A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: slow fast (D) H 2 O
|
|
|
- Emery Booth
- 6 years ago
- Views:
Transcription
1 Chemistry 112, Spring 2007 Prof. Metz Exam 2 Practice Use the following information to answer questions 1 through 3 A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: H 2 O 2 + I - H 2 O + OI - H 2 O 2 + OI - H 2 O + O 2 + I - slow fast 1. The rate law consistent with this mechanism is: (A) R = k[h 2 O 2 2 (D) R = k[h 2 O 2 2 [I - /[H 2 O (B) R = k[h 2 O 2 [I - (E) R = k[h 2 O[OI - /[H 2 O 2 [I - (C) R = k[h 2 O[OI - The rate limiting step is the first reaction, in which one H 2 O 2 reacts with one I A catalyst in this mechanism is: (A) I - (C) O 2 (E) there is none (B) OI - (D) H 2 O I - is the catalyst. Overall, it is not produced or consumed, but it is added to the reaction and is involved in the rate-limiting step. 3. An intermediate in this mechanism is: (A) I - (C) O 2 (E) there is none (B) OI - (D) H 2 O OI - is an intermediate. Overall, it s not produced or consumed, and it s not added to the reaction, but is produced by one reaction and consumed by another. 4. Methanogens are a class of bacteria that metabolize methane. They have an enzyme that allows them to convert methane to methanol. A very simplified mechanism has two steps: First, oxygen binds to the enzyme (E) to produce an enzyme-o 2 complex (E O 2 ), then methane reacts with the E O 2 complex to produce methanol: k 1 O 2 + E E O 2 fast, equilibrium k -1 k 2 E O CH 4 E + 2 CH 3 OH slow What is the rate law for this reaction? (Note: k is a combination of k 1, k -1 and k 2 ) (A) Rate = k[o 2 [CH 4 [E (C) Rate = k[o 2 [E (E) Rate = k[ch 4 2 (B) Rate = k[o 2 [CH 4 2 [E (D) Rate = k[e[ch 4 2 Rate law is determined by rate-limiting (slow) step, so Rate = k 2 [E O 2 [CH 4 2 However, E O 2 is an intermediate, and it s best to write rate laws in terms of reactants (and products, if necessary), so express [E O 2 in terms of other concentrations. From reaction 1, k 1 [O 2 [E = k -1 [E O 2, so [E O 2 = (k 1 /k -1 ) [O 2 [E 1
2 Plug this in to the rate equation to get Rate = k 2 (k 1 /k -1 ) [O 2 [E[CH A reaction with an activation energy of E a =100 kj/mol has a rate constant k=10 s -1 at a temperature of 20 C. What is the rate of this reaction (in s -1 ) at 50 C? (A) 7.1 (B) 10.0 (C) 14.0 (D)450 (E) 3300! ln# k 2 $ E = ' a! # 1 ' 1 $ " k 1 R " T 2 T 1 T 1 is 20 C = 293 K, and T 2 is 50 C = 323 K. # k ln kj /mol # 1 ( = " $ 10 s "1 ' 8.314x10 "3 kj /(molk) 323K " 1 ( $ 293K ' # k ln 2 $ 10 s "1 ' ( = "1.203x10 4 K # K " $ K ( ' # k ln 2 $ 10 s "1 ' ( = "1.203x10 4 K # " $ K ( ' # k ln 2 ( = $ 10 s "1 ' k 2 10 s = "1 e = 45.3 k 2 = (10 s -1 ) (45.3) = 453 s Predict the sign of ΔS o rxn for the three reactions below: I. 2NO 2 (g) N 2 O 4 (liq) II. NH 4 NO 3 (s) N 2 O (g) + 2H 2 O (g) III. B (s) + 3 / 2 F 2 (g) BF 3 (g) I II III I II III (A) (D) (B) (E) (C) A reaction that produces more moles of gas than it consumes is entropy favored (ΔS>0), so reactions I and III have ΔS<0, reaction II has ΔS>0 7. Under what circumstances will ΔG for a chemical reaction always be positive? (A) An endothermic reaction that generates fewer moles of gaseous products than gaseous reactants. (B) An exothermic reaction that generates solids from liquid reactants. (C) An exothermic reaction that generates fewer moles of gaseous products than gaseous reactants. (D) An endothermic reaction that generates more moles of gaseous products than gaseous 2
3 reactant. (E) both (B) and (C) above. ΔG=ΔH TΔS For ΔG to be positive (at all temperatures), ΔH must be positive (endothermic reaction) and TΔS must be positive, so ΔS must be negative (entropy disfavored: generate fewer moles of gas in products than in reactants) For questions 8 and 9 use the thermodynamic data at 298 K given below and the following reaction. Mg (s) O 2 (g) MgO (s) ΔH o f (kj/mol) ΔGo f (kj/mol) So (J/mol K) Mg (s) O 2 (g) MgO (s) What is ΔS o rxn in J/mol K? (A) -175 (C) -27 (E) -108 (B) 27 (D) 108!S 0 = " S 0 (products) # " S 0 (reactan ts) ΔS 0 rxn = ( 1 / 2 )(205) = J/(mol K) Note that ΔS 0 rxn is negative, as reaction is entropy disfavored (consume ½ mol gas, produce 0 moles gas 9. If magnesium metal is ignited in oxygen atmosphere in an isolated system at 300 K: (A) no reaction will take place because ΔS o rxn is negative. (B) no reaction will take place because ΔS surroundings = 0. (C) a reaction will take place because ΔS universe is positive. (D) no reaction will take place because ΔG o reaction is positive. (E) a reaction will take place because ΔS surroundings is negative. A reaction is spontaneous if ΔS universe is positive (or equivalently, if ΔG o reaction is negative). Recall that ΔS universe = ΔS reaction + ΔS surroundings and "S surroundings = # "H rxn T ΔH rxn = -602 (0) (1/2)(0) = -602 kj/mol ΔS surroundings = -(-602 kj/mol)/300 K = kj/(mol K) = 2007 J/(mol K) So, ΔS universe = -108 J/(mol K) J/(mol K) = 1899 J/(mol K) The reaction is so exothermic that ΔS surroundings is very large and positive, completely overwhelming (small, negative) ΔS universe Questions 10 and 11 refer to the reaction NO (g) O 2 (g) NO 2 (g) for which ΔH rxn = -57 kj/mol and ΔS rxn = -73 J/(mol K) at 298 K. 3
4 10. What is ΔG rxn in kj/mol at 25 C for this reaction? (A) -35 (B) -79 (C) -22 (D) 68 (E) 85 ΔG = ΔH TΔS ΔG = -57 kj/mol (298 K) (-73 x 10-3 kj/(mol K)) ΔG = -35 kj/mol 11. What is the temperature in C above/below which this reaction would have K p greater than one? (A) below 508 C (D) above 128 C (B) above 508 C (E) There is none. (C) below 128 C K p = 1 if ΔG = 0, and K p > 1 when ΔG is negative. Find T for which ΔG = 0: ΔG = ΔH TΔS = 0-57 kj/mol T(-73 x 10-3 kj/(mol K)) = 0 (0.073 kj/mol K) T = 57 kj/mol T = (57 kj/mol) / (0.073 kj/mol K) = 781 K, or 508 C If T is below 508 C, then ΔG is negative (see #10), and K p > 1. 4
5 Thermodynamic values for Problems 12 and 13: Species ΔH 0 f (298 K) S 0 (298 K) ΔG 0 f (298 K) kj/mol J/(K mol) kj/mol C(s, graphite) CH 4 (g) C 2 H 4 (g) C 2 H 6 (g) CO(g) CO 2 (g) H 2 (g) H 2 O(g) H 2 O(l) NO(g) NO 2 (g) O 2 (g) Use the thermodynamic values above to calculate ΔG rxn in kj/mol at a temperature of 500 K for the reaction CH 4 (g) + 3 / 2 O 2 (g) CO (g) + 2 H 2 O (g) (A) 275 (B) 280 (C) 479 (D) 544 (E) 560 ΔG = ΔH TΔS, so calculate ΔH and ΔS for the reaction using the table, then calculate ΔG. Note that the ΔG values given in the table are at 298 K, so you can t just use them (as ΔG depends on temperature!) ΔH = ( ) [ (3/2)(0) = kj/mol ΔS = (188.84) [ (3/2)(205.07) = J/mol K ΔG = ΔH TΔS ΔG = kj/mol (500 K) (81.49 x 10-3 kj/mol K) ΔG = kj/mol 13. Use the thermodynamic values at the top of this page to calculate the equilibrium constant K p at 298 K for the reaction O 2 (g) + 2 NO (g) 2 NO 2 (g) (A) 4.0 x (B) 6.4 x 10-7 (C) 4.8 x 10-4 (D) 1.6 x 10 6 (E) 2.5 x First calculate ΔG for the reaction. Since you want a value at 298 K, can use ΔG 0 f (298 K) values directly: 0 0 0!G rxn = " G f (products) # " G f (reactan ts) ΔG rxn = 2(51.23) [0 + 2(86.58) = kj/mol K p = e -ΔG/RT -ΔG/RT= -(-70.7 kj/mol)/[(8.314 x 10-3 kj/mol)(298 K) = So, K p = e = 2.5 x Recall that ΔG is negative, so K p > The equilibrium constant K c for the reaction U (s) + 3 F 2(g) UF 6(g) is (A) K c = [UF 6 [U[F 2 (D) K c = [UF 6 [F 2 5
6 (B) K c = [UF 6 [U[3F 2 (E) K c = [UF 6 [F 2 3 (C) K c = [UF 6 [U[F 2 3 Don t include U, as it s a solid, so the answer is (E) 15. At a particular temperature, an equilibrium mixture contains the following concentrations of gases: [NO 2 = 0.32 M, [NO = 10-4 M, [O 2 = M. What is the equilibrium constant K c for the reaction 2 NO 2(g) 2 NO (g) + O 2(g) (A) 4.5 x (D) 1.4 x 10-5 (B) 1.4 x 10-9 (E) 4.5 x 10-5 (C) 4.4 x 10-9 K c = [NO2 [O 2 [NO 2 2 K c = (10-4 ) 2 (0.045)/(0.32) 2 = 4.4 x The reaction H 2(g) + I 2(g) 2 HI (g) has K c =56 at 300 K. A 1 liter flask is filled with 0.2 moles of H 2, 0.2 moles of I 2 and 0.4 moles of HI. Will any reaction occur? If so, is HI produced or consumed? (A) No reaction will occur (B) A reaction will occur; HI will be produced (C) A reaction will occur; HI will be consumed Q = [HI2 [H 2 [I 2 Q = (0.4) 2 /[(0.2)(0.2) = 4 Q < K c, so as the reaction proceeds, Q will become larger (eventually reaching K c at equilibrium), for Q to increase, need to make more HI product, less H 2 and I 2 reactant moles of NH 4 Cl (s) are put into an evacuated 1 liter container at 550 K, and the following reaction occurs: NH 4 Cl (s) NH 3(g) + HCl (g) At equilibrium, [NH 3(g) = 2.2 x 10-3 M. What is K c for the reaction? (A) 2.2 x 10-3 (B) 2.4 x 10-6 (C) 4.8 x 10-6 (D) 9.6 x 10-6 (E) 1.9 x 10-5 K c = [NH 3 [HCl (note that NH 4 Cl isn t in K c, as it s a solid). NH 4 Cl NH 3 HCl Initial Change -x x x Equil. 2-x x x So, if [NH 3 = 2.2 x 10-3 M at equilibrium, x = [HCl = 2.2 x 10-3 M K c = (2.2 x 10-3 )(2.2 x 10-3 ) = 4.84 x
7 moles of NOCl (g) are placed in a 1 liter container at 372 K. The following reaction occurs: 2 NOCl (g) 2 NO (g) + Cl 2(g) K c = 1.78 x 10-9 What is the Cl 2 concentration at equilibrium? (Hint: K c is so small that very little of the NOCl decomposes) (A) 6.67 x 10-6 (D) 1.64 x 10-4 (B) 1.33 x 10-5 (E) 3.28 x 10-4 (C) 2.66 x 10-5 NOCl NO Cl 2 Initial Change -2x 2x x Equil x 2x x K c = [NO 2 [Cl 2 /[NOCl 2 K c = (2x) 2 (x)/(0.1-2x) 2 = 1.78 x 10-9 Because Kc is so small, it s probably a good approximation to assume that x is small (note that that s what the hint says). If x is small, then 0.1 2x is approximately 0.1, so (2x) 2 (x)/(0.1) 2 = 1.78 x x 3 /0.01 = 1.78 x x 3 = 1.78 x x 3 = 4.45 x x = 1.64 x 10-4 Note that this is much less than 0.1, so our approximation is good. Since [Cl 2 = x, [Cl 2 = 1.64 x 10-4 M 19. At high temperatures, graphite reacts with CO 2 to produce CO: C (s) + CO 2(g) 2 CO (g) K c = 0.10 If 0.07 moles of CO 2 are placed in a 1 liter container, what is the CO concentration at equilibrium? (Note: K c is fairly large, so a significant amount of the CO 2 reacts) (A) M (D) M (B) M (E) 0.13 M (C) M CO 2 CO Initial Change -x 2x Equil x 2x K c = [CO 2 /[CO 2 (note that C is a solid, so it s not in K c ) K c = (2x) 2 /(0.07 x) = 0.10 Could assume that x is small, but this turns out to be a poor assumption (note that K c is not very small; also, the hint says that a significant amount of CO 2 reacts, so x isn t small). So, solve the quadratic equation without approximations: 4x 2 = 0.10 (0.07 x) 4x x = 0 Ax 2 + Bx + C = 0 x = "B± B2 " 4AC 2A x = "0.1± (0.1)2 " 4(4)("0.007) 2(4) x = "0.1±
8 x = , The negative root is unphysical, so x = M [CO = 2x = M (I gave partial credit for (B)) Questions 20 through 22 refer to the following gas phase equilibrium for which K c = 12 at 1100K and ΔH = -198 kj/mol. 2SO 2(g) + O 2(g) 2SO 3(g) 20. Increasing the concentration (the pressure) on an equilibrium mixture by decreasing the volume at constant temperature would cause: (A) K to decrease and the amount of O 2(g) to increase. (B) K to decrease and the amount of O 2(g) to decrease. (C) K to increase and the amount of O 2(g) to decrease. (D) no change in K but an increase in the amount of O 2(g). (E) no change in K but a decrease in the amount of O 2(g). Don t change temperature, so don t change K. Increase pressure, so reaction proceeds in a direction that decreases pressure, reacting to make fewer moles of gas, so make more SO 3 (consuming SO 2 and O 2 ) 21. Addition of SO 3 (g) to an equilibrium mixture of the three gases at constant volume and temperature would cause: (A) K to decrease and the amount of O 2 (g) to increase. (B) K to decrease and the amount of O 2 (g) to decrease. (C) K to increase and the amount of O 2 (g) to decrease. (D) no change in K but an increase in the amount of O 2(g). (E) no change in K but a decrease in the amount of O 2 (g). Don t change temperature, so don t change K. Add product, so reaction proceeds in a direction that consumes product (SO 3 ), forming more O 2 and SO An equilibrium mixture of the three gases is initially at 1100K. The temperature is increased to 1300K, at constant volume. This would cause: (A) K to decrease and the amount of O 2 (g) to increase. (B) K to decrease and the amount of O 2 (g) to decrease. (C) K to increase and the amount of O 2 (g) to decrease. (D) no change in K but an increase in the amount of O 2(g). (E) no change in K but a decrease in the amount of O 2 (g). Change temperature, so change K. Increase temperature, so reaction proceeds in a direction that uses heat (in the endothermic direction). Production of SO 3 is exothermic, so consume SO 3, producing SO 2 and O 2. Make less product, more reactant, so K decreases. 8
Chemistry 112, Spring 2007 Prof. Metz Exam 2 Solutions April 5, 2007 Each question is worth 5 points, unless otherwise indicated
 Chemistry 11, Spring 007 Prof. Metz Exam Solutions April 5, 007 Each question is worth 5 points, unless otherwise indicated 1. A proposed mechanism for the reaction of NO with Br to give BrNO is NO + NO
Chemistry 11, Spring 007 Prof. Metz Exam Solutions April 5, 007 Each question is worth 5 points, unless otherwise indicated 1. A proposed mechanism for the reaction of NO with Br to give BrNO is NO + NO
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16
 Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Second Law of Thermodynamics
 Second Law of Thermodynamics First Law: the total energy of the universe is a constant Second Law: The entropy of the universe increases in a spontaneous process, and remains unchanged in a process at
Second Law of Thermodynamics First Law: the total energy of the universe is a constant Second Law: The entropy of the universe increases in a spontaneous process, and remains unchanged in a process at
Chem 1B Dr. White 1 Chapter 17: Thermodynamics. Review From Chem 1A (Chapter 6, section 1) A. The First Law of Thermodynamics
 Chem 1B Dr. White 1 Chapter 17: Thermodynamics Review From Chem 1A (Chapter 6, section 1) A. The First Law of Thermodynamics 17.1 Spontaneous Processes and Entropy A. Spontaneous Change Chem 1B Dr. White
Chem 1B Dr. White 1 Chapter 17: Thermodynamics Review From Chem 1A (Chapter 6, section 1) A. The First Law of Thermodynamics 17.1 Spontaneous Processes and Entropy A. Spontaneous Change Chem 1B Dr. White
Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book)
 Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 34 multiple choice questions. Fill
Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 34 multiple choice questions. Fill
Chem 401 Unit 1 (Kinetics & Thermo) Review
 KINETICS 1. For the equation 2 H 2(g) + O 2(g) 2 H 2 O (g) How is the rate of formation of H 2 O mathematically related to the rate of disappearance of O 2? 1 Δ [H2O] Δ[O 2] = 2 Δt Δt 2. Determine the
KINETICS 1. For the equation 2 H 2(g) + O 2(g) 2 H 2 O (g) How is the rate of formation of H 2 O mathematically related to the rate of disappearance of O 2? 1 Δ [H2O] Δ[O 2] = 2 Δt Δt 2. Determine the
Entropy. Spontaneity. Entropy. Entropy mol of N 2 at 1 atm or 1 mol of N 2 at atm. process a process that occurs without intervention
 Entropy Spontaneity process a process that occurs without intervention can be fast or slow Entropy (s) the measure of molecular randomness or disorder Think of entropy as the amount of chaos Entropy Predict
Entropy Spontaneity process a process that occurs without intervention can be fast or slow Entropy (s) the measure of molecular randomness or disorder Think of entropy as the amount of chaos Entropy Predict
Collision Theory. Unit 12: Chapter 18. Reaction Rates. Activation Energy. Reversible Reactions. Reversible Reactions. Reaction Rates and Equilibrium
 Collision Theory For reactions to occur collisions between particles must have Unit 12: Chapter 18 Reaction Rates and Equilibrium the proper orientation enough kinetic energy See Both In Action 1 2 Activation
Collision Theory For reactions to occur collisions between particles must have Unit 12: Chapter 18 Reaction Rates and Equilibrium the proper orientation enough kinetic energy See Both In Action 1 2 Activation
UNIT ONE BOOKLET 6. Thermodynamic
 DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT ONE BOOKLET 6 Thermodynamic Can we predict if a reaction will occur? What determines whether a reaction will be feasible or not? This is a question that
DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT ONE BOOKLET 6 Thermodynamic Can we predict if a reaction will occur? What determines whether a reaction will be feasible or not? This is a question that
Lecture #13. Chapter 17 Enthalpy and Entropy
 Lecture #13 Chapter 17 Enthalpy and Entropy First Law of Thermodynamics Energy cannot be created or destroyed The total energy of the universe cannot change Energy can be transferred from one place to
Lecture #13 Chapter 17 Enthalpy and Entropy First Law of Thermodynamics Energy cannot be created or destroyed The total energy of the universe cannot change Energy can be transferred from one place to
Thermodynamics: Free Energy and Entropy. Suggested Reading: Chapter 19
 Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
Free-energy change ( G) and entropy change ( S)
 Free-energy change ( G) and entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result
Free-energy change ( G) and entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result
Chem 401 Unit 1 (Kinetics & Thermo) Review
 KINETICS 1. For the equation 2 H 2(g) + O 2(g) 2 H 2 O (g) How is the rate of formation of H 2 O mathematically related to the rate of disappearance of O 2? 2. Determine the relative reaction rates of
KINETICS 1. For the equation 2 H 2(g) + O 2(g) 2 H 2 O (g) How is the rate of formation of H 2 O mathematically related to the rate of disappearance of O 2? 2. Determine the relative reaction rates of
EQUILIBRIA. e Q = a D B
 I. Basis of Equilibrium. A. Q and equilibrium. EQUILIBRIA 1. Consider the general reaction bb + cc dd + ee a. Αs time elapses, [B] and [C] decrease causing the rate of the forward reaction to decrease.
I. Basis of Equilibrium. A. Q and equilibrium. EQUILIBRIA 1. Consider the general reaction bb + cc dd + ee a. Αs time elapses, [B] and [C] decrease causing the rate of the forward reaction to decrease.
Equilibrium & Reaction Rate
 Equilibrium & Reaction Rate 1. One of the important reactions in coal gasification is the catalytic methanation reaction: CO(g) + H (g) H O(g) + CH 4 (g) H 06 kj a) Predict the direction in which this
Equilibrium & Reaction Rate 1. One of the important reactions in coal gasification is the catalytic methanation reaction: CO(g) + H (g) H O(g) + CH 4 (g) H 06 kj a) Predict the direction in which this
Name Chem 161, Section: Group Number: ALE 27. Hess s Law. (Reference: Chapter 6 - Silberberg 5 th edition)
 Name Chem 161, Section: Group Number: ALE 27. Hess s Law (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a calculation you must show your work neatly using dimensional
Name Chem 161, Section: Group Number: ALE 27. Hess s Law (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a calculation you must show your work neatly using dimensional
CHAPTER 20 THERMODYNAMICS: ENTROPY, FREE ENERGY, AND THE DIRECTION OF CHEMICAL REACTIONS
 CHAPTER 0 THERMODYNAMICS: ENTROPY, FREE ENERGY, AND THE DIRECTION OF CHEMICAL REACTIONS FOLLOW UP PROBLEMS 0.1A Plan: Particles with more freedom of motion have higher entropy. In general the entropy of
CHAPTER 0 THERMODYNAMICS: ENTROPY, FREE ENERGY, AND THE DIRECTION OF CHEMICAL REACTIONS FOLLOW UP PROBLEMS 0.1A Plan: Particles with more freedom of motion have higher entropy. In general the entropy of
Thermodynamics. 1. Which of the following processes causes an entropy decrease?
 Thermodynamics 1. Which of the following processes causes an entropy decrease? A. boiling water to form steam B. dissolution of solid KCl in water C. mixing of two gases in one container D. beach erosion
Thermodynamics 1. Which of the following processes causes an entropy decrease? A. boiling water to form steam B. dissolution of solid KCl in water C. mixing of two gases in one container D. beach erosion
(E) half as fast as methane.
 Name AP Chem / / AP Chem Practice Exam #2 Part I: 40 Questions, 40 minutes, Multiple Choice, No Calculator Allowed Bubble the correct answer on the BLUE SIDE of your scantron for each of the following.
Name AP Chem / / AP Chem Practice Exam #2 Part I: 40 Questions, 40 minutes, Multiple Choice, No Calculator Allowed Bubble the correct answer on the BLUE SIDE of your scantron for each of the following.
Free Energy and Spontaneity
 Free Energy and Spontaneity CHEM 107 T. Hughbanks Free Energy One more state function... We know S universe > 0 for a spontaneous change, but... We are still looking for a state function of the system
Free Energy and Spontaneity CHEM 107 T. Hughbanks Free Energy One more state function... We know S universe > 0 for a spontaneous change, but... We are still looking for a state function of the system
B 2 Fe(s) O 2(g) Fe 2 O 3 (s) H f = -824 kj mol 1 Iron reacts with oxygen to produce iron(iii) oxide as represented above. A 75.
 1 2004 B 2 Fe(s) + 3 2 O 2(g) Fe 2 O 3 (s) H f = -824 kj mol 1 Iron reacts with oxygen to produce iron(iii) oxide as represented above. A 75.0 g sample of Fe(s) is mixed with 11.5 L of O 2 (g) at 2.66
1 2004 B 2 Fe(s) + 3 2 O 2(g) Fe 2 O 3 (s) H f = -824 kj mol 1 Iron reacts with oxygen to produce iron(iii) oxide as represented above. A 75.0 g sample of Fe(s) is mixed with 11.5 L of O 2 (g) at 2.66
Chemical Equilibrium
 Chemical Equilibrium Concept of Equilibrium Equilibrium Constant Equilibrium expressions Applications of equilibrium constants Le Chatelier s Principle The Concept of Equilibrium The decomposition of N
Chemical Equilibrium Concept of Equilibrium Equilibrium Constant Equilibrium expressions Applications of equilibrium constants Le Chatelier s Principle The Concept of Equilibrium The decomposition of N
BCIT Fall Chem Exam #2
 BCI Fall 2016 Chem 3310 Exam #2 Name: Attempt all questions in this exam. Read each question carefully and give a complete answer in the space provided. Part marks given for wrong answers with partially
BCI Fall 2016 Chem 3310 Exam #2 Name: Attempt all questions in this exam. Read each question carefully and give a complete answer in the space provided. Part marks given for wrong answers with partially
3 A (aq) + 2 D (aq) 4 C (g) + B (s) + 2 E (l)
 AP Chemistry Test (Chapter 13) Multiple Choice (20%) 1) Which one best describes the K C for this reaction? 3 A (aq) + 2 D (aq) 4 C (g) + B (s) + 2 E (l) A) K c = [A] 3 [D] 2 B) K c = [C] 4 [B][E] 2 [C]
AP Chemistry Test (Chapter 13) Multiple Choice (20%) 1) Which one best describes the K C for this reaction? 3 A (aq) + 2 D (aq) 4 C (g) + B (s) + 2 E (l) A) K c = [A] 3 [D] 2 B) K c = [C] 4 [B][E] 2 [C]
REACTION EQUILIBRIUM
 REACTION EQUILIBRIUM A. REVERSIBLE REACTIONS 1. In most spontaneous reactions the formation of products is greatly favoured over the reactants and the reaction proceeds to completion (one direction). In
REACTION EQUILIBRIUM A. REVERSIBLE REACTIONS 1. In most spontaneous reactions the formation of products is greatly favoured over the reactants and the reaction proceeds to completion (one direction). In
CHEMISTRY 102 FALL 2010 EXAM 2 FORM C SECTION 502 DR. KEENEY-KENNICUTT PART 1
 NAME CHEMISTRY 102 FALL 2010 EXAM 2 FORM C SECTION 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
NAME CHEMISTRY 102 FALL 2010 EXAM 2 FORM C SECTION 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
The Extent of Chemical Reactions
 Equilibrium: The Extent of Chemical Reactions The Equilibrium State and the Equilibrium Constant The Reaction Quotient and the Equilibrium Constant Equilibrium: The Extent of Chemical Reactions Expressing
Equilibrium: The Extent of Chemical Reactions The Equilibrium State and the Equilibrium Constant The Reaction Quotient and the Equilibrium Constant Equilibrium: The Extent of Chemical Reactions Expressing
Exam 4, Ch 14 and 15 December 7, Points
 Chem 130 Name Exam 4, Ch 14 and 15 December 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct
Chem 130 Name Exam 4, Ch 14 and 15 December 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct
6. Which expression correctly describes the equilibrium constant for the following reaction? 4NH 3 (g) + 5O 2 (g) 4NO(g) + 6H 2 O(g)
 1. Which of the following can we predict from an equilibrium constant for a reaction? 1. The extent of a reaction 2. Whether the reaction is fast or slow 3. Whether a reaction is exothermic or endothermic
1. Which of the following can we predict from an equilibrium constant for a reaction? 1. The extent of a reaction 2. Whether the reaction is fast or slow 3. Whether a reaction is exothermic or endothermic
CHAPTER 16 REVIEW. Reaction Energy. SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 16 REVIEW Reaction Energy SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. For elements in their standard state, the value of H 0 f is 0. 2. The formation and decomposition
CHAPTER 16 REVIEW Reaction Energy SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. For elements in their standard state, the value of H 0 f is 0. 2. The formation and decomposition
Entropy, Free Energy, and Equilibrium
 Entropy, Free Energy, and Equilibrium Chapter 17 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs
Entropy, Free Energy, and Equilibrium Chapter 17 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs
Gibbs Free Energy. Evaluating spontaneity
 Gibbs Free Energy Evaluating spontaneity Predicting Spontaneity An increase in entropy; Changing from a more structured to less structured physical state: Solid to liquid Liquid to gas Increase in temperature
Gibbs Free Energy Evaluating spontaneity Predicting Spontaneity An increase in entropy; Changing from a more structured to less structured physical state: Solid to liquid Liquid to gas Increase in temperature
H = DATA THAT YOU MAY USE. Units Conventional Volume ml or cm 3 = cm 3 or 10-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr = 1.
 DATA THAT YOU MAY USE Units Conventional S.I. Volume ml or cm 3 = cm 3 or 10-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr = 1.013 10 5 Pa torr = 133.3 Pa Temperature C 0 C = 73.15 K PV L-atm = 1.013
DATA THAT YOU MAY USE Units Conventional S.I. Volume ml or cm 3 = cm 3 or 10-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr = 1.013 10 5 Pa torr = 133.3 Pa Temperature C 0 C = 73.15 K PV L-atm = 1.013
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Chemistry 12: Dynamic Equilibrium Practice Test
 Chemistry 12: Dynamic Equilibrium Practice Test A. Multiple Choice: For each question, select the best answer and record your choice on the answer key provided. /25 1) A system at equilibrium is said to
Chemistry 12: Dynamic Equilibrium Practice Test A. Multiple Choice: For each question, select the best answer and record your choice on the answer key provided. /25 1) A system at equilibrium is said to
b. Free energy changes provide a good indication of which reactions are favorable and fast, as well as those that are unfavorable and slow.
 Chem 130 Name Exam 3, Ch 7, 19, 14 November 9, 2018 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct
Chem 130 Name Exam 3, Ch 7, 19, 14 November 9, 2018 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct
ALE 27. Hess s Law. (Reference: Chapter 6 - Silberberg 5 th edition)
 Answer Key ALE 27. Hess s Law (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a calculation you must show your work neatly using dimensional analysis with correct
Answer Key ALE 27. Hess s Law (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a calculation you must show your work neatly using dimensional analysis with correct
Homework 11 - Second Law & Free Energy
 HW11 - Second Law & Free Energy Started: Nov 1 at 9:0am Quiz Instructions Homework 11 - Second Law & Free Energy Question 1 In order for an endothermic reaction to be spontaneous, endothermic reactions
HW11 - Second Law & Free Energy Started: Nov 1 at 9:0am Quiz Instructions Homework 11 - Second Law & Free Energy Question 1 In order for an endothermic reaction to be spontaneous, endothermic reactions
Thermodynamics. Thermodynamics of Chemical Reactions. Enthalpy change
 Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes
 Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
The Equilibrium State. Chapter 13 - Chemical Equilibrium. The Equilibrium State. Equilibrium is Dynamic! 5/29/2012
 Chapter 13 - Chemical Equilibrium The Equilibrium State Not all chemical reactions go to completion; instead they attain a state of equilibrium. When you hear equilibrium, what do you think of? Example:
Chapter 13 - Chemical Equilibrium The Equilibrium State Not all chemical reactions go to completion; instead they attain a state of equilibrium. When you hear equilibrium, what do you think of? Example:
Exam 3, Ch 7, 19, 14 November 9, Points
 Chem 30 Name Exam 3, Ch 7, 9, 4 November 9, 206 00 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units
Chem 30 Name Exam 3, Ch 7, 9, 4 November 9, 206 00 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units
CHEMISTRY 102 FALL 2009 EXAM 2 FORM B SECTION 501 DR. KEENEY-KENNICUTT PART 1
 NAME CHEMISTRY 102 FALL 2009 EXAM 2 FORM B SECTION 501 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
NAME CHEMISTRY 102 FALL 2009 EXAM 2 FORM B SECTION 501 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
AP Chem Chapter 12 Notes: Gaseous Equilibrium
 AP Chem Chapter 12 Notes: Gaseous Equilibrium Equilibrium I. Equilibrium is reached when both the and reactions are occurring at. A. Dynamic Equilibrium: reactions are still occurring but the of reactants
AP Chem Chapter 12 Notes: Gaseous Equilibrium Equilibrium I. Equilibrium is reached when both the and reactions are occurring at. A. Dynamic Equilibrium: reactions are still occurring but the of reactants
(i.e., equilibrium is established) leads to: K = k 1
 CHEMISTRY 104 Help Sheet #8 Chapter 12 Equilibrium Do the topics appropriate for your lecture http://www.chem.wisc.edu/areas/clc (Resource page) Prepared by Dr. Tony Jacob Nuggets: Equilibrium Constant
CHEMISTRY 104 Help Sheet #8 Chapter 12 Equilibrium Do the topics appropriate for your lecture http://www.chem.wisc.edu/areas/clc (Resource page) Prepared by Dr. Tony Jacob Nuggets: Equilibrium Constant
Advanced Chemistry Practice Problems
 Thermodynamics: Review of Thermochemistry 1. Question: What is the sign of DH for an exothermic reaction? An endothermic reaction? Answer: ΔH is negative for an exothermic reaction and positive for an
Thermodynamics: Review of Thermochemistry 1. Question: What is the sign of DH for an exothermic reaction? An endothermic reaction? Answer: ΔH is negative for an exothermic reaction and positive for an
and mol of Cl 2 was heated in a vessel of fixed volume to a constant temperature, the following reaction reached equilibrium.
 Q1. When a mixture of 0.45 mol of PCl and 0.68 mol of Cl was heated in a vessel of fixed volume to a constant temperature, the following reaction reached equilibrium. PCl + Cl PCl 5 H = 9 kj mol 1 At equilibrium,
Q1. When a mixture of 0.45 mol of PCl and 0.68 mol of Cl was heated in a vessel of fixed volume to a constant temperature, the following reaction reached equilibrium. PCl + Cl PCl 5 H = 9 kj mol 1 At equilibrium,
OCR Chemistry A H432
 All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
Chapter Seven. Chemical Reactions: Energy, Rates, and Equilibrium
 Chapter Seven Chemical Reactions: Energy, Rates, and Equilibrium Endothermic vs. Exothermic 2 Endothermic: A process or reaction that absorbs heat and has a positive ΔH. Exothermic: A process or reaction
Chapter Seven Chemical Reactions: Energy, Rates, and Equilibrium Endothermic vs. Exothermic 2 Endothermic: A process or reaction that absorbs heat and has a positive ΔH. Exothermic: A process or reaction
Practice Test F.1 (pg 1 of 7) Unit F - General Equilibrium Kp and Kc Name Per
 Practice Test F. (pg of 7) Unit F - General Equilibrium Kp and Kc Name Per This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions
Practice Test F. (pg of 7) Unit F - General Equilibrium Kp and Kc Name Per This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions
CHEM 102 Final Mock Exam
 CHEM 102 Final Mock Exam 1. A system releases 300 J of heat and does 650 J of work on the surroundings. What is the change in internal energy of the system? a. -950 J b. 350 J c. 950 J d. -350 J 2. Which
CHEM 102 Final Mock Exam 1. A system releases 300 J of heat and does 650 J of work on the surroundings. What is the change in internal energy of the system? a. -950 J b. 350 J c. 950 J d. -350 J 2. Which
Chemistry and the material world Unit 4, Lecture 4 Matthias Lein
 Chemistry and the material world 123.102 Unit 4, Lecture 4 Matthias Lein Gibbs ree energy Gibbs ree energy to predict the direction o a chemical process. Exergonic and endergonic reactions. Temperature
Chemistry and the material world 123.102 Unit 4, Lecture 4 Matthias Lein Gibbs ree energy Gibbs ree energy to predict the direction o a chemical process. Exergonic and endergonic reactions. Temperature
Review Unit #11. Review Unit # H 2 O (g) + CO (g) H 2(g) + CO 2(g) H>1
 Review Unit #11 1. H 2 O (g) + CO (g) H 2(g) + CO 2(g) H>1 K c = 1.6 What effect would these changes have on the equilibrium position? a. Cool the mixture b. Increase the volume of the flask c. Add H 2(g)
Review Unit #11 1. H 2 O (g) + CO (g) H 2(g) + CO 2(g) H>1 K c = 1.6 What effect would these changes have on the equilibrium position? a. Cool the mixture b. Increase the volume of the flask c. Add H 2(g)
Thermochemistry. Chapter 6. Dec 19 8:52 AM. Thermochemistry. Energy: The capacity to do work or to produce heat
 Chapter 6 Dec 19 8:52 AM Intro vocabulary Energy: The capacity to do work or to produce heat Potential Energy: Energy due to position or composition (distance and strength of bonds) Kinetic Energy: Energy
Chapter 6 Dec 19 8:52 AM Intro vocabulary Energy: The capacity to do work or to produce heat Potential Energy: Energy due to position or composition (distance and strength of bonds) Kinetic Energy: Energy
Name period AP Unit 8: equilibrium
 Name period AP Unit 8: equilibrium 1. What is equilibrium? Rate of the forward reaction equals the rate of the reverse reaction 2. How can you tell when equilibrium has been reached? The concentrations
Name period AP Unit 8: equilibrium 1. What is equilibrium? Rate of the forward reaction equals the rate of the reverse reaction 2. How can you tell when equilibrium has been reached? The concentrations
Unit 5: Spontaneity of Reaction. You need to bring your textbooks everyday of this unit.
 Unit 5: Spontaneity of Reaction You need to bring your textbooks everyday of this unit. THE LAWS OF THERMODYNAMICS 1 st Law of Thermodynamics Energy is conserved ΔE = q + w 2 nd Law of Thermodynamics A
Unit 5: Spontaneity of Reaction You need to bring your textbooks everyday of this unit. THE LAWS OF THERMODYNAMICS 1 st Law of Thermodynamics Energy is conserved ΔE = q + w 2 nd Law of Thermodynamics A
Chapter 16. Thermodynamics. Thermochemistry Review. Calculating H o rxn. Predicting sign for H o rxn. Creative Commons License
 Chapter 16 Thermodynamics GCC CHM152 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution License 4.0. ChemWiki (CC
Chapter 16 Thermodynamics GCC CHM152 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution License 4.0. ChemWiki (CC
1 A reaction that is spontaneous.
 Slide 1 / 55 1 A reaction that is spontaneous. A B C D E is very rapid will proceed without outside intervention is also spontaneous in the reverse direction has an equilibrium position that lies far to
Slide 1 / 55 1 A reaction that is spontaneous. A B C D E is very rapid will proceed without outside intervention is also spontaneous in the reverse direction has an equilibrium position that lies far to
AP* Thermodynamics Free Response Questions page 1. Essay Questions
 AP* Thermodynamics Free Response Questions page 1 Essay Questions 1991 The reaction represented above is a reversible reaction. BCl 3 (g) + NH 3 (g) Cl 3 BNH 3 (s) (a) Predict the sign of the entropy change,
AP* Thermodynamics Free Response Questions page 1 Essay Questions 1991 The reaction represented above is a reversible reaction. BCl 3 (g) + NH 3 (g) Cl 3 BNH 3 (s) (a) Predict the sign of the entropy change,
UNIT II - REVIEW EQUILIBRIA. Part I - Multiple Choice. 1. In which of the following does the entropy decrease?
 CHEMISTRY 12 UNIT II - REVIEW EQUILIBRIA Part I - Multiple Choice 1. In which of the following does the entropy decrease? A. NaCl (s) Na + (aq) + Cl (aq) B. 4 NO (g) + 6 H 2 O (g) 4 NH 3 (g) + 5 O 2 (g)
CHEMISTRY 12 UNIT II - REVIEW EQUILIBRIA Part I - Multiple Choice 1. In which of the following does the entropy decrease? A. NaCl (s) Na + (aq) + Cl (aq) B. 4 NO (g) + 6 H 2 O (g) 4 NH 3 (g) + 5 O 2 (g)
Ch 10 Practice Problems
 Ch 10 Practice Problems 1. Which of the following result(s) in an increase in the entropy of the system? I. (See diagram.) II. Br 2(g) Br 2(l) III. NaBr(s) Na + (aq) + Br (aq) IV. O 2(298 K) O 2(373 K)
Ch 10 Practice Problems 1. Which of the following result(s) in an increase in the entropy of the system? I. (See diagram.) II. Br 2(g) Br 2(l) III. NaBr(s) Na + (aq) + Br (aq) IV. O 2(298 K) O 2(373 K)
Chemical Thermodynamics
 Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Chemical Equilibrium: Ch Dynamic Equilibrium. Dynamic Equilibrium. Three Approaches to Equilibrium The Equilibrium Constant Expression
 Chemical Equilibrium: Ch. 15 15-1 Dynamic Equilibrium 15- The Equilibrium Constant Expression 15- Relationships Involving Equilibrium Constants 15-4 The Magnitude of an Equilibrium Constant 15-5 The Reaction
Chemical Equilibrium: Ch. 15 15-1 Dynamic Equilibrium 15- The Equilibrium Constant Expression 15- Relationships Involving Equilibrium Constants 15-4 The Magnitude of an Equilibrium Constant 15-5 The Reaction
Chem 401 Unit 1 Exam: Thermodynamics & Kinetics (Nuss: Spr 2018)
 Date: Exam # Chem 401 Unit 1 Exam: Thermodynamics & Kinetics (Nuss: Spr 2018) Multiple Choice Identify the choice that best completes the statement or answers the question. (3 pts each) 1. Which of the
Date: Exam # Chem 401 Unit 1 Exam: Thermodynamics & Kinetics (Nuss: Spr 2018) Multiple Choice Identify the choice that best completes the statement or answers the question. (3 pts each) 1. Which of the
Chemistry 30: Reaction Kinetics. Practice Problems
 Name: Period: Chemistry 30: Reaction Kinetics Practice Problems Date: Measuring Reaction Rates 1. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. The equation for the
Name: Period: Chemistry 30: Reaction Kinetics Practice Problems Date: Measuring Reaction Rates 1. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. The equation for the
CHEM Dr. Babb s Sections Lecture Problem Sheets
 CHEM 116 - Dr. Babb s Sections Lecture Problem Sheets Kinetics: Integrated Form of Rate Law 61. Give the integrated form of a zeroth order reaction. Define the half-life and find the halflife for a general
CHEM 116 - Dr. Babb s Sections Lecture Problem Sheets Kinetics: Integrated Form of Rate Law 61. Give the integrated form of a zeroth order reaction. Define the half-life and find the halflife for a general
Chem 116 POGIL Worksheet - Week 12 - Solutions Second & Third Laws of Thermodynamics Balancing Redox Equations
 Chem 116 POGIL Worksheet - Week 12 - Solutions Second & Third Laws of Thermodynamics Balancing Redox Equations Key Questions 1. Does the entropy of the system increase or decrease for the following changes?
Chem 116 POGIL Worksheet - Week 12 - Solutions Second & Third Laws of Thermodynamics Balancing Redox Equations Key Questions 1. Does the entropy of the system increase or decrease for the following changes?
CHEMpossible. Final Exam Review
 CHEMpossible Final Exam Review 1. Given the following pair of reactions and their equilibrium constants: 2NO 2 (g) 2NO (g) + O 2 (g) K c = 15.5 2NO (g) + Cl 2 (g) 2 NOCl (g) K c = 3.20 10-3 Calculate a
CHEMpossible Final Exam Review 1. Given the following pair of reactions and their equilibrium constants: 2NO 2 (g) 2NO (g) + O 2 (g) K c = 15.5 2NO (g) + Cl 2 (g) 2 NOCl (g) K c = 3.20 10-3 Calculate a
Thermodynamics II. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics II Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs downhill A lump of sugar dissolves
Thermodynamics II Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs downhill A lump of sugar dissolves
3. Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g) a) [X] 2 [Y][W] 3 [V] [W] 3 [V] [X] 2 [Y] [3W][V] [2X][Y]
![3. Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g) a) [X] 2 [Y][W] 3 [V] [W] 3 [V] [X] 2 [Y] [3W][V] [2X][Y] 3. Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g) a) [X] 2 [Y][W] 3 [V] [W] 3 [V] [X] 2 [Y] [3W][V] [2X][Y]](/thumbs/75/71520563.jpg) 1. Which of the following statements concerning equilibrium is not true? a) A system that is disturbed from an equilibrium condition responds in a manner to restore equilibrium. b) Equilibrium in molecular
1. Which of the following statements concerning equilibrium is not true? a) A system that is disturbed from an equilibrium condition responds in a manner to restore equilibrium. b) Equilibrium in molecular
(03) WMP/Jun10/CHEM4
 Thermodynamics 3 Section A Answer all questions in the spaces provided. 1 A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may
Thermodynamics 3 Section A Answer all questions in the spaces provided. 1 A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may
Entropy is a measure of the number of equivalent ways in which a system may exist.
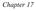 Chapter 17 1 ENTROPY (S) Entropy is a measure of the number of equivalent ways in which a system may exist. S = Units: -23 k = Boltzmann constant ( 1.38 x 10 J/K) Ù = number of equivalent ways for the
Chapter 17 1 ENTROPY (S) Entropy is a measure of the number of equivalent ways in which a system may exist. S = Units: -23 k = Boltzmann constant ( 1.38 x 10 J/K) Ù = number of equivalent ways for the
Chapter 6: Chemical Equilibrium
 Chapter 6: Chemical Equilibrium 6.1 The Equilibrium Condition 6. The Equilibrium Constant 6.3 Equilibrium Expressions Involving Pressures 6.4 The Concept of Activity 6.5 Heterogeneous Equilibria 6.6 Applications
Chapter 6: Chemical Equilibrium 6.1 The Equilibrium Condition 6. The Equilibrium Constant 6.3 Equilibrium Expressions Involving Pressures 6.4 The Concept of Activity 6.5 Heterogeneous Equilibria 6.6 Applications
ASSIGNMENT SHEET #11 APQ ANSWERS
 ASSIGNMENT SHEET #11 APQ ANSWERS #1 a. The unit for q must be an energy unit, typically Joules or calories. The unit for mass is the gram. The unit for specific heat is J per gram-degree or calorie per
ASSIGNMENT SHEET #11 APQ ANSWERS #1 a. The unit for q must be an energy unit, typically Joules or calories. The unit for mass is the gram. The unit for specific heat is J per gram-degree or calorie per
Kinetics. 1. Consider the following reaction: 3 A 2 B How is the average rate of appearance of B related to the average rate of disappearance of A?
 Kinetics 1. Consider the following reaction: 3 A 2 B How is the average rate of appearance of B related to the average rate of disappearance of A? A. [A]/ t = [B]/ t B. [A]/ t = (2/3)( [B]/ t) C. [A]/
Kinetics 1. Consider the following reaction: 3 A 2 B How is the average rate of appearance of B related to the average rate of disappearance of A? A. [A]/ t = [B]/ t B. [A]/ t = (2/3)( [B]/ t) C. [A]/
OAT General Chemistry Problem Drill 15: Thermochemistry & Thermodynamics
 OAT General Chemistry Problem Drill 15: Thermochemistry & Thermodynamics Question No. 1 of 10 1. A metal with a high heat capacity is put on a hot plate. What will happen? Question #01 (A) The temperature
OAT General Chemistry Problem Drill 15: Thermochemistry & Thermodynamics Question No. 1 of 10 1. A metal with a high heat capacity is put on a hot plate. What will happen? Question #01 (A) The temperature
January 03, Ch 13 SB equilibrium.notebook
 Ch 13: Chemical Equilibrium exists when 2 opposing reactions occur simultaneously at the same rate (dynamic rather than static) Forward rate = reverse rate https://www.youtube.com/watch?v=wld_imyqagq The
Ch 13: Chemical Equilibrium exists when 2 opposing reactions occur simultaneously at the same rate (dynamic rather than static) Forward rate = reverse rate https://www.youtube.com/watch?v=wld_imyqagq The
UNIT 8 KINETICS & EQ: NOTE & PRACTICE PACKET
 UNIT 8 KINETICS & EQ: NOTE & PRACTICE PACKET 1 2 Lesson 1: Kinetics = study of the RATE or SPEED at which REACTIONS occur A REACTION is the Reaction Mechanism = STEP BY STEP PROCESS needed to make a product;
UNIT 8 KINETICS & EQ: NOTE & PRACTICE PACKET 1 2 Lesson 1: Kinetics = study of the RATE or SPEED at which REACTIONS occur A REACTION is the Reaction Mechanism = STEP BY STEP PROCESS needed to make a product;
THERMODYNAMICS. Dr. Sapna Gupta
 THERMODYNAMICS Dr. Sapna Gupta FIRST LAW OF THERMODYNAMICS Thermodynamics is the study of heat and other forms of energy involved in chemical or physical processes. First Law of Thermodynamics Energy cannot
THERMODYNAMICS Dr. Sapna Gupta FIRST LAW OF THERMODYNAMICS Thermodynamics is the study of heat and other forms of energy involved in chemical or physical processes. First Law of Thermodynamics Energy cannot
15/04/2018 EQUILIBRIUM- GENERAL CONCEPTS
 15/04/018 EQUILIBRIUM- GENERAL CONCEPTS When a system is at equilibrium, the forward and reverse reactions are proceeding at the same rate. The concentrations of all species remain constant over time,
15/04/018 EQUILIBRIUM- GENERAL CONCEPTS When a system is at equilibrium, the forward and reverse reactions are proceeding at the same rate. The concentrations of all species remain constant over time,
AP Chemistry Chapter 16 Assignment. Part I Multiple Choice
 Page 1 of 7 AP Chemistry Chapter 16 Assignment Part I Multiple Choice 1984 47. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O(l) H = 889.1 kj H f H 2 O(l) = 285.8 kj mol 1 H f CO 2 (g) = 393.3 kj mol 1 What is
Page 1 of 7 AP Chemistry Chapter 16 Assignment Part I Multiple Choice 1984 47. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O(l) H = 889.1 kj H f H 2 O(l) = 285.8 kj mol 1 H f CO 2 (g) = 393.3 kj mol 1 What is
Spontaneity, Entropy, and Free Energy
 Spontaneity, Entropy, and Free Energy A ball rolls spontaneously down a hill but not up. Spontaneous Processes A reaction that will occur without outside intervention; product favored Most reactants are
Spontaneity, Entropy, and Free Energy A ball rolls spontaneously down a hill but not up. Spontaneous Processes A reaction that will occur without outside intervention; product favored Most reactants are
CHEM 1423 Chapter 17 Homework Questions TEXTBOOK HOMEWORK
 CHEM 1423 Chapter 17 Homework Questions TEXTBOOK HOMEWORK 17.29 At 425 o C, Kp = 4.18x10-9 for the reaction 2HBr(g) H 2 (g) + Br 2 (g) In one experiment, 0.20 atm of HBr(g), 0.010 atm of H 2 (g), and 0.010
CHEM 1423 Chapter 17 Homework Questions TEXTBOOK HOMEWORK 17.29 At 425 o C, Kp = 4.18x10-9 for the reaction 2HBr(g) H 2 (g) + Br 2 (g) In one experiment, 0.20 atm of HBr(g), 0.010 atm of H 2 (g), and 0.010
MCAT General Chemistry Discrete Question Set 19: Thermochemistry & Thermodynamics
 MCAT General Chemistry Discrete Question Set 19: Thermochemistry & Thermodynamics Question No. 1 of 10 1: A metal with a high heat capacity is put on a hot plate. What will happen? Question #01 A. The
MCAT General Chemistry Discrete Question Set 19: Thermochemistry & Thermodynamics Question No. 1 of 10 1: A metal with a high heat capacity is put on a hot plate. What will happen? Question #01 A. The
EQUILIBRIUM GENERAL CONCEPTS
 017-11-09 WHEN THE REACTION IS IN EQUILIBRIUM EQUILIBRIUM GENERAL CONCEPTS The concentrations of all species remain constant over time, but both the forward and reverse reaction never cease When a system
017-11-09 WHEN THE REACTION IS IN EQUILIBRIUM EQUILIBRIUM GENERAL CONCEPTS The concentrations of all species remain constant over time, but both the forward and reverse reaction never cease When a system
Chapter 9. Chemical Equilibrium
 Chapter 9. Chemical Equilibrium 9.1 The Nature of Chemical Equilibrium -Approach to Equilibrium [Co(H 2 O) 6 ] 2+ + 4 Cl- [CoCl 4 ] 2- + 6 H 2 O Characteristics of the Equilibrium State example) H 2 O(l)
Chapter 9. Chemical Equilibrium 9.1 The Nature of Chemical Equilibrium -Approach to Equilibrium [Co(H 2 O) 6 ] 2+ + 4 Cl- [CoCl 4 ] 2- + 6 H 2 O Characteristics of the Equilibrium State example) H 2 O(l)
Chemical Equilibrium Basics
 Chemical Equilibrium Basics Reading: Chapter 16 of Petrucci, Harwood and Herring (8th edition) Problem Set: Chapter 16 questions 25, 27, 31, 33, 35, 43, 71 York University CHEM 1001 3.0 Chemical Equilibrium
Chemical Equilibrium Basics Reading: Chapter 16 of Petrucci, Harwood and Herring (8th edition) Problem Set: Chapter 16 questions 25, 27, 31, 33, 35, 43, 71 York University CHEM 1001 3.0 Chemical Equilibrium
Entropy and Free Energy
 Page 1 Entropy and Free Energy How to predict if a reaction can occur at a reasonable rate? KINEICS Chapter 17 How to predict if a reaction can occur, given enough time? HERMODYNAMICS 1 Objectives Spontaneity
Page 1 Entropy and Free Energy How to predict if a reaction can occur at a reasonable rate? KINEICS Chapter 17 How to predict if a reaction can occur, given enough time? HERMODYNAMICS 1 Objectives Spontaneity
Name ID# Section # CH 1020 EXAM 2 Spring Form A
 Name ID# Section # CH EXAM Spring 7 - Form A Fill in your name, ID#, and section on this test booklet. Fill in and bubble in your name, ID# (bubble for C ), and section on the scantron form. For question
Name ID# Section # CH EXAM Spring 7 - Form A Fill in your name, ID#, and section on this test booklet. Fill in and bubble in your name, ID# (bubble for C ), and section on the scantron form. For question
CHEMISTRY 202 Practice Hour Exam II. Dr. D. DeCoste T.A (60 pts.) 21 (40 pts.) 22 (20 pts.)
 CHEMISTRY 202 Practice Hour Exam II Fall 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 22 questions on 7 numbered pages. Check now to make sure you have a complete exam. You have two hours
CHEMISTRY 202 Practice Hour Exam II Fall 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 22 questions on 7 numbered pages. Check now to make sure you have a complete exam. You have two hours
In previous chapters we have studied: Why does a change occur in the first place? Methane burns but not the reverse CH 4 + 2O 2 CO 2 + 2H 2 O
 Chapter 19. Spontaneous Change: Entropy and Free Energy In previous chapters we have studied: How fast does the change occur How is rate affected by concentration and temperature How much product will
Chapter 19. Spontaneous Change: Entropy and Free Energy In previous chapters we have studied: How fast does the change occur How is rate affected by concentration and temperature How much product will
12A Entropy. Entropy change ( S) N Goalby chemrevise.org 1. System and Surroundings
 12A Entropy Entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result in products that
12A Entropy Entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result in products that
Chp 13, 14, 15 SHOW ALL WORK AND CIRCLE FINAL ANSWERS. a) 1 only b) 2 only c) 3 only d) 1 and 2 only e) 1, 2, and H 2
 Chp 13, 14, 15 Name: SHOW ALL WORK AND CIRCLE FINAL ANSWERS 1. Which of the following factors affect the initial rate of a reaction? 1) The nature of the reactants. 2) The concentration of the reactants.
Chp 13, 14, 15 Name: SHOW ALL WORK AND CIRCLE FINAL ANSWERS 1. Which of the following factors affect the initial rate of a reaction? 1) The nature of the reactants. 2) The concentration of the reactants.
Chapter 13 Kinetics: Rates and Mechanisms of Chemical Reactions
 Chapter 13 Kinetics: Rates and Mechanisms of Chemical Reactions 14.1 Focusing on Reaction Rate 14.2 Expressing the Reaction Rate 14.3 The Rate Law and Its Components 14.4 Integrated Rate Laws: Concentration
Chapter 13 Kinetics: Rates and Mechanisms of Chemical Reactions 14.1 Focusing on Reaction Rate 14.2 Expressing the Reaction Rate 14.3 The Rate Law and Its Components 14.4 Integrated Rate Laws: Concentration
AQA A2 CHEMISTRY TOPIC 4.2 EQUILIBRIA BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.2 EQUILIBRIA BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. (a) The diagram below shows the effect of temperature and pressure on the equilibrium yield of the product in a gaseous
AQA A2 CHEMISTRY TOPIC 4.2 EQUILIBRIA BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. (a) The diagram below shows the effect of temperature and pressure on the equilibrium yield of the product in a gaseous
Chapter 7 Chemical Reactions: Energy, Rates, and Equilibrium
 Chapter 7 Chemical Reactions: Energy, Rates, and Equilibrium Introduction This chapter considers three factors: a) Thermodynamics (Energies of Reactions) a reaction will occur b) Kinetics (Rates of Reactions)
Chapter 7 Chemical Reactions: Energy, Rates, and Equilibrium Introduction This chapter considers three factors: a) Thermodynamics (Energies of Reactions) a reaction will occur b) Kinetics (Rates of Reactions)
7/19/2011. Models of Solution. State of Equilibrium. State of Equilibrium Chemical Reaction
 Models of Solution Chemistry- I State of Equilibrium A covered cup of coffee will not be colder than or warmer than the room temperature Heat is defined as a form of energy that flows from a high temperature
Models of Solution Chemistry- I State of Equilibrium A covered cup of coffee will not be colder than or warmer than the room temperature Heat is defined as a form of energy that flows from a high temperature
Slide 1 / Objects can possess energy as: (a) endothermic energy (b) potential energy (c) kinetic energy. a only b only c only a and c b and c
 Slide 1 / 84 1 Objects can possess energy as: (a) endothermic energy (b) potential energy (c) kinetic energy A B C D E a only b only c only a and c b and c Slide 2 / 84 2 The internal energy of a system
Slide 1 / 84 1 Objects can possess energy as: (a) endothermic energy (b) potential energy (c) kinetic energy A B C D E a only b only c only a and c b and c Slide 2 / 84 2 The internal energy of a system
The partial pressure of sulphur dioxide in the equilibrium mixture was 24 kpa and the total pressure in the flask was 104 kpa.
 Q1. Sulphur dioxide and oxygen were mixed in a 2:1 mol ratio and sealed in a flask with a catalyst. The following equilibrium was established at temperature T 1 2SO 2(g) + O 2(g) 2SO 3(g) ΔH = 196 kj mol
Q1. Sulphur dioxide and oxygen were mixed in a 2:1 mol ratio and sealed in a flask with a catalyst. The following equilibrium was established at temperature T 1 2SO 2(g) + O 2(g) 2SO 3(g) ΔH = 196 kj mol
Gummy Bear Demonstration:
 Name: Unit 8: Chemical Kinetics Date: Regents Chemistry Aim: _ Do Now: a) Using your glossary, define chemical kinetics: b) Sort the phrases on the SmartBoard into the two columns below. Endothermic Rxns
Name: Unit 8: Chemical Kinetics Date: Regents Chemistry Aim: _ Do Now: a) Using your glossary, define chemical kinetics: b) Sort the phrases on the SmartBoard into the two columns below. Endothermic Rxns
