Today: Finite box wavefunctions
|
|
|
- Brook George
- 6 years ago
- Views:
Transcription
1 Toay: Finite bo wavefunctions 1.Think outsie the bo!.solving the S.E. 3.Unerstaning the results. HWK1 ue We. 1AM. Reaing for Monay.: TZ&D Chap. 8
2 Reaing Quiz Classically forbien regions are where A. a particle s total energy is less than its kinetic energy B. a particle s total energy is greater than its kinetic energy C. a particle s total energy is less than its potential energy D. a particle s total energy is greater than its potential energy E. None of the above. Answer is c.
3 Ψ(, t) = L nπ sin( ) e L iet / n= Quantize: k=nπ/l π ml E = n = Quantize: 1 n E What you epect classically: What you get quantum mech.: Electron can have any energy Lowest energy in the bo has zero KE. Particle at rest. Electron can only have specific energies. (quantize) Lowest energy in the bo still has KE! ZERO POINT MOTION Aitional new physics appears when the bo sies are not infinitely high (all real cases!).
4 V() Goo Approimation: Electrons never got out of (< or >L) =. (OK when << work function) L What happens if electron bigger? Eact Potential curve (V): small chance electrons get out of (< or >L)~, but not eactly! What if two s very close to each other? E_total Then whether leaks out a little or not, is very important. How much coupling to other?
5 Nee to solve for eact Potential curve: V(): small chance electrons get out of (< or >L)~, but not eactly! V() L Important for thinking about Quantum tunneling : Raioactive ecay Scanning tunneling microscope to stuy surfaces Bining of molecules Behavior or soli state materials really important ieas. Finite Square Well Work function
6 In Region II, when the wavefunction is negative, it: (A) curves up. (B) curves own. (C) can curve either way (D) Not enough info 4.7eV V() Nee to solve Schroinger Eqn: m + V = E L Region I Region II Region III In Region II total energy E > potential energy V m = ( V E) = k Negative number k is real
7 4.7eV V() Nee to solve Schroinger Eqn: m + V = E L Region I Region II Region III In Region II total energy E > potential energy V m = ( V E) = k Negative number When E>V: Solutions = sin(k), cos(k), e ik. Always epect sinusoial functions k is real
8 4.7eV V() Nee to solve Schroinger Eqn: m + V = E Region II L Region II Region III In Region I an III total energy E < potential energy V m = ( V E) Positive = α α is real What functional forms of () work? a. e iα b. sin(α) c. e α. more than one of these
9 4.7eV V() L Region I Region II Region III III = Ae α + Be α In Region III total energy E < potential energy V m = ( V E) Positive Answer is C: e α coul also be e -α. Eponential ecay or growth = α α is real Why not e iα? LHS RHS α α
10 Back to case of with workfunction of 4.7 ev 4.7 ev = V E particle ev L Positive number m = ( E V ) = α Answer is c coul also be e -α. Eponential ecay or growth = Ae α + Be α > < > < (curves upwar) (curves ownwar)
11 4.7eV V() = I L Region I Region II Region III Ee α + Fe α = C sin( k) D cos( k) II + III = Ae α + Be What will wave function in Region III look like? What makes sense for constants A an B? a. A must be b. B must be c. A an B must be equal. A= an B= e. A an B can be anything, nee more info. α
12 What will wave function in Region III look like? What makes sense for constants A an B? Answer is a. A must be.. otherwise blows up as gets bigger. This oesn t make sense! an probability shoul go to at large! Nee to be able to normalize 4.7eV V() L Region I Region II Region III I = Ee α + Fe α = C sin( k) D cos( k) II + III = Ae α + Be α
13 Outsie well (E<V): (Region I) On HW net week 4.7 ev Insie well (E>V): (Region II) II = k II = C sin( k) D cos( k) II + Outsie well (E<V): (Region III) III = α III = Be III α V= ev L Bounary Conitions: ( L) = continuous ( L) = continuous ( L) = ( L) II III ( L) III ( L = II ) as
14 m = ( V E) 4.7 ev Insie well (E>V): Outsie well (E<V): V= ev L Electron is elocalize sprea out. Some small part of wave is where Total is less than potential energy! Classically forbien regions. The particle tunnels into the forbien regions. TUNNELING is a new quantum effect.
15 L If very very long gets closer an closer, what will happen? a. electron is share between s, with fraction in each constant over time b. the electron will flow away through c. electron will jump back an forth between 1 an. electron stays in 1. e. something else happens.
16 L wi If very very long gets closer an closer, what will happen? a. electron is share between s, with fraction in each constant over time b. the electron will flow away through c. electron will jump back an forth between 1 an. electron stays in 1. e. something else happens.
17 L If gets closer an closer, what will happen? a. electron is share between s, with fraction in each constant over time b. the electron will flow away through c. electron will jump back an forth between 1 an. electron stays in 1. e. something else happens.
18
Today: Particle-in-a-Box wavefunctions
 Today: Particle-in-a-Bo wavefunctions 1.Potential wells.solving the S.E. 3.Understanding the results. HWK11 due Wed. 5PM. Reading for Fri.: TZ&D Chap. 7.1 PE for electrons with most PE. On top work function
Today: Particle-in-a-Bo wavefunctions 1.Potential wells.solving the S.E. 3.Understanding the results. HWK11 due Wed. 5PM. Reading for Fri.: TZ&D Chap. 7.1 PE for electrons with most PE. On top work function
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
Lecture 10: The Schrödinger Equation. Lecture 10, p 2
 Quantum mechanics is the description of the behavior of matter and light in all its details and, in particular, of the happenings on an atomic scale. Things on a very small scale behave like nothing that
Quantum mechanics is the description of the behavior of matter and light in all its details and, in particular, of the happenings on an atomic scale. Things on a very small scale behave like nothing that
Lecture 10: The Schrödinger Equation. Lecture 10, p 2
 Quantum mechanics is the description of the behavior of matter and light in all its details and, in particular, of the happenings on an atomic scale. Things on a very small scale behave like nothing that
Quantum mechanics is the description of the behavior of matter and light in all its details and, in particular, of the happenings on an atomic scale. Things on a very small scale behave like nothing that
Lecture 10: The Schrödinger Equation Lecture 10, p 1
 Lecture 10: The Schrödinger Equation Lecture 10, p 1 Overview Probability distributions Schrödinger s Equation Particle in a Bo Matter waves in an infinite square well Quantized energy levels y() U= n=1
Lecture 10: The Schrödinger Equation Lecture 10, p 1 Overview Probability distributions Schrödinger s Equation Particle in a Bo Matter waves in an infinite square well Quantized energy levels y() U= n=1
Schrödinger s equation.
 Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
Topic 4: The Finite Potential Well
 Topic 4: The Finite Potential Well Outline: The quantum well The finite potential well (FPW) Even parity solutions of the TISE in the FPW Odd parity solutions of the TISE in the FPW Tunnelling into classically
Topic 4: The Finite Potential Well Outline: The quantum well The finite potential well (FPW) Even parity solutions of the TISE in the FPW Odd parity solutions of the TISE in the FPW Tunnelling into classically
Hydrogen atom energies. Friday Honors lecture. Quantum Particle in a box. Classical vs Quantum. Quantum version
 Friday onors lecture Prof. Clint Sprott takes us on a tour of fractals. ydrogen atom energies Quantized energy levels: Each corresponds to different Orbit radius Velocity Particle wavefunction Energy Each
Friday onors lecture Prof. Clint Sprott takes us on a tour of fractals. ydrogen atom energies Quantized energy levels: Each corresponds to different Orbit radius Velocity Particle wavefunction Energy Each
Modern Physics. Unit 3: Operators, Tunneling and Wave Packets Lecture 3.3: The Momentum Operator
 Modern Physics Unit 3: Operators, Tunneling and Wave Packets Lecture 3.3: The Momentum Operator Ron Reifenberger Professor of Physics Purdue University 1 There are many operators in QM H Ψ= EΨ, or ˆop
Modern Physics Unit 3: Operators, Tunneling and Wave Packets Lecture 3.3: The Momentum Operator Ron Reifenberger Professor of Physics Purdue University 1 There are many operators in QM H Ψ= EΨ, or ˆop
Separation of Variables
 Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
It illustrates quantum mechanical principals. It illustrates the use of differential eqns. & boundary conditions to solve for ψ
 MODEL SYSTEM: PARTICLE IN A BOX Important because: It illustrates quantum mechanical principals It illustrates the use of differential eqns. & boundary conditions to solve for ψ It shows how discrete energy
MODEL SYSTEM: PARTICLE IN A BOX Important because: It illustrates quantum mechanical principals It illustrates the use of differential eqns. & boundary conditions to solve for ψ It shows how discrete energy
Nuclear Physics and Astrophysics
 Nuclear Physics an Astrophysics PHY-302 Dr. E. Rizvi Lecture 2 - Introuction Notation Nuclies A Nuclie is a particular an is esignate by the following notation: A CN = Atomic Number (no. of Protons) A
Nuclear Physics an Astrophysics PHY-302 Dr. E. Rizvi Lecture 2 - Introuction Notation Nuclies A Nuclie is a particular an is esignate by the following notation: A CN = Atomic Number (no. of Protons) A
Unit #6 - Families of Functions, Taylor Polynomials, l Hopital s Rule
 Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
Physics 256: Lecture Schrodinger Equation. The Effective Wavelength Time Independent Schrodinger Equation
 Physics 56: Lecture Schroinger Equation The Effective Wavelength Time Inepenent Schroinger Equation Examples Clicker Question 0: What is true about a particle in the first energy eigenfunction for the
Physics 56: Lecture Schroinger Equation The Effective Wavelength Time Inepenent Schroinger Equation Examples Clicker Question 0: What is true about a particle in the first energy eigenfunction for the
The Schrödinger Equation in One Dimension
 The Schrödinger Equation in One Dimension Introduction We have defined a comple wave function Ψ(, t) for a particle and interpreted it such that Ψ ( r, t d gives the probability that the particle is at
The Schrödinger Equation in One Dimension Introduction We have defined a comple wave function Ψ(, t) for a particle and interpreted it such that Ψ ( r, t d gives the probability that the particle is at
Lecture 12: Particle in 1D boxes, Simple Harmonic Oscillators
 Lecture 1: Particle in 1D boes, Simple Harmonic Oscillators U U() ψ() U n= n=0 n=1 n=3 Lecture 1, p 1 This week and last week are critical for the course: Week 3, Lectures 7-9: Week 4, Lectures 10-1: Light
Lecture 1: Particle in 1D boes, Simple Harmonic Oscillators U U() ψ() U n= n=0 n=1 n=3 Lecture 1, p 1 This week and last week are critical for the course: Week 3, Lectures 7-9: Week 4, Lectures 10-1: Light
Math 1271 Solutions for Fall 2005 Final Exam
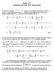 Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
Part 1. The Quantum Particle
 CONTACT CONTACT Introuction to Nanoelectronics Part. The Quantum Particle This class is concerne with the propagation of electrons in conuctors. Here in Part, we will begin by introucing the tools from
CONTACT CONTACT Introuction to Nanoelectronics Part. The Quantum Particle This class is concerne with the propagation of electrons in conuctors. Here in Part, we will begin by introucing the tools from
Section 7.1: Integration by Parts
 Section 7.1: Integration by Parts 1. Introuction to Integration Techniques Unlike ifferentiation where there are a large number of rules which allow you (in principle) to ifferentiate any function, the
Section 7.1: Integration by Parts 1. Introuction to Integration Techniques Unlike ifferentiation where there are a large number of rules which allow you (in principle) to ifferentiate any function, the
Calculations on the One-dimensional H-Atom in Coordinate and Momentum Space
 Calculations on the One-imensional H-Atom in Coorinate an Momentum Space Frank Riou Chemistry Department CSB SJU The following eercises pertain to several of the l = states of the one-imensional hyrogen
Calculations on the One-imensional H-Atom in Coorinate an Momentum Space Frank Riou Chemistry Department CSB SJU The following eercises pertain to several of the l = states of the one-imensional hyrogen
is even and ψ 1 ( ξ 2 1)e ξ 2 dξ = 2 p 2 dx =! 2 2α 2! 2 π ξ 4 e ξ 2 dξ =
 Physics 0 Homework #6 Spring 06 Due Friay 5/3/6. Griffith s. a. The expectation value of the position (an momentum by virtue of Ehrenfest s theorem) will be zero. Since ψ 0 is even an ψ is o, ψ will be
Physics 0 Homework #6 Spring 06 Due Friay 5/3/6. Griffith s. a. The expectation value of the position (an momentum by virtue of Ehrenfest s theorem) will be zero. Since ψ 0 is even an ψ is o, ψ will be
Numerical Solution of the Time-Independent 1-D Schrodinger Equation
 Numerical Solution of the Time-Independent -D Schrodinger Equation Gavin Cheung 932873 December 4, 2 Abstract The -D time independent Schrodinger Equation is solved numerically using the Numerov algorithm.
Numerical Solution of the Time-Independent -D Schrodinger Equation Gavin Cheung 932873 December 4, 2 Abstract The -D time independent Schrodinger Equation is solved numerically using the Numerov algorithm.
Today: Examples of Tunneling
 Today: Examples of Tunneling 1. Last time: Scanning tunneling microscope. 2. Next: Alpha particle tunneling HWK13 Postponed until next week. STM (picture with reversed voltage, works exactly the same)
Today: Examples of Tunneling 1. Last time: Scanning tunneling microscope. 2. Next: Alpha particle tunneling HWK13 Postponed until next week. STM (picture with reversed voltage, works exactly the same)
:C O: σorbitals of CO. πorbitals of CO. Assumed knowledge. Chemistry 2. Learning outcomes. Lecture 2 Particle in a box approximation. C 2p.
 Chemistry 2 Lecture 2 Particle in a bo approimation Assumed knowledge Be able to predict the geometry of a hydrocarbon from its structure and account for each valence electron. Predict the hybridization
Chemistry 2 Lecture 2 Particle in a bo approimation Assumed knowledge Be able to predict the geometry of a hydrocarbon from its structure and account for each valence electron. Predict the hybridization
Answers to Coursebook questions Chapter 5.6
 Answers to Courseook questions Chapter 56 Questions marke with a star (*) use the formula for the magnetic fiel create y a current μi ( = ) which is not on the syllaus an so is not eaminale See Figure
Answers to Courseook questions Chapter 56 Questions marke with a star (*) use the formula for the magnetic fiel create y a current μi ( = ) which is not on the syllaus an so is not eaminale See Figure
Probability, Expectation Values, and Uncertainties
 Chapter 5 Probability, Epectation Values, and Uncertainties As indicated earlier, one of the remarkable features of the physical world is that randomness is incarnate, irreducible. This is mirrored in
Chapter 5 Probability, Epectation Values, and Uncertainties As indicated earlier, one of the remarkable features of the physical world is that randomness is incarnate, irreducible. This is mirrored in
Problem Set 5: Solutions
 University of Alabama Department of Physics and Astronomy PH 53 / eclair Spring 1 Problem Set 5: Solutions 1. Solve one of the exam problems that you did not choose.. The Thompson model of the atom. Show
University of Alabama Department of Physics and Astronomy PH 53 / eclair Spring 1 Problem Set 5: Solutions 1. Solve one of the exam problems that you did not choose.. The Thompson model of the atom. Show
Problem Set 2: Solutions
 UNIVERSITY OF ALABAMA Department of Physics an Astronomy PH 102 / LeClair Summer II 2010 Problem Set 2: Solutions 1. The en of a charge rubber ro will attract small pellets of Styrofoam that, having mae
UNIVERSITY OF ALABAMA Department of Physics an Astronomy PH 102 / LeClair Summer II 2010 Problem Set 2: Solutions 1. The en of a charge rubber ro will attract small pellets of Styrofoam that, having mae
Integration by Parts
 Integration by Parts 6-3-207 If u an v are functions of, the Prouct Rule says that (uv) = uv +vu Integrate both sies: (uv) = uv = uv + u v + uv = uv vu, vu v u, I ve written u an v as shorthan for u an
Integration by Parts 6-3-207 If u an v are functions of, the Prouct Rule says that (uv) = uv +vu Integrate both sies: (uv) = uv = uv + u v + uv = uv vu, vu v u, I ve written u an v as shorthan for u an
Electric Charge and Electrostatic Force
 PHY 049 Lecture Notes Chapter : Page 1 of 8 Electric Charge an Electrostatic Force Contemporary vision: all forces of nature can be viewe as interaction between "charges", specific funamental properties
PHY 049 Lecture Notes Chapter : Page 1 of 8 Electric Charge an Electrostatic Force Contemporary vision: all forces of nature can be viewe as interaction between "charges", specific funamental properties
Lecture 12: Particle in 1D boxes & Simple Harmonic Oscillator
 Lecture 12: Particle in 1D boxes & Simple Harmonic Oscillator U(x) E Dx y(x) x Dx Lecture 12, p 1 Properties of Bound States Several trends exhibited by the particle-in-box states are generic to bound
Lecture 12: Particle in 1D boxes & Simple Harmonic Oscillator U(x) E Dx y(x) x Dx Lecture 12, p 1 Properties of Bound States Several trends exhibited by the particle-in-box states are generic to bound
The Quantum Theory of Atoms and Molecules
 The Quantum Theory of Atoms and Molecules The postulates of quantum mechanics Dr Grant Ritchie The postulates.. 1. Associated with any particle moving in a conservative field of force is a wave function,
The Quantum Theory of Atoms and Molecules The postulates of quantum mechanics Dr Grant Ritchie The postulates.. 1. Associated with any particle moving in a conservative field of force is a wave function,
Module FP2. Further Pure 2. Cambridge University Press Further Pure 2 and 3 Hugh Neill and Douglas Quadling Excerpt More information
 5548993 - Further Pure an 3 Moule FP Further Pure 5548993 - Further Pure an 3 Differentiating inverse trigonometric functions Throughout the course you have graually been increasing the number of functions
5548993 - Further Pure an 3 Moule FP Further Pure 5548993 - Further Pure an 3 Differentiating inverse trigonometric functions Throughout the course you have graually been increasing the number of functions
The Description of the microscopic world
 The Description of the microscopic worl This Friay Honor lecture Previous Lecture: Quantization of light, photons Photoelectric effect Particle-Wave ualism Catherine Woowar Botany Photosynthesis This Lecture:
The Description of the microscopic worl This Friay Honor lecture Previous Lecture: Quantization of light, photons Photoelectric effect Particle-Wave ualism Catherine Woowar Botany Photosynthesis This Lecture:
PHYS Concept Tests Fall 2009
 PHYS 30 Concept Tests Fall 009 In classical mechanics, given the state (i.e. position and velocity) of a particle at a certain time instant, the state of the particle at a later time A) cannot be determined
PHYS 30 Concept Tests Fall 009 In classical mechanics, given the state (i.e. position and velocity) of a particle at a certain time instant, the state of the particle at a later time A) cannot be determined
CHAPTER 6 Quantum Mechanics II
 CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
Physics 505 Electricity and Magnetism Fall 2003 Prof. G. Raithel. Problem Set 3. 2 (x x ) 2 + (y y ) 2 + (z + z ) 2
 Physics 505 Electricity an Magnetism Fall 003 Prof. G. Raithel Problem Set 3 Problem.7 5 Points a): Green s function: Using cartesian coorinates x = (x, y, z), it is G(x, x ) = 1 (x x ) + (y y ) + (z z
Physics 505 Electricity an Magnetism Fall 003 Prof. G. Raithel Problem Set 3 Problem.7 5 Points a): Green s function: Using cartesian coorinates x = (x, y, z), it is G(x, x ) = 1 (x x ) + (y y ) + (z z
Lecture 4: Solution of Schrodinger Equation
 NCN www.nanohub.org EE 66: Solid State Devices Lecture 4: Solution of Schrodinger Equation Muhammad Ashraful Alam alam@purdue.edu Alam ECE 66 S9 1 Outline 1) Time independent independent Schrodinger Equation
NCN www.nanohub.org EE 66: Solid State Devices Lecture 4: Solution of Schrodinger Equation Muhammad Ashraful Alam alam@purdue.edu Alam ECE 66 S9 1 Outline 1) Time independent independent Schrodinger Equation
Calculus in the AP Physics C Course The Derivative
 Limits an Derivatives Calculus in the AP Physics C Course The Derivative In physics, the ieas of the rate change of a quantity (along with the slope of a tangent line) an the area uner a curve are essential.
Limits an Derivatives Calculus in the AP Physics C Course The Derivative In physics, the ieas of the rate change of a quantity (along with the slope of a tangent line) an the area uner a curve are essential.
ECE606: Solid State Devices Lecture 3
 ECE66: Solid State Devices Lecture 3 Gerhard Klimeck gekco@purdue.edu Motivation Periodic Structure E Time-independent Schrodinger Equation ħ d Ψ dψ + U ( x) Ψ = iħ m dx dt Assume Ψ( x, t) = ψ( x) e iet/
ECE66: Solid State Devices Lecture 3 Gerhard Klimeck gekco@purdue.edu Motivation Periodic Structure E Time-independent Schrodinger Equation ħ d Ψ dψ + U ( x) Ψ = iħ m dx dt Assume Ψ( x, t) = ψ( x) e iet/
Based on transitions between bands electrons delocalized rather than bound to particular atom
 EE31 Lasers I 1/01/04 #6 slie 1 Review: Semiconuctor Lasers Base on transitions between bans electrons elocalize rather than boun to particular atom transitions between bans Direct electrical pumping high
EE31 Lasers I 1/01/04 #6 slie 1 Review: Semiconuctor Lasers Base on transitions between bans electrons elocalize rather than boun to particular atom transitions between bans Direct electrical pumping high
What are these waves?
 PH300 Modern Phsics SP11 Is the state of the cat to be created onl when a phsicist inestigates the situation at some definite time? Nobod reall doubts that the presence or absence of a dead cat is something
PH300 Modern Phsics SP11 Is the state of the cat to be created onl when a phsicist inestigates the situation at some definite time? Nobod reall doubts that the presence or absence of a dead cat is something
Math 210 Midterm #1 Review
 Math 20 Miterm # Review This ocument is intene to be a rough outline of what you are expecte to have learne an retaine from this course to be prepare for the first miterm. : Functions Definition: A function
Math 20 Miterm # Review This ocument is intene to be a rough outline of what you are expecte to have learne an retaine from this course to be prepare for the first miterm. : Functions Definition: A function
The Simple Harmonic Oscillator
 The Simple Harmonic Oscillator Michael Fowler, University of Virginia Einstein s Solution of the Specific Heat Puzzle The simple harmonic oscillator, a nonrelativistic particle in a potential ½C, is a
The Simple Harmonic Oscillator Michael Fowler, University of Virginia Einstein s Solution of the Specific Heat Puzzle The simple harmonic oscillator, a nonrelativistic particle in a potential ½C, is a
Physics 121 for Majors
 Physics 121 for Majors Scheule Do Post-Class Quiz #3 Do Pre-Class Quiz #4 HW #2 is ue Wenesay Quiz #1 is ue Saturay, Sept. 16 Lab #1 is set up now, ue Monay Some Department Resources Computers N-212 ESC
Physics 121 for Majors Scheule Do Post-Class Quiz #3 Do Pre-Class Quiz #4 HW #2 is ue Wenesay Quiz #1 is ue Saturay, Sept. 16 Lab #1 is set up now, ue Monay Some Department Resources Computers N-212 ESC
STEP Support Programme. Pure STEP 3 Solutions
 STEP Support Programme Pure STEP 3 Solutions S3 Q6 Preparation Completing the square on gives + + y, so the centre is at, and the radius is. First draw a sketch of y 4 3. This has roots at and, and you
STEP Support Programme Pure STEP 3 Solutions S3 Q6 Preparation Completing the square on gives + + y, so the centre is at, and the radius is. First draw a sketch of y 4 3. This has roots at and, and you
There is light at the end of the tunnel. -- proverb. The light at the end of the tunnel is just the light of an oncoming train. --R.
 A vast time bubble has been projected into the future to the precise moment of the end of the universe. This is, of course, impossible. --D. Adams, The Hitchhiker s Guide to the Galaxy There is light at
A vast time bubble has been projected into the future to the precise moment of the end of the universe. This is, of course, impossible. --D. Adams, The Hitchhiker s Guide to the Galaxy There is light at
Basic methods to solve equations
 Roberto s Notes on Prerequisites for Calculus Chapter 1: Algebra Section 1 Basic methods to solve equations What you need to know already: How to factor an algebraic epression. What you can learn here:
Roberto s Notes on Prerequisites for Calculus Chapter 1: Algebra Section 1 Basic methods to solve equations What you need to know already: How to factor an algebraic epression. What you can learn here:
RFSS: Lecture 4 Alpha Decay
 RFSS: Lecture 4 Alpha Decay Reaings Nuclear an Raiochemistry: Chapter 3 Moern Nuclear Chemistry: Chapter 7 Energetics of Alpha Decay Geiger Nuttall base theory Theory of Alpha Decay Hinrance Factors Different
RFSS: Lecture 4 Alpha Decay Reaings Nuclear an Raiochemistry: Chapter 3 Moern Nuclear Chemistry: Chapter 7 Energetics of Alpha Decay Geiger Nuttall base theory Theory of Alpha Decay Hinrance Factors Different
1 dx. where is a large constant, i.e., 1, (7.6) and Px is of the order of unity. Indeed, if px is given by (7.5), the inequality (7.
 Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
Final Exam. Tuesday, May 8, Starting at 8:30 a.m., Hoyt Hall.
 Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Summary of Chapter 38 In Quantum Mechanics particles are represented by wave functions Ψ. The absolute square of the wave function Ψ 2
Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Summary of Chapter 38 In Quantum Mechanics particles are represented by wave functions Ψ. The absolute square of the wave function Ψ 2
Hydrogen atom energies. From Last Time. Today. Another question. Hydrogen atom question. Compton scattering and Photoelectric effect
 From ast Time Observation of atoms indicated quantized energy states. Atom only emitted certain wavelengths of light Structure of the allowed wavelengths indicated the what the energy structure was Quantum
From ast Time Observation of atoms indicated quantized energy states. Atom only emitted certain wavelengths of light Structure of the allowed wavelengths indicated the what the energy structure was Quantum
From Last time. Exam 3 results. Probability. The wavefunction. Example wavefunction. Discrete vs continuous. De Broglie wavelength
 From ast time Eam 3 results De Broglie wavelength Uncertainty principle Eam average ~ 70% Scores posted on learn@uw D C BC B AB A Wavefunction of a particle Course evaluations: Tuesday, Dec. 9 Tue. Dec.
From ast time Eam 3 results De Broglie wavelength Uncertainty principle Eam average ~ 70% Scores posted on learn@uw D C BC B AB A Wavefunction of a particle Course evaluations: Tuesday, Dec. 9 Tue. Dec.
Physics 443, Solutions to PS 4
 Physics, Solutions to PS. Neutrino Oscillations a Energy eigenvalues and eigenvectors The eigenvalues of H are E E + A, and E E A and the eigenvectors are ν, ν And ν ν b Similarity transformation S S ν
Physics, Solutions to PS. Neutrino Oscillations a Energy eigenvalues and eigenvectors The eigenvalues of H are E E + A, and E E A and the eigenvectors are ν, ν And ν ν b Similarity transformation S S ν
4. Important theorems in quantum mechanics
 TFY4215 Kjemisk fysikk og kvantemekanikk - Tillegg 4 1 TILLEGG 4 4. Important theorems in quantum mechanics Before attacking three-imensional potentials in the next chapter, we shall in chapter 4 of this
TFY4215 Kjemisk fysikk og kvantemekanikk - Tillegg 4 1 TILLEGG 4 4. Important theorems in quantum mechanics Before attacking three-imensional potentials in the next chapter, we shall in chapter 4 of this
Quantum Theory. Thornton and Rex, Ch. 6
 Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Non-Equilibrium Continuum Physics TA session #10 TA: Yohai Bar Sinai Dislocations
 Non-Equilibrium Continuum Physics TA session #0 TA: Yohai Bar Sinai 0.06.206 Dislocations References There are countless books about islocations. The ones that I recommen are Theory of islocations, Hirth
Non-Equilibrium Continuum Physics TA session #0 TA: Yohai Bar Sinai 0.06.206 Dislocations References There are countless books about islocations. The ones that I recommen are Theory of islocations, Hirth
Chapter. 5 Bound States: Simple Case
 Announcement Course webpage http://highenergy.phys.ttu.edu/~slee/2402/ Textbook PHYS-2402 Lecture 12 HW3 (due 3/2) 13, 15, 20, 31, 36, 41, 48, 53, 63, 66 ***** Exam: 3/12 Ch.2, 3, 4, 5 Feb. 26, 2015 Physics
Announcement Course webpage http://highenergy.phys.ttu.edu/~slee/2402/ Textbook PHYS-2402 Lecture 12 HW3 (due 3/2) 13, 15, 20, 31, 36, 41, 48, 53, 63, 66 ***** Exam: 3/12 Ch.2, 3, 4, 5 Feb. 26, 2015 Physics
Chapter 1 Overview: Review of Derivatives
 Chapter Overview: Review of Derivatives The purpose of this chapter is to review the how of ifferentiation. We will review all the erivative rules learne last year in PreCalculus. In the net several chapters,
Chapter Overview: Review of Derivatives The purpose of this chapter is to review the how of ifferentiation. We will review all the erivative rules learne last year in PreCalculus. In the net several chapters,
Sample Quantum Chemistry Exam 1 Solutions
 Chemistry 46 Fall 217 Dr Jean M Standard September 27, 217 Name SAMPE EXAM Sample Quantum Chemistry Exam 1 Solutions 1 (24 points Answer the following questions by selecting the correct answer from the
Chemistry 46 Fall 217 Dr Jean M Standard September 27, 217 Name SAMPE EXAM Sample Quantum Chemistry Exam 1 Solutions 1 (24 points Answer the following questions by selecting the correct answer from the
ECE 487 Lecture 5 : Foundations of Quantum Mechanics IV Class Outline:
 ECE 487 Lecture 5 : Foundations of Quantum Mechanics IV Class Outline: Linearly Varying Potential Triangular Potential Well Time-Dependent Schrödinger Equation Things you should know when you leave Key
ECE 487 Lecture 5 : Foundations of Quantum Mechanics IV Class Outline: Linearly Varying Potential Triangular Potential Well Time-Dependent Schrödinger Equation Things you should know when you leave Key
PHYS 3313 Section 001 Lecture #20
 PHYS 3313 Section 001 ecture #0 Monday, April 10, 017 Dr. Amir Farbin Infinite Square-well Potential Finite Square Well Potential Penetration Depth Degeneracy Simple Harmonic Oscillator 1 Announcements
PHYS 3313 Section 001 ecture #0 Monday, April 10, 017 Dr. Amir Farbin Infinite Square-well Potential Finite Square Well Potential Penetration Depth Degeneracy Simple Harmonic Oscillator 1 Announcements
Electron in a Box. A wave packet in a square well (an electron in a box) changing with time.
 Electron in a Box A wave packet in a square well (an electron in a box) changing with time. Last Time: Light Wave model: Interference pattern is in terms of wave intensity Photon model: Interference in
Electron in a Box A wave packet in a square well (an electron in a box) changing with time. Last Time: Light Wave model: Interference pattern is in terms of wave intensity Photon model: Interference in
Chap. 3. Elementary Quantum Physics
 Chap. 3. Elementary Quantum Physics 3.1 Photons - Light: e.m "waves" - interference, diffraction, refraction, reflection with y E y Velocity = c Direction of Propagation z B z Fig. 3.1: The classical view
Chap. 3. Elementary Quantum Physics 3.1 Photons - Light: e.m "waves" - interference, diffraction, refraction, reflection with y E y Velocity = c Direction of Propagation z B z Fig. 3.1: The classical view
Probability and Normalization
 Probability and Normalization Although we don t know exactly where the particle might be inside the box, we know that it has to be in the box. This means that, ψ ( x) dx = 1 (normalization condition) L
Probability and Normalization Although we don t know exactly where the particle might be inside the box, we know that it has to be in the box. This means that, ψ ( x) dx = 1 (normalization condition) L
Rutherford s Gold Foil Experiment. Quantum Theory Max Planck (1910)
 Neils Bohr Niels Bohr (1913) developed the planetary model of the atom based upon the following: Rutherford s Gold Foil Experiment E = mc 2 Albert Einstein (1905) Quantum Theory Max Planck (1910) He postulated
Neils Bohr Niels Bohr (1913) developed the planetary model of the atom based upon the following: Rutherford s Gold Foil Experiment E = mc 2 Albert Einstein (1905) Quantum Theory Max Planck (1910) He postulated
Lecture 6: Calculus. In Song Kim. September 7, 2011
 Lecture 6: Calculus In Song Kim September 7, 20 Introuction to Differential Calculus In our previous lecture we came up with several ways to analyze functions. We saw previously that the slope of a linear
Lecture 6: Calculus In Song Kim September 7, 20 Introuction to Differential Calculus In our previous lecture we came up with several ways to analyze functions. We saw previously that the slope of a linear
Kinetic Energy Is Important in the Nanoscale World
 Kinetic Energy Is Important in the Nanoscale Worl Frank Riou Department of Chemistry College of St. Beneict & St. John's University St. Joseph, MN 56374 Most eplanations of atomic an molecular phenomena
Kinetic Energy Is Important in the Nanoscale Worl Frank Riou Department of Chemistry College of St. Beneict & St. John's University St. Joseph, MN 56374 Most eplanations of atomic an molecular phenomena
Physics 43 Chapter 41 Homework #11 Key
 Physics 43 Chapter 4 Homework # Key π sin. A particle in an infinitely deep square well has a wave function given by ( ) for and zero otherwise. Determine the epectation value of. Determine the probability
Physics 43 Chapter 4 Homework # Key π sin. A particle in an infinitely deep square well has a wave function given by ( ) for and zero otherwise. Determine the epectation value of. Determine the probability
QUANTUM CHEMISTRY PROJECT 1
 Chemistry 460 Fall 2017 Dr. Jean M. Standard September 11, 2017 QUANTUM CHEMISTRY PROJECT 1 OUTLINE This project focuses on applications and solutions of quantum mechanical problems involving one-dimensional
Chemistry 460 Fall 2017 Dr. Jean M. Standard September 11, 2017 QUANTUM CHEMISTRY PROJECT 1 OUTLINE This project focuses on applications and solutions of quantum mechanical problems involving one-dimensional
Advanced Quantum Mechanics, Notes based on online course given by Leonard Susskind - Lecture 8
 Advanced Quantum Mechanics, Notes based on online course given by Leonard Susskind - Lecture 8 If neutrinos have different masses how do you mix and conserve energy Mass is energy. The eigenstates of energy
Advanced Quantum Mechanics, Notes based on online course given by Leonard Susskind - Lecture 8 If neutrinos have different masses how do you mix and conserve energy Mass is energy. The eigenstates of energy
18 EVEN MORE CALCULUS
 8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
Vectors in two dimensions
 Vectors in two imensions Until now, we have been working in one imension only The main reason for this is to become familiar with the main physical ieas like Newton s secon law, without the aitional complication
Vectors in two imensions Until now, we have been working in one imension only The main reason for this is to become familiar with the main physical ieas like Newton s secon law, without the aitional complication
MTH 133 Solutions to Exam 1 October 10, Without fully opening the exam, check that you have pages 1 through 11.
 MTH 33 Solutions to Eam October 0, 08 Name: Section: Recitation Instructor: INSTRUCTIONS Fill in your name, etc. on this first page. Without fully opening the eam, check that you have pages through. Show
MTH 33 Solutions to Eam October 0, 08 Name: Section: Recitation Instructor: INSTRUCTIONS Fill in your name, etc. on this first page. Without fully opening the eam, check that you have pages through. Show
Relevant self-assessment exercises: [LIST SELF-ASSESSMENT EXERCISES HERE]
![Relevant self-assessment exercises: [LIST SELF-ASSESSMENT EXERCISES HERE] Relevant self-assessment exercises: [LIST SELF-ASSESSMENT EXERCISES HERE]](/thumbs/89/100348917.jpg) Chapter 5 Fourier Analysis of Finite Difference Methods In this lecture, we determine the stability of PDE discretizations using Fourier analysis. First, we begin with Fourier analysis of PDE s, and then
Chapter 5 Fourier Analysis of Finite Difference Methods In this lecture, we determine the stability of PDE discretizations using Fourier analysis. First, we begin with Fourier analysis of PDE s, and then
Quantum Mechanics. incorporate the phenomenon of quantum tunneling, which we explore in this theory for explaining the behavior of
 chapter 41 Quantum Mechanics 41.1 The Wave Function 41. Analysis Model: Quantum Particle Under Boundary Conditions 41.3 The Schrödinger Equation 41.4 A Particle in a Well of Finite Height 41.5 Tunneling
chapter 41 Quantum Mechanics 41.1 The Wave Function 41. Analysis Model: Quantum Particle Under Boundary Conditions 41.3 The Schrödinger Equation 41.4 A Particle in a Well of Finite Height 41.5 Tunneling
Quantum Mechanics Basics II
 Quantum Mechanics Basics II VBS/MRC Quantum Mechanics Basics II 0 Basic Postulates of Quantum Mechanics 1. State of a Particle Wave Functions 2. Physical Observables Hermitian Operators 3. Outcome of Experiment
Quantum Mechanics Basics II VBS/MRC Quantum Mechanics Basics II 0 Basic Postulates of Quantum Mechanics 1. State of a Particle Wave Functions 2. Physical Observables Hermitian Operators 3. Outcome of Experiment
The Schrodinger Equation
 The Schrodinger quation Dae Yong JONG Inha University nd week Review lectron device (electrical energy traducing) Understand the electron properties energy point of view To know the energy of electron
The Schrodinger quation Dae Yong JONG Inha University nd week Review lectron device (electrical energy traducing) Understand the electron properties energy point of view To know the energy of electron
Lectures - Week 10 Introduction to Ordinary Differential Equations (ODES) First Order Linear ODEs
 Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
AP CALCULUS AB Summer Work. The following are guidelines for completing the summer work packet
 Name: Perio: AP CALCULUS AB Summer Work For stuents to successfully complete the objectives of the AP Calculus curriculum, the stuent must emonstrate a high level of inepenence, capability, eication, an
Name: Perio: AP CALCULUS AB Summer Work For stuents to successfully complete the objectives of the AP Calculus curriculum, the stuent must emonstrate a high level of inepenence, capability, eication, an
De Broglie s Pilot Waves
 De Broglie s Pilot Waves Bohr s Moel of the Hyrogen tom: One way to arrive at Bohr s hypothesis is to think of the electron not as a particle but as a staning wave at raius r aroun the proton. Thus, nλ
De Broglie s Pilot Waves Bohr s Moel of the Hyrogen tom: One way to arrive at Bohr s hypothesis is to think of the electron not as a particle but as a staning wave at raius r aroun the proton. Thus, nλ
MTH 133 Solutions to Exam 1 February 21, Without fully opening the exam, check that you have pages 1 through 11.
 MTH Solutions to Eam February, 8 Name: Section: Recitation Instructor: INSTRUCTIONS Fill in your name, etc. on this first page. Without fully opening the eam, check that you have pages through. Show all
MTH Solutions to Eam February, 8 Name: Section: Recitation Instructor: INSTRUCTIONS Fill in your name, etc. on this first page. Without fully opening the eam, check that you have pages through. Show all
CHM 671. Homework set # 4. 2) Do problems 2.3, 2.4, 2.8, 2.9, 2.10, 2.12, 2.15 and 2.19 in the book.
 CHM 67 Homework set # 4 Due: Thursday, September 28 th ) Read Chapter 2 in the 4 th edition Atkins & Friedman's Molecular Quantum Mechanics book. 2) Do problems 2.3, 2.4, 2.8, 2.9, 2.0, 2.2, 2.5 and 2.9
CHM 67 Homework set # 4 Due: Thursday, September 28 th ) Read Chapter 2 in the 4 th edition Atkins & Friedman's Molecular Quantum Mechanics book. 2) Do problems 2.3, 2.4, 2.8, 2.9, 2.0, 2.2, 2.5 and 2.9
Derivatives and the Product Rule
 Derivatives an the Prouct Rule James K. Peterson Department of Biological Sciences an Department of Mathematical Sciences Clemson University January 28, 2014 Outline Differentiability Simple Derivatives
Derivatives an the Prouct Rule James K. Peterson Department of Biological Sciences an Department of Mathematical Sciences Clemson University January 28, 2014 Outline Differentiability Simple Derivatives
Model Problems 09 - Ch.14 - Engel/ Particle in box - all texts. Consider E-M wave 1st wave: E 0 e i(kx ωt) = E 0 [cos (kx - ωt) i sin (kx - ωt)]
![Model Problems 09 - Ch.14 - Engel/ Particle in box - all texts. Consider E-M wave 1st wave: E 0 e i(kx ωt) = E 0 [cos (kx - ωt) i sin (kx - ωt)] Model Problems 09 - Ch.14 - Engel/ Particle in box - all texts. Consider E-M wave 1st wave: E 0 e i(kx ωt) = E 0 [cos (kx - ωt) i sin (kx - ωt)]](/thumbs/79/80354810.jpg) VI 15 Model Problems 09 - Ch.14 - Engel/ Particle in box - all texts Consider E-M wave 1st wave: E 0 e i(kx ωt) = E 0 [cos (kx - ωt) i sin (kx - ωt)] magnitude: k = π/λ ω = πc/λ =πν ν = c/λ moves in space
VI 15 Model Problems 09 - Ch.14 - Engel/ Particle in box - all texts Consider E-M wave 1st wave: E 0 e i(kx ωt) = E 0 [cos (kx - ωt) i sin (kx - ωt)] magnitude: k = π/λ ω = πc/λ =πν ν = c/λ moves in space
Physics 2112 Unit 5: Electric Potential Energy
 Physics 11 Unit 5: Electric Potential Energy Toay s Concept: Electric Potential Energy Unit 5, Slie 1 Stuff you aske about: I on't like this return to mechanics an the potential energy concept, but this
Physics 11 Unit 5: Electric Potential Energy Toay s Concept: Electric Potential Energy Unit 5, Slie 1 Stuff you aske about: I on't like this return to mechanics an the potential energy concept, but this
Final Exam - Solutions PHYS/ECE Fall 2011
 Final Exam - Solutions PHYS/ECE 34 - Fall 211 Problem 1 Cosmic Rays The telescope array project in Millard County, UT can detect cosmic rays with energies up to E 1 2 ev. The cosmic rays are of unknown
Final Exam - Solutions PHYS/ECE 34 - Fall 211 Problem 1 Cosmic Rays The telescope array project in Millard County, UT can detect cosmic rays with energies up to E 1 2 ev. The cosmic rays are of unknown
One Dimensional Convection: Interpolation Models for CFD
 One Dimensional Convection: Interpolation Moels for CFD ME 448/548 Notes Geral Recktenwal Portlan State University Department of Mechanical Engineering gerry@p.eu ME 448/548: D Convection-Di usion Equation
One Dimensional Convection: Interpolation Moels for CFD ME 448/548 Notes Geral Recktenwal Portlan State University Department of Mechanical Engineering gerry@p.eu ME 448/548: D Convection-Di usion Equation
Harmonic Oscillator Eigenvalues and Eigenfunctions
 Chemistry 46 Fall 217 Dr. Jean M. Standard October 4, 217 Harmonic Oscillator Eigenvalues and Eigenfunctions The Quantum Mechanical Harmonic Oscillator The quantum mechanical harmonic oscillator in one
Chemistry 46 Fall 217 Dr. Jean M. Standard October 4, 217 Harmonic Oscillator Eigenvalues and Eigenfunctions The Quantum Mechanical Harmonic Oscillator The quantum mechanical harmonic oscillator in one
Chem 452 Mega Practice Exam 1
 Last Name: First Name: PSU ID #: Chem 45 Mega Practice Exam 1 Cover Sheet Closed Book, Notes, and NO Calculator The exam will consist of approximately 5 similar questions worth 4 points each. This mega-exam
Last Name: First Name: PSU ID #: Chem 45 Mega Practice Exam 1 Cover Sheet Closed Book, Notes, and NO Calculator The exam will consist of approximately 5 similar questions worth 4 points each. This mega-exam
Quantum Theory. Thornton and Rex, Ch. 6
 Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Mathematics 116 HWK 25a Solutions 8.6 p610
 Mathematics 6 HWK 5a Solutions 8.6 p6 Problem, 8.6, p6 Fin a power series representation for the function f() = etermine the interval of convergence. an Solution. Begin with the geometric series = + +
Mathematics 6 HWK 5a Solutions 8.6 p6 Problem, 8.6, p6 Fin a power series representation for the function f() = etermine the interval of convergence. an Solution. Begin with the geometric series = + +
Lecture 16: 3D Potentials and the Hydrogen Atom. 1 = π. r = a 0. P(r) ( ) h E. E n. Lecture 16, p 2
 It was almost as incredible as if ou fired a 15-inch shell at a piece of tissue paper, and it came back to hit ou! --E. Rutherford (on the discover of the nucleus) ecture 16, p 1 ecture 16, p ecture 16:
It was almost as incredible as if ou fired a 15-inch shell at a piece of tissue paper, and it came back to hit ou! --E. Rutherford (on the discover of the nucleus) ecture 16, p 1 ecture 16, p ecture 16:
Math 342 Partial Differential Equations «Viktor Grigoryan
 Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
Lecture 2 - First order linear PDEs and PDEs from physics
 18.15 - Introuction to PEs, Fall 004 Prof. Gigliola Staffilani Lecture - First orer linear PEs an PEs from physics I mentione in the first class some basic PEs of first an secon orer. Toay we illustrate
18.15 - Introuction to PEs, Fall 004 Prof. Gigliola Staffilani Lecture - First orer linear PEs an PEs from physics I mentione in the first class some basic PEs of first an secon orer. Toay we illustrate
Solving the Schrödinger Equation for the 1 Electron Atom (Hydrogen-Like)
 Stockton Univeristy Chemistry Program, School of Natural Sciences an Mathematics 101 Vera King Farris Dr, Galloway, NJ CHEM 340: Physical Chemistry II Solving the Schröinger Equation for the 1 Electron
Stockton Univeristy Chemistry Program, School of Natural Sciences an Mathematics 101 Vera King Farris Dr, Galloway, NJ CHEM 340: Physical Chemistry II Solving the Schröinger Equation for the 1 Electron
UNDERSTANDING INTEGRATION
 UNDERSTANDING INTEGRATION Dear Reaer The concept of Integration, mathematically speaking, is the "Inverse" of the concept of result, the integration of, woul give us back the function f(). This, in a way,
UNDERSTANDING INTEGRATION Dear Reaer The concept of Integration, mathematically speaking, is the "Inverse" of the concept of result, the integration of, woul give us back the function f(). This, in a way,
Problem Set 6: Workbook on Operators, and Dirac Notation Solution
 Moern Physics: Home work 5 Due ate: 0 March. 014 Problem Set 6: Workbook on Operators, an Dirac Notation Solution 1. nswer 1: a The cat is being escribe by the state, ψ >= ea > If we try to observe it
Moern Physics: Home work 5 Due ate: 0 March. 014 Problem Set 6: Workbook on Operators, an Dirac Notation Solution 1. nswer 1: a The cat is being escribe by the state, ψ >= ea > If we try to observe it
2.13 Linearization and Differentials
 Linearization and Differentials Section Notes Page Sometimes we can approimate more complicated functions with simpler ones These would give us enough accuracy for certain situations and are easier to
Linearization and Differentials Section Notes Page Sometimes we can approimate more complicated functions with simpler ones These would give us enough accuracy for certain situations and are easier to
Physics 8 Friday, October 25, 2013
 Physics 8 Friday, October 25, 2013 Hold on to your HW8 paper. Don t turn it in yet! What we covered in class this week went much more slowly than I had expected. We spent a lot of time discussing the ideas
Physics 8 Friday, October 25, 2013 Hold on to your HW8 paper. Don t turn it in yet! What we covered in class this week went much more slowly than I had expected. We spent a lot of time discussing the ideas
