Introduction to geochemical modeling
|
|
|
- Annis Quinn
- 6 years ago
- Views:
Transcription
1 Introducton to geochemcal modelng Prof. Dr. Broder J. Merkel Char of Hydrogeology Technsche Unverstät Bergakademe Freberg Germany What determnes the dstrbuton of aquatc speces? Interactons of aquatc speces among themselves (complexaton, redox reactons) of aquatc speces wth gases of aquatc speces wth mneral phases (dssoluton / precptaton, sorpton) Transport processes Decay processes (bologcal degradaton, radoactve decay) 1
2 What determnes the dstrbuton of aquatc speces? Defnton of chemcal reactons thermodynamcal approach mass acton law (MAL) Gbbs free energy knetc approach 2
3 mass acton law aa + bb cc + dd K c d {} C {} D a { A} { B} b mass acton law aa + bb cc + dd cc + dd aa + bb K c d {} C {} D a { A} { B} b a b K* { A} { B} 1 c d {} C {} D K 3
4 Gbbs free energy G H S 0 * T G Gbbs free energy H enthalpy S 0 entropy T temperature Gbbs free energy G 0 G + R T ln c d {} C {} D a { A} { B} b R general gas constant (8.315 J/Kmol) G 0 standard Gbbs free energy at 25 C and 100 kpa 4
5 actvty / concentraton Ionc strenght ( I mol/l ) a f c f onspecfc correcton, < 1 (.e. a < c) I 0.5 m z 2 wth m molalty of a speces z valence of a speces Relatonshp onc strength and actvtycoeffcent f
6 Ion dssocaton theory Debye-Hückel-equaton 2 log(f ) A z I I < mol/kg z valence, A temp.dependng parameter extended Debye-Hückel-equaton A z I log(f ) I < 1 + B a I mol/kg B temp.dependng param., a on spec. param. (dependng on on radus) Güntelberg-equaton 2 I log(f ) 0.5z I < 0.1 mol/kg 1 + 1,4 I Ion dssocaton theory Daves-equaton 2 I log(f ) A z ( 1+ I - 0.3I) I < 0.5mol/kg WATQ-Debye-Hückel-equaton 2 A z I log(f ) + b I I < 1mol/kg 1 + B a I A, B temp.dependng param., a on spec. param. (dependng on on radus) Ion dssocaton theory ends at 1 mol/kg at the latest! 6
7 Specfc Ion Interacton (SIP) model at onc strengths > 1 mol/l (e.g. Ptzer equaton) Ionc strenght mol/l Actvty coeffcent Specff Ion Interacton (SIP) theory onc strenghts > 1 mol/kg e.g. salne waters For comparson: 1 kg water ~ 55.3 mol water Ptzer-equaton Debye-Hückel-equaton + Vral equaton 7
8 Comparson on dssocaton vs specfc on nteracton (SIP Ptzer) theory 1 actvty coeffcent Ptzer DebyeHückel DebyeHückelx ext.debyehückel ext.debyehückelx Güntelberg Güntelbergx Daves Davesx WATQ WATQx Na onc strength [mol/kg] What determnes the dstrbuton of aquatc speces? Interactons of aquatc speces among themselves (complexaton, redox reactons) of aquatc speces wth gases of aquatc speces wth mneral phases (dssoluton / precptaton, sorpton) Transport processes Decay processes (bologcal degradaton, radoactve decay) 8
9 Dssoluton / Precptaton AB A + K { A} { B} { AB} B snce {AB} sold phase 1 Ion actvty product IAP {A} {B} Dependency of the on actvty product temperature partal pressure ph H complex formaton / complex destructon 9
10 Saturaton Index SI SI log(iap) - log (K) log (IAP/K) IAP > K IAP/K > 1 IAP < K IAP/K < 1 Defnes whether a water s over- or under saturated wth respect to a certan mneral Saturaton Index SI SI > 0? Water s super saturated wth respect to a certan mneral. Wll the mneral precptate? Not necessarly, depends on knetcs and boundary condtons SI < 0? Water s under saturated wth respect to certan mneral Wll the mneral dssolve? yes, but dssoluton mght be very slowly 10
11 Autoprotolyss of water H 2 O H + + OH K w + { H }{ OH } { H O} 2 + K W {H } {OH } SATP Standard Ambent Temperature and Pressure ph-value neutral: {H + } {OH - }.e. K W {H + } {OH - } {H + } resp. {H + } 10-7 snce ph -log {H + } -log ph < 7 acd ph > 7 alkalne 11
12 What determnes the dstrbuton of aquatc speces? Interactons of aquatc speces among themselves (complexaton, redox reactons) of aquatc speces wth gases of aquatc speces wth mneral phases (dssoluton / precptaton, sorpton) Transport processes Decay processes (bologcal degradaton, radoactve decay) Henry-Law c K H p xample Ntrogen N 2 : p(n 2 ) 0.78 bar, Henry constant mol/(kg. kpa) at 25 C c mol/kg/kpa kpa 0.5 mmol/kg c 0.5 mmol/kg 2 14 mg/mmol 14 mg/kg temperature [ C] Henry-const. N 2 [ 10-6 mol/kg/kpa] conc. mg/l
13 Henry-Law xample Carbon-Doxde CO 2 Henry constant mol/kg/bar at 25 C (log K H ) 3 Vol% CO 2 n sol gas (0.03 bar) c bar c 1 mmol/kg 44 mg/kg CO 2 rght? CO 2(g) CO 2(aq) CO 2(aq) + H 2 O HCO 3- + H + Ca 2+ + HCO - 3 CaHCO + 3 CO H + HCO - 3 CaCO 3(s) Ca 2+ + CO 2-3 etc. open closed systems open system closed system CO 2 CO 2 CO 2 Wasser Calct Wasser Calct 13
14 What determnes the dstrbuton of aquatc speces? Interactons of aquatc speces among themselves (complexaton, redox reactons) of aquatc speces wth gases of aquatc speces wth mneral phases (dssoluton / precptaton, sorpton) Transport processes Decay processes (bologcal degradaton, radoactve decay) Redox potental - H -value [oxdzed speces] + n[e ] [reduced speces] H 0 + R T [ox] ln n F [red] 0 normal redox potental (V) R general gas constant (8.315 J/K mol) T absolute temperature (K) n number of transfered electrons (e) F FARADAY-constant (96484 C/mol J/V mol) [ox] actvty of oxdzed partners [red] actvty of reduced partners 14
15 15 Redox potental at standard condtons 25 C [ox] [red] log n H [red] [ox] ln F n T R 0 H + [ox] [red] log F n T R H NRNST-potental Redox potental of ph-dependng reactons [ox] [red] log F n T R ph F n T R m H [ox] [red] log F n T R H
16 Redox potental n dependency of temperature H M (T 25 C) Potental varatons at ph-varatons of 1 ph-unt mv be 25 C mv be 20 C mv be 10 C mv be 0 p - concept [oxdzed speces] + n[e ] [reduced speces] log K log [red] [ox] [e - ] n [red] 1 log K log + log n [ox] [e ] log K - log[e ] - [red] log [ox] - n log[e 1 1 [red] logk - log n n [ox] ] - n p - [red] log[e ] logk - log [ox] 1 n log K 1 n log [red] [ox] 16
17 Transformaton pe - H - p - log[e ] F 2.303R T H at standard condtons (25 C) approxmately: p 16.9 H H n Volt measured whth H 2 - probe! at ph : Ranges of H n natural groundwater -400 mv to +800 mv oxdzed condtons partly reduced condtons reduced condtons 17
18 pe-ph-dagram ron (predomnance-dagram) Vald for: The system Fe-H + -OH - Certan onc strenght Certan temeperature Certan ron concentraton 18
Chapter 18, Part 1. Fundamentals of Atmospheric Modeling
 Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
If two volatile and miscible liquids are combined to form a solution, Raoult s law is not obeyed. Use the experimental data in Table 9.
 9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
CHEMISTRY Midterm #2 answer key October 25, 2005
 CHEMISTRY 123-01 Mdterm #2 answer key October 25, 2005 Statstcs: Average: 70 pts (70%); Hghest: 97 pts (97%); Lowest: 33 pts (33%) Number of students performng at or above average: 62 (63%) Number of students
CHEMISTRY 123-01 Mdterm #2 answer key October 25, 2005 Statstcs: Average: 70 pts (70%); Hghest: 97 pts (97%); Lowest: 33 pts (33%) Number of students performng at or above average: 62 (63%) Number of students
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Table 1. Solubility of gypsum and anhydrite adopted in this research and the predicted SI values
 Supporting information: Table 1. Solubility of gypsum and anhydrite adopted in this research and the predicted SI values Sample T ID # ( o C) 16, 65, 74 P NaCl CaSO 4 nh 2 O SI System (psi) (mol/kg H 2
Supporting information: Table 1. Solubility of gypsum and anhydrite adopted in this research and the predicted SI values Sample T ID # ( o C) 16, 65, 74 P NaCl CaSO 4 nh 2 O SI System (psi) (mol/kg H 2
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Mass Transfer Processes
 Mass Transfer Processes S. Majd Hassanzadeh Department of Earth Scences Faculty of Geoscences Utrecht Unversty Outlne: 1. Measures of Concentraton 2. Volatlzaton and Dssoluton 3. Adsorpton Processes 4.
Mass Transfer Processes S. Majd Hassanzadeh Department of Earth Scences Faculty of Geoscences Utrecht Unversty Outlne: 1. Measures of Concentraton 2. Volatlzaton and Dssoluton 3. Adsorpton Processes 4.
Chemistry 163B Free Energy and Equilibrium E&R ( ch 6)
 Chemstry 163B Free Energy and Equlbrum E&R ( ch 6) 1 ΔG reacton and equlbrum (frst pass) 1. ΔG < spontaneous ( natural, rreversble) ΔG = equlbrum (reversble) ΔG > spontaneous n reverse drecton. ΔG = ΔHΔS
Chemstry 163B Free Energy and Equlbrum E&R ( ch 6) 1 ΔG reacton and equlbrum (frst pass) 1. ΔG < spontaneous ( natural, rreversble) ΔG = equlbrum (reversble) ΔG > spontaneous n reverse drecton. ΔG = ΔHΔS
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
between standard Gibbs free energies of formation for products and reactants, ΔG! R = ν i ΔG f,i, we
 hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Be true to your work, your word, and your friend.
 Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
PART I: MULTIPLE CHOICE (32 questions, each multiple choice question has a 2-point value, 64 points total).
 CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
Solution Thermodynamics
 CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
CH2351 Chemcal Engneerng Thermodynamcs II Unt I, II www.msubbu.n Soluton Thermodynamcs www.msubbu.n Dr. M. Subramanan Assocate Professor Department of Chemcal Engneerng Sr Svasubramanya Nadar College of
Gasometric Determination of NaHCO 3 in a Mixture
 60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
Topic 3 : Thermodynamics
 GEOL360 LECTURE NOTES: T3 : THERMODYNAMICS 1/20 GEOL360 Topc 3 : Thermodynamcs 3.1 Introducton and vocabulary Thermodynamcs deals wth the physcal and chemcal changes of matter due to work and hear flow
GEOL360 LECTURE NOTES: T3 : THERMODYNAMICS 1/20 GEOL360 Topc 3 : Thermodynamcs 3.1 Introducton and vocabulary Thermodynamcs deals wth the physcal and chemcal changes of matter due to work and hear flow
Chem 2A Exam 1. First letter of your last name
 Frst letter of your last name NAME: PERM# INSTRUCTIONS: Fll n your name, perm number and frst ntal of your last name above. Be sure to show all of your work for full credt. Use the back of the page f necessary.
Frst letter of your last name NAME: PERM# INSTRUCTIONS: Fll n your name, perm number and frst ntal of your last name above. Be sure to show all of your work for full credt. Use the back of the page f necessary.
Name: SID: Discussion Session:
 Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Solutions Review Worksheet
 Solutons Revew Worksheet NOTE: Namng acds s ntroduced on pages 163-4 and agan on pages 208-9.. You learned ths and were quzzed on t, but snce acd names are n the Data Booklet you wll not be tested on ths
Solutons Revew Worksheet NOTE: Namng acds s ntroduced on pages 163-4 and agan on pages 208-9.. You learned ths and were quzzed on t, but snce acd names are n the Data Booklet you wll not be tested on ths
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
II. Equilibrium Thermodynamics. Lecture 8: The Nernst Equation
 II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
Phase equilibria for the oxygen-water system up to elevated temperatures and pressures
 Phase equlbra for the oxygen-water system up to elevated temperatures and pressures Xaoyan J 1, 2, Xaohua Lu 2, Jnyue Yan 1,3* 1 Department of Chemcal Engneerng and Technology / Energy Processes, Royal
Phase equlbra for the oxygen-water system up to elevated temperatures and pressures Xaoyan J 1, 2, Xaohua Lu 2, Jnyue Yan 1,3* 1 Department of Chemcal Engneerng and Technology / Energy Processes, Royal
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Environmental Chemodynamics
 Envronmental Chemodynamcs Instructor: Gregory Möller, Ph.D. Unversty of Idaho Learnng Objectves Lst the thermodynamc functons used to descrbe the energy status of molecules n an envronmental system. Understand
Envronmental Chemodynamcs Instructor: Gregory Möller, Ph.D. Unversty of Idaho Learnng Objectves Lst the thermodynamc functons used to descrbe the energy status of molecules n an envronmental system. Understand
3) Thermodynamic equation to characterize interfaces
 3) Thermodynamc equaton to characterze nterfaces 3.1) Gbbs Model Realty: rapd contnuous change of chemcal and thermodynamc propertes Replaced by model (constant propertes up to the surface) uv bulk uv
3) Thermodynamc equaton to characterze nterfaces 3.1) Gbbs Model Realty: rapd contnuous change of chemcal and thermodynamc propertes Replaced by model (constant propertes up to the surface) uv bulk uv
Lecture # 9. Chapter 8. Activity and Systematic Treatment of Equilibrium. K = [Fe(SCN) 2+ ] [Fe 3+ ] [SCN - ] Fe 3+ + SCN - Fe(SCN) 2+
![Lecture # 9. Chapter 8. Activity and Systematic Treatment of Equilibrium. K = [Fe(SCN) 2+ ] [Fe 3+ ] [SCN - ] Fe 3+ + SCN - Fe(SCN) 2+ Lecture # 9. Chapter 8. Activity and Systematic Treatment of Equilibrium. K = [Fe(SCN) 2+ ] [Fe 3+ ] [SCN - ] Fe 3+ + SCN - Fe(SCN) 2+](/thumbs/74/70057063.jpg) Lecture # 9 Chapter 8 Activity and Systematic Treatment of Equilibrium K = [Fe(SCN) ] [Fe 3 ] [SCN ] Fe 3 SCN Fe(SCN) CaCO 3 (s) Ca (aq) CO K sp =.5 x 10 9 CaCO 3 (s) CO H O Ca HCO 3 CaCO 3 (s) Ca (aq)
Lecture # 9 Chapter 8 Activity and Systematic Treatment of Equilibrium K = [Fe(SCN) ] [Fe 3 ] [SCN ] Fe 3 SCN Fe(SCN) CaCO 3 (s) Ca (aq) CO K sp =.5 x 10 9 CaCO 3 (s) CO H O Ca HCO 3 CaCO 3 (s) Ca (aq)
EXAM I Comparative Animal Physiology ZOO 424 Fall 2002
 EXAM I Comparatve Anmal Physology ZOO 424 Fall 2002 V Eq = RT X o. ln( [ zf [ X ) RT p K[K o pna[na o pcl[cl V = m ln F pk[k pna[na pcl[cl o I = g(v m V eq. ) Q = C m V m Drvng Force = V m V eq. Ionc Speces
EXAM I Comparatve Anmal Physology ZOO 424 Fall 2002 V Eq = RT X o. ln( [ zf [ X ) RT p K[K o pna[na o pcl[cl V = m ln F pk[k pna[na pcl[cl o I = g(v m V eq. ) Q = C m V m Drvng Force = V m V eq. Ionc Speces
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Model. formulation. procedure
 nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
V T for n & P = constant
 Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Determination of Structure and Formation Conditions of Gas Hydrate by Using TPD Method and Flash Calculations
 nd atonal Iranan Conference on Gas Hydrate (ICGH) Semnan Unersty Determnaton of Structure and Formaton Condtons of Gas Hydrate by Usng TPD Method and Flash Calculatons H. Behat Rad, F. Varamnan* Department
nd atonal Iranan Conference on Gas Hydrate (ICGH) Semnan Unersty Determnaton of Structure and Formaton Condtons of Gas Hydrate by Usng TPD Method and Flash Calculatons H. Behat Rad, F. Varamnan* Department
Available online at Energy Procedia 4 (2011) Energy Procedia 00 (2010) GHGT-10
 Avalable onlne at www.scencedrect.com Energy Proceda 4 (011) 179 186 Energy Proceda 00 (010) 000 000 Energy Proceda www.elsever.com/locate/xxx www.elsever.com/locate/proceda GHGT10 Solublty of Carbon Doxde
Avalable onlne at www.scencedrect.com Energy Proceda 4 (011) 179 186 Energy Proceda 00 (010) 000 000 Energy Proceda www.elsever.com/locate/xxx www.elsever.com/locate/proceda GHGT10 Solublty of Carbon Doxde
A Modulated Hydrothermal (MHT) Approach for the Facile. Synthesis of UiO-66-Type MOFs
 Supplementary Informaton A Modulated Hydrothermal (MHT) Approach for the Facle Synthess of UO-66-Type MOFs Zhgang Hu, Yongwu Peng, Zx Kang, Yuhong Qan, and Dan Zhao * Department of Chemcal and Bomolecular
Supplementary Informaton A Modulated Hydrothermal (MHT) Approach for the Facle Synthess of UO-66-Type MOFs Zhgang Hu, Yongwu Peng, Zx Kang, Yuhong Qan, and Dan Zhao * Department of Chemcal and Bomolecular
Chapter 3 Thermochemistry of Fuel Air Mixtures
 Chapter 3 Thermochemstry of Fuel Ar Mxtures 3-1 Thermochemstry 3- Ideal Gas Model 3-3 Composton of Ar and Fuels 3-4 Combuston Stochometry t 3-5 The1 st Law of Thermodynamcs and Combuston 3-6 Thermal converson
Chapter 3 Thermochemstry of Fuel Ar Mxtures 3-1 Thermochemstry 3- Ideal Gas Model 3-3 Composton of Ar and Fuels 3-4 Combuston Stochometry t 3-5 The1 st Law of Thermodynamcs and Combuston 3-6 Thermal converson
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
j) = 1 (note sigma notation) ii. Continuous random variable (e.g. Normal distribution) 1. density function: f ( x) 0 and f ( x) dx = 1
 Random varables Measure of central tendences and varablty (means and varances) Jont densty functons and ndependence Measures of assocaton (covarance and correlaton) Interestng result Condtonal dstrbutons
Random varables Measure of central tendences and varablty (means and varances) Jont densty functons and ndependence Measures of assocaton (covarance and correlaton) Interestng result Condtonal dstrbutons
Electrochemistry Thermodynamics
 CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Thermo-Calc Software. Modelling Multicomponent Precipitation Kinetics with CALPHAD-Based Tools. EUROMAT2013, September 8-13, 2013 Sevilla, Spain
 Modellng Multcomponent Precptaton Knetcs wth CALPHAD-Based Tools Kasheng Wu 1, Gustaf Sterner 2, Qng Chen 2, Åke Jansson 2, Paul Mason 1, Johan Bratberg 2 and Anders Engström 2 1 Inc., 2 AB EUROMAT2013,
Modellng Multcomponent Precptaton Knetcs wth CALPHAD-Based Tools Kasheng Wu 1, Gustaf Sterner 2, Qng Chen 2, Åke Jansson 2, Paul Mason 1, Johan Bratberg 2 and Anders Engström 2 1 Inc., 2 AB EUROMAT2013,
a for save as PDF Chemistry 163B Introduction to Multicomponent Systems and Partial Molar Quantities
 a for save as PDF Chemstry 163B Introducton to Multcomponent Systems and Partal Molar Quanttes 1 the problem of partal mmolar quanttes mx: 10 moles ethanol C 2 H 5 OH (580 ml) wth 1 mole water H 2 O (18
a for save as PDF Chemstry 163B Introducton to Multcomponent Systems and Partal Molar Quanttes 1 the problem of partal mmolar quanttes mx: 10 moles ethanol C 2 H 5 OH (580 ml) wth 1 mole water H 2 O (18
CHAPTER 7 ENERGY BALANCES SYSTEM SYSTEM. * What is energy? * Forms of Energy. - Kinetic energy (KE) - Potential energy (PE) PE = mgz
 SYSTM CHAPTR 7 NRGY BALANCS 1 7.1-7. SYSTM nergy & 1st Law of Thermodynamcs * What s energy? * Forms of nergy - Knetc energy (K) K 1 mv - Potental energy (P) P mgz - Internal energy (U) * Total nergy,
SYSTM CHAPTR 7 NRGY BALANCS 1 7.1-7. SYSTM nergy & 1st Law of Thermodynamcs * What s energy? * Forms of nergy - Knetc energy (K) K 1 mv - Potental energy (P) P mgz - Internal energy (U) * Total nergy,
Lecture 8. Chapter 7. - Thermodynamic Web - Departure Functions - Review Equations of state (chapter 4, briefly)
 Lecture 8 Chapter 5 - Thermodynamc Web - Departure Functons - Revew Equatons of state (chapter 4, brefly) Chapter 6 - Equlbrum (chemcal potental) * Pure Component * Mxtures Chapter 7 - Fugacty (chemcal
Lecture 8 Chapter 5 - Thermodynamc Web - Departure Functons - Revew Equatons of state (chapter 4, brefly) Chapter 6 - Equlbrum (chemcal potental) * Pure Component * Mxtures Chapter 7 - Fugacty (chemcal
Groundwater chemistry
 Read: Ch. 3, sections 1, 2, 3, 5, 7, 9; Ch. 7, sections 2, 3 PART 14 Groundwater chemistry Introduction Matter present in water can be divided into three categories: (1) Suspended solids (finest among
Read: Ch. 3, sections 1, 2, 3, 5, 7, 9; Ch. 7, sections 2, 3 PART 14 Groundwater chemistry Introduction Matter present in water can be divided into three categories: (1) Suspended solids (finest among
The ChemSep Book. Harry A. Kooijman Consultant. Ross Taylor Clarkson University, Potsdam, New York University of Twente, Enschede, The Netherlands
 The ChemSep Book Harry A. Koojman Consultant Ross Taylor Clarkson Unversty, Potsdam, New York Unversty of Twente, Enschede, The Netherlands Lbr Books on Demand www.bod.de Copyrght c 2000 by H.A. Koojman
The ChemSep Book Harry A. Koojman Consultant Ross Taylor Clarkson Unversty, Potsdam, New York Unversty of Twente, Enschede, The Netherlands Lbr Books on Demand www.bod.de Copyrght c 2000 by H.A. Koojman
Introductory Inorganic Chemistry
 Introductory Inorganic Chemistry What is Inorganic Chemistry? As: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3 Classes of Inorganic Substances Elements Ionic Compounds Covalent Compounds Atomic/Molecular
Introductory Inorganic Chemistry What is Inorganic Chemistry? As: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3 Classes of Inorganic Substances Elements Ionic Compounds Covalent Compounds Atomic/Molecular
Chapter 2: Equilibrium Thermodynamics and Kinetics
 Chapter 2: Equilibrium Thermodynamics and Kinetics Equilibrium Thermodynamics: predicts the concentrations (or more precisely, activities) of various species and phases if a reaction reaches equilibrium.
Chapter 2: Equilibrium Thermodynamics and Kinetics Equilibrium Thermodynamics: predicts the concentrations (or more precisely, activities) of various species and phases if a reaction reaches equilibrium.
General Thermodynamics for Process Simulation. Dr. Jungho Cho, Professor Department of Chemical Engineering Dong Yang University
 General Thermodynamcs for Process Smulaton Dr. Jungho Cho, Professor Department of Chemcal Engneerng Dong Yang Unversty Four Crtera for Equlbra μ = μ v Stuaton α T = T β α β P = P l μ = μ l1 l 2 Thermal
General Thermodynamcs for Process Smulaton Dr. Jungho Cho, Professor Department of Chemcal Engneerng Dong Yang Unversty Four Crtera for Equlbra μ = μ v Stuaton α T = T β α β P = P l μ = μ l1 l 2 Thermal
Electrical double layer: revisit based on boundary conditions
 Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Chapter 2. Electrode/electrolyte interface: ----Structure and properties
 Chapter 2 Electrode/electrolyte nterface: ----Structure and propertes Electrochemcal reactons are nterfacal reactons, the structure and propertes of electrode / electrolytc soluton nterface greatly nfluences
Chapter 2 Electrode/electrolyte nterface: ----Structure and propertes Electrochemcal reactons are nterfacal reactons, the structure and propertes of electrode / electrolytc soluton nterface greatly nfluences
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
Thermodynamics II. Department of Chemical Engineering. Prof. Kim, Jong Hak
 Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
VAPOR LIQUID EQUILIBRIUM DATA GENERATION FOR ACETIC ACID AND p-xylene AT ATMOSPHERIC PRESSURE
 Int. J. Chem. Sc.: 14(3), 2016, 1511-1519 ISSN 0972-768X www.sadgurupublcatons.com VAPOR LIQUID EQUILIBRIUM DATA GENERATION FOR ACETIC ACID AND p-xylene AT ATMOSPHERIC PRESSURE PAWAN KIRAN MALI *,a and
Int. J. Chem. Sc.: 14(3), 2016, 1511-1519 ISSN 0972-768X www.sadgurupublcatons.com VAPOR LIQUID EQUILIBRIUM DATA GENERATION FOR ACETIC ACID AND p-xylene AT ATMOSPHERIC PRESSURE PAWAN KIRAN MALI *,a and
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
Wilbur and Ague 4 WILBUR AND AGUE; APPENDIX DR1. Two-dimensional chemical maps as well as chemical profiles were done at 15 kv using
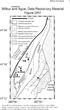 DR2006139 Wlbur and Ague 4 WILBUR AND AGUE; APPENDIX DR1 MINERAL ANALYSES Two-dmensonal chemcal maps as well as chemcal profles were done at 15 kv usng the JEOL JXA-8600 electron mcroprobe at Yale Unversty
DR2006139 Wlbur and Ague 4 WILBUR AND AGUE; APPENDIX DR1 MINERAL ANALYSES Two-dmensonal chemcal maps as well as chemcal profles were done at 15 kv usng the JEOL JXA-8600 electron mcroprobe at Yale Unversty
V. Electrostatics. Lecture 25: Diffuse double layer structure
 V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
Diffusion Mass Transfer
 Dffuson Mass Transfer General onsderatons Mass transfer refers to mass n transt due to a speces concentraton gradent n a mture. Must have a mture of two or more speces for mass transfer to occur. The speces
Dffuson Mass Transfer General onsderatons Mass transfer refers to mass n transt due to a speces concentraton gradent n a mture. Must have a mture of two or more speces for mass transfer to occur. The speces
Name ID # For relatively dilute aqueous solutions the molality and molarity are approximately equal.
 Name ID # 1 CHEMISTRY 212, Lect. Sect. 002 Dr. G. L. Roberts Exam #1/Sprng 2000 Thursday, February 24, 2000 CLOSED BOOK EXM No notes or books allowed. Calculators may be used. tomc masses of nterest are
Name ID # 1 CHEMISTRY 212, Lect. Sect. 002 Dr. G. L. Roberts Exam #1/Sprng 2000 Thursday, February 24, 2000 CLOSED BOOK EXM No notes or books allowed. Calculators may be used. tomc masses of nterest are
1st Year Thermodynamic Lectures Dr Mark R. Wormald BIBLIOGRAPHY
 M.R.W. -- Thermodynamcs 5 1st Year Thermodynamc Lectures Dr Mark R. Wormald Soluton thermodynamcs :- Lecture 1. Behavour of deal solutons (and solvents). Defnton of dealty. Solvent n dlute solutons, collgatve
M.R.W. -- Thermodynamcs 5 1st Year Thermodynamc Lectures Dr Mark R. Wormald Soluton thermodynamcs :- Lecture 1. Behavour of deal solutons (and solvents). Defnton of dealty. Solvent n dlute solutons, collgatve
Factors that Effect the Rate of Solvation
 Factors that Effect the Rate of Solvation Rate of Solvation there are three ways to increase collisions between the solvent and the solute. agitating the mixture increasing the surface area of the solute
Factors that Effect the Rate of Solvation Rate of Solvation there are three ways to increase collisions between the solvent and the solute. agitating the mixture increasing the surface area of the solute
The Dissolution and Transport of Radionuclides from Used Nuclear Fuel in an Underground Repository
 Presented at the COMSOL Conference 2010 Boston The Dssoluton and Transport of adonucldes from Used Nuclear Fuel n an Underground epostory Y A N N I C K B E A U E G A D 1 M A K G O B I E N 2 F A N K G A
Presented at the COMSOL Conference 2010 Boston The Dssoluton and Transport of adonucldes from Used Nuclear Fuel n an Underground epostory Y A N N I C K B E A U E G A D 1 M A K G O B I E N 2 F A N K G A
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Vapor-Liquid Equilibria for Water+Hydrochloric Acid+Magnesium Chloride and Water+Hydrochloric Acid+Calcium Chloride Systems at Atmospheric Pressure
 Chnese J. Chem. Eng., 4() 76 80 (006) RESEARCH OES Vapor-Lqud Equlbra for Water+Hydrochlorc Acd+Magnesum Chlorde and Water+Hydrochlorc Acd+Calcum Chlorde Systems at Atmospherc Pressure ZHAG Yng( 张颖 ) and
Chnese J. Chem. Eng., 4() 76 80 (006) RESEARCH OES Vapor-Lqud Equlbra for Water+Hydrochlorc Acd+Magnesum Chlorde and Water+Hydrochlorc Acd+Calcum Chlorde Systems at Atmospherc Pressure ZHAG Yng( 张颖 ) and
CHEMICAL ENGINEERING
 Postal Correspondence GATE & PSUs -MT To Buy Postal Correspondence Packages call at 0-9990657855 1 TABLE OF CONTENT S. No. Ttle Page no. 1. Introducton 3 2. Dffuson 10 3. Dryng and Humdfcaton 24 4. Absorpton
Postal Correspondence GATE & PSUs -MT To Buy Postal Correspondence Packages call at 0-9990657855 1 TABLE OF CONTENT S. No. Ttle Page no. 1. Introducton 3 2. Dffuson 10 3. Dryng and Humdfcaton 24 4. Absorpton
Assignment 4. Adsorption Isotherms
 Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Redox reactions.
 Redox reactions http://eps.mcgill.ca/~courses/c220/ Redox reactions In a similar way that acids and bases have been defined as proton donors and proton acceptors, reductants and oxidants are defined as
Redox reactions http://eps.mcgill.ca/~courses/c220/ Redox reactions In a similar way that acids and bases have been defined as proton donors and proton acceptors, reductants and oxidants are defined as
ACCRETE - An explicit algorithm to solve kinetically constrained CO 2 -water-rock interactions
 Proceedngs of the 3rd WSEAS Internatonal Conference on Mathematcal Bology and Ecology, Gold Coast, Queensland, Australa, January 17-19, 2007 67 ACCRETE - An explct algorthm to solve knetcally constraned
Proceedngs of the 3rd WSEAS Internatonal Conference on Mathematcal Bology and Ecology, Gold Coast, Queensland, Australa, January 17-19, 2007 67 ACCRETE - An explct algorthm to solve knetcally constraned
GEOL 414/514 ACTIVITY COEFFICIENTS OF DISSOLVED SPECIES
 GEOL 414/514 ACTIVITY COEFFICIENTS OF DISSOLVED SPECIES Chapter 4 LANGMUIR ACTIVITY & ACTIVITY COEFFICIENTS Earlier we studied common ion effect on decreasing the solubility CaCO 3 Ca +2 + CO 3 Add Ca
GEOL 414/514 ACTIVITY COEFFICIENTS OF DISSOLVED SPECIES Chapter 4 LANGMUIR ACTIVITY & ACTIVITY COEFFICIENTS Earlier we studied common ion effect on decreasing the solubility CaCO 3 Ca +2 + CO 3 Add Ca
Tutorials : Corrosion Part 1: Theory and basics
 Tutorials : Corrosion Part 1: Theory and basics Outline A. Definition and effects of corrosion B. General thermodynamics and kinetics in electrochemistry C. Thermodynamics and kinetics in corrosion 2 2/21
Tutorials : Corrosion Part 1: Theory and basics Outline A. Definition and effects of corrosion B. General thermodynamics and kinetics in electrochemistry C. Thermodynamics and kinetics in corrosion 2 2/21
Homework Chapter 21 Solutions!!
 Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Learning Outcomes: At the end of this assignment, students will be able to:
 Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Computers&Concrete, An International Journal, Vo. 4, No. 4, August 2007 MECHANISMS OF SULFATE IONIC DIFFUSION IN POROUS CEMENT BASED COMPOSITES
 Computers&Concrete, An Internatonal Journal, Vo. 4, No. 4, August 27 MECHANISMS OF SULFATE IONIC DIFFUSION IN POROUS CEMENT BASED COMPOSITES P. Gospodnov, M. Mronova 2, R. Kaandjev * Insttute of Mechancs,
Computers&Concrete, An Internatonal Journal, Vo. 4, No. 4, August 27 MECHANISMS OF SULFATE IONIC DIFFUSION IN POROUS CEMENT BASED COMPOSITES P. Gospodnov, M. Mronova 2, R. Kaandjev * Insttute of Mechancs,
Introduction to Interfacial Segregation. Xiaozhe Zhang 10/02/2015
 Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
SUPPLEMENTARY INFORMATION
 Hghly monodsperse core-shell partcles created by sold-state reactons Supplementary Materals Velmr Radmlovc 1,2, Coln Ophus 1,, Emmanuelle A Marqus 3, Marta D Rossell 4, Alfredo Tolley 1,5, Abhay Gautam
Hghly monodsperse core-shell partcles created by sold-state reactons Supplementary Materals Velmr Radmlovc 1,2, Coln Ophus 1,, Emmanuelle A Marqus 3, Marta D Rossell 4, Alfredo Tolley 1,5, Abhay Gautam
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Estimation of the composition of the liquid and vapor streams exiting a flash unit with a supercritical component
 Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
( ) Phase equilibrium Some basic principles for phase calculations
 Chapter From Fundamentals to Propertes 6 Table. Total propertes from an excess approach V U H A G S Pure component Real mxture Ideal mxture Mxng contrbuton Excess property = * v + 0 + * v v (.0) = * u
Chapter From Fundamentals to Propertes 6 Table. Total propertes from an excess approach V U H A G S Pure component Real mxture Ideal mxture Mxng contrbuton Excess property = * v + 0 + * v v (.0) = * u
Numerical Analysis on Si Deoxidation of Molten Ni and Ni Cu Alloy by Quadratic Formalism
 Materals ransactons, Vol. 44, No. 9 (2003) pp. 1817 to 1823 #2003 he Japan Insttute of Metals Numercal Analyss on S Deoxdaton of Molten N and N Cu Alloy by Quadratc Formalsm akahro Mk, Fuo Ish and Mtsutaka
Materals ransactons, Vol. 44, No. 9 (2003) pp. 1817 to 1823 #2003 he Japan Insttute of Metals Numercal Analyss on S Deoxdaton of Molten N and N Cu Alloy by Quadratc Formalsm akahro Mk, Fuo Ish and Mtsutaka
Modeling the binding of fulvic acid by goethite: The speciation of adsorbed FA molecules
 Pergamon PII S0016-7037(02)01042-6 Geochmca et Cosmochmca Acta, Vol. 67, No. 8, pp. 1463 1474, 2003 Copyrght 2003 Elsever Scence Ltd Prnted n the USA. All rghts reserved 0016-7037/03 $30.00.00 Modelng
Pergamon PII S0016-7037(02)01042-6 Geochmca et Cosmochmca Acta, Vol. 67, No. 8, pp. 1463 1474, 2003 Copyrght 2003 Elsever Scence Ltd Prnted n the USA. All rghts reserved 0016-7037/03 $30.00.00 Modelng
I wish to publish my paper on The International Journal of Thermophysics. A Practical Method to Calculate Partial Properties from Equation of State
 I wsh to publsh my paper on The Internatonal Journal of Thermophyscs. Ttle: A Practcal Method to Calculate Partal Propertes from Equaton of State Authors: Ryo Akasaka (correspondng author) 1 and Takehro
I wsh to publsh my paper on The Internatonal Journal of Thermophyscs. Ttle: A Practcal Method to Calculate Partal Propertes from Equaton of State Authors: Ryo Akasaka (correspondng author) 1 and Takehro
CHEMISTRY Topic #2: Thermochemistry and Electrochemistry What Makes Reactions Go? Fall 2018 Dr. Susan Findlay See Exercises in Topic 8
 CHEMISTRY 2000 Topic #2: Thermochemistry and Electrochemistry What Makes Reactions Go? Fall 208 Dr. Susan Findlay See Exercises in Topic 8 Vapour Pressure of Pure Substances When you leave wet dishes on
CHEMISTRY 2000 Topic #2: Thermochemistry and Electrochemistry What Makes Reactions Go? Fall 208 Dr. Susan Findlay See Exercises in Topic 8 Vapour Pressure of Pure Substances When you leave wet dishes on
First Law: A body at rest remains at rest, a body in motion continues to move at constant velocity, unless acted upon by an external force.
 Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
1. Mathematical models of the chromatographic process
 1. athematcal models of the chromatographc process - What determnes retenton tme n L? - What causes pea broadenng n L? - Why are the L peas often asymmetrc? - Why s partton chromatography much more popular
1. athematcal models of the chromatographc process - What determnes retenton tme n L? - What causes pea broadenng n L? - Why are the L peas often asymmetrc? - Why s partton chromatography much more popular
Using Spectrophotometric Methods to Determine an Equilibrium Constant Prelab
 Usng Spectrophotometrc Methods to Determne an Equlbrum Constant Prelab 1. What s the purpose of ths experment? 2. Wll the absorbance of the ulbrum mxture (at 447 nm) ncrease or decrease as Fe soluton s
Usng Spectrophotometrc Methods to Determne an Equlbrum Constant Prelab 1. What s the purpose of ths experment? 2. Wll the absorbance of the ulbrum mxture (at 447 nm) ncrease or decrease as Fe soluton s
1) Silicon oxide has a typical surface potential in an aqueous medium of ϕ,0
 1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
Andrzej Anderko OLI Systems Inc. 108 American Road Morris Plains, NJ ABSTRACT
 SIMULATION OF CO 3 / S SCALE FORMATION USING TERMODYNAMIC AND ELECTROCEMICAL MODELS Andrzej Anderko OLI Systems Inc. 18 Amercan Road Morrs Plans, NJ 795 ABSTRACT A comprehensve model has been developed
SIMULATION OF CO 3 / S SCALE FORMATION USING TERMODYNAMIC AND ELECTROCEMICAL MODELS Andrzej Anderko OLI Systems Inc. 18 Amercan Road Morrs Plans, NJ 795 ABSTRACT A comprehensve model has been developed
CALCULATION OF DIFFUSION COEFFICIENT OF ION IN MULTICOMPONENT SOLUTION FOR ION MOVEMENT IN CONCRETE
 CALCULATION OF DIFFUSION COEFFICIENT OF ION IN MULTICOMPONENT SOLUTION FOR ION MOVEMENT IN CONCRETE Worapatt RITTHICHAUY *1, Takafum SUGIYAMA * and Yukkazu TSUJI *3 ABSTRACT: A method for calculatng the
CALCULATION OF DIFFUSION COEFFICIENT OF ION IN MULTICOMPONENT SOLUTION FOR ION MOVEMENT IN CONCRETE Worapatt RITTHICHAUY *1, Takafum SUGIYAMA * and Yukkazu TSUJI *3 ABSTRACT: A method for calculatng the
Non-Ideality Through Fugacity and Activity
 Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Problem Set #6 solution, Chem 340, Fall 2013 Due Friday, Oct 11, 2013 Please show all work for credit
 Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Properties of the living organism. Interaction between living organism and the enviroment. Processing informations. Definitions
 thermodynamcs energy materal Interacton between lvng organsm and the envroment Propertes of the lvng organsm Separaton from the envroment: Strctly controlled energy and materal transport. Changng n the
thermodynamcs energy materal Interacton between lvng organsm and the envroment Propertes of the lvng organsm Separaton from the envroment: Strctly controlled energy and materal transport. Changng n the
Chemistry 2000 Lecture 12: Temperature dependence of the equilibrium constant
 Chemistry 2000 Lecture 12: Temperature dependence of the equilibrium constant Marc R. Roussel February 12, 2019 Marc R. Roussel Temperature dependence of equilibrium February 12, 2019 1 / 15 Temperature
Chemistry 2000 Lecture 12: Temperature dependence of the equilibrium constant Marc R. Roussel February 12, 2019 Marc R. Roussel Temperature dependence of equilibrium February 12, 2019 1 / 15 Temperature
ON A DETERMINATION OF THE INITIAL FUNCTIONS FROM THE OBSERVED VALUES OF THE BOUNDARY FUNCTIONS FOR THE SECOND-ORDER HYPERBOLIC EQUATION
 Advanced Mathematcal Models & Applcatons Vol.3, No.3, 2018, pp.215-222 ON A DETERMINATION OF THE INITIAL FUNCTIONS FROM THE OBSERVED VALUES OF THE BOUNDARY FUNCTIONS FOR THE SECOND-ORDER HYPERBOLIC EUATION
Advanced Mathematcal Models & Applcatons Vol.3, No.3, 2018, pp.215-222 ON A DETERMINATION OF THE INITIAL FUNCTIONS FROM THE OBSERVED VALUES OF THE BOUNDARY FUNCTIONS FOR THE SECOND-ORDER HYPERBOLIC EUATION
Oxidation-reduction (redox) reactions
 Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
