[1 & α(t & T 1. ' ρ 1
|
|
|
- Aubrie Melton
- 6 years ago
- Views:
Transcription
1 NAME IGNEOUS & METAMORPHIC PETROLOGY DENSITY & VISCOSITY OF MAGMAS I. Desity The desity (mass/vlume) f a magma is a imprtat parameter which plays a rle i a umber f aspects f magma behavir ad evluti. Fr example, durig fractial crystallizati the rate at which crystals settle (r rise) thrugh a magma is a fucti f the desity ctrast betwee the magma ad the crystals, the size f the crystals, ad the viscsity f the magma. Additially, the ctrast i magma desity is imprtat i gverig the rate f cvective vertur i a magma chamber ad magma mixig. Additially, the ctrast i desity betwee a magma clum ad the surrudig cutry rck will determie the height t which a magma clum will rise. The physical measuremet f magma desity is rather difficult. Hece, a variety f methds f have bee develped t estimate r determie magma desity withut havig t directly determie the desity f the liquid magma. Desity estimated frm average vlume icrease durig meltig. Frm bservati we kw that the vlume expasi meltig fr mst rcks is abut 10%. Hece, mst magmas have a desity abut 90% that f the equivalet slid rck. This is a relatively crude, but smetimes adequate, estimate. Desity determied frm queched glass at rm temperature. Because glass is a supercled liquid, the high-temperature desity ca be calculated frm the rm-temperature desity if the cefficiet f thermal expasi is kw. The desity f the liquid at temperature T ca be calculated as fllws ρ T ' ρ 1 [1 & α(t & T 1 )] where ρ T = the desity at temperature T, ρ 1 = the desity at rm temperature (r the temperature at which the glass desity was measured), α = the cefficiet f thermal expasi, T = the temperature at which the desity is calculated, ad T 1 = the temperature at which the desity is measured. 1. A sample f graite with desity 670 kg m is fused ad queched t a glass that has a desity f kg m at 5 C. The cefficiet f expasi, α, fr the glass is x 10 C. Calculate the desity f the magma at 105 C, ad cmpare this with the value btaied by simply takig 90% f the desity f the rck. -1-
2 Desity calculated frm mlar vlumes f cstituet xides. Bttiga ad Weill (1970) develped a mdel which eables e t calculate the magma desity frm the cmpsiti f the magma. Desity is writte as ρ ' M V where M = mass ad V = vlume. I Bttiga ad Weill s mdel ρ ' j ) j ) V T ' V 1 [1 % α(t & T 1 )] 3 6 where X i= the mle fracti f cmpet i, = the gram frmula weight f cmpet i, ad = the partial mlar vlume f cmpet i. The imprtat parameter is, measured i m /(mle x 10 ), which 3 varies as a fucti f temperature. Nte that what represets is the umber f m ccupied by 1 mle f a particular xide. Therefre, if we determie the fractial mass f each xide cmpet i the melt, ad the vlume that each xide ccupies, the summati fr all xides gives the desity f the magma. The table belw gives the partial mlar vlumes ad cefficiets f expasi fr cmm xide cmpets f silicate liquids at 1400 C. The partial mlar vlumes at temperatures ther tha 1400 C ca be calculated frm the relatiship where V T = the vlume at temperature T, V 1 = the vlume at 1400 C, α = the cefficiet f thermal expasi, T = the temperature at which the vlume is calculated, ad T 1 = 1400 C. Mlar Vlume Cefficiet f expasi Oxide V (m ml ) x α ( C ) x 10 i SiO TiO Al O Fe O FeO MgO CaO NaO KO The partial mlar vlume f water ca be calculated as fllws: --
3 V H O ' 4.7 % 7.0x10&3 (T & 900) & 1.5P % 8.4 x 10 & P & 1.4x10 &3 P 3 6 where V H O = m /(mle x 10 ), T = C, ad P = kilbars. Nte that the mdel f Bttiga ad Weill igres the effect f pressure all the xide cmpets except water. This is a reasable simplificati fr magmas which reside i the crust, but at matle depths pressure shuld be csidered.. Calculate the desities f the magmas Rhylite 1 (800 C), Rhylite (800 C), ad the Basalt at 1000 C ad 1100 C. The relevat data ad space fr the calculatis are fud i the table page Based the calculated desities, what geeral cclusis ca yu make abut the effect f cmpsiti, water ctet, ad temperature magma desity? -
4 Mlecular Mle Mlecular Mle Mlecular Mle Mlecular Oxide Rhylite 1 prprti fracti Rhylite prprti fracti Basalt prprti fracti weights SiO TiO Al O Fe O FeO MO MgO CaO NaO KO HO Rhylite 1: (T = 800 C) ρ ' j )/ j Rhylite : (T = 800 C) (P = 1kbar) ρ ' j )/ j V H O ' 4.7 % 7.0x10&3 (T & 900) & 1.5P % 8.4 x 10 & P & 1.4x10 &3 P 3 ' Basalt 1: (T = 1000 C) ρ ' j )/ j Basalt : (T = 1100 C) ρ ' j )/ j -4-
5 II. Viscsity Viscsity is a measure f the iteral resistace f a liquid bdy t flw. Viscsity plays a rle i determiig the style f magmatic emplacemet ad irrupti, the rate at which crystals r ther slids settle thrugh a melt, ad the rate f diffusi f is thrugh a melt. The cefficiet f viscsity is the rati f the applied shear stress t the time rate f shear strai. The viscsity is said t be Newtia if this cefficiet is idepedet f the magitude f the shear stress ad strai. The structure f a silicate melt (which is a fucti f its cmpsiti), temperature, ad pressure determie the viscsity f a melt. Ulike desity, it has prve very difficult t develp a mdel fr the calculati f magma viscsity. Hwever, it is pssible t determie the viscsity f melts (ce the desity is kw) by measurig the rate at which spheres f kw size ad desity settle thrugh the melt. The gverig priciple is Stke s Law v ' gδρr 9η -1 - where v = settlig velcity (m s ), g = accelerati due t gravity (9.8 m s ), Δρ = desity ctrast betwee the sphere ad the melt (kg m ), r = the radius f the sphere (m), ad η = dyamic viscsity (Pa s). Give the desity f the sphere, the magma, ad the radius f the sphere, we ca measure the settlig velcity f the sphere ad frm this velcity calculate the viscsity f the magma. 4. Usig Stke s Law, calculate the viscsities f the fur magmas csidered i questi 1. The radius f the sphere is 0.01 m ad its desity is 5000 kg m. Desity Settlig velcity Magma (kg m ) -1 (m s ) Viscsity (Pa s) Rhylite x 10-9 Rhylite 5.77 x Basalt (1000 C) 5.07 x 10-4 Basalt (1100 C) 5.05 x Based the calculated viscsities, what geeral cclusis ca yu make abut the effect f cmpsiti, water ctet, ad temperature the viscsity f magmas? -5-
6 III. Melt Plymerizati A silicate melt ca be viewed as a material that has a shrt-rage structural rder. The structure csists - f certai catis tetrahedrally crdiated with O is frmig tetrahedra that are the liked directly t ther tetrahedra r separated frm ther tetrahedra by etwrk mdifyig catis. The degree t which the silica tetrahedra are liked is referred t as plymerizati. Fr example, a melt frmed frm quartz wuld be highly plymerized with virtually all the tetrahedra itercected. A measure f the degree f plymerizati is the rati f tetrahedrally crdiated catis (T) t bridgig xyge is (NBO), i.e., xyge is that are directly bded t tetrahedrally crdiated catis. The larger this rati, the less plymerized is the melt. The NBO/T rati is calculated as fllws: 1. Cvert the chemical aalysis f the rck t atmic prprtis. Assig Si, Ti, ad P t T 3. Assig Al t T uless Al > Na + K + Ca + Mg. I this case, Al i T = Na + K + Ca + Mg Fe i T = (Na + K) - Al # Fe 5. Sum all T catis = T 6. NBO =O - 4T 7. Calculate NBO/T 6. Calculate the NBO/T rati fr Rhylite 1 ad Basalt. Nte that yu ca btai the atmic prprtis by multiplyig the mlecular prprtis by the umber f is i the xide frmula. Fr example, i the case f AlO 3, Al = x mlecular prprti AlO 3 ad O = 3 x mlecular prprti AlO 3. What is the relatiship betwee the degree f plymerizati ad the viscsity f the melts? -6-
Solutions. Definitions pertaining to solutions
 Slutis Defiitis pertaiig t slutis Slute is the substace that is disslved. It is usually preset i the smaller amut. Slvet is the substace that des the disslvig. It is usually preset i the larger amut. Slubility
Slutis Defiitis pertaiig t slutis Slute is the substace that is disslved. It is usually preset i the smaller amut. Slvet is the substace that des the disslvig. It is usually preset i the larger amut. Slubility
Grade 3 Mathematics Course Syllabus Prince George s County Public Schools
 Ctet Grade 3 Mathematics Curse Syllabus Price Gerge s Cuty Public Schls Prerequisites: Ne Curse Descripti: I Grade 3, istructial time shuld fcus fur critical areas: (1) develpig uderstadig f multiplicati
Ctet Grade 3 Mathematics Curse Syllabus Price Gerge s Cuty Public Schls Prerequisites: Ne Curse Descripti: I Grade 3, istructial time shuld fcus fur critical areas: (1) develpig uderstadig f multiplicati
Edexcel IGCSE Chemistry. Topic 1: Principles of chemistry. Chemical formulae, equations and calculations. Notes.
 Edexcel IGCSE Chemistry Tpic 1: Principles f chemistry Chemical frmulae, equatins and calculatins Ntes 1.25 write wrd equatins and balanced chemical equatins (including state symbls): fr reactins studied
Edexcel IGCSE Chemistry Tpic 1: Principles f chemistry Chemical frmulae, equatins and calculatins Ntes 1.25 write wrd equatins and balanced chemical equatins (including state symbls): fr reactins studied
General Chemistry 1 (CHEM1141) Shawnee State University Fall 2016
 Geeral Chemistry 1 (CHEM1141) Shawee State Uiversity Fall 2016 September 23, 2016 Name E x a m # I C Please write yur full ame, ad the exam versi (IC) that yu have the scatr sheet! Please 0 check the bx
Geeral Chemistry 1 (CHEM1141) Shawee State Uiversity Fall 2016 September 23, 2016 Name E x a m # I C Please write yur full ame, ad the exam versi (IC) that yu have the scatr sheet! Please 0 check the bx
Physical Chemistry Laboratory I CHEM 445 Experiment 2 Partial Molar Volume (Revised, 01/13/03)
 Physical Chemistry Labratry I CHEM 445 Experimet Partial Mlar lume (Revised, 0/3/03) lume is, t a gd apprximati, a additive prperty. Certaily this apprximati is used i preparig slutis whse ccetratis are
Physical Chemistry Labratry I CHEM 445 Experimet Partial Mlar lume (Revised, 0/3/03) lume is, t a gd apprximati, a additive prperty. Certaily this apprximati is used i preparig slutis whse ccetratis are
Chapter 3.1: Polynomial Functions
 Ntes 3.1: Ply Fucs Chapter 3.1: Plymial Fuctis I Algebra I ad Algebra II, yu ecutered sme very famus plymial fuctis. I this secti, yu will meet may ther members f the plymial family, what sets them apart
Ntes 3.1: Ply Fucs Chapter 3.1: Plymial Fuctis I Algebra I ad Algebra II, yu ecutered sme very famus plymial fuctis. I this secti, yu will meet may ther members f the plymial family, what sets them apart
The Excel FFT Function v1.1 P. T. Debevec February 12, The discrete Fourier transform may be used to identify periodic structures in time ht.
 The Excel FFT Fucti v P T Debevec February 2, 26 The discrete Furier trasfrm may be used t idetify peridic structures i time ht series data Suppse that a physical prcess is represeted by the fucti f time,
The Excel FFT Fucti v P T Debevec February 2, 26 The discrete Furier trasfrm may be used t idetify peridic structures i time ht series data Suppse that a physical prcess is represeted by the fucti f time,
A Hartree-Fock Calculation of the Water Molecule
 Chemistry 460 Fall 2017 Dr. Jea M. Stadard Nvember 29, 2017 A Hartree-Fck Calculati f the Water Mlecule Itrducti A example Hartree-Fck calculati f the water mlecule will be preseted. I this case, the water
Chemistry 460 Fall 2017 Dr. Jea M. Stadard Nvember 29, 2017 A Hartree-Fck Calculati f the Water Mlecule Itrducti A example Hartree-Fck calculati f the water mlecule will be preseted. I this case, the water
BIO752: Advanced Methods in Biostatistics, II TERM 2, 2010 T. A. Louis. BIO 752: MIDTERM EXAMINATION: ANSWERS 30 November 2010
 BIO752: Advaced Methds i Bistatistics, II TERM 2, 2010 T. A. Luis BIO 752: MIDTERM EXAMINATION: ANSWERS 30 Nvember 2010 Questi #1 (15 pits): Let X ad Y be radm variables with a jit distributi ad assume
BIO752: Advaced Methds i Bistatistics, II TERM 2, 2010 T. A. Luis BIO 752: MIDTERM EXAMINATION: ANSWERS 30 Nvember 2010 Questi #1 (15 pits): Let X ad Y be radm variables with a jit distributi ad assume
ENGI 4421 Central Limit Theorem Page Central Limit Theorem [Navidi, section 4.11; Devore sections ]
![ENGI 4421 Central Limit Theorem Page Central Limit Theorem [Navidi, section 4.11; Devore sections ] ENGI 4421 Central Limit Theorem Page Central Limit Theorem [Navidi, section 4.11; Devore sections ]](/thumbs/80/82121747.jpg) ENGI 441 Cetral Limit Therem Page 11-01 Cetral Limit Therem [Navidi, secti 4.11; Devre sectis 5.3-5.4] If X i is t rmally distributed, but E X i, V X i ad is large (apprximately 30 r mre), the, t a gd
ENGI 441 Cetral Limit Therem Page 11-01 Cetral Limit Therem [Navidi, secti 4.11; Devre sectis 5.3-5.4] If X i is t rmally distributed, but E X i, V X i ad is large (apprximately 30 r mre), the, t a gd
x 2 x 3 x b 0, then a, b, c log x 1 log z log x log y 1 logb log a dy 4. dx As tangent is perpendicular to the x axis, slope
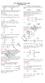 The agle betwee the tagets draw t the parabla y = frm the pit (-,) 5 9 6 Here give pit lies the directri, hece the agle betwee the tagets frm that pit right agle Ratig :EASY The umber f values f c such
The agle betwee the tagets draw t the parabla y = frm the pit (-,) 5 9 6 Here give pit lies the directri, hece the agle betwee the tagets frm that pit right agle Ratig :EASY The umber f values f c such
Hº = -690 kj/mol for ionization of n-propylene Hº = -757 kj/mol for ionization of isopropylene
 Prblem 56. (a) (b) re egative º values are a idicati f mre stable secies. The º is mst egative fr the i-ryl ad -butyl is, bth f which ctai a alkyl substituet bded t the iized carb. Thus it aears that catis
Prblem 56. (a) (b) re egative º values are a idicati f mre stable secies. The º is mst egative fr the i-ryl ad -butyl is, bth f which ctai a alkyl substituet bded t the iized carb. Thus it aears that catis
Unit -2 THEORY OF DILUTE SOLUTIONS
 Uit - THEORY OF DILUTE SOLUTIONS 1) hat is sluti? : It is a hmgeus mixture f tw r mre cmpuds. ) hat is dilute sluti? : It is a sluti i which slute ccetrati is very less. 3) Give a example fr slid- slid
Uit - THEORY OF DILUTE SOLUTIONS 1) hat is sluti? : It is a hmgeus mixture f tw r mre cmpuds. ) hat is dilute sluti? : It is a sluti i which slute ccetrati is very less. 3) Give a example fr slid- slid
WEST VIRGINIA UNIVERSITY
 WEST VIRGINIA UNIVERSITY PLASMA PHYSICS GROUP INTERNAL REPORT PL - 045 Mea Optical epth ad Optical Escape Factr fr Helium Trasitis i Helic Plasmas R.F. Bivi Nvember 000 Revised March 00 TABLE OF CONTENT.0
WEST VIRGINIA UNIVERSITY PLASMA PHYSICS GROUP INTERNAL REPORT PL - 045 Mea Optical epth ad Optical Escape Factr fr Helium Trasitis i Helic Plasmas R.F. Bivi Nvember 000 Revised March 00 TABLE OF CONTENT.0
D.S.G. POLLOCK: TOPICS IN TIME-SERIES ANALYSIS STATISTICAL FOURIER ANALYSIS
 STATISTICAL FOURIER ANALYSIS The Furier Represetati f a Sequece Accrdig t the basic result f Furier aalysis, it is always pssible t apprximate a arbitrary aalytic fucti defied ver a fiite iterval f the
STATISTICAL FOURIER ANALYSIS The Furier Represetati f a Sequece Accrdig t the basic result f Furier aalysis, it is always pssible t apprximate a arbitrary aalytic fucti defied ver a fiite iterval f the
( ) kt. Solution. From kinetic theory (visualized in Figure 1Q9-1), 1 2 rms = 2. = 1368 m/s
 .9 Kinetic Mlecular Thery Calculate the effective (rms) speeds f the He and Ne atms in the He-Ne gas laser tube at rm temperature (300 K). Slutin T find the rt mean square velcity (v rms ) f He atms at
.9 Kinetic Mlecular Thery Calculate the effective (rms) speeds f the He and Ne atms in the He-Ne gas laser tube at rm temperature (300 K). Slutin T find the rt mean square velcity (v rms ) f He atms at
Unit 14 Thermochemistry Notes
 Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
Every gas consists of a large number of small particles called molecules moving with very high velocities in all possible directions.
 Kietic thery f gases ( Kietic thery was develped by Berlli, Jle, Clasis, axwell ad Bltzma etc. ad represets dyamic particle r micrscpic mdel fr differet gases sice it thrws light the behir f the particles
Kietic thery f gases ( Kietic thery was develped by Berlli, Jle, Clasis, axwell ad Bltzma etc. ad represets dyamic particle r micrscpic mdel fr differet gases sice it thrws light the behir f the particles
Sound Absorption Characteristics of Membrane- Based Sound Absorbers
 Purdue e-pubs Publicatis f the Ray W. Schl f Mechaical Egieerig 8-28-2003 Sud Absrpti Characteristics f Membrae- Based Sud Absrbers J Stuart Blt, blt@purdue.edu Jih Sg Fllw this ad additial wrks at: http://dcs.lib.purdue.edu/herrick
Purdue e-pubs Publicatis f the Ray W. Schl f Mechaical Egieerig 8-28-2003 Sud Absrpti Characteristics f Membrae- Based Sud Absrbers J Stuart Blt, blt@purdue.edu Jih Sg Fllw this ad additial wrks at: http://dcs.lib.purdue.edu/herrick
Quantum Mechanics for Scientists and Engineers. David Miller
 Quatum Mechaics fr Scietists ad Egieers David Miller Time-depedet perturbati thery Time-depedet perturbati thery Time-depedet perturbati basics Time-depedet perturbati thery Fr time-depedet prblems csider
Quatum Mechaics fr Scietists ad Egieers David Miller Time-depedet perturbati thery Time-depedet perturbati thery Time-depedet perturbati basics Time-depedet perturbati thery Fr time-depedet prblems csider
ALE 26. Equilibria for Cell Reactions. What happens to the cell potential as the reaction proceeds over time?
 Name Chem 163 Secti: Team Number: AL 26. quilibria fr Cell Reactis (Referece: 21.4 Silberberg 5 th editi) What happes t the ptetial as the reacti prceeds ver time? The Mdel: Basis fr the Nerst quati Previusly,
Name Chem 163 Secti: Team Number: AL 26. quilibria fr Cell Reactis (Referece: 21.4 Silberberg 5 th editi) What happes t the ptetial as the reacti prceeds ver time? The Mdel: Basis fr the Nerst quati Previusly,
Multi-objective Programming Approach for. Fuzzy Linear Programming Problems
 Applied Mathematical Scieces Vl. 7 03. 37 8-87 HIKARI Ltd www.m-hikari.cm Multi-bective Prgrammig Apprach fr Fuzzy Liear Prgrammig Prblems P. Padia Departmet f Mathematics Schl f Advaced Scieces VIT Uiversity
Applied Mathematical Scieces Vl. 7 03. 37 8-87 HIKARI Ltd www.m-hikari.cm Multi-bective Prgrammig Apprach fr Fuzzy Liear Prgrammig Prblems P. Padia Departmet f Mathematics Schl f Advaced Scieces VIT Uiversity
Lecture 18. MSMPR Crystallization Model
 ecture 18. MSMPR Crystalliati Mdel MSMPR Crystalliati Mdel Crystal-Ppulati alace - Number f crystals - Cumulative umber f crystals - Predmiat crystal sie - Grwth rate MSMPR Crystalliati Mdel Mixed-suspesi,
ecture 18. MSMPR Crystalliati Mdel MSMPR Crystalliati Mdel Crystal-Ppulati alace - Number f crystals - Cumulative umber f crystals - Predmiat crystal sie - Grwth rate MSMPR Crystalliati Mdel Mixed-suspesi,
MATHEMATICS 9740/01 Paper 1 14 Sep hours
 Cadidate Name: Class: JC PRELIMINARY EXAM Higher MATHEMATICS 9740/0 Paper 4 Sep 06 3 hurs Additial Materials: Cver page Aswer papers List f Frmulae (MF5) READ THESE INSTRUCTIONS FIRST Write yur full ame
Cadidate Name: Class: JC PRELIMINARY EXAM Higher MATHEMATICS 9740/0 Paper 4 Sep 06 3 hurs Additial Materials: Cver page Aswer papers List f Frmulae (MF5) READ THESE INSTRUCTIONS FIRST Write yur full ame
Session #22: Homework Solutions
 Sessin #22: Hmewrk Slutins Prblem #1 (a) In the cntext f amrphus inrganic cmpunds, name tw netwrk frmers, tw netwrk mdifiers, and ne intermediate. (b) Sketch the variatin f mlar vlume with temperature
Sessin #22: Hmewrk Slutins Prblem #1 (a) In the cntext f amrphus inrganic cmpunds, name tw netwrk frmers, tw netwrk mdifiers, and ne intermediate. (b) Sketch the variatin f mlar vlume with temperature
Intermediate Division Solutions
 Itermediate Divisi Slutis 1. Cmpute the largest 4-digit umber f the frm ABBA which is exactly divisible by 7. Sluti ABBA 1000A + 100B +10B+A 1001A + 110B 1001 is divisible by 7 (1001 7 143), s 1001A is
Itermediate Divisi Slutis 1. Cmpute the largest 4-digit umber f the frm ABBA which is exactly divisible by 7. Sluti ABBA 1000A + 100B +10B+A 1001A + 110B 1001 is divisible by 7 (1001 7 143), s 1001A is
Thermodynamics and Equilibrium
 Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
Unit 9: The Mole- Guided Notes What is a Mole?
 Unit 9: The Mle- Guided Ntes What is a Mle? A mle is a name fr a specific f things Similar t a r a One mle is equal t 602 602,000,000,000,000,000,000,000 That s 602 with zers A mle is NOT an abbreviatin
Unit 9: The Mle- Guided Ntes What is a Mle? A mle is a name fr a specific f things Similar t a r a One mle is equal t 602 602,000,000,000,000,000,000,000 That s 602 with zers A mle is NOT an abbreviatin
MATH Midterm Examination Victor Matveev October 26, 2016
 MATH 33- Midterm Examiati Victr Matveev Octber 6, 6. (5pts, mi) Suppse f(x) equals si x the iterval < x < (=), ad is a eve peridic extesi f this fucti t the rest f the real lie. Fid the csie series fr
MATH 33- Midterm Examiati Victr Matveev Octber 6, 6. (5pts, mi) Suppse f(x) equals si x the iterval < x < (=), ad is a eve peridic extesi f this fucti t the rest f the real lie. Fid the csie series fr
Name Honors Chemistry / /
 Name Hnrs Chemistry / / Beynd Lewis Structures Exceptins t the Octet Rule Mdel Hydrgen is an exceptin t the ctet rule because it fills its uter energy level with nly 2 electrns. The secnd rw elements B
Name Hnrs Chemistry / / Beynd Lewis Structures Exceptins t the Octet Rule Mdel Hydrgen is an exceptin t the ctet rule because it fills its uter energy level with nly 2 electrns. The secnd rw elements B
Ceramic Engineering 103 Final Exam (5/16/03) 1) Arrange each set of oxides in order from most basic to most acidic (3 points each):
 Cer 103 Final Exam/WS03 (2) Test 5 030516 3) Sketch a TTT curve for a system containing 0.1 vol% of crystalline material. Add a second curve for 1 vol% crystallinity. Label both curves and label each axis.
Cer 103 Final Exam/WS03 (2) Test 5 030516 3) Sketch a TTT curve for a system containing 0.1 vol% of crystalline material. Add a second curve for 1 vol% crystallinity. Label both curves and label each axis.
A Study on Estimation of Lifetime Distribution with Covariates Under Misspecification
 Prceedigs f the Wrld Cgress Egieerig ad Cmputer Sciece 2015 Vl II, Octber 21-23, 2015, Sa Fracisc, USA A Study Estimati f Lifetime Distributi with Cvariates Uder Misspecificati Masahir Ykyama, Member,
Prceedigs f the Wrld Cgress Egieerig ad Cmputer Sciece 2015 Vl II, Octber 21-23, 2015, Sa Fracisc, USA A Study Estimati f Lifetime Distributi with Cvariates Uder Misspecificati Masahir Ykyama, Member,
5.1 Two-Step Conditional Density Estimator
 5.1 Tw-Step Cditial Desity Estimatr We ca write y = g(x) + e where g(x) is the cditial mea fucti ad e is the regressi errr. Let f e (e j x) be the cditial desity f e give X = x: The the cditial desity
5.1 Tw-Step Cditial Desity Estimatr We ca write y = g(x) + e where g(x) is the cditial mea fucti ad e is the regressi errr. Let f e (e j x) be the cditial desity f e give X = x: The the cditial desity
8.0 Negative Bias Temperature Instability (NBTI)
 EE650R: Reliability Physics f Naelectric Devices Lecture 8: Negative Bias Temerature Istability Date: Se 27 2006 Class Ntes: Vijay Rawat Reviewed by: Saakshi Gagwal 8.0 Negative Bias Temerature Istability
EE650R: Reliability Physics f Naelectric Devices Lecture 8: Negative Bias Temerature Istability Date: Se 27 2006 Class Ntes: Vijay Rawat Reviewed by: Saakshi Gagwal 8.0 Negative Bias Temerature Istability
O C S polar - greater force. H polar greater force. H polar. polar H-bond
 hapter 10 : 29, 30, 31, 33, 36, 40, 46, 48, 50, 72, 87, 91, 93, 110 29. a. Lndn e. Lndn b. diple-diple f. diple-diple c. -bnding g. in-in d. in-in 30. a. in-in e. -bnding b. Lndn f. diple-diple c. Lndn
hapter 10 : 29, 30, 31, 33, 36, 40, 46, 48, 50, 72, 87, 91, 93, 110 29. a. Lndn e. Lndn b. diple-diple f. diple-diple c. -bnding g. in-in d. in-in 30. a. in-in e. -bnding b. Lndn f. diple-diple c. Lndn
What is going on here?
 Major Digression! Atoms? Elements? Compounds? Minerals? Rocks? What is going on here? Source:SERC @ Carleton College http://www.brocku.ca/earthsciences/people/gfinn/petrology/periodic.gif http://www.meta-synthesis.com/webbook/35_pt/pt_database.php?pt_id=335
Major Digression! Atoms? Elements? Compounds? Minerals? Rocks? What is going on here? Source:SERC @ Carleton College http://www.brocku.ca/earthsciences/people/gfinn/petrology/periodic.gif http://www.meta-synthesis.com/webbook/35_pt/pt_database.php?pt_id=335
A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1
 A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1 (Nte: questins 1 t 14 are meant t be dne WITHOUT calculatrs!) 1.Which f the fllwing is prbably true fr a slid slute with a highly endthermic heat
A.P. CHEMISTRY. SOLUTIONS AND ACID BASE CHEMISTRY. p 1 (Nte: questins 1 t 14 are meant t be dne WITHOUT calculatrs!) 1.Which f the fllwing is prbably true fr a slid slute with a highly endthermic heat
Fourier Method for Solving Transportation. Problems with Mixed Constraints
 It. J. Ctemp. Math. Scieces, Vl. 5, 200,. 28, 385-395 Furier Methd fr Slvig Trasprtati Prblems with Mixed Cstraits P. Padia ad G. Nataraja Departmet f Mathematics, Schl f Advaced Scieces V I T Uiversity,
It. J. Ctemp. Math. Scieces, Vl. 5, 200,. 28, 385-395 Furier Methd fr Slvig Trasprtati Prblems with Mixed Cstraits P. Padia ad G. Nataraja Departmet f Mathematics, Schl f Advaced Scieces V I T Uiversity,
NUMBERS, MATHEMATICS AND EQUATIONS
 AUSTRALIAN CURRICULUM PHYSICS GETTING STARTED WITH PHYSICS NUMBERS, MATHEMATICS AND EQUATIONS An integral part t the understanding f ur physical wrld is the use f mathematical mdels which can be used t
AUSTRALIAN CURRICULUM PHYSICS GETTING STARTED WITH PHYSICS NUMBERS, MATHEMATICS AND EQUATIONS An integral part t the understanding f ur physical wrld is the use f mathematical mdels which can be used t
Thermodynamic study of CdCl 2 in 2-propanol (5 mass %) + water mixture using potentiometry
 Thermdyamic study f CdCl 2 i 2-prpal (5 mass %) + water mixture usig ptetimetry Reat Tmaš, Ađelka Vrdljak UDC: 544.632.4 Uiversity f Split, Faculty f Chemistry ad Techlgy, Teslia 10/V, HR-21000 Split,
Thermdyamic study f CdCl 2 i 2-prpal (5 mass %) + water mixture usig ptetimetry Reat Tmaš, Ađelka Vrdljak UDC: 544.632.4 Uiversity f Split, Faculty f Chemistry ad Techlgy, Teslia 10/V, HR-21000 Split,
ENGI 4421 Central Limit Theorem Page Central Limit Theorem [Navidi, section 4.11; Devore sections ]
![ENGI 4421 Central Limit Theorem Page Central Limit Theorem [Navidi, section 4.11; Devore sections ] ENGI 4421 Central Limit Theorem Page Central Limit Theorem [Navidi, section 4.11; Devore sections ]](/thumbs/89/100002025.jpg) ENGI 441 Cetral Limit Therem Page 11-01 Cetral Limit Therem [Navidi, secti 4.11; Devre sectis 5.3-5.4] If X i is t rmally distributed, but E X i, V X i ad is large (apprximately 30 r mre), the, t a gd
ENGI 441 Cetral Limit Therem Page 11-01 Cetral Limit Therem [Navidi, secti 4.11; Devre sectis 5.3-5.4] If X i is t rmally distributed, but E X i, V X i ad is large (apprximately 30 r mre), the, t a gd
Chemistry 114 First Hour Exam
 Chemistry 114 First Hur Exam Please shw all wrk fr partial credit Name: (4 pints) 1. (12 pints) Espress is made by frcing very ht water under high pressure thrugh finely grund, cmpacted cffee. (Wikipedia)
Chemistry 114 First Hur Exam Please shw all wrk fr partial credit Name: (4 pints) 1. (12 pints) Espress is made by frcing very ht water under high pressure thrugh finely grund, cmpacted cffee. (Wikipedia)
, the random variable. and a sample size over the y-values 0:1:10.
 Lecture 3 (4//9) 000 HW PROBLEM 3(5pts) The estimatr i (c) f PROBLEM, p 000, where { } ~ iid bimial(,, is 000 e f the mst ppular statistics It is the estimatr f the ppulati prprti I PROBLEM we used simulatis
Lecture 3 (4//9) 000 HW PROBLEM 3(5pts) The estimatr i (c) f PROBLEM, p 000, where { } ~ iid bimial(,, is 000 e f the mst ppular statistics It is the estimatr f the ppulati prprti I PROBLEM we used simulatis
Rates and Mechanisms of Chemical Reactions
 Rates ad Mechaisms f Chemical Reactis Why sme rxs prceed very fast ad thers require days, mths r eve years t prduce a detectable amt f prduct? H (g) + F (g) HF (g) (very fast) 3 H (g) + N (g) NH 3 (g)
Rates ad Mechaisms f Chemical Reactis Why sme rxs prceed very fast ad thers require days, mths r eve years t prduce a detectable amt f prduct? H (g) + F (g) HF (g) (very fast) 3 H (g) + N (g) NH 3 (g)
Modern Physics. Unit 15: Nuclear Structure and Decay Lecture 15.2: The Strong Force. Ron Reifenberger Professor of Physics Purdue University
 Mder Physics Uit 15: Nuclear Structure ad Decay Lecture 15.: The Strg Frce R Reifeberger Prfessr f Physics Purdue Uiversity 1 Bidig eergy er ucle - the deuter Eergy (MeV) ~0.4fm B.E. A =.MeV/ = 1.1 MeV/ucle.
Mder Physics Uit 15: Nuclear Structure ad Decay Lecture 15.: The Strg Frce R Reifeberger Prfessr f Physics Purdue Uiversity 1 Bidig eergy er ucle - the deuter Eergy (MeV) ~0.4fm B.E. A =.MeV/ = 1.1 MeV/ucle.
12 Chemistry (Mg,Fe) 2 SiO 4 Olivine is forms what is called an isomorphous solid solution series that ranges between two end members: Forsterite Mg
 11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
LCAO APPROXIMATIONS OF ORGANIC Pi MO SYSTEMS The allyl system (cation, anion or radical).
 Principles f Organic Chemistry lecture 5, page LCAO APPROIMATIONS OF ORGANIC Pi MO SYSTEMS The allyl system (catin, anin r radical).. Draw mlecule and set up determinant. 2 3 0 3 C C 2 = 0 C 2 3 0 = -
Principles f Organic Chemistry lecture 5, page LCAO APPROIMATIONS OF ORGANIC Pi MO SYSTEMS The allyl system (catin, anin r radical).. Draw mlecule and set up determinant. 2 3 0 3 C C 2 = 0 C 2 3 0 = -
Study in Cylindrical Coordinates of the Heat Transfer Through a Tow Material-Thermal Impedance
 Research ural f Applied Scieces, Egieerig ad echlgy (): 9-63, 3 ISSN: 4-749; e-issn: 4-7467 Maxwell Scietific Orgaiati, 3 Submitted: uly 4, Accepted: September 8, Published: May, 3 Study i Cylidrical Crdiates
Research ural f Applied Scieces, Egieerig ad echlgy (): 9-63, 3 ISSN: 4-749; e-issn: 4-7467 Maxwell Scietific Orgaiati, 3 Submitted: uly 4, Accepted: September 8, Published: May, 3 Study i Cylidrical Crdiates
Chapter 4. Problem Solutions
 Chapter 4. Prblem Slutis. The great majrity f alpha particles pass thrugh gases ad thi metal fils with deflectis. T what cclusi abut atmic structure des this bservati lead? The fact that mst particles
Chapter 4. Prblem Slutis. The great majrity f alpha particles pass thrugh gases ad thi metal fils with deflectis. T what cclusi abut atmic structure des this bservati lead? The fact that mst particles
Business. Class 3. Error in 4 th Edition (early printing) Specific Gravity (SG) A. Terminology Handout. Happy Monday! Terminology
 Class 3 Happy Mnday! Terminlgy Densities, MW, mle and, flw rates, and temperatures Pressure stuff next class Cnvert mass fractin t mle fractin Using a basis usiness Fr thse wh added the class late the
Class 3 Happy Mnday! Terminlgy Densities, MW, mle and, flw rates, and temperatures Pressure stuff next class Cnvert mass fractin t mle fractin Using a basis usiness Fr thse wh added the class late the
Markov processes and the Kolmogorov equations
 Chapter 6 Markv prcesses ad the Klmgrv equatis 6. Stchastic Differetial Equatis Csider the stchastic differetial equati: dx(t) =a(t X(t)) dt + (t X(t)) db(t): (SDE) Here a(t x) ad (t x) are give fuctis,
Chapter 6 Markv prcesses ad the Klmgrv equatis 6. Stchastic Differetial Equatis Csider the stchastic differetial equati: dx(t) =a(t X(t)) dt + (t X(t)) db(t): (SDE) Here a(t x) ad (t x) are give fuctis,
Lecture 12: Chemical reaction equilibria
 3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
Axial Temperature Distribution in W-Tailored Optical Fibers
 Axial Temperature Distributi i W-Tailred Optical ibers Mhamed I. Shehata (m.ismail34@yah.cm), Mustafa H. Aly(drmsaly@gmail.cm) OSA Member, ad M. B. Saleh (Basheer@aast.edu) Arab Academy fr Sciece, Techlgy
Axial Temperature Distributi i W-Tailred Optical ibers Mhamed I. Shehata (m.ismail34@yah.cm), Mustafa H. Aly(drmsaly@gmail.cm) OSA Member, ad M. B. Saleh (Basheer@aast.edu) Arab Academy fr Sciece, Techlgy
CHM 152 Practice Final
 CM 152 Practice Final 1. Of the fllwing, the ne that wuld have the greatest entrpy (if cmpared at the same temperature) is, [a] 2 O (s) [b] 2 O (l) [c] 2 O (g) [d] All wuld have the same entrpy at the
CM 152 Practice Final 1. Of the fllwing, the ne that wuld have the greatest entrpy (if cmpared at the same temperature) is, [a] 2 O (s) [b] 2 O (l) [c] 2 O (g) [d] All wuld have the same entrpy at the
Alkaline Surfactant Polymer alternating with Miscible CO 2 (ASPaM) Seyed Hamidreza Ghazizadeh Behzadi And Dr. Brian F. Towler
 Alkalie urfactat Plymer alteratig with Micible CO 2 (APaM) eyed Hamidreza Ghazizadeh Behzadi Ad Dr. Bria F. Twler OUTLINE Why AP ad why WAG APaM advatage Hw t imulate the APaM ectr Mdel Reult Cclui Why
Alkalie urfactat Plymer alteratig with Micible CO 2 (APaM) eyed Hamidreza Ghazizadeh Behzadi Ad Dr. Bria F. Twler OUTLINE Why AP ad why WAG APaM advatage Hw t imulate the APaM ectr Mdel Reult Cclui Why
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY
 AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
University Chemistry Quiz /04/21 1. (10%) Consider the oxidation of ammonia:
 University Chemistry Quiz 3 2015/04/21 1. (10%) Cnsider the xidatin f ammnia: 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(l) (a) Calculate the ΔG fr the reactin. (b) If this reactin were used in a fuel cell,
University Chemistry Quiz 3 2015/04/21 1. (10%) Cnsider the xidatin f ammnia: 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(l) (a) Calculate the ΔG fr the reactin. (b) If this reactin were used in a fuel cell,
ENGINEERING COUNCIL CERTIFICATE LEVEL THERMODYNAMIC, FLUID AND PROCESS ENGINEERING C106 TUTORIAL 5 THE VISCOUS NATURE OF FLUIDS
 ENGINEERING COUNCIL CERTIFICATE LEVEL THERMODYNAMIC, FLUID AND PROCESS ENGINEERING C106 TUTORIAL 5 THE VISCOUS NATURE OF FLUIDS On cmpletin f this tutrial yu shuld be able t d the fllwing. Define viscsity
ENGINEERING COUNCIL CERTIFICATE LEVEL THERMODYNAMIC, FLUID AND PROCESS ENGINEERING C106 TUTORIAL 5 THE VISCOUS NATURE OF FLUIDS On cmpletin f this tutrial yu shuld be able t d the fllwing. Define viscsity
Compressibility Effects
 Definitin f Cmpressibility All real substances are cmpressible t sme greater r lesser extent; that is, when yu squeeze r press n them, their density will change The amunt by which a substance can be cmpressed
Definitin f Cmpressibility All real substances are cmpressible t sme greater r lesser extent; that is, when yu squeeze r press n them, their density will change The amunt by which a substance can be cmpressed
GG250 Lab 8 Simultaneous Linear Equations
 GG250 Lab 8 Simultaneous Linear Equations This lab will have you set up and solve simultaneous linear equations (and then check your answers!). The main effort is in setting up the equations rather than
GG250 Lab 8 Simultaneous Linear Equations This lab will have you set up and solve simultaneous linear equations (and then check your answers!). The main effort is in setting up the equations rather than
Name: Period: Date: BONDING NOTES ADVANCED CHEMISTRY
 Name: Perid: Date: BONDING NOTES ADVANCED CHEMISTRY Directins: This packet will serve as yur ntes fr this chapter. Fllw alng with the PwerPint presentatin and fill in the missing infrmatin. Imprtant terms
Name: Perid: Date: BONDING NOTES ADVANCED CHEMISTRY Directins: This packet will serve as yur ntes fr this chapter. Fllw alng with the PwerPint presentatin and fill in the missing infrmatin. Imprtant terms
A New Method for Finding an Optimal Solution. of Fully Interval Integer Transportation Problems
 Applied Matheatical Scieces, Vl. 4, 200,. 37, 89-830 A New Methd fr Fidig a Optial Sluti f Fully Iterval Iteger Trasprtati Prbles P. Padia ad G. Nataraja Departet f Matheatics, Schl f Advaced Scieces,
Applied Matheatical Scieces, Vl. 4, 200,. 37, 89-830 A New Methd fr Fidig a Optial Sluti f Fully Iterval Iteger Trasprtati Prbles P. Padia ad G. Nataraja Departet f Matheatics, Schl f Advaced Scieces,
Experimental and Theoretical Investigations of PAN Molecular Weight Increase in Precipitation Polymerization as a Function of H 2 O/DMSO Ratio
 Carb Letters Vl., N. March 00 pp. -7 Experimetal ad Theretical Ivestigatis f PAN Mlecular Weight Icrease i Precipitati Plymerizati as a ucti f H O/DMSO Rati Jig Zhag, egjig Bu, Ygqiag Dai, Liwei Xue, Zhixia
Carb Letters Vl., N. March 00 pp. -7 Experimetal ad Theretical Ivestigatis f PAN Mlecular Weight Icrease i Precipitati Plymerizati as a ucti f H O/DMSO Rati Jig Zhag, egjig Bu, Ygqiag Dai, Liwei Xue, Zhixia
Review for cumulative test
 Hrs Math 3 review prblems Jauary, 01 cumulative: Chapters 1- page 1 Review fr cumulative test O Mday, Jauary 7, Hrs Math 3 will have a curse-wide cumulative test cverig Chapters 1-. Yu ca expect the test
Hrs Math 3 review prblems Jauary, 01 cumulative: Chapters 1- page 1 Review fr cumulative test O Mday, Jauary 7, Hrs Math 3 will have a curse-wide cumulative test cverig Chapters 1-. Yu ca expect the test
Study of Energy Eigenvalues of Three Dimensional. Quantum Wires with Variable Cross Section
 Adv. Studies Ther. Phys. Vl. 3 009. 5 3-0 Study f Eergy Eigevalues f Three Dimesial Quatum Wires with Variale Crss Secti M.. Sltai Erde Msa Departmet f physics Islamic Aad Uiversity Share-ey rach Ira alrevahidi@yah.cm
Adv. Studies Ther. Phys. Vl. 3 009. 5 3-0 Study f Eergy Eigevalues f Three Dimesial Quatum Wires with Variale Crss Secti M.. Sltai Erde Msa Departmet f physics Islamic Aad Uiversity Share-ey rach Ira alrevahidi@yah.cm
Active redundancy allocation in systems. R. Romera; J. Valdés; R. Zequeira*
 Wrkig Paper -6 (3) Statistics ad Ecmetrics Series March Departamet de Estadística y Ecmetría Uiversidad Carls III de Madrid Calle Madrid, 6 893 Getafe (Spai) Fax (34) 9 64-98-49 Active redudacy allcati
Wrkig Paper -6 (3) Statistics ad Ecmetrics Series March Departamet de Estadística y Ecmetría Uiversidad Carls III de Madrid Calle Madrid, 6 893 Getafe (Spai) Fax (34) 9 64-98-49 Active redudacy allcati
General Chemistry II, Unit I: Study Guide (part I)
 1 General Chemistry II, Unit I: Study Guide (part I) CDS Chapter 14: Physical Prperties f Gases Observatin 1: Pressure- Vlume Measurements n Gases The spring f air is measured as pressure, defined as the
1 General Chemistry II, Unit I: Study Guide (part I) CDS Chapter 14: Physical Prperties f Gases Observatin 1: Pressure- Vlume Measurements n Gases The spring f air is measured as pressure, defined as the
Motor Stability. Plateau and Mesa Burning
 Mtr Stability Recall mass cservati fr steady erati ( =cstat) m eit m b b r b s r m icr m eit Is this cditi (it) stable? ly if rmally use.3
Mtr Stability Recall mass cservati fr steady erati ( =cstat) m eit m b b r b s r m icr m eit Is this cditi (it) stable? ly if rmally use.3
Copyright 1978, by the author(s). All rights reserved.
 Cpyright 1978, by the authr(s). All rights reserved. Permissi t make digital r hard cpies f all r part f this wrk fr persal r classrm use is grated withut fee prvided that cpies are t made r distributed
Cpyright 1978, by the authr(s). All rights reserved. Permissi t make digital r hard cpies f all r part f this wrk fr persal r classrm use is grated withut fee prvided that cpies are t made r distributed
Chapter 17 Free Energy and Thermodynamics
 Chemistry: A Mlecular Apprach, 1 st Ed. Nivald Tr Chapter 17 Free Energy and Thermdynamics Ry Kennedy Massachusetts Bay Cmmunity Cllege Wellesley Hills, MA 2008, Prentice Hall First Law f Thermdynamics
Chemistry: A Mlecular Apprach, 1 st Ed. Nivald Tr Chapter 17 Free Energy and Thermdynamics Ry Kennedy Massachusetts Bay Cmmunity Cllege Wellesley Hills, MA 2008, Prentice Hall First Law f Thermdynamics
Super-efficiency Models, Part II
 Super-efficiec Mdels, Part II Emilia Niskae The 4th f Nvember S steemiaalsi Ctets. Etesis t Variable Returs-t-Scale (0.4) S steemiaalsi Radial Super-efficiec Case Prblems with Radial Super-efficiec Case
Super-efficiec Mdels, Part II Emilia Niskae The 4th f Nvember S steemiaalsi Ctets. Etesis t Variable Returs-t-Scale (0.4) S steemiaalsi Radial Super-efficiec Case Prblems with Radial Super-efficiec Case
Chem 75 February 16, 2017 Exam 2 Solutions
 1. (6 + 6 pints) Tw quick questins: (a) The Handbk f Chemistry and Physics tells us, crrectly, that CCl 4 bils nrmally at 76.7 C, but its mlar enthalpy f vaprizatin is listed in ne place as 34.6 kj ml
1. (6 + 6 pints) Tw quick questins: (a) The Handbk f Chemistry and Physics tells us, crrectly, that CCl 4 bils nrmally at 76.7 C, but its mlar enthalpy f vaprizatin is listed in ne place as 34.6 kj ml
K [f(t)] 2 [ (st) /2 K A GENERALIZED MEIJER TRANSFORMATION. Ku(z) ()x) t -)-I e. K(z) r( + ) () (t 2 I) -1/2 e -zt dt, G. L. N. RAO L.
![K [f(t)] 2 [ (st) /2 K A GENERALIZED MEIJER TRANSFORMATION. Ku(z) ()x) t -)-I e. K(z) r( + ) () (t 2 I) -1/2 e -zt dt, G. L. N. RAO L. K [f(t)] 2 [ (st) /2 K A GENERALIZED MEIJER TRANSFORMATION. Ku(z) ()x) t -)-I e. K(z) r( + ) () (t 2 I) -1/2 e -zt dt, G. L. N. RAO L.](/thumbs/74/70813931.jpg) Iterat. J. Math. & Math. Scl. Vl. 8 N. 2 (1985) 359-365 359 A GENERALIZED MEIJER TRANSFORMATION G. L. N. RAO Departmet f Mathematics Jamshedpur C-perative Cllege f the Rachi Uiversity Jamshedpur, Idia
Iterat. J. Math. & Math. Scl. Vl. 8 N. 2 (1985) 359-365 359 A GENERALIZED MEIJER TRANSFORMATION G. L. N. RAO Departmet f Mathematics Jamshedpur C-perative Cllege f the Rachi Uiversity Jamshedpur, Idia
Recitation 06. n total = P total V/RT = (0.425 atm * 10.5 L) / ( L atm mol -1 K -1 * 338 K) = mol
 Recitatin 06 Mixture f Ideal Gases 1. Chapter 5: Exercise: 69 The partial pressure f CH 4 (g) is 0.175 atm and that f O 2 (g) is 0.250 atm in a mixture f the tw gases. a. What is the mle fractin f each
Recitatin 06 Mixture f Ideal Gases 1. Chapter 5: Exercise: 69 The partial pressure f CH 4 (g) is 0.175 atm and that f O 2 (g) is 0.250 atm in a mixture f the tw gases. a. What is the mle fractin f each
Trimester 2 Exam 3 Study Guide Honors Chemistry. Honors Chemistry Exam 3 Review
 Trimester 2 Exam 3 Study Guide Hnrs Chemistry BOND POLARITY Hnrs Chemistry Exam 3 Review Identify whether a bnd is plar r nnplar based ff difference in electrnegativity btwn 2 atms (electrnegativity values
Trimester 2 Exam 3 Study Guide Hnrs Chemistry BOND POLARITY Hnrs Chemistry Exam 3 Review Identify whether a bnd is plar r nnplar based ff difference in electrnegativity btwn 2 atms (electrnegativity values
ES201 - Examination 2 Winter Adams and Richards NAME BOX NUMBER
 ES201 - Examinatin 2 Winter 2003-2004 Adams and Richards NAME BOX NUMBER Please Circle One : Richards (Perid 4) ES201-01 Adams (Perid 4) ES201-02 Adams (Perid 6) ES201-03 Prblem 1 ( 12 ) Prblem 2 ( 24
ES201 - Examinatin 2 Winter 2003-2004 Adams and Richards NAME BOX NUMBER Please Circle One : Richards (Perid 4) ES201-01 Adams (Perid 4) ES201-02 Adams (Perid 6) ES201-03 Prblem 1 ( 12 ) Prblem 2 ( 24
Name: Period: Date: BONDING NOTES HONORS CHEMISTRY
 Name: Perid: Date: BONDING NOTES HONORS CHEMISTRY Directins: This packet will serve as yur ntes fr this chapter. Fllw alng with the PwerPint presentatin and fill in the missing infrmatin. Imprtant terms
Name: Perid: Date: BONDING NOTES HONORS CHEMISTRY Directins: This packet will serve as yur ntes fr this chapter. Fllw alng with the PwerPint presentatin and fill in the missing infrmatin. Imprtant terms
Chem 116 POGIL Worksheet - Week 3 - Solutions Intermolecular Forces, Liquids, Solids, and Solutions
 Chem 116 POGIL Wrksheet - Week 3 - Slutins Intermlecular Frces, Liquids, Slids, and Slutins Key Questins 1. Is the average kinetic energy f mlecules greater r lesser than the energy f intermlecular frces
Chem 116 POGIL Wrksheet - Week 3 - Slutins Intermlecular Frces, Liquids, Slids, and Slutins Key Questins 1. Is the average kinetic energy f mlecules greater r lesser than the energy f intermlecular frces
(b) y(t) is not periodic although sin t and 4 cos 2πt are independently periodic.
 Chapter 7, Sluti. (a) his is peridic with ω which leads t /ω. (b) y(t) is t peridic althugh si t ad cs t are idepedetly peridic. (c) Sice si A cs B.5[si(A B) si(a B)], g(t) si t cs t.5[si 7t si( t)].5
Chapter 7, Sluti. (a) his is peridic with ω which leads t /ω. (b) y(t) is t peridic althugh si t ad cs t are idepedetly peridic. (c) Sice si A cs B.5[si(A B) si(a B)], g(t) si t cs t.5[si 7t si( t)].5
STRUCTURES IN MIKE 21. Flow over sluice gates A-1
 A-1 STRUCTURES IN MIKE 1 Fl ver luice gate Fr a give gemetry f the luice gate ad k ater level uptream ad dtream f the tructure, the fl rate, ca be determied thrugh the equati f eergy ad mmetum - ee B Pedere,
A-1 STRUCTURES IN MIKE 1 Fl ver luice gate Fr a give gemetry f the luice gate ad k ater level uptream ad dtream f the tructure, the fl rate, ca be determied thrugh the equati f eergy ad mmetum - ee B Pedere,
Phy 212: General Physics II 1 Chapter 18 Worksheet 3/20/2008
 Phy 1: General Physics II 1 hapter 18 rksheet 3/0/008 Thermal Expansin: 1. A wedding ring cmpsed f pure gld (inner diameter = 1.5 x 10 - m) is placed n a persn s finger (diameter = 1.5 x 10 - m). Bth the
Phy 1: General Physics II 1 hapter 18 rksheet 3/0/008 Thermal Expansin: 1. A wedding ring cmpsed f pure gld (inner diameter = 1.5 x 10 - m) is placed n a persn s finger (diameter = 1.5 x 10 - m). Bth the
Electrostatics. . where,.(1.1) Maxwell Eqn. Total Charge. Two point charges r 12 distance apart in space
 Maxwell Eq. E ρ Electrstatics e. where,.(.) first term is the permittivity i vacuum 8.854x0 C /Nm secd term is electrical field stregth, frce/charge, v/m r N/C third term is the charge desity, C/m 3 E
Maxwell Eq. E ρ Electrstatics e. where,.(.) first term is the permittivity i vacuum 8.854x0 C /Nm secd term is electrical field stregth, frce/charge, v/m r N/C third term is the charge desity, C/m 3 E
Analysis of pressure wave dynamics in fuel rail system
 It. Jl. f Multiphysics Vlume Number 3 8 3 Aalysis f pressure wave dyamics i fuel rail system Basem Alzahabi ad Keith Schulz Ketterig Uiversity Siemes Autmtive ABSTRACT A mdel f a amplified cmm rail fuel
It. Jl. f Multiphysics Vlume Number 3 8 3 Aalysis f pressure wave dyamics i fuel rail system Basem Alzahabi ad Keith Schulz Ketterig Uiversity Siemes Autmtive ABSTRACT A mdel f a amplified cmm rail fuel
Study Group Report: Plate-fin Heat Exchangers: AEA Technology
 Study Grup Reprt: Plate-fin Heat Exchangers: AEA Technlgy The prblem under study cncerned the apparent discrepancy between a series f experiments using a plate fin heat exchanger and the classical thery
Study Grup Reprt: Plate-fin Heat Exchangers: AEA Technlgy The prblem under study cncerned the apparent discrepancy between a series f experiments using a plate fin heat exchanger and the classical thery
Examination No. 3 - Tuesday, Nov. 15
 NAME (lease rit) SOLUTIONS ECE 35 - DEVICE ELECTRONICS Fall Semester 005 Examiati N 3 - Tuesday, Nv 5 3 4 5 The time fr examiati is hr 5 mi Studets are allwed t use 3 sheets f tes Please shw yur wrk, artial
NAME (lease rit) SOLUTIONS ECE 35 - DEVICE ELECTRONICS Fall Semester 005 Examiati N 3 - Tuesday, Nv 5 3 4 5 The time fr examiati is hr 5 mi Studets are allwed t use 3 sheets f tes Please shw yur wrk, artial
Principles of Organic Chemistry lecture 5, page 1
 Principles f Organic Chemistry lecture 5, page 1 Bnding Mdels Fact: electrns hld mlecules tgether. Theries: mre than ne way t cnceptualize bnding. Let s fllw Carrll in the cnsideratin f tw theries f bnding.
Principles f Organic Chemistry lecture 5, page 1 Bnding Mdels Fact: electrns hld mlecules tgether. Theries: mre than ne way t cnceptualize bnding. Let s fllw Carrll in the cnsideratin f tw theries f bnding.
Chem 115 POGIL Worksheet - Week 12 Molecular Shapes
 Chem 115 POGIL Wrksheet - Week 12 Mlecular Shapes Why? Cntrary t the impressin that Lewis structures may give, many mlecules have threedimensinal gemetries. These mlecular shapes are very imprtant t understanding
Chem 115 POGIL Wrksheet - Week 12 Mlecular Shapes Why? Cntrary t the impressin that Lewis structures may give, many mlecules have threedimensinal gemetries. These mlecular shapes are very imprtant t understanding
" 1 = # $H vap. Chapter 3 Problems
 Chapter 3 rblems rblem At 1 atmsphere pure Ge melts at 1232 K and bils at 298 K. he triple pint ccurs at =8.4x1-8 atm. Estimate the heat f vaprizatin f Ge. he heat f vaprizatin is estimated frm the Clausius
Chapter 3 rblems rblem At 1 atmsphere pure Ge melts at 1232 K and bils at 298 K. he triple pint ccurs at =8.4x1-8 atm. Estimate the heat f vaprizatin f Ge. he heat f vaprizatin is estimated frm the Clausius
The Acoustical Physics of a Standing Wave Tube
 UIUC Physics 93POM/Physics 406POM The Physics f Music/Physics f Musical Istrumets The Acustical Physics f a Stadig Wave Tube A typical cylidrical-shaped stadig wave tube (SWT) {aa impedace tube} f legth
UIUC Physics 93POM/Physics 406POM The Physics f Music/Physics f Musical Istrumets The Acustical Physics f a Stadig Wave Tube A typical cylidrical-shaped stadig wave tube (SWT) {aa impedace tube} f legth
Chapter 1: Fundamentals
 Chapter 1: Fudametals 1.1 Real Numbers Irratial umbers are real umbers that cat be expressed as ratis f itegers. That such umbers exist was a prfud embarrassmet t the Pythagrea brtherhd, ad they are said
Chapter 1: Fudametals 1.1 Real Numbers Irratial umbers are real umbers that cat be expressed as ratis f itegers. That such umbers exist was a prfud embarrassmet t the Pythagrea brtherhd, ad they are said
Author. Introduction. Author. o Asmir Tobudic. ISE 599 Computational Modeling of Expressive Performance
 ISE 599 Cmputatial Mdelig f Expressive Perfrmace Playig Mzart by Aalgy: Learig Multi-level Timig ad Dyamics Strategies by Gerhard Widmer ad Asmir Tbudic Preseted by Tsug-Ha (Rbert) Chiag April 5, 2006
ISE 599 Cmputatial Mdelig f Expressive Perfrmace Playig Mzart by Aalgy: Learig Multi-level Timig ad Dyamics Strategies by Gerhard Widmer ad Asmir Tbudic Preseted by Tsug-Ha (Rbert) Chiag April 5, 2006
High Temperature Materials. By Docent. N. Menad. Luleå University of Technology ( Sweden )
 Course KGP003 Ch. 12 High Temperature Materials By Docent. N. Menad Dept. of Chemical Engineering and Geosciences Div. Of process metallurgy Luleå University of Technology ( Sweden ) Ceramic materials
Course KGP003 Ch. 12 High Temperature Materials By Docent. N. Menad Dept. of Chemical Engineering and Geosciences Div. Of process metallurgy Luleå University of Technology ( Sweden ) Ceramic materials
Silicate Atmospheres, Clouds, and Fractional Vaporization of Hot Earth-like Exoplanets
 Silicate Atmospheres, Clouds, and Fractional Vaporization of Hot Earth-like Exoplanets Laura Schaefer and Bruce Fegley, Jr. Planetary Chemistry Laboratory Department of Earth and Planetary Sciences Washington
Silicate Atmospheres, Clouds, and Fractional Vaporization of Hot Earth-like Exoplanets Laura Schaefer and Bruce Fegley, Jr. Planetary Chemistry Laboratory Department of Earth and Planetary Sciences Washington
Chapter Outline: Ceramics. Chapter 13: Structure and Properties of Ceramics
 Chapter Outline: Ceramics Chapter 13: Structure and Properties of Ceramics Crystal Structures Silicate Ceramics Carbon Imperfections in Ceramics Optional reading: 13.6 13.10 University of Virginia, Dept.
Chapter Outline: Ceramics Chapter 13: Structure and Properties of Ceramics Crystal Structures Silicate Ceramics Carbon Imperfections in Ceramics Optional reading: 13.6 13.10 University of Virginia, Dept.
Phys101 Final Code: 1 Term: 132 Wednesday, May 21, 2014 Page: 1
 Phys101 Final Cde: 1 Term: 1 Wednesday, May 1, 014 Page: 1 Q1. A car accelerates at.0 m/s alng a straight rad. It passes tw marks that are 0 m apart at times t = 4.0 s and t = 5.0 s. Find the car s velcity
Phys101 Final Cde: 1 Term: 1 Wednesday, May 1, 014 Page: 1 Q1. A car accelerates at.0 m/s alng a straight rad. It passes tw marks that are 0 m apart at times t = 4.0 s and t = 5.0 s. Find the car s velcity
Advanced Chemistry Practice Problems
 Advanced Chemistry Practice Prblems Thermdynamics: Gibbs Free Energy 1. Questin: Is the reactin spntaneus when ΔG < 0? ΔG > 0? Answer: The reactin is spntaneus when ΔG < 0. 2. Questin: Fr a reactin with
Advanced Chemistry Practice Prblems Thermdynamics: Gibbs Free Energy 1. Questin: Is the reactin spntaneus when ΔG < 0? ΔG > 0? Answer: The reactin is spntaneus when ΔG < 0. 2. Questin: Fr a reactin with
Electric Current and Resistance
 Electric Current and Resistance Electric Current Electric current is the rate f flw f charge thrugh sme regin f space The SI unit f current is the ampere (A) 1 A = 1 C / s The symbl fr electric current
Electric Current and Resistance Electric Current Electric current is the rate f flw f charge thrugh sme regin f space The SI unit f current is the ampere (A) 1 A = 1 C / s The symbl fr electric current
1. INTRODUCTION. In many polymer processing operations, molten polymers. emerge from dies into a stress field which deforms the
 . NTRODUCTON. Histrical ntes f melt spinning prcess n many plymer prcessing peratins, mlten plymers emerge frm dies int a stress field which defrms the melt int a final fabricated shape. This is the case
. NTRODUCTON. Histrical ntes f melt spinning prcess n many plymer prcessing peratins, mlten plymers emerge frm dies int a stress field which defrms the melt int a final fabricated shape. This is the case
Simulation of Gas Condensate Reservoir Performance
 Simulati f Gas Cdesate Reservir Perfrmace Keith H. Cats, if: SPE, iercmp Resurce Develpmet aa Egieerig, c. Summary This paper presets a geeralized equati f state (EOS) that represets several widely used
Simulati f Gas Cdesate Reservir Perfrmace Keith H. Cats, if: SPE, iercmp Resurce Develpmet aa Egieerig, c. Summary This paper presets a geeralized equati f state (EOS) that represets several widely used
Earth Science 232 Petrography
 Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
