MOLECULAR SPECTROSCOPY: -Molecular Absorption (UV-Vis and IR) -Molecular Emission
|
|
|
- Erica Peters
- 5 years ago
- Views:
Transcription
1 MOLECULAR SPECTROSCOPY: -Molecular Absorption (UV-Vis and IR) -Molecular Emission
2 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry
3 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation find widespread application for the quantitative determination of a large variety of species. Ordinarily, the concentration of an absorbing analyte is linearly related to absorbance as given by Beer's law: Beer s Law: A = -logt = logp 0 /P = εbc A = absorbance ε = molar absorptivity [M -1 cm -1 ] c = concentration [M] P 0 = incident power P = transmitted power (after passing through sample)
4 Transmittance and absorbance, as defined in Table 13-1, cannot normally be measured in the laboratory because the analyte solution must be held in a transparent container or cell.
5 Reflection occurs at the two air-wall interfaces as well as at the two wall-solution interfaces. The resulting beam attenuation is substantial, as we demonstrated in Example 6-2, where it was shown that about 8.5% of a beam of yellow light is lost by reflection in passing through a glass cell containing water. In addition, attenuation of a beam may occur as a result of scattering by large molecules and sometimes from absorption by the container walls. FIGURE 13-1 Reflection and scattering losses with a solution contained in a typical glass cell. Losses by reflection can occur at all the boundaries that separate the diffferent materials. In this example, the light passes through the air-glass, glasssolution, solution-glass, and glass-air interfaces.
6 Measurement of Transmittance and Absorbance: The power of the beam transmitted by the analyte solution is usually compared with the power of the beam transmitted by an identical cell containing only solvent. An experimental transmittance and absorbance are then obtained with the equations. P 0 and P refers to the power of radiation after it has passed through the solvent and the analyte. T Psolution = = Psolvent P P 0 A Psolvent P = log log 0 Psolution P
7 Derivation of Beer s Law: Sample cell with absorbing molecules P 0 P b=0 b=b db P P-dP
8 dp P Incremental power lost power in; i.e., increase power in, increase power absorbed dp db Longer pathlength, greater number of molecules in incremental slice and more power absorbed Therefore, dp Pdb dp = -kpdb k = proportionality constant (function of λ, c) negative sign: because power is lost (i.e., absorbed) Rearrange: dp P = kdb
9 Integrate: P P 1 P dp b = k db 0 0 InP InP0 = kb ( k)( 0) In P P 0 = kb Factor out concentration part of k: k = k c In P P 0 = k" bc Convert fraction (remove sign) and change In to log: P0 1 log = k:" bc P P0 A = log = εbc P (1/2.303)k = ε
10 13B-1 Application of Beer s Law to Mixtures Beer s law applies to a medium containing more than one kind of absorbing substance. Provided there is no interaction among the various species, the total absorbance for a multicomponent system is given by A total = A 1 + A A n =ε 1 bc 1 + ε 2 bc ε n bc n where, the subscripts refer to absorbing components 1, 2,, n.
11 Assumptions in derivation of Beer s Law incident radiation is Monochromatic(all molecules absorb light of one λ) Absorbing molecules act independently of one another i.e, low c Pathlength is uniform (all rays travel the same distance in sample) No scattering Absorbing medium is optically homogeneous Incident beam is not large enough to cause saturation All rays should be parallel to each other and perpendicular to surface of medium.
12 13B-2 Limitations (deviations) to Beer s Law * Real Limitations High concentration > 0.01 M the extent of solute-solvent interactions, solute-solute interactions, or hydrogen bonding can affect the analyte environment and its absorptivity. * Chemical Deviations Analyte dissociates, associates or reacts to give molecule with different absorption characteristics (e.g., ph-dependent indicators) Example 13-1 * Instrumental Deviations Polychromatic radiation Stray Radiation
13 Instrumental Deviations : In the presence of Polychromatic radiation (i.e., light of more than one λ) A meas = log ( p' + P" ) 0 0 ( ) P' + P" Where P and P are powers for λ and λ, respectively - Negative deviation = lower absorbance than predicted because higher transmittance - Higher T because molecules don t absorb one λ as well as other The absorber has the indicated molar absorptivities at the two wavelengths λ' and
14 The effect of polychromatic radiation on Beer's law. In the spectrum on the left, the absorptivity of the analyte is nearly constant over band A from the source. Note in the Beer's law plot at the bottom that using band A gives a linear relationship. In the spectrum, band B corresponds to a region where the absorptivity shows substantial changes. In the lower plot, note the dramatic deviation from Beer's law that results. To avoid deviations, it is advisable to select a wavelength band near the wavelength of maximum absorption where the analyte absorptivity changes little with wavelength
15 Instrumental Deviations : In the presence of Stray radiation the radiation exiting from a monochromator is usually contaminated with small amounts of scattered or stray radiation. This radiation, commonlv called stray light, is defined as radiation from the instrument that is outside the nominal wavelength band chosen for the determination. This stray radiation often is the result of scattering and reflection off the surfaces of gratings, lenses or mirrors. filters, and windows. The wavelength of stray radiation often differs greatly from that of the principal radiation and, in addition, the radiation may not have passed through the sample.
16 FIGURE 13-6 Apparent deviation from Beer's law brought about by various amounts of stray radiation. Note that the absorbance begins to level off with concentration at high stray-light levels. Stray light always limitsthe maximum absorbance that can be obtained because when the absorbance is high, the radiant power transmitted through Instrumental Deviations : In the presence of Stray radiation ( p' P ) 0 + s A' = log ( P' + Ps) Ps = power from stray radiation Extra light hits detector higher T; lower A
17 13.C Effect of Slit Width on Absorbance Measurements Figure 13-8 illustrates the loss of detail that occurs when slit widths are increased from small values on the left to larger values in the middle and right. In this example, the absorption spectrum of benzene vapor was obtained at slit settings that provided effective bandwidths of 1.6, 4, and 10 nm. For qualitative studies, the loss of resolution that accompanies the use of wider slits is often important because the details of spectra are useful for identifying species. Narrow slits widths are required to resolve complex spectra
18 13.C Effect of Slit Width on Absorbance Measurements quantitative measurement of narrow absorption bands requires using narrow slit widths or, alternatively, very reproducible slit-width settings. Unfortunately, a decrease in slit width by a factor of 10 reduces the radiant power by a factor of 100 because the radiant power is proportional to the square of the slit width. There is thus a trade-off between resolution and signal-to-noise ratio. For quantitative measurements, slit width needs to be kept large enough
19 Instrumentation Light source λ - selection Sample container Detector Signal processing Light Sources (commercial instruments) D 2 lamp (UV: nm) W lamp (vis: nm)
20 Deuterium and Hydrogen Lamps Tungsten Filament Lamps
21 λ - selection (monochromators) Sample holders Cuvettes (b = 1 cm typically) Glass (Vis) Fused silica (UV 350 nm) Detectors Photodiodes PMTs
22 Types of Instruments Single beam Place cuvette with blank (i.e., solvent) in instrument and take a reading 100% T Replace cuvette with sample and take reading % T for analyte (from which absorbance is calc d) Double beam (most commercial instruments) Light is split and directed towards both reference cell (blank) and sample cell Two detectors; electronics measure ratio (i.e., measure/calculate absorbance) Advantages: Compensates for fluctuations in source and drift in detector Better design for continuous recording of spectra
23 Single beam double beam in space double beam in time
24 Multichannel Instruments Photodiode array detectors used (multichannel detector, can measure all wavelengths dispersed by grating simultaneously). Advantage: scan spectrum very quickly snapshot < 1 sec. Powerful tool for studies of transient intermediates in moderately fast reactions. Useful for kinetic studies. Useful for qualitative and quantitative determination of the components exiting from a liquid chromatographic column.
25 Multichannel Instruments
26 Instruments for the Visible Region.
27 FIGURE Schematic of a typical manual double-beam spectrophotometer for the UVvisible region.
28 FIGURE Schematic of the VarianCary 100 double-beam spectrophotometer for the UV-visible region
29 FIGURE A multichannel diode-array spectrophotometer, the Agilent Technologies 8453.
30 Chapter 14: Applications of UV-Vis Molecular Absorption Spectrometry Characteristics of UV/Vis Methods: -Wide applicability to organic and inorganic systems -Sensitivities to 10-4 to 10-7 M -Moderate to high selectivity -Good accuracy, about 1-3% relative uncertainty -Ease and convenient data acquisition
31 UV/Vis Absorbance: Results from excitation of bonding electrons. So, can correlate wavelength of absorption peaks with types of bonds. Types of electronic transitions relative to UV/Vis absorbance: 1) p, s, and n electrons 2) d and f electrons 3) Charge-transfer electrons Absorbing Electrons: Electrons that contribute to absorbance in organic molecules: 1) Those that directly participate in bond formation between atoms and are associated with more than one atom. 2) Nonbonding or unshared outer electrons largely localized about atoms like oxygen, sulfur, nitrogen, halogens.
32 - Most applications of absorption spectroscopy to organic molecules are based on n or p to p * transition which require unsaturated functional groups. Source: Skoog, Holler, and Nieman, Principles of Instrumental Analysis, 5 th edition, Saunders College Publishing.
33 Effect of solvents in reducing fine structure in absorbance spectra Source: Skoog, Holler, and Nieman, Principles of Instrumental Analysis, 5 th edition, Saunders College Publishing.
34 - If chromophores are separated by more than one single bond, absorbance of multichromophores in a single organic molecule are approximately additive. Source: Skoog, Holler, and Nieman, Principles of Instrumental Analysis, 5 th edition, Saunders College Publishing.
35 Absorption of d and f electrons: -Most transition-metal ions absorb in UV/Vis. -Lanthanides and actinides have narrow, welldefined, characteristic absorption peaks. - Ions or complexes from first and second transition series tend to absorb in all oxidation states, tend to be broad bands that are dependent on chemical environment. Sample spectra from lanthanide ions Sample spectra from transition-metal ions Source: Skoog, Holler, and Nieman, Principles of Instrumental Analysis, 5 th edition, Saunders College Publishing.
36 Effect of Solvent on Absorption Spectra - Polar solvents eliminate fine detail. Nonpolar solvent keep spectral details more similar to gas phase measurements. Source: Skoog, Holler, and Nieman, Principles of Instrumental Analysis, 5 th edition, Saunders College Publishing.
37 Absorbance Spectra of Mixtures: - The absorbance of a solution at a given wavelength is the sum of the absorbances of the individual components. To analyze the mixture, molar absorptivities for M and N are first determined at wavelengths λ1, and λ2 with sufficient concentrations of the two standard solutions to be sure that Beer's law is obeyed over an absorbance range that encompasses the absorbance of the sample. Note that the wavelengths selected are ones at which the molar absorptivities of the two components differ significantly. Thus, at λ1 the molar absorptivity of component M is much larger than that for component N. The greatest accuracy is obtained by choosing wavelengths at which the differences in molar absorptivities are large. Source: Skoog, Holler, and Nieman, Principles of Instrumental Analysis, 5 th edition, Saunders College Publishing.
38 Photometric Titration: -Plot absorbance (corrected for change in volume) as function of volume of titrant. Analyte, titrant, product, or indicator must absorb. High accuracy since multiple measurements are pooled. - Photometric titrations are ordinarily performed with a spectrophotometer or a photometer that has been modified so that the titration vessel is held stationary in the light path Source: Skoog, Holler, and Nieman, Principles of Instrumental Analysis, 5 th edition, Saunders College Publishing.
39 Chapter 15 Molecular Luminescence Spectrometry Two types of Luminescence methods are: 1) Photoluminescence, Light is directed onto a sample, where it is absorbed and imparts excess energy into the material in a process called "photo-excitation." One way this excess energy can be dissipated by the sample is through the emission of light, or luminescence. (i) fluorescence (ii) phosphorescence 2) Chemiluminescence, based on an excited species formed by a chemical reaction. - no excitation source In each, molecules of the analyte are excited to give a species whose emission spectrum provides information for qualitative or quantitative analysis. Luminescence methods are used as detectors for HPLC & CE.
40 Theory Of Fluorescence And Phosphorescence Types of Fluorescence: Resonance Fluorescence (emitted λ = excitation λ; e.g., AF) Stokes shift (emitted λ > excitation λ; e.g., molecular fluorescence) Electron spin and excited states: Excited, paired = excited singlet state fluorescence Excited, unpaired = excited triplet state phosphorescence FIGURE 15-1 Electronic spin states of molecules. In (a) the ground electronic state is shown. In the lowest energy or ground state, the spins are always paired, and the state is said to be a singlet state. In (b) and (c), excited electronic states are shown. If the spins remain paired in the excited state, the molecule is in an excited singlet state (b). If the spins become unpaired, the molecule is in an excited triplet state (c).
41 Term Symbols Example: Na ground state 1s 2 2s 2 2p 6 3s 1 s=1/2, 2S+1=2, ground state doublet s electron written 3( 2 S) Two spin states of equal energy (up/down) Na 1 st excited state 1s 2 2s 2 2p 6 3p 1 Doublet written 3( 2 P) BUT two spins states? J (total ang. mom)=l+s or L-S now 1s 2 2s 2 2p 6 3s 1 = 3( 2 P 1/2 ) and 3( 2 P 3/2 ) Term Symbol 2S+1 L J Na 3p 3s fluorescence two lines : at nm ( 2 P 3/2 ) and at nm ( 2 P 1/2 )
42 Energy-level diagram for typical photoluminescent system
43 Deactivation Processes: Fluorescence: absorption of photon, short-lived excited state (singlet), emission of photon. Phosphorescence: absorption of photon, long-lived excited state (triplet), emission of photon. Vibration Relaxation Internal Conversion External Conversion Intersystem Crossing Deactivation process by which an excited molecule returns to the ground state by minimizing lifetime of electronic state is preferred (i.e., the deactivation process with the faster rate constant will predominate) Radiationless Deactivation Without emission of a photon (i.e., without radiation)
44 Terms From Energy-level Diagram Term: Absorption Effect: Excitation Process: Analyte molecule absorbs photon (very fast ~ s); electron is promoted to higher energy state. Slightly different wavelength excitation into different vibrational energy levels. Term: Vibrational Relaxation Effect: Radiationless Deactivation Process: Collisions of excited state analyte molecules with other molecules loss of excess vibrational energy and relaxation to lower vibrational levels (within the excited electronic state) Term: Internal conversion Effect:RadiationlessDeactivation Process: Molecule passes to a lower energy state vibrational energy levels of the two electronic states overlap (see diagram) and molecules passes from one electronic state to the other. Term: Intersystem Crossing Effect: Radiationless Deactivation Process: Spin of electron is reversed leading to change from singlet to triplet state. Occurs more readily if vibrational levels of the two states overlap. Common in molecules with heavy atoms (e.g., I or Br) Term: External Conversion Effect: Radiationless Deactivation Process: Collisions of excited state analyte molecules with other molecules molecule relaxes to the ground state without emission of a photon.
45 Term: Fluorescence Effect: Raditive Deactivation Process: Emission of a photon via a singlet to singlet transition (short lived excited state ~ s). Term: Phosphorescence Effect: Radiative Deactivation Process: Emission of a photon via a triplet to single transition (long lived excited state ~ s)
46 Quantum Efficiency or Quantum Yield: The quantum yield or quantum efficiency for fluorescence or phosphorescence is the ratio of the number of molecules that luminesce to the total number of excited molecule. It gives a measure of how efficient a fluorophore (i.e., fluorescing molecule) is. A quantum yield = 1 means that every excited molecules deactivates by emitting a photon such a molecule is considered a very good fluorophore. We can express quantum yield as a function of rate constants Quantum Yield, φ = φ = kf k + k + k + k + k + k f i ec ic pd d total # luminescing molecules total # of excited molecules [ k = rate constant]
47 What Factors Affect fluorescence? 1) Excitation wavelength Short λs break bonds, increase k pre-dis and k dis σ σ photochemical decomposition (seldom observed) Transitions mostly occur from n π* or Low energy π π* (aromatic, most intense fluorescence) 2) Molecular structure Conjugated double bond structures exhibit fluorescence. Most unsubstituted aromatic hydrocarbons fluoresce in solution, the quantum efficiency usually increasing with the number of rings and their degree of condensation. The simple heterocyclics such as pyridine, furan, thiophene, and pyrrole do not fluoresce; heterocyclics fused to other rings fluoresce. Heteroatom increases ISC then φ f decreases. (pyridine-quinoline)
48 3) Structural rigidity fluorescence is particularly favored in molecules with rigid structures.(e.g., fluorene vs biphenyl). If flexibility increases, φ f decreases. Lack of rigidity in a molecule probably causes an enhanced internal conversion rate and a consequent increase in the likelihood for radiationless deactivation. 4) Temperature and Solvent effect The quantum efficiency of fluorescence in most molecules decreases with increasing temperature because the increased frequency of collisions at elevated temperatures improves the probability for deactivation by external conversion increased fluorescence with increased viscosity (decreased likelihood of external conversion radiationless deactivation) The fluorescence of a molecule is decreased by solvents containing heavy atoms or other solutes with such atoms in their structure: Heavy atoms such as I, Br, Th increases ISC, as a consequence φ f decreases. Compounds containing heavy atoms are frequently incorporated into solvents when enhanced phosphorescence is desired.
49 5) Effect of ph on Fluorescence The fluorescence of an aromatic compound with acidic or basic ring substituents is usually ph dependent. Both the wavelength and the emission intensity are likely to be different for the protonated and unprotonated forms of the compound. Increased resonance structures (protonation or deprotonation) stable excited state and greater quantum yield analytical procedures based on fluorescence frequently require close control of ph. 6) Dissolved oxygen; Presence of dissolved oxygen reduces fluorescence yield due to oxidation of the fluorescent specie. Also, paramagnetic properties of the oxygen promotes ISC and transition to triplet state.
50 Fluorescence Intensity And Concentration Of Analyte The power of fluorescence emission F is proportional to the radiant power of the excitation beam that is absorbed by the system. fluorescence intensity depends linearly on concentration. F = Kc Deviations occur at high concentrations Self absorption: neighboring molecule absorbs emitted photon from other molecule happens if there is overlap between the excitation and emission spectra Quenching: collisions of excited state molecule with other excited state molecules radiationless deactivation Photobleaching, Photochemical Decomposition: Excited state molecule absorbs another photon and is destroyed destroyed excited state molecule is not able to emit fluorescent photon
51 Excitation And Emission Spectra Excitation spectrum: - Emission wavelength is fixed; excitation wavelength is scanned - Monochromator or filters selected to allow only one λ of fluorescent light to pass through to the detector. -Excitation wavelength is varied at each excitation λ increment, fluorescent photons at the fixed emission λ are collected. - The emission intensity (i.e., the number of fluorescent photons collected) at each λ increment varies as the excitation λ comes closer to or goes further from the λ of maximum absorption this is why an excitation spectrum looks like an absorption spectrum. anthracene in alcohol Emission spectrum: - Excitation wavelength is fixed; emission wavelength is scanned - Monochromator or filter is selected to allow only one λ of excitation light to pass onto the sample. - Emission λ is varied fluorescent photons are collected at each incremental emission λ. - The emission intensity (i.e., the number of fluorescent photons collected) at each λ increment varies as the emission λ is changed. - Spectrum shows at what λ the fluorescence intensity is a maximum for a given excitation λ.
52 INSTRUMENTATION Sources Low pressure Hg lamp (254, 302, 313, 546, 578 nm) line source Xe lamp ( nm) continum source Lasers (tunable dye lasers) Filter/monochromator Isolate excitation λ Scan excitation λ Isolate emission λ from excitation λ Scan emission λ Both cylindrical and rectangular cells fabricated of glass or silica are employed for fluorescence measurements. Care must be taken in the design of the cell compartment to reduce the amount of scattered radiation reaching the detector. Baffles are often introduced into the compartment for this purpose. Even more than in absorbance measurements, it is important to avoid fingerprints on cells because skin oils often fluoresce, Detector Usually PMT: very low light levels are measured. Transducers are sometimes cooled to improve signal-to-noise ratios. Charge-transfer devices such as charge-coupled devices (CCDs), are also used for spectrofluorometry. This type of transducer permits the rapid recording of both excitation and emission spectra and is particularly useful in chromatography and electrophoresis.
53
54 Fluorometer: Filter fluorometers provide a relatively simple, lowcost way of performing quantitative fluorescence analyses.
55 Spectrofluorometer Several instrument manufacturers offer spectrofluorometers capable of providing both excitation and emission spectra. The optical design of these instruments employs two grating monochromators. Radiation from the excitation monochromator is split, part passing to a reference photomultiplier and part to the sample. The resulting fluorescence radiation, after dispersion by the emission monochromator is detected by a second photomultiplier.
56 Phosphorescence Instrumentation Similar in design to the fluorometers and spectrofluorometers except that two additional components are required. The first is a device that alternately irradiates the sample and, after a suitable time delay, measures the intensity of phosphorescence. The time delay is required to differentiate between long-lived phosphorescence emission and short-lived fluorescence emission, both of which would originate from the same sample. Both mechanical and electronic devices are used, and many commercial fluorescence instruments have accessories for phosphorescence measurements. Many of the current instruments use a gated scheme for the delay. A pulsed xenon arc lamp is often used to excite the sample. After a delay time, specified by the user, the dataacquisition system is activated to obtain the phosphorescence signal. Often, the signal is integrated during this period when the lamp is off and fluorescence has decayed to a very small value. The second new component is needed because phosphorescence measurements are usually performed at liquid nitrogen tcmperature in a rigid medium to minimize collisional deactivation of the long-lived triplet state. Usually, a Dewar flask with quartz windows, as shown in Figure 15-13, is a part of a phosphorimeter. At the temperature used, the analyte exists as a solute in a glass or solid solvent. A common solvent for this purpose is a mixture of diethylether, pentane, and ethanol.
57
58 Chapter An Introduction and Application to Infrared Spectrometry The infrared region of the spectrum encompasses radiation with wavenumbers ranging from about 12,800 to 10 cm -1 or wavelengths from 0.78 to 1000 µm. The infrared spectrum is divided into near-, mid-, and farinfrared radiation.
59 Infrared Spectroscopy A) Introduction A) 1.)Infrared (IR) spectroscopy: based on IR absorption by molecules as undergo vibrational and rotational transitions. Absorption of radiation in this region by a typical organic molecule results in the excitation of vibrational, rotational and bending modes, while the molecule itself remains in its electronic ground state. rotational transitions Potential Energy (E) Vibrational transitions Interatomic Distance (r) Potential energy resembles classic Harmonic Oscillator
60 2.) IR radiation is in the range of 12, cm -1 or λ = µm - rotational transitions have small energy differences 100 cm -1, λ > 100 µm - vibrational transitions occur at higher energies - rotational and vibrational transitions often occur together 3.) Typical IR spectrum for Organic Molecule % Transmittance Wavenumber (cm -1 )
61 E=hν = hc/λ Wide Range of Types of Electromagnetic Radiation in nature. 1. Only a small fraction ( nm is visible light). 2. The complete variety of electromagnetic radiation is used throughout spectroscopy. 3. Different energies allow monitoring of different types of interactions with matter.
62 3.) Typical IR spectrum for Organic Molecule - many more bands then in UV-vis, fluorescence or phosphorescence - bands are also much sharper - pattern is distinct for given molecule except for optical isomers - good qualitative tool can be used for compound identification group analysis - also quantitative tool intensity of bands related to amount of compound present - spectra usually shown as percent transmittance (instead of absorbance) vs. wavenumber (instead of λ) for convenience Hexane Hexene Hexyne
63 B) Theory of IR Absorption 1.) Molecular Vibrations i.) Harmonic Oscillator Model: - approximate representation of atomic stretching - two masses attached by a spring E = ½ ky 2 where: y is spring displacement k is spring constant
64 Vibrational frequency given by: ν = 1/2 π k / m where: ν : frequency k: force constant (measure of bond stiffness) µ: reduced mass m 1 m 2 /m 1 +m 2 If know ν and atoms in bond, can get k: Single bonds: k ~ 3x10 2 to 8 x10 2 N/m (Avg ~ 5x10 2 ) double and triple bonds ~ 2x and 3x k for single bond. ν k So, vibration ν occur in order: single < double < triple
65 ii.) Anharmonic oscillation: - harmonic oscillation model good at low energy levels (ν 0, ν 1, ν 2, ) - not good at high energy levels due to atomic repulsion & attraction as atoms approach, coulombic repulsion force adds to the bond force making energy increase greater then harmonic as atoms separate, approach dissociation energy and the harmonic function rises quicker Harmonic oscillation Anharmonic oscillation Because of anharmonics: at low E, ν =±2, ±3 are observed which cause the appearance of overtone lines at frequencies at ~ 2-3 times the fundamental frequency. Normally ν = ± 1
66 iii.) Types of Molecular Vibrations Bond Stretching Bond Bending symmetric In-plane rocking asymmetric In-plane scissoring Out-of-plane wagging Out-of-plane twisting
67 symmetric asymmetric In-plane scissoring Out-of-plane twisting In-plane rocking Out-of-plane wagging
68 iv.) Number of Vibrational Modes: - for non-linear molecules, number of types of vibrations: 3N-6 - for linear molecules, number of types of vibrations: 3N-5 - why so many peaks in IR spectra - observed vibration can be less then predicted because symmetry ( no change in dipole) energies of vibration are identical absorption intensity too low frequency beyond range of instrument Examples: 1) HCl: 3(2)-5 = 1 mode 2) CO 2 : 3(3)-5 = 4 modes moving in-out of plane See web site for 3D animations of vibrational modes for a variety of molecules
69 v.) IR Active Vibrations: - In order for molecule to absorb IR radiation: vibration at same frequency as in light but also, must have a change in its net dipole moment as a result of the vibration Examples: 1) CO 2 : 3(3)-5 = 4 modes degenerate identical energy single IR peak δ - 2δ + δ - δ - 2δ + δ δ - 2δ + δ - 2δ + δ - δ - µ = 0; IR inactive µ > 0; IR active µ > 0; IR active µ > 0; IR active
70 Example 8: Calculate the absorption frequency for the C-H stretch with a force constant of k = 5.0x10 2 N/m.
71 C) Instrumentation 1.) Basic Design - normal IR instrument similar to UV-vis - main differences are light source & detector
72 i.) Light Source: - must produce IR radiation - can t use glass since absorbs IR radiation - several possible types a) Nernst Glower Zr, Ce, Th V - rare earth metal oxides (Zr, Ce, Th) heated electrically - apply current to cylinder, has resistance to current flow generates heat (1200 o 2200 o C). - causes light production similar to blackbody radiation - range of use ~ ,000cm -1 - need good current control or overheats and damaged b) Globar - similar to Nernst Glower but uses silicon carbide rod instead of rare earth oxides - similar usable range
73 c) Incandescent Wire Source - tightly wound nichrome or rodium wire that is electrically heated - same principal as Nernst Glower - lower intensity then Nernst Glower or Globar, but longer lifetime d) CO 2 Laser - CO 2 laser gas mixture consists of 70% He, 15% CO 2, and 15% N 2 - a voltage is placed across the gas, exciting N 2 to lowest vibrational levels. - the excited N 2 populate the asymmetric vibrational states in the CO 2 through collisions. - infrared output of the laser is the result of transitions between rotational states of the CO 2 molecule of the first asymmetric vibrational mode to rotational states of both the first symmetric stretch mode and the second bending mode - gives off band of ~ 100 cm -1 s in range of cm -1 - small range but can choose which band used & many compounds have IR absorbance in this region - much more intense than Blackbody sources e) Others - mercury arc (λ > 50 µm) (far IR) - tungsten lamp ( ,800cm -1 ) (near IR)
74 ii.) Detectors: - two main types in common IR instruments a) Thermal Detectors 1.) Thermocouple - two pieces of dissimilar metals fused together at the ends - when heated, metals heat at different rates - potential difference is created between two metals that varies with their difference in temperature - usually made with blackened surface (to improve heat absorption) - placed in evacuated tube with window transparent to IR (not glass or quartz) - IR hits and heats one of the two wires. - can use several thermocouples to increase sensitivity. hν metal 1 metal V IR transparent material (NaCl)
75 2.) Bolometer - strips of metal (Pt, Ni) or semiconductor that has a large change in resistance to current with temperature. - as light is absorbed by blackened surface, resistance increases and current decreases - very sensitive i hν A b) Photoconducting Detectors - thin film of semiconductor (ex. PbS) on a nonconducting glass surface and sealed in a vacuum. - absorption of light by semiconductor moves from non-conducting to conducting state - decrease in resistance increase in current hν - range: 10, cm -1 vacuum at room temperature semiconductor glass Transparent to IR
76 c) Pyroelectric Detectors - pyroelectric (ceramic, lithium tantalate) material get polarized (separation of (+) and (-) charges) in presence of electric field. - temperature dependent polarization - measure degree of polarization related to temperature of crystal - fast response, good for FTIR
77 iii.) Other Components a.) Sample Cell - must be made of IR transparent material (KBr pellets or NaCl) Liguid Sample Holder NaCl plates b.) monochromator - reflective grating is common - can t use glass prism, since absorbs IR
78 iv.) Overall Instrument Design -Need chopper to discriminate source light from background IR radiation -Monochromator after sample cell -Not done in UV-Vis since letting in all hν to sample may cause photdegradation (too much energy) -IR lower energy -Advantage that allows monochromator to be used to screen out more background IR light -Problems: -Source weak, need long scans -Detector response slow rounded peaks
79 v.) Fourier Transfer IR (FTIR) alternative to Normal IR - Based on Michelson Interferometer Principal: 1) light from source is split by central mirror into 2 beams of equal intensity 2) beams go to two other mirrors, reflected by central mirror, recombine and pass through sample to detector 3) two side mirrors. One fixed and other movable a) move second mirror, light in two-paths travel different distances before recombined b) constructive & destructive interference c) as mirror is moved, get a change in signal
80 Light enters the spectrometer and is split by the beam splitter. The figure above shows what is referred to as the Michelson interferometer
81 Remember Destructive Interference can be created when two waves from the same source travel different paths to get to a point. This may cause a difference in the phase between the two waves. If the paths differ by an integer multiple of a wavelength, the waves will also be in phase. If the waves differ by an odd multiple of half a wave then the waves will be 180 degrees out of phase and cancel out.
82 - observe a plot of Intensity vs. Distance (interferograms) - convert to plot of Intensity vs. Frequency by doing a Fourier Transform - resolution ν = 1/ δ (interval of distance traveled by mirror)
83 Advantages of FTIR compared to Normal IR: 1) much faster, seconds vs. minutes 2) use signal averaging to increase signal-to-noise (S/N) increase S / N number scans 3) higher inherent S/N no slits, less optical equipment, higher light intensity 4) high resolution (<0.1 cm -1 ) Disadvantages of FTIR compared to Normal IR: 1) single-beam, requires collecting blank 2) can t use thermal detectors too slow In normal IR, scan through frequency range. In FTIR collect all frequencies at once.
84 Advantages of FTIR: Enhanced signal-to-noise Rapid scanning High resolution (<0.1 cm -1 ) Accurate and reproducible frequency determinations Larger energy throughput Free from problems of stray radiation
85 D) Application of IR 1.) Qualitative Analysis (Compound Identification) - main application - Use of IR, with NMR and MS, in late 1950 s revolutionized organic chemistry decreased the time to confirm compound identification fold i.) General Scheme 1) examine what functional groups are present by looking at group frequency region cm -1 to 1200 cm -1
86 ii.) Group Frequency Region - approximate frequency of many functional groups (C=O,C=C,C-H,O-H) can be calculated from atomic masses & force constants - positions changes a little with neighboring atoms, but often in same general region - serves as a good initial guide to compound identity, but not positive proof.
87 Abbreviated Table of Group Frequencies for Organic Groups Bond Type of Compound Frequency Range, cm -1 Intensity C-H Alkanes Strong C-H Alkenes H C C Medium strong C-H Alkynes C C H 3300 Strong C-H Aromatic rings Medium strong 0-H Monomeric alcohols, phenols Hydrogen-bonded alchohols, phenols Monomeric carboxylic acids Hydrogen-bonded carboxylic acids Variable Variable, sometimes broad Medium broad N-H Amines, amides medium C=C Alkenes Variable C=C Aromatic rings Variable C C Alkynes Variable C-N Amines, amides Strong C N Nitriles Strong C-O Alcohols, ethers,carboxylic acids, esters Strong C=O Aldehydes, ketones, carboxylic acids, esters Strong NO 2 Nitro compounds Strong
88 iii.) Fingerprint Region ( cm -1 ) - region of most single bond signals - many have similar frequencies, so affect each other & give pattern characteristics of overall skeletal structure of a compound - exact interpretation of this region of spectra seldom possible because of complexity - complexity uniqueness Fingerprint Region
89 iv.) Computer Searches - many modern instruments have reference IR spectra on file (~100,000 compounds) - matches based on location of strongest band, then 2 nd strongest band, etc overall skeletal structure of a compound - exact interpretation of this region of spectra seldom possible because of complexity - complexity uniqueness Bio-Rad SearchIT database of ~200,000 IR spectra
90 2.) Quantitative Analysis - not as good as UV/Vis in terms of accuracy and precision more complex spectra narrower bands (Beer s Law deviation) limitations of IR instruments (lower light throughput, weaker detectors) high background IR difficult to match reference and sample cells changes in ε (A=εbc) common - potential advantage is good selectivity, since so many compounds have different IR spectra one common application is determination of air contaminants. Contaminants Concn, ppm Found, ppm Relative error, % Carbon Monoxide Methylethyl ketone Methyl alcohol Ethylene oxide chloroform
91 Example 9: The spectrum is for a substance with an empirical formula of C 3 H 5 N. What is the compound? Aliphatic hydrogens Nitrile or alkyne group No aromatics One or more alkane groups
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry
 Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Chapter 15 Molecular Luminescence Spectrometry
 Chapter 15 Molecular Luminescence Spectrometry Two types of Luminescence methods are: 1) Photoluminescence, Light is directed onto a sample, where it is absorbed and imparts excess energy into the material
Chapter 15 Molecular Luminescence Spectrometry Two types of Luminescence methods are: 1) Photoluminescence, Light is directed onto a sample, where it is absorbed and imparts excess energy into the material
25 Instruments for Optical Spectrometry
 25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Spectroscopy. Page 1 of 8 L.Pillay (2012)
 Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Molecular Luminescence Spectroscopy
 Molecular Luminescence Spectroscopy In Molecular Luminescence Spectrometry ( MLS ), molecules of the analyte in solution are excited to give a species whose emission spectrum provides information for qualitative
Molecular Luminescence Spectroscopy In Molecular Luminescence Spectrometry ( MLS ), molecules of the analyte in solution are excited to give a species whose emission spectrum provides information for qualitative
10/2/2008. hc λ. νλ =c. proportional to frequency. Energy is inversely proportional to wavelength And is directly proportional to wavenumber
 CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
Introduction to Spectroscopic methods
 Introduction to Spectroscopic methods Spectroscopy: Study of interaction between light* and matter. Spectrometry: Implies a quantitative measurement of intensity. * More generally speaking electromagnetic
Introduction to Spectroscopic methods Spectroscopy: Study of interaction between light* and matter. Spectrometry: Implies a quantitative measurement of intensity. * More generally speaking electromagnetic
Chapter 12 Mass Spectrometry and Infrared Spectroscopy
 Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Reference literature. (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters )
 September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
Infra Red Spectroscopy
 CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
levels. The signal is either absorbance vibrational and rotational energy levels or percent transmittance of the analyte
 1 In this chapter, absorption by molecules, rather than atoms, is considered. Absorption in the ultraviolet and visible regions occurs due to electronic transitions from the ground state to excited state.
1 In this chapter, absorption by molecules, rather than atoms, is considered. Absorption in the ultraviolet and visible regions occurs due to electronic transitions from the ground state to excited state.
Application of IR Raman Spectroscopy
 Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
1901 Application of Spectrophotometry
 1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
2001 Spectrometers. Instrument Machinery. Movies from this presentation can be access at
 2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
Infrared Spectroscopy
 Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
Instrumental Analysis: Spectrophotometric Methods
 Instrumental Analysis: Spectrophotometric Methods 2007 By the end of this part of the course, you should be able to: Understand interaction between light and matter (absorbance, excitation, emission, luminescence,fluorescence,
Instrumental Analysis: Spectrophotometric Methods 2007 By the end of this part of the course, you should be able to: Understand interaction between light and matter (absorbance, excitation, emission, luminescence,fluorescence,
Chem Homework Set Answers
 Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
Infrared Spectroscopy
 Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Spectroscopy Problem Set February 22, 2018
 Spectroscopy Problem Set February, 018 4 3 5 1 6 7 8 1. In the diagram above which of the following represent vibrational relaxations? 1. Which of the following represent an absorbance? 3. Which of following
Spectroscopy Problem Set February, 018 4 3 5 1 6 7 8 1. In the diagram above which of the following represent vibrational relaxations? 1. Which of the following represent an absorbance? 3. Which of following
Spectroscopy: Introduction. Required reading Chapter 18 (pages ) Chapter 20 (pages )
 Spectroscopy: Introduction Required reading Chapter 18 (pages 378-397) Chapter 20 (pages 424-449) Spectrophotometry is any procedure that uses light to measure chemical concentrations Properties of Light
Spectroscopy: Introduction Required reading Chapter 18 (pages 378-397) Chapter 20 (pages 424-449) Spectrophotometry is any procedure that uses light to measure chemical concentrations Properties of Light
Analytical Spectroscopy Review
 Analytical Spectroscopy Review λ = wavelength ν = frequency V = velocity = ν x λ = 2.998 x 10 8 m/sec = c (in a vacuum) ν is determined by source and does not change as wave propogates, but V can change
Analytical Spectroscopy Review λ = wavelength ν = frequency V = velocity = ν x λ = 2.998 x 10 8 m/sec = c (in a vacuum) ν is determined by source and does not change as wave propogates, but V can change
Ultraviolet-Visible and Infrared Spectrophotometry
 Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER
 SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
Molecular Spectroscopy. H 2 O e -
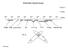 Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
Ch 313 FINAL EXAM OUTLINE Spring 2010
 Ch 313 FINAL EXAM OUTLINE Spring 2010 NOTE: Use this outline at your own risk sometimes a topic is omitted that you are still responsible for. It is meant to be a study aid and is not meant to be a replacement
Ch 313 FINAL EXAM OUTLINE Spring 2010 NOTE: Use this outline at your own risk sometimes a topic is omitted that you are still responsible for. It is meant to be a study aid and is not meant to be a replacement
Chemistry Instrumental Analysis Lecture 3. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 3 Quantum Transitions The energy of a photon can also be transferred to an elementary particle by adsorption if the energy of the photon exactly matches the
Chemistry 4631 Instrumental Analysis Lecture 3 Quantum Transitions The energy of a photon can also be transferred to an elementary particle by adsorption if the energy of the photon exactly matches the
PAPER No. 12: ORGANIC SPECTROSCOPY MODULE No. 4: Basic principles and Instrumentation for IR spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Module 4: Basic principles and Instrumentation for IR spectroscopy CHE_P12_M4_e-Text TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Module 4: Basic principles and Instrumentation for IR spectroscopy CHE_P12_M4_e-Text TABLE OF CONTENTS
Reflection = EM strikes a boundary between two media differing in η and bounces back
 Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Compact Knowledge: Absorbance Spectrophotometry. Flexible. Reliable. Personal.
 L A B O R A T O R Y C O M P E T E N C E Compact Knowledge: Absorbance Spectrophotometry Flexible. Reliable. Personal. The interaction of light with molecules is an essential and well accepted technique
L A B O R A T O R Y C O M P E T E N C E Compact Knowledge: Absorbance Spectrophotometry Flexible. Reliable. Personal. The interaction of light with molecules is an essential and well accepted technique
Singlet. Fluorescence Spectroscopy * LUMO
 Fluorescence Spectroscopy Light can be absorbed and re-emitted by matter luminescence (photo-luminescence). There are two types of luminescence, in this discussion: fluorescence and phosphorescence. A
Fluorescence Spectroscopy Light can be absorbed and re-emitted by matter luminescence (photo-luminescence). There are two types of luminescence, in this discussion: fluorescence and phosphorescence. A
Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different types are classified by frequency or
 CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
9/28/10. Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Valence Electronic Structure. n σ* transitions
 Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Electromagnetic Spectrum - Molecular transitions Widely used in chemistry. Perhaps the most widely used in Biological Chemistry.
Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Electromagnetic Spectrum - Molecular transitions Widely used in chemistry. Perhaps the most widely used in Biological Chemistry.
24 Introduction to Spectrochemical Methods
 24 Introduction to Spectrochemical Methods Spectroscopic method: based on measurement of the electromagnetic radiation produced or absorbed by analytes. electromagnetic radiation: include γ-ray, X-ray,
24 Introduction to Spectrochemical Methods Spectroscopic method: based on measurement of the electromagnetic radiation produced or absorbed by analytes. electromagnetic radiation: include γ-ray, X-ray,
Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry
 Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry Properties of light Electromagnetic radiation and electromagnetic spectrum Absorption of light Beer s law Limitation of Beer s
Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry Properties of light Electromagnetic radiation and electromagnetic spectrum Absorption of light Beer s law Limitation of Beer s
Analytical Technologies in Biotechnology Prof. Dr. Ashwani K Sharma Department of Biotechnology Indian Institute of Technology, Roorkee
 Analytical Technologies in Biotechnology Prof. Dr. Ashwani K Sharma Department of Biotechnology Indian Institute of Technology, Roorkee Module - 6 Spectroscopic Techniques Lecture - 2 UV-Visible Spectroscopy
Analytical Technologies in Biotechnology Prof. Dr. Ashwani K Sharma Department of Biotechnology Indian Institute of Technology, Roorkee Module - 6 Spectroscopic Techniques Lecture - 2 UV-Visible Spectroscopy
R O Y G B V. Spin States. Outer Shell Electrons. Molecular Rotations. Inner Shell Electrons. Molecular Vibrations. Nuclear Transitions
 Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
Lecture 0. NC State University
 Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
LABORATORY OF ELEMENTARY BIOPHYSICS
 LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
Ultraviolet-Visible Spectroscopy
 Ultraviolet-Visible Spectroscopy Introduction to UV-Visible Absorption spectroscopy from 160 nm to 780 nm Measurement of transmittance Conversion to absorbance * A=-logT=εbc Measurement of transmittance
Ultraviolet-Visible Spectroscopy Introduction to UV-Visible Absorption spectroscopy from 160 nm to 780 nm Measurement of transmittance Conversion to absorbance * A=-logT=εbc Measurement of transmittance
Learning Guide for Chapter 3 - Infrared Spectroscopy
 Learning Guide for hapter 3 - Infrared Spectroscopy I. Introduction to spectroscopy - p 1 II. Molecular vibrations - p 3 III. Identifying functional groups - p 6 IV. Interpreting an IR spectrum - p 12
Learning Guide for hapter 3 - Infrared Spectroscopy I. Introduction to spectroscopy - p 1 II. Molecular vibrations - p 3 III. Identifying functional groups - p 6 IV. Interpreting an IR spectrum - p 12
Ultraviolet-Visible and Infrared Spectrophotometry
 Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Chemistry 524--Final Exam--Keiderling Dec. 12, pm SES
 Chemistry 524--Final Exam--Keiderling Dec. 12, 2002 --4-8 pm -- 238 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted plus one 8.5 x 11 sheet
Chemistry 524--Final Exam--Keiderling Dec. 12, 2002 --4-8 pm -- 238 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted plus one 8.5 x 11 sheet
Course Details. Analytical Techniques Based on Optical Spectroscopy. Course Details. Textbook. SCCH 211: Analytical Chemistry I
 SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Chapter 17: Fundamentals of Spectrophotometry
 Chapter 17: Fundamentals of Spectrophotometry Spectroscopy: the science that deals with interactions of matter with electromagnetic radiation or other forms energy acoustic waves, beams of particles such
Chapter 17: Fundamentals of Spectrophotometry Spectroscopy: the science that deals with interactions of matter with electromagnetic radiation or other forms energy acoustic waves, beams of particles such
Molecular Luminescence. Absorption Instrumentation. UV absorption spectrum. lg ε. A = εbc. monochromator. light source. Rotating mirror (beam chopper)
 Molecular Luminescence Absorption Instrumentation light source I 0 sample I detector light source Rotating mirror (beam chopper) motor b sample I detector reference I 0 UV absorption spectrum lg ε A =
Molecular Luminescence Absorption Instrumentation light source I 0 sample I detector light source Rotating mirror (beam chopper) motor b sample I detector reference I 0 UV absorption spectrum lg ε A =
NPTEL/IITM. Molecular Spectroscopy Lectures 1 & 2. Prof.K. Mangala Sunder Page 1 of 15. Topics. Part I : Introductory concepts Topics
 Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Chapter 17: Fundamentals of Spectrophotometry
 Chapter 17: Fundamentals of Spectrophotometry Spectroscopy: the science that deals with interactions of matter with electromagnetic radiation or other forms energy acoustic waves, beams of particles such
Chapter 17: Fundamentals of Spectrophotometry Spectroscopy: the science that deals with interactions of matter with electromagnetic radiation or other forms energy acoustic waves, beams of particles such
Chapter 6 Photoluminescence Spectroscopy
 Chapter 6 Photoluminescence Spectroscopy Course Code: SSCP 4473 Course Name: Spectroscopy & Materials Analysis Sib Krishna Ghoshal (PhD) Advanced Optical Materials Research Group Physics Department, Faculty
Chapter 6 Photoluminescence Spectroscopy Course Code: SSCP 4473 Course Name: Spectroscopy & Materials Analysis Sib Krishna Ghoshal (PhD) Advanced Optical Materials Research Group Physics Department, Faculty
Electronic Excitation by UV/Vis Spectroscopy :
 SPECTROSCOPY Light interacting with matter as an analytical tool III Pharm.D Department of Pharmaceutical Analysis SRM College Of Pharmacy,Katankulathur Electronic Excitation by UV/Vis Spectroscopy : X-ray:
SPECTROSCOPY Light interacting with matter as an analytical tool III Pharm.D Department of Pharmaceutical Analysis SRM College Of Pharmacy,Katankulathur Electronic Excitation by UV/Vis Spectroscopy : X-ray:
Infrared Spectroscopy: Identification of Unknown Substances
 Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
CHEM*3440. Photon Energy Units. Spectrum of Electromagnetic Radiation. Chemical Instrumentation. Spectroscopic Experimental Concept.
 Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy. Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy
 Topic 1: Atomic Spectroscopy Text: Chapter 12,13 & 14 Rouessac (~2 weeks) 1.0 Review basic concepts in Spectroscopy 2.0 Atomic Absorption and Graphite Furnace Instruments 3.0 Inductively Coupled Plasmas
Topic 1: Atomic Spectroscopy Text: Chapter 12,13 & 14 Rouessac (~2 weeks) 1.0 Review basic concepts in Spectroscopy 2.0 Atomic Absorption and Graphite Furnace Instruments 3.0 Inductively Coupled Plasmas
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy
 Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Chemistry Instrumental Analysis Lecture 15. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
Skoog Chapter 7 Components of Optical Instruments
 Skoog Chapter 7 Components of Optical Instruments General Design of Optical Instruments Sources of Radiation Wavelength Selectors (Filters, Monochromators, Interferometers) Sample Containers Radiation
Skoog Chapter 7 Components of Optical Instruments General Design of Optical Instruments Sources of Radiation Wavelength Selectors (Filters, Monochromators, Interferometers) Sample Containers Radiation
m m lighter 'atom' dominates 2 INFRARED SPECTROSCOPY All modes of vibrations are not IR active.
 INFRARED SPECTROSCOPY Infrared spectroscopy probes the interaction of infrared radiation with covalent bonds in molecules. Absorption of IR radiation results in the transitions between vibrational energy
INFRARED SPECTROSCOPY Infrared spectroscopy probes the interaction of infrared radiation with covalent bonds in molecules. Absorption of IR radiation results in the transitions between vibrational energy
Welcome to Organic Chemistry II
 Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Chem 310 rd. 3 Homework Set Answers
 -1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
-1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
Introduction. The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants
 Introduction The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants Spectroscopy and the Electromagnetic Spectrum Unlike mass spectrometry,
Introduction The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants Spectroscopy and the Electromagnetic Spectrum Unlike mass spectrometry,
Symmetric Stretch: allows molecule to move through space
 BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
FTIR Spectrometer. Basic Theory of Infrared Spectrometer. FTIR Spectrometer. FTIR Accessories
 FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
Spectrochemical methods
 Spectrochemical methods G. Galbács The interactions of radiations and matter are the subject of spectroscopy py or spectrochemical methods (also called spectrometry). Spectrochemical methods usually measure
Spectrochemical methods G. Galbács The interactions of radiations and matter are the subject of spectroscopy py or spectrochemical methods (also called spectrometry). Spectrochemical methods usually measure
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
IR Spectrography - Absorption. Raman Spectrography - Scattering. n 0 n M - Raman n 0 - Rayleigh
 RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
Fourier Transform IR Spectroscopy
 Fourier Transform IR Spectroscopy Absorption peaks in an infrared absorption spectrum arise from molecular vibrations Absorbed energy causes molecular motions which create a net change in the dipole moment.
Fourier Transform IR Spectroscopy Absorption peaks in an infrared absorption spectrum arise from molecular vibrations Absorbed energy causes molecular motions which create a net change in the dipole moment.
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
CHM 223 Organic Chemistry I Prof. Chad Landrie. Lecture 10: September 20, 2018 Ch. 12: Spectroscopy mass spectrometry infrared spectroscopy
 M 223 Organic hemistry I Prof. had Landrie Lecture 10: September 20, 2018 h. 12: Spectroscopy mass spectrometry infrared spectroscopy i>licker Question onsider a solution that contains 65g R enantiomer
M 223 Organic hemistry I Prof. had Landrie Lecture 10: September 20, 2018 h. 12: Spectroscopy mass spectrometry infrared spectroscopy i>licker Question onsider a solution that contains 65g R enantiomer
Lecture- 08 Emission and absorption spectra
 Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture- 08 Emission and absorption spectra
Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture- 08 Emission and absorption spectra
Chemistry 524--Final Exam--Keiderling May 4, :30 -?? pm SES
 Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Infrared spectroscopy Basic theory
 Infrared spectroscopy Basic theory Dr. Davide Ferri Paul Scherrer Institut 056 310 27 81 davide.ferri@psi.ch Importance of IR spectroscopy in catalysis IR Raman NMR XAFS UV-Vis EPR 0 200 400 600 800 1000
Infrared spectroscopy Basic theory Dr. Davide Ferri Paul Scherrer Institut 056 310 27 81 davide.ferri@psi.ch Importance of IR spectroscopy in catalysis IR Raman NMR XAFS UV-Vis EPR 0 200 400 600 800 1000
Fourier transform infrared spectroscopy (FTIR) is a method used to obtain an infrared
 Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
09/05/40 MOLECULAR ABSORPTION METHODS
 MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY
 EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
Instrumental Chemical Analysis
 L11 page 1 Instrumental Chemical Analysis Infrared Spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017 Infrared Spectroscopy
L11 page 1 Instrumental Chemical Analysis Infrared Spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017 Infrared Spectroscopy
Complete the following. Clearly mark your answers. YOU MUST SHOW YOUR WORK TO RECEIVE CREDIT.
 CHEM 322 Name Exam 3 Spring 2013 Complete the following. Clearly mark your answers. YOU MUST SHOW YOUR WORK TO RECEIVE CREDIT. Warm-up (3 points each). 1. In Raman Spectroscopy, molecules are promoted
CHEM 322 Name Exam 3 Spring 2013 Complete the following. Clearly mark your answers. YOU MUST SHOW YOUR WORK TO RECEIVE CREDIT. Warm-up (3 points each). 1. In Raman Spectroscopy, molecules are promoted
Fourier Transform Infrared Spectroscopy of Metal Ligand Complexes *
 OpenStax-CNX module: m34660 1 Fourier Transform Infrared Spectroscopy of Metal Ligand Complexes * Jiebo Li Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons
OpenStax-CNX module: m34660 1 Fourier Transform Infrared Spectroscopy of Metal Ligand Complexes * Jiebo Li Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons
1.1. IR is part of electromagnetic spectrum between visible and microwave
 CH2SWK 44/6416 IR Spectroscopy 2013Feb5 1 1. Theory and properties 1.1. IR is part of electromagnetic spectrum between visible and microwave 1.2. 4000 to 400 cm -1 (wave numbers) most interesting to organic
CH2SWK 44/6416 IR Spectroscopy 2013Feb5 1 1. Theory and properties 1.1. IR is part of electromagnetic spectrum between visible and microwave 1.2. 4000 to 400 cm -1 (wave numbers) most interesting to organic
MOLECULAR AND ATOMIC SPECTROSCOPY
 MOLECULAR AND ATOMIC SPECTROSCOPY 1. General Background on Molecular Spectroscopy 3 1.1. Introduction 3 1.2. Beer s Law 5 1.3. Instrumental Setup of a Spectrophotometer 12 1.3.1. Radiation Sources 13 1.3.2.
MOLECULAR AND ATOMIC SPECTROSCOPY 1. General Background on Molecular Spectroscopy 3 1.1. Introduction 3 1.2. Beer s Law 5 1.3. Instrumental Setup of a Spectrophotometer 12 1.3.1. Radiation Sources 13 1.3.2.
Structure Determination. How to determine what compound that you have? One way to determine compound is to get an elemental analysis
 Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Educational experiment package Volume 1. Molecular spectroscopy
 Educational experiment package Volume 1 Molecular spectroscopy Overview Thermo Fisher Scientific is proud to offer a variety of educational experiments for use with Fourier transform infrared (FTIR) spectrometers.
Educational experiment package Volume 1 Molecular spectroscopy Overview Thermo Fisher Scientific is proud to offer a variety of educational experiments for use with Fourier transform infrared (FTIR) spectrometers.
Spectroscopy. Fourier Transform Infrared (FT-IR) Spectroscopy
 Fourier Transform Infrared (FT-IR) Spectroscopy Learning objectives Learning outcomes After completing this course, the student will be able to: Recognize the concept and principle of FT-IR Spectroscopy
Fourier Transform Infrared (FT-IR) Spectroscopy Learning objectives Learning outcomes After completing this course, the student will be able to: Recognize the concept and principle of FT-IR Spectroscopy
Lecture 3: Light absorbance
 Lecture 3: Light absorbance Perturbation Response 1 Light in Chemistry Light Response 0-3 Absorbance spectrum of benzene 2 Absorption Visible Light in Chemistry S 2 S 1 Fluorescence http://www.microscopyu.com
Lecture 3: Light absorbance Perturbation Response 1 Light in Chemistry Light Response 0-3 Absorbance spectrum of benzene 2 Absorption Visible Light in Chemistry S 2 S 1 Fluorescence http://www.microscopyu.com
Instrumental Chemical Analysis
 L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
Instrumental Chemical Analysis
 L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
Lecture 13 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 13 rganic hemistry 1 Professor Duncan Wardrop February 23, 2010 1 EM 232 rganic hemistry I at hicago Spectroscopy & Spectrometry hapter 13 2 EM 232 rganic hemistry
EM 232 rganic hemistry I at hicago Lecture 13 rganic hemistry 1 Professor Duncan Wardrop February 23, 2010 1 EM 232 rganic hemistry I at hicago Spectroscopy & Spectrometry hapter 13 2 EM 232 rganic hemistry
two slits and 5 slits
 Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Lecture 11. IR Theory. Next Class: Lecture Problem 4 due Thin-Layer Chromatography
 Lecture 11 IR Theory Next Class: Lecture Problem 4 due Thin-Layer Chromatography This Week In Lab: Ch 6: Procedures 2 & 3 Procedure 4 (outside of lab) Next Week in Lab: Ch 7: PreLab Due Quiz 4 Ch 5 Final
Lecture 11 IR Theory Next Class: Lecture Problem 4 due Thin-Layer Chromatography This Week In Lab: Ch 6: Procedures 2 & 3 Procedure 4 (outside of lab) Next Week in Lab: Ch 7: PreLab Due Quiz 4 Ch 5 Final
An Introduction to Ultraviolet-Visible Molecular Spectrometry (Chapter 13)
 An Introduction to Ultraviolet-Visible Molecular Spectrometry (Chapter 13) Beer s Law: A = -log T = -logp 0 / P = e x b x C See Table 13-1 for terms. In measuring absorbance or transmittance, one should
An Introduction to Ultraviolet-Visible Molecular Spectrometry (Chapter 13) Beer s Law: A = -log T = -logp 0 / P = e x b x C See Table 13-1 for terms. In measuring absorbance or transmittance, one should
2. Separate the ions based on their mass to charge (m/e) ratio. 3. Measure the relative abundance of the ions that are produced
 I. Mass spectrometry: capable of providing both quantitative and qualitative information about samples as small as 100 pg (!) and with molar masses in the 10 4-10 5 kdalton range A. The mass spectrometer
I. Mass spectrometry: capable of providing both quantitative and qualitative information about samples as small as 100 pg (!) and with molar masses in the 10 4-10 5 kdalton range A. The mass spectrometer
高等食品分析 (Advanced Food Analysis) I. SPECTROSCOPIC METHODS *Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation
 *Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation (EMR): γ-ray emission X-Ray absorption, emission, fluorescence and diffraction Vacuum ultraviolet (UV) absorption
*Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation (EMR): γ-ray emission X-Ray absorption, emission, fluorescence and diffraction Vacuum ultraviolet (UV) absorption
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions
 CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions I. General Features of Electronic spectroscopy. A. Visible and ultraviolet photons excite electronic state transitions. ε photon = 120 to 1200
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions I. General Features of Electronic spectroscopy. A. Visible and ultraviolet photons excite electronic state transitions. ε photon = 120 to 1200
Chapter 10. Spectroscopic Methods. An early example of a colorimetric analysis is Nessler s method for ammonia, which was.
 Chapter 10 Spectroscopic Methods Chapter Overview Section 10A Overview of Spectroscopy Section 10B Spectroscopy Based on Absorption Section 10C UV/Vis and IR Spectroscopy Section 10D Atomic Absorption
Chapter 10 Spectroscopic Methods Chapter Overview Section 10A Overview of Spectroscopy Section 10B Spectroscopy Based on Absorption Section 10C UV/Vis and IR Spectroscopy Section 10D Atomic Absorption
CHEM Outline (Part 15) - Luminescence 2013
 CHEM 524 -- Outline (Part 15) - Luminescence 2013 XI. Molecular Luminescence Spectra (Chapter 15) Kinetic process, competing pathways fluorescence, phosphorescence, non-radiative decay Jablonski diagram
CHEM 524 -- Outline (Part 15) - Luminescence 2013 XI. Molecular Luminescence Spectra (Chapter 15) Kinetic process, competing pathways fluorescence, phosphorescence, non-radiative decay Jablonski diagram
Reflection = EM strikes a boundary between two media differing in η and bounces back
 Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
C101-E111. Talk Letter. Vol.2 February 2009
 C101-E111 UV Talk Letter Vol.2 February 2009 UV Talk Letter UV Talk Letter The Structure of a Spectrophotometer Vol.2 February 2009 1.The Measurement Principle Used by a Spectrophotometer The basic measurement
C101-E111 UV Talk Letter Vol.2 February 2009 UV Talk Letter UV Talk Letter The Structure of a Spectrophotometer Vol.2 February 2009 1.The Measurement Principle Used by a Spectrophotometer The basic measurement
Spectroscopic techniques: why, when, where,and how Dr. Roberto GIANGIACOMO
 Spectroscopic techniques: why, when, where,and how Dr. Roberto GIANGIACOMO BASIC INFORMATION Spectroscopy uses light to analyze substances or products by describing the energy transfer between light and
Spectroscopic techniques: why, when, where,and how Dr. Roberto GIANGIACOMO BASIC INFORMATION Spectroscopy uses light to analyze substances or products by describing the energy transfer between light and
Infrared Spectroscopy
 x-rays ultraviolet (UV) visible Infrared (I) microwaves radiowaves near I middle I far I λ (cm) 8 x 10-5 2.5 x 10-4 2.5 x 10-3 2.5 x 10-2 µ 0.8 2.5 25 250 ν (cm -1 ) 13,000 4,000 400 40 ν (cm -1 1 ) =
x-rays ultraviolet (UV) visible Infrared (I) microwaves radiowaves near I middle I far I λ (cm) 8 x 10-5 2.5 x 10-4 2.5 x 10-3 2.5 x 10-2 µ 0.8 2.5 25 250 ν (cm -1 ) 13,000 4,000 400 40 ν (cm -1 1 ) =
