Second order reactions. Second order, class I reactions 2A P A + B P. class I. class II. Nov 17, 2010!
|
|
|
- Maximillian Booth
- 6 years ago
- Views:
Transcription
1 Nov 7, ½ Exam 5 Friday /9/ opics & Old Exam problems o sudy (also sudy OWLs & noes): Ligand binding including cooperaiviy HE, 5 HE5 7 E, 8 E, 9 E Cenrifugaion & Elecrophoresis 6 Final abc, 7 E 5 8 E abc, 9 E abc Kineics hrough.7 HE5, 6 Final 7 E abc, 9 E abc Second order reacions Second order, class I reacions A P A + B P A P class I class II
2 A + B P Second order, class II reacions ( ) coninues [P] [B] e ( [B] )k ( ([B] )e [B] )k ( [B] e [B] )k ( [B] e [ A] [ B] )k Zero order Reacion half-life / is he ime a which half of he reacan concenraion is consumed Firs order Second order, class I 5 6 Summary of rae laws order law linear plo half ime unis of rae cons. [ A] k M/sec sec - Deermining reacion order Plo, ln and / vs. Measure v vs. Use iniial reacion velociy (I) k M - sec - [ A] (II) messy M - sec - n(i) n [ A] M -n sec - k n 7 8
3 Example: hypochlorie reducion he iniial velociy of he reacion I- + OCl- OI- + Clin basic soluion was sudied a differen soluion concenraions as shown. Wha are he orders of I-, OCland OH- and he value of he rae consan? [I-] (mm) [OCl-] (mm) [OH-] (M) v (mm/s of iodide) R 8.5 J/K mol.85 [I-] (mm) [OCl-] (mm).5 [OH-] (M) coninues v (mm/s of iodide) Formulas and consans, /5/ L am/k mol.987 cal/k mol kb.8 - J/K J Nm kgm/s.9 cal L am cal/k eu, Pa N/ m am. 5 Pa 76 orr, F 96,5 J/mol V PV nr E nr mu E q + w -.7 Srxn CP d CP,cons ln µ A µ a, µ a, R ln H rxn C p (9 ) P P Grxn V (P P ) dg VdP Sd + µadna + Grxn n gasr ln f asolv, asolv, K A [L] [L] N + K A [L] K D +[L] µa µa + RlnaA agas gaspgas/ am asolven solvenxsolven asolue soluecsolue/ M ; G G + RlnQ H E + PV Srxn CP d CP,cons ln µ A µ a, µ a, R ln H rxn C p ( ) ds G dqrev / n P rxn P Grxn V (P P ) dg VdP Sd + µadna + gas R ln f G G + RlnQ G H S V & dg Gdw v% ( non-pv -R lnk + VdP Sd $ w ' G -nef R lnq ne F G H S dg dwnon-pv + VdP Sd P,,K Rs D( v ) x (b a log M ) v dc/d f(, [B], [C], ) k ln ln k M ln ds dqrev/ G -nef R lnq ne F H vap $ ' P & ) P R % ( Psolven XsolvenPsolven,pure Pgas kxdissolved gas µa µa + RlnaA agas gaspgas/ am asolven solvenxsolven asolue soluecsolue/ M ; asolv, asolv, K A [L] [L] N + K A [L] K D +[L] H E + PV Veff V & v% ( $ w 'P,,K m G -R lnk Formulas and consans, /5/ R 8.5 J/K mol.85 L am/k mol.987 cal/k mol kb.8 - J/K J Nm kgm/s.9 cal L am cal/k eu, Pa N/ m am. 5 Pa 76 orr, F 96,5 J/mol V PV nr E nr mu E q + w cr / Veff m A k Rs D( v ) x (b a log M ) v dc/d f(, [B], [C], ) k ln ln k M H vap $ ' P ln & ) P R % ( Psolven XsolvenPsolven,pure Pgas kxdissolved gas cr / A k
4 C S rxn P d CP,cons ln H rxn C p ( ) µ A µ a, µ a, R ln a solv, a solv, ln µa µ a, µ a, R ln a solv, a solv, G rxn n gas R ln P P G rxn V (P P ) dg VdP Sd + µadna + µa µ A + RlnaA agas gaspgas/ am asolven solvenxsolven asolue soluecsolue/ M f N K A[L] + K A [L] [L] K D +[L] f N K A[L] + K A [L] [L] K D +[L] G G + RlnQ G -R lnk v V & % ( $ w ' P,,K V eff m G -nef R ds dqrev/ n e F lnq Rs M D( v ) ln P H x (b a log M ) $ vap ' & ) v dc/d f(, [B], [C], ) P R % ( k Psolven XsolvenPsolven,pure ln ln k v % V & ( V Pgas kxdissolved gas eff $ w ' P,,K m G H S cr / A k dg dwnon-pv + VdP Sd Chem/Biochem 7 Half Exam /5/ Page of Name:. Suppose you are sudying a proein (P) wih binding sies for is ligand (L). You perform an equilibrium dialysis experimen wih $% &'inside he dialysis bag, placed in a beaker conaining a()*+,-).)/., )9(,):+-*-5;-+9<,;-( $% & )+,(-6,5>7>.6>,),>*)/?@ $% &-.(-6,5>7Assume he emperaure is 5 C and ha P canno cross he dialysis membrane. a) >,-(,8).8.,;>,-).)/,>,-(5)+.6,)'-.,5>7AB)CD)+;;>().-.7 5E>,-(,)(9),-8F;((+;>8;)((,995;>.A c) If he binding sies are idenical and independen, wha is he dissociaion consan for L binding o a single sie on P? $%&'()*+,-*./*/$*+* -565+* $%&'() 7/$ * *+%,%-./+%.+'++-$%, * 6:56%%78%('.8+%.69.'('+%,6%,%%8-+',:569.'+,/,'('.8.;'< /.'$%;'9;%%%%.69.'('+%9('.8%>56%%78%6,('?.;/9/$,/%,('. CD E F <C */(?/,.%/+?9%9+/G% ;/'6%8/,,+/G%'9'99''/%./6%6'6%.H6'%./''(:5/++ 6+('(6%%78%,/%,;%'?-/%9;:5I ;@(6%('?.:5;/9/$,/%,.%-',//J%+''-%./J%/6K/++'%((//%'(&>LH6 '%./''(:5/++6+('(6%%78%,/%,;%'?-/%9;:5I M6%$.-6;%+'H-+'6%(./''(%78%,/%,;'?9J%.,?,N:5O('.6%,%',%,> P%,?.%'+;%+6%Q%,/6--.'-./%?8;%.,> (./';'?9 d) Again assuming he sies are idenical and independen, >,C>,8).8.,;>,-).)/C-**>*/ )/,F;),-.(-,(5)88+F-65DA 5 N:5O 6
5 Chem/Biochem 7 Exam /5/9 $%&'() Name: *+%,%-./+%.+'++-$%,.56%///+7%+'/'(6%$,8-6,%.%/' P:9*H P<*H va*hr,'(<9 k ACASA ACA9 9CU SA 8V 9:9;9< 9:9;<9 ACAA ACA9 SC9 SA 8V >,,?@/%@)AA BCD/6%E+%./$6 ACASA ACAS9 ACVA SA 8V,6'>,6%///+.%'(.%/'?@%.@/((%.%.%/''%./''@//',C F6/,6%'.@%.'(6/,.%/'('.P<G EF./%@'>6%.%+>('.6/,.%/'C*56/,/,('.H?+C HL%?-6%-.'-',%@H%6/,H('.6/,.%/'C k F6/,6%7+?%'(6%.%',/-.E/%.H, H + NO N + HO + O, slow '(6%%M?/+/E./?H@.%',,/6%%+%H%. k.%/',g*56/,/,('.h?+c O + H HO, fas k- 7
Northern Arizona University Exam #1. Section 2, Spring 2006 February 17, 2006
 Norhern Arizona Universiy Exam # CHM 52, General Chemisry II Dr. Brandon Cruickshank Secion 2, Spring 2006 February 7, 2006 Name ID # INSTRUCTIONS: Code he answers o he True-False and Muliple-Choice quesions
Norhern Arizona Universiy Exam # CHM 52, General Chemisry II Dr. Brandon Cruickshank Secion 2, Spring 2006 February 7, 2006 Name ID # INSTRUCTIONS: Code he answers o he True-False and Muliple-Choice quesions
Chapter 13 Homework Answers
 Chaper 3 Homework Answers 3.. The answer is c, doubling he [C] o while keeping he [A] o and [B] o consan. 3.2. a. Since he graph is no linear, here is no way o deermine he reacion order by inspecion. A
Chaper 3 Homework Answers 3.. The answer is c, doubling he [C] o while keeping he [A] o and [B] o consan. 3.2. a. Since he graph is no linear, here is no way o deermine he reacion order by inspecion. A
2) Of the following questions, which ones are thermodynamic, rather than kinetic concepts?
 AP Chemisry Tes (Chaper 12) Muliple Choice (40%) 1) Which of he following is a kineic quaniy? A) Enhalpy B) Inernal Energy C) Gibb s free energy D) Enropy E) Rae of reacion 2) Of he following quesions,
AP Chemisry Tes (Chaper 12) Muliple Choice (40%) 1) Which of he following is a kineic quaniy? A) Enhalpy B) Inernal Energy C) Gibb s free energy D) Enropy E) Rae of reacion 2) Of he following quesions,
Chapter 15: Phenomena. Chapter 15 Chemical Kinetics. Reaction Rates. Reaction Rates R P. Reaction Rates. Rate Laws
 Chaper 5: Phenomena Phenomena: The reacion (aq) + B(aq) C(aq) was sudied a wo differen emperaures (98 K and 35 K). For each emperaure he reacion was sared by puing differen concenraions of he 3 species
Chaper 5: Phenomena Phenomena: The reacion (aq) + B(aq) C(aq) was sudied a wo differen emperaures (98 K and 35 K). For each emperaure he reacion was sared by puing differen concenraions of he 3 species
AP Chemistry--Chapter 12: Chemical Kinetics
 AP Chemisry--Chaper 12: Chemical Kineics I. Reacion Raes A. The area of chemisry ha deals wih reacion raes, or how fas a reacion occurs, is called chemical kineics. B. The rae of reacion depends on he
AP Chemisry--Chaper 12: Chemical Kineics I. Reacion Raes A. The area of chemisry ha deals wih reacion raes, or how fas a reacion occurs, is called chemical kineics. B. The rae of reacion depends on he
Chapter 14 Chemical Kinetics
 /5/4 Chaper 4 Chemical Kineics Chemical Kineics Raes of Reacions Chemical Kineics is he sudy of he rae of reacion. How fas does i ake place? Very Fas Reacions Very Slow Reacions Acid/Base Combusion Rusing
/5/4 Chaper 4 Chemical Kineics Chemical Kineics Raes of Reacions Chemical Kineics is he sudy of he rae of reacion. How fas does i ake place? Very Fas Reacions Very Slow Reacions Acid/Base Combusion Rusing
Chapter 14 Homework Answers
 4. Suden responses will vary. (a) combusion of gasoline (b) cooking an egg in boiling waer (c) curing of cemen Chaper 4 Homework Answers 4. A collision beween only wo molecules is much more probable han
4. Suden responses will vary. (a) combusion of gasoline (b) cooking an egg in boiling waer (c) curing of cemen Chaper 4 Homework Answers 4. A collision beween only wo molecules is much more probable han
CHEMICAL KINETICS: 1. Rate Order Rate law Rate constant Half-life Temperature Dependence
 CHEMICL KINETICS: Rae Order Rae law Rae consan Half-life Temperaure Dependence Chemical Reacions Kineics Chemical ineics is he sudy of ime dependence of he change in he concenraion of reacans and producs.
CHEMICL KINETICS: Rae Order Rae law Rae consan Half-life Temperaure Dependence Chemical Reacions Kineics Chemical ineics is he sudy of ime dependence of he change in he concenraion of reacans and producs.
Chapter 14 Chemical Kinetics
 # of paricles 5/9/4 Chemical Kineics Raes of Reacions Chemical Kineics is he sudy of he rae of reacion. How fas does i ae place? Very Fas Reacions Very Slow Reacions Chaper 4 Chemical Kineics Acid/Base
# of paricles 5/9/4 Chemical Kineics Raes of Reacions Chemical Kineics is he sudy of he rae of reacion. How fas does i ae place? Very Fas Reacions Very Slow Reacions Chaper 4 Chemical Kineics Acid/Base
Chapter 12. Chemical Kinetics
 Chaper. Chemical Kineics Chemisry: The Sudy of Change FCT ORY Processes => Chemical Reacions (Redox, cid-base, Precipiaion in q. Soln) Two facors of chemical reacions How fas? => Chemical Kineics (Mechanism,
Chaper. Chemical Kineics Chemisry: The Sudy of Change FCT ORY Processes => Chemical Reacions (Redox, cid-base, Precipiaion in q. Soln) Two facors of chemical reacions How fas? => Chemical Kineics (Mechanism,
A First Course on Kinetics and Reaction Engineering. Class 19 on Unit 18
 A Firs ourse on Kineics and Reacion Engineering lass 19 on Uni 18 Par I - hemical Reacions Par II - hemical Reacion Kineics Where We re Going Par III - hemical Reacion Engineering A. Ideal Reacors B. Perfecly
A Firs ourse on Kineics and Reacion Engineering lass 19 on Uni 18 Par I - hemical Reacions Par II - hemical Reacion Kineics Where We re Going Par III - hemical Reacion Engineering A. Ideal Reacors B. Perfecly
04. Kinetics of a second order reaction
 4. Kineics of a second order reacion Imporan conceps Reacion rae, reacion exen, reacion rae equaion, order of a reacion, firs-order reacions, second-order reacions, differenial and inegraed rae laws, Arrhenius
4. Kineics of a second order reacion Imporan conceps Reacion rae, reacion exen, reacion rae equaion, order of a reacion, firs-order reacions, second-order reacions, differenial and inegraed rae laws, Arrhenius
Reaction Order Molecularity. Rate laws, Reaction Orders. Determining Reaction Order. Determining Reaction Order. Determining Reaction Order
 Rae laws, Reacion Orders The rae or velociy of a chemical reacion is loss of reacan or appearance of produc in concenraion unis, per uni ime d[p] d[s] The rae law for a reacion is of he form Rae d[p] k[a]
Rae laws, Reacion Orders The rae or velociy of a chemical reacion is loss of reacan or appearance of produc in concenraion unis, per uni ime d[p] d[s] The rae law for a reacion is of he form Rae d[p] k[a]
General Chemistry Exam 3 Chem 211 Section 03, Spring 2017
 General Chemistry Exam 3 Chem 211 Section 03, Spring 2017 Exam Information You have 50 minutes to complete this exam. Be sure to put your name on the next page. Please ask for clarification of questions
General Chemistry Exam 3 Chem 211 Section 03, Spring 2017 Exam Information You have 50 minutes to complete this exam. Be sure to put your name on the next page. Please ask for clarification of questions
Nuclear Decay kinetics : Transient and Secular Equilibrium. What can we say about the plot to the right?
 uclear Decay kineics : Transien and Secular Equilibrium Wha can we say abou he plo o he righ? IV. Paren-Daugher Relaionships Key poin: The Rae-Deermining Sep. Case of Radioacive Daugher (Paren) 1/2 ()
uclear Decay kineics : Transien and Secular Equilibrium Wha can we say abou he plo o he righ? IV. Paren-Daugher Relaionships Key poin: The Rae-Deermining Sep. Case of Radioacive Daugher (Paren) 1/2 ()
המחלקה : ביולוגיה מולקולרית הפקולטה למדעי הטבע
 טל : 03-9066345 פקס : 03-9374 ראש המחלקה: ד"ר אלברט פנחסוב המחלקה : ביולוגיה מולקולרית הפקולטה למדעי הטבע Course Name: Physical Chemisry - (for Molecular Biology sudens) כימיה פיזיקאלית )לסטודנטים לביולוגיה
טל : 03-9066345 פקס : 03-9374 ראש המחלקה: ד"ר אלברט פנחסוב המחלקה : ביולוגיה מולקולרית הפקולטה למדעי הטבע Course Name: Physical Chemisry - (for Molecular Biology sudens) כימיה פיזיקאלית )לסטודנטים לביולוגיה
Unsteady Mass- Transfer Models
 See T&K Chaper 9 Unseady Mass- Transfer Models ChEn 6603 Wednesday, April 4, Ouline Conex for he discussion Soluion for ransien binary diffusion wih consan c, N. Soluion for mulicomponen diffusion wih
See T&K Chaper 9 Unseady Mass- Transfer Models ChEn 6603 Wednesday, April 4, Ouline Conex for he discussion Soluion for ransien binary diffusion wih consan c, N. Soluion for mulicomponen diffusion wih
CHEMICAL KINETICS Rate Order Rate law Rate constant Half-life Molecularity Elementary. Complex Temperature dependence, Steady-state Approximation
 CHEMICL KINETICS Rae Order Rae law Rae consan Half-life Moleculariy Elemenary Complex Temperaure dependence, Seady-sae pproximaion Chemical Reacions Kineics Chemical ineics is he sudy of ime dependence
CHEMICL KINETICS Rae Order Rae law Rae consan Half-life Moleculariy Elemenary Complex Temperaure dependence, Seady-sae pproximaion Chemical Reacions Kineics Chemical ineics is he sudy of ime dependence
2. For a one-point fixed time method, a pseudo-first order reaction obeys the equation 0.309
 Chaper 3. To derive an appropriae equaion we firs noe he following general relaionship beween he concenraion of A a ime, [A], he iniial concenraion of A, [A], and he concenraion of P a ime, [P] [ P] Subsiuing
Chaper 3. To derive an appropriae equaion we firs noe he following general relaionship beween he concenraion of A a ime, [A], he iniial concenraion of A, [A], and he concenraion of P a ime, [P] [ P] Subsiuing
Module 3: The Damped Oscillator-II Lecture 3: The Damped Oscillator-II
 Module 3: The Damped Oscillaor-II Lecure 3: The Damped Oscillaor-II 3. Over-damped Oscillaions. This refers o he siuaion where β > ω (3.) The wo roos are and α = β + α 2 = β β 2 ω 2 = (3.2) β 2 ω 2 = 2
Module 3: The Damped Oscillaor-II Lecure 3: The Damped Oscillaor-II 3. Over-damped Oscillaions. This refers o he siuaion where β > ω (3.) The wo roos are and α = β + α 2 = β β 2 ω 2 = (3.2) β 2 ω 2 = 2
8. Basic RL and RC Circuits
 8. Basic L and C Circuis This chaper deals wih he soluions of he responses of L and C circuis The analysis of C and L circuis leads o a linear differenial equaion This chaper covers he following opics
8. Basic L and C Circuis This chaper deals wih he soluions of he responses of L and C circuis The analysis of C and L circuis leads o a linear differenial equaion This chaper covers he following opics
δs > 0 predicts spontaneous processes. U A + U B = constant U A = U B ds = ds A + ds B Case 1: TB > TA (-) (-) (+)(+) ds = C v(t )
 ---onight: Lecture 5 July 23 δs > 0 predicts spontaneous processes. ---Assignment 2 (do not include 1-3 done in-class): Due Friday July 30: Class time ---Assignment 3 posted ater class tonight. Whatever
---onight: Lecture 5 July 23 δs > 0 predicts spontaneous processes. ---Assignment 2 (do not include 1-3 done in-class): Due Friday July 30: Class time ---Assignment 3 posted ater class tonight. Whatever
MA Study Guide #1
 MA 66 Su Guide #1 (1) Special Tpes of Firs Order Equaions I. Firs Order Linear Equaion (FOL): + p() = g() Soluion : = 1 µ() [ ] µ()g() + C, where µ() = e p() II. Separable Equaion (SEP): dx = h(x) g()
MA 66 Su Guide #1 (1) Special Tpes of Firs Order Equaions I. Firs Order Linear Equaion (FOL): + p() = g() Soluion : = 1 µ() [ ] µ()g() + C, where µ() = e p() II. Separable Equaion (SEP): dx = h(x) g()
Chemical Engineering Thermodynamics
 Engi-3434 Chemical Engineering Thermodynamics Dr. Charles Xu @ Chemical Engineering, Lakehead Universiy Chemical Engineering Thermodynamics Insrucor: Dr. Charles Xu, P.Eng. Associae Professor Deparmen
Engi-3434 Chemical Engineering Thermodynamics Dr. Charles Xu @ Chemical Engineering, Lakehead Universiy Chemical Engineering Thermodynamics Insrucor: Dr. Charles Xu, P.Eng. Associae Professor Deparmen
Area A 0 level is h 0, assuming the pipe flow to be laminar. D, L and assuming the pipe flow to be highly turbulent.
 Pipe Flows (ecures 5 o 7). Choose he crec answer (i) While deriving an expression f loss of head due o a sudden expansion in a pipe, in addiion o he coninuiy and impulse-momenum equaions, one of he following
Pipe Flows (ecures 5 o 7). Choose he crec answer (i) While deriving an expression f loss of head due o a sudden expansion in a pipe, in addiion o he coninuiy and impulse-momenum equaions, one of he following
where R = universal gas constant R = PV/nT R = atm L mol R = atm dm 3 mol 1 K 1 R = J mol 1 K 1 (SI unit)
 Ideal Gas Law PV = nrt where R = universal gas constant R = PV/nT R = 0.0821 atm L mol 1 K 1 R = 0.0821 atm dm 3 mol 1 K 1 R = 8.314 J mol 1 K 1 (SI unit) Standard molar volume = 22.4 L mol 1 at 0 C and
Ideal Gas Law PV = nrt where R = universal gas constant R = PV/nT R = 0.0821 atm L mol 1 K 1 R = 0.0821 atm dm 3 mol 1 K 1 R = 8.314 J mol 1 K 1 (SI unit) Standard molar volume = 22.4 L mol 1 at 0 C and
Physical Chemistry I CHEM 4641 Final Exam 13 questions, 30 points
 Physical Chemistry I CHEM 4641 Final Exam 13 questions, 30 points Name: KEY Gas constant: R = 8.314 J mol -1 K -1 = 0.008314 kj mol -1 K -1. Boltzmann constant k = 1.381 10-23 J/K = 0.6950 cm -1 /K h =
Physical Chemistry I CHEM 4641 Final Exam 13 questions, 30 points Name: KEY Gas constant: R = 8.314 J mol -1 K -1 = 0.008314 kj mol -1 K -1. Boltzmann constant k = 1.381 10-23 J/K = 0.6950 cm -1 /K h =
Review - Quiz # 1. 1 g(y) dy = f(x) dx. y x. = u, so that y = xu and dy. dx (Sometimes you may want to use the substitution x y
 Review - Quiz # 1 (1) Solving Special Tpes of Firs Order Equaions I. Separable Equaions (SE). d = f() g() Mehod of Soluion : 1 g() d = f() (The soluions ma be given implicil b he above formula. Remember,
Review - Quiz # 1 (1) Solving Special Tpes of Firs Order Equaions I. Separable Equaions (SE). d = f() g() Mehod of Soluion : 1 g() d = f() (The soluions ma be given implicil b he above formula. Remember,
PHYS 1401 General Physics I Test 3 Review Questions
 PHYS 1401 General Physics I Tes 3 Review Quesions Ch. 7 1. A 6500 kg railroad car moving a 4.0 m/s couples wih a second 7500 kg car iniially a res. a) Skech before and afer picures of he siuaion. b) Wha
PHYS 1401 General Physics I Tes 3 Review Quesions Ch. 7 1. A 6500 kg railroad car moving a 4.0 m/s couples wih a second 7500 kg car iniially a res. a) Skech before and afer picures of he siuaion. b) Wha
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
04. Kinetics of a second order reaction
 4. Kineics of a second order reacion Imporan conceps Reacion rae, reacion exen, reacion rae equaion, order of a reacion, firs-order reacions, second-order reacions, differenial and inegraed rae laws, rrhenius
4. Kineics of a second order reacion Imporan conceps Reacion rae, reacion exen, reacion rae equaion, order of a reacion, firs-order reacions, second-order reacions, differenial and inegraed rae laws, rrhenius
ASTR415: Problem Set #5
 ASTR45: Problem Se #5 Curran D. Muhlberger Universi of Marland (Daed: April 25, 27) Three ssems of coupled differenial equaions were sudied using inegraors based on Euler s mehod, a fourh-order Runge-Kua
ASTR45: Problem Se #5 Curran D. Muhlberger Universi of Marland (Daed: April 25, 27) Three ssems of coupled differenial equaions were sudied using inegraors based on Euler s mehod, a fourh-order Runge-Kua
Chapter 1 Electric Circuit Variables
 Chaper 1 Elecric Circui Variables Exercises Exercise 1.2-1 Find he charge ha has enered an elemen by ime when i = 8 2 4 A, 0. Assume q() = 0 for < 0. 8 3 2 Answer: q () = 2 C 3 () 2 i = 8 4 A 2 8 3 2 8
Chaper 1 Elecric Circui Variables Exercises Exercise 1.2-1 Find he charge ha has enered an elemen by ime when i = 8 2 4 A, 0. Assume q() = 0 for < 0. 8 3 2 Answer: q () = 2 C 3 () 2 i = 8 4 A 2 8 3 2 8
Q2.1 This is the x t graph of the motion of a particle. Of the four points P, Q, R, and S, the velocity v x is greatest (most positive) at
 Q2.1 This is he x graph of he moion of a paricle. Of he four poins P, Q, R, and S, he velociy is greaes (mos posiive) a A. poin P. B. poin Q. C. poin R. D. poin S. E. no enough informaion in he graph o
Q2.1 This is he x graph of he moion of a paricle. Of he four poins P, Q, R, and S, he velociy is greaes (mos posiive) a A. poin P. B. poin Q. C. poin R. D. poin S. E. no enough informaion in he graph o
Solutions to Assignment 1
 MA 2326 Differenial Equaions Insrucor: Peronela Radu Friday, February 8, 203 Soluions o Assignmen. Find he general soluions of he following ODEs: (a) 2 x = an x Soluion: I is a separable equaion as we
MA 2326 Differenial Equaions Insrucor: Peronela Radu Friday, February 8, 203 Soluions o Assignmen. Find he general soluions of he following ODEs: (a) 2 x = an x Soluion: I is a separable equaion as we
PHYSICS 220 Lecture 02 Motion, Forces, and Newton s Laws Textbook Sections
 PHYSICS 220 Lecure 02 Moion, Forces, and Newon s Laws Texbook Secions 2.2-2.4 Lecure 2 Purdue Universiy, Physics 220 1 Overview Las Lecure Unis Scienific Noaion Significan Figures Moion Displacemen: Δx
PHYSICS 220 Lecure 02 Moion, Forces, and Newon s Laws Texbook Secions 2.2-2.4 Lecure 2 Purdue Universiy, Physics 220 1 Overview Las Lecure Unis Scienific Noaion Significan Figures Moion Displacemen: Δx
MA 366 Review - Test # 1
 MA 366 Review - Tes # 1 Fall 5 () Resuls from Calculus: differeniaion formulas, implici differeniaion, Chain Rule; inegraion formulas, inegraion b pars, parial fracions, oher inegraion echniques. (1) Order
MA 366 Review - Tes # 1 Fall 5 () Resuls from Calculus: differeniaion formulas, implici differeniaion, Chain Rule; inegraion formulas, inegraion b pars, parial fracions, oher inegraion echniques. (1) Order
Chapter 5: Control Volume Approach and Continuity Principle Dr Ali Jawarneh
 Chaper 5: Conrol Volume Approach and Coninuiy Principle By Dr Ali Jawarneh Deparmen of Mechanical Engineering Hashemie Universiy 1 Ouline Rae of Flow Conrol volume approach. Conservaion of mass he coninuiy
Chaper 5: Conrol Volume Approach and Coninuiy Principle By Dr Ali Jawarneh Deparmen of Mechanical Engineering Hashemie Universiy 1 Ouline Rae of Flow Conrol volume approach. Conservaion of mass he coninuiy
CHAPTER 12 DIRECT CURRENT CIRCUITS
 CHAPTER 12 DIRECT CURRENT CIUITS DIRECT CURRENT CIUITS 257 12.1 RESISTORS IN SERIES AND IN PARALLEL When wo resisors are conneced ogeher as shown in Figure 12.1 we said ha hey are conneced in series. As
CHAPTER 12 DIRECT CURRENT CIUITS DIRECT CURRENT CIUITS 257 12.1 RESISTORS IN SERIES AND IN PARALLEL When wo resisors are conneced ogeher as shown in Figure 12.1 we said ha hey are conneced in series. As
APPM 2360 Homework Solutions, Due June 10
 2.2.2: Find general soluions for he equaion APPM 2360 Homework Soluions, Due June 10 Soluion: Finding he inegraing facor, dy + 2y = 3e µ) = e 2) = e 2 Muliplying he differenial equaion by he inegraing
2.2.2: Find general soluions for he equaion APPM 2360 Homework Soluions, Due June 10 Soluion: Finding he inegraing facor, dy + 2y = 3e µ) = e 2) = e 2 Muliplying he differenial equaion by he inegraing
1. Consider a pure-exchange economy with stochastic endowments. The state of the economy
 Answer 4 of he following 5 quesions. 1. Consider a pure-exchange economy wih sochasic endowmens. The sae of he economy in period, 0,1,..., is he hisory of evens s ( s0, s1,..., s ). The iniial sae is given.
Answer 4 of he following 5 quesions. 1. Consider a pure-exchange economy wih sochasic endowmens. The sae of he economy in period, 0,1,..., is he hisory of evens s ( s0, s1,..., s ). The iniial sae is given.
Exam I. Name. Answer: a. W B > W A if the volume of the ice cubes is greater than the volume of the water.
 Name Exam I 1) A hole is punched in a full milk caron, 10 cm below he op. Wha is he iniial veloci of ouflow? a. 1.4 m/s b. 2.0 m/s c. 2.8 m/s d. 3.9 m/s e. 2.8 m/s Answer: a 2) In a wind unnel he pressure
Name Exam I 1) A hole is punched in a full milk caron, 10 cm below he op. Wha is he iniial veloci of ouflow? a. 1.4 m/s b. 2.0 m/s c. 2.8 m/s d. 3.9 m/s e. 2.8 m/s Answer: a 2) In a wind unnel he pressure
Selective peracetic acid determination in the presence of hydrogen peroxide using the molecular absorption properties of catalase
 Analyical and Bioanalyical Chemisry Elecronic Supplemenary Maerial Selecive peraceic acid deerminaion in he presence of hydrogen peroxide using he molecular absorpion properies of caalase Javier Galbán,
Analyical and Bioanalyical Chemisry Elecronic Supplemenary Maerial Selecive peraceic acid deerminaion in he presence of hydrogen peroxide using he molecular absorpion properies of caalase Javier Galbán,
dt = C exp (3 ln t 4 ). t 4 W = C exp ( ln(4 t) 3) = C(4 t) 3.
 Mah Rahman Exam Review Soluions () Consider he IVP: ( 4)y 3y + 4y = ; y(3) = 0, y (3) =. (a) Please deermine he longes inerval for which he IVP is guaraneed o have a unique soluion. Soluion: The disconinuiies
Mah Rahman Exam Review Soluions () Consider he IVP: ( 4)y 3y + 4y = ; y(3) = 0, y (3) =. (a) Please deermine he longes inerval for which he IVP is guaraneed o have a unique soluion. Soluion: The disconinuiies
Math 333 Problem Set #2 Solution 14 February 2003
 Mah 333 Problem Se #2 Soluion 14 February 2003 A1. Solve he iniial value problem dy dx = x2 + e 3x ; 2y 4 y(0) = 1. Soluion: This is separable; we wrie 2y 4 dy = x 2 + e x dx and inegrae o ge The iniial
Mah 333 Problem Se #2 Soluion 14 February 2003 A1. Solve he iniial value problem dy dx = x2 + e 3x ; 2y 4 y(0) = 1. Soluion: This is separable; we wrie 2y 4 dy = x 2 + e x dx and inegrae o ge The iniial
Relaxation in Glass. Transition
 Relaxaion in Glass Lecure 2: he Glass ransiion as a Kineic Lecure 2: he Glass ransiion as a Kineic ransiion Enhalpy Changes in he Glass ransiion Range H decreases coninuously wih cooling Slope of he H
Relaxaion in Glass Lecure 2: he Glass ransiion as a Kineic Lecure 2: he Glass ransiion as a Kineic ransiion Enhalpy Changes in he Glass ransiion Range H decreases coninuously wih cooling Slope of he H
First Order RC and RL Transient Circuits
 Firs Order R and RL Transien ircuis Objecives To inroduce he ransiens phenomena. To analyze sep and naural responses of firs order R circuis. To analyze sep and naural responses of firs order RL circuis.
Firs Order R and RL Transien ircuis Objecives To inroduce he ransiens phenomena. To analyze sep and naural responses of firs order R circuis. To analyze sep and naural responses of firs order RL circuis.
MPOC Ch8. Forthe aboveelimination 1 7. Dissociation. than C D. If C HID bond is breaking in the RDS Kitshouldbe than KD
 MPOC Ch8 KineicisoopeEffec soopeshave he same chemical properies Labeling will no changehemechanism H o Ph Br v ph H D D O D Ph rbr ph u soopes can minimally perurb he rim leadingo a change in An rae KE
MPOC Ch8 KineicisoopeEffec soopeshave he same chemical properies Labeling will no changehemechanism H o Ph Br v ph H D D O D Ph rbr ph u soopes can minimally perurb he rim leadingo a change in An rae KE
Solutionbank Edexcel AS and A Level Modular Mathematics
 Page of 4 Soluionbank Edexcel AS and A Level Modular Mahemaics Exercise A, Quesion Quesion: Skech he graphs of (a) y = e x + (b) y = 4e x (c) y = e x 3 (d) y = 4 e x (e) y = 6 + 0e x (f) y = 00e x + 0
Page of 4 Soluionbank Edexcel AS and A Level Modular Mahemaics Exercise A, Quesion Quesion: Skech he graphs of (a) y = e x + (b) y = 4e x (c) y = e x 3 (d) y = 4 e x (e) y = 6 + 0e x (f) y = 00e x + 0
EEEB113 CIRCUIT ANALYSIS I
 9/14/29 1 EEEB113 CICUIT ANALYSIS I Chaper 7 Firs-Order Circuis Maerials from Fundamenals of Elecric Circuis 4e, Alexander Sadiku, McGraw-Hill Companies, Inc. 2 Firs-Order Circuis -Chaper 7 7.2 The Source-Free
9/14/29 1 EEEB113 CICUIT ANALYSIS I Chaper 7 Firs-Order Circuis Maerials from Fundamenals of Elecric Circuis 4e, Alexander Sadiku, McGraw-Hill Companies, Inc. 2 Firs-Order Circuis -Chaper 7 7.2 The Source-Free
Advanced Organic Chemistry
 Lalic, G. Chem 53A Chemisry 53A Advanced Organic Chemisry Lecure noes 1 Kineics: A racical Approach Simple Kineics Scenarios Fiing Experimenal Daa Using Kineics o Deermine he Mechanism Doughery, D. A.,
Lalic, G. Chem 53A Chemisry 53A Advanced Organic Chemisry Lecure noes 1 Kineics: A racical Approach Simple Kineics Scenarios Fiing Experimenal Daa Using Kineics o Deermine he Mechanism Doughery, D. A.,
Economics 6130 Cornell University Fall 2016 Macroeconomics, I - Part 2
 Economics 6130 Cornell Universiy Fall 016 Macroeconomics, I - Par Problem Se # Soluions 1 Overlapping Generaions Consider he following OLG economy: -period lives. 1 commodiy per period, l = 1. Saionary
Economics 6130 Cornell Universiy Fall 016 Macroeconomics, I - Par Problem Se # Soluions 1 Overlapping Generaions Consider he following OLG economy: -period lives. 1 commodiy per period, l = 1. Saionary
Suggested Problem Solutions Associated with Homework #5
 Suggesed Problem Soluions Associaed wih Homework #5 431 (a) 8 Si has proons and neurons (b) 85 3 Rb has 3 proons and 48 neurons (c) 5 Tl 81 has 81 proons and neurons 43 IDENTIFY and SET UP: The ex calculaes
Suggesed Problem Soluions Associaed wih Homework #5 431 (a) 8 Si has proons and neurons (b) 85 3 Rb has 3 proons and 48 neurons (c) 5 Tl 81 has 81 proons and neurons 43 IDENTIFY and SET UP: The ex calculaes
IB Physics Kinematics Worksheet
 IB Physics Kinemaics Workshee Wrie full soluions and noes for muliple choice answers. Do no use a calculaor for muliple choice answers. 1. Which of he following is a correc definiion of average acceleraion?
IB Physics Kinemaics Workshee Wrie full soluions and noes for muliple choice answers. Do no use a calculaor for muliple choice answers. 1. Which of he following is a correc definiion of average acceleraion?
Structural Dynamics and Earthquake Engineering
 Srucural Dynamics and Earhquae Engineering Course 1 Inroducion. Single degree of freedom sysems: Equaions of moion, problem saemen, soluion mehods. Course noes are available for download a hp://www.c.up.ro/users/aurelsraan/
Srucural Dynamics and Earhquae Engineering Course 1 Inroducion. Single degree of freedom sysems: Equaions of moion, problem saemen, soluion mehods. Course noes are available for download a hp://www.c.up.ro/users/aurelsraan/
NYU CHEM GA 2600: Statistical Mechanics Midterm
 NYU CHEM GA 2600: Saisical Mechanics Miderm Glen Hocky Ocober 18, 2018 nsrucions: This miderm is open book/open noe No elecronic devices besides a calculaor Please wrie your name on each page and ry o
NYU CHEM GA 2600: Saisical Mechanics Miderm Glen Hocky Ocober 18, 2018 nsrucions: This miderm is open book/open noe No elecronic devices besides a calculaor Please wrie your name on each page and ry o
Math 2214 Solution Test 1A Spring 2016
 Mah 14 Soluion Tes 1A Spring 016 sec Problem 1: Wha is he larges -inerval for which ( 4) = has a guaraneed + unique soluion for iniial value (-1) = 3 according o he Exisence Uniqueness Theorem? Soluion
Mah 14 Soluion Tes 1A Spring 016 sec Problem 1: Wha is he larges -inerval for which ( 4) = has a guaraneed + unique soluion for iniial value (-1) = 3 according o he Exisence Uniqueness Theorem? Soluion
Module 2 F c i k c s la l w a s o s f dif di fusi s o i n
 Module Fick s laws of diffusion Fick s laws of diffusion and hin film soluion Adolf Fick (1855) proposed: d J α d d d J (mole/m s) flu (m /s) diffusion coefficien and (mole/m 3 ) concenraion of ions, aoms
Module Fick s laws of diffusion Fick s laws of diffusion and hin film soluion Adolf Fick (1855) proposed: d J α d d d J (mole/m s) flu (m /s) diffusion coefficien and (mole/m 3 ) concenraion of ions, aoms
Elementary Differential Equations and Boundary Value Problems
 Elemenar Differenial Equaions and Boundar Value Problems Boce. & DiPrima 9 h Ediion Chaper 1: Inroducion 1006003 คณ ตศาสตร ว ศวกรรม 3 สาขาว ชาว ศวกรรมคอมพ วเตอร ป การศ กษา 1/2555 ผศ.ดร.อร ญญา ผศ.ดร.สมศ
Elemenar Differenial Equaions and Boundar Value Problems Boce. & DiPrima 9 h Ediion Chaper 1: Inroducion 1006003 คณ ตศาสตร ว ศวกรรม 3 สาขาว ชาว ศวกรรมคอมพ วเตอร ป การศ กษา 1/2555 ผศ.ดร.อร ญญา ผศ.ดร.สมศ
Initial Value Problems
 Iniial Value Problems ChEn 2450 d d f(, ) (0) 0 6 ODE.key - November 26, 2014 Example - Cooking a Lobser Assumpions: The lobser remains a a uniform emperaure. This implies ha he hermal conduciviy of he
Iniial Value Problems ChEn 2450 d d f(, ) (0) 0 6 ODE.key - November 26, 2014 Example - Cooking a Lobser Assumpions: The lobser remains a a uniform emperaure. This implies ha he hermal conduciviy of he
2.1: What is physics? Ch02: Motion along a straight line. 2.2: Motion. 2.3: Position, Displacement, Distance
 Ch: Moion along a sraigh line Moion Posiion and Displacemen Average Velociy and Average Speed Insananeous Velociy and Speed Acceleraion Consan Acceleraion: A Special Case Anoher Look a Consan Acceleraion
Ch: Moion along a sraigh line Moion Posiion and Displacemen Average Velociy and Average Speed Insananeous Velociy and Speed Acceleraion Consan Acceleraion: A Special Case Anoher Look a Consan Acceleraion
Advanced Placement. Chemistry. Integrated Rates
 Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
1 st order ODE Initial Condition
 Mah-33 Chapers 1-1 s Order ODE Sepember 1, 17 1 1 s order ODE Iniial Condiion f, sandard form LINEAR NON-LINEAR,, p g differenial form M x dx N x d differenial form is equivalen o a pair of differenial
Mah-33 Chapers 1-1 s Order ODE Sepember 1, 17 1 1 s order ODE Iniial Condiion f, sandard form LINEAR NON-LINEAR,, p g differenial form M x dx N x d differenial form is equivalen o a pair of differenial
RANDOM PROCESS: Identical to Unimolecular Decomposition. ΔE a
 7 Lecure 13: Radioacive Decay Kineics I. Kineics of Firs-Order Processes. Mechanism: 1. ucleus has Inernal Srucure Z X Z Decay involves inernal + Y + Q ; Q =+ rearrangemen of sysem. RDOM PROCESS: Idenical
7 Lecure 13: Radioacive Decay Kineics I. Kineics of Firs-Order Processes. Mechanism: 1. ucleus has Inernal Srucure Z X Z Decay involves inernal + Y + Q ; Q =+ rearrangemen of sysem. RDOM PROCESS: Idenical
u(x) = e x 2 y + 2 ) Integrate and solve for x (1 + x)y + y = cos x Answer: Divide both sides by 1 + x and solve for y. y = x y + cos x
 . 1 Mah 211 Homework #3 February 2, 2001 2.4.3. y + (2/x)y = (cos x)/x 2 Answer: Compare y + (2/x) y = (cos x)/x 2 wih y = a(x)x + f(x)and noe ha a(x) = 2/x. Consequenly, an inegraing facor is found wih
. 1 Mah 211 Homework #3 February 2, 2001 2.4.3. y + (2/x)y = (cos x)/x 2 Answer: Compare y + (2/x) y = (cos x)/x 2 wih y = a(x)x + f(x)and noe ha a(x) = 2/x. Consequenly, an inegraing facor is found wih
Homework-8(1) P8.3-1, 3, 8, 10, 17, 21, 24, 28,29 P8.4-1, 2, 5
 Homework-8() P8.3-, 3, 8, 0, 7, 2, 24, 28,29 P8.4-, 2, 5 Secion 8.3: The Response of a Firs Order Circui o a Consan Inpu P 8.3- The circui shown in Figure P 8.3- is a seady sae before he swich closes a
Homework-8() P8.3-, 3, 8, 0, 7, 2, 24, 28,29 P8.4-, 2, 5 Secion 8.3: The Response of a Firs Order Circui o a Consan Inpu P 8.3- The circui shown in Figure P 8.3- is a seady sae before he swich closes a
Stat 601 The Design of Experiments
 Sa 601 The Design of Experimens Yuqing Xu Deparmen of Saisics Universiy of Wisconsin Madison, WI 53706, USA December 1, 2016 Yuqing Xu (UW-Madison) Sa 601 Week 12 December 1, 2016 1 / 17 Lain Squares Definiion
Sa 601 The Design of Experimens Yuqing Xu Deparmen of Saisics Universiy of Wisconsin Madison, WI 53706, USA December 1, 2016 Yuqing Xu (UW-Madison) Sa 601 Week 12 December 1, 2016 1 / 17 Lain Squares Definiion
Ch.1. Group Work Units. Continuum Mechanics Course (MMC) - ETSECCPB - UPC
 Ch.. Group Work Unis Coninuum Mechanics Course (MMC) - ETSECCPB - UPC Uni 2 Jusify wheher he following saemens are rue or false: a) Two sreamlines, corresponding o a same insan of ime, can never inersec
Ch.. Group Work Unis Coninuum Mechanics Course (MMC) - ETSECCPB - UPC Uni 2 Jusify wheher he following saemens are rue or false: a) Two sreamlines, corresponding o a same insan of ime, can never inersec
What s free about Gibbs free energy?
 What s free about Gibbs free energy? The change in free energy for a process equals the maximum work that can be done by the system on the surroundings in a spontaneous process occurring at constant temperature
What s free about Gibbs free energy? The change in free energy for a process equals the maximum work that can be done by the system on the surroundings in a spontaneous process occurring at constant temperature
Math 10B: Mock Mid II. April 13, 2016
 Name: Soluions Mah 10B: Mock Mid II April 13, 016 1. ( poins) Sae, wih jusificaion, wheher he following saemens are rue or false. (a) If a 3 3 marix A saisfies A 3 A = 0, hen i canno be inverible. True.
Name: Soluions Mah 10B: Mock Mid II April 13, 016 1. ( poins) Sae, wih jusificaion, wheher he following saemens are rue or false. (a) If a 3 3 marix A saisfies A 3 A = 0, hen i canno be inverible. True.
KINEMATICS IN ONE DIMENSION
 KINEMATICS IN ONE DIMENSION PREVIEW Kinemaics is he sudy of how hings move how far (disance and displacemen), how fas (speed and velociy), and how fas ha how fas changes (acceleraion). We say ha an objec
KINEMATICS IN ONE DIMENSION PREVIEW Kinemaics is he sudy of how hings move how far (disance and displacemen), how fas (speed and velociy), and how fas ha how fas changes (acceleraion). We say ha an objec
G f. 1 Electric double layer effect for the redox species in solution. Electric double layer effect (EDL) for the redox species in solution
 M M Elecric doule layer effec EDL for he redox species in soluion Masahiro Yamamoo April 8, 008 9 : 0 am In his repor I will reformulae he elecric doule layer effec of ulk redox reacion. 1 Elecric doule
M M Elecric doule layer effec EDL for he redox species in soluion Masahiro Yamamoo April 8, 008 9 : 0 am In his repor I will reformulae he elecric doule layer effec of ulk redox reacion. 1 Elecric doule
ln y t 2 t c where c is an arbitrary real constant
 SOLUTION TO THE PROBLEM.A y y subjec o condiion y 0 8 We recognize is as a linear firs order differenial equaion wi consan coefficiens. Firs we sall find e general soluion, and en we sall find one a saisfies
SOLUTION TO THE PROBLEM.A y y subjec o condiion y 0 8 We recognize is as a linear firs order differenial equaion wi consan coefficiens. Firs we sall find e general soluion, and en we sall find one a saisfies
Math 1b. Calculus, Series, and Differential Equations. Final Exam Solutions
 Mah b. Calculus, Series, and Differenial Equaions. Final Exam Soluions Spring 6. (9 poins) Evaluae he following inegrals. 5x + 7 (a) (x + )(x + ) dx. (b) (c) x arcan x dx x(ln x) dx Soluion. (a) Using
Mah b. Calculus, Series, and Differenial Equaions. Final Exam Soluions Spring 6. (9 poins) Evaluae he following inegrals. 5x + 7 (a) (x + )(x + ) dx. (b) (c) x arcan x dx x(ln x) dx Soluion. (a) Using
AP CALCULUS AB 2003 SCORING GUIDELINES (Form B)
 SCORING GUIDELINES (Form B) Quesion A ank conains 15 gallons of heaing oil a ime =. During he ime inerval 1 hours, heaing oil is pumped ino he ank a he rae 1 H ( ) = + ( 1 + ln( + 1) ) gallons per hour.
SCORING GUIDELINES (Form B) Quesion A ank conains 15 gallons of heaing oil a ime =. During he ime inerval 1 hours, heaing oil is pumped ino he ank a he rae 1 H ( ) = + ( 1 + ln( + 1) ) gallons per hour.
Summary:Linear Motion
 Summary:Linear Moion D Saionary objec V Consan velociy D Disance increase uniformly wih ime D = v. a Consan acceleraion V D V = a. D = ½ a 2 Velociy increases uniformly wih ime Disance increases rapidly
Summary:Linear Moion D Saionary objec V Consan velociy D Disance increase uniformly wih ime D = v. a Consan acceleraion V D V = a. D = ½ a 2 Velociy increases uniformly wih ime Disance increases rapidly
Exam Thermodynamics 12 April 2018
 1 Exam Thermodynamics 12 April 2018 Please, hand in your answers to problems 1, 2, 3 and 4 on separate sheets. Put your name and student number on each sheet. The examination time is 12:30 until 15:30.
1 Exam Thermodynamics 12 April 2018 Please, hand in your answers to problems 1, 2, 3 and 4 on separate sheets. Put your name and student number on each sheet. The examination time is 12:30 until 15:30.
Math 36. Rumbos Spring Solutions to Assignment #6. 1. Suppose the growth of a population is governed by the differential equation.
 Mah 36. Rumbos Spring 1 1 Soluions o Assignmen #6 1. Suppose he growh of a populaion is governed by he differenial equaion where k is a posiive consan. d d = k (a Explain why his model predics ha he populaion
Mah 36. Rumbos Spring 1 1 Soluions o Assignmen #6 1. Suppose he growh of a populaion is governed by he differenial equaion where k is a posiive consan. d d = k (a Explain why his model predics ha he populaion
Differential Equations
 Mah 21 (Fall 29) Differenial Equaions Soluion #3 1. Find he paricular soluion of he following differenial equaion by variaion of parameer (a) y + y = csc (b) 2 y + y y = ln, > Soluion: (a) The corresponding
Mah 21 (Fall 29) Differenial Equaions Soluion #3 1. Find he paricular soluion of he following differenial equaion by variaion of parameer (a) y + y = csc (b) 2 y + y y = ln, > Soluion: (a) The corresponding
ph = pk a + log 10{[base]/[acid]}
![ph = pk a + log 10{[base]/[acid]} ph = pk a + log 10{[base]/[acid]}](/thumbs/91/105823399.jpg) FORMULA SHEET (tear off) N A = 6.022 x 10 23 C = ( 5 / 9) ( F - 32) F = ( 9 / 5)( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013 bar pv = nrt
FORMULA SHEET (tear off) N A = 6.022 x 10 23 C = ( 5 / 9) ( F - 32) F = ( 9 / 5)( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013 bar pv = nrt
KEY. Math 334 Midterm I Fall 2008 sections 001 and 003 Instructor: Scott Glasgow
 1 KEY Mah 4 Miderm I Fall 8 secions 1 and Insrucor: Sco Glasgow Please do NOT wrie on his eam. No credi will be given for such work. Raher wrie in a blue book, or on our own paper, preferabl engineering
1 KEY Mah 4 Miderm I Fall 8 secions 1 and Insrucor: Sco Glasgow Please do NOT wrie on his eam. No credi will be given for such work. Raher wrie in a blue book, or on our own paper, preferabl engineering
6.003 Homework 1. Problems. Due at the beginning of recitation on Wednesday, February 10, 2010.
 6.003 Homework Due a he beginning of reciaion on Wednesday, February 0, 200. Problems. Independen and Dependen Variables Assume ha he heigh of a waer wave is given by g(x v) where x is disance, v is velociy,
6.003 Homework Due a he beginning of reciaion on Wednesday, February 0, 200. Problems. Independen and Dependen Variables Assume ha he heigh of a waer wave is given by g(x v) where x is disance, v is velociy,
LAPLACE TRANSFORM AND TRANSFER FUNCTION
 CHBE320 LECTURE V LAPLACE TRANSFORM AND TRANSFER FUNCTION Professor Dae Ryook Yang Spring 2018 Dep. of Chemical and Biological Engineering 5-1 Road Map of he Lecure V Laplace Transform and Transfer funcions
CHBE320 LECTURE V LAPLACE TRANSFORM AND TRANSFER FUNCTION Professor Dae Ryook Yang Spring 2018 Dep. of Chemical and Biological Engineering 5-1 Road Map of he Lecure V Laplace Transform and Transfer funcions
EXERCISES FOR SECTION 1.5
 1.5 Exisence and Uniqueness of Soluions 43 20. 1 v c 21. 1 v c 1 2 4 6 8 10 1 2 2 4 6 8 10 Graph of approximae soluion obained using Euler s mehod wih = 0.1. Graph of approximae soluion obained using Euler
1.5 Exisence and Uniqueness of Soluions 43 20. 1 v c 21. 1 v c 1 2 4 6 8 10 1 2 2 4 6 8 10 Graph of approximae soluion obained using Euler s mehod wih = 0.1. Graph of approximae soluion obained using Euler
Solutions of Sample Problems for Third In-Class Exam Math 246, Spring 2011, Professor David Levermore
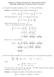 Soluions of Sample Problems for Third In-Class Exam Mah 6, Spring, Professor David Levermore Compue he Laplace ransform of f e from is definiion Soluion The definiion of he Laplace ransform gives L[f]s
Soluions of Sample Problems for Third In-Class Exam Mah 6, Spring, Professor David Levermore Compue he Laplace ransform of f e from is definiion Soluion The definiion of he Laplace ransform gives L[f]s
Polymerization Technology Laboratory
 Versuch eacion Calorimery Polymerizaion Technology Laboraory eacion Calorimery 1. Subjec Isohermal and adiabaic emulsion polymerizaion of mehyl mehacrylae in a bach reacor. 2. Theory 2.1 Isohermal and
Versuch eacion Calorimery Polymerizaion Technology Laboraory eacion Calorimery 1. Subjec Isohermal and adiabaic emulsion polymerizaion of mehyl mehacrylae in a bach reacor. 2. Theory 2.1 Isohermal and
Displacement ( x) x x x
 Kinemaics Kinemaics is he branch of mechanics ha describes he moion of objecs wihou necessarily discussing wha causes he moion. 1-Dimensional Kinemaics (or 1- Dimensional moion) refers o moion in a sraigh
Kinemaics Kinemaics is he branch of mechanics ha describes he moion of objecs wihou necessarily discussing wha causes he moion. 1-Dimensional Kinemaics (or 1- Dimensional moion) refers o moion in a sraigh
I. OBJECTIVE OF THE EXPERIMENT.
 I. OBJECTIVE OF THE EXPERIMENT. Swissmero raels a high speeds hrough a unnel a low pressure. I will hereore undergo ricion ha can be due o: ) Viscosiy o gas (c. "Viscosiy o gas" eperimen) ) The air in
I. OBJECTIVE OF THE EXPERIMENT. Swissmero raels a high speeds hrough a unnel a low pressure. I will hereore undergo ricion ha can be due o: ) Viscosiy o gas (c. "Viscosiy o gas" eperimen) ) The air in
AP CALCULUS AB/CALCULUS BC 2016 SCORING GUIDELINES. Question 1. 1 : estimate = = 120 liters/hr
 AP CALCULUS AB/CALCULUS BC 16 SCORING GUIDELINES Quesion 1 (hours) R ( ) (liers / hour) 1 3 6 8 134 119 95 74 7 Waer is pumped ino a ank a a rae modeled by W( ) = e liers per hour for 8, where is measured
AP CALCULUS AB/CALCULUS BC 16 SCORING GUIDELINES Quesion 1 (hours) R ( ) (liers / hour) 1 3 6 8 134 119 95 74 7 Waer is pumped ino a ank a a rae modeled by W( ) = e liers per hour for 8, where is measured
Math 334 Test 1 KEY Spring 2010 Section: 001. Instructor: Scott Glasgow Dates: May 10 and 11.
 1 Mah 334 Tes 1 KEY Spring 21 Secion: 1 Insrucor: Sco Glasgow Daes: Ma 1 and 11. Do NOT wrie on his problem saemen bookle, excep for our indicaion of following he honor code jus below. No credi will be
1 Mah 334 Tes 1 KEY Spring 21 Secion: 1 Insrucor: Sco Glasgow Daes: Ma 1 and 11. Do NOT wrie on his problem saemen bookle, excep for our indicaion of following he honor code jus below. No credi will be
C From Faraday's Law, the induced voltage is, C The effect of electromagnetic induction in the coil itself is called selfinduction.
 Inducors and Inducanc C For inducors, v() is proporional o h ra of chang of i(). Inducanc (con d) C Th proporionaliy consan is h inducanc, L, wih unis of Hnris. 1 Hnry = 1 Wb / A or 1 V sc / A. C L dpnds
Inducors and Inducanc C For inducors, v() is proporional o h ra of chang of i(). Inducanc (con d) C Th proporionaliy consan is h inducanc, L, wih unis of Hnris. 1 Hnry = 1 Wb / A or 1 V sc / A. C L dpnds
6. 6 v ; degree = 7; leading coefficient = 6; 7. The expression has 3 terms; t p no; subtracting x from 3x ( 3x x 2x)
 70. a =, r = 0%, = 0. 7. a = 000, r = 0.%, = 00 7. a =, r = 00%, = 7. ( ) = 0,000 0., where = ears 7. ( ) = + 0.0, where = weeks 7 ( ) =,000,000 0., where = das 7 = 77. = 9 7 = 7 geomeric 0. geomeric arihmeic,
70. a =, r = 0%, = 0. 7. a = 000, r = 0.%, = 00 7. a =, r = 00%, = 7. ( ) = 0,000 0., where = ears 7. ( ) = + 0.0, where = weeks 7 ( ) =,000,000 0., where = das 7 = 77. = 9 7 = 7 geomeric 0. geomeric arihmeic,
Physics 221 Fall 2008 Homework #2 Solutions Ch. 2 Due Tues, Sept 9, 2008
 Physics 221 Fall 28 Homework #2 Soluions Ch. 2 Due Tues, Sep 9, 28 2.1 A paricle moving along he x-axis moves direcly from posiion x =. m a ime =. s o posiion x = 1. m by ime = 1. s, and hen moves direcly
Physics 221 Fall 28 Homework #2 Soluions Ch. 2 Due Tues, Sep 9, 28 2.1 A paricle moving along he x-axis moves direcly from posiion x =. m a ime =. s o posiion x = 1. m by ime = 1. s, and hen moves direcly
Mathcad Lecture #8 In-class Worksheet Curve Fitting and Interpolation
 Mahcad Lecure #8 In-class Workshee Curve Fiing and Inerpolaion A he end of his lecure, you will be able o: explain he difference beween curve fiing and inerpolaion decide wheher curve fiing or inerpolaion
Mahcad Lecure #8 In-class Workshee Curve Fiing and Inerpolaion A he end of his lecure, you will be able o: explain he difference beween curve fiing and inerpolaion decide wheher curve fiing or inerpolaion
Outline. 9. The Second Law of Thermodynamics: Entropy. 10.Entropy and the Third law of thermodynamics 11.Spontaneous change: Free energy
 hermochemistry opic 6. hermochemistry hermochemistry Outline. Getting Started: Some terminology. State functions 3. Pressure-Volume Work 4. he First Law of hermodynamics: Heat, work and enthalpy 5. Heat
hermochemistry opic 6. hermochemistry hermochemistry Outline. Getting Started: Some terminology. State functions 3. Pressure-Volume Work 4. he First Law of hermodynamics: Heat, work and enthalpy 5. Heat
WEEK-3 Recitation PHYS 131. of the projectile s velocity remains constant throughout the motion, since the acceleration a x
 WEEK-3 Reciaion PHYS 131 Ch. 3: FOC 1, 3, 4, 6, 14. Problems 9, 37, 41 & 71 and Ch. 4: FOC 1, 3, 5, 8. Problems 3, 5 & 16. Feb 8, 018 Ch. 3: FOC 1, 3, 4, 6, 14. 1. (a) The horizonal componen of he projecile
WEEK-3 Reciaion PHYS 131 Ch. 3: FOC 1, 3, 4, 6, 14. Problems 9, 37, 41 & 71 and Ch. 4: FOC 1, 3, 5, 8. Problems 3, 5 & 16. Feb 8, 018 Ch. 3: FOC 1, 3, 4, 6, 14. 1. (a) The horizonal componen of he projecile
UNIT #4 TEST REVIEW EXPONENTIAL AND LOGARITHMIC FUNCTIONS
 Name: Par I Quesions UNIT #4 TEST REVIEW EXPONENTIAL AND LOGARITHMIC FUNCTIONS Dae: 1. The epression 1 is equivalen o 1 () () 6. The eponenial funcion y 16 could e rewrien as y () y 4 () y y. The epression
Name: Par I Quesions UNIT #4 TEST REVIEW EXPONENTIAL AND LOGARITHMIC FUNCTIONS Dae: 1. The epression 1 is equivalen o 1 () () 6. The eponenial funcion y 16 could e rewrien as y () y 4 () y y. The epression
A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: slow fast (D) H 2 O
 Chemistry 112, Spring 2007 Prof. Metz Exam 2 Practice Use the following information to answer questions 1 through 3 A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: H 2
Chemistry 112, Spring 2007 Prof. Metz Exam 2 Practice Use the following information to answer questions 1 through 3 A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: H 2
Notes 8B Day 1 Doubling Time
 Noes 8B Day 1 Doubling ime Exponenial growh leads o repeaed doublings (see Graph in Noes 8A) and exponenial decay leads o repeaed halvings. In his uni we ll be convering beween growh (or decay) raes and
Noes 8B Day 1 Doubling ime Exponenial growh leads o repeaed doublings (see Graph in Noes 8A) and exponenial decay leads o repeaed halvings. In his uni we ll be convering beween growh (or decay) raes and
Physics Equation List :Form 4 Introduction to Physics
 Physics Equaion Lis :Form 4 Inroducion o Physics Relaive Deviaion Relaive Deviaion Mean Deviaion 00% Mean Value Prefixes Unis for Area and Volume Prefixes Value Sandard form Symbol Tera 000 000 000 000
Physics Equaion Lis :Form 4 Inroducion o Physics Relaive Deviaion Relaive Deviaion Mean Deviaion 00% Mean Value Prefixes Unis for Area and Volume Prefixes Value Sandard form Symbol Tera 000 000 000 000
