Stereochemistry. Conformers: Compounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds.
|
|
|
- Patricia Webster
- 5 years ago
- Views:
Transcription
1 Stereochemistry Terms onformers: ompounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds. onstitutional isomers (also called structural isomers): ompounds with the same chemical formula, but differ in their connectivity. They cannot be interconverted without breaking covalent bonds. Stereoisomers: ompounds with the same constitution (same numbering in name), but differ by the spatial arrangement of atoms. They cannot be interconverted without breaking covalent bonds. Enantiomers (optical isomers): A pair of molecules that are mirror images of each other, but are not identical (no matter how they are rotated). Enantiomers are one type of stereoisomer. Stereogenic enter: A carbon atom (or other atom) in a molecule that is substituted in such a way that a new stereoisomer is produced when two substituents (on the same carbon/atom) are switched. A hirality center is a carbon atom (or other atom) that is attached to four different substituents. All chirality centers are stereogenic centers. onfiguration: A chirality center can have one of two configurations, or S (to be defined later). Switching any two substituents on a chirality center changes the configuration (from to S or from S to ). Likewise, the mirror image of an center is S, and the mirror image of an S center is. ommon abbreviations: Me = methyl; Et = ethyl; Pr = propyl; Ph = phenyl (a benzene ring). hirality enters? Stereochemistry and rings: Treat rings a flat; ignore conformations because they interconvert rapidly.
2 hirality enter?: ings Treat the rings themsleves as flat; ignore conformers as they interconvert rapidly ortisone: circle all the chirality centers.
3 Be sure you draw molecules well and clearly: Good Drawings: ' ' ' Bad Drawings: ' ' ' hiral (handedness) compounds: ompounds that exhibit enantiomerism. hiral compounds are optically active (optically activity will be discussed later). mirror A B B A D D Achiral compounds: ompounds whose mirror images are superimposable. Achiral compounds are optically inactive. hiral Molecules: Is Molecule X hiral? A chiral molecule: (1) as handedness, (2) can be separated into enantiomers, and (3) is not superimposable on its mirror image. A molecule that contains only one stereogenic center is chiral. A molecule that contains a plane of symmetry (or mirror plane; not to be confused with mirror image) is achiral. A molecule with two or more stereogenic centers may or may not be chiral. [A compound that contains no stereogenic centers is likely to be achiral, but could be chiral: helical molecules and atropisomers are examples of chiral molecules with no stereogenic centers.]
4 3? ? Allenes l l Atropisomers Me F F Me l l Problem: Draw all the constitutional and stereoisomers named bromochlorocyclopentane. n an exam, you will lose points if you draw the same structure twice. Structural Determination: ow do you know what you have? If you make a compound or isolate a compound, how do you know what its structure is? ne large set of methods of structural analysis or characterization is by spectroscopic methods. Photons over the range of the electromagnetic spectrum interact with molecules in various ways. X-rays can be diffracted, which gives direct structural information, based on electron densities. UV absorption relates to electronic excitation (e.g., raising an electron from a pi to a pi* orbital). Microwave absorption relates to rotational excitation. Infrared
5 relates to vibrational excitation. adiowave absorption relates to nuclear excitation (NM) and distinguishes different types of hydrogens (e.g., N 3 from 3 ). The photons can be absorbed, scattered, or emitted, among other forms of interactions. Enantiomers of a molecule can only be distinuished by their structure (visually), by recognition by other molecules, or by optical rotation. Nearly all other properties are the same for two enantiomers: NM, I, UV, rotation, mp, bp, etc. Light interacts with chiral molecules in such as way that the light wave rotates. Picture a sine wave along an x-axis rotating about this axis. Enantiomers of a molecule rotate light in equal but opposite directions. To observe the rotation, the light must be plane polarized. therwise, the randomness of the light waves would appear to average out to zero rotation. The more concentrated the solution, the more chiral molecules each photon will be rotated by, the more overall rotation will be observed. Likewise for the pathlength: the longer the pathlength, the more molecules each photon will encounter. Both pathlength and sample concentration are normalized by specific rotation. ptical Activity: otation of plane polarized light that occurs on passing through a sample containing one (or a majority of one) enantiomer. otation is measured using a polarimeter. otation of light in the right-hand direction (clockwise) is called dextrorotatory, d, or (+). otation of light in the left-hand direction (counter-clockwise) is called levorotatory, l, or (-). Specific otation: Defined for an optically pure sample as: α [ ] D 20 = α lx[x] 20: 20 (temperature of the experiment) D: D-line of sodium (wavelength of light used in the experiment) α: observed rotation from polarimeter l: pathlength of cell containing sample (often 10 cm) [X]: concentration of sample (in g/ml) A acemic mixture is a 1:1 mixture of corresponding enantiomers. otation = 0 because enantiomers have equal but opposite optical activity. ptically Pure: ne and only one enantiomer is present. ptically active: a majority of one enantiomer is present. ptically inactive: either (1) no chiral compounds are present, or (2) there is a racemic mixture. Problem: [ ]-α-carvone has a specific rotation of 72. A solution of α-carvone has a rotation of +50. What is the % of [ } and [+] enantiomers?
6 3 3 2 [ ]-α-carvone Solution: Let x = the fraction of [ ]; 1 x = fraction of [+]. 72x + 72(1 x) = x x = x = 22 x = 0.153; the solution contains 15.3% [ ] and 84.7% [+]. heck: 15.3%( 72) $(+72) =? (+50 ) Another method: = 22; 22/(72+72) = 15.3% ahn-ingold Prelog ules: Enantiomers can be distinguished on paper (and thus, by name) using a system of nomenclature called the ahn-ingold-prelog rules. These rules assign onfiguration (i.e., or S; see below) to each chiral carbon. First, assign priority to each substituent on the stereogenic carbon using rules 1 and 2: 1. ompare the atomic number of the atoms that are attached directly to the stereogenic carbon. The atoms with the highest atomic number have the highest priority. The second-highest atomic number has the next highest priority, etc. 2. If there is a tie, go to the atoms that are two bonds away from the stereogenic carbon. Again, assign priorities by atomic numbers. For example, ethyl has higher priority than methyl: There is a tie at the first carbon, but then there are 3 next (i.e., two bonds away) in the case of the methyl group, but 2 and a next in the case of the ethyl group. The in the ethyl case "beats" all three 's in the methyl case: >, >, > ; the 's are not "added together". 3. Draw the molecule such that the lowest-priority substituent (usually ) is sticking into the page and the other three substituents form a steering wheel. ount going either clockwise or counter-clockwise, depending on the priorities. lockwise is assigned "" (for rectus, Latin for right) and counter clockwise is assigned "S" (for sinister, Latin for left).
7 F l 1 1 F l F 3 4 l 2 F 3 4 l 2 For the compound below, ties in priorities have to be broken. We can do this with a table (eventually, you can do this in your head): l l l Substituent l Methyl Ethyl At one bond l away: Two bonds away: ,,,, We can see from one bond away that l is the number 1 priority and is number 4. For methyl and ethyl, we need to go two bonds away. The one carbon (the green one) of ethyl beats each of the three s of the methyl. The center is. The compound is 2-()-chlorobutane or ()-2-chlorobutane. Problem: draw (S)-2-chlorobutane. 1 l arbonyls: For assigning the priorities of carbonyls, treat the carbonyl carbon as if it is bound to two oxygens: Treat: as: Assign /S configuration to alanine, below: N
8 N Substituent N 2 2 Methyl At one bond N away: Two bonds away: ,,,, The nitrogen wins in round one as does the hydrogen lose. ound two goes to the carboxylic acid. The compound is (S)-alanine. 1 N ings: Assign priorities the same way. 3 Substituent Methyl At one bond away: Two bonds away:,, ---,,,, loses. Methyl is third. For the ring, going to the right as drawn we get to 2, then an. Going to the left, we get to 2 then another 2. From the table, we can see that the winner is the side with the.
9 What if there are two or more chirality centers in a molecule? Simply assign each center, independently For the compound above, first assign prioritied to the carbon on the left (in red). Note that the other chirality center is included as the second priority group (dotted circle). The configuration will be S because the hydrogen (not drawn) is pointing toward you. Next, assign priorities for the second carbon (middle structure, carbon in red). Likewise, this carbon is S. ptions on viewing the 4 th priority group: If it is pointing away from you, the molecule is setup for assigning configuration. If not, there a few things you can do; choose the option you find easiest. (1) Use a model: be sure the model is consistent with the drawing, then assign configuration. You can simplify the model to a tetrahedron with four different colored balls, where you assign appropriate priorities to the colors. This way all such assignments can be done using one or two models. You can bring these pre-built to exams; just don t write on them. (2) edraw the molecule so that the 4 th substituent is pointing away from you. Be careful to redraw the molecule properly. (3) Visualize the molecule so that you are looking down the 4 th bond properly. This may entail visualizing from behind. (4) Simply switch the rule: if looking down the 4 th bond the wrong way (i.e., it is pointing toward you), the rule becomes: clockwise = S; counterclockwise =. Enantiomerism in nature. Most physical and chemical properties of enantiomers are identical (optical activity being a key exception). owever, nearly all reactions of chiral compounds that occur in nature involve only one enantiomer. Enzymes are chiral and recognize one enantiomer over the other. The recognition is like two right hands shaking together. A right and a left just won t do. The enantiomers of α-carvone, which we encountered earlier, have different properties that we can detect: one smells like caraway seeds (rye bread) and the other smells like spearmint. Some people S S
10 have a mutation and cannot make the distinction. Such folk usually do not like rye bread as it tastes minty to them [ ]-α-carvone A much more somber story is that of thalidomide. This compound was used as an anti-nausea drug in the 1950 s. Sadly, it led to birth defects. Turns out that one enantiomer is responsible for the antinausea activity while the other is responsible for the birth defects. The drug was sold as a racemic mixture. Later there was the idea that had only the active enantiomer be used, no side-effects would have occurred. owever, the two enantiomers interconvert in the body, so there was no way around the side effects other than avoiding the drug altogether. N Thalidomide N "" and "S" configuration cannot be used to predict optical rotation. d(+) and l(-) are observables. and S are conventions. There is no relationship between /S and d/l, except if a molecule has only one chiral center and if = d, then S = l (and vice versa). [D and L refer to amino acid and carbohydrate nomenclature and are not a large part of hem 260.] In a pair of enantiomers, the configurations about all stereogenic centers are opposite. In creating the mirror image of a molecule, all configurations become S, and all S become. If you switch two substituents on a chiral center, becomes S, and S becomes. Diastereomers: Stereoisomers that are not enantiomers. Diastereomers have different properties. Meso compounds: ompounds that have more than one stereogenic center, but are achiral (and have stereoisomers that are chiral).
11 2 N 2 2 N 2 2 N 2 2 N A B D Enantiomers Enantiomers Diastereomers: A and ; A and D; B and ; B and D. Tartaric Acid (+) ( ) Enantiomers (meso)-tartaric Acid Identical, Achiral, Meso (+) and ( ) tartaric acid are enantiomers. (+) and meso tartaric acid are diastereomers. ( ) and meso tartaric acid are diastereomers. amifications of hanging onfiguration of Stereogenic enters: (1) If a compound has only one stereogenic center, and you change the configuration (from S, or from S ), you create the enanatiomer of that compound. (2) If a chiral compound has more than one stereogenic center, and you change the configuration of all of its stereogenic centers, you create the enantiomer of that compound. (3) If a compound has more than one stereogenic center, and you change the configuration of any but not all of its stereogenic centers, you create a diastereomer of that compound. is-trans isomers: Substituted alkenes can exist as either cis or trans stereoisomers (sometimes called geometrical isomer), as the substituents can be either on the same side (cis) of the double bond or on opposite sides (trans). is and trans stereoisomers are a type of diastereomers. As the substituents get more complex and/or more than one double bond exists in a molecule, complications arise. A system of nomenclature based on the ahn-ingold-prelog rules has been developed. First, assign priorities for each substituent on each alkene carbon. If the two highest-priority substituents are on the same side of the double bond (as in cis), it is called a Z double bond for zusammen, together ("Zee same side"); if the substituents are on opposite sides (as in trans), the double bond is E for entgegen, opposite. For alkenes, always use E/Z nomenclature, not cis/trans. Note that E/Z isomers are indeed isomers, as the energy barrier for interconversion (breaking of a π-bond) is 65 kcal/mol.
12 For the example below, the ethyl has the higher priority on the left hand carbon; the methyl has the higher priority on the right hand carbon (middle figure). Next (structure on right), the two winners are on the same side of the double bond (on the top, as drawn), so the compound is (Z)-3-methyl-2- pentene Disubstituted cycloalkanes can have the two substituents either on the same side of the ring, cis, or on opposite sides of the ring, trans. The isomers cannot interconvert without breaking a σ-bond, which is around 80 kcal/mol. Always use cis/trans for cycloalkanes. For stereochemical determinations (e.g., is compound X chiral?), treat cycloalkanes as flat since the conformers interconvert rapidly. If compounds differ in their numbering (and have the same name otherwise), they are onstitutional isomers. If they have the same numbering (and name, outside of stereochemical designations), they are stereoisomers. ow many stereoisomers are there for dimethylcyclohexane? All Isomers Named Dimethylcyclohexane onstitutional Isomer Stereoisomers with denoted onstitution Number of Stereoisomers 1,1 1 1,2 3 1,3 3 1,4 2 All 9 There is only one 1,1-isomer. There are three 1,2-isomers, one cis and two trans. The two trans are enantiomers; the cis is a diastereomer of the trans. The case for 1,3 is similar to 1,2. For 1,4, there are only one cis and one trans isomer. ow many stereoisomers are there for 1-ethyl-3-methylcyclohexane? (ans: 4) ow many for 1-ethyl-4- methylcyclohexane? (ans: 2)
13 Naming: If there are two or more stereocenters, name each or S with the appropriate number indicating which carbon refers to the designation. For example, (1,2)-trans-1,2- dimethylcyclohexane, (1S,2S)-trans-1,2-dimethylcyclohexane, and cis-1,2-dimethylcyclohexane. Although it doesn t matter with the first two examples where you start the numbering, you should include the numbers. For the cis example, the stereocenters are,s, which is the same as S, (you can start at either stereocenter), but you don t need to indicate this because the molecule is achiral (meso). More detail: The compounds below are both trans-1,2-dimethylcyclohexane. They have opposite configuration at both stereogenic centers. They have the same constitution, are mirror images of one another, and are not identical: they are enantiomers. They are named trans-(,)-1,2- dimethylcyclohexane and trans-(s,s)-1,2-dimethylcyclohexane. You can leave out the trans if you like, as that is defined in the -(,)- or -(S,S)-. You can write -(1,2)- or -(1S,2S)-, but that is unnecessary. The numbering can go before or after the word trans. You do need to indicate the configuration, as that is how to distinguish one enantiomer from the other. In principle, the word trans can be left out in this case, but it is left in for convenience. S S The compounds below are cis-1,2-dimethylcyclohexane. They have the same constitution and although they are mirror images of one another, they are identical, so they cannot be enantiomers. You can include (,S), (1,2S), or (1S,2) in the name, but it is unnecessary. Note that cis-1,2- dimethylcyclohexane is a meso compound. S S For the compound below, the has priority, so it is carbon-1. You can name it 2S-methyl-bromocyclopentane or (1, 2S)-2-methylbromocyclopentane. 3 For each constitutional and stereoisomer of dimethylcyclohexane that contains the name dimethylcyclohexane, draw the chair conformations. Draw the chair conformations from different perspectives (e.g., attach the first substituent to each of the 12 positions).
14 For the 1,1-isomer, there is only one stereoisomer. Upon ring-flipping, the new chair is indistinguishable from the first. The conformers of cis-1,2-dimethylcyclohexane are in rapid equilibrium, just as any two chair conformations are. The two conformers of cis-1,2-dimethylcyclohexane can be considered to be conformational enantiomers, and as such, a kind of racemic mixture. But due to the rapid equilibration, we simply consider the molecule to be achiral. The two conformers are of equal energy and thus exist as a 50:50 mixture. For the 1,2-trans isomer, the analysis for each enantiomer is the same. Draw either conformer first. Generate the other conformer by switching axial to equatorial, and equatorial to axial. Keep up as up and down as down. The two conformers in this case are different in energy by 2.7 kcal/mol. This is the difference between two axial versus two equatorial substituents (3.6 kcal) less 0.9 kcal. What accounts for the 0.9 kcal? For the 1,3-isomer, let s start with the cis-stereoisomer. Two axial versus two equatorial substituents yields a ΔG = 3.6 kcal/mol. For the 1,3-trans, the two conformers are indistinguishable. Keep looking until you can see this. The case is the same for the enantiomer.
15 For 1,4-trans, ΔG = 3.6 kcal/mol. For cis-1,4, the two conformers are indistinguishable.
geometric isomers (diastereomers)
 Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
STEREOGENIC CENTER (Chiral Center,Asymmetric Center) Atom (usually carbon) to which 4 different groups are attached: W Z C X Y
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENIC CENTER (Chiral Center,Asymmetric Center)
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
C 4 H 10 O. butanol. diethyl ether. different carbon skeleton different functional group different position of FG
 hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
240 Chem. Stereochemistry. Chapter 5
 240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
Lesson 4. Molecular Geometry and Isomers II. Lesson 4 CH 3 HO H OH
 Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Stereochemistry. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects.
 Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Stereochemistry. 3-dimensional Aspects of Tetrahedral Atoms
 Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
Chapter 5 Stereochemistry. Stereoisomers
 Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Lecture Topics: I. Stereochemistry Stereochemistry is the study of the three dimensional structure of molecules
 Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
1. Make two superimposable models of bromochloroiodomethane. Position your models on your desk to prove that they are superimposable.
 HM 204 Organic hemistry Introduction to Stereochemistry Recall that two models are identical if they can be superimposed without breaking bonds. Recall that conformations (conformers) are structures that
HM 204 Organic hemistry Introduction to Stereochemistry Recall that two models are identical if they can be superimposed without breaking bonds. Recall that conformations (conformers) are structures that
Stereochemistry. Based on McMurry s Organic Chemistry, 6 th edition
 Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry CHAPTER SUMMARY
 2 7 2 7. Introduction APTER SUMMARY Isomers are compounds with identical molecular formulas but different structural formulas. Structural or constitutional isomers differ in the bonding arrangement of
2 7 2 7. Introduction APTER SUMMARY Isomers are compounds with identical molecular formulas but different structural formulas. Structural or constitutional isomers differ in the bonding arrangement of
ORGANIC - BROWN 8E CH.3 - STEREOISOMERISM AND CHIRALITY.
 !! www.clutchprep.com CONCEPT: TYPES OF ISOMERS Isomers are used to describe relationships between similar molecules. We can order these relationships in order of increasing similarity Page 2 CONCEPT:
!! www.clutchprep.com CONCEPT: TYPES OF ISOMERS Isomers are used to describe relationships between similar molecules. We can order these relationships in order of increasing similarity Page 2 CONCEPT:
IN-CLASS PROBLEM. ChemistryOnline, STEREOCHEMISTRY OF TETRAHEDRAL CENTERS. ChemistryOnline, No Plane of Symmetry
 hapter 5 Draw the structure of bromocyclopentane. Stereochemistry Reproduction or distribution of any of the content, or any of the images in this presentation is strictly prohibited without the expressed
hapter 5 Draw the structure of bromocyclopentane. Stereochemistry Reproduction or distribution of any of the content, or any of the images in this presentation is strictly prohibited without the expressed
Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional)
 Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional) Stereoisomers... have the same connectivity but a different
Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional) Stereoisomers... have the same connectivity but a different
Assigning Stereochemistry I What is stereochemistry?
 S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
Chem 341 Jasperse Ch. 9 Handouts 1
 Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Basic Stereochemical Considerations
 Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Chapter 4: Stereochemistry
 Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
It is possible for organic molecules with the same molecular formula to have different structures
 Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
Stereochemistry Terminology for two pure isomeric compounds, both of which are chiral? A pair of stereoisomers
 Name Last, irst STEECEMISTY This handout will help you understand stereoisomerism, naming conventions and relationships between stereoisomers. I hope that you will use this to help you study for exam 1.
Name Last, irst STEECEMISTY This handout will help you understand stereoisomerism, naming conventions and relationships between stereoisomers. I hope that you will use this to help you study for exam 1.
9. Stereochemistry. Stereochemistry
 9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
a. Does the model have a plane of symmetry? Yes No The central carbon is said to be a stereocenter, stereogenic center, or chiral carbon.
 Name: TA Name Lab Section: Day Time OPTICAL ISOMERISM 1. Construct a model that has a central carbon atom with 4 different colored spheres attached to it, representing four different atoms or groups. Draw
Name: TA Name Lab Section: Day Time OPTICAL ISOMERISM 1. Construct a model that has a central carbon atom with 4 different colored spheres attached to it, representing four different atoms or groups. Draw
Chapter 5: Stereoisomerism
 hapter 5: Stereoisomerism [Sections: 5.1-5.9] 1. dentifying Types of somers Same MolecularFormula? A B compounds are not isomers Same onnectivity? D E constitutional isomers have different names (parent
hapter 5: Stereoisomerism [Sections: 5.1-5.9] 1. dentifying Types of somers Same MolecularFormula? A B compounds are not isomers Same onnectivity? D E constitutional isomers have different names (parent
Chapter 5 Stereochemistry
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Chapter 5 Stereochemistry
 Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
02/07/2017. Isomerism. Structural isomerism. 1. Structural isomerism different linkages of atoms. Same molecular formula Different structural formulae
 hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
Three-Dimensional Structures of Drugs
 Three-Dimensional Structures of Drugs Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Chiral drugs are sometimes sold as one enantiomer (pure
Three-Dimensional Structures of Drugs Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Chiral drugs are sometimes sold as one enantiomer (pure
Enantiomers. nonsuperimposable mirror image Both Configuration will be opposite. Both Configuration will be opposite
 Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
CHAPTER 5. Stereoisomers
 CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
E30 ENANTIOMERS Chirality in organic chemistry
 E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
CHEM 263 Oct 18, Do they have the same molecular formula?
 EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
STEREOCHEMISTRY CHIRALITY
 TERECEMITRY CIRALITY A A C C B C D D C B Chirality - tereoisomers 2 Chirality Enantiomers - molecules that are not superimposable on their mirror image. Molecules that can exist as enantiomers are called
TERECEMITRY CIRALITY A A C C B C D D C B Chirality - tereoisomers 2 Chirality Enantiomers - molecules that are not superimposable on their mirror image. Molecules that can exist as enantiomers are called
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
Chapter 6. Isomers and Stereochemistry
 hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
9. Stereochemistry: Introduction to Using Molecular Models
 9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
Organic Chemistry. Chemical Bonding and Structure (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
(1) Check to see if the two compounds are identical. (2) Recall the definitions of stereoisomers, conformational isomers, and constitutional isomers.
 MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Introduction to Organic Chemistry, Unit 3: Chirality at Carbon Centers Bring your model kits to class!
 Introduction to rganic hemistry, Unit 3: hirality at arbon enters Bring your model kits to class! rganic hemistry #3 1 bjectives: by the end of this unit, you should be able to... Identify the chiral centres
Introduction to rganic hemistry, Unit 3: hirality at arbon enters Bring your model kits to class! rganic hemistry #3 1 bjectives: by the end of this unit, you should be able to... Identify the chiral centres
MOLECULAR MODELS : STEREOISOMERS
 MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
(S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer
 C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
CHEM 261 Feb. 2, Stereochemistry and Chirality
 70 EM 261 eb. 2, 2017 Stereochemistry and hirality hiral object or molecule: has a non-superimposable mirror image Achiral object: not chiral, has a superimposable mirror image 1848 - Louis Pasteur separated
70 EM 261 eb. 2, 2017 Stereochemistry and hirality hiral object or molecule: has a non-superimposable mirror image Achiral object: not chiral, has a superimposable mirror image 1848 - Louis Pasteur separated
10/4/2010. Sequence Rules for Specifying Configuration. Sequence Rules for Specifying Configuration. 5.5 Sequence Rules for Specifying.
 5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
Chapter 7 Cyclic Compounds. Stereochemistry of Reactions
 Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 7 Cyclic Compounds. Stereochemistry of Reactions Solutions to In-Text Problems 7.3 Following the procedure in the
Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 7 Cyclic Compounds. Stereochemistry of Reactions Solutions to In-Text Problems 7.3 Following the procedure in the
Chapter 6. Isomers and Stereochemistry
 Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Essentials of Chapter 6
 Essentials of Chapter 6 (Videos: conformational Analysis, Conformational Analysis of Cycloalkanes, Chirality, /S Nomenclature [Basic Advanced], ptical Activity) A. Stereochemical Structures Wedge Bond
Essentials of Chapter 6 (Videos: conformational Analysis, Conformational Analysis of Cycloalkanes, Chirality, /S Nomenclature [Basic Advanced], ptical Activity) A. Stereochemical Structures Wedge Bond
Organic Chemistry Chapter 5 Stereoisomers H. D. Roth
 Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Chem 201 Midterm Winter, 2013 Beauchamp
 hem 0 Midterm Winter, 0 Beauchamp Name Problems Points redit. Functional Group Nomenclature. Degrees of Unsaturation & Functional Groups or Various Nomenclature Terms. D structure, Functional Groups 0.
hem 0 Midterm Winter, 0 Beauchamp Name Problems Points redit. Functional Group Nomenclature. Degrees of Unsaturation & Functional Groups or Various Nomenclature Terms. D structure, Functional Groups 0.
Topic 5 Stereochemistry and optical isomers Isomerism
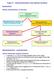 Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
(2/94)(6,7,9/95)(8,9/97)(12/99)(1/00) Neuman Chapter 4
 4: Stereochemistry Tetrahedral Carbon Configurations Stereoisomers and R,S Assignments The Number and Types of Stereoisomers Drawing Structures of Stereoisomers Cyclic Molecules Optical Activity Preview
4: Stereochemistry Tetrahedral Carbon Configurations Stereoisomers and R,S Assignments The Number and Types of Stereoisomers Drawing Structures of Stereoisomers Cyclic Molecules Optical Activity Preview
STEREOCHEMISTRY A STUDENT WHO HAS MASTERED THE MATERIAL IN THIS SECTION SHOULD BE ABLE TO:
 STEREOEMISTRY A STUDENT WO AS MASTERED TE MATERIAL IN TIS SETION SOULD BE ABLE TO: 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of
STEREOEMISTRY A STUDENT WO AS MASTERED TE MATERIAL IN TIS SETION SOULD BE ABLE TO: 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of
Stereochemistry Terminology
 Stereochemistry Terminology Axis of symmetry: When an operation on an axis C n, where n = 360 /rotation, leads to a structure indistinguishable from the original. C 2 180 Plane of symmetry: (σ) A plane
Stereochemistry Terminology Axis of symmetry: When an operation on an axis C n, where n = 360 /rotation, leads to a structure indistinguishable from the original. C 2 180 Plane of symmetry: (σ) A plane
L35 REVIEW OUTLINE. 1. Reactions. 2. Spectroscopy and Stereochemistry. 3. Preview of Final. CHEM2312: Spring 2008
 L35 REVIEW UTLINE 1. Reactions 2. Spectroscopy and Stereochemistry 3. Preview of Final EM2312: Spring 2008 REVIEW F REATINS While the following schemes group reactions by type of functional group, recognize
L35 REVIEW UTLINE 1. Reactions 2. Spectroscopy and Stereochemistry 3. Preview of Final EM2312: Spring 2008 REVIEW F REATINS While the following schemes group reactions by type of functional group, recognize
CHEM 261 Oct 11, Diastereomers. Enantiomers. Pheromones: from Greek pherein horman meaning to carry excitement. Discovered by Adolf Butenanot.
 EM 26 ct, 208 REALL: is Trans Trans Diastereomers Enantiomers Enantiomers have opposite stereochemistry at every stereocenter (chiral center) Diastereomers are all stereoisomers that are not enantiomers
EM 26 ct, 208 REALL: is Trans Trans Diastereomers Enantiomers Enantiomers have opposite stereochemistry at every stereocenter (chiral center) Diastereomers are all stereoisomers that are not enantiomers
comes forward STEREOISOMERS ISOMERS THAT ARE DIFFERENT BECAUSE OF THEIR ORIENTATION IN SPACE
 STEREOCEMISTRY SOME DEFINITIONS WIT EXAMPLES PRESENTING STEREO STRUCTURES CIRAL CENTER REPRESENTAITON goes back goes back in the plane of the paper comes forward comes forward DOTTED LINE - WEDGE goes
STEREOCEMISTRY SOME DEFINITIONS WIT EXAMPLES PRESENTING STEREO STRUCTURES CIRAL CENTER REPRESENTAITON goes back goes back in the plane of the paper comes forward comes forward DOTTED LINE - WEDGE goes
Chapter 06 Chirality: The Handedness of Molecules
 tereoisomers A A B D D tereoisomers: Isomers (different compounds) that have all the same number and kind of atoms that are all connected the same, but differ in their arrangement in three dimensions.
tereoisomers A A B D D tereoisomers: Isomers (different compounds) that have all the same number and kind of atoms that are all connected the same, but differ in their arrangement in three dimensions.
4Types of Isomers. 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B.
 4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
ISOMERISM - A general survey
 Isomerism 1 ISOMERISM - A general survey STRUTURAL ISOMERS have the same molecular formula but different structural formulae They occur due to variations in... the carbon skeleton AIN ISOMERISM 2 2 positions
Isomerism 1 ISOMERISM - A general survey STRUTURAL ISOMERS have the same molecular formula but different structural formulae They occur due to variations in... the carbon skeleton AIN ISOMERISM 2 2 positions
CSUS - CH6B Fischer projection and R/S configurations Instructor: J.T., P: 1. a) Fischer Projection can be rotated by 180 only!
 CSUS - C6B Fischer projection and R/S configurations Instructor: J.T., P: () Fischer Projection: orizontal line is coming out of the plane of the page. Vertical line is going back behind of the plane of
CSUS - C6B Fischer projection and R/S configurations Instructor: J.T., P: () Fischer Projection: orizontal line is coming out of the plane of the page. Vertical line is going back behind of the plane of
Names. Chiral: A chiral object is not superimposable upon its mirror image. A chiral object contains the property of "handedness.
 CEM 241 IN-CLASS #3 MOLECULAR MODELS EXERCISE Names Stereoisomerism Construct a model containing a tetrahedral carbon (black ball) that is attached to four different atoms (use the green, orange, purple
CEM 241 IN-CLASS #3 MOLECULAR MODELS EXERCISE Names Stereoisomerism Construct a model containing a tetrahedral carbon (black ball) that is attached to four different atoms (use the green, orange, purple
STEREOISOMERS ARRANGEMENTS IN 3D- SPACE
 STEREOISOMERS ARRANGEMENTS IN 3D- SPACE 1 Isomers 2 Physiological Proper@es of Stereoisomers (Enan@omers) Enan@omers can have very different physiological proper@es. 3 Oranges and Lemons found in oranges
STEREOISOMERS ARRANGEMENTS IN 3D- SPACE 1 Isomers 2 Physiological Proper@es of Stereoisomers (Enan@omers) Enan@omers can have very different physiological proper@es. 3 Oranges and Lemons found in oranges
Química Orgânica I 2008/09. w3.ualg.pt\~abrigas QOI 0809 A3 1
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A3 1 O O O 3 C 3 C O O O C 3 N O C 3 O O O O O O C 3 O w3.ualg.pt\~abrigas QOI 0809 A3 2 Adaptado de: Jo Blackburn; 2006, Prentice all; Organic Chemistry,
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A3 1 O O O 3 C 3 C O O O C 3 N O C 3 O O O O O O C 3 O w3.ualg.pt\~abrigas QOI 0809 A3 2 Adaptado de: Jo Blackburn; 2006, Prentice all; Organic Chemistry,
STEREOCHEMISTRY. 2. Define the following, and tell whether or not a given compound or structure fits the description or possesses the feature.
 A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
Copyright 2009 James K Whitesell
 Copyright 2009 James K Whitesell 5-1 These two molecules, cyclopropylcyclopentane and cyclobutycyclobutane have the same number of carbon and hydrogen atoms and thus they are constitutional isomers. 5-2
Copyright 2009 James K Whitesell 5-1 These two molecules, cyclopropylcyclopentane and cyclobutycyclobutane have the same number of carbon and hydrogen atoms and thus they are constitutional isomers. 5-2
CHEM 261 Feb. 2, Pheromone: from Greek pherein horman meaning to carry excitement. Only about 50 % of the population can smell this compound
 70 EM 61 eb., 017 Pheromone: from Greek pherein horman meaning to carry excitement O Only about 50 % of the population can smell this compound omenclature of Alkynes Rules: - ind longest chain with max
70 EM 61 eb., 017 Pheromone: from Greek pherein horman meaning to carry excitement O Only about 50 % of the population can smell this compound omenclature of Alkynes Rules: - ind longest chain with max
Due Date: 2) What is the relationship between the following compounds?
 Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
Unit One Part 8: stereochemistry
 Unit ne Part 8: stereochemistry 1 Describe the difference between stereoisomers & structural isomers omenclature used for double bonds (cis-trans or E-Z) Predict conformations of cyclohexanes Define chirality
Unit ne Part 8: stereochemistry 1 Describe the difference between stereoisomers & structural isomers omenclature used for double bonds (cis-trans or E-Z) Predict conformations of cyclohexanes Define chirality
LECTURE #09 Thurs., March 06, in Lect.07 s notes actually Biological relevance of chirality
 EM 221 section 52 LETURE #09 Thurs., March 06, 2008 ASSIGNED READINGS: TDAY S LASS: 5.1-5.4 in Lect.07 s notes actually 5.21 Biological relevance of chirality 5.5-5.14 Drawing & naming enantiomers & diastereomers
EM 221 section 52 LETURE #09 Thurs., March 06, 2008 ASSIGNED READINGS: TDAY S LASS: 5.1-5.4 in Lect.07 s notes actually 5.21 Biological relevance of chirality 5.5-5.14 Drawing & naming enantiomers & diastereomers
Lecture 8: September 13, 2012
 CHM 223 Organic Chemistry I Fall 2012, Des Plaines Prof. Chad Landrie Lecture 8: September 13, 2012 Skillbuilder 2 Ch. 5: Stereochemistry (Sec8ons 5.1-5.5) CHM 223 Organic Chemistry I Fall 2012, Des Plaines
CHM 223 Organic Chemistry I Fall 2012, Des Plaines Prof. Chad Landrie Lecture 8: September 13, 2012 Skillbuilder 2 Ch. 5: Stereochemistry (Sec8ons 5.1-5.5) CHM 223 Organic Chemistry I Fall 2012, Des Plaines
Organic Chemistry Unit #2: Structure of Alkanes, Cycloalkanes, and Alkenes
 Organic hemistry Unit #2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this unit, you should be able to... Interconvert
Organic hemistry Unit #2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this unit, you should be able to... Interconvert
Counterclockwise (Leftward turning) S. 3 2 Clockwise (Rightward turning) R
 Stereochemistry I and S in Alkanes A chiral carbon has four different groups attached to it, the differences may be several bonds away, but so long as they are different in any way the groups are considered
Stereochemistry I and S in Alkanes A chiral carbon has four different groups attached to it, the differences may be several bonds away, but so long as they are different in any way the groups are considered
A. Structure and Nomenclature. Introduction of Organic Chemistry. Unit 2: Structure of Alkanes, Cycloalkanes, and Alkenes
 Organic hemistry #2 1 Introduction of Organic hemistry. Unit 2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this
Organic hemistry #2 1 Introduction of Organic hemistry. Unit 2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this
Please provide clear and concise answers to all of the following questions. Use equations and/or drawings to support your answers where appropriate.
 Organic hemistry I (230-001) Examination II October 27, 2004 Key Name (PINT LEGIBLY): Please provide clear and concise answers to all of the following questions. Use equations and/or drawings to support
Organic hemistry I (230-001) Examination II October 27, 2004 Key Name (PINT LEGIBLY): Please provide clear and concise answers to all of the following questions. Use equations and/or drawings to support
Experiment 8 Optical Isomers. In this experiment you will be given the opportunity to see the 3-dimensional aspects of
 Experiment 8 Optical Isomers In this experiment you will be given the opportunity to see the 3-dimensional aspects of stereochemistry and optical isomers. Previously in class you were exposed to the concept
Experiment 8 Optical Isomers In this experiment you will be given the opportunity to see the 3-dimensional aspects of stereochemistry and optical isomers. Previously in class you were exposed to the concept
18 Isomerism and stereochemistry
 s manual for Burrows et.al. hemistry Third edition 8 Isomerism and stereochemistry s to worked examples WE 8. Structural isomers (on p. 88 in hemistry ) For the following four compounds, A D, identify
s manual for Burrows et.al. hemistry Third edition 8 Isomerism and stereochemistry s to worked examples WE 8. Structural isomers (on p. 88 in hemistry ) For the following four compounds, A D, identify
stereochemistry 8.1 stereoisomers 8.2 mirror image objects, mirror image molecules, and chirality M. C. Escher, Drawing Hands, 1948
 8 stereochemistry M.. Escher, Drawing ands, 1948 8.1 stereoisomers Molecules that have different arrangements in three-dimensional space are stereoisomers. Stereoisomers have different configurations.
8 stereochemistry M.. Escher, Drawing ands, 1948 8.1 stereoisomers Molecules that have different arrangements in three-dimensional space are stereoisomers. Stereoisomers have different configurations.
Alkanes. Introduction
 Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
1. Chirality & Stereochemistry
 hapter 5 Stereochemistry hiral Molecules reated by Professor William Tam & Dr. illis hang h. 5 - About The Authors These Powerpoint Lecture Slides were created and prepared by Professor William Tam and
hapter 5 Stereochemistry hiral Molecules reated by Professor William Tam & Dr. illis hang h. 5 - About The Authors These Powerpoint Lecture Slides were created and prepared by Professor William Tam and
Enantiomers 2:22 PM 1
 1 Definition (Revisited) Enantiomers are stereoisomers that are nonsuperimposable mirror images. Note that all the chiral centres in a pair of enantiomers are mirror images of each other. 2 Practice Questions
1 Definition (Revisited) Enantiomers are stereoisomers that are nonsuperimposable mirror images. Note that all the chiral centres in a pair of enantiomers are mirror images of each other. 2 Practice Questions
Solutions 80 CHAPTER a) trans b) not stereoisomeric c) trans d) trans e) trans f) not stereoisomeric g) cis
 80 CAPTE 5 killbuilder 5.9 Assigning configuration from a Fischer projection AIG TE CFIGUATI F TE CIALITY CETE I TE FLLWIG CMPUD C 2 olutions 5.1. trans not stereoisomeric trans trans trans f) not stereoisomeric
80 CAPTE 5 killbuilder 5.9 Assigning configuration from a Fischer projection AIG TE CFIGUATI F TE CIALITY CETE I TE FLLWIG CMPUD C 2 olutions 5.1. trans not stereoisomeric trans trans trans f) not stereoisomeric
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
4 1,2,3 - Clockwise 1,2,3 - Counterclockwise S
 Assigning Stereochemistry using Fischer Projections: Fischer projections can be used to assign stereochemistry. If the th (lowest) priority group is vertical the other three groups will show clockwise
Assigning Stereochemistry using Fischer Projections: Fischer projections can be used to assign stereochemistry. If the th (lowest) priority group is vertical the other three groups will show clockwise
Exam Analysis: Organic Chemistry, Midterm 1
 Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Dr. Steven Pedersen July 28, Chemistry 3A. Midterm 2
 Dr. Steven Pedersen July 28, 2015 hemistry A Midterm 2 Student name: ANSWER KEY Student signature: Problem 1 Problem 2 Problem Problem 4 Problem 5 Problem 6 Problem 7 Total Points (16 pts) (4 pts) (2 pts)
Dr. Steven Pedersen July 28, 2015 hemistry A Midterm 2 Student name: ANSWER KEY Student signature: Problem 1 Problem 2 Problem Problem 4 Problem 5 Problem 6 Problem 7 Total Points (16 pts) (4 pts) (2 pts)
Experiment 6. Stereochemistry
 Experiment 6. Stereochemistry Introduction Organic molecules with the same molecular formula but different arrangements of atoms are called isomers. Structural isomers are different because the location
Experiment 6. Stereochemistry Introduction Organic molecules with the same molecular formula but different arrangements of atoms are called isomers. Structural isomers are different because the location
Optical Isomerism. Types of isomerism. chemrevise.org 20/08/2013. N Goalby Chemrevise.org. Isomerism. Structural isomerism.
 ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. KH (1 equiv.) + KCl THF. + HBr.
 1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
Chapter 6 Principles of Stereochemistry
 6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
Chapter 3: Stereochemistry & Chirality
 Chapter 3: Stereochemistry & Chirality 1. Chiral & Achiral Compounds - Identifying Stereocenters 2. Assigning R & S configurations 3. Diastereomers - Molecules with two or more stereocenters 4. Properties
Chapter 3: Stereochemistry & Chirality 1. Chiral & Achiral Compounds - Identifying Stereocenters 2. Assigning R & S configurations 3. Diastereomers - Molecules with two or more stereocenters 4. Properties
HO C. Explain briefly (in one or two short sentences) the meaning of the following basic stereochemical terms.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 3 Answers Question 1. Four compounds, each having the molecular formula C 3 5, have the I spectra summarized below. What are their structures? a. ne sharp
Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 3 Answers Question 1. Four compounds, each having the molecular formula C 3 5, have the I spectra summarized below. What are their structures? a. ne sharp
STEREOCHEMISTRY A STUDENT SHOULD BE ABLE TO:
 A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
10/4/2010. Chapter 5 Stereochemistry at Tetrahedral Centers. Handedness. 5.1 Enantiomers and the Tetrahedral Carbon
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 5 Stereochemistry at Tetrahedral Centers Richard Morrison University of Georgia, Athens Handedness Right and left hands are not identical
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 5 Stereochemistry at Tetrahedral Centers Richard Morrison University of Georgia, Athens Handedness Right and left hands are not identical
Loudon Chapter 7 Review: Cyclic Compounds Jacquie Richardson, CU Boulder Last updated 8/24/2017
 Compounds with a single ring are monocyclic. For example: Assuming they have no double or triple bonds, they each have one degree of unsaturation. This means that their formulas follow the pattern C nh
Compounds with a single ring are monocyclic. For example: Assuming they have no double or triple bonds, they each have one degree of unsaturation. This means that their formulas follow the pattern C nh
Stereochemistry Tutorials: Assigning R/S and E/Z
 Stereochemistry Tutorials: Assigning R/S and E/Z Definitions for vocabulary words can be found in the llustrated Glossary of rganic hemistry, available at the course web site. Discussion: Every organic
Stereochemistry Tutorials: Assigning R/S and E/Z Definitions for vocabulary words can be found in the llustrated Glossary of rganic hemistry, available at the course web site. Discussion: Every organic
Assign (R) or (S) configurations to the chiral carbons in the following molecules: enantiomers
 CAPTER 5: STERECEMISTRY (cont.) Assign (R) or (S) configurations to the chiral carbons in the following molecules: 3 C 3 C N 2 Molecules With Multiple Chiral Atoms. 1-chloro-2-methylcyclohexane has four
CAPTER 5: STERECEMISTRY (cont.) Assign (R) or (S) configurations to the chiral carbons in the following molecules: 3 C 3 C N 2 Molecules With Multiple Chiral Atoms. 1-chloro-2-methylcyclohexane has four
Practice Hour Examination # 1-2
 CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-2 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-2 Monday,
CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-2 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-2 Monday,
