The Environmental Smart Card
|
|
|
- Brett Griffith
- 5 years ago
- Views:
Transcription
1 The Environmental Smart Card
2 NIKOLA TESLA Alpha Waves in the human brain are between 6 and 8 Hz, the wave frequency in the human cavity, modulates between 6 and 8 Hz. All biological systems operate in the same frequency range. The human brains important Alpha waves is harmonised by the same frequency waves from EARTH WHICH IS 6-8 Hz. Thus our brain and our entire biological system is in unison with the earths own frequency of 6-8 Hz. IF WE CAN CONTROL THE FREQUENCY AND RESONANCES ELECTRICALLY WE CAN IMPROVE THE STATE OF MANKIND. Copyright Cell Wellbeing Ltd
3 The Earth Frequency HAS CHANGED CONSIDERABLY FROM TESLAS TIME The Earth Spins Counter clockwise. In Teslas time it had a Schuman Resonance of 7.83 hz. It is now thought to be at a frequency of between 12.8 and 14.3 hz. The SMART CARD reduces the effects of this negative shift in frequency.
4 ALL LIVING THINGS ARE BIO ELECTRIC 12.8 and 14.3 Hz Hz Hz DISTURBING FREQUENCY FACTORS 4-7 Hz 1-3 Hz. Copyright Cell Wellbeing Ltd
5 What has changed Copyright Cell Wellbeing Ltd
6 Environmental Disharmony Caused by Interference from Negative Frequencies Radiation from EMFs interfere with our body s natural processes including sleep, hormone production, our immune system and our ability to self-heal. Radiation from EMFs from outside the body disrupt our body s natural balance and can play havoc with the millions of electrical impulses that the body uses to regulate all cellular activity. We are sensitive natural bioelectric beings. ( Copyright Cell Wellbeing Ltd
7 Copyright Cell Wellbeing Ltd Environmental Pollution at night!!
8 Can we protect our Children and Family! Exposure to EMFs is dangerous to your health as it can cause adverse health conditions such as chronic fatigue, depression, headaches, abnormal behaviour in children, autism, attention deficit disorder, concentration and memory problems, learning and behavioral disorders, etc. (Source British Department of Health, Health Protection Agency and World Health Organisation) Did you know that the thinner the skull the more radiation damages the brain? Source : ( 3.full) Copyright Cell Wellbeing Ltd
9 How early is early for autism to hit home Copyright Cell Wellbeing Ltd
10 The Smart Card Has Proprietary Frequency and a Powerful Resonance capable of creating a positive energy field. A quantum energy field is established. Harmonize Electronic Magnetic Frequencies Radiations, restructure Water Molecules to Smaller Clusters, and other Wellness Benefits such as producing Energy Water, Reflexology, Beauty Application, etc. The resonance and vibration of frequencies is transferred through the card to Water, Light and Air. Natural resonance of the Smart Card is similar to that found in many water springs around the world. Copyright Cell Wellbeing Ltd
11 Frequency Field Theory The vortex starts with a twist in a counterclockwise direction. The counterclockwise direction allows for expansion. The counterclockwise spin accelerates or speeds up processes. Smart Card was designed taking into account the effect of the sine waves that are formed when Frequency moves counterclockwise and clockwise.
12 EFFECT OF THE QUANTUM ENERGY FIELD ON THE RADIATION EMITTED BY A MOBILE PHONE Mobile phone without quantum energy field Mobile phone with quantum energy field RF Strength 13 dbm. Warning RF Strength Minus-25 dbm. Good
13 EFFECT OF THE SMART CARD ON THE MOBILE PHONE Non Energized Mobile Phone Energized mobile phone RF Strength 10 dbm. Warning RF Strength Minus-25 dbm. Normal
14 MAKE A SMART CARD PHONE CALL! Non Energized mobile phone Energized mobile phone RF Strength + 7 dbm. Warning RF Strength Minus -7 dbm. Normal
15 SAFER LIVING SPACE AND ROOMS Effects of a Vortex in the Quantum Energy Field. Created using 4 cards or 4 bottles of Energized water Demo : Quantum Energy Field Protection The quantum energy field shapes as a tetrahedron with angles of o. Just as the atom of Carbon undergoes SP 3 hybridization does. Frequency is then expressed as a result of the conversion of the four vortexes resulting in the contraction of the quantum energy field, meaning it gets smaller.
16 Reduce Harmful Electromagnetic Frequencies such as E.M.F and E.L.F Emanating from : TV, Radio (FM, AM) Mobile Phones * Wi-Fi Micro Waves Copyright Cell Wellbeing Ltd
17 Simple Solution SWITCH THEM ALL OFF Can you stop using the Wi-Fi, mobile phone, video games and computers The simple answer is no. In fact the usage of these modern items will keep on increasing along with the parallel development of technology. Basically the radiation emitted from Wi-Fi, computers and mobile phones is a silent threat to our health with potentially drastic consequences. Copyright Cell Wellbeing Ltd
18 THE SMART CARD ALSO CREATES SMART WATER Softer Reduced Surface Tension Improved Hydration Qualities Increased Oxygen Levels The Perfect carrier for supplements and nutrition
19 Copyright Cell Wellbeing Ltd
20 Copyright Cell Wellbeing Ltd
21 Copyright Cell Wellbeing Ltd
22 Blood Analysis Tests Before Drinking Smart Card Structured Water After Drinking Smart Card Structured Water shows an increase amount of oxygen in the blood.
23 Skin and Beauty Application By spraying structured water generated by the Smart Card, it has been observed that the hydration of the skin improves allowing activation of collagen and elastin fibers in the skin. The result is a youthful look. Placing skincare products on top of the Smart Card for just a few seconds, has been shown to improve absorption through the skin.
24 Skin and Beauty Application By spraying Smart Card water on the wrinkles on the face and using the Smart Card. Rotate the card counterclockwise over the wrinkles for at least 1 minutes, your skin becomes smoother and wrinkles start to fade, as the oxygen enriched water is absorbed by the skin.
25 Skin and Beauty Applications By spraying Smart Card water on the wrinkles on the face and using the Smart Card. Rotate the card counterclockwise over the wrinkles for at least 1 minutes, your skin becomes smoother and wrinkles start to fade, as the oxygen enriched water is absorbed by the skin.
26 All of them received the Nobel Prize in Physics, for their significant contributions to the world of frequency and energy History of Frequency Frequency is the energy of everything that exists and was first described by Thales de Miletus ( BC) Max Planck (1918) received the Nobel Prize for his work on Quantum Theory Walther Nernst received the Nobel Prize in 1920 for his work in Thermo-chemistry Albert Einstein received the Noble Prize in 1921 for his work in the Photoelectric effect and his now famous theory of relativity, where Energy and matter become interconvertible Otto Stern received the Noble Prize in 1943 for his work on Spin Quantization
27 The Environmental Smart Card Your partner in Life Thank You
Frequently Asked Questions. 1. What is AlphaSpin?
 Frequently Asked Questions 1. What is AlphaSpin? AlphaSpin is a powerful holistic wellness tool infused with Proprietary Spinning Frequency that transfers spinning energy so that a quantum energy field
Frequently Asked Questions 1. What is AlphaSpin? AlphaSpin is a powerful holistic wellness tool infused with Proprietary Spinning Frequency that transfers spinning energy so that a quantum energy field
BioZen. Reduce the harmful effects of electrosmog! Low Frequency Magnetic Field Protection. BioZen. Extreme Low Frequency Wave Shield
 BioZen Static and Extreme Low Frequency Magnetic Field Protection BioZen Extreme Low Frequency Wave Shield d Technology- escientifically Proven - Certifi Reduce the harmful effects of electrosmog! HOW
BioZen Static and Extreme Low Frequency Magnetic Field Protection BioZen Extreme Low Frequency Wave Shield d Technology- escientifically Proven - Certifi Reduce the harmful effects of electrosmog! HOW
CRHS Academic Chemistry Unit 4 Electrons. Notes. Key Dates
 Name Period CRHS Academic Chemistry Unit 4 Electrons Notes Key Dates Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name Period CRHS Academic Chemistry Unit 4 Electrons Notes Key Dates Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
progressive electromagnetic wave
 LECTURE 11 Ch17 A progressive electromagnetic wave is a self-supporting, energy-carrying disturbance that travels free of its source. The light from the Sun travels through space (no medium) for only 8.3
LECTURE 11 Ch17 A progressive electromagnetic wave is a self-supporting, energy-carrying disturbance that travels free of its source. The light from the Sun travels through space (no medium) for only 8.3
LIGHT WAVES AND PARTICLES
 LIGHT WAVES AND PARTICLES THE ELECTROMAGNETIC SPECTRUM The light we see is only a tiny part of a much larger set of transverse waves. Like all waves, these carry energy without moving matter Although they
LIGHT WAVES AND PARTICLES THE ELECTROMAGNETIC SPECTRUM The light we see is only a tiny part of a much larger set of transverse waves. Like all waves, these carry energy without moving matter Although they
Frequency: the number of complete waves that pass a point in a given time. It has the symbol f. 1) SI Units: Hertz (Hz) Wavelength: The length from
 Frequency: the number of complete waves that pass a point in a given time. It has the symbol f. 1) SI Units: Hertz (Hz) Wavelength: The length from the one crest of a wave to the next. I. Electromagnetic
Frequency: the number of complete waves that pass a point in a given time. It has the symbol f. 1) SI Units: Hertz (Hz) Wavelength: The length from the one crest of a wave to the next. I. Electromagnetic
Photoelectric Effect Worksheet
 Photoelectric Effect Worksheet The photoelectric effect refers to the emission of electrons from metallic surfaces usually caused by incident light. The incident light is absorbed by electrons thus giving
Photoelectric Effect Worksheet The photoelectric effect refers to the emission of electrons from metallic surfaces usually caused by incident light. The incident light is absorbed by electrons thus giving
Recall: The Importance of Light
 Key Concepts: Lecture 19: Light Light: wave-like behavior Light: particle-like behavior Light: Interaction with matter - Kirchoff s Laws The Wave Nature of Electro-Magnetic Radiation Visible light is just
Key Concepts: Lecture 19: Light Light: wave-like behavior Light: particle-like behavior Light: Interaction with matter - Kirchoff s Laws The Wave Nature of Electro-Magnetic Radiation Visible light is just
Honors Ch3 and Ch4. Atomic History and the Atom
 Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
Quantum Model Einstein s Hypothesis: Photoelectric Effect
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT Quantum Model Einstein s Hypothesis: Photoelectric Effect The photoelectric effect was discovered by Hertz in 1887 as he confirmed Maxwell s electromagnetic
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT Quantum Model Einstein s Hypothesis: Photoelectric Effect The photoelectric effect was discovered by Hertz in 1887 as he confirmed Maxwell s electromagnetic
Nuclear Magnetic Resonance (NMR) Spectroscopy Introduction:
 Nuclear Magnetic Resonance (NMR) Spectroscopy Introduction: Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for organic structure determination. Like IR spectroscopy,
Nuclear Magnetic Resonance (NMR) Spectroscopy Introduction: Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for organic structure determination. Like IR spectroscopy,
29:006 FINAL EXAM FRIDAY MAY 11 3:00 5:00 PM IN LR1 VAN
 L 33 Modern Physics [1] 29:006 FINAL EXAM FRIDAY MAY 11 3:00 5:00 PM IN LR1 VAN Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices
L 33 Modern Physics [1] 29:006 FINAL EXAM FRIDAY MAY 11 3:00 5:00 PM IN LR1 VAN Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices
L 35 Modern Physics [1]
![L 35 Modern Physics [1] L 35 Modern Physics [1]](/thumbs/90/102858198.jpg) L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
Modern Physics- Introduction. L 35 Modern Physics [1] ATOMS and classical physics. Newton s Laws have flaws! accelerated charges radiate energy
![Modern Physics- Introduction. L 35 Modern Physics [1] ATOMS and classical physics. Newton s Laws have flaws! accelerated charges radiate energy Modern Physics- Introduction. L 35 Modern Physics [1] ATOMS and classical physics. Newton s Laws have flaws! accelerated charges radiate energy](/thumbs/87/96907347.jpg) L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
Radiation Protection Fundamentals and Biological Effects: Session 1
 Radiation Protection Fundamentals and Biological Effects: Session 1 Reading assignment: LLE Radiological Controls Manual (LLEINST 6610): Part 1 UR Radiation Safety Training Manual and Resource Book: Parts
Radiation Protection Fundamentals and Biological Effects: Session 1 Reading assignment: LLE Radiological Controls Manual (LLEINST 6610): Part 1 UR Radiation Safety Training Manual and Resource Book: Parts
ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM When white light passes through a prism, it spreads out into a rainbow of colours, with red at one end and
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM When white light passes through a prism, it spreads out into a rainbow of colours, with red at one end and
Chemistry Instrumental Analysis Lecture 2. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 2 Electromagnetic Radiation Can be described by means of a classical sinusoidal wave model. Oscillating electric and magnetic field. (Wave model) wavelength,
Chemistry 4631 Instrumental Analysis Lecture 2 Electromagnetic Radiation Can be described by means of a classical sinusoidal wave model. Oscillating electric and magnetic field. (Wave model) wavelength,
Keeping well and healthy when it is really cold
 Cold Weather Plan for England 2012 Keeping well and healthy when it is really cold Easy Read version of: Cold Weather Plan for England 2012: Protecting health and reducing harm from severe cold. What
Cold Weather Plan for England 2012 Keeping well and healthy when it is really cold Easy Read version of: Cold Weather Plan for England 2012: Protecting health and reducing harm from severe cold. What
Sunlight is a combination of light-waves of various frequencies. Some
 96 The Electromagnetic Spectrum r e a d i n g Sunlight is a combination of light-waves of various frequencies. Some of the frequencies can be seen and some cannot be seen by the human eye. The reading
96 The Electromagnetic Spectrum r e a d i n g Sunlight is a combination of light-waves of various frequencies. Some of the frequencies can be seen and some cannot be seen by the human eye. The reading
Measuring Planck s Constant By Martin Hackworth
 Measuring Planck s Constant By Martin Hackworth Historical Perspective and Physics Theory Max Planck (1858-1947) was born in Kiel Germany and attended schools in Munich and Berlin. Planck was an early
Measuring Planck s Constant By Martin Hackworth Historical Perspective and Physics Theory Max Planck (1858-1947) was born in Kiel Germany and attended schools in Munich and Berlin. Planck was an early
Physics 1C. Lecture 27A
 Physics 1C Lecture 27A "Any other situation in quantum mechanics, it turns out, can always be explained by saying, You remember the experiment with the two holes? It s the same thing. " --Richard Feynman
Physics 1C Lecture 27A "Any other situation in quantum mechanics, it turns out, can always be explained by saying, You remember the experiment with the two holes? It s the same thing. " --Richard Feynman
Quantum Theory of the Atom
 The Wave Nature of Light Quantum Theory of the Atom Electromagnetic radiation carries energy = radiant energy some forms are visible light, x rays, and radio waves Wavelength ( λ) is the distance between
The Wave Nature of Light Quantum Theory of the Atom Electromagnetic radiation carries energy = radiant energy some forms are visible light, x rays, and radio waves Wavelength ( λ) is the distance between
Chapter 27. Quantum Physics
 Chapter 27 Quantum Physics Need for Quantum Physics Problems remained from classical mechanics that relativity didn t explain Blackbody Radiation The electromagnetic radiation emitted by a heated object
Chapter 27 Quantum Physics Need for Quantum Physics Problems remained from classical mechanics that relativity didn t explain Blackbody Radiation The electromagnetic radiation emitted by a heated object
The Photoelectric Effect
 The Photoelectric Effect Light can strike the surface of some metals causing an electron to be ejected No matter how brightly the light shines, electrons are ejected only if the light has sufficient energy
The Photoelectric Effect Light can strike the surface of some metals causing an electron to be ejected No matter how brightly the light shines, electrons are ejected only if the light has sufficient energy
Electromagnetic Radiation (EMR)
 Electromagnetic Radiation (EMR) It is kind of energy with wave character ( exactly as sea waves ) that can be characterized by : Wavelength ( ) : The distance between two identical points on the wave.
Electromagnetic Radiation (EMR) It is kind of energy with wave character ( exactly as sea waves ) that can be characterized by : Wavelength ( ) : The distance between two identical points on the wave.
Chemistry 6A F2007. Dr. J.A. Mack 12/3/07. What do I need to bring? Exam 3: Friday 12/7/07 (here in lecture)
 Chemistry 6A F2007 Dr. J.A. Mack Exam 3: Friday 12/7/07 (here in lecture) What will be covered on the exam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab as well
Chemistry 6A F2007 Dr. J.A. Mack Exam 3: Friday 12/7/07 (here in lecture) What will be covered on the exam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab as well
CVB102 Lecture 1 - Chemical Structure and Reactivity. Contact Information: Dr. Bill Lot Electronic Structure of Atoms
 CVB102 Lecture 1 - Chemical Structure and Reactivity Contact Information: Dr. Bill Lot b.lott@qut.edu.au Electronic Structure of Atoms Text: Blackman, et al Pp. 127-147 (Pp. 148-159 recommended) The periodic
CVB102 Lecture 1 - Chemical Structure and Reactivity Contact Information: Dr. Bill Lot b.lott@qut.edu.au Electronic Structure of Atoms Text: Blackman, et al Pp. 127-147 (Pp. 148-159 recommended) The periodic
SPH4U UNIVERSITY PHYSICS
 SPH4U UNIVERSITY PHYSICS REVOLUTIONS IN MODERN PHYSICS:... L Photons & the Quantum Theory of... (P.620-623) The Work Function Around 1800, Thomas Young performed his double-slit interference experiment
SPH4U UNIVERSITY PHYSICS REVOLUTIONS IN MODERN PHYSICS:... L Photons & the Quantum Theory of... (P.620-623) The Work Function Around 1800, Thomas Young performed his double-slit interference experiment
Modern Physics, summer Modern physics. Historical introduction to quantum mechanics
 1 Modern physics 2 Gustav Kirchhoff (1824-1887) Surprisingly, the path to quantum mechanics begins with the work of German physicist Gustav Kirchhoff in 1859. Electron was discovered by J.J.Thomson in
1 Modern physics 2 Gustav Kirchhoff (1824-1887) Surprisingly, the path to quantum mechanics begins with the work of German physicist Gustav Kirchhoff in 1859. Electron was discovered by J.J.Thomson in
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level *5719133184* PHYSICS 9702/43 Paper 4 A2 Structured Questions October/November 2011 2 hours Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level *5719133184* PHYSICS 9702/43 Paper 4 A2 Structured Questions October/November 2011 2 hours Candidates
Georgia Institute of Technology CHEM 1310 revised 10/8/09 Spring The Development of Quantum Mechanics. ν (nu) = frequency (in s -1 or hertz)
 The Development of Quantum Mechanics Early physicists used the properties of electromagnetic radiation to develop fundamental ideas about the structure of the atom. A fundamental assumption for their work
The Development of Quantum Mechanics Early physicists used the properties of electromagnetic radiation to develop fundamental ideas about the structure of the atom. A fundamental assumption for their work
Dr. Friedhelm Schneider
 Dr. Friedhelm Schneider memon-technology measurable effects 1 Dr. Friedhelm Schneider Content memon-technology measurable effects 3 magnetic flux density [µt, micro Tesla], 0 to 15 Hz, memonizercombi,
Dr. Friedhelm Schneider memon-technology measurable effects 1 Dr. Friedhelm Schneider Content memon-technology measurable effects 3 magnetic flux density [µt, micro Tesla], 0 to 15 Hz, memonizercombi,
Modern physics. Historical introduction to quantum mechanics
 2012-0-08 Modern physics dr hab. inż. Katarzyna ZAKRZEWSKA, prof. AGH KATEDRA ELEKTRONIKI, C-1, office 17, rd floor, phone 617 29 01, mobile phone 0 601 51 5 e-mail: zak@agh.edu.pl, Internet site http://home.agh.edu.pl/~zak
2012-0-08 Modern physics dr hab. inż. Katarzyna ZAKRZEWSKA, prof. AGH KATEDRA ELEKTRONIKI, C-1, office 17, rd floor, phone 617 29 01, mobile phone 0 601 51 5 e-mail: zak@agh.edu.pl, Internet site http://home.agh.edu.pl/~zak
Early Quantum Theory & Models of the Atom (Ch 27) Discovery of electron. Blackbody Radiation. Blackbody Radiation. J. J. Thomson ( )
 Early Quantum Theory & Models of the Atom (Ch 27) Discovery of electron Modern physics special relativity quantum theory J. J. Thomson (1856-1940) measured e/m directly set-up was similar to mass spectrometer
Early Quantum Theory & Models of the Atom (Ch 27) Discovery of electron Modern physics special relativity quantum theory J. J. Thomson (1856-1940) measured e/m directly set-up was similar to mass spectrometer
The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation
 The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation Electromagnetic Radiation (How we get most of our information about the cosmos) Examples of electromagnetic
The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation Electromagnetic Radiation (How we get most of our information about the cosmos) Examples of electromagnetic
Classical and Planck picture. Planck s constant. Question. Quantum explanation for the Wein Effect.
 6.1 Quantum Physics. Particle Nature of Light Particle nature of Light Blackbody Radiation Photoelectric Effect Properties of photons Ionizing radiation Radiation damage x-rays Compton effect X-ray diffraction
6.1 Quantum Physics. Particle Nature of Light Particle nature of Light Blackbody Radiation Photoelectric Effect Properties of photons Ionizing radiation Radiation damage x-rays Compton effect X-ray diffraction
Chapter 7: The Quantum-Mechanical Model of the Atom
 C h e m i s t r y 1 A : C h a p t e r 7 P a g e 1 Chapter 7: The Quantum-Mechanical Model of the Atom Homework: Read Chapter 7. Work out sample/practice exercises Check for the MasteringChemistry.com assignment
C h e m i s t r y 1 A : C h a p t e r 7 P a g e 1 Chapter 7: The Quantum-Mechanical Model of the Atom Homework: Read Chapter 7. Work out sample/practice exercises Check for the MasteringChemistry.com assignment
Light. October 16, Chapter 5: Electrons in Atoms Honors Chemistry. Bohr Model
 Chapter 5: Electrons in Atoms Honors Chemistry Bohr Model Niels Bohr, a young Danish physicist and a student of Rutherford improved Rutherford's model. Bohr proposed that an electron is found only in specific
Chapter 5: Electrons in Atoms Honors Chemistry Bohr Model Niels Bohr, a young Danish physicist and a student of Rutherford improved Rutherford's model. Bohr proposed that an electron is found only in specific
Radiation Safety Training Session 1: Radiation Protection Fundamentals and Biological Effects
 Radiation Safety Training Session 1: Radiation Protection Fundamentals and Biological Effects Reading Assignment: LLE Radiological Controls Manual (LLEINST 6610) Part 1 UR Radiation Safety Training Manual
Radiation Safety Training Session 1: Radiation Protection Fundamentals and Biological Effects Reading Assignment: LLE Radiological Controls Manual (LLEINST 6610) Part 1 UR Radiation Safety Training Manual
Chem 325 NMR Intro. The Electromagnetic Spectrum. Physical properties, chemical properties, formulas Shedding real light on molecular structure:
 Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
Lecture 16 Quantum Physics Chapter 28
 Lecture 16 Quantum Physics Chapter 28 Particles vs. Waves Physics of particles p = mv K = ½ mv2 Particles collide and do not pass through each other Conservation of: Momentum Energy Electric Charge Physics
Lecture 16 Quantum Physics Chapter 28 Particles vs. Waves Physics of particles p = mv K = ½ mv2 Particles collide and do not pass through each other Conservation of: Momentum Energy Electric Charge Physics
1. Historical perspective
 Atomic and Molecular Physics/Lecture notes presented by Dr. Fouad Attia Majeed/Third year students/college of Education (Ibn Hayyan)/Department of Physics/University of Babylon. 1. Historical perspective
Atomic and Molecular Physics/Lecture notes presented by Dr. Fouad Attia Majeed/Third year students/college of Education (Ibn Hayyan)/Department of Physics/University of Babylon. 1. Historical perspective
Chapter 7. Quantum Theory and the Electronic Structure of Atoms
 Chapter 7 Quantum Theory and the Electronic Structure of Atoms This chapter introduces the student to quantum theory and the importance of this theory in describing electronic behavior. Upon completion
Chapter 7 Quantum Theory and the Electronic Structure of Atoms This chapter introduces the student to quantum theory and the importance of this theory in describing electronic behavior. Upon completion
Honors Chemistry Unit 3 ELECTRONS IN ATOMS
 Honors Chemistry Unit 3 ELECTRONS IN ATOMS I. RADIATION A. Particles 1. alpha particle - helium nucleus with 2 protons, 2 neutrons 2. beta particle - electron or positron ejected from nucleus B. Energy
Honors Chemistry Unit 3 ELECTRONS IN ATOMS I. RADIATION A. Particles 1. alpha particle - helium nucleus with 2 protons, 2 neutrons 2. beta particle - electron or positron ejected from nucleus B. Energy
This watermark does not appear in the registered version - Laser- Tissue Interaction
 S S d Laser- Tissue Interaction Types of radiation ionizing radiation Non - ionizing radiation You may click on any of the types of radiation for more detail about its particular type of interaction
S S d Laser- Tissue Interaction Types of radiation ionizing radiation Non - ionizing radiation You may click on any of the types of radiation for more detail about its particular type of interaction
College Physics B - PHY2054C
 College - PHY2054C Physics - Radioactivity 11/24/2014 My Office Hours: Tuesday 10:00 AM - Noon 206 Keen Building Review Question 1 Isotopes of an element A have the same number of protons and electrons,
College - PHY2054C Physics - Radioactivity 11/24/2014 My Office Hours: Tuesday 10:00 AM - Noon 206 Keen Building Review Question 1 Isotopes of an element A have the same number of protons and electrons,
Thermo-elastic Response of Cutaneous and Subcutaneous Tissues to Noninvasive Radiofrequency Heating
 Thermo-elastic Response of Cutaneous and Subcutaneous Tissues to Noninvasive Radiofrequency Heating Joel N. Jiménez Lozano, Paulino Vacas-Jacques, Walfre Franco. Excerpt from the Proceedings of the 2012
Thermo-elastic Response of Cutaneous and Subcutaneous Tissues to Noninvasive Radiofrequency Heating Joel N. Jiménez Lozano, Paulino Vacas-Jacques, Walfre Franco. Excerpt from the Proceedings of the 2012
Chapter 5 Light and Matter
 Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
General Physics (PHY 2140)
 General Physics (PHY 2140) Lecture 27 Modern Physics Quantum Physics Blackbody radiation Plank s hypothesis http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 27 1 Quantum Physics 2 Introduction: Need
General Physics (PHY 2140) Lecture 27 Modern Physics Quantum Physics Blackbody radiation Plank s hypothesis http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 27 1 Quantum Physics 2 Introduction: Need
Lecture 1 Bioradiation
 1 1 Radiation definition: Radiation, when broadly defined, includes the entire spectrum of electromagnetic waves : radiowaves, microwaves, infrared, visible light, ultraviolet, and x-rays and particles.
1 1 Radiation definition: Radiation, when broadly defined, includes the entire spectrum of electromagnetic waves : radiowaves, microwaves, infrared, visible light, ultraviolet, and x-rays and particles.
Lesson Plan: Introduction to Quantum Mechanics via Wave Theory and the Photoelectric Effect
 Lesson Plan: Introduction to Quantum Mechanics via Wave Theory and the Photoelectric Effect Will Stoll, Norcross High School Problem: To understand the basic principles of Quantum Mechanics through an
Lesson Plan: Introduction to Quantum Mechanics via Wave Theory and the Photoelectric Effect Will Stoll, Norcross High School Problem: To understand the basic principles of Quantum Mechanics through an
CAMI - Science. CAPS - Physics Links Grade 10
 CAMI - Science CAPS - Physics Links Grade 10 TERM 1 TOPICS CONTENT, CONCEPTS & SKILLS CAMI - KEYSTROKES Transverse pulses on a string or spring Pulse, amplitude Define a pulse Define a transverse pulse
CAMI - Science CAPS - Physics Links Grade 10 TERM 1 TOPICS CONTENT, CONCEPTS & SKILLS CAMI - KEYSTROKES Transverse pulses on a string or spring Pulse, amplitude Define a pulse Define a transverse pulse
10/27/2017 [pgs ]
![10/27/2017 [pgs ] 10/27/2017 [pgs ]](/thumbs/82/85139288.jpg) Objectives SWBAT explain the relationship between energy and frequency. SWBAT predict the behavior of and/or calculate quantum and photon energy from frequency. SWBAT explain how the quantization of energy
Objectives SWBAT explain the relationship between energy and frequency. SWBAT predict the behavior of and/or calculate quantum and photon energy from frequency. SWBAT explain how the quantization of energy
Ch. 7 The Quantum Mechanical Atom. Brady & Senese, 5th Ed.
 Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
Name Period. Practice Problems
 Name Period CRHS Academic Chemistry Unit 4 Electrons Practice Problems Due Date Assignment On-Time (100) Late (70) 4.1 4.2 4.3 4.4 4.5 Warm-Up EC Notes, Homework, Exam Reviews and Their KEYS located on
Name Period CRHS Academic Chemistry Unit 4 Electrons Practice Problems Due Date Assignment On-Time (100) Late (70) 4.1 4.2 4.3 4.4 4.5 Warm-Up EC Notes, Homework, Exam Reviews and Their KEYS located on
Nuclear Physics and Astrophysics
 Nuclear Physics and Astrophysics PHY-302 Dr. E. Rizvi Lecture 24 Medical Imaging Effects of Radiation We now know what radiation is But what does it mean for our bodies? Radioactivity is quantified in
Nuclear Physics and Astrophysics PHY-302 Dr. E. Rizvi Lecture 24 Medical Imaging Effects of Radiation We now know what radiation is But what does it mean for our bodies? Radioactivity is quantified in
The ELECTRON: Wave Particle Duality. chapter 4
 The ELECTRON: Wave Particle Duality chapter 4 What do we know about light? Before 1900 s scientists thought light behaved as a wave. This belief changed when it was discovered that light also has particle
The ELECTRON: Wave Particle Duality chapter 4 What do we know about light? Before 1900 s scientists thought light behaved as a wave. This belief changed when it was discovered that light also has particle
Magnetic Resonance Imaging
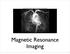 http://www.qldxray.com.au/filelibrary/mri_cardiovascular_system_ca_0005.jpg Magnetic Resonance Imaging 1 Overview 1. The magnetic properties of nuclei, and how they behave in strong magnetic fields. 2.
http://www.qldxray.com.au/filelibrary/mri_cardiovascular_system_ca_0005.jpg Magnetic Resonance Imaging 1 Overview 1. The magnetic properties of nuclei, and how they behave in strong magnetic fields. 2.
Which type of electromagnetic wave has a wavelength longer than that of yellow light? A. Infrared radiation C. X-rays B. Gamma Rays D.
 Which type of electromagnetic wave has a wavelength longer than that of yellow light? A. Infrared radiation C. X-rays B. Gamma Rays D. UV Rays Science Starter! 10.14-15.13! THE UNIVERSE AND ELECTROMAGNETIC
Which type of electromagnetic wave has a wavelength longer than that of yellow light? A. Infrared radiation C. X-rays B. Gamma Rays D. UV Rays Science Starter! 10.14-15.13! THE UNIVERSE AND ELECTROMAGNETIC
Chapter 4. Development of a New Model
 Chapter 4 Development of a New Model Electrons behave like particles in some experiments, and like waves in others. The electron's 'wave/particle duality' has no real analogy in the everyday world. The
Chapter 4 Development of a New Model Electrons behave like particles in some experiments, and like waves in others. The electron's 'wave/particle duality' has no real analogy in the everyday world. The
Problems with the atomic model?
 Modern Atomic Theory- Electronic Structure of Atoms DR HNIMIR-CH7 Where should (-) electrons be found? Problems with the atomic model? First, a Little About Electromagnetic Radiation- Waves Another Look
Modern Atomic Theory- Electronic Structure of Atoms DR HNIMIR-CH7 Where should (-) electrons be found? Problems with the atomic model? First, a Little About Electromagnetic Radiation- Waves Another Look
Ozone: Earth s shield from UV radiation
 Outline Ozone: Earth s shield from UV radiation Review electromagnetic radiation absorptivity by selective gases temperature vs. height in atmosphere Ozone production and destruction natural balance anthropogenic
Outline Ozone: Earth s shield from UV radiation Review electromagnetic radiation absorptivity by selective gases temperature vs. height in atmosphere Ozone production and destruction natural balance anthropogenic
Module 5 : MODERN PHYSICS Lecture 23 : Particle and Waves
 Module 5 : MODERN PHYSICS Lecture 23 : Particle and Waves Objectives In this lecture you will learn the following Radiation (light) exhibits both wave and particle nature. Laws governing black body radiation,
Module 5 : MODERN PHYSICS Lecture 23 : Particle and Waves Objectives In this lecture you will learn the following Radiation (light) exhibits both wave and particle nature. Laws governing black body radiation,
Chapter 13 Spectroscopy
 hapter 13 Spectroscopy Infrared spectroscopy Ultraviolet-Visible spectroscopy Nuclear magnetic resonance spectroscopy Mass Spectrometry 13.1 Principles of Molecular Spectroscopy: Electromagnetic Radiation
hapter 13 Spectroscopy Infrared spectroscopy Ultraviolet-Visible spectroscopy Nuclear magnetic resonance spectroscopy Mass Spectrometry 13.1 Principles of Molecular Spectroscopy: Electromagnetic Radiation
Advanced Physics in Creation Table of Contents
 Module #1: Units and Vectors Revisited Advanced Physics in Creation Table of Contents Introduction.. 1 Units Revisited.. 1 A Review of Vectors.... 5 Unit Vectors.. 12 The Dot Product... 15 The Physical
Module #1: Units and Vectors Revisited Advanced Physics in Creation Table of Contents Introduction.. 1 Units Revisited.. 1 A Review of Vectors.... 5 Unit Vectors.. 12 The Dot Product... 15 The Physical
Professor Stuart Bunt 217
 Professor Stuart Bunt 217 Traditional Anatomy Phrenology, the study of bumps on the skull. Measuring brain weights and size (still being done..see the fuss about Einstein s brain). Little link between
Professor Stuart Bunt 217 Traditional Anatomy Phrenology, the study of bumps on the skull. Measuring brain weights and size (still being done..see the fuss about Einstein s brain). Little link between
Modern Physics (Lec. 1)
 Modern Physics (Lec. 1) Physics Fundamental Science Concerned with the fundamental principles of the Universe Foundation of other physical sciences Has simplicity of fundamental concepts Divided into five
Modern Physics (Lec. 1) Physics Fundamental Science Concerned with the fundamental principles of the Universe Foundation of other physical sciences Has simplicity of fundamental concepts Divided into five
Chapter 21
 Chapter 21 http://youtu.be/kwasz59f8ga Nuclear reactions involve the nucleus The nucleus opens, and protons and neutrons are rearranged. The opening of the nucleus releases a tremendous amount of energy
Chapter 21 http://youtu.be/kwasz59f8ga Nuclear reactions involve the nucleus The nucleus opens, and protons and neutrons are rearranged. The opening of the nucleus releases a tremendous amount of energy
Card Appendix Quantum Concepts
 1 Physics 310 Card Appendix Quantum Concepts Table of Contents 0. Blackbody Radiation 2 3. Normalize 2 4. Angular Momentum 3 4. Hydrogen 4 5. Wavefunction 4 6. Photoelectric Effect 5 7. Lowering Operator
1 Physics 310 Card Appendix Quantum Concepts Table of Contents 0. Blackbody Radiation 2 3. Normalize 2 4. Angular Momentum 3 4. Hydrogen 4 5. Wavefunction 4 6. Photoelectric Effect 5 7. Lowering Operator
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level *8055009334* PHYSICS 9702/43 Paper 4 A2 Structured Questions May/June 2012 2 hours Candidates answer on
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level *8055009334* PHYSICS 9702/43 Paper 4 A2 Structured Questions May/June 2012 2 hours Candidates answer on
Explain how Planck resolved the ultraviolet catastrophe in blackbody radiation. Calculate energy of quanta using Planck s equation.
 Objectives Explain how Planck resolved the ultraviolet catastrophe in blackbody radiation. Calculate energy of quanta using Planck s equation. Solve problems involving maximum kinetic energy, work function,
Objectives Explain how Planck resolved the ultraviolet catastrophe in blackbody radiation. Calculate energy of quanta using Planck s equation. Solve problems involving maximum kinetic energy, work function,
UNCORRECTED PROOF. Table of Contents
 00-Stabin-Prelims SNY001-Stabin (Typeset by spi publisher services, Delhi) vii of xvi June 1, 2007 17:15 Preface xiii Acknowledgments xv Chapter 1. Introduction to Health Physics 1 1.1 Definition of Health
00-Stabin-Prelims SNY001-Stabin (Typeset by spi publisher services, Delhi) vii of xvi June 1, 2007 17:15 Preface xiii Acknowledgments xv Chapter 1. Introduction to Health Physics 1 1.1 Definition of Health
FI 3103 Quantum Physics
 FI 3103 Quantum Physics Alexander A. Iskandar Physics of Magnetism and Photonics Research Group Institut Teknologi Bandung General Information Lecture schedule 17 18 9136 51 5 91 Tutorial Teaching Assistant
FI 3103 Quantum Physics Alexander A. Iskandar Physics of Magnetism and Photonics Research Group Institut Teknologi Bandung General Information Lecture schedule 17 18 9136 51 5 91 Tutorial Teaching Assistant
Principles of Molecular Spectroscopy: Electromagnetic Radiation and Molecular structure. Nuclear Magnetic Resonance (NMR)
 Principles of Molecular Spectroscopy: Electromagnetic Radiation and Molecular structure Nuclear Magnetic Resonance (NMR) !E = h" Electromagnetic radiation is absorbed when the energy of photon corresponds
Principles of Molecular Spectroscopy: Electromagnetic Radiation and Molecular structure Nuclear Magnetic Resonance (NMR) !E = h" Electromagnetic radiation is absorbed when the energy of photon corresponds
Properties of Light and Atomic Structure. Chapter 7. So Where are the Electrons? Electronic Structure of Atoms. The Wave Nature of Light!
 Properties of Light and Atomic Structure Chapter 7 So Where are the Electrons? We know where the protons and neutrons are Nuclear structure of atoms (Chapter 2) The interaction of light and matter helps
Properties of Light and Atomic Structure Chapter 7 So Where are the Electrons? We know where the protons and neutrons are Nuclear structure of atoms (Chapter 2) The interaction of light and matter helps
Photochemical principles
 Chapter 1 Photochemical principles Dr. Suzan A. Khayyat 1 Photochemistry Photochemistry is concerned with the absorption, excitation and emission of photons by atoms, atomic ions, molecules, molecular
Chapter 1 Photochemical principles Dr. Suzan A. Khayyat 1 Photochemistry Photochemistry is concerned with the absorption, excitation and emission of photons by atoms, atomic ions, molecules, molecular
UNIT 7 ATOMIC AND NUCLEAR PHYSICS
 1 UNIT 7 ATOMIC AND NUCLEAR PHYSICS PHYS:1200 LECTURE 33 ATOMIC AND NUCLEAR PHYSICS (1) The physics that we have presented thus far in this course is classified as Classical Physics. Classical physics
1 UNIT 7 ATOMIC AND NUCLEAR PHYSICS PHYS:1200 LECTURE 33 ATOMIC AND NUCLEAR PHYSICS (1) The physics that we have presented thus far in this course is classified as Classical Physics. Classical physics
The Properties of Light. Our Window on the Universe
 The Properties of Light Chapter 11 Our Window on the Universe Light! And God said, Let there be light: and there was light. And God saw the light, that it was good Genesis 1:3-4 Standing Waves We can create
The Properties of Light Chapter 11 Our Window on the Universe Light! And God said, Let there be light: and there was light. And God saw the light, that it was good Genesis 1:3-4 Standing Waves We can create
Modern Physics. Overview
 Modern Physics Overview History ~1850s Classical (Newtonian) mechanics could not explain the new area of investigation atomic physics Macro vs Micro New field of Quantum Mechanics, focused on explaining
Modern Physics Overview History ~1850s Classical (Newtonian) mechanics could not explain the new area of investigation atomic physics Macro vs Micro New field of Quantum Mechanics, focused on explaining
Light carries energy. Lecture 5 Understand Light. Is light. Light as a Particle. ANSWER: Both.
 Light carries energy Lecture 5 Understand Light Reading: Chapter 6 You feel energy carried by light when light hits your skin. Energy Conservation: Radiation energy will be given to molecules making your
Light carries energy Lecture 5 Understand Light Reading: Chapter 6 You feel energy carried by light when light hits your skin. Energy Conservation: Radiation energy will be given to molecules making your
The greater the frequency the greater the energy. Thus ordering in increasing frequency is equivalent to ordering in increasing energy;
 Exercise F.1.1 Answers 1. Radio Waves λ = 100m f = c/λ = 3 10 8 /100 = 3 10 6 Hz X-rays λ = 1nm f = c/λ = 3 10 8 /1 10-9 = 3 10 17 Hz Gamma rays Infrared f = 3 10 19 Hz f = 100GHz = 100 10 9 Hz The greater
Exercise F.1.1 Answers 1. Radio Waves λ = 100m f = c/λ = 3 10 8 /100 = 3 10 6 Hz X-rays λ = 1nm f = c/λ = 3 10 8 /1 10-9 = 3 10 17 Hz Gamma rays Infrared f = 3 10 19 Hz f = 100GHz = 100 10 9 Hz The greater
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space.
 Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
Ch 7 Quantum Theory of the Atom (light and atomic structure)
 Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
OPTI510R: Photonics. Khanh Kieu College of Optical Sciences, University of Arizona Meinel building R.626
 OPTI510R: Photonics Khanh Kieu College of Optical Sciences, University of Arizona kkieu@optics.arizona.edu Meinel building R.626 Important announcements Homework #1 assigned, due Jan 24 No class Monday,
OPTI510R: Photonics Khanh Kieu College of Optical Sciences, University of Arizona kkieu@optics.arizona.edu Meinel building R.626 Important announcements Homework #1 assigned, due Jan 24 No class Monday,
The Photoelectric Effect Can Be Explained Without Quantum Theory
 The Photoelectric Effect Can Be Explained Without Quantum Theory Quantum theory is a field of physics that is required to understand An object that absorbs all the radiation can also perfectly emit all
The Photoelectric Effect Can Be Explained Without Quantum Theory Quantum theory is a field of physics that is required to understand An object that absorbs all the radiation can also perfectly emit all
HALF LIFE. NJSP HMRU June 10, Student Handout CBRNE AWARENESS Module 4 1. Objectives. Student will
 June 10, 2004 Radiological/Nuclear Overview 1 Student will demonstrate a knowledge of self protection techniques identify types of radiation and their associated hazards demonstrate a knowledge of terminology
June 10, 2004 Radiological/Nuclear Overview 1 Student will demonstrate a knowledge of self protection techniques identify types of radiation and their associated hazards demonstrate a knowledge of terminology
January 29, 2019 Chemistry 328N
 Lecture 3 NMR Spectroscopy January 29, 2019 Molecular Spectroscopy Molecular spectroscopy: the study of the frequencies of electromagnetic radiation that are absorbed or emitted by substances and the correlation
Lecture 3 NMR Spectroscopy January 29, 2019 Molecular Spectroscopy Molecular spectroscopy: the study of the frequencies of electromagnetic radiation that are absorbed or emitted by substances and the correlation
3. Particle-like properties of E&M radiation
 3. Particle-like properties of E&M radiation 3.1. Maxwell s equations... Maxwell (1831 1879) studied the following equations a : Gauss s Law of Electricity: E ρ = ε 0 Gauss s Law of Magnetism: B = 0 Faraday
3. Particle-like properties of E&M radiation 3.1. Maxwell s equations... Maxwell (1831 1879) studied the following equations a : Gauss s Law of Electricity: E ρ = ε 0 Gauss s Law of Magnetism: B = 0 Faraday
Light Quanta. Particle-Wave History 11/2/2008. Particle-Wave Nature Continued s
 Light Quanta Particle-Wave History 1700 s Corpuscular Model -- Newton Wave Model Huygens 1801 Thomas Young s double slit experiment waves 1862 Maxwell s prediction that light carried energy as oscillating
Light Quanta Particle-Wave History 1700 s Corpuscular Model -- Newton Wave Model Huygens 1801 Thomas Young s double slit experiment waves 1862 Maxwell s prediction that light carried energy as oscillating
Unit 13: Nuclear Chemistry
 Name Unit 13: Nuclear Chemistry Skills: 1. Review Atomic Structure 2. Determining Nuclear Stability 3. Naming and Drawing Hydrocarbons 4. Using N + O to Write Decay Equations Period 5. Solve Various Half
Name Unit 13: Nuclear Chemistry Skills: 1. Review Atomic Structure 2. Determining Nuclear Stability 3. Naming and Drawing Hydrocarbons 4. Using N + O to Write Decay Equations Period 5. Solve Various Half
Computation of Electromagnetic Energy Absorption in the Human Body Tissues by High Frequency Structure Simulator
 Computation of Electromagnetic Energy Absorption in the Human... Computation of Electromagnetic Energy Absorption in the Human Body Tissues by High requency Structure Simulator Md. Selim Hossain 1 and
Computation of Electromagnetic Energy Absorption in the Human... Computation of Electromagnetic Energy Absorption in the Human Body Tissues by High requency Structure Simulator Md. Selim Hossain 1 and
Physics Spring 2010 Lab 1 - Electron Spin Resonance
 Physics 24 -- Spring 2010 Lab 1 - Electron Spin Resonance Theory The application of an external magnetic field to an atom will split the atomic energy levels due to an interaction between the magnetic
Physics 24 -- Spring 2010 Lab 1 - Electron Spin Resonance Theory The application of an external magnetic field to an atom will split the atomic energy levels due to an interaction between the magnetic
Electron Spin Resonance, Basic principle of NMR, Application of NMR in the study of Biomolecules, NMR imaging and in vivo NMR spectromicroscopy
 Electron Spin Resonance, Basic principle of NMR, Application of NMR in the study of Biomolecules, NMR imaging and in vivo NMR spectromicroscopy Mitesh Shrestha Electron Spin Resonance Electron paramagnetic
Electron Spin Resonance, Basic principle of NMR, Application of NMR in the study of Biomolecules, NMR imaging and in vivo NMR spectromicroscopy Mitesh Shrestha Electron Spin Resonance Electron paramagnetic
Modeling Effect of Electromagnetic Interference on Cardiovascular System
 ISSN: 5-68 Modeling Effect of Electromagnetic Interference on Cardiovascular System Anil Sirswal Electronics & Communication Engineering, School of Engineering and Technology, Noida International University,
ISSN: 5-68 Modeling Effect of Electromagnetic Interference on Cardiovascular System Anil Sirswal Electronics & Communication Engineering, School of Engineering and Technology, Noida International University,
Yellow. Strontium red white. green. yellow violet. green. red. Chapter 4. Arrangement of Electrons in Atoms. Table of Contents
 Chapter 4 Arrangement of Electrons in Atoms Table of Contents Section 1 Section 2 Section 3 The Development of a New Atomic Model The Quantum Model of the Atom Electron Configurations Sodium Yellow Strontium
Chapter 4 Arrangement of Electrons in Atoms Table of Contents Section 1 Section 2 Section 3 The Development of a New Atomic Model The Quantum Model of the Atom Electron Configurations Sodium Yellow Strontium
Unit 5 Physical Science Radioactivity Answer Key
 Unit 5 Physical Science Radioactivity Answer Key Page 198 1. True 2. False: The higher the frequency is, the higher the energy is. 3. True 4. False: Energy increases as frequency increases. 5. False: The
Unit 5 Physical Science Radioactivity Answer Key Page 198 1. True 2. False: The higher the frequency is, the higher the energy is. 3. True 4. False: Energy increases as frequency increases. 5. False: The
Introduction. Electromagnetic Waves. Electromagnetic Waves
 Introduction Much of the information we know about electrons comes from studies of interactions of light and matter. In the early 1900 s, scientists discovered that light has properties of both a wave
Introduction Much of the information we know about electrons comes from studies of interactions of light and matter. In the early 1900 s, scientists discovered that light has properties of both a wave
Globe Institute- October 2016 CRYSTALS HEALING & VIBRATION
 Globe Institute- October 2016 CRYSTALS HEALING & VIBRATION I. Introductory notes: Every living thing on this planet has a life force of energy. Energy in its simplest form is vibration. This vibration
Globe Institute- October 2016 CRYSTALS HEALING & VIBRATION I. Introductory notes: Every living thing on this planet has a life force of energy. Energy in its simplest form is vibration. This vibration
Chapter 28: Quantum Physics. Don t Copy This. Quantum Physics 3/16/13
 Chapter 28: Quantum Physics Key Terms: Photoelectric effect Photons de Broglie wavelength Energy level diagram Wave-particle duality Don t Copy This Except for relativity, everything we have studied up
Chapter 28: Quantum Physics Key Terms: Photoelectric effect Photons de Broglie wavelength Energy level diagram Wave-particle duality Don t Copy This Except for relativity, everything we have studied up
Chapter 5. The Electromagnetic Spectrum. What is visible light? What is visible light? Which of the following would you consider dangerous?
 Which of the following would you consider dangerous? X-rays Radio waves Gamma rays UV radiation Visible light Microwaves Infrared radiation Chapter 5 Periodicity and Atomic Structure 2 The Electromagnetic
Which of the following would you consider dangerous? X-rays Radio waves Gamma rays UV radiation Visible light Microwaves Infrared radiation Chapter 5 Periodicity and Atomic Structure 2 The Electromagnetic
