b) For this ground state, obtain all possible J values and order them from lowest to highest in energy.
|
|
|
- Leslie Henry
- 5 years ago
- Views:
Transcription
1 Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible J values and order them from lowest to highest in energy. L = 3 S = 3/2 J = {9/2, 7/2, 5/2, 3/2} 3/2 < 5/2 < 7/2 < 9/2 c) The first excited state for the d 3 ion is the 4 P state. How many microstates does the 4 P state contain? 4 3 = 12 microstates Splitting of terms and orbitals in a chemical environment: Consider an octahedral ligand field on a set of atomic wave functions. The full symmetry of the octahedron is Oh but we can work with the rotational subgroup O. An atomic orbital can be represented as follows (radial, angular (θ, φ), and spin wavefunctions): Ψ = R(r) Θ(θ) Φ(φ) ψ s We can ignore ψs assuming negligible spin-orbit coupling. The radial function R has no directionality, so it can also be ignored. The angular function Θ is invariant with respect to rotation in the z axis (principal rotation axis), so it can be ignored as well. Working only with Φ, we show without derivation that the character of the reducible representation under Cα for a basis set in which the orbital angular momentum is l is given by: For example, for the set of f orbitals (l = 3) : χ(α) = sin [(l ) α] sin (α/2) 1
2 In the limit where α = 0, χ(e) = 2l+1. χ(c 3 ) = sin [( ) (2π 3 )] sin (π/3) = sin (7π/3) sin (π/3) = 1 These characters belong to irreducible representations of the spherical point group of atoms (K). The table below allows conversion to the lower symmetry point group, O. a) By applying the formula, complete the table below. E 6 C4 3 C2 (= C4 2 ) 8 C3 6 C2ꞌ S (l = 0) P (l = 1) D (l = 2) F (l = 3) G (l = 4) H (l = 5) I (l = 6) b) Each row is a reducible representation in the O point group. Represent each row as a sum of irreducible representations. S: A1 P: T1 D: E + T2 F: A2 + T1 + T2 G: A1 + E + T1 + T2 H: E + 2 T1 + T2 I: A1 + A2 + E + T1 + 2 T2 2
3 Part B In class, we derived the electronic states for a carbon atom (1s 2 2s 2 2p 2 ). They are, in ascending order, 3 P, 1 D, and 1 S. Below is a more complete table of the energy states of carbon (with total angular momentum, J, left out): Electron configuration Term Symbol Rel. Energy (cm -1 ) 2s 2 2p 2 3 P 0 2s 2 2p 2 1 D 10,193 2s 2 2p 2 1 S 21,648 2s 1 2p 3 5 S 33,735 2s 2 2p 1 3s 1 3 P 60,333 2s 2 2p 1 3s 1 1 P 61,982 2s 1 2p 3 3 D 64,087 2s 2 2p 1 3p 1 1 P 68,856 2s 2 2p 1 3p 1 3 D 69,689 2s 2 2p 1 3p 1 3 S 70,744 a) For each of the excited state electron configurations in the table, fill in the electronic ground state with the appropriate term symbol. Note, the ground state of the 2s 2 2p 1 3p 1 electron configuration for carbon is not what you would predict with Hund s rules, instead the first excited state of this configuration matches the prediction using the shortcut presented in class. b) Give the two lowest energy electronic transitions that are spin allowed (high intensity). 3 P (2s 2 2p 2 ) 3 P (2s 2 2p 1 3s 1 ) 3 P (2s 2 2p 2 ) 3 D (2s 1 2p 3 ) 3
4 Problem 2 (1 point) Three center four electron (3c/4e) bonds were introduced in class. John F. Berry (Dalton Trans. 2012, 41, ) discusses the effect of the larger density of states for the 3c/4e interaction than for the 2c/2e case. The 3c/4e system is expected to be not only more reactive than the 2c/2e system, but also more diverse in reactivity. To illustrate this concept, consider H2 and [H3]. 1. Sketch the MO diagram of H2 and assign Mulliken symbols for all molecular orbitals. Write down all possible electronic configurations of H2. Obtain all electronic states clearly indicating the configuration they correspond to and predict their relative energy. (Σu + ) 2 : 1 Σg + (Σg + ) 1 (Σu + ) 1 : 1 Σu Σu + (Σg + ) 2 : 1 Σg+ Total: 2( 1 Σg + ) + 1 Σu Σu + 2. Sketch the MO diagram of [H3] and assign Mulliken symbols for all molecular orbitals. Write down all possible electronic configurations of [H3]. Obtain all electronic states clearly indicating the configuration they correspond to and predict their relative energy. (highest energy) (nb) 2 (σ*) 2 : 1 Σg + (σ) 1 (nb) 1 (σ*) 2 : 1 Σu Σu + (σ) 2 (σ*) 2 : 1 Σg + (σ) 1 (nb) 2 (σ*) 1 : 1 Σg Σg + (σ) 2 (nb) 1 (σ*) 1 : 1 Σu Σu + (lowest energy) (σ) 2 (nb) 2 : 1 Σg + Total: 4( 1 Σg + ) + ( 3 Σg + ) + 2( 1 Σu + ) + 2( 3 Σu + ) 4
5 3. Indicate the lowest energy spin allowed transition from the ground state to an excited state for [H3] -. Indicate the lowest energy spin forbidden transition from the ground state to an excited state for [H3] -. Spin allowed: ((σ) 2 (nb) 2 ) 1 Σg + ((σ) 1 (nb) 2 (σ*) 1 ) 1 Σu + Spin forbidden: ((σ) 2 (nb) 2 ) 1 Σg + ((σ) 1 (nb) 2 (σ*) 1 ) 3 Σu + Problem 3 (1 point) The electronic spectroscopy of metallocenes has been studied extensively. For this problem, you will analyze, in part, the electronic spectrum of ferrocene and its one electron analogue, ferricenium. We ve already derived an MO diagram for ferrocene in lecture, however that was in the D5h point group. a) Provide the MO diagram of ferrocene in D5d point group. Metal-ligand interactions do not change, just the Mulliken symbol of the MOs. Occupy the MO with electrons and provide the lowest energy electronic configuration. What is the term symbol for this electronic state? Lowest energy configuration: (1e2g) 4 (2a1g) 2 Term Symbol: 1 A1g 5
6 b) What are the two lowest d-d electron configuration transitions expected, based on your MO diagram? What are the term symbols of these excited states? (Assume symmetry of (1e2g) 3 =(1e2g) 1 ) (1e2g) 4 (2a1g) 2 (1e2g) 4 (2a1g) 1 (2e1g) 1 : 1 E1g, 3 E1g (1e2g) 4 (2a1g) 2 (1e2g) 3 (2a1g) 2 (2e1g) 1 : 1 E1g, 3 E1g, 1 E2g, 3 E2g c) Based on selection rules, what are the highest intensity d-d transitions you expect to observe for ferrocene? 1 A1g 1 E1g [(1e2g) 4 (2a1g) 1 (2e1g) 1 ] 1 A1g 1 E1g [(1e2g) 3 (2a1g) 2 (2e1g) 1 ] 1 A1g 1 E2g [(1e2g) 3 (2a1g) 2 (2e1g) 1 ] The ferricenium cation is considered to have a (1e2g) 3 (2a1g) 2 ground state. d) What is the term symbol for the ground state configuration? 2 E2g 6
7 e) Give the term symbols for the excited states of: (1e2g) 3 (2a1g) 2 (1e2g) 4 (2a1g) 1 d-d transition, LMCT from 1e1u MO to 1e2g. (1e2g) 3 (2a1g) 2 (1e2g) 4 (2a1g) 1 d-d transition: 2 A1g LMCT: 2 E1u f) Based on the absorption spectrum of ferricenium above, which bands do you attribute to d-d transitions, and which do you attribute to charge transfer? Bands I-V consistent with d-d transitions (ε<10 3 [d-d transitions inherently orbitally forbidden), lower energy transitions) Bands VI-VIII consistent with LMCT (ε>10 3, higher energy transitions) 7
8 Problem 4 (3 Points) Consider the following electronic spectrum of [PtCl6] 2-. For this question, consider only the spectrum collected at 77 K (dashed line). Vertical scales are different for the left and right sides (in M -1 cm -1 ). [PtCl 6] 2- Solid line: 300 K Dashed line: 77 K a. Based on intensity, assign the three features as spin forbidden, spin allowed orbitally forbidden, or fully allowed. What is the origin of these transitions? (d-d, MLCT, LMCT) 26,400 cm -1 : spin allowed-orbitally forbidden (d-d) 28,300 cm -1 : spin allowed-orbitally forbidden (d-d) 36,900 cm -1 : fully allowed (LMCT) b. Determine the Pt oxidation state and d-electron count of this ion. List the first four (if applicable) spin-allowed d-d transitions that you expect for this ion in the correct field strength limit (using the appropriate Tanabe-Sugano diagram). Pt 4+, d 6, five d-d transitions (low spin). 1 A1 1 T1 1 A1 1 T2 1 A1 1 E 1 A1 1 T2 c. What is the lowest-energy d-d transition (represented in state symbols) that you might see if the spin-selection rule is ignored? 1 A1 3 T1. d. To explain the high intensity band do the following. Consider that the highest occupied ligandbased orbitals are of T1u and T2g symmetry. Determine the electron configuration for the lowest energy transition that is orbitally allowed. Identify the ground and excited states for this transition. 8
9 Electron configuration: (t1u) 6 (t2g) 6 (eg) 0 (t1u) 5 (t2g) 6 (eg) 1 Ground state: 1 A1g Possible excited states: Eg x T1u = T1u + T2u (both singlet and triplet) Spin-allowed: 1 T1u + 1 T2u e. The spectrum of [PtBr6] 2- is shown below. Propose an explanation for the energy difference compared to [PtCl6] 2- of the highest intensity bands based on the properties of the ligands. Cl - (1.36 V reduction potential for Cl2) is less reducing than Br - (1.07 V reduction potential for Br2). Chlorine s high-energy p-orbitals are lower energy than bromine s high-energy p-orbitals, so the LMCT band is higher in energy (Pt d-based orbitals roughly the same in energy). [PtBr 6] 2- Solid line: 300 K Dashed line: 77 K f. Using the appropriate Tanabe-Sugano diagram, list the first four (if applicable) spin allowed d- d transitions for the following two ions: i. [Mn(CN)6] 3- ii. [MnF6] 3-3 T1 3 E 5 E 5 T2 3 T1 3 T1 3 T1 3 T2 3 T1 3 A1 9
10 g. Consider octahedral [Cu(H2O)6] 2+. Draw the d-orbital splitting diagram, label with Mulliken symbols, and populate with electrons. How many spin-allowed d-d transitions do you expect for this ion? One e g t 2g h. Three features attributable to d-d transitions can be resolved in the electronic spectrum for [Cu(H2O)6] 2+. Propose a structural distortion that lowers the symmetry of this ion, and assign the new point group. Elongation along the z axis. D4h. i. Draw the new d-orbital splitting diagram corresponding to this new structure (consider aquo ligands as σ-donors and very weak π-donors). b 1g, x2 -y 2 a 1g, z2 b 2g, xy e g, xz, yz 10
11 Problem 5 (3 points) Consider the vanadyl ion [VO(H2O)5] 2+ a) Assign the point group of this ion and the oxidation state and d-electron count of vanadium. C4v, V IV, d 1 b) Draw the complete d-orbital splitting diagram from the following basis set: Five d-orbitals on vanadium. Three p orbitals on the oxo ligand. A single σ-donor orbital for each aquo ligand c) Populate the diagram with electrons. Assign the ground state symbol of this ion. Assign the V- oxo bond order. Ground state: 2 B2 V-O BO: 3 11
12 d) The four features observed in the electronic spectra for [VO(H2O)5] 2+ are listed in the table below. They correspond to the two lowest energy d-d transitions and the two lowest energy ligand to metal charge transfers. The charge transfer bands, which are also the most intense, correspond to the highest energy transitions observed. Fill in the chart corresponding to the following information. i. Assign the electron configuration (using Mulliken symbols) corresponding to the excited states for these transitions. Assume that charge transfer transitions involving ligands only take place with the oxo ligand. ii. Assign the symbols for the excited states. iii. Indicate the transitions that are orbitally allowed with z and (x,y) polarized light, respectively. υ (cm -1 ) i. Excited state configuration ii. Excited state symbol iii. Orbitally allowed? z iii. Orbitally allowed? (x,y) 1. 13,060..(1b2) 0 (3e) 1 2 E N Y 2. 16,000 (1b2) 0 (2b1) 1 2 B1 N N 3. 41,700 (2e) 3 (1b2) 2 2 E N Y 4. 50,000 (2e) 3 (1b2) 1 (3e) 1 * 2 B1** Y N *Did not go through in lecture how to assign state symbols to this configuration. The transition going to the (1a1) 1 (1b2) 2 excited state configuration was accepted. However, based on the MO diagram, this transition will be much higher in energy and is actually orbitally forbidden (so will not be an intense band in the spectrum as this band is described to be) ** Possible Mulliken symbols would be (E)(B2)(E) = B2 + B1 + A1 + A2 with multiplicities 4, 3, 2. Transition to 2 B1 will be spin- and orbitally allowed. 12
13 13
14 14
15 15
16 16
17 17
18 18
19 19
b) For this ground state, obtain all possible J values and order them from lowest to highest in energy.
 Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
indicating the configuration they correspond to and predict their relative energy.
 Problem 1 (1 point) Three center four electron (3c/4e) bonds were introduced in class. John F. Berry (Dalton Trans. 2012, 41, 700-713) discusses the effect of the larger density of states for the 3c/4e
Problem 1 (1 point) Three center four electron (3c/4e) bonds were introduced in class. John F. Berry (Dalton Trans. 2012, 41, 700-713) discusses the effect of the larger density of states for the 3c/4e
2018 Ch112 problem set 6 Due: Thursday, Dec. 6th. Problem 1 (2 points)
 Problem 1 (2 points) a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions (υ1 and
Problem 1 (2 points) a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions (υ1 and
In the fourth problem set, you derived the MO diagrams for two complexes containing Cr-Cr bonds:
 Problem 1 (2 points) Part 1 a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions
Problem 1 (2 points) Part 1 a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions
Electronic Selection Rules (II)
 Term Symbols Electronic Selection Rules (II) IMPORTANT now we are finally ready to clearly define our electronic states! microstates for a particular atomic configuration are grouped into what are called
Term Symbols Electronic Selection Rules (II) IMPORTANT now we are finally ready to clearly define our electronic states! microstates for a particular atomic configuration are grouped into what are called
Mo 2+, Mo 2+, Cr electrons. Mo-Mo quadruple bond.
 Problem 1 (2 points) 1. Consider the MoMoCr heterotrimetallic complex shown below (Berry, et. al. Inorganica Chimica Acta 2015, p. 241). Metal-metal bonds are not drawn. The ligand framework distorts this
Problem 1 (2 points) 1. Consider the MoMoCr heterotrimetallic complex shown below (Berry, et. al. Inorganica Chimica Acta 2015, p. 241). Metal-metal bonds are not drawn. The ligand framework distorts this
Electronic Spectra of Coordination Compounds
 Electronic Spectra of Coordination Compounds Microstates and free-ion terms for electron configurations Identify the lowest-energy term Electronic Spectra of Coordination Compounds Identify the lowest-energy
Electronic Spectra of Coordination Compounds Microstates and free-ion terms for electron configurations Identify the lowest-energy term Electronic Spectra of Coordination Compounds Identify the lowest-energy
6.2. Introduction to Spectroscopic states and term symbols
 Chemistry 3820 Lecture Notes Dr. M. Gerken Page62 6.2. Introduction to Spectroscopic states and term symbols From the number of absorption bands we have already seen that usually more d-d transitions are
Chemistry 3820 Lecture Notes Dr. M. Gerken Page62 6.2. Introduction to Spectroscopic states and term symbols From the number of absorption bands we have already seen that usually more d-d transitions are
Electronic Spectra of Complexes
 Electronic Spectra of Complexes Interpret electronic spectra of coordination compounds Correlate with bonding Orbital filling and electronic transitions Electron-electron repulsion Application of MO theory
Electronic Spectra of Complexes Interpret electronic spectra of coordination compounds Correlate with bonding Orbital filling and electronic transitions Electron-electron repulsion Application of MO theory
11-1 Absorption of Light Quantum Numbers of Multielectron Atoms Electronic Spectra of Coordination Compounds
 Chapter 11 Coordination Chemistry III: Electronic Spectra 11-1 Absorption of Light 11-2 Quantum Numbers of Multielectron Atoms 11-3 Electronic Spectra of Coordination Compounds Chapter 11 Coordination
Chapter 11 Coordination Chemistry III: Electronic Spectra 11-1 Absorption of Light 11-2 Quantum Numbers of Multielectron Atoms 11-3 Electronic Spectra of Coordination Compounds Chapter 11 Coordination
Inorganic Chemistry with Doc M. Fall Semester, 2011 Day 19. Transition Metals Complexes IV: Spectroscopy
 Inorganic Chemistry with Doc M. Fall Semester, 011 Day 19. Transition Metals Complexes IV: Spectroscopy Name(s): lement: Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields. Term symbols
Inorganic Chemistry with Doc M. Fall Semester, 011 Day 19. Transition Metals Complexes IV: Spectroscopy Name(s): lement: Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields. Term symbols
Perhaps the most striking aspect of many coordination compounds of transition metals is that they have vivid colors. The UV-vis spectra of
 1 Perhaps the most striking aspect of many coordination compounds of transition metals is that they have vivid colors. The UV-vis spectra of coordination compounds of transition metals involve transitions
1 Perhaps the most striking aspect of many coordination compounds of transition metals is that they have vivid colors. The UV-vis spectra of coordination compounds of transition metals involve transitions
Ligand Field Theory Notes
 Ligand Field Theory Notes Read: Hughbanks, Antisymmetry (Handout). Carter, Molecular Symmetry..., Sections 7.4-6. Cotton, Chemical Applications..., Chapter 9. Harris & Bertolucci, Symmetry and Spectroscopy...,
Ligand Field Theory Notes Read: Hughbanks, Antisymmetry (Handout). Carter, Molecular Symmetry..., Sections 7.4-6. Cotton, Chemical Applications..., Chapter 9. Harris & Bertolucci, Symmetry and Spectroscopy...,
How to identify types of transition in experimental spectra
 17 18 19 How to identify types of transition in experimental spectra 1. intensity 2. Band width 3. polarization Intensities are governed by how well the selection rules can be applied to the molecule under
17 18 19 How to identify types of transition in experimental spectra 1. intensity 2. Band width 3. polarization Intensities are governed by how well the selection rules can be applied to the molecule under
Chem 673, Problem Sets 4 & 5 Due Tuesday, December 3, Problems from Carter: Chapter 6: 6.1, 6.3, 6.7, 6.9 Chapter 7: 7.2a,b,e,g,i,j, 7.
 Chem 673, Problem Sets 4 & 5 Due Tuesday, December 3, 2013 Problems from Carter: Chapter 6: 6.1, 6.3, 6.7, 6.9 Chapter 7: 7.2a,b,e,g,i,j, 7.6, (1) Use the angular overlap table given on the back page of
Chem 673, Problem Sets 4 & 5 Due Tuesday, December 3, 2013 Problems from Carter: Chapter 6: 6.1, 6.3, 6.7, 6.9 Chapter 7: 7.2a,b,e,g,i,j, 7.6, (1) Use the angular overlap table given on the back page of
Crystal Field Theory History
 Crystal Field Theory History 1929 Hans Bethe - Crystal Field Theory (CFT) Developed to interpret color, spectra, magnetism in crystals 1932 J. H. Van Vleck - CFT of Transition Metal Complexes Champions
Crystal Field Theory History 1929 Hans Bethe - Crystal Field Theory (CFT) Developed to interpret color, spectra, magnetism in crystals 1932 J. H. Van Vleck - CFT of Transition Metal Complexes Champions
Chem 673, Problem Set 5 Due Thursday, December 1, 2005
 otton, Problem 9.3 (assume D 4h symmetry) Additional Problems: hem 673, Problem Set 5 Due Thursday, December 1, 2005 (1) Infrared and Raman spectra of Benzene (a) Determine the symmetries (irreducible
otton, Problem 9.3 (assume D 4h symmetry) Additional Problems: hem 673, Problem Set 5 Due Thursday, December 1, 2005 (1) Infrared and Raman spectra of Benzene (a) Determine the symmetries (irreducible
Colors of Co(III) solutions. Electronic-Vibrational Coupling. Vibronic Coupling
 Colors of Co(III) solutions Electronic-Vibrational Coupling Vibronic Coupling Because they have g g character, the d-d transitions of complees of the transition metals are forbidden (LaPorte forbidden).
Colors of Co(III) solutions Electronic-Vibrational Coupling Vibronic Coupling Because they have g g character, the d-d transitions of complees of the transition metals are forbidden (LaPorte forbidden).
Chem 673, Problem Set 5 Due Thursday, November 29, 2007
 Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy
 Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields 2. Term symbols and the method of microstates
Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields 2. Term symbols and the method of microstates
LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES
 SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
LECTURE 2 DEGENERACY AND DESCENT IN SYMMETRY: LIGAND FIELD SPLITTINGS AND RELATED MATTERS
 SYMMETRY II. J. M. GOICOECHEA. LECTURE 2. 1 LECTURE 2 DEGENERACY AND DESCENT IN SYMMETRY: LIGAND FIELD SPLITTINGS AND RELATED MATTERS 2.1 Degeneracy When dealing with non-degenerate symmetry adapted wavefunctions
SYMMETRY II. J. M. GOICOECHEA. LECTURE 2. 1 LECTURE 2 DEGENERACY AND DESCENT IN SYMMETRY: LIGAND FIELD SPLITTINGS AND RELATED MATTERS 2.1 Degeneracy When dealing with non-degenerate symmetry adapted wavefunctions
Transition Metal Complexes Electronic Spectra 2
 Transition Metal Complexes Electronic Spectra 2 Electronic Spectra of Transition Metal Complexes Cr[(NH 3 ) 6 ] 3+ d 3 complex Molecular Term Symbols Quartet states Doublet state Different Ways of Transitions
Transition Metal Complexes Electronic Spectra 2 Electronic Spectra of Transition Metal Complexes Cr[(NH 3 ) 6 ] 3+ d 3 complex Molecular Term Symbols Quartet states Doublet state Different Ways of Transitions
Absorption Spectra. ! Ti(H 2 O) 6 3+ appears purple (red + blue) because it absorbs green light at ~500 nm = ~20,000 cm 1.
 Absorption Spectra! Colors of transition metal complexes result from absorption of a small portion of the visible spectrum with transmission of the unabsorbed frequencies. Visible Spectra of [M(H 2 O)
Absorption Spectra! Colors of transition metal complexes result from absorption of a small portion of the visible spectrum with transmission of the unabsorbed frequencies. Visible Spectra of [M(H 2 O)
Free-Ion Terms to Ligand-field Terms
 Free-Ion Terms to Ligand-field Terms! Orbital term symbols for free atoms and ions are identical to symbols for irreducible representations in R 3. " The irreducible representations of R 3 include all
Free-Ion Terms to Ligand-field Terms! Orbital term symbols for free atoms and ions are identical to symbols for irreducible representations in R 3. " The irreducible representations of R 3 include all
Problem 1 (4 points) D2h. C2v. Part A.
 Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
5.04, Principles of Inorganic Chemistry II MIT Department of Chemistry Lecture 29: Weak and Strong Field Approximations
 5.4, Principles of Inorganic Chemistry II MIT Department of Chemistry ecture 9: Weak and Strong Field Approximations Weak Field In the weak field, the e - energies arreater than the e - energies, i.e.
5.4, Principles of Inorganic Chemistry II MIT Department of Chemistry ecture 9: Weak and Strong Field Approximations Weak Field In the weak field, the e - energies arreater than the e - energies, i.e.
L L Ch112 Problem Set 3 Due: Thursday, October 22 before class. Problem 1 (3 points)
 Problem 1 (3 points) Part A. In problem set 2, the π-system of bicyclo[2.2.2]octa-2,5,7-triene was analyzed. 1. Starting from the MO diagram of the π-system of barrelene, show how the energy of each molecular
Problem 1 (3 points) Part A. In problem set 2, the π-system of bicyclo[2.2.2]octa-2,5,7-triene was analyzed. 1. Starting from the MO diagram of the π-system of barrelene, show how the energy of each molecular
Coordination Chemistry: Bonding Theories. Molecular Orbital Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Assignment 2 Due Thursday, February 25, (1) The free-ion terms are split in an octahedral field in the manner given in this table.
 Assignment Due Thursday, February 5, 010 (1) The free-ion terms are split in an octahedral field in the manner given in this table. Splitting of the Free-Ion terms of d n configurations in an Octahedral
Assignment Due Thursday, February 5, 010 (1) The free-ion terms are split in an octahedral field in the manner given in this table. Splitting of the Free-Ion terms of d n configurations in an Octahedral
1) For molecules A and B, list the symmetry operations and point groups.
 Problem 1 (2 points) The MO diagrams of complicated molecules can be constructed from the interactions of molecular fragments. The point groups of isolated fragments are often of higher symmetry that the
Problem 1 (2 points) The MO diagrams of complicated molecules can be constructed from the interactions of molecular fragments. The point groups of isolated fragments are often of higher symmetry that the
Chem 634, Assignment 2 Due Tuesday, February 27, 2018
 Chem 634, Assignment Due Tuesday, February 7, 018 The following two questions were given on the January 018 cumulative eam. They are not assigned here, but you should be able to fully answer them for study
Chem 634, Assignment Due Tuesday, February 7, 018 The following two questions were given on the January 018 cumulative eam. They are not assigned here, but you should be able to fully answer them for study
Chapter 21. d-block metal chemistry: coordination complexes
 Inorganic Chemistry B Chapter 21 d-block metal chemistry: coordination complexes Dr. Said El-Kurdi 1 21.1 Introduction In this chapter, we discuss complexes of the d-block metals and we consider bonding
Inorganic Chemistry B Chapter 21 d-block metal chemistry: coordination complexes Dr. Said El-Kurdi 1 21.1 Introduction In this chapter, we discuss complexes of the d-block metals and we consider bonding
Chapter 21 d-block metal chemistry: coordination complexes
 Chapter 21 d-block metal chemistry: coordination complexes Bonding: valence bond, crystal field theory, MO Spectrochemical series Crystal field stabilization energy (CFSE) Electronic Spectra Magnetic Properties
Chapter 21 d-block metal chemistry: coordination complexes Bonding: valence bond, crystal field theory, MO Spectrochemical series Crystal field stabilization energy (CFSE) Electronic Spectra Magnetic Properties
Chem 673, Problem Set 5 Due Tuesday, December 2, 2008
 Chem 673, Problem Set 5 Due Tuesday, December 2, 2008 (1) (a) Trigonal bipyramidal (tbp) coordination is fairly common. Calculate the group overlaps of the appropriate SALCs for a tbp with the 5 d-orbitals
Chem 673, Problem Set 5 Due Tuesday, December 2, 2008 (1) (a) Trigonal bipyramidal (tbp) coordination is fairly common. Calculate the group overlaps of the appropriate SALCs for a tbp with the 5 d-orbitals
Inorganic Chemistry with Doc M. Day 18. Transition Metals Complexes IV: Ligand Field Theory continued
 Inorganic Chemistry with Doc M. Day 18. Transition Metals Complexes IV: Ligand Field Theory continued Topics: 1. The three scenarios 2. Scenario 3: π-back bonding 1. The three scenarios for the MO energy
Inorganic Chemistry with Doc M. Day 18. Transition Metals Complexes IV: Ligand Field Theory continued Topics: 1. The three scenarios 2. Scenario 3: π-back bonding 1. The three scenarios for the MO energy
Coordination Chemistry: Bonding Theories. Crystal Field Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry. Zachary Chin, Alex Li, Alex Liu
 A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
Chapter 6 Answers to Problems
 Chapter 6 Answers to Problems 6.1 (a) NH 3 C3v E 2C3 3 v 4 1 2 3 0 1 12 0 2 3n = 3A 1 A 2 4E trans = A 1 E rot = A 2 E = 2A 2E = 4 frequencies 3n-6 1 Infrared 4 (2A 1 2E) Raman 4 (2A 1 2E) Polarized 2
Chapter 6 Answers to Problems 6.1 (a) NH 3 C3v E 2C3 3 v 4 1 2 3 0 1 12 0 2 3n = 3A 1 A 2 4E trans = A 1 E rot = A 2 E = 2A 2E = 4 frequencies 3n-6 1 Infrared 4 (2A 1 2E) Raman 4 (2A 1 2E) Polarized 2
ECEN 5005 Crystals, Nanocrystals and Device Applications Class 20 Group Theory For Crystals
 ECEN 5005 Crystals, Nanocrystals and Device Applications Class 20 Group Theory For Crystals Laporte Selection Rule Polarization Dependence Spin Selection Rule 1 Laporte Selection Rule We first apply this
ECEN 5005 Crystals, Nanocrystals and Device Applications Class 20 Group Theory For Crystals Laporte Selection Rule Polarization Dependence Spin Selection Rule 1 Laporte Selection Rule We first apply this
RDCH 702 Lecture 4: Orbitals and energetics
 RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
right (A, B, and C Example A corresponds to the structure reported by the Chirik group).
 Problem 1 (3 points) In 2004, a bimetallic Zr compound exhibiting side-on 2 binding was reported by Chirik and coworkers (ature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Problem 1 (3 points) In 2004, a bimetallic Zr compound exhibiting side-on 2 binding was reported by Chirik and coworkers (ature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Chemistry 120A 2nd Midterm. 1. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (1-electron):
 April 6th, 24 Chemistry 2A 2nd Midterm. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (-electron): E n = m e Z 2 e 4 /2 2 n 2 = E Z 2 /n 2, n =, 2, 3,... where Ze is
April 6th, 24 Chemistry 2A 2nd Midterm. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (-electron): E n = m e Z 2 e 4 /2 2 n 2 = E Z 2 /n 2, n =, 2, 3,... where Ze is
Name CHM 4610/5620 Fall 2016 December 15 FINAL EXAMINATION SOLUTIONS
 Name CHM 4610/5620 Fall 2016 December 15 FINAL EXAMINATION SOLUTIONS I. (80 points) From the literature... A. The synthesis and properties of copper(ii) complexes with ligands containing phenanthroline
Name CHM 4610/5620 Fall 2016 December 15 FINAL EXAMINATION SOLUTIONS I. (80 points) From the literature... A. The synthesis and properties of copper(ii) complexes with ligands containing phenanthroline
Electronic Spectroscopy of Transition Metal Ions (continued)
 Electronic Spectroscopy of Transition Metal Ions (continued) What about the spectroscopy! First some selection rules are found to apply: 1) Spin selection rule: S = 0 theory: transitions can only occur
Electronic Spectroscopy of Transition Metal Ions (continued) What about the spectroscopy! First some selection rules are found to apply: 1) Spin selection rule: S = 0 theory: transitions can only occur
Electronic structure / bonding in d-block complexes
 LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
CHEMISTRY. Electronic Spectra and Magnetic Properties of Transition Metal Complexes)
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 7: Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 16.
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 7: Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 16.
Construction of the C 2v character table
 Construction of the C 2v character table The character table C 2v has the following form: C 2v E C 2 σ v (xz) σ v '(yz) Α 1 1 1 1 1 z x 2, y 2, z 2 Α 2 1 1-1 -1 R z xy Β 1 1-1 1-1 x, R y xz Β 2 1-1 -1
Construction of the C 2v character table The character table C 2v has the following form: C 2v E C 2 σ v (xz) σ v '(yz) Α 1 1 1 1 1 z x 2, y 2, z 2 Α 2 1 1-1 -1 R z xy Β 1 1-1 1-1 x, R y xz Β 2 1-1 -1
Chm December 2008
 Inorganic Exam 3 Chm 451 4 December 2008 Name: Instructions. Always show your work where required for full credit. 1. (15 pts) True/False a T F Ionization energy decreases as one moves down from Li to
Inorganic Exam 3 Chm 451 4 December 2008 Name: Instructions. Always show your work where required for full credit. 1. (15 pts) True/False a T F Ionization energy decreases as one moves down from Li to
CH 612 Advanced Inorganic Chemistry Structure and Reactivity. Exam # 2 10/25/2010. Print name:
 CH 612 dvanced Inorganic Chemistry Structure and Reactivity Print name: 1. (25 points) a) Given the set of operations C 4, h determine the other operations that must be present to form a complete point
CH 612 dvanced Inorganic Chemistry Structure and Reactivity Print name: 1. (25 points) a) Given the set of operations C 4, h determine the other operations that must be present to form a complete point
Electronic Microstates & Term Symbols. Suggested reading: Shriver and Atkins, Chapter 20.3 or Douglas,
 Lecture 4 Electronic Microstates & Term Symbols Suggested reading: Shriver and Atkins, Chapter 20.3 or Douglas, 1.4-1.5 Recap from last class: Quantum Numbers Four quantum numbers: n, l, m l, and m s Or,
Lecture 4 Electronic Microstates & Term Symbols Suggested reading: Shriver and Atkins, Chapter 20.3 or Douglas, 1.4-1.5 Recap from last class: Quantum Numbers Four quantum numbers: n, l, m l, and m s Or,
Molecular Orbital Theory and Charge Transfer Excitations
 Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
Template for the d 2 Correlation Diagram
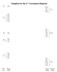 S A g Template for the d Correlation Diagram (,3)A g e g (,3)A g G T g T g A g T g 3 P 3T g T g 3T g t g e g 3T g D T g 3A g 3F 3T g 3T g A g (,3)T g t g (,3)T g Free Ion Weak Strong Extreme Making the
S A g Template for the d Correlation Diagram (,3)A g e g (,3)A g G T g T g A g T g 3 P 3T g T g 3T g t g e g 3T g D T g 3A g 3F 3T g 3T g A g (,3)T g t g (,3)T g Free Ion Weak Strong Extreme Making the
Orbitals and energetics
 Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Molecular Orbital Theory and Charge Transfer Excitations
 Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H 2 Antibonding Molecular Orbital (Orbitals interfere destructively) H 1s Orbital
PAPER No. 7: Inorganic chemistry II MODULE No. 5: Molecular Orbital Theory
 Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
Chm 363. Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra. Mr. Linck. Version 1.5 March 9, 2017
 Chm 363 Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra Mr. Linck Version 1.5 March 9, 2017 3.1 Transition Metal Bonding in Octahedral Compounds How do the metal 3d, 4s, and 4p orbitals
Chm 363 Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra Mr. Linck Version 1.5 March 9, 2017 3.1 Transition Metal Bonding in Octahedral Compounds How do the metal 3d, 4s, and 4p orbitals
Other Crystal Fields
 Other Crystal Fields! We can deduce the CFT splitting of d orbitals in virtually any ligand field by " Noting the direct product listings in the appropriate character table to determine the ways in which
Other Crystal Fields! We can deduce the CFT splitting of d orbitals in virtually any ligand field by " Noting the direct product listings in the appropriate character table to determine the ways in which
Bonding in Octahedral and Tetrahedral Metal Complexes. Predict how the d orbitals are affected by the Metal- Ligand Bonding
 Bonding in Octahedral and Tetrahedral Metal Complexes 327 Molecular Orbital Theory and Crystal Field/Ligand Field Theory Predict how the d orbitals are affected by the Metal- Ligand Bonding d z 2, d x
Bonding in Octahedral and Tetrahedral Metal Complexes 327 Molecular Orbital Theory and Crystal Field/Ligand Field Theory Predict how the d orbitals are affected by the Metal- Ligand Bonding d z 2, d x
Chemistry 324 Midterm 1 KEY Wednesday, October 19, 2011 Instructor: D. J. Berg
 Chem 324 Midterm 1 Fall 2011 Version 1 Page 1 of 9 Chemistry 324 Midterm 1 KEY Wednesday, October 19, 2011 Instructor: D. J. Berg Name: Answer all questions on the paper (use the back if necessary). There
Chem 324 Midterm 1 Fall 2011 Version 1 Page 1 of 9 Chemistry 324 Midterm 1 KEY Wednesday, October 19, 2011 Instructor: D. J. Berg Name: Answer all questions on the paper (use the back if necessary). There
light is absorbed, the complex appears green; If
 Color of Transition Metal Complexes The variety of color among transition metal complexes has long fascinated the chemists. For example, aqueous solutions of [Fe(H 2 O) 6 ] 3+ are red, [Co(H 2 O) 6 ] 2+
Color of Transition Metal Complexes The variety of color among transition metal complexes has long fascinated the chemists. For example, aqueous solutions of [Fe(H 2 O) 6 ] 3+ are red, [Co(H 2 O) 6 ] 2+
B7 Symmetry : Questions
 B7 Symmetry 009-10: Questions 1. Using the definition of a group, prove the Rearrangement Theorem, that the set of h products RS obtained for a fixed element S, when R ranges over the h elements of the
B7 Symmetry 009-10: Questions 1. Using the definition of a group, prove the Rearrangement Theorem, that the set of h products RS obtained for a fixed element S, when R ranges over the h elements of the
THE BONDING IN VANADYL ION COMPLEXES
 THE BONDING IN VANADYL ION COMPLEXES Abstract This chapter describes how the degeneracy of a d ion is split in octahedral crystal field. The energy levels of a vanadyl ion water complex have further been
THE BONDING IN VANADYL ION COMPLEXES Abstract This chapter describes how the degeneracy of a d ion is split in octahedral crystal field. The energy levels of a vanadyl ion water complex have further been
Advanced Inorganic Chemistry
 Advanced Inorganic Chemistry Orgel Diagrams Correlation of spectroscopic terms for d n configuration in O h complexes Atomic Term Splitting of the weak field d n ground state terms in an octahedral ligand
Advanced Inorganic Chemistry Orgel Diagrams Correlation of spectroscopic terms for d n configuration in O h complexes Atomic Term Splitting of the weak field d n ground state terms in an octahedral ligand
Symmetry. Chemistry 481(01) Spring 2017 Instructor: Dr. Upali Siriwardane Office: CTH 311 Phone Office Hours:
 Chemistry 481(01) Spring 2017 Instructor: Dr. Upali Siriwardane e-mail: upali@latech.edu Office: CT 311 Phone 257-4941 Office ours: M,W 8:00-9:00 & 11:00-12:00 am; Tu,Th, F 9:30-11:30 a.m. April 4, 2017:
Chemistry 481(01) Spring 2017 Instructor: Dr. Upali Siriwardane e-mail: upali@latech.edu Office: CT 311 Phone 257-4941 Office ours: M,W 8:00-9:00 & 11:00-12:00 am; Tu,Th, F 9:30-11:30 a.m. April 4, 2017:
Chapter 11 Coordination Chemistry III: Electronic Spectra
 Chapter Coordination Chemistry III: Electronic Spectra - Absorption of Light -2 Quantum Numbers of Multielectron Atoms - Electronic Spectra of Coordination Compounds Chapter Coordination Chemistry III:
Chapter Coordination Chemistry III: Electronic Spectra - Absorption of Light -2 Quantum Numbers of Multielectron Atoms - Electronic Spectra of Coordination Compounds Chapter Coordination Chemistry III:
Lecture 9 Electronic Spectroscopy
 Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
( ) dσ 1 dσ 2 + α * 2
 Chemistry 36 Dr. Jean M. Standard Problem Set Solutions. The spin up and spin down eigenfunctions for each electron in a many-electron system are normalized and orthogonal as given by the relations, α
Chemistry 36 Dr. Jean M. Standard Problem Set Solutions. The spin up and spin down eigenfunctions for each electron in a many-electron system are normalized and orthogonal as given by the relations, α
- an approach to bonding that is useful for making estimates of E of orbitals in coordination complexes
 10.4 Angular Overlap - an approach to bonding that is useful for making estimates of E of orbitals in coordination complexes - estimate the strength of interaction b/w ligand orbitals & metal d orbitals
10.4 Angular Overlap - an approach to bonding that is useful for making estimates of E of orbitals in coordination complexes - estimate the strength of interaction b/w ligand orbitals & metal d orbitals
Molecular orbitals for σbonding in T d complexes
 Molecular orbitals for σbonding in T d complexes The set of n A B σ bonds in AB n (T d n = 4) molecules are often thought of as independent entities. The concept of MO s allows us to begin with a very
Molecular orbitals for σbonding in T d complexes The set of n A B σ bonds in AB n (T d n = 4) molecules are often thought of as independent entities. The concept of MO s allows us to begin with a very
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Tables for Group Theory
 Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Molecular Orbital Theory (MOT)
 Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
PAPER No.7 : Inorganic Chemistry-II MODULE No.1 : Crystal Field Theory
 Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic Chemistry II 1, Crystal Field Theory CHE_P7_M1 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Crystal Field Theory
Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic Chemistry II 1, Crystal Field Theory CHE_P7_M1 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Crystal Field Theory
5.61 Physical Chemistry Exam III 11/29/12. MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry Physical Chemistry.
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry - 5.61 Physical Chemistry Exam III (1) PRINT your name on the cover page. (2) It is suggested that you READ THE ENTIRE EXAM before
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry Chemistry - 5.61 Physical Chemistry Exam III (1) PRINT your name on the cover page. (2) It is suggested that you READ THE ENTIRE EXAM before
Inorganic Chemistry with Doc M. Fall Semester, 2012 Day 21. Transition Metals Complexes V: Reaction Mechanisms
 Inorganic Chemistry with Doc M. Fall Semester, 2012 Day 21. Transition Metals Complexes V: Reaction Mechanisms Name(s): Element: Topics: 1. Substitution reactions: dissociative v. associative 4. Pseudorotation
Inorganic Chemistry with Doc M. Fall Semester, 2012 Day 21. Transition Metals Complexes V: Reaction Mechanisms Name(s): Element: Topics: 1. Substitution reactions: dissociative v. associative 4. Pseudorotation
Tables for Group Theory
 Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Symmetry: Translation and Rotation
 Symmetry: Translation and Rotation The sixth column of the C 2v character table indicates the symmetry species for translation along (T) and rotation about (R) the Cartesian axes. y y y C 2 F v (x) T x
Symmetry: Translation and Rotation The sixth column of the C 2v character table indicates the symmetry species for translation along (T) and rotation about (R) the Cartesian axes. y y y C 2 F v (x) T x
5.4. Electronic structure of water
 5.4. Electronic structure of water Water belongs to C 2v point group, we have discussed the corresponding character table. Here it is again: C 2v E C 2 σ v (yz) σ v (xz) A 1 1 1 1 1 A 2 1 1-1 -1 B 1 1-1
5.4. Electronic structure of water Water belongs to C 2v point group, we have discussed the corresponding character table. Here it is again: C 2v E C 2 σ v (yz) σ v (xz) A 1 1 1 1 1 A 2 1 1-1 -1 B 1 1-1
Chemistry 543--Final Exam--Keiderling May 5, pm SES
 Chemistry 543--Final Exam--Keiderling May 5,1992 -- 1-5pm -- 174 SES Please answer all questions in the answer book provided. Make sure your name is clearly indicated and that the answers are clearly numbered,
Chemistry 543--Final Exam--Keiderling May 5,1992 -- 1-5pm -- 174 SES Please answer all questions in the answer book provided. Make sure your name is clearly indicated and that the answers are clearly numbered,
2015 Ch112 problem set 4 Due: Thursday, Oct. 29 before class Remember to turn in your second paper critique with this problem set!
 Problem 1 (4 points) In 2004, a bimetallic compound exhibiting side-on 2 binding was reported b Chirik and coworkers (ature, 2004, 427, pp. 527-530). The crstal structure of this compound was obtained,
Problem 1 (4 points) In 2004, a bimetallic compound exhibiting side-on 2 binding was reported b Chirik and coworkers (ature, 2004, 427, pp. 527-530). The crstal structure of this compound was obtained,
QUANTUM MECHANICS AND ATOMIC STRUCTURE
 5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
Inorganic Chemistry with Doc M. Fall Semester, 2012 Day 9. Molecular Orbitals, Part 4. Beyond Diatomics, continued
 Inorganic Chemistry with Doc M. Fall Semester, 2012 Day 9. Molecular Orbitals, Part 4. Beyond Diatomics, continued Topics: Name(s): Element: 1. Using p-orbitals for σ-bonding: molecular orbital diagram
Inorganic Chemistry with Doc M. Fall Semester, 2012 Day 9. Molecular Orbitals, Part 4. Beyond Diatomics, continued Topics: Name(s): Element: 1. Using p-orbitals for σ-bonding: molecular orbital diagram
Ch120 - Study Guide 10
 Ch120 - Study Guide 10 Adam Griffith October 17, 2005 In this guide: Symmetry; Diatomic Term Symbols; Molecular Term Symbols Last updated October 27, 2005. 1 The Origin of m l States and Symmetry We are
Ch120 - Study Guide 10 Adam Griffith October 17, 2005 In this guide: Symmetry; Diatomic Term Symbols; Molecular Term Symbols Last updated October 27, 2005. 1 The Origin of m l States and Symmetry We are
H atom solution. 1 Introduction 2. 2 Coordinate system 2. 3 Variable separation 4
 H atom solution Contents 1 Introduction 2 2 Coordinate system 2 3 Variable separation 4 4 Wavefunction solutions 6 4.1 Solution for Φ........................... 6 4.2 Solution for Θ...........................
H atom solution Contents 1 Introduction 2 2 Coordinate system 2 3 Variable separation 4 4 Wavefunction solutions 6 4.1 Solution for Φ........................... 6 4.2 Solution for Θ...........................
Ψ t = ih Ψ t t. Time Dependent Wave Equation Quantum Mechanical Description. Hamiltonian Static/Time-dependent. Time-dependent Energy operator
 Time Dependent Wave Equation Quantum Mechanical Description Hamiltonian Static/Time-dependent Time-dependent Energy operator H 0 + H t Ψ t = ih Ψ t t The Hamiltonian and wavefunction are time-dependent
Time Dependent Wave Equation Quantum Mechanical Description Hamiltonian Static/Time-dependent Time-dependent Energy operator H 0 + H t Ψ t = ih Ψ t t The Hamiltonian and wavefunction are time-dependent
Quiz 5 R = lit-atm/mol-k 1 (25) R = J/mol-K 2 (25) 3 (25) c = X 10 8 m/s 4 (25)
 ADVANCED INORGANIC CHEMISTRY QUIZ 5 and FINAL December 18, 2012 INSTRUCTIONS: PRINT YOUR NAME > NAME. QUIZ 5 : Work 4 of 1-5 (The lowest problem will be dropped) FINAL: #6 (10 points ) Work 6 of 7 to 14
ADVANCED INORGANIC CHEMISTRY QUIZ 5 and FINAL December 18, 2012 INSTRUCTIONS: PRINT YOUR NAME > NAME. QUIZ 5 : Work 4 of 1-5 (The lowest problem will be dropped) FINAL: #6 (10 points ) Work 6 of 7 to 14
Molecular-Orbital Theory
 Prof. Dr. I. Nasser atomic and molecular physics -551 (T-11) April 18, 01 Molecular-Orbital Theory You have to explain the following statements: 1- Helium is monatomic gas. - Oxygen molecule has a permanent
Prof. Dr. I. Nasser atomic and molecular physics -551 (T-11) April 18, 01 Molecular-Orbital Theory You have to explain the following statements: 1- Helium is monatomic gas. - Oxygen molecule has a permanent
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
Chemistry 431. Lecture 14. Wave functions as a basis Diatomic molecules Polyatomic molecules Huckel theory. NC State University
 Chemistry 431 Lecture 14 Wave functions as a basis Diatomic molecules Polyatomic molecules Huckel theory NC State University Wave functions as the basis for irreducible representations The energy of the
Chemistry 431 Lecture 14 Wave functions as a basis Diatomic molecules Polyatomic molecules Huckel theory NC State University Wave functions as the basis for irreducible representations The energy of the
Magnetism in low dimensions from first principles. Atomic magnetism. Gustav Bihlmayer. Gustav Bihlmayer
 IFF 10 p. 1 Magnetism in low dimensions from first principles Atomic magnetism Gustav Bihlmayer Institut für Festkörperforschung, Quantum Theory of Materials Gustav Bihlmayer Institut für Festkörperforschung
IFF 10 p. 1 Magnetism in low dimensions from first principles Atomic magnetism Gustav Bihlmayer Institut für Festkörperforschung, Quantum Theory of Materials Gustav Bihlmayer Institut für Festkörperforschung
Nuclear Quadrupole Resonance Spectroscopy. Some examples of nuclear quadrupole moments
 Nuclear Quadrupole Resonance Spectroscopy Review nuclear quadrupole moments, Q A negative value for Q denotes a distribution of charge that is "football-shaped", i.e. a sphere elongated at the poles; a
Nuclear Quadrupole Resonance Spectroscopy Review nuclear quadrupole moments, Q A negative value for Q denotes a distribution of charge that is "football-shaped", i.e. a sphere elongated at the poles; a
d 3 r d 3 vf( r, v) = N (2) = CV C = n where n N/V is the total number of molecules per unit volume. Hence e βmv2 /2 d 3 rd 3 v (5)
 LECTURE 12 Maxwell Velocity Distribution Suppose we have a dilute gas of molecules, each with mass m. If the gas is dilute enough, we can ignore the interactions between the molecules and the energy will
LECTURE 12 Maxwell Velocity Distribution Suppose we have a dilute gas of molecules, each with mass m. If the gas is dilute enough, we can ignore the interactions between the molecules and the energy will
Name CHM 4610/5620 Fall 2017 December 14 FINAL EXAMINATION SOLUTIONS Part I, from the Literature Reports
 Name CHM 4610/5620 Fall 2017 December 14 FINAL EXAMINATION SOLUTIONS Part I, from the Literature Reports I II III IV V VI VII VIII IX X Total This exam consists of several problems. Rough point values
Name CHM 4610/5620 Fall 2017 December 14 FINAL EXAMINATION SOLUTIONS Part I, from the Literature Reports I II III IV V VI VII VIII IX X Total This exam consists of several problems. Rough point values
Molecular Symmetry. Symmetry is relevant to: spectroscopy, chirality, polarity, Group Theory, Molecular Orbitals
 Molecular Symmetry Symmetry is relevant to: spectroscopy, chirality, polarity, Group Theory, Molecular Orbitals - A molecule has a symmetry element if it is unchanged by a particular symmetry operation
Molecular Symmetry Symmetry is relevant to: spectroscopy, chirality, polarity, Group Theory, Molecular Orbitals - A molecule has a symmetry element if it is unchanged by a particular symmetry operation
PAPER :8, PHYSICAL SPECTROSCOPY MODULE: 29, MOLECULAR TERM SYMBOLS AND SELECTION RULES FOR DIATOMIC MOLECULES
 Subject Chemistry Paper No and Title Module No and Title Module Tag 8: Physical Spectroscopy 29: Molecular Term Symbols and Selection Rules for Diatomic Molecules. CHE_P8_M29 TLE OF CONTENTS 1. Learning
Subject Chemistry Paper No and Title Module No and Title Module Tag 8: Physical Spectroscopy 29: Molecular Term Symbols and Selection Rules for Diatomic Molecules. CHE_P8_M29 TLE OF CONTENTS 1. Learning
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics
 Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
d 2 Correlation Diagram
 d 2 Correlation Diagram 1 S 1A 1g 1A 1g 1E g e g 2 3A 2g 1E g 1 G 1T 1g 1T 2g 1A 1g 1T 1g 3 P 3T 1g 1T 2g 3T 1g t 2g 1e g 1 3T 2g 1 D 1E g 1T 2g 3A 2g 3 F 3T 2g 3T 1g 1A 1g 1E g 1T 2g t 2g 2 3T 1g Free
d 2 Correlation Diagram 1 S 1A 1g 1A 1g 1E g e g 2 3A 2g 1E g 1 G 1T 1g 1T 2g 1A 1g 1T 1g 3 P 3T 1g 1T 2g 3T 1g t 2g 1e g 1 3T 2g 1 D 1E g 1T 2g 3A 2g 3 F 3T 2g 3T 1g 1A 1g 1E g 1T 2g t 2g 2 3T 1g Free
wbt Λ = 0, 1, 2, 3, Eq. (7.63)
 7.2.2 Classification of Electronic States For all diatomic molecules the coupling approximation which best describes electronic states is analogous to the Russell- Saunders approximation in atoms The orbital
7.2.2 Classification of Electronic States For all diatomic molecules the coupling approximation which best describes electronic states is analogous to the Russell- Saunders approximation in atoms The orbital
Electronic structure Crystal-field theory Ligand-field theory. Electronic-spectra electronic spectra of atoms
 Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
