A CONVENIENT NUCLEUS PARAMETER FOR CONSIDERATIONS OF DROPLET GROWTH
|
|
|
- Theresa Hodge
- 6 years ago
- Views:
Transcription
1 A CONVENIENT NUCLEUS PARAMETER FOR CONSIDERATIONS OF DROPLET GROWTH by E. X BERRY Desert Research Institute, Reno, Nevada, U.S.A. RESUME La quantite r 0 est definie comme le rayon d'une gouttelette quand elle est en equilibre avec son entourage a une humiditl de 100 % On montre ici que r 0 est un parametre fondamental des noyaux et que r 0 permet une reprhentation de la croissance dts gouttelettes servant d'exemple. Cette representation e8t utile a de8 fins pedagogiques. ABSTRACT The quantity r 0 is defined as the radius of a droplet when it is in equilibrium with its environment at 100 % humidity. It is shown here that r 0 is a fundamental nucleus parameter and that r 0 allows an illustrative repre8entation of droplet growth. This representation is useful for pedagogical purposes. INTRODU CTION. In dealing with the growth of cloud droplets by condensation it is illustrative to formulate the growth equation in terms of the equilibrium radius r 0 of the droplet at 100 % humidity. This can easily be done with the equations as used by Howell [l], Mordy [2], and several textbooks, where the assumption is made that a spherical water surface has already formed about the condensation nucleus. When the droplet radius is large enough the adjustment of the droplet's vapor pressure by surface tension and dissolved foreign molecules may be simply approximated. This approximation leads directly to the use of r 0, which results in a picturesque illustration of the growth of a distribution of droplets on a distribution of condensation nuclei. Following in a general way the derivation of the droplet growth equation by Mordy [2], we define Surface tension term, A = 2cr / prt =i= 10-3 (µ.), Raoult's law term, B and U = A /r - B /r 3, (I) (2) (3) and write the applicable equations for droplet condensation, vapor diffusion: rr = (D /prtj (e.n - e,.), droplet vapor pressure: e,. = e r (1 + U), (4) (5)
2 412 JOURNAL DE RECHERCHES ATMOSPHERIQUES Clausius-Clapeyron : et = es - (Les /T 2,,,R) (T,, - T), heat diffusion : (T,, - T) = (1 /47trk) q, 1966 (6) (7) where heat continuity: (1 /47tr) q = (pc /3) r2t - a p R J\'I r D T "' T e "' e,. e... es L k Lprr, surface tension water density gas constant van't Hoff factor mass of nucleus in gm. radius of drop in microns vapor diffusion coefficient temperature of the environment temperature of the drop vapor pressure of the environment vapor pressure near droplet surface saturation vapor pressure at temperature T saturation vapor pressure at temperature T % latent heat of condensation heat diffusion coefficient c specific heat of water q heat content of droplet The dot above a quantity indicates its time derivative. Using (4), (5) and (6) to eliminate e,. and en and (7) and (8) to eliminate q, we have rr = (Des/pRT "" ) {e,,. /es - (1 + U] [l -- (I./RT2) (T ~ - T)] }. (9) r2t + (3k/pc) (T,, - T) + (3L /c) rf. (10) From (10) we find that the time response -r of the temperature (T) of the droplet, to changes in the temperature (T,,) of its environment is "T = pcr2 /3k ~ l0-4 r2 (sec), (11) assuming f = 0. Reasoning from this we conclude that the term (L /RT 2 } (T,,. - T) ~ 20 (T,,. - T) /Tin (9) has an absolute value much less than 1 under all regular conditions in cloud and laboratory. Also the terms in U are taken to be much smaller than 1 since they result originally from an expansion requiring just this. Therefore (9) may be rewritten to give (8) where rf D' [E - U + (L /RT2) (T,, - T)J, supersaturation, (12) (13) D' := Des/pRT (µ2 /sec) (14)
3 E.X BERRY 413 The supersaturation of the environment is denoted by E. It is independent of the parameters of the droplet but is affected as nearby droplets grow. The term U is a function only of the droplet parameters and is independent of the environment. The (T~ - T) term in {12) is eliminated by (10). We neglect the T term for our present purposes and re-solve for r?' to get r?' = H (E - U), (15) where H is a combination of parameters that change slowly and is of the order of 10 2 (µ 2 /sec.). The rf term which appeared. on the right before re-solving to get (15) accounts for the negative feedback upon droplet growth due to the raising of the droplet's vapor pressure by the heat of condensation. This feedback term is now included in H. Rooth (3] reasoned that 1' cannot increase without limit as r is decreased and suggested a correction to the diffusion coefficient. When this is accounted for the r on the left of (15) is replaced by (r + l'), where l' is approximately 2[1.. Thus we have (r + l') 1' = H (E - U). (16) The use of ro. If we re-write (3) in the form U := (A /r) (1 - r 0 2 /r2) (17) (18) we notice that the r 0 so defined is just the equilibrium radius for E 0 and includes all of the parameters of the condensation nucleus used in the preceding, common account of condensation theory. For the viewpoint of condensation theory, the value of r 0 is more convenient and representative than the various properties of the nucleus itself. while r 0 has here evolved from a form of condensation theory designed to treat salt nuclei, its usefulness is not so restricted. The fundamental consideration in B (equation 2, above), is how the nucleus lowers the droplet's vapor pressure by replacing some of the water molecules at the droplet's surface. The term U is not really valid down to r = 0 since it is made up of the leading terms of the expansions of the Raoult's law and the surface curvature effects. Each of the terms of U must therefore be small compared to 1. If we set the maximum value of each of the terms in equation (3) equal to 1 /3, then the minimum radius at which U is valid is given by the larger of l (3B) 3 BA = 3 x I0-3. (19) (20) For all but the smallest salt nuclei (19) prevails, and in all but very dry atmospheric conditions the above criteria are satisfied so that the term U in the form of equation (3) is valid.
4 414 JOURNAL DE RECHERCHES ATMOSPHERIQUES 1966 An Illustrative Representation of Droplet Growth. Since the value of r 0 (along with rrnin) contains all necessary information about a nucleus once condensation has begun, it seems natural to plot the function ro (r) and watch its behavior during condensation. Solving (17) for r 0 we have ro = r y 1 - U' r, U' = u I A ~ J03U (21) (22) 10. / ~r= r. v'3 IS THE LOCUS OF MAXIMUM r 0 FOR.U>O Fw The iso-u lines; which are also iso-e lines of the same value for E ~ - 1. In Figure 1 are sketched the iso-u lines, with r 0 and r plotted on logarithmic coordinates since several orders of magnitude need to be encompassed. This plot has several conveniences. U = 0 is just the line r = r 0 As U increases above zero its curve keeps the same shape while it slides down the line U = 0. The maximum ro for U > 0 lies on the line r = r 0 y 3. The value of U j A for a given curve is just the recipricol of the r approached by the right leg of U for small r 0 when U < 0 its curve lies above lj = 0 and its change of position occurs only slowly with change in U. The curves of U are also the curves of E, so that for a given E, U = E is an equilibrium curve. Equilibrium is stable when r < r 0 y 3 and unstable when r ~ roy3. Supersaturation is undefined for values less than - 1, corresponding to perfectly dry air, and therefore we must have E ~ - 1. It is apparent then that U may be much less than E even in dry air, which suggests a strong «potential» for droplet growth up to U = - 1. It must be remembered, however, that the formulation used here ignores the important aspect of the initial water condensation on the nucleus and therefore tells nothing about the initiation of condensation. The formulation applies only after a complete water surface has formed and sufficient water has condensed to make the solution at the droplets surface reasonably dilute. The changes in the van't Hoff factor [ 4] as the solution goes from concentrated to dilute could be accounted for by lines on this graph showing how r 0 changes with r. Here we have assumed that r 0 is a constant for a given nucleus.
5 E.X BERRY EXPANSION OF FOR 1.0 r. EFFECT OF NUCLEUS IS NEGLIGIBLE 0.1 FOR r > r.:; FrG The regions where surface tension or nucleus effect become insignificant are shown along with the area where the dilute solution approximation becomes incorrect. Figure 2 outlines the significant regions of the r, r 0 -plane. The region excluded by (19) is shown in the upper left corner while the region excluded by (20) is far to the left of the range of r shown here. Notice that the term r 0 2 /r 2 in (17) gives the ratio of the nucleus effect to the surface tension effect. By putting this term either greater than or less than 9 we can define the regions where one effect or the other becomes negligible. These areas are also sketched in Figure 2. Another requirement on r is that it be no smaller than the average radius of the nucleus itself. For salt, r 0 is large, and thus the r,,,; 11 of (19) will always be much larger than the nucleus. However, once a water surface has formed around an insoluble nucleus, B = 0 and therefore r 0 = 0. Then (20) will haye no effect and rmin will be determined by the size of the nucleus. The quantity r 0, even though less than r..,i,., will still govern the subsequent growth of the droplet by condensation. A large insoluble nucleus will be represented by a point at low r 0 and large rmin We see from Figure 2 that the nucleus effect would be negligible in condensation considerations, but also we see from Figure 1 that the rmin is very important. For if E were raised enough for its curve to go below the point (rmin r 0 ) for this nucleus, the droplet on it may begin to grow. Thus the fact that a large r ; may initiate droplet growth is shown clearly on this diagram. A population of nuclei is represented by some curve r 0 (r). Suppose that the t = 0 line in Figure 3 gives the initial size of the droplets about each nucleus. The line ro (r) is really a locus of points, each point representing a droplet and its nucleus. The projection of these points on either the r 0 or r axes gives a density of points which is proportional to the density functions f 0 (r 0 ) and f (r) of the nuclei and the droplets respectively.
6 416 JOURNAL DE RECHERCHES ATMOSPHERIQUES r. 0.1 ' \ \ \ I I I I I,22,16,10 - I I I C ~~~~~---'t--... ~~~-t~~~~~~-+-~~~~~~+-~~~~~~ r FIG A plot of r 0 (r) for the droplet growth by condensation as computed by Mordy (2] for his distribution I at 1 m /sec vertical velocity. Figure 3 illustrates the growth of droplets at a cloud base as computed by Mordy [2]. The results shown in his Figure 12 for 1 m /sec vertical velocity are here plotted on the r, r 0 -plane. Curves of r 0 (r) and E are labeled with the elapsed time in seconds. The fact that as E is increased the droplets with lower r 0 are freed to grow is clearly shown. Those droplets with r > r 0 Va have grown beyond the crest of their U and continued growth brings them into a region of lower U. They will continue to grow until E is decreased enough for its curve to lie above the point (r, r 0 ). SUMMARY. The equilibrium radius r 0 is a convenient parameter for the classification of condensation nuclei. This information must be supplemented by the nucleus radius itself when this has a greater value than r 0 The plot of r 0 (r) shows clearly the characteristics of condensation growth, including the regions where surface tension or nuclei effects predominate. The use of f 0 (r 0 ) is useful to the cloud physicist in the study and teaching of condensation and evaporation as it occurs in both the real cloud and the laboratory. REFERENCES (1] HowELI. W.E. (1949). - The growth of cloud drops in uniformly cooled air. J. of Met., 6, pp (2] l\fordy W.A. (1959). - Computations of the growth by condensation of a population of cloud droplets. Tellus, 11, pp (3] ROOTH C. (1957). - On a special aspect of the condensation process and its importance in the treatment of cloud particle growth. Tellus, 9, pp [4] Mc DONALD J.E. (1953). - Erroneous cloud physics applications of Raoult's law. J. of Met., IO, pp
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Generating cloud drops from CCN. Wallace & Hobbs (1977)
 Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Diffusional Growth of Liquid Phase Hydrometeros.
 Diffusional Growth of Liquid Phase Hydrometeros. I. Diffusional Growth of Liquid Phase Hydrometeors A. Basic concepts of diffusional growth. 1. To understand the diffusional growth of a droplet, we must
Diffusional Growth of Liquid Phase Hydrometeros. I. Diffusional Growth of Liquid Phase Hydrometeors A. Basic concepts of diffusional growth. 1. To understand the diffusional growth of a droplet, we must
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
Exam 2: Cloud Physics April 16, 2008 Physical Meteorology Questions 1-10 are worth 5 points each. Questions are worth 10 points each.
 Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Convective Heat and Mass Transfer Prof. A.W. Date Department of Mechanical Engineering Indian Institute of Technology, Bombay
 Convective Heat and Mass Transfer Prof. A.W. Date Department of Mechanical Engineering Indian Institute of Technology, Bombay Module No. # 01 Lecture No. # 32 Stefan Flow Model We are now familiar with
Convective Heat and Mass Transfer Prof. A.W. Date Department of Mechanical Engineering Indian Institute of Technology, Bombay Module No. # 01 Lecture No. # 32 Stefan Flow Model We are now familiar with
Surface Tension Kelvin Effect
 Lecture Ch. 5a Surface tension (Kelvin effect) Hygroscopic growth (subsaturated humidity) Saturation Chemical potential (Raoult effect) (partly from 4.5.1) Curry and Webster, Ch. 5 (skip 5.6, 5.7); also
Lecture Ch. 5a Surface tension (Kelvin effect) Hygroscopic growth (subsaturated humidity) Saturation Chemical potential (Raoult effect) (partly from 4.5.1) Curry and Webster, Ch. 5 (skip 5.6, 5.7); also
Precipitation Processes METR σ is the surface tension, ρ l is the water density, R v is the Gas constant for water vapor, T is the air
 Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Lecture 7: quick review
 Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
Sec Water vapour variables each has its own usefulness 2/11 The ideal gas law inter-relates vapour pressure (e) & absolute humidity ( ρv) 1 e
 Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
Simplified Microphysics. condensation evaporation. evaporation
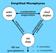 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Chapter 9 Generation of (Nano)Particles by Growth
 Chapter 9 Generation of (Nano)Particles by Growth 9.1 Nucleation (1) Supersaturation Thermodynamics assumes a phase change takes place when there reaches Saturation of vapor in a gas, Saturation of solute
Chapter 9 Generation of (Nano)Particles by Growth 9.1 Nucleation (1) Supersaturation Thermodynamics assumes a phase change takes place when there reaches Saturation of vapor in a gas, Saturation of solute
Melting of ice particles:
 Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
Chapter 5. Atmospheric Moisture
 Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
Chapter 17. Fundamentals of Atmospheric Modeling
 Overhead Slides for Chapter 17 of Fundamentals of Atmospheric Modeling by Mark Z. Jacobson Department of Civil & Environmental Engineering Stanford University Stanford, CA 94305-4020 August 21, 1998 Mass
Overhead Slides for Chapter 17 of Fundamentals of Atmospheric Modeling by Mark Z. Jacobson Department of Civil & Environmental Engineering Stanford University Stanford, CA 94305-4020 August 21, 1998 Mass
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
9 Condensation. Learning Goals. After studying this chapter, students should be able to:
 9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.
![P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C. P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.](/thumbs/79/79184286.jpg) Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Chemical Potential. Combining the First and Second Laws for a closed system, Considering (extensive properties)
 Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM
 Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM 7.1 INTRODUCTION The intensive thermodynamic properties of a system are temperature, pressure, and the chemical potentials of the various species occurring
Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM 7.1 INTRODUCTION The intensive thermodynamic properties of a system are temperature, pressure, and the chemical potentials of the various species occurring
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
12.009/ Problem Set 2
 12.009/18.352 Problem Set 2 Due Thursday, 26 February 2015 100 points total Problem 1: 15 pts (a,b)=(10,5) Problem 2: 45 pts (a,b,c,d,e,f)=(5,5,5,10,10,10) Problem 3: 40 pts (a,b,c,d,e,f)=(5,5,5,5,10,10)
12.009/18.352 Problem Set 2 Due Thursday, 26 February 2015 100 points total Problem 1: 15 pts (a,b)=(10,5) Problem 2: 45 pts (a,b,c,d,e,f)=(5,5,5,10,10,10) Problem 3: 40 pts (a,b,c,d,e,f)=(5,5,5,5,10,10)
Aircraft Icing Icing Physics
 Aircraft Icing Icing Physics Prof. Dr. Dept. Aerospace Engineering, METU Fall 2015 Outline Formation of ice in the atmosphere Supercooled water droplets Mechanism of aircraft icing Icing variations Ice
Aircraft Icing Icing Physics Prof. Dr. Dept. Aerospace Engineering, METU Fall 2015 Outline Formation of ice in the atmosphere Supercooled water droplets Mechanism of aircraft icing Icing variations Ice
Solutions to questions from chapter 8 in GEF Cloud Physics
 Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
EARTH SCIENCE. Prentice Hall Water in the Atmosphere Water in the Atmosphere Water in the Atmosphere.
 Prentice Hall EARTH SCIENCE Tarbuck Lutgens Water s Changes of State 1. Precipitation is any form of water that falls from a cloud. a. Examples: Snow, rain, hail, sleet 3 States of matter of water: 1.
Prentice Hall EARTH SCIENCE Tarbuck Lutgens Water s Changes of State 1. Precipitation is any form of water that falls from a cloud. a. Examples: Snow, rain, hail, sleet 3 States of matter of water: 1.
Chapter 5 - Atmospheric Moisture
 Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Geol. 656 Isotope Geochemistry
 STABLE ISOTOPE THEORY: KINETIC FRACTIONATION AND THE HYDROLOGIC SYSTEM KINETIC FRACTIONATION Kinetic effects are normally associated with fast, incomplete, or unidirectional processes like evaporation,
STABLE ISOTOPE THEORY: KINETIC FRACTIONATION AND THE HYDROLOGIC SYSTEM KINETIC FRACTIONATION Kinetic effects are normally associated with fast, incomplete, or unidirectional processes like evaporation,
Lecture 20. Phase Transitions. Phase diagrams. Latent heats. Phase-transition fun. Reading for this Lecture: Elements Ch 13.
 Lecture 20 Phase ransitions Phase diagrams Latent heats Phase-transition fun Reading for this Lecture: Elements Ch 13 Lecture 20, p 1 Solid-gas equilibrium: vapor pressure Consider solid-gas equilibrium
Lecture 20 Phase ransitions Phase diagrams Latent heats Phase-transition fun Reading for this Lecture: Elements Ch 13 Lecture 20, p 1 Solid-gas equilibrium: vapor pressure Consider solid-gas equilibrium
Parametrizing cloud and precipitation in today s NWP and climate models. Richard Forbes
 Parametrizing cloud and precipitation in today s NWP and climate models Richard Forbes (ECMWF) with thanks to Peter Bechtold and Martin Köhler RMetS National Meeting on Clouds and Precipitation, 16 Nov
Parametrizing cloud and precipitation in today s NWP and climate models Richard Forbes (ECMWF) with thanks to Peter Bechtold and Martin Köhler RMetS National Meeting on Clouds and Precipitation, 16 Nov
Mid High Latitude Cirrus Precipitation Processes. Jon Sauer, Dan Crocker, Yanice Benitez
 Mid High Latitude Cirrus Precipitation Processes Jon Sauer, Dan Crocker, Yanice Benitez Department of Chemistry and Biochemistry, University of California, San Diego, CA 92093, USA *To whom correspondence
Mid High Latitude Cirrus Precipitation Processes Jon Sauer, Dan Crocker, Yanice Benitez Department of Chemistry and Biochemistry, University of California, San Diego, CA 92093, USA *To whom correspondence
Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17
 Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17 Stanley C. Hatfield Southwestern Illinois College Changes of state of water, H 2 O Water is the only substance in atmosphere that exists
Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17 Stanley C. Hatfield Southwestern Illinois College Changes of state of water, H 2 O Water is the only substance in atmosphere that exists
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
General Physical Chemistry I
 General Physical Chemistry I Lecture 14 Aleksey Kocherzhenko April 9, 2015" Last time " Chemical potential " Partial molar property the contribution per mole that a substance makes to an overall property
General Physical Chemistry I Lecture 14 Aleksey Kocherzhenko April 9, 2015" Last time " Chemical potential " Partial molar property the contribution per mole that a substance makes to an overall property
Name Class Date. What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
Business. Business. Multiphase Systems Ch. 6. P vs T Diagram: Water (pure component) P vs T Diagram: CO 2 LYNN ORR
 Business LYNN ORR Izatt-Christensen Lecturer Former Assistant Secretary of Energy Public lecture Thursday 11 am, JSB Auditorium Technical lecture, Friday, 11 am, Varsity Theater Courtesy Corbin Critchfield
Business LYNN ORR Izatt-Christensen Lecturer Former Assistant Secretary of Energy Public lecture Thursday 11 am, JSB Auditorium Technical lecture, Friday, 11 am, Varsity Theater Courtesy Corbin Critchfield
Solution formation. The nature (polarity, or composition) of the solute and the solvent will determine. Factors determining rate of solution...
 Solutions Solution formation The nature (polarity, or composition) of the solute and the solvent will determine 1. Whether a substance will dissolve 2. How much will dissolve Factors determining rate of
Solutions Solution formation The nature (polarity, or composition) of the solute and the solvent will determine 1. Whether a substance will dissolve 2. How much will dissolve Factors determining rate of
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur
 Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
Chem/Biochem 471 Exam 2 11/14/07 Page 1 of 7 Name:
 Page 1 of 7 Please leave the exam pages stapled together. The formulas are on a separate sheet. This exam has 5 questions. You must answer at least 4 of the questions. You may answer all 5 questions if
Page 1 of 7 Please leave the exam pages stapled together. The formulas are on a separate sheet. This exam has 5 questions. You must answer at least 4 of the questions. You may answer all 5 questions if
1. Droplet Growth by Condensation
 1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
PRECIPITATION PROCESSES
 PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics
 Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio Stel (1)
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
Dew Point and Cloud Formation
 Name: Date: ES: 1 2 3 4 Dew Point and Cloud Formation Part A: Run some water over your hands. After shaking off the excess, wave them back and forth until they are dry. 1. Describe the change in temperature
Name: Date: ES: 1 2 3 4 Dew Point and Cloud Formation Part A: Run some water over your hands. After shaking off the excess, wave them back and forth until they are dry. 1. Describe the change in temperature
Name Class Date STUDY GUIDE FOR CONTENT MASTERY
 Atmosphere SECTION 11.1 Atmospheric Basics In your textbook, read about the composition of the atmosphere. Circle the letter of the choice that best completes the statement. 1. Most of Earth s atmosphere
Atmosphere SECTION 11.1 Atmospheric Basics In your textbook, read about the composition of the atmosphere. Circle the letter of the choice that best completes the statement. 1. Most of Earth s atmosphere
Measurement Matter and Density. Name: Period:
 Measurement Matter and Density Name: Period: Studying Physics and Chemistry Physics Tells us how fast objects move or how much it takes to get objects to, turn or stop. Chemistry Explains how different
Measurement Matter and Density Name: Period: Studying Physics and Chemistry Physics Tells us how fast objects move or how much it takes to get objects to, turn or stop. Chemistry Explains how different
Chemistry: The Central Science
 Chemistry: The Central Science Fourteenth Edition Chapter 11 Liquids and Intermolecular Forces Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions
Chemistry: The Central Science Fourteenth Edition Chapter 11 Liquids and Intermolecular Forces Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions
(Refer Slide Time: 00:00:43 min) Welcome back in the last few lectures we discussed compression refrigeration systems.
 Refrigeration and Air Conditioning Prof. M. Ramgopal Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No. # 14 Vapour Absorption Refrigeration Systems (Refer Slide
Refrigeration and Air Conditioning Prof. M. Ramgopal Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No. # 14 Vapour Absorption Refrigeration Systems (Refer Slide
Collision and Coalescence 3/3/2010. ATS 351 Lab 7 Precipitation. Droplet Growth by Collision and Coalescence. March 7, 2006
 ATS 351 Lab 7 Precipitation March 7, 2006 Droplet Growth by Collision and Coalescence Growth by condensation alone takes too long ( 15 C -) Occurs in clouds with tops warmer than 5 F Greater the speed
ATS 351 Lab 7 Precipitation March 7, 2006 Droplet Growth by Collision and Coalescence Growth by condensation alone takes too long ( 15 C -) Occurs in clouds with tops warmer than 5 F Greater the speed
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Vector equations of lines in the plane and 3-space (uses vector addition & scalar multiplication).
 Boise State Math 275 (Ultman) Worksheet 1.6: Lines and Planes From the Toolbox (what you need from previous classes) Plotting points, sketching vectors. Be able to find the component form a vector given
Boise State Math 275 (Ultman) Worksheet 1.6: Lines and Planes From the Toolbox (what you need from previous classes) Plotting points, sketching vectors. Be able to find the component form a vector given
Lecture Presentation. Chapter 11. Liquids and Intermolecular Forces Pearson Education, Inc.
 Lecture Presentation Chapter 11 Liquids and States of Matter The fundamental difference between states of matter is the strength of the intermolecular forces of attraction. Stronger forces bring molecules
Lecture Presentation Chapter 11 Liquids and States of Matter The fundamental difference between states of matter is the strength of the intermolecular forces of attraction. Stronger forces bring molecules
A.% by mass (like % composition)
 Solutions; Colloids Key Words Solute Solvent Solubility effervescence Miscible saturated Supersaturated (metastable system)- a cooled solution contains more solute than it would at equilibrium, desolvation=
Solutions; Colloids Key Words Solute Solvent Solubility effervescence Miscible saturated Supersaturated (metastable system)- a cooled solution contains more solute than it would at equilibrium, desolvation=
Habitable Planets. Much of it stolen from. Yutaka ABE University of Tokyo
 Habitable Planets Much of it stolen from Yutaka ABE University of Tokyo 1. Habitability and Water Why water? Importance of Liquid Gas: highly mobile, but low material density. Solid: high density but very
Habitable Planets Much of it stolen from Yutaka ABE University of Tokyo 1. Habitability and Water Why water? Importance of Liquid Gas: highly mobile, but low material density. Solid: high density but very
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
MIXTURES, COMPOUNDS, & SOLUTIONS
 MIXTURES, COMPOUNDS, & SOLUTIONS As with elements, few compounds are found pure in nature and usually found as mixtures with other compounds. A mixture is a combination of two or more substances that are
MIXTURES, COMPOUNDS, & SOLUTIONS As with elements, few compounds are found pure in nature and usually found as mixtures with other compounds. A mixture is a combination of two or more substances that are
Per 5 Activity Solutions: Thermal Energy, the Microscopic Picture
 er 5 Activity Solutions: Thermal Energy, the Microscopic icture 5. How Is Temperature Related to Molecular Motion? ) Temperature Your instructor will discuss molecular motion and temperature. a) Watch
er 5 Activity Solutions: Thermal Energy, the Microscopic icture 5. How Is Temperature Related to Molecular Motion? ) Temperature Your instructor will discuss molecular motion and temperature. a) Watch
End-of-Chapter Exercises
 End-of-Chapter Exercises Exercises 1 12 are primarily conceptual questions designed to see whether you understand the main concepts of the chapter. 1. (a) If the electric field at a particular point is
End-of-Chapter Exercises Exercises 1 12 are primarily conceptual questions designed to see whether you understand the main concepts of the chapter. 1. (a) If the electric field at a particular point is
Revision Sheet Final Exam Term
 Revision Sheet Final Exam Term-1 2018-2019 Name: Subject: Chemistry Grade: 11 A, B, C Required Materials: Chapter: 10 Section: 1,2,3,4,5 (Textbook pg. 311-333) Chapter: 11 Section: 1,2, (Textbook pg. 341-355)
Revision Sheet Final Exam Term-1 2018-2019 Name: Subject: Chemistry Grade: 11 A, B, C Required Materials: Chapter: 10 Section: 1,2,3,4,5 (Textbook pg. 311-333) Chapter: 11 Section: 1,2, (Textbook pg. 341-355)
Silent Card Shuffle. Dump out the word strips onto your desk.
 Silent Card Shuffle Dump out the word strips onto your desk. With a partner, silently work to arrange the strips into 8 groups. Each group should have a term (purple paper), its definition (white paper),
Silent Card Shuffle Dump out the word strips onto your desk. With a partner, silently work to arrange the strips into 8 groups. Each group should have a term (purple paper), its definition (white paper),
The two major assumptions required to reduce VLE calculations to Raoult's law are:
 10.4 SIMPLE MODELS FOR VAPOR/LIQUID EQUILIBRIUM The preceding section has described what is observed through experimental observation. When thermodynamics is applied to vapour/liquid equilibrium, the goal
10.4 SIMPLE MODELS FOR VAPOR/LIQUID EQUILIBRIUM The preceding section has described what is observed through experimental observation. When thermodynamics is applied to vapour/liquid equilibrium, the goal
Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of
 Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of phase without change of chemical composition. In this chapter
Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of phase without change of chemical composition. In this chapter
2. As gas P increases and/or T is lowered, intermolecular forces become significant, and deviations from ideal gas laws occur (van der Waal equation).
 A. Introduction. (Section 11.1) CHAPTER 11: STATES OF MATTER, LIQUIDS AND SOLIDS 1. Gases are easily treated mathematically because molecules behave independently. 2. As gas P increases and/or T is lowered,
A. Introduction. (Section 11.1) CHAPTER 11: STATES OF MATTER, LIQUIDS AND SOLIDS 1. Gases are easily treated mathematically because molecules behave independently. 2. As gas P increases and/or T is lowered,
Precipitation. GEOG/ENST 2331 Lecture 12 Ahrens: Chapter 7
 Precipitation GEOG/ENST 2331 Lecture 12 Ahrens: Chapter 7 Last lecture! Atmospheric stability! Condensation! Cloud condensation nuclei (CCN)! Types of clouds Precipitation! Why clouds don t fall! Terminal
Precipitation GEOG/ENST 2331 Lecture 12 Ahrens: Chapter 7 Last lecture! Atmospheric stability! Condensation! Cloud condensation nuclei (CCN)! Types of clouds Precipitation! Why clouds don t fall! Terminal
Lecture 7: The Monash Simple Climate
 Climate of the Ocean Lecture 7: The Monash Simple Climate Model Dr. Claudia Frauen Leibniz Institute for Baltic Sea Research Warnemünde (IOW) claudia.frauen@io-warnemuende.de Outline: Motivation The GREB
Climate of the Ocean Lecture 7: The Monash Simple Climate Model Dr. Claudia Frauen Leibniz Institute for Baltic Sea Research Warnemünde (IOW) claudia.frauen@io-warnemuende.de Outline: Motivation The GREB
(Refer Slide Time: 2:11)
 Control Engineering Prof. Madan Gopal Department of Electrical Engineering Indian institute of Technology, Delhi Lecture - 40 Feedback System Performance based on the Frequency Response (Contd.) The summary
Control Engineering Prof. Madan Gopal Department of Electrical Engineering Indian institute of Technology, Delhi Lecture - 40 Feedback System Performance based on the Frequency Response (Contd.) The summary
Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena
 Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena Saurabh Nath and Jonathan B. Boreyko Department of Biomedical Engineering and Mechanics, Virginia
Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena Saurabh Nath and Jonathan B. Boreyko Department of Biomedical Engineering and Mechanics, Virginia
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1
 Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
SEMESTER I EXAMINATION 2009/2010. MAPH Physical Meteorology
 SEMESTER I EXAMINATION 2009/2010 MAPH 40240 Physical Meteorology Extern examiner: Professor Keith Shine Head of School: Professor Micheal O Searcoid Examiner: Dr. Rodrigo Caballero Time Allowed: 2 hours
SEMESTER I EXAMINATION 2009/2010 MAPH 40240 Physical Meteorology Extern examiner: Professor Keith Shine Head of School: Professor Micheal O Searcoid Examiner: Dr. Rodrigo Caballero Time Allowed: 2 hours
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES
 THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
m m 3 mol Pa = Pa or bar At this pressure the system must also be at approximately 1000 K.
 5. PHASES AND SOLUTIONS n Thermodynamics of Vapor Pressure 5.. At equilibrium, G(graphite) G(diamond); i.e., G 2 0. We are given G 2900 J mol. ( G/ P) T V V 2.0 g mol.95 0 6 m 3 mol Holding T constant
5. PHASES AND SOLUTIONS n Thermodynamics of Vapor Pressure 5.. At equilibrium, G(graphite) G(diamond); i.e., G 2 0. We are given G 2900 J mol. ( G/ P) T V V 2.0 g mol.95 0 6 m 3 mol Holding T constant
1/2/2016 WEATHER DEFINITION
 WEATHER DEFINITION Weather state or condition of the variables of the atmosphere at a given time Weather variables temperature, air pressure, wind, moisture, cloud cover, precipitation, storms Weather
WEATHER DEFINITION Weather state or condition of the variables of the atmosphere at a given time Weather variables temperature, air pressure, wind, moisture, cloud cover, precipitation, storms Weather
Precipitation Processes
 Precipitation Processes Dave Rahn Precipitation formation processes may be classified into two categories. These are cold and warm processes, where cold processes can only occur below 0 C and warm processes
Precipitation Processes Dave Rahn Precipitation formation processes may be classified into two categories. These are cold and warm processes, where cold processes can only occur below 0 C and warm processes
Cloud Condensation Nuclei Hygroscopic Parameter Kappa
 Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus
 Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus US Department of Energy, ARM http://www.arm.gov/science/highlights/rntm3/view Typical EPMS Characteristics
Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus US Department of Energy, ARM http://www.arm.gov/science/highlights/rntm3/view Typical EPMS Characteristics
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
2. Conservation laws and basic equations
 2. Conservation laws and basic equations Equatorial region is mapped well by cylindrical (Mercator) projection: eastward, northward, upward (local Cartesian) coordinates:,, velocity vector:,,,, material
2. Conservation laws and basic equations Equatorial region is mapped well by cylindrical (Mercator) projection: eastward, northward, upward (local Cartesian) coordinates:,, velocity vector:,,,, material
C12 technical supplement
 C12 technical supplement scientific notation 2 density, unit conversion 3 gravitational force 4 light:wavelengths, etc. 5 thermal radiation (overview) 6 thermal radiation (stefan-boltzmann law) 7 thermal
C12 technical supplement scientific notation 2 density, unit conversion 3 gravitational force 4 light:wavelengths, etc. 5 thermal radiation (overview) 6 thermal radiation (stefan-boltzmann law) 7 thermal
THIRD GRADE WATER 1 WEEK LESSON PLANS AND ACTIVITIES
 THIRD GRADE WATER 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen peroxide.
THIRD GRADE WATER 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen peroxide.
SUPPLEMENT I. Example. Graph the vector 4, 3. Definition. Given two points A(x 1, y 1 ) and B(x 2, y 2 ), the vector represented by # AB is # AB =,
 SUPPLEMENT I 1. Vectors Definition. A vector is a quantity that has both a magnitude and a direction. A twodimensional vector is an ordered pair a = a 1, a 2 of real numbers. The numbers a 1 and a 2 are
SUPPLEMENT I 1. Vectors Definition. A vector is a quantity that has both a magnitude and a direction. A twodimensional vector is an ordered pair a = a 1, a 2 of real numbers. The numbers a 1 and a 2 are
Multiphase Flow and Heat Transfer
 Multiphase Flow and Heat Transfer Liquid-Vapor Interface Sudheer Siddapuredddy sudheer@iitp.ac.in Department of Mechanical Engineering Indian Institution of Technology Patna Multiphase Flow and Heat Transfer
Multiphase Flow and Heat Transfer Liquid-Vapor Interface Sudheer Siddapuredddy sudheer@iitp.ac.in Department of Mechanical Engineering Indian Institution of Technology Patna Multiphase Flow and Heat Transfer
water Plays dominant role in radiation All three phases emit and absorb in longwave radiation
 4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
8.2 Surface phenomenon of liquid. Out-class reading: Levine p Curved interfaces
 Out-class reading: Levine p. 387-390 13.2 Curved interfaces https://news.cnblogs.com/n/559867/ 8.2.1 Some interesting phenomena 8.2.1 Some interesting phenomena Provided by Prof. Yu-Peng GUO of Jilin
Out-class reading: Levine p. 387-390 13.2 Curved interfaces https://news.cnblogs.com/n/559867/ 8.2.1 Some interesting phenomena 8.2.1 Some interesting phenomena Provided by Prof. Yu-Peng GUO of Jilin
Chapter 12 Intermolecular Forces and Liquids
 Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Problem # 1 Determine the kinds of intermolecular forces present in each element or compound:
 Problem # 1 Determine the kinds of interecular forces present in each element or compound: A. Kr B. NCl 3 C. SiH 4 D. HF SOLUTION: Kr is a single atom, hence it can have no permanent dipole. The only possible
Problem # 1 Determine the kinds of interecular forces present in each element or compound: A. Kr B. NCl 3 C. SiH 4 D. HF SOLUTION: Kr is a single atom, hence it can have no permanent dipole. The only possible
Steady-State Molecular Diffusion
 Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS
 CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Particle nature of Matter *** 2. States of Matter **** 3. Interchange in states of Matter
CHAPTER 1 Matter in our Surroundings CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Particle nature of Matter *** 2. States of Matter **** 3. Interchange in states of Matter
Intermolecular Forces and Liquids and Solids
 Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 A phase is a homogeneous part of the system in contact
Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 A phase is a homogeneous part of the system in contact
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Put sufficient ice cubes into water (1 M) and wait for equilibrium (both exist) (1 M)
 NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
9.7 Freezing Point Depression & Boiling Point Elevation
 Figure 9.11 9.7 Freezing Point Depression & Boiling Point Elevation If the solution is in equilibrium with the pure solid solvent, (9.25) μ solution = chemical potential of the solvent in the solution
Figure 9.11 9.7 Freezing Point Depression & Boiling Point Elevation If the solution is in equilibrium with the pure solid solvent, (9.25) μ solution = chemical potential of the solvent in the solution
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
