Originaldokument gespeichert auf dem Dokumentenserver der Universität Basel edoc.unibas.ch
|
|
|
- Rudolph Brooks
- 5 years ago
- Views:
Transcription
1 riginaldokument gespeichert auf dem Dokumentenserver der Universität Basel edoc.unibas.ch Dieses Werk ist unter dem Vertrag Creative Commons amensnennung-keine kommerzielle utzung-keine Bearbeitung 3.0 Schweiz (CC BY-C-D 3.0 CH) lizenziert. Die vollständige Lizenz kann unter creativecommons.org/licenses/by-nc-nd/3.0/ch/ eingesehen werden
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17 π
18
19
20
21
22
23 δ α C C ω C α 1 cis δ α C C ω C α 1 trans H C α ω C α 1 cis H C α ω C α 1 trans
24 π π
25 π π π π β π = = H Me
26 H Me H 3 C Ktrans/cis in D > 98% trans Me H 3 C H 3 C H Me CH 3 HMe
27 Me 3 (S) Me 3 (R) 2 5S 5R 6S 6R Me F (S) Me F (R) Me γ Ktrans/cis in D Hγ 3 H β' H δ' H H β δ C 2 CH 3 Ac H α C β 3 H δ H δ' Hγ Hγ' H β' H δ' H β H δ 3 C 2 CH 3 Ac H α C β H δ' 3 Hγ' H δ φφφ
28 σσ σσ Hδ 3 H δ' Cβ Hγ Cβ σ C- 3 H δ' Hγ H δ σ C-H anti gauche σ σ π γ
29 π
30 ω ω α φψω φψω
31
32 γ
33 CH 3 Ph C 2 CH 3 Ph C 2 CH 3 H H 2 a) 3 H C 2 CH 3 CuS 4 5H 2 a ascorbate H 2 : t-buh 8:1 RT, overnight C 2 CH 3 H 6 6 b) 3 3 H 3 Pd/C, HCl (aq), H 2 (g), MeH RT, 20 h Cl Cl H 3 H 3 H 3
34
35
36
37
38
39 γ
40 H 2 H H 2 H 3 H 2 H H 2 H 2 H H 2 H 2 H H 2 CF H H H H H H H H H H H H 2 H H 2 H H 3 H 2 H 2 H 2 H 2 H 2
41
42 β β H 2 H 3 H 2 H β Fl H H 2 7 Fl H H 2 7
43 R H H S H H S H H S H CF 4 PF 6 2 H 2 H H H H H B Fl H H H 8 H 2 H 2 H H B H H 2 H 2 H H 2 H 2 H H 2 H 2 H H 2 H
44 H H R 1 H R 2 a) proline-derived peptides R 3 H 2 * H H H cis-γ-amino-l-proline H H imidazolidine-2-carboxylic acid H * H H hydroxy proline R 2 R 3 R 1 b) amphiphatic oligoproline helices containing modified side chains H H L-proline c) (PPR) n, (PRR) n miniature proteins e) (VXLPPP) n = SAP amphiphatic proline-rich peptides d) (PX) n, proline-rich peptides derived from the antimicrobial peptide bactenicin 7 f) proline-based dendrimers γ
45 γ CF H H H H H H H 2 3 γ
46 H 3 H 2 H H 2 CF H H 2 CF H H 2 n = 2, 3 n = 2, 3 H 3 H H 2 H 2
47
48
49
50 hydrophobic hydrophilic a) b)
51
52
53 β
54
55 δ ε ε
56
57 a) H 2 H 2 H 2 H 2 H 3 H 3 H H CF H 3 H 2 CF H 3 H 2 b) H 2 H 2 H 2 H 2 H H CF H H 2 CF H H c) H 2 H H 2 CF H H 2 6
58 3 * Me BocH * Me X H 3 * Me BocH Boc H * Me H 2 X H 2 H * Me 7 8 X = CF 3 C 2 9 X = Cl X = CF 3 C 2 12 X = Cl 13 H 3 H 3 H 3 H 3 H H 3 3
59
60
61 a) b) H 2 H 2 H 2 H H H 2 H 2 H H 2 HC H 3 H 2 HC H H 2 n = 3 m = 6 H H
62 a) b) H 2 H 2 H 2 H 2 H 3 H 3 H H CF H H 2 n = 2,3,4 CF H H 2 n = 2,3,4
63 a) b) H 2 H 2 H 2 H 2 H 2 H 2 H 2 H 2 H H H H CF H H 2 CF H H 2 n = 2,3,4 n = 2,3,4 c) H 2 H 2 d) H H 2 H H 2 CF H H 2 CF H H 2 n = 6,8 m = 3,4 6 3 e) H 2 H 2 f) H H 2 H H 2 CF H H 2 CF H H 2 n = 3,4 m = 6,8 3 6 a) b) H 2 H 2 H CF H H 2 * = R or S n = 6,8,10,12
64 a) H 2 H 2 H 2 H 2 H H * * CF H * = R or S 3 H 2 b) c) H 2 H 2 H * H 2 H * H 2 CF H H 2 CF H H 2 * = R or S 6 3 * = R or S 3 6
65 γ H H $$39 7 H H2 H FmocH H Strategy A H Staudinger reduction, cleavage from solid support CF 3 * Fmoc H 16 G SPPS, 5(6)-carboxyfluorescein labeling (3)n Strategy B Staudinger reduction BocH Boc H * C 2 H Fmoc 17 Strategy C H2 H FmocH H H SPPS, 5(6)-carboxyfluorescein labeling CF 3 C 2 H 3 n (H2)n CF G CPP CH2 CF G CF G BocH H Boc n Et3, Tf BocH HBoc Et3, Tf BocH HBoc BocH H Boc n BocH H Boc n CF G CH2 CF G Boc deprotection cleavage from solid support Boc deprotection & cleavage from solid support CF G CF 3 C 2 H 2 H 2 H n CPP CH2 ligoproline-based Cell Penetrating Peptides
66
67 H2 SPPS Fmoc G (3)n a), b) CF G (3)n CH2 CF 3 C 2 BocH H 2 CF 3 C 2 Boc H 2 H 3 n H n H n c) d) e) CF G CPP CH2 CF G CH2 CF G CPP CH2
68 H2 SPPS Fmoc G (3)n a) CF G (3)n CF 3 C 2 BocH H 2 Boc H 2 (H2)n H n H n b) c) d) CF G CF G CF G CPP CH2
69 SPPS BocH H Boc n H2 Fmoc G CF 3 C 2 a) CF G BocH H Boc n b) CF G H 2 H CPP H 2 n CH2
70
71
72
73
74 γ H HBoc BocH Boc H Pbf H H 3 H C 2 Me H C 2 Me H C 2 Me H 2 C 2 Me H C 2 Me pkb (calculated*) pkb (determined**) pkb ucleophilicity
75
76 H H H Boc 2 (1.2 eq.), SCl 2 (1.5 eq.), MeH ahc 3 (2.3 eq.), dioxane/h 2 reflux, 19 h; quant. rt, 19 h; 77% H H H 2 Me Boc Cl Me a) Et 3 (1.2 eq.), MsCl (1.2 eq.), DCM, 0 C to rt, 2 h b) a 3 (5.0 eq.), DMF, 80 C, 17 h; 95% 3 Boc 56S Me ah (2.0 eq.), THF/H 2 /MeH rt, 3.5 h, 86% 3 Boc 57S H HCl/dioxane (4 M) rt, 5 h; 90% 3 Cl H 2 58S H FmocCl (1.2 eq.), ahc 3 (2.5 eq.), dioxane/h 2, rt, 3 h; quant. Fmoc H 3 16S
77 H Boc Me a) Methanesulfonic acid (1.2 eq.), Et 3 (0.4 eq.), PPh 3 (1.8 eq.), DIAD (1.9 eq.), toluene, 0 C - 70 C, 3.0 h b) a 3 (5.0 eq), DMF, 80 C, overnight; 95% 3 Boc Me ah (2.0 eq.), THF/H 2 /MeH, rt, 4.0 h; 95% 55 56R 57R 3 Boc H HCl/dioxane (4 M) rt, 2 h; quant. 3 Cl H 2 58R H Fmoc-Cl (1.2 eq.), ahc 3 (2.7 eq.), dioxane/h 2, rt, 6.5 h; 80% 3 Fmoc H 16R
78 H ah (2.0 eq), H H BnBr (1.1 eq), CbzCl (1.0 eq), H 2 Et 3 (1.1 eq), THF C 2 H C 2 H 0 C to rt, 2 d; H 0 C to rt, 5.5 h; 94% Cbz 68% Cbz C 2 Bn MsCl (1.2 eq), Et 3 (1.2 eq), DCM 0 C to rt, 2 h PPh 3 (2.0 eq), H 2 (2.0 eq), THF reflux, 5.5 h; quant. Ms H 2 a 3 (5.0 eq), DMF C 2 Bn 80 C, 22 h; C 2 Bn Cbz 92 % Cbz BocH Tf H BocH HBoc (1.0 eq) 18 C 2 Bn Et 3 (1.0 eq), DCM, Cbz 0 C to rt, 3 d; 89% 3 Boc Cbz C 2 Bn H 2 (1atm), Pd/C (5% w/w), MeH rt, overnight BocH H BocH Boc Boc Fmoc-Su (1.5 eq), H a 2 C 3 (2.0 eq), DMF/H 2 C 2 H C 2 H 0 C to rt, 2 h; 24% H Fmoc 65 17
79
80 Cl Boc H 2 (1.7 eq), 2 H Tf 2 (1.1 eq), Tf ah (4.0 eq) Hünig's base (1.1 eq), DCM H 2 H 2 0 C to rt, 22 h; BocH HBoc BocH HBoc -78 C to -20 C, 3.5 h; 66 55% 67 75% 18
81 10 Θ /10 3 deg cm 2 dmol PPII conformation Min 206 nm Max 226 nm ZPZ ZPZ ZPZ (24) PPP ZZZ ZZZ (29) λ [nm]
82 a) 10 b) Θ /10 3 deg cm 2 dmol λ [nm] ZPZ ZPZ ZPZ (24) UPU UPU UPU (21) PPP PPP PPP (50) Θ /10 3 deg cm 2 dmol zpz zpz zpz (27) 20 xpx xpx xpx (28) 30 ZPZ ZPZ ZPZ (24) XPX XPX XPX (26) λ [nm]
83 a) 5 b) Θ /10 3 deg cm 2 dmol αβ 0 λ [nm] RRR RRR RR (45) Tat(48 60) (42) Penetratin (43)
84 Hela MCF-7 PC-3 HT-29
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102 A B C D A B C D
103
104 .2 7 %!! ) $&! $ "! # "! + ) 15 2-,) # $ #!!!!! %! + # " # $ $! & 0).)1).)., % # $ " #,) (! # * * * * * / +, ,'! * # 0001/ +, " $ +,) (! # * * * * * / +, ,'! * # 0001/ +, " $ +,) (! # * * 4* 036 +,'! * # 0001/ +, " $ +,) 4/ / +,.2 7.
105
106
107
108
109 Tat(48-60) CF-G-RKK-RRQ-RRR-PPQ-CH2 (42) CF-G-RRR-RRR-RR-CH2 (45) mau Control mau Control min min mau 0 min mau 0 min min min mau 1 h mau 1 h min min mau 48 h mau 48 h min min
110 CF-G-ZZZ-ZZZ-PPP-CH2 (30) CF-G-ZZZ-ZZZ-ZZ-CH2 (39) mau 0 min mau 0 min min min mau 1 h mau 1 h min min mau 48 h mau 48 h min min
111 CF-G-PPP-PPP-PPP-CH2 (50) CF-G-ppp-ppp-ppp-CH2 (52) mau 0 min mau 0 min min min mau 1 h mau 1 h min min mau 48 h mau 48 h min min
112
113 Tat(48-60) CF-G-RKK-RRQ-RRR-PPQ-CH2 (42) CF-G-RRR-RRR-RR-CH2 (45) mau 0 min mau 0 min min min mau 1 h mau 1 h min min mau 48 h mau 48 h min min
114 CF-G-ZZZ-ZZZ-PPP-CH2 (30) CF-G-ZZZ-ZZZ-ZZ-CH2 (39) mau 0 min mau 0 min min min mau 1 h mau 1 h min min mau 48 h mau 48 h min min
115 CF-G-PPP-PPP-PPP-CH2 (50) CF-G-ppp-ppp-ppp-CH2 (52) mau 0 min mau 0 min min min mau 1 h mau 1 h min min mau 48 h mau 48 h min min
116
117 Mitochondrial dehydrogenase Br S ADH AD+ H S MTT pale yellow Formazan purple Mitochondrion of a viable cell
118 Cell Viability (%)
119 Cell Viability (%)
120
121 ± ± ± a) Hemolysis (%) b) Hemolysis (%)
122
123
124
125 a) siren Transfection via CPPs b) c) psicheck-2 vector Transfection via jetpei Beetle luciferin Celenterazine
126 a) H S S CH + ATP + 2 Recombinant Firefly Luciferase Mg 2+ S S + AMP + PP i + C 2 Beetle Luciferin hv xyluciferin b) H H H Renilla Luciferase + 2 hv H H H + C 2 Coelenterazine Coelenteramide
127
128
129 3 * Me BocH * Me X H 3 * Me BocH Boc H * Me H 2 X H 2 H * Me 7 8 X = CF 3 C 2 9 X = Cl X = CF 3 C 2 12 X = Cl 13
130 H 3 H 3 H 3 H 3 H H 3 3
131 3 a) Methansulfonic acid, a) Et H 3, MsCl, Et 3 3, PPh 3, DIAD, DCM, rt, 2h toluene, 70 C, 5 h C 2 Me C 2 Me b) a C 2 Me 3, DMF, b) a 3, DMF, Boc 80 C, 26 h; 95% 80 C, 17 h; 95% Boc Boc 56S 55 56R
132 3 * Boc 56R 56S Lindlar cat., Boc 2, H 2, C 2 Me HCl/dioxane (4 M) rt, 5 h; 69S: quant.; 69R: 87% BocH * 3 H 2 Cl C 2 Me Ac 2, Et 3, DCM rt, 4 h a) DCM/TFA 3:2, 1 h, rt or b) HCl in dioxane, 5 h, rt MeH, rt, 7 h C 2 Me X = CF 3 C 2 : 9S: 85%; 8S: quant.; 9R: 85% 8R: quant. X = Cl: 10S: quant.; 10R: quant. Tf BocH Boc BocH HBoc H a) DCM/TFA 1:1, 1.5 h, rt or 18 b) HCl in dioxane, 5 h, rt Et 3, DCM, rt, 5 h C 2 Me X = CF 3 C 2 : 12S: quant.; 11S: quant.; 12R: quant. 11R: 94% X = Cl: 13S: 70%; 13R: 73% * * 7S: 95%; 7R: 71% 3 H 3 H 2 H * * * X H 2 C 2 Me C 2 Me X C 2 Me
133 ψ
134 a) b) (R) Hγ X i Ψ ~145 C i C i-1 n π* stabilization repulsion X (S) Hγ CH3 Ψ ~145 C(4)-exo conformation X = e. g. F or 3 C(4)-endo conformation
135
136 a) H-bonding b) H Y CH3 Ψ ~145 n π* Hγ stabilization
137
138
139
140
141 20 20 Θ /10 3 deg cm 2 dmol R Θ /10 3 deg cm 2 dmol S λ [nm] λ [nm] 30 73R 2R 2S 73S [Θ] (10 3 deg cm 2 dmol -1 ) Wavelength (nm) Wavelength (nm)
142
143
144
145
146 π
147
148
149 H Cl H 2 Me
150 H Boc Me
151 3 Boc Me
152 3 Boc H
153 H 2 H Cl 3
154 3 Fmoc H
155
156 3 Boc Me
157
158 3 Boc H
159 H 2 H Cl 3
160 3 Fmoc H
161 H Cbz C 2 H
162 H Cbz C 2 Bn
163 Ms Cbz C 2 Bn
164 3 Cbz C 2 Bn
165 H 2 Cbz C 2 Bn
166 BocH H Boc C 2 Bn Cbz
167 BocH H Boc C 2 H H
168 BocH H Boc C 2 H Fmoc
169 BocH H HBoc
170 BocH Tf HBoc
171 3 Cl H 2 Me
172 3 Me
173 3 Cl H 2 Me
174 3 Me
175 BocH Me
176 BocH Me
177 CF 3 C 2 H 3 Me
178
179 Me CF 3 C 2 H 3
180 Cl H 3 Me
181 Me Cl H 3
182 BocH H Boc Me
183
184 BocH Boc H Me
185 H 2 H CF 3 C 2 H 2 Me
186
187 H 2 H CF 3 C 2 H 2 Me
188 H 2 H Cl H 2 Me
189 H 2 H Cl H 2 Me
190
191
192
193
194
195
196
197
198
199
200
201
202
203 = = = =
204
205
206
207
208
209
210
211
212
213
214
215
216
217
218
219
220
221
222
223
224
225
226
227
228
229
230
231
232
233 Θ /10 3 deg cm 2 dmol PPP PPP PPP PPP PPP Θ /10 3 deg cm 2 dmol ppp ppp ppp ppp ppp λ [nm] λ [nm]
234 BocH * Me
235 CF 3 C 2 H 3 * Me
236 Cl H 3 * Me
237 Boc H * BocH Me
238 CF 3 C 2 H 2 H 2 H * Me
239 Cl H 2 H 2 H * Me
240 a) b) c)
241 a)
242
Azidoproline containing Helices Stabilization of the Polyproline II Structure by a Functionalizable Group
 Azidoproline containing Helices Stabilization of the Polyproline II Structure by a Functionalizable Group Michael Kümin, Louis-Sebastian Sonntag, Helma Wennemers* Department of Chemistry, University of
Azidoproline containing Helices Stabilization of the Polyproline II Structure by a Functionalizable Group Michael Kümin, Louis-Sebastian Sonntag, Helma Wennemers* Department of Chemistry, University of
1. Draw the structure of oxazolone formed upon activation of N-Acetylvaline
 Peptide quiz 1 (5 questions for 10 minutes) 10 points max 1. Draw the structure of oxazolone formed upon activation of -Acetylvaline 3 C 3 C 2. The following DKP (1) is prepared by cyclisation of dipeptide
Peptide quiz 1 (5 questions for 10 minutes) 10 points max 1. Draw the structure of oxazolone formed upon activation of -Acetylvaline 3 C 3 C 2. The following DKP (1) is prepared by cyclisation of dipeptide
Cis Trans Proline Isomerization Effects on Collagen Triple-Helix Stability are Limited
 Cis Trans Proline Isomerization Effects on Collagen Triple-Helix Stability are Limited an Dai and Felicia A. Etzkorn* Department of Chemistry, Virginia Tech, Blacksburg, VA 24061-0212 Supporting Information.
Cis Trans Proline Isomerization Effects on Collagen Triple-Helix Stability are Limited an Dai and Felicia A. Etzkorn* Department of Chemistry, Virginia Tech, Blacksburg, VA 24061-0212 Supporting Information.
Short Access to (+)-Lupinine and (+)-Epiquinamide via Double Hydroformylation
 Short Access to (+)-Lupinine and (+)-Epiquinamide via Double Hydroformylation Kai Gao Checker: Changbin Yu Mann, A.* et al rg. Lett. 2009, ASAP Strategy for the formation of quinolizidine alkaloids R H
Short Access to (+)-Lupinine and (+)-Epiquinamide via Double Hydroformylation Kai Gao Checker: Changbin Yu Mann, A.* et al rg. Lett. 2009, ASAP Strategy for the formation of quinolizidine alkaloids R H
MULTICOMPONENT REACTIONS AND ORGANOCATALYSIS: A SUITABLE COMBINATION FOR STEREOSELECTIVE SYNTHESIS OF HIGHLY SUBSTITUTED BENZAZEPINES
 MULTICMPET REACTIS AD RGACATALYSIS: A SUITABLE CMBIATI FR STERESELECTIVE SYTHESIS F HIGHLY SUBSTITUTED BEZAZEPIES Martina Spallarossa Department of Chemistry and Industrial Chemistry, University of Genoa
MULTICMPET REACTIS AD RGACATALYSIS: A SUITABLE CMBIATI FR STERESELECTIVE SYTHESIS F HIGHLY SUBSTITUTED BEZAZEPIES Martina Spallarossa Department of Chemistry and Industrial Chemistry, University of Genoa
A Divalent Protecting Group for Benzoxaboroles
 A Divalent Protecting Group for Benzoxaboroles Brett VanVeller, Matthew R. Aronoff, and Ronald T. Raines* Departments of Chemistry and Biochemistry, University of Wisconsin Madison, Madison, Wisconsin
A Divalent Protecting Group for Benzoxaboroles Brett VanVeller, Matthew R. Aronoff, and Ronald T. Raines* Departments of Chemistry and Biochemistry, University of Wisconsin Madison, Madison, Wisconsin
Nickel-Catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides
 ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization
![Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization](/thumbs/82/85235286.jpg) Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Peptides And Proteins
 Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
Protein Structure Basics
 Protein Structure Basics Presented by Alison Fraser, Christine Lee, Pradhuman Jhala, Corban Rivera Importance of Proteins Muscle structure depends on protein-protein interactions Transport across membranes
Protein Structure Basics Presented by Alison Fraser, Christine Lee, Pradhuman Jhala, Corban Rivera Importance of Proteins Muscle structure depends on protein-protein interactions Transport across membranes
Structures in equilibrium at point A: Structures in equilibrium at point B: (ii) Structure at the isoelectric point:
 ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
Electronic Supplementary Information for
 Electronic Supplementary Information for Sequence Selective Dual-Emission Detection of (i, i+1) Bis-Phosphorylated Peptide Using Diazastilbene-Type Zn(II)-Dpa Chemosensor Yoshiyuki Ishida, Masa-aki Inoue,
Electronic Supplementary Information for Sequence Selective Dual-Emission Detection of (i, i+1) Bis-Phosphorylated Peptide Using Diazastilbene-Type Zn(II)-Dpa Chemosensor Yoshiyuki Ishida, Masa-aki Inoue,
Total Syntheses of Nominine
 Total Syntheses of ominine i Literature t Group eting Lizzie Bryan ctober 17, 2007 Aconite Alkaloids Atidane V eatchane Cycloveatchane Aconitane etisan Muratake,. M.; atsume, M.; akai,. Tetrahedron, 2006,
Total Syntheses of ominine i Literature t Group eting Lizzie Bryan ctober 17, 2007 Aconite Alkaloids Atidane V eatchane Cycloveatchane Aconitane etisan Muratake,. M.; atsume, M.; akai,. Tetrahedron, 2006,
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
The Azido Gauche Effect Implications for the Conformation of Azidoprolines
 The Azido Gauche Effect Implications for the Conformation of Azidoprolines Louis-Sebastian Sonntag, a Sabine Schweizer, b Christian Ochsenfeld, b * Helma Wennemers a * [a] [b] Department of Chemistry,
The Azido Gauche Effect Implications for the Conformation of Azidoprolines Louis-Sebastian Sonntag, a Sabine Schweizer, b Christian Ochsenfeld, b * Helma Wennemers a * [a] [b] Department of Chemistry,
C-O C-O C-N A A. (KHMDS) C-N (scheme 1) C-O C-N (1)
 C- C- C- A A 2007 2008 -t- (Boc)-- (MM)- () (KMDS) C- (scheme ) C- C- () C- C- C- C- C- C- B C- B (scheme 2) C- B (2) C- manzacidin A Manzacidine A (5) - ATP (fig. ) C- 5 Ph MM Boc ethyl lactate X n=,2
C- C- C- A A 2007 2008 -t- (Boc)-- (MM)- () (KMDS) C- (scheme ) C- C- () C- C- C- C- C- C- B C- B (scheme 2) C- B (2) C- manzacidin A Manzacidine A (5) - ATP (fig. ) C- 5 Ph MM Boc ethyl lactate X n=,2
Literature Report IX. Cho, S. H. et al. Org. Lett. 2016, 18, Cho, S. H. et al. Angew. Chem. Int. Ed. 2017, 56,
 Literature Report IX ynthesis of β-aminoboronates by Copper(I)- Catalyzed Addition of Diborylalkanes to Imines Reporter Checker Date : hubo Hu : Xiaoyong Zhai : 2017-9-1818 Cho,. H. et al. rg. Lett. 2016,
Literature Report IX ynthesis of β-aminoboronates by Copper(I)- Catalyzed Addition of Diborylalkanes to Imines Reporter Checker Date : hubo Hu : Xiaoyong Zhai : 2017-9-1818 Cho,. H. et al. rg. Lett. 2016,
Silenes in organic synthesis: a concise synthesis of (±)-epi-picropodophyllin
 lenes in organic synthesis: a concise synthesis of (±)-epi-picropodophyllin obert D. C. Pullin, Jonathan D. Sellars, and Patrick G. Steel* rg. Biomol. Chem., 2007, 5, 3201-3206 H H C Adam Hoye Current
lenes in organic synthesis: a concise synthesis of (±)-epi-picropodophyllin obert D. C. Pullin, Jonathan D. Sellars, and Patrick G. Steel* rg. Biomol. Chem., 2007, 5, 3201-3206 H H C Adam Hoye Current
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Content Synthesis of compounds 2a, 2b in Scheme
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Content Synthesis of compounds 2a, 2b in Scheme
SECTION 12. «POT-POURRI» in Organic Synthesis (2018)
 SECTIN 12 «PT-PURRI» in rganic Synthesis (2018) 1 Total Synthesis of Erythromycin A via a Spiroketal ERYTRMYCIN A DESLNGCAMPS et al. Can. J. Chem. 1985, 63, 2810-2820. 2 Tetrahydropyran Derivative as Starting
SECTIN 12 «PT-PURRI» in rganic Synthesis (2018) 1 Total Synthesis of Erythromycin A via a Spiroketal ERYTRMYCIN A DESLNGCAMPS et al. Can. J. Chem. 1985, 63, 2810-2820. 2 Tetrahydropyran Derivative as Starting
RNA-Ligand Interactions: Affinity and Specificity of Aminoglycoside Dimers and Acridine Conjugates to the HIV-1 Rev Response Element
 RA-Ligand Interactions: Affinity and pecificity of Aminoglycoside Dimers and Acridine Conjugates to the HIV-1 Rev Response Element athan W. Luedtke, Qi Liu, and Yitzhak Tor* *Department of Chemistry and
RA-Ligand Interactions: Affinity and pecificity of Aminoglycoside Dimers and Acridine Conjugates to the HIV-1 Rev Response Element athan W. Luedtke, Qi Liu, and Yitzhak Tor* *Department of Chemistry and
Solid phase peptide synthesis (SPPS)
 Tapio Nevalainen Drug synthesis II 2012 Solid phase peptide synthesis (SPPS) Solid phase peptide synthesis (SPPS) R. Bruce Merrifield, Professor at Rockefeller University, has been awarded the Nobel Prize
Tapio Nevalainen Drug synthesis II 2012 Solid phase peptide synthesis (SPPS) Solid phase peptide synthesis (SPPS) R. Bruce Merrifield, Professor at Rockefeller University, has been awarded the Nobel Prize
Electronic Supplementary Information
 Electronic upplementary Material (EI) for Green Chemistry This journal is The Royal ociety of Chemistry 2012 I1 Electronic upplementary Information Cysteine as sustainable sulfur-reagent for the protecting-group-free
Electronic upplementary Material (EI) for Green Chemistry This journal is The Royal ociety of Chemistry 2012 I1 Electronic upplementary Information Cysteine as sustainable sulfur-reagent for the protecting-group-free
BCMP 201 Protein biochemistry
 BCMP 201 Protein biochemistry BCMP 201 Protein biochemistry with emphasis on the interrelated roles of protein structure, catalytic activity, and macromolecular interactions in biological processes. The
BCMP 201 Protein biochemistry BCMP 201 Protein biochemistry with emphasis on the interrelated roles of protein structure, catalytic activity, and macromolecular interactions in biological processes. The
Total Synthesis of Oxazolomycin A
 Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Arylhalide-Tolerated Electrophilic Amination of Arylboronic Acids with N-Chloroamides Catalyzed by CuCl at Room Temperature
 Current Literature July 19, 08 Jitendra Mishra Arylhalide-Tolerated Electrophilic Amination of Arylboronic Acids with -Chloroamides Catalyzed by CuCl at Room Temperature Aiwen Lei et.al. College of the
Current Literature July 19, 08 Jitendra Mishra Arylhalide-Tolerated Electrophilic Amination of Arylboronic Acids with -Chloroamides Catalyzed by CuCl at Room Temperature Aiwen Lei et.al. College of the
Department of Chemistry, Colorado State University, Fort Collins, Colorado University of Colorado Cancer Center, Aurora, Colorado 80045
 Improved Biomimetic Total Synthesis of d,l-stephacidin A Thomas J. Greshock 1 and Robert M. Williams 1,2 * 1 Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523 2 University
Improved Biomimetic Total Synthesis of d,l-stephacidin A Thomas J. Greshock 1 and Robert M. Williams 1,2 * 1 Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523 2 University
Comparison of diffusion coefficients for matched pairs of macrocyclic and linear molecules
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry Supporting Information for: Comparison of diffusion coefficients for matched pairs of macrocyclic and linear molecules over a
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry Supporting Information for: Comparison of diffusion coefficients for matched pairs of macrocyclic and linear molecules over a
Total Syntheses of Minfiensine
 Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Organic Tutorials 3 rd Year Michaelmas Transition Metals in Organic Synthesis: (General paper level) ! 1! Reading
 rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
Self-Assembly of Single Amino acid-pyrene Conjugates with Unique Structure-Morphology Relationship
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2017 Self-Assembly of Single Amino acid-pyrene Conjugates with Unique Structure-Morphology Relationship
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2017 Self-Assembly of Single Amino acid-pyrene Conjugates with Unique Structure-Morphology Relationship
"Friendship is one mind in two bodies." Mencius
 California State Polytechnic University, Pomona 1 Fall, 2014 Midterm Exam Chem 314 Beauchamp Chem 314 Name Problem Points Credit 1. Nomenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal Charge
California State Polytechnic University, Pomona 1 Fall, 2014 Midterm Exam Chem 314 Beauchamp Chem 314 Name Problem Points Credit 1. Nomenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal Charge
Catalytic Reactions in Organic Synthesis
 17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
Synthesis of the Stenine Ring System from Pyrrole
 Current Literature Presentation gor psenica 06/18/2011 Synthesis of the Stenine Ring System from Pyrrole Bates, R. W.; Sridhar, S. J. rg. Chem., 2011, 76, 5026 5035 gor psenica @ Wipf Group Page 1 of 16
Current Literature Presentation gor psenica 06/18/2011 Synthesis of the Stenine Ring System from Pyrrole Bates, R. W.; Sridhar, S. J. rg. Chem., 2011, 76, 5026 5035 gor psenica @ Wipf Group Page 1 of 16
Firefly Luciferase 1. ATP + Luciferin AMP + Oxyluciferin + Light (565 nm)
 Berthold Detection Systems GmbH Bleichstrasse 56 68 D-75173 Pforzheim/Germany Phone: +49(0)7231/9206-0 Fax: +49(0)7231/9206-50 E-Mail: contact@berthold-ds.com Internet: www.berthold-ds.com Dual Luciferase
Berthold Detection Systems GmbH Bleichstrasse 56 68 D-75173 Pforzheim/Germany Phone: +49(0)7231/9206-0 Fax: +49(0)7231/9206-50 E-Mail: contact@berthold-ds.com Internet: www.berthold-ds.com Dual Luciferase
Wilkinson s other (ruthenium) catalyst
 Wilkinson s other (ruthenium) catalyst Cl 3 ; 2 h 3, reflux 3h h 3 Cl h 3 h Cl 3 Good catalyst especially for 2 1-alkenes 2, base toluene Cl h 3 h 3 h 3 Et 3 Cl h 3 Cl h 3 h 3 R h 3 h 3 Cl h 3 R RC 2 C
Wilkinson s other (ruthenium) catalyst Cl 3 ; 2 h 3, reflux 3h h 3 Cl h 3 h Cl 3 Good catalyst especially for 2 1-alkenes 2, base toluene Cl h 3 h 3 h 3 Et 3 Cl h 3 Cl h 3 h 3 R h 3 h 3 Cl h 3 R RC 2 C
Research Article Synthesis of Hemopressin Peptides by Classical Solution Phase Fragment Condensation
 Peptides Volume 2012, Article ID 186034, 5 pages doi:10.1155/2012/186034 Research Article Synthesis of Hemopressin Peptides by Classical Solution Phase Fragment Condensation P. Anantha Reddy, Sean T. Jones,
Peptides Volume 2012, Article ID 186034, 5 pages doi:10.1155/2012/186034 Research Article Synthesis of Hemopressin Peptides by Classical Solution Phase Fragment Condensation P. Anantha Reddy, Sean T. Jones,
Crystal structure of DL-Tryptophan at 173K
 Cryst. Res. Technol. 39, No. 3, 274 278 (2004) / DOI 10.1002/crat.200310182 Crystal structure of DL-Tryptophan at 173K Ch. B. Hübschle, M. Messerschmidt, and P. Luger* Institut für Chemie / Kristallographie,
Cryst. Res. Technol. 39, No. 3, 274 278 (2004) / DOI 10.1002/crat.200310182 Crystal structure of DL-Tryptophan at 173K Ch. B. Hübschle, M. Messerschmidt, and P. Luger* Institut für Chemie / Kristallographie,
Supplementary Information
 Supplementary Information Ammonium-directed dihydroxylation of 3-aminocyclohex-1-enes: development of a metal-free dihydroxylation protocol Caroline Aciro, a Timothy D. W. Claridge, a Stephen G. Davies,
Supplementary Information Ammonium-directed dihydroxylation of 3-aminocyclohex-1-enes: development of a metal-free dihydroxylation protocol Caroline Aciro, a Timothy D. W. Claridge, a Stephen G. Davies,
Supporting Information
 Supporting Information Construction of Highly Functional α-amino itriles via a ovel Multicomponent Tandem rganocatalytic Reaction: a Facile Access to α-methylene γ-lactams Feng Pan, Jian-Ming Chen, Zhe
Supporting Information Construction of Highly Functional α-amino itriles via a ovel Multicomponent Tandem rganocatalytic Reaction: a Facile Access to α-methylene γ-lactams Feng Pan, Jian-Ming Chen, Zhe
Supporting Information For Structure-Based Design of Pseudopeptidic Inhibitors for SIRT1 and SIRT2
 Supporting Information For Structure-Based Design of Pseudopeptidic Inhibitors for SIRT1 and SIRT2 Tero Huhtiniemi,, Heikki S. Salo,*,, Tiina Suuronen, Antti Poso, Antero Salminen,, Jukka Leppänen, Elina
Supporting Information For Structure-Based Design of Pseudopeptidic Inhibitors for SIRT1 and SIRT2 Tero Huhtiniemi,, Heikki S. Salo,*,, Tiina Suuronen, Antti Poso, Antero Salminen,, Jukka Leppänen, Elina
Protecting Groups. Tactical Considerations
 Tactical Considerations Cheap & commercially available Easy & efficient introduction Should not create any stereogenic center Stable throughout reaction, work-up & purification Efficient removal By-products
Tactical Considerations Cheap & commercially available Easy & efficient introduction Should not create any stereogenic center Stable throughout reaction, work-up & purification Efficient removal By-products
Development of Chiral Phosphine Olefin Ligands and Their Use in Asymmetric Catalysis
 Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
A Lanthanide(III) Triflate-mediated Macrolactonization / Solid-Phase Synthesis Approach for Depsipeptide Synthesis
 A Lanthanide(III) Triflate-mediated Macrolactonization / Solid-Phase Synthesis Approach for Depsipeptide Synthesis Jordan D. Goodreid, Eduardo da Silveira dos Santos and Robert A. Batey* Davenport Research
A Lanthanide(III) Triflate-mediated Macrolactonization / Solid-Phase Synthesis Approach for Depsipeptide Synthesis Jordan D. Goodreid, Eduardo da Silveira dos Santos and Robert A. Batey* Davenport Research
Secondary and sidechain structures
 Lecture 2 Secondary and sidechain structures James Chou BCMP201 Spring 2008 Images from Petsko & Ringe, Protein Structure and Function. Branden & Tooze, Introduction to Protein Structure. Richardson, J.
Lecture 2 Secondary and sidechain structures James Chou BCMP201 Spring 2008 Images from Petsko & Ringe, Protein Structure and Function. Branden & Tooze, Introduction to Protein Structure. Richardson, J.
CHAPTER I. Synthesis and conformational analysis of mannose-derived furanoid sugar amino acid based linear peptides
 The thesis entitled Design, Synthesis, Conformational Analysis and Biological Studies of Peptides containing ucleoside and Sugar Amino Acids consists of three chapters. CAPTER I: Describes the synthesis
The thesis entitled Design, Synthesis, Conformational Analysis and Biological Studies of Peptides containing ucleoside and Sugar Amino Acids consists of three chapters. CAPTER I: Describes the synthesis
Huang, C.; Gevorgyan, V. J. Am. Chem. Soc. 2009, 131, Daniel Tzvi Cohen Short Literature Feb. 23, MeO HO OH. COOH ( )-Plicatic Acid OH OH
 Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Supplementary Information
 Catalytically Efficient Palladium anoparticles Stabilized by Click rrocenyl Dendrimers Cátia rnelas, Lionel Salmon, Jaime Ruiz Aranzaes, Didier Astruc Supplementary Information Cyclic Voltammetry (CV),
Catalytically Efficient Palladium anoparticles Stabilized by Click rrocenyl Dendrimers Cátia rnelas, Lionel Salmon, Jaime Ruiz Aranzaes, Didier Astruc Supplementary Information Cyclic Voltammetry (CV),
Rapid Synthesis and Purification of Sulfahydantion Library Using Microwave Assisted Organic Synthesis and Automated Flash Chromatography
 Rapid ynthesis and Purification of ulfahydantion Library Using Microwave Assisted rganic ynthesis and Automated Flash Chromatography hahnaz Ghassemi, Ph.D. March 17, 2005 Discovery Chemistry Group 1725
Rapid ynthesis and Purification of ulfahydantion Library Using Microwave Assisted rganic ynthesis and Automated Flash Chromatography hahnaz Ghassemi, Ph.D. March 17, 2005 Discovery Chemistry Group 1725
Overview. The peptide bond. Page 1
 Overview Secondary structure: the conformation of the peptide backbone The peptide bond, steric implications Steric hindrance and sterically allowed conformations. Ramachandran diagrams Side chain conformations
Overview Secondary structure: the conformation of the peptide backbone The peptide bond, steric implications Steric hindrance and sterically allowed conformations. Ramachandran diagrams Side chain conformations
Prof. Ang Li. Literature Seminar Kosuke Minagawa (D2)
 Prof. Ang Li Literature Seminar 2017. 10. 28 Kosuke Minagawa (D2) 1 2 3 aspidodasycarpine 1) 2 C sespenine 3) atural Products Synthesized by Ang Li group clostrubin 6) drimentine A 7) i-bu rubriflordilactone
Prof. Ang Li Literature Seminar 2017. 10. 28 Kosuke Minagawa (D2) 1 2 3 aspidodasycarpine 1) 2 C sespenine 3) atural Products Synthesized by Ang Li group clostrubin 6) drimentine A 7) i-bu rubriflordilactone
Development of [ 18 F]Fluoro-C-glycosides to radiolabel peptides for PET application
![Development of [ 18 F]Fluoro-C-glycosides to radiolabel peptides for PET application Development of [ 18 F]Fluoro-C-glycosides to radiolabel peptides for PET application](/thumbs/78/77494838.jpg) Development of [ ]luoro-c-glycosides to radiolabel peptides for PET application Dr Charlotte Collet, Petry., Chrétien., Karcher G., Pellegrini-Moïse., Lamandé-Langle. ancyclotep, Plateforme d imagerie
Development of [ ]luoro-c-glycosides to radiolabel peptides for PET application Dr Charlotte Collet, Petry., Chrétien., Karcher G., Pellegrini-Moïse., Lamandé-Langle. ancyclotep, Plateforme d imagerie
Aziridine Carboxylates, Carboxamides and Lactones: New Methods for Their Preparation and Their Transformation into α- and β-amino Acid Derivatives
 Molecules 2000, 5 293 Aziridine Carboxylates, Carboxamides and Lactones: ew Methods for Their Preparation and Their Transformation into α- and β-amino Acid Derivatives obert H. Dodd Institut de Chimie
Molecules 2000, 5 293 Aziridine Carboxylates, Carboxamides and Lactones: ew Methods for Their Preparation and Their Transformation into α- and β-amino Acid Derivatives obert H. Dodd Institut de Chimie
Molecular Modelling. part of Bioinformatik von RNA- und Proteinstrukturen. Sonja Prohaska. Leipzig, SS Computational EvoDevo University Leipzig
 part of Bioinformatik von RNA- und Proteinstrukturen Computational EvoDevo University Leipzig Leipzig, SS 2011 Protein Structure levels or organization Primary structure: sequence of amino acids (from
part of Bioinformatik von RNA- und Proteinstrukturen Computational EvoDevo University Leipzig Leipzig, SS 2011 Protein Structure levels or organization Primary structure: sequence of amino acids (from
SUPPORTING INFORMATION
 S1 SUPPRTIG IFRMATI Discovery of a Selective Aurora A Kinase Inhibitor by Virtual Screening Falco Kilchmann,a) Maria J. Marcaida,a)c) Sachin Kotak,b)d) Thomas Schick,a) Silvan D. Boss,a) Mahendra Awale,a)
S1 SUPPRTIG IFRMATI Discovery of a Selective Aurora A Kinase Inhibitor by Virtual Screening Falco Kilchmann,a) Maria J. Marcaida,a)c) Sachin Kotak,b)d) Thomas Schick,a) Silvan D. Boss,a) Mahendra Awale,a)
Supporting Information
 Supporting Information Synthesis of End-functionalized Phosphate and Phosphonate- polypeptides by Ring- pening Polymerization of their Corresponding N-carboxyanhydride (NCA) Soumen Das, Mrityunjoy Kar
Supporting Information Synthesis of End-functionalized Phosphate and Phosphonate- polypeptides by Ring- pening Polymerization of their Corresponding N-carboxyanhydride (NCA) Soumen Das, Mrityunjoy Kar
Synthesis of Pyridazine-Based α-helix Mimetics
 Synthesis of Pyridazine-Based α-helix Mimetics Allyn T. Londregan, David W. Piotrowski, Liuqing Wei Pfizer Inc. Medicine Design, Eastern Point Rd., Groton, Connecticut, 06340 USA Materials and Methods...
Synthesis of Pyridazine-Based α-helix Mimetics Allyn T. Londregan, David W. Piotrowski, Liuqing Wei Pfizer Inc. Medicine Design, Eastern Point Rd., Groton, Connecticut, 06340 USA Materials and Methods...
Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril
![Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril](/thumbs/94/119194061.jpg) SUPPORTING INFORMATION Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril Lidia Strimbu Berbeci, Wei Wang and Angel E. Kaifer* Center for Supramolecular Science and
SUPPORTING INFORMATION Drastically Decreased Reactivity of Thiols and Disulfides Complexed by Cucurbit[6]uril Lidia Strimbu Berbeci, Wei Wang and Angel E. Kaifer* Center for Supramolecular Science and
Chiral Bronsted Acids as Catalysts
 Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
A Review of Total Synthesis of Spirotryprostatin A and B. Jinglong Chen Supergroup meeting Princeton University June
 A Review of Total Synthesis of Spirotryprostatin A and B Jinglong Chen Supergroup meeting Princeton University June 28 2006 ovel Mammalian Cell Cycle Inhibitors, Spirotryprostatins A and B Me Spirotryprostatin
A Review of Total Synthesis of Spirotryprostatin A and B Jinglong Chen Supergroup meeting Princeton University June 28 2006 ovel Mammalian Cell Cycle Inhibitors, Spirotryprostatins A and B Me Spirotryprostatin
Protein Structure Bioinformatics Introduction
 1 Swiss Institute of Bioinformatics Protein Structure Bioinformatics Introduction Basel, 27. September 2004 Torsten Schwede Biozentrum - Universität Basel Swiss Institute of Bioinformatics Klingelbergstr
1 Swiss Institute of Bioinformatics Protein Structure Bioinformatics Introduction Basel, 27. September 2004 Torsten Schwede Biozentrum - Universität Basel Swiss Institute of Bioinformatics Klingelbergstr
Prof. Jason Kahn Your Signature: Exams written in pencil or erasable ink will not be re-graded under any circumstances.
 1 Biochemistry 461 February 16, 1995 Exam #1 Prof. Jason Kahn Your Printed Name: Your SS#: Your Signature: You have 75 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded
1 Biochemistry 461 February 16, 1995 Exam #1 Prof. Jason Kahn Your Printed Name: Your SS#: Your Signature: You have 75 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded
Iridium-Catalyzed Hydrogenation with Chiral P,N Ligands
 Iridium-Catalyzed Hydrogenation with Chiral P, Ligands 贾佳 utline Brief Introduction Hydrogenation of C=C Bonds Hydrogenation of C= Bonds Hydrogenation of C= Bonds Conclusion Brief Introduction First example
Iridium-Catalyzed Hydrogenation with Chiral P, Ligands 贾佳 utline Brief Introduction Hydrogenation of C=C Bonds Hydrogenation of C= Bonds Hydrogenation of C= Bonds Conclusion Brief Introduction First example
Studies toward the Total Synthesis of Caribbean Ciguatoxin C-CTX-1: Synthesis of the LMN-Ring Fragment through Reductive Olefin Cross-Coupling
 S1 Studies toward the Total Synthesis of Caribbean Ciguatoxin C-CTX-1: Synthesis of the LMN-Ring Fragment through Reductive lefin Cross-Coupling Makoto Sasaki,* Kotaro Iwasaki, Keisuke Arai Graduate School
S1 Studies toward the Total Synthesis of Caribbean Ciguatoxin C-CTX-1: Synthesis of the LMN-Ring Fragment through Reductive lefin Cross-Coupling Makoto Sasaki,* Kotaro Iwasaki, Keisuke Arai Graduate School
Chapter 2: Synthesis of 2,3 and 3,4 disubstituted pyrrolidine based peptide nucleic acid monomers
 Chapter 2: Synthesis of 2,3 and 3,4 disubstituted pyrrolidine based peptide nucleic acid monomers Well your DA made you do it, i m sentencing your DA to 10yrs prison 2.1 Introduction Highly stable analogues
Chapter 2: Synthesis of 2,3 and 3,4 disubstituted pyrrolidine based peptide nucleic acid monomers Well your DA made you do it, i m sentencing your DA to 10yrs prison 2.1 Introduction Highly stable analogues
Total Synthesis of (+)-Suaveolindole
 1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
Total Synthesis of Cyclosporine: Access to N-Methylated Peptides via Isonitrile Coupling Reactions
 Total Synthesis of Cyclosporine: Access to -thylated Peptides via Isonitrile Coupling Reactions Xiangyang Wu, Jennifer L. Stockdill, Ping Wang, Samuel J. Danishefsky* J. Am. Chem. Soc. 2010,132, 4098-4100
Total Synthesis of Cyclosporine: Access to -thylated Peptides via Isonitrile Coupling Reactions Xiangyang Wu, Jennifer L. Stockdill, Ping Wang, Samuel J. Danishefsky* J. Am. Chem. Soc. 2010,132, 4098-4100
Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe
 Supporting Information for Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe Ho Yu Au-Yeung, Jefferson Chan, Teera Chantarojsiri and Christopher J. Chang* Departments
Supporting Information for Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe Ho Yu Au-Yeung, Jefferson Chan, Teera Chantarojsiri and Christopher J. Chang* Departments
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Energetics and Thermodynamics
 DNA/Protein structure function analysis and prediction Protein Folding and energetics: Introduction to folding Folding and flexibility (Ch. 6) Energetics and Thermodynamics 1 Active protein conformation
DNA/Protein structure function analysis and prediction Protein Folding and energetics: Introduction to folding Folding and flexibility (Ch. 6) Energetics and Thermodynamics 1 Active protein conformation
N,N -linked urea oligomers : synthesis, conformational studies and selfassembly
 V. Semetey --- IUPAC Prize for Young Chemists --- 2003 onorable Mention, -linked urea oligomers : synthesis, conformational studies and selfassembly properties. The functional diversity in proteins, although
V. Semetey --- IUPAC Prize for Young Chemists --- 2003 onorable Mention, -linked urea oligomers : synthesis, conformational studies and selfassembly properties. The functional diversity in proteins, although
Ultraviolet Spectroscopy. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
 Ultraviolet Spectroscopy CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan Ultraviolet Spectroscopy UV light can be absorbed by molecules to excite higher
Ultraviolet Spectroscopy CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan Ultraviolet Spectroscopy UV light can be absorbed by molecules to excite higher
Bicyclic helical peptides as dual inhibitors selective. for Bcl2A1 and Mcl-1 proteins
 Supporting Inormation Bicyclic helical peptides as dual inhibitors selective or Bcl2A1 and Mcl-1 proteins de Araujo A.D.; Lim, J.; Wu, K-C.; Xiang, Y.; Good, A. C.; Skerlj, R.; Fairlie, D. P.* Contents
Supporting Inormation Bicyclic helical peptides as dual inhibitors selective or Bcl2A1 and Mcl-1 proteins de Araujo A.D.; Lim, J.; Wu, K-C.; Xiang, Y.; Good, A. C.; Skerlj, R.; Fairlie, D. P.* Contents
Synthesis of two novel indolo[3,2-b]carbazole derivatives with aggregation-enhanced emission property
![Synthesis of two novel indolo[3,2-b]carbazole derivatives with aggregation-enhanced emission property Synthesis of two novel indolo[3,2-b]carbazole derivatives with aggregation-enhanced emission property](/thumbs/95/126281026.jpg) Supporting Information for: Synthesis of two novel indolo[3,2-b]carbazole derivatives with aggregation-enhanced emission property Wen-Bin Jia, Hao-Wei Wang, Long-Mei Yang, Hong-Bo Lu, Lin Kong, Yu-Peng
Supporting Information for: Synthesis of two novel indolo[3,2-b]carbazole derivatives with aggregation-enhanced emission property Wen-Bin Jia, Hao-Wei Wang, Long-Mei Yang, Hong-Bo Lu, Lin Kong, Yu-Peng
ROADMAP FOR REACTIONS Chapter 6
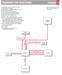 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
Problem session (3) Daiki Kuwana. Please fill in the blank and explain reaction mechanisms and stereoselectivities.
 Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Organic Cumulative Exam February 22, 2018
 rganic Cumulative Exam February 22, 2018 Answer only three of the five questions. o more than three question answers will be graded and any work not to be considered must be clearly marked as such. early
rganic Cumulative Exam February 22, 2018 Answer only three of the five questions. o more than three question answers will be graded and any work not to be considered must be clearly marked as such. early
One-pot synthesis of dual functional peptides by Sortase A-mediated on-resin cleavage and ligation
 Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2017 One-pot synthesis of dual functional peptides by Sortase A-mediated on-resin cleavage
Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2017 One-pot synthesis of dual functional peptides by Sortase A-mediated on-resin cleavage
Supporting Information For:
 Supporting Information For: Peptidic α-ketocarboxylic Acids and Sulfonamides as Inhibitors of Protein Tyrosine Phosphatases Yen Ting Chen, Jian Xie, and Christopher T. Seto* Department of Chemistry, Brown
Supporting Information For: Peptidic α-ketocarboxylic Acids and Sulfonamides as Inhibitors of Protein Tyrosine Phosphatases Yen Ting Chen, Jian Xie, and Christopher T. Seto* Department of Chemistry, Brown
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Literature Report 3. Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A. Date :
 Literature Report 3 Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A Reporter : Xiao-Yong Zhai Checker : Shubo u Date : 2017-10-30 Pritchett, B. P.; Donckele, E. J.; Stoltz, B. M. Angew.
Literature Report 3 Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A Reporter : Xiao-Yong Zhai Checker : Shubo u Date : 2017-10-30 Pritchett, B. P.; Donckele, E. J.; Stoltz, B. M. Angew.
Computational Analysis of Protein-Ligand Binding: From single continuous trajectories to multiple parallel simulations
 Computational Analysis of Protein-Ligand Binding: From single continuous trajectories to multiple parallel simulations Inauguraldissertation zur Erlangung der Würde eines Doktors der Philosophie vorgelegt
Computational Analysis of Protein-Ligand Binding: From single continuous trajectories to multiple parallel simulations Inauguraldissertation zur Erlangung der Würde eines Doktors der Philosophie vorgelegt
CEM 852 Exam What is the ratio of (S) / (R) alcohol formed during this reaction? (2 pts) baker's yeast. H 2 O, sucrose 25 C
 CEM 852 Exam-1 February 19, 2005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. What is the ratio
CEM 852 Exam-1 February 19, 2005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. What is the ratio
Supporting Information
 Supporting Information Responsive Prodrug Self-Assembled Vesicles for Targeted Chemotherapy in Combination with Intracellular Imaging Hongzhong Chen, Huijun Phoebe Tham,, Chung Yen Ang, Qiuyu Qu, Lingzhi
Supporting Information Responsive Prodrug Self-Assembled Vesicles for Targeted Chemotherapy in Combination with Intracellular Imaging Hongzhong Chen, Huijun Phoebe Tham,, Chung Yen Ang, Qiuyu Qu, Lingzhi
Supporting Information
 Supporting Information Novel diphenylmethyl-derived amide protecting group for efficient liquid-phase peptide synthesis; AJIPHASE Daisuke Takahashi*, Tatsuya Yano and Tatsuya Fukui Research Institute For
Supporting Information Novel diphenylmethyl-derived amide protecting group for efficient liquid-phase peptide synthesis; AJIPHASE Daisuke Takahashi*, Tatsuya Yano and Tatsuya Fukui Research Institute For
Haiyang Sun, Huimin Guo,* Wenting Wu, Xin Liu, Jianzhang Zhao*
 Electronic Supplementary Information for: Coumarin phosphorescence observed with ^ Pt(II) bisacetylide complex and its applications for luminescent oxygen sensing and triplet-triplet-annihilation based
Electronic Supplementary Information for: Coumarin phosphorescence observed with ^ Pt(II) bisacetylide complex and its applications for luminescent oxygen sensing and triplet-triplet-annihilation based
(A) 10 (B) 10 (C) 140 (D) 140 (E) 70 7.Calculate the ionization energy, in kj/mol, of a mole of hydrogen atoms in the ground state.
 80 1.The term that describes the following series Ne, F -, 2-, and N 3- is (A) Isotopic (C) isobar anionic 2.Which oxide of chlorine is unstable? (B) isoelectronic allotropic (A) Cl 2 (B) Cl 2 3 (C) Cl
80 1.The term that describes the following series Ne, F -, 2-, and N 3- is (A) Isotopic (C) isobar anionic 2.Which oxide of chlorine is unstable? (B) isoelectronic allotropic (A) Cl 2 (B) Cl 2 3 (C) Cl
1-N-(3,4,6-tri-O-acetyl-2-deoxy-2-N-acetyl-β-D-glucopyranosylamide)-4-(N -methylidenyl -2 - bromoacetamido)-4,5-anhydro-triazole (5) AcO AcO
 upporting Information -(propargyl)-bromacetamide (4) ac 3, 2 2 Propargylamine (0.1 ml, 1.45 mmol) was dissolved in water (15.0 ml) and was treated with bromoacetic anhydride (1.85 g, 7.25 mmol) in the
upporting Information -(propargyl)-bromacetamide (4) ac 3, 2 2 Propargylamine (0.1 ml, 1.45 mmol) was dissolved in water (15.0 ml) and was treated with bromoacetic anhydride (1.85 g, 7.25 mmol) in the
Supporting Information
 Supporting Information Discovery of PF-06463922, a macrocyclic inhibitor of ALK/RS1 with pre-clinical brain exposure and broad spectrum potency against ALK-resistant mutations Ted W. Johnson,* Paul F.
Supporting Information Discovery of PF-06463922, a macrocyclic inhibitor of ALK/RS1 with pre-clinical brain exposure and broad spectrum potency against ALK-resistant mutations Ted W. Johnson,* Paul F.
Supplementary Methods
 Supplementary Methods Synthesis overview 4 a-c C 5 TBS d H 2 TBS 6 H 2 H 2 (Boc) 2 e f-h i Br TBS TBS TBS 7 8 9 j,k (Boc) 2 l H 2 H H 10 fmk (1) Scheme 1. Reagents and conditions: a. malononitrile, MeH,
Supplementary Methods Synthesis overview 4 a-c C 5 TBS d H 2 TBS 6 H 2 H 2 (Boc) 2 e f-h i Br TBS TBS TBS 7 8 9 j,k (Boc) 2 l H 2 H H 10 fmk (1) Scheme 1. Reagents and conditions: a. malononitrile, MeH,
9701 CHEMISTRY. Mark schemes should be read in conjunction with the question paper and the Principal Examiner Report for Teachers.
 CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Level MARK SCHEME f the May/June 2013 series 9701 CHEMISTRY 9701/42 Paper 4 (A2 Structured Questions), maximum raw mark 100 This mark scheme is published
CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Level MARK SCHEME f the May/June 2013 series 9701 CHEMISTRY 9701/42 Paper 4 (A2 Structured Questions), maximum raw mark 100 This mark scheme is published
Supporting Information
 Supporting Information An L-proline Functionalized Metallo-organic Triangle as Size-Selective Homogeneous Catalyst for Asymmertry Catalyzing Aldol Reactions Xiao Wu, Cheng He, Xiang Wu, Siyi Qu and Chunying
Supporting Information An L-proline Functionalized Metallo-organic Triangle as Size-Selective Homogeneous Catalyst for Asymmertry Catalyzing Aldol Reactions Xiao Wu, Cheng He, Xiang Wu, Siyi Qu and Chunying
Practice Final for Chem 104
 Practice Final for Chem 104 These questions cover the material that will be covered on the Final Exam. The distribution of the questions is about the same as the Final Exam. The forms of the questions
Practice Final for Chem 104 These questions cover the material that will be covered on the Final Exam. The distribution of the questions is about the same as the Final Exam. The forms of the questions
A CONFORMATIONAL STUDY OF PROLINE DERIVATIVES. M.E. Kamwaya * and F.N. Ngassapa
 , 199-206. ISSN 1011-3924 Printed in Ethiopia 2002 Chemical Society of Ethiopia A CONFORMATIONAL STUDY OF PROLINE DERIVATIVES M.E. Kamwaya * and F.N. Ngassapa Chemistry Department, University of Dar es
, 199-206. ISSN 1011-3924 Printed in Ethiopia 2002 Chemical Society of Ethiopia A CONFORMATIONAL STUDY OF PROLINE DERIVATIVES M.E. Kamwaya * and F.N. Ngassapa Chemistry Department, University of Dar es
Literature Report I. Total Synthesis of (+)-Piperarborenine B. Reporter: Zheng Gu. Date:
 Literature Report I Total Synthesis of (+)-Piperarborenine B Reporter: Zheng Gu Checker: Bo Song Date: 2016-12-12 Hu, J. L.; Xie, Z. W.; Tang, Y. J. Am. Chem. Soc. 2016, 138, 13151. Panish, R. A.; Chintala,
Literature Report I Total Synthesis of (+)-Piperarborenine B Reporter: Zheng Gu Checker: Bo Song Date: 2016-12-12 Hu, J. L.; Xie, Z. W.; Tang, Y. J. Am. Chem. Soc. 2016, 138, 13151. Panish, R. A.; Chintala,
SUPPORTING INFORMATION. Reversible and versatile on-tether modification of chiral. content
 UPPRTIG IFRMATI Reversible and versatile on-tether modification of chiral center-induced helical peptides Kuan u,,# Chengjie un,,# Zigang Li #,* # chool of Chemical Biology and Biotechnology, Peking University
UPPRTIG IFRMATI Reversible and versatile on-tether modification of chiral center-induced helical peptides Kuan u,,# Chengjie un,,# Zigang Li #,* # chool of Chemical Biology and Biotechnology, Peking University
Peptide functionalized Polydiacetylene Liposomes act as a Fluorescent Turn-On Sensor for Bacterial Lipopolysaccharide
 SUPPRTIG IFRMATI FR: Peptide functionalized Polydiacetylene Liposomes act as a Fluorescent Turn-n Sensor for Bacterial Lipopolysaccharide Junchen Wu, Adam Zawistowski, Michael Ehrmann, Tao Yi and Carsten
SUPPRTIG IFRMATI FR: Peptide functionalized Polydiacetylene Liposomes act as a Fluorescent Turn-n Sensor for Bacterial Lipopolysaccharide Junchen Wu, Adam Zawistowski, Michael Ehrmann, Tao Yi and Carsten
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for uminum complexes containing salicylbenzoxazole
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for uminum complexes containing salicylbenzoxazole
Supporting Information
 Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany rganocatalytic Conjugate Addition of Malonates to a,ß-unsaturated Aldehydes: Asymmetric Formal Synthesis of (-)-Paroxetine, Chiral Lactams
Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany rganocatalytic Conjugate Addition of Malonates to a,ß-unsaturated Aldehydes: Asymmetric Formal Synthesis of (-)-Paroxetine, Chiral Lactams
Radical Reactions. Radical Stability!!! bond dissociation energies X Y X + Y. bond BDE (kcal/mol) bond BDE (kcal/mol) CH 3 CH 3 CH 2 95 O H R 2 C H
 adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
