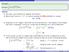
1) [16 points] The light intensity of a particular light source (not an ideal blackbody) is given by the equation. p = - iħ d (2.
|
|
|
- Valentine Brooks
- 5 years ago
- Views:
Transcription
1 CHM 3411 First hour exam February 8, 2019 There are 5 problems on the exam. Do all of the problems. Show your work. 1) [16 points] The light intensity of a particular light source (not an ideal blackbody) is given by the equation M(,T) d = A exp( - B/ T) d (1.1) where A and B are constants. a) Find an expression for max, the wavelength of maximum intensity, for a light source whose intensity is given by eq 1.1 b) Find an expression for M(,T) that is correct for long wavelengths ( ). 2) [18 points] Consider the momentum operator p = - iħ d (2.1) dx For each of the following functions determine whether the function is or is not an eigenfunction of p. Clearly indicate your answer and give work to show your answer is correct. For cases where the function is an eigenfunction, give the corresponding eigenvalue. a) f(x) = 1 sin(4x) (2.2) (2) 1/2 b) f(x) = 1 exp(2ix) (2.3) c) f(x) = 2 [ sin(3 x) + i cos(3 x) ] (2.4) 3) [12 points] What is the value for the commutator [x 2, p], where x is the operator for position and p = - iħ d/dx is the operator for momentum in one dimension. 4) [36 points] The n = 4 solution to the particle in a one dimensional box TISE is (x) = (2/) 1/2 sin(4 x/) 0 x (4.1) (x) = 0 x < 0 or x > box. a) Find <x> and <x 2 >, the expectation values for x and x 2, for a particle in the n = 4 state of the particle in a b) Is the n = 4 solution to the particle in a box orthogonal to the function f(x) = A (x 2 x) 0 x (4.2) f(x) = 0 x < 0 or x > c) Find the value for the constant A in eq 4.2 that makes f(x) a normalized function.
2 5) [18 points] The value for the vibrational constant for the molecule NaF (sodium fluoride) is = 536. cm -1. a) What is the energy of the v = 2 state of the NaF molecule? Assume the molecule is correctly described as a harmonic oscillator. Give your answer in units of cm -1 and J/molecule. b) What is the value for k, the force constant, for the chemical bond in NaF? Give your answer in units of N/m (note that 1 N/m = 1 kg/s 2 ). The masses of the most common isotopes of Na and F (in atomic mass units) are m(na) = amu, m(f) = amu.
3 Solutions. 1) a) To find an extreme point we set d/d M(,T) = 0 But d = A exp(- B/ T) = - 3 A exp(-b/ T) + A exp(-b/ T) B = 0 d 4 2 T If we multiply both sides by 4 exp(b/ T) we get A B = 0 T 3 = B or max = B T 3T b) M(,T) = A exp(-b/ T) The power series expansion of the exponential is exp(- B/ T) = 1 B + T If we substitute into the expression for M(,T), we get M(,T) = A [ 1 B/ T + ] As, all the terms in the brackets become negligibly small except the leading term, and so lim ( ) M(,T) = A 2) a) iħ d/dx [ (1/2) 1/2 sin(4x) ] = - iħ (4) [ (1/2) 1/2 cos(4x) ], so NO, not an eigenfunction b) iħ d/dx [ (1/ ) exp(2ix) ] = - iħ (2i) [ (1/ ) exp(2ix) ] = 2ħ [ (1/ ) exp(2ix) ] so YES, an eigenfunction, eigenvalue is 2ħ c) iħ d/dx { 2 [ sin(3 x) + i cos(3 x) ] } = - iħ{ (2 ) [ (3 ) cos(3 x) (3 i) sin(3 x) ] } If we remove the common factor of 3 from inside the square brackets, we get = - iħ (3 ) { (2 ) [ cos(3 x) i sin(3 x) ] } If we move i to inside the square brackets, then = - ħ (3 ) { (2 ) [i cos(3 x) i 2 sin(3 x) ] } = - 3 ħ { 2 [ sin(3 x) + i cos(3 x) ] } so YES, eigenfunction, eigenvalue is 3 ħ 3) The easiest way to find the value for a commutator is to let it operate on some arbitrary function f(x) So [x 2,p] f(x) = (x 2 p p x 2 ) f(x) = x 2 (- iħ d/dx) f(x) - (- iħ d/dx) x 2 f(x) Therefore [x 2,p] = 2iħx = - iħ x 2 (df(x)/dx) + iħ x 2 (df(x)/dx) + iħ (2x) f(x) = 2iħx f(x)
4 4) a) <x> = 0 [(2/) 1/2 sin(4 x/)] x [(2/) 1/2 sin(4 x/)] dx The general form for this integral is = (2/) 0 x [sin(4 x/)] 2 dx x [sin(ax)] 2 dx = x 2 - x sin(2ax) - cos(2ax) 4 4a 8a 2 If we let a = 4 /, then we get = (2/) { (x 2 /4) (/16 ) x sin(8 x/) ( 2 /128 2 ) cos(8 x/} 0 Since sin(0) = sin(8 ) = 0, and cos(0) = cos(8 ) = 1, this gives us The general form for this integral is = (2/) ( 2 /4) = /2 <x 2 > = 0 [(2/) 1/2 sin(4 x/)] x 2 [(2/) 1/2 sin(4 x/)] dx = (2/) 0 x 2 [sin(4 x/)] 2 dx x 2 [sin(ax)] 2 dx = (x 3 /6) - [ (x 2 /4a) (1/8a 3 ) ] sin(2ax) - (x/4a 2 ) cos(2ax) ] If we let a = 4 /, then we get = (2/) { (x 3 /6) [ (x 2 /16 ) ( 3 /512 ) ] sin(8 x/) (x 2 /64 2 ) cos(8 x) } 0 Since sin(0) = sin(8 ) = 0, and cos(0) = cos(8 ) = 1, this gives us = (2/) { ( 3 /6) ( 3 /64 2 ) } = 2 { (1/3) (1/32 2 ) } b) To check for orthonormality we need to find the value for the integral 0 [(2/) 1/2 sin(4 x/)] [ A (x 2 x) ] dx = A(2/) 1/2 0 sin(4 x/)] [ (x 2 x) ] dx = A(2/) 1/2 { 0 x 2 sin(4 x/) dx 0 x sin(4 x/) dx } There are two integrals we need here x 2 sin(ax) dx = (2x/a 2 ) sin(ax) (x 2 /a) cos(ax) + (2/a 3 ) cos(ax) x sin(ax) dx = (1/a 2 ) sin(ax) (x/a) cos(ax) If we let a = 4 /, then we get = A(2/) 1/2 { (x 2 /8 2 ) sin(4 x/) (x 2 /4 ) cos(4 x/) + ( 3 /32 ) cos(4 x/) ] - [ ( 2 /16 2 sin(4 x/) (x/4 ) cos(4 x/) ] } 0
5 Since sin(0) = sin(4 ) = 0, and cos(0) = cos(4 ) = 1, this gives us = A(2/) 1/2 { - ( 3 /4 ) + ( 2 /4 ) } = 0 So the two functions are orthonormal. Note that there is an easier way of showing these two functions are orthogonal based on symmetry. c) Normalization is the condition 1 = 0 [A (x 2 x) ] [A (x 2 x) ] dx = A 2 0 [ x 4 2x 3 + x 2 2 ] dx = A 2 [ (x 5 /5) (2x 4 /4) + (x 3 /3) ] 0 = A 2 5 [ (1/5) (1/2) + (1/3) ] = A Solving for A gives A = (30/ 5 ) 1/2 5) a) E(in cm -1 ) = (v + ½) = (2.5) (536. cm -1 ) = cm -1 Using the conversion between cm -1 and J, we get E(in J) = (1340. cm -1 ) (1.986 x J/cm -1 ) = x J/molecule b) = (1/2 c) (k/ ) 1/2 If we square both sides of this equation and solve for k, we get k = c 2 = (536. cm -1 ) (100 cm/m) = m -1 = m N am F = ( amu)( amu) = amu ( x kg/amu) = x kg (m Na + m F) ( ) amu So k = (4 2 ) ( m -1 ) 2 (2.998 x 10 8 m/s) 2 (1.728 x kg) = 176. kg/s 2 = 176. N/m
6
Chemistry 3502/4502. Exam I. February 6, ) Circle the correct answer on multiple-choice problems.
 D Chemistry 3502/4502 Exam I February 6, 2006 1) Circle the correct answer on multiple-choice problems. 2) There is one correct answer to every multiple-choice problem. There is no partial credit. On the
D Chemistry 3502/4502 Exam I February 6, 2006 1) Circle the correct answer on multiple-choice problems. 2) There is one correct answer to every multiple-choice problem. There is no partial credit. On the
Chemistry 3502/4502. Exam I. February 6, ) Circle the correct answer on multiple-choice problems.
 A Chemistry 3502/4502 Exam I February 6, 2006 1) Circle the correct answer on multiple-choice problems. 2) There is one correct answer to every multiple-choice problem. There is no partial credit. On the
A Chemistry 3502/4502 Exam I February 6, 2006 1) Circle the correct answer on multiple-choice problems. 2) There is one correct answer to every multiple-choice problem. There is no partial credit. On the
Physical Chemistry II Exam 2 Solutions
 Chemistry 362 Spring 208 Dr Jean M Standard March 9, 208 Name KEY Physical Chemistry II Exam 2 Solutions ) (4 points) The harmonic vibrational frequency (in wavenumbers) of LiH is 4057 cm Based upon this
Chemistry 362 Spring 208 Dr Jean M Standard March 9, 208 Name KEY Physical Chemistry II Exam 2 Solutions ) (4 points) The harmonic vibrational frequency (in wavenumbers) of LiH is 4057 cm Based upon this
Physics 250 Green s functions for ordinary differential equations
 Physics 25 Green s functions for ordinary differential equations Peter Young November 25, 27 Homogeneous Equations We have already discussed second order linear homogeneous differential equations, which
Physics 25 Green s functions for ordinary differential equations Peter Young November 25, 27 Homogeneous Equations We have already discussed second order linear homogeneous differential equations, which
Last Name or Student ID
 12/05/18, Chem433 Final Exam Last Name or Student ID 1. (2 pts) 12. (3 pts) 2. (6 pts) 13. (3 pts) 3. (3 pts) 14. (2 pts) 4. (3 pts) 15. (3 pts) 5. (4 pts) 16. (3 pts) 6. (2 pts) 17. (15 pts) 7. (9 pts)
12/05/18, Chem433 Final Exam Last Name or Student ID 1. (2 pts) 12. (3 pts) 2. (6 pts) 13. (3 pts) 3. (3 pts) 14. (2 pts) 4. (3 pts) 15. (3 pts) 5. (4 pts) 16. (3 pts) 6. (2 pts) 17. (15 pts) 7. (9 pts)
Last Name or Student ID
 12/05/18, Chem433 Final Exam answers Last Name or Student ID 1. (2 pts) 12. (3 pts) 2. (6 pts) 13. (3 pts) 3. (3 pts) 14. (2 pts) 4. (3 pts) 15. (3 pts) 5. (4 pts) 16. (3 pts) 6. (2 pts) 17. (15 pts) 7.
12/05/18, Chem433 Final Exam answers Last Name or Student ID 1. (2 pts) 12. (3 pts) 2. (6 pts) 13. (3 pts) 3. (3 pts) 14. (2 pts) 4. (3 pts) 15. (3 pts) 5. (4 pts) 16. (3 pts) 6. (2 pts) 17. (15 pts) 7.
Chem 452 Mega Practice Exam 1
 Last Name: First Name: PSU ID #: Chem 45 Mega Practice Exam 1 Cover Sheet Closed Book, Notes, and NO Calculator The exam will consist of approximately 5 similar questions worth 4 points each. This mega-exam
Last Name: First Name: PSU ID #: Chem 45 Mega Practice Exam 1 Cover Sheet Closed Book, Notes, and NO Calculator The exam will consist of approximately 5 similar questions worth 4 points each. This mega-exam
( )( s 1
 Chemistry 362 Dr Jean M Standard Homework Problem Set 6 Solutions l Calculate the reduced mass in kg for the OH radical The reduced mass for OH is m O m H m O + m H To properly calculate the reduced mass
Chemistry 362 Dr Jean M Standard Homework Problem Set 6 Solutions l Calculate the reduced mass in kg for the OH radical The reduced mass for OH is m O m H m O + m H To properly calculate the reduced mass
Spin Dynamics Basic Theory Operators. Richard Green SBD Research Group Department of Chemistry
 Spin Dynamics Basic Theory Operators Richard Green SBD Research Group Department of Chemistry Objective of this session Introduce you to operators used in quantum mechanics Achieve this by looking at:
Spin Dynamics Basic Theory Operators Richard Green SBD Research Group Department of Chemistry Objective of this session Introduce you to operators used in quantum mechanics Achieve this by looking at:
CHM320 EXAM #2 USEFUL INFORMATION
 CHM30 EXAM # USEFUL INFORMATION Constants mass of electron: m e = 9.11 10 31 kg. Rydberg constant: R H = 109737.35 cm 1 =.1798 10 18 J. speed of light: c = 3.00 10 8 m/s Planck constant: 6.66 10 34 Js
CHM30 EXAM # USEFUL INFORMATION Constants mass of electron: m e = 9.11 10 31 kg. Rydberg constant: R H = 109737.35 cm 1 =.1798 10 18 J. speed of light: c = 3.00 10 8 m/s Planck constant: 6.66 10 34 Js
Last Name or Student ID
 12/9/15, Chem433 Final Exam Last Name or Student ID 1. (2 pts) 11. (4 pts) 2. (6 pts) 12. (3 pts) 3. (2 pts) 13. (4 pts) 4. (3 pts) 14. (3 pts) 5. (5 pts) 15. (3 pts) 6. (3 pts) 16. (7 pts) 7. (12 pts)
12/9/15, Chem433 Final Exam Last Name or Student ID 1. (2 pts) 11. (4 pts) 2. (6 pts) 12. (3 pts) 3. (2 pts) 13. (4 pts) 4. (3 pts) 14. (3 pts) 5. (5 pts) 15. (3 pts) 6. (3 pts) 16. (7 pts) 7. (12 pts)
Math 113 (Calculus 2) Exam 4
 Math 3 (Calculus ) Exam 4 November 0 November, 009 Sections 0, 3 7 Name Student ID Section Instructor In some cases a series may be seen to converge or diverge for more than one reason. For such problems
Math 3 (Calculus ) Exam 4 November 0 November, 009 Sections 0, 3 7 Name Student ID Section Instructor In some cases a series may be seen to converge or diverge for more than one reason. For such problems
df(x) = h(x) dx Chemistry 4531 Mathematical Preliminaries Spring 2009 I. A Primer on Differential Equations Order of differential equation
 Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Physical Chemistry II Exam 2 Solutions
 Chemistry 362 Spring 2017 Dr Jean M Standard March 10, 2017 Name KEY Physical Chemistry II Exam 2 Solutions 1) (14 points) Use the potential energy and momentum operators for the harmonic oscillator to
Chemistry 362 Spring 2017 Dr Jean M Standard March 10, 2017 Name KEY Physical Chemistry II Exam 2 Solutions 1) (14 points) Use the potential energy and momentum operators for the harmonic oscillator to
Indicate if the statement is True (T) or False (F) by circling the letter (1 pt each):
 Indicate if the statement is (T) or False (F) by circling the letter (1 pt each): False 1. In order to ensure that all observables are real valued, the eigenfunctions for an operator must also be real
Indicate if the statement is (T) or False (F) by circling the letter (1 pt each): False 1. In order to ensure that all observables are real valued, the eigenfunctions for an operator must also be real
2m dx 2. The particle in a one dimensional box (of size L) energy levels are
 Name: Chem 3322 test #1 solutions, out of 40 marks I want complete, detailed answers to the questions. Show all your work to get full credit. indefinite integral : sin 2 (ax)dx = x 2 sin(2ax) 4a (1) with
Name: Chem 3322 test #1 solutions, out of 40 marks I want complete, detailed answers to the questions. Show all your work to get full credit. indefinite integral : sin 2 (ax)dx = x 2 sin(2ax) 4a (1) with
Lecture 6. Four postulates of quantum mechanics. The eigenvalue equation. Momentum and energy operators. Dirac delta function. Expectation values
 Lecture 6 Four postulates of quantum mechanics The eigenvalue equation Momentum and energy operators Dirac delta function Expectation values Objectives Learn about eigenvalue equations and operators. Learn
Lecture 6 Four postulates of quantum mechanics The eigenvalue equation Momentum and energy operators Dirac delta function Expectation values Objectives Learn about eigenvalue equations and operators. Learn
Chemistry 3502/4502. Exam I. September 19, ) This is a multiple choice exam. Circle the correct answer.
 D Chemistry 350/450 Exam I September 9, 003 ) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of useful
D Chemistry 350/450 Exam I September 9, 003 ) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of useful
Problem Set 5 Solutions
 Chemistry 362 Dr Jean M Standard Problem Set 5 Solutions ow many vibrational modes do the following molecules or ions possess? [int: Drawing Lewis structures may be useful in some cases] In all of the
Chemistry 362 Dr Jean M Standard Problem Set 5 Solutions ow many vibrational modes do the following molecules or ions possess? [int: Drawing Lewis structures may be useful in some cases] In all of the
Reading: Mathchapters F and G, MQ - Ch. 7-8, Lecture notes on hydrogen atom.
 Chemistry 356 017: Problem set No. 6; Reading: Mathchapters F and G, MQ - Ch. 7-8, Lecture notes on hydrogen atom. The H atom involves spherical coordinates and angular momentum, which leads to the shapes
Chemistry 356 017: Problem set No. 6; Reading: Mathchapters F and G, MQ - Ch. 7-8, Lecture notes on hydrogen atom. The H atom involves spherical coordinates and angular momentum, which leads to the shapes
Examples of the Fourier Theorem (Sect. 10.3). The Fourier Theorem: Continuous case.
 s of the Fourier Theorem (Sect. 1.3. The Fourier Theorem: Continuous case. : Using the Fourier Theorem. The Fourier Theorem: Piecewise continuous case. : Using the Fourier Theorem. The Fourier Theorem:
s of the Fourier Theorem (Sect. 1.3. The Fourier Theorem: Continuous case. : Using the Fourier Theorem. The Fourier Theorem: Piecewise continuous case. : Using the Fourier Theorem. The Fourier Theorem:
Overview of Fourier Series (Sect. 6.2). Origins of the Fourier Series.
 Overview of Fourier Series (Sect. 6.2. Origins of the Fourier Series. Periodic functions. Orthogonality of Sines and Cosines. Main result on Fourier Series. Origins of the Fourier Series. Summary: Daniel
Overview of Fourier Series (Sect. 6.2. Origins of the Fourier Series. Periodic functions. Orthogonality of Sines and Cosines. Main result on Fourier Series. Origins of the Fourier Series. Summary: Daniel
CHEM 301: Homework assignment #5
 CHEM 30: Homework assignment #5 Solutions. A point mass rotates in a circle with l =. Calculate the magnitude of its angular momentum and all possible projections of the angular momentum on the z-axis.
CHEM 30: Homework assignment #5 Solutions. A point mass rotates in a circle with l =. Calculate the magnitude of its angular momentum and all possible projections of the angular momentum on the z-axis.
Higher. Further Calculus 149
 hsn.uk.net Higher Mathematics UNIT 3 OUTCOME 2 Further Calculus Contents Further Calculus 49 Differentiating sinx an cosx 49 2 Integrating sinx an cosx 50 3 The Chain Rule 5 4 Special Cases of the Chain
hsn.uk.net Higher Mathematics UNIT 3 OUTCOME 2 Further Calculus Contents Further Calculus 49 Differentiating sinx an cosx 49 2 Integrating sinx an cosx 50 3 The Chain Rule 5 4 Special Cases of the Chain
Physics 137A Quantum Mechanics Fall 2012 Midterm II - Solutions
 Physics 37A Quantum Mechanics Fall 0 Midterm II - Solutions These are the solutions to the exam given to Lecture Problem [5 points] Consider a particle with mass m charge q in a simple harmonic oscillator
Physics 37A Quantum Mechanics Fall 0 Midterm II - Solutions These are the solutions to the exam given to Lecture Problem [5 points] Consider a particle with mass m charge q in a simple harmonic oscillator
Sample Quantum Chemistry Exam 2 Solutions
 Chemistry 46 Fall 7 Dr. Jean M. Standard Name SAMPE EXAM Sample Quantum Chemistry Exam Solutions.) ( points) Answer the following questions by selecting the correct answer from the choices provided. a.)
Chemistry 46 Fall 7 Dr. Jean M. Standard Name SAMPE EXAM Sample Quantum Chemistry Exam Solutions.) ( points) Answer the following questions by selecting the correct answer from the choices provided. a.)
This practice exam is intended to help you prepare for the final exam for MTH 142 Calculus II.
 MTH 142 Practice Exam Chapters 9-11 Calculus II With Analytic Geometry Fall 2011 - University of Rhode Island This practice exam is intended to help you prepare for the final exam for MTH 142 Calculus
MTH 142 Practice Exam Chapters 9-11 Calculus II With Analytic Geometry Fall 2011 - University of Rhode Island This practice exam is intended to help you prepare for the final exam for MTH 142 Calculus
Simple Harmonic Oscillator
 Classical harmonic oscillator Linear force acting on a particle (Hooke s law): F =!kx From Newton s law: F = ma = m d x dt =!kx " d x dt + # x = 0, # = k / m Position and momentum solutions oscillate in
Classical harmonic oscillator Linear force acting on a particle (Hooke s law): F =!kx From Newton s law: F = ma = m d x dt =!kx " d x dt + # x = 0, # = k / m Position and momentum solutions oscillate in
Physics 505 Homework No. 1 Solutions S1-1
 Physics 505 Homework No s S- Some Preliminaries Assume A and B are Hermitian operators (a) Show that (AB) B A dx φ ABψ dx (A φ) Bψ dx (B (A φ)) ψ dx (B A φ) ψ End (b) Show that AB [A, B]/2+{A, B}/2 where
Physics 505 Homework No s S- Some Preliminaries Assume A and B are Hermitian operators (a) Show that (AB) B A dx φ ABψ dx (A φ) Bψ dx (B (A φ)) ψ dx (B A φ) ψ End (b) Show that AB [A, B]/2+{A, B}/2 where
School of Mathematics & Statistics MT 4507/ Classical Mechanics - Solutions Sheet 1
 T. Neukirch Sem /3 School of Mathematics & Statistics MT 457/587 - Classical Mechanics - Solutions Sheet. (i) A = (,, ), B = (4,, 3) C = (,, ) (a) The magnitudes of A, B and C are A A = A = + + = 9 = A
T. Neukirch Sem /3 School of Mathematics & Statistics MT 457/587 - Classical Mechanics - Solutions Sheet. (i) A = (,, ), B = (4,, 3) C = (,, ) (a) The magnitudes of A, B and C are A A = A = + + = 9 = A
An operator is a transformation that takes a function as an input and produces another function (usually).
 Formalism of Quantum Mechanics Operators Engel 3.2 An operator is a transformation that takes a function as an input and produces another function (usually). Example: In QM, most operators are linear:
Formalism of Quantum Mechanics Operators Engel 3.2 An operator is a transformation that takes a function as an input and produces another function (usually). Example: In QM, most operators are linear:
Lecture 12. The harmonic oscillator
 Lecture 12 The harmonic oscillator 107 108 LECTURE 12. THE HARMONIC OSCILLATOR 12.1 Introduction In this chapter, we are going to find explicitly the eigenfunctions and eigenvalues for the time-independent
Lecture 12 The harmonic oscillator 107 108 LECTURE 12. THE HARMONIC OSCILLATOR 12.1 Introduction In this chapter, we are going to find explicitly the eigenfunctions and eigenvalues for the time-independent
( ) = 9φ 1, ( ) = 4φ 2.
 Chemistry 46 Dr Jean M Standard Homework Problem Set 6 Solutions The Hermitian operator A ˆ is associated with the physical observable A Two of the eigenfunctions of A ˆ are and These eigenfunctions are
Chemistry 46 Dr Jean M Standard Homework Problem Set 6 Solutions The Hermitian operator A ˆ is associated with the physical observable A Two of the eigenfunctions of A ˆ are and These eigenfunctions are
Physics 741 Graduate Quantum Mechanics 1 Solutions to Midterm Exam, Fall x i x dx i x i x x i x dx
 Physics 74 Graduate Quantum Mechanics Solutions to Midterm Exam, Fall 4. [ points] Consider the wave function x Nexp x ix (a) [6] What is the correct normaliation N? The normaliation condition is. exp,
Physics 74 Graduate Quantum Mechanics Solutions to Midterm Exam, Fall 4. [ points] Consider the wave function x Nexp x ix (a) [6] What is the correct normaliation N? The normaliation condition is. exp,
= ( Prove the nonexistence of electron in the nucleus on the basis of uncertainty principle.
 Worked out examples (Quantum mechanics). A microscope, using photons, is employed to locate an electron in an atom within a distance of. Å. What is the uncertainty in the momentum of the electron located
Worked out examples (Quantum mechanics). A microscope, using photons, is employed to locate an electron in an atom within a distance of. Å. What is the uncertainty in the momentum of the electron located
Problem #1 30 points Problem #2 30 points Problem #3 30 points Problem #4 30 points Problem #5 30 points
 Name ME 5 Exam # November 5, 7 Prof. Lucht ME 55. POINT DISTRIBUTION Problem # 3 points Problem # 3 points Problem #3 3 points Problem #4 3 points Problem #5 3 points. EXAM INSTRUCTIONS You must do four
Name ME 5 Exam # November 5, 7 Prof. Lucht ME 55. POINT DISTRIBUTION Problem # 3 points Problem # 3 points Problem #3 3 points Problem #4 3 points Problem #5 3 points. EXAM INSTRUCTIONS You must do four
Appendix A. The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System
 Appendix A The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System Real quantum mechanical systems have the tendency to become mathematically
Appendix A The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System Real quantum mechanical systems have the tendency to become mathematically
Convergence Tests. Academic Resource Center
 Convergence Tests Academic Resource Center Series Given a sequence {a 0, a, a 2,, a n } The sum of the series, S n = A series is convergent if, as n gets larger and larger, S n goes to some finite number.
Convergence Tests Academic Resource Center Series Given a sequence {a 0, a, a 2,, a n } The sum of the series, S n = A series is convergent if, as n gets larger and larger, S n goes to some finite number.
Math Review for Exam Answer each of the following questions as either True or False. Circle the correct answer.
 Math 22 - Review for Exam 3. Answer each of the following questions as either True or False. Circle the correct answer. (a) True/False: If a n > 0 and a n 0, the series a n converges. Soln: False: Let
Math 22 - Review for Exam 3. Answer each of the following questions as either True or False. Circle the correct answer. (a) True/False: If a n > 0 and a n 0, the series a n converges. Soln: False: Let
Chemistry 3502/4502. Exam I Key. September 19, ) This is a multiple choice exam. Circle the correct answer.
 D Chemistry 350/450 Exam I Key September 19, 003 1) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of
D Chemistry 350/450 Exam I Key September 19, 003 1) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of
PY 351 Modern Physics - Lecture notes, 3
 PY 351 Modern Physics - Lecture notes, 3 Copyright by Claudio Rebbi, Boston University, October 2016. These notes cannot be duplicated and distributed without explicit permission of the author. Time dependence
PY 351 Modern Physics - Lecture notes, 3 Copyright by Claudio Rebbi, Boston University, October 2016. These notes cannot be duplicated and distributed without explicit permission of the author. Time dependence
Page 404. Lecture 22: Simple Harmonic Oscillator: Energy Basis Date Given: 2008/11/19 Date Revised: 2008/11/19
 Page 404 Lecture : Simple Harmonic Oscillator: Energy Basis Date Given: 008/11/19 Date Revised: 008/11/19 Coordinate Basis Section 6. The One-Dimensional Simple Harmonic Oscillator: Coordinate Basis Page
Page 404 Lecture : Simple Harmonic Oscillator: Energy Basis Date Given: 008/11/19 Date Revised: 008/11/19 Coordinate Basis Section 6. The One-Dimensional Simple Harmonic Oscillator: Coordinate Basis Page
Quantum Chemistry Exam 2 Solutions
 Chemistry 46 Fall 17 Dr. Jean M. Standard November 8, 17 Name KEY Quantum Chemistry Exam Solutions 1.) ( points) Answer the following questions by selecting the correct answer from the choices provided.
Chemistry 46 Fall 17 Dr. Jean M. Standard November 8, 17 Name KEY Quantum Chemistry Exam Solutions 1.) ( points) Answer the following questions by selecting the correct answer from the choices provided.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 8, February 3, 2006 & L "
 Chem 352/452 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 26 Christopher J. Cramer Lecture 8, February 3, 26 Solved Homework (Homework for grading is also due today) Evaluate
Chem 352/452 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 26 Christopher J. Cramer Lecture 8, February 3, 26 Solved Homework (Homework for grading is also due today) Evaluate
If electrons moved in simple orbits, p and x could be determined, but this violates the Heisenberg Uncertainty Principle.
 CHEM 2060 Lecture 18: Particle in a Box L18-1 Atomic Orbitals If electrons moved in simple orbits, p and x could be determined, but this violates the Heisenberg Uncertainty Principle. We can only talk
CHEM 2060 Lecture 18: Particle in a Box L18-1 Atomic Orbitals If electrons moved in simple orbits, p and x could be determined, but this violates the Heisenberg Uncertainty Principle. We can only talk
Math 106 Fall 2014 Exam 2.1 October 31, ln(x) x 3 dx = 1. 2 x 2 ln(x) + = 1 2 x 2 ln(x) + 1. = 1 2 x 2 ln(x) 1 4 x 2 + C
 Math 6 Fall 4 Exam. October 3, 4. The following questions have to do with the integral (a) Evaluate dx. Use integration by parts (x 3 dx = ) ( dx = ) x3 x dx = x x () dx = x + x x dx = x + x 3 dx dx =
Math 6 Fall 4 Exam. October 3, 4. The following questions have to do with the integral (a) Evaluate dx. Use integration by parts (x 3 dx = ) ( dx = ) x3 x dx = x x () dx = x + x x dx = x + x 3 dx dx =
df(x) dx = h(x) Chemistry 4531 Mathematical Preliminaries Spring 2009 I. A Primer on Differential Equations Order of differential equation
 Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Degree Master of Science in Mathematical Modelling and Scientific Computing Mathematical Methods I Thursday, 12th January 2012, 9:30 a.m.- 11:30 a.m.
 Degree Master of Science in Mathematical Modelling and Scientific Computing Mathematical Methods I Thursday, 12th January 2012, 9:30 a.m.- 11:30 a.m. Candidates should submit answers to a maximum of four
Degree Master of Science in Mathematical Modelling and Scientific Computing Mathematical Methods I Thursday, 12th January 2012, 9:30 a.m.- 11:30 a.m. Candidates should submit answers to a maximum of four
Basic Quantum Mechanics
 Frederick Lanni 10feb'12 Basic Quantum Mechanics Part I. Where Schrodinger's equation comes from. A. Planck's quantum hypothesis, formulated in 1900, was that exchange of energy between an electromagnetic
Frederick Lanni 10feb'12 Basic Quantum Mechanics Part I. Where Schrodinger's equation comes from. A. Planck's quantum hypothesis, formulated in 1900, was that exchange of energy between an electromagnetic
Introduction to Quantum Mechanics PVK - Solutions. Nicolas Lanzetti
 Introduction to Quantum Mechanics PVK - Solutions Nicolas Lanzetti lnicolas@student.ethz.ch 1 Contents 1 The Wave Function and the Schrödinger Equation 3 1.1 Quick Checks......................................
Introduction to Quantum Mechanics PVK - Solutions Nicolas Lanzetti lnicolas@student.ethz.ch 1 Contents 1 The Wave Function and the Schrödinger Equation 3 1.1 Quick Checks......................................
22. Periodic Functions and Fourier Series
 November 29, 2010 22-1 22. Periodic Functions and Fourier Series 1 Periodic Functions A real-valued function f(x) of a real variable is called periodic of period T > 0 if f(x + T ) = f(x) for all x R.
November 29, 2010 22-1 22. Periodic Functions and Fourier Series 1 Periodic Functions A real-valued function f(x) of a real variable is called periodic of period T > 0 if f(x + T ) = f(x) for all x R.
Physical Chemistry Quantum Mechanics, Spectroscopy, and Molecular Interactions. Solutions Manual. by Andrew Cooksy
 Physical Chemistry Quantum Mechanics, Spectroscopy, and Molecular Interactions Solutions Manual by Andrew Cooksy February 4, 2014 Contents Contents i Objectives Review Questions 1 Chapter Problems 11 Notes
Physical Chemistry Quantum Mechanics, Spectroscopy, and Molecular Interactions Solutions Manual by Andrew Cooksy February 4, 2014 Contents Contents i Objectives Review Questions 1 Chapter Problems 11 Notes
EXAM INFORMATION. Radial Distribution Function: B is the normalization constant. d dx. p 2 Operator: Heisenberg Uncertainty Principle:
 EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance
 IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance The foundation of electronic spectroscopy is the exact solution of the time-independent Schrodinger equation for the hydrogen atom.
IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance The foundation of electronic spectroscopy is the exact solution of the time-independent Schrodinger equation for the hydrogen atom.
1. For the case of the harmonic oscillator, the potential energy is quadratic and hence the total Hamiltonian looks like: d 2 H = h2
 15 Harmonic Oscillator 1. For the case of the harmonic oscillator, the potential energy is quadratic and hence the total Hamiltonian looks like: d 2 H = h2 2mdx + 1 2 2 kx2 (15.1) where k is the force
15 Harmonic Oscillator 1. For the case of the harmonic oscillator, the potential energy is quadratic and hence the total Hamiltonian looks like: d 2 H = h2 2mdx + 1 2 2 kx2 (15.1) where k is the force
Electronic structure of solids
 Electronic structure of solids Eigenvalue equation: Áf(x) = af(x) KNOWN: Á is an operator. UNKNOWNS: f(x) is a function (and a vector), an eigenfunction of Á; a is a number (scalar), the eigenvalue. Ackowledgement:
Electronic structure of solids Eigenvalue equation: Áf(x) = af(x) KNOWN: Á is an operator. UNKNOWNS: f(x) is a function (and a vector), an eigenfunction of Á; a is a number (scalar), the eigenvalue. Ackowledgement:
Chapter 3. Integration. 3.1 Indefinite Integration
 Chapter 3 Integration 3. Indefinite Integration Integration is the reverse of differentiation. Consider a function f(x) and suppose that there exists another function F (x) such that df f(x). (3.) For
Chapter 3 Integration 3. Indefinite Integration Integration is the reverse of differentiation. Consider a function f(x) and suppose that there exists another function F (x) such that df f(x). (3.) For
Problems and Multiple Choice Questions
 Problems and Multiple Choice Questions 1. A momentum operator in one dimension is 2. A position operator in 3 dimensions is 3. A kinetic energy operator in 1 dimension is 4. If two operator commute, a)
Problems and Multiple Choice Questions 1. A momentum operator in one dimension is 2. A position operator in 3 dimensions is 3. A kinetic energy operator in 1 dimension is 4. If two operator commute, a)
Midterm Solution
 18303 Midterm Solution Problem 1: SLP with mixed boundary conditions Consider the following regular) Sturm-Liouville eigenvalue problem consisting in finding scalars λ and functions v : [0, b] R b > 0),
18303 Midterm Solution Problem 1: SLP with mixed boundary conditions Consider the following regular) Sturm-Liouville eigenvalue problem consisting in finding scalars λ and functions v : [0, b] R b > 0),
Lecture 12: Particle in 1D boxes & Simple Harmonic Oscillator
 Lecture 12: Particle in 1D boxes & Simple Harmonic Oscillator U(x) E Dx y(x) x Dx Lecture 12, p 1 Properties of Bound States Several trends exhibited by the particle-in-box states are generic to bound
Lecture 12: Particle in 1D boxes & Simple Harmonic Oscillator U(x) E Dx y(x) x Dx Lecture 12, p 1 Properties of Bound States Several trends exhibited by the particle-in-box states are generic to bound
For a system with more than one electron, we can t solve the Schrödinger Eq. exactly. We must develop methods of approximation, such as
 VARIATIO METHOD For a system with more than one electron, we can t solve the Schrödinger Eq. exactly. We must develop methods of approximation, such as Variation Method Perturbation Theory Combination
VARIATIO METHOD For a system with more than one electron, we can t solve the Schrödinger Eq. exactly. We must develop methods of approximation, such as Variation Method Perturbation Theory Combination
Chemistry 881 Lecture Topics Fall 2001
 Chemistry 881 Lecture Topics Fall 2001 Texts PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon MATHEMATICS for PHYSICAL CHEMISTRY, Mortimer i. Mathematics Review (M, Chapters 1,2,3 & 4; M&S,
Chemistry 881 Lecture Topics Fall 2001 Texts PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon MATHEMATICS for PHYSICAL CHEMISTRY, Mortimer i. Mathematics Review (M, Chapters 1,2,3 & 4; M&S,
Section 9 Variational Method. Page 492
 Section 9 Variational Method Page 492 Page 493 Lecture 27: The Variational Method Date Given: 2008/12/03 Date Revised: 2008/12/03 Derivation Section 9.1 Variational Method: Derivation Page 494 Motivation
Section 9 Variational Method Page 492 Page 493 Lecture 27: The Variational Method Date Given: 2008/12/03 Date Revised: 2008/12/03 Derivation Section 9.1 Variational Method: Derivation Page 494 Motivation
Massachusetts Institute of Technology Physics Department
 Massachusetts Institute of Technology Physics Department Physics 8.32 Fall 2006 Quantum Theory I October 9, 2006 Assignment 6 Due October 20, 2006 Announcements There will be a makeup lecture on Friday,
Massachusetts Institute of Technology Physics Department Physics 8.32 Fall 2006 Quantum Theory I October 9, 2006 Assignment 6 Due October 20, 2006 Announcements There will be a makeup lecture on Friday,
Spring 2017 Midterm 1 04/26/2017
 Math 2B Spring 2017 Midterm 1 04/26/2017 Time Limit: 50 Minutes Name (Print): Student ID This exam contains 10 pages (including this cover page) and 5 problems. Check to see if any pages are missing. Enter
Math 2B Spring 2017 Midterm 1 04/26/2017 Time Limit: 50 Minutes Name (Print): Student ID This exam contains 10 pages (including this cover page) and 5 problems. Check to see if any pages are missing. Enter
The Quantum Theory of Atoms and Molecules
 The Quantum Theory of Atoms and Molecules The postulates of quantum mechanics Dr Grant Ritchie The postulates.. 1. Associated with any particle moving in a conservative field of force is a wave function,
The Quantum Theory of Atoms and Molecules The postulates of quantum mechanics Dr Grant Ritchie The postulates.. 1. Associated with any particle moving in a conservative field of force is a wave function,
Introduction Derivation General formula List of series Convergence Applications Test SERIES 4 INU0114/514 (MATHS 1)
 MACLAURIN SERIES SERIES 4 INU0114/514 (MATHS 1) Dr Adrian Jannetta MIMA CMath FRAS Maclaurin Series 1/ 21 Adrian Jannetta Recap: Binomial Series Recall that some functions can be rewritten as a power series
MACLAURIN SERIES SERIES 4 INU0114/514 (MATHS 1) Dr Adrian Jannetta MIMA CMath FRAS Maclaurin Series 1/ 21 Adrian Jannetta Recap: Binomial Series Recall that some functions can be rewritten as a power series
Math Exam II Review
 Math 114 - Exam II Review Peter A. Perry University of Kentucky March 6, 2017 Bill of Fare 1. It s All About Series 2. Convergence Tests I 3. Convergence Tests II 4. The Gold Standards (Geometric Series)
Math 114 - Exam II Review Peter A. Perry University of Kentucky March 6, 2017 Bill of Fare 1. It s All About Series 2. Convergence Tests I 3. Convergence Tests II 4. The Gold Standards (Geometric Series)
Harmonic Oscillator with raising and lowering operators. We write the Schrödinger equation for the harmonic oscillator in one dimension as follows:
 We write the Schrödinger equation for the harmonic oscillator in one dimension as follows: H ˆ! = "!2 d 2! + 1 2µ dx 2 2 kx 2! = E! T ˆ = "! 2 2µ d 2 dx 2 V ˆ = 1 2 kx 2 H ˆ = ˆ T + ˆ V (1) where µ is
We write the Schrödinger equation for the harmonic oscillator in one dimension as follows: H ˆ! = "!2 d 2! + 1 2µ dx 2 2 kx 2! = E! T ˆ = "! 2 2µ d 2 dx 2 V ˆ = 1 2 kx 2 H ˆ = ˆ T + ˆ V (1) where µ is
Math Practice Exam 3 - solutions
 Math 181 - Practice Exam 3 - solutions Problem 1 Consider the function h(x) = (9x 2 33x 25)e 3x+1. a) Find h (x). b) Find all values of x where h (x) is zero ( critical values ). c) Using the sign pattern
Math 181 - Practice Exam 3 - solutions Problem 1 Consider the function h(x) = (9x 2 33x 25)e 3x+1. a) Find h (x). b) Find all values of x where h (x) is zero ( critical values ). c) Using the sign pattern
Introduction Derivation General formula Example 1 List of series Convergence Applications Test SERIES 4 INU0114/514 (MATHS 1)
 MACLAURIN SERIES SERIES 4 INU0114/514 (MATHS 1) Dr Adrian Jannetta MIMA CMath FRAS Maclaurin Series 1/ 19 Adrian Jannetta Background In this presentation you will be introduced to the concept of a power
MACLAURIN SERIES SERIES 4 INU0114/514 (MATHS 1) Dr Adrian Jannetta MIMA CMath FRAS Maclaurin Series 1/ 19 Adrian Jannetta Background In this presentation you will be introduced to the concept of a power
Understand the basic principles of spectroscopy using selection rules and the energy levels. Derive Hund s Rule from the symmetrization postulate.
 CHEM 5314: Advanced Physical Chemistry Overall Goals: Use quantum mechanics to understand that molecules have quantized translational, rotational, vibrational, and electronic energy levels. In a large
CHEM 5314: Advanced Physical Chemistry Overall Goals: Use quantum mechanics to understand that molecules have quantized translational, rotational, vibrational, and electronic energy levels. In a large
The goal of today is to determine what u-substitution to use for trigonometric integrals. The most common substitutions are the following:
 Trigonometric Integrals The goal of today is to determine what u-substitution to use for trigonometric integrals. The most common substitutions are the following: Substitution u sinx u cosx u tanx u secx
Trigonometric Integrals The goal of today is to determine what u-substitution to use for trigonometric integrals. The most common substitutions are the following: Substitution u sinx u cosx u tanx u secx
Ma 530 Power Series II
 Ma 530 Power Series II Please note that there is material on power series at Visual Calculus. Some of this material was used as part of the presentation of the topics that follow. Operations on Power Series
Ma 530 Power Series II Please note that there is material on power series at Visual Calculus. Some of this material was used as part of the presentation of the topics that follow. Operations on Power Series
1 Commutators (10 pts)
 Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
5.61 FIRST HOUR EXAM ANSWERS Fall, 2013
 5.61 FIRST HOUR EXAM ANSWERS Fall, 013 I. A. Sketch ψ 5(x)ψ 5 (x) vs. x, where ψ 5 (x) is the n = 5 wavefunction of a particle in a box. Describe, in a few words, each of the essential qualitative features
5.61 FIRST HOUR EXAM ANSWERS Fall, 013 I. A. Sketch ψ 5(x)ψ 5 (x) vs. x, where ψ 5 (x) is the n = 5 wavefunction of a particle in a box. Describe, in a few words, each of the essential qualitative features
1 f. result from periodic disturbance same period (frequency) as source Longitudinal or Transverse Waves Characterized by
 result from periodic disturbance same period (frequency) as source Longitudinal or Transverse Waves Characterized by amplitude (how far do the bits move from their equilibrium positions? Amplitude of MEDIUM)
result from periodic disturbance same period (frequency) as source Longitudinal or Transverse Waves Characterized by amplitude (how far do the bits move from their equilibrium positions? Amplitude of MEDIUM)
(Refer Slide Time: 1:20) (Refer Slide Time: 1:24 min)
 Engineering Chemistry - 1 Prof. K. Mangala Sunder Department of Chemistry Indian Institute of Technology, Madras Lecture - 5 Module 1: Atoms and Molecules Harmonic Oscillator (Continued) (Refer Slide Time:
Engineering Chemistry - 1 Prof. K. Mangala Sunder Department of Chemistry Indian Institute of Technology, Madras Lecture - 5 Module 1: Atoms and Molecules Harmonic Oscillator (Continued) (Refer Slide Time:
Chemistry 483 Lecture Topics Fall 2009
 Chemistry 483 Lecture Topics Fall 2009 Text PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon A. Background (M&S,Chapter 1) Blackbody Radiation Photoelectric effect DeBroglie Wavelength Atomic
Chemistry 483 Lecture Topics Fall 2009 Text PHYSICAL CHEMISTRY A Molecular Approach McQuarrie and Simon A. Background (M&S,Chapter 1) Blackbody Radiation Photoelectric effect DeBroglie Wavelength Atomic
Quantum Harmonic Oscillator
 Quantum Harmonic Oscillator Chapter 13 P. J. Grandinetti Chem. 4300 Oct 20, 2017 P. J. Grandinetti (Chem. 4300) Quantum Harmonic Oscillator Oct 20, 2017 1 / 26 Kinetic and Potential Energy Operators Harmonic
Quantum Harmonic Oscillator Chapter 13 P. J. Grandinetti Chem. 4300 Oct 20, 2017 P. J. Grandinetti (Chem. 4300) Quantum Harmonic Oscillator Oct 20, 2017 1 / 26 Kinetic and Potential Energy Operators Harmonic
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 7, February 1, 2006
 Chem 350/450 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 006 Christopher J. Cramer ecture 7, February 1, 006 Solved Homework We are given that A is a Hermitian operator such that
Chem 350/450 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 006 Christopher J. Cramer ecture 7, February 1, 006 Solved Homework We are given that A is a Hermitian operator such that
Chapter 4 Sequences and Series
 Chapter 4 Sequences and Series 4.1 Sequence Review Sequence: a set of elements (numbers or letters or a combination of both). The elements of the set all follow the same rule (logical progression). The
Chapter 4 Sequences and Series 4.1 Sequence Review Sequence: a set of elements (numbers or letters or a combination of both). The elements of the set all follow the same rule (logical progression). The
One-dimensional harmonic oscillator. -motivation. -equation, energy levels. -eigenfunctions, Hermite polynomials. -classical analogy
 One-dimensional harmonic oscillator -motivation -equation, energy levels -eigenfunctions, Hermite polynomials -classical analogy One-dimensional harmonic oscillator 05/0 Harmonic oscillator = potential
One-dimensional harmonic oscillator -motivation -equation, energy levels -eigenfunctions, Hermite polynomials -classical analogy One-dimensional harmonic oscillator 05/0 Harmonic oscillator = potential
Qualifying Exam for Ph.D. Candidacy Department of Physics October 11, 2014 Part I
 Qualifying Exam for Ph.D. Candidacy Department of Physics October 11, 214 Part I Instructions: The following problems are intended to probe your understanding of basic physical principles. When answering
Qualifying Exam for Ph.D. Candidacy Department of Physics October 11, 214 Part I Instructions: The following problems are intended to probe your understanding of basic physical principles. When answering
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 9, February 8, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 9, February 8, 2006 The Harmonic Oscillator Consider a diatomic molecule. Such a molecule
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 9, February 8, 2006 The Harmonic Oscillator Consider a diatomic molecule. Such a molecule
2m r2 (~r )+V (~r ) (~r )=E (~r )
 Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Problem set 3: Solutions Math 207B, Winter Suppose that u(x) is a non-zero solution of the eigenvalue problem. (u ) 2 dx, u 2 dx.
 Problem set 3: Solutions Math 27B, Winter 216 1. Suppose that u(x) is a non-zero solution of the eigenvalue problem u = λu < x < 1, u() =, u(1) =. Show that λ = (u ) 2 dx u2 dx. Deduce that every eigenvalue
Problem set 3: Solutions Math 27B, Winter 216 1. Suppose that u(x) is a non-zero solution of the eigenvalue problem u = λu < x < 1, u() =, u(1) =. Show that λ = (u ) 2 dx u2 dx. Deduce that every eigenvalue
Lecture-XXVI. Time-Independent Schrodinger Equation
 Lecture-XXVI Time-Independent Schrodinger Equation Time Independent Schrodinger Equation: The time-dependent Schrodinger equation: Assume that V is independent of time t. In that case the Schrodinger equation
Lecture-XXVI Time-Independent Schrodinger Equation Time Independent Schrodinger Equation: The time-dependent Schrodinger equation: Assume that V is independent of time t. In that case the Schrodinger equation
Example. Evaluate. 3x 2 4 x dx.
 3x 2 4 x 3 + 4 dx. Solution: We need a new technique to integrate this function. Notice that if we let u x 3 + 4, and we compute the differential du of u, we get: du 3x 2 dx Going back to our integral,
3x 2 4 x 3 + 4 dx. Solution: We need a new technique to integrate this function. Notice that if we let u x 3 + 4, and we compute the differential du of u, we get: du 3x 2 dx Going back to our integral,
Exam 3. MA Exam 3 Fall Solutions. 1. (10 points) Find where the following functions are increasing and decreasing.
 Exam 3 Solutions. (0 points) Find where the following functions are increasing and decreasing. (a) (5 points) f(x) = x2 + 2x 3, x 3 2. x2 f(x) = + 2x 3 f (x) = 2x( x3 ) ( + 2x 3 ) 2 Critical points: x
Exam 3 Solutions. (0 points) Find where the following functions are increasing and decreasing. (a) (5 points) f(x) = x2 + 2x 3, x 3 2. x2 f(x) = + 2x 3 f (x) = 2x( x3 ) ( + 2x 3 ) 2 Critical points: x
Solution to the exam in TFY4275/FY8907 CLASSICAL TRANSPORT THEORY May 20, 2011
 NTNU Page 1 of 5 Institutt for fysikk Fakultet for fysikk, informatikk og matematikk Solution to the exam in TFY4275/FY8907 CLASSICAL TRANSPORT THEORY May 20, 2011 This solution consists of 5 pages. Problem
NTNU Page 1 of 5 Institutt for fysikk Fakultet for fysikk, informatikk og matematikk Solution to the exam in TFY4275/FY8907 CLASSICAL TRANSPORT THEORY May 20, 2011 This solution consists of 5 pages. Problem
Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor
 Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor It turns out that the boundary condition of the wavefunction going to zero at infinity is sufficient to quantize the value of energy that
Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor It turns out that the boundary condition of the wavefunction going to zero at infinity is sufficient to quantize the value of energy that
SIMPLE QUANTUM SYSTEMS
 SIMPLE QUANTUM SYSTEMS Chapters 14, 18 "ceiiinosssttuu" (anagram in Latin which Hooke published in 1676 in his "Description of Helioscopes") and deciphered as "ut tensio sic vis" (elongation of any spring
SIMPLE QUANTUM SYSTEMS Chapters 14, 18 "ceiiinosssttuu" (anagram in Latin which Hooke published in 1676 in his "Description of Helioscopes") and deciphered as "ut tensio sic vis" (elongation of any spring
Opinions on quantum mechanics. CHAPTER 6 Quantum Mechanics II. 6.1: The Schrödinger Wave Equation. Normalization and Probability
 CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6. Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite- 6.6 Simple Harmonic
CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6. Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite- 6.6 Simple Harmonic
Math 115 ( ) Yum-Tong Siu 1. Derivation of the Poisson Kernel by Fourier Series and Convolution
 Math 5 (006-007 Yum-Tong Siu. Derivation of the Poisson Kernel by Fourier Series and Convolution We are going to give a second derivation of the Poisson kernel by using Fourier series and convolution.
Math 5 (006-007 Yum-Tong Siu. Derivation of the Poisson Kernel by Fourier Series and Convolution We are going to give a second derivation of the Poisson kernel by using Fourier series and convolution.
The polar coordinates
 The polar coordinates 1 2 3 4 Graphing in polar coordinates 5 6 7 8 Area and length in polar coordinates 9 10 11 Partial deravitive 12 13 14 15 16 17 18 19 20 Double Integral 21 22 23 24 25 26 27 Triple
The polar coordinates 1 2 3 4 Graphing in polar coordinates 5 6 7 8 Area and length in polar coordinates 9 10 11 Partial deravitive 12 13 14 15 16 17 18 19 20 Double Integral 21 22 23 24 25 26 27 Triple
Chemistry 3502/4502. Exam III. All Hallows Eve/Samhain, ) This is a multiple choice exam. Circle the correct answer.
 B Chemistry 3502/4502 Exam III All Hallows Eve/Samhain, 2003 1) This is a multiple choice exam. Circle the correct answer. 2) There is one correct answer to every problem. There is no partial credit. 3)
B Chemistry 3502/4502 Exam III All Hallows Eve/Samhain, 2003 1) This is a multiple choice exam. Circle the correct answer. 2) There is one correct answer to every problem. There is no partial credit. 3)
Topic 9-1: Bloch Theorem and the Central Equation Kittel Pages:
 Topic 9-1: Bloch Theorem and the Central Equation Kittel Pages: 167-174 Summary: We begin here by postulating Bloch s theorems which develop the form of the wavefunction in a periodic solid. We then show
Topic 9-1: Bloch Theorem and the Central Equation Kittel Pages: 167-174 Summary: We begin here by postulating Bloch s theorems which develop the form of the wavefunction in a periodic solid. We then show
MATH 1207 R02 MIDTERM EXAM 2 SOLUTION
 MATH 7 R MIDTERM EXAM SOLUTION FALL 6 - MOON Name: Write your answer neatly and show steps. Except calculators, any electronic devices including laptops and cell phones are not allowed. () (5 pts) Find
MATH 7 R MIDTERM EXAM SOLUTION FALL 6 - MOON Name: Write your answer neatly and show steps. Except calculators, any electronic devices including laptops and cell phones are not allowed. () (5 pts) Find
If y = f (u) is a differentiable function of u and u = g(x) is a differentiable function of x then dy dx = dy. du du. If y = f (u) then y = f (u) u
 Section 3 4B The Chain Rule If y = f (u) is a differentiable function of u and u = g(x) is a differentiable function of x then dy dx = dy du du dx or If y = f (u) then f (u) u The Chain Rule with the Power
Section 3 4B The Chain Rule If y = f (u) is a differentiable function of u and u = g(x) is a differentiable function of x then dy dx = dy du du dx or If y = f (u) then f (u) u The Chain Rule with the Power
