FIG. 1. (a) Crystal structure of K2MgF4 type and (b) the first Brillouin zone of body-centered tetragonal unit cell.
|
|
|
- Alvin Lynch
- 5 years ago
- Views:
Transcription
1 PHYSICAL REVIEW 8 VOLUME 39, NUMBER MAY 989 Phase transitions in the perovskitelike A 2BX structure Dorian M. Hatch and Harold T. Stokes Brigham Young Uniuersity, Provo, Utah 802 K. S. Aleksandrov and S. V. Misyul L. V. Kirenskii Institute of Physics, Academy ofsciences of the U.S.S.R, Sibe. rian Branch, Krasnoyarsk, U.S S.R.. (Received 23 September 988; revised manuscript received 23 January 989) A comparison is made of two different approaches to the description of phase transitions in the A2BX structure (space group D&, I/mmm) due to rigid octahedral tiltings. The lower-symmetry subgroups due to coupling of the K»f (X2) and E&2~ (P) representations ( tilts) to other tilting modes are listed. All observed transitions in the A2BX structure are of the octahedral tilting types. These are listed and correlated with the driving representations. I. INTRODUCTION A2BX crystals with the structure of K2MgF type are layered perovskitelike compounds. One of the main features of the perovskite structure, namely the flat square-layers of BX octahedra corner linked within the layer plane, is preserved in the AzBX structure. Layers neighboring along the c axis in these crystals are shifted by half of the body diagonal of the unit cell G&=Ilmmm which has two formula units per conventional cell [Fig. (a)]. As a rule the A cations are arranged almost in the plane formed by the X atoms at the top (or bottom) of the octahedra and have nine nearest neighbors. Crystals with the structure of K2MgF type are widely exhibited by fluorides and oxides A 2 B +0 and Az B +O with large A cations. A large group of chlorides and bromides is also known, where the sites of the A cations in the general formula are occupied by organic chain alkylammonium cations (C Hz +&NH3)'+, n =,...,.' Many of the compounds listed above undergo phase transitions (PT's) or sequences of PT's. It has been shown recently' that by considering the mutual tilts of BX octahedra (rotational distortions), one may describe all experimentally observed types of successive distortions within the A2BX structure. These investigations were performed independently by two research groups and the results were published in Refs. 3. However, each of these works has omissions and the philosophies of approach taken by the two groups are different. In this publication, we indicate the distinct approaches of the two groups, we provide data on the symmetry of rotationally distorted phases, and we make a comparison of the calculated results with existing experimental data. The latter two sets of information correct omissions of the previous papers. which consists of the determination of the space group for the distorted phase G; by means of actually depicting its structure. The advantage of this method is physical clarity in the determination of the symmetry elements of the distorted structure when superpositions of definite types of distortions (in our case rotational ones) take place. Earlier, such a procedure was successfully used in the analysis of rotationa distortions in perovskites and X Y "K ~ K 0 ~ F II. PHILOSOPHIES OF APPROACH Aleksandrov et al.' have solved the problem of listing the possible symmetry changes of the A2BX crystal structure using mainly a direct crystallographic method, FIG.. (a) Crystal structure of K2MgF type and (b) the first Brillouin zone of body-centered tetragonal unit cell The American Physical Society
2 39 PHASE TRANSITIONS IN THE PEROVSKITELIKE A28X. ~ elapsolites. It was assumed that in the first approximation the BX octahedra for small tilts do not undergo any additional distortion, and the complex distortions may be treated as a superposition of either tilts of different kinds, or of those of the same kind but around the different axes of Gp. For the layered perovskitelike crystals A2BX, it was shown' that in the square layers of octahedra three kinds of tilts are possible: a tilt around the normal to the layer plane (0 tilts) [Fig. 2(a)], and two kinds of tilts (N and ) which can both occur around the ao and/or bo axes of Go. For tilts around bo, all octahedra in a column (looking down bo) are turned in the same direction [Fig. 2(b)]. The symbol of such a distortion is (00), i.e., tilts around the bo axis. The simultaneous + tilts around both ap and bp are also possible, which is indicated by the distortion symbol (O'%0). tilts are characterized by alternating tilt angles along the axis of rotation. Figure 2(c) corresponds to (0@0). Besides the symmetry equivalent (%00) and (0&0) distortions, also (C&0), (C&&@20), etc., are possible. In all, variants result from distortions in a single layer. All of these were listed by Aleksandrov et al.' This method allowed the detection of one peculiarity inherent in layered crystals which is called polydistortion or distortional polytypism. It distinguishes the variety of spatial symmetry variants in such crystals due to different combinations of kinds and amplitudes (or angles) of distortions in nearby layers, which are linked together comparatively weakly. An example of the phenomenon was found in the studied group of crystals. Experimental data on A2BX structures (given in Refs. and 2) indicated the absence of unit-cell multiplication along the c~ axis of the Go phase [Fig. (a)]. As a result, Aleksandrov et al.' considered only the distortions G; leading to unit-cell multiplication in the basic plane (00). This means that the irreducible representations responsible for PT's Gp +G; were restricted to the two-armed K, 3 star (in terms of Kovalev ) or to the X point of the Brillouin zone of Go (in terms of Miller and Love ) [Fig. (b)]. The partial group-theoretical analysis of distortions in A2BX crystals was performed in Refs. and 2 in order to obtain the correspondence between the kinds of tilts and the irreducible representations of Gp belonging to the K ] 3 (X ) star of the wave vector. Those changes due to rotational distortions of N and tilts were left out in the first paper. ' Only after acquaintance with the work later published as Ref. 0 was this omission partially corrected in Ref. 2. Hatch and Stokes obtained the permissible rotational distortions in a manner distinct from Refs. and 2. Previously they had implemented on computer a systematic method for obtaining subgroups resulting from a single irreducible physical distortion. This procedure is an extension of the Landau theory of continuous phase transitions (see Ref. and references therein). The usual assumption in the Landau theory is that only one irreducible representation (irrep) drives the transition to the lower-symmetry (subgroup) phase. A listing of the allowed subgroups for all Brillouin-zone points of symmetry had been obtained and the order parameter direction had been matched with the resulting lower-symmetry phase and the multiple domains. ' Together with the subgroup and order parameter listing, Hatch, Stokes, and Putnam' had also obtained a connection between the Wyckoff point-group representations and the resulting representations of Gp which could be induced from the representations of the Wyckoff point group. Thus, for a given group-subgroup change, the compatibility of this change with an assumed site distortion could be checked. By using projection operator techniques the specific sublattice displacements within a unit cell could then be obtained. ' However, for the perovskitelike structures, coupled parameters (direct sums of representations) are of interest. If G denotes the subgroup symmetry for the order parameter g' ', and G~ denotes the subgroup symmetry for the order parameter g'~', then an allowed subgroup for the bicoupled parametric transition will be the grouptheoretical intersection of G and G~. By considering all possible domains for the Gp ~G and Gp + G~ transitions, a complete listing of all bicoupled (bireducible) representations can be found for these transitions. In Refs. 3 and 0, Hatch and Stokes published a selected listing of coupled irreps. In Ref. 0 the subgroups of D& (P/mmm ) were published restricting attention to the irreducible representations K,sr (X3 ) K]87 (~3 ) K+ (Ms ) K2or ( ~ s ) and K&'r (R 3 ). These were the only representations which those authors determined to be associated with tilting modes in the perovskitelike ABX structure, and all tilting configurations were obtained by projection operator methods. Specific examples of the tilting configurations were given in Ref. 0. In Ref. 3 they published similar work for the perovskitelike A 2BX structure. However, the twodimensional representations K r (X2+ ) and K,2r (P) corresponding to the tilts were omitted in that publication. III. NEW RESULTS Recognizing the need to include these representations (e tilts) in the listing of bicoupled transitions, and the need to give a more complete listing of the experimentally observed transitions in the A2BX crystals due to the X XXY, XXX FICx. 2. Three types of octahedral tilting in layers of linked octahedra: (a) (008); (b) (0%'0); (c) (0+0). (c)
3 HATCH, STOKES, ALEKSANDROV, AND MISYUL 39 coupling of 8 tilts with N and tilts, in this section we remedy the omissions of Refs. 3. Using the approach of Hatch and Stokes, ' we give in Table I the listing of transitions driven by uncoupled representations of Dh. We list only those corresponding to octahedral tilting modes. In Table II we list the transitions resulting from the eouphng of the E&3r (X2+ ) and K,2r (P) representations with K, 37 (X3 ), K,3r TABLE I. Irreducible representations allowing octahedral tiltings in the A~BX~ structure are listed. The subgroups resulting from the tilting are shown along with the conventional "basis" vectors and origins (in terms of the original conventional vectors of D J } Numbers in parentheses are from Ref.. The order parameter orbit is also indicated for each subgroup. Irrep X2+ Xq P P, DI, (27) D, 'q () D~i, () D~g(8) D2~(} D~I, () D ' () D, z () a,', (8) q(2) D~q(} C~p (2) q {0) C {2} D/, ( ) D 'g(9) a",, (7) C,h(} C,' (2) C,'h(2} C,'(2) DI, (2) Dq (0) a," (2o) a," (22) D2d(2) D,",(7) D~I, (72) D," (7o) D~q (9) S(82} 2 (), (3) C~, (2) D q(2) D'(23) D2(22) C,' () C', g, (2) () P P 0 C8 S D P P (,0),(,0),{0,0, ) (0,0, ),(,0),(,0) (,0),(0,0, ),(,,0) (,0),{,0),(0,0, ) (0,0, ),(,0)(,0) (0, ),(,0,0),(0, ) (0,2,0),(0,0,2),(,0,0) ) (0,),(,0,0),(0,, ) (, ),(, ',, ', ', (2 0 0),(0 2 0) (0,0,2) ),(, &, ) {0,0, 2),( 2, 2,0),(,) (0,0, 2),( 2, 2,0),(,) (2,0, 2),(0,2,0),(0,0,2) (,),(, ),(, ) (0),(,0)(0,0,2) (,0),(,,0),(0,0,2) {0,0,2),(,,0),(,0) (0,0,2),(,,0),(,0) (2,0,0),(0,2,0),{0,0,2) (,,0),(,0,),(0,0,2) (0,2,0),(0,0,2),(,,0) (0,2,0),(0,0,2),(,,0} (0,2,0),(0,0,2),(,,0) (0,0,0} (o,o,o} (0,0,0} ( ', 0,0) (0, ', 0) (0,, ', 0} (0, ', 0)
4 39 PHASE TRANSITIONS IN THE PEROVSKITELIKE AzBX TABLE II. The subgroups obtained from reducible representations of DI, are listed. The irreps being coupled are shown as well as the order parameter orbits and stratum subspaces. The subgroup conventional basis vectors and origin are expressed in terms of the original conventional basis vectors of D&. Irreps Subspace Xz+ ex3+ Xz+ X+ Xz P Xz+ P PX3+ 3 Cz Dza Cz~ CzI Cz~ 3 Dz~ 0 CzI 28 Dz~ 2 3 Czi Czs 3 D D 7 Dz~ 8 Dz~ C8 Dz~ 7 Dz~ 7 2 Dz~ 0 Dz~ C D3 Dz~ D Cz 2 C9 D Dzi D D2I, Dz~ CzI D Cz, Pl,,,,,, Pl, Pl,,,,, Pl, pl Pl, Pl, Pl, S, C 0, cl,d Pl, Pl, Pl,,,, Pl, pl P Pi, C8 P,0, 2, 2, C9,, P,, 2, 3 C,D Pl, Pi, Pl,,,,, pl,,3, l, l l, l l, l (0,2,0,)(2,0,0)(0,0, l) ( ', ', '), (,,0)(0,0, ) (,0)(,0){0,0, ) (,0),(,0),(0,0,) (0,0,),(,,0),(,0) (,0),(,0),(0,0,) (0,2,0),(2,0,0),{0,0, ) (0,0, ),(,0),(,0) (0,0,l),(,,0),(l,l,o) (,,0),(,0),(,) (0,0,),(,0),(,0) (,0),(,0),(0,0,) (2,,0,0),(0,0,2),(0, 2,0) (2,0, 2),(0,2,0),(0,0,2) (0,0, 2),( 2, 2,0),(,) ( 2,2,0),(0,0,2),(2,0,0) (,),(l, ),(, ) {,,0),(,,0),(0,0,2) (,,0),(,,0),(0,0,2) (,0),(,,0)(0,0,2) (,0),(0,0, 2),(,,0) {,,0),(,0),(0,0, 2) {,,0), (,,0),(0,0, 2) (0, 2,0),(2,0,0),(0,0,2) (0,0,2),(,,0),(,0) (0,0,2),(,0),(,0) (,0),(0,0,2),(,0) {0,0,2),( I,,0),(0) (,0),(0,0,2),(,0) (,0),(0,0,2),(,0) (,,0),(,0),{0,0,2) (,0),(0,0,2),(,0) (,,0),(,,0),(0,0,2) (,,0),(0,0, 2), ( l,,0) (,,0),(0,0, 2), (,,0) {0, 2,0),(2,0,0),(0,0, 2) (,,0), (,,0),(0,0, 2) (, l, o),{0,0, 2), (0, 2,0) ( 2,0,0),(0,2,0),(0,0, 2) (,,0), (,,0),(0,0, 2) ( 27 2) 2 ) ' (o,o,, ) {0,0,0) ( 2, 0,0) 3 ) 3) ' ' { 2,0,0)
5 928 HATCH, STOKES, ALEKSANDROV, AND MISYUL 39 TABLE II. (Continued). Irreps Subspace PEBX PXi+ PPs D7 D2h D2 D2 D, D8 C 7 D2h 7 Dh D D2h D2 2 2 h 2 D D2d D3 D C' h 9 2, C C h, Pl, pl Pl, Pl,,,,,,,,P Pl, P PI, P,o P,,,,,,,,, C8,S,, D,,,,,, C8,S,D Pl, Pl, P P P P Pl, Pl, C9 P,C9 Pl, Pl,,P,3, 22 2,,3 2 (,,0),(0,0, 2), (0, 2,0) {,,0),(,,0),{0,0,2) (0,0, 2), (,,0), (,,0) (,,0),(,,0),(0,0, 2) {0, 2,0),(2,0,0),(0,0,2) (,,0), (,,0),(0,0, 2) (,,0),{0,0, 2), (0, 2, 0) ( 2, 0,0),{0,2,0),(0,0, 2) (,,O), {,,0),(o,o, 2) (,,0),(0,0, 2), (0, 2,0) { 2, 0,0),(0, 2,0),(0,0, 2) (0,, ),( 2,0,0),(0,, ) (2, 2,0), ( 2, 2, 0),(0,0, 2) (2, 2,0), ( 2, 2,0),(0,0, 2) (0, 2, 0),(0,0, 2), (2, 2,0) (0, 2,0),{0,0,2),( 2,0,0) (0,0, 2), (0, 2, 0), ( 2,0,0) ( 2,0,0),(0, 2, 0),(0,0, 2) ( 2, 0,0),(0, 2, 0),{0,0,2) ( 2, 0,0),(0, 2, 0),(0,0,2) ( 2,0,0),(0, 2,0},(0,0, 2) ( 2,0,0),(0, 2,0),(0,0, 2) (0,, ),( 2,0,0),(0,, ) (2, 2, 0), { 2, 2,0},(0,0, 2) (0,0, 2), (0, 2, 0),{ 2,0,0) (0,0, 2), (0, 2, 0), ( 2,0, 2) (0, 2,0),(0,0, 2), (2, 2, 0) (0,0, 2), (0, 2,0), ( 2,0,0) ( 2, 0,0),(0, 2,0),(0,0, 2) ( 2,0,0),(0, 2, 0),(0,0,2) (0,0, 2), (0, 2, 0), ( 2,0,0) (0,, ), ( 2, 0,0),(0,, ) (2, 2,0), ( 2, 2,0),(0,0, 2) (0,0, 2), (0, 2, 0), ( 2,0,0) (0, 2,0),(0,0, 2), (2, 2,0) (0,0, 2), (0, 2,0), (,0, ) (0,0, 2), (0, 2,0), ( 2, 0,0) (, 2), (,,0),(0,0, 2} (,,0),{0,0,2),(,0) {,,0),(0,0,2), (,,0) (,, 2), (,,0), (,,0) (0,0, 2), (0, 2, 0), (,0, ) (0,, ),(0,0, 2), (,,0) (0,2,0),(0,0,2),{,,0) (, 2), (,,0),(0,0, 2) (,, 2), (,,0), (,,0) {,, 2},(,,0), (,,0} ( (0, 2,0) 3 ) ~ ' (0,,0) (o -' o) 3 & & (O, 2, ) (2,0, ) (,0,0) (0,,0) (0,,0) (0 0 ) ) 0 ) )
6 39 PHASE TRANSITIONS IN THE PEROVSKITELIKE A28X TABLE II. (Continued). Irreps Subspace 2 2U C C C,,P,,, 0, 2., 2,C8, 3,D, 2,,9 (0,0,2),{,,0),(,,0) (,, 2), (,,0), (,,0) {,, 2),(,,0),(0,0, 2) (0,0, 2), (0, 2,0), (,0, ) (0, ),(0,0, 2), (,,0) (0,2,0),(0,0,2),(,,0) (0,0, 2), (0, 2,0), (,0, ) (,, 2),{,,0), (,,0) (0,, ),(0,0, 2),(,,0) (0 ) ( 0 ) TABLE III. Symmetry of distorted phases arising due to octahedral tilts in crystals of K2MgF type. Distortions connected with representations N +,P are not covered. MA is CH3NH3, EA is HNH3;PA is H7NH3. No 'Ref.. Ref.. 'Ref. 7. Ref.. I layer (00) (00 } {ooe) (ooe, ) (00) {@%20} (0%0) {0+0) (0) {CO) {0) (N, 0) '', 0) {e, (e, 'e2'0) (N, +20} (owe) (COB} (0) {+,%2) (owe) (e,e,e) {,e,e) (@) (N+e) (ewe) {eve) (@+) Tilts (in layers) II layer (ooe) (00) (000) (ooe, ) (0+0) {e,e 20) {00) {00) (0) ( 0} (%0} (N2+, 0) {&+20) (@2+,0} ( 2,0) (ore) (a oe) (+0) (+q+, ) (0) {+2@,e) {a,e,e) {eve} (&+) (e ee) (wee} (N@e) ; D2h 9 Dh Dh 2 D20 2h Ch D2h 0 2 C D 0 h Space group Irreducible representations of E» (X) star z &, (X,+) ~7 (X2+ ) w7 (X2+ ) &,+& (X3+ +X+ } ~, (X3+) ~ (X+ ) w3 {X3+ ) ~ (X+ } ~3& {X3+@X+ ) &3EB& (X3+ EpX+ ) ~, (X, ) v (X+ ) ~,e~er, (X3+ ex+ +X2+ ) ~ (X+) ~,e~, (X3+@X2+ ) 'Ref. 9. Ref. 20. ~Ref. 2. ~~7 (X+ +X2+ ) ~+~, ) ~,+~, (X, ) ~,e~, (X,++X2 ) ~W7 ) ~e~, (X+ SX2+ ) ~, ) Examples (MA)2Mn, ' (MA)2Cd b [(MA)zMn~, '(MA)zCd~" (EA) Mn, (PA) Mn {PA),Cd,,CaYCrO ' Rb2Cd8 (EA }2Mn, (PA)2CdCI' CaYCrG ( MA)2Mn, '( MA )2Cd
7 HATCH, STOKES, ALEKSANDROV, AND MISYUL 39 (X ), K,, r (N&+ ), and K,2r (P) representations. In Tables I and II we give the subgroup, the orbit, and domain orientations (i.e., stratum subspace, see Ref. 3 for more details), the new lattice basis vectors in terms of the parent conventional basis, and the origin of the subgroup in terms of the parent converitional basis. The irrep labels in both tables follow the convention of Miller and Love and we use the space-group settings of Hahn. ' In Table III we list experimentally observed transitions for the A2BX structure. In this table we give the subgroup, the primitive cell size change, the representation corresponding to the transition (see Tables I and II), and in the last column we give the experimentally observed crystals undergoing the associated transition. Tables I, II, and III augment the information given in Refs. 3. Tables I and II, when used with similar tables in Ref. 3, give the complete listing of tilting modes in the A2BX structure. Table III, when used with a similar table in Ref. 2, gives the complete correspondence with the experimentally observed transitions in this structure. 'K. S. Aleksandrov, Kristallografiya 32, 937 (987). K. S. Aleksandrov, B. V. Beznosikov, and S. V. Misyul, Phys. Status Solidi A 0, 29 (987). 3D. M. Hatch and H. T. Stokes, Phys. Rev. B 3, 809 (987). ~K. S. Aleksandrov, A. T. Anistratov, B. V. Beznosikov, and N. V. Fedoseeva, Phase Transitions in Crystals of Halide AI3X3 Compounds (Nauka, Novosibirsk, 98),p. 2. K. S. Aleksandrov, V. N. Voronov, S. V. Misyul, and I. N. Flerov, in Problems of Crystallography (Nauka, Moscow, 987), p. 27. K. S. Aleksandrov and S. V. Misyul, Kristallografiya 2, 07 (98). 7K. S. Aleksandrov, Kristallografiya 32, (987). O. V. Kovalev, Irreducible and Induced Representations and Co representatio-ns of Fedoroo Groups (Nauka, Moscow, 98), p S. C. Miller and W. F. Love, Tables of Irreducible Representa tions of Space Groups and Co Representation-s of Magnetic Space Groups (Pruett, Boulder, 97). D. M. Hatch and H. T. Stokes, in the XV International Colloquium on Group Theoretical Methods in Physics, edited by R. Gilmore (World-Scientific, Singapore, 987), p. 70. D. M. Hatch and H. T. Stokes, Phys. Rev. B 3, 2908 (98); 30, 92 (98). '2H. T. Stokes and D. M. Hatch, Isotropy s of the 230 Crystallographic Space Groups (World-Scientific, Singapore, 988). D. M. Hatch, H. T. Stokes, and R. M. Putnam, Phys. Rev. B 3, 93 (987); in the XVI International Colloquium on Group Theoretical Methods in Physics, edited by H. Doebner and f. D. Palev (World-Scientific, Singapore, in press). 'T. Hahn, International Tables for Crystallography (Reidel, Boston, 983), Vol. A. G. Heger, D. Mullen, and K. Knorr, Phys. Status Solidi A 3, 27 (97). 'R. Kind, G. Chapius, and H. Arend, Phys. Status Solidi A 3, 9 (97). '7G. Heger, D. Mullen, K. Knorr, Phys. Status Solidi A 3, (97). ' W. J. Depmeier, Solid State Chem. 29, (979). W. J. Depmeier, J. Felsche, and G. J. Widdermuth, Solid State Chem. 2, 7 (977). P. Gungaly, and C. J. Rao, Solid State Chem. 3, 93 (98). ia. I. Kruglik, A. D. Vasilyev, and K. S. Aleksandrov (unpublished).
I. INTRODUCTION. Classification of octahedral tilting phases in the perovskitelike A2BX4 structure
 PHYSICAL REVIEW B VOLUME 35, NUMBER 1 1 JUNE 1987 assification of octahedral tilting phases in the perovskitelike A2BX4 structure Dorian M. Hatch and Harold T. Stokes Department of Physics and Astronomy,
PHYSICAL REVIEW B VOLUME 35, NUMBER 1 1 JUNE 1987 assification of octahedral tilting phases in the perovskitelike A2BX4 structure Dorian M. Hatch and Harold T. Stokes Department of Physics and Astronomy,
Part 1. Theory. Space-Group Symmetry and Atomic Displacements
 Introduction to Isotropy Subgroups and Displacive Phase Transitions Harold T. Stokes and Dorian M. Hatch Brigham Young University, Provo, Utah April 2006 This is an introduction to the concepts of isotropy
Introduction to Isotropy Subgroups and Displacive Phase Transitions Harold T. Stokes and Dorian M. Hatch Brigham Young University, Provo, Utah April 2006 This is an introduction to the concepts of isotropy
Landau-Ginzburg model for antiferroelectric phase transitions based on microscopic symmetry
 PHYSICAL REVIEW B VOLUME 62, NUMBER 2 1 JULY 2000-II Landau-Ginzburg model for antiferroelectric phase transitions based on microscopic symmetry Richard A. Hatt Materials Research Laboratory, The Pennsylvania
PHYSICAL REVIEW B VOLUME 62, NUMBER 2 1 JULY 2000-II Landau-Ginzburg model for antiferroelectric phase transitions based on microscopic symmetry Richard A. Hatt Materials Research Laboratory, The Pennsylvania
Procedure for obtaining microscopic mechanisms of reconstructive phase transitions in crystalline solids
 PHYSICAL REVIEW B, VOLUME 65, 144114 Procedure for obtaining microscopic mechanisms of reconstructive phase transitions in crystalline solids Harold T. Stokes and Dorian M. Hatch Department of Physics
PHYSICAL REVIEW B, VOLUME 65, 144114 Procedure for obtaining microscopic mechanisms of reconstructive phase transitions in crystalline solids Harold T. Stokes and Dorian M. Hatch Department of Physics
Molecular-Dynamics Simulations of Some BaXF4 Compounds
 University of Nebraska at Omaha DigitalCommons@UNO Physics Faculty Publications Department of Physics 1994 Molecular-Dynamics Simulations of Some BaXF4 Compounds John Flocken University of Nebraska at
University of Nebraska at Omaha DigitalCommons@UNO Physics Faculty Publications Department of Physics 1994 Molecular-Dynamics Simulations of Some BaXF4 Compounds John Flocken University of Nebraska at
Preparation for the workshop. Part 1. Introduction to the Java applet
 ISODISPLACE Workshop Harold T. Stokes International School in the Use and Applications of the Bilbao Crystallographic Server June 2009, Lekeitio, Spain isodisplace is a tool for exploring the structural
ISODISPLACE Workshop Harold T. Stokes International School in the Use and Applications of the Bilbao Crystallographic Server June 2009, Lekeitio, Spain isodisplace is a tool for exploring the structural
Tables of crystallographic properties of double antisymmetry space groups
 Tables of crystallographic properties of double antisymmetry space groups Mantao Huang a, Brian K. VanLeeuwen a, Daniel B. Litvin b and Venkatraman Gopalan a * a Department of Materials Science and Engineering,
Tables of crystallographic properties of double antisymmetry space groups Mantao Huang a, Brian K. VanLeeuwen a, Daniel B. Litvin b and Venkatraman Gopalan a * a Department of Materials Science and Engineering,
ISOTROPY. Tutorial. July 2013
 ISOTROPY Tutorial July 2013 Harold T. Stokes, Dorian M. Hatch, and Branton J. Campbell Department of Physics and Astronomy Brigham Young University with a contribution by Christopher J. Howard University
ISOTROPY Tutorial July 2013 Harold T. Stokes, Dorian M. Hatch, and Branton J. Campbell Department of Physics and Astronomy Brigham Young University with a contribution by Christopher J. Howard University
Symmetry considerations in structural phase transitions
 EPJ Web of Conferences 22, 00008 (2012) DOI: 10.1051/epjconf/20122200008 C Owned by the authors, published by EDP Sciences, 2012 Symmetry considerations in structural phase transitions J.M. Perez-Mato,
EPJ Web of Conferences 22, 00008 (2012) DOI: 10.1051/epjconf/20122200008 C Owned by the authors, published by EDP Sciences, 2012 Symmetry considerations in structural phase transitions J.M. Perez-Mato,
On conservation of the of crystal lattice symmetry in transition at Curie point in exchange magnets Khisa Sh. Borlakov * and Albert Kh.
 On conservation of the of crystal lattice symmetry in transition at Curie point in exchange magnets Khisa Sh. Borlakov * and Albert Kh. Borlakov North Caucasian State Humanitarian and Technological Academy,
On conservation of the of crystal lattice symmetry in transition at Curie point in exchange magnets Khisa Sh. Borlakov * and Albert Kh. Borlakov North Caucasian State Humanitarian and Technological Academy,
Condensed Matter A Week 2: Crystal structure (II)
 QUEEN MARY, UNIVERSITY OF LONDON SCHOOL OF PHYSICS AND ASTRONOMY Condensed Matter A Week : Crystal structure (II) References for crystal structure: Dove chapters 3; Sidebottom chapter. Last week we learnt
QUEEN MARY, UNIVERSITY OF LONDON SCHOOL OF PHYSICS AND ASTRONOMY Condensed Matter A Week : Crystal structure (II) References for crystal structure: Dove chapters 3; Sidebottom chapter. Last week we learnt
Proper ferroelastic transitions in two dimensions: Anisotropic long-range kernels, domain wall orientations, and microstructure
 Proper ferroelastic transitions in two dimensions: Anisotropic long-range kernels, domain wall orientations, and microstructure Dorian M. Hatch, 1 Turab Lookman, 2 Avadh Saxena, 2 and Subodh R. Shenoy
Proper ferroelastic transitions in two dimensions: Anisotropic long-range kernels, domain wall orientations, and microstructure Dorian M. Hatch, 1 Turab Lookman, 2 Avadh Saxena, 2 and Subodh R. Shenoy
Mechanisms for the reconstructive phase transition between the B1 and B2 structure types in NaCl and PbS
 Mechanisms for the reconstructive phase transition between the B1 and B2 structure types in NaCl and PbS Harold T. Stokes, 1 Dorian M. Hatch, 1 Jianjun Dong, 2 and James P. Lewis 1 1 Department of Physics
Mechanisms for the reconstructive phase transition between the B1 and B2 structure types in NaCl and PbS Harold T. Stokes, 1 Dorian M. Hatch, 1 Jianjun Dong, 2 and James P. Lewis 1 1 Department of Physics
ISODISPLACE: a web-based tool for exploring structural distortions
 Journal of Applied Crystallography ISSN 0021-8898 Editor: Gernot Kostorz ISODISPLACE: a web-based tool for exploring structural distortions Branton J. Campbell, Harold T. Stokes, David E. Tanner and Dorian
Journal of Applied Crystallography ISSN 0021-8898 Editor: Gernot Kostorz ISODISPLACE: a web-based tool for exploring structural distortions Branton J. Campbell, Harold T. Stokes, David E. Tanner and Dorian
Chem 728 Introduction to Solid Surfaces
 Chem 728 Introduction to Solid Surfaces Solids: hard; fracture; not compressible; molecules close to each other Liquids: molecules mobile, but quite close to each other Gases: molecules very mobile; compressible
Chem 728 Introduction to Solid Surfaces Solids: hard; fracture; not compressible; molecules close to each other Liquids: molecules mobile, but quite close to each other Gases: molecules very mobile; compressible
Tutorial on the use of the program AMPLIMODES of the Bilbao Crystallographic Server (www.cryst.ehu.es).
 Tutorial on the use of the program AMPLIMODES of the Bilbao Crystallographic Server (www.cryst.ehu.es). J. Manuel Perez-Mato, D. Orobengoa, Mois I. Aroyo and C. Capillas Dept. de Fisica de la Materia Condensada,
Tutorial on the use of the program AMPLIMODES of the Bilbao Crystallographic Server (www.cryst.ehu.es). J. Manuel Perez-Mato, D. Orobengoa, Mois I. Aroyo and C. Capillas Dept. de Fisica de la Materia Condensada,
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS
 A11046W1 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2015 Wednesday, 17 June, 2.30
A11046W1 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2015 Wednesday, 17 June, 2.30
Symmetry mode analysis in the Bilbao Crystallographic Server: The program AMPLIMODES
 Symmetry mode analysis in the Bilbao Crystallographic Server: The program AMPLIMODES Acknowledgements: Bilbao: M. Aroyo, D. Orobengoa, J.M. Igartua Grenoble: J. Rodriguez-Carvajal Provo, Utah: H.T. Stokes,
Symmetry mode analysis in the Bilbao Crystallographic Server: The program AMPLIMODES Acknowledgements: Bilbao: M. Aroyo, D. Orobengoa, J.M. Igartua Grenoble: J. Rodriguez-Carvajal Provo, Utah: H.T. Stokes,
by the resonant atoms. Thus NQR becomes an important experimental method in analyzing phase transitions.
 PHYSICL RVIW B VOLUM 35, NUMBR 0 PRIL 987 Symmetry analysis of the microstructure and phase transitions of a crystallographic space group: pplications Dorian M. Hatch, Harold T. Stokes, and Rand M. Putnam*
PHYSICL RVIW B VOLUM 35, NUMBR 0 PRIL 987 Symmetry analysis of the microstructure and phase transitions of a crystallographic space group: pplications Dorian M. Hatch, Harold T. Stokes, and Rand M. Putnam*
CHAPTER 4. Crystal Structure
 CHAPTER 4 Crystal Structure We can assume minerals to be made of orderly packing of atoms or rather ions or molecules. Many mineral properties like symmetry, density etc are dependent on how the atoms
CHAPTER 4 Crystal Structure We can assume minerals to be made of orderly packing of atoms or rather ions or molecules. Many mineral properties like symmetry, density etc are dependent on how the atoms
Molecular Symmetry 10/25/2018
 Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy). Predict IR spectra or Interpret UV-Vis spectra Predict optical activity
Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy). Predict IR spectra or Interpret UV-Vis spectra Predict optical activity
2.3 Band structure and lattice symmetries: example of diamond
 2.2.9 Product of representaitons Besides the sums of representations, one can also define their products. Consider two groups G and H and their direct product G H. If we have two representations D 1 and
2.2.9 Product of representaitons Besides the sums of representations, one can also define their products. Consider two groups G and H and their direct product G H. If we have two representations D 1 and
PART 1 Introduction to Theory of Solids
 Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
Materials 218/UCSB: Phase transitions and polar materials
 Materials 218/UCSB: Phase transitions and polar materials Ram Seshadri (seshadri@mrl.ucsb.edu) Background: Intrinsic stability of thermodynamic systems (after H. B. Callen, Thermodynamics and an introduction
Materials 218/UCSB: Phase transitions and polar materials Ram Seshadri (seshadri@mrl.ucsb.edu) Background: Intrinsic stability of thermodynamic systems (after H. B. Callen, Thermodynamics and an introduction
Roger Johnson Structure and Dynamics: Displacive phase transition Lecture 9
 9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
2 ( º ) Intensity (a.u.) Supplementary Figure 1. Crystal structure for composition Bi0.75Pb0.25Fe0.7Mn0.05Ti0.25O3. Highresolution
 Intensity (a.u.) Y Obs Y Cal Y Obs - Y Cal Bragg position Cc 20 40 60 80 100 2 ( º ) Supplementary Figure 1. Crystal structure for composition Bi0.75Pb0.25Fe0.7Mn0.05Ti0.25O3. Highresolution X-ray diffraction
Intensity (a.u.) Y Obs Y Cal Y Obs - Y Cal Bragg position Cc 20 40 60 80 100 2 ( º ) Supplementary Figure 1. Crystal structure for composition Bi0.75Pb0.25Fe0.7Mn0.05Ti0.25O3. Highresolution X-ray diffraction
Constructing Landau-Ginzburg-Devonshire Type Models for Ferroelectric Systems Based on Symmetry
 701 GFER A 3395 October 18, 008 11:19 GFER #3395, VOL 375, ISS 1 Constructing Landau-Ginzburg-Devonshire Type Models for Ferroelectric Systems Based on Symmetry WENWU CAO QUERY SHEET This page lists questions
701 GFER A 3395 October 18, 008 11:19 GFER #3395, VOL 375, ISS 1 Constructing Landau-Ginzburg-Devonshire Type Models for Ferroelectric Systems Based on Symmetry WENWU CAO QUERY SHEET This page lists questions
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
PROOF COPY [LD9110BJ] PRB
![PROOF COPY [LD9110BJ] PRB PROOF COPY [LD9110BJ] PRB](/thumbs/84/91030401.jpg) Effect of oxygen stoichiometry on spin, charge, and orbital ordering in manganites R. Vidya,* P. Ravindran, P. Vajeeston, A. Kjekshus, and H. Fjellvåg Department of Chemistry, University of Oslo, P.O.
Effect of oxygen stoichiometry on spin, charge, and orbital ordering in manganites R. Vidya,* P. Ravindran, P. Vajeeston, A. Kjekshus, and H. Fjellvåg Department of Chemistry, University of Oslo, P.O.
INTERNATIONAL SCHOOL ON FUNDAMENTAL CRYSTALLOGRAPHY AND WORKSHOP ON STRUCTURAL PHASE TRANSITIONS. 30 August - 4 September 2017
 INTERNATIONAL SCHOOL ON FUNDAMENTAL CRYSTALLOGRAPHY AND WORKSHOP ON STRUCTURAL PHASE TRANSITIONS 30 August - 4 September 2017 ROURKELA INTERNATIONAL CRYSTALLOGRAPHY SCHOOL BILBAO CRYSTALLOGRAPHIC SERVER
INTERNATIONAL SCHOOL ON FUNDAMENTAL CRYSTALLOGRAPHY AND WORKSHOP ON STRUCTURAL PHASE TRANSITIONS 30 August - 4 September 2017 ROURKELA INTERNATIONAL CRYSTALLOGRAPHY SCHOOL BILBAO CRYSTALLOGRAPHIC SERVER
The structure of planar defects in tilted perovskites
 The structure of planar defects in tilted perovskites RichardBeanland Department of Physics, University of Warwick, Coventry, CV4 7AL, UK. E-mail: r.beanland@warwick.ac.uk Synopsis Continuity of octahedral
The structure of planar defects in tilted perovskites RichardBeanland Department of Physics, University of Warwick, Coventry, CV4 7AL, UK. E-mail: r.beanland@warwick.ac.uk Synopsis Continuity of octahedral
Atomic Theory and Atomic structure. Part A. d) Distance of electrons from the nucleus
 Grade Subject Topic : AP : Science : Atomic Theory and Atomic Structure Atomic Theory and Atomic structure Part A (1) The Principal quantum number represents a) Shape of an orbital b) Number of electrons
Grade Subject Topic : AP : Science : Atomic Theory and Atomic Structure Atomic Theory and Atomic structure Part A (1) The Principal quantum number represents a) Shape of an orbital b) Number of electrons
Algorithm for conversion between geometric algebra versor notation and conventional crystallographic symmetry-operation symbols
 Algorithm for conversion between geometric algebra versor notation and conventional crystallographic symmetry-operation symbols Eckhard Hitzer and Christian Perwass June, 2009 Introduction This paper establishes
Algorithm for conversion between geometric algebra versor notation and conventional crystallographic symmetry-operation symbols Eckhard Hitzer and Christian Perwass June, 2009 Introduction This paper establishes
130 points on 6 pages + a useful page Circle the element/compound most likely to have the desired property. Briefly explain your choice
 Name Chemistry 35 Spring 212 Exam #2, March 3, 212 5 minutes 13 points on 6 pages + a useful page 7 1. Circle the element/compound most likely to have the desired property. Briefly explain your choice
Name Chemistry 35 Spring 212 Exam #2, March 3, 212 5 minutes 13 points on 6 pages + a useful page 7 1. Circle the element/compound most likely to have the desired property. Briefly explain your choice
Little Orthogonality Theorem (LOT)
 Little Orthogonality Theorem (LOT) Take diagonal elements of D matrices in RG * D R D R i j G ij mi N * D R D R N i j G G ij ij RG mi mi ( ) By definition, D j j j R TrD R ( R). Sum GOT over β: * * ( )
Little Orthogonality Theorem (LOT) Take diagonal elements of D matrices in RG * D R D R i j G ij mi N * D R D R N i j G G ij ij RG mi mi ( ) By definition, D j j j R TrD R ( R). Sum GOT over β: * * ( )
EPR in Kagome Staircase Compound Mg Co V 2 O 8
 Vol. 111 (2007) ACTA PHYSICA POLONICA A No. 1 Proceedings of the Symposium K: Complex Oxide Materials for New Technologies of E-MRS Fall Meeting 2006, Warsaw, September 4 8, 2006 EPR in Kagome Staircase
Vol. 111 (2007) ACTA PHYSICA POLONICA A No. 1 Proceedings of the Symposium K: Complex Oxide Materials for New Technologies of E-MRS Fall Meeting 2006, Warsaw, September 4 8, 2006 EPR in Kagome Staircase
arxiv:physics/ v1 [physics.chem-ph] 14 Nov 2005
![arxiv:physics/ v1 [physics.chem-ph] 14 Nov 2005 arxiv:physics/ v1 [physics.chem-ph] 14 Nov 2005](/thumbs/88/117666865.jpg) A NEW APPROACH to ANALYSE H (h g) JAHN-TELLER SYSTEM for C 60 Ramazan Koç and Hayriye Tütüncüler Department of Physics, Faculty of Engineering University of Gaziantep, 710 Gaziantep, Turkey arxiv:physics/0111v1
A NEW APPROACH to ANALYSE H (h g) JAHN-TELLER SYSTEM for C 60 Ramazan Koç and Hayriye Tütüncüler Department of Physics, Faculty of Engineering University of Gaziantep, 710 Gaziantep, Turkey arxiv:physics/0111v1
[1-3] was originally thought to be second order. Grouptheoretical. Phonon Softening and High-Pressure Low-Symmetry Phases of Cesium Iodide
![[1-3] was originally thought to be second order. Grouptheoretical. Phonon Softening and High-Pressure Low-Symmetry Phases of Cesium Iodide [1-3] was originally thought to be second order. Grouptheoretical. Phonon Softening and High-Pressure Low-Symmetry Phases of Cesium Iodide](/thumbs/91/105597537.jpg) VOLUME 69, NUMBER 7 PH YSCAL R EV EW LETTERS 17 AVr. UST 1992 Phonon Softening and High-Pressure Low-Symmetry Phases of Cesium odide Marco Buongiorno Nardelli and Stefano Baroni Scuola nternazionale Superiore
VOLUME 69, NUMBER 7 PH YSCAL R EV EW LETTERS 17 AVr. UST 1992 Phonon Softening and High-Pressure Low-Symmetry Phases of Cesium odide Marco Buongiorno Nardelli and Stefano Baroni Scuola nternazionale Superiore
PREDICTING NEW STRUCTURES: THE SIMPLE CUBIC AND PEROVSKITE CASE
 PREDICTING NEW STRUCTURES: THE SIMPLE CUBIC AND PEROVSKITE CASE by Matthew Lords advisor Dr. Gus Hart Physics 492R Capstone Project Report Department of Physics and Astronomy Brigham Young University March
PREDICTING NEW STRUCTURES: THE SIMPLE CUBIC AND PEROVSKITE CASE by Matthew Lords advisor Dr. Gus Hart Physics 492R Capstone Project Report Department of Physics and Astronomy Brigham Young University March
M.S. Dresselhaus G. Dresselhaus A. Jorio. Group Theory. Application to the Physics of Condensed Matter. With 131 Figures and 219 Tables.
 M.S. Dresselhaus G. Dresselhaus A. Jorio Group Theory Application to the Physics of Condensed Matter With 131 Figures and 219 Tables 4) Springer Contents Part I Basic Mathematics 1 Basic Mathematical Background:
M.S. Dresselhaus G. Dresselhaus A. Jorio Group Theory Application to the Physics of Condensed Matter With 131 Figures and 219 Tables 4) Springer Contents Part I Basic Mathematics 1 Basic Mathematical Background:
PHASE TRANSITIONS IN (CH3NH3)5B12X11 (X = Cl, Br) CRYSTALS H. PYKACZ, J. MRÓZ
 Vol. 84 (1993) ACTA PHYSICA POLONICA A Νo. 6 PHASE TRANSITIONS IN (CH3NH3)5B12X11 (X = Cl, Br) CRYSTALS H. PYKACZ, J. MRÓZ Institute of Physics, Technical University of Wroclaw Wybrzeże Wyspiańskiego 27,
Vol. 84 (1993) ACTA PHYSICA POLONICA A Νo. 6 PHASE TRANSITIONS IN (CH3NH3)5B12X11 (X = Cl, Br) CRYSTALS H. PYKACZ, J. MRÓZ Institute of Physics, Technical University of Wroclaw Wybrzeże Wyspiańskiego 27,
Chapter 11. Non-rigid Molecules
 Chapter. Non-rigid olecules Notes: ost of the material presented in this chapter is taken from Bunker and Jensen (998), Chap. 5, and Bunker and Jensen (2005), Chap. 3.. The Hamiltonian As was stated before,
Chapter. Non-rigid olecules Notes: ost of the material presented in this chapter is taken from Bunker and Jensen (998), Chap. 5, and Bunker and Jensen (2005), Chap. 3.. The Hamiltonian As was stated before,
Chem Spring, 2018 Test II - Part 1 April 9, (30 points; 3 points each) Circle the correct answer to each of the following.
 Chem 370 - Spring, 2018 Test II - Part 1 April 9, 2018 Page 1 of 5 1. (30 points; 3 points each) Circle the correct answer to each of the following. a. Which one of the following aqueous solutions would
Chem 370 - Spring, 2018 Test II - Part 1 April 9, 2018 Page 1 of 5 1. (30 points; 3 points each) Circle the correct answer to each of the following. a. Which one of the following aqueous solutions would
Analytical Methods for Materials
 Analytical Methods for Materials Lesson 11 Crystallography and Crystal Structures, Part 3 Suggested Reading Chapter 6 in Waseda Chapter 1 in F.D. Bloss, Crystallography and Crystal Chemistry: An Introduction,
Analytical Methods for Materials Lesson 11 Crystallography and Crystal Structures, Part 3 Suggested Reading Chapter 6 in Waseda Chapter 1 in F.D. Bloss, Crystallography and Crystal Chemistry: An Introduction,
Metallic and Ionic Structures and Bonding
 Metallic and Ionic Structures and Bonding Ionic compounds are formed between elements having an electronegativity difference of about 2.0 or greater. Simple ionic compounds are characterized by high melting
Metallic and Ionic Structures and Bonding Ionic compounds are formed between elements having an electronegativity difference of about 2.0 or greater. Simple ionic compounds are characterized by high melting
Chapter 10: Chemical Bonding II. Bonding Theories
 Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
b) For this ground state, obtain all possible J values and order them from lowest to highest in energy.
 Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
b) For this ground state, obtain all possible J values and order them from lowest to highest in energy.
 Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
Ceramic Bonding. CaF 2 : large SiC: small
 Recall ceramic bonding: - Mixed ionic and covalent. - % ionic character ( f ) increases with difference in electronegativity Large vs small ionic bond character: Ceramic Bonding CaF : large SiC: small
Recall ceramic bonding: - Mixed ionic and covalent. - % ionic character ( f ) increases with difference in electronegativity Large vs small ionic bond character: Ceramic Bonding CaF : large SiC: small
Crystallographic structure Physical vs Chemical bonding in solids
 Crystallographic structure Physical vs Chemical bonding in solids Inert gas and molecular crystals: Van der Waals forces (physics) Water and organic chemistry H bonds (physics) Quartz crystal SiO 2 : covalent
Crystallographic structure Physical vs Chemical bonding in solids Inert gas and molecular crystals: Van der Waals forces (physics) Water and organic chemistry H bonds (physics) Quartz crystal SiO 2 : covalent
Atomic Arrangement. Primer in Materials Spring
 Atomic Arrangement Primer in Materials Spring 2017 30.4.2017 1 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling the volume to
Atomic Arrangement Primer in Materials Spring 2017 30.4.2017 1 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling the volume to
research papers Homogeneous sphere packings with triclinic symmetry 1. Introduction 2. Sphere packings corresponding to lattice complex P11 1a
 Acta Crystallographica Section A Foundations of Crystallography ISSN 008-7673 Received 3 May 2002 Accepted 7 July 2002 Homogeneous sphere packings with triclinic symmetry W. Fischer and E. Koch* Institut
Acta Crystallographica Section A Foundations of Crystallography ISSN 008-7673 Received 3 May 2002 Accepted 7 July 2002 Homogeneous sphere packings with triclinic symmetry W. Fischer and E. Koch* Institut
Rutile TiO 2 tetragonal unit cell with a = b = Å, c = Å Fig. 1.32a: Ti positions, 2 per cell, corner(0,0,0) and body center( 21
 1 f) Rutile (TiO 2 ), cadmium iodide (CdI 2 ), cadmium chloride (CdCl 2 ) and caesium oxide (Cs 2 O) Together with fluorite, they represent the main AX 2 structure types. Rutile TiO 2 tetragonal unit cell
1 f) Rutile (TiO 2 ), cadmium iodide (CdI 2 ), cadmium chloride (CdCl 2 ) and caesium oxide (Cs 2 O) Together with fluorite, they represent the main AX 2 structure types. Rutile TiO 2 tetragonal unit cell
Colossal magnetoresistance:
 Colossal magnetoresistance: Ram Seshadri (seshadri@mrl.ucsb.edu) The simplest example of magnetoresistance is transverse magnetoresistance associated with the Hall effect: H + + + + + + + + + + E y - -
Colossal magnetoresistance: Ram Seshadri (seshadri@mrl.ucsb.edu) The simplest example of magnetoresistance is transverse magnetoresistance associated with the Hall effect: H + + + + + + + + + + E y - -
Atomic Arrangement. Primer Materials For Science Teaching Spring
 Atomic Arrangement Primer Materials For Science Teaching Spring 2016 31.3.2015 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling
Atomic Arrangement Primer Materials For Science Teaching Spring 2016 31.3.2015 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling
Chapter 10 Theories of Covalent Bonding
 Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Phase Transitions in Strontium Titanate
 Phase Transitions in Strontium Titanate Xinyue Fang Department of Physics, University of Illinois at Urbana-Champaign Abstract Strontium Titanate SrTiO 3 (STO) is known to undergo an antiferrodistortive
Phase Transitions in Strontium Titanate Xinyue Fang Department of Physics, University of Illinois at Urbana-Champaign Abstract Strontium Titanate SrTiO 3 (STO) is known to undergo an antiferrodistortive
SPACE GROUPS. International Tables for Crystallography, Volume A: Space-group Symmetry. Mois I. Aroyo Universidad del Pais Vasco, Bilbao, Spain
 SPACE GROUPS International Tables for Crystallography, Volume A: Space-group Symmetry Mois I. Aroyo Universidad del Pais Vasco, Bilbao, Spain SPACE GROUPS Crystal pattern: Space group G: A model of the
SPACE GROUPS International Tables for Crystallography, Volume A: Space-group Symmetry Mois I. Aroyo Universidad del Pais Vasco, Bilbao, Spain SPACE GROUPS Crystal pattern: Space group G: A model of the
Tight-binding molecular dynamics study of palladium
 PHYSICAL REVIEW B 79, 064107 2009 Tight-binding molecular dynamics study of palladium A. Shabaev and D. A. Papaconstantopoulos George Mason University, Fairfax, Virginia 22030, USA Received 24 September
PHYSICAL REVIEW B 79, 064107 2009 Tight-binding molecular dynamics study of palladium A. Shabaev and D. A. Papaconstantopoulos George Mason University, Fairfax, Virginia 22030, USA Received 24 September
Tables of crystallographic properties of magnetic space groups
 Acta Crystallographica Section A Foundations of Crystallography ISSN 0108-7673 Editor: D. Schwarzenbach Tables of crystallographic properties of magnetic space groups D. B. Litvin Acta Cryst. (2008). A64,
Acta Crystallographica Section A Foundations of Crystallography ISSN 0108-7673 Editor: D. Schwarzenbach Tables of crystallographic properties of magnetic space groups D. B. Litvin Acta Cryst. (2008). A64,
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
CHEM 121 Introduction to Fundamental Chemistry. Summer Quarter 2008 SCCC. Lecture 5.
 CHEM 121 Introduction to Fundamental Chemistry Summer Quarter 2008 SCCC Lecture 5 http://seattlecentral.edu/faculty/lcwest/che121 Forces Between Particles Noble Gas Configurations Ionic Bonding Ionic Compounds
CHEM 121 Introduction to Fundamental Chemistry Summer Quarter 2008 SCCC Lecture 5 http://seattlecentral.edu/faculty/lcwest/che121 Forces Between Particles Noble Gas Configurations Ionic Bonding Ionic Compounds
Lecture 2: Bonding in solids
 Lecture 2: Bonding in solids Electronegativity Van Arkel-Ketalaar Triangles Atomic and ionic radii Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonds in Reciprocal space
Lecture 2: Bonding in solids Electronegativity Van Arkel-Ketalaar Triangles Atomic and ionic radii Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonds in Reciprocal space
EPSC501 Crystal Chemistry WEEK 5
 EPSC501 Crystal Chemistry WEEK 5 Oxidation states of transition elements (many more in aqueous solutions than in the common rock-forming minerals) Notice that almost every transition metal has a +2 oxidation
EPSC501 Crystal Chemistry WEEK 5 Oxidation states of transition elements (many more in aqueous solutions than in the common rock-forming minerals) Notice that almost every transition metal has a +2 oxidation
Representation analysis vs. Magnetic Symmetry
 Representation analysis vs. Magnetic Symmetry J. Manuel Perez-Mato Facultad de Ciencia y Tecnología Universidad del País Vasco, UPV-EHU BILBAO, SPAIN WHAT IS SYMMETRY? A symmetry operation in a solid IS
Representation analysis vs. Magnetic Symmetry J. Manuel Perez-Mato Facultad de Ciencia y Tecnología Universidad del País Vasco, UPV-EHU BILBAO, SPAIN WHAT IS SYMMETRY? A symmetry operation in a solid IS
Supporting Information. Ising-Type Magnetic Ordering in Atomically Thin
 Supporting Information Ising-Type Magnetic Ordering in Atomically Thin FePS3 Jae-Ung Lee, Sungmin Lee,, Ji Hoon Ryoo,,Soonmin Kang,, Tae Yun Kim, Pilkwang Kim, Cheol-Hwan Park, *,, Je-Geun Park, *,, and
Supporting Information Ising-Type Magnetic Ordering in Atomically Thin FePS3 Jae-Ung Lee, Sungmin Lee,, Ji Hoon Ryoo,,Soonmin Kang,, Tae Yun Kim, Pilkwang Kim, Cheol-Hwan Park, *,, Je-Geun Park, *,, and
INTERNATIONAL SCHOOL ON FUNDAMENTAL CRYSTALLOGRAPHY AND WORKSHOP ON STRUCTURAL PHASE TRANSITIONS. 30 August - 4 September 2017
 INTERNATIONAL SCHOOL ON FUNDAMENTAL CRYSTALLOGRAPHY AND WORKSHOP ON STRUCTURAL PHASE TRANSITIONS 30 August - 4 September 2017 ROURKELA INTERNATIONAL CRYSTALLOGRAPHY SCHOOL BILBAO CRYSTALLOGRAPHIC SERVER
INTERNATIONAL SCHOOL ON FUNDAMENTAL CRYSTALLOGRAPHY AND WORKSHOP ON STRUCTURAL PHASE TRANSITIONS 30 August - 4 September 2017 ROURKELA INTERNATIONAL CRYSTALLOGRAPHY SCHOOL BILBAO CRYSTALLOGRAPHIC SERVER
In situ growth of nanoparticles through control of non-stoichiometry
 DOI: 10.1038/NCHEM.1773 In situ growth of nanoparticles through control of non-stoichiometry Dragos Neagu 1 *, George Tsekouras 1, David N. Miller 1, Hervé Ménard 2 and John T.S. Irvine 1 * 1 University
DOI: 10.1038/NCHEM.1773 In situ growth of nanoparticles through control of non-stoichiometry Dragos Neagu 1 *, George Tsekouras 1, David N. Miller 1, Hervé Ménard 2 and John T.S. Irvine 1 * 1 University
Order parameters for phase transitions to structures with one-dimensional incommensurate modulations
 Acta rystallographica Section A Foundations of rystallography ISSN 8-7673 Editor: D. Schwarzenbach Order parameters for phase transitions to structures with one-dimensional incommensurate modulations Harold
Acta rystallographica Section A Foundations of rystallography ISSN 8-7673 Editor: D. Schwarzenbach Order parameters for phase transitions to structures with one-dimensional incommensurate modulations Harold
Factors Affecting the Magnitude of the Metal-Insulator Transition Temperature Bull. Korean Chem. Soc. 2004, Vol. 25, No
 Factors Affecting the Magnitude of the Metal-Insulator Transition Temperature Bull. Korean Chem. Soc. 2004, Vol. 25, No. 7 959 Articles Factors Affecting the Magnitude of the Metal-Insulator Transition
Factors Affecting the Magnitude of the Metal-Insulator Transition Temperature Bull. Korean Chem. Soc. 2004, Vol. 25, No. 7 959 Articles Factors Affecting the Magnitude of the Metal-Insulator Transition
Lead-Free MA 2 CuCl x Br 4-x Hybrid Perovskites
 Supporting Information Lead-Free MA 2 CuCl x Br 4-x Hybrid Perovskites Daniele Cortecchia, 1,2 Herlina Arianita Dewi, 2 Jun Yin, 3 Annalisa Bruno, 2,3 Shi Chen, 3 Tom Baikie, 2 Pablo P. Boix, 2 Michael
Supporting Information Lead-Free MA 2 CuCl x Br 4-x Hybrid Perovskites Daniele Cortecchia, 1,2 Herlina Arianita Dewi, 2 Jun Yin, 3 Annalisa Bruno, 2,3 Shi Chen, 3 Tom Baikie, 2 Pablo P. Boix, 2 Michael
Geometry of Crystal Lattice
 0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
Antiphase structures of an improper ferroelastic phase transition driven by an M 5 À zone boundary phonon in RAg 1Àx In x
 PHYSICAL REVIEW B, VOLUME 64, 04106 Antiphase structures of an improper ferroelastic phase transition driven by an M 5 À zone boundary phonon in RAg 1Àx In x Wenwu Cao, 1, * Avadh Saxena,, and Dorian M.
PHYSICAL REVIEW B, VOLUME 64, 04106 Antiphase structures of an improper ferroelastic phase transition driven by an M 5 À zone boundary phonon in RAg 1Àx In x Wenwu Cao, 1, * Avadh Saxena,, and Dorian M.
Chemistry 431. Lecture 14. Wave functions as a basis Diatomic molecules Polyatomic molecules Huckel theory. NC State University
 Chemistry 431 Lecture 14 Wave functions as a basis Diatomic molecules Polyatomic molecules Huckel theory NC State University Wave functions as the basis for irreducible representations The energy of the
Chemistry 431 Lecture 14 Wave functions as a basis Diatomic molecules Polyatomic molecules Huckel theory NC State University Wave functions as the basis for irreducible representations The energy of the
NMR, STRUCTURE AND SPECTROSCOPIC INVESTIGATIONS ON A CESIUM-AMMONIUM CADMIUM TRICHLORIDE
 Research and Reviews in Materials Science and Chemistry Vol. 4, Issue 1, 2014, Pages 17-34 ISSN 2319-6920 Published Online on July 19, 2014 2014 Jyoti Academic Press http://jyotiacademicpress.net NMR,
Research and Reviews in Materials Science and Chemistry Vol. 4, Issue 1, 2014, Pages 17-34 ISSN 2319-6920 Published Online on July 19, 2014 2014 Jyoti Academic Press http://jyotiacademicpress.net NMR,
Self-compensating incorporation of Mn in Ga 1 x Mn x As
 Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
Mode Crystallography of distorted structures J. M. Perez-Mato, D. Orobengoa and M. Aroyo Departamento de Fisica de la Materia Condensada, Facultad de
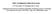 Mode Crystallography of distorted structures J. M. Perez-Mato, D. Orobengoa and M. Aroyo Departamento de Fisica de la Materia Condensada, Facultad de Ciencia y Tecnologia, Universidad del Pais Vasco (UPV-EHU),
Mode Crystallography of distorted structures J. M. Perez-Mato, D. Orobengoa and M. Aroyo Departamento de Fisica de la Materia Condensada, Facultad de Ciencia y Tecnologia, Universidad del Pais Vasco (UPV-EHU),
The ability of the scanning tunneling microscope (STM) to record real-space, atomicscale
 EXPERIMENTAL AND SIMULATED SCANNING TUNNELING MICROSCOPY OF THE CLEAVED Rb 1 / 3 WO 3 (0001) SURFACE WEIER LU AND GREGORY S. ROHRER Carnegie Mellon University Department of Materials Science and Engineering
EXPERIMENTAL AND SIMULATED SCANNING TUNNELING MICROSCOPY OF THE CLEAVED Rb 1 / 3 WO 3 (0001) SURFACE WEIER LU AND GREGORY S. ROHRER Carnegie Mellon University Department of Materials Science and Engineering
Symmetry in 2D. 4/24/2013 L. Viciu AC II Symmetry in 2D
 Symmetry in 2D 1 Outlook Symmetry: definitions, unit cell choice Symmetry operations in 2D Symmetry combinations Plane Point groups Plane (space) groups Finding the plane group: examples 2 Symmetry Symmetry
Symmetry in 2D 1 Outlook Symmetry: definitions, unit cell choice Symmetry operations in 2D Symmetry combinations Plane Point groups Plane (space) groups Finding the plane group: examples 2 Symmetry Symmetry
Earth Materials Lab 2 - Lattices and the Unit Cell
 Earth Materials Lab 2 - Lattices and the Unit Cell Unit Cell Minerals are crystallographic solids and therefore are made of atoms arranged into lattices. The average size hand specimen is made of more
Earth Materials Lab 2 - Lattices and the Unit Cell Unit Cell Minerals are crystallographic solids and therefore are made of atoms arranged into lattices. The average size hand specimen is made of more
The Goldstone theorem and the Jahn-Teller effect
 PROC. PHYS. SOC., 1967, VOL. 91. PRINTED IN GREAT BRITAIX The Goldstone theorem and the Jahn-Teller effect J. SARFATTt and A. M. STONEHAM$ t Department of Physics, University of California, La Jolla, U.S.A.
PROC. PHYS. SOC., 1967, VOL. 91. PRINTED IN GREAT BRITAIX The Goldstone theorem and the Jahn-Teller effect J. SARFATTt and A. M. STONEHAM$ t Department of Physics, University of California, La Jolla, U.S.A.
Lecture 6 - Bonding in Crystals
 Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Phase Transitions and Crystal Symmetry
 Phase Transitions and Crystal Symmetry by Yu. A. IZYUMOV and V. N. SYROMYATNIKOV Institute of Metal Physics, Ural Division of the U.S.S.R. Academy of Sciences, Sverdlovsk, U.S.S.R. KLUWER ACADEMIC PUBLISHERS
Phase Transitions and Crystal Symmetry by Yu. A. IZYUMOV and V. N. SYROMYATNIKOV Institute of Metal Physics, Ural Division of the U.S.S.R. Academy of Sciences, Sverdlovsk, U.S.S.R. KLUWER ACADEMIC PUBLISHERS
Inorganic Exam 1 Chm October 2010
 Inorganic Exam 1 Chm 451 28 October 2010 Name: Instructions. Always show your work where required for full credit. 1. In the molecule CO 2, the first step in the construction of the MO diagram was to consider
Inorganic Exam 1 Chm 451 28 October 2010 Name: Instructions. Always show your work where required for full credit. 1. In the molecule CO 2, the first step in the construction of the MO diagram was to consider
MANY BILLS OF CONCERN TO PUBLIC
 - 6 8 9-6 8 9 6 9 XXX 4 > -? - 8 9 x 4 z ) - -! x - x - - X - - - - - x 00 - - - - - x z - - - x x - x - - - - - ) x - - - - - - 0 > - 000-90 - - 4 0 x 00 - -? z 8 & x - - 8? > 9 - - - - 64 49 9 x - -
- 6 8 9-6 8 9 6 9 XXX 4 > -? - 8 9 x 4 z ) - -! x - x - - X - - - - - x 00 - - - - - x z - - - x x - x - - - - - ) x - - - - - - 0 > - 000-90 - - 4 0 x 00 - -? z 8 & x - - 8? > 9 - - - - 64 49 9 x - -
Chemistry Higher level Paper 1
 N15/4/EMI/PM/ENG/TZ0/XX hemistry igher level Paper 1 Friday 13 November 2015 (afternoon) 1 hour Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions.
N15/4/EMI/PM/ENG/TZ0/XX hemistry igher level Paper 1 Friday 13 November 2015 (afternoon) 1 hour Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions.
CHEM 251 (Fall-2003) Final Exam (100 pts)
 CEM 251 (Fall-2003) Final Exam (100 pts) Name: -------------------------------------------------------------------------------, SSN -------------------------------- LAST NAME, First (Circle the alphabet
CEM 251 (Fall-2003) Final Exam (100 pts) Name: -------------------------------------------------------------------------------, SSN -------------------------------- LAST NAME, First (Circle the alphabet
* motif: a single or repeated design or color
 Chapter 2. Structure A. Electronic structure vs. Geometric structure B. Clean surface vs. Adsorbate covered surface (substrate + overlayer) C. Adsorbate structure - how are the adsorbed molecules bound
Chapter 2. Structure A. Electronic structure vs. Geometric structure B. Clean surface vs. Adsorbate covered surface (substrate + overlayer) C. Adsorbate structure - how are the adsorbed molecules bound
First principles computer simulations of Li 10 GeP 2 S 12 and related lithium superionic conductors
 First principles computer simulations of Li 10 GeP 2 S 12 and related lithium superionic conductors N. A. W. Holzwarth Department of Physics, Wake Forest University, Winston-Salem, NC, 27109, USA Introduction
First principles computer simulations of Li 10 GeP 2 S 12 and related lithium superionic conductors N. A. W. Holzwarth Department of Physics, Wake Forest University, Winston-Salem, NC, 27109, USA Introduction
Lecture Note on Crystal structures Masatsugu Sei Suzuki and Itsuko S. Suzuki Department of Physics, SUNY at Binghamton (Date: February 03, 2012)
 Lecture Note on Crystal structures Masatsugu Sei Suzuki and Itsuko S. Suzuki Department of Physics, SUNY at Binghamton (Date: February 03, 2012) This is a part of lecture note on solid state physics (Phys.472/572)
Lecture Note on Crystal structures Masatsugu Sei Suzuki and Itsuko S. Suzuki Department of Physics, SUNY at Binghamton (Date: February 03, 2012) This is a part of lecture note on solid state physics (Phys.472/572)
LOWELL WEEKI.Y JOURINAL
 / $ 8) 2 {!»!» X ( (!!!?! () ~ x 8» x /»!! $?» 8! ) ( ) 8 X x /! / x 9 ( 2 2! z»!!»! ) / x»! ( (»»!» [ ~!! 8 X / Q X x» ( (!»! Q ) X x X!! (? ( ()» 9 X»/ Q ( (X )!» / )! X» x / 6!»! }? ( q ( ) / X! 8 x»
/ $ 8) 2 {!»!» X ( (!!!?! () ~ x 8» x /»!! $?» 8! ) ( ) 8 X x /! / x 9 ( 2 2! z»!!»! ) / x»! ( (»»!» [ ~!! 8 X / Q X x» ( (!»! Q ) X x X!! (? ( ()» 9 X»/ Q ( (X )!» / )! X» x / 6!»! }? ( q ( ) / X! 8 x»
Chapter 20 d-metal complexes: electronic structures and properties
 CHEM 511 Chapter 20 page 1 of 21 Chapter 20 d-metal complexes: electronic structures and properties Recall the shape of the d-orbitals... Electronic structure Crystal Field Theory: an electrostatic approach
CHEM 511 Chapter 20 page 1 of 21 Chapter 20 d-metal complexes: electronic structures and properties Recall the shape of the d-orbitals... Electronic structure Crystal Field Theory: an electrostatic approach
EXERCISE SET 5.1. = (kx + kx + k, ky + ky + k ) = (kx + kx + 1, ky + ky + 1) = ((k + )x + 1, (k + )y + 1)
 EXERCISE SET 5. 6. The pair (, 2) is in the set but the pair ( )(, 2) = (, 2) is not because the first component is negative; hence Axiom 6 fails. Axiom 5 also fails. 8. Axioms, 2, 3, 6, 9, and are easily
EXERCISE SET 5. 6. The pair (, 2) is in the set but the pair ( )(, 2) = (, 2) is not because the first component is negative; hence Axiom 6 fails. Axiom 5 also fails. 8. Axioms, 2, 3, 6, 9, and are easily
Space Group: translations and point Group
 Space Group: translations and point Group Fyodorov and Schönflies in 1891 listed the 230 space Groups in 3d Group elements: r ' r a with a traslation rotation matrix ( 1 No rotation). The ope ration denoted
Space Group: translations and point Group Fyodorov and Schönflies in 1891 listed the 230 space Groups in 3d Group elements: r ' r a with a traslation rotation matrix ( 1 No rotation). The ope ration denoted
THE TRANSLATION PLANES OF ORDER 49 AND THEIR AUTOMORPHISM GROUPS
 MATHEMATICS OF COMPUTATION Volume 67, Number 223, July 1998, Pages 1207 1224 S 0025-5718(98)00961-2 THE TRANSLATION PLANES OF ORDER 49 AND THEIR AUTOMORPHISM GROUPS C. CHARNES AND U. DEMPWOLFF Abstract.
MATHEMATICS OF COMPUTATION Volume 67, Number 223, July 1998, Pages 1207 1224 S 0025-5718(98)00961-2 THE TRANSLATION PLANES OF ORDER 49 AND THEIR AUTOMORPHISM GROUPS C. CHARNES AND U. DEMPWOLFF Abstract.
Chemical bonding, elasticity, and valence force field models: A case study for -Pt 2 Si and PtSi
 PHYSICAL REVIEW B, VOLUME 64, 550 Chemical bonding, elasticity, and valence force field models: A case study for -Pt 2 Si and PtSi J. E. Klepeis, O. Beckstein, 2 O. Pankratov, 2 and G. L. W. Hart 3 Lawrence
PHYSICAL REVIEW B, VOLUME 64, 550 Chemical bonding, elasticity, and valence force field models: A case study for -Pt 2 Si and PtSi J. E. Klepeis, O. Beckstein, 2 O. Pankratov, 2 and G. L. W. Hart 3 Lawrence
Ionic Bond, Latice energy, characteristic of Ionic compounds
 Ionic Bond, Latice energy, characteristic of Ionic compounds 1. The strong electrostatic attraction between two oppositely charged ions which are formed due to transfer of electrons from one atom to another
Ionic Bond, Latice energy, characteristic of Ionic compounds 1. The strong electrostatic attraction between two oppositely charged ions which are formed due to transfer of electrons from one atom to another
130 points on 6 pages + a useful page 7
 Name KEY Chemistry 350 Spring 2012 Exam #2, March 30, 2012 50 minutes 130 points on 6 pages + a useful page 7 1. Circle the element/compound most likely to have the desired property. Briefly explain your
Name KEY Chemistry 350 Spring 2012 Exam #2, March 30, 2012 50 minutes 130 points on 6 pages + a useful page 7 1. Circle the element/compound most likely to have the desired property. Briefly explain your
