Modeling and Analysis for Control of Reactant and Water Distributions in Fuel Cells
|
|
|
- Peter Simpson
- 5 years ago
- Views:
Transcription
1 Modeling and Analysis for Control of Reactant and Water Distributions in Fuel Cells by Buz A. McCain A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Mechanical Engineering) in The Uniersity of Michigan 28 Doctoral Committee: Professor Anna G. Stefanopoulou, Chair Professor N. Harris McClamroch Professor Jeffrey L. Stein Research Scientist Hosam K. Fathy Kenneth R. Butts, Toyota Engineering and Manufacturing, North America
2 c Buz A. McCain All Rights Resered 28
3 I dedicate this work to my father, Eerd A. McCain, the greatest influence on the person I hae become. And to my mother, Iona I. McCain, who has always been my greatest supporter. ii
4 Acknowledgments I owe a huge debt of gratitude to my adisor, Professor Anna G. Stefanopoulou, for her guidance and patience during the last three years. I do not know how she managed to put up with me, but I know better than to ask. Dr. Kenneth R. Butts saed me from a financial abyss with his support of my research at Toyota, and from spiritual obliion with his friendship and liing by example. There s really no way to pay him back, and he s the first person to get me to understand that it is not necessary to repay those kinds of debts. Dr. Hosam K. Fathy was more than a committee member, his explanations of the academic world helped me make sense of an unfamiliar arena, and his explanations of modeling and model order reduction were indispensable. I hae Professor Jeffrey L. Stein to thank for getting me interested in modeling, starting me down the path to my current specialty. If I eer hae a teaching opportunity, my goal will be to imitate his lecture style. Professor Harris McClamroch is the epitome of the ideal academic. Wellrespected and well-informed, high-energy and non-judgmental, helpful and friendly; meeting him through class was proidence. I am thankful for his participation in my academic career. A number of Toyota coworkers and superisors share the credit for my meager accomplishments, including my last pre-phd superisor, Mr. Hiroyuki Koba, who supported my unorthodox plan respectfully. Mr. Chikuo Kubota and Mr. Akio Nishimura taught me that I respond best to trust and responsibility, and without their support, I would not hae been able to study at the Uniersity of Michigan. Special thanks are owed to Ms. Melissa Spaid, Mr. John Baylis and Mr. James Griffith of Toyota Administration for their open-minded outlook on my return to school. My labmates made the difference during my time at the Powertrain Control Lab. I am grateful to Dr. Ardalan Vahidi for his warm welcome during my first months, and to Dr. Ray Chiang for his sense of humor and kindness. Dr. Amey Karnik was inaluable to me for his knowledge of fuel cells, linear algebra, and strong logical ability. Dr. Denise McKay, thank you for sharing your towering knowledge of fuel cell operation and experimentation. Finally, I am thankful to Jason Siegel for his willingness to share his knowledge and understanding of the theoretical side of mathematics, as well as a helping hand for just about anything else. I am ery appreciatie of the effort made by my study partner Gayathri Seenumani to stay friends with me, since that is not an easy task. Together we conquered iii
5 Quals, and made it through many classes. Thank you for sticking with me. Chris Vermillion deseres special mention for his willingness to share both his impressie knowledge of control theory as well as his optimistic opinions on politics during our nearly two years of internship together. Finally, I would like to express my appreciation of my wife, Yoshimi, who definitely had reserations, but neer complained loudly or directly. I would also like to apologize to my children Tyler, Lyle, and Mia for being around so much, yet playing with you so little. The hardest part of the last three years has been answering your requests with sorry, Daddy has too much to do. I hope I can make it up to you all. i
6 Table of Contents Dedication Acknowledgments List of Figures List of Tables List of Appendices ii iii i ii iii Chapter 1 Introduction Motiation Fuel Cell Operation About Water in the Fuel Cell Objectie of the Research Literature Surey Fuel Cell Modeling Fuel Cell Control Model Order Reduction Contributions and Outline Chapter 2 Full-Order Model Formulation Model of the Gas Diffusion Layer Model Assumptions Continuous 1D Model Formulation Boundary Conditions Channel Equations Discretization and Numerical Solution Difference Equations Chapter 3 Model Order Reduction Deriation of the Bond Graph Equations Gas Species Concentration (Capacitance Model) Gas Species Diffusion (Resistance Model)
7 3.1.3 Liquid Water Storage (Capacitance Model) Liquid Water Transport (Resistance Model) The Bond Graph Model The Model Order Reduction Algorithm Background of the MORA Application of the MORA Time-Scale Decomposition Chapter 4 The Semi-Analytic Solution Deelopment of the Semi-Analytic Model Sensitiity of Diffusiity to Liquid States Gas Constituent Solutions Liquid Water Solution Vapor Solution Transition Simulation of Vapor Solution Transition Verification of the Semi-Analytic Model Applications of the SAS Model About the Along-the-Channel Dimension Model Extension to 1+1D Maximizing Hydrogen Utilization by Control Chapter 5 Control Analysis Analysis of Equilibria Stability Stability of Liquid Water in the GDL Linearization of the Continuous PDE Transformation of the Linearized PDE Stability of the Transformed PDE Stabilizing Equilibrium Control Objectie Concept Controllability On the Choice of Controllability-Obserability Test Aailable Control Inputs Surrogate States for Reduced Models Controllability-Obserability Results Controllability-Obserability Tables Controllability of the GDL Liquid Chapter 6 Conclusions Fuel Cell Control-Oriented Modeling Control Analysis Appendices Bibliography i
8 List of Figures Figure 1.1 Basic concept of fuel cell electricity generation ESEM images of GDL porous media in arious stages of flooding Water generation, transport, and phase change occur throughout the fuel cell Oeriew of research content, leading to the control-oriented model and its control analysis Basic concept of water transport in a fuel cell Capillary flow of liquid water through the gas diffusion layer Boundary conditions for anode and cathode GDL Bond Graph Building Block for one Discrete GDL Section Complete Bond Graph Representation of a 3-Section Discrete Water Dynamics Model Actiity of resistie elements hae information regarding degree of linearity of distribution from state in discretized PDEs Small actiity leads to residualization of the state associated with the capacitie element Steady-state error in (c,an c sat ) x= for arying choices of constant liquid water fraction Use of s = s im has negligible effect on solution Transient solutions of c H2 and c,an for a step in current density (.15.3 A/cm 2 ) Water apor distribution for the switching analytic solution with the mobile front in the transition from two-phase to single-phase during a step change in λ H The semi-analytic model is able to capture static and transient trends in a direct comparison to experimental oltage output, and generates a oltage estimate ery similar to the numeric solution The oltage prediction captures fluctuations due to changing conditions, and bias is within the cell-to-cell ariation Neutron imaging shows liquid water accumulating near the anode channel exit (horizontal liquid images are from cathode side) ii
9 4.8 Schematic of along the channel model, with two-phase front for channel, and within the GDL for each unit model discretization along the channel Distribution along-the-channel of water apor at the membrane, as well as liquid, water apor and hydrogen in the channel (λ H2 = 25%) Distribution along-the-channel of water apor at the membrane, as well as liquid, water apor and hydrogen in the channel (λ H2 = 4%) Addition of a temperature gradient turns model prediction from flooding to non-flooding for otherwise identical conditions Hydrogen waste comparison between dead-ended and flow-through anode control Simple P-control drien by exhaust humidity error ersus reference preents oltage degradation due to liquid accumulation Experimental results support the hypothesis that anode flooding causes oltage degradation that is recoered through anode channel purging The transition from almost complete liquid transport to complete apor transport across the GDL-channel boundary Channel water apor concentration boundary alue can shape the liquid water distribution D.1 Solution flow the case where the GDL has s > for all sections (x fr = L).113 D.2 Solution flow for the case where there may be some s = sections (x fr < L) iii
10 List of Tables Table 3.1 Actiity Summary for Borderline and Flooding Cases Options for element reduction based upon actiity analysis Gas and liquid state coupling ersus current density i GDL State Response Speeds (Time Constants (τ c ) ) Cases Studied for Controllability/Obserability States in the output matrix remoed by analytic solutions are replaced by remaining surrogate states Controllability PBH Eigenector Test Result Obserability PBH Eigenector Test Result A.1 Constant alues and notation A.2 Variable descriptions A.3 Parameter descriptions ix
11 List of Appendices Appendix A Abbreiations and Nomenclature B Separation of Variables C Liquid Steady-State Solution Deriation D Simultaneous Solution of SAS Boundary Conditions and Coefficients. 16 E Voltage Model x
12 Chapter 1 Introduction 1.1 Motiation Fuel cell technology has been on the horizon since its successful implementation in the space programs of the 195s, with business and technology pundits as far back as 196 proclaiming that...a score of non-utility companies are well adanced toward deeloping a powerful chemical fuel cell, which could sit in some hidden closet of eery home silently ticking off electric power ([1]). Oer 47 years since that hopeful prediction, and oer 16 years since the fuel cell was discoered, there are still a number of significant obstacles to widescale implementation. Commonly listed barriers include hydrogen generation and storage, component durability, cost, thermal and water management, system integration, poisoning, startup/shutdown, and public perception. In spite of the great deal of work that remains to be done, fuel cells represent an important part of the proposed hydrogen economy, and an immense opportunity to impact the socioeconomic structure of society. The last seeral years hae seen a significant increase in budgeting for fuel cell research and hydrogen economy deelopment, with examples including General Motors funding of oer $1B in fuel cell research through 23 ([2]), President Bush s pledge of $1.7B oer 5 years in research support during the 23 State of the Union address, and California Go. Schwarzenegger s promise to install a hydrogen highway refueling system, estimated at $6 million, by 21. 1
13 1.1.1 Fuel Cell Operation Polymer electrolyte membrane fuel cells (PEMFC) are electrochemical energy conersion deices that conert the chemical energy of supplied reactants (hydrogen and oxygen) into electricity. Of the many types of fuel cells, the PEMFC is considered a commercially implementable frontrunner for its low temperature operation and efficiency. Shown pictorially in Fig.1.1, each fuel cell consists of an anode side and a cathode side, commonly referred to as the electrodes. The main components on each electrode are the channel through which the reactants enter/exit the fuel cell assembly and a gas diffusion layer (GDL) that receies all or a portion of the flow and physically distributes the reactants oer the catalytic material. The catalyst is typically a coating on the part of the GDL that is in contact with the proton exchange membrane (PEM). The heart of the fuel cell is the PEM, which is a thin proton- and water-permeable material that requires sufficient humidification in order to function efficiently and safely (without damage) (Cross-sectional iew) Chemical Reactions Anode: H 2 2H + + 2e - (Oxidation) Cathode: O 2 + 4H + + 4e - 2H 2 O (Reduction) O H 2 O O Polymer Electrolyte Membrane H 2 H 2 H Cathode Channel (O 2 supply) Gas Diffusion Layers Anode Channel (H 2 supply) Catalyst Catalytic Layer Figure 1.1: Basic concept of fuel cell electricity generation. 2
14 To summarize the operation simply, reactant gases are supplied to both electrodes of the fuel cell ia the channels, the gas diffusion layer facilitates een distribution to the catalyst-coated membrane, and the catalyst accelerates the oxidation and reduction of the reactants, which are the primary reactions desired for fuel cell operation (Fig. 1.1). The inputs to the system are the reactants and water (in the form of humidified reactant flows), while the outputs are electricity and more water. Water is mentioned as an input and an output because water is required for operation of the cell, and water is generated as a result of operation of the cell. Oxygen is aailable as a component of air, and the hydrogen must be processed and proided in pure form to the cell stack. The topic of hydrogen reformation is a complex research topic on its own. The H 2 oxidation on the anode catalyst of the membrane releases two electrons, which traerse the circuit to satisfy the load required of the cell, while the remaining protons (H + ) trael through the membrane to the cathode side. The cathode side catalyst splits the oxygen molecules, which then join with the electrons completing the circuit and the protons from the membrane to form product water. On both sides of the membrane, the hydrophobic GDL porous material draws the water away from the membrane and catalyst to the respectie channels for remoal. The production, phase transformation, and transport of water within the fuel cell is critical for efficient performance and long life. As a prime example, the efficiency of membrane proton transport (the proton conductiity) is directly dependent upon the water content of the membrane About Water in the Fuel Cell Water management is the fuel cell implementation barrier addressed in this thesis. At issue is the need to balance the detrimental effect of liquid water accumulation against the requirement of a sufficient leel of water in the membrane. High membrane humidity is desirable for proton conductiity, yet excess liquid water has been experimentally shown to be a cause of output oltage degradation [3, 4]. Specifically, liquid water occupies pore space in the porous material of the GDL, impedes the diffusion of reactant flow towards the membrane, and ultimately reduces the actie fuel cell area. The pore blocking phenomenon is shown clearly in the enironmental scanning electron micrograph (ESEM) image of liquid accumulation in Fig Transport, generation, and phase change of water are critical aspects of the 3
15 Figure 1.2: ESEM images of GDL porous media in arious stages of flooding [5]. modeling of fuel cell dynamics. As shown in Fig. 1.3, eaporation/condensation occurs throughout the GDL and channels, water is generated on the cathode catalyst, and transport across the boundaries between both GDLs and the membrane as well as across the GDL-channel interfaces must be considered. Membrane water transport occurs as a result of competing phenomena, the osmotic drag and back diffusion. Osmotic drag is the process by which protons traeling across the membrane drag water molecules with them. Osmotic drag is a function of water content and current drawn, and flows from anode to cathode. The direction of back diffusion is typically opposite to that of osmotic drag because it is drien by a concentration gradient and the cathode commonly has higher water apor concentration than the anode. Also shown in Fig. 1.3 is the ale controlling the anode outlet flow. In deadended anode architecture, the ale is closed, and is opened for only a short period of time (e.g. 1 sec in 18 sec) to remoe water. In a flow-through arrangement, the control ale position ū is aried between zero and one based on some control logic. To aoid damage associated with membrane and GDL drying, fuel cells may be operated under flooding conditions (i.e., a net build-up of liquid water). Remoal of liquid water is necessary to regain performance lost when reaction sites become flooded. Recoery is typically accomplished by a massie and brief inlet flow increase (e.g. anode purge), which lowers efficiency due to the large reactant flow out. Additionally, durability can be compromised by the cycling of the GDL through saturated and sub-saturated conditions that arise from these substantial periodic inlet flow rate changes. The management of water within the fuel cell stack is critical for optimal stack 4
16 in W ca Cathode GDL Catalyst Layers Anode GDL in W an Water Generated membrane channel z = GDL W O2 osmotic drag GDL W H2 GDL W w,an GDL W w,an back diffusion x = Closed: Dead-end Open: Flow-thru Flow-thru out W ca r =γ sat ( c c ) (Eaporation/condensation) out W an u : Position Figure 1.3: Water generation, transport, and phase change occur throughout the fuel cell. A dead-ended anode channel has the outlet closed except during intermittent high elocity flow purges. performance. The oerall goal is to use control to strike a balance between hydrogen and oxygen deliery, and water supply/remoal. In this work, anode water management is addressed (instead of cathode) for four reasons. First, anode components significantly increase the cost, weight, and size of a fuel cell system. Efficient anode water management will enable the elimination or reduction in equipment for anode inlet humidification and/or recirculation systems. Second, aoidance of excessie flooding allows higher hydrogen concentrations to be attained, preenting staration and fuel cell damage due to carbon corrosion. Third, we desire to implement a flow-through anode channel arrangement to aoid the detrimental effect on GDL material cycling through liquid saturation and drying that occurs during purge cycles in a dead-ended system. Finally, we seek to aoid the preiously mentioned cell oltage degradation associated with anode channel flooding. 5
17 1.1.3 Objectie of the Research Figure 1.4 proides an oeriew of the research content of this thesis. The specific objectie of this work is to derie a control-oriented fuel cell model where the states used hae physical meaning, the computational cost is minimized, and the performance is equialent to an existing 24-state numeric fuel cell dynamics model [3]. The objectie is approached as a combination of model reduction and model deriation. The preexisting work includes a physics-based continuous model with six 2 nd -order PDEs, ordinary differential equations for channel mass balance, and a set of algebraic equations related mostly to membrane transport and orifice flows. The pre-existing work also includes an implementation of the continuous model as a series of cascaded firstorder difference equations, with alidation. In this thesis, a control-oriented model that maintains the physically-intuitie characteristics is reached ia a combination of nonlinear model order reduction, modal analysis, analytic solutions to the PDEs, and a control study of both the continuous PDE model and the difference equation approximation. The OVERVIEW Pre-existing work START Continuous domain physics-based model Six 2 nd -order PDEs Mass balances (ODEs) Algebraic equations Cascaded difference equations Full (Coarse) Numeric model Experimentally alidated 1 24 states Semi-Analytic Solution Deriation 4 of 6 analytic solutions Liq. steady-state solution PDE Analysis PDE stability Equilibria range Control objectie Nonlinear Model Order Reduction MORA application Solution form info Time separation (Model Order Reduction Algorithm) Linear System Analysis Modal decomposition Contr-Obser-ability Time separation Validation & Application Experiment Full-order model comparison Features Control-oriented physics based model Simplified (computational cost) Physical intuition (resolution) Water management System response GOAL Figure 1.4: Oeriew of research content, leading to the control-oriented model and its control analysis. The full-order model was shown to predict the experimentally-obsered oltage degradation during prolonged operation in a dead-ended anode condition [3], and the mass of water that accumulates in the anode channels measured ia neutron 6
18 imaging [4]. Though 24 states might seem sufficiently low order, a 3-section discretization is quite coarse, proiding limited or ague physical insights. For analysis, finer spatial resolution is desired, but for each section added to the spatial discretization, a sixfold increase in the number of states is generated. Model order reduction is pursued for the dual purposes of simplification for control and for increased resolution at similar computational cost. The model order reduction methodology created here is a combination of an energy-based state contribution analysis, model assumption error study, empirical model reformulation, singular perturbation inestigation, and application of PDE analytic solutions to reduce the computational load associated with simulation of dynamic state equations. Model simplification is accomplished by deriation of a semi-analytic solution (SAS) model which replaces the majority of the dynamic states with their analytic solutions, thereby reducing computational effort. The SAS model is alidated using experimental data under dead-ended operation conditions, and is applicable to flowthrough since the on-off dead-ended arrangement is a subset of flow-through. System behaior is studied through an analysis of the range of possible equilibria. Further, though the total system shows unbounded growth for bounded inputs, the GDL portion of the liquid water distribution is found to be stable using a Lyapuno stability analysis, implying that it is the anode channel liquid mode causing the unbounded growth. Further, this mode is shown to be controllable, a necessary condition for system stabilizability. Based on stability, maximization of hydrogen utilization, equilibrium analysis, and controllability-obserability arguments, a hypothesis on an appropriate control setpoint is made. It will be the equilibrium condition such that net water flow into the anode GDL is zero and where the water mass transport across the GDL-channel interface is only in apor phase. For conditions where a lack of liquid water disrupts eaporation, switching water apor solutions are proided. This is necessary since the setpoint will be on the cusp of GDL drying. This condition is termed a borderline drying condition because it is the point where the boundary between the single-phase and two-phase water distribution lies on the GDL-channel interface and there is no liquid flow from GDL to channel. 7
19 1.2 Literature Surey Fuel Cell Modeling Fuel cell modeling is a widely researched field, as eidenced by the plurality of published work on reiew of the models themseles (e.g. [6]). For the purpose of proiding perspectie for the work herein, it is helpful to classify FC models by motiation (as accurate as possible by some experimental measure or control-oriented deriation with sufficient accuracy), by formulation (analytic, low-order numeric, high-order numeric), by dimension (D, 1D, 1+1D, 2D, 3D), and by dynamic conditions (transient or steady-state). The zero-dimensional models consider only the aerage alues of the species pressures within the fuel cell, whereas the one-dimensional model adds spatial ariation either in the through-membrane direction or along-the-channel. The union of the two types of 1D model produces the 1+1D model, so named because though the channel may be serpentine, it is assumed to be stretched out into a straight line. There is ariety in the definition of a 2D model, with some mapping the ariation in the plane of the membrane ([7]), some addressing the plane normal to the membrane ([8]), and still others adding a dimension along the channel width to the through-membrane spatial ariation ([9]). The D models are for control applications, usually with the fuel cell as a component in an oerall system (FC plus parasitic support deices or fuel reformation [1],[11],[12]). For the most part, fuel cell modeling has become more complex since Springer et al ([13]) and Bernardi et al ([14]) published early 1D work on the topic in D work was pioneered by Fuller et al ([15]) in 1993, followed by Dannenberg s ([16]) steady-state mathematical model reealing ariation in membrane water content along the channel. Recent along-the-channel work includes Kulikosky s ([17]) inestigation of semi-analytic means to determine ariation in reactant concentration and current density along a straightened channel and Berg et al ([18]) applying channel spatial ariation for water management. All the aforementioned 1+1D models are steady-state. Most models published implement numeric solutions, with the 2D and higher dimension models exclusiely employing intense computational numeric algorithms ([19],[2]). Again, steady-state solutions comprise the majority of the higher dimension models, though [9] includes transients. The work herein would be classified as a 1D, dynamic, control-oriented model by way of model-order reduction of a medium-order numeric model, with experimental 8
20 alidation of sufficient accuracy and supplemental inclusion of analysis for control. Similar practical application of mathematical modeling work is also being performed by Promislow [21, 22], though without the focus on simplification to prepare for a control-oriented application. For example, though Promislow includes conectie transport, our assumption of a diffusion-dominated GDL gas transport model allows a simple steady-state analytic solution for the gas constituent distributions to be found, which facilitates stability analysis. Similar to [22], we apply quasi steadystate solutions for the fast gas species, and focus on the slow transients of the liquid. Another difference can be found in our assumption that water apor concentration ariation from saturation within the membrane electrode assembly has an important role to play in liquid and apor water transport through the gas diffusion layers. Use of this apor concentration model proides for diffusie transport under isothermal conditions. The ery recently published work in [23] has the similar goal of a two-phase PEMFC model for control by reducing the complexity. The assumptions and focus areas are significantly different, howeer, with the analysis of the water spatial distributions within the GDLs, inclusion of membrane water apor transport, and emphasis on aoidance of flooding-induced degradation of oltage output as the focus of this work as opposed to their simplifying assumptions of lumped alues for constituents in the GDL, liquid membrane transport, and emphasis on capturing the multiplicities predicted by their high-order model Fuel Cell Control Fuel cell control is a growing research interest area, with work expanding in both component and system leels. Modeling and control by [24] and [25] focused on issues associated with control of fuel cells and support components such as compressors run parasitically by the fuel cell. Further, when discussing fuel cell control, the typical meaning is fuel cell power control and the goal is operating the fuel cell efficiently while striing to meet the power demands placed on it. Controlled ariables for safe and efficient fuel cell operation include oxygen concentration, relatie humidity, and power output. In [26], the control methodology attempted to address all of these aried control objecties by nesting multiple loops. Ideas in the literature for power control include setting a oltage output command to obtain cell current output to meet the power requirements (or ice ersa due to the current-oltage dependency) [26], modulation of oxygen excess ratio ia airflow [27], and application 9
21 of controlled DC/DC conerters to conert the fuel cell output to a desired power leel [28]. Airflow rate and stack current are the typical control inputs for oxygen staration preention [29], while temperature was used as the manipulated ariable for humidity control in [26] Model Order Reduction Model order reduction has a long and well-documented history. While the list of articles releant to model order reduction is dauntingly long, a ery brief summary of some of the major points is included here to aid in the understanding of the proposed direction. Linear MOR: The ast majority of model order reduction is done on linear or linearized systems, in the state space, and has as a fundamental aspect a change of coordinates. A basic example of this is the eigenector-based method of using a similarity transformation matrix with columns formed by the unit eigenectors of the system. Each eigenalue of the system proides information regarding the speed of its respectie mode. In modal truncation, the fastest modes are remoed from the system. At issue, howeer, is whether the modes should be remoed in order from fastest to slowest, or if there might be slower modes that contribute less than one of the faster modes. In Moore s classic work [3](1981), he introduced the usage of Hankel singular alues (which he termed second-order modes ) and the balanced realization, which is, again, a similarity transformation of the original system. This balanced ersion of the system is obtained by determining the transformation that renders the obserability and controllability grammians diagonal and equal. The diagonal elements of these grammians are the Hankel singular alues of the system. The magnitude of the Hankel SVs indicates the significance of that mode to the input-output dynamics. The modes judged to be non-contributing can be reduced through either truncation or residualization. This method represents a ariation on eigen-based concepts, because the Hankel SVs are the square root of the eigenalues of the product of the controllability and obserability grammians. The idea of an internally balanced system is that if a realization can be found where the input-output relationships are equialent within each mode, then it will be possible to determine which modes contribute the least to both input and output simultaneously. The adantage oer the modal truncation is that the balanced realization considers the inputs and outputs selected, and uses that information to rank the mode contribution. 1
22 Other important work in the field includes Wilson s historic work [31](197) focusing on minimizing a quadratically-weighted output error (optimality), as well as Skelton s [32] use of a quadratic optimality criterion to decompose the error contribution of each state, then truncating those that contribute least to the error [33]. Loee [34] is credited with creation of the data-based Karhunen-Loee method (aka POD - Proper Orthogonal Decomposition). Gloer [35] has become synonymous with analysis of the Optimal Hankel Norm approximation, and its infinity-norm bound. An attractie aspect of the Optimal Hankel Norm approximation method is that its error bound is half that of the balanced realization methods. It would not be unusual to specify a classification of model order reduction by its usage of projections onto a subspace spanned by a basis of modes as the foundation of the method. This classification would coer the majority of methods used in practice. Within this lower-order-subspace projection classification, modal truncation, balanced truncation, the POD, and the moment-matching Krylo subspace methods are the most commonly applied. The remaining group of model order reduction techniques is much harder to classify than the subspace-projection methods described aboe. For lack of a better term, these methods might be called the non-subspace-projection methods. They include singular perturbation (within which balanced residualization would be included), the Optimal Hankel norm model reduction [35], and the equally applicable to linear and nonlinear systems model order reduction algorithm (MORA) [36]. In balanced residualization, the first step is, like balanced truncation, to find a balanced realization of the system and determine which states of the new system contribute least to the input-output relationships by analysis of the Hankel singular alues (typically, these states are the fast states of the new system). These fast states are not truncated, howeer. They are re-introduced into the reduced system, maintaining the DC gain, by setting their deriaties to zero. A somewhat unique method of model order deduction (MODA), created by Stein and Wilson [37], takes a notably different tack to determine a sufficient model order for the application. In MODA, instead of reducing a model, gien the desired range of frequency of operation, the model is built-up, mode by mode, until sufficient accuracy of the highest frequency mode within the range of interest is obtained. Accuracy is judged by how much the eigenalues of the modes change each time the order of the model is increased through addition of the model accuracy-improing modes. The error bound for MODA, set in terms of allowable eigenalue ariation, must be set as a design specification prior to model creation. 11
23 Work in linear system order reduction is still an actie topic of research, as eidenced by recent papers from Lall [38] and Rowley [39]. With Lall working on applications of POD to mechanical systems and Rowley presenting a combined Balanced POD method of determining approximate balanced truncations to gain computational efficiency. Nonlinear MOR: The application of model order reduction to nonlinear systems is still in the steep learning cure phase, and much of the work seeks to draw upon the large number of linear systems tools aailable. Lall [4],[41] and Wilson [42] quasilinearize (through describing functions or Taylor series expansion), and then apply POD or balanced realization techniques. The Trajectory Piecewise Linear (TPWL) approach is also seeing significant research actiity ([43],[44]) in conjunction with truncated balanced realization. It should be noted that the MORA of Stein and Louca is applicable to nonlinear systems, adds minimal additional computation effort, but does not hae clearly defined error bounds. For the purposes of foreshadowing the direction of this research, it is important to note that most MOR methods do not presere the meaning of the states of models created from first principles. The reduced system states exist as combinations of the original states. After transformations and truncations/projections, it is, in general, not possible to assign physical meanings to the reduced model states (though in chance applications, it is sometimes the case that a state chosen from first principles happens to be close to a remaining mode of the reduced system) Contributions and Outline The contributions to the areas of fuel cell modeling, model simplification, and control are as follows: A bond graph representation of a channel-membrane-channel model is created to deelop system operation physical intuition and to implement the Model Order Reduction Algorithm. The energy-based MORA is used in a noel way to predict the form of the analytic solutions of a set of interdependent 2 nd -order PDEs. In a sense, predicting the degree of linearity of the solutions. MORA results on the nonlinear system are shown to collaborate well with a modal analysis performed on the linearized system to show the time separation between the liquid and gas species modes. Error, sensitiity, and singular perturbation analyses are performed to ealuate the coupling effect on gas diffusiity as a function of liquid water present. 12
24 Justified approximations for effectie diffusiity and capillary pressure are made to create a semi-analytic solution model that is shown to predict oltage degradation due to anode flooding and reactant response to input changes. Control analyses for stability and controllability-obserability are performed on the SAS model showing that the system is stabilizable. A control objectie placing the actie two-phase water front at x = L, with proof of concept simulation, is proposed that reduces hydrogen waste and eliminates liquid accumulation in the anode channel. Gien the control objectie proposed, the SAS is expanded to include a spatially and temporally arying two-phase water front within the GDL (water apor switching solution). The SAS model is structured such that it can be modified easily to include along-the-channel spatial ariation and temperature ariation. Computational cost has been decreased by oer 44%, allowing increased spatial resolution of the numeric portion of the solution. The remainder of this dissertation is organized as follows. Chapter 2 contains the background information for the continuous model formulation, including the first-principles based PDEs and the boundary conditions. Chapter 3 explains the model order reduction methodology used, including deriation of the the channelmembrane-channel bond graph and interpretation of the applied MORA and modal decomposition parallel results. Chapter 4 coers the deriation of the semi-analytic solution model (including the switching solution), model alidation, and a brief reiew of potential applications. Chapter 5 has the control analyses showing the stability of liquid water in the GDL, the stabilizability of the oerall anode system, and the range of possible equilibria for liquid and water apor. This chapter concludes with the control objectie concept. Finally, conclusions and suggested next steps are listed in Chapter 6, followed by the Appendix. 13
25 Chapter 2 Full-Order Model Formulation The fuel cell model described in this chapter is a slightly modified ersion from McKay, et al ([3]). It combines GDL, channel, and membrane transport models using boundary conditions to relate each domain. The channel constituent dynamics are included because it is through the channels (lumped with the inlet and outlet manifolds) that a controller will influence the GDL states (liquid water olume, reactant and water apor concentrations). The water (liquid and apor) dynamics within the gas diffusion layers (GDL) are tightly coupled through the eaporation/condensation rate. Additionally, the liquid water becomes a nonlinearly distributed parameter that inhibits reactant gas and water apor diffusion. The channel conditions are the boundary conditions for the PDEs that describe the behaior of the GDL constituents. GDL dynamics are important since the GDL represents the path by which the channel conditions influence the membrane states, and thus the cell performance. The first principles based constituent dynamics within the GDL used herein, including the capillary flow and porous media gas transport mechanisms, as well as the boundary conditions, follow closely to that of [5]. 2.1 Model of the Gas Diffusion Layer Because creation of a physically intuitie model facilitates controller design and tuning, and since it is currently infeasible to obtain direct, realtime measurements of the critical ariables at the membrane and in the GDL, a low-order and compact model of the multi-component (reactants, water), two-phase (apor and liquid water), spatially-distributed and dynamic behaior across the gas diffusion layer (Fig. 2.1) has been inestigated. The time-arying constituent distributions in the GDL of each 14
26 electrode are described by three second-order parabolic PDEs for reactant (oxygen in the cathode and hydrogen in the anode) concentration, water apor concentration, and liquid water. Instantaneous electrochemical reactions on, and the mass transport through, the catalyst-coered membrane couple the anode and cathode behaiors and, together with the channel conditions, proide the time-arying boundary alues for these PDEs Model Assumptions The Semi-Analytic Solution (SAS) model deeloped herein has the same capability of a completely numeric model to capture the effects of changes to inputs/outputs on oltage estimation. The model combines analytic solutions for the spatial distributions of gases with a numeric solution for the liquid water and was obtained using the following assumptions: The model is spatially isothermal, but temperature is allowed to ary in time as this affects inlet and outlet water flow rates as well as the oltage model output. Although the effect of spatial temperature gradients are shown to be important [45], the simple tunable isothermal model considered was shown capable of predicting behaior for a reasonable range of conditions. Conectie (bulk) flow of the gases in the GDL is neglected due to the assumption of ery low elocities normal to the membrane. Mass transport is in 1D, normal to the membrane, and we neglect the GDLchannel interface ariations due to backing plate land and channel interaction. Variation along the channel is also neglected (except as an example of a potential model application in Sec ) Due to the generation of product water in the cathode GDL, and the fully humidified inlet flow, it is assumed that two-phase water conditions and liquid capillary flow are always present in the cathode. Therefore, the single-phase water regime is not considered on the cathode side. Water transport out of the anode channel is assumed to be in apor form due to the low flow rates that will be employed to ultimately achiee high efficiency through high hydrogen utilization during small flow-through conditions. For a high-elocity channel flow system, this assumption may not be alid [46]. Though the eaporation model of [47] includes eaporation een under fully saturated apor conditions due to the inclusion of compressibility, we employ the simplifying eaporation/condensation model from [5], where mass transfer between the liquid and apor phases is proportional to the difference between the water apor concentration and the concentration at the saturation pressure, i.e. incompressibility is assumed. 15
27 It is assumed that the front between one and two-phase water will always be two-phase on the membrane side of the front, and if the front is within the GDL, the entire range on the channel side of the front will be single-phase (i.e. there are no islands of two-phase water). There is assumed to be a small, constant, and negligible resistance to liquid flow across the GDL-channel border, represented by a constant S δ in subsequent equations. Though a modification of this boundary condition would shift the liquid water distribution, it is not expected to significantly alter the qualitatie findings of this research. Assuming a one-dimensional treatment of the GDL processes, let x denote the spatial ariable, with x = corresponding to the membrane location and x = ±L corresponding to the channel locations (+L at anode channel, -L at cathode channel) per Fig. 2.1, and let t denote the time ariable. The model includes channel and GDL for both anode and cathode, with differences between the electrodes appearing only in sign, rate of reactant consumption, and the generation of water apor on the cathode side. This water generation is lumped into the membrane transport to form the x = cathode boundary condition, and thus does not change the form of the equations, only the specific boundary alue. The state ariables are as follows: c,an (x, t) and c,ca (x, t) are the concentrations of water apor at time t at a cross-section of GDL located at x, x L (anode) or L x (cathode); c H2 (x, t) and c O2 (x, t) are the reactant concentrations at time t at a cross-section of GDL located at x, x L (anode-h 2 ) or L x (cathode-o 2 ); s(x, t) is the anode GDL fraction of liquid water olume V L to the total pore olume V p, s = V L Vp, commonly referred to as the water saturation. The ariable s is thus a concentration-like ariable for the liquid water at time t, at a crosssection of GDL located at x, x L. The following intermediate ariables are useful: N (x, t) is the water apor molar flux (mol/m 2 /s) at time t at a cross-section of the GDL located at x, x L (anode) or L x (cathode); W l (x, t) is the liquid water mass flow (kg/s) at time t at a cross-section of the GDL located at x, x L. 16
28 x= x=l Channel Ox Catalyst Layer Membrane Channel Gas Diffusion Layer Liquid Water C Figure 2.1: Basic concept of water transport in a fuel cell Continuous 1D Model Formulation The molar fluxes are drien entirely by the presence of a concentration gradient (i.e. diffusion), since bulk flow (conection) is neglected: N j,e = D j,e (s) c j,e x, (2.1) where the j subscript refers to any of the gas constituents within the fuel cell, and the subscript e indicates that the equation is applicable for both anode (an) and cathode (ca) electrodes. The D j,e (s) are the effectie diffusiities for the gases which depend on the liquid fraction, s, since liquid water reduces diffusiity in the GDL by occupying pore space, and are defined by D j,e (s) = D j,ε (1 s) m. D j,ε captures the influence of porosity on diffusiity, D j,ε = D j ε ( ).785 ε.11, (2.2) 1.11 where D j is the gas diffusion coefficient and m = 2 based on [5]. 17
29 The reactant gas conseration equations are of the form, c j t = N j x, (2.3) where j in (2.3) is limited to O 2 or H 2. The water apor conseration equations are of the form, c,e t where r is the eaporation rate defined as, r (c,e ) = { = N x + r (c,e ), (2.4) γ(c sat c,e ) for s > min {, γ(c sat c,e )} for s = where γ is the olumetric eaporation coefficient and c sat is the apor concentration associated with the water apor saturation pressure. Note that eaporation can only occur if there is liquid water (s > ) in the GDL, yet condensation can occur een if s = (under supersaturated conditions). Under the isothermal conditions assumed in this model, once the production or transport of apor exceeds the ability of the apor to diffuse through the GDL to the channel, the apor condenses at the rate determined by γ, hence supersaturated conditions (c (x) > c sat ) are allowed. The mass flow of liquid water is drien by the gradient in capillary pressure (p c ) due to build-up of liquid water in the porous medium, W l = εa fc ρ l KK rl µ l p c x, (2.5) where µ l is the liquid iscosity, ρ l is the liquid water density, A fc is the fuel cell actie area, and K is the material-dependent absolute permeability. The relatie liquid permeability is a cubic function of the reduced water saturation (K rl = S 3 ) [5], and p c is a function of a third-order polynomial in S(x, t) (Leerett J-function J(S) = 1.417S 2.12S S 3 ) [5]), where S(x, t) { s(x,t) sim 1 s im for s s im, for s < s im. (2.6) The immobile saturation, s im, works as stiction, i.e. there is no liquid flow unless the water saturation exceeds s im. It should be noted that the electrode subscript e on S and s is hereafter dropped for conenience, since the deelopment is the same for both anode and cathode. 18
30 Figure 2.2: Capillary flow of liquid water through the gas diffusion layer [5] Combining (2.1) with (2.4) proides the two second-order parabolic PDEs that goern the water apor concentrations in the cathode and anode, c,e t = ( D,e (s) c ),e + r (c,e ). (2.7) x x It is shown in Sec that an approximation of the time arying D,e (s) with D,e (s im ) yielded negligible error, and since this makes the diffusiity independent of x, it can be represented by D s im water apor PDEs become: D,e (s im ) for both cathode and anode, and the c,e t = D s 2 c im,e + r x 2 (c,e ). (2.8) Under appropriate conditions, liquid accumulates in the GDL until it has surpassed the immobile saturation threshold (s im ), at which point capillary flow will carry it to an area of lower capillary pressure (toward the GDL-channel interface as shown in Fig. 2.2). To facilitate the analytic solution, (2.5) is rewritten as, Using a least squares fit approximation, K W l = εa fc ρ l S 3 p c S µ l S x. (2.9) K µ l S 3 p c S = K µ l S 3 ( S S 2 ) b 1 S b 2, (2.1) 19
31 where b 1 = and b 2 =2.88, results in Conseration of liquid mass is employed to determine the rate of liquid accumulation, W l = εa fc ρ l b 1 S b 2 S x. (2.11) where M is the molar mass of water. s t = 1 W l εa fc ρ l x M r (c,e ), (2.12) ρ l Substitution of (2.11) into (2.12) yields the water saturation PDE, Boundary Conditions s t = ( b 1 S b S ) 2 M r (c,e ). (2.13) x x ρ l The choice of boundary conditions (BC) is important for the solution of the PDE system described in the preious section. For c,e (x, t), mixed Neumann-Dirichlet type BC are imposed. The channel (ch) boundary condition is, c,e x=±l = c ch,e = p ch,e/ (RT st ), (2.14) where R is the uniersal gas constant, T st is the stack temperature, and the total pressure in the anode channel, p ch an, is a key state of the model as it is influenced by fuel cell flow in, flow out, and GDL-channel transport. The anode membrane water apor BC is: c,an x = Nmb, (2.15) x= D s im which represents the assumption that water enters the GDLs in apor form only due to the presence of microporous layers between the membrane and GDLs [5, 48]. The membrane water molar flux N mb is goerned by electrosmotic drag and back diffusion, which are drien by current density i(t) (A/m 2 ) and water apor concentration ariation across the membrane, respectiely, and depend on stack temperature. The model for N mb used here is taken from [3], and takes the general form: with back diffusion {}}{ osmotic drag {}}{ N mb = β w (λ ca λ an ) 2.5 λmb 22 ρ mb i(t) F, (2.16) β w = λ mb α w M mb t mb 14 e 2436 T st, (2.17) 2
32 where α w is a tuned parameter, λ mb is the membrane water content ([13],[49]), λ e are the water contents on either side of the membrane, and F represents Faradays constant. The λ e are polynomial functions of the c mb,e, which are the water apor concentrations on either side of the membrane (x = ). The material and physical parameters of the membrane enter through its density (ρ mb ), molecular weight (M mb ), and thickness (t mb ). At the catalyst layer of the cathode side, product water is generated as a function of i(t), and thus the Neumann BC contains the net of N rct and N mb, where N rct gien by, c,ca x = Nrct N mb, (2.18) x= D s im represents the generation of product water at the cathode catalyst layer N rct = i(t) 2F. (2.19) From the assumption that water transport into the GDL at x = has no liquid component, Neumann boundary conditions are imposed for s(x, t): s x =. (2.2) x= The setting of a physically meaningful liquid water boundary condition at the GDLchannel interface has been a challenging issue [5, 51] with the choice of either zero water saturation or zero liquid flow being typically assumed [5, 52, 53], though channel water saturation BCs of.1,.4, and.6 were considered in [54]. A lack of liquid flow into the channel seems physically unlikely due to the hydrophobic nature of the GDL material, and the assumption of zero water saturation (S x= = S ch = ) is conenient for analysis, but does not hae a solid physical interpretation. Here we consider the simplifying and conenient form of: S(L, t) { Sδ for m ch l,an >, for m ch l,an =, (2.21) where S δ represents the effect of the liquid water that accumulates on the GDLchannel interface, and the inclusion of S δ essentially adds a means to proide resistance to flow due to accumulation of liquid in the channel. The alue S δ =.3 was assumed for simulation. Based on the oltage output experimental erification, 21
33 this assumption does not impair the model s estimation ability. Howeer, as more information becomes aailable from measurement methods such as neutron imaging, this assumption may be reealuated. Channel Cathode GDL mb Anode GDL Channel ch c ca = c, x= L, ca c x rct N, ca = x= D sim N mb c, an x x= N = D mb sim ch c an = c, x= L, an ch co = c 2 x= L O2 c O 2 x i( t) = sim D F c i( t) H 2 sim 4 x D 2F x= O H 2 x= 2 = ch ch = c 2 x= L H2 S = ca S an = = = δ S ca x= L δ x x= x an x= L x= x= L S S x = x= L Figure 2.3: Boundary conditions for anode and cathode GDL. Time-arying Neumann BC are placed at the membrane, with time-arying Dirchlet BC at the channels (not to scale). A graphical representation of the boundary conditions described, including appropriately similar boundary conditions imposed on the reactants H 2 and O 2, is shown in Fig S 2.2 Channel Equations To determine the water apor channel dynamics, the total channel pressure must be found. The pressures, which represent the channel boundary conditions, are calculated from: p ch e = j p ch j, (2.22a) p ch j p ch = mch j RT st M j V ch (j ), (2.22b) j,e = min { m ch w,e RT st M j V ch, psat } (j = ), (2.22c) where the subscript j represents each of the gaseous elements present (H 2 and water apor for the anode, and O 2, N 2, and water apor for the cathode). 22
34 The goerning equations for the reactants and water in the channel are: dm ch j dt = W in j + W GDL j W out j, (2.23a) where dm ch w,e dt = W,e in + Ww,e GDL W,e out, W in j (2.23b) = λ j i(t) 2ηF A fcm j, (2.24) with the in and out subscripts referring to channel inlets and outlets, and η is a factor that depends on the electrochemical reaction so that η = 1 for H 2 and η = 2 for O 2, and λ j is the excess ratio of the reactant j. In the general case, both anode and cathode will hae humidified inlet streams, with the relatie humidity (RHe in in in ) and the inlet flow rates (Wair and WH 2 ) prescribed, thus, W in,ca Pair in,ca = P in M M air W in air and W in,an PH in 2,an = P in M M H2 W in H 2 (2.25) where P in,e = RH in e P sat, and P in H 2, P in air are the pressures of the dry inlet gases. The cathode exit flow rate to the ambient (amb) is modeled as a linearly proportional nozzle equation, W out ca whereas the anode exit flow rate, = k out ca (p ch ca p amb ), (2.26) W out an = ū kout an (pch an pamb ), (2.27) has a controllable ale flow ū(t) 1 to remoe water and, unfortunately, hydrogen. ū = represents a dead-ended anode arrangement, which is commonly paired with a periodic purge cycle (ū = 1) for water remoal. For < ū < 1, this becomes a flow through anode water management system. The model erification of [55] was from experimentation that employed the dead-end/purge system, the analysis in this work is applicable for both dead-end and flow through conditions. The constituent exit mass flow rates are found from, W out j = mch j W m ch e out, (2.28a) gas,e 23
35 W,e out = W e out j W out j, (2.28b) where m ch gas,e = m ch j + p ch,evan ch M /(RT st ), and j is H 2 for the anode, but addresses both O 2 and N 2 for the cathode. The reactant and water mass flow rate from the GDL to the channel are: ( Wj GDL = εa fc M j D j (s) c ) j, (2.29a) x x=±l ( Ww,e GDL = εa fc ρ l b 1 S b S 2 x + M D (s) c ),e. (2.29b) x x=±l Using the dynamic water mass balance in the channel (2.23b), the liquid water in the channel is found by assuming that any water in the channel in excess of the maximum that can be held in apor is liquid, [ ] m ch l,e = max, m ch w,e csat M Ve ch. (2.3) This is equialent to assuming an instantaneous eaporation rate for the channel, and a maximum channel relatie humidity of 1%. The channel model structure differs from that of the porous medium GDL, which has an eaporation/condensation rate, and water apor concentration in excess of saturation is allowed. This differing treatment of the eaporation is simplifying since the channel then does not require separate water apor and liquid states. For the oltage model of [3], liquid water mass is of interest, and the approximation of instantaneous eaporation has a ery negligible effect on the oltage estimation. 2.3 Discretization and Numerical Solution The one-dimensional system of interconnected parabolic PDEs and support equations of (2.3)-(2.13), combined with three similar PDEs describing the spatial and temporal eolution of the reactants (H 2, O 2 ), water apor, and water saturation was modeled and discretized into 3 sections for each electrode and parameterized using data from an experimental fuel cell [3]. The discretization procedure is described here because analysis of the linearized numeric model is key background work and allows direct comparison with the deeloped semi-analytic model. 24
36 The 24 states of the 3-section discretized fuel cell model (includes anode and cathode) can be grouped as follows: Concentrations of Gas constituents in the GDLs: 13 3 each sections of O 2, H 2, anode water apor, and cathode water apor, plus 1 lumped section for N 2. Liquid constituents in GDL: 6 3 sections of liquid water fraction for each GDL. Dynamics states in channels: 5 Concentrations of O 2, N 2, and water apor in the cathode channel and concentrations of H 2, and water apor in the anode channel Difference Equations It is because the boundary conditions for each of the constituents are Neumann type that the discretization is performed on three pairs of first-order DEs (2.1)-(2.9) instead of three second-order PDEs. The forward-difference method is used for the discretizations of the flux and flow equations, N H2 [k] D H2 (s) c H 2 [k + 1] c H2 [k], (2.31) δx N [k] D (s) c,an[k + 1] c,an [k], (2.32) δx W l [k] b 1 S[k] b 2 s[k + 1] s[k], (2.33) δx where δx = x[k+1] x[k], and the k represents a counter for the discretized sections. Next, since the fluxes across the membrane are included in the model, and form the BC, the difference equations relating the states to the fluxes are formed using the backward-difference method: dc H2 [k] dt N H 2 [k] N H2 [k 1], (2.34) δx 25
37 dc,an [k] N [k] N [k 1] + r (c,an [k]), (2.35) dt δx ds[k] dt W l[k] W l [k 1] δx M ρ l r (c,an [k]). (2.36) Dymola TM software is used to implement the resulting lumped-parameter, ordinary differential equations of the discretized system, which will be referred to as the Coarse Numeric Solution (CNS), and will be compared with respect to accuracy and computational load to the semi-analytic solution presented in Chap
38 Chapter 3 Model Order Reduction The model order reduction methodology implemented was chosen based on the criteria of applicability to nonlinear systems and the capability to maintain the physical meanings of the states. The former requirement is simply because our system is nonlinear, and the latter because it is desirable to obtain physical insights into the operation of the fuel cell to be able to justify assumptions and explain results. For baseline comparison purposes, balanced truncation model order reduction was performed on the linearized system with results showing that a 56% order reduction to 11 states with negligible loss in estimation accuracy. It is therefore expected that this research can create a control-oriented model with less than 12 states for equialent spatial discretization. 3.1 Deriation of the Bond Graph Equations The aboe requirements suggest application of the Model Order Reduction Algorithm (MORA) from [36], which requires both an understanding of the model on an element-by-element basis and knowledge of the effort and flow of each element oer time. The bond graph is a modeling tool that lends itself coneniently to the MORA s energy-based analysis because effort and flow are easily obtained. Howeer, for bond graph implementation, each equation in the mathematical model must first be cast in a form that is applicable to the building blocks of the bond graph. The deriation and background for the following physical model equations can be found in Chap. 2. The discrete model therein consists of equations for gas diffusion, gas concentration time rate of change, capillary pressure-drien liquid water flow, eaporation, chemical reactions, and a number of conditional and empirical 27
39 equations goerning membrane mass transport. In this section, the equations modeling the physical system are used as the starting point for the bond graph model creation. Each subsection in this section will explain how the bond graph for each model phenomenon is deried Gas Species Concentration (Capacitance Model) The rate of change of molar concentration (c j = p j ) of gas species j is: RT dc j dt = N j x + r j (3.1) where the general electrode subscript e has been dropped for conenience and the species denoted by j correspond to the oxygen O 2 and apor from the cathode, and to the hydrogen H 2 and apor from the anode. The reaction term r j is zero for the oxygen and hydrogen cases, whereas for the apor case it captures the eaporation rate r j = γ (p sat /(RT) c ), where p sat In discrete form (3.1) is written: is the saturation apor pressure. dc j [k] dt = 1 δx (N j[k] N j [k 1]) + r j, (3.2) where [k] and [k-1] represent any two sequential sections of the discrete model. As can be seen from (3.2), the rate of change of concentration (mol/m 3 /s) becomes a function of the difference in molar fluxes across the section boundaries for the particular gas species. Additionally, the local reaction (eaporation) rate r j is included for the water apor model. The O 2 and H 2 reactions are calculated as molar fluxes (mol/m 2 s), and thus enter the equation through the across-boundary flow. The bond graph constitutie law for a capacitance is: de[k] dt = 1 C (f[k] f[k 1] + MSf rct), (3.3) where for our case C = δx = (t GDL /3) (the length one section), MSf rct is a modulated flow source that can represent a ariety of flow inputs, but is non-zero for this application only when j is water apor,. From these obserations, a choice of molar flux as the flow ariable, concentration as the effort ariable, and section length for the capacitance for the gas mass transport is logical, and preseres physical meaning. 28
40 Finally, while a choice of pressure for the effort ariable would also be acceptable, concentration was chosen simply because this is an existing state ariable Gas Species Diffusion (Resistance Model) Diffusion of gases takes place in the anode and cathode gas diffusion layers. On the anode side, relatie diffusion of hydrogen and apor must be considered, while the cathode side model must take into account the relatie diffusion of oxygen, nitrogen, and apor. This model is simplified by assuming that the presence of N 2 in the mixture does not significantly affect the diffusiity of O 2 and apor. The diffusion of gas species in the diffusion layer is a function of the concentration gradient, transferring gas from regions of higher concentration to regions of lower concentration: N j = D j (s) c j x. (3.4) As shown in (3.4), the molar flux, N j (mol/m 2 /s), depends on the diffusion coefficient, D j (s). The discrete ersion of (3.4) is realized with N j being a function of the difference in the concentration between neighboring sections: N j [k] = D j(s[k]) (c j [k + 1] c j [k]). (3.5) δx The general resistance constitutie law utilized in bond graphs is: f[k] = 1 (e[k] e[k + 1]) (3.6) R[k] where for this application the flow resistance is represented by a modulated resistance: R[k] = MR[k] = δx D j (s[k]). (3.7) A modulated resistance is necessary because the diffusiity of gas constituents in the GDL is affected by the olume of liquid water present, represented by s[k], giing rise to the discrete effectie diffusiity [5]: D j (s[k]) = D j ε ( ).785 ε.11 (1 s[k]) 2, (3.8) 1.11 where s[k] = V l[k] V p, D j is the diffusiity constant for the species j (which is dependent 29
41 upon the molecular size of j) Liquid Water Storage (Capacitance Model) The olume of liquid water (V l ) in each GDL section is determined by the capillary liquid water olumetric flow rate, Q l, and the eaporation rate, r : dv l dt = Qin l Q out l r V p M, (3.9) ρ l where the eaporation (condensation) rate goerns the creation of liquid water microdroplets (considered to be eenly distributed at any x), and where conditions necessary for water droplet formation are assumed to hae been met [5]. For simplification of the model, dynamics associated with the formation of the liquid water droplets hae been neglected. In order to cast our model into a form that is conducie to calculation of power equals the product of effort and flow, the time rate of change of liquid water olume should be translated into a dynamic capillary pressure relationship with olumetric flow. As a pore fills with liquid water, the capillary pressure increases, causing the water to flow to an adjacent pore with less water. This process creates a flow of liquid water through the GDL, finally resulting in the incursion of liquid into the channel. Capillary pressure results from surface tension of the water droplets, and is calculated as follows: p c = β pc g NL (S), (3.1) where β pc is a constant that captures the geometry of the surface tension between the water and air, the porosity, and the permeability of the GDL (details can be found in [5]). The nonlinear function g NL (S) is an empirically-fit third-order polynomial in S commonly used to describe the relationship between capillary pressure and the amount of liquid water present, g NL (S) = 1.417S 2.12S S 3. (3.11) In this model, graitational effects on the liquid water are considered negligible due to the liquid water surface tension interaction within the fibers of the GDL. Taking the time deriatie of (3.1), it can be shown that the time rate of change of the capillary pressure is related to the olumetric flow by a discrete equation of 3
42 the form: dp c [k] dt = β Q g NL (S, k)(q l[k] Q l [k 1] r l [k]), (3.12) where β Q represents a constant that depends upon surface tension, permeability, and GDL porosity. The nonlinear function g NL (S, k) is the deriatie of g NL(S, k) from (3.1) with respect to S. Further, the condensation rate r l is a conersion from r to relate molar to mass condensation rates (m 3 /s)(3.15). Following the pattern of the preious subsections, the next step is to translate (3.12) into a bond graph compatible form. While (3.12) could be implemented using a modulated capacitance, simulations hae shown that a simplification can be obtained by using the mean alue for the applicable range of g NL (S). This direction is justified by noting first that per [5], our model assumes no liquid water flow until 1% of the pore olume is filled with liquid water. At that point capillary action causes the liquid to flow into the next section, significantly reducing the liquid water fill rate for the original section, while notably increasing the fill rate for the next. The practical range of S is small enough after liquid flow begins to justify use of the mean alue, therefore g NL (S, k) becomes ḡ. Then, with the eaporation modeled as a modulated flow source, the capillary pressure equation can now be modeled as a capacitance in a bond graph model: de[k] dt = 1 C (f[k 1] f[k] MSf r). (3.13) Where the capacitance becomes: 1 C = β Qḡ, (3.14) and the molar liquid eaporation rate is represented by a modulated flow source: MSf r = r l = M V p ρ l r. (3.15) Liquid Water Transport (Resistance Model) The remaining equation to be modeled in bond graph form is the liquid water flow. Parameters that affect the mass flow rate of liquid water are flow area, permeability, iscosity and density of liquid water, and the section thickness. These factors will naturally influence the resistance to flow for this element. It is, howeer, the presence 31
43 of a liquid water olume gradient that dries the liquid water flow. Capillary pressure and liquid water olume are related through (3.1). The physics of the liquid flow phenomenon are described by: where p c is capillary pressure, S = ( ) Q l = β wl S 3 pc, (3.16) x ( ) s s im 1 s im is the reduced water saturation, β wl is a constant that embodies flow area, permeability, density, and iscosity, and p c / x describes the influence spatial ariation of capillary pressure has on olumetric flow rate. Similar to the gas species diffusion bond graph equation, an application of a modulated resistance, in discrete form, is appropriate: f[k] = 1 (e[k] e[k + 1]). (3.17) MR[k] In the bond graph model, the MR will be a function of seeral system parameters (listed below in the explanation of β R ), and the continuously arying liquid water olume: 1 MR[k] = β RS 3, (3.18) where β R is a constant that depends upon flow area, permeability, water iscosity, section thickness and porosity, and surface tension([5]). The olumetric flow rate is calculated from: Q l [k] = 1 MR[k] (p c[k] p c [k + 1]). (3.19) Capillary pressure as the effort ariable and olumetric flow rate as the flow ariable were natural choices, as was the liquid water olume as the modulus. Finally, a conditional statement must be included to preent capillary action from starting unless the liquid water olume reaches 1% of the pore olume (the minimum condition for flow suggested in [5]). 3.2 The Bond Graph Model The goal of this model order reduction is to determine the minimum number of sections required to accurately model flooding. Therefore, a repeatable bond graph model for one section would be useful. 32
44 MR Liq Volume Signal C 1 1 Liquid Water One discretized GDL section MSf:Cond MR MSf:Eap 1 1 Vapor Species One discretized GDL section C Figure 3.1: Bond Graph Building Block for one Discrete GDL Section Using the relationships from Section 3.1, a single section bond graph submodel was created (Fig. 3.1). With this submodel, a GDL discretization for a ariety of resolutions can be easily accomplished. Flow through the GDL is bounded on one side by the channel, and on the other by the catalyst-coated membrane. These boundary conditions are clarified in this section. The conditional and empirical equations for the membrane water transport do not fit into standard bond graph form, so function blocks were built to accommodate them. The air, hydrogen and water apor supplied to the gas distribution channels are easily modeled as flow sources, where the amount supplied can be controlled to supply the desired O 2 and H 2 excess ratios. Similarly, eaporation is modeled as a modulated flow source, with presence of liquid water as the fundamental prerequisite and the modulus being the product of the eaporation coefficient and water apor pressure relatie to the saturation pressure. The boundary conditions for the molar fluxes at the GDL-catalyst interface are equal to the chemical reaction rates and the membrane water transport, which depends on the GDL water concentrations at the catalyst c mb and the current drawn from the stack I st (2.16,2.19). The membrane water transport and reaction rates are thus modeled as modulated flow sources. The actie signals are stack current for reaction rates, including generation of water at the cathode catalyst, and stack current and c mb for the membrane water transport flow source. 33
45 Stacking three of the discretized sections from Fig. 3.1 for each constituent of interest, including function submodels for the membrane physics, adding eaporation flow sources, and putting in flow sources for the supply of air and hydrogen gies the model shown in Fig In this full-order bond graph model, the channels are modeled as dynamic boundary conditions and shown on the far left and right sides of the bond graph. The flow into and out of the fuel cell occurs in the channels. The cathode side of the model is the left half of the model, the anode side the right half. The membrane is located in the middle of the model. Positie flow in the model is defined to be from cathode channel to anode channel. Finally, for the purpose of H2O,liquid Ca_C_Liq_Ch Ca_MR_Liq_sec3 C MR Ca_C_Liq_sec3 Ca_MR_Liq_sec2 C MR Ca_C_Liq_sec2 Ca_MR_Liq_sec1 C MR C Ca_C_Liq_sec1 An_C_Liq_sec1 C An_MR_Liq_sec1 An_C_Liq_sec2 MR C An_MR_Liq_sec2 An_C_Liq_sec3 MR C An_MR_Liq_sec3 An_C_Liq_Ch MR C H2O,liquid Ca_Eap_Ch MSf Ca_Pres_Liq_Ch f 1 Ca_flow_Liq_sec3 Ca_Pres_Liq_sec3 1 Ca_flow_Liq_sec2 Ca_Pres_Liq_sec2 1 Ca_flow_Liq_sec1 Ca_Pres_Liq_sec1 An_Pres_Liq_sec1 1 An_Flow_Liq_sec1 An_Pres_Liq_sec2 1 An_Flow_Liq_sec2 An_Pres_Liq_sec3 1 An_Flow_Liq_sec3 An_Pres_Liq_Ch f An_Eap_Ch MSf H2O, apor Ca_H2O_Flow_In O2 Ca_O2_Flow_In N2 MSf H2O_MolFlowRate_In_Ca Ca_N2_Flow_In Ca_Ch_Liq2Vap MSf MSf O2_MolFlowRate_In flow (m^3/s) Ca_conc_H2O_Ch Ca_C_H2O_ChC Ca_C_O2_ChC Ca_conc_O2_Ch Ca_Flux_O2_sec3 Ca_FlowOut_O2MSf Ca_C_N2_ChC Ca_conc_N2_Ch MSf N2_MolFlowRate_In Ca_R_H2O_sec3 R 1 Ca_Flow_H2O_sec3 Ca_FlowOut_H2O MSf Ca_Eap_sec3MSf 1 R Ca_R_O2_Sec3 Ca_conc_H2O_sec3 Ca_C_H2O_sec3 C Ca_C_O2_sec3C MSf Ca_FlowOut_N2 MSf Ca_Cond_sec3 Ca_conc_O2_sec3 Ca_Eap_sec2MSf Ca_R_H2O_sec2 R 1 Ca_Flow_H2O_sec2 Ca_Flux_O2_sec2 1 R Ca_R_O2_sec2 Ca_conc_H2O_sec2 Ca_C_H2O_sec2 C Ca_C_O2_sec2C MSf Ca_Cond_sec2 Ca_conc_O2_sec2 Ca_Eap_sec1MSf Ca_R_H2O_sec1 R 1 Ca_Flow_H2O_sec1 Ca_Flux_O2_sec1 1 R Ca_R_O2_sec1 MSf Ca_Cond_sec1 Ca_conc_H2O_sec1 Ca_C_H2O_sec1C Ca_C_O2_sec1C Ca_conc_O2_sec1 MSf O2_Reacted MSf Ist Ca_H2O_Reacted Ca_wateract MSf Membrane iapp Vap_across_memb Global Parameters Global Variables Conc_Tot_Ch An_Cond_sec1 MSf An_wateract An_conc_H2O_sec1 An_conc_H2_sec1 C An_C_H2O_sec1 C An_C_H2_sec1 MSf H2_reacted An_Cond_sec2 MSf An_R_H2O_sec1 R 1 An_Flow_H2O_sec1 An_Flow_H2_sec1 1 R An_R_H2_sec1 An_conc_H2O_sec2 An_conc_H2_sec2 An_R_H2O_sec2 R C An_C_H2O_sec2 C An_C_H2_sec2 An_Cond_sec3 MSf MSf An_Eap_sec1 MSf An_Eap_sec2 MSf An_Eap_sec3 1 An_Flow_H2O_sec2 An_Flow_H2_sec2 1 R An_R_H2_sec2 An_conc_H2O_sec3 C An_C_H2O_sec3 C An_C_H2_sec3 An_conc_H2_sec3 An_R_H2O_sec3 R 1 An_Flow_H2O_sec3 An_Flow_H2_sec3 1 R An_R_H2_sec3 An_Ch_Liq2Vap MSf An_conc_H2O_Ch An_conc_H2_Ch H2O C An_C_H2O_Ch C An_C_H2_Ch An_Vapor_Purge MSf An_H2O_Purge H2_MolFlowRate_In MSf An_H2_FlowIn H2 Cathode Channel Cathode GDL Membrane Anode GDL Anode Channel Figure 3.2: Complete Bond Graph Representation of a 3-Section Discrete Water Dynamics Model. model simplification, the concentration of nitrogen throughout the cathode diffusion 34
46 layer is assumed to be identical to the concentration in the channel. 3.3 The Model Order Reduction Algorithm Analysis ia MORA proceeds with the aboe-deried bond graph ersion of the fullorder numeric model Background of the MORA The Model Order Reduction Algorithm (MORA) deried by Louca et al. [36] is applied and analyzed in this chapter. The MORA is a method that seeks to simplify complex models by creating a metric named actiity to determine the energetic contribution of eery element of a model. Based on the fact that Power = P = effort flow, the actiity is defined: actiity j = T P j (t) dt = T e j f j dt, (3.2) where the effort and flow ariables for this application, (deried in 3.1), are concentration (mol/m 3 ) and molar flux rate (mol/m 2 /sec) for the gas constituents, and capillary pressure, p cap (Pa) and olumetric flow rate (m 3 /sec) for the liquid states, respectiely. Use of pressure and olumetric flow for the liquid constituents is typical, resulting in units of Watts for the power and Joules for the actiity. Howeer, for comparison between gas and liquid elements, it is necessary to scale the results of the power calculations for the gaseous elements since the molar flux needs to be conerted to olumetric flow rate (m 3 /s) and the concentration to pressure (N/m 2 ). Once the actiity of an element is determined, it is normalized by an Actiity Index(AI) to determine whether the element has significant contribution to the oerall energy of the system. The definition of the Actiity Index(AI) is: AI j = Actiity j m 1 (Actiity 1%, (3.21) j) where m is the total number of energetic (energy-utilizing) elements in the model. As a function of the sum total of all the indiidual element actiites, the AI 35
47 proides a means to compare elements from any part of the model. An important aspect of the MORA is that it reduces model complexity while maintaining the physical meaning of the ariables and parameters. It is also applicable to nonlinear systems, which, due to the nonlinear nature of this model, makes it attractie for use here Application of the MORA Actiity results are strongly dependent upon the duration of the simulation and the inputs to the system. For this analysis, an attempt was made to systematically determine the influence on the actiity for appropriate durations and inputs. Inputs considered were a step changes in I st, purge eents, and a sum of sinusoids stack temperature. Actiity calculations were performed for all the possible combinations of input mentioned aboe, and a range of durations that were tied to mode speeds of the system. Reiew of the results showed that using the longer time-constants of the system gae a disadantage to the concentration states, that reach equilibrium quickly, as their net flow goes to zero early in the time window. At the opposite extreme, using the time constant of the fastest mode as the duration underalues the slow modes. As can be seen from (3.2), it is necessary to assign a time duration for the actiity calculation. This is a critical aspect of the method, with influence on the results as great as ariations in the system inputs. Excitation of all elements can be obtained by arying the main disturbance inputs of I st and T st at frequencies significantly higher than normally experienced during fuel cell operation. It was determined that the equitable input/duration combination would be to implement high-frequency stack current and temperature changes while taking energy measurements oer an extended period of time. This allows the fast gas and apor capacitance elements to be frequently excited, while giing the slow diffusion actiities time to grow. To address the full potential of each group of states, an actiity calculation was made for flooded and non-flooded conditions, with results listed in Table 3.1. An example of an actiity simulation using the resistie elements of the H 2 and the anode water apor an is shown in Fig In this plot, the H 2 actiities show only slight ariation from section to section, while the water apor actiities show significant section to section ariation. In a typical actiity analysis, it is common to compare actiity magnitudes, 36
48 Table 3.1: Actiity Summary for Borderline and Flooding Cases : Threshold =.1 pink Small sec-to-sec ariation orange Large sec-to-sec ariation blue Negligible actiity Borderline Flooding sec Element AI% Element AI% Sec Element AI% Element AI% 1 H 2 storage.2 H 2 flow H 2 storage.2 H 2 flow Capacitance.2 Resistance Capacitance.2 H 2 Resistance an storage.1 an flow.1 1 an storage.1 an flow.87 2 Capacitance.1 Resistance.3 2 Capacitance.1 Resistance O 2 storage.1 O 2 flow O 2 storage.1 O 2 flow Capacitance.1 Resistance Capacitance.1 Resistance ca storage.1 ca flow ca storage.1 ca flow Capacitance.1 Resistance.23 2 Capacitance.1 Resistance GDL N 2 storage. N 2 flow.77 GDL N 2 storage. N 2 flow.77 Capacitance Resistance Capacitance Resistance 1 s an storage.15 s an flow s an storage.87 s an flow.11 2 Capacitance.14 Resistance Capacitance.3 Resistance s ca storage.13 s ca flow s ca storage.13 s ca flow.42 2 Capacitance.1 Resistance Capacitance.1 Resistance S eap f,an.479 Sf,an cond S eap f,an.488 Sf,an cond Flow Source.7 Flow Source.13 2 Flow Source.16 Flow Source S eap f,ca 4.18 Sf,ca cond S eap f,ca 4.21 Sf,ca cond Flow Source.129 Flow Source.85 2 Flow Source.13 Flow Source
49 an R Actiity.1.5 Flooding Borderline sec1 sec2 sec H 2 R Actiity 3 Flooding 2 1 Borderline Time (s) Current (A) 7 4 I st Temp (K) T st Time (s) Figure 3.3: Actiity of resistie elements hae information regarding degree of linearity of distribution from state in discretized PDEs. and conclude that either an element is negligible and can be eliminated, or that it s dynamics are fast enough to justify assignment of instantaneous equilibrium. The threshold for such considerations is a judgment call, with <.1% being common. Howeer, the AI for this application will be dominated by the O 2 resistie elements, suggesting a more conseratie threshold of.1% because a reduction in one order of magnitude will bring those O 2 resistie elements in line with other contributors to the AI. As can be seen from the data in Table 3.1, the small actiities of the capacitance elements for apors and reactants imply that their dynamics are relatiely inconsequential to the system. This suggests that it is appropriate to take the steady-state solutions for the gas and apor states. From the bond graph point of iew, remoal of a capacitance element will leae a zero junction without it s capacitie element as shown in Fig The figure also includes the remoal of the actie modification signal for the gas flow resistance, which will be justified in Sec A zero junction, representing a sum of flows, is the equation of mass balance 38
50 C Liq Volume Signal MR C Liq Volume Signal MR MR MSf:Cond MSf:Eap MR MR R R MSf:Cond MSf:Eap R C C Figure 3.4: Small actiity leads to residualization of the state associated with the capacitie element. in the discretized k th section for the j th element, dc j [k] dt = 1 C ( flow in flow out). (3.22) Thus, elimination of the capacitie element is identical to setting flow-in equal to flow-out, resulting in an equation that proides the steady-state solution. Beyond the traditional element elimination, the actiity analysis holds important information regarding degree of nonlinearity in a discretized PDE application, and thus aids in a judgment regarding sufficient spatial resolution. From this iew, comparing the actiity leels, it can be seen that the elements of some species hae small ariation in actiity from section to section. It is hypothesized that small ariation in diffusion actiity implies oer-discretization. To ealuate the hypothesis, study of the definition of actiity for a diffusion element shows that similar diffusion actiity implies associated state reduction potential. As described in Sec , the diffusion of a gas species through the liquid water-filled GDL pores can be modeled as resistance elements, and therefore hae the power (P) through them determined by the concentration across multiplied by 39
51 the flow through, or P = (c j [k] c j [k + 1])N j [k]. For the non-apor gases O 2 and H 2, once equilibrium in the section is reached, the flow into a section equals the flow out. Since the concentrations reach equilibrium ery fast, the flow through each section is nearly equialent. It is only ariations in concentration that separate the diffusion actities. Therefore, similar actiity results in diffusion elements imply a linear distribution of concentration in the GDL, which indicates a single section is sufficient to describe the distribution (i.e. lumped olume approximation). The conclusion of the actiity analysis is a reduction proposal with two options. The first is state residualization due to the sufficiency of steady-state solutions implied by low actiity. These states are listed in the left side of Table 3.2. The second option is to keep the dynamics of the states, yet reduce the discretization because the small ariation in section to section actiity is small. The states with potential for reduced spatial discretization are shown on the right column of Table 3.2. Table 3.2: Options for element reduction based upon actiity analysis Use Steady-State Solution Lumped Volume c O2 c O2 c H2 c N2 c,an c H2 s ca s an c,ca Due to a lack of defensible metric, it is unclear if the degree of section to section ariation for the liquid water flows can be judged similar or not. Howeer, the results can be interpreted to indicate that the distribution of the liquid water in the GDL is more nonlinear than the distributions of the H 2 and O 2, and less nonlinear than than the water apor distributions (using the slope of a least squares best fit to the data as the metric). Results from Chap. 4 will confirm this interpretation. 3.4 Time-Scale Decomposition The actiity study of Sec suggests that the water apor and reactant states hae ery fast dynamics. In this section, a modal analysis is applied to erify the MORA result and attempt to quantify the time scale separation between liquid and gas states. 4
52 Insight into the relatie response speeds of the system states is gained by linear time-scale decomposition techniques. Linearization of the numeric 24-state system of Sec. 2.3 was performed for operating points differentiated by λ H2 to cause the system to experience both flooding and drying conditions. The other parameters of the system were kept at alues typical of mid-leel operation for the fuel cell stack; T st =6 o C, i=45 A/cm 2, RH in an and RH in ca equal to nearly dry H 2 (.1%) and fully humidified air, respectiely, and O 2 excess ratio at 3%. This operating point represents the midrange of the normal operation of the experimental fuel cell on which the model was tuned and consequently alidated ( i.45 A/cm 2, 15% λ O2 3%, 4 o C T st 7 o C) [3]. The linearization was performed numerically using the linearization function within Dymola TM on the full-order model. The linear system was then exported to Matlab TM,1 for analysis using the Controls System Toolbox TM,1. The analysis begins by letting be the eigeneectors of the discretized and linearized system matrix A (24 24). It is assumed that there is a similarity transformation A T = TAT 1 that will partition the system by modes into subsystems that can be analyzed separately. This transformation was accomplished by applying the balancing algorithm of [56], where each row-column pair of a matrix is scaled ia similarity transformation T to hae equal norms. The standard form of a similaritytransformed (x T = Tx) system is: ẋ T y = TAT 1 x T + TBu = CT 1 x T + Du (3.23) The new system matrices can be fit into an oerall matrix for purposes of performing scaling on one entity (D is omitted because the transformation does not affect it): [ ] TAT 1 TB M = (3.24) CT 1 The net result of the balancing transformation is that the off-diagonal elements are scaled-to-minimize according to their row and column position, moing the representation close to block diagonal. Thus, the resulting eigenectors are grouped by blocks, which in the physical system tend to be elements with strong mutual interaction (such as a discretized PDE or states sharing a olume). For this two-phase application, this results in a pair of subsystems with one consisting only of gas states, 1 Trademark of the MathWorks 41
53 and the other only of liquid states. Because T is diagonal, the diagonal elements of the A matrix are unchanged, and the physical meanings of the states are presered. After applying the transformation, the new system of eigenectors are practically decoupled with T = T, and V T = [ T,1 T,2... T,n ] defining the eigenspace of the transformed system. Normalizing those eigenectors, and eliminating components below a negligible threshold, it is found that the eigenspace has the form: [ V T V T,l,6 6 V T,g,18 18 ], (3.25) where the subscripts g and l indicate gas or liquid states. This shows that, though not a perfect mathematical decoupling, with reasonable thresholds a transformation can be found that results in a practical decoupling of the liquid and gas states. Note that the resulting eigenectors cannot be associated with indiidual states of the original system, only that each eigenector can be assigned to a linear combination of either only gas states or only liquid states. We measure the gas/liquid coupling by the infinity norm of the components that correspond to liquid states in the unit normalized, predominantly gas state eigenectors, and likewise of the gas states in the predominantly liquid state eigenectors. Repetition of the process for low and high end operating points of the typical range, as well as for flooding and drying conditions, showed that the strength of the gas/liquid coupling grows with the current density (i) and is greater during drying conditions. At a typical current density, the coupling is 4%, and is still only a minor influence at maximum load. (Table 3.3). Table 3.3: Gas and liquid state coupling ersus current density i. i (A/cm) Coupling.5%.3% 3.9% 11.3% As the anode inlet flow increases due to the high current density, the channel states emerge as components of the anode liquid modes, indicating a channel gas- GDL liquid coupling on the anode side. Further, the coupling is entirely one-way, i.e. certain gas constituents show up in the predominantly liquid modes, but liquid states are nearly absent from the predominantly gas modes. Modal analysis proides an understanding of the time constants associated with the system. For this model, the time constants all relate to combinations of filling dynamics for gases and/or liquid water. Using a flooding condition as an example, 42
54 the time constants for the GDL states within the full-order system are listed in Table 3.4. Table 3.4: GDL State Response Speeds (Time Constants (τ c ) ) Reactants Water Vapor Liquid Water Mode τ c [s] Mode τ c [s] Mode τ c [s] c H2 3.x1 4 c,an 7.5x1 4 s an 2.91 c H2 5.3x1 4 c,an 8.5x1 4 s an 5.61 c H2 4.x1 3 c,an 1.x1 3 s an 25.6 c O2 1.2x1 3 c,ca 4.8x1 4 s ca 1.3 c O2 2.2x1 3 c,ca 5.5x1 4 s ca 2.48 c O2 1.2x1 2 c,ca.9x1 3 s ca 12.5 The most significant information to be gathered from this table of 18 discretized PDE states within the GDL is the separating line that can be drawn between fast and slow modes of this system. An analysis of the eigenectors for the 24 modes reeals that the slowest six modes are related to the filling dynamics of the six states associated with liquid water in the GDL. The slow mode time constants (liquid) range from 1.3 to 26 seconds, while the fast modes (gas) hae time constants ranging from.3 ms to 12 ms. These results for the liquid are on the same order as the qualitatie conclusions reached by Natarajan et al [9]. This large time-scale separation indicates that application of a singular perturbation analysis is appropriate, i.e. (i) treat the liquid states as time-inariant (slowly-arying) parameters when we seek the analytic solutions for the gaseous states, and (ii) treat the gaseous states as haing reached their steady-state alues during the calculation of the liquid states. The main conclusion taken to the analytic solution stage is that the MORA analysis (Sec. 3.3) indicates that the gas/water apor-related capacitance elements of the model (which represent the dynamic characteristics of the gas/apor states) can be neglected due to their low actiity. This implies that steady-state solutions can be used for the GDL hydrogen, oxygen, and water apor concentration states when soling for the slow GDL liquid states. This result is confirmed with a modal analysis on the linearized system, which shows additionally that there is a significant separation between the GDL liquid and gaseous state time constants. 43
55 Chapter 4 The Semi-Analytic Solution In this chapter, significant model simplification is obtained by deriation and implementation of analytic solutions for those states associated with the low-energy modes found through the Actiity analysis of Sec A time scale decomposition erified the selection of the GDL gas constituent states for residualization to algebraic equations due to large time scale separation. This fact justifies use of the quasi steady-state solutions for H 2, O 2, and water apor when soling for the slowly arying liquid water distributions. The SAS model must accommodate a mobile boundary front between single and two-phase water conditions in the GDL, and x fr is defined as the location nearest the membrane such that s(x e,fr, t) =. Inclusion of the moing front necessitates the addition of a set of equations to establish the analytic solution for the range where only water apor is present (i.e. x fr < x L). The behaior throughout the GDL will then be described with a combined analytic solution that allows switching between the range {s(x, t) > for x [, x e,fr ]} and {s(x, t) = for x [x e,fr, ±L]}. 4.1 Deelopment of the Semi-Analytic Model In this section the deriation of a semi-analytic model capable of predicting fuel cell oltage output response to changes in input is presented. The oltage estimation capability of this model is equialent to that of the discretized numeric model presented in [3], with the notable improements being: Reduced computational effort due to implementation of steady-state analytic solutions for gaseous constituents, 44
56 Improed understanding of constituent distributions due to finer model resolution, Improed understanding of the physics of liquid flow conditions at the capillary flow boundary and two-phase water interaction Sensitiity of Diffusiity to Liquid States There are two phenomena responsible for the coupling between the liquid and the gas states. The first is the direct coupling between water apor concentration and water fraction due to eaporation/condensation, which is clear from (2.12), and is addressed in Sec Second, the amount of liquid water present has an influence on the gas fluxes ia the diffusiity s dependence on s(x, t) (2.1), the liquid water states. The diffusiity coupling must be addressed in order to reach an analytic solution for the gas constituents. An assumption of negligible liquid water influence on diffusiity (i.e. s = ) can alter estimation significantly as shown in Fig Further, an inappropriate choice of constant liquid water fraction s can hae a significantly detrimental affect on the model predictions of (c,an c sat ) x=, which is an important alue for calculation of membrane water transport. The plot shows that if the aerage liquid ( water fraction (s ag ) was known and used to calculate diffusiity, D sag 1 L =D ), sdx the error would be at a minimum. Unfortunately, s L ag is not known a priori, so a logical choice that can be made a priori is s=s im, thus = D (s) is used as an approximation for the effectie diffusiity. The error D s im caused by this approximation is considered negligible since s(x, t) is generally in the icinity of s im. A releant exception to this is during GDL drying (s = ), which will be addressed by switching the water apor concentration model to one without eaporation, and using the effectie diffusiity for zero water saturation (D,ε ()). The case where a section of the GDL is drying requires a more comprehensie analysis of the system equations since it inoles a dynamically arying boundary, where the two-phase water flow transitions to only water apor flow. The experimental data used here to alidate the soon-to-be-presented semi-analytic model and the coarse numeric models do not correspond to conditions that cause the GDL section nearest the channel to reach s =. Were the discretization to hae greater resolution (perhaps n >1), then GDL section drying may hae occurred during purges of the dead-ended system used in the experiments. Howeer, the effect of this ery short term drying on the oltage prediction is expected to be negligible. 45
57 Error [%] 8 6 Typical Range of s s=s im 4 2 s ag Water Saturation s Figure 4.1: Steady-state error in (c,an c sat ) x= for arying choices of constant liquid water fraction. Approximation of the temporally and spatially-arying s(x, t) with s(x, t) s im decouples the liquid and apor PDEs. This enables an analytic solution for the gas species. To ealuate the effect of this assumption on the c,an (x) distribution, the results of Sec. 3.4 suggest that the diffusiities can be treated as slowly arying parameters for the purpose of soling the gas PDEs because they are functions of only the slowly arying s. For error comparison purposes, the numerically determined s(x[k], t) is used at each time step and in each discretized section to calculate the effectie diffusiity. Assuming the liquid saturation as constant while the water apor concentration PDE is soled leads to Fig It shows that the assumption of s = s im does not result in a significant change in the water apor concentration distribution. With the assumption of constant diffusiities, denoted D s im and D s im j for the water apor and reactant cases respectiely, it is possible to sole the c j (x, t) and c,e (x, t) second-order PDEs analytically using separation of ariables Gas Constituent Solutions It is assumed that the water phase mix will always be two-phase on the membrane side of the mobile front, and if the front is within the GDL, the entire range on the channel 46
58 7.2 w/const D w/d(s(x)) 7.18 Concentration (mol/m 3 ) Position in GDL (m) x 1 4 Figure 4.2: Use of s = s im has negligible effect on solution. side of the front will be single-phase. An undesirable example where this would not be true is if the cathode side experienced sudden and significant drying such that the back diffusion not only ceased, but changed direction. That case will not be considered here. It should be noted that the assumption of consistent cathode liquid water presence implies that the cathode channel water apor concentration is c sat (T st) for all t. Further, the transient solution of the gas constituent solutions is presented in order to identify any/which of the modes from the linearization correspond to the analytic solutions. The separation of ariables methodology is used to find the solution to the gas/apor PDEs (shown in Appendix B). The explicit solution for the anode (similar for the cathode) gas constituents when liquid water is present throughout the GDL (i.e. under the assumption that s(x, t) > for x [, L]) is found to be: and, c H2 (x, t) = c,an (x, t) = n= e Ds im H2 η nt A n cos( η n x) + NH rct 2 D s (L x) + c ch im H2, (4.1) H 2 e ζnt B n cos( η n x) + n= (α 1 e βx + α 2 e βx ) + c sat, (4.2) 47
59 where η n = (n + 1/2) 2 (π/l) 2, and β = γ/d s im ζ n = (D s im η n + γ). (4.3) A n and B n are coefficients of the infinite series approximations in (4.1) and (4.2) for the shape of the distribution of the respectie concentrations at time t =. Upon a change in the system conditions (e.g. a step in I st ), the solution will begin a transition from the steady-state distribution at t = (let this state be ss ) to the steady-state solution dictated by the new inputs (identified as ss + ). For the H 2 solution, the coefficients A n are A n = 2 L [ N rct H 2 t=ss NH rct 2 t=ss + D s + (4.4) im H 2 η n ( 1) n (c ch H 2 t=ss c ch H 2 t=ss +) ]. ηn Note: Substitution of NO rct 2 and D s im O 2 for similar terms in the H 2 solution represented by (4.1) and (4.4) will result in the analytic solution for O 2. From (4.2) it can be seen that the water apor transient response solution is a transition from one exponential form steady-state solution to the next, described by the product of a decaying exponential in time and an infinite Fourier series. The coefficients for the water apor transient solution are, B n = 2 L(β 2 + η n ) [β(ϕ 2 ϕ 1 ) + (4.5) ηn ( 1) n (ϕ 1 e βl + ϕ 2 e βl )], where ϕ i = α i t=ss α i t=ss +. (4.6) Figure 4.3 shows the transitions from an initial distribution to the next steadystate distribution required due to a change in BC. The changes in slopes at the membrane are caused by a step current density input. The fast time constants deried in Sec. 3.4 are apparent. Note here that the solution for the apor concentration is contingent upon the assumption that liquid water extends throughout the GDL length, so (4.2) holds for x L. Considering the time scale separation, only the steady-state solutions of the gas constituent PDEs are of interest hereafter. For H 2 and O 2, the only difference in the analytic solutions between single and two-phase 48
60 t = [.3ms 2ms 4ms ] 7.17 H 2 Concentration (mol/m 3 ) t = [.1ms.2ms 1ms ] Initial Steady State Transients Water Vapor Concentration (mol/m 3 ) Membrane GDL Position (m) Channel x 1 4 Figure 4.3: Transient solutions of c H2 and c,an for a step in current density (.15.3 A/cm 2 ). regions is in the diffusiity coefficient. The steady-state solutions for the reactants can be expanded to include the moing boundary at x fr : c H2 (x) = Ds im H 2 i(t) (x L) + cch H D 2 [x fr x L], (4.7a) H2,ε2F c H2 (x) = i(t) D s im H 2 2F (x x fr) + c H2 (x fr ) [ x < x fr ], (4.7b) c O2 (x) = i(t) D s (x L) + cch im O 2 4F O2 [ L x ], (4.7c) where c H2 (x fr ) is determined from (4.7a), then applied in (4.7b). Determination of the Vapor Solution Coefficients The steady-state water apor distributions when s(x, t) > are gien by: c,an (x) = α 1 e βx + α 2 e βx + c sat [ x < x fr ], (4.8a) 49
61 c,ca (x) = ν 1 e βx + ν 2 e βx + c sat [ L x ], (4.8b) where, β = γ/d s im. (4.9) The α i are functions of N mb and the anode channel condition (i.e., the anode GDL BC), α 1 e βx fr + α2 e βx fr α 1 α 2 = c (x fr ) c sat, = N mb /βd s im. (4.1) The ν i, similar to the α i, are dependent upon N mb and the cathode channel condition, but are additionally influenced by the water apor reaction term N rct formation of H 2 O at the cathode catalyst, from the ν 1 e βl + ν 2 e βl ν 1 ν 2 = c ch,ca c sat = (N rct N mb )/βd s im. (4.11) The membrane water transport, N mb, can be found from (2.16), and requires knowledge of the water apor concentrations on both sides of the membrane, obtained from (4.8a) at x =, c mb,an c mb,ca = (α 1 + α 2 ) + c sat, = (ν 1 + ν 2 ) + c sat. (4.12) The cathode side water apor concentration is modeled to determine the c mb,ca, which is necessary to find N mb. Howeer, the focus of our model is anode side water management (though the process deeloped is equally applicable to the cathode), thus for notation simplification the subscript an will be understood and omitted in subsequent formulation and discussion. Since water apor concentration on one side of the membrane influences the water apor concentration on the opposite, a complex cathode channel inlet/outletmembrane-anode channel inlet/outlet system of equations was deried and soled to determine the alues of the four boundary conditions needed to simultaneously sole both the anode and cathode water apor concentration second-order PDEs. These boundary conditions are influenced by inlet flow reactant excess ratios, temperatures, and relatie humidities, as well as by the stack current, which is an exogenous signal defined by the power required. The details of this simultaneous channel-to-channel solution method are gien in Appendix D. The eaporation/condensation present in the two-phase regions acts as a dis- 5
62 tributed source. This source disappears in the single-phase water region. Implementation of the solution for alues of x such that s = for x > x fr (single-phase GDL conditions) requires determination of the front location (which is found numerically from the solution of s(x, t) as expressed in Sec ) and a method of mating the solutions on either side of the front (discussed in Sec ). Within the single-phase water flow condition, the effectie diffusiity is constant, so considering (2.8) when the eaporation reaction term has gone to zero, c t = ( ) c 2 c D,ε = D,ε x x x, (4.13) 2 where D,ε = D () is the apor effectie diffusiity when s =. The steady-state solution of = D,ε 2 c x 2, (4.14) is c 1p (x) = m1p (x L) + c ch (x x fr ), (4.15) where the slope mating the single-phase (1p) solution, m 1p, with the two-phase (2p) water region will be deried in Sec Liquid Water Solution Since it has been shown that the time constant of the water apor is multiple orders of magnitude faster than that of the liquid water, it is acceptable to replace the c coupling term in (2.13) by its steady-state solution (4.8a). The PDE for liquid water distribution in the porous medium is then, for (x, t) such that s(x, t) s im, and s t = ( b 1 S b s ) 2 + M γ (α 1 e βx + α 2 e βx ), (4.16) x x ρ l s t = M γ (α 1 e βx + α 2 e βx ), (4.17) ρ l for (x, t) such that < s(x, t) < s im where S = per (2.6), and β and α i are as defined in the c,e (x) solution preiously (4.8a). For the s(x, t) s im case, (4.16) can be integrated twice (details in Appendix C) 51
63 to obtain the steady-state liquid water saturation, with s ss (x) = β z [ β(α 1 α 2 )(x L) + c ch csat (4.18) (α 1 e βx + α 2 e βx ) + ( Sδ β z ) b2 +1 ] 1 b sim, ( ) 1 M b γ(b 2 + 1) 2 +1 β z = (1 s im ). (4.19) ρ l β 2 b 1 For the < s(x, t) < s im case, because it is required for equilibria analysis, the unsteady solution of (4.17) can be found easily by integrating with respect to time, s(x, t) = M ρ l γ(α 1 e βx + α 2 e βx )t + s (x), (4.2) where s (x) is the water saturation distribution at t = t. The s (x) term represents the influence of the initial conditions on the water saturation. Since the complete analytic solution of (4.16) has not been found, a Semi- Analytic Solution (SAS) is defined that combines the quasi steady-state analytic solutions for the gas constituents (c H2 (x), c O2 (x), c,an (x), c,ca (x)) with the anode liquid water ratio (s(x, t)) numeric differential algebraic equation (DAE) Vapor Solution Transition In this section the transition between the two-phase exponential solution (4.8a) and the linear single-phase solution (4.15) is analyzed. It is assumed that the water mass flow is presered at the transition from two-phase to single-phase and use mass continuity across the transition point to establish the necessary flow rate on the single-phase side of the mobile front. To satisfy mass flow continuity, Ww 1p = M εa fc N 1p = M εa fc N 2p + W l (x 2p fr, t) = Ww, (4.21) where N 1p dc 1p = D,ε dx = D,εm 1p for x < x fr, (4.22) 52
64 and N 2p = D s im dc 2p dx = Ds im β(α 1 e βx α 2 e βx ) for x x fr. (4.23) Rearranging to find the slope for the single-phase water concentration of (4.15), m 1p = Ds im β ( ) W α 1 e βx fr α 2 e βx l (x fr fr, t). (4.24) D,ε M εa fc D,ε Here x fr indicates the spatial coordinate of the mobile front that is part of the two-phase water solution, where x fr is defined as the smallest alue of x such that s(x fr, t) = from the numeric solution of (4.16). The apor-only water mass flow rate on the single-phase side of x fr must match the sum of the liquid and apor water mass flow rates on the two-phase side, hence the W l (x fr, t) term in (4.24), which is found by soling (2.11) with S(x fr, t). Finally, the α i are found from (4.1) and (4.12) Simulation of Vapor Solution Transition A simulation result of the reaction-diffusion to diffusion-only switching solution for a 1-section GDL discretization is shown in Fig The solution switches as the s(x, t)= boundary traels, section-to-section, from channel toward the membrane. The transition points are aligned to section boundaries due to application of the numeric solution. The graph shows the time progression of the water apor concentration distribution as the liquid water mobile front recedes into the GDL, where sec. 1 is the lumped parameter olume closest to the membrane, and sec. 1 is closest to the channel. The system is modeled as an aeraged single cell of the 24-cell, 3 cm 2 actie area, 1.2kW experimental fuel cell stack set-up used in the model erification. The current density is.15a/cm 2, the cathode inlet stream is fully-humidified air, the anode inlet stream is pure, nearly dry hydrogen supplied at 2.63 times stoichiometry, and the stack temperature is set at 333K. For this result, an initially flooded anode at t=s experiences a change to a drying condition due to a 9% increase in H 2 excess ratio. By t =5s, the channel liquid water has been remoed as eidenced by the drop in apor concentration at x = L, but since there is still liquid water in sec. 1, the exponential solution is temporarily alid throughout the GDL ( x L). Howeer, s(1) < s im indicates that liquid flow into the channel has ceased, implying that GDL-channel water transport 53
65 mb sec 1 sec 2 sec 3 sec 4 sec 5 sec 6 sec 7 sec 8 sec 9 sec 1 ch c,an (x): Concentration (mol/m 3 ) Liquid Ratio (m 3 /m 3 ) c,sat t = 1 s t = s Steady state : transition point t=s s x 1 4 im 5s.1 25s 6s.5 1s Position (m) x 1 4 Figure 4.4: Water apor distribution for the switching analytic solution with the mobile front in the transition from two-phase to single-phase during a step change in λ H2. m o = lin dc dx 5s 25s 6s dc exp dx is in apor phase only, with the mass flow rate gien by, W 2p w (x fr, t) = M εa fc D s im (x) x c 2p + W l (x fr, t), (4.25) x=x fr and W l (L, t) =. This GDL to channel water flow rate is smaller than the apor flow rate out of the channel because the two-phase front recedes toward the membrane during the time period 5s < t < 1s, finding equilibrium when the front reaches sec. 7. Thus, Fig. 4.4 depicts the modeled water apor distribution of the combined diffusion-reaction/diffusion-only SAS, with the c (x) in x < x fr taking the two-phase exponential solution, and the c (x) in x x fr taking the single-phase linear solution. 4.2 Verification of the Semi-Analytic Model The model predicts oltage degradation by establishing a causal relationship between anode channel flooding and the fuel cell oltage deterioration. The experiments were performed using a 1.2KW, 24-cell stack with an actie area of 3 cm 2. The experiment operated with a dead-ended anode, and the nec- 54
66 Voltage (V) Experiment CNS SAS Current (A) I st 335 Temp (K) T st Time (sec) Figure 4.5: The semi-analytic model is able to capture static and transient trends in a direct comparison to experimental oltage output, and generates a oltage estimate ery similar to the numeric solution essary purges executed at 18 second interals for a duration of 1 second. The SAS model used the same parameter alues identified for the CNS model. For further experiment and parameter identification details, readers are requested to see [3]. In the first experiment, the stack current was aried from 75A 9A 75A while temperature was thermostatically controlled from 5C 6C. Comparing the coarse numeric model to the semi-analytic model in Fig. 4.5 illustrates that little, if any, predictie accuracy has been lost by implementing the semi-analytic solution to the GDL dynamics, and the important trends related to transient manifold pressure dynamics and stack current changes are captured. A desirable aspect of the model s control-oriented goal is estimation of the behaior of a 24-cell stack using a single cell model. In the set of alidation experimental results shown in Fig. 4.6 a (2% 3%) stoichiometry change is added to a series of stack current steps (4A 6A 15A). This data illustrates the range of oltage outputs from the indiidual cells in the stack. Though the model oltage prediction departs from the stack aerage alue for some conditions, trends are still captured and the results are still within the range of indiidual cell alues. The semi-analytic model output again tracks the coarse numeric model closely. 55
67 SAS Model CNS Model Voltage (V) Aeraged cell oltage Selected indiidual cell oltages (A/cm 2 ) I st (mg/s) 1 5 O 2 Flow Time (sec) Figure 4.6: The oltage prediction captures fluctuations due to changing conditions, and bias is within the cell-to-cell ariation. 4.3 Applications of the SAS Model Three examples of applications of the SAS model to extend understanding of PEM fuel cell operation are briefly discussed in this section. The potential for future work related to addition of an along-the-channel dimension, inclusion of a temperature gradient in the channel, and studies of hydrogen utilization is shown by simulation of modified ersions of the SAS model About the Along-the-Channel Dimension It is commonly understood that the portion of the channel nearest the inlet will be significantly drier than the channel portion nearest the outlet. Anode channel flow determination is a balance between preention of membrane drying at the inlet, while aoiding the flooding generated at the exit due to the accumulation of water from the 56
68 length of the channel. Numerous articles on the topic hae been published, but the neutron imaging work is most dramatic. Fig. 4.7, borrowed from [4] indicates clearly the dry-inlet, wet-outlet phenomenon. If it were desirable for the outlet humidity to be less than c sat, in the interest of membrane protection, it is necessary to consider the resulting membrane humidity near the channel inlet. Channel Inlet Increasing liquid Channel Outlet Figure 4.7: Neutron imaging shows liquid water accumulating near the anode channel exit (horizontal liquid images are from cathode side). Modeling of along-the-channel concentration ariation is implemented by considering the SAS model as a unit, and stacking it to stretch from inlet to outlet at the desired discretization degree. Fig. 4.8 demonstrates the concept. Using this methodology it is discoered that H 2 excess ratio must be increased significantly to make the water apor concentration nearest the channel exit fall below c sat. For a single lumped olume model under flow-control, flow-through conditions, a hydrogen excess ration of about 2% is required to keep the anode exhaust sub-saturated. As shown in Fig. 4.1, for a 9-section channel discretization, that excess ratio doubles to 4%, raising a concern for membrane drying near the inlet. Such a large excess ratio appears to indicate that the SAS model will hae a lower efficiency than the numeric model because of the high hydrogen pass-through. The issue of hydrogen utilization is addressed in Sec , where it will be shown that a controlled flow-through model uses significantly less hydrogen than the purge- 57
Stability Analysis for Liquid Water Accumulation in Low Temperature Fuel Cells
 Proceedings of the 47th IEEE Conference on Decision and Control Cancun Mexico Dec. 9-11 28 TuB7.5 Stability Analysis for Liquid Water Accumulation in Low Temperature Fuel Cells B.A. McCain and A.G. Stefanopoulou
Proceedings of the 47th IEEE Conference on Decision and Control Cancun Mexico Dec. 9-11 28 TuB7.5 Stability Analysis for Liquid Water Accumulation in Low Temperature Fuel Cells B.A. McCain and A.G. Stefanopoulou
Controllability and Observability Analysis of the Liquid Water Distribution Inside the Gas Diffusion Layer of a Unit Fuel Cell Model
 Buz A. McCain e-mail: bmccain@umi.edu Anna G. Stefanopoulou e-mail: annastef@umi.edu Department of Meanical Engineering, Fuel Cell Control Laboratory, Uniersity of Miigan, Ann Arbor, MI 4819 Jason B. Siegel
Buz A. McCain e-mail: bmccain@umi.edu Anna G. Stefanopoulou e-mail: annastef@umi.edu Department of Meanical Engineering, Fuel Cell Control Laboratory, Uniersity of Miigan, Ann Arbor, MI 4819 Jason B. Siegel
Introduction to Thermodynamic Cycles Part 1 1 st Law of Thermodynamics and Gas Power Cycles
 Introduction to Thermodynamic Cycles Part 1 1 st Law of Thermodynamics and Gas Power Cycles by James Doane, PhD, PE Contents 1.0 Course Oeriew... 4.0 Basic Concepts of Thermodynamics... 4.1 Temperature
Introduction to Thermodynamic Cycles Part 1 1 st Law of Thermodynamics and Gas Power Cycles by James Doane, PhD, PE Contents 1.0 Course Oeriew... 4.0 Basic Concepts of Thermodynamics... 4.1 Temperature
Water equilibria and management using a two-volume model of a polymer electrolyte fuel cell
 Journal of Power Sources 164 (2007) 590 605 Water equilibria and management using a two-volume model of a polymer electrolyte fuel cell Amey Y. Karnik a,, Anna G. Stefanopoulou a, Jing Sun b a Department
Journal of Power Sources 164 (2007) 590 605 Water equilibria and management using a two-volume model of a polymer electrolyte fuel cell Amey Y. Karnik a,, Anna G. Stefanopoulou a, Jing Sun b a Department
Astrometric Errors Correlated Strongly Across Multiple SIRTF Images
 Astrometric Errors Correlated Strongly Across Multiple SIRTF Images John Fowler 28 March 23 The possibility exists that after pointing transfer has been performed for each BCD (i.e. a calibrated image
Astrometric Errors Correlated Strongly Across Multiple SIRTF Images John Fowler 28 March 23 The possibility exists that after pointing transfer has been performed for each BCD (i.e. a calibrated image
SELECTION, SIZING, AND OPERATION OF CONTROL VALVES FOR GASES AND LIQUIDS Class # 6110
 SELECTION, SIZIN, AND OERATION OF CONTROL VALVES FOR ASES AND LIUIDS Class # 6110 Ross Turbiille Sales Engineer Fisher Controls International Inc. 301 S. First Aenue Marshalltown, Iowa USA Introduction
SELECTION, SIZIN, AND OERATION OF CONTROL VALVES FOR ASES AND LIUIDS Class # 6110 Ross Turbiille Sales Engineer Fisher Controls International Inc. 301 S. First Aenue Marshalltown, Iowa USA Introduction
The Kinetic Theory of Gases
 978-1-107-1788-3 Classical and Quantum Thermal Physics The Kinetic Theory of Gases CHAPTER 1 1.0 Kinetic Theory, Classical and Quantum Thermodynamics Two important components of the unierse are: the matter
978-1-107-1788-3 Classical and Quantum Thermal Physics The Kinetic Theory of Gases CHAPTER 1 1.0 Kinetic Theory, Classical and Quantum Thermodynamics Two important components of the unierse are: the matter
Two Phase Pressure Drop of CO2, Ammonia, and R245fa in Multiport Aluminum Microchannel Tubes
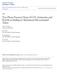 Purdue Uniersity Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 6 Two Phase Pressure Drop of CO, Ammonia, and R45fa in Multiport Aluminum Microchannel
Purdue Uniersity Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 6 Two Phase Pressure Drop of CO, Ammonia, and R45fa in Multiport Aluminum Microchannel
Modeling of Liquid Water Distribution at Cathode Gas Flow Channels in Proton Exchange Membrane Fuel Cell - PEMFC
 Modeling of Liquid Water Distribution at Cathode Gas Flow Channels in Proton Exchange Membrane Fuel Cell - PEMFC Sandro Skoda 1*, Eric Robalinho 2, André L. R. Paulino 1, Edgar F. Cunha 1, Marcelo Linardi
Modeling of Liquid Water Distribution at Cathode Gas Flow Channels in Proton Exchange Membrane Fuel Cell - PEMFC Sandro Skoda 1*, Eric Robalinho 2, André L. R. Paulino 1, Edgar F. Cunha 1, Marcelo Linardi
NUMERICAL ANALYSIS ON 36cm 2 PEM FUEL CELL FOR PERFORMANCE ENHANCEMENT
 NUMERICAL ANALYSIS ON 36cm 2 PEM FUEL CELL FOR PERFORMANCE ENHANCEMENT Lakshminarayanan V 1, Karthikeyan P 2, D. S. Kiran Kumar 1 and SMK Dhilip Kumar 1 1 Department of Mechanical Engineering, KGiSL Institute
NUMERICAL ANALYSIS ON 36cm 2 PEM FUEL CELL FOR PERFORMANCE ENHANCEMENT Lakshminarayanan V 1, Karthikeyan P 2, D. S. Kiran Kumar 1 and SMK Dhilip Kumar 1 1 Department of Mechanical Engineering, KGiSL Institute
IMECE DRAFT
 Proceedings of IMECE2006 2006 ASME International Mechanical Engineering Congress and Exposition November 5-10, 2006, Chicago, Illinois USA IMECE2006-14509-DRAFT A STUDY TOWARD MINIMUM SPATIAL DISCRETIZATION
Proceedings of IMECE2006 2006 ASME International Mechanical Engineering Congress and Exposition November 5-10, 2006, Chicago, Illinois USA IMECE2006-14509-DRAFT A STUDY TOWARD MINIMUM SPATIAL DISCRETIZATION
ME224 Lab 5 - Thermal Diffusion
 ME4 Lab 5 ME4 Lab 5 - hermal Diffusion (his lab is adapted from IBM-PC in the laboratory by B G homson & A F Kuckes, Chapter 5) 1. Introduction he experiments which you will be called upon to do in this
ME4 Lab 5 ME4 Lab 5 - hermal Diffusion (his lab is adapted from IBM-PC in the laboratory by B G homson & A F Kuckes, Chapter 5) 1. Introduction he experiments which you will be called upon to do in this
Brake applications and the remaining velocity Hans Humenberger University of Vienna, Faculty of mathematics
 Hans Humenberger: rake applications and the remaining elocity 67 rake applications and the remaining elocity Hans Humenberger Uniersity of Vienna, Faculty of mathematics Abstract It is ery common when
Hans Humenberger: rake applications and the remaining elocity 67 rake applications and the remaining elocity Hans Humenberger Uniersity of Vienna, Faculty of mathematics Abstract It is ery common when
Transmission lines using a distributed equivalent circuit
 Cambridge Uniersity Press 978-1-107-02600-1 - Transmission Lines Equialent Circuits, Electromagnetic Theory, and Photons Part 1 Transmission lines using a distributed equialent circuit in this web serice
Cambridge Uniersity Press 978-1-107-02600-1 - Transmission Lines Equialent Circuits, Electromagnetic Theory, and Photons Part 1 Transmission lines using a distributed equialent circuit in this web serice
MATHEMATICAL MODELLING AND IDENTIFICATION OF THE FLOW DYNAMICS IN
 MATHEMATICAL MOELLING AN IENTIFICATION OF THE FLOW YNAMICS IN MOLTEN GLASS FURNACES Jan Studzinski Systems Research Institute of Polish Academy of Sciences Newelska 6-447 Warsaw, Poland E-mail: studzins@ibspan.waw.pl
MATHEMATICAL MOELLING AN IENTIFICATION OF THE FLOW YNAMICS IN MOLTEN GLASS FURNACES Jan Studzinski Systems Research Institute of Polish Academy of Sciences Newelska 6-447 Warsaw, Poland E-mail: studzins@ibspan.waw.pl
Real Gas Thermodynamics. and the isentropic behavior of substances. P. Nederstigt
 Real Gas Thermodynamics and the isentropic behaior of substances. Nederstigt ii Real Gas Thermodynamics and the isentropic behaior of substances by. Nederstigt in partial fulfillment of the requirements
Real Gas Thermodynamics and the isentropic behaior of substances. Nederstigt ii Real Gas Thermodynamics and the isentropic behaior of substances by. Nederstigt in partial fulfillment of the requirements
Purpose of the experiment
 Impulse and Momentum PES 116 Adanced Physics Lab I Purpose of the experiment Measure a cart s momentum change and compare to the impulse it receies. Compare aerage and peak forces in impulses. To put the
Impulse and Momentum PES 116 Adanced Physics Lab I Purpose of the experiment Measure a cart s momentum change and compare to the impulse it receies. Compare aerage and peak forces in impulses. To put the
Modelling fuel cells in start-up and reactant starvation conditions
 Modelling fuel cells in start-up and reactant starvation conditions Brian Wetton Radu Bradean Keith Promislow Jean St Pierre Mathematics Department University of British Columbia www.math.ubc.ca/ wetton
Modelling fuel cells in start-up and reactant starvation conditions Brian Wetton Radu Bradean Keith Promislow Jean St Pierre Mathematics Department University of British Columbia www.math.ubc.ca/ wetton
Direct Energy Conversion: Fuel Cells
 Direct Energy Conversion: Fuel Cells References and Sources: Direct Energy Conversion by Stanley W. Angrist, Allyn and Beacon, 1982. Fuel Cell Systems, Explained by James Larminie and Andrew Dicks, Wiley,
Direct Energy Conversion: Fuel Cells References and Sources: Direct Energy Conversion by Stanley W. Angrist, Allyn and Beacon, 1982. Fuel Cell Systems, Explained by James Larminie and Andrew Dicks, Wiley,
Analysis of cylindrical heat pipes incorporating the e ects of liquid±vapor coupling and non-darcian transportða closed form solution
 International Journal of Heat and Mass Transfer 42 (1999) 3405±341 Analysis of cylindrical heat pipes incorporating the e ects of liquid±apor coupling and non-darcian transportða closed form solution N.
International Journal of Heat and Mass Transfer 42 (1999) 3405±341 Analysis of cylindrical heat pipes incorporating the e ects of liquid±apor coupling and non-darcian transportða closed form solution N.
Chapter 7: The Second Law of Thermodynamics
 Chapter 7: he Second Law of hermodynamics he second law of thermodynamics asserts that processes occur in a certain direction and that the energy has quality as well as quantity he first law places no
Chapter 7: he Second Law of hermodynamics he second law of thermodynamics asserts that processes occur in a certain direction and that the energy has quality as well as quantity he first law places no
SLIP MODEL PERFORMANCE FOR MICRO-SCALE GAS FLOWS
 3th AIAA Thermophysics Conference 3- June 3, Orlando, Florida AIAA 3-5 SLIP MODEL PERFORMANCE FOR MICRO-SCALE GAS FLOWS Matthew J. McNenly* Department of Aerospace Engineering Uniersity of Michigan, Ann
3th AIAA Thermophysics Conference 3- June 3, Orlando, Florida AIAA 3-5 SLIP MODEL PERFORMANCE FOR MICRO-SCALE GAS FLOWS Matthew J. McNenly* Department of Aerospace Engineering Uniersity of Michigan, Ann
Residual migration in VTI media using anisotropy continuation
 Stanford Exploration Project, Report SERGEY, Noember 9, 2000, pages 671?? Residual migration in VTI media using anisotropy continuation Tariq Alkhalifah Sergey Fomel 1 ABSTRACT We introduce anisotropy
Stanford Exploration Project, Report SERGEY, Noember 9, 2000, pages 671?? Residual migration in VTI media using anisotropy continuation Tariq Alkhalifah Sergey Fomel 1 ABSTRACT We introduce anisotropy
Management of Power Systems With In-line Renewable Energy Sources
 Management of Power Systems With In-line Renewable Energy Sources Haoliang Shi, Xiaoyu Shi, Haozhe Xu, Ziwei Yu Department of Mathematics, Uniersity of Arizona May 14, 018 Abstract Power flowing along
Management of Power Systems With In-line Renewable Energy Sources Haoliang Shi, Xiaoyu Shi, Haozhe Xu, Ziwei Yu Department of Mathematics, Uniersity of Arizona May 14, 018 Abstract Power flowing along
January 21, 2004 Fuel Cell Engineering Course CHEG 320 Taught at UTC Fuel Cells. Fuel Cells
 January 21, 2004 Fuel Cell Engineering Course CHEG 320 Taught at UTC Fuel Cells Fuel Cells Instructor James M. Fenton, Professor, Chemical Engineering University of Connecticut Teaching Assistants: 1.
January 21, 2004 Fuel Cell Engineering Course CHEG 320 Taught at UTC Fuel Cells Fuel Cells Instructor James M. Fenton, Professor, Chemical Engineering University of Connecticut Teaching Assistants: 1.
THE ANALYSIS OF THE CONVECTIVE-CONDUCTIVE HEAT TRANSFER IN THE BUILDING CONSTRUCTIONS. Zbynek Svoboda
 THE NLSIS OF THE CONECTIE-CONDUCTIE HET TRNSFER IN THE BUILDING CONSTRUCTIONS Zbynek Soboda Czech Technical Uniersity in Prague Faculty of Ciil Engineering 166 29 Prague 6 - Czech Republic BSTRCT The numerical
THE NLSIS OF THE CONECTIE-CONDUCTIE HET TRNSFER IN THE BUILDING CONSTRUCTIONS Zbynek Soboda Czech Technical Uniersity in Prague Faculty of Ciil Engineering 166 29 Prague 6 - Czech Republic BSTRCT The numerical
Performance Analysis of a Two phase Non-isothermal PEM Fuel Cell
 Performance Analysis of a Two phase Non-isothermal PEM Fuel Cell A. H. Sadoughi 1 and A. Asnaghi 2 and M. J. Kermani 3 1, 2 Ms Student of Mechanical Engineering, Sharif University of Technology Tehran,
Performance Analysis of a Two phase Non-isothermal PEM Fuel Cell A. H. Sadoughi 1 and A. Asnaghi 2 and M. J. Kermani 3 1, 2 Ms Student of Mechanical Engineering, Sharif University of Technology Tehran,
0 a 3 a 2 a 3 0 a 1 a 2 a 1 0
 Chapter Flow kinematics Vector and tensor formulae This introductory section presents a brief account of different definitions of ector and tensor analysis that will be used in the following chapters.
Chapter Flow kinematics Vector and tensor formulae This introductory section presents a brief account of different definitions of ector and tensor analysis that will be used in the following chapters.
NUMERICAL EVALUATION OF THE THERMAL PERFORMANCES OF ROOF-MOUNTED RADIANT BARRIERS
 NUMERICAL EVALUATION OF THE THERMAL PERFORMANCES OF ROOF-MOUNTED RADIANT BARRIERS F. Miranille, H. Boyer, F. Lucas, J. Sériacaroupin Building Physics and Systems Laboratory 40, aenue de Soweto - 97410
NUMERICAL EVALUATION OF THE THERMAL PERFORMANCES OF ROOF-MOUNTED RADIANT BARRIERS F. Miranille, H. Boyer, F. Lucas, J. Sériacaroupin Building Physics and Systems Laboratory 40, aenue de Soweto - 97410
FINITE ELEMENT METHOD MODELLING OF A HIGH TEMPERATURE PEM FUEL CELL
 CONDENSED MATTER FINITE ELEMENT METHOD MODELLING OF A HIGH TEMPERATURE PEM FUEL CELL V. IONESCU 1 1 Department of Physics and Electronics, Ovidius University, Constanta, 900527, Romania, E-mail: ionescu.vio@gmail.com
CONDENSED MATTER FINITE ELEMENT METHOD MODELLING OF A HIGH TEMPERATURE PEM FUEL CELL V. IONESCU 1 1 Department of Physics and Electronics, Ovidius University, Constanta, 900527, Romania, E-mail: ionescu.vio@gmail.com
Modeling as a tool for understanding the MEA. Henrik Ekström Utö Summer School, June 22 nd 2010
 Modeling as a tool for understanding the MEA Henrik Ekström Utö Summer School, June 22 nd 2010 COMSOL Multiphysics and Electrochemistry Modeling The software is based on the finite element method A number
Modeling as a tool for understanding the MEA Henrik Ekström Utö Summer School, June 22 nd 2010 COMSOL Multiphysics and Electrochemistry Modeling The software is based on the finite element method A number
ADVANCEMENT OF SMALL-SCALE THERMOACOUSTIC ENGINE
 ADVANCEMENT OF SMALL-SCALE THERMOACOUSTIC ENGINE By SUNGMIN JUNG Masters in Mechanical Engineering WASHINGTON STATE UNIVERSITY School of Mechanical and Material Engineering MAY 2009 To the Faculty of Washington
ADVANCEMENT OF SMALL-SCALE THERMOACOUSTIC ENGINE By SUNGMIN JUNG Masters in Mechanical Engineering WASHINGTON STATE UNIVERSITY School of Mechanical and Material Engineering MAY 2009 To the Faculty of Washington
Control of Proton Electrolyte Membrane Fuel Cell Systems. Dr. M. Grujicic Department of Mechanical Engineering
 Control of Proton Electrolyte Membrane Fuel Cell Systems Dr. M. Grujicic 4 Department of Mechanical Engineering OUTLINE. Feedforward Control, Fuel Cell System. Feedback Control, Fuel Cell System W Cp Supply
Control of Proton Electrolyte Membrane Fuel Cell Systems Dr. M. Grujicic 4 Department of Mechanical Engineering OUTLINE. Feedforward Control, Fuel Cell System. Feedback Control, Fuel Cell System W Cp Supply
FREEWAY WEAVING. Highway Capacity Manual 2000 CHAPTER 24 CONTENTS EXHIBITS
 CHAPTER 24 FREEWAY WEAVING CONTENTS I. INTRODUCTION... 24-1 Scope of the Methodology...24-1 Limitations of the Methodology...24-1 II. METHODOLOGY...24-1 LOS...24-2 Weaing Segment Parameters...24-3 Determining
CHAPTER 24 FREEWAY WEAVING CONTENTS I. INTRODUCTION... 24-1 Scope of the Methodology...24-1 Limitations of the Methodology...24-1 II. METHODOLOGY...24-1 LOS...24-2 Weaing Segment Parameters...24-3 Determining
Modeling, Simulation and Optimization of a Cross Flow Molten Carbonate Fuel Cell
 Modeling, Simulation and Optimization of a Cross Flow Molten Carbonate Fuel Cell P. Heidebrecht 1, M. Mangold 2, M. Gundermann 1, A. Kienle 2,3, K. Sundmacher 1,2 1 Otto-von-Guericke-University Magdeburg,
Modeling, Simulation and Optimization of a Cross Flow Molten Carbonate Fuel Cell P. Heidebrecht 1, M. Mangold 2, M. Gundermann 1, A. Kienle 2,3, K. Sundmacher 1,2 1 Otto-von-Guericke-University Magdeburg,
CONDENSING EJECTOR FOR SECOND STEP COMPRESSION IN REFRIGERATION CYCLES
 Paper 2174, Page 1 CONDENSING EJECTOR FOR SECOND STEP COMPRESSION IN REFRIGERATION CYCES Mark J. BERGANDER 1, PhD, P.E., Prof. Daid P. SCHMIDT 2, Daid A. HEBERT 2, Dr. Jerzy WOJCIECHOWSKI 3, Mateusz SZKARZ
Paper 2174, Page 1 CONDENSING EJECTOR FOR SECOND STEP COMPRESSION IN REFRIGERATION CYCES Mark J. BERGANDER 1, PhD, P.E., Prof. Daid P. SCHMIDT 2, Daid A. HEBERT 2, Dr. Jerzy WOJCIECHOWSKI 3, Mateusz SZKARZ
Probabilistic Engineering Design
 Probabilistic Engineering Design Chapter One Introduction 1 Introduction This chapter discusses the basics of probabilistic engineering design. Its tutorial-style is designed to allow the reader to quickly
Probabilistic Engineering Design Chapter One Introduction 1 Introduction This chapter discusses the basics of probabilistic engineering design. Its tutorial-style is designed to allow the reader to quickly
Ugur Pasaogullari, Chao-Yang Wang Electrochemical Engine Center The Pennsylvania State University University Park, PA, 16802
 Computational Fluid Dynamics Modeling of Proton Exchange Membrane Fuel Cells using Fluent Ugur Pasaogullari, Chao-Yang Wang Electrochemical Engine Center The Pennsylvania State University University Park,
Computational Fluid Dynamics Modeling of Proton Exchange Membrane Fuel Cells using Fluent Ugur Pasaogullari, Chao-Yang Wang Electrochemical Engine Center The Pennsylvania State University University Park,
Tools for Investigation of Dynamics of DC-DC Converters within Matlab/Simulink
 Tools for Inestigation of Dynamics of DD onerters within Matlab/Simulink Riga Technical Uniersity, Riga, Latia Email: pikulin03@inbox.l Dmitry Pikulin Abstract: In this paper the study of complex phenomenon
Tools for Inestigation of Dynamics of DD onerters within Matlab/Simulink Riga Technical Uniersity, Riga, Latia Email: pikulin03@inbox.l Dmitry Pikulin Abstract: In this paper the study of complex phenomenon
( t) Fuel Cell Stack System. Fuel Cells. Reactant Flow Subsystem. Fuel Cell Characteristics: Polarization
 Stack Syem ater, electril energy and heat arise through the controlled coination of hydrogen and oxygen. s Polarization Cure V cell Anna Stefanopoulou Control Laboratory Uniersity of Michigan i H + H O
Stack Syem ater, electril energy and heat arise through the controlled coination of hydrogen and oxygen. s Polarization Cure V cell Anna Stefanopoulou Control Laboratory Uniersity of Michigan i H + H O
Chapter 1 Solutions Engineering and Chemical Thermodynamics 2e Wyatt Tenhaeff Milo Koretsky
 Chapter 1 Solutions Engineering and Chemical Thermodynamics 2e Wyatt Tenhaeff Milo Koretsky School of Chemical, Biological, and Enironmental Engineering Oregon State Uniersity 1.1 (b) The olume of water
Chapter 1 Solutions Engineering and Chemical Thermodynamics 2e Wyatt Tenhaeff Milo Koretsky School of Chemical, Biological, and Enironmental Engineering Oregon State Uniersity 1.1 (b) The olume of water
ELECTROPHORESIS SLAB (THIN LAYER GEL) AND CAPILLARY METHODS. A. General Introduction
 ELECTROPHORESIS SLAB (THIN LAYER GEL) AND CAPILLARY METHODS A. General Introduction Electrophoresis: a saration method based on differential rate of migration of charged species in an applied dc electric
ELECTROPHORESIS SLAB (THIN LAYER GEL) AND CAPILLARY METHODS A. General Introduction Electrophoresis: a saration method based on differential rate of migration of charged species in an applied dc electric
D DAVID PUBLISHING. 1. Introduction. Akira Nishimura 1, Masashi Baba 1, Kotaro Osada 1, Takenori Fukuoka 1, Masafumi Hirota 1 and Eric Hu 2
 Journal of Energy and Power Engineering () - doi:./-/.. D DAVID PUBLISHING Temperature Distributions in Single Cell of Polymer Electrolyte Fuel Cell Simulated by an D Multi-plate Heat-Transfer Model and
Journal of Energy and Power Engineering () - doi:./-/.. D DAVID PUBLISHING Temperature Distributions in Single Cell of Polymer Electrolyte Fuel Cell Simulated by an D Multi-plate Heat-Transfer Model and
Dr. V.LAKSHMINARAYANAN Department of Mechanical Engineering, B V Raju Institute of Technology, Narsapur, Telangana,, India
 Parametric analysis performed on 49 cm 2 serpentine flow channel of PEM fuel cell by Taguchi method (Parametric analysis performed on PEMFC by Taguchi method) Dr. V.LAKSHMINARAYANAN Department of Mechanical
Parametric analysis performed on 49 cm 2 serpentine flow channel of PEM fuel cell by Taguchi method (Parametric analysis performed on PEMFC by Taguchi method) Dr. V.LAKSHMINARAYANAN Department of Mechanical
Modeling the Behaviour of a Polymer Electrolyte Membrane within a Fuel Cell Using COMSOL
 Modeling the Behaviour of a Polymer Electrolyte Membrane within a Fuel Cell Using COMSOL S. Beharry 1 1 University of the West Indies, St. Augustine, Trinidad and Tobago Abstract: In recent years, scientists
Modeling the Behaviour of a Polymer Electrolyte Membrane within a Fuel Cell Using COMSOL S. Beharry 1 1 University of the West Indies, St. Augustine, Trinidad and Tobago Abstract: In recent years, scientists
Chapter 4: Properties of Pure Substances. Pure Substance. Phases of a Pure Substance. Phase-Change Processes of Pure Substances
 Chapter 4: roperties o ure Substances ure Substance A substance that has a ixed chemical composition throughout is called a pure substance such as water, air, and nitrogen A pure substance does not hae
Chapter 4: roperties o ure Substances ure Substance A substance that has a ixed chemical composition throughout is called a pure substance such as water, air, and nitrogen A pure substance does not hae
Appendix A Electric Vehicle PEM Fuel Cell Stack Parameters
 Appendix A Electric Vehicle PEM Fuel Cell Stack Parameters A.1 Return Manifold Polynomial Fitting Table A.1 Return manifold polynomial fitting Parameter Value Return manifold parameter p 0 0.001248 kg/s
Appendix A Electric Vehicle PEM Fuel Cell Stack Parameters A.1 Return Manifold Polynomial Fitting Table A.1 Return manifold polynomial fitting Parameter Value Return manifold parameter p 0 0.001248 kg/s
Inelastic Collapse in One Dimensional Systems with Ordered Collisions
 Lake Forest College Lake Forest College Publications Senior Theses Student Publications 4-4-05 Inelastic Collapse in One Dimensional Systems with Ordered Collisions Brandon R. Bauerly Lake Forest College,
Lake Forest College Lake Forest College Publications Senior Theses Student Publications 4-4-05 Inelastic Collapse in One Dimensional Systems with Ordered Collisions Brandon R. Bauerly Lake Forest College,
Basic overall reaction for hydrogen powering
 Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Mathematical Modeling for a PEM Fuel Cell.
 Mathematical Modeling for a PEM Fuel Cell. Mentor: Dr. Christopher Raymond, NJIT. Group Jutta Bikowski, Colorado State University Aranya Chakrabortty, RPI Kamyar Hazaveh, Georgia Institute of Technology
Mathematical Modeling for a PEM Fuel Cell. Mentor: Dr. Christopher Raymond, NJIT. Group Jutta Bikowski, Colorado State University Aranya Chakrabortty, RPI Kamyar Hazaveh, Georgia Institute of Technology
Review of temperature distribution in cathode of PEMFC
 Project Report 2008 MVK 160 Heat and Mass Transport May 08, 2008, Lund, Sweden Review of temperature distribution in cathode of PEMFC Munir Ahmed Khan Department of Energy Sciences, Lund Institute of Technology,
Project Report 2008 MVK 160 Heat and Mass Transport May 08, 2008, Lund, Sweden Review of temperature distribution in cathode of PEMFC Munir Ahmed Khan Department of Energy Sciences, Lund Institute of Technology,
A matrix Method for Interval Hermite Curve Segmentation O. Ismail, Senior Member, IEEE
 International Journal of Video&Image Processing Network Security IJVIPNS-IJENS Vol:15 No:03 7 A matrix Method for Interal Hermite Cure Segmentation O. Ismail, Senior Member, IEEE Abstract Since the use
International Journal of Video&Image Processing Network Security IJVIPNS-IJENS Vol:15 No:03 7 A matrix Method for Interal Hermite Cure Segmentation O. Ismail, Senior Member, IEEE Abstract Since the use
Activity. Modeling the Fuel Cell Reaction. Overview. Advance Preparation. Background Information
 4 Activity 1-2 class sessions Modeling the uel Cell Reaction 2011 Regents of the University of California Overview n order to understand the chemistry of fuel cells, students are introduced to oxidation-reduction
4 Activity 1-2 class sessions Modeling the uel Cell Reaction 2011 Regents of the University of California Overview n order to understand the chemistry of fuel cells, students are introduced to oxidation-reduction
UNDERSTAND MOTION IN ONE AND TWO DIMENSIONS
 SUBAREA I. COMPETENCY 1.0 UNDERSTAND MOTION IN ONE AND TWO DIMENSIONS MECHANICS Skill 1.1 Calculating displacement, aerage elocity, instantaneous elocity, and acceleration in a gien frame of reference
SUBAREA I. COMPETENCY 1.0 UNDERSTAND MOTION IN ONE AND TWO DIMENSIONS MECHANICS Skill 1.1 Calculating displacement, aerage elocity, instantaneous elocity, and acceleration in a gien frame of reference
Computational Analysis of Heat Transfer in Air-cooled Fuel Cells
 Proceedings of ASME 2011, 5th International Conference on Energy Sustainability & 9th Fuel Cell Science, Engineering and Technology Conference, ESFuelCell2011 August 7-10, 2011, Washington, DC, USA ESFuelCell2011-54794
Proceedings of ASME 2011, 5th International Conference on Energy Sustainability & 9th Fuel Cell Science, Engineering and Technology Conference, ESFuelCell2011 August 7-10, 2011, Washington, DC, USA ESFuelCell2011-54794
Cross Section of Proton Exchange Membrane Fuel Cell
 PEMFC Electrodes 1 Cross Section of Proton Exchange Membrane Fuel Cell Anode Cathode 2 Typical PEMFC Electrodes: - Anode Hydrogen Oxidation - Pt Ru / C - Cathode Oxygen reduction - Pt / C Pt is alloyed
PEMFC Electrodes 1 Cross Section of Proton Exchange Membrane Fuel Cell Anode Cathode 2 Typical PEMFC Electrodes: - Anode Hydrogen Oxidation - Pt Ru / C - Cathode Oxygen reduction - Pt / C Pt is alloyed
V. Transistors. 3.1 III. Bipolar-Junction (BJT) Transistors
 V. Transistors 3.1 III. Bipolar-Junction (BJT) Transistors A bipolar junction transistor is formed by joining three sections of semiconductors with alternatiely different dopings. The middle section (base)
V. Transistors 3.1 III. Bipolar-Junction (BJT) Transistors A bipolar junction transistor is formed by joining three sections of semiconductors with alternatiely different dopings. The middle section (base)
ICON. The Icosahedral Nonhydrostatic model: Formulation of the dynamical core and physics-dynamics coupling
 ICON The Icosahedral Nonhydrostatic model: Formulation of the dynamical core and physics-dynamics coupling Günther Zängl and the ICON deelopment team PDEs on the sphere 2012 Outline Introduction: Main
ICON The Icosahedral Nonhydrostatic model: Formulation of the dynamical core and physics-dynamics coupling Günther Zängl and the ICON deelopment team PDEs on the sphere 2012 Outline Introduction: Main
Through the Membrane & Along the Channel Flooding in PEMFCs
 2009 American Control Conference Hyatt Regency Riverfront, St. Louis, MO, USA June 10-12, 2009 ThA20.4 Through the Membrane & Along the Channel Flooding in PEMFCs Jason B. Siegel and Anna G. Stefanopoulou
2009 American Control Conference Hyatt Regency Riverfront, St. Louis, MO, USA June 10-12, 2009 ThA20.4 Through the Membrane & Along the Channel Flooding in PEMFCs Jason B. Siegel and Anna G. Stefanopoulou
Experimental Characterization Methodology for the Identification of Voltage Losses of PEMFC: Applied to an Open Cathode Stack
 Experimental Characterization Methodology for the Identification of Voltage Losses of PEMFC: Applied to an Open Cathode Stack A. Husar *, S. Strahl, J. Riera Institut de Robòtica i Informàtica Industrial
Experimental Characterization Methodology for the Identification of Voltage Losses of PEMFC: Applied to an Open Cathode Stack A. Husar *, S. Strahl, J. Riera Institut de Robòtica i Informàtica Industrial
Two Phase Transport in Porous Media
 Two Phase Transport in Porous Media Lloyd Bridge Iain Moyles Brian Wetton Mathematics Department University of British Columbia www.math.ubc.ca/ wetton CRM CAMP Seminar, October 19, 2011 Overview Two phase
Two Phase Transport in Porous Media Lloyd Bridge Iain Moyles Brian Wetton Mathematics Department University of British Columbia www.math.ubc.ca/ wetton CRM CAMP Seminar, October 19, 2011 Overview Two phase
ANALYTICAL INVESTIGATION AND IMPROVEMENT OF PERFORMANCE OF A PROTON EXCHANGE MEMBRANE (PEM) FUEL CELL IN MOBILE APPLICATIONS
 Int. J. of Applied Mechanics and Engineering, 015, vol.0, No., pp.319-38 DOI: 10.1515/ijame-015-001 ANALYTICAL INVESTIGATION AND IMPROVEMENT OF PERFORMANCE OF A PROTON EXCHANGE MEMBRANE (PEM) FUEL CELL
Int. J. of Applied Mechanics and Engineering, 015, vol.0, No., pp.319-38 DOI: 10.1515/ijame-015-001 ANALYTICAL INVESTIGATION AND IMPROVEMENT OF PERFORMANCE OF A PROTON EXCHANGE MEMBRANE (PEM) FUEL CELL
Performance Investigation on Electrochemical Compressor with Ammonia
 Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 2016 Performance Investigation on Electrochemical Compressor with Ammonia Ye Tao University
Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 2016 Performance Investigation on Electrochemical Compressor with Ammonia Ye Tao University
Fuel Cell Activities in MME Waterloo
 Fuel Cell Activities in MME Waterloo Xianguo Li and Roydon Fraser Fuel Cells and Green Energy Research Group Department of Mechanical & Mechatronics Engineering University of Waterloo, Waterloo, Ontario,
Fuel Cell Activities in MME Waterloo Xianguo Li and Roydon Fraser Fuel Cells and Green Energy Research Group Department of Mechanical & Mechatronics Engineering University of Waterloo, Waterloo, Ontario,
Min Chen Department of Mathematics Purdue University 150 N. University Street
 EXISTENCE OF TRAVELING WAVE SOLUTIONS OF A HIGH-ORDER NONLINEAR ACOUSTIC WAVE EQUATION Min Chen Department of Mathematics Purdue Uniersity 150 N. Uniersity Street 47907-067 chen@math.purdue.edu Monica
EXISTENCE OF TRAVELING WAVE SOLUTIONS OF A HIGH-ORDER NONLINEAR ACOUSTIC WAVE EQUATION Min Chen Department of Mathematics Purdue Uniersity 150 N. Uniersity Street 47907-067 chen@math.purdue.edu Monica
Interpreting enzyme and receptor kinetics: keeping it simple, but not too simple 1
 Nuclear Medicine and Biology 30 (2003) 819 826 www.elseier.com/locate/nucmedbio Interpreting enzyme and receptor kinetics: keeping it simple, but not too simple 1 Kenneth A. Krohn *, Jeanne M. Link Department
Nuclear Medicine and Biology 30 (2003) 819 826 www.elseier.com/locate/nucmedbio Interpreting enzyme and receptor kinetics: keeping it simple, but not too simple 1 Kenneth A. Krohn *, Jeanne M. Link Department
A. unchanged increased B. unchanged unchanged C. increased increased D. increased unchanged
 IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS CHAPTER B TEST REVIEW. A rocket is fired ertically. At its highest point, it explodes. Which one of the following describes what happens
IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS CHAPTER B TEST REVIEW. A rocket is fired ertically. At its highest point, it explodes. Which one of the following describes what happens
Advanced Analytical Chemistry Lecture 12. Chem 4631
 Advanced Analytical Chemistry Lecture 12 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Advanced Analytical Chemistry Lecture 12 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Outline. Example. Solution. Property evaluation examples Specific heat Internal energy, enthalpy, and specific heats of solids and liquids Examples
 Outline Property ealuation examples Specific heat Internal energy, enthalpy, and specific heats of solids and liquids s A piston-cylinder deice initially contains 0.5m of saturated water apor at 00kPa.
Outline Property ealuation examples Specific heat Internal energy, enthalpy, and specific heats of solids and liquids s A piston-cylinder deice initially contains 0.5m of saturated water apor at 00kPa.
A Geometric Review of Linear Algebra
 A Geometric Reiew of Linear Algebra The following is a compact reiew of the primary concepts of linear algebra. I assume the reader is familiar with basic (i.e., high school) algebra and trigonometry.
A Geometric Reiew of Linear Algebra The following is a compact reiew of the primary concepts of linear algebra. I assume the reader is familiar with basic (i.e., high school) algebra and trigonometry.
A Geometric Review of Linear Algebra
 A Geometric Reiew of Linear Algebra The following is a compact reiew of the primary concepts of linear algebra. The order of presentation is unconentional, with emphasis on geometric intuition rather than
A Geometric Reiew of Linear Algebra The following is a compact reiew of the primary concepts of linear algebra. The order of presentation is unconentional, with emphasis on geometric intuition rather than
SIMULATIONS OF CHARACTERISTICS OF TUNED LIQUID COLUMN DAMPER USING AN ELLIPTICAL FLOW PATH ESTIMATION METHOD
 October -7, 008, Beijing, China SIMULATIONS OF CHARACTERISTICS OF TUNED LIQUID COLUMN DAMPER USING AN ELLIPTICAL FLOW PATH ESTIMATION METHOD P. Chaiiriyawong, S. Limkatanyu and T. Pinkaew 3 Lecturer, Dept.
October -7, 008, Beijing, China SIMULATIONS OF CHARACTERISTICS OF TUNED LIQUID COLUMN DAMPER USING AN ELLIPTICAL FLOW PATH ESTIMATION METHOD P. Chaiiriyawong, S. Limkatanyu and T. Pinkaew 3 Lecturer, Dept.
DMFC Models and Applications - A Literature Survey, Part I
 Proceedings of the 2014 International Conference on Industrial Engineering and Operations Management Bali, Indonesia, January 7 9, 2014 DMFC Models and Applications - A Literature Survey, Part I S. Patrabansh,
Proceedings of the 2014 International Conference on Industrial Engineering and Operations Management Bali, Indonesia, January 7 9, 2014 DMFC Models and Applications - A Literature Survey, Part I S. Patrabansh,
4 Fundamentals of Continuum Thermomechanics
 4 Fundamentals of Continuum Thermomechanics In this Chapter, the laws of thermodynamics are reiewed and formulated for a continuum. The classical theory of thermodynamics, which is concerned with simple
4 Fundamentals of Continuum Thermomechanics In this Chapter, the laws of thermodynamics are reiewed and formulated for a continuum. The classical theory of thermodynamics, which is concerned with simple
sensors ISSN by MDPI
 Sensors 008, 8, 1475-1487 Full Research Paper sensors ISSN 144-80 008 by MDPI www.mdpi.org/sensors Three-Dimensional Transport Modeling for Proton Exchange Membrane(PEM) Fuel Cell with Micro Parallel Flow
Sensors 008, 8, 1475-1487 Full Research Paper sensors ISSN 144-80 008 by MDPI www.mdpi.org/sensors Three-Dimensional Transport Modeling for Proton Exchange Membrane(PEM) Fuel Cell with Micro Parallel Flow
Performance Simulation of Passive Direct Methanol Fuel Cell
 International Journal of Advanced Mechanical Engineering. ISSN 50-334 Volume 8, Number 1 (018), pp. 05-1 Research India Publications http://www.ripublication.com Performance Simulation of Passive Direct
International Journal of Advanced Mechanical Engineering. ISSN 50-334 Volume 8, Number 1 (018), pp. 05-1 Research India Publications http://www.ripublication.com Performance Simulation of Passive Direct
Motors and Generators
 Physics Motors and Generators New Reised Edition Brian Shadwick Contents Use the table of contents to record your progress through this book. As you complete each topic, write the date completed, then
Physics Motors and Generators New Reised Edition Brian Shadwick Contents Use the table of contents to record your progress through this book. As you complete each topic, write the date completed, then
TRANSIENTS IN POLYMER ELECTROLYTE MEMBRANE (PEM) FUEL CELLS
 TRANSIENTS IN POLYMER ELECTROLYTE MEMBRANE (PEM) FUEL CELLS Atul Verma Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements
TRANSIENTS IN POLYMER ELECTROLYTE MEMBRANE (PEM) FUEL CELLS Atul Verma Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements
ME 200 Exam 2 October 22, :30 p.m. to 7:30 p.m.
 CIRCLE YOUR LECTURE BELOW: First Name Solution Last Name 7:0 a.m. 8:0 a.m. 10:0 a.m. 11:0 a.m. Boregowda Boregowda Braun Bae :0 p.m. :0 p.m. 4:0 p.m. Meyer Naik Hess ME 00 Exam October, 015 6:0 p.m. to
CIRCLE YOUR LECTURE BELOW: First Name Solution Last Name 7:0 a.m. 8:0 a.m. 10:0 a.m. 11:0 a.m. Boregowda Boregowda Braun Bae :0 p.m. :0 p.m. 4:0 p.m. Meyer Naik Hess ME 00 Exam October, 015 6:0 p.m. to
are applied to ensure that physical principles are not iolated in the definition of the discrete transition model. The oerall goal is to use this fram
 A Comprehensie Methodology for Building Hybrid Models of Physical Systems Pieter J. Mosterman Λ Institute of Robotics and System Dynamics DLR Oberpfaffenhofen P.O. Box 1116 D-8223 Wessling Germany Gautam
A Comprehensie Methodology for Building Hybrid Models of Physical Systems Pieter J. Mosterman Λ Institute of Robotics and System Dynamics DLR Oberpfaffenhofen P.O. Box 1116 D-8223 Wessling Germany Gautam
Notes on Linear Minimum Mean Square Error Estimators
 Notes on Linear Minimum Mean Square Error Estimators Ça gatay Candan January, 0 Abstract Some connections between linear minimum mean square error estimators, maximum output SNR filters and the least square
Notes on Linear Minimum Mean Square Error Estimators Ça gatay Candan January, 0 Abstract Some connections between linear minimum mean square error estimators, maximum output SNR filters and the least square
State-space Modelling of Hysteresis-based Control Schemes
 European Control Conference (ECC) July 7-9,, Zürich, Switzerland. State-space Modelling of Hysteresis-based Control Schemes Soumya Kundu Ian A. Hiskens Abstract The paper deelops a state-space model for
European Control Conference (ECC) July 7-9,, Zürich, Switzerland. State-space Modelling of Hysteresis-based Control Schemes Soumya Kundu Ian A. Hiskens Abstract The paper deelops a state-space model for
Modeling Hydraulic Accumulators for use in Wind Turbines
 The 13th Scandinaian International Conference on Fluid Power, SICFP213, une 3-5, 213, Linköping, Sweden Modeling Hydraulic Accumulators for use in Wind Turbines Henrik Brun Hansen and Peter Windfeld Rasmussen
The 13th Scandinaian International Conference on Fluid Power, SICFP213, une 3-5, 213, Linköping, Sweden Modeling Hydraulic Accumulators for use in Wind Turbines Henrik Brun Hansen and Peter Windfeld Rasmussen
e - Galvanic Cell 1. Voltage Sources 1.1 Polymer Electrolyte Membrane (PEM) Fuel Cell
 Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
LINEAR ALGEBRA. and VECTOR GEOMETRY Volume 2 of 2 September 2014 edition
 LINEAR ALGEBRA and VECTOR GEOMETRY Volume 2 of 2 September 2014 edition Because the book is so large, the entire Linear Algebra course has been split into two olumes. Grant Skene for Grant s Tutoring (www.grantstutoring.com)
LINEAR ALGEBRA and VECTOR GEOMETRY Volume 2 of 2 September 2014 edition Because the book is so large, the entire Linear Algebra course has been split into two olumes. Grant Skene for Grant s Tutoring (www.grantstutoring.com)
LECTURE NOTE THERMODYNAMICS (GEC 221)
 LETURE NOTE ON THERMODYNAMIS (GE ) Thermodynamics is the branch of science that treats the arious phenomena of energy and related properties of matter especially the relationship between heat, work and
LETURE NOTE ON THERMODYNAMIS (GE ) Thermodynamics is the branch of science that treats the arious phenomena of energy and related properties of matter especially the relationship between heat, work and
Parameters Identification of Equivalent Circuit Diagrams for Li-Ion Batteries
 Parameters Identification of Equialent Circuit Diagrams for Li-Ion eries Ahmad ahmoun, Helmuth Biechl Uniersity of Applied ciences Kempten Ahmad.ahmoun@stud.fh-empten.de, biechl@fh-empten.de Abstract-eries
Parameters Identification of Equialent Circuit Diagrams for Li-Ion eries Ahmad ahmoun, Helmuth Biechl Uniersity of Applied ciences Kempten Ahmad.ahmoun@stud.fh-empten.de, biechl@fh-empten.de Abstract-eries
Relativity in Classical Mechanics: Momentum, Energy and the Third Law
 Relatiity in Classical Mechanics: Momentum, Energy and the Third Law Roberto Assumpção, PUC-Minas, Poços de Caldas- MG 37701-355, Brasil assumpcao@pucpcaldas.br Abstract Most of the logical objections
Relatiity in Classical Mechanics: Momentum, Energy and the Third Law Roberto Assumpção, PUC-Minas, Poços de Caldas- MG 37701-355, Brasil assumpcao@pucpcaldas.br Abstract Most of the logical objections
E : Ground-penetrating radar (GPR)
 Geophysics 3 March 009 E : Ground-penetrating radar (GPR) The EM methods in section D use low frequency signals that trael in the Earth by diffusion. These methods can image resistiity of the Earth on
Geophysics 3 March 009 E : Ground-penetrating radar (GPR) The EM methods in section D use low frequency signals that trael in the Earth by diffusion. These methods can image resistiity of the Earth on
Chem 4521 Kinetic Theory of Gases PhET Simulation
 Chem 451 Kinetic Theory of Gases PhET Simulation http://phet.colorado.edu/get_phet/simlauncher.php The discussion in the first lectures centered on the ideal gas equation of state and the modifications
Chem 451 Kinetic Theory of Gases PhET Simulation http://phet.colorado.edu/get_phet/simlauncher.php The discussion in the first lectures centered on the ideal gas equation of state and the modifications
A possible mechanism to explain wave-particle duality L D HOWE No current affiliation PACS Numbers: r, w, k
 A possible mechanism to explain wae-particle duality L D HOWE No current affiliation PACS Numbers: 0.50.-r, 03.65.-w, 05.60.-k Abstract The relationship between light speed energy and the kinetic energy
A possible mechanism to explain wae-particle duality L D HOWE No current affiliation PACS Numbers: 0.50.-r, 03.65.-w, 05.60.-k Abstract The relationship between light speed energy and the kinetic energy
Prof. Mario L. Ferrari
 Sustainable Energy Mod.1: Fuel Cells & Distributed Generation Systems Dr. Ing. Mario L. Ferrari Thermochemical Power Group (TPG) - DiMSET University of Genoa, Italy Lesson II Lesson II: fuel cells (electrochemistry)
Sustainable Energy Mod.1: Fuel Cells & Distributed Generation Systems Dr. Ing. Mario L. Ferrari Thermochemical Power Group (TPG) - DiMSET University of Genoa, Italy Lesson II Lesson II: fuel cells (electrochemistry)
DISCLAIMER. Portions of this document may be illegible in electronic image products. Images are produced from the best available original document.
 ; i i : L4 0 t DSCLAMER Portions of this document may be illegible in electronic image products. mages are produced from the best available original document. EVALUATON OF THE HUMDFCATON REQTJREMENTS OF
; i i : L4 0 t DSCLAMER Portions of this document may be illegible in electronic image products. mages are produced from the best available original document. EVALUATON OF THE HUMDFCATON REQTJREMENTS OF
Section 6: PRISMATIC BEAMS. Beam Theory
 Beam Theory There are two types of beam theory aailable to craft beam element formulations from. They are Bernoulli-Euler beam theory Timoshenko beam theory One learns the details of Bernoulli-Euler beam
Beam Theory There are two types of beam theory aailable to craft beam element formulations from. They are Bernoulli-Euler beam theory Timoshenko beam theory One learns the details of Bernoulli-Euler beam
Fuel Cell System Model: Auxiliary Components
 2 Fuel Cell System Model: Auxiliary Components Models developed specifically for control studies have certain characteristics. Important characteristics such as dynamic (transient) effects are included
2 Fuel Cell System Model: Auxiliary Components Models developed specifically for control studies have certain characteristics. Important characteristics such as dynamic (transient) effects are included
MOTION OF FALLING OBJECTS WITH RESISTANCE
 DOING PHYSICS WIH MALAB MECHANICS MOION OF FALLING OBJECS WIH RESISANCE Ian Cooper School of Physics, Uniersity of Sydney ian.cooper@sydney.edu.au DOWNLOAD DIRECORY FOR MALAB SCRIPS mec_fr_mg_b.m Computation
DOING PHYSICS WIH MALAB MECHANICS MOION OF FALLING OBJECS WIH RESISANCE Ian Cooper School of Physics, Uniersity of Sydney ian.cooper@sydney.edu.au DOWNLOAD DIRECORY FOR MALAB SCRIPS mec_fr_mg_b.m Computation
Quantum confined materials
 Quantum confined materials Quantum Wells Quantum wells structures can be fabricated by seeral methods in semiconductors alloys. A quantum well is fabricated by depositing ery thin layers of different materials
Quantum confined materials Quantum Wells Quantum wells structures can be fabricated by seeral methods in semiconductors alloys. A quantum well is fabricated by depositing ery thin layers of different materials
Development of Bifunctional Electrodes for Closed-loop Fuel Cell Applications. Pfaffenwaldring 6, Stuttgart, Germany
 Development of Bifunctional Electrodes for Closed-loop Fuel Cell Applications S. Altmann a,b, T. Kaz b, K. A. Friedrich a,b a Institute of Thermodynamics and Thermal Engineering, University Stuttgart,
Development of Bifunctional Electrodes for Closed-loop Fuel Cell Applications S. Altmann a,b, T. Kaz b, K. A. Friedrich a,b a Institute of Thermodynamics and Thermal Engineering, University Stuttgart,
Online Companion to Pricing Services Subject to Congestion: Charge Per-Use Fees or Sell Subscriptions?
 Online Companion to Pricing Serices Subject to Congestion: Charge Per-Use Fees or Sell Subscriptions? Gérard P. Cachon Pnina Feldman Operations and Information Management, The Wharton School, Uniersity
Online Companion to Pricing Serices Subject to Congestion: Charge Per-Use Fees or Sell Subscriptions? Gérard P. Cachon Pnina Feldman Operations and Information Management, The Wharton School, Uniersity
The Pennsylvania State University. The Graduate School. College of Engineering A COMPUTATIONAL MODEL FOR ASSESSING IMPACT OF INTERFACIAL
 The Pennsylvania State University The Graduate School College of Engineering A COMPUTATIONAL MODEL FOR ASSESSING IMPACT OF INTERFACIAL MORPHOLOGY ON POLYMER ELECTROLYTE FUEL CELL PERFORMANCE A Thesis in
The Pennsylvania State University The Graduate School College of Engineering A COMPUTATIONAL MODEL FOR ASSESSING IMPACT OF INTERFACIAL MORPHOLOGY ON POLYMER ELECTROLYTE FUEL CELL PERFORMANCE A Thesis in
