Lecture 2: Rotational and Vibrational Spectra
|
|
|
- Emily Webster
- 5 years ago
- Views:
Transcription
1 Lctur : Rotational and Vibrational Spctra 1. Light-mattr intraction. Rigid-rotor modl for diatomic molcul 3. Non-rigid rotation 4. Vibration-rotation for diatomics
2 H O 1. Light-mattr intraction Possibilitis of intraction Prmannt lctric dipol momnt Rotation and vibration produc oscillating dipol (Emission/Absorption) HCl Enrgy ΔE Absorption = qd Emission What if Homonuclar? Elastic scattring (Rayligh), s = Inlastic scattring (Raman), s = / Virtual Stat v s m or as s < Inlastic scattring as >
3 1. Light-mattr intraction Elmnts of spctra: Lin position Lin strngth Lin shaps Intrnal Enrgy: E int = E lc (n)+e vib ()+ E rot (J) Lin position () is dtrmind by diffrnc btwn nrgy lvls What dtrmins th nrgy lvls? Quantum Mchanics! μ Rotation: Microwav Rgion (ΔJ) Elctric dipol momnt: + q r i i i E vib ΔE E rot μ x C O E μ x T rot 1/ν v s E lc Ar som molculs Microwav inactiv? YES,.g., H, Cl, CO Tim 3
4 1. Light-mattr intraction Elmnts of spctra: Lin position Lin strngth Lin shaps Intrnal Enrgy : E int = E lc (n)+e vib ()+ E rot (J) μ Rotation: Microwav Rgion (ΔJ) Vibration: Infrard Rgion (Δv, J) E vib ΔE E rot μ x C O δ+ δ- μ x t= v s E lc Htronuclar diatomic cas is IR-activ Ar som vibrations Infra-rd inactiv? Ys,.g., symmtric strtch of CO 4
5 1. Light-mattr intraction Summary E int = E lc (n)+e vib (v)+ E rot (J) ΔE rot < ΔE vib < ΔE lc E vib ΔE E rot Currnt intrst Enrgy lvls ar discrt Optically allowd transitions may occur only in crtain cass Absorption/mission spctra ar discrt Rotation Rigid Rotor Non-rigid Rotor E lc Vibration Simpl Harmonic Oscillator (SHO) Anharmonic Oscillator (AHO) 5
6 . Rigid-Rotor modl of diatomic molcul Rigid Rotor Axs of rotation m 1 m C + - ~ cm r 1 r C: r Cntr of mass C 1 m 1 = r m r 1 +r = r ~ 10-8 cm Assum: Point masss (d nuclus ~ cm, r ~ 10-8 cm) r = const. ( rigid rotor ) Rlax this latr 6
7 . Rigid-Rotor modl of diatomic molcul Classical Mchanics Momnt of Inrtia I miri r m1m rducd mass m m 1 -body problm changd to singl point mass Rotational Enrgy h Erot Irot Irot J J 1 J J 1 I I 8 I Convntion is to dnot rotational nrgy as F(J), cm -1 F E hc h 8 Ic 1 rot J cm J J 1 BJ J 1, Quantum Mchanics Valu of ω rot is quantizd I rot J 1 h / J Rot. quantum numbr = 0,1,, E rot is quantizd! hc Not : E, J h hc(, cm -1 ) so (nrgy,cm -1 ) = (nrgy,j)/hc 7
8 . Rigid-Rotor modl of diatomic molcul Rotational spctrum Schrödingr s Equation: Transition probability d m dx m d n E U x x 0 J 1 Slction Ruls for rotational transitions (uppr) (lowr) ΔJ = J J = +1 Rcall: F J BJ J 1 Wav function Complx conjugat Dipol momnt.g., J 1 FJ 0 B 0 B J 1 J 0 F 8
9 . Rigid-Rotor modl of diatomic molcul Rotational spctrum Rmmbr that: F J BJ J 1 E.g., J 1 FJ 0 B 0 B J 1 J 0 F J F 1 st diff = ν nd diff = spacing 1B B 6B 3 1B 4 0B B 4B 6B 8B B B B Lins vry B! 6B B F=0 B 4B 6B 1 J=0 In gnral: J 1 J J ' J " BJ" 1J " BJ" J" 1 J ' J ", cm 1 B J" 1 Lt s look at absorption spctrum 9
10 . Rigid-Rotor modl of diatomic molcul Rotational spctrum 1B Rcall: E.g., F J BJ J 1 0 FJ 1 FJ 0 B 0 B J 1 J B B F=0 B 4B 6B 1 J=0 T λ Htronuclar molculs only! λ J =0 ~.5mm rot for J=0 1 ~10 11 Hz (frquncis of rotation) J ν/b=j +1 Not: 1. Uniform spacing (asy to idntify/intrprt). B CO ~ cm -1 λ J =0 = 1/ν = ¼ cm =.5mm (microwav/mm wavs) rot,j=1 = c/λ = 3x10 10 /0.5 Hz = 1.x10 11 Hz (microwav) 10
11 . Rigid-Rotor modl of diatomic molcul Usfulnss of rotational spctra Masurd spctra Lin spacing = B Physical charactristics of molcul h B I r r Accuratly! 8 Ic Exampl: CO B = cm -1 r CO = Å 10-6 Å = m! 11
12 . Rigid-Rotor modl of diatomic molcul Intnsitis of spctral lins Equal probability assumption (crud but usful) Abs. (or miss.) probability pr molcul is (crudly) indpndnt of J Abs. (or miss.) spctrum varis w/ J lik Boltzmann distribution Rcall: J 1xp E kt N J J / N Q E J k hcf k Partition function: J Q rot Dgnracy is a QM rsult associatd w/ possibl dirctions of Angular Momntum vctor rot hc BJ J k 1 kt hcb hc k Dfin rotational T: K B r 1 J 1xp J J 1 r J 1 T r J 1 Symmtric no. (ways of rotating to achiv sam orintation) = 1 for microwav activ T r / r / T CO: σ=1 microwav activ! N : σ= microwav inactiv! 1
13 . Rigid-Rotor modl of diatomic molcul Intnsitis of spctral lins Rotational Charactristic Tmpratur: K Spcis θ rot [K] O.1 N.9 NO.5 Cl N J J 1 xp r J J 1 / T N T / r Strongst pak: occurs whr th population is at a local maximum dn J / N 1/ 0 J T / 1/ f T / dj max rot r B hc k hc k 1.44K / cm rot 1 13
14 . Rigid-Rotor modl of diatomic molcul Effct of isotopic substitution h Rcall: B 8 Ic Changs in nuclar mass (nutrons) do not chang r 0 r dpnds on binding forcs, associatd w/ chargd particls Can dtrmin mass from B Thrfor, for xampl: B B 1 13 C C O O m m 13 C C Agrs to 0.0% of othr dtrminations 14
15 3. Non-Rigid Rotation Two ffcts; follows from B 1/ r Vibrational strtching r(v) v r B Cntrifugal distortion r(j) J r B Effcts shrink lin spacings/nrgis Nots: Rsult: J ", v v v 3 4B 1. But D is small; whr D B sinc J B J J 1 D J J 1 F v v v D B NO J" 1 4D J" 3 J ' B 1 B Cntrifugal distribution constant D/B smallr for stiff/hi-frq bonds 15
16 3. Non-Rigid Rotation Nots: E.g., NO B 6 1. D is small;.g., D B. v dpndnc is givn by 1 3 4B D B B D/B smallr for stiff/hi-frq bonds NO / D ~ D cm D x 13.97cm ; / 1 1/ ~ 810 / B 9 cm 1 ~ / B D v v Asid: B D v v 8 x 1/ 1/ / D 3 B 4B Hrzbrg, Vol. I 5 1 dnots valuatd at quilibrium intr-nuclar sparation r 16
17 4. Vibration-Rotation Spctra (IR) (oftn trmd Rovibrational) 1. Diatomic Molculs Simpl Harmonic Oscillator (SHO) Anharmonic Oscillator (AHO). Vibration-Rotation spctra Simpl modl R-branch / P-branch Absorption spctrum 3. Vibration-Rotation spctra Improvd modl 4. Combustion Gas Spctra Vibration-Rotation spctrum of CO (from FTIR) 17
18 4.1. Diatomic Molculs Simpl Harmonic Oscillator (SHO) Δ/ r min m 1 m r Molcul at instant of gratst comprssion Equilibrium position (balanc btwn attractiv + rpulsiv forcs) i.. min nrgy position As usual, w bgin w. classical mchanics + incorporat QM only as ndd 18
19 4.1. Diatomic Molculs Simpl Harmonic Oscillator (SHO) Classical mchanics Forc k r - Linar forc law / Hook s law Fundamntal Frq. Potntial Enrgy Quantum mchanics v = vib. quantum no. = 0,1,,3, Vibration nrgy G=U/hc G Slction Ruls: s r 1 v, cm v / cv 1/ v v' v" 1 vib only! 1 1 vib k s /, cm / c 1 U kr r Parabola cntrd at distanc of min. potntial nrgy Equal nrgy spacing ral Zro nrgy = diss. nrgy 19
20 4.1. Diatomic Molculs Anharmonic Oscillator (AHO) SHO AHO 1 1 Gv, cm v 1/ Gv, cm v 1/ x v 1/... H. O. T. Dcrass nrgy spacing 1 st anharmonic corrction ral Δν=+1 Fundamntal Band (.g., 1 0, 1) 1 10 G 1 G 0 1x 1 4x Δν=+ 1 st Ovrton (.g., 0,3 1) 0 1 3x Δν=+3 nd Ovrton (.g., 3 0,4 1) x In addition, brakdown in slction ruls 0
21 4.1. Diatomic Molculs Vibrational Partition Function hc hc Qvib 1 xp xp kt kt Or choos rfrnc (zro) nrgy at v=0, so Gv v thn Vibrational Tmpratur Q vib 1 hc 1 xp kt 1 Th sam zro nrgy must b usd in spcifying molcular nrgis E i for lvl i and in valuating th associatd partition function hc k vibk N N vib g vib xp xp v Q v T vib vib vib / T 1 xp T whr g vib vib 1 Spcis θ vib [K] θ rot [K] O 70.1 N NO Cl
22 4.1. Diatomic Molculs Som typical valus (Banwll, p.63, Tabl 3.1) Gas Molcular Wight Vibration ω [cm -1 ] Anharmonicity constant x Forc constant k s [dyns/cm] Intrnuclar distanc r [Å] Dissociation nrgy D q [V] CO x NO x H x Br x Not IR-activ, us Raman spctroscopy! k / m / for homonuclar molculs D / 4x larg k, larg D Wak, long bond loos spring constant low frquncy
23 4.1. Diatomic Molculs Som usful convrsions Enrgy Forc Lngth 1cal J 1cm cal/mol 1V cm 1 N 10 1A o 5 0.1nm dyns kcal/mol J How many HO lvls in a molcul? (Considr CO) D o 56 kcal N no. of HO lvls 56 kcal/mol 1.86 cal/mol cm 170 cm 1 41 Actual numbr is?greater as AHO shrinks lvl spacing 3
24 4.. Vib-Rot spctra simpl modl Born-Oppnhimr Approximation Vibration and Rotation ar rgardd as indpndnt Vibrating rigid rotor Enrgy: Slction Ruls: Lin Positions: v'=1 v"=0 P R T v, J RR SHO FJ Gv BJ J 1 v 1/ v 1 J 1 T ' T" T J'=J"+1 J'= J" J'= J"-1 J"+1 J" v', J ' T v", J" Transition Probabilitis P branch Two Branchs: P (ΔJ = -1) R(ΔJ=+1) Asid: Nomnclatur for branchs Branch O P Q R S ΔJ Null Gap R() ΔJ = J' - J" R branch R(0) P(1) o B 4
25 4.. Vib-Rot spctra simpl modl R-branch R 1 J", cm G v' Gv" BJ" 1J " BJ" J" 1 R v o o (SHO)... 1 x 1 4x J" 0 BJ" 1 = Rotationlss transition wavnumbr (AHO,1 0) (AHO, 1) Not: spacing = B, sam as RR spctra P-branch P J" 0 BJ" Not: ω o =f(v")foraho v'=1 P R J'=J"+1 J'= J" J'= J"-1 P-R Branch pak sparation _ 8BkT hc v"=0 J"+1 J" Largr nrgy 5
26 4.. Vib-Rot spctra simpl modl Absorption spctrum (for molcul in v" = 0) Width, shap dpnds on masuring instrumnt and xprimntal conditions Transition Probabilitis Null Gap R() P branch R branch Lin (sum of all lins is a band ) R(0) P(1) o B Hight of lin amount of absorption N J /N Equal probability approximation indpndnt of J (as with RR) What if w rmov RR limit? Improvd tratmnt 6
27 4.3. Vib-Rot spctra improvd modl Brakdown of Born-Oppnhimr Approximation Allows non-rigid rotation, anharmonic vibration, vib-rot intraction T v, J Gv Fv, J B(v) v 1/ x v 1/ B J J 1 D J J 1 R-branch P-branch SHO Anharm. corr. RR(v) Cnt. dist. trm v", J" v" Bv ' 3Bv ' Bv" J" Bv ' Bv" J" v", J" v" B ' B " J" B ' B " J R o P o v v v v v " Bv B v 1/ Bv ' B ' v' 1/ B " " v" 1/ Transition Probabilitis P branch B v v B ' B " v 0 Null Gap P(1) R(0) R() v B ' B " v v Spacing on P sid, on R sid R branch o B 7
28 4.3. Vib-Rot spctra improvd modl Bandhad Transition Probabilitis P branch Null Gap R() P branch R branch R(0) P(1) J" R branch 4 Bandhad 3 1 o B dr dj J B' B" B' B" J" 0 B' E.g., CO B o B B J" ' bandhad B not oftn obsrvd Incrasing spacing Dcrasing spacing 8
29 4.3. Vib-Rot spctra improvd modl Finding ky paramtrs: B, α, ω, x 1 st Approach: Us masurd band origin data for th fundamntal and first ovrton, i.., ΔG 1 0, ΔG 0, to gt ω, x nd Approach: G G 10 0 G G 1 G0 1 x G0 1 3x Fit rotational transitions to th lin spacing quation to gt B and α B' B v' 1/ B" B v" 1/ o V V B' B" m B' B" m m J 1 in R - branch m J in P - branch B', B", x B, α 9
30 4.3. Vib-Rot spctra improvd modl Finding ky paramtrs: B, α, ω, x 3 rd Approach: Us th mthod of common stats v' v" ΔE P(J+1) R(J-1) J'= J" J'= J"-1 J"+1 J" J"- 1 Common uppr-stat In gnral E F R B FJ BJ J 1 J 1 FJ 1 J 1 PJ 1 " J 1J B" J 1J E B" 4J B" v' ΔE P(J) R(J) J'=J"+1 J'= J" J'= J"-1 E E F B J 1 FJ 1 ' J 1J B' J 1J B' 4J B' B, v" J"+1 J" Common lowr-stat 30
31 4.3. Vib-Rot spctra improvd modl Isotopic ffcts 1 B I ks 1 1 Lin spacing changs as μ changs Band origin changs as μ changs 1 st Exampl: CO Isotop 13 C 16 O 13 1 C C O O B 13 C C O 16 O B 13 C 16 O C 16 O B cm / 50cm 1 31
32 4.3. Vib-Rot spctra improvd modl Isotopic ffcts CO fundamntal band Not vidnc of 1.1% natural abundanc of 13 C 3
33 4.3. Vib-Rot spctra improvd modl Isotopic ffcts 1 B I ks 1 1 Lin spacing changs as μ changs Band origin changs as μ changs nd Exampl: HCl Isotop H 35 Cl and H 37 Cl H Cl 3H Cl / / / Shift in ω is.00075ω =.cm -1 Small! 33
34 4.3. Vib-Rot spctra improvd modl Isotopic ffcts HCl fundamntal band Not isotropic splitting du to H 35 Cl and H 37 Cl 34
35 4.3. Vib-Rot spctra improvd modl Hot bands Whn ar hot bands (bands involving xcitd stats) important? v v g xp Nv T v v v xp 1 xp N Q vib T T 10 N 1 E.g. v, CO 3000K 1 1 N 1 Hot bands bcom important whn tmpratur is comparabl to th charactristic vibrational tmpratur hc / kt 1 Gas 01 cm N1/ N0 300K 1000K H x x 10-3 HCl x x 10 - N x x 10 - CO x x 10 - O x x 10-1 S x x 10-1 Cl x x 10-1 I x x
36 4.3. Vib-Rot spctra improvd modl Exampls of intnsity distribution within th rotation-vibration band B = 10.44cm -1 (HCl) B = cm -1 (CO) 36
37 4.4. Absorption Spctra for Combustion Gass TDL Snsors Provid Accss to a Wid Rang of Combustion Spcis/Applications Small spcis such as NO, CO, CO, and H O hav discrt rotational transitions in th vibrational bands Largr molculs,.g., hydrocarbon fuls, hav blndd spctral faturs 37
38 Nxt: Diatomic Molcular Spctra Elctronic (Rovibronic) Spctra (UV, Visibl)
22/ Breakdown of the Born-Oppenheimer approximation. Selection rules for rotational-vibrational transitions. P, R branches.
 Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
Lecture 28 Title: Diatomic Molecule : Vibrational and Rotational spectra
 Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
Title: Vibrational structure of electronic transition
 Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
15 Lecture Short Course at Tsinghua University
 15 Lctur Short Cours at Tsinghua Univrsity Lcturr: Ronald K. Hanson Woodard Profssor, Dpt. of Mchanical Enginring Ph.D. Stanford, Aro/Astro; at Stanford sinc 197 Undrlying Scinc: Molcular Spctroscopy Diagnostic
15 Lctur Short Cours at Tsinghua Univrsity Lcturr: Ronald K. Hanson Woodard Profssor, Dpt. of Mchanical Enginring Ph.D. Stanford, Aro/Astro; at Stanford sinc 197 Undrlying Scinc: Molcular Spctroscopy Diagnostic
Hydrogen Atom and One Electron Ions
 Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpnCoursWar http://ocw.mit.du 5.80 Small-Molcul Spctroscopy and Dynamics Fall 008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. Lctur # 3 Supplmnt Contnts
MIT OpnCoursWar http://ocw.mit.du 5.80 Small-Molcul Spctroscopy and Dynamics Fall 008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. Lctur # 3 Supplmnt Contnts
Classical Magnetic Dipole
 Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
Molecular Orbitals in Inorganic Chemistry
 Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
Numerical Problem Set for Atomic and Molecular Spectroscopy. Yr 2 HT SRM
 Numrical Problm St for Atomic and Molcular Spctroscopy Yr HT SRM Sction 1: Atomic Spctra 1. For ach of th atomic trm symbols 1 S, P, 3 P, 3 D, 4 D, writ down: a) Th associatd valus of th total spin and
Numrical Problm St for Atomic and Molcular Spctroscopy Yr HT SRM Sction 1: Atomic Spctra 1. For ach of th atomic trm symbols 1 S, P, 3 P, 3 D, 4 D, writ down: a) Th associatd valus of th total spin and
Background: We have discussed the PIB, HO, and the energy of the RR model. In this chapter, the H-atom, and atomic orbitals.
 Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
2. Laser physics - basics
 . Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
. Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
Lecture 37 (Schrödinger Equation) Physics Spring 2018 Douglas Fields
 Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
Determination of Vibrational and Electronic Parameters From an Electronic Spectrum of I 2 and a Birge-Sponer Plot
 5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
Phys 402: Nonlinear Spectroscopy: SHG and Raman Scattering
 Rquirmnts: Polariation of Elctromagntic Wavs Phys : Nonlinar Spctroscopy: SHG and Scattring Gnral considration of polariation How Polarirs work Rprsntation of Polariation: Jons Formalism Polariation of
Rquirmnts: Polariation of Elctromagntic Wavs Phys : Nonlinar Spctroscopy: SHG and Scattring Gnral considration of polariation How Polarirs work Rprsntation of Polariation: Jons Formalism Polariation of
Ch. 24 Molecular Reaction Dynamics 1. Collision Theory
 Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Coupled Pendulums. Two normal modes.
 Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
Schrodinger Equation in 3-d
 Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
Contemporary, atomic, nuclear, and particle physics
 Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
The pn junction: 2 Current vs Voltage (IV) characteristics
 Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
The van der Waals interaction 1 D. E. Soper 2 University of Oregon 20 April 2012
 Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
Exam 2 Thursday (7:30-9pm) It will cover material through HW 7, but no material that was on the 1 st exam.
 Exam 2 Thursday (7:30-9pm) It will covr matrial through HW 7, but no matrial that was on th 1 st xam. What happns if w bash atoms with lctrons? In atomic discharg lamps, lots of lctrons ar givn kintic
Exam 2 Thursday (7:30-9pm) It will covr matrial through HW 7, but no matrial that was on th 1 st xam. What happns if w bash atoms with lctrons? In atomic discharg lamps, lots of lctrons ar givn kintic
The failure of the classical mechanics
 h failur of th classical mchanics W rviw som xprimntal vidncs showing that svral concpts of classical mchanics cannot b applid. - h blac-body radiation. - Atomic and molcular spctra. - h particl-li charactr
h failur of th classical mchanics W rviw som xprimntal vidncs showing that svral concpts of classical mchanics cannot b applid. - h blac-body radiation. - Atomic and molcular spctra. - h particl-li charactr
On the Hamiltonian of a Multi-Electron Atom
 On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
Chapter 1 Late 1800 s Several failures of classical (Newtonian) physics discovered
 Chaptr 1 Lat 1800 s Svral failurs of classical (Nwtonian) physics discovrd 1905 195 Dvlopmnt of QM rsolvd discrpancis btwn xpt. and classical thory QM Essntial for undrstanding many phnomna in Chmistry,
Chaptr 1 Lat 1800 s Svral failurs of classical (Nwtonian) physics discovrd 1905 195 Dvlopmnt of QM rsolvd discrpancis btwn xpt. and classical thory QM Essntial for undrstanding many phnomna in Chmistry,
Why is a E&M nature of light not sufficient to explain experiments?
 1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
6. The Interaction of Light and Matter
 6. Th Intraction of Light and Mattr - Th intraction of light and mattr is what maks lif intrsting. - Light causs mattr to vibrat. Mattr in turn mits light, which intrfrs with th original light. - Excitd
6. Th Intraction of Light and Mattr - Th intraction of light and mattr is what maks lif intrsting. - Light causs mattr to vibrat. Mattr in turn mits light, which intrfrs with th original light. - Excitd
Atomic and Laser Spectroscopy
 L-E B, OL, MOV 83 Atomic and Lasr Spctroscopy Th aim of this xrcis is to giv an ovrviw of th fild of lasr spctroscopy and to show modrn spctroscopic mthods usd in atomic, molcular and chmical physics.
L-E B, OL, MOV 83 Atomic and Lasr Spctroscopy Th aim of this xrcis is to giv an ovrviw of th fild of lasr spctroscopy and to show modrn spctroscopic mthods usd in atomic, molcular and chmical physics.
de/dx Effectively all charged particles except electrons
 de/dx Lt s nxt turn our attntion to how chargd particls los nrgy in mattr To start with w ll considr only havy chargd particls lik muons, pions, protons, alphas, havy ions, Effctivly all chargd particls
de/dx Lt s nxt turn our attntion to how chargd particls los nrgy in mattr To start with w ll considr only havy chargd particls lik muons, pions, protons, alphas, havy ions, Effctivly all chargd particls
Introduction to Condensed Matter Physics
 Introduction to Condnsd Mattr Physics pcific hat M.P. Vaughan Ovrviw Ovrviw of spcific hat Hat capacity Dulong-Ptit Law Einstin modl Dby modl h Hat Capacity Hat capacity h hat capacity of a systm hld at
Introduction to Condnsd Mattr Physics pcific hat M.P. Vaughan Ovrviw Ovrviw of spcific hat Hat capacity Dulong-Ptit Law Einstin modl Dby modl h Hat Capacity Hat capacity h hat capacity of a systm hld at
Brief Introduction to Statistical Mechanics
 Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
Experiment 6, page 1 Version of March 17, 2015
 Exprimnt 6, pag 1 Vrsion of March 17, 015 Exprimnt 6 VIBRATION-ROTATION SPECTROSCOPY OF DIATOMIC MOLECULES IMPORTANT: Th cll should b stord in th dssicator whn not in us to prvnt moistur in th atmosphr
Exprimnt 6, pag 1 Vrsion of March 17, 015 Exprimnt 6 VIBRATION-ROTATION SPECTROSCOPY OF DIATOMIC MOLECULES IMPORTANT: Th cll should b stord in th dssicator whn not in us to prvnt moistur in th atmosphr
orbiting electron turns out to be wrong even though it Unfortunately, the classical visualization of the
 Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
CE 530 Molecular Simulation
 CE 53 Molcular Simulation Lctur 8 Fr-nrgy calculations David A. Kofk Dpartmnt of Chmical Enginring SUNY Buffalo kofk@ng.buffalo.du 2 Fr-Enrgy Calculations Uss of fr nrgy Phas quilibria Raction quilibria
CE 53 Molcular Simulation Lctur 8 Fr-nrgy calculations David A. Kofk Dpartmnt of Chmical Enginring SUNY Buffalo kofk@ng.buffalo.du 2 Fr-Enrgy Calculations Uss of fr nrgy Phas quilibria Raction quilibria
Lecture 4: Polyatomic Spectra
 Lecture 4: Polyatomic Spectra 1. From diatomic to polyatomic Ammonia molecule A-axis. Classification of polyatomic molecules 3. Rotational spectra of polyatomic molecules N 4. Vibrational bands, vibrational
Lecture 4: Polyatomic Spectra 1. From diatomic to polyatomic Ammonia molecule A-axis. Classification of polyatomic molecules 3. Rotational spectra of polyatomic molecules N 4. Vibrational bands, vibrational
PRINCIPLES OF PLASMA PROCESSING Course Notes: Prof. J. P. Chang Part B3: ATOMIC COLLISIONS AND SPECTRA
 Atomic Collisions and Spctra 125 PRINCIPLES OF PLASMA PROCESSING Cours Nots: Prof. J. P. Chang Part B3: ATOMIC COLLISIONS AND SPECTRA I. ATOMIC ENERGY LEVELS Atoms and molculs mit lctromagntic radiation
Atomic Collisions and Spctra 125 PRINCIPLES OF PLASMA PROCESSING Cours Nots: Prof. J. P. Chang Part B3: ATOMIC COLLISIONS AND SPECTRA I. ATOMIC ENERGY LEVELS Atoms and molculs mit lctromagntic radiation
Forces. Quantum ElectroDynamics. α = = We have now:
 W hav now: Forcs Considrd th gnral proprtis of forcs mdiatd by xchang (Yukawa potntial); Examind consrvation laws which ar obyd by (som) forcs. W will nxt look at thr forcs in mor dtail: Elctromagntic
W hav now: Forcs Considrd th gnral proprtis of forcs mdiatd by xchang (Yukawa potntial); Examind consrvation laws which ar obyd by (som) forcs. W will nxt look at thr forcs in mor dtail: Elctromagntic
Elements of Statistical Thermodynamics
 24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
High Energy Physics. Lecture 5 The Passage of Particles through Matter
 High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
Collisions between electrons and ions
 DRAFT 1 Collisions btwn lctrons and ions Flix I. Parra Rudolf Pirls Cntr for Thortical Physics, Unirsity of Oxford, Oxford OX1 NP, UK This rsion is of 8 May 217 1. Introduction Th Fokkr-Planck collision
DRAFT 1 Collisions btwn lctrons and ions Flix I. Parra Rudolf Pirls Cntr for Thortical Physics, Unirsity of Oxford, Oxford OX1 NP, UK This rsion is of 8 May 217 1. Introduction Th Fokkr-Planck collision
PH300 Modern Physics SP11 Final Essay. Up Next: Periodic Table Molecular Bonding
 PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
Extraction of Doping Density Distributions from C-V Curves
 Extraction of Doping Dnsity Distributions from C-V Curvs Hartmut F.-W. Sadrozinski SCIPP, Univ. California Santa Cruz, Santa Cruz, CA 9564 USA 1. Connction btwn C, N, V Start with Poisson quation d V =
Extraction of Doping Dnsity Distributions from C-V Curvs Hartmut F.-W. Sadrozinski SCIPP, Univ. California Santa Cruz, Santa Cruz, CA 9564 USA 1. Connction btwn C, N, V Start with Poisson quation d V =
Davisson Germer experiment Announcements:
 Davisson Grmr xprimnt Announcmnts: Homwork st 7 is du Wdnsday. Problm solving sssions M3-5, T3-5. Th 2 nd midtrm will b April 7 in MUEN E0046 at 7:30pm. BFFs: Davisson and Grmr. Today w will go ovr th
Davisson Grmr xprimnt Announcmnts: Homwork st 7 is du Wdnsday. Problm solving sssions M3-5, T3-5. Th 2 nd midtrm will b April 7 in MUEN E0046 at 7:30pm. BFFs: Davisson and Grmr. Today w will go ovr th
Intro to Nuclear and Particle Physics (5110)
 Intro to Nuclar and Particl Physics (5110) March 09, 009 Frmi s Thory of Bta Dcay (continud) Parity Violation, Nutrino Mass 3/9/009 1 Final Stat Phas Spac (Rviw) Th Final Stat lctron and nutrino wav functions
Intro to Nuclar and Particl Physics (5110) March 09, 009 Frmi s Thory of Bta Dcay (continud) Parity Violation, Nutrino Mass 3/9/009 1 Final Stat Phas Spac (Rviw) Th Final Stat lctron and nutrino wav functions
Chemical Physics II. More Stat. Thermo Kinetics Protein Folding...
 Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
DIELECTRIC AND MAGNETIC PROPERTIES OF MATERIALS
 DILCTRIC AD MAGTIC PROPRTIS OF MATRIALS Dilctric Proprtis: Dilctric matrial Dilctric constant Polarization of dilctric matrials, Typs of Polarization (Polarizability). quation of intrnal filds in liquid
DILCTRIC AD MAGTIC PROPRTIS OF MATRIALS Dilctric Proprtis: Dilctric matrial Dilctric constant Polarization of dilctric matrials, Typs of Polarization (Polarizability). quation of intrnal filds in liquid
5.62 Physical Chemistry II Spring 2008
 MIT OpnCoursWar http://ocw.mit.du 5.62 Physical Chmistry II Spring 2008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. 5.62 Lctur #7: Translational Part of
MIT OpnCoursWar http://ocw.mit.du 5.62 Physical Chmistry II Spring 2008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. 5.62 Lctur #7: Translational Part of
Chapter 8: Electron Configurations and Periodicity
 Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
fiziks Institute for NET/JRF, GATE, IIT JAM, M.Sc. Entrance, JEST, TIFR and GRE in Physics
 Atoic and olcular Physics JEST Q. Th binding nrgy of th hydrogn ato (lctron bound to proton) is.6 V. Th binding nrgy of positroniu (lctron bound to positron) is (a).6 / V (b).6 / 8 V (c).6 8 V (d).6 V.6
Atoic and olcular Physics JEST Q. Th binding nrgy of th hydrogn ato (lctron bound to proton) is.6 V. Th binding nrgy of positroniu (lctron bound to positron) is (a).6 / V (b).6 / 8 V (c).6 8 V (d).6 V.6
Modern Physics. Unit 5: Schrödinger s Equation and the Hydrogen Atom Lecture 5.6: Energy Eigenvalues of Schrödinger s Equation for the Hydrogen Atom
 Mdrn Physics Unit 5: Schrödingr s Equatin and th Hydrgn Atm Lctur 5.6: Enrgy Eignvalus f Schrödingr s Equatin fr th Hydrgn Atm Rn Rifnbrgr Prfssr f Physics Purdu Univrsity 1 Th allwd nrgis E cm frm th
Mdrn Physics Unit 5: Schrödingr s Equatin and th Hydrgn Atm Lctur 5.6: Enrgy Eignvalus f Schrödingr s Equatin fr th Hydrgn Atm Rn Rifnbrgr Prfssr f Physics Purdu Univrsity 1 Th allwd nrgis E cm frm th
Chapter 7b Electron Spin and Spin- Orbit Coupling
 Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
E hf. hf c. 2 2 h 2 2 m v f ' f 2f ' f cos c
 EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
Constants and Conversions:
 EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
How can I control light? (and rule the world?)
 How can I control light? (and rul th world?) "You know, I hav on simpl rqust. And that is to hav sharks with frickin' lasr bams attachd to thir hads! - Dr. Evil Phys 230, Day 35 Qustions? Spctra (colors
How can I control light? (and rul th world?) "You know, I hav on simpl rqust. And that is to hav sharks with frickin' lasr bams attachd to thir hads! - Dr. Evil Phys 230, Day 35 Qustions? Spctra (colors
Phys 446: Solid State Physics / Optical Properties. Lattice vibrations: Thermal, acoustic, and optical properties. Fall v =
 Phys 446: Solid Stat Physics / Optical Proprtis Lattic vibrations: Thrmal, acoustic, and optical proprtis Solid Stat Physics Last wk: (Ch. 3) Phonons Today: Einstin and Dby modls for thrmal capacity Thrmal
Phys 446: Solid Stat Physics / Optical Proprtis Lattic vibrations: Thrmal, acoustic, and optical proprtis Solid Stat Physics Last wk: (Ch. 3) Phonons Today: Einstin and Dby modls for thrmal capacity Thrmal
Today. Wave-Matter Duality. Quantum Non-Locality. What is waving for matter waves?
 Today Wav-Mattr Duality HW 7 and Exam 2 du Thurs. 8pm 0 min rcap from last lctur on QM Finish QM odds and nds from ch.4 Th Standard Modl 4 forcs of Natur Fundamntal particls of Natur Fynman diagrams EM
Today Wav-Mattr Duality HW 7 and Exam 2 du Thurs. 8pm 0 min rcap from last lctur on QM Finish QM odds and nds from ch.4 Th Standard Modl 4 forcs of Natur Fundamntal particls of Natur Fynman diagrams EM
There is an arbitrary overall complex phase that could be added to A, but since this makes no difference we set it to zero and choose A real.
 Midtrm #, Physics 37A, Spring 07. Writ your rsponss blow or on xtra pags. Show your work, and tak car to xplain what you ar doing; partial crdit will b givn for incomplt answrs that dmonstrat som concptual
Midtrm #, Physics 37A, Spring 07. Writ your rsponss blow or on xtra pags. Show your work, and tak car to xplain what you ar doing; partial crdit will b givn for incomplt answrs that dmonstrat som concptual
fiziks Institute for NET/JRF, GATE, IIT JAM, M.Sc. Entrance, JEST, TIFR and GRE in Physics NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-2011)
 NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-) 64 Q. Th radius of a 9Cu nuclus is masurd to b 4.8 - cm. (A). Th radius of a 7 Mg nuclus can b stimatd to b.86 - cm (b) 5. - cm (c).6 - cm (d) 8.6 - cm (c)
NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-) 64 Q. Th radius of a 9Cu nuclus is masurd to b 4.8 - cm. (A). Th radius of a 7 Mg nuclus can b stimatd to b.86 - cm (b) 5. - cm (c).6 - cm (d) 8.6 - cm (c)
Statistical Thermodynamics: Sublimation of Solid Iodine
 c:374-7-ivap-statmch.docx mar7 Statistical Thrmodynamics: Sublimation of Solid Iodin Chm 374 For March 3, 7 Prof. Patrik Callis Purpos:. To rviw basic fundamntals idas of Statistical Mchanics as applid
c:374-7-ivap-statmch.docx mar7 Statistical Thrmodynamics: Sublimation of Solid Iodin Chm 374 For March 3, 7 Prof. Patrik Callis Purpos:. To rviw basic fundamntals idas of Statistical Mchanics as applid
1.2 Faraday s law A changing magnetic field induces an electric field. Their relation is given by:
 Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
Outline. Thanks to Ian Blockland and Randy Sobie for these slides Lifetimes of Decaying Particles Scattering Cross Sections Fermi s Golden Rule
 Outlin Thanks to Ian Blockland and andy obi for ths slids Liftims of Dcaying Particls cattring Cross ctions Frmi s Goldn ul Physics 424 Lctur 12 Pag 1 Obsrvabls want to rlat xprimntal masurmnts to thortical
Outlin Thanks to Ian Blockland and andy obi for ths slids Liftims of Dcaying Particls cattring Cross ctions Frmi s Goldn ul Physics 424 Lctur 12 Pag 1 Obsrvabls want to rlat xprimntal masurmnts to thortical
ECE507 - Plasma Physics and Applications
 ECE507 - Plasma Physics and Applications Lctur 7 Prof. Jorg Rocca and Dr. Frnando Tomasl Dpartmnt of Elctrical and Computr Enginring Collisional and radiativ procsss All particls in a plasma intract with
ECE507 - Plasma Physics and Applications Lctur 7 Prof. Jorg Rocca and Dr. Frnando Tomasl Dpartmnt of Elctrical and Computr Enginring Collisional and radiativ procsss All particls in a plasma intract with
Neutrino Mass and Forbidden Beta Decays
 NUCLEAR THEORY Vol. 35 016) ds. M. Gaidarov N. Minkov Hron Prss Sofia Nutrino Mass and Forbiddn Bta Dcays R. Dvornický 1 D. Štfánik F. Šimkovic 3 1 Dzhlpov Laboratory of Nuclar Problms JINR 141980 Dubna
NUCLEAR THEORY Vol. 35 016) ds. M. Gaidarov N. Minkov Hron Prss Sofia Nutrino Mass and Forbiddn Bta Dcays R. Dvornický 1 D. Štfánik F. Šimkovic 3 1 Dzhlpov Laboratory of Nuclar Problms JINR 141980 Dubna
A coupled-channel calculation of the B 3 Σ u X 3 Σ g spectrum of S 2
 A coupldchannl calculation o th B 3 Σ u X 3 Σ g spctrum o S 2 Stv Gibson Stvn Cavanagh Brnton Lwis Glnn Stark 2 Rsarch School o Physical Scincs and Enginring, Th Australian National Univrsity, Canbrra
A coupldchannl calculation o th B 3 Σ u X 3 Σ g spctrum o S 2 Stv Gibson Stvn Cavanagh Brnton Lwis Glnn Stark 2 Rsarch School o Physical Scincs and Enginring, Th Australian National Univrsity, Canbrra
Physics 2D Lecture Slides Lecture 12: Jan 28 th 2004
 Brian Wcht, th TA, is away this wk. I will substitut for his offic hours (in my offic 3314 Mayr Hall, discussion and PS sssion. Pl. giv all rgrad rqusts to m this wk (only) Quiz 3 Will Covr Sctions.1-.5
Brian Wcht, th TA, is away this wk. I will substitut for his offic hours (in my offic 3314 Mayr Hall, discussion and PS sssion. Pl. giv all rgrad rqusts to m this wk (only) Quiz 3 Will Covr Sctions.1-.5
Where k is either given or determined from the data and c is an arbitrary constant.
 Exponntial growth and dcay applications W wish to solv an quation that has a drivativ. dy ky k > dx This quation says that th rat of chang of th function is proportional to th function. Th solution is
Exponntial growth and dcay applications W wish to solv an quation that has a drivativ. dy ky k > dx This quation says that th rat of chang of th function is proportional to th function. Th solution is
Finite element discretization of Laplace and Poisson equations
 Finit lmnt discrtization of Laplac and Poisson quations Yashwanth Tummala Tutor: Prof S.Mittal 1 Outlin Finit Elmnt Mthod for 1D Introduction to Poisson s and Laplac s Equations Finit Elmnt Mthod for 2D-Discrtization
Finit lmnt discrtization of Laplac and Poisson quations Yashwanth Tummala Tutor: Prof S.Mittal 1 Outlin Finit Elmnt Mthod for 1D Introduction to Poisson s and Laplac s Equations Finit Elmnt Mthod for 2D-Discrtization
The Matrix Exponential
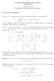 Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
1 Isoparametric Concept
 UNIVERSITY OF CALIFORNIA BERKELEY Dpartmnt of Civil Enginring Spring 06 Structural Enginring, Mchanics and Matrials Profssor: S. Govindj Nots on D isoparamtric lmnts Isoparamtric Concpt Th isoparamtric
UNIVERSITY OF CALIFORNIA BERKELEY Dpartmnt of Civil Enginring Spring 06 Structural Enginring, Mchanics and Matrials Profssor: S. Govindj Nots on D isoparamtric lmnts Isoparamtric Concpt Th isoparamtric
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2014 Lecture 20: Transition State Theory. ERD: 25.14
 Univrsity of Wasinton Dpartmnt of Cmistry Cmistry 453 Wintr Quartr 04 Lctur 0: Transition Stat Tory. ERD: 5.4. Transition Stat Tory Transition Stat Tory (TST) or ctivatd Complx Tory (CT) is a raction mcanism
Univrsity of Wasinton Dpartmnt of Cmistry Cmistry 453 Wintr Quartr 04 Lctur 0: Transition Stat Tory. ERD: 5.4. Transition Stat Tory Transition Stat Tory (TST) or ctivatd Complx Tory (CT) is a raction mcanism
Pair (and Triplet) Production Effect:
 Pair (and riplt Production Effct: In both Pair and riplt production, a positron (anti-lctron and an lctron (or ngatron ar producd spontanously as a photon intracts with a strong lctric fild from ithr a
Pair (and riplt Production Effct: In both Pair and riplt production, a positron (anti-lctron and an lctron (or ngatron ar producd spontanously as a photon intracts with a strong lctric fild from ithr a
Physics 2D Lecture Slides Lecture 14: Feb 1 st 2005
 Physics D Lctur Slids Lctur 14: Fb 1 st 005 Vivk Sharma UCSD Physics Compton Effct: what should Happn Classically? Plan wav [f,λ] incidnt on a surfac with loosly bound lctrons intraction of E fild of EM
Physics D Lctur Slids Lctur 14: Fb 1 st 005 Vivk Sharma UCSD Physics Compton Effct: what should Happn Classically? Plan wav [f,λ] incidnt on a surfac with loosly bound lctrons intraction of E fild of EM
Quasi-Classical States of the Simple Harmonic Oscillator
 Quasi-Classical Stats of th Simpl Harmonic Oscillator (Draft Vrsion) Introduction: Why Look for Eignstats of th Annihilation Oprator? Excpt for th ground stat, th corrspondnc btwn th quantum nrgy ignstats
Quasi-Classical Stats of th Simpl Harmonic Oscillator (Draft Vrsion) Introduction: Why Look for Eignstats of th Annihilation Oprator? Excpt for th ground stat, th corrspondnc btwn th quantum nrgy ignstats
Addition of angular momentum
 Addition of angular momntum April, 0 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat th
Addition of angular momntum April, 0 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat th
Lecture 19: Free Energies in Modern Computational Statistical Thermodynamics: WHAM and Related Methods
 Statistical Thrmodynamics Lctur 19: Fr Enrgis in Modrn Computational Statistical Thrmodynamics: WHAM and Rlatd Mthods Dr. Ronald M. Lvy ronlvy@tmpl.du Dfinitions Canonical nsmbl: A N, V,T = k B T ln Q
Statistical Thrmodynamics Lctur 19: Fr Enrgis in Modrn Computational Statistical Thrmodynamics: WHAM and Rlatd Mthods Dr. Ronald M. Lvy ronlvy@tmpl.du Dfinitions Canonical nsmbl: A N, V,T = k B T ln Q
I. The Connection between Spectroscopy and Quantum Mechanics
 I. Th Connction twn Spctroscopy nd Quntum Mchnics On of th postults of quntum mchnics: Th stt of systm is fully dscrid y its wvfunction, Ψ( r1, r,..., t) whr r 1, r, tc. r th coordints of th constitunt
I. Th Connction twn Spctroscopy nd Quntum Mchnics On of th postults of quntum mchnics: Th stt of systm is fully dscrid y its wvfunction, Ψ( r1, r,..., t) whr r 1, r, tc. r th coordints of th constitunt
Middle East Technical University Department of Mechanical Engineering ME 413 Introduction to Finite Element Analysis
 Middl East Tchnical Univrsity Dpartmnt of Mchanical Enginring ME 43 Introduction to Finit Elmnt Analysis Chaptr 3 Computr Implmntation of D FEM Ths nots ar prpard by Dr. Cünyt Srt http://www.m.mtu.du.tr/popl/cunyt
Middl East Tchnical Univrsity Dpartmnt of Mchanical Enginring ME 43 Introduction to Finit Elmnt Analysis Chaptr 3 Computr Implmntation of D FEM Ths nots ar prpard by Dr. Cünyt Srt http://www.m.mtu.du.tr/popl/cunyt
Introduction to the quantum theory of matter and Schrödinger s equation
 Introduction to th quantum thory of mattr and Schrödingr s quation Th quantum thory of mattr assums that mattr has two naturs: a particl natur and a wa natur. Th particl natur is dscribd by classical physics
Introduction to th quantum thory of mattr and Schrödingr s quation Th quantum thory of mattr assums that mattr has two naturs: a particl natur and a wa natur. Th particl natur is dscribd by classical physics
the electrons. Expanding the exponential and neglecting the constant term Ze 2 λ, we have
 LECTURE.8 Prof.R.Parthasarathy Atomic Structur - Elmntary Tratmnt Th ground stat of hydrogn atom has bn solvd xactly in th nonrlativistic tratmnt. Th ground stat of hlium atom has bn handld in variational
LECTURE.8 Prof.R.Parthasarathy Atomic Structur - Elmntary Tratmnt Th ground stat of hydrogn atom has bn solvd xactly in th nonrlativistic tratmnt. Th ground stat of hlium atom has bn handld in variational
Problem Set 4 Solutions Distributed: February 26, 2016 Due: March 4, 2016
 Probl St 4 Solutions Distributd: Fbruary 6, 06 Du: March 4, 06 McQuarri Probls 5-9 Ovrlay th two plots using Excl or Mathatica. S additional conts blow. Th final rsult of Exapl 5-3 dfins th forc constant
Probl St 4 Solutions Distributd: Fbruary 6, 06 Du: March 4, 06 McQuarri Probls 5-9 Ovrlay th two plots using Excl or Mathatica. S additional conts blow. Th final rsult of Exapl 5-3 dfins th forc constant
AS 5850 Finite Element Analysis
 AS 5850 Finit Elmnt Analysis Two-Dimnsional Linar Elasticity Instructor Prof. IIT Madras Equations of Plan Elasticity - 1 displacmnt fild strain- displacmnt rlations (infinitsimal strain) in matrix form
AS 5850 Finit Elmnt Analysis Two-Dimnsional Linar Elasticity Instructor Prof. IIT Madras Equations of Plan Elasticity - 1 displacmnt fild strain- displacmnt rlations (infinitsimal strain) in matrix form
(most) due to long range e m forces i.e. via atomic collisions or due to short range nuclear collisions or through decay ( = weak interactions)
 Spring 01, P67, YK Monday January 30, 01 8 Obsrvabl particl dtction ffcts ar : (most) du to long rang m forcs i.. via atomic collisions or du to short rang nuclar collisions or through dcay ( = wak intractions)
Spring 01, P67, YK Monday January 30, 01 8 Obsrvabl particl dtction ffcts ar : (most) du to long rang m forcs i.. via atomic collisions or du to short rang nuclar collisions or through dcay ( = wak intractions)
Optics and Non-Linear Optics I Non-linear Optics Tutorial Sheet November 2007
 Optics and Non-Linar Optics I - 007 Non-linar Optics Tutorial Sht Novmbr 007 1. An altrnativ xponntial notion somtims usd in NLO is to writ Acos (") # 1 ( Ai" + A * $i" ). By using this notation and substituting
Optics and Non-Linar Optics I - 007 Non-linar Optics Tutorial Sht Novmbr 007 1. An altrnativ xponntial notion somtims usd in NLO is to writ Acos (") # 1 ( Ai" + A * $i" ). By using this notation and substituting
APP-IV Introduction to Astro-Particle Physics. Maarten de Jong
 APP-IV Introduction to Astro-Particl Physics Maartn d Jong 1 cosmology in a nut shll Hubbl s law cosmic microwav background radiation abundancs of light lmnts (H, H, ) Hubbl s law (199) 1000 vlocity [km/s]
APP-IV Introduction to Astro-Particl Physics Maartn d Jong 1 cosmology in a nut shll Hubbl s law cosmic microwav background radiation abundancs of light lmnts (H, H, ) Hubbl s law (199) 1000 vlocity [km/s]
EXST Regression Techniques Page 1
 EXST704 - Rgrssion Tchniqus Pag 1 Masurmnt rrors in X W hav assumd that all variation is in Y. Masurmnt rror in this variabl will not ffct th rsults, as long as thy ar uncorrlatd and unbiasd, sinc thy
EXST704 - Rgrssion Tchniqus Pag 1 Masurmnt rrors in X W hav assumd that all variation is in Y. Masurmnt rror in this variabl will not ffct th rsults, as long as thy ar uncorrlatd and unbiasd, sinc thy
Structure of the Atom. Thomson s Atomic Model. Knowledge of atoms in Experiments of Geiger and Marsden 2. Experiments of Geiger and Marsden
 CHAPTER 4 Structur of th Atom 4.1 Th Atomic Modls of Thomson and Ruthrford 4. Ruthrford Scattring 4.3 Th Classic Atomic Modl 4.4 Th Bohr Modl of th Hydrogn Atom 4.5 Succsss & Failurs of th Bohr Modl 4.6
CHAPTER 4 Structur of th Atom 4.1 Th Atomic Modls of Thomson and Ruthrford 4. Ruthrford Scattring 4.3 Th Classic Atomic Modl 4.4 Th Bohr Modl of th Hydrogn Atom 4.5 Succsss & Failurs of th Bohr Modl 4.6
Exam 1. It is important that you clearly show your work and mark the final answer clearly, closed book, closed notes, no calculator.
 Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
4E : The Quantum Universe. Lecture 5, April 5 Vivek Sharma
 4E : Th Quantum Univrs Lctur 5, April 5 Vivk Sharma modphys@hpmail.ucsd.du An X-ray Tub from 0 th Cntury Xray Th High Enrgy Acclrator of 1900s: producd nrgtic light : X Ray, gav nw optic to subatomic world
4E : Th Quantum Univrs Lctur 5, April 5 Vivk Sharma modphys@hpmail.ucsd.du An X-ray Tub from 0 th Cntury Xray Th High Enrgy Acclrator of 1900s: producd nrgtic light : X Ray, gav nw optic to subatomic world
Addition of angular momentum
 Addition of angular momntum April, 07 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat
Addition of angular momntum April, 07 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat
Neutrino Probes of Dark Energy and Dark Matter
 SNOWPAC@Snowbird March 25, 2010 Nutrino Probs of Dark Enrgy and Dark Mattr Shin ichiro Ando California Institut of Tchnology Dark Enrgy and Dark Mattr 2.0 1.5 1.0 No Big Bang SN Most of th nrgy in th Univrs
SNOWPAC@Snowbird March 25, 2010 Nutrino Probs of Dark Enrgy and Dark Mattr Shin ichiro Ando California Institut of Tchnology Dark Enrgy and Dark Mattr 2.0 1.5 1.0 No Big Bang SN Most of th nrgy in th Univrs
2008 AP Calculus BC Multiple Choice Exam
 008 AP Multipl Choic Eam Nam 008 AP Calculus BC Multipl Choic Eam Sction No Calculator Activ AP Calculus 008 BC Multipl Choic. At tim t 0, a particl moving in th -plan is th acclration vctor of th particl
008 AP Multipl Choic Eam Nam 008 AP Calculus BC Multipl Choic Eam Sction No Calculator Activ AP Calculus 008 BC Multipl Choic. At tim t 0, a particl moving in th -plan is th acclration vctor of th particl
The Matrix Exponential
 Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
Studies of Turbulence and Transport in Alcator C-Mod Ohmic Plasmas with Phase Contrast Imaging and Comparisons with GYRO*
 Studis of Turbulnc and Transport in Ohmic Plasmas with Phas Contrast Imaging and Comparisons with GYRO* L. Lin 1, M. Porkolab 1, E.M. Edlund 1, J.C. Rost 1, M. Grnwald 1, D.R. Mikklsn 2, N. Tsujii 1 1
Studis of Turbulnc and Transport in Ohmic Plasmas with Phas Contrast Imaging and Comparisons with GYRO* L. Lin 1, M. Porkolab 1, E.M. Edlund 1, J.C. Rost 1, M. Grnwald 1, D.R. Mikklsn 2, N. Tsujii 1 1
Brief Notes on the Fermi-Dirac and Bose-Einstein Distributions, Bose-Einstein Condensates and Degenerate Fermi Gases Last Update: 28 th December 2008
 Brif ots on th Frmi-Dirac and Bos-Einstin Distributions, Bos-Einstin Condnsats and Dgnrat Frmi Gass Last Updat: 8 th Dcmbr 8 (A)Basics of Statistical Thrmodynamics Th Gibbs Factor A systm is assumd to
Brif ots on th Frmi-Dirac and Bos-Einstin Distributions, Bos-Einstin Condnsats and Dgnrat Frmi Gass Last Updat: 8 th Dcmbr 8 (A)Basics of Statistical Thrmodynamics Th Gibbs Factor A systm is assumd to
Spectra of Diatomic Molecules: From Cold to Hot
 Spctra of Diatomic Molculs: From Cold to Hot obrt M. uc rislav Horvatić Mladn Movr Zagrb Croatia Outlin Diatomic molculs hav bn an objct of vry intnsiv invstigation for diffrnt physical conditions. I shall
Spctra of Diatomic Molculs: From Cold to Hot obrt M. uc rislav Horvatić Mladn Movr Zagrb Croatia Outlin Diatomic molculs hav bn an objct of vry intnsiv invstigation for diffrnt physical conditions. I shall
Atomic energy levels. Announcements:
 Atomic nrgy lvls Announcmnts: Exam solutions ar postd. Problm solving sssions ar M3-5 and Tusday 1-3 in G-140. Will nd arly and hand back your Midtrm Exam at nd of class. http://www.colorado.du/physics/phys2170/
Atomic nrgy lvls Announcmnts: Exam solutions ar postd. Problm solving sssions ar M3-5 and Tusday 1-3 in G-140. Will nd arly and hand back your Midtrm Exam at nd of class. http://www.colorado.du/physics/phys2170/
λ = 2L n Electronic structure of metals = 3 = 2a Free electron model Many metals have an unpaired s-electron that is largely free
 5.6 4 Lctur #4-6 pag Elctronic structur of mtals r lctron modl Many mtals av an unpaird s-lctron tat is largly fr Simplst modl: Particl in a box! or a cubic box of lngt L, ψ ( xyz) 8 xπ ny L L L n x π
5.6 4 Lctur #4-6 pag Elctronic structur of mtals r lctron modl Many mtals av an unpaird s-lctron tat is largly fr Simplst modl: Particl in a box! or a cubic box of lngt L, ψ ( xyz) 8 xπ ny L L L n x π
Principles of Humidity Dalton s law
 Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Physics 2D Lecture Slides. Oct 21. UCSD Physics. Vivek Sharma
 Physics D Lctur Slids Oct 1 Vivk Sharma UCSD Physics Modrn Viw of Photolctric Effct E = hf = KE+ ϕ Is h sam in Photolctric Effct as in BBQ Radiation? Slop h = 6.66 x 10-34 JS Einstin Nobl Priz! No mattr
Physics D Lctur Slids Oct 1 Vivk Sharma UCSD Physics Modrn Viw of Photolctric Effct E = hf = KE+ ϕ Is h sam in Photolctric Effct as in BBQ Radiation? Slop h = 6.66 x 10-34 JS Einstin Nobl Priz! No mattr
IYPT 2000 Problem No. 3 PLASMA
 IYPT 000 Problm No. 3 PLASMA Tam Austria Invstigat th lctrical conducivity of th flam of a candl. Examin th influnc of rlvant paramtrs, in particular, th shap and polarity of th lctrods. Th xprimnts should
IYPT 000 Problm No. 3 PLASMA Tam Austria Invstigat th lctrical conducivity of th flam of a candl. Examin th influnc of rlvant paramtrs, in particular, th shap and polarity of th lctrods. Th xprimnts should
Chapter. 3 Wave & Particles I
 Announcmnt Cours wbpag http://highnrgy.phys.ttu.du/~sl/2402/ Txtbook PHYS-2402 Lctur 8 Quiz 1 Class avrag: 14.2 (out of 20) ~ 70% Fb. 10, 2015 HW2 (du 2/19) 13, 17, 23, 25, 28, 31, 37, 38, 41, 44 Chaptr.
Announcmnt Cours wbpag http://highnrgy.phys.ttu.du/~sl/2402/ Txtbook PHYS-2402 Lctur 8 Quiz 1 Class avrag: 14.2 (out of 20) ~ 70% Fb. 10, 2015 HW2 (du 2/19) 13, 17, 23, 25, 28, 31, 37, 38, 41, 44 Chaptr.
