CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
|
|
|
- Grant Lyons
- 6 years ago
- Views:
Transcription
1 CEM J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C O Which of the following best describes ( )-linalool? achiral compound, racemic mixture, (R)-enantiomer, or (S)-enantiomer (R)-enantiomer What functional groups are present in ( )-linalool? Tertiary alcohol and alkene Is it possible to obtain (Z) and (E) isomers of ( )-linalool? Give a reason for your answer. No. One end of each double bond has two identical groups (methyl or hydrogen) attached to it.
2 CEM N-9 November 2014 Draw the structure of (S)-pent-4-en-2-ol. 3 O When (S)-pent-4-en-2-ol reacts with bromine, 2, two stereoisomers are formed. Draw the structure of both products. O O
3 CEM N-10 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute configuration of alcohol A. Show your working. 2 O 1 C 4 3 (R) (S) There are 2 chiral centres. On the diagram on the left, the priorities are as shown and are in an (R) configuration. On the diagram on the right, the priorities are in an (S) arrangement. 3 4 C 2 O 1 Name compound B fully. (Z)-3-methylpent-2-ene A diastereoisomer of B is also formed in these reactions. Draw the enantiomer of A and the diastereoisomer of B. enantiomer of A diastereoisomer of B O ANSWERS CONTINUES ON TE NEXT PAGE
4 CEM N-10 November 2014 Propose a mechanism for the formation of B from A under the conditions shown. Use curly arrows and draw the structures of any intermediates. O 2 O + TIS QUESTION CONTINUES ON TE NEXT PAGE.
5 CEM N-11 November 2014 Explain why compound C is the minor product of this reaction. 4 C has the new C=C bond with fewer substituents. This is an example of Zeitsev s rule: the more substituted alkene is more thermodynamically stable. Propose a mechanism for the formation of C from D under the conditions shown. Use curly arrows and draw the structures of any intermediates. O t Bu O t Bu Compound C is the major product formed from D under these conditions. What would be the major product if the enantiomer of D were exposed to the same reaction conditions? TE REMAINDER OF TIS PAGE IS FOR ROUG WORKING ONLY.
6 CEM J-11 June 2013 Shown below is a reaction sequence beginning with the chiral alcohol, F. 7 Draw the enantiomer of F. The specific optical rotation of F is +24. What is the optical rotation of a mixture consisting of equal amounts of F and its enantiomer? 0 o Assign the stereochemistry of the atom in alcohol F indicated by the asterisk (*), showing how you arrived at your answer. The order of priority is: O > C(C,C,) > C(C,,) > With lowest priority (d) at back, the order of the groups goes anticlockwise as shown. Therefore the stereochemistry is (S). Alcohol F is oxidised to give the corresponding ketone, G. Is this molecule still chiral? Why/why not? Explain your answer. The molecule is still chiral as the molecule still contains a stereogenic centre. Ketone G is reduced with sodium borohydride, to give a mixture of two alcohols, F and. is a diastereomer of F. Draw the diastereomer. What is the expected ratio of alcohols F and in this mixture? Why? The approximate ratio will be 1:1 as attack by hydride ion is equally likely from above or below the ring. (There may be a slight excess of F as the methyl group may slightly inhibit attack from above by steric interference.)
7 CEM J-12 June 2013 Below is the structure of an ether, J. 5 Draw a constitutional isomer of J. Any of the following: Draw a conformational isomer of J. There is an infinite number of conformational isomers. Only one is given There are no configurational isomers of J. Why not? As there are no rings, double bonds or stereogenic centres, the molecule does not have any diastereomers or enantiomers. Below is the structure of an alkene, K, which does have a configurational isomer. Draw this configurational isomer. Name K, making sure your name distinguishes K from its isomer. (E)-2-butene
8 CEM N-10 November 2013 Shown below is a reaction sequence beginning with the chiral alcohol, A. 7 Draw the enantiomer of A. The specific optical rotation of A is +30. If equal amounts of A and its enantiomer are mixed, what is the optical rotation of the mixture? 0 o Assign the stereochemistry of the atom in alcohol A indicated by the asterisk (*), showing how you arrived at your answer. The order of priority is: O > C(C,C,C) > C(C,,) > C(,,) With lowest priority (d) at back, the order of the groups goes anticlockwise as shown. Therefore the stereochemistry is (S). Alcohol A is dehydrated to give the alkene B. Is alkene B chiral? Why/why not? The molecule is still chiral as the molecule still contains a stereogenic centre. Alkene B is hydrated with dilute sulfuric acid, to give a sample that contains A and a diastereomer of A. Draw this diastereomer. In this sample, what do you expect to be the ratio of A and its diastereomer? Why? Ratio A : diastereomer is approximately 1 : 1. The tertiary carbocation intermediate has trigonal planar geometry, so the attacking nucleophile ( 2 O) is equally likely to attack from above or below the plane of the ring.
9 CEM J-12 June 2012 Consider the following molecule (M) isolated from a natural source. N COO 2 N O (M) 6 N 2 Indicate on the above structure all stereogenic centres in molecule (M). Use numbered asterisks (*1, *2, etc.). Select one of these stereogenic centres and determine its absolute configuration. Show your working. Around C*1, the priority of the groups are a > b > c > d. Looking down the C- bond the groups a b c go anticlockwise. Therefore configuration is (S)-. Around C*2, the priority of the groups are a' > b' > c' > d'. Looking down the C- bond (i.e. from behind the plane of the paper) the groups a' b' c' go anticlockwise. Therefore configuration is (S)-.
10 CEM J-15 June 2012 Give the major product from the following reaction. 6 Show the mechanism of the reaction. Make sure you show structural formulas for all relevant intermediate species and the final product, as well as using curly arrows to indicate the movement of electrons (i.e. the breaking and formation of bonds). What is the appropriate stereochemical descriptor for the major product of this reaction? Give a reason for your answer. Racemic mixture. The carbon where the is attached has 4 different groups around it, so is stereogenic. The carbocation from which it forms is planar and so attack by the is equally likely from either the top or bottom side. This results in equal amounts of both enantiomers being formed. Give the structure of the minor product of this reaction and explain why very little of it forms. This product is derived from the primary carbocation intermediate. Secondary carbocations are more stable than primary carbocations, so little of this product forms.
11 CEM N-12 November 2012 Consider compound (P), whose structure is shown below. 4 Give the full name of compound (P) that unambiguously describes its stereochemistry. (R)-3-methyl-1-pentene When compound (P) reacts with bromine ( 2 ), two stereoisomers are formed. Draw the structure of both products and label all stereogenic centres appropriately.
12 CEM N-15 November 2012 When reacts with 1-pentene, three products, L, M and N, are formed. L and M are enantiomers, whilst L and N (and M and N) are constitutional isomers. Give the structures of these products and explain how they form? Discuss the relative amounts of each product, paying attention to the regioselectivity and stereoselectivity of the reaction. int: You need to discuss important aspects of the reaction mechanism, including the relative stabilities of any intermediates, but you do not need to give the full mechanism using curly arrows. 6 L M N Electrophilic addition of + to the double bond gives 2 possible carbocations. The more stable carbocation leads to the major products L and M, which are formed in equal amounts as attack by ion on the planar carbocation is equally likely from either top or bottom. The minor product, N, comes from the less stable carbocation. TE REMAINDER OF TIS PAGE IS FOR ROUG WORKING ONLY.
13 CEM J-9 June 2010 Consider the following pairs of compounds. Indicate the isomeric relationship that exists between the compounds in each set. 8 constitutional isomers (different connectivity) C 3 C 3 3 C C 3 conformational isomers (related by a rotation about a C-C bond) NMe 2 O enantiomers O NMe 2 (A) (non-superimposable mirror images) diastereoisomers (B) (different arrangement in space but not enantiomers) CO 2 Et CO CO 2 Et CO (C) diastereoisomers (different arrangement in space but not enantiomers the molecules are not mirror images of one another) What is the configuration of the stereogenic centre in compound (A)? (S). The groups have the priorities shown below. With the lowest priority group at the back, the other groups are in an anticlockwise order. Give the full name of compound (B) that unambiguously describes its stereochemistry. (Z)-3-bromo-4-methylpent-2-ene Is compound (C) a meso isomer? Give a reason for your answer. No. It has no plane of symmetry.
14 CEM J-12 June 2010 Draw the constitutional formulas of all isomers of C bromo-3-chloropropane 1-bromo-2-chloropropane 2-bromo-1-chloropropane 2-bromo-2-chloropropane 1-bromo-1-chloropropane A number of the above isomers are optically active. For all such compounds, draw the two enantiomers. (S)-1-bromo-1-chloropropane (S)-1-bromo-2-chloropropane (S)-2-bromo-1-chloropropane (R)-1-bromo-1-chloropropane (R)-1-bromo-2-chloropropane (R)-2-bromo-1-chloropropane Select any one of the structures you have drawn on this page and write its full systematic name just below it. See above.
15 CEM N-12 November 2010 The structure of a chiral molecule, P, is shown below. P has a specific optical rotation of P O Assign the stereochemistry at the two stereogenic centres, showing your working. The priorities around the first stereogenic centre are shown. With the lowest priority group at the back, the other groups are related in a clockwise manner: (R) The priorities around the second stereogenic centre are shown. With the lowest priority group at the back, the other groups are related in a clockwise manner: (R) Draw the structure of a molecule that will have a specific optical rotation of 26. Draw a diastereoisomer of P. The addition of hot concentrated sulfuric acid causes P to transform into another molecule, Q (C 6 12 ) that is optically inactive. What is the structure of molecule Q and why is it optically inactive? or (E)-3-methyl-2-pentene (Z)-3-methyl-2-pentene Neither compound has a stereogenic centre, so neither is optically active. Name molecule Q. See above.
16 CEM J-7 June 2009 Consider the following pairs of compounds. Indicate the isomeric relationship that exists between the compounds in each set. 8 constitutional isomers (different connectivity) (A) diastereoisomers (same connectivity but different 3D arrangement) NMe 2 O (B) O NMe 2 enantiomers (non-superimposable mirror images) O C 3 O C 3 conformational isomers (can be interconverted by rotation of the central C-C bond) O CO O CO 2 Et O O CO CO 2 Et diastereoisomers (same connectivity but different 3D arrangement. Not enantiomers) (C) Give the full name of compound (A) that unambiguously describes its stereochemistry. (E)-2-pentene. (The alkyl groups are on opposite sides of the C=C hence (E)). What is the configuration of the stereogenic centre in compound (B)? 1 4 N M e O (B) The priorities are indicated on the figure. With the lowest priority at the back, the sequence is in a clockwise direction: (R). Is compound (C) a meso isomer? Give a reason for your answer. No. C does not have a plane of symmetry so is optically active.
17 CEM J-10 June ,2-Dichloropropane can exist in two enantiomeric forms, compounds I and II. In the boxes below draw structures of the two enantiomers of 1,2-dichloropropane clearly showing the stereochemistry at the chiral carbon. compound I compound II 5 ( S)- e nantiomer (R )-enantiomer There are three other compounds, III, IV and V with molecular formula C In the boxes below, give the constitutional formulas and names of these compounds. Structure compound III Name 2,2-dichloropropane compound IV 1,1-dichloropropane compound V 1,3-dichloropropane ANSWER CONTINUES ON TE NEXT PAGE
18 CEM J-10 June 2009 Compounds I, II, III, IV and V are isomers. From the list enantiomers, diastereomers, conformers, constitutional isomers complete the following table. 4 PAIR OF COMPOUNDS I and III ISOMERIC RELATIONSIP BETWEEN PAIR OF COMPOUNDS constitutional isomers I and IV constitutional isomers II and IV constitutional isomers 1,2-Dichloropropane can be synthesised in the laboratory by treatment of propene with chlorine as is shown in the following equation. C 3 C C C 3 2 C2 C Which of the following best describes the product: (R)-enantiomer, (S)-enantiomer, racemate? racemate (equal amounts of (R) and (S) will be formed)
19 CEM N-7 November 2009 Consider compound F shown below. 8 Assign the stereocentre in compound F as (R) or (S), explaining your reasoning. F (S) The four groups at the stereogenic centre are assigned priorities based on atomic numbers. has highest priority, the lowest. The carbon labelled b, C(C,C,C) has higher priority than the carbon labelled c C(C,,) by examining the atoms attached to them. With d at the back, a b c is anticlockwise, so the configuration is (S). c b d a Assign the double bond stereochemistry in compound F, explaining your reasoning. (Z) Compare the priorities of the two groups at each end of the double bond: i.e. a1 with b1 and a2 with b2. The two low priority groups (b) are on the same side of the double bond, so the configuration is (Z). Draw the enantiomer of compound F. b1 C a1 b2 C 3 C a2 or When compound F is reacted with hydrogen gas in the presence of a palladium catalyst, two stereoisomeric products, G and, are formed. Draw these products. + What word is used to describe the stereochemical relationship between G and? They are diastereomers. They differ in the arrangement of the bonds in space but are not mirror images.
20 CEM J-7 June 2008 Consider the following pairs of compounds. Indicate the isomeric relationship that exists between the compounds in each set. 8 Constitutional isomers. They differ in the interatomic connectivity. C 3 C 3 Conformational isomers. C 3 They differ only be rotation about the central C-C bond. C3 3 C (L) C 3 3 C C 3 Diastereomers. They differ in the arrangement of the atoms in space. They are not mirrorimage stereoisomers. C 3 CO C 3 (M) CO Identical. They are superimposable mirror images of each other. C 3 C C C 3 C 3 C C C 3 Diastereomers. They differ in the arrangement of the atoms in space. They are not mirrorimage stereoisomers. (N) Give the name of compound (L) that unambiguously describes its stereochemistry. 3 C (L) C 3 (E)-but-2-ene The two C 3 groups on either end of the double bond have higher priority than the two groups. As they are located on opposite sides of the double bond, the stereochemistry is designated as (E). ANSWER CONTINUES ON TE NEXT PAGE
21 CEM J-7 June 2008 Give the name of compound (M) that unambiguously describes its stereochemistry. (3) C 3 (1) (4) CO (2) The priority of the groups is > CO > C 3 >. With the lowest priority () at the back, the path from highest to lowest ((1)-(2)-(3)) is clockwise. The stereochemistry is designed as (R). (M) (R)-2-bromopropanal Is compound (N) optically active? Give a reason for your answer. Compound (N) is meso and is not optically active. It is superimposable on its mirror image. Simple rotation of the mirror image on the right generates the molecule on the left. (N) possesses an internal mirror plane (between the carbon atoms) and is thus not chiral. C 3 C C C 3 (N) C 3 C C C 3
22 CEM N-11 November 2008 Consider compound F shown below F Assign the stereocentre in compound F as (R) or (S), explaining your reasoning. The priority of the groups around the stereocentre in F is shown above. has the highest atomic number and has the highest priority. has the lowest atomic number and has the lowest priority. It is placed at the back. The other two groups both have carbon bonded to the stereocentre but the group on the left has a higher priority as it has C atoms attached whereas the group on the right has atoms attached. With the atom at the back, the groups are arranged anti-clockwise and so the stereocentre is assigned as (S). Draw the enantiomer of compound F. When compound F is reacted with hot KO solution, a product (G) is formed that shows three peaks in the 1 NMR spectrum in the region 7-8 ppm and three peaks in the region 5-6 ppm. Draw the structure of this product The signals due to atoms 1, 2 and 3 are all different and occur in the region 5-6 ppm. The signals due to the aromatic atoms appear in the 7-8 ppm region and are due to the groups 4, 5 and 6. ANSWER CONTINUES ON TE PAGE
23 CEM N-11 November 2008 When G is reacted with dilute sulfuric acid, a further product,, is formed. has a peak at 3300 cm 1 in its IR spectrum. Draw the structure of product. O Markovnikov addition of - O across double bond, with O adding to more substituted end of double bond. IR peak at 3300 cm -1 is due to O- stretch. Is formed as a single enantiomer, as a racemate, or is achiral? The alcohol C is stereocentre but the addition reaction generates equal amounts of both enantiomers: a racemic mixture is formed. Assuming an S N 2 mechanism, draw the product of the substitution reaction between F and (C 3 ) 2 N, indicating stereochemistry where appropriate. The S N 2 reaction involves attack from the back, leading to inversion of the stereochemistry.
24 CEM N-12 November 2008 Consider the compound J below. 5 What is the systematic name for compound J. J (E)-hex-2-ene Draw a constitutional isomer of J. Constitutional isomers have different atomic connectivities. Possible constitutional isomers include those below: Draw a configurational isomer of J. Configurational isomers have the same atomic connectivity but different spatial arrangements of the atoms. Because of the restricted rotation about a C=C double bond, the following compound is a configurational isomer of J: Draw the structure of the product formed when compound J is reacted with hydrogen gas ( 2 ) and a palladium on carbon (Pd/C) catalyst. Treatment with 2 a Pd/C catalyst will lead to addition of - across the C=C bond:
25 CEM N-9 November 2006 Propionaldehyde (propanal) is treated first with phenylmagnesium bromide in dry diethyl ether and then with dilute aqueous acid, to yield alcohol G. 5 O G C 2 C 3 State whether G is obtained as the (R)-enantiomer, the (S)-enantiomer, a racemic mixture, or is achiral. Racemic mixture List below, the substituents on the stereogenic carbon atom in G, in decreasing priority (i.e. from highest to lowest priority), as determined by the sequence rules. highest priority lowest priority -O -C 2 C 3 - Draw the (R) enantiomer of G, showing the correct absolute stereochemistry. (a) O 3 C 2 C (c) (b) The incomplete proposed mechanism for the reaction of (E)-but-2-ene with aqueous acid is shown below. Complete the mechanism by adding curly arrows and relevant lone pairs to illustrate the bonding changes that take place. 5 O O O O What two-word description may be used for the name of this mechanism? electrophilic addition
26 CEM J-6 June 2003 Adrenaline (A) is produced by the body as part of its flight or fight response. 6 * (i) On the above diagram, clearly mark the stereogenic centre in (A) with an asterisk (*). (ii) List the substituents attached to the stereogenic centre in descending order of priority according to the sequence rules. highest priority lowest priority O C 2 NC 3 aromatic ring (iii) What is the absolute stereochemistry of adrenaline (A)? Write (R) or (S). (R) (iv) Name the functional groups a, b and c, present in adrenaline (A)? a = alcohol b = amine c = aromatic Give the stick representation of the product formed when bromine reacts with 2-methylbutene. 2 2 * State whether the product formed by this reaction is achiral, the (S)-enantiomer, the (R)-enantiomer, a meso-compound or a racemic mixture? There is a single stereogenic centre in the product. Although addition of 2 is stereospecific, either enantiomer can be formed: a racemic mixture
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
Topics in the June 2009 Exam Paper for CHEM1102
 June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
CHEM1902/ N-8 November Consider the following reaction sequences beginning with the carboxylic acid, E.
 CEM1902/4 2014--8 ovember 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 ame compounds E and G. E: propionic acid G: methyl propionate Propose structures for compounds
CEM1902/4 2014--8 ovember 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 ame compounds E and G. E: propionic acid G: methyl propionate Propose structures for compounds
It is possible for organic molecules with the same molecular formula to have different structures
 Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Enantiomers. nonsuperimposable mirror image Both Configuration will be opposite. Both Configuration will be opposite
 Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
CHAPTER 5. Stereoisomers
 CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
Chemistry 201. MW 12:00pm 1:15pm Examination #2 August 15 th Bronco ID. Question Score Possible Points. 1 (12pts) 2 (24pts) 3 (25pts)
 Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
(S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer
 C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
E30 ENANTIOMERS Chirality in organic chemistry
 E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
Topics in the November 2014 Exam Paper for CHEM1102
 November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
3.2.8 Haloalkanes. Elimination. 148 minutes. 145 marks. Page 1 of 22
 3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
REVIEW PROBLEMS Key. 1. Draw a complete orbital picture for the molecule shown below. Is this molecule chiral? Explain. H H.
 rganic hemistry II (E325) REVIEW PRBLEMS Key 1. Draw a complete orbital picture for the molecule shown below. Is this molecule chiral? Explain. 3 3 sp3 orbital p orbital sp2 orbital s orbital molecule
rganic hemistry II (E325) REVIEW PRBLEMS Key 1. Draw a complete orbital picture for the molecule shown below. Is this molecule chiral? Explain. 3 3 sp3 orbital p orbital sp2 orbital s orbital molecule
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Lecture 4: 12.4 Isomerism
 Lecture 4: 12.4 Isomerism Learning Outcomes: At the end of the lesson the students should be able to : Define isomerism. Explain constitutional isomerism. chain isomers positional isomers functional group
Lecture 4: 12.4 Isomerism Learning Outcomes: At the end of the lesson the students should be able to : Define isomerism. Explain constitutional isomerism. chain isomers positional isomers functional group
Organic Chemistry HL IB CHEMISTRY HL
 Organic Chemistry HL IB CHEMISTRY HL Understandings: Nucleophilic Substitution Reactions: SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular
Organic Chemistry HL IB CHEMISTRY HL Understandings: Nucleophilic Substitution Reactions: SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Optical Isomerism. Types of isomerism. chemrevise.org 20/08/2013. N Goalby Chemrevise.org. Isomerism. Structural isomerism.
 ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
CHEM 263 Oct 18, Do they have the same molecular formula?
 EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
CHE 321 Summer 2010 Exam 2 Form Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III
 CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
Chapter 4: Stereochemistry
 Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
Number of d electrons CO 2 carbon dioxide +IV or +4 0
 CEM1611 2007-J-2 June 2007 Complete the following table. Give, as required, the formula, the systematic name, the oxidation number of the underlined atom and, where indicated, the number of d electrons
CEM1611 2007-J-2 June 2007 Complete the following table. Give, as required, the formula, the systematic name, the oxidation number of the underlined atom and, where indicated, the number of d electrons
Chapter 6 Principles of Stereochemistry
 6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
Stereochemistry. Based on McMurry s Organic Chemistry, 6 th edition
 Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Lesson 4. Molecular Geometry and Isomers II. Lesson 4 CH 3 HO H OH
 Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Names. Chiral: A chiral object is not superimposable upon its mirror image. A chiral object contains the property of "handedness.
 CEM 241 IN-CLASS #3 MOLECULAR MODELS EXERCISE Names Stereoisomerism Construct a model containing a tetrahedral carbon (black ball) that is attached to four different atoms (use the green, orange, purple
CEM 241 IN-CLASS #3 MOLECULAR MODELS EXERCISE Names Stereoisomerism Construct a model containing a tetrahedral carbon (black ball) that is attached to four different atoms (use the green, orange, purple
Chapter 5 Stereochemistry. Stereoisomers
 Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
STEREOGENIC CENTER (Chiral Center,Asymmetric Center)
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
Stereochemistry. 3-dimensional Aspects of Tetrahedral Atoms
 Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
(1) Check to see if the two compounds are identical. (2) Recall the definitions of stereoisomers, conformational isomers, and constitutional isomers.
 MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
Stereochemistry. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects.
 Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Topics in the November 2008 Exam Paper for CHEM1102
 November 2008 Topics in the November 2008 Exam Paper for CEM1102 Click on the links for resources on each topic. 2008-N-2: 2008-N-3: 2008-N-4: 2008-N-5: 2008-N-6: 2008-N-7: 2008-N-8: 2008-N-9: Periodic
November 2008 Topics in the November 2008 Exam Paper for CEM1102 Click on the links for resources on each topic. 2008-N-2: 2008-N-3: 2008-N-4: 2008-N-5: 2008-N-6: 2008-N-7: 2008-N-8: 2008-N-9: Periodic
Solutions 80 CHAPTER a) trans b) not stereoisomeric c) trans d) trans e) trans f) not stereoisomeric g) cis
 80 CAPTE 5 killbuilder 5.9 Assigning configuration from a Fischer projection AIG TE CFIGUATI F TE CIALITY CETE I TE FLLWIG CMPUD C 2 olutions 5.1. trans not stereoisomeric trans trans trans f) not stereoisomeric
80 CAPTE 5 killbuilder 5.9 Assigning configuration from a Fischer projection AIG TE CFIGUATI F TE CIALITY CETE I TE FLLWIG CMPUD C 2 olutions 5.1. trans not stereoisomeric trans trans trans f) not stereoisomeric
Chem 341 Jasperse Ch. 9 Handouts 1
 Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
O N N. electrons in ring
 ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
ame I. ( points) Page 1 A. The compound shown below is a commonly prescribed antifungal drug. It belongs to a category of compounds known as triazoles. Analyze the geometry and other properties for various
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
a. Does the model have a plane of symmetry? Yes No The central carbon is said to be a stereocenter, stereogenic center, or chiral carbon.
 Name: TA Name Lab Section: Day Time OPTICAL ISOMERISM 1. Construct a model that has a central carbon atom with 4 different colored spheres attached to it, representing four different atoms or groups. Draw
Name: TA Name Lab Section: Day Time OPTICAL ISOMERISM 1. Construct a model that has a central carbon atom with 4 different colored spheres attached to it, representing four different atoms or groups. Draw
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22
 Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Due Date: 2) What is the relationship between the following compounds?
 Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Option II: Chiral + Achiral = Optically Active Diastereomers
 Option II: Chiral + Achiral = Optically Active Diastereomers What about additions to chiral alkenes? The previous examples were reactions done on achiral alkenes. What is the difference when an alkene
Option II: Chiral + Achiral = Optically Active Diastereomers What about additions to chiral alkenes? The previous examples were reactions done on achiral alkenes. What is the difference when an alkene
1. (3 pts) Circle the highest priority substituent of the following list:
 Ch 334 Midterm #3 November 17, 2006 Code 1. (3 pts) Circle the highest priority substituent of the following list: 2. (4 pts) Rank the following groups in order of increasing priority. Place the letter
Ch 334 Midterm #3 November 17, 2006 Code 1. (3 pts) Circle the highest priority substituent of the following list: 2. (4 pts) Rank the following groups in order of increasing priority. Place the letter
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Chapter 10 Lecture Outline
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Organic Chemistry 1 CHM 2210 Exam 3 (November 9, 2001)
 Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
Answer Marks Guidance
 Question number (a) molecular formula: C 4 H 8 Answer Marks Guidance empirical formula: CH 2 This is a revision of earlier chapters. (b) (i) name of mechanism: electrophilic addition Remember that reactions
Question number (a) molecular formula: C 4 H 8 Answer Marks Guidance empirical formula: CH 2 This is a revision of earlier chapters. (b) (i) name of mechanism: electrophilic addition Remember that reactions
CHEM 2423 Unit 8 Homework Answers
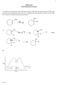 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Unless otherwise stated, all images in this file have been reproduced from:
 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 hemistry 1B EM1002 Lecture
Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 hemistry 1B EM1002 Lecture
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Exam Analysis: Organic Chemistry, Midterm 1
 Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
STEREOCHEMISTRY A STUDENT SHOULD BE ABLE TO:
 A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
2.9 ALKENES EXTRA QUESTIONS
 .9 ALKENES EXTRA QUESTIONS 1. (a) Most of the ethene used by industry is produced when ethane is heated to 900 C in the absence of air. Write an equation for this reaction.... Name the type of polymerisation
.9 ALKENES EXTRA QUESTIONS 1. (a) Most of the ethene used by industry is produced when ethane is heated to 900 C in the absence of air. Write an equation for this reaction.... Name the type of polymerisation
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
CHEM1102 Worksheet 4 Answers to Critical Thinking Questions Model 1: Infrared (IR) Spectroscopy
 CEM1102 Worksheet 4 Answers to Critical Thinking Questions Model 1: Infrared (IR) Spectroscopy 1. See below. Model 2: UV-Visible Spectroscopy 1. See below. 2. All of the above. 3. Restricted to the identification
CEM1102 Worksheet 4 Answers to Critical Thinking Questions Model 1: Infrared (IR) Spectroscopy 1. See below. Model 2: UV-Visible Spectroscopy 1. See below. 2. All of the above. 3. Restricted to the identification
Problem Set 8: Substitution Reactions-ANSWER KEY. (b) nucleophile NH 3 H C. (d) H 3 N
 Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Chem 232. Problem Set 9. Question 1. D. J. Wardrop
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
Organic Chemistry. Alkenes (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
General Glossary. General Glossary
 General Glossary Absolute configuration The actual three-dimensional structure of a chiral molecule. Absolute configurations are specified verbally by the Cahn-Ingold-Prelog R,S convention and are represented
General Glossary Absolute configuration The actual three-dimensional structure of a chiral molecule. Absolute configurations are specified verbally by the Cahn-Ingold-Prelog R,S convention and are represented
Chapter 5 Stereochemistry
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Elimination Reactions The E2 Mechanism
 Elimination Reactions The E2 Mechanism The E2 Mechanism X X- B: B- δ- B:- δ+ R 1 δ- R 2 δ+ X δ- The E2 Mechanism R 3 R 4 transition state Free energy (G) Eact B:- B R 1 R 2 X R 1 R 2 R 3 R 4 R 4 R 3 X:-
Elimination Reactions The E2 Mechanism The E2 Mechanism X X- B: B- δ- B:- δ+ R 1 δ- R 2 δ+ X δ- The E2 Mechanism R 3 R 4 transition state Free energy (G) Eact B:- B R 1 R 2 X R 1 R 2 R 3 R 4 R 4 R 3 X:-
C 4 H 10 O. butanol. diethyl ether. different carbon skeleton different functional group different position of FG
 hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
STEREOGENIC CENTER (Chiral Center,Asymmetric Center) Atom (usually carbon) to which 4 different groups are attached: W Z C X Y
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
CHEM J-2 June 2012
 CHEM1102 2012-J-2 June 2012 Explain why HClO 4 is a stronger Brønsted acid than HBrO 4, but HCl is a weaker acid than HBr. 2 In Group 17 oxyacids, electron density is drawn away from the O atom as the
CHEM1102 2012-J-2 June 2012 Explain why HClO 4 is a stronger Brønsted acid than HBrO 4, but HCl is a weaker acid than HBr. 2 In Group 17 oxyacids, electron density is drawn away from the O atom as the
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
2.0 h; 240 points please print clearly Dr. Kathleen Nolta Signature. Problem Points Score GSI I 42 II 35 III 36 IV 46 V 42 VI 39 Total 240
 hemistry 10 irst ame inal Examination Last ame.0 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # Problem Points core GI I II 5 III IV V VI 9 Total 0 Precision in drawing counts. heck
hemistry 10 irst ame inal Examination Last ame.0 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # Problem Points core GI I II 5 III IV V VI 9 Total 0 Precision in drawing counts. heck
KEY Page 1. Name H H H 3 C C 3. Cl CH. AlCl 3 H H. + enantiomer H 3 C CH 3. Cl CH3 HNO 3. AlCl NO 2 CH 3 1) O 3 2) H 2 O 2 OH H 3 C C.
 ame Page 1 I. (8 points) omplete the following reactions as directed. Transformations requiring sequential experimental steps should be numbered appropriately. Show the major organic product(s) unless
ame Page 1 I. (8 points) omplete the following reactions as directed. Transformations requiring sequential experimental steps should be numbered appropriately. Show the major organic product(s) unless
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
If Compound 2 (below) was used in the above reaction, how might things change? Name Page 1. I. (23 points)
 I. (2 points) ame Page itrogen atoms in amines can undergo substitution reactions repeatedly under mildly basic conditions. Provide the structure of the starting amine and supply the missing reagent(s).
I. (2 points) ame Page itrogen atoms in amines can undergo substitution reactions repeatedly under mildly basic conditions. Provide the structure of the starting amine and supply the missing reagent(s).
Chapter 5 Stereochemistry
 Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional)
 Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional) Stereoisomers... have the same connectivity but a different
Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional) Stereoisomers... have the same connectivity but a different
HO HO. 1) BH 3 HCl. + enantiomer either one may be drawn 4. + enantiomer either one may be drawn 4 1) O 3
 . (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
. (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
CEM 351 2nd EXAM/Version A Friday, October 17, :50 2:40 p.m. Room 138, Chemistry
 Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
20 Halogenoalkanes:substitution and elimination reactions
 20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
9. Stereochemistry. Stereochemistry
 9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
240 Chem. Stereochemistry. Chapter 5
 240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
Worksheet Chapter 10: Organic chemistry glossary
 Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Organic Chemistry Chapter 5 Stereoisomers H. D. Roth
 Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
CHEM Exam 2 ANSWER KEY
 CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
MOLECULAR MODELS : STEREOISOMERS
 MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
