Molecular Vibrations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology
|
|
|
- Stephanie Boone
- 6 years ago
- Views:
Transcription
1 Molecular Vibrations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology
2 Why Estimate Molecular Vibrations? Simulation of vibrational spectrum (identification of molecules) Vibrational corrections to enthalpy (Small) vibrational corrections to polarizability and other properties Understanding of vibrational motion could assist dynamics experiments and mode-selective chemistry
3 Small Vibrations in Classical Mechanics The classic reference is Wilson, Decius, and Cross, Molecular Vibrations (Dover, New York, 1980). Cheap book, makes a good reference. Let us focus on purely classical systems at first; all the results carry over to quantum mechanics. For small vibrations, the motion of atom α away from its equilibrium value may be described by x α, y α, z α, with kinetic energy T = 1 2 N M α α=1 ( d xα dt ) 2 + ( d yα dt ) 2 + ( d zα dt ) 2
4 If we switch to mass-weighted coordinates, such as q 1 = M1 x 1, q 2 = M 1 y 1, q 3 = M 1 z 1, q 4 = M 2 x 2, etc., then the kinetic energy operator becomes simpler since the mass factors are now absorbed 3N T = 1 2 i=1 V = V 0 + q 2 i 3N i=1 ( V q i ) 0q i N i=1 ( 2 V q i q j ) 0 q i q j + (1) Remember that at equilibrium, ( V/ q i ) 0 = 0; we can also set V 0 = 0. Also abbreviate ( 2 V/ q i q j ) 0 as just f ij.
5 Newton s Equations of Motion We can rewrite Newton s equations of motion as or d dt T q j + V q j = 0 j = 1,2,,3N q j + 3N i=1 f ij q i = 0 A possible solution to this equation is q i = a i cos ( λt+φ ) where the angular frequency is λ; this is just k/m in harmonic oscillator the m has been absorbed by the massweighted coordinate system used here!
6 Substitute the last expression into the differential equations to get 3N i=1 (f ij δ ij λ)a i = 0 j = 1,2,,3N or in matrix notation, just F a = λ a. This is an eigenvalue equation! We have a solution to this system of 3N linear equations only if λ has special values obtainable from the secular determinant f 11 λ f 12 f 13 f 1,3N f 21 f 22 λ f 23 f 2,3N... f 3N,1 f 3N,2 f 3N,3 f 3N,3N λ.. = 0
7 Normal Modes of Vibration The matrix eigenvalue equation is equivalent to matrix diagonalization which is equivalent to solving the secular determinant for each λ (N of them). Once we have the eigenvalues λ k we can get the corresponding eigenvectors a k, giving the motion of each atom for the given eigenvalue λ k : ) q ik = a ik cos ( λ k t+φ k. The eigenvectors a k are the normal modes of vibration. For each normal mode, all the atoms move with the same frequency and phase, but with different amplitudes.
8 Normal Coordinates We can define a new set of coordinates using the normal modes. This gives us the normal coordinates Q k = 3N i=1 a ik q i k = 1,2,,3N Since the eigenvectors of a real, symmetric matrix (F) are orthogonal, T and V become diagonal (no cross terms): 3N T = 1 2 k=1 3N Q 2 k V = 1 2 k=1 λ k Q 2 k The Hamiltonian is separable in this representation!
9
10 Polyatomic Molecules What happens for quantum mechanics, and for polyatomic molecules? Use Harmonic Oscillator model. 3N-6 frequencies (3N-5 for linear molecules); the rest are translations and rotations with zero frequency In normal mode coordinates, Hamiltonian is separable: wavefunction is a product and energy is a sum. Total vibrational energy is iω i h(v i +1/2) Minimum energy (due to uncertainty principle) is zero point vibrational energy (ZPVE or ZPE), where v i = 0 for all i. ZPVE = 2 h 1 iω i
11 How Would We Get Harmonic Frequencies for a Molecule? Easy just diagonalize the second derivative matrix F, called the Hessian. The frequencies ω i are the square roots of the eigenvalues, λ i. Where do we get F? Recall f ij = ( 2 V/ q i q j ) Potential energy V is just E e (B.O. approximation!): Need E 2 e/ q i q j. Compute second derivative of E e in terms of of Cartesian displacements (x α, y α, z α, call them q i ) and it s easy to transform to mass-weighted coordinates, using
12 F = M 1/2 F M 1/2. How do we get 2 E e / x α y β, etc? Need second derivative of electronic energy vs nuclear coordinates. Can compute analytically (using formula) or numerically from finite differences of energies or gradients: 2 [( ) ( ) ] E e Ee Ee / x x α y β y β α. xα =x α0 + x α y β xα =x α0 x α
13 Analytic Hessian Better than Numerical Analytic Hessian might cost 10-30x cost of energy; analytic gradient costs maybe 1.5-2x. Can need gradients from many displaced geometries up to 6N (+ and - for each of 3N coordinates) unless reduced by point group symmetry Numerical Hessian contains numerical errors (divide small number by small number) (Can land on wrong solution if displacement drops symmetry)
14 Availability of Analytic Derivatives Method Gradient Hessian HF, DFT Y Y CI Y N CCSD, CCSD(T) Y S MP2 Y S CASSCF Y S CIS Y Y EOM-CCSD S N TD-DFT S N S = available in some packages; Y = widely available
15 Approximate Average Errors in Harmonic Frequencies (Using polarized double and triple zeta basis sets) Method Error HF 11% CISD 4-6% CCSD 1-4% CCSD(T) 1-3% Anharmonicity accounts for another 2-3% difference from experimental fundamental frequencies. Many workers employ scaling factors for each level of theory to better predict fundamental frequencies.
16 100 Spectra of SiC2H2 isomers: DZP CCSD(T) Silapropadienylidene Silacyclopropyne Experiment Intensity Frequency (1/cm)
17 Scaling ZPVE s In an enlightening paper, Grev, Janssen, and Schaefer[J. Chem. Phys. 95, 5128 (1991)] showed that using scaled fundamental frequencies to estimate the ZPVE is not necessarily better than using unscaled frequencies. The reason is anharmonicity. If ZPVE s use scaling, they should have a different scaling factor than the individual frequencies. G(v) = ( ω r v r + 1 ) ( χ rs v r + 1 )( v s + 1 ) +, r r s harm = G(0) ZPVE harm = 1 4 fund = G(0) ZPVE fund = 3 4 r r χ rr χ rr 1 4 r>s r>s χ rs. χ rs.
18 Characterization of Stationary Points A stationary point is a geometry q for which the gradient E e ( q)/ q i for all coordinates q i : can be a (global or local) PES minimum, transition state, or higher order saddle point The Hessian Index is the number of negative force constants (corresponding to imaginary vibrational frequencies, often printed as negative frequencies) For a minimum, verify that there are no imaginary frequencies For a transition state, verify there is exactly one unique imaginary frequency
19 Printed by David Sherrill Dec 15, 00 14:54 h2o.freq.out Page 1/5 *************************************************************** Running Q Chem cicero.chemistry.gatech.edu on Fri Dec 15 14:51:29 EST Version: /usr/local/qchem /bin/qchem /usr/local/qchem /exe/progman.exe *************************************************************** Welcome to Q Chem A Quantum Leap Into The Future Of Chemistry J. Kong, C. A. White, A. I. Krylov, C. D. Sherrill, R. D. Adamson, T. R. Furlani, M. S. Lee, A. M. Lee, S. R. Gwaltney, T. R. Adams, H. Dachsel, W. M. Zhang, P. P. Korambath, C. Ochsenfeld, A. T. B. Gilbert, G. S. Kedziora, D. R. Maurice, N. Nair, Y. Shao, N. A. Besley, P. E. Maslen, J. P. Dombroski, J. Baker, E. F. C. Byrd, T. Van Voorhis, M. Oumi, S. Hirata, C. P. Hsu, N. Ishikawa, J. Florian, A. Warshel, B. G. Johnson, P. M. W. Gill, M. Head Gordon, J. A. Pople, Q Chem, Version 2.0, Q Chem, Inc., Export, PA (2000). Intel x86 Linux Version ====================================================================== User input: ====================================================================== $comment Water $end $molecule 0 1 O H1 O OH H2 O OH H1 HOH OH = HOH = $end $rem JOBTYPE FREQ EXCHANGE HF CORRELATION NONE BASIS STO 3G SCF_FINAL_PRINT 2 Energies plus MOS $end ====================================================================== Processing $rem in the input. # Entering fldman.exe on Fri Dec 15 14:51: # Standard Nuclear Orientation (Angstroms) I Atom X Y Z 1 O H H Molecular Point Group C2v NOp = 4 Largest Abelian Subgroup C2v NOp = 4 Nuclear Repulsion Energy = hartrees h2o.freq.out Dec 15, 00 14:54 Page 2/5 There are 5 alpha and 5 beta electrons Requested basis set is STO 3G There are 4 shells and 7 basis functions A cutoff of 1.0D 10 yielded 10 shell pairs There are 34 function pairs # Entering gesman.exe on Fri Dec 15 14:51: # Smallest overlap matrix eigenvalue = 3.63E 01 Multipole matrices computed through 2nd order Guess from superposition of atomic densities Warning: Energy on first SCF cycle will be non variational # Entering scfman.exe on Fri Dec 15 14:51: # A restricted Hartree Fock SCF calculation will be performed using Pulay DIIS extrapolation SCF converges when DIIS error is below 1.0E 08 Cycle Energy DIIS Error E E E E E E E E E E Convergence criterion met SCF time: CPU 0.08 s wall 0.00 s Final Alpha MO Eigenvalues Final Alpha MO Coefficients Final Alpha density matrix Monday April 23, 2001 h2o.freq.out 1/1
20 Printed by David Sherrill Dec 15, 00 14:54 Page 3/ # Entering anlman.exe on Fri Dec 15 14:51: # Analysis of SCF Wavefunction Orbital Energies (a.u.) and Symmetries Alpha MOs, Restricted Occupied A1 2 A1 1 B1 3 A1 1 B2 Virtual A1 2 B1 Beta MOs, Restricted Occupied A1 2 A1 1 B1 3 A1 1 B2 Virtual A1 2 B1 Mulliken Net Atomic Charges Atom Charge (a.u.) 1 O H H Sum of atomic charges = h2o.freq.out Cartesian Multipole Moments Charge (ESU x 10^10) Dipole Moment (Debye) X Y Z Tot Quadrupole Moments (Debye Ang) XX XY YY XZ YZ ZZ Octapole Moments (Debye Ang^2) XXX XXY XYY YYY XXZ XYZ YYZ XZZ YZZ ZZZ Hexadecapole Moments (Debye Ang^3) XXXX XXXY XXYY XYYY YYYY XXXZ XXYZ XYYZ YYYZ XXZZ XYZZ YYZZ XZZZ YZZZ ZZZZ Dec 15, 00 14:54 Page 4/5 # Entering drvman.exe on Fri Dec 15 14:51: # Calculating MO derivatives via CPHF Roots Converged Calculating analytic Hessian of the SCF energy Polarizability Matrix (a.u.) Direct stationary perturbation theory relativistic correction: rels = relv = rel2e = E_rel = h2o.freq.out Hessian of the SCF Energy Gradient time: CPU 0.59 s wall 1.00 s # Entering vibman.exe on Fri Dec 15 14:51: # ********************************************************************** ** ** ** VIBRATIONAL ANALYSIS ** ** ** ** ** ** VIBRATIONAL FREQUENCIES (CM** 1) AND NORMAL MODES ** ** INFRARED INTENSITIES (KM/MOL) ** ** ** ********************************************************************** Frequency: IR Active: YES YES YES IR Intens: Raman Active: YES YES YES X Y Z X Y Z X Y Z O H H STANDARD THERMODYNAMIC QUANTITIES AT K AND 1.00 ATM This Molecule has 0 Imaginary Frequencies Monday April 23, 2001 h2o.freq.out 1/1
Hong Zhou, Guang-Xiang Liu,* Xiao-Feng Wang and Yan Wang * Supporting Information
 Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
MO Calculation for a Diatomic Molecule. /4 0 ) i=1 j>i (1/r ij )
 MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
Simulated Quantum Computation of Molecular. Energies
 Simulated Quantum Computation of Molecular Energies Alán Aspuru-Guzik* a, Anthony D. Dutoi* a, Peter J. Love c and Martin Head-Gordon a,b a Department of Chemistry, University of California, Berkeley b
Simulated Quantum Computation of Molecular Energies Alán Aspuru-Guzik* a, Anthony D. Dutoi* a, Peter J. Love c and Martin Head-Gordon a,b a Department of Chemistry, University of California, Berkeley b
Chemistry 4681 Module: Electronic Structure of Small Molecules Background Handout
 Chemistry 4681 Module: Electronic Structure of Small Molecules Background Handout C. David Sherrill Last Revised: 6 January 2000 1 Computational Chemistry The term computational chemistry is used to mean
Chemistry 4681 Module: Electronic Structure of Small Molecules Background Handout C. David Sherrill Last Revised: 6 January 2000 1 Computational Chemistry The term computational chemistry is used to mean
Computer Laboratory DFT and Frequencies
 Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
An Introduction to Quantum Chemistry and Potential Energy Surfaces. Benjamin G. Levine
 An Introduction to Quantum Chemistry and Potential Energy Surfaces Benjamin G. Levine This Week s Lecture Potential energy surfaces What are they? What are they good for? How do we use them to solve chemical
An Introduction to Quantum Chemistry and Potential Energy Surfaces Benjamin G. Levine This Week s Lecture Potential energy surfaces What are they? What are they good for? How do we use them to solve chemical
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
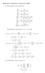 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Electric properties of molecules
 Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
The mechanism for singlet fission in pentacene and tetracene: from single exciton to two triplets
 The mechanism for singlet fission in pentacene and tetracene: from single exciton to two triplets Paul M. Zimmerman*, Franziska Bell, David Casanova, Martin Head-Gordon* Dr. P. M. Zimmerman,* Franziska
The mechanism for singlet fission in pentacene and tetracene: from single exciton to two triplets Paul M. Zimmerman*, Franziska Bell, David Casanova, Martin Head-Gordon* Dr. P. M. Zimmerman,* Franziska
Performance of Hartree-Fock and Correlated Methods
 Chemistry 460 Fall 2017 Dr. Jean M. Standard December 4, 2017 Performance of Hartree-Fock and Correlated Methods Hartree-Fock Methods Hartree-Fock methods generally yield optimized geomtries and molecular
Chemistry 460 Fall 2017 Dr. Jean M. Standard December 4, 2017 Performance of Hartree-Fock and Correlated Methods Hartree-Fock Methods Hartree-Fock methods generally yield optimized geomtries and molecular
Programming Project 2: Harmonic Vibrational Frequencies
 Programming Project 2: Harmonic Vibrational Frequencies Center for Computational Chemistry University of Georgia Athens, Georgia 30602 Summer 2012 1 Introduction This is the second programming project
Programming Project 2: Harmonic Vibrational Frequencies Center for Computational Chemistry University of Georgia Athens, Georgia 30602 Summer 2012 1 Introduction This is the second programming project
A BODIPY-luminol chemiluminescent resonance energy-transfer (CRET) cassette for imaging of cellular superoxide. Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 214 A BODIPY-luminol chemiluminescent resonance energy-transfer (CRET) cassette
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 214 A BODIPY-luminol chemiluminescent resonance energy-transfer (CRET) cassette
Chemistry 3502/4502. Final Exam Part I. May 14, 2005
 Advocacy chit Chemistry 350/450 Final Exam Part I May 4, 005. For which of the below systems is = where H is the Hamiltonian operator and T is the kinetic-energy operator? (a) The free particle
Advocacy chit Chemistry 350/450 Final Exam Part I May 4, 005. For which of the below systems is = where H is the Hamiltonian operator and T is the kinetic-energy operator? (a) The free particle
Chemistry 4560/5560 Molecular Modeling Fall 2014
 Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
Molecular Simulation I
 Molecular Simulation I Quantum Chemistry Classical Mechanics E = Ψ H Ψ ΨΨ U = E bond +E angle +E torsion +E non-bond Jeffry D. Madura Department of Chemistry & Biochemistry Center for Computational Sciences
Molecular Simulation I Quantum Chemistry Classical Mechanics E = Ψ H Ψ ΨΨ U = E bond +E angle +E torsion +E non-bond Jeffry D. Madura Department of Chemistry & Biochemistry Center for Computational Sciences
Gaussian: Basic Tutorial
 Input file: # hf sto-g pop=full Water - Single Point Energy 0 H.0 H.0 H 04.5 Route Section Start with # Contains the keywords Gaussian: Basic Tutorial Route Section Title Section Charge-Multiplicity Molecule
Input file: # hf sto-g pop=full Water - Single Point Energy 0 H.0 H.0 H 04.5 Route Section Start with # Contains the keywords Gaussian: Basic Tutorial Route Section Title Section Charge-Multiplicity Molecule
EXAM INFORMATION. Radial Distribution Function: B is the normalization constant. d dx. p 2 Operator: Heisenberg Uncertainty Principle:
 EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
Q-Chem, Inc., 5001 Baum Blvd., Suite 690, Pittsburgh, PA 15213, USA
 Chemical Physics Letters 1 (2) 9 95 www.elsevier.com/locate/cplett Interpolation density values on a cartesian grid: Improving the efficiency of Lebedev based numerical integration in Kohn Sham density
Chemical Physics Letters 1 (2) 9 95 www.elsevier.com/locate/cplett Interpolation density values on a cartesian grid: Improving the efficiency of Lebedev based numerical integration in Kohn Sham density
Supporting Information for Transition State Charge-Transfer Reveals Electrophilic, Ambiphilic, and Nucleophilic Carbon-Hydrogen Bond Activation
 Supporting Information for Transition State Charge-Transfer Reveals Electrophilic, Ambiphilic, and Nucleophilic Carbon-Hydrogen Bond Activation by Daniel H. Ess, Robert J. Nielsen, William A. Goddard,
Supporting Information for Transition State Charge-Transfer Reveals Electrophilic, Ambiphilic, and Nucleophilic Carbon-Hydrogen Bond Activation by Daniel H. Ess, Robert J. Nielsen, William A. Goddard,
Practical Advice for Quantum Chemistry Computations. C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology
 Practical Advice for Quantum Chemistry Computations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Choice of Basis Set STO-3G is too small 6-31G* or 6-31G** 6 probably
Practical Advice for Quantum Chemistry Computations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Choice of Basis Set STO-3G is too small 6-31G* or 6-31G** 6 probably
Quantum Chemical and Dynamical Tools for Solving Photochemical Problems
 2.165430 3.413060 3.889592 9 H 3.413060 2.165430 1.099610 2.165430 3.413060 10 H 3.889592 3.413060 2.165430 1.099610 2.165430 11 H 3.413060 3.889592 3.413060 2.165430 1.099610 12 H 2.165430 3.413060 3.889592
2.165430 3.413060 3.889592 9 H 3.413060 2.165430 1.099610 2.165430 3.413060 10 H 3.889592 3.413060 2.165430 1.099610 2.165430 11 H 3.413060 3.889592 3.413060 2.165430 1.099610 12 H 2.165430 3.413060 3.889592
Introduction to Hartree-Fock Molecular Orbital Theory
 Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Origins of Mathematical Modeling in Chemistry Plato (ca. 428-347
Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Origins of Mathematical Modeling in Chemistry Plato (ca. 428-347
Chemistry 3502/4502. Final Exam Part I. May 14, 2005
 Chemistry 3502/4502 Final Exam Part I May 14, 2005 1. For which of the below systems is = where H is the Hamiltonian operator and T is the kinetic-energy operator? (a) The free particle (e) The
Chemistry 3502/4502 Final Exam Part I May 14, 2005 1. For which of the below systems is = where H is the Hamiltonian operator and T is the kinetic-energy operator? (a) The free particle (e) The
Beyond the Hartree-Fock Approximation: Configuration Interaction
 Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Chemistry 416 Spectroscopy Fall Semester 1997 Dr. Rainer Glaser
 Chemistry 416 Spectroscopy Fall Semester 1997 Dr. Rainer Glaser Third 1-Hour Examination Vibrational Spectroscopy Monday, November 24, 1997, 8:40-9:30 Name: Answer Key Question 1 (Combination) 25 Question
Chemistry 416 Spectroscopy Fall Semester 1997 Dr. Rainer Glaser Third 1-Hour Examination Vibrational Spectroscopy Monday, November 24, 1997, 8:40-9:30 Name: Answer Key Question 1 (Combination) 25 Question
Chemistry 334 Part 2: Computational Quantum Chemistry
 Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Introduction to Electronic Structure Theory
 Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Chemistry 431. NC State University. Lecture 17. Vibrational Spectroscopy
 Chemistry 43 Lecture 7 Vibrational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule vibrates.
Chemistry 43 Lecture 7 Vibrational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule vibrates.
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi. Lecture 28, December 08, 2014
 Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
CHEM6085: Density Functional Theory Lecture 10
 CHEM6085: Density Functional Theory Lecture 10 1) Spin-polarised calculations 2) Geometry optimisation C.-K. Skylaris 1 Unpaired electrons So far we have developed Kohn-Sham DFT for the case of paired
CHEM6085: Density Functional Theory Lecture 10 1) Spin-polarised calculations 2) Geometry optimisation C.-K. Skylaris 1 Unpaired electrons So far we have developed Kohn-Sham DFT for the case of paired
Vibrational Analysis in Gaussian
 Vibrational Analysis in Gaussian Joseph W. Ochterski, Ph.D. help@gaussian.com October 9, 1999 Minor corrections 14 June 018 Abstract One of the most commonly asked questions about Gaussian is What is the
Vibrational Analysis in Gaussian Joseph W. Ochterski, Ph.D. help@gaussian.com October 9, 1999 Minor corrections 14 June 018 Abstract One of the most commonly asked questions about Gaussian is What is the
Introduction to Computational Chemistry
 Introduction to Computational Chemistry Vesa Hänninen Laboratory of Physical Chemistry Chemicum 4th floor vesa.hanninen@helsinki.fi September 10, 2013 Lecture 3. Electron correlation methods September
Introduction to Computational Chemistry Vesa Hänninen Laboratory of Physical Chemistry Chemicum 4th floor vesa.hanninen@helsinki.fi September 10, 2013 Lecture 3. Electron correlation methods September
Lecture 10 Diatomic Vibration Spectra Harmonic Model
 Chemistry II: Introduction to Molecular Spectroscopy Prof. Mangala Sunder Department of Chemistry and Biochemistry Indian Institute of Technology, Madras Lecture 10 Diatomic Vibration Spectra Harmonic
Chemistry II: Introduction to Molecular Spectroscopy Prof. Mangala Sunder Department of Chemistry and Biochemistry Indian Institute of Technology, Madras Lecture 10 Diatomic Vibration Spectra Harmonic
Adrian W. Lange and John M. Herbert Department of Chemistry, The Ohio State University, Columbus, OH February 3, 2009
 Supporting Information for: Both intra- and interstrand charge-transfer excited states in aqueous B-DNA are present at energies comparable to, or just above, the 1 ππ excitonic bright states Adrian W.
Supporting Information for: Both intra- and interstrand charge-transfer excited states in aqueous B-DNA are present at energies comparable to, or just above, the 1 ππ excitonic bright states Adrian W.
I. CSFs Are Used to Express the Full N-Electron Wavefunction
 Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
DFT and beyond: Hands-on Tutorial Workshop Tutorial 1: Basics of Electronic Structure Theory
 DFT and beyond: Hands-on Tutorial Workshop 2011 Tutorial 1: Basics of Electronic Structure Theory V. Atalla, O. T. Hofmann, S. V. Levchenko Theory Department, Fritz-Haber-Institut der MPG Berlin July 13,
DFT and beyond: Hands-on Tutorial Workshop 2011 Tutorial 1: Basics of Electronic Structure Theory V. Atalla, O. T. Hofmann, S. V. Levchenko Theory Department, Fritz-Haber-Institut der MPG Berlin July 13,
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry.
 1 Computational Chemistry (Quantum Chemistry) Primer This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry. TABLE OF CONTENTS Methods...1 Basis
1 Computational Chemistry (Quantum Chemistry) Primer This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry. TABLE OF CONTENTS Methods...1 Basis
AN INTRODUCTION TO QUANTUM CHEMISTRY. Mark S. Gordon Iowa State University
 AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
Jack Simons, Henry Eyring Scientist and Professor Chemistry Department University of Utah
 1. Born-Oppenheimer approx.- energy surfaces 2. Mean-field (Hartree-Fock) theory- orbitals 3. Pros and cons of HF- RHF, UHF 4. Beyond HF- why? 5. First, one usually does HF-how? 6. Basis sets and notations
1. Born-Oppenheimer approx.- energy surfaces 2. Mean-field (Hartree-Fock) theory- orbitals 3. Pros and cons of HF- RHF, UHF 4. Beyond HF- why? 5. First, one usually does HF-how? 6. Basis sets and notations
EJL R Sβ. sum. General objective: Reduce the complexity of the analysis by exploiting symmetry. Garth J. Simpson
 e sum = EJL R Sβ General objective: Reduce the complexity of the analysis by exploiting symmetry. Specific Objectives: 1. The molecular symmetry matrix S. How to populate it.. Relationships between the
e sum = EJL R Sβ General objective: Reduce the complexity of the analysis by exploiting symmetry. Specific Objectives: 1. The molecular symmetry matrix S. How to populate it.. Relationships between the
Coupled-Cluster Perturbative Triples for Bond Breaking
 Coupled-Cluster Perturbative Triples for Bond Breaking Andrew G. Taube and Rodney J. Bartlett Quantum Theory Project University of Florida INT CC Meeting Seattle July 8, 2008 Why does chemistry need triples?
Coupled-Cluster Perturbative Triples for Bond Breaking Andrew G. Taube and Rodney J. Bartlett Quantum Theory Project University of Florida INT CC Meeting Seattle July 8, 2008 Why does chemistry need triples?
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL
 CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r)
 Born Oppenheimer Approximation: Ĥ el ( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r) For a molecule with N electrons and M nuclei: Ĥ el What is E el (R)? s* potential surface Reaction Barrier Unstable intermediate
Born Oppenheimer Approximation: Ĥ el ( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r) For a molecule with N electrons and M nuclei: Ĥ el What is E el (R)? s* potential surface Reaction Barrier Unstable intermediate
NMR and IR spectra & vibrational analysis
 Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Example: H 2 O (the car file)
 Example: H 2 O (the car file) As a practical example of DFT methods we calculate the energy and electronic properties of the water molecule. In order to carry out the DFT calculation you will need a set
Example: H 2 O (the car file) As a practical example of DFT methods we calculate the energy and electronic properties of the water molecule. In order to carry out the DFT calculation you will need a set
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory
 Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
DFT calculations of NMR indirect spin spin coupling constants
 DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
Electron Correlation
 Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Advanced Spectroscopy. Dr. P. Hunt Rm 167 (Chemistry) web-site:
 Advanced Spectroscopy Dr. P. Hunt p.hunt@imperial.ac.uk Rm 167 (Chemistry) web-site: http://www.ch.ic.ac.uk/hunt Maths! Coordinate transformations rotations! example 18.1 p501 whole chapter on Matrices
Advanced Spectroscopy Dr. P. Hunt p.hunt@imperial.ac.uk Rm 167 (Chemistry) web-site: http://www.ch.ic.ac.uk/hunt Maths! Coordinate transformations rotations! example 18.1 p501 whole chapter on Matrices
IFM Chemistry Computational Chemistry 2010, 7.5 hp LAB2. Computer laboratory exercise 1 (LAB2): Quantum chemical calculations
 Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES
 SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
Phonons and lattice dynamics
 Chapter Phonons and lattice dynamics. Vibration modes of a cluster Consider a cluster or a molecule formed of an assembly of atoms bound due to a specific potential. First, the structure must be relaxed
Chapter Phonons and lattice dynamics. Vibration modes of a cluster Consider a cluster or a molecule formed of an assembly of atoms bound due to a specific potential. First, the structure must be relaxed
Molecular Symmetry 10/25/2018
 Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy). Predict IR spectra or Interpret UV-Vis spectra Predict optical activity
Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy). Predict IR spectra or Interpret UV-Vis spectra Predict optical activity
( ) R kj. = y k y j. y A ( ) z A. y a. z a. Derivatives of the second order electrostatic tensor with respect to the translation of ( ) δ yβ.
 Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Chem 442 Review for Exam 2. Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative (3D) components.
 Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
P. W. Atkins and R. S. Friedman. Molecular Quantum Mechanics THIRD EDITION
 P. W. Atkins and R. S. Friedman Molecular Quantum Mechanics THIRD EDITION Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1997 Introduction and orientation 1 Black-body radiation 1 Heat capacities 2 The
P. W. Atkins and R. S. Friedman Molecular Quantum Mechanics THIRD EDITION Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1997 Introduction and orientation 1 Black-body radiation 1 Heat capacities 2 The
QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C
 Chemistry 460 Fall 2017 Dr. Jean M. Standard November 6, 2017 QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C PART B: POTENTIAL CURVE, SPECTROSCOPIC CONSTANTS, AND DISSOCIATION ENERGY OF DIATOMIC HYDROGEN (20
Chemistry 460 Fall 2017 Dr. Jean M. Standard November 6, 2017 QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C PART B: POTENTIAL CURVE, SPECTROSCOPIC CONSTANTS, AND DISSOCIATION ENERGY OF DIATOMIC HYDROGEN (20
( ) + ( ) + ( ) = 0.00
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 32, April 14, 2006 (Some material in this lecture has been adapted from Cramer, C.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 32, April 14, 2006 (Some material in this lecture has been adapted from Cramer, C.
Rotations and vibrations of polyatomic molecules
 Rotations and vibrations of polyatomic molecules When the potential energy surface V( R 1, R 2,..., R N ) is known we can compute the energy levels of the molecule. These levels can be an effect of: Rotation
Rotations and vibrations of polyatomic molecules When the potential energy surface V( R 1, R 2,..., R N ) is known we can compute the energy levels of the molecule. These levels can be an effect of: Rotation
Before we start: Important setup of your Computer
 Before we start: Important setup of your Computer change directory: cd /afs/ictp/public/shared/smr2475./setup-config.sh logout login again 1 st Tutorial: The Basics of DFT Lydia Nemec and Oliver T. Hofmann
Before we start: Important setup of your Computer change directory: cd /afs/ictp/public/shared/smr2475./setup-config.sh logout login again 1 st Tutorial: The Basics of DFT Lydia Nemec and Oliver T. Hofmann
The effect of substituents on electronic states ordering in meta-xylylene diradicals: Qualitative insights from quantitative studies
 THE JOURNAL OF CHEMICAL PHYSICS 123, 104304 2005 The effect of substituents on electronic states ordering in meta-xylylene diradicals: Qualitative insights from quantitative studies Tao Wang and Anna I.
THE JOURNAL OF CHEMICAL PHYSICS 123, 104304 2005 The effect of substituents on electronic states ordering in meta-xylylene diradicals: Qualitative insights from quantitative studies Tao Wang and Anna I.
2 Electronic structure theory
 Electronic structure theory. Generalities.. Born-Oppenheimer approximation revisited In Sec..3 (lecture 3) the Born-Oppenheimer approximation was introduced (see also, for instance, [Tannor.]). We are
Electronic structure theory. Generalities.. Born-Oppenheimer approximation revisited In Sec..3 (lecture 3) the Born-Oppenheimer approximation was introduced (see also, for instance, [Tannor.]). We are
Lecture 9. Hartree Fock Method and Koopman s Theorem
 Lecture 9 Hartree Fock Method and Koopman s Theorem Ψ(N) is approximated as a single slater determinant Φ of N orthogonal One electron spin-orbitals. One electron orbital φ i = φ i (r) χ i (σ) χ i (σ)
Lecture 9 Hartree Fock Method and Koopman s Theorem Ψ(N) is approximated as a single slater determinant Φ of N orthogonal One electron spin-orbitals. One electron orbital φ i = φ i (r) χ i (σ) χ i (σ)
Born-Oppenheimer Approximation
 Born-Oppenheimer Approximation Adiabatic Assumption: Nuclei move so much more slowly than electron that the electrons that the electrons are assumed to be obtained if the nuclear kinetic energy is ignored,
Born-Oppenheimer Approximation Adiabatic Assumption: Nuclei move so much more slowly than electron that the electrons that the electrons are assumed to be obtained if the nuclear kinetic energy is ignored,
Example questions for Molecular modelling (Level 4) Dr. Adrian Mulholland
 Example questions for Molecular modelling (Level 4) Dr. Adrian Mulholland 1) Question. Two methods which are widely used for the optimization of molecular geometies are the Steepest descents and Newton-Raphson
Example questions for Molecular modelling (Level 4) Dr. Adrian Mulholland 1) Question. Two methods which are widely used for the optimization of molecular geometies are the Steepest descents and Newton-Raphson
Figure 1: Transition State, Saddle Point, Reaction Pathway
 Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Potential Energy Surfaces
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Potential Energy Surfaces
Chemistry 483 Lecture Topics Fall 2009
 Chemistry 483 Lecture Topics Fall 2009 Text PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon A. Background (M&S,Chapter 1) Blackbody Radiation Photoelectric effect DeBroglie Wavelength Atomic
Chemistry 483 Lecture Topics Fall 2009 Text PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon A. Background (M&S,Chapter 1) Blackbody Radiation Photoelectric effect DeBroglie Wavelength Atomic
A One-Slide Summary of Quantum Mechanics
 A One-Slide Summary of Quantum Mechanics Fundamental Postulate: O! = a! What is!?! is an oracle! operator wave function (scalar) observable Where does! come from?! is refined Variational Process H! = E!
A One-Slide Summary of Quantum Mechanics Fundamental Postulate: O! = a! What is!?! is an oracle! operator wave function (scalar) observable Where does! come from?! is refined Variational Process H! = E!
Chemistry 881 Lecture Topics Fall 2001
 Chemistry 881 Lecture Topics Fall 2001 Texts PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon MATHEMATICS for PHYSICAL CHEMISTRY, Mortimer i. Mathematics Review (M, Chapters 1,2,3 & 4; M&S,
Chemistry 881 Lecture Topics Fall 2001 Texts PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon MATHEMATICS for PHYSICAL CHEMISTRY, Mortimer i. Mathematics Review (M, Chapters 1,2,3 & 4; M&S,
Application of Computational Chemistry Methods to the Prediction of Electronic Structure in Nickel Dithiolene Complexes
 Application of Computational Chemistry Methods to the Prediction of Electronic Structure in Nickel Dithiolene Complexes Philip Chang Advisor: Kenneth L. Marshall Laboratory for Laser Energetics University
Application of Computational Chemistry Methods to the Prediction of Electronic Structure in Nickel Dithiolene Complexes Philip Chang Advisor: Kenneth L. Marshall Laboratory for Laser Energetics University
ARTICLES. Normal-mode analysis without the Hessian: A driven molecular-dynamics approach
 JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 2 8 JULY 2003 ARTICLES Normal-mode analysis without the Hessian: A driven molecular-dynamics approach Joel M. Bowman, a) Xiubin Zhang, and Alex Brown Cherry
JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 2 8 JULY 2003 ARTICLES Normal-mode analysis without the Hessian: A driven molecular-dynamics approach Joel M. Bowman, a) Xiubin Zhang, and Alex Brown Cherry
13, Applications of molecular symmetry and group theory
 Subject Paper No and Title Module No and Title Module Tag Chemistry 13, Applications of molecular symmetry and group theory 27, Group theory and vibrational spectroscopy: Part-IV(Selection rules for IR
Subject Paper No and Title Module No and Title Module Tag Chemistry 13, Applications of molecular symmetry and group theory 27, Group theory and vibrational spectroscopy: Part-IV(Selection rules for IR
Chem 442 Review of Spectroscopy
 Chem 44 Review of Spectroscopy General spectroscopy Wavelength (nm), frequency (s -1 ), wavenumber (cm -1 ) Frequency (s -1 ): n= c l Wavenumbers (cm -1 ): n =1 l Chart of photon energies and spectroscopies
Chem 44 Review of Spectroscopy General spectroscopy Wavelength (nm), frequency (s -1 ), wavenumber (cm -1 ) Frequency (s -1 ): n= c l Wavenumbers (cm -1 ): n =1 l Chart of photon energies and spectroscopies
Demonstration of the Coupled Evolution Rules 163 APPENDIX F: DEMONSTRATION OF THE COUPLED EVOLUTION RULES
 Demonstration of the Coupled Evolution Rules 163 APPENDIX F: DEMONSTRATION OF THE COUPLED EVOLUTION RULES Before going into the demonstration we need to point out two limitations: a. It assumes I=1/2 for
Demonstration of the Coupled Evolution Rules 163 APPENDIX F: DEMONSTRATION OF THE COUPLED EVOLUTION RULES Before going into the demonstration we need to point out two limitations: a. It assumes I=1/2 for
NWChem: Coupled Cluster Method (Tensor Contraction Engine)
 NWChem: Coupled Cluster Method (Tensor Contraction Engine) Why CC is important?! Correlation effects are important!! CC is size-extensive theory: can be used to describe dissociation processes.! Higher-order
NWChem: Coupled Cluster Method (Tensor Contraction Engine) Why CC is important?! Correlation effects are important!! CC is size-extensive theory: can be used to describe dissociation processes.! Higher-order
A contribution to the understanding of the structure of xenon hexafluoride
 A contribution to the understanding of the structure of xenon hexafluoride T. Daniel Crawford, a) Kristen W. Springer, b) and Henry F. Schaefer III Center for Computational Quantum Chemistry, University
A contribution to the understanding of the structure of xenon hexafluoride T. Daniel Crawford, a) Kristen W. Springer, b) and Henry F. Schaefer III Center for Computational Quantum Chemistry, University
Session 7 Overview: Part A I. Prediction of Vibrational Frequencies (IR) Part B III. Prediction of Electronic Transitions (UV-Vis) IV.
 Session 7 Overview: Part A I. Prediction of Vibrational Frequencies (IR) II. Thermochemistry Part B III. Prediction of Electronic Transitions (UV-Vis) IV. NMR Predictions 1 I. Prediction of Vibrational
Session 7 Overview: Part A I. Prediction of Vibrational Frequencies (IR) II. Thermochemistry Part B III. Prediction of Electronic Transitions (UV-Vis) IV. NMR Predictions 1 I. Prediction of Vibrational
QUANTUM CHEMISTRY FOR TRANSITION METALS
 QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
Q-Chem 4.0: Expanding the Frontiers. Jing Kong Q-Chem Inc. Pittsburgh, PA
 Q-Chem 4.0: Expanding the Frontiers Jing Kong Q-Chem Inc. Pittsburgh, PA Q-Chem: Profile Q-Chem is a high performance quantum chemistry program; Contributed by best quantum chemists from 40 universities
Q-Chem 4.0: Expanding the Frontiers Jing Kong Q-Chem Inc. Pittsburgh, PA Q-Chem: Profile Q-Chem is a high performance quantum chemistry program; Contributed by best quantum chemists from 40 universities
If the symmetry axes of a uniform symmetric body coincide with the coordinate axes, the products of inertia (Ixy etc.
 Prof. O. B. Wright, Autumn 007 Mechanics Lecture 9 More on rigid bodies, coupled vibrations Principal axes of the inertia tensor If the symmetry axes of a uniform symmetric body coincide with the coordinate
Prof. O. B. Wright, Autumn 007 Mechanics Lecture 9 More on rigid bodies, coupled vibrations Principal axes of the inertia tensor If the symmetry axes of a uniform symmetric body coincide with the coordinate
THE VIBRATIONAL SPECTRUM OF A POLYATOMIC MOLECULE (Revised 4/7/2004)
 INTRODUCTION THE VIBRATIONAL SPECTRUM OF A POLYATOMIC MOLECULE (Revised 4/7/2004) The vibrational motion of a molecule is quantized and the resulting energy level spacings give rise to transitions in the
INTRODUCTION THE VIBRATIONAL SPECTRUM OF A POLYATOMIC MOLECULE (Revised 4/7/2004) The vibrational motion of a molecule is quantized and the resulting energy level spacings give rise to transitions in the
5.61 Physical Chemistry Final Exam 12/16/09. MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry Physical Chemistry
 5.6 Physical Chemistry Final Exam 2/6/09 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry - 5.6 Physical Chemistry Final Examination () PRINT your name on the cover page. (2) It
5.6 Physical Chemistry Final Exam 2/6/09 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry - 5.6 Physical Chemistry Final Examination () PRINT your name on the cover page. (2) It
Basis Set for Molecular Orbital Theory
 Basis Set for Molecular Orbital Theory! Different Types of Basis Functions! Different Types of Atom Center Basis Functions! Classifications of Gaussian Basis Sets! Pseudopotentials! Molecular Properties
Basis Set for Molecular Orbital Theory! Different Types of Basis Functions! Different Types of Atom Center Basis Functions! Classifications of Gaussian Basis Sets! Pseudopotentials! Molecular Properties
MOLECULAR SPECTROSCOPY
 MOLECULAR SPECTROSCOPY First Edition Jeanne L. McHale University of Idaho PRENTICE HALL, Upper Saddle River, New Jersey 07458 CONTENTS PREFACE xiii 1 INTRODUCTION AND REVIEW 1 1.1 Historical Perspective
MOLECULAR SPECTROSCOPY First Edition Jeanne L. McHale University of Idaho PRENTICE HALL, Upper Saddle River, New Jersey 07458 CONTENTS PREFACE xiii 1 INTRODUCTION AND REVIEW 1 1.1 Historical Perspective
Lecture 5: More about one- Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory.
 Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Electron Correlation - Methods beyond Hartree-Fock
 Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
The Overhauser Instability
 The Overhauser Instability Zoltán Radnai and Richard Needs TCM Group ESDG Talk 14th February 2007 Typeset by FoilTEX Introduction Hartree-Fock theory and Homogeneous Electron Gas Noncollinear spins and
The Overhauser Instability Zoltán Radnai and Richard Needs TCM Group ESDG Talk 14th February 2007 Typeset by FoilTEX Introduction Hartree-Fock theory and Homogeneous Electron Gas Noncollinear spins and
Appendix D Simulating Spectroscopic Bands Using Gaussian and PGopher
 429 Appendix D Simulating Spectroscopic Bands Using Gaussian and PGopher This appendix contains methods for using Gaussian 09 121 and PGopher 120 to simulate vibrational and electronic bands of molecules.
429 Appendix D Simulating Spectroscopic Bands Using Gaussian and PGopher This appendix contains methods for using Gaussian 09 121 and PGopher 120 to simulate vibrational and electronic bands of molecules.
Principles of Molecular Spectroscopy
 Principles of Molecular Spectroscopy What variables do we need to characterize a molecule? Nuclear and electronic configurations: What is the structure of the molecule? What are the bond lengths? How strong
Principles of Molecular Spectroscopy What variables do we need to characterize a molecule? Nuclear and electronic configurations: What is the structure of the molecule? What are the bond lengths? How strong
diradicals, and triradicals
 The spin-flip electronic structure method: A shortcut in the Fock space towards accurate description of bond-breaking, diradicals, and triradicals Anna I. Krylov February 18, 2005 Abstract The spin-flip
The spin-flip electronic structure method: A shortcut in the Fock space towards accurate description of bond-breaking, diradicals, and triradicals Anna I. Krylov February 18, 2005 Abstract The spin-flip
QUANTUM CHEMISTRY WITH GAUSSIAN : A VERY BRIEF INTRODUCTION (PART 2)
 QUANTUM CHEMISTRY WITH GAUSSIAN : A VERY BRIEF INTRODUCTION (PART 2) TARAS V. POGORELOV AND MIKE HALLOCK SCHOOL OF CHEMICAL SCIENCES, UIUC This tutorial continues introduction to Gaussian [2]. Here we
QUANTUM CHEMISTRY WITH GAUSSIAN : A VERY BRIEF INTRODUCTION (PART 2) TARAS V. POGORELOV AND MIKE HALLOCK SCHOOL OF CHEMICAL SCIENCES, UIUC This tutorial continues introduction to Gaussian [2]. Here we
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.76 Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.76 Lecture
THE VIBRATIONAL SPECTRA OF A POLYATOMIC MOLECULE (Revised 3/27/2006)
 THE VIBRATIONAL SPECTRA OF A POLYATOMIC MOLECULE (Revised 3/27/2006) 1) INTRODUCTION The vibrational motion of a molecule is quantized and the resulting energy level spacings give rise to transitions in
THE VIBRATIONAL SPECTRA OF A POLYATOMIC MOLECULE (Revised 3/27/2006) 1) INTRODUCTION The vibrational motion of a molecule is quantized and the resulting energy level spacings give rise to transitions in
CHEM3023: Spins, Atoms and Molecules
 CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
Computational Spectroscopy III. Spectroscopic Hamiltonians
 Computational Spectroscopy III. Spectroscopic Hamiltonians (e) Elementary operators for the harmonic oscillator (f) Elementary operators for the asymmetric rotor (g) Implementation of complex Hamiltonians
Computational Spectroscopy III. Spectroscopic Hamiltonians (e) Elementary operators for the harmonic oscillator (f) Elementary operators for the asymmetric rotor (g) Implementation of complex Hamiltonians
Magic. The Lattice Boltzmann Method
 Magic The Lattice Boltzmann Method Lattice Boltzmann: secret ingredients Kn(Ma t f + v x x f + v y y f + v z z f ) = Ω( f ) Analysis Modeling Kn(Ma t π α + x π αx + y π αy + z π αz ) = ω α ( α π α ) ω
Magic The Lattice Boltzmann Method Lattice Boltzmann: secret ingredients Kn(Ma t f + v x x f + v y y f + v z z f ) = Ω( f ) Analysis Modeling Kn(Ma t π α + x π αx + y π αy + z π αz ) = ω α ( α π α ) ω
Electron Correlation Methods
 Electron Correlation Methods HF method: electron-electron interaction is replaced by an average interaction E HF c = E 0 E HF E 0 exact ground state energy E HF HF energy for a given basis set HF E c
Electron Correlation Methods HF method: electron-electron interaction is replaced by an average interaction E HF c = E 0 E HF E 0 exact ground state energy E HF HF energy for a given basis set HF E c
Population Analysis. Mulliken Population Analysis APPENDIX S
 APPENDIX S Population Analysis On p. 665, electronic density ρ is defined. If the wave function is a Slater determinant p. 397) and assuming the double occupancy of orbitals ϕ i, we have see 11.7) ρ r
APPENDIX S Population Analysis On p. 665, electronic density ρ is defined. If the wave function is a Slater determinant p. 397) and assuming the double occupancy of orbitals ϕ i, we have see 11.7) ρ r
where, c is the speed of light, ν is the frequency in wave numbers (cm -1 ) and µ is the reduced mass (in amu) of A and B given by the equation: ma
 Vibrational Spectroscopy A rough definition of spectroscopy is the study of the interaction of matter with energy (radiation in the electromagnetic spectrum). A molecular vibration is a periodic distortion
Vibrational Spectroscopy A rough definition of spectroscopy is the study of the interaction of matter with energy (radiation in the electromagnetic spectrum). A molecular vibration is a periodic distortion
