Mass. Time. Dimension is a property that can be: Length. By the end of this lecture you should be able to do the following: Dimension:
|
|
|
- Paula Morgan
- 6 years ago
- Views:
Transcription
1 Unit: B the end of this lecture ou should be able to do the following: Bapco Refiner has the capacit to refine 6, barrel/da of crude oil value + unit. Understand the meaning of unit and dimension.. Identif the meaning of a quantit. 3. Understand the Difference between measured and calculated dimension. 4. Understand the meaning of conversion factor. 5. Use the conversion factors table. 6. Convert between different units 7. Define Sstems of units, compound units, derived units, and unit prefi. 8. Know Unit prefies. Dimension: is a propert that can be: Measured Calculated Dimension is a propert that can be: Measured dimensions such as Calculated dimensions such as Units can be treated like algebraic variables when quantities are added, subtracted, multiplied, or divided: 4 cm cm = 3 cm 4 = 3 measured calculated Mass velocit 3 cm + mm =?? 3 + =?? Time volume 3 N 4 m = N. m 3 4 =. m m = 4 m = 4 Length densit 5 m / s = 5 m/s 6 m / 3 m/s = s Temperature???? g / g = (dimensionless)
2 A measured quantit can be epressed in terms of an units having the appropriate dimension: 6 km/h = km/min = 37.3 mile/h = 7 m/s = 55 ft/s Convert cm to its equivalent value epressed in mm? mm cm = cm X cm = cm X cm / cm = mm / Conversion Factor Quantit Equivalent values Mass kg lb m = g =. metric ton =.46 lb m = oz = 6 oz = 5 4 ton = g = kg Length m ft = cm = mm = 6 microns = angstroms = in = 3.88 ft =.936 d =.64 mile = in. = /3 d =.348 m = 3.48 cm Volume m 3 ft 3 = L = 6 cm 3 = 6 ml = ft 3 =.83 imperial gallons = 64.7 gal = qt = 78 in 3 = gal =.837 m 3 = 8.37 L = 837 cm 3 Force N lb f = kg.m/s = 5 dnes = 5 g.cm/s =.48 lb f = 3.74 lb m.ft/s = N = dnes Pressure atm =.35 5 N/m (Pa) =.35 kpa =.35 bar =.35 6 dnes/cm = 76 mm Hg at o C (torr) =.333 m H O at 4 o C = lb f /in (psi) Energ J = N. m = 7 ergs = 7 dne.cm = kwh =.39 cal =.7376 ft. lb f = Btu Power W = J/s =.39 cal/s =.7376 ft.lb f /s = Btu/s =.34 3 hp Eample.- (TXB) Convert an acceleration of cm/s to its equivalent in km/r cm km 36 s 4 h 365 da s cm m h da r Common Sstems of Units: SI Units CGS Sstem English Units Write the given quantit (36 X 4 X 365 ) km = = 9.95 X ( X ) r 9 km/r Determine the proper conversion factor Cancel the original units Multipl and divide numbers to get quantit with new units International Sstem of Units: m, kg, s. Centimeter gram second sstem: cm, g, s American Engineering Sstem: ft, lb m, s Base units units for the dimensions of mass, length, time, absolute temperature, electric current, luminous intensit, and amount of substance. Multiple units which are multiples or fractions of base units used for convenience (ears instead of seconds, kilometers instead of meters, etc.). Derived units Compound units: obtained b multipling and dividing base or multiple units ( cm, ft/min, kg.m/s ). As defined equivalents of compound units (N= kg.m/s, pascal = N/m ) A sstem of units has the following components: Sstem of units Base Units Multiple Units Derived Units Compound Units Defined Equivalents of Compound units
3 A sstem of units has the following components:. Base units: for mass, length, time, temperature, electrical charge and light intensit.. Multiple Units: multiple or fractions of base units; Second Dimension SI sstem CGS sstem Mass Kilogram (kg) American Engineering Sstem Gram (g) Pound mass (lb m ) Length Meter (m) Centimeter(cm Foot (ft) ) Time Second (s) Second (s) Second (s) millisecond minutes hour Temperature Kelvin (K) Kelvin (K) Rankin ( R) 3. Derived units: a. Compound Units: b multipling and dividing base or multiple units; e.g. cm, ft/min, m/s b. Defined Equivalents of Compound units: e.g. newton kg.m/s, J N.m = kg.m /s Electric current Intensit of light Ampere (A) Candela (cd) Ampere (A) Candela (cd) Ampere (A) Candela (cd) /8/6 4 Table.3 (TXB) Table.3 (TXB) Multiple unit Prefies Table.3 (TXB) Wh mastering unit conversion skills is important? Human error blamed for Orbiter loss The Mars Climate Orbiter now in pieces on the planet's surface B BBC News Online Science Editor Dr David Whitehouse. September 4, 999. On later article b the same author: The Mars Climate Orbiter burnt up when entering the planet's orbit: a mi-up of English and metric units used in calculating its trajector sent the spacecraft too close to Mars. /8/6 8 3
4 Conversion Between Sstem of Units Eample.3 (TXB) Convert 3 lb m.ft/min to its equivalent in kg.cm/s 3 lb m.ft kg in.54 cm min min. lb m ft in 6 s (3 X X.54) kg.cm = (. X 6 ) s =.885 kg.cm/s Newton s second law of motion F = m. a Natural force units kg.m/s (SI) g.cm/s (CGS) lb m.ft/s (American Eng Ss) Derived force units have been defined: SI: Newton (N) kg.m/s, N is the force required to accelerate a mass of kg at a rate of m/s N a = m/s Object Mass = kg dne a = Object cm/s Mass = g Derived force units have been defined: SI: Newton (N) kg.m/s, N is the force required to accelerate a mass of kg at a rate of m/s CGS: dne g.cm/s dne is the force required to accelerate a mass of g at a rate of cm/s lbf a = 3.74 ft/s Object Mass = lbm American engineering sstem: lb f (pound force) is the force eerted b earth on an object that has a mass of lb m and falling at acceleration of gravit at sea level and 45 o latitude (which is 3.74 ft/s ). lb f 3.74 lb m.ft/s Derived Force Units Derived force units have been defined: SI: Newton (N) kg.m/s N is the force required to accelerate a mass of kg at a rate of m/s CGS: dne g.cm/s dne is the force required to accelerate a mass of g at a rate of cm/s American engineering sstem: lb f (pound force) is the force eerted b earth on an object that has a mass of lb m and falling at acceleration of gravit at sea level and 45 o latitude (which is 3.74 ft/s ). lb f 3.74 lb m.ft/s 4
5 Derived Force Units Derived force units have been defined: SI: Newton (N) kg.m/s N is the force required to accelerate a mass of kg at a rate of m/s Derived Force Units American engineering sstem: lb f 3.74 lb m.ft/s CGS: dne g.cm/s dne is the force required to accelerate a mass of g at a rate of cm/s Eample: The force required to accelerate a mass of 4. lb m at a rate of ft/s is 3.74 lb m.ft/s. Epress the force in the unit of lb f. lb f American engineering sstem: lb f 3.74 lb m.ft/s lb f (pound force) is the force eerted b earth on an object that has a mass of lb m and falling at acceleration of gravit at sea level and 45 o latitude (which is 3.74 ft/s ). or 3.74 lb m.ft l lb f s 3.74 lb m.ft/s = lb f The weight of an object is the force eerted on the object b gravitational attraction. Mass vs. Weight.. Eample: If ou have a mass of 7 kg then how much ou weigh? Assume g = m/s = cm/s = 3.74 ft/s W = m. g = 7 kg m/s = kg.m/s l N = N kg.m/s Mass vs. Weight Eample: Water has a densit of 6.4 lb m /ft 3. How much does. ft 3 of water weigh at a location where the gravitational acceleration is 3.39 ft/s? (Epress the answer in lb f ) Ans: 4.7 lb f 5
6 B the end of this lecture understand the following: ou should be able to. Mathematical Operation and Significant Figures.. and accurac 3. Validating Results. 4. Estimation of Measured Values: Sample Mean. 5. Sample Variance of Scattered Data. 6. Process Data Representation and Analsis 7. Two Point Linear Interpolation.5a Scientific Notation, Significant Figures, and Scientific Notation: Ver large or ver small numbers are not convenient 5,,.5 =.5 8 =.5-5 Scientific Notation Scientific notation is used in which the number is epressed as the product of another number(usuall between. and ) and a power of..5a Scientific Notation, Significant Figures, and Significant Figures: of a number are the digits from the first nonzero digit on the left of the number to either: - the last digit (zero or nonzero) on the right if there is a decimal point (e.g or 8.63 X has 5 sig. fig.) Significant Figures Significant Figures: of a number are the digits from the first nonzero digit on the left of the number to either: or - the last nonzero digit of the number if there is no decimal point (e.g., has sig. fig.) the last digit (zero or nonzero) on the right if there is a decimal point (e.g or 8.63 has 5 sig. fig.) the last nonzero digit of the number if there is no decimal point (e.g., has sig. fig.) /8/6 34 Eample.5a Scientific Notation, Significant Figures, and Wh a number is reported as 3. instead of 3??,. 4 : The No. of significant figures in a given value is an indication of the precision with which the quantit is known... 3 The more significant figures the more precise is the value.. 4, /8/6 36 6
7 .5a Scientific Notation, Significant Figures, and So Ahmed Can sa that he is 68.8 kg But Can Not claim that he is 68.8 kg In other words, his mass Could Be somewhere between kg.5a Scientific Notation, Significant Figures, and Mathematical Operation and Significant Figures =.5,.5,.59, or.59? = 6.7, 6.74, 6.744, or 6.744? The of the final answer can be no greater than the least precise measurement Multiplication and Division: =.5 (3 sig. fig.) (4 sig. fig.) (3 sig. fig.) lowest sig. fig. No. of sig. fig. of the result = the lowest sig. fig..5a Scientific Notation, Significant Figures, and Mathematical Operation and Significant Figures Eample.53X -3 X 3.5 = 46.3 =?????? 46 (5 sig. fig.) ( sig. fig.) (3 sig. fig.) lowest sig. fig..5a Scientific Notation, Significant Figures, and Mathematical Operation and Significant Figures Addition and Subtraction: The result has lowest =??? No. of digits after D.P digits after the D.P. 3 digits after the D.P = 6.74 lowest No. of digits after D.P. D.P. = Decimal Point digits after the D.P..5a Scientific Notation, Significant Figures, and Mathematical Operation and Significant Figures Eample:. +.3 = 3. The precision of the final answer can be no greater than the least precise measurement Eample =.76 =?????? ( digits after D.P.) (No digits after D.P.).5a Scientific Notation, Significant Figures, and Eample =.94 =??????.9 (5 digits after D.P.) (4 digits after D.P.) ( digits after D.P.) Eample =??????. +.4 =.34 =.3?????? =.3 - ( digits after D.P.) (3 digits after D.P.) or =?????? =.34 - =??????.3 - ( digits after D.P.) ( digits after D.P.) A good rule to follow is to epress all numbers in the highest power of ten. 7
8 Accurac vs..5a Scientific Notation, Significant Figures, and Mathematical Operation and Significant Figures Note If ou are rounding off numbers in which the digit to be dropped off is a 5 -make the last digit even: Accurac vs. : YouTube movie In other cases, rounded to the closest number: Accurac vs. In the fields of engineering, industr and statistics: The accurac of a measurement sstem is the degree of closeness of measurements of a quantit to its actual true value. Accurac How well a measurement agrees with an accepted value. The precision of a measurement sstem, also called reproducibilit or repeatabilit, is the degree to which repeated measurements under unchanged conditions show the same results. How well a series of measurements agree with each other. Precise /8/6 45.5b Validating Results If ou have a problem: before solving the problem ou should ask: How should I solve the problem? after solving the problem ou should ask: Is the answer right? How do I know it s right? Validate our answer b: Back Substitution Accurate Precise Not accurate Precise Not accurate Not precise Order-of-Magnitude Estimation Test of Reasonableness 8
9 .5b Validating Results.5b Validating Results Back Substitution before solving the problem ou should ask: How should I solve the problem? after solving the problem ou should ask: How do I know it s right? Validate our answer Order-of-Magnitude Estimation If ou have a problem: Is the answer right? Test of Reasonableness /8/6 49 /8/6 5.5b Validating Results.5b Validating Results Back Substitution 3. = I found that = Order-of-magnitude: obtain an approimate answer and compare to our solution = calculator solution is = 4. 3() = I substitute back 3. = I make crude calculation = So I am right = /3 = 4 So I am right Procedure for checking an arithmetic calculation b Order Of Magnitude Estimation is as follows:. Substitute simple integers for all numerical quantities, using powers of ten(scientific Notation) for ver large and ver small quantities.. If a number is added to a second, much smaller, drop the second number in the approimation. 3. Do the resulting arithmetic calculation b hand, continuing to round off intermediate answers. 4. If the correct answer (obtained using a calculator) is of the same magnitude as the estimate, ou can be reasonabl confident that ou haven t made a gross error in calculation..5b Validating Results Order-of-magnitude: Some rules Eample.5-: The Calculator Solution = , or 43, You were calculating volume flow rate of a stream and ou ended up with the following equation: The correct answer is.3 or.3x 3 9
10 .5b Validating Results b Validating Results Reasonabilit of the Number: You calculated: the area of a countr to be m. Is that right? the temperature in the street to be 5 o C. Is that right? Atmospheric pressure on top of a mountain to be.8 atm. Is that right?.. B the end of this lecture understand the following:. Estimation of Measured Values: Sample Mean.. Sample Variance of Scattered Data. 3. Process Data Representation and Analsis 4. Two Point Linear Interpolation 5. Fitting a straight line. 6. Fitting nonlinear data. 7. Logarithmic coordinates. ou should be able to The conversion after 4 min X is a random variable changing in an unpredictable manner from one run to another run at the same eperimental conditions. Run X (%) We estimate the true value of X for the given eperimental conditions as the sample mean X= ( X X... X N ) N X= ( ) 69.3% Conversion (X%) Mean (69.3%) Run
11 .5c Estimation of Measured Values: Sample Mean The mean is 69.3%. How scattered are the values around their mean value? Run X (%) Range: R = X ma X min R = = 6% Sample Variance: S X [( ) ( )... ( ) ] 3.7 Sample Standard Deviation: S X [( X X ) ( X X )...( X N X ) ] N.5d Sample Variance of Scattered Data Three quantities -the range, the sample variance, and the sample standard deviation-used to epress the etent to which values of a random variable scatter about their mean value. Range Sample Variance Sample Standard Deviation S X S X Measure of Scatter:. Sample Variance :. Range: is a much better measure for the degree of scatter. is the difference between maimum and minimum measured variable 3. Sample Standard Deviation S : However, range : a) is the crudest measure of scatter. b) gives no indication whether or not most of the values cluster close to the mean or scatter widel around it. isanothermeasureforthedegreeof scatter epressed as the square root of the sample variance. /8/6 63.5d Sample Variance of Scattered Data Run X (%) The mean is How scattered are the values around their mean value? Range: Sample Variance: S X % Sample Standard Deviation: S X [( X X ) ( X X )...( X N X ) ] N [( ) ( )... ( ) ] 3.7 S X S X Conversion (X) Conversion (X) Set Run Set Run Range: R = 75-55=% Mean: 65% Sample Standard Deviation: S = 5. % Range: R = 75-55=% Mean: 65% Sample Standard Deviation: S = 8. %
12 Consistent units Length Length Length Dimensionall Homogeneous Consistent units Inconsistent unit Dimensionall Homogeneous Dimensionall Homogeneous Quantities can be added and subtracted ONLY if their units are the same. Dimensional Homogeneit in Equations: Ever valid equation must be dimensionall homogeneous; that is all additive terms on both sides of the equation must have the same dimensions. Consistent in its unit v(m/s) = v (m/s) + a(m/s ).t(s) Dimensionall Homogeneous Length/time = Length/time + Length/time Inconsistent in its unit v(m/s) = v (cm/s) + a(m/s ).t(s) Dimensionall Homogeneous Length/time = Length/time + Length/time v(m/s)= v (cm/s) m + a(m/s ).t(s) cm v(m/s)= v (cm/s)/ + a(m/s Can be fied b appling the ).t(s) appropriate conversion Factor Dimensionless Quantities: Relative Values: e.g. relative densities: (the ratio of densit of a substance to a densit of a standard) 3 D ( cm ) u ( cm / s ) ( g / cm ) Dimensionless Group: e.g. Renolds Number: Re ( g / cms) Eponents: e.g.in Transcendental Functions: e.g. log, ln, ep e, sin Arguments of Transcendental Functions: e.g.xinlog(x)orsin(x) ft ft Log () log( min), or log () m Sin (3.4) sin (3.4 N)
13 General Procedure for rewriting an equation in terms of new variables having the same dimensions but different units: Eample : Convert 3 s to min.. Eample : Convert 5 m s to ft Eample.6 (REF) Microchip etching roughl follows the following relation: where d is the depth of the etch in micron () and t is the time of the etch in seconds.. What are the units associated with the numbers 6., 6.,.?. Convert the relation so that d becomes epressed in inches and t can be used in min. Solution 6.. d = 6.38 X 4 [ ep(.6. t)] where d is in (in) and t in (min)..... & The operation of an chemical process is ultimatel based on the measurements of process variables: T, P, F flow rates, C i Generall: Indirect Techniques are used to measure those variables. Eample: FTIR measures light absorbance Then, absorbance is correlated to concentration. Fourier Transform Infrared Spectroscop (FTIR) The relationship between C A and light absorbance is determined b calibration eperiment in which solutions of known concentration are prepared and absorbance is measured for each solution. Concentration Absorption 4 35 mole/l Need C A when Abs. =.? Have to do interpolation Conc. of Acetic Anhdride, mole/l Absorption Intensit Need C A when Abs. =.95? Have to do etrapolation 3
14 Two Point Linear Interpolation Graphical Interpolation Interpolation and Etrapolation Methods Curve fitting Depends on the nature of the relationship between the two variables (e.g. C A and Abs., or an and ).7a Two-Point Linear Interpolation The equation of the line through (, ) and (, ) on a plot of versus is: Concentration Absorption ( ) () mole/l () Eample: What is the value of C A if the absorbance is.? (93) 4 (.95.6, ) C A = = 5.98 mole/l Conc. of Acetic Anhdride, mole/l (, )..4.6 Absorption Intensit.7a Two-Point Linear Interpolation The equation of the line through (, ) and (, ) on a plot of versus is: ( ) 5 (, ) Conc. of Acetic Anhdride, mole/l 5 (, )..4.6 Absorption Intensit.7b Fitting a Straight Line. Slope intercept Intercept b Slope a.7b Fitting a Straight Line The equation of straight line is: a slope b intercept a a Eample: (, ) = (.8, 5) ( Fit the C A vs. Abs. data to a straight line equation., ) = (.95, 9) 5 9 a b 9 (3.5)(.95). (, ) CA 3.5 ( abs). What is C A at abs. =.? C A Conc. of Acetic Anhdride, mole/l 3.48 (.). 6. mole/l ab (, ) Absorption Intensit.7c Fitting Nonlinear Data If the data looks like: Series And if I want to find a value of using a value of that is not in the Data Table: Can I use a fitted linear equation? Can I use linear interpolation or etrapolation? 4
15 .7c Fitting Nonlinear Data You could still fit some nonlinear data to a straight line..7c Fitting Nonlinear Data You could still fit some nonlinear data to a straight line. : Series : ( 8.5) Series : c Fitting Nonlinear Data You could still fit some nonlinear data to a straight line ( 8.5) 75 5 Series c Fitting Nonlinear Data Conclusion: If an Two Quantities are related b an equation of the form:. Slope intercept Intercept b Slope a.7c Fitting Nonlinear Data Eample The following equations ield straight lines: a b If ( ) is plotted vs. ( ) a b If ( ) is plotted vs. ( ) If ( ) is plotted vs. ( ) ae a b b If ( ln ) is plotted vs. ( ) If ( ln ) is plotted vs. ( ln ).7c Fitting Nonlinear Data Eample The chemical engineering department bought a device that produces fresh water from tap water. The device manual states that the production rate of fresh water [ ]isaffectedbthe ambient temperature [T ( o C)] according to the following equation:.. Can ou using straight-line plot verif this formula and determine the constants a and b. 5
16 .7c Fitting Nonlinear Data Eample.. T ( o C) What 3.6to plot? d Logarithmic Coordinates Eample: a ep(b) ln () What to plot? , , d Logarithmic Coordinates Eample: 5 ln a ep(b) ln ln a b 3 5 ln () What to plot? , , , , d Logarithmic Coordinates Eample: a ep(b) ln ln a b Y. Ln Parallel ais.7d Logarithmic Coordinates Eample: a ep(b) Log Y-ais Rectangular X-ais 6
17 .7d Logarithmic Coordinates Eample: a ep(b) Rectangular Y-ais Rectangular X-ais Log Y-ais Rectangular X-ais.7d Logarithmic Coordinates Eample (Problem.3 (a)) Aplotof versus ields a straight line on a semilog plot (the vertical ais is a logarithmic scale while the horizontal ais is a rectangular scale). The line passes through (8.68,.) and (,). Sketch the plot and calculate the equation (). a ep(b) ln ln a b Solution: =4e d Logarithmic Coordinates = 3.97e.689 R² = Eample (Problem.3 (a)) Aplotof versus ields a straight line on a semilog plot (the vertical ais is a logarithmic scale while the horizontal ais is a rectangular scale). The line passes through (8.68,.) and (,).. Sketch the plot and. calculate the equation () Solution: =4e :.,. &, Use Eq. to calculate a d Logarithmic Coordinates Eample: Log Y-ais Use Eq. for checking Finall give the equation with calculated values of a and b.. Log X-ais 7
18 e Fitting a Line to a Scattered Data The fitting of a straight line to a series of versus data points (linear regression) can be accomplished using different techniques:. method of least squares, R. robust regression 8
Physical Measurement
 Order of Magnitude Estimations: estimations to the nearest power of ten The ranges of magnitude that occur in the universe: Sizes: 10-15 m to 10 +25 m (subnuclear particles to etent of the visible universe).
Order of Magnitude Estimations: estimations to the nearest power of ten The ranges of magnitude that occur in the universe: Sizes: 10-15 m to 10 +25 m (subnuclear particles to etent of the visible universe).
CHAPTER 1 INTRODUCTION TO ENGINEERING CALCULATIONS
 CHAPTER 1 INTRODUCTION TO ENGINEERING CALCULATIONS Sem 1, 2016/2017 ERT 214 Material and Energy Balance / Imbangan Bahan dan Tenaga After completing this chapter, you should be able to do the following:
CHAPTER 1 INTRODUCTION TO ENGINEERING CALCULATIONS Sem 1, 2016/2017 ERT 214 Material and Energy Balance / Imbangan Bahan dan Tenaga After completing this chapter, you should be able to do the following:
CHAPTER 1 MEASUREMENTS AND VECTORS
 CHPTER 1 MESUREMENTS ND VECTORS 1 CHPTER 1 MESUREMENTS ND VECTORS 1.1 UNITS ND STNDRDS n phsical quantit must have, besides its numerical value, a standard unit. It will be meaningless to sa that the distance
CHPTER 1 MESUREMENTS ND VECTORS 1 CHPTER 1 MESUREMENTS ND VECTORS 1.1 UNITS ND STNDRDS n phsical quantit must have, besides its numerical value, a standard unit. It will be meaningless to sa that the distance
Units and Dimensions. Lecture 1. Introduction to Chemical Engineering Calculations
 Introduction to Chemical Engineering Calculations Lecture 1. Mathematics and Engineering In mathematics, If x = 500 and y = 100, then (x + y) = 600 In engineering, If x = 500m and y = 100m, then (x + y)
Introduction to Chemical Engineering Calculations Lecture 1. Mathematics and Engineering In mathematics, If x = 500 and y = 100, then (x + y) = 600 In engineering, If x = 500m and y = 100m, then (x + y)
FDE 211 MATERIAL & ENERGY BALANCES. Instructor: Dr. Ilgin Paker Yikici Fall 2015
 FDE 211 MATERIAL & ENERGY BALANCES Instructor: Dr. Ilgin Paker Yikici Fall 2015 Meet & Greet Hello! My name is I am from 2 Class Overview Units & Conversions Process & Process Variables Process Units &
FDE 211 MATERIAL & ENERGY BALANCES Instructor: Dr. Ilgin Paker Yikici Fall 2015 Meet & Greet Hello! My name is I am from 2 Class Overview Units & Conversions Process & Process Variables Process Units &
Summary of common Pressure Units Version 1.00, 12/15/2003
 Summary of common Pressure Units Version.00, /5/003 Portland State Aerospace Society There are too many pressure units in common use. This is not nearly all of them. For PSAS,
Summary of common Pressure Units Version.00, /5/003 Portland State Aerospace Society There are too many pressure units in common use. This is not nearly all of them. For PSAS,
Chapter 1B. Measurement CHAPTER OUTLINE
 Chapter 1B Measurement 1 CHAPTER OUTLINE SI Units Scientific Notation Error in Measurements Significant Figures Rounding Off Numbers Conversion of Factors Conversion of Units Volume & Density 2 1 SI UNITS
Chapter 1B Measurement 1 CHAPTER OUTLINE SI Units Scientific Notation Error in Measurements Significant Figures Rounding Off Numbers Conversion of Factors Conversion of Units Volume & Density 2 1 SI UNITS
Chapter One: Dimensions, Units, and Their Conversion
 Lecture # 1, 2/8/2012 Chapter One: Dimensions, Units, and Their Conversion Objectives: 1. Understand and explain the difference between dimensions and units. 2. Add, subtract, multiply and divide units
Lecture # 1, 2/8/2012 Chapter One: Dimensions, Units, and Their Conversion Objectives: 1. Understand and explain the difference between dimensions and units. 2. Add, subtract, multiply and divide units
Chapter 1 (Part 2) Measurements in Chemistry 1.6 Physical Quantities
 Chapter 1 (Part 2) Measurements in Chemistry 1.6 Physical Quantities This is a property that can by physically measured. It consists of a number and a unit of measure. (e.g. ) Units Units are very important.
Chapter 1 (Part 2) Measurements in Chemistry 1.6 Physical Quantities This is a property that can by physically measured. It consists of a number and a unit of measure. (e.g. ) Units Units are very important.
Chemistry 104 Chapter Two PowerPoint Notes
 Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
General Chemistry I Introductory Concepts. Units, dimensions, and mathematics for problem solving
 General Chemistry I Introductory Concepts Units, dimensions, and mathematics for problem solving Unit Conversion What is the value of S in cm per second? S = 5x10 3 furlongs fortnight Conversion Factor:
General Chemistry I Introductory Concepts Units, dimensions, and mathematics for problem solving Unit Conversion What is the value of S in cm per second? S = 5x10 3 furlongs fortnight Conversion Factor:
Unit I: Measurements A. Significant figures B. Rounding numbers C. Scientific notation D. Using electronic calculators E.
 Unit I: Measurements A. Significant figures B. Rounding numbers C. Scientific notation D. Using electronic calculators E. Using sig figs in arithmetic operations F. The metric system G. Problem solving
Unit I: Measurements A. Significant figures B. Rounding numbers C. Scientific notation D. Using electronic calculators E. Using sig figs in arithmetic operations F. The metric system G. Problem solving
Math for CH 301 Workshop
 Welcome to the Math for Chemistry workshop! We are going to cover some mathrelated topics that you are expected to be proficient in for your first semester chemistry course. We will often refer to your
Welcome to the Math for Chemistry workshop! We are going to cover some mathrelated topics that you are expected to be proficient in for your first semester chemistry course. We will often refer to your
Name: Chapter 2: Analyzing Data Note Taking Guide This worksheet is meant to help us learn some of the basic terms and concepts of chemistry.
 Chemistry Name: Section ANALYZE DATA KEY Date: Chapter 2: Analyzing Data Note Taking Guide This worksheet is meant to help us learn some of the basic terms and concepts of chemistry. Most, but not all,
Chemistry Name: Section ANALYZE DATA KEY Date: Chapter 2: Analyzing Data Note Taking Guide This worksheet is meant to help us learn some of the basic terms and concepts of chemistry. Most, but not all,
Introduction to Mechanical Engineering Measurements Two Main Purposes of Measurements Engineering experimentation Operational systems
 Introduction, Page 1 Introduction to Mechanical Engineering Measurements Author: John M. Cimbala, Penn State University Latest revision, 19 August 011 Two Main Purposes of Measurements Engineering experimentation
Introduction, Page 1 Introduction to Mechanical Engineering Measurements Author: John M. Cimbala, Penn State University Latest revision, 19 August 011 Two Main Purposes of Measurements Engineering experimentation
Chapter 2: Standards for Measurement. 2.1 Scientific Notation
 Chapter 2: Standards for Measurement 2.1 Scientific Notation A measurement (quantitative observation) consists of two parts: o Numerical value which gives magnitude, and o Unit which gives the scale used
Chapter 2: Standards for Measurement 2.1 Scientific Notation A measurement (quantitative observation) consists of two parts: o Numerical value which gives magnitude, and o Unit which gives the scale used
Regular Physics - Notes Ch. 1
 Regular Phsics - Notes Ch. 1 What is Phsics? the stud of matter and energ and their relationships; the stud of the basic phsical laws of nature which are often stated in simple mathematical equations.
Regular Phsics - Notes Ch. 1 What is Phsics? the stud of matter and energ and their relationships; the stud of the basic phsical laws of nature which are often stated in simple mathematical equations.
oz ounce (mass) = L = cm 3
 Memorize relationships shown in each box! NOTE: Exact quantities are specified as exact. Also, consider 1 as exact. mass (M) Common unit abbreviations (singular) 1 kg = 2.20462 lb m = 35.27392 oz L liter
Memorize relationships shown in each box! NOTE: Exact quantities are specified as exact. Also, consider 1 as exact. mass (M) Common unit abbreviations (singular) 1 kg = 2.20462 lb m = 35.27392 oz L liter
APPENDIX B ABBREVIATIONS, SYMBOLS AND CONVERSION FACTORS Abbreviations
 APPENDIX B ABBREVIATIONS, SYMBOLS AND CONVERSION FACTORS Abbreviations A ampere AASHTO American Association of State Highway & Transportation Officials ABS (%) Percent of Absorbed Moisture Abs. Vol. Absolute
APPENDIX B ABBREVIATIONS, SYMBOLS AND CONVERSION FACTORS Abbreviations A ampere AASHTO American Association of State Highway & Transportation Officials ABS (%) Percent of Absorbed Moisture Abs. Vol. Absolute
Chapter 2 Dimensions, Units, and Unit Conversion
 AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri Chapter 2 Dimensions, Units, and Unit Conversion Dimensions Dimensions are concepts of measurement in engineering works. The basic
AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri Chapter 2 Dimensions, Units, and Unit Conversion Dimensions Dimensions are concepts of measurement in engineering works. The basic
APPENDIX H CONVERSION FACTORS
 APPENDIX H CONVERSION FACTORS A ampere American Association of State AASHTO Highway & Transportation Officials ABS (%) Percent of Absorbed Moisture Abs. Vol. Absolute Volume ACI American Concrete Institute
APPENDIX H CONVERSION FACTORS A ampere American Association of State AASHTO Highway & Transportation Officials ABS (%) Percent of Absorbed Moisture Abs. Vol. Absolute Volume ACI American Concrete Institute
hp calculators HP 9g Solving Problems Involving Unit Conversions Metric Units and Imperial Units The CONV Menu
 Metric Units and Imperial Units The CONV Menu Practice Working Problems Involving Conversions Metric units and Imperial units The Longman Mathematics Handbook (York Press, 1990) defines the unit as a conventional
Metric Units and Imperial Units The CONV Menu Practice Working Problems Involving Conversions Metric units and Imperial units The Longman Mathematics Handbook (York Press, 1990) defines the unit as a conventional
Introduction to Engineering ENGR System of Units
 Introduction to Engineering ENGR 1100 - System of Units System of Units The SI system of units (Le Systeme International d unites) is the system used worldwide except for the United States, Liberia and
Introduction to Engineering ENGR 1100 - System of Units System of Units The SI system of units (Le Systeme International d unites) is the system used worldwide except for the United States, Liberia and
Practice A ( 1, 3 ( 0, 1. Match the function with its graph. 3 x. Explain how the graph of g can be obtained from the graph of f. 5 x.
 8. Practice A For use with pages 65 7 Match the function with its graph.. f. f.. f 5. f 6. f f Lesson 8. A. B. C. (, 6) (0, ) (, ) (0, ) ( 0, ) (, ) D. E. F. (0, ) (, 6) ( 0, ) (, ) (, ) (0, ) Eplain how
8. Practice A For use with pages 65 7 Match the function with its graph.. f. f.. f 5. f 6. f f Lesson 8. A. B. C. (, 6) (0, ) (, ) (0, ) ( 0, ) (, ) D. E. F. (0, ) (, 6) ( 0, ) (, ) (, ) (0, ) Eplain how
Today is Thursday, February 11 th, 2016
 In This Lesson: Scientific Notation and Unit Analysis (Lesson 4 of 6) Today is Thursday, February 11 th, 2016 Stuff You Need: Calculator Paper Towel Pre-Class: By now you ve probably heard of scientific
In This Lesson: Scientific Notation and Unit Analysis (Lesson 4 of 6) Today is Thursday, February 11 th, 2016 Stuff You Need: Calculator Paper Towel Pre-Class: By now you ve probably heard of scientific
Unit Conversions, Important Constants and Relationships
 NOTE: Exact quantities are specified as exact. Consider 1 as exact! mass (M) 1 kg = 2.20462 lb m = 35.27392 oz 1 lb m = 16 oz (exact)= 453.593 g length (L) 1 m = 10 10 (exact) angstroms (Å) = 100 cm =
NOTE: Exact quantities are specified as exact. Consider 1 as exact! mass (M) 1 kg = 2.20462 lb m = 35.27392 oz 1 lb m = 16 oz (exact)= 453.593 g length (L) 1 m = 10 10 (exact) angstroms (Å) = 100 cm =
Unit 1: Fundamentals of Chemistry
 Significant Digits and Scientific Notation Activities of Science Describing Matter Unit 1: Fundamentals of Chemistry Significant Digits and Scientific Notation Qualitative and quantitative measurements
Significant Digits and Scientific Notation Activities of Science Describing Matter Unit 1: Fundamentals of Chemistry Significant Digits and Scientific Notation Qualitative and quantitative measurements
CHEM 2: An Introduction to Inorganic Chemistry
 Dimensional Analysis: Numbers and Units: The English System of units The Metric System of units (SI) Prefixes (kilo-, centi-, milli-, etc.) A systematic method for performing unit conversions Formulating
Dimensional Analysis: Numbers and Units: The English System of units The Metric System of units (SI) Prefixes (kilo-, centi-, milli-, etc.) A systematic method for performing unit conversions Formulating
CHEE 221: Chemical Processes and Systems What is a process?
 CHEE 221: Chemical Processes and Systems What is a process? An operation (or series of operations) by which a particular objective is accomplished Chem Eng: operations that cause a physical or chemical
CHEE 221: Chemical Processes and Systems What is a process? An operation (or series of operations) by which a particular objective is accomplished Chem Eng: operations that cause a physical or chemical
Measurement and Chemical Calculations. Measurement and Chemical Calculations
 Measurement and Chemical Calculations. Chapter 3 Measurement and Chemical Calculations Very large and very small numbers: exponential notation Metric system and SI base units Mass, length, temperature,
Measurement and Chemical Calculations. Chapter 3 Measurement and Chemical Calculations Very large and very small numbers: exponential notation Metric system and SI base units Mass, length, temperature,
ChE 201: Introduction to Chemical Engineering. CHE 201: Introduction to Chemical Engineering Calculations
 بسم االله الرحمن الرحيم CHE 201: Introduction to Chemical Engineering Calculations Dr. Saad Al-Shahrani Text Book: Basic Principles and Calculations in Chemical Engineering, by David M. Himmelblau and
بسم االله الرحمن الرحيم CHE 201: Introduction to Chemical Engineering Calculations Dr. Saad Al-Shahrani Text Book: Basic Principles and Calculations in Chemical Engineering, by David M. Himmelblau and
2 Standards for Measurement. Careful and accurate measurements of ingredients are important both when cooking and in the chemistry laboratory!
 2 Standards for Measurement Careful and accurate measurements of ingredients are important both when cooking and in the chemistry laboratory! Chapter Outline 2.1 Scientific Notation 2.2 Measurement and
2 Standards for Measurement Careful and accurate measurements of ingredients are important both when cooking and in the chemistry laboratory! Chapter Outline 2.1 Scientific Notation 2.2 Measurement and
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin The Metric System by Christopher G. Hamaker Illinois State University Basic Units and Symbols The English
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin The Metric System by Christopher G. Hamaker Illinois State University Basic Units and Symbols The English
Table One. Mass of a small package using three different measurement methods
 MS20 Laboratory Scientific Measurements and the Metric System Objectives To understand how to make measurements utilizing various instruments To learn how to use the metric system To convert between the
MS20 Laboratory Scientific Measurements and the Metric System Objectives To understand how to make measurements utilizing various instruments To learn how to use the metric system To convert between the
Chapter 1 Dimensions, Units, and Their Conversion
 1.1 Units and Dimensions Chemical Engineering principles First Year/ Chapter One Chapter 1 Dimensions, Units, and Their Conversion Dimensions are our basic concepts of measurement such as length, time,
1.1 Units and Dimensions Chemical Engineering principles First Year/ Chapter One Chapter 1 Dimensions, Units, and Their Conversion Dimensions are our basic concepts of measurement such as length, time,
Introduction to Vectors
 Introduction to Vectors 1. Which of the following natural phenomena would serve as the best time standard? a. The mean orbit radius of the earth. b. The period of audible sound waves. c. The time for one
Introduction to Vectors 1. Which of the following natural phenomena would serve as the best time standard? a. The mean orbit radius of the earth. b. The period of audible sound waves. c. The time for one
2.1 The Rectangular Coordinate System
 . The Rectangular Coordinate Sstem In this section ou will learn to: plot points in a rectangular coordinate sstem understand basic functions of the graphing calculator graph equations b generating a table
. The Rectangular Coordinate Sstem In this section ou will learn to: plot points in a rectangular coordinate sstem understand basic functions of the graphing calculator graph equations b generating a table
PREFIXES AND SYMBOLS SI Prefixes you need to know by heart
 PREFIXES AND SYMBOLS SI Prefixes you need to know by heart Prefix Symbol In 10 n in Decimal Forms Giga G 10 9 1,000,000,000 Mega M 10 6 1,000,000 kilo k 10 3 1,000 deci d 10 1 0.1 centi c 10 2 0.01 milli
PREFIXES AND SYMBOLS SI Prefixes you need to know by heart Prefix Symbol In 10 n in Decimal Forms Giga G 10 9 1,000,000,000 Mega M 10 6 1,000,000 kilo k 10 3 1,000 deci d 10 1 0.1 centi c 10 2 0.01 milli
US Customary System (USC)
 What is the length of this line? US Customary System (USC) Based on things that made sense to people Previously known as English (or British) inch = 3 dry, round, barleycorns end-to-end foot = length of
What is the length of this line? US Customary System (USC) Based on things that made sense to people Previously known as English (or British) inch = 3 dry, round, barleycorns end-to-end foot = length of
Measurements in Chemistry
 Measurements in Chemistry Measurements are part of our daily lives. We measure our weight, driving distances and gallons of gasoline. A health professional might measure blood pressure, temperature and
Measurements in Chemistry Measurements are part of our daily lives. We measure our weight, driving distances and gallons of gasoline. A health professional might measure blood pressure, temperature and
# x 10. Quiz 1next Monday, June 18 Names/symbols of common elements Two math problems from chapter 2
 Announcements Wednesday, June 13, 2012 Quiz 1next Monday, June 18 Names/symbols of common elements Two math problems from chapter 2 MasteringChemistry assignments (due at 11:59 pm): Ch 1-2a: this Fri,
Announcements Wednesday, June 13, 2012 Quiz 1next Monday, June 18 Names/symbols of common elements Two math problems from chapter 2 MasteringChemistry assignments (due at 11:59 pm): Ch 1-2a: this Fri,
Measurement. Scientific Notation. Measurements and Problem Solving. Writing Numbers in Scientific Notation
 Measurement Chapter 2 Measurements and Problem Solving Quantitative observation Comparison based on an accepted scale e.g. Meter stick Has 2 parts number and unit Number tells comparison Unit tells scale
Measurement Chapter 2 Measurements and Problem Solving Quantitative observation Comparison based on an accepted scale e.g. Meter stick Has 2 parts number and unit Number tells comparison Unit tells scale
3.1 Exponential Functions and Their Graphs
 .1 Eponential Functions and Their Graphs Sllabus Objective: 9.1 The student will sketch the graph of a eponential, logistic, or logarithmic function. 9. The student will evaluate eponential or logarithmic
.1 Eponential Functions and Their Graphs Sllabus Objective: 9.1 The student will sketch the graph of a eponential, logistic, or logarithmic function. 9. The student will evaluate eponential or logarithmic
Everyday Conversion: Money
 Everyday Conversion: Money Everyday Measurement: Water Everyday Measurement: Water Everyday Accuracy: Weighing Scales The need to measure correctly and convert! Some Interesting Quantities Length Volume
Everyday Conversion: Money Everyday Measurement: Water Everyday Measurement: Water Everyday Accuracy: Weighing Scales The need to measure correctly and convert! Some Interesting Quantities Length Volume
James Chickos Room B435. Introductory Chemistry 1111
 James Chickos Room B435 Introductory Chemistry 1111 What is Chemistry? Chemistry is the study of substances in terms of Composition of Matter What a material it made of Structure of Matter How the elementary
James Chickos Room B435 Introductory Chemistry 1111 What is Chemistry? Chemistry is the study of substances in terms of Composition of Matter What a material it made of Structure of Matter How the elementary
Let s try an example of Unit Analysis. Your friend gives you this formula: x=at. You have to figure out if it s right using Unit Analysis.
 Lecture 1 Introduction to Measurement - SI sstem Dimensional nalsis / Unit nalsis Unit Conversions Vectors and Mathematics International Sstem of Units (SI) Table 1.1, p.5 The Seven Base Units What is
Lecture 1 Introduction to Measurement - SI sstem Dimensional nalsis / Unit nalsis Unit Conversions Vectors and Mathematics International Sstem of Units (SI) Table 1.1, p.5 The Seven Base Units What is
UNIT 1 UNITS AND DIMENSIONS
 UNIT 1 UNITS AND DIMENSIONS Unit is any measure or amount used as a standard for measurement. It is a means of the measurable extent of a physical quantity. The derived unit is a combination of primary
UNIT 1 UNITS AND DIMENSIONS Unit is any measure or amount used as a standard for measurement. It is a means of the measurable extent of a physical quantity. The derived unit is a combination of primary
US Customary System (USC) Systeme Internationale (SI) Prefixes. Units & Significant Figures
 Units & Significant Figures US Customary System (USC) What is the length of this line? Based on things that made sense to people Previously known as English (or British) 1 inch = 3 dry, round, barleycorns
Units & Significant Figures US Customary System (USC) What is the length of this line? Based on things that made sense to people Previously known as English (or British) 1 inch = 3 dry, round, barleycorns
I. Qualit a Qualit t a ive iv vs. Quantit Quan a tit tiv a e tiv Measurements
 I. Qualitative vs. Quantitative Measurements Qualitative Measurement 1) Qualitative measurement = a measurement that gives descriptive, NONnumeric results a)ex: Jillian ran a fast race. b)ex: The light
I. Qualitative vs. Quantitative Measurements Qualitative Measurement 1) Qualitative measurement = a measurement that gives descriptive, NONnumeric results a)ex: Jillian ran a fast race. b)ex: The light
UNIT CONVERSIONS User Guide & Disclaimer
 v.5.4 www.hvacnotebook.com UNIT CONVERSIONS User Guide & Disclaimer (FREE SAMPLE VERSION) Conversion Spreadsheets Distance Weight 34 Simple User Interface Click On Any Yellow Cells And Enter (Replace With)
v.5.4 www.hvacnotebook.com UNIT CONVERSIONS User Guide & Disclaimer (FREE SAMPLE VERSION) Conversion Spreadsheets Distance Weight 34 Simple User Interface Click On Any Yellow Cells And Enter (Replace With)
Name Period Date. Measurements. Fill-in the blanks during the PowerPoint presentation in class.
 Name Period Date Measurements Fill-in the blanks during the PowerPoint presentation in class. What is Scientific Notation? Scientific notation is a way of expressing big numbers and small numbers. It is
Name Period Date Measurements Fill-in the blanks during the PowerPoint presentation in class. What is Scientific Notation? Scientific notation is a way of expressing big numbers and small numbers. It is
1.4 Perform the following unit conversions: (b) (c) s. g s. lb min. (d) (e) in. ft s. m 55 h. (f) ft s. km h. (g)
 1.4 Perform the following unit conversions: 0.05 ft 1 in. (a) 1L 61in. 1L 1ft (b) 1kJ 650 J 10 J 1Btu 1.0551kJ 0.616 Btu (c) 41 Btu/h 0.15 kw 1kW 1h 600 s 778.17 ft lbf 1Btu ft lbf 99.596 s (d) g 78 s
1.4 Perform the following unit conversions: 0.05 ft 1 in. (a) 1L 61in. 1L 1ft (b) 1kJ 650 J 10 J 1Btu 1.0551kJ 0.616 Btu (c) 41 Btu/h 0.15 kw 1kW 1h 600 s 778.17 ft lbf 1Btu ft lbf 99.596 s (d) g 78 s
Chapter 2. Measurements in Chemistry. 2.1 Measurement Systems. Prefixes used in abbreviating measurements
 Chapter 2. Measurements in Chemistry 2.1 Measurement Systems Measuring system is an integral part of any commerce or science for people to agree on the quantities of in their business and practices. In
Chapter 2. Measurements in Chemistry 2.1 Measurement Systems Measuring system is an integral part of any commerce or science for people to agree on the quantities of in their business and practices. In
MEASUREMENTS. Significant Figures
 SIGNIFICANT FIGURES MEASUREMENTS Significant Figures Every measured value, that you record on paper, reflects the precision of the measuring device used to obtain that value. Every calculated value that
SIGNIFICANT FIGURES MEASUREMENTS Significant Figures Every measured value, that you record on paper, reflects the precision of the measuring device used to obtain that value. Every calculated value that
Chapter 1. Introduction: Matter and Measurement
 Chapter 1 Introduction: Matter and Measurement Steps in the Scientific Method 1. Observations - quantitative - qualitative 2. Formulating hypotheses - possible explanation for the observation 3. Performing
Chapter 1 Introduction: Matter and Measurement Steps in the Scientific Method 1. Observations - quantitative - qualitative 2. Formulating hypotheses - possible explanation for the observation 3. Performing
What are these standards? Who decides what they are? How many Standards should we have?
 AP Physics Summer Work: Read the following notes from our first unit. Answer any questions. Do the Algebra Review Sheet. This will allow us to go through this unit very quickly upon your return. PHYSICAL
AP Physics Summer Work: Read the following notes from our first unit. Answer any questions. Do the Algebra Review Sheet. This will allow us to go through this unit very quickly upon your return. PHYSICAL
13) = 4 36 = ) = 5-8 = -3 =3 15) = = -58 = 58 16) = 81-9 = 72 = 72
 Practice Practice Practice 3 ) (-3) + (-6) = -9 ) () + (-5) = -3 3) (-7) + (-) = -8 4) (-3) - (-6) = (-3) + 6 = + 3 5) (+) - (+5) = -3 6) (-7) - (-4) = (-7) + 4 = -3 7) (5)(-4) = - 8) (-3)(-6) = +8 9)
Practice Practice Practice 3 ) (-3) + (-6) = -9 ) () + (-5) = -3 3) (-7) + (-) = -8 4) (-3) - (-6) = (-3) + 6 = + 3 5) (+) - (+5) = -3 6) (-7) - (-4) = (-7) + 4 = -3 7) (5)(-4) = - 8) (-3)(-6) = +8 9)
MOTOR WIRING DATA From National Electrical Code 3 PHASE SQUIRREL CAGE INDUCTION MOTORS 230 Volt 460 Volt Min. # Max. Rating
 MOTOR WIRING DATA From National Electrical Code PHASE SQUIRREL CAGE INDUCTION MOTORS 20 Volt 0 Volt Min. # Max. Rating Min. Size Size of Full Size Wire Conduit Branch Circuit Load Wire AWG (inches) Fuses
MOTOR WIRING DATA From National Electrical Code PHASE SQUIRREL CAGE INDUCTION MOTORS 20 Volt 0 Volt Min. # Max. Rating Min. Size Size of Full Size Wire Conduit Branch Circuit Load Wire AWG (inches) Fuses
In recording measurements, it is necessary to understand 1. SIGNIFICANCE of numbers 2. importance of UNITS.
 CHEMISTRY IS LARGELY A QUANTITATIVE SCIENCE Theories and ideas are tested by measurement Measurements are usually quantitative have numbers Science is built on a foundation of mathematics. In recording
CHEMISTRY IS LARGELY A QUANTITATIVE SCIENCE Theories and ideas are tested by measurement Measurements are usually quantitative have numbers Science is built on a foundation of mathematics. In recording
Introduction. The Scientific Method and Measurement
 Introduction The Scientific Method and Measurement Defining How We Look At The Universe Observation: seeing an event or process in nature we wish to explain Hypothesis: a tentative explanation based on
Introduction The Scientific Method and Measurement Defining How We Look At The Universe Observation: seeing an event or process in nature we wish to explain Hypothesis: a tentative explanation based on
UNITS AND DIMENSIONS. M.Sathyanarayanan AP/CIvil-VCET
 UNITS AND DIMENSIONS UNITS AND DIMENSION Measurement and Units Fundamental units Systems of units Dimensional Analysis UNITS AND DIMENSION :: Why do we need units? We need units because we want to measure
UNITS AND DIMENSIONS UNITS AND DIMENSION Measurement and Units Fundamental units Systems of units Dimensional Analysis UNITS AND DIMENSION :: Why do we need units? We need units because we want to measure
Appendix. Using Your Calculator. Squares, Square Roots, Reciprocals, and Logs. Addition, Subtraction, Multiplication, and Division
 370770_app.qxd 1/9/03 7:2 PM Page A1 mac114 Mac 114:2nd shift:4_rst: Using Your Calculator In this section we will review how to use your calculator to perform common mathematical operations. This discussion
370770_app.qxd 1/9/03 7:2 PM Page A1 mac114 Mac 114:2nd shift:4_rst: Using Your Calculator In this section we will review how to use your calculator to perform common mathematical operations. This discussion
Linear and Nonlinear Systems of Equations. The Method of Substitution. Equation 1 Equation 2. Check (2, 1) in Equation 1 and Equation 2: 2x y 5?
 3330_070.qd 96 /5/05 Chapter 7 7. 9:39 AM Page 96 Sstems of Equations and Inequalities Linear and Nonlinear Sstems of Equations What ou should learn Use the method of substitution to solve sstems of linear
3330_070.qd 96 /5/05 Chapter 7 7. 9:39 AM Page 96 Sstems of Equations and Inequalities Linear and Nonlinear Sstems of Equations What ou should learn Use the method of substitution to solve sstems of linear
Chemistry: The Study of Change Chang & Goldsby 12 th edition
 Chemistry: The Study of Change Chang & Goldsby 12 th edition modified by Dr. Hahn Chapter 1 Example 1.4 Determine the number of significant figures in the following measurements: (a)478 cm (b)6.01 g end
Chemistry: The Study of Change Chang & Goldsby 12 th edition modified by Dr. Hahn Chapter 1 Example 1.4 Determine the number of significant figures in the following measurements: (a)478 cm (b)6.01 g end
Metric Prefixes UNITS & MEASUREMENT 10/6/2015 WHY DO UNITS AND MEASUREMENT MATTER?
 UNITS & MEASUREMENT WHY DO UNITS AND MEASUREMENT MATTER? Chemistry In Action On 9/3/99, $15,000,000 Mars Climate Orbiter entered Mar s atmosphere 100 km (6 miles) lower than planned and was destroyed by
UNITS & MEASUREMENT WHY DO UNITS AND MEASUREMENT MATTER? Chemistry In Action On 9/3/99, $15,000,000 Mars Climate Orbiter entered Mar s atmosphere 100 km (6 miles) lower than planned and was destroyed by
Chapter 2 Measurements & Calculations. Quantity: A thing that can be measured. ex. Length (6.3 ft), mass (35 kg), and time (7.2 s)
 Chapter 2 Measurements & Calculations Quantity: A thing that can be measured. ex. Length (6.3 ft), mass (35 kg), and time (7.2 s) Measurements can be expressed in a variety of units: Example: length(cm,
Chapter 2 Measurements & Calculations Quantity: A thing that can be measured. ex. Length (6.3 ft), mass (35 kg), and time (7.2 s) Measurements can be expressed in a variety of units: Example: length(cm,
Ch. 2 Notes: ANALYZING DATA MEASUREMENT NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 2 Notes: ANALYZING DATA MEASUREMENT NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Units and Measurement - Metrics A. The International System of Units
Ch. 2 Notes: ANALYZING DATA MEASUREMENT NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Units and Measurement - Metrics A. The International System of Units
Engineering Physics CUPY 106 Dr C Sumanya. Office 8 Block 9
 Engineering Phsics CUPY 106 Dr C Sumana Office 8 lock 9 csumana@cut.ac.zw Outline Measurements and Vectors Kinematics and Forces Work and Energ Momentum Impulse and Collisions Fluid Mechanics Oscillation
Engineering Phsics CUPY 106 Dr C Sumana Office 8 lock 9 csumana@cut.ac.zw Outline Measurements and Vectors Kinematics and Forces Work and Energ Momentum Impulse and Collisions Fluid Mechanics Oscillation
CHAPTER 2 Data Analysis
 CHAPTER 2 Data Analysis 2.1 Units of Measurement The standard of measurement used in science are those of the metric system. All the units are based on 10 or multiples of 10. SI Units: The International
CHAPTER 2 Data Analysis 2.1 Units of Measurement The standard of measurement used in science are those of the metric system. All the units are based on 10 or multiples of 10. SI Units: The International
Chem 115 POGIL Worksheet - Week 1 Units, Measurement Uncertainty, and Significant Figures
 Chem 115 POGIL Worksheet - Week 1 Units, Measurement Uncertainty, and Significant Figures Why? All scientists the world over use metric units. Since 1960, the metric system in use has been the Système
Chem 115 POGIL Worksheet - Week 1 Units, Measurement Uncertainty, and Significant Figures Why? All scientists the world over use metric units. Since 1960, the metric system in use has been the Système
Law vs. Theory. Steps in the Scientific Method. Outcomes Over the Long-Term. Measuring Matter in Two Ways
 Law vs. Theory A law summarizes what happens A theory (model) is an attempt to explain why it happens. Unit 2: (Chapter 5) Measurements and Calculations Cartoon courtesy of NearingZero.net Steps in the
Law vs. Theory A law summarizes what happens A theory (model) is an attempt to explain why it happens. Unit 2: (Chapter 5) Measurements and Calculations Cartoon courtesy of NearingZero.net Steps in the
Reference Guide. Science Reference 9/25/ Copyright 1996 Gary Lewis Revisions 2007 by John Pratte
 Reference Guide Contents...1 1. General Scientific Terminology...2 2. Types of Errors...3 3. Scientific Notation...4 4. Significant Figures...6 5. Graphs...7 6. Making Measurements...8 7. Units...9 8.
Reference Guide Contents...1 1. General Scientific Terminology...2 2. Types of Errors...3 3. Scientific Notation...4 4. Significant Figures...6 5. Graphs...7 6. Making Measurements...8 7. Units...9 8.
PREFIXES AND SYMBOLS SI Prefixes you need to know by heart
 PREFIXES AND SYMBOLS SI Prefixes you need to know by heart Prefix Symbol In 10 n in Decimal Forms Giga G 10 9 1,000,000,000 Mega M 10 6 1,000,000 kilo k 10 3 1,000 deci d 10 1 0.1 centi c 10 2 0.01 milli
PREFIXES AND SYMBOLS SI Prefixes you need to know by heart Prefix Symbol In 10 n in Decimal Forms Giga G 10 9 1,000,000,000 Mega M 10 6 1,000,000 kilo k 10 3 1,000 deci d 10 1 0.1 centi c 10 2 0.01 milli
Example 1: The mass of the earth is 5.98 x kg. What is its order of magnitude? What is the order of magnitude for 400?
 Physics 11 Realms of Physics Physics attempts to model the behavior of the universe from the very large scale (entire universe, 10 52 kg, 10 26 m, 10 19 s) to the very small (components of a proton, 10-28
Physics 11 Realms of Physics Physics attempts to model the behavior of the universe from the very large scale (entire universe, 10 52 kg, 10 26 m, 10 19 s) to the very small (components of a proton, 10-28
6.4 graphs OF logarithmic FUnCTIOnS
 SECTION 6. graphs of logarithmic functions 9 9 learning ObjeCTIveS In this section, ou will: Identif the domain of a logarithmic function. Graph logarithmic functions. 6. graphs OF logarithmic FUnCTIOnS
SECTION 6. graphs of logarithmic functions 9 9 learning ObjeCTIveS In this section, ou will: Identif the domain of a logarithmic function. Graph logarithmic functions. 6. graphs OF logarithmic FUnCTIOnS
Today is Tuesday, February 13 th, 2018
 In This Lesson: Scientific Notation and Unit Analysis (Lesson 4 of 6) Today is Tuesday, February 13 th, 2018 Stuff You Need: Calculator Pre-Class: By now you ve probably heard of scientific notation. What
In This Lesson: Scientific Notation and Unit Analysis (Lesson 4 of 6) Today is Tuesday, February 13 th, 2018 Stuff You Need: Calculator Pre-Class: By now you ve probably heard of scientific notation. What
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed.
 Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl, Donald J. DeCoste University of Illinois Chapter 2 Measurements and Calculations
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl, Donald J. DeCoste University of Illinois Chapter 2 Measurements and Calculations
APPENDIX D UNIT CONVERSION TABLES. Sl SYMBOLS AND PREFIXES
 UNIT CONVERSION TABLES Sl SYMBOLS AND PREFIXES BASE UNITS Quantity Unit Symbol Length Meter m Mass Kilogram kg Time Second s Electric current Ampere A Thermodynamic temperature Kelvin K Amount of substance
UNIT CONVERSION TABLES Sl SYMBOLS AND PREFIXES BASE UNITS Quantity Unit Symbol Length Meter m Mass Kilogram kg Time Second s Electric current Ampere A Thermodynamic temperature Kelvin K Amount of substance
1 centimeter (cm) 5 10 millimeters (mm) 1 meter (m) centimeters. 1 kilometer (km) 5 1,000 meters. Set up equivalent ratios and cross multiply.
 Domain 2 Lesson 16 Convert Measurements Common Core State Standard: 6.RP.3.d Getting the Idea The tables below show some conversions for units of length in both the customary system and the metric system.
Domain 2 Lesson 16 Convert Measurements Common Core State Standard: 6.RP.3.d Getting the Idea The tables below show some conversions for units of length in both the customary system and the metric system.
Chemistry Day 39. Friday, December 14 th Monday, December 17 th, 2018
 Chemistry Day 39 Friday, December 14 th Monday, December 17 th, 2018 Do-Now: Reactions Quiz Do-Now 1. Write down today s FLT 2. Copy: KCl + H 2 O à? 3. Identify the type of reaction in #2. 4. Predict the
Chemistry Day 39 Friday, December 14 th Monday, December 17 th, 2018 Do-Now: Reactions Quiz Do-Now 1. Write down today s FLT 2. Copy: KCl + H 2 O à? 3. Identify the type of reaction in #2. 4. Predict the
f 0 ab a b: base f
 Precalculus Notes: Unit Eponential and Logarithmic Functions Sllabus Objective: 9. The student will sketch the graph of a eponential, logistic, or logarithmic function. 9. The student will evaluate eponential
Precalculus Notes: Unit Eponential and Logarithmic Functions Sllabus Objective: 9. The student will sketch the graph of a eponential, logistic, or logarithmic function. 9. The student will evaluate eponential
Unit 1 Part 1: Significant Figures and Scientific Notation. Objective understand significant figures and their rules. Be able to use scientific
 Unit 1 Part 1: Significant Figures and Scientific Notation. Objective understand significant figures and their rules. Be able to use scientific notation in calculations. Significant figures - consist of
Unit 1 Part 1: Significant Figures and Scientific Notation. Objective understand significant figures and their rules. Be able to use scientific notation in calculations. Significant figures - consist of
Chapter 2 Measurement and Problem Solving
 Measurement and Problem Solving What Is a Measurement? Quantitative observation. Comparison to an agreed upon standard. Every measurement has a number and a unit. 2 A Measurement The unit tells you to
Measurement and Problem Solving What Is a Measurement? Quantitative observation. Comparison to an agreed upon standard. Every measurement has a number and a unit. 2 A Measurement The unit tells you to
Ch. 2 Notes: ANALYZING DATA MEASUREMENT NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 2 Notes: ANALYZING DATA MEASUREMENT NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Units and Measurement - Metrics A. The International System of Units
Ch. 2 Notes: ANALYZING DATA MEASUREMENT NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Units and Measurement - Metrics A. The International System of Units
READ ONLY. Adopting Agency BSC SFM. Adopt Entire Chapter X X X X X X X X X Adopt Entire Chapter as amended (amended sections listed below)
 CALIFORNIA MECHANICAL CODE MATRIX ADOPTION TABLE APPENDIX D UNIT CONVERSION TABLES (Matrix Adoption Tables are non-regulatory, intended only as an aid to the user. See Chapter 1 for state agency authority
CALIFORNIA MECHANICAL CODE MATRIX ADOPTION TABLE APPENDIX D UNIT CONVERSION TABLES (Matrix Adoption Tables are non-regulatory, intended only as an aid to the user. See Chapter 1 for state agency authority
Notes Chapter 2: Measurements and Calculations. It is used to easily and simply write very large numbers, and very small numbers.
 Scientific Notation Notes Chapter 2: Measurements and Calculations It is used to easily and simply write very large numbers, and very small numbers. It begins with a number greater than zero & less than
Scientific Notation Notes Chapter 2: Measurements and Calculations It is used to easily and simply write very large numbers, and very small numbers. It begins with a number greater than zero & less than
CHAPTER TWO: MEASUREMENTS AND PROBLEM SOLVING
 CHAPTER TWO: MEASUREMENTS AND PROBLEM SOLVING Measurements: Our Starting Point! Why should we begin our study of chemistry with the topic of measurement?! Much of the laboratory work in this course is
CHAPTER TWO: MEASUREMENTS AND PROBLEM SOLVING Measurements: Our Starting Point! Why should we begin our study of chemistry with the topic of measurement?! Much of the laboratory work in this course is
Chemistry and Measurement
 Chemistry and Measurement What Is Chemistry? Chemistry is the study of the composition, structure, and properties of matter and energy and changes that matter undergoes. Matter is anything that occupies
Chemistry and Measurement What Is Chemistry? Chemistry is the study of the composition, structure, and properties of matter and energy and changes that matter undergoes. Matter is anything that occupies
Math 122 Fall Solutions to Homework #5. ( ) 2 " ln x. y = x 2
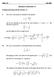 Math 1 Fall 8 Solutions to Homework #5 Problems from Pages 383-38 (Section 7.) 6. The curve in this problem is defined b the equation: = ( ) " ln and we are interested in the part of the curve between
Math 1 Fall 8 Solutions to Homework #5 Problems from Pages 383-38 (Section 7.) 6. The curve in this problem is defined b the equation: = ( ) " ln and we are interested in the part of the curve between
General Chemistry Unit 8 Measurement ( )
 General Chemistry Unit 8 Measurement (2017-2018) Significant Figures Scientific Notation Unit Analysis Unit of Measure Accuracy and Precision Density Percent Error 1 Adding Numbers: Add numbers as you
General Chemistry Unit 8 Measurement (2017-2018) Significant Figures Scientific Notation Unit Analysis Unit of Measure Accuracy and Precision Density Percent Error 1 Adding Numbers: Add numbers as you
International System of Units (SI)
 Measurement International System of Units (SI) revised metric system proposed in 1960 widely used in science 7 base units SI Base Units Length Meter m Mass Kilogram kg Time Second s or sec Electrical current
Measurement International System of Units (SI) revised metric system proposed in 1960 widely used in science 7 base units SI Base Units Length Meter m Mass Kilogram kg Time Second s or sec Electrical current
Chapter 2 Measurement and Problem Solving. What Is a Measurement? Scientific Notation 8/20/09. Introductory Chemistry, 3 rd Edition Nivaldo Tro
 Introductory Chemistry, 3 rd Edition Nivaldo Tro Measurement and Problem Solving Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA 2009, Prentice Hall What Is a Measurement? Quantitative
Introductory Chemistry, 3 rd Edition Nivaldo Tro Measurement and Problem Solving Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA 2009, Prentice Hall What Is a Measurement? Quantitative
ab is shifted horizontally by h units. ab is shifted vertically by k units.
 Algera II Notes Unit Eight: Eponential and Logarithmic Functions Sllaus Ojective: 8. The student will graph logarithmic and eponential functions including ase e. Eponential Function: a, 0, Graph of an
Algera II Notes Unit Eight: Eponential and Logarithmic Functions Sllaus Ojective: 8. The student will graph logarithmic and eponential functions including ase e. Eponential Function: a, 0, Graph of an
Text book. Tenth edition. Walker, Halliday and Resnick. Principles of physics.
 Text book Principles of physics. Tenth edition Walker, Halliday and Resnick Chapter 1 Measurement In this chapter we will explore the following concepts: 1. Measurement of a physical parameter 2. Units,
Text book Principles of physics. Tenth edition Walker, Halliday and Resnick Chapter 1 Measurement In this chapter we will explore the following concepts: 1. Measurement of a physical parameter 2. Units,
Chem 140 Section C Instructor: Ken Marr. Chem 140 Section A Instructor: Ken Marr. Chem 140 Section E Instructor: Ken Marr. Day 1 Activities CHEMISTRY
 Chem 140 Section A Instructor: Ken Marr Weekly Schedule Lecture 9-10, MWF in STB-2 Lab 8-10, Tu in STB-2 8-10, Th in STB-5 Chem 140 Section C Instructor: Ken Marr Weekly Schedule Lecture 10 11, MWF in
Chem 140 Section A Instructor: Ken Marr Weekly Schedule Lecture 9-10, MWF in STB-2 Lab 8-10, Tu in STB-2 8-10, Th in STB-5 Chem 140 Section C Instructor: Ken Marr Weekly Schedule Lecture 10 11, MWF in
Engineering Calculations
 Engineering Calculations (EC) Engineering Calculations In order to properly communicate with other engineers / scientists you must use the correct language.how can you use concise language to convey not
Engineering Calculations (EC) Engineering Calculations In order to properly communicate with other engineers / scientists you must use the correct language.how can you use concise language to convey not
International System of Units (SI)
 Measurement International System of Units (SI) revised metric system proposed in 1960 widely used in science 7 base units SI Base Units Length Meter m Mass Kilogram kg Time Electrical current Second Ampere
Measurement International System of Units (SI) revised metric system proposed in 1960 widely used in science 7 base units SI Base Units Length Meter m Mass Kilogram kg Time Electrical current Second Ampere
3.2 LOGARITHMIC FUNCTIONS AND THEIR GRAPHS
 Section. Logarithmic Functions and Their Graphs 7. LOGARITHMIC FUNCTIONS AND THEIR GRAPHS Ariel Skelle/Corbis What ou should learn Recognize and evaluate logarithmic functions with base a. Graph logarithmic
Section. Logarithmic Functions and Their Graphs 7. LOGARITHMIC FUNCTIONS AND THEIR GRAPHS Ariel Skelle/Corbis What ou should learn Recognize and evaluate logarithmic functions with base a. Graph logarithmic
Chem 115 POGIL Worksheet - Week 1 Units, Measurement Uncertainty, and Significant Figures - Solutions
 Chem 115 POGIL Worksheet - Week 1 Units, Measurement Uncertainty, and Significant Figures - Solutions Key Questions & Exercises 1. Give the names and their abbreviations for the SI units of length, mass,
Chem 115 POGIL Worksheet - Week 1 Units, Measurement Uncertainty, and Significant Figures - Solutions Key Questions & Exercises 1. Give the names and their abbreviations for the SI units of length, mass,
2053 College Physics. Chapter 1 Introduction
 2053 College Physics Chapter 1 Introduction 1 Fundamental Quantities and Their Dimension Length [L] Mass [M] Time [T] other physical quantities can be constructed from these three 2 Systems of Measurement
2053 College Physics Chapter 1 Introduction 1 Fundamental Quantities and Their Dimension Length [L] Mass [M] Time [T] other physical quantities can be constructed from these three 2 Systems of Measurement
