Fuel & Advanced Combustion. Lecture Chemical Reaction
|
|
|
- Alfred Parsons
- 5 years ago
- Views:
Transcription
1 Fuel & Advaced Cmbust Lecture Cemcal eact
2 Ideal Gas Mdel e deal gas equat f state s: V m m M were s te Uversal Gas Cstat (8.34 kj/kml K, M s te mlecular wegt ad s te umber f mles. Secfc teral eergy (uts: kj/kg Secfc etaly (uts: kj/kg u( cv ( d ( c ( d Secfc etry (uts: kj/kg K s, s ( l( ( ( = bar, s etry at
3 Ideal Gas Mdel fr Mxtures e mass m f a mxture s equal t te sum f te mass f cmets m m e mass fract, x, f ay gve seces s defed as: x m ad m x e mxture teral eergy U ad etaly H (uts: kj s: were u ad U mu m u H m m are mass secfc values (uts: kj/kg e mxture secfc teral eergy u ad etaly s: u U m m u xu m H m m x m 3
4 4 e mxture teral eergy U ad etaly H (uts: kj s: H u U were are mlar secfc values (uts: kj/kml u ad e ttal umber f mles te mxture s: Ideal Gas Mdel fr Mxtures e mle fract, y, f ay gve seces s defed as: y y ad y y u u e mxture mlar secfc teral eergy ad etaly (uts kj/ kml s:
5 5 Ideal Gas Mdel fr Mxtures e artal ressure f a cmet,, te mxture (uts: ka s: y V V y r Mass secfc etry (kj/kg K mlar secfc mxture etry (kj/ml K : s x s l / l( y s y s l l e mxture mlecular wegt, M, s gve by: M y M m m M
6 Cmst f Stadard Dry Ar Ar s a mxture f gases cludg xyge (O, trge(n, arg (Ar, carb dxde (CO, water vaur (H 0. Fr cmbust dry ar s take t be cmsed f % O ad 79% N by vlume (y O =0., y N =0.79. N O tt N tt O y y N O 3.76 Fr every mle f O tere are 3.76 mles f N. Mlecular wegt f ar s M ar y M y O M O y N M N 0.(3 0.79( kg/kml e amut f water mst ar at temerature s secfed by te secfc umdty (w r te relatve umdty (F defed as fllws: w mh O H O F 0 F m ( ar sat 6
7 Cmbust Stcmetry If suffcet xyge s avalable, a ydrcarb fuel ca be cmletely xdzed, te carb s cverted t carb dxde (CO ad te ydrge s cverted t water (H O. e verall cemcal equat fr te cmlete cmbust f e mle f rae (C 3 H 8 wt xyge s: C3H8 ao bco cho Elemets cat be created r destryed, s C balace: 3 = b b= 3 H balace: 8 = c c= 4 O balace: a = b + c a= 5 # f mles seces us te stcmetrc react s: C3H8 5O 3CO 4HO 7
8 Cmbust Stcmetry Ar ctas mlecular trge N, f rducts are at a lw temerature te trge s t sgfcatly affected by te react, t s csdered ert. e cmlete react f a geeral ydrcarb C H wt ar s: C H a( O bco ch O dn 3.76N C balace: = b b = H balace: = c c = / O balace: a = b + c a = b + c/ a = + /4 N balace: (3.76a = d d = 3.76a/ d = 3.76( + /4 C H ( O N CO H O 3.76 N 4 Examle: e stcmetrc react f ctae (C 8 H 8 = 8 ad = 8 C.5( O 3.76N 8CO 9HO N 8H8 47 8
9 Cmbust Stcmetry e stcmetrc mass based ar/fuel rat fr C H fuel s: A/ F s m m ar fuel O M ar 4 M M C M H fuel M 3.76 M 4 N Substtutg te resectve mlecular wegts ad dvdg t ad bttm by e gets te fllwg exress tat ly deeds te rat f te umber f ydrge atms t ydrge atms (/ te fuel. A/ F s ( F / A s ( Nte abve equat ly ales t stcmetrc mxture Examle: Fr ctae (C 8 H 8, / =.5 (A/F s = 5. (gasle (A/F s 4.6 9
10 Fuel Lea Mxture Fuel-ar mxtures wt mre ta stcmetrc ar (excess ar ca bur Wt excess ar yu ave fuel lea cmbust At lw cmbust temeratures, te extra ar aears te rducts as O ad N : C H g ( ( O 3.76N CO HO dn eo 4 a fuel lea mxture as excess ar, s g > Abve react equat as tw ukws (d, e ad we ave tw atm balace equats (O, N s ca slve fr te ukws 0
11 Fuel-ar mxtures wt less ta stcmetrc ar (excess fuel ca bur. Wt less ta stcmetrc ar yu ave fuel rc cmbust, tere s suffcet xyge t xdze all te C ad H te fuel t CO ad H O. C H Fuel c Mxture Get cmlete cmbust were carb mxde (CO ad mlecular ydrge (H als aear te rducts. g ( ( O 3.76N CO HO dn eco fh 4 a fuel rc mxture as suffcet ar g < Abve react equat as tree ukws (d, e, f ad we ly ave tw atm balace equats (O, N s cat slve fr te ukws uless addtal frmat abut te rducts s gve.
12 Off-Stcmetrc Mxtures e equvalece rat, f, s cmmly used t dcate f a mxture s stcmetrc, fuel lea, r fuel rc. f A/ F A/ F s mxture F / A F / A mxture s Stcmetrc mxture: C H stcmetrc f = fuel lea f < fuel rc f > Off-stcmetrc mxture: ( O 3.76N rducts 4 C H f ( O 3.76N 4 rducts g f
13 Off-stcmetrc Cdts Oter termlgy used t descrbe w muc ar s used cmbust: 0% stcmetrc ar = 0% teretcal ar = 0% excess ar C3H8 g ( ( O 3.76N g. 4 Examle: Csder a react f ctae wt 0% excess ar, wat s f? Stcmetrc : C8H8.5( O 3.76N 8CO 9HO 47N 0% excess ar s: C8H8.(.5( O 3.76N 8CO 9HO ao bn O balace:.(.5( = a a =.5, N balace:.(.5(3.76( = b b = 5.7 mxture s fuel lea A/ F s.5(4.76 / f 0.9. A/ F.(.5(4.76 / f.9 mxture 3
14 Frst Law Aalyss fr eactg System Csder a cstat ressure rcess wc f mles f fuel react wt a mles f ar t rduce mles f rduct: f F a A eactats rducts W eactats Q rducts State State eact Alyg Frst Law wt state beg te reactats at, ad state beg rducts at, : 4
15 5 Q H H H H V U V U ( ( ( ( Frst Law Aalyss fr eactg System ( ( V V U U Q W U Q
16 Etaly f eact Csder te case were te fal temerature f te rducts s te same as te tal temerature f te reactats (e.g., calrmeter s used t measure Q. W eact Q = = = = e eat released uder ts stuat s referred t as te etaly f react, H, Q H ( ( ( ( uts : kj er kg r kml f fuel 6
17 Heat f Cmbust e maxmum amut f eergy s released frm a fuel we reacted wt a stcmetrc amut f ar ad all te ydrge ad carb ctaed te fuel s cverted t CO ad H O C H ( O N CO H O 3.76 N 4 s maxmum eergy s cmmly referred t as te eat f cmbust, r te eatg value, gve er ut mass f fuel r ar H = H H < 0 (extermc H = H H > 0 (edtermc 7
18 Heat f Cmbust ere are tw ssble values fr te eat f cmbust deedg weter te water te rducts s take t be saturated lqud r vaur. f g Frm steam tables: fg = g f > 0 S e term ger eat f cmbust ad eatg value (HHV s used we te water te rducts s take t be te lqud state ( H0 = f e term lwer eat f cmbust ad eatg value (LHV s used we te water te rducts s take t be te vaur state ( H0 = g 8
19 Heat f Cmbust, gracal (kj/kg fuel eactats 0 rducts wt HO (g lw rducts wt HO (l g fg,ho er kg fuel 98K 9
20 Surce: ulkrabek 0
21 Fuel A/F Eergy desty (MJ/L fuel Secfc eergy (MJ/kg ar Gasle* Butal Etal Metal Hydrge 34.3 ** 4. * Desel abut 0% mre eergy er vlume ta gasle ** lquefed ydrge as rugly ¼ te eergy desty f gasle
22 Heat f Frmat Csder te fllwg reacts takg lace at atmserc ressure ad wt = = 98K / O C( s O ( g H ( g H ( g CO ( g O( l Q 86,000 kj / kml H Q 394,000 kj / kml CO O I tese reacts H O ad CO are frmed frm ter elemets ter atural state at stadard temerature ad ressure (S atm ad 98K. eacts f ts tye are called frmat reacts ad te crresdg measured eat release Q s referred t as te stadard eat f frmat ( s: f f, H O f, CO Q Q 86,000 kj / kml 394,000 kj / kml Values fr stadard eat f frmat fr dfferet seces are tabulated
23 Heat f Frmat fr Dfferet Fuels 3
24 Etaly Scale fr a eactg System By teratal cvet, te etaly f every elemet ts atural state (e.g., O (g, N (g, H (g, C(s at S as bee set t zer.e, ( atm,98k f 0 Csder te fllwg detty: (, (atm,98k [ (, (atm,98k] erefre, te etaly f te t cmet a mxture s: (,, [ (, (atm,98k] f cemcal etaly 98K sesble etaly K c d 98, 4
25 -5000 Etaly (kj/kg CO H O f, emerature K e data s als fud te JANNAF tables rvded at curse web ste 5
26 6
27 Adabatc Flame emerature Csder te fllwg adabatc cstat ressure rcess: eactats ( Fuel Ar rducts ( a Fr a cstat ressure rcess, te fal rducts temerature, a, s kw as te adabatc flame temerature (AF. Q ( ( a ( ( 0 Fr a gve react were te s are kw fr bt te reactats ad te rducts, a ca be calculated exlctly. 7
28 8 Adabatc Flame emerature a ( ( f f d c d c a,, 98, 98, O f a f K K (98 ( (98 (,, H Sesble eat f reactats (equal t 0 f = 98K Sesble eat f rducts f f a K K,, (98 ( (98 (
29 Adabatc Flame emerature, examle C 8H8 l.5( O 3.76N 8CO 9HO 47 ( N Csder cstat ressure cmlete cmbust f stcmetrc lqud butae-ar tally at 98K ad atm { ( a (98K} ( (98K f, f, 8 Nte: f, O f, N 0 CO ( a CO (98K 9 H O ( a H O (98K 47 N ( a N (98K 8 f, CO 9 f, H O Lk u etaly f frmat values ad terate a 40K f, C H 8 8 9
30 Adabatc Flame emerature wt rducts at equlbrum a, 30
31 Adabatc Flame emerature, examle Nw csder ctae ar wt 0% excess ar C 8H8.(.5( O 3.76N 8CO 9HO.5O 5. 7 N 8 CO ( a CO (98K 9 H O ( a H O (98K.5 O ( a O (98K 5.7 ( (98K 8 Lk u values ad terat gves a = 6K N a N f, CO 9 f, H O f, C H same as fr f = Excess ar adds 6 mles f datmc mlecules (O ad N t te rducts tat des t ctrbute t eat release just saks t u. 8 H 8 3
32 Adabatc Flame emerature wt rducts at equlbrum trmetae ydrge ctae etal 3
33 Cstat Vlume AF Csder te case were te st s fxed ad te cylder s erfectly sulated s te rcess s adabatc (Q = 0 W eact Q Q u u ( ( u a u ( ( 0 Nte = u + v = u +, s ( ( ( ( a 33
34 34 a f f a K K,, (98 ( (98 ( Extra term cmared t cstat ressure AF ( term > 0 Cstat Vlume AF f a f K K (98 ( (98 (,, e AF fr a cstat vlume rcess s larger ta fr a cstat ressure rcess. e AF s lwer fr cstat ressure rcess sce tere s dv wrk de
35 35 a CV V V Cstat Vlume Cmbust ressure Assumg deal gas beavur: Fr large ydrcarbs lke ctae te mle rat term s clse t e ( ( N O H CO N O l H C (
36 Stcmetrc ctae-ar (H = 47.9 MJ/kg-fuel: C 8H8 N CV.5( O 3.76N 8CO 9HO 47 a Ege Fuel Cmars Stcmetrc ydrge-ar (H = 4.6 MJ/kg-fuel: H CV 0.5( O 3.76N H O.88 N a Stcmetrc etal-ar (H = 9.7 MJ/kg-fuel: 6.8 C H6O.5( O 3.76N CO 3HO 3. CV 3 N a
General Method for Calculating Chemical Equilibrium Composition
 AE 6766/Setzma Sprg 004 Geeral Metod for Calculatg Cemcal Equlbrum Composto For gve tal codtos (e.g., for gve reactats, coose te speces to be cluded te products. As a example, for combusto of ydroge wt
AE 6766/Setzma Sprg 004 Geeral Metod for Calculatg Cemcal Equlbrum Composto For gve tal codtos (e.g., for gve reactats, coose te speces to be cluded te products. As a example, for combusto of ydroge wt
Difference of 2 kj per mole of propane! E = kj
 Ethaly, H Fr rcesses measured uder cstat ressure cditi, the heat the reacti is q. E = q + w = q P ext V he subscrit remids is that the heat measured is uder cstat ressure cditi. hermdyamics Slve r q q
Ethaly, H Fr rcesses measured uder cstat ressure cditi, the heat the reacti is q. E = q + w = q P ext V he subscrit remids is that the heat measured is uder cstat ressure cditi. hermdyamics Slve r q q
Lecture 2. Basic Semiconductor Physics
 Lecture Basc Semcductr Physcs I ths lecture yu wll lear: What are semcductrs? Basc crystal structure f semcductrs Electrs ad hles semcductrs Itrsc semcductrs Extrsc semcductrs -ded ad -ded semcductrs Semcductrs
Lecture Basc Semcductr Physcs I ths lecture yu wll lear: What are semcductrs? Basc crystal structure f semcductrs Electrs ad hles semcductrs Itrsc semcductrs Extrsc semcductrs -ded ad -ded semcductrs Semcductrs
6-5. H 2 O 200 kpa 200 C Q. Entropy Changes of Pure Substances
 Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
ELABORATING AND ANALYSING THE REAL BALANCE OF HEAT FOR THE STEAM GENERATOR RGL10/D-D
 Annals f te Unversty f Petrşan, Mecancal Engneerng, 10 (2008), 155-160 155 ELABORATING AND ANALYSING THE REAL BALANCE OF HEAT FOR THE STEAM GENERATOR RGL10/D-D DAN CODRUŢ PETRILEAN 1 Abstract: Te real
Annals f te Unversty f Petrşan, Mecancal Engneerng, 10 (2008), 155-160 155 ELABORATING AND ANALYSING THE REAL BALANCE OF HEAT FOR THE STEAM GENERATOR RGL10/D-D DAN CODRUŢ PETRILEAN 1 Abstract: Te real
Lecture 14 Chapter 16, Sections 3-4 Equilibrium. Nifty K eq math Q and K eq Connection with G Le Chatelier
 Lecture 14 Chater 16, Sectins 3-4 Equilibrium Nifty K math Q and K Cnnectin with G Le Chatelier Remember In general fr a reactin like aa + bb dd + ee K [ ] d D [ E] e [ ] a A [ ] b B K s can be cmbined
Lecture 14 Chater 16, Sectins 3-4 Equilibrium Nifty K math Q and K Cnnectin with G Le Chatelier Remember In general fr a reactin like aa + bb dd + ee K [ ] d D [ E] e [ ] a A [ ] b B K s can be cmbined
CHEM 1001 Problem Set #3: Entropy and Free Energy
 CHEM 1001 Prblem Set #3: Entry and Free Energy 19.7 (a) Negative; A liquid (mderate entry) cmbines with a slid t frm anther slid. (b)psitive; One mle f high entry gas frms where n gas was resent befre.
CHEM 1001 Prblem Set #3: Entry and Free Energy 19.7 (a) Negative; A liquid (mderate entry) cmbines with a slid t frm anther slid. (b)psitive; One mle f high entry gas frms where n gas was resent befre.
Solutions. Definitions pertaining to solutions
 Slutis Defiitis pertaiig t slutis Slute is the substace that is disslved. It is usually preset i the smaller amut. Slvet is the substace that des the disslvig. It is usually preset i the larger amut. Slubility
Slutis Defiitis pertaiig t slutis Slute is the substace that is disslved. It is usually preset i the smaller amut. Slvet is the substace that des the disslvig. It is usually preset i the larger amut. Slubility
Byeong-Joo Lee
 yeg-j Lee OSECH - MSE calphad@pstech.ac.r yeg-j Lee www.pstech.ac.r/~calphad Fudametals Mcrscpc vs. Macrscpc Vew t State fuct vs. rcess varable Frst Law f hermdyamcs Specal prcesses 1. Cstat-Vlume rcess:
yeg-j Lee OSECH - MSE calphad@pstech.ac.r yeg-j Lee www.pstech.ac.r/~calphad Fudametals Mcrscpc vs. Macrscpc Vew t State fuct vs. rcess varable Frst Law f hermdyamcs Specal prcesses 1. Cstat-Vlume rcess:
Exergy Analysis of Large ME-TVC Desalination System
 Exergy Aalyss f arge ME-V esalat System Awar O. Bamer Water & Eergy Prgram\Research rectrate Kuwat udat fr the Advacemet f Sceces (KAS) he 0 th Gulf Water ferece, -4 Aprl 0, ha- Qatar Outles Itrduct Prcess
Exergy Aalyss f arge ME-V esalat System Awar O. Bamer Water & Eergy Prgram\Research rectrate Kuwat udat fr the Advacemet f Sceces (KAS) he 0 th Gulf Water ferece, -4 Aprl 0, ha- Qatar Outles Itrduct Prcess
Chemical Equilibrium
 0.110/5.60 Fall 005 Lecture #10 age 1 Chemical Equilibrium Ideal Gases Questin: What is the cmsitin f a reacting miture f ideal gases? e.g. ½ N (g, T, ) + 3/ H (g, T, ) = NH 3 (g, T, ) What are N,, and
0.110/5.60 Fall 005 Lecture #10 age 1 Chemical Equilibrium Ideal Gases Questin: What is the cmsitin f a reacting miture f ideal gases? e.g. ½ N (g, T, ) + 3/ H (g, T, ) = NH 3 (g, T, ) What are N,, and
ALE 26. Equilibria for Cell Reactions. What happens to the cell potential as the reaction proceeds over time?
 Name Chem 163 Secti: Team Number: AL 26. quilibria fr Cell Reactis (Referece: 21.4 Silberberg 5 th editi) What happes t the ptetial as the reacti prceeds ver time? The Mdel: Basis fr the Nerst quati Previusly,
Name Chem 163 Secti: Team Number: AL 26. quilibria fr Cell Reactis (Referece: 21.4 Silberberg 5 th editi) What happes t the ptetial as the reacti prceeds ver time? The Mdel: Basis fr the Nerst quati Previusly,
Approach: (Equilibrium) TD analysis, i.e., conservation eqns., state equations Issues: how to deal with
 Schl f Aerspace Chemcal D: Mtvatn Prevus D Analyss cnsdered systems where cmpstn f flud was frzen fxed chemcal cmpstn Chemcally eactng Flw but there are numerus stuatns n prpulsn systems where chemcal
Schl f Aerspace Chemcal D: Mtvatn Prevus D Analyss cnsdered systems where cmpstn f flud was frzen fxed chemcal cmpstn Chemcally eactng Flw but there are numerus stuatns n prpulsn systems where chemcal
We have already referred to a certain reaction, which takes place at high temperature after rich combustion.
 ME 41 Day 13 Topcs Chemcal Equlbrum - Theory Chemcal Equlbrum Example #1 Equlbrum Costats Chemcal Equlbrum Example #2 Chemcal Equlbrum of Hot Bured Gas 1. Chemcal Equlbrum We have already referred to a
ME 41 Day 13 Topcs Chemcal Equlbrum - Theory Chemcal Equlbrum Example #1 Equlbrum Costats Chemcal Equlbrum Example #2 Chemcal Equlbrum of Hot Bured Gas 1. Chemcal Equlbrum We have already referred to a
ALE 21. Gibbs Free Energy. At what temperature does the spontaneity of a reaction change?
 Name Chem 163 Sectin: Team Number: ALE 21. Gibbs Free Energy (Reference: 20.3 Silberberg 5 th editin) At what temperature des the spntaneity f a reactin change? The Mdel: The Definitin f Free Energy S
Name Chem 163 Sectin: Team Number: ALE 21. Gibbs Free Energy (Reference: 20.3 Silberberg 5 th editin) At what temperature des the spntaneity f a reactin change? The Mdel: The Definitin f Free Energy S
Chemistry 114 First Hour Exam
 Chemistry 114 First Hur Exam Please shw all wrk fr partial credit Name: (4 pints) 1. (12 pints) Espress is made by frcing very ht water under high pressure thrugh finely grund, cmpacted cffee. (Wikipedia)
Chemistry 114 First Hur Exam Please shw all wrk fr partial credit Name: (4 pints) 1. (12 pints) Espress is made by frcing very ht water under high pressure thrugh finely grund, cmpacted cffee. (Wikipedia)
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY
 AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
PY3101 Optics. Learning objectives. Wave propagation in anisotropic media Poynting walk-off The index ellipsoid Birefringence. The Index Ellipsoid
 The Ide Ellpsd M.P. Vaugha Learg bjectves Wave prpagat astrpc meda Ptg walk-ff The de ellpsd Brefrgece 1 Wave prpagat astrpc meda The wave equat Relatve permttvt I E. Assumg free charges r currets E. Substtutg
The Ide Ellpsd M.P. Vaugha Learg bjectves Wave prpagat astrpc meda Ptg walk-ff The de ellpsd Brefrgece 1 Wave prpagat astrpc meda The wave equat Relatve permttvt I E. Assumg free charges r currets E. Substtutg
Hº = -690 kj/mol for ionization of n-propylene Hº = -757 kj/mol for ionization of isopropylene
 Prblem 56. (a) (b) re egative º values are a idicati f mre stable secies. The º is mst egative fr the i-ryl ad -butyl is, bth f which ctai a alkyl substituet bded t the iized carb. Thus it aears that catis
Prblem 56. (a) (b) re egative º values are a idicati f mre stable secies. The º is mst egative fr the i-ryl ad -butyl is, bth f which ctai a alkyl substituet bded t the iized carb. Thus it aears that catis
enthalpies of formation for a few thousand compounds can be used for thermochemical calculations for millions of different chemical reactions
 hater 4. hermchemistry Summary thermchemistry: branch f thermdynamics dealing with energy changes f chemical reactins, w, U, and are calculated fr chemical reactin rcesses enthalies f frmatin are intrduced
hater 4. hermchemistry Summary thermchemistry: branch f thermdynamics dealing with energy changes f chemical reactins, w, U, and are calculated fr chemical reactin rcesses enthalies f frmatin are intrduced
GASES. PV = nrt N 2 CH 4 CO 2 O 2 HCN N 2 O NO 2. Pressure & Boyle s Law Temperature & Charles s Law Avogadro s Law IDEAL GAS LAW
 GASES Pressure & Byle s Law Temperature & Charles s Law Avgadr s Law IDEAL GAS LAW PV = nrt N 2 CH 4 CO 2 O 2 HCN N 2 O NO 2 Earth s atmsphere: 78% N 2 21% O 2 sme Ar, CO 2 Sme Cmmn Gasses Frmula Name
GASES Pressure & Byle s Law Temperature & Charles s Law Avgadr s Law IDEAL GAS LAW PV = nrt N 2 CH 4 CO 2 O 2 HCN N 2 O NO 2 Earth s atmsphere: 78% N 2 21% O 2 sme Ar, CO 2 Sme Cmmn Gasses Frmula Name
Chapters 29 and 35 Thermochemistry and Chemical Thermodynamics
 Chapters 9 and 35 Thermchemistry and Chemical Thermdynamics 1 Cpyright (c) 011 by Michael A. Janusa, PhD. All rights reserved. Thermchemistry Thermchemistry is the study f the energy effects that accmpany
Chapters 9 and 35 Thermchemistry and Chemical Thermdynamics 1 Cpyright (c) 011 by Michael A. Janusa, PhD. All rights reserved. Thermchemistry Thermchemistry is the study f the energy effects that accmpany
Recitation 06. n total = P total V/RT = (0.425 atm * 10.5 L) / ( L atm mol -1 K -1 * 338 K) = mol
 Recitatin 06 Mixture f Ideal Gases 1. Chapter 5: Exercise: 69 The partial pressure f CH 4 (g) is 0.175 atm and that f O 2 (g) is 0.250 atm in a mixture f the tw gases. a. What is the mle fractin f each
Recitatin 06 Mixture f Ideal Gases 1. Chapter 5: Exercise: 69 The partial pressure f CH 4 (g) is 0.175 atm and that f O 2 (g) is 0.250 atm in a mixture f the tw gases. a. What is the mle fractin f each
Unit -2 THEORY OF DILUTE SOLUTIONS
 Uit - THEORY OF DILUTE SOLUTIONS 1) hat is sluti? : It is a hmgeus mixture f tw r mre cmpuds. ) hat is dilute sluti? : It is a sluti i which slute ccetrati is very less. 3) Give a example fr slid- slid
Uit - THEORY OF DILUTE SOLUTIONS 1) hat is sluti? : It is a hmgeus mixture f tw r mre cmpuds. ) hat is dilute sluti? : It is a sluti i which slute ccetrati is very less. 3) Give a example fr slid- slid
"NEET / AIIMS " SOLUTION (6) Avail Video Lectures of Experienced Faculty.
 07 NEET EXAMINATION SOLUTION (6) Avail Vide Lectures f Exerienced Faculty Page Sl. The lean exressin which satisfies the utut f this lgic gate is C = A., Whichindicates fr AND gate. We can see, utut C
07 NEET EXAMINATION SOLUTION (6) Avail Vide Lectures f Exerienced Faculty Page Sl. The lean exressin which satisfies the utut f this lgic gate is C = A., Whichindicates fr AND gate. We can see, utut C
Lecture 12: Chemical reaction equilibria
 3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1
 A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1 (Nte: questins 1 t 14 are meant t be dne WITHOUT calculatrs!) 1.Which f the fllwing is prbably true fr a slid slute with a highly endthermic heat
A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1 (Nte: questins 1 t 14 are meant t be dne WITHOUT calculatrs!) 1.Which f the fllwing is prbably true fr a slid slute with a highly endthermic heat
SAFE HANDS & IIT-ian's PACE EDT-04 (JEE) Solutions
 ED- (JEE) Slutins Answer : Optin () ass f the remved part will be / I Answer : Optin () r L m (u csθ) (H) Answer : Optin () P 5 rad/s ms - because f translatin ωr ms - because f rtatin Cnsider a thin shell
ED- (JEE) Slutins Answer : Optin () ass f the remved part will be / I Answer : Optin () r L m (u csθ) (H) Answer : Optin () P 5 rad/s ms - because f translatin ωr ms - because f rtatin Cnsider a thin shell
Chapter 8 Balances on Nonreactive Processes 8.1 Elements of Energy Balances Calculations 8.1a Reference States A Review
 Chater 8 Balances on Nonreactve Processes 8.1 Elements of Energy Balances Calculatons 8.1a Reference States A Revew We can never know the absolute values of U and H for a seces at a gven state. t Fortunately,
Chater 8 Balances on Nonreactve Processes 8.1 Elements of Energy Balances Calculatons 8.1a Reference States A Revew We can never know the absolute values of U and H for a seces at a gven state. t Fortunately,
11. Ideal Gas Mixture
 . Ideal Ga xture. Geeral oderato ad xture of Ideal Gae For a geeral xture of N opoet, ea a pure ubtae [kg ] te a for ea opoet. [kol ] te uber of ole for ea opoet. e al a ( ) [kg ] N e al uber of ole (
. Ideal Ga xture. Geeral oderato ad xture of Ideal Gae For a geeral xture of N opoet, ea a pure ubtae [kg ] te a for ea opoet. [kol ] te uber of ole for ea opoet. e al a ( ) [kg ] N e al uber of ole (
Advanced Chemistry Practice Problems
 Advanced Chemistry Practice Prblems Thermdynamics: Gibbs Free Energy 1. Questin: Is the reactin spntaneus when ΔG < 0? ΔG > 0? Answer: The reactin is spntaneus when ΔG < 0. 2. Questin: Fr a reactin with
Advanced Chemistry Practice Prblems Thermdynamics: Gibbs Free Energy 1. Questin: Is the reactin spntaneus when ΔG < 0? ΔG > 0? Answer: The reactin is spntaneus when ΔG < 0. 2. Questin: Fr a reactin with
Idea is to sample from a different distribution that picks points in important regions of the sample space. Want ( ) ( ) ( ) E f X = f x g x dx
 Importace Samplg Used for a umber of purposes: Varace reducto Allows for dffcult dstrbutos to be sampled from. Sestvty aalyss Reusg samples to reduce computatoal burde. Idea s to sample from a dfferet
Importace Samplg Used for a umber of purposes: Varace reducto Allows for dffcult dstrbutos to be sampled from. Sestvty aalyss Reusg samples to reduce computatoal burde. Idea s to sample from a dfferet
Types of Energy COMMON MISCONCEPTIONS CHEMICAL REACTIONS INVOLVE ENERGY
 CHEMICAL REACTIONS INVOLVE ENERGY The study energy and its transrmatins is knwn as thermdynamics. The discussin thermdynamics invlve the cncepts energy, wrk, and heat. Types Energy Ptential energy is stred
CHEMICAL REACTIONS INVOLVE ENERGY The study energy and its transrmatins is knwn as thermdynamics. The discussin thermdynamics invlve the cncepts energy, wrk, and heat. Types Energy Ptential energy is stred
Module 7: Solved Problems
 Mdule 7: Slved Prblems 1 A tn-walled nentr tube eat exanger f 019-m lengt s t be used t eat denzed water frm 40 t 60 at a flw rate f 5 kg/s te denzed water flws trug te nner tube f 30-mm dameter wle t
Mdule 7: Slved Prblems 1 A tn-walled nentr tube eat exanger f 019-m lengt s t be used t eat denzed water frm 40 t 60 at a flw rate f 5 kg/s te denzed water flws trug te nner tube f 30-mm dameter wle t
Periodic Table of Elements. EE105 - Spring 2007 Microelectronic Devices and Circuits. The Diamond Structure. Electronic Properties of Silicon
 EE105 - Srg 007 Mcroelectroc Devces ad Crcuts Perodc Table of Elemets Lecture Semcoductor Bascs Electroc Proertes of Slco Slco s Grou IV (atomc umber 14) Atom electroc structure: 1s s 6 3s 3 Crystal electroc
EE105 - Srg 007 Mcroelectroc Devces ad Crcuts Perodc Table of Elemets Lecture Semcoductor Bascs Electroc Proertes of Slco Slco s Grou IV (atomc umber 14) Atom electroc structure: 1s s 6 3s 3 Crystal electroc
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
CHAPTER 5 ENTROPY GENERATION Instructor: Prof. Dr. Uğur Atikol
 CAPER 5 ENROPY GENERAION Istructr: Pr. Dr. Uğur Atkl Chapter 5 Etrpy Geerat (Exergy Destruct Outle st Avalable rk Cycles eat ege cycles Rergerat cycles eat pump cycles Nlw Prcesses teady-flw Prcesses Exergy
CAPER 5 ENROPY GENERAION Istructr: Pr. Dr. Uğur Atkl Chapter 5 Etrpy Geerat (Exergy Destruct Outle st Avalable rk Cycles eat ege cycles Rergerat cycles eat pump cycles Nlw Prcesses teady-flw Prcesses Exergy
[1 & α(t & T 1. ' ρ 1
 NAME 89.304 - IGNEOUS & METAMORPHIC PETROLOGY DENSITY & VISCOSITY OF MAGMAS I. Desity The desity (mass/vlume) f a magma is a imprtat parameter which plays a rle i a umber f aspects f magma behavir ad evluti.
NAME 89.304 - IGNEOUS & METAMORPHIC PETROLOGY DENSITY & VISCOSITY OF MAGMAS I. Desity The desity (mass/vlume) f a magma is a imprtat parameter which plays a rle i a umber f aspects f magma behavir ad evluti.
Unit 14 Thermochemistry Notes
 Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
When a substance heats up (absorbs heat) it is an endothermic reaction with a (+)q
 Chemistry Ntes Lecture 15 [st] 3/6/09 IMPORTANT NOTES: -( We finished using the lecture slides frm lecture 14) -In class the challenge prblem was passed ut, it is due Tuesday at :00 P.M. SHARP, :01 is
Chemistry Ntes Lecture 15 [st] 3/6/09 IMPORTANT NOTES: -( We finished using the lecture slides frm lecture 14) -In class the challenge prblem was passed ut, it is due Tuesday at :00 P.M. SHARP, :01 is
NEWCOMEN ATMOSPHERIC ENGINE
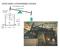 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
The Simple Linear Regression Model: Theory
 Chapter 3 The mple Lear Regress Mdel: Ther 3. The mdel 3.. The data bservats respse varable eplaatr varable : : Plttg the data.. Fgure 3.: Dsplag the cable data csdered b Che at al (993). There are 79
Chapter 3 The mple Lear Regress Mdel: Ther 3. The mdel 3.. The data bservats respse varable eplaatr varable : : Plttg the data.. Fgure 3.: Dsplag the cable data csdered b Che at al (993). There are 79
New Trade Theory (1979)
 Ne Trade Theory 979 Ne Trade Theory Krugma, 979: - Ecoomes of scale as reaso for trade - Elas trade betee smlar coutres Ituto of model: There s a trade-off betee ecoomes of scale the roducto of good tyes
Ne Trade Theory 979 Ne Trade Theory Krugma, 979: - Ecoomes of scale as reaso for trade - Elas trade betee smlar coutres Ituto of model: There s a trade-off betee ecoomes of scale the roducto of good tyes
MECH6661 lectures 10/1 Dr. M. Medraj Mech. Eng. Dept. - Concordia University
 Outle Revew ublattce Mdel Example ublattce Mdel Thermdyamc Mdelg Itrduct Example lly Desg Terary Phase Dagrams Gbbs Phase Rule Thermdyamcs f Multcmpet ystems Mech. Eg. Dept. - crda Uversty Revew: ublattce
Outle Revew ublattce Mdel Example ublattce Mdel Thermdyamc Mdelg Itrduct Example lly Desg Terary Phase Dagrams Gbbs Phase Rule Thermdyamcs f Multcmpet ystems Mech. Eg. Dept. - crda Uversty Revew: ublattce
Chemistry 1A Fall 2000
 Chemistry 1A Fall 2000 Midterm Exam III, versin B Nvember 14, 2000 (Clsed bk, 90 minutes, 155 pints) Name: SID: Sectin Number: T.A. Name: Exam infrmatin, extra directins, and useful hints t maximize yur
Chemistry 1A Fall 2000 Midterm Exam III, versin B Nvember 14, 2000 (Clsed bk, 90 minutes, 155 pints) Name: SID: Sectin Number: T.A. Name: Exam infrmatin, extra directins, and useful hints t maximize yur
Main components of the above cycle are: 1) Boiler (steam generator) heat exchanger 2) Turbine generates work 3) Condenser heat exchanger 4) Pump
 Introducton to Terodynacs, Lecture -5 Pro. G. Cccarell (0 Applcaton o Control olue Energy Analyss Most terodynac devces consst o a seres o coponents operatng n a cycle, e.g., stea power plant Man coponents
Introducton to Terodynacs, Lecture -5 Pro. G. Cccarell (0 Applcaton o Control olue Energy Analyss Most terodynac devces consst o a seres o coponents operatng n a cycle, e.g., stea power plant Man coponents
Thermochemistry. Thermochemistry
 Thermchemistry Petrucci, Harwd and Herring: Chapter 7 CHEM 1000A 3.0 Thermchemistry 1 Thermchemistry The study energy in chemical reactins A sub-discipline thermdynamics Thermdynamics studies the bulk
Thermchemistry Petrucci, Harwd and Herring: Chapter 7 CHEM 1000A 3.0 Thermchemistry 1 Thermchemistry The study energy in chemical reactins A sub-discipline thermdynamics Thermdynamics studies the bulk
MATHEMATICAL LITERACY
 MATBUS NOVEMBER 2013 EXAMINATION DATE: 8 NOVEMBER 2013 TIME: 14H00 16H00 TOTAL: 100 MARKS DURATION: 2 HOURS PASS MARK: 40% (UC-02) MATHEMATICAL LITERACY THIS EXAMINATION PAPER CONSISTS OF 11 QUESTIONS:
MATBUS NOVEMBER 2013 EXAMINATION DATE: 8 NOVEMBER 2013 TIME: 14H00 16H00 TOTAL: 100 MARKS DURATION: 2 HOURS PASS MARK: 40% (UC-02) MATHEMATICAL LITERACY THIS EXAMINATION PAPER CONSISTS OF 11 QUESTIONS:
Lecture 17: Free Energy of Multi-phase Solutions at Equilibrium
 Lecture 17: 11.07.05 Free Energy f Multi-phase Slutins at Equilibrium Tday: LAST TIME...2 FREE ENERGY DIAGRAMS OF MULTI-PHASE SOLUTIONS 1...3 The cmmn tangent cnstructin and the lever rule...3 Practical
Lecture 17: 11.07.05 Free Energy f Multi-phase Slutins at Equilibrium Tday: LAST TIME...2 FREE ENERGY DIAGRAMS OF MULTI-PHASE SOLUTIONS 1...3 The cmmn tangent cnstructin and the lever rule...3 Practical
8.0 Negative Bias Temperature Instability (NBTI)
 EE650R: Reliability Physics f Naelectric Devices Lecture 8: Negative Bias Temerature Istability Date: Se 27 2006 Class Ntes: Vijay Rawat Reviewed by: Saakshi Gagwal 8.0 Negative Bias Temerature Istability
EE650R: Reliability Physics f Naelectric Devices Lecture 8: Negative Bias Temerature Istability Date: Se 27 2006 Class Ntes: Vijay Rawat Reviewed by: Saakshi Gagwal 8.0 Negative Bias Temerature Istability
CHEM Thermodynamics. Change in Gibbs Free Energy, G. Review. Gibbs Free Energy, G. Review
 Review Accrding t the nd law f Thermdynamics, a prcess is spntaneus if S universe = S system + S surrundings > 0 Even thugh S system
Review Accrding t the nd law f Thermdynamics, a prcess is spntaneus if S universe = S system + S surrundings > 0 Even thugh S system
Ordinary Differential Equations. Orientation. Lesson Objectives. Ch. 25. ODE s. Runge-Kutta Methods. Motivation Mathematical Background
 Ordar Deretal Equats C. 5 Oretat ODE s Mtvat Matematcal Bacgrud Ruge-Kutta Metds Euler s Metd Hue ad Mdpt metds Less Objectves Be able t class ODE s ad dstgus ODE s rm PDE s. Be able t reduce t rder ODE
Ordar Deretal Equats C. 5 Oretat ODE s Mtvat Matematcal Bacgrud Ruge-Kutta Metds Euler s Metd Hue ad Mdpt metds Less Objectves Be able t class ODE s ad dstgus ODE s rm PDE s. Be able t reduce t rder ODE
Unit 9: The Mole- Guided Notes What is a Mole?
 Unit 9: The Mle- Guided Ntes What is a Mle? A mle is a name fr a specific f things Similar t a r a One mle is equal t 602 602,000,000,000,000,000,000,000 That s 602 with zers A mle is NOT an abbreviatin
Unit 9: The Mle- Guided Ntes What is a Mle? A mle is a name fr a specific f things Similar t a r a One mle is equal t 602 602,000,000,000,000,000,000,000 That s 602 with zers A mle is NOT an abbreviatin
Kelvin Planck Statement of the Second Law. Clausius Statement of the Second Law
 Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Goal of the Lecture. Lecture Structure. FWF 410: Analysis of Habitat Data I: Definitions and Descriptive Statistics
 FWF : Aalyss f Habtat Data I: Defts ad Descrptve tatstcs Number f Cveys A A B Bur Dsk Bur/Dsk Habtat Treatmet Matthew J. Gray, Ph.D. Cllege f Agrcultural ceces ad Natural Resurces Uversty f Teessee-Kvlle
FWF : Aalyss f Habtat Data I: Defts ad Descrptve tatstcs Number f Cveys A A B Bur Dsk Bur/Dsk Habtat Treatmet Matthew J. Gray, Ph.D. Cllege f Agrcultural ceces ad Natural Resurces Uversty f Teessee-Kvlle
Basics of heteroskedasticity
 Sect 8 Heterskedastcty ascs f heterskedastcty We have assumed up t w ( ur SR ad MR assumpts) that the varace f the errr term was cstat acrss bservats Ths s urealstc may r mst ecmetrc applcats, especally
Sect 8 Heterskedastcty ascs f heterskedastcty We have assumed up t w ( ur SR ad MR assumpts) that the varace f the errr term was cstat acrss bservats Ths s urealstc may r mst ecmetrc applcats, especally
" 1 = # $H vap. Chapter 3 Problems
 Chapter 3 rblems rblem At 1 atmsphere pure Ge melts at 1232 K and bils at 298 K. he triple pint ccurs at =8.4x1-8 atm. Estimate the heat f vaprizatin f Ge. he heat f vaprizatin is estimated frm the Clausius
Chapter 3 rblems rblem At 1 atmsphere pure Ge melts at 1232 K and bils at 298 K. he triple pint ccurs at =8.4x1-8 atm. Estimate the heat f vaprizatin f Ge. he heat f vaprizatin is estimated frm the Clausius
HANSEN SOLUBILITY PARAMETERS IN CHROMATOGRAPHIC SCIENCES ADAM VOELKEL, K. ADAMSKA POZNAŃ UNIVERSITY OF TECHNOLOGY, POLAND
 HANSN SOLUBILITY PARAMTRS IN CHROMATOGRAPHIC SCINCS ADAM OLKL, K. ADAMSKA POZNAŃ UNIRSITY OF TCHNOLOGY, POLAND SOLUBILITY PARAMTR THORY nergy f varzatn g l U ar U T d Chesve energy densty c c ch H w RT
HANSN SOLUBILITY PARAMTRS IN CHROMATOGRAPHIC SCINCS ADAM OLKL, K. ADAMSKA POZNAŃ UNIRSITY OF TCHNOLOGY, POLAND SOLUBILITY PARAMTR THORY nergy f varzatn g l U ar U T d Chesve energy densty c c ch H w RT
Chapter 6 : Gibbs Free Energy
 Wnter 01 Chem 54: ntrductry hermdynamcs Chapter 6 : Gbbs Free Energy... 64 Defntn f G, A... 64 Mawell Relatns... 65 Gbbs Free Energy G(,) (ure substances)... 67 Gbbs Free Energy fr Mtures... 68 ΔG f deal
Wnter 01 Chem 54: ntrductry hermdynamcs Chapter 6 : Gbbs Free Energy... 64 Defntn f G, A... 64 Mawell Relatns... 65 Gbbs Free Energy G(,) (ure substances)... 67 Gbbs Free Energy fr Mtures... 68 ΔG f deal
CHEE 221: Chemical Processes and Systems
 CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
CH 221: Chemical Processes and Systems Module 4. nergy Balances without Reaction Part a: Introduction to nergy Balances (Felder & Rousseau Ch 7.0 7.4 nergy and nergy Balances: very chemical rocess volves
CHAPTER PRACTICE PROBLEMS CHEMISTRY
 Chemical Kinetics Name: Batch: Date: Rate f reactin. 4NH 3 (g) + 5O (g) à 4NO (g) + 6 H O (g) If the rate f frmatin f NO is 3.6 0 3 ml L s, calculate (i) the rate f disappearance f NH 3 (ii) rate f frmatin
Chemical Kinetics Name: Batch: Date: Rate f reactin. 4NH 3 (g) + 5O (g) à 4NO (g) + 6 H O (g) If the rate f frmatin f NO is 3.6 0 3 ml L s, calculate (i) the rate f disappearance f NH 3 (ii) rate f frmatin
O C S polar - greater force. H polar greater force. H polar. polar H-bond
 hapter 10 : 29, 30, 31, 33, 36, 40, 46, 48, 50, 72, 87, 91, 93, 110 29. a. Lndn e. Lndn b. diple-diple f. diple-diple c. -bnding g. in-in d. in-in 30. a. in-in e. -bnding b. Lndn f. diple-diple c. Lndn
hapter 10 : 29, 30, 31, 33, 36, 40, 46, 48, 50, 72, 87, 91, 93, 110 29. a. Lndn e. Lndn b. diple-diple f. diple-diple c. -bnding g. in-in d. in-in 30. a. in-in e. -bnding b. Lndn f. diple-diple c. Lndn
Asymptotic Formulas Composite Numbers II
 Iteratoal Matematcal Forum, Vol. 8, 203, o. 34, 65-662 HIKARI Ltd, www.m-kar.com ttp://d.do.org/0.2988/mf.203.3854 Asymptotc Formulas Composte Numbers II Rafael Jakmczuk Dvsó Matemátca, Uversdad Nacoal
Iteratoal Matematcal Forum, Vol. 8, 203, o. 34, 65-662 HIKARI Ltd, www.m-kar.com ttp://d.do.org/0.2988/mf.203.3854 Asymptotc Formulas Composte Numbers II Rafael Jakmczuk Dvsó Matemátca, Uversdad Nacoal
Answer Key THERMODYNAMICS TEST (a) 33. (d) 17. (c) 1. (a) 25. (a) 2. (b) 10. (d) 34. (b) 26. (c) 18. (d) 11. (c) 3. (d) 35. (c) 4. (d) 19.
 HERMODYNAMICS ES Answer Key. (a) 9. (a) 7. (c) 5. (a). (d). (b) 0. (d) 8. (d) 6. (c) 4. (b). (d). (c) 9. (b) 7. (c) 5. (c) 4. (d). (a) 0. (b) 8. (b) 6. (b) 5. (b). (d). (a) 9. (a) 7. (b) 6. (a) 4. (d).
HERMODYNAMICS ES Answer Key. (a) 9. (a) 7. (c) 5. (a). (d). (b) 0. (d) 8. (d) 6. (c) 4. (b). (d). (c) 9. (b) 7. (c) 5. (c) 4. (d). (a) 0. (b) 8. (b) 6. (b) 5. (b). (d). (a) 9. (a) 7. (b) 6. (a) 4. (d).
Motor Stability. Plateau and Mesa Burning
 Mtr Stability Recall mass cservati fr steady erati ( =cstat) m eit m b b r b s r m icr m eit Is this cditi (it) stable? ly if rmally use.3
Mtr Stability Recall mass cservati fr steady erati ( =cstat) m eit m b b r b s r m icr m eit Is this cditi (it) stable? ly if rmally use.3
Modern Physics. Unit 15: Nuclear Structure and Decay Lecture 15.2: The Strong Force. Ron Reifenberger Professor of Physics Purdue University
 Mder Physics Uit 15: Nuclear Structure ad Decay Lecture 15.: The Strg Frce R Reifeberger Prfessr f Physics Purdue Uiversity 1 Bidig eergy er ucle - the deuter Eergy (MeV) ~0.4fm B.E. A =.MeV/ = 1.1 MeV/ucle.
Mder Physics Uit 15: Nuclear Structure ad Decay Lecture 15.: The Strg Frce R Reifeberger Prfessr f Physics Purdue Uiversity 1 Bidig eergy er ucle - the deuter Eergy (MeV) ~0.4fm B.E. A =.MeV/ = 1.1 MeV/ucle.
Chem 111 Summer 2013 Key III Whelan
 Chem 111 Summer 2013 Key III Whelan Questin 1 6 Pints Classify each f the fllwing mlecules as plar r nnplar? a) NO + : c) CH 2 Cl 2 : b) XeF 4 : Questin 2 The hypthetical mlecule PY 3 Z 2 has the general
Chem 111 Summer 2013 Key III Whelan Questin 1 6 Pints Classify each f the fllwing mlecules as plar r nnplar? a) NO + : c) CH 2 Cl 2 : b) XeF 4 : Questin 2 The hypthetical mlecule PY 3 Z 2 has the general
Thermodynamics and Equilibrium
 Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
Ch5 Appendix Q-factor and Smith Chart Matching
 h5 Appedx -factr ad mth hart Matchg 5B-1 We-ha a udwg, F rcut Desg Thery ad Applcat, hapter 8 Frequecy espse f -type Matchg Netwrks 5B- Fg.8-8 Tw desg realzats f a -type matchg etwrk.65pf, 80 f 1 GHz Fg.8-9
h5 Appedx -factr ad mth hart Matchg 5B-1 We-ha a udwg, F rcut Desg Thery ad Applcat, hapter 8 Frequecy espse f -type Matchg Netwrks 5B- Fg.8-8 Tw desg realzats f a -type matchg etwrk.65pf, 80 f 1 GHz Fg.8-9
Chapter 14 Logistic Regression Models
 Chapter 4 Logstc Regresso Models I the lear regresso model X β + ε, there are two types of varables explaatory varables X, X,, X k ad study varable y These varables ca be measured o a cotuous scale as
Chapter 4 Logstc Regresso Models I the lear regresso model X β + ε, there are two types of varables explaatory varables X, X,, X k ad study varable y These varables ca be measured o a cotuous scale as
Combustion Chamber. (0.1 MPa)
 ME 354 Tutial #10 Winte 001 Reacting Mixtues Pblem 1: Detemine the mle actins the pducts cmbustin when ctane, C 8 18, is buned with 00% theetical ai. Als, detemine the dew-pint tempeatue the pducts i the
ME 354 Tutial #10 Winte 001 Reacting Mixtues Pblem 1: Detemine the mle actins the pducts cmbustin when ctane, C 8 18, is buned with 00% theetical ai. Als, detemine the dew-pint tempeatue the pducts i the
Autumn 2012 CHEM452B Bruce H. Robinson 322 Gould Hall HW 10(A) Homework 10A KEY (there will not be a 10B) 2
 Autumn 0 CHEM45B Bruce H. Rbinsn Guld Hall HW 0(A) Hmewrk 0A KEY (there will nt be a 0B) QA) Let c be the speed f sund in air. he square f the speed f sund, () f the gas with respect t the change in the
Autumn 0 CHEM45B Bruce H. Rbinsn Guld Hall HW 0(A) Hmewrk 0A KEY (there will nt be a 0B) QA) Let c be the speed f sund in air. he square f the speed f sund, () f the gas with respect t the change in the
Data Mining: Concepts and Techniques
 Data Mg: cepts ad Techques 3 rd ed. hapter 10 1 Evaluat f lusterg lusterg evaluat assesses the feasblty f clusterg aalyss a data set ad the qualty f the results geerated by a clusterg methd. Three mar
Data Mg: cepts ad Techques 3 rd ed. hapter 10 1 Evaluat f lusterg lusterg evaluat assesses the feasblty f clusterg aalyss a data set ad the qualty f the results geerated by a clusterg methd. Three mar
Chapter 3. Differentiation 3.3 Differentiation Rules
 3.3 Dfferetato Rules 1 Capter 3. Dfferetato 3.3 Dfferetato Rules Dervatve of a Costat Fucto. If f as te costat value f(x) = c, te f x = [c] = 0. x Proof. From te efto: f (x) f(x + ) f(x) o c c 0 = 0. QED
3.3 Dfferetato Rules 1 Capter 3. Dfferetato 3.3 Dfferetato Rules Dervatve of a Costat Fucto. If f as te costat value f(x) = c, te f x = [c] = 0. x Proof. From te efto: f (x) f(x + ) f(x) o c c 0 = 0. QED
Thermochemistry. The study of energy changes that occur during chemical : at constant volume ΔU = q V. no at constant pressure ΔH = q P
 Thermchemistry The study energy changes that ccur during chemical : at cnstant vlume ΔU = q V n at cnstant pressure = q P nly wrk Fr practical reasns mst measurements are made at cnstant, s thermchemistry
Thermchemistry The study energy changes that ccur during chemical : at cnstant vlume ΔU = q V n at cnstant pressure = q P nly wrk Fr practical reasns mst measurements are made at cnstant, s thermchemistry
E o and the equilibrium constant, K
 lectrchemical measuremets (Ch -5 t 6). T state the relati betwee ad K. (D x -b, -). Frm galvaic cell vltage measuremet (a) K sp (D xercise -8, -) (b) K sp ad γ (D xercise -9) (c) K a (D xercise -G, -6)
lectrchemical measuremets (Ch -5 t 6). T state the relati betwee ad K. (D x -b, -). Frm galvaic cell vltage measuremet (a) K sp (D xercise -8, -) (b) K sp ad γ (D xercise -9) (c) K a (D xercise -G, -6)
Gestão de Sistemas Energéticos 2017/2018
 Gestão de Sistemas Energéticos 2017/2018 Exergy Analysis Prof. Tânia Sousa taniasousa@tecnico.ulisboa.pt Conceptualizing Chemical Exergy C a H b O c enters the control volume at T 0, p 0. O 2 and CO 2,
Gestão de Sistemas Energéticos 2017/2018 Exergy Analysis Prof. Tânia Sousa taniasousa@tecnico.ulisboa.pt Conceptualizing Chemical Exergy C a H b O c enters the control volume at T 0, p 0. O 2 and CO 2,
Experiment #3. Graphing with Excel
 Experiment #3. Graphing with Excel Study the "Graphing with Excel" instructins that have been prvided. Additinal help with learning t use Excel can be fund n several web sites, including http://www.ncsu.edu/labwrite/res/gt/gt-
Experiment #3. Graphing with Excel Study the "Graphing with Excel" instructins that have been prvided. Additinal help with learning t use Excel can be fund n several web sites, including http://www.ncsu.edu/labwrite/res/gt/gt-
Chapter 3.1: Polynomial Functions
 Ntes 3.1: Ply Fucs Chapter 3.1: Plymial Fuctis I Algebra I ad Algebra II, yu ecutered sme very famus plymial fuctis. I this secti, yu will meet may ther members f the plymial family, what sets them apart
Ntes 3.1: Ply Fucs Chapter 3.1: Plymial Fuctis I Algebra I ad Algebra II, yu ecutered sme very famus plymial fuctis. I this secti, yu will meet may ther members f the plymial family, what sets them apart
th th th The air-fuel ratio is determined by taking the ratio of the mass of the air to the mass of the fuel,
 Cheical Reactins 14-14 rpane is burned wi 75 percent excess during a cbustin prcess. The AF rati is t be deterined. Assuptins 1 Cbustin is cplete. The cbustin prducts cntain CO, H O, O, and N nly. rperties
Cheical Reactins 14-14 rpane is burned wi 75 percent excess during a cbustin prcess. The AF rati is t be deterined. Assuptins 1 Cbustin is cplete. The cbustin prducts cntain CO, H O, O, and N nly. rperties
BIT Chapters = =
 BIT Chapters 17-0 1. K w = [H + ][OH ] = 9.5 10 14 [H + ] = [OH ] =.1 10 7 ph = 6.51 The slutin is neither acidic nr basic because the cncentratin f the hydrnium in equals the cncentratin f the hydride
BIT Chapters 17-0 1. K w = [H + ][OH ] = 9.5 10 14 [H + ] = [OH ] =.1 10 7 ph = 6.51 The slutin is neither acidic nr basic because the cncentratin f the hydrnium in equals the cncentratin f the hydride
Reacting Gas Mixtures
 Reacting Gas Mixtures Reading Problems 15-1 15-7 15-21, 15-32, 15-51, 15-61, 15-74 15-83, 15-91, 15-93, 15-98 Introduction thermodynamic analysis of reactive mixtures is primarily an extension of the principles
Reacting Gas Mixtures Reading Problems 15-1 15-7 15-21, 15-32, 15-51, 15-61, 15-74 15-83, 15-91, 15-93, 15-98 Introduction thermodynamic analysis of reactive mixtures is primarily an extension of the principles
Pipe Networks - Hardy Cross Method Page 1. Pipe Networks
 Pie Netwrks - Hardy Crss etd Page Pie Netwrks Itrducti A ie etwrk is a itercected set f ies likig e r mre surces t e r mre demad (delivery) its, ad ca ivlve ay umber f ies i series, bracig ies, ad arallel
Pie Netwrks - Hardy Crss etd Page Pie Netwrks Itrducti A ie etwrk is a itercected set f ies likig e r mre surces t e r mre demad (delivery) its, ad ca ivlve ay umber f ies i series, bracig ies, ad arallel
A Chemical Reaction occurs when the of a substance changes.
 Perid: Unit 8 Chemical Reactin- Guided Ntes Chemical Reactins A Chemical Reactin ccurs when the f a substance changes. Chemical Reactin: ne r mre substances are changed int ne r mre new substances by the
Perid: Unit 8 Chemical Reactin- Guided Ntes Chemical Reactins A Chemical Reactin ccurs when the f a substance changes. Chemical Reactin: ne r mre substances are changed int ne r mre new substances by the
ES201 - Examination 2 Winter Adams and Richards NAME BOX NUMBER
 ES201 - Examinatin 2 Winter 2003-2004 Adams and Richards NAME BOX NUMBER Please Circle One : Richards (Perid 4) ES201-01 Adams (Perid 4) ES201-02 Adams (Perid 6) ES201-03 Prblem 1 ( 12 ) Prblem 2 ( 24
ES201 - Examinatin 2 Winter 2003-2004 Adams and Richards NAME BOX NUMBER Please Circle One : Richards (Perid 4) ES201-01 Adams (Perid 4) ES201-02 Adams (Perid 6) ES201-03 Prblem 1 ( 12 ) Prblem 2 ( 24
Part One: Heat Changes and Thermochemistry. This aspect of Thermodynamics was dealt with in Chapter 6. (Review)
 CHAPTER 18: THERMODYNAMICS AND EQUILIBRIUM Part One: Heat Changes and Thermchemistry This aspect f Thermdynamics was dealt with in Chapter 6. (Review) A. Statement f First Law. (Sectin 18.1) 1. U ttal
CHAPTER 18: THERMODYNAMICS AND EQUILIBRIUM Part One: Heat Changes and Thermchemistry This aspect f Thermdynamics was dealt with in Chapter 6. (Review) A. Statement f First Law. (Sectin 18.1) 1. U ttal
General Chemistry 1 (CHEM1141) Shawnee State University Fall 2016
 Geeral Chemistry 1 (CHEM1141) Shawee State Uiversity Fall 2016 September 23, 2016 Name E x a m # I C Please write yur full ame, ad the exam versi (IC) that yu have the scatr sheet! Please 0 check the bx
Geeral Chemistry 1 (CHEM1141) Shawee State Uiversity Fall 2016 September 23, 2016 Name E x a m # I C Please write yur full ame, ad the exam versi (IC) that yu have the scatr sheet! Please 0 check the bx
LCAO APPROXIMATIONS OF ORGANIC Pi MO SYSTEMS The allyl system (cation, anion or radical).
 Principles f Organic Chemistry lecture 5, page LCAO APPROIMATIONS OF ORGANIC Pi MO SYSTEMS The allyl system (catin, anin r radical).. Draw mlecule and set up determinant. 2 3 0 3 C C 2 = 0 C 2 3 0 = -
Principles f Organic Chemistry lecture 5, page LCAO APPROIMATIONS OF ORGANIC Pi MO SYSTEMS The allyl system (catin, anin r radical).. Draw mlecule and set up determinant. 2 3 0 3 C C 2 = 0 C 2 3 0 = -
Edexcel IGCSE Chemistry. Topic 1: Principles of chemistry. Chemical formulae, equations and calculations. Notes.
 Edexcel IGCSE Chemistry Tpic 1: Principles f chemistry Chemical frmulae, equatins and calculatins Ntes 1.25 write wrd equatins and balanced chemical equatins (including state symbls): fr reactins studied
Edexcel IGCSE Chemistry Tpic 1: Principles f chemistry Chemical frmulae, equatins and calculatins Ntes 1.25 write wrd equatins and balanced chemical equatins (including state symbls): fr reactins studied
Every gas consists of a large number of small particles called molecules moving with very high velocities in all possible directions.
 Kietic thery f gases ( Kietic thery was develped by Berlli, Jle, Clasis, axwell ad Bltzma etc. ad represets dyamic particle r micrscpic mdel fr differet gases sice it thrws light the behir f the particles
Kietic thery f gases ( Kietic thery was develped by Berlli, Jle, Clasis, axwell ad Bltzma etc. ad represets dyamic particle r micrscpic mdel fr differet gases sice it thrws light the behir f the particles
UNIVERSITY OF OSLO DEPARTMENT OF ECONOMICS
 UNIVERSITY OF OSLO DEPARTMENT OF ECONOMICS Exam: ECON430 Statstcs Date of exam: Frday, December 8, 07 Grades are gve: Jauary 4, 08 Tme for exam: 0900 am 00 oo The problem set covers 5 pages Resources allowed:
UNIVERSITY OF OSLO DEPARTMENT OF ECONOMICS Exam: ECON430 Statstcs Date of exam: Frday, December 8, 07 Grades are gve: Jauary 4, 08 Tme for exam: 0900 am 00 oo The problem set covers 5 pages Resources allowed:
CHAPTER 19 ELECTROCHEMISTRY
 CHAPTR 19 LCTROCHMISTRY 19.1 We fllw the steps are described i detail i Secti 19.1 f the text. (a) The prblem is give i iic frm, s cmbiig Steps 1 ad, the half-reactis are: xidati: Fe Fe 3+ reducti: H O
CHAPTR 19 LCTROCHMISTRY 19.1 We fllw the steps are described i detail i Secti 19.1 f the text. (a) The prblem is give i iic frm, s cmbiig Steps 1 ad, the half-reactis are: xidati: Fe Fe 3+ reducti: H O
If σis unknown. Properties of t distribution. 6.3 One and Two Sample Inferences for Means. What is the correct multiplier? t
 /8/009 6.3 Oe a Tw Samle Iferece fr Mea If i kw a 95% Cfiece Iterval i 96 ±.96 96.96 ± But i ever kw. If i ukw Etimate by amle taar eviati The etimate taar errr f the mea will be / Uig the etimate taar
/8/009 6.3 Oe a Tw Samle Iferece fr Mea If i kw a 95% Cfiece Iterval i 96 ±.96 96.96 ± But i ever kw. If i ukw Etimate by amle taar eviati The etimate taar errr f the mea will be / Uig the etimate taar
CHAPTER Read Chapter 17, sections 1,2,3. End of Chapter problems: 25
 CHAPTER 17 1. Read Chapter 17, sectins 1,2,3. End f Chapter prblems: 25 2. Suppse yu are playing a game that uses tw dice. If yu cunt the dts n the dice, yu culd have anywhere frm 2 t 12. The ways f prducing
CHAPTER 17 1. Read Chapter 17, sectins 1,2,3. End f Chapter prblems: 25 2. Suppse yu are playing a game that uses tw dice. If yu cunt the dts n the dice, yu culd have anywhere frm 2 t 12. The ways f prducing
Name: Period: Date: BONDING NOTES ADVANCED CHEMISTRY
 Name: Perid: Date: BONDING NOTES ADVANCED CHEMISTRY Directins: This packet will serve as yur ntes fr this chapter. Fllw alng with the PwerPint presentatin and fill in the missing infrmatin. Imprtant terms
Name: Perid: Date: BONDING NOTES ADVANCED CHEMISTRY Directins: This packet will serve as yur ntes fr this chapter. Fllw alng with the PwerPint presentatin and fill in the missing infrmatin. Imprtant terms
Lecture 4. The First Law of Thermodynamics
 Lecture 4. The First Law f Thermdynamics THERMODYNAMICS: Basic Cncepts Thermdynamics: (frm the Greek therme, meaning "heat" and, dynamis, meaning "pwer") is the study f energy cnversin between heat and
Lecture 4. The First Law f Thermdynamics THERMODYNAMICS: Basic Cncepts Thermdynamics: (frm the Greek therme, meaning "heat" and, dynamis, meaning "pwer") is the study f energy cnversin between heat and
SOLUTION. The reactor thermal output is related to the maximum heat flux in the hot channel by. Z( z ). The position of maximum heat flux ( z max
 Te verpwer trip set pit i PWRs is desiged t isure te iu fuel eterlie teperature reais belw a give value T, ad te iiu rati reais abve a give value MR. Fr te give ifrati give a step by step predure, iludig
Te verpwer trip set pit i PWRs is desiged t isure te iu fuel eterlie teperature reais belw a give value T, ad te iiu rati reais abve a give value MR. Fr te give ifrati give a step by step predure, iludig
Thermodynamics of Materials
 Thermdynamcs f Materals 14th Lecture 007. 4. 8 (Mnday) FUGACITY dg = Vd SdT dg = Vd at cnstant T Fr an deal gas dg = (RT/)d = RT dln Ths s true fr deal gases nly, but t wuld be nce t have a smlar frm fr
Thermdynamcs f Materals 14th Lecture 007. 4. 8 (Mnday) FUGACITY dg = Vd SdT dg = Vd at cnstant T Fr an deal gas dg = (RT/)d = RT dln Ths s true fr deal gases nly, but t wuld be nce t have a smlar frm fr
MATH Midterm Examination Victor Matveev October 26, 2016
 MATH 33- Midterm Examiati Victr Matveev Octber 6, 6. (5pts, mi) Suppse f(x) equals si x the iterval < x < (=), ad is a eve peridic extesi f this fucti t the rest f the real lie. Fid the csie series fr
MATH 33- Midterm Examiati Victr Matveev Octber 6, 6. (5pts, mi) Suppse f(x) equals si x the iterval < x < (=), ad is a eve peridic extesi f this fucti t the rest f the real lie. Fid the csie series fr
Examination No. 3 - Tuesday, Nov. 15
 NAME (lease rit) SOLUTIONS ECE 35 - DEVICE ELECTRONICS Fall Semester 005 Examiati N 3 - Tuesday, Nv 5 3 4 5 The time fr examiati is hr 5 mi Studets are allwed t use 3 sheets f tes Please shw yur wrk, artial
NAME (lease rit) SOLUTIONS ECE 35 - DEVICE ELECTRONICS Fall Semester 005 Examiati N 3 - Tuesday, Nv 5 3 4 5 The time fr examiati is hr 5 mi Studets are allwed t use 3 sheets f tes Please shw yur wrk, artial
