Chapter 9 Long range perturbation theory
|
|
|
- Poppy Dorsey
- 5 years ago
- Views:
Transcription
1 Chapter 9 Long range perturbation theory 9.1 Induction energy Substitution of the expanded interaction operator leads at the first order to the multipole expression of the electrostatic interaction energy. At the second order, we have in the Cartesian tensor formulation E (2) (ind,b) = b 0 1 E B 0b 00 T ˆq Aˆq B + T α (ˆq Aˆµ B α ˆµ A α ˆq B ) + T αβ (ˆq A ˆΘB αβ + ˆΘ A αβ ˆq B ˆµ A α ˆµ B β ) b 0b T ˆq Aˆq B + T α (ˆq Aˆµ B α ˆµA α ˆqB ) + T α β (ˆqA ˆΘB α β + ˆΘ A α β ˆqB ˆµ A α ˆµB β ) (9.1) Performing the integrations on the A subsystem, we obtain the corresponding ground state multipole moments. Grouping the terms according to the multipole rank of the B subsystem, and taking into account that ψ B 0 ˆq B ψ B b = 0, E (2) (ind,b) = (T α q A T αβ µ A α +...) b 0 0 ˆµ B α b b ˆµ B α 0 (T E0b B α q A T α β µa α +...) (T αβ q A + T αβγ µ A γ +...) b 0 0 ˆΘ B αβ b b ˆµB α 0 (T α q A T α β µa β +...) E B 0b (T α q A T αβ µ A α +...) b 0 0 ˆµ B α b b ˆΘ B α β 0 (T α β qa + T α β γ µa γ +...) E B 0b (T αβ q A + T αβγ µ A γ +...) b 0 0 ˆΘ B αβ b b ˆΘ B α β 0 (T α β qa T α β γ µa γ +...)... E B 0b (9.2) 75
2 E (2) (ind,b) = 1 2 F A α α B α,α F A α 1 3 F A α A B α,α β F A α β 1 6 F A αβc B αβ,α β F A α β +... (9.5) Introduce the multipole polarizabilities α αβ = n 0 0 ˆµ α n n ˆµ β ˆµ β n n ˆµ α 0 A α,βγ = n 0 C αβ,γδ = 1 3 n 0 0 ˆµ α n n ˆΘ βγ ˆΘ βγ n n ˆµ α 0 0 ˆΘ αβ n n ˆΘ γδ ˆΘ γδ n n ˆΘ αβ 0 (9.3) and identify the parentheses as the electric field, field gradient, etc. F A α = (T α q A T αβ µ A α +...) F A αβ = (T αβ q A + T αβγ µ A γ +...) (9.4) The induction energy in terms of the field (and its gradients) and the multipolar polarizabilities In the spherical tensor formalism, the general polarizability component α lκ,l κ = n 0 and the induction energy takes the form E (2) (ind,b) = ˆQ lκ n n ˆQ l κ ˆQ l κ n n ˆQ lκ 0 (9.6) lκ l κ α B lκl κ V A lκv A l κ = 1 2 lκ Q B lκlv A lκ (9.7) where Q B lκl are the induced moments of B Q B lκ = l κ α B lκl κ V A l κ (9.8) The multipolar induction energy can be truncated according to the powers of (1/R) N does not ensure that the induction energy is negative according to the maximum rank of the multipole operators (L) always negative 76
3 9.2 Multipole polarizabilities Dipole polarizability is a symmetric tensor α xx α xy α xz α = α yy α yz (9.9) α zz Units are volume (Å 3 or bohr 3 ); the SI units are Fm 2. 1 a.u. = Å 3 = Fm 2. Proportional to the volume, as it can be seen by introducing an average excitation energy and using the resolution of identity α = n ˆr α n n ˆr α 0 2 U ( 0 ˆr 2 α 0 0 ˆr α 0 0 ˆr α 0 ) (9.10) and inversely proportional to the excitation energy. As any second-rank tensor, it can be decomposed into irreducible parts, according to α = α (0) + α (1) + α (2) (9.11) where α (0) αβ = 1 3 Tr(α)δ αβ α (1) αβ = 1 2 (α αβ α βα ) = 0 (9.12) α (2) αβ = 1 2 (α αβ + α βα ) 1 3 Tr(α)δ αβ α (1) vanishes, since α is symmetric. In a principal-axis system we have the decomposition α 0 0 α xx α 0 0 α = 0 α α yy α 0 (9.13) 0 0 α 0 0 α zz α The first trace-invariant quantity that can be formed is the mean polarizability α = 1 3 Trα = 1 3 (α xx + α yy + α zz ) (9.14) and the second trace-invariant is the polarizability anisotropy, γ, defined as the positive root of γ 2 = 1 2 [3Tr(α2 ) Tr(α) 2 ] (9.15) 77
4 or in components which can be written in a principal axis system γ 2 = 1 2 [3α αβα βα α αα α ββ ] (9.16) γ 2 = 1 2 [(α xx α yy ) 2 + (α yy α zz ) 2 + (α zz α xx ) 2 ] (9.17) and makes clear that this quantity vanishes for spherically symmetric systems. For linear molecules α zz = α and α xx = α yy = α and the polarizability anisotropy, γ γ = α α (9.18) For linear molecules the dipole-quadrupole polarizability can be written in terms of the unit vector along the symmetry axis, e = (e x, e y, e z ) as A αβγ = 1 2 A (3e α e β e γ e α δ βγ ) + A (e β δ αγ + e γ δ αβ 2e α e β e γ ) (9.19) where A = A zzz and A = A xxz = A yyz. For tetrahedral molecules the dipole-dipole polarizability is isotropic, the dipolequadrupole polarizability contains only one independent principal axis component, A = A xyz. This is the first anisotropic polarizability for tetrahedral symmetry Translational invariance The dipole-dipole polarizability is always origin-independent. Higher-rank multipole polarizabilities are (always) origin-dependent. For instance, the A z,zz component involves the ˆΘ zz operator. After a shift of the origin by c = (0, 0, c) ˆΘ C zz = ˆΘ O zz 2cˆµ O z (9.20) and the dipole-quadrupole polarizability at the new origin is A C z,zz = n 0 0 ˆµ O z n n ˆΘ O zz 2cˆµ O z 0 = A O z,zz 2cα zz (9.21) This means that the origin (and the dipole polarizability) should always be specified when A α,βγ is given. 78
5 9.2.2 Multipole polarizabilities spherical tensors The general multipole polarizabilities, defined in terms of the (irreducible) multipole moment operators, ˆQlm α l1 m 1,l 2 m 2 = n 0 0 ˆQ l1 m 1 n n ˆQ l2 m ˆQ l2 m 2 n n ˆQ l1 m 1 0 (9.22) is a reducible spherical tensor quantity, which can be decomposed into irreducible parts as α l1 l 2 :kq = m 1 m 2 C(l 1 l 2 k; m 1 m 2 q)α l1 m 1,l 2 m 2 (9.23) where C(l 1 l 2 k; m 1 m 2 q) are the Clebsch-Gordan coefficients. Since C = 0, unless k = l 1 +l 2, l 1 +l 2 1,..., l 1 l 2, the nonzero irreducible parts of the dipole-dipole polarizability can be only with k = 0, 2, for the dipole-quadrupole polarizability, k = 1, 3, etc. For instance, 1 α 11:00 = 3 (α xx + α yy + α zz ) 2 α 11:20 = 3 γ (9.24) 9.3 Distance dependence of the induction energy Interaction of a spherically symmetric polarizability α αβ = αδ αβ with multipoles: charge E(ind, q) = 1 2 qt αα αβ T β q = 1 2 q2 T α T α α = 1 2 q2 α αrα 2 R 3 R = 1 q 2 α 3 2 R 4 (9.25) dipole E(ind, µ) = 1 2 µ αt αβ α ββ T β α µ α = 1 µ 2 α(3 cos 2 θ + 1) (9.26) 2 R 6 quadrupole E(ind, Θ) R 8 (9.27) 79
6 9.4 Convergence of the multipolar induction energy The multipole-expanded interaction Hamiltonian of a H-atom + proton system is V mult = 1 [ ( ) n r 1 P n (cos θ)] (9.28) R R The exact second order multipole energy (Dalgarno & Lynn, 1957) The ratio of successive terms E (2) = n=1 n=0 (2n + 2)!(n + 2) n(n + 1)R (2n+2) (9.29) 2n(n + 3)(2n + 3) lim = (9.30) n (n + 2)R 2 indicates that for any value of R the series is divergent. 9.5 Dispersion energy Dispersion interaction energy in spherical tensor formalism E (2) (disp) = a 0 b 0 l A m A l B m B 00 ˆQ A l A m A T la m A,l B m B ˆQB lb m B ab ab ˆQ A l T A m l A A m ˆQ B A,l B m B l B B 00 m E0a A + E0b B Applying the Casimir-Polder method (9.31) E (2) (disp) = 2 π T l A m A,l B m B T l A m A,l B m B 0 ˆQ A l A m A a a ˆQ A l dω A A 0 ω m 0a 0 ˆQ B l B m B b b ˆQ B l B B 0 ω m 0b (9.32) ω0a 2 + ω 2 ω0b 2 + ω2 a 0 b 0 Dynamic (frequency-dependent) multipole polarizabilities in molecule-fixed frame α lm,l m (ω) = n 0 2ω 0n 0 ˆQ lm a a ˆQ l m 0 ω 2 0a ω 2 (9.33) related to the charge density susceptibility α lm,l m (ω) = drdr R lm (r)α(r, r ω)r l m (r ) (9.34) 80
7 Dispersion energy E (2) (disp) = T la m A,l B m B T l A m A,l B m B XAB l A m A l B m B l A m A,l B m B (9.35) The Casimir-Polder or dispersion integrals are defined as X AB l A m A l B m B l = dωα A A m A,l B l 2π A m A,l A m (iω)αb A l B m B,l (iω) (9.36) B m B The dispersion integrals can be calculated by numerical quadrature as X AB = M w(ω j )α A (iω j )α B (iω j ) (9.37) 2π j=1 With a Gauss-Chebyshev quadrature scheme the grid points are chosen as ( ω j = cot π 2j 1 ) 4M and the weights w(ω j ) = 2π 4M sin 2 (π(2j 1)/4M) (9.38) (9.39) Typically, with 5 and 7 grid points one has an accuracy of 0.1 %. Numerically more efficient procedure (for high-rank calculations) is to contract first the polarizabilities with the interaction tensors (in local frame) and perform the numerical integration afterwards (Hättig, 1996). In this form the interaction tensors and X AB are both reducible: they transform according to the double and quadruple product group of SO(3). In terms of irreducible tensors, the dispersion coefficients are of the form (Wormer) C L AL B L l A l A l Bl B = m A m A m B m B X AB l A m A l B m B l factor (9.40) A m A,l B m B The algebraic factor depends on the Wigner 3j and 9j coefficients. These coefficients are coupled to the following form C L AL B L n (n = l A + l B + l A + l B + 2) For atoms in S-state, for example, E (2) disp = C 2n R 2n n=3 n 2 C 2n = C(l; n k 1) l=1 C(l; l ) = (2l + 2l )! (2l)!(2l )! 1 2π dωα A l (iω)α B l (iω) (9.41) 81
8 The dispersion integral depends on the properties of the individual molecules and independent of the intermolecular geometry. The complete expression can be expressed in terms of irreducible spherical tensor components. dipole-dipole leading term R 3 R 3 R 6 dipole-quadrupole would be R 3 R 4 R 7, after averaging over all orientations, it gives zero quadrupole-quadrupole term R 4 R 4 R 8 dipole-octopole and quadrupole-qudrupole R Dipolar dispersion energy The leading multipolar term in the dispersion interaction energy E (2) (disp) = a 0 00 ˆµ A αt αβ ˆµ B β ab ab ˆµA γ T γδ ˆµ B δ 00 b 0 E A 0a + E B 0b (9.42) Casimir-Polder formula E (2) (disp) = T αβ T γδ 2 π dω a 0 0 ˆµ A α a a ˆµ A γ 0 ω 0a ω 2 0a + ω 2 0 ˆµ B β b b ˆµB δ 0 ω 0b ω0b 2 + ω2 (9.43) b 0 Unsöld approximation E (2) E (disp) = T αβ T 0a E A B 0 ˆµ A 0b α a a ˆµ A γ 0 0 ˆµ B β b b ˆµB δ 0 γδ E A a 0 b 0 0a + E0b B ω0a A ω0b B (9.44) In order to factor this latter expression, we use the identity with E0a E A 0b B E0a A + E0b B = U AU B U A + U B (1 + ab ) (9.45) ab = 1/U A 1/E A 0a + 1/U B 1/E B 0b 1/E A 0a + 1/E B 0b (9.46) that can be made negligibly small by choosing appropriate average excitation energies U A and U B E (2) (disp) U AU B 4(U A + U B ) T αβt γδ ααγα A βδ B (9.47) 82
9 In both expressions we can separate the orientation-independent spherically averaged and various orientation-dependent components, by using the decomposition of the polarizabilities to irreducible parts. The spherically averaged component becomes in both cases leading to the general expression with the C 6 coefficient C 6 = 3 T αβ T γδ α A δ αγ α B δ βδ = α A α B T αβ T αβ = α A α B 6 R 6 (9.48) C 6 E (2) (disp) C 6 R 6 (9.49) dωα A (iω)α B (iω) (Casimir-Polder) 3U AU B 2(U A + U B ) αa α B (London) (9.50) Experimental oscillator strength distributions can be used to determine experimental C 6 coefficients. Roughly proportional to the square of the polarizability/volume. Some typical values system C 6 C 8 C 10 H H He He Ne Ne Ar Ar Kr Kr Xe Xe 286 Combining rules can be deduced from the London-formula C AB 6 C AA 6 C BB 6 (9.51) Approximate dispersion formulae The average excitation energy of the London formula is an empirical parameter, either the first ionization potential (quite bad) or the first excitation energy (somewhat better) is used. 83
10 Similar expressions can be derived from the Casimir-Polder expression, by considering some general properties of the average dynamic dipole-dipole polarizabilities α(iω) = 1 k 0 2ω 0k 0 ˆx k 2 ω 2 0k + ω2 (9.52) We are looking for the simplest, one-term approximation in the form α(iω) ω 2 ω 2 + ω 2 a (9.53) Parameters a and ω will be found from the asymptotic behaviour of α(iω). For ω = 0 one gets the static polarizability, a = α(0). For ω one gets the Thomas-Reiche-Kuhn sum rule, the number of electrons α(iω) 1 2 E 2 ω 2 0n x 2 0n = n 0 n 2 ω 2 (9.54) In this limit we have a ω 2 /ω 2 = n 2 ω 2 ω 2 = n 2 α(0) ω = 1 n α(0) (9.55) which leads to the Mavroyannis-Stephen (Slater-Kirkwood) approximation C 6 3α A α B (α A /n A ) 1/2 + (α B /n B ) 1/2 (9.56) Take another, equivalent form of the dynamic polarizability α(ω) = α + (ω) + α + ( ω) = 1 3 n 0 r on 2 ω 0n + ω n 0 r on 2 ω 0n ω (9.57) For ω = 0 α + (ω) = 1 α(0) (9.58) 2 For ω ωα + (ω) 1 r 0n 2 = 1 0 r 3 3[ r 0 2 = 1 3 ( r)2 (9.59) n 0 84
11 Considering a one-term approximation α + (ω) = Taking the limiting cases one obtains a (ω + ω) a = 1 2 α(0) ω = 2 ( r) 2 3 α and we get the Salem-Tang-Karplus approximation C 6 = For dimers it is identical to the Alexander upper bound (9.60) (9.61) α A α B α A /( r A ) 2 + α B ( r B ) 2 (9.62) C S( 2)S( 1) = 1 2 α( r B) 2 (9.63) Solutions of the H + H dispersion problem The multipolar interaction Hamiltonian for two H atoms lying on the z axis, separated by a distance R 0 is ˆV = 2[R 3 0 ξ 1 ξ 2 cos θ 1 cos θ 2 + βξ 1 ξ 2 2 cos θ 1 (3 cos 2 θ 2 1) + γξ 2 1ξ 2 2(3 cos 2 θ 1 1)(3 cos 2 θ 2 1) +... (9.64) with polar coordinates ξ 1,2 = r 1,2, α = ( 6/2)R 3 0, β = ( (30)/4)R 4 0 and γ = ( 70/8)R 5. By direct summation over the excited states, Eisenchitz and London obtained for the dipolar dispersion energy E (2) = 12 z0n E 2 0m z0m 2 ) (9.65) R0 6 n,m (1 )(1 1n2 )(2 1m2 1n2 1m2 It is difficult to make converge this expression, because of the discrete-continuum matrix elements. The best value is C 6 = The difficulties of the sum-over-states solution can be avoided by solving directly for the first-order wave function using the Ansatz proposed by Slater and Kirkwood, ψ(r 1, r 2 ) = ψ 0 (r 1, r 2 )[1 + φ(r 1, r 2 )] (9.66) with the ground state unperturbed wave function of the dimer, ψ 0 (r 1, r 2 ). two-particle correlation function, φ, satisfies the differential equation The φ + ( ln ψ 0 ) φ) v = 0 (9.67) 85
12 where v can be one of the multipolar terms of the interaction Hamiltonian. the correlation function in the form leads to a differential equation for R(ξ, ξ ). Taking φ = vr(ξ, ξ ) E 0 (9.68) Slater and Kirkwood (1931) obtained an approximate solution, leading to C 6 = Pauling and Beach (1935) used special orbitals to construct the Hamiltonian matrix and obtained C 6 = , C 8 = and C 10 = Recent exact solutions obtained by Choy (P.R.A. 62 (2000) ) using orthogonal polynomials, confirm these values. 9.6 Convergence of the 1/R-expansion The 1/R expansion of the total interaction energy converges asymptotically to the ground state interaction energy. For each finite R, however, the 1/R expansion may diverge. It had been proven for H 2 molecule and H + 2 ion. The 1/R expansion of the second-order polarization energy for H + 2 ion (induction energy) diverges for all finite R. The 1/R expansion of the second-order polarization energy for H 2 molecule, i.e. its dispersion energy, diverges for all finite R. Given a finite one-particle GTF or STF basis set, the multipole expansion of each order of the polarization series converges, as long as the smallest spheres around the respective nuclei and basis function centers do not touch. However, the limit of this convergence may be quite different from the exact interaction energy. 86
13 87
Electric properties of molecules
 Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Multipole moments. Dipole moment. The second moment µ is more commonly called the dipole moment, of the charge. distribution and is a vector
 Dipole moment Multipole moments The second moment µ is more commonly called the dipole moment, of the charge distribution and is a vector µ = µ x ˆx + µ y ŷ + µ z ẑ where the α component is given by µ
Dipole moment Multipole moments The second moment µ is more commonly called the dipole moment, of the charge distribution and is a vector µ = µ x ˆx + µ y ŷ + µ z ẑ where the α component is given by µ
Many-body dispersion interactions between semiconducting wires
 Many-body dispersion interactions between semiconducting wires Alston J. Misquitta TCM Group, Cavendish Laboratory University of Cambridge 26 th July, 2010 Collaborators James Spencer (Imperial College,
Many-body dispersion interactions between semiconducting wires Alston J. Misquitta TCM Group, Cavendish Laboratory University of Cambridge 26 th July, 2010 Collaborators James Spencer (Imperial College,
Intermolecular Forces
 Natural Sciences Tripos: Part III Chemistry course M4 Intermolecular Forces Alston J. Misquitta Michaelmas Term 2007 Copyright A. J. Misquitta 2007 Contents 1 Introduction 1 2 Basic ideas 4 3 Pair potentials
Natural Sciences Tripos: Part III Chemistry course M4 Intermolecular Forces Alston J. Misquitta Michaelmas Term 2007 Copyright A. J. Misquitta 2007 Contents 1 Introduction 1 2 Basic ideas 4 3 Pair potentials
Module I: Electromagnetic waves
 Module I: Electromagnetic waves Lectures 10-11: Multipole radiation Amol Dighe TIFR, Mumbai Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation
Module I: Electromagnetic waves Lectures 10-11: Multipole radiation Amol Dighe TIFR, Mumbai Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation
Electrodynamics II: Lecture 9
 Electrodynamics II: Lecture 9 Multipole radiation Amol Dighe Sep 14, 2011 Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation Outline 1 Multipole
Electrodynamics II: Lecture 9 Multipole radiation Amol Dighe Sep 14, 2011 Outline 1 Multipole expansion 2 Electric dipole radiation 3 Magnetic dipole and electric quadrupole radiation Outline 1 Multipole
Spin Interactions. Giuseppe Pileio 24/10/2006
 Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Short-Ranged Central and Tensor Correlations. Nuclear Many-Body Systems. Reaction Theory for Nuclei far from INT Seattle
 Short-Ranged Central and Tensor Correlations in Nuclear Many-Body Systems Reaction Theory for Nuclei far from Stability @ INT Seattle September 6-, Hans Feldmeier, Thomas Neff, Robert Roth Contents Motivation
Short-Ranged Central and Tensor Correlations in Nuclear Many-Body Systems Reaction Theory for Nuclei far from Stability @ INT Seattle September 6-, Hans Feldmeier, Thomas Neff, Robert Roth Contents Motivation
Convergence of the multipole expansions of the polarization and dispersion interactions for atoms under confinement
 Convergence of the multipole expansions of the polarization and dispersion interactions for atoms under confinement Yong-Hui Zhang 1,2, Li-Yan Tang 2, Xian-Zhou Zhang 1, Jun Jiang 3 and J. Mitroy 3 1 Department
Convergence of the multipole expansions of the polarization and dispersion interactions for atoms under confinement Yong-Hui Zhang 1,2, Li-Yan Tang 2, Xian-Zhou Zhang 1, Jun Jiang 3 and J. Mitroy 3 1 Department
Problem Set 2 Due Tuesday, September 27, ; p : 0. (b) Construct a representation using five d orbitals that sit on the origin as a basis: 1
 Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
Supporting Information. Local decomposition of imaginary polarizabilities. and dispersion coefficients
 Electronic Supplementary Material (ESI) for Physical hemistry hemical Physics. This journal is the Owner Societies 27 Supporting Information Local decomposition of imaginary polarizabilities and dispersion
Electronic Supplementary Material (ESI) for Physical hemistry hemical Physics. This journal is the Owner Societies 27 Supporting Information Local decomposition of imaginary polarizabilities and dispersion
Physics 221A Fall 1996 Notes 21 Hyperfine Structure in Hydrogen and Alkali Atoms
 Physics 221A Fall 1996 Notes 21 Hyperfine Structure in Hydrogen and Alkali Atoms Hyperfine effects in atomic physics are due to the interaction of the atomic electrons with the electric and magnetic multipole
Physics 221A Fall 1996 Notes 21 Hyperfine Structure in Hydrogen and Alkali Atoms Hyperfine effects in atomic physics are due to the interaction of the atomic electrons with the electric and magnetic multipole
Nuclear models: Collective Nuclear Models (part 2)
 Lecture 4 Nuclear models: Collective Nuclear Models (part 2) WS2012/13: Introduction to Nuclear and Particle Physics,, Part I 1 Reminder : cf. Lecture 3 Collective excitations of nuclei The single-particle
Lecture 4 Nuclear models: Collective Nuclear Models (part 2) WS2012/13: Introduction to Nuclear and Particle Physics,, Part I 1 Reminder : cf. Lecture 3 Collective excitations of nuclei The single-particle
B. PHENOMENOLOGICAL NUCLEAR MODELS
 B. PHENOMENOLOGICAL NUCLEAR MODELS B.0. Basic concepts of nuclear physics B.0. Binding energy B.03. Liquid drop model B.04. Spherical operators B.05. Bohr-Mottelson model B.06. Intrinsic system of coordinates
B. PHENOMENOLOGICAL NUCLEAR MODELS B.0. Basic concepts of nuclear physics B.0. Binding energy B.03. Liquid drop model B.04. Spherical operators B.05. Bohr-Mottelson model B.06. Intrinsic system of coordinates
University of Illinois at Chicago Department of Physics
 University of Illinois at Chicago Department of Physics Electromagnetism Qualifying Examination January 4, 2017 9.00 am - 12.00 pm Full credit can be achieved from completely correct answers to 4 questions.
University of Illinois at Chicago Department of Physics Electromagnetism Qualifying Examination January 4, 2017 9.00 am - 12.00 pm Full credit can be achieved from completely correct answers to 4 questions.
( ) R kj. = y k y j. y A ( ) z A. y a. z a. Derivatives of the second order electrostatic tensor with respect to the translation of ( ) δ yβ.
 Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
B7 Symmetry : Questions
 B7 Symmetry 009-10: Questions 1. Using the definition of a group, prove the Rearrangement Theorem, that the set of h products RS obtained for a fixed element S, when R ranges over the h elements of the
B7 Symmetry 009-10: Questions 1. Using the definition of a group, prove the Rearrangement Theorem, that the set of h products RS obtained for a fixed element S, when R ranges over the h elements of the
Electric and magnetic multipoles
 Electric and magnetic multipoles Trond Saue Trond Saue (LCPQ, Toulouse) Electric and magnetic multipoles Virginia Tech 2017 1 / 22 Multipole expansions In multipolar gauge the expectation value of the
Electric and magnetic multipoles Trond Saue Trond Saue (LCPQ, Toulouse) Electric and magnetic multipoles Virginia Tech 2017 1 / 22 Multipole expansions In multipolar gauge the expectation value of the
Vibrational states of molecules. Diatomic molecules Polyatomic molecules
 Vibrational states of molecules Diatomic molecules Polyatomic molecules Diatomic molecules V v 1 v 0 Re Q Birge-Sponer plot The solution of the Schrödinger equation can be solved analytically for the
Vibrational states of molecules Diatomic molecules Polyatomic molecules Diatomic molecules V v 1 v 0 Re Q Birge-Sponer plot The solution of the Schrödinger equation can be solved analytically for the
Expansion of 1/r potential in Legendre polynomials
 Expansion of 1/r potential in Legendre polynomials In electrostatics and gravitation, we see scalar potentials of the form V = K d Take d = R r = R 2 2Rr cos θ + r 2 = R 1 2 r R cos θ + r R )2 Use h =
Expansion of 1/r potential in Legendre polynomials In electrostatics and gravitation, we see scalar potentials of the form V = K d Take d = R r = R 2 2Rr cos θ + r 2 = R 1 2 r R cos θ + r R )2 Use h =
Notes: Most of the material presented in this chapter is taken from Jackson, Chap. 2, 3, and 4, and Di Bartolo, Chap. 2. 2π nx i a. ( ) = G n.
 Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 2017
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.07: Electromagnetism II November 5, 207 Prof. Alan Guth FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 207 A few items below are marked
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.07: Electromagnetism II November 5, 207 Prof. Alan Guth FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 207 A few items below are marked
Multipole Fields in the Vacuum Gauge. June 26, 2016
 Multipole Fields in the Vacuum Gauge June 26, 2016 Whatever you call them rubber bands, or Poincaré stresses, or something else there have to be other forces in nature to make a consistent theory of this
Multipole Fields in the Vacuum Gauge June 26, 2016 Whatever you call them rubber bands, or Poincaré stresses, or something else there have to be other forces in nature to make a consistent theory of this
Problem Set 2 Due Thursday, October 1, & & & & # % (b) Construct a representation using five d orbitals that sit on the origin as a basis:
 Problem Set 2 Due Thursday, October 1, 29 Problems from Cotton: Chapter 4: 4.6, 4.7; Chapter 6: 6.2, 6.4, 6.5 Additional problems: (1) Consider the D 3h point group and use a coordinate system wherein
Problem Set 2 Due Thursday, October 1, 29 Problems from Cotton: Chapter 4: 4.6, 4.7; Chapter 6: 6.2, 6.4, 6.5 Additional problems: (1) Consider the D 3h point group and use a coordinate system wherein
This is the author s version of a work that was submitted for publication prior to peer-review. This is known as the pre-print.
 This is the author s version of a work that was submitted for publication prior to peer-review. This is known as the pre-print. Citation for author s submitted version Zhang, Yong-Hui, Tang, Li-Yan, Zhang,
This is the author s version of a work that was submitted for publication prior to peer-review. This is known as the pre-print. Citation for author s submitted version Zhang, Yong-Hui, Tang, Li-Yan, Zhang,
PEAT SEISMOLOGY Lecture 2: Continuum mechanics
 PEAT8002 - SEISMOLOGY Lecture 2: Continuum mechanics Nick Rawlinson Research School of Earth Sciences Australian National University Strain Strain is the formal description of the change in shape of a
PEAT8002 - SEISMOLOGY Lecture 2: Continuum mechanics Nick Rawlinson Research School of Earth Sciences Australian National University Strain Strain is the formal description of the change in shape of a
The Raman Effect. A Unified Treatment of the Theory of Raman Scattering by Molecules. DerekA. Long
 The Raman Effect A Unified Treatment of the Theory of Raman Scattering by Molecules DerekA. Long Emeritus Professor ofstructural Chemistry University of Bradford Bradford, UK JOHN WILEY & SONS, LTD Vll
The Raman Effect A Unified Treatment of the Theory of Raman Scattering by Molecules DerekA. Long Emeritus Professor ofstructural Chemistry University of Bradford Bradford, UK JOHN WILEY & SONS, LTD Vll
Multipole moments. November 9, 2015
 Multipole moments November 9, 5 The far field expansion Suppose we have a localized charge distribution, confined to a region near the origin with r < R. Then for values of r > R, the electric field must
Multipole moments November 9, 5 The far field expansion Suppose we have a localized charge distribution, confined to a region near the origin with r < R. Then for values of r > R, the electric field must
The AKLT Model. Lecture 5. Amanda Young. Mathematics, UC Davis. MAT290-25, CRN 30216, Winter 2011, 01/31/11
 1 The AKLT Model Lecture 5 Amanda Young Mathematics, UC Davis MAT290-25, CRN 30216, Winter 2011, 01/31/11 This talk will follow pg. 26-29 of Lieb-Robinson Bounds in Quantum Many-Body Physics by B. Nachtergaele
1 The AKLT Model Lecture 5 Amanda Young Mathematics, UC Davis MAT290-25, CRN 30216, Winter 2011, 01/31/11 This talk will follow pg. 26-29 of Lieb-Robinson Bounds in Quantum Many-Body Physics by B. Nachtergaele
Problem Set #4: 4.1,4.7,4.9 (Due Monday, March 25th)
 Chapter 4 Multipoles, Dielectrics Problem Set #4: 4.,4.7,4.9 (Due Monday, March 5th 4. Multipole expansion Consider a localized distribution of charges described by ρ(x contained entirely in a sphere of
Chapter 4 Multipoles, Dielectrics Problem Set #4: 4.,4.7,4.9 (Due Monday, March 5th 4. Multipole expansion Consider a localized distribution of charges described by ρ(x contained entirely in a sphere of
QUANTUM MECHANICS AND ATOMIC STRUCTURE
 5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
Chem8028(1314) - Spin Dynamics: Spin Interactions
 Chem8028(1314) - Spin Dynamics: Spin Interactions Malcolm Levitt see also IK m106 1 Nuclear spin interactions (diamagnetic materials) 2 Chemical Shift 3 Direct dipole-dipole coupling 4 J-coupling 5 Nuclear
Chem8028(1314) - Spin Dynamics: Spin Interactions Malcolm Levitt see also IK m106 1 Nuclear spin interactions (diamagnetic materials) 2 Chemical Shift 3 Direct dipole-dipole coupling 4 J-coupling 5 Nuclear
An introduction to Solid State NMR and its Interactions
 An introduction to Solid State NMR and its Interactions From tensor to NMR spectra CECAM Tutorial September 9 Calculation of Solid-State NMR Parameters Using the GIPAW Method Thibault Charpentier - CEA
An introduction to Solid State NMR and its Interactions From tensor to NMR spectra CECAM Tutorial September 9 Calculation of Solid-State NMR Parameters Using the GIPAW Method Thibault Charpentier - CEA
Coupling of Angular Momenta Isospin Nucleon-Nucleon Interaction
 Lecture 5 Coupling of Angular Momenta Isospin Nucleon-Nucleon Interaction WS0/3: Introduction to Nuclear and Particle Physics,, Part I I. Angular Momentum Operator Rotation R(θ): in polar coordinates the
Lecture 5 Coupling of Angular Momenta Isospin Nucleon-Nucleon Interaction WS0/3: Introduction to Nuclear and Particle Physics,, Part I I. Angular Momentum Operator Rotation R(θ): in polar coordinates the
Multipole Expansion for Radiation;Vector Spherical Harmonics
 Multipole Expansion for Radiation;Vector Spherical Harmonics Michael Dine Department of Physics University of California, Santa Cruz February 2013 We seek a more systematic treatment of the multipole expansion
Multipole Expansion for Radiation;Vector Spherical Harmonics Michael Dine Department of Physics University of California, Santa Cruz February 2013 We seek a more systematic treatment of the multipole expansion
Tutorial Session - Exercises. Problem 1: Dipolar Interaction in Water Moleclues
 Tutorial Session - Exercises Problem 1: Dipolar Interaction in Water Moleclues The goal of this exercise is to calculate the dipolar 1 H spectrum of the protons in an isolated water molecule which does
Tutorial Session - Exercises Problem 1: Dipolar Interaction in Water Moleclues The goal of this exercise is to calculate the dipolar 1 H spectrum of the protons in an isolated water molecule which does
The Central Force Problem: Hydrogen Atom
 The Central Force Problem: Hydrogen Atom B. Ramachandran Separation of Variables The Schrödinger equation for an atomic system with Z protons in the nucleus and one electron outside is h µ Ze ψ = Eψ, r
The Central Force Problem: Hydrogen Atom B. Ramachandran Separation of Variables The Schrödinger equation for an atomic system with Z protons in the nucleus and one electron outside is h µ Ze ψ = Eψ, r
Nucleons in Nuclei: Interactions, Geometry, Symmetries
 Nucleons in Nuclei: Interactions, Geometry, Symmetries Jerzy DUDEK Department of Subatomic Research, CNRS/IN 2 P 3 and University of Strasbourg, F-67037 Strasbourg, FRANCE September 28, 2010 Mathematial
Nucleons in Nuclei: Interactions, Geometry, Symmetries Jerzy DUDEK Department of Subatomic Research, CNRS/IN 2 P 3 and University of Strasbourg, F-67037 Strasbourg, FRANCE September 28, 2010 Mathematial
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
NMR: Formalism & Techniques
 NMR: Formalism & Techniques Vesna Mitrović, Brown University Boulder Summer School, 2008 Why NMR? - Local microscopic & bulk probe - Can be performed on relatively small samples (~1 mg +) & no contacts
NMR: Formalism & Techniques Vesna Mitrović, Brown University Boulder Summer School, 2008 Why NMR? - Local microscopic & bulk probe - Can be performed on relatively small samples (~1 mg +) & no contacts
13, Applications of molecular symmetry and group theory
 Subject Paper No and Title Module No and Title Module Tag Chemistry 13, Applications of molecular symmetry and group theory 27, Group theory and vibrational spectroscopy: Part-IV(Selection rules for IR
Subject Paper No and Title Module No and Title Module Tag Chemistry 13, Applications of molecular symmetry and group theory 27, Group theory and vibrational spectroscopy: Part-IV(Selection rules for IR
Polarization Effects and Ionic Bonding in a Polar Diatomic : the CaF + X 1 Σ + state
 Polarization Effects and Ionic Bonding in a Polar Diatomic : the CaF + X 1 Σ + state Stephen L. Coy, Joshua H. Baraban, Robert W. Field (Chemistry, MIT) and Bryan M. Wong (Materials Chemistry, Sandia)
Polarization Effects and Ionic Bonding in a Polar Diatomic : the CaF + X 1 Σ + state Stephen L. Coy, Joshua H. Baraban, Robert W. Field (Chemistry, MIT) and Bryan M. Wong (Materials Chemistry, Sandia)
QUALIFYING EXAMINATION, Part 1. Solutions. Problem 1: Mathematical Methods. x 2. 1 (1 + 2x 2 /3!) ] x 2 1 2x 2 /3
![QUALIFYING EXAMINATION, Part 1. Solutions. Problem 1: Mathematical Methods. x 2. 1 (1 + 2x 2 /3!) ] x 2 1 2x 2 /3 QUALIFYING EXAMINATION, Part 1. Solutions. Problem 1: Mathematical Methods. x 2. 1 (1 + 2x 2 /3!) ] x 2 1 2x 2 /3](/thumbs/95/124550008.jpg) QUALIFYING EXAMINATION, Part 1 Solutions Problem 1: Mathematical Methods (a) Keeping only the lowest power of x needed, we find 1 x 1 sin x = 1 x 1 (x x 3 /6...) = 1 ) 1 (1 x 1 x /3 = 1 [ 1 (1 + x /3!)
QUALIFYING EXAMINATION, Part 1 Solutions Problem 1: Mathematical Methods (a) Keeping only the lowest power of x needed, we find 1 x 1 sin x = 1 x 1 (x x 3 /6...) = 1 ) 1 (1 x 1 x /3 = 1 [ 1 (1 + x /3!)
PH 451/551 Quantum Mechanics Capstone Winter 201x
 These are the questions from the W7 exam presented as practice problems. The equation sheet is PH 45/55 Quantum Mechanics Capstone Winter x TOTAL POINTS: xx Weniger 6, time There are xx questions, for
These are the questions from the W7 exam presented as practice problems. The equation sheet is PH 45/55 Quantum Mechanics Capstone Winter x TOTAL POINTS: xx Weniger 6, time There are xx questions, for
Nucleon-nucleon interaction
 Nucleon-nucleon interaction Shell structure in nuclei and lots more to be explained on the basis of how nucleons interact with each other in free space QCD Lattice calculations Effective field theory Exchange
Nucleon-nucleon interaction Shell structure in nuclei and lots more to be explained on the basis of how nucleons interact with each other in free space QCD Lattice calculations Effective field theory Exchange
Phys 622 Problems Chapter 5
 1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
CE 530 Molecular Simulation
 1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
Ket space as a vector space over the complex numbers
 Ket space as a vector space over the complex numbers kets ϕ> and complex numbers α with two operations Addition of two kets ϕ 1 >+ ϕ 2 > is also a ket ϕ 3 > Multiplication with complex numbers α ϕ 1 >
Ket space as a vector space over the complex numbers kets ϕ> and complex numbers α with two operations Addition of two kets ϕ 1 >+ ϕ 2 > is also a ket ϕ 3 > Multiplication with complex numbers α ϕ 1 >
Coherent isotropic averaging in zero-field nuclear magnetic resonance. II. Cubic sequences and time-reversal of spin couplings
 Coherent isotropic averaging in zero-field nuclear magnetic resonance. II. Cubic sequences and time-reversal of spin couplings A. Llor, a) Z. Olejniczak, b) and A. Pines Materials Sciences Division, Lawrence
Coherent isotropic averaging in zero-field nuclear magnetic resonance. II. Cubic sequences and time-reversal of spin couplings A. Llor, a) Z. Olejniczak, b) and A. Pines Materials Sciences Division, Lawrence
Electron States of Diatomic Molecules
 IISER Pune March 2018 Hamiltonian for a Diatomic Molecule The hamiltonian for a diatomic molecule can be considered to be made up of three terms Ĥ = ˆT N + ˆT el + ˆV where ˆT N is the kinetic energy operator
IISER Pune March 2018 Hamiltonian for a Diatomic Molecule The hamiltonian for a diatomic molecule can be considered to be made up of three terms Ĥ = ˆT N + ˆT el + ˆV where ˆT N is the kinetic energy operator
Dispersion Forces in Méthane
 Dispersion Forces in Méthane BY HENDRIK N. W. LEKKERKERKER,* PHILIPPE COULON AND RENE LUYCKX Faculteit van de Wetenschappen, Vrije Universiteit Brussel, 1050 Brussels, Belgium Received 1 6th February,
Dispersion Forces in Méthane BY HENDRIK N. W. LEKKERKERKER,* PHILIPPE COULON AND RENE LUYCKX Faculteit van de Wetenschappen, Vrije Universiteit Brussel, 1050 Brussels, Belgium Received 1 6th February,
Tensor Operators and the Wigner Eckart Theorem
 November 11, 2009 Tensor Operators and the Wigner Eckart Theorem Vector operator The ket α transforms under rotation to α D(R) α. The expectation value of a vector operator in the rotated system is related
November 11, 2009 Tensor Operators and the Wigner Eckart Theorem Vector operator The ket α transforms under rotation to α D(R) α. The expectation value of a vector operator in the rotated system is related
Solutions to exam : 1FA352 Quantum Mechanics 10 hp 1
 Solutions to exam 6--6: FA35 Quantum Mechanics hp Problem (4 p): (a) Define the concept of unitary operator and show that the operator e ipa/ is unitary (p is the momentum operator in one dimension) (b)
Solutions to exam 6--6: FA35 Quantum Mechanics hp Problem (4 p): (a) Define the concept of unitary operator and show that the operator e ipa/ is unitary (p is the momentum operator in one dimension) (b)
Chemistry 120A 2nd Midterm. 1. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (1-electron):
 April 6th, 24 Chemistry 2A 2nd Midterm. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (-electron): E n = m e Z 2 e 4 /2 2 n 2 = E Z 2 /n 2, n =, 2, 3,... where Ze is
April 6th, 24 Chemistry 2A 2nd Midterm. (36 pts) For this question, recall the energy levels of the Hydrogenic Hamiltonian (-electron): E n = m e Z 2 e 4 /2 2 n 2 = E Z 2 /n 2, n =, 2, 3,... where Ze is
Classical Field Theory: Electrostatics-Magnetostatics
 Classical Field Theory: Electrostatics-Magnetostatics April 27, 2010 1 1 J.D.Jackson, Classical Electrodynamics, 2nd Edition, Section 1-5 Electrostatics The behavior of an electrostatic field can be described
Classical Field Theory: Electrostatics-Magnetostatics April 27, 2010 1 1 J.D.Jackson, Classical Electrodynamics, 2nd Edition, Section 1-5 Electrostatics The behavior of an electrostatic field can be described
Electric fields : Stark effect, dipole & quadrupole polarizability.
 Electric fields : Stark effect, dipole & quadrupole polarizability. We are often interested in the effect of an external electric field on the energy levels and wavefunction of H and other one-electron
Electric fields : Stark effect, dipole & quadrupole polarizability. We are often interested in the effect of an external electric field on the energy levels and wavefunction of H and other one-electron
Quantization of the E-M field
 April 6, 20 Lecture XXVI Quantization of the E-M field 2.0. Electric quadrupole transition If E transitions are forbidden by selection rules, then we consider the next term in the expansion of the spatial
April 6, 20 Lecture XXVI Quantization of the E-M field 2.0. Electric quadrupole transition If E transitions are forbidden by selection rules, then we consider the next term in the expansion of the spatial
Quantum Mechanics Solutions
 Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
Chemistry 431. NC State University. Lecture 17. Vibrational Spectroscopy
 Chemistry 43 Lecture 7 Vibrational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule vibrates.
Chemistry 43 Lecture 7 Vibrational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule vibrates.
Dielectrics - III. Lecture 22: Electromagnetic Theory. Professor D. K. Ghosh, Physics Department, I.I.T., Bombay
 Dielectrics - III Lecture 22: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We continue with our discussion of dielectric medium. Example : Dielectric Sphere in a uniform
Dielectrics - III Lecture 22: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We continue with our discussion of dielectric medium. Example : Dielectric Sphere in a uniform
ICD - THE DECAY WIDTHS I
 CD - THE DECAY WDTHS 1 st ORDER THEORY AND GENERAL CHARACTERSTCS Přemysl Kolorenč nstitute of Theoretical Physics, Charles University in Prague CD Summer School Bad Honnef September 1 st 5 th P. Kolorenč
CD - THE DECAY WDTHS 1 st ORDER THEORY AND GENERAL CHARACTERSTCS Přemysl Kolorenč nstitute of Theoretical Physics, Charles University in Prague CD Summer School Bad Honnef September 1 st 5 th P. Kolorenč
Group Representations
 Group Representations Alex Alemi November 5, 2012 Group Theory You ve been using it this whole time. Things I hope to cover And Introduction to Groups Representation theory Crystallagraphic Groups Continuous
Group Representations Alex Alemi November 5, 2012 Group Theory You ve been using it this whole time. Things I hope to cover And Introduction to Groups Representation theory Crystallagraphic Groups Continuous
Practical Manual. General outline to use the structural information obtained from molecular alignment
 Practical Manual General outline to use the structural information obtained from molecular alignment 1. In order to use the information one needs to know the direction and the size of the tensor (susceptibility,
Practical Manual General outline to use the structural information obtained from molecular alignment 1. In order to use the information one needs to know the direction and the size of the tensor (susceptibility,
Lecture 33: Intermolecular Interactions
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.61 Physical Chemistry I Fall, 2017 Professors Robert W. Field Lecture 33: Intermolecular Interactions Recent Lectures Non-degenerate Perturbation Theory vs. Variational
MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.61 Physical Chemistry I Fall, 2017 Professors Robert W. Field Lecture 33: Intermolecular Interactions Recent Lectures Non-degenerate Perturbation Theory vs. Variational
NIELINIOWA OPTYKA MOLEKULARNA
 NIELINIOWA OPTYKA MOLEKULARNA chapter 1 by Stanisław Kielich translated by:tadeusz Bancewicz http://zon8.physd.amu.edu.pl/~tbancewi Poznan,luty 2008 ELEMENTS OF THE VECTOR AND TENSOR ANALYSIS Reference
NIELINIOWA OPTYKA MOLEKULARNA chapter 1 by Stanisław Kielich translated by:tadeusz Bancewicz http://zon8.physd.amu.edu.pl/~tbancewi Poznan,luty 2008 ELEMENTS OF THE VECTOR AND TENSOR ANALYSIS Reference
Pure and zero-point vibrational corrections to molecular properties
 Pure and zero-point vibrational corrections to molecular properties Kenneth Ruud UiT The Arctic University of Norway I UNIVERSITETET TROMSØ June 30 2015 Outline Why consider vibrational effects? General
Pure and zero-point vibrational corrections to molecular properties Kenneth Ruud UiT The Arctic University of Norway I UNIVERSITETET TROMSØ June 30 2015 Outline Why consider vibrational effects? General
Quantum Theory of Angular Momentum and Atomic Structure
 Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
NMR Dynamics and Relaxation
 NMR Dynamics and Relaxation Günter Hempel MLU Halle, Institut für Physik, FG Festkörper-NMR 1 Introduction: Relaxation Two basic magnetic relaxation processes: Longitudinal relaxation: T 1 Relaxation Return
NMR Dynamics and Relaxation Günter Hempel MLU Halle, Institut für Physik, FG Festkörper-NMR 1 Introduction: Relaxation Two basic magnetic relaxation processes: Longitudinal relaxation: T 1 Relaxation Return
Intermolecular interactions
 Intermolecular interactions János Ángyán Équipe Modélisaton Quantique et Cristallographique, LCM3B 2008 J. Ángyán (EMQC) IMF 1 / 201 Part I Introduction J. Ángyán (EMQC) IMF 2 / 201 What are intermolecular
Intermolecular interactions János Ángyán Équipe Modélisaton Quantique et Cristallographique, LCM3B 2008 J. Ángyán (EMQC) IMF 1 / 201 Part I Introduction J. Ángyán (EMQC) IMF 2 / 201 What are intermolecular
Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014
 Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014 Question 1: Basis sets Consider the split valence SV3-21G one electron basis set for formaldehyde
Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014 Question 1: Basis sets Consider the split valence SV3-21G one electron basis set for formaldehyde
Jackson 6.4 Homework Problem Solution Dr. Christopher S. Baird University of Massachusetts Lowell
 Jackson 6.4 Homework Problem Solution Dr. Christopher S. Baird University of Massachusetts Lowell PROBLEM: A uniformly magnetized and conducting sphere of radius R and total magnetic moment m = 4πMR 3
Jackson 6.4 Homework Problem Solution Dr. Christopher S. Baird University of Massachusetts Lowell PROBLEM: A uniformly magnetized and conducting sphere of radius R and total magnetic moment m = 4πMR 3
Hartree-Fock. It can also be viewed as the lowest order term in perturbative expansion (linear in U) with respect to interaction between electrons.
 Hartree-Fock It is probably the simplest method to treat the many-particle system. The dynamic many particle problem is replaced by an effective one-electron problem: electron is moving in an effective
Hartree-Fock It is probably the simplest method to treat the many-particle system. The dynamic many particle problem is replaced by an effective one-electron problem: electron is moving in an effective
Multiple Integrals and Vector Calculus (Oxford Physics) Synopsis and Problem Sets; Hilary 2015
 Multiple Integrals and Vector Calculus (Oxford Physics) Ramin Golestanian Synopsis and Problem Sets; Hilary 215 The outline of the material, which will be covered in 14 lectures, is as follows: 1. Introduction
Multiple Integrals and Vector Calculus (Oxford Physics) Ramin Golestanian Synopsis and Problem Sets; Hilary 215 The outline of the material, which will be covered in 14 lectures, is as follows: 1. Introduction
Representation theory and quantum mechanics tutorial Spin and the hydrogen atom
 Representation theory and quantum mechanics tutorial Spin and the hydrogen atom Justin Campbell August 3, 2017 1 Representations of SU 2 and SO 3 (R) 1.1 The following observation is long overdue. Proposition
Representation theory and quantum mechanics tutorial Spin and the hydrogen atom Justin Campbell August 3, 2017 1 Representations of SU 2 and SO 3 (R) 1.1 The following observation is long overdue. Proposition
1 r 2 sin 2 θ. This must be the case as we can see by the following argument + L2
 PHYS 4 3. The momentum operator in three dimensions is p = i Therefore the momentum-squared operator is [ p 2 = 2 2 = 2 r 2 ) + r 2 r r r 2 sin θ We notice that this can be written as sin θ ) + θ θ r 2
PHYS 4 3. The momentum operator in three dimensions is p = i Therefore the momentum-squared operator is [ p 2 = 2 2 = 2 r 2 ) + r 2 r r r 2 sin θ We notice that this can be written as sin θ ) + θ θ r 2
Problem 1: Spin 1 2. particles (10 points)
 Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Chimica Inorganica 3
 A symmetry operation carries the system into an equivalent configuration, which is, by definition physically indistinguishable from the original configuration. Clearly then, the energy of the system must
A symmetry operation carries the system into an equivalent configuration, which is, by definition physically indistinguishable from the original configuration. Clearly then, the energy of the system must
Multiple Integrals and Vector Calculus: Synopsis
 Multiple Integrals and Vector Calculus: Synopsis Hilary Term 28: 14 lectures. Steve Rawlings. 1. Vectors - recap of basic principles. Things which are (and are not) vectors. Differentiation and integration
Multiple Integrals and Vector Calculus: Synopsis Hilary Term 28: 14 lectures. Steve Rawlings. 1. Vectors - recap of basic principles. Things which are (and are not) vectors. Differentiation and integration
0 + E (1) and the first correction to the ground state energy is given by
 1 Problem set 9 Handout: 1/24 Due date: 1/31 Problem 1 Prove that the energy to first order for the lowest-energy state of a perturbed system is an upper bound for the exact energy of the lowest-energy
1 Problem set 9 Handout: 1/24 Due date: 1/31 Problem 1 Prove that the energy to first order for the lowest-energy state of a perturbed system is an upper bound for the exact energy of the lowest-energy
Rotational Motion. Chapter 4. P. J. Grandinetti. Sep. 1, Chem P. J. Grandinetti (Chem. 4300) Rotational Motion Sep.
 Rotational Motion Chapter 4 P. J. Grandinetti Chem. 4300 Sep. 1, 2017 P. J. Grandinetti (Chem. 4300) Rotational Motion Sep. 1, 2017 1 / 76 Angular Momentum The angular momentum of a particle with respect
Rotational Motion Chapter 4 P. J. Grandinetti Chem. 4300 Sep. 1, 2017 P. J. Grandinetti (Chem. 4300) Rotational Motion Sep. 1, 2017 1 / 76 Angular Momentum The angular momentum of a particle with respect
LoProp: local property calculations with quantum chemical methods
 LoProp: local property calculations with quantum chemical methods Laura Gagliardi Dipartimento di Chimica Fisica F. Accascina Università degli Studi di Palermo Italy Gunnar Karlström, Jesper Krogh, and
LoProp: local property calculations with quantum chemical methods Laura Gagliardi Dipartimento di Chimica Fisica F. Accascina Università degli Studi di Palermo Italy Gunnar Karlström, Jesper Krogh, and
which implies that we can take solutions which are simultaneous eigen functions of
 Module 1 : Quantum Mechanics Chapter 6 : Quantum mechanics in 3-D Quantum mechanics in 3-D For most physical systems, the dynamics is in 3-D. The solutions to the general 3-d problem are quite complicated,
Module 1 : Quantum Mechanics Chapter 6 : Quantum mechanics in 3-D Quantum mechanics in 3-D For most physical systems, the dynamics is in 3-D. The solutions to the general 3-d problem are quite complicated,
GROUP THEORY IN PHYSICS
 GROUP THEORY IN PHYSICS Wu-Ki Tung World Scientific Philadelphia Singapore CONTENTS CHAPTER 1 CHAPTER 2 CHAPTER 3 CHAPTER 4 PREFACE INTRODUCTION 1.1 Particle on a One-Dimensional Lattice 1.2 Representations
GROUP THEORY IN PHYSICS Wu-Ki Tung World Scientific Philadelphia Singapore CONTENTS CHAPTER 1 CHAPTER 2 CHAPTER 3 CHAPTER 4 PREFACE INTRODUCTION 1.1 Particle on a One-Dimensional Lattice 1.2 Representations
Hyperfine interactions Mössbauer, PAC and NMR Spectroscopy: Quadrupole splittings, Isomer shifts, Hyperfine fields (NMR shifts)
 Hyperfine interactions Mössbauer, PAC and NMR Spectroscopy: Quadrupole splittings, Isomer shifts, Hyperfine fields (NMR shifts) Peter Blaha Institute of Materials Chemistry TU Wien Definition of Hyperfine
Hyperfine interactions Mössbauer, PAC and NMR Spectroscopy: Quadrupole splittings, Isomer shifts, Hyperfine fields (NMR shifts) Peter Blaha Institute of Materials Chemistry TU Wien Definition of Hyperfine
20 The Hydrogen Atom. Ze2 r R (20.1) H( r, R) = h2 2m 2 r h2 2M 2 R
 20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
Phonons and lattice dynamics
 Chapter Phonons and lattice dynamics. Vibration modes of a cluster Consider a cluster or a molecule formed of an assembly of atoms bound due to a specific potential. First, the structure must be relaxed
Chapter Phonons and lattice dynamics. Vibration modes of a cluster Consider a cluster or a molecule formed of an assembly of atoms bound due to a specific potential. First, the structure must be relaxed
Fun With Carbon Monoxide. p. 1/2
 Fun With Carbon Monoxide p. 1/2 p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results C V (J/K-mole) 35 30 25
Fun With Carbon Monoxide p. 1/2 p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results C V (J/K-mole) 35 30 25
Intermolecular forces and their representation in electronic structure calculations
 Intermolecular forces and their representation in electronic structure calculations János Ángyán 1 2 Contents 1 Introduction 7 1.1 What are intermolecular forces?.................. 7 1.2 Intermolecular
Intermolecular forces and their representation in electronic structure calculations János Ángyán 1 2 Contents 1 Introduction 7 1.1 What are intermolecular forces?.................. 7 1.2 Intermolecular
CHAPTER 3 POTENTIALS 10/13/2016. Outlines. 1. Laplace s equation. 2. The Method of Images. 3. Separation of Variables. 4. Multipole Expansion
 CHAPTER 3 POTENTIALS Lee Chow Department of Physics University of Central Florida Orlando, FL 32816 Outlines 1. Laplace s equation 2. The Method of Images 3. Separation of Variables 4. Multipole Expansion
CHAPTER 3 POTENTIALS Lee Chow Department of Physics University of Central Florida Orlando, FL 32816 Outlines 1. Laplace s equation 2. The Method of Images 3. Separation of Variables 4. Multipole Expansion
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
B.Sc. MATHEMATICS I YEAR
 B.Sc. MATHEMATICS I YEAR DJMB : ALGEBRA AND SEQUENCES AND SERIES SYLLABUS Unit I: Theory of equation: Every equation f(x) = 0 of n th degree has n roots, Symmetric functions of the roots in terms of the
B.Sc. MATHEMATICS I YEAR DJMB : ALGEBRA AND SEQUENCES AND SERIES SYLLABUS Unit I: Theory of equation: Every equation f(x) = 0 of n th degree has n roots, Symmetric functions of the roots in terms of the
d 1 µ 2 Θ = 0. (4.1) consider first the case of m = 0 where there is no azimuthal dependence on the angle φ.
 4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom
 Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
Absorption of Radiation due to Collisions of Hydrogen Molecules with Helium Atoms at High Temperatures
 Absorption of Radiation due to Collisions of Hydrogen Molecules with Helium Atoms at High Temperatures Xiaoping Li, Anirban Mandal, Evangelos Miliordos, Katharine L. C. Hunt, Martin Abel and Lothar Frommhold
Absorption of Radiation due to Collisions of Hydrogen Molecules with Helium Atoms at High Temperatures Xiaoping Li, Anirban Mandal, Evangelos Miliordos, Katharine L. C. Hunt, Martin Abel and Lothar Frommhold
eigenvalues eigenfunctions
 Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Chem 442 Review for Exam 2. Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative (3D) components.
 Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
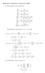 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
l=0 The expansion coefficients can be determined, for example, by finding the potential on the z-axis and expanding that result in z.
 Electrodynamics I Exam - Part A - Closed Book KSU 15/11/6 Name Electrodynamic Score = 14 / 14 points Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try
Electrodynamics I Exam - Part A - Closed Book KSU 15/11/6 Name Electrodynamic Score = 14 / 14 points Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try
Chiroptical Spectroscopy
 Chiroptical Spectroscopy Theory and Applications in Organic Chemistry Lecture 3: (Crash course in) Theory of optical activity Masters Level Class (181 041) Mondays, 8.15-9.45 am, NC 02/99 Wednesdays, 10.15-11.45
Chiroptical Spectroscopy Theory and Applications in Organic Chemistry Lecture 3: (Crash course in) Theory of optical activity Masters Level Class (181 041) Mondays, 8.15-9.45 am, NC 02/99 Wednesdays, 10.15-11.45
