) 26
27 6. (a) The apparatus shown below is used to investigate photoelectric emission from the metal surface X when the electromagnetic radiation is shone on the surface. The frequency of the electromagnetic radiation can be varied. (i) When radiation of a certain frequency is shone on the metal surface X, a reading is obtained on the ammeter. Sketch a graph to show how the current in the circuit varies with the irradiance of the radiation. 2 (ii) Explain why there is no reading on the ammeter when the frequency of the radiation is decreased below a particular value. 1 (b) The maximum kinetic energy of the photoelectrons emitted from X is measured for a number of different frequencies of the radiation. The graph shows how this kinetic energy varies with frequency. (i) Use the graph to find the threshold frequency for metal X. 1 (ii) The table below gives the work function of different metals. Metal Potassium Calcium Zinc Gold Work function/j Which one of these metals was used in the investigation? You must justify your answer using the information given in the table. 3 (7) 27
28 7. (a) It is quoted in a text book that the work function of caesium is J. Explain what is meant by the above statement. 1 (b) In an experiment to investigate the photoelectric effect, a glass vacuum tube is arranged as shown below. The tube has two electrodes, one of which is coated with caesium. Light of frequency Hz is shone on to the caesium coated electrode. (i) Calculate the maximum kinetic energy of a photoelectron leaving the caesium coated electrode. 6 (ii) An electron leaves the caesium coated electrode with this maximum kinetic energy. Calculate its kinetic energy as it reaches the upper electrode when the p.d. across the electrode is 0 80 V. 6 (c) The polarity of the supply voltage is now reversed. Calculate the minimum voltage which should be supplied across the electrodes to stop photoelectrons from reaching the upper electrode. 3 (13) 28
29 INTERFERENCE AND DIFFRACTION 1. A student is carrying out an experiment to investigate the interference of sound waves. She sets up the following apparatus. The microphone is initially placed at point X which is the same distance from each loudspeaker. A maximum is detected at X. (a) The microphone is now moved to the first minimum Y as shown. Calculate the wavelength of the sound waves. 3 (b) Loudspeaker 1 is now disconnected. What happens to the amplitude of the sound detected by the microphone at Y? Explain your answer. 2 (5) 29
30 2. Two identical loudspeakers X and Y are set up in a room which has been designed to eliminate the reflection of sound. The loudspeakers are connected to the same signal generator as shown. (a) (i) When a sound level meter is moved from P to T, maxima and minima of sound intensity are detected. Explain, in terms of waves, why the maxima and minima are produced. 2 (ii) The sound level meter detects a maximum at P. As the sound level meter is moved from P, it detects a minimum then a maximum then another minimum when it reaches Q. Calculate the wavelength of the sound used. 3 (b) The sound level meter is now fixed at Q. The frequency of the output from the signal generator is increased steadily from 200 Hz to 1000 Hz. (i) What happens to the wavelength of the sound as the frequency of the output is increased? 1 (ii) Explain why the sound level meter detects a series of maxima and minima as the frequency of the output is increased. 2 (8) 30
31 3. Loudspeakers 1 and 2 are both connected to the same signal generator which is set to produce a 1 00 khz signal. Loudspeaker 1 is switched on but loudspeaker 2 is switched off. The speed of sound in air is 340 m s 1. State and explain, including an appropriate calculation, what happens to the amplitude of the signal picked up by the microphone when loudspeaker 2 is switched on. 3 (3) 4. A laser produces a narrow beam of monochromatic light. (a) Red light from a laser passes through a grating as shown. A series of maxima and minima are observed. Explain in terms of waves how a minimum is produced. 1 (b) The laser is now replaced by a second laser, which emits blue light. Explain why the observed maxima are now closer together. 1 (c) The wavelength of the blue light from the second laser is m. The spacing between the lines on the grating is m. Calculate the angle between the central maximum and the second order maximum. 3 (5) 31
32 5. In an experiment, laser light of wavelength 633 nm is incident on a grating. A series of bright spots are seen on a screen placed some distance from the grating. The distance between these spots and the central spot is shown. (a) Calculate the number of lines per metre on the grating. 4 (b) The laser is replaced with another laser and the experiment repeated. With this laser the bright spots are closer together. How does the wavelength of the light from this laser compare with that from the original laser? You must justify your answer. 2 (6) 32
33 6. Light from a laser is shone onto a grating. The separation of the slits on the grating is m. A pattern is produced on a screen as shown below. (a) (i) The angle between the central maximum and the 2nd order maximum is 14. Calculate the wavelength of the light produced by the laser. 3 (ii) A pupil suggests that a more accurate value for the wavelength of the laser light can be found if a grating with a slit separation of m is used. Explain why this suggestion is correct. 2 (b) The laser is replaced by a source of white light and the pattern on the screen changes to a white central maximum with other maxima in the form of continuous spectra on each side of the central maximum. Explain: (i) why the central maximum is white; 1 (ii) why the other maxima are in the form of continuous spectra. 2 (8) 33
34 1. A laser beam is used to investigate the refraction of light from water into air. A waterproof laser is placed in within a tank of water and the laser beam is directed towards the water surface as shown below. REFRACTION OF LIGHT (a) The water in the tank has a refractive index of Describe what happens to the light at the water surface. You must justify your answer by calculation. 4 (b) The water in the tank is replaced by another liquid. The position of the laser is altered so that the laser beam follows the path shown in the diagram below. The angle 1 and the angle 2, as shown in the diagram, are measured. The measurements are repeated for different values of 1 and the corresponding values of 2. The values of sin 1 and sin 2 are used to plot the graph shown below. Use information from the graph to calculate the refractive index of the liquid. 2 (c) Light from the laser has a wavelength of m. Calculate the wavelength of the laser light when passing through a liquid which has a refractive index of (9) 34
35 2. A ray of red light is incident on a semicircular block of glass at the midpoint of XY as shown. The refractive index of the block is 1 50 for this red light. (a) Calculate the angle shown on the diagram. 3 (b) The wavelength of the red light in the glass is 420 nm. Calculate the wavelength of the light in air. 3 (c) The ray of red light is replaced by a ray of blue light incident at the same angle. The blue light enters the block at the same point. Explain why the path taken by the blue light in the block is different to that taken by the red light. 1 (7) 3. A ray of red light is directed at a glass prism of side 80 mm as shown in the diagram below. (a) Using information from this diagram, show that the refractive index of the glass for this red light is (b) What is meant by the term critical angle? 1 (c) Calculate the critical angle for the red light in the prism. 3 (d) Sketch a diagram showing the path of the ray of red light until it leaves the prism. Mark on your diagram the values of all relevant angles. 3 (10) 35
36 4. (a) A ray of red light of frequency Hz is incident on a glass lens as shown. The ray passes through point Y after leaving the lens. The refractive index of glass is 1 61 for this red light. (i) Calculate the value of the angle shown in the diagram. 3 (ii) Calculate the wavelength of this light inside the lens. 6 (b) The ray of red light is now replaced by a ray of blue light. The ray is incident on the lens at the same point as in part (a). Through which point, X, Y or Z, will this ray pass after leaving the lens? You must justify your answer. 1 (8) 36
37 5. A decorative lamp has a transparent liquid in the space above a bulb. Light from the bulb passes through rotating coloured filters giving red or blue light in the liquid. (a) A ray of red light is incident on the liquid surface as shown. (i) Calculate the refractive index of the liquid for the red light. 3 (ii) A ray of blue light is incident on the liquid surface at the same angle as the ray of red light. The refractive index of the liquid for blue light is greater than that for red light. Is the angle of refraction greater than, equal to or less than 82 for the blue light? You must justify your answer. 2 (b) A similar lamp contains a liquid which has a refractive index of 1 44 for red light. A ray of red light in the liquid is incident on the surface at an angle of 45 as before. Sketch a diagram to show the path of this ray after it is incident on the liquid surface. Mark on your diagram the value of all appropriate angles. All relevant calculations must be shown. 3 (8) 37
38 6. (a) The diagram below shows the refraction of a ray of red light as it passes through a plastic prism. Calculate the refractive index of the plastic for this red light. 3 (b) The refractive index of a glass block is found to be 1 44 when red light is used. (i) Calculate the value of the critical angle for this red light in the glass. 3 (ii) The diagram shows the paths of two rays of this red light, PO and QO, in the glass block. When rays PO and QO strike the glass-air boundary, three further rays of light are observed. Copy and complete the diagram to show all five rays. Clearly indicate which of the three rays came from P and which came from Q. The values of all angles should be shown on the diagram. 4 (10) 38
39 7. A swimming pool is illuminated by a lamp built into the bottom of the pool. Three rays of light from the same point in the lamp are incident on the water-air boundary with angles of incidence of 30, 40 and 50, as shown above. The refractive index of the water in the pool is (a) Draw a diagram to show clearly what happens to each ray at the boundary. Indicate on your diagram the sizes of appropriate angles. All necessary calculations must be shown. 5 (b) An observer stands at the side of the pool and looks into the water. Explain, with the aid of a diagram, why the image of the lamp appears to be at a shallower depth than the actual depth of the bottom of the pool. 2 (7) 39
40 SPECTRA 1. The diagram shows a light sensor connected to a voltmeter. The reading on the voltmeter is 20 mv for each 1 0 mw of power incident on the sensor. (a) The reading on the voltmeter is 40 mv. The area of the light sensor is m 2. Calculate the irradiance of light on the sensor. 4 (b) The small lamp is replaced by a different source of light. The results show how the irradiance varies with distance. Distance/m Irradiance/Wm Can this new source be considered to be a point source of light? Use all the data to justify your answer. 2 (6) 2. A student carries out an experiment to investigate how irradiance on a surface varies with distance from a small lamp. Irradiance is measured with a light meter. The distance between the small lamp and the light meter is measured with a metre stick. The apparatus is set up as shown in a darkened laboratory. The following results are obtained. Distance from source/m Irradiance/units (a) What is meant by the term irradiance? 1 (b) Use all the data to find the relationship between irradiance I and the distance d from the source. 2 (c) What is the purpose of the black cloth on top of the bench. 1 (d) The small lamp is replaced by a laser. Light from the laser is shone on the light meter. A reading is taken from the light meter when the distance between it and the laser is 0 50 m. The distance is now increased to 1 00 m. Compare the new reading on the light meter with the one taken at 0 50 m. Justify your answer. 2 (6) 40
41 3. A particular atom has energy levels as shown below. Transitions are possible between all these levels to produce emission lines in the electromagnetic spectrum. (a) How many lines are in the spectrum of this atom? 1 (b) Between which two energy levels does an electron transition lead to the emission of radiation of the lowest frequency? 1 (c) Explain why some lines in the spectrum are more intense than other. 1 (3) 41
42 4. The line emission spectrum of hydrogen has four lines in the visible spectrum as shown in the following diagram. These four lines are caused by electron transitions in a hydrogen atom from high energy levels to a low energy level E 2 as shown below. (a) From the information above, state which spectral line W, X, Y or Z is produced by an electron transition from E 3 to E 2. 1 (b) Explain why lines Y and Z in the line emission spectrum are brighter than the other two lines. 1 (c) Infrared radiation of frequency Hz is emitted from a hydrogen atom. (i) Calculate the energy of one photon of this radiation. 3 (ii) Show by calculation which electron transition produces this radiation. 4 (9) 42
43 5. (a) A sodium vapour lamp emits bright yellow light when electrons make transitions from one energy level to another within the sodium atom. (i) State whether electrons are moving to a higher or lower energy level when the light is emitted. 1 (ii) Using information provided in the data sheet, calculate the energy difference between these two electron energy levels in the sodium atom. 5 (b) A Bunsen flame contains vaporised sodium is placed between a sodium vapour lamp and a screen as shown. (i) Explain why a dark shadow of the flame is seen on the screen. 1 (ii) The sodium vapour lamp is replaced with a cadmium vapour lamp. Explain why there is now no dark shadow of the flame on the screen. 1 (8) 43
44 UNCERTAINTIES AND EXPERIMENTS IN PARTICLES AND WAVES 1. A student is investigating the effect that a semicircular block has on a ray of monochromatic light. She observes that at point X the incident ray splits into two rays: T a transmitted ray; R a reflected ray. The student uses a light meter to measure the irradiance of ray R as angle is changed. (a) State what is meant by the irradiance of a radiation. 1 (b) Explain why, as angle q is changed, it is important to keep the light meter at a constant distance from point X for each measurement of irradiance. 1 (c) The graph below is obtained from the student s results. (i) What is the value of the critical angle in the glass for this light? 1 (ii) Calculate the refractive index of the glass for this light. 3 (iii) As the angle is increased, what happens to the irradiance of ray T? 1 44 (7)
45 2. An experiment to determine the wavelength of light from a laser is shown. A second order maximum is observed at B. (a) Explain in terms of waves how a maximum is formed. 1 (b) Distance AB is measured six times. The results are shown: 1 11 m 1 08 m 1 10 m 1 13 m 1 11 m 1 07 m (i) Calculate: (A) the mean value for distance AB; 1 (B) the approximate random uncertainty in this value. 1 (ii) Distance BC is measured as (270 ± 10) mm. Show whether AB or BC has the largest percentage uncertainty. 2 (iii) The spacing between the lines on the gating is m. Calculate the wavelength of the light from the laser. Express your answer in the form wavelength ± absolute uncertainty. 4 (9) 45
46 3. The apparatus shown below is set up to determine the wavelength of light from a laser. The wavelength of the light is calculated using the equations: = d sin and sin = x L where angle and distances x and L are shown in the diagram. (a) Seven students measure the distance L with a tape measure. Their results are as follows m m m m m m m Calculate the mean value for L and the approximate random uncertainty in the mean. 2 (b) The best estimate of the distance x is (91 ± 1) mm. Show by calculation whether L or x has the largest percentage uncertainty. 2 (c) Calculate the wavelength, in nanometres, of the laser light. You must give your answer in the form final value ± absolute uncertainty. 4 (d) Suggest an improvement which could be made so that a more accurate estimate of the wavelength could be made. You must use only the same equipment and make the same number of measurements. 1 (9) 46
47 4. An extract from a student s investigation diary is shown. Investigation Preventing damage from earthquakes. 9 th November We found on the web that most of the energy of seismic waves from earthquakes is from waves close to a certain frequency. We carried out an experiment to find how the amplitude of vibratio n of the top of a building depended on the frequency of vibration of the ground. We used a flexible model of a building which was stuck to a horizontal plate that we vibrated at different frequencies. Here are the results. Vibration frequency / Hz Amplitude of vibration / mm The student has taken six sets of readings and used the results to reach a conclusion. (a) Sketch a graph of the results. 2 (b) Using the information from your sketch graph write a conclusion for the experiment. 2 (c) Suggest two improvements that the student could have made to the collection of data for this investigation. 2 (d) Suggest any changes or extensions to the experiment that would make it more useful in determining the effect of earthquakes on buildings. 2 (8) 47
48 OPEN-ENDED QUESTIONS IN PARTICLES AND WAVES 1. Monochromatic light is shone onto a diffraction grating to produce an interference pattern on a screen. Describe the significance of diffraction on the production of an interference pattern A ray of light of wavelength m is shone onto a diffraction grating. The spacing between the lines of the grating is m. An interference pattern is observed on a screen. A student observing the pattern states that there is no limit to the number of maxima of intensity produced on the screen. Use your knowledge of physics to comment on this statement about the number of maxima In an experiment a ray of red light and a ray of blue light pass through a triangular glass prism as shown. red light blue light red light blue light A student viewing this experiment makes the following statement about the refractive index of the glass and the frequency of the light. The refractive index of the glass depends on the frequency of light. Use your knowledge of physics to comment on this statement A beam of blue light and a beam of red light are shone onto a screen. A student states that a beam of blue light will always produce a greater irradiance on the screen than the beam of red light. Use your knowledge of physics to comment on this statement. 3 48
Particles and Waves Final Revision Exam Questions Part 1
 Particles and Waves Final Revision Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN Version 2013 P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model 1 Section
Particles and Waves Final Revision Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN Version 2013 P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model 1 Section
CfE Higher Physics. Particles and Waves
 Wallace Hall Academy CfE Higher Physics Particles and Waves Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model
Wallace Hall Academy CfE Higher Physics Particles and Waves Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model
Farr High School HIGHER PHYSICS. Unit 2 Particles and Waves
 Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Exam Question Booklet June 2017 1 2 THE STANDARD MODEL 1. Three students each make a comment about antiparticles. I An antiparticle has the same
Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Exam Question Booklet June 2017 1 2 THE STANDARD MODEL 1. Three students each make a comment about antiparticles. I An antiparticle has the same
Higher Physics. Particles and Waves
 Perth Academy Physics Department Higher Physics Particles and Waves Particles and Waves Homework Standard Model 1 Electric Fields and Potential Difference 2 Radioactivity 3 Fusion & Fission 4 The Photoelectric
Perth Academy Physics Department Higher Physics Particles and Waves Particles and Waves Homework Standard Model 1 Electric Fields and Potential Difference 2 Radioactivity 3 Fusion & Fission 4 The Photoelectric
Higher -o-o-o- Past Paper questions o-o-o- 3.3 Photoelectric
 Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.3 Photoelectric 1996 Q36 The work function for sodium metal is 2.9x10-19 J. Light of wavelength 5.4x10-7 m strikes the surface of this metal. What
Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.3 Photoelectric 1996 Q36 The work function for sodium metal is 2.9x10-19 J. Light of wavelength 5.4x10-7 m strikes the surface of this metal. What
Farr High School HIGHER PHYSICS. Unit 2 Particles and Waves. Question Booklet
 Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Question Booklet 1 THE STANDARD MODEL Orders of magnitude 1. The diagram shows a simple model of the atom. A B + + + + + C D Match each of the
Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Question Booklet 1 THE STANDARD MODEL Orders of magnitude 1. The diagram shows a simple model of the atom. A B + + + + + C D Match each of the
Cathkin High School Physics Particles and Waves Questions and Solutions
 NATIONAL QUALIFICATIONS CURRICULUM SUPPORT Cathkin High School Physics Particles and Waves Questions and Solutions James Page Arthur Baillie [HIGHER] The Scottish Qualifications Authority regularly reviews
NATIONAL QUALIFICATIONS CURRICULUM SUPPORT Cathkin High School Physics Particles and Waves Questions and Solutions James Page Arthur Baillie [HIGHER] The Scottish Qualifications Authority regularly reviews
Homework 2: Forces on Charged Particles
 Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Particles and Waves Homework One (Target mark 13 out of 15)
 Particles and Waves Homework One (Target mark 13 out of 15) Display all answers to 2 significant figures. 1. A car covers a distance of 170m in a time of 18s. Calculate the average speed of the car. 2.
Particles and Waves Homework One (Target mark 13 out of 15) Display all answers to 2 significant figures. 1. A car covers a distance of 170m in a time of 18s. Calculate the average speed of the car. 2.
Higher Physics Particles and Waves 2 Notes
 Higher Physics Particles and Waves 2 Notes Teachers Booklet Learning Outcomes Interference and diffraction This builds on information from N5 Waves and Radiation H Particles and Waves book 1 At the end
Higher Physics Particles and Waves 2 Notes Teachers Booklet Learning Outcomes Interference and diffraction This builds on information from N5 Waves and Radiation H Particles and Waves book 1 At the end
Bannerman High School Physics Department. Making Accurate Statements. Higher Physics. Quanta and Waves
 Bannerman High School Physics Department Making Accurate Statements Higher Physics Quanta and Waves Mandatory Key Area: Particle Physics 1. Use your knowledge of physics to estimate the ratio of the smallest
Bannerman High School Physics Department Making Accurate Statements Higher Physics Quanta and Waves Mandatory Key Area: Particle Physics 1. Use your knowledge of physics to estimate the ratio of the smallest
ATOMIC WORLD P.1. ejected photoelectrons. current amplifier. photomultiplier tube (PMT)
 ATOMIC WORLD P. HKAL PAPER I 0 8 The metal Caesium has a work function of.08 ev. Given: Planck constant h = 6.63 0 34 J s, charge of an electron e =.60 0 9 C (a) (i) Calculate the longest wavelength of
ATOMIC WORLD P. HKAL PAPER I 0 8 The metal Caesium has a work function of.08 ev. Given: Planck constant h = 6.63 0 34 J s, charge of an electron e =.60 0 9 C (a) (i) Calculate the longest wavelength of
Higher -o-o-o- Past Paper questions o-o-o- 3.6 Radiation
 Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.6 Radiation 2000 Q29 Radium (Ra) decays to radon (Rn) by the emission of an alpha particle. Some energy is also released by this decay. The decay
Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.6 Radiation 2000 Q29 Radium (Ra) decays to radon (Rn) by the emission of an alpha particle. Some energy is also released by this decay. The decay
EXAMINATION QUESTIONS (6)
 1. What is a beta-particle? A a helium nucleus B a high-energy electron C four protons D two neutrons EXAMINATION QUESTIONS (6) 2. The diagram shows part of a circuit used to switch street lamps on and
1. What is a beta-particle? A a helium nucleus B a high-energy electron C four protons D two neutrons EXAMINATION QUESTIONS (6) 2. The diagram shows part of a circuit used to switch street lamps on and
Unit Three. Mesons can only be made from matter/anti-matter combinations. This makes mesons Unstable Short lived
 Unit Three The Standard Model Particle models of matter have existed from the early recorded history. At the start of the 20 th century the model that was being developed consisted of a central nucleus
Unit Three The Standard Model Particle models of matter have existed from the early recorded history. At the start of the 20 th century the model that was being developed consisted of a central nucleus
Unit 2 - Particles and Waves - Part 2
 WAVE-PARTICLE DUALITY Unit - Particles and Waves - Part 8. The photoelectric effect and wave particle duality Photoelectric effect as evidence for the particulate nature of light. Photons of sufficient
WAVE-PARTICLE DUALITY Unit - Particles and Waves - Part 8. The photoelectric effect and wave particle duality Photoelectric effect as evidence for the particulate nature of light. Photons of sufficient
Radioactivity. (b) Fig shows two samples of the same radioactive substance. The substance emits β-particles. Fig. 12.1
 112 (a) What is meant by radioactive decay? Radioactivity [2] (b) Fig. 12.1 shows two samples of the same radioactive substance. The substance emits β-particles. Fig. 12.1 Put a tick alongside any of the
112 (a) What is meant by radioactive decay? Radioactivity [2] (b) Fig. 12.1 shows two samples of the same radioactive substance. The substance emits β-particles. Fig. 12.1 Put a tick alongside any of the
Higher -o-o-o- Past Paper questions o-o-o- 3.4 Spectra
 Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
RED. BLUE Light. Light-Matter
 1 Light-Matter This experiment demonstrated that light behaves as a wave. Essentially Thomas Young passed a light of a single frequency ( colour) through a pair of closely spaced narrow slits and on the
1 Light-Matter This experiment demonstrated that light behaves as a wave. Essentially Thomas Young passed a light of a single frequency ( colour) through a pair of closely spaced narrow slits and on the
CHAPTER 12 TEST REVIEW
 IB PHYSICS Name: Period: Date: # Marks: 76 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 12 TEST REVIEW 1. An alpha particle is accelerated through a potential difference of 10 kv.
IB PHYSICS Name: Period: Date: # Marks: 76 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 12 TEST REVIEW 1. An alpha particle is accelerated through a potential difference of 10 kv.
Topic 7 &13 Review Atomic, Nuclear, and Quantum Physics
 Name: Date:. Isotopes provide evidence for the existence of A. protons. B. electrons. C. nuclei. Topic 7 &3 Review Atomic, Nuclear, and Quantum Physics D. neutrons.. The atomic line spectra of elements
Name: Date:. Isotopes provide evidence for the existence of A. protons. B. electrons. C. nuclei. Topic 7 &3 Review Atomic, Nuclear, and Quantum Physics D. neutrons.. The atomic line spectra of elements
1. (a) An ion of plutonium Pu has an overall charge of C. (iii) electrons... (3) (2) (Total 5 marks)
 AQA Questions from 2004 to 2006 Particle Physics 239 94 1. (a) An ion of plutonium Pu has an overall charge of +1.6 10 19 C. For this ion state the number of (i) protons... neutrons... (iii) electrons...
AQA Questions from 2004 to 2006 Particle Physics 239 94 1. (a) An ion of plutonium Pu has an overall charge of +1.6 10 19 C. For this ion state the number of (i) protons... neutrons... (iii) electrons...
PHYSICS HIGHER LEVEL
 PRE-LEAVING CERTIFICATE EXAMINATION, 2009 PHYSICS HIGHER LEVEL Ti m e : 3 h o u r s Answer three questions from section A and five questions from section B. Page 1 of 8 SECTION A (120 marks) Answer three
PRE-LEAVING CERTIFICATE EXAMINATION, 2009 PHYSICS HIGHER LEVEL Ti m e : 3 h o u r s Answer three questions from section A and five questions from section B. Page 1 of 8 SECTION A (120 marks) Answer three
PHYSICS ADVANCED HIGHER. Unit 3 Electromagnetism Homework
 PHYSICS ADVANCED HIGHER Unit 3 Electromagnetism Homework 1 DATA SHEET COMMON PHYSICAL QUANTITIES Quantity Symbol Value Quantity Symbol Value Gravitational acceleration on Earth Radius of Earth Mass of
PHYSICS ADVANCED HIGHER Unit 3 Electromagnetism Homework 1 DATA SHEET COMMON PHYSICAL QUANTITIES Quantity Symbol Value Quantity Symbol Value Gravitational acceleration on Earth Radius of Earth Mass of
PHYSICS ORDINARY LEVEL
 *B16* PRE-LEAVING CERTIFICATE EXAMINATION, 2011 PHYSICS ORDINARY LEVEL TIME: 3 HOURS Answer three questions from section A and five questions from section B. Page 1 of 10 SECTION A (120 marks) Answer three
*B16* PRE-LEAVING CERTIFICATE EXAMINATION, 2011 PHYSICS ORDINARY LEVEL TIME: 3 HOURS Answer three questions from section A and five questions from section B. Page 1 of 10 SECTION A (120 marks) Answer three
Higher -o-o-o- Past Paper questions o-o-o- 3.6 Radiation
 Higher -o-o-o- Past Paper questions 1991-2001 -o-o-o- 3.6 Radiation 1992 Q35 A typical reaction produced in the core of a nuclear reactor can be described by the following equation: (a) State the name
Higher -o-o-o- Past Paper questions 1991-2001 -o-o-o- 3.6 Radiation 1992 Q35 A typical reaction produced in the core of a nuclear reactor can be described by the following equation: (a) State the name
VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION. 23/9/ h 1600h
 Name: Class: 13S VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION PHYSICS 9646/3 Higher 2 Paper 3 23/9/2014 1400h 1600h TUESDAY (2 Hours) This paper consists of two sections: Section A (40 marks)
Name: Class: 13S VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION PHYSICS 9646/3 Higher 2 Paper 3 23/9/2014 1400h 1600h TUESDAY (2 Hours) This paper consists of two sections: Section A (40 marks)
THE INDIAN COMMUNITY SCHOOL, KUWAIT SECOND SEMESTER EXAMINATION PHYSICS (Theory)
 CLASS:XII Marks : 70 General Instructions: THE INDIAN COMMUNITY SCHOOL, KUWAIT SECOND SEMESTER EXAMINATION 2016-17 PHYSICS (Theory) Time : Hrs (i) (ii) (iii) (iv) (v) All questions are compulsory. This
CLASS:XII Marks : 70 General Instructions: THE INDIAN COMMUNITY SCHOOL, KUWAIT SECOND SEMESTER EXAMINATION 2016-17 PHYSICS (Theory) Time : Hrs (i) (ii) (iii) (iv) (v) All questions are compulsory. This
Cumulative Review 1 Use the following information to answer the next two questions.
 Cumulative Review 1 Use the following information to answer the next two questions. 1. At what distance from the mirror is the image located? a. 0.10 m b. 0.20 m c. 0.30 m d. 0.40 m 2. At what distance
Cumulative Review 1 Use the following information to answer the next two questions. 1. At what distance from the mirror is the image located? a. 0.10 m b. 0.20 m c. 0.30 m d. 0.40 m 2. At what distance
Physics. Student Materials Advanced Higher. Tutorial Problems Electrical Phenomena HIGHER STILL. Spring 2000
 Spring 2000 HIGHER STILL Physics Student Materials Advanced Higher Tutorial Problems Electrical Phenomena TUTORIAL 1 Coulomb's Inverse Square Law 1 A charge of 2.0 x 10-8 C is placed a distance of 2.0
Spring 2000 HIGHER STILL Physics Student Materials Advanced Higher Tutorial Problems Electrical Phenomena TUTORIAL 1 Coulomb's Inverse Square Law 1 A charge of 2.0 x 10-8 C is placed a distance of 2.0
Name the region of the electromagnetic radiation emitted by the laser. ...
 1. An argon-laser emits electromagnetic radiation of wavelength 5.1 10 7 m. The radiation is directed onto the surface of a caesium plate. The work function energy for caesium is 1.9 ev. (i) Name the region
1. An argon-laser emits electromagnetic radiation of wavelength 5.1 10 7 m. The radiation is directed onto the surface of a caesium plate. The work function energy for caesium is 1.9 ev. (i) Name the region
tip conducting surface
 PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
SECTION A Quantum Physics and Atom Models
 AP Physics Multiple Choice Practice Modern Physics SECTION A Quantum Physics and Atom Models 1. Light of a single frequency falls on a photoelectric material but no electrons are emitted. Electrons may
AP Physics Multiple Choice Practice Modern Physics SECTION A Quantum Physics and Atom Models 1. Light of a single frequency falls on a photoelectric material but no electrons are emitted. Electrons may
A beam of coherent monochromatic light from a distant galaxy is used in an optics experiment on Earth.
 Waves_P2 [152 marks] A beam of coherent monochromatic light from a distant galaxy is used in an optics experiment on Earth. The beam is incident normally on a double slit. The distance between the slits
Waves_P2 [152 marks] A beam of coherent monochromatic light from a distant galaxy is used in an optics experiment on Earth. The beam is incident normally on a double slit. The distance between the slits
Explain how line spectra are produced. In your answer you should describe:
 The diagram below shows the line spectrum of a gas. Explain how line spectra are produced. In your answer you should describe: how the collisions of charged particles with gas atoms can cause the atoms
The diagram below shows the line spectrum of a gas. Explain how line spectra are produced. In your answer you should describe: how the collisions of charged particles with gas atoms can cause the atoms
Subject: PHYSICS Level: ADVANCED Time: 3 hrs
 SIR MICHELANGELO REFALO CENTRE FOR FURTHER STUDIES VICTORIA GOZO Annual Exam 2013 Subject: PHYSICS Level: ADVANCED Time: 3 hrs Take the acceleration due to gravity g = 10m/s 2 Section A Answer all questions
SIR MICHELANGELO REFALO CENTRE FOR FURTHER STUDIES VICTORIA GOZO Annual Exam 2013 Subject: PHYSICS Level: ADVANCED Time: 3 hrs Take the acceleration due to gravity g = 10m/s 2 Section A Answer all questions
Larbert High School. Quanta and Waves. Homework Exercises ADVANCED HIGHER PHYSICS
 Larbert High School ADVANCED HIGHER PHYSICS Quanta and Waves Homework Exercises 3.1 3.6 3.1 Intro to Quantum Theory HW 1. (a) Explain what is meant by term black body. (1) (b) State two observations that
Larbert High School ADVANCED HIGHER PHYSICS Quanta and Waves Homework Exercises 3.1 3.6 3.1 Intro to Quantum Theory HW 1. (a) Explain what is meant by term black body. (1) (b) State two observations that
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *6182103596* PHYSICS 5054/21 Paper 2 Theory May/June 2015 1 hour 45 minutes Candidates answer on the Question Paper. No Additional Materials
Cambridge International Examinations Cambridge Ordinary Level *6182103596* PHYSICS 5054/21 Paper 2 Theory May/June 2015 1 hour 45 minutes Candidates answer on the Question Paper. No Additional Materials
FALL 2004 Final Exam, Part A
 Physics 152 FALL 2004 Final Exam, Part A Roster No.: Score: 23 pts. possible Exam time limit: 2 hours. You may use a calculator and both sides of 2 sheets of notes, handwritten only. Closed book; no collaboration.
Physics 152 FALL 2004 Final Exam, Part A Roster No.: Score: 23 pts. possible Exam time limit: 2 hours. You may use a calculator and both sides of 2 sheets of notes, handwritten only. Closed book; no collaboration.
(Total for Question = 5 marks) PhysicsAndMathsTutor.com
 1 Rutherford designed an experiment to see what happened when alpha particles were directed at a piece of gold foil. Summarise the observations and state the conclusions Rutherford reached about the structure
1 Rutherford designed an experiment to see what happened when alpha particles were directed at a piece of gold foil. Summarise the observations and state the conclusions Rutherford reached about the structure
λ = h = h p mv λ = h mv FXA 2008 Candidates should be able to :
 1 Candidates should be able to : Explain electron diffraction as evidence for the wave nature of particles like electrons. Explain that electrons travelling through polycrystalline graphite will be diffracted
1 Candidates should be able to : Explain electron diffraction as evidence for the wave nature of particles like electrons. Explain that electrons travelling through polycrystalline graphite will be diffracted
0 ev. first excited state 3.4 ev. ground state 13.6 ev
 1. Below is a simplified energy level diagram for atomic hydrogen. 0 ev first excited state 3.4 ev ground state 13.6 ev (a) A free electron with 12 ev of kinetic energy collides with an atom of hydrogen.
1. Below is a simplified energy level diagram for atomic hydrogen. 0 ev first excited state 3.4 ev ground state 13.6 ev (a) A free electron with 12 ev of kinetic energy collides with an atom of hydrogen.
Photoelectric Effect
 Photoelectric Effect Teacher s Handbook In association with the Cherenkov Telescope Array Goldleaf Electroscope Experiment Goldleaf Electroscope Experiment Duration: 50mins Prerequisites: Knowledge of
Photoelectric Effect Teacher s Handbook In association with the Cherenkov Telescope Array Goldleaf Electroscope Experiment Goldleaf Electroscope Experiment Duration: 50mins Prerequisites: Knowledge of
Bi β + Po Bismuth-214 is radioactive. It has a half-life of 20 minutes. (a) The nuclide notation for bismuth-214 is Bi.
 1 Bismuth-214 is radioactive. It has a half-life of 20 minutes. (a) The nuclide notation for bismuth-214 is Bi. State the composition of the nucleus of bismuth-214. [2] (b) Bismuth-214 decays by β-decay
1 Bismuth-214 is radioactive. It has a half-life of 20 minutes. (a) The nuclide notation for bismuth-214 is Bi. State the composition of the nucleus of bismuth-214. [2] (b) Bismuth-214 decays by β-decay
Mock Examination Physics 2 nd Book Time 3 Hrs MM : 70
 Mock Examination Physics 2 nd Book Time 3 Hrs MM : 70 SECTION A 1. How does the angle of minimum deviation of a glass prism vary, If the incident violet light is replaced with red light? 2. What is principle
Mock Examination Physics 2 nd Book Time 3 Hrs MM : 70 SECTION A 1. How does the angle of minimum deviation of a glass prism vary, If the incident violet light is replaced with red light? 2. What is principle
An ion follows a circular path in a uniform magnetic field. Which single change decreases the radius of the path?
 T5-1 [237 marks] 1. A circuit is formed by connecting a resistor between the terminals of a battery of electromotive force (emf) 6 V. The battery has internal resistance. Which statement is correct when
T5-1 [237 marks] 1. A circuit is formed by connecting a resistor between the terminals of a battery of electromotive force (emf) 6 V. The battery has internal resistance. Which statement is correct when
KCSE 2009 PHYSICS PAPER 2
 KCSE 2009 PHYSICS PAPER 2 SECTION A (25 MARKS) Answer all the questions in this section in the spaces provided 1. State the number of images formed when an object is between two plane mirror placed in
KCSE 2009 PHYSICS PAPER 2 SECTION A (25 MARKS) Answer all the questions in this section in the spaces provided 1. State the number of images formed when an object is between two plane mirror placed in
Care should be taken to give an appropriate number of significant figures in the final answers to calculations.
 X069/70 NATIONAL QUALIFICATIONS 007 WEDNESDAY, 6 MAY.00 PM 3.30 PM PHYSICS ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the
X069/70 NATIONAL QUALIFICATIONS 007 WEDNESDAY, 6 MAY.00 PM 3.30 PM PHYSICS ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the
1. What is the minimum energy required to excite a mercury atom initially in the ground state? ev ev ev
 Page 1 of 10 modern bank Name 25-MAY-05 1. What is the minimum energy required to excite a mercury atom initially in the ground state? 1. 4.64 ev 3. 10.20 ev 2. 5.74 ev 4. 10.38 ev 2. The diagram represents
Page 1 of 10 modern bank Name 25-MAY-05 1. What is the minimum energy required to excite a mercury atom initially in the ground state? 1. 4.64 ev 3. 10.20 ev 2. 5.74 ev 4. 10.38 ev 2. The diagram represents
A level Physics (7407/7408)
 rayton Manor High School level Physics (7407/7408) S MQ 1 Name: lass: uthor: ate: Time: Marks: omments: Page 1 rayton Manor High School Q1. nucleus of a particular element decays, emitting a series of
rayton Manor High School level Physics (7407/7408) S MQ 1 Name: lass: uthor: ate: Time: Marks: omments: Page 1 rayton Manor High School Q1. nucleus of a particular element decays, emitting a series of
A motion of particles motion of particles
 PHYSICS 2.3 Externally assessed 4 credits Demonstrate understanding of waves Unit 1: Wave properties Energy transfer Wave motion, in general, is a process in which kinetic energy is transferred from one
PHYSICS 2.3 Externally assessed 4 credits Demonstrate understanding of waves Unit 1: Wave properties Energy transfer Wave motion, in general, is a process in which kinetic energy is transferred from one
Physics Assessment Unit AS 2
 Centre Number 71 Candidate Number ADVANCED SUBSIDIARY General Certificate of Education January 2011 Physics Assessment Unit AS 2 assessing Module 2: Waves, Photons and Medical Physics AY121 [AY121] MONDAY
Centre Number 71 Candidate Number ADVANCED SUBSIDIARY General Certificate of Education January 2011 Physics Assessment Unit AS 2 assessing Module 2: Waves, Photons and Medical Physics AY121 [AY121] MONDAY
London Examinations IGCSE
 Centre No. Candidate No. Surname Signature Initial(s) Paper Reference(s) 4420/2H London Examinations IGCSE Physics Paper 2H Higher Tier Tuesday 2 May 2006 Morning Time: 2 hours Materials required for examination
Centre No. Candidate No. Surname Signature Initial(s) Paper Reference(s) 4420/2H London Examinations IGCSE Physics Paper 2H Higher Tier Tuesday 2 May 2006 Morning Time: 2 hours Materials required for examination
Which of the following classes of electromagnetic waves will not ionise neutral atoms?
 1 In an experiment to demonstrate the photoelectric effect, a charged metal plate is illuminated with light from different sources. The plate loses its charge when an ultraviolet light source is used but
1 In an experiment to demonstrate the photoelectric effect, a charged metal plate is illuminated with light from different sources. The plate loses its charge when an ultraviolet light source is used but
Topic 4 &11 Review Waves & Oscillations
 Name: Date: Topic 4 &11 Review Waves & Oscillations 1. A source produces water waves of frequency 10 Hz. The graph shows the variation with horizontal position of the vertical displacement of the surface
Name: Date: Topic 4 &11 Review Waves & Oscillations 1. A source produces water waves of frequency 10 Hz. The graph shows the variation with horizontal position of the vertical displacement of the surface
H2 Physics Set A Paper 3 H2 PHYSICS. Exam papers with worked solutions. (Selected from Top JC) SET A PAPER 3.
 H2 PHYSICS Exam papers with worked solutions (Selected from Top JC) SET A PAPER 3 Compiled by THE PHYSICS CAFE 1 P a g e Candidates answer on the Question Paper. No Additional Materials are required. READ
H2 PHYSICS Exam papers with worked solutions (Selected from Top JC) SET A PAPER 3 Compiled by THE PHYSICS CAFE 1 P a g e Candidates answer on the Question Paper. No Additional Materials are required. READ
Physics 30: Chapter 5 Exam Wave Nature of Light
 Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
CBSE XII Physics 2016
 Time: 3 hours; Maximum Marks: 70 General Instructions: 1. All questions are compulsory. There are 26 questions in all. 2. This question paper has five sections: Section A, Section B, Section, Section D
Time: 3 hours; Maximum Marks: 70 General Instructions: 1. All questions are compulsory. There are 26 questions in all. 2. This question paper has five sections: Section A, Section B, Section, Section D
EXTRA NOTES FOR IGCSE PHYSICS. Calculate the orbital speed of the Earth around the Sun. (Earth orbital radius = 150 million km)
 EXTRA NOTES FOR IGCSE PHYSICS #1.33 use the relationship between orbital speed, orbital radius and time period: orbital speed = 2 π orbital radius time period or v = 2 π r T Example 1: Calculate the orbital
EXTRA NOTES FOR IGCSE PHYSICS #1.33 use the relationship between orbital speed, orbital radius and time period: orbital speed = 2 π orbital radius time period or v = 2 π r T Example 1: Calculate the orbital
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
U n 3 n Ba Kr (D) Br (C) Kr (B) Rb (E) 94 37
 1984 36. The critical angle for a transparent material in air is 30. The index of refraction of the material is most nearly (A) 0.33 (B) 0.50 (C) 1.0 (D) 1.5 (E) 2.0 37. An object is placed as shown in
1984 36. The critical angle for a transparent material in air is 30. The index of refraction of the material is most nearly (A) 0.33 (B) 0.50 (C) 1.0 (D) 1.5 (E) 2.0 37. An object is placed as shown in
Answer three questions from Section A and five questions from Section B.
 L.36 PRE-LEAVING CERTIFICATE EXAMINATION, 2016 PHYSICS HIGHER LEVEL TIME 3 HOURS Answer three questions from Section A and five questions from Section B. Relevant data are listed in the Formulae and Tables
L.36 PRE-LEAVING CERTIFICATE EXAMINATION, 2016 PHYSICS HIGHER LEVEL TIME 3 HOURS Answer three questions from Section A and five questions from Section B. Relevant data are listed in the Formulae and Tables
5 Atomic Physics. 1 of the isotope remains. 1 minute, 4. Atomic Physics. 1. Radioactivity 2. The nuclear atom
 5 Atomic Physics 1. Radioactivity 2. The nuclear atom 1. In a fission reactor, which particle causes a Uranium-235 nucleus to split? A. alpha-particle B. gamma ray C. neutron D. proton 2. A radioactive
5 Atomic Physics 1. Radioactivity 2. The nuclear atom 1. In a fission reactor, which particle causes a Uranium-235 nucleus to split? A. alpha-particle B. gamma ray C. neutron D. proton 2. A radioactive
REVISION: WAVES, SOUND & LIGHT 11 JUNE 2013
 REVISION: WAVES, SOUND & LIGHT 11 JUNE 2013 Lesson Description In this lesson we revise: the Doppler Effect, Huygens Principle, Diffraction of Light & the Photoelectric Effect Key Concepts The Doppler
REVISION: WAVES, SOUND & LIGHT 11 JUNE 2013 Lesson Description In this lesson we revise: the Doppler Effect, Huygens Principle, Diffraction of Light & the Photoelectric Effect Key Concepts The Doppler
WAVES AND PARTICLES. (c)
 WAVES AND PARTICLES 1. An electron and a proton are accelerated through the same potential difference. The ration of their De Broglie wave length will be -- (a) (b) (c) (d) 1 2. What potential must be
WAVES AND PARTICLES 1. An electron and a proton are accelerated through the same potential difference. The ration of their De Broglie wave length will be -- (a) (b) (c) (d) 1 2. What potential must be
Exam 2 Development of Quantum Mechanics
 PHYS40 (Spring 00) Riq Parra Exam # (Friday, April 1 th, 00) Exam Development of Quantum Mechanics Do NOT write your name on this exam. Write your class ID number on the top right hand corner of each problem
PHYS40 (Spring 00) Riq Parra Exam # (Friday, April 1 th, 00) Exam Development of Quantum Mechanics Do NOT write your name on this exam. Write your class ID number on the top right hand corner of each problem
FOR EXAMINER S USE ONLY. Section Question (s) Max. Score Candidates Score A
 Name. School Index No.. Candidates Signature Date.... 232/2 PHYSICS Paper 2 (Theory) July/August 2010 2 Hours BOMET/CHEPALUNGU JOINT EVALUATION TEST - 2010 Kenya Certificate of Secondary Education (K.C.S.E)
Name. School Index No.. Candidates Signature Date.... 232/2 PHYSICS Paper 2 (Theory) July/August 2010 2 Hours BOMET/CHEPALUNGU JOINT EVALUATION TEST - 2010 Kenya Certificate of Secondary Education (K.C.S.E)
Wednesday 21 June 2017 Morning Time allowed: 2 hours 15 minutes
 Oxford Cambridge and RSA A Level Physics A H556/02 Exploring physics Wednesday 21 June 2017 Morning Time allowed: 2 hours 15 minutes *6829545160* You must have: the Data, Formulae and Relationship Booklet
Oxford Cambridge and RSA A Level Physics A H556/02 Exploring physics Wednesday 21 June 2017 Morning Time allowed: 2 hours 15 minutes *6829545160* You must have: the Data, Formulae and Relationship Booklet
Unit 3 Electromagnetism. Class Questions
 Unit 3 Electromagnetism Class Questions Contents TUTORIAL 1 TUTORIAL 2 TUTORIAL 3 Coulomb's Inverse Square Law Electric Field Strength Electrostatic Potential PAST PAPERS 2012 Q5, 2011 Q5, 2010 Q6, 2009
Unit 3 Electromagnetism Class Questions Contents TUTORIAL 1 TUTORIAL 2 TUTORIAL 3 Coulomb's Inverse Square Law Electric Field Strength Electrostatic Potential PAST PAPERS 2012 Q5, 2011 Q5, 2010 Q6, 2009
P.M. THURSDAY, 21 May hours. Write your name, centre number and candidate number in the spaces at the top of this page.
 Candidate Name Centre Number Candidate Number GCE AS/A level 1322/01 New AS PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. THURSDAY, 21 May 2009 1 1 4 hours ADDITIONAL MATERIALS In addition to this
Candidate Name Centre Number Candidate Number GCE AS/A level 1322/01 New AS PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. THURSDAY, 21 May 2009 1 1 4 hours ADDITIONAL MATERIALS In addition to this
State the position of protons, neutrons and electrons in the atom
 2.1 The Atom 2.1.1 - State the position of protons, neutrons and electrons in the atom Atoms are made up of a nucleus containing positively charged protons and neutral neutrons, with negatively charged
2.1 The Atom 2.1.1 - State the position of protons, neutrons and electrons in the atom Atoms are made up of a nucleus containing positively charged protons and neutral neutrons, with negatively charged
1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. ...[1]
![1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. ...[1] 1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. ...[1]](/thumbs/81/83978040.jpg) 1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. 1 (a) (b) Name the effect described above....[1] The variation with frequency f of the maximum
1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. 1 (a) (b) Name the effect described above....[1] The variation with frequency f of the maximum
Spring Not-Break Review Assignment
 Name AP Physics B Spring Not-Break Review Assignment Date Mrs. Kelly. A kilogram block is released from rest at the top of a curved incline in the shape of a quarter of a circle of radius R. The block
Name AP Physics B Spring Not-Break Review Assignment Date Mrs. Kelly. A kilogram block is released from rest at the top of a curved incline in the shape of a quarter of a circle of radius R. The block
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space.
 Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
L-35 Modern Physics-3 Nuclear Physics 29:006 FINAL EXAM. Structure of the nucleus. The atom and the nucleus. Nuclear Terminology
 9:006 FINAL EXAM L-5 Modern Physics- Nuclear Physics The final exam is on Monday MAY 7:0 AM - 9:0 AM in W90 CB The FE is not cumulative, and will cover lectures through 6. (50 questions) The last regular
9:006 FINAL EXAM L-5 Modern Physics- Nuclear Physics The final exam is on Monday MAY 7:0 AM - 9:0 AM in W90 CB The FE is not cumulative, and will cover lectures through 6. (50 questions) The last regular
Care should be taken to give an appropriate number of significant figures in the final answers to calculations.
 X069//0 NATIONAL QUALIFICATIONS 0 MONDAY, 7 MAY PHYSICS.00 PM.0 PM ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the Data Sheet
X069//0 NATIONAL QUALIFICATIONS 0 MONDAY, 7 MAY PHYSICS.00 PM.0 PM ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the Data Sheet
Modern Physics, Waves, Electricity
 Name: Date: 1. Metal sphere has a charge of +12 elementary charges and identical sphere has a charge of +16 elementary charges. fter the two spheres are brought into contact, the charge on sphere is 4.
Name: Date: 1. Metal sphere has a charge of +12 elementary charges and identical sphere has a charge of +16 elementary charges. fter the two spheres are brought into contact, the charge on sphere is 4.
Care should be taken to give an appropriate number of significant figures in the final answers to calculations.
 X069/3/0 NATIONAL QUALIFICATIONS 0 MONDAY, 8 MAY PHYSICS.00 PM 3.30 PM ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the Data
X069/3/0 NATIONAL QUALIFICATIONS 0 MONDAY, 8 MAY PHYSICS.00 PM 3.30 PM ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the Data
GCE AS/A level 1322/01 PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES
 Surname Centre Number Candidate Number Other Names 2 GCE AS/A level 1322/01 PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. FRIDAY, 25 May 2012 1½ hours ADDITIONAL MATERIALS In addition to this paper,
Surname Centre Number Candidate Number Other Names 2 GCE AS/A level 1322/01 PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. FRIDAY, 25 May 2012 1½ hours ADDITIONAL MATERIALS In addition to this paper,
Please write down the Serial Number of the question before attempting it. PHYSICS (Theory) Time allowed : 3 hours Maximum Marks : 70
 Series ONS SET-1 Roll No. Candiates must write code on the title page of the answer book Please check that this question paper contains 16 printed pages. Code number given on the right hand side of the
Series ONS SET-1 Roll No. Candiates must write code on the title page of the answer book Please check that this question paper contains 16 printed pages. Code number given on the right hand side of the
Coimisiún na Scrúduithe Stáit State Examinations Commission
 2009. M 35 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION, 2009 PHYSICS ORDINARY LEVEL MONDAY 15 JUNE MORNING 9:30 TO 12:30 Answer three questions from Section
2009. M 35 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION, 2009 PHYSICS ORDINARY LEVEL MONDAY 15 JUNE MORNING 9:30 TO 12:30 Answer three questions from Section
PSI AP Physics How was it determined that cathode rays possessed a negative charge?
 PSI AP Physics 2 Name Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently named
PSI AP Physics 2 Name Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently named
Name: Class: Date: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Name: Class: Date: AP REVIEW 3 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.. For a mass hanging from a spring, the maximum displacement the
Name: Class: Date: AP REVIEW 3 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.. For a mass hanging from a spring, the maximum displacement the
Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes
 St Ninian s High School Chemistry Department National 5 Chemistry Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes Name Learning Outcomes After completing this topic you should be able to :
St Ninian s High School Chemistry Department National 5 Chemistry Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes Name Learning Outcomes After completing this topic you should be able to :
MODERN PHYSICS. A. s c B. dss C. u
 MODERN PHYSIS Name: Date: 1. Which color of light has the greatest energy per photon? 4. The composition of a meson with a charge of 1 elementary charge could be. red. green. blue D. violet. s c. dss.
MODERN PHYSIS Name: Date: 1. Which color of light has the greatest energy per photon? 4. The composition of a meson with a charge of 1 elementary charge could be. red. green. blue D. violet. s c. dss.
Care should be taken to give an appropriate number of significant figures in the final answers to calculations.
 X069/3/0 NATIONAL QUALIFICATIONS 05 TUESDAY, 5 MAY PHYSICS.00 PM 3.30 PM ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the
X069/3/0 NATIONAL QUALIFICATIONS 05 TUESDAY, 5 MAY PHYSICS.00 PM 3.30 PM ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the
Answer three questions from Section A and five questions from Section B.
 L.35 PRE-LEAVING CERTIFICATE EXAMINATION, 2016 PHYSICS ORDINARY LEVEL TIME 3 HOURS Answer three questions from Section A and five questions from Section B. Relevant data are listed in the Formulae and
L.35 PRE-LEAVING CERTIFICATE EXAMINATION, 2016 PHYSICS ORDINARY LEVEL TIME 3 HOURS Answer three questions from Section A and five questions from Section B. Relevant data are listed in the Formulae and
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Wave Nature of Matter
 Wave Nature of Matter Wave-Particle Duality de Broglie proposed that particles with momentum could have an associated wavelength (converse of photons having momentum) de Broglie wavelength h λ = p or p
Wave Nature of Matter Wave-Particle Duality de Broglie proposed that particles with momentum could have an associated wavelength (converse of photons having momentum) de Broglie wavelength h λ = p or p
PhysicsAndMathsTutor.com 1
 Q1. When a clean metal surface in a vacuum is irradiated with ultraviolet radiation of a certain frequency, electrons are emitted from the metal. (a) Explain why the kinetic energy of the emitted electrons
Q1. When a clean metal surface in a vacuum is irradiated with ultraviolet radiation of a certain frequency, electrons are emitted from the metal. (a) Explain why the kinetic energy of the emitted electrons
The Final Exam (Exam 4) will be on FRIDAY MAY 11 From 3 5 PM in LR1 VAN
 1 --------------------------------------------------------------------------------------------------------------------- 29:006 SPRING 2012 PRACTICE EXAM 4 ---------------------------------------------------------------------------------------------------------------------
1 --------------------------------------------------------------------------------------------------------------------- 29:006 SPRING 2012 PRACTICE EXAM 4 ---------------------------------------------------------------------------------------------------------------------
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
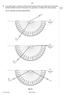 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
Coimisiún na Scrúduithe Stáit State Examinations Commission
 M 36 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION, 2004 PHYSICS HIGHER LEVEL MONDAY, 21 JUNE MORNING 9.30 TO 12.30 Answer three questions from section A and
M 36 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION, 2004 PHYSICS HIGHER LEVEL MONDAY, 21 JUNE MORNING 9.30 TO 12.30 Answer three questions from section A and
N13/4/PHYSI/HPM/ENG/TZ0/XX. Physics Higher level Paper 1. Wednesday 6 November 2013 (morning) 1 hour INSTRUCTIONS TO CANDIDATES
 N13/4/PHYSI/HPM/ENG/TZ/XX 8813651 Physics Higher level Paper 1 Wednesday 6 November 213 (morning) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
N13/4/PHYSI/HPM/ENG/TZ/XX 8813651 Physics Higher level Paper 1 Wednesday 6 November 213 (morning) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Physics 3204 UNIT 3 Test Matter Energy Interface
 Physics 3204 UNIT 3 Test Matter Energy Interface 2005 2006 Time: 60 minutes Total Value: 33 Marks Formulae and Constants v = f λ E = hf h f = E k + W 0 E = m c 2 p = h λ 1 A= A T 0 2 t 1 2 E k = ½ mv 2
Physics 3204 UNIT 3 Test Matter Energy Interface 2005 2006 Time: 60 minutes Total Value: 33 Marks Formulae and Constants v = f λ E = hf h f = E k + W 0 E = m c 2 p = h λ 1 A= A T 0 2 t 1 2 E k = ½ mv 2
1 The Cathode Rays experiment is associated. with: Millikan A B. Thomson. Townsend. Plank Compton
 1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
Revision Guide. Chapter 7 Quantum Behaviour
 Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
GraspIT AQA Atomic Structure Questions
 A. Atomic structure Atoms and isotopes 1. a) The diagram shows an atom of Beryllium. Name the parts labelled a, b and c. (3) electron (1) neutron (1) proton (1) b) What is the atomic mass of this atom?
A. Atomic structure Atoms and isotopes 1. a) The diagram shows an atom of Beryllium. Name the parts labelled a, b and c. (3) electron (1) neutron (1) proton (1) b) What is the atomic mass of this atom?
Chapter 1: Electrostatics
 1.1 Coulomb s law a) State Coulomb s law, Chapter 1: Electrostatics b) Sketch the electric force diagram and apply Coulomb s law for a system of point charges. 1.2 Electric field a) Define and use electric
1.1 Coulomb s law a) State Coulomb s law, Chapter 1: Electrostatics b) Sketch the electric force diagram and apply Coulomb s law for a system of point charges. 1.2 Electric field a) Define and use electric
Core Questions Physics unit 4 - Atomic Structure
 Core Questions Physics unit 4 - Atomic Structure No. Question Answer 1 What did scientists think about atoms before the discovery of the They were tiny spheres that could not be broken up electron? 2 Which
Core Questions Physics unit 4 - Atomic Structure No. Question Answer 1 What did scientists think about atoms before the discovery of the They were tiny spheres that could not be broken up electron? 2 Which
