Effective Scientific Presentations
|
|
|
- Giles Banks
- 5 years ago
- Views:
Transcription
1 Effective Scientific Presentations
2 The best retention occurs for presentations that are both vocal and visual Hear See Hear and See Recall (%) Data from the Wharton Research Center
3 Research shows that the brain is good at reading, good at listening, but not doing both simultaneously.! Reading + Listening John Sweller, University of South Wales
4 To resolve the problem we first have to understand how the brain works verbal visual Cognitive scientists say the mind processes information in 2 channels
5 The mind pays attention to only a few pieces of information in each channel Then it must select, organize, and! integrate what s important
6 To be effective, the audience must grasp the content quickly Use short statements Water has special thermal properties It helps to controls the climate on our planet Use images to increase comprehension It helps to maintain our body temperature Use blank spaces to enhance readership
7 Too much information and distractions can confuse and annoy your listeners Theta oscillations in the BLA Many figures Data This data is truly pioneering!! COOL DATA!!!!
8 Don t force your audience to choose between listening to you - OR reading your slides!
9 ! The slides should follow several rules! Use a sentence headline to state the slide s purpose MD simulations show that nitric acid readily dissociates in water O Molecular Bond Lengths Use images to support the sentence O N H O Bondlength (Angstroms) O H N O N O The OH bond breaks upon dissociation Timestep (fs)
10 The statement at the top should have no more than two lines The surface spectroscopy shows nitric acid in two different forms at a water surface Call-out, if necessary: keep to 1-2 lines Pictu 1662 cm cm -1 The two HNO 3 molecules differ by the number of bonds to water. If necessary, identify key assumption or background for audience keep to two lines (18 24 point type)
11 Use typography that is quickly and easily read Use a readable simple font (Arial, Gill Sans) MD simulations show that nitric acid does not dissociate when on a water surface Molecular Bond Lengths Use a high contrast between words and background Bondlength (Angstroms) O H Pictu The proton does not dissociate N O N O Timestep (fs)
12 Some fonts work for manuscripts but not for presentations Times Roman Font is harder to read quickly MD simulations show that nitric acid does not dissociate when on a water surface Molecular Bond Lengths Bondlength (Angstroms) O H Pictu The proton does not dissociate N O N O Timestep (fs)
13 Even italics can slow the reading and comprehension MD simulations show that nitric acid does not dissociate when on a water surface Molecular Bond Lengths Bondlength (Angstroms) O H Pictu The proton does not dissociate N O N O Timestep (fs)
14 ! The title slide should draw interest Understanding Environmentally Important Processes at Liquid Surfaces Geri Richmond Department of Chemistry University of Oregon Eugene, OR ACS National Meeting April 20, 2010 Understanding Environmentally Important Processes at Liquid Surfaces Geri Richmond University of Oregon ACS National Meeting Use the title slide to connect with your audience
15 The outline slide should be a visual roadmap Image&for& Topic&1& & Topic& 1& Image&for& Topic&2& & Topic& 2& Image&for& Topic&3& & Topic& 3&
16 The focus slide should be a visual roadmap Presentation Outline 1. Introduction 2. Background 3. Methods - experimental - theoretical 4. VSFS studies of water surfaces 5. Studies of how gases adsorb on a water surface - room temperature studies - low temperatures studies 6. Studies of nitric acid at a water surface 7. Conclusions and future studies 8. Acknowledgements This presentation shows the unique structure and reactivity that is present at water surfaces hydrogen bonding at water surfaces gaseous adsorption at water surfaces surface acidity of HNO 3 solutions
17 The methods slide should follow the same format To probe the water surface we use surface vibrational sum frequency spectroscopy (VSFS) The technique selectively probes the topmost layers of the interface Tunable pulsed lasers probe the surface species The resulting vibration spectrum measures surface molecules
18 The most difficult part is to consider what to include and what to exclude on each slide
19 The summary slide headline states the most important assertion of the presentation This&sentence&summarizes&the&most& important&conclusion&of&the&presenta-on)& Suppor-ng&point&(no& more&than&two&lines)& & & Another&suppor-ng&point& (parallel&to&the&first)& & Image&that&supports& conclusion& &
20 Don t use long lists that limit comprehension Summary of the This Presentation! The amazing discovery that no one knew about! Another remarkable discovery that you maybe knew! A third fact that you might not have noticed! And a fourth finding that only few people ever heard of! Throw in a fifth discovery that I particularly like! A sixth discovery that I didn t have time to talk about! And two final smaller discoveries that are also important - the one found in the noise - a second found by turning the data upside down Avoid lists with more than four items.
21 The summary slide headline states the most important assertion of the presentation The surface of water has unique properties that control its chemical properties Water participates in weak H-bonding at the topmost surface layers SO 2 adsorbs at the surface whereas CO 2 quickly absorbs Nitric acid is a weak acid at aqueous surfaces
22 Keep your audience engaged Don t read your slides! Don t talk to your slides! Don t apologize for your slides!
23 Limit the number of slides Max: 1 slide per minute
24 And finally, rehearse, rehearse and rehearse
The Power of Persuasion
 The Power of Persuasion Geri Richmond University of Oregon COACh http://coach.uoregon.edu The presentation today is comprised of three parts: Enhancing your communication skills Persuasive Scientific Presentations
The Power of Persuasion Geri Richmond University of Oregon COACh http://coach.uoregon.edu The presentation today is comprised of three parts: Enhancing your communication skills Persuasive Scientific Presentations
Energy, Work, and Simple Machines
 CHAPTER 3 Energy, Work, and Simple Machines Types of Energy What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree
CHAPTER 3 Energy, Work, and Simple Machines Types of Energy What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree
No Math. ASTR/PHYS 109 at. Texas A&M University November Big Bang, Black Holes, No Math ASTR/PHYS 109 at Texas A&M 1
 Big Bang, Black Holes, No Math ASTR/PHYS 109 at Texas A&M David Toback Texas A&M University November 2009 ASTR/PHYS 109 at Texas A&M 1 Talk Outline Motivation, Intended Audience and Class Goals Course
Big Bang, Black Holes, No Math ASTR/PHYS 109 at Texas A&M David Toback Texas A&M University November 2009 ASTR/PHYS 109 at Texas A&M 1 Talk Outline Motivation, Intended Audience and Class Goals Course
Today s Catalyst. Complete the Exit Ticket from yesterday
 Today s Catalyst Complete the Exit Ticket from yesterday 1. What is the difference between Mendeleev and Moseley s periodic tables? Write your answer in complete sentences. 2. How is the periodic table
Today s Catalyst Complete the Exit Ticket from yesterday 1. What is the difference between Mendeleev and Moseley s periodic tables? Write your answer in complete sentences. 2. How is the periodic table
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
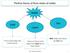 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
A is capable of donating one or more H+
 Slide 1 / 48 1 According to the Arrhenius concept, an acid is a substance that. A is capable of donating one or more H+ B C D E causes an increase in the concentration of H+ in aqueous solutions can accept
Slide 1 / 48 1 According to the Arrhenius concept, an acid is a substance that. A is capable of donating one or more H+ B C D E causes an increase in the concentration of H+ in aqueous solutions can accept
Lecture 08 Born Oppenheimer Approximation
 Chemistry II: Introduction to Molecular Spectroscopy Prof. Mangala Sunder Department of Chemistry and Biochemistry Indian Institute of Technology, Madras Lecture 08 Born Oppenheimer Approximation Welcome
Chemistry II: Introduction to Molecular Spectroscopy Prof. Mangala Sunder Department of Chemistry and Biochemistry Indian Institute of Technology, Madras Lecture 08 Born Oppenheimer Approximation Welcome
States of Matter. Solids, Liquids, and Gases
 States of Matter Solids, Liquids, and Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
States of Matter Solids, Liquids, and Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
Strong and Weak Acids
 Strong and Weak Acids 1 of 21 Boardworks Ltd 2016 Strong and Weak Acids 2 of 21 Boardworks Ltd 2016 What are acids? 3 of 21 Boardworks Ltd 2016 All acids contain a positive hydrogen ion (H + ) bound to
Strong and Weak Acids 1 of 21 Boardworks Ltd 2016 Strong and Weak Acids 2 of 21 Boardworks Ltd 2016 What are acids? 3 of 21 Boardworks Ltd 2016 All acids contain a positive hydrogen ion (H + ) bound to
PPT PRESENTATION. What works, and what doesn t. Associate Dean USF Graduate School. Revised
 PPT PRESENTATION TUTORIAL What works, and what doesn t RS Pollenz, PhD Associate Dean USF Graduate School Revised 5.5.10 Acknowledgment (This tutorial was based on a previous document constructed by by
PPT PRESENTATION TUTORIAL What works, and what doesn t RS Pollenz, PhD Associate Dean USF Graduate School Revised 5.5.10 Acknowledgment (This tutorial was based on a previous document constructed by by
The rest of topic 11 INTRODUCTION TO ORGANIC SPECTROSCOPY
 The rest of topic 11 INTRODUCTION TO ORGANIC SPECTROSCOPY 1. Mass spectrometry: SPECTROSCOPIC TECHNIQUES - A technique capable of identifying the presence of various mass segments of organic molecules.
The rest of topic 11 INTRODUCTION TO ORGANIC SPECTROSCOPY 1. Mass spectrometry: SPECTROSCOPIC TECHNIQUES - A technique capable of identifying the presence of various mass segments of organic molecules.
Gases: Properties and Behaviour
 SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
SECTION 11.1 Gases: Properties and Behaviour Key Terms kinetic molecular theory of gases ideal gas On Earth, matter typically exists in three physical states: solid, liquid, and gas. All three states of
States of Matter in Food
 Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Page 2. Q1.The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements.
 Q1.The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
Q1.The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion.
 Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
The Singapore Copyright Act applies to the use of this document.
 Title Use of analogy in teaching the particulate theory of matter Author(s) Boo Hong Kwen & Toh Kok Aun Source Teaching and Learning, 17(2),79-85 Published by Institute of Education (Singapore) This document
Title Use of analogy in teaching the particulate theory of matter Author(s) Boo Hong Kwen & Toh Kok Aun Source Teaching and Learning, 17(2),79-85 Published by Institute of Education (Singapore) This document
Properties and Structure of Matter
 Properties and Structure of Matter Chapter 10 You can use a spider map to organize the main ideas and supporting details of a topic such as properties of matter. Look at the example shown below. The central
Properties and Structure of Matter Chapter 10 You can use a spider map to organize the main ideas and supporting details of a topic such as properties of matter. Look at the example shown below. The central
Chemistry 20 Course Outline
 Chemistry 20 Course Outline Textbook: Frank Jenkins, et al, Nelson Canada. Unit Outline (approximate page numbers included) Review Unit A. Chapter 1: Elements and Compounds (p. 6-39) B. Chapter 2: Chemical
Chemistry 20 Course Outline Textbook: Frank Jenkins, et al, Nelson Canada. Unit Outline (approximate page numbers included) Review Unit A. Chapter 1: Elements and Compounds (p. 6-39) B. Chapter 2: Chemical
Acidic Water Monolayer on Ruthenium(0001)
 Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
Nonmetals, Inert Gases, and Semimetals
 Chapter 4 Elements and the Periodic Table Section 4 Summary Nonmetals, Inert Gases, and Semimetals Key Concepts What are the properties of nonmetals and inert gases? How are semimetals useful? Nonmetals
Chapter 4 Elements and the Periodic Table Section 4 Summary Nonmetals, Inert Gases, and Semimetals Key Concepts What are the properties of nonmetals and inert gases? How are semimetals useful? Nonmetals
Reaction Rates and Equilibrium
 CHAPTER 7 14 SECTION Chemical Reactions Reaction Rates and Equilibrium KEY IDEAS As you read this section, keep these questions in mind: How can you increase the rate of a reaction? What does a catalyst
CHAPTER 7 14 SECTION Chemical Reactions Reaction Rates and Equilibrium KEY IDEAS As you read this section, keep these questions in mind: How can you increase the rate of a reaction? What does a catalyst
S8P1C. STATES OF MATTER E.Q. WHAT ARE THE STATES OF MATTER? WHAT ARE THE CHARACTERISTICS OF EACH STATE OF MATTER?
 S8P1C. STATES OF MATTER E.Q. WHAT ARE THE STATES OF MATTER? WHAT ARE THE CHARACTERISTICS OF EACH STATE OF MATTER? ATOMIC MODEL PROJECT BE CREATIVE!!! MIXTURES VS. SUBSTANCES TASK DUE BY FRIDAY, NOVEMBER
S8P1C. STATES OF MATTER E.Q. WHAT ARE THE STATES OF MATTER? WHAT ARE THE CHARACTERISTICS OF EACH STATE OF MATTER? ATOMIC MODEL PROJECT BE CREATIVE!!! MIXTURES VS. SUBSTANCES TASK DUE BY FRIDAY, NOVEMBER
LEVEL ZERO VOICE CATALYST (4 minutes, individual work): How do plants grow? What do plants eat?
 Assignment #5 Photosynthesis LO: To understand the process of photosynthesis. EQ: Explain the light dependent and light independent reactions. Include where they take place. (4-5 sentences) AGENDA 2/6-2/7
Assignment #5 Photosynthesis LO: To understand the process of photosynthesis. EQ: Explain the light dependent and light independent reactions. Include where they take place. (4-5 sentences) AGENDA 2/6-2/7
Chemical Reactions and Equations
 Chemical Reactions and Equations Changes and Chemical Reactions Key Concepts Why do chemical reactions always involve a change in? What is the difference between an endothermic reaction and an exothermic
Chemical Reactions and Equations Changes and Chemical Reactions Key Concepts Why do chemical reactions always involve a change in? What is the difference between an endothermic reaction and an exothermic
1
 1 Index: Page 3: Covalent Bonding Knowledge Card Page 4: Separation Techniques Knowledge Card Page 5: Exam Descriptors/Sample Questions Page 6: Covalent Bonding & How to draw them Page 7: Simple & Giant
1 Index: Page 3: Covalent Bonding Knowledge Card Page 4: Separation Techniques Knowledge Card Page 5: Exam Descriptors/Sample Questions Page 6: Covalent Bonding & How to draw them Page 7: Simple & Giant
MITOCW watch?v=ws1mx-c2v9w
 MITOCW watch?v=ws1mx-c2v9w The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=ws1mx-c2v9w The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
This activity has been used in an introductory chemistry course (prep chemistry or GOB course) Learning Goals: Prerequisite knowledge
 This activity has been used in an introductory chemistry course (prep chemistry or GOB course) Learning Goals: Name phase changes Identify phase changes at molecular (particulate) level Name intermolecular
This activity has been used in an introductory chemistry course (prep chemistry or GOB course) Learning Goals: Name phase changes Identify phase changes at molecular (particulate) level Name intermolecular
(g) + 3H 2. (g) 2NH 3. (g) (a) Explain what is meant by a dynamic equilibrium. (2)
 1 When nitrogen and hydrogen react to form ammonia, the reaction can reach a dynamic equilibrium. (g) + 3H 2 (g) 2NH 3 (g) (a) Explain what is meant by a dynamic equilibrium. (b) In industry, the reaction
1 When nitrogen and hydrogen react to form ammonia, the reaction can reach a dynamic equilibrium. (g) + 3H 2 (g) 2NH 3 (g) (a) Explain what is meant by a dynamic equilibrium. (b) In industry, the reaction
Madras High School Unit Plan. Subject: Environmental Science Grades: Semester: 2
 Madras High School Unit Plan Subject: Environmental Science Grades: 11 12 Semester: 2 Unit of Study: Geologic Time Pacing Teaching: Interactive Notes: 4 class periods Practice work assignments: 5 class
Madras High School Unit Plan Subject: Environmental Science Grades: 11 12 Semester: 2 Unit of Study: Geologic Time Pacing Teaching: Interactive Notes: 4 class periods Practice work assignments: 5 class
Winter Break Packet Absence makes the mind go blank. You will thank me for this later.
 Name Date Period Winter Break Packet Absence makes the mind go blank. You will thank me for this later. 1. Describe a physical property. Give three examples of physical properties. 2. Describe a chemical
Name Date Period Winter Break Packet Absence makes the mind go blank. You will thank me for this later. 1. Describe a physical property. Give three examples of physical properties. 2. Describe a chemical
STAIRWAY TO WELDING SCHOOL
 STAIRWAY TO WELDING SCHOOL There's a premed who's sure That counterclockwise is "R" And his grades will make him perfect For welding school When he gets there he'll say What was that rule of the day Maybe
STAIRWAY TO WELDING SCHOOL There's a premed who's sure That counterclockwise is "R" And his grades will make him perfect For welding school When he gets there he'll say What was that rule of the day Maybe
ENERGY IN CHEMISTRY. R. Ashby Duplication by permission only.
 CH 11 TOPIC 28 CHANGING STATES OF MATTER 1 You have mastered this topic when you can: 1) define or describe: ENERGY, POTENTIAL ENERGY, KINETIC ENERGY & KINETIC MOLECULAR THEORY 2) define or describe HEAT
CH 11 TOPIC 28 CHANGING STATES OF MATTER 1 You have mastered this topic when you can: 1) define or describe: ENERGY, POTENTIAL ENERGY, KINETIC ENERGY & KINETIC MOLECULAR THEORY 2) define or describe HEAT
Parenting Tip of the Month. April. Lower Elementary Teachers
 Parenting Tip of the Month April Lower Elementary Teachers Why Use Higher Order Thinking Skills Everyday? Research tells us that Higher Order Thinking Skills help to build life long interaction and communication
Parenting Tip of the Month April Lower Elementary Teachers Why Use Higher Order Thinking Skills Everyday? Research tells us that Higher Order Thinking Skills help to build life long interaction and communication
Physics Lab #2: Spectroscopy
 Physics 10263 Lab #2: Spectroscopy Introduction This lab is meant to serve as an introduction to the science of spectroscopy. In this lab, we ll learn about how emission and absorption works, and we ll
Physics 10263 Lab #2: Spectroscopy Introduction This lab is meant to serve as an introduction to the science of spectroscopy. In this lab, we ll learn about how emission and absorption works, and we ll
XYZ file format Protein Data Bank (pdb) file format Z - matrix
 Chemistry block (exercise 1) In this exercise, students will be introduced how to preform simple quantum chemical calculations. Input files for Gaussian09. Output file structure. Geometry optimization,
Chemistry block (exercise 1) In this exercise, students will be introduced how to preform simple quantum chemical calculations. Input files for Gaussian09. Output file structure. Geometry optimization,
COMMON CORE Lessons & Activities SAMPLE
 COMMON CORE Lessons & Activities T T TEACH IT TODAY! Common Core Lessons & Activities: States of Matter By Carole Marsh Carole Marsh/Gallopade Published by Gallopade International, Inc. Printed in the
COMMON CORE Lessons & Activities T T TEACH IT TODAY! Common Core Lessons & Activities: States of Matter By Carole Marsh Carole Marsh/Gallopade Published by Gallopade International, Inc. Printed in the
AP BIOLOGY CHAPTERS 1-3 WORKSHEET
 Name Date AP BIOLOGY CHAPTERS 1-3 WORKSHEET MULTIPLE CHOICE. 33 pts. Place the letter of the choice that best completes the statement or answers the question in the blank. 1. Which of the following sequences
Name Date AP BIOLOGY CHAPTERS 1-3 WORKSHEET MULTIPLE CHOICE. 33 pts. Place the letter of the choice that best completes the statement or answers the question in the blank. 1. Which of the following sequences
The lower the energy of a substance, the interaction between its atoms and molecules.
 PHYSICAL STATES OF MATTER Kinetic Molecular Theory To understand the different states in which matter can exist, we need to understand something called the Kinetic Molecular Theory of Matter. Kinetic Molecular
PHYSICAL STATES OF MATTER Kinetic Molecular Theory To understand the different states in which matter can exist, we need to understand something called the Kinetic Molecular Theory of Matter. Kinetic Molecular
Activation Energy is the minimum amount of energy needed to start a chemical reaction.
 5.3 Controlling Chemical Reactions Vocabulary: Activation energy Concentration Catalyst Enzyme Inhibitor - How do reactions get started? Chemical reactions won t begin until the reactants have enough energy.
5.3 Controlling Chemical Reactions Vocabulary: Activation energy Concentration Catalyst Enzyme Inhibitor - How do reactions get started? Chemical reactions won t begin until the reactants have enough energy.
Chemistry Day 17. Friday, October 5 th Monday, October 8 th, 2018
 Chemistry Day 17 Friday, October 5 th Monday, October 8 th, 2018 Do-Now: Unit 1 Test Day Do-Now 1. Write down today s FLT 2. Write down one question you have about the test (topic-wise or format-wise).
Chemistry Day 17 Friday, October 5 th Monday, October 8 th, 2018 Do-Now: Unit 1 Test Day Do-Now 1. Write down today s FLT 2. Write down one question you have about the test (topic-wise or format-wise).
ASTRO 114 Lecture Okay. We re now gonna continue discussing and conclude discussing the entire
 ASTRO 114 Lecture 55 1 Okay. We re now gonna continue discussing and conclude discussing the entire universe. So today we re gonna learn about everything, everything that we know of. There s still a lot
ASTRO 114 Lecture 55 1 Okay. We re now gonna continue discussing and conclude discussing the entire universe. So today we re gonna learn about everything, everything that we know of. There s still a lot
The Do s and Don ts of Giving a Math Talk
 The of Giving a Math Talk Lakehead University November 2011 Caveats What follows is mostly for short math talks, e.g., one hour or less. Some of advice doesn t apply to a lecture series (2 or more lectures)
The of Giving a Math Talk Lakehead University November 2011 Caveats What follows is mostly for short math talks, e.g., one hour or less. Some of advice doesn t apply to a lecture series (2 or more lectures)
N10/4/CHEMI/SP2/ENG/TZ0/XX CHEMISTRY STANDARD LEVEL PAPER 2. Thursday 11 November 2010 (afternoon) Candidate session number.
 N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
1. Let s quickly review some of the phosphorus fixation reactions in soils. 2. At low ph (acidic conditons below 6.0), phosphorus fixation occurs
 1 1. Let s quickly review some of the phosphorus fixation reactions in soils. 2. At low ph (acidic conditons below 6.0), phosphorus fixation occurs between phosphates and iron or aluminum in the soil solution
1 1. Let s quickly review some of the phosphorus fixation reactions in soils. 2. At low ph (acidic conditons below 6.0), phosphorus fixation occurs between phosphates and iron or aluminum in the soil solution
Kinetic Molecular Theory, Gases, and Phase Changes (Answer Key)
 Kinetic Molecular Theory, Gases, and Phase Changes (Answer Key) solid liquid melting sublimation freezing evaporation condensation deposition gas 8. See above. 9. Endothermic: melting, evaporation, sublimation.
Kinetic Molecular Theory, Gases, and Phase Changes (Answer Key) solid liquid melting sublimation freezing evaporation condensation deposition gas 8. See above. 9. Endothermic: melting, evaporation, sublimation.
Section 16.3 Phase Changes
 Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Sample Pages from. Leveled Texts for Science: Physical Science
 Sample Pages from Leveled Texts for Science: Physical Science The following sample pages are included in this download: Table of Contents Readability Chart Sample Passage For correlations to Common Core
Sample Pages from Leveled Texts for Science: Physical Science The following sample pages are included in this download: Table of Contents Readability Chart Sample Passage For correlations to Common Core
FEMTOSECOND MID-INFRARED SPECTROSCOPY OF HYDROGEN-BONDED LIQUIDS
 Laser Chem., 1999, Vol. 19, pp. 83-90 Reprints available directly from the publisher Photocopying permitted by license only (C) 1999 OPA (Overseas Publishers Association) N.V. Published by license under
Laser Chem., 1999, Vol. 19, pp. 83-90 Reprints available directly from the publisher Photocopying permitted by license only (C) 1999 OPA (Overseas Publishers Association) N.V. Published by license under
Electrolysis of Water. fossil fuels such as coal. This process has worked so well many people ask, Why
 Kimberly H. Garske Chemistry 1046 Honors Project Chipola College Spring 2005 Electrolysis of Water For hundreds of years our main source of energy has come from burning carbonbased fossil fuels such as
Kimberly H. Garske Chemistry 1046 Honors Project Chipola College Spring 2005 Electrolysis of Water For hundreds of years our main source of energy has come from burning carbonbased fossil fuels such as
CHM Solids, Liquids, and Phase Changes (r15) Charles Taylor 1/9
 CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
Name Date Block LESSON CLUSTER 6: Expansion and Contraction
 LESSON CLUSTER 6: Expansion and Contraction Did you know that when you say that something is hot or cold, you are actually saying something about the molecules of that substance? Words like hot and cold
LESSON CLUSTER 6: Expansion and Contraction Did you know that when you say that something is hot or cold, you are actually saying something about the molecules of that substance? Words like hot and cold
Describe how the inter-conversion of solids, liquids and gases are achieved and recall names used for these inter-conversions
 Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
Cell Compounds, Bonds, Reactions - 1
 Biology 12 Name: KEY Cell Compounds, Bonds, Reactions - 1 Order of Subtopics Covered: 1. Chemical Bonds a. Ionic b. Covalent 2. Water a. Hydrogen Bonds b. Importance 3. Acids & Bases a. ph b. Buffers 4.
Biology 12 Name: KEY Cell Compounds, Bonds, Reactions - 1 Order of Subtopics Covered: 1. Chemical Bonds a. Ionic b. Covalent 2. Water a. Hydrogen Bonds b. Importance 3. Acids & Bases a. ph b. Buffers 4.
Theoretical Models for Chemical Kinetics
 Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
Chemistry 141 Samuel A. Abrash Chemical Reactions Lab Lecture 9/5/2011
 Chemistry 141 Samuel A. Abrash Chemical Reactions Lab Lecture 9/5/2011 Q: Before we start discussing this week s lab, can we talk about our lab notebooks? Sure. Q: What makes a lab notebook a good notebook?
Chemistry 141 Samuel A. Abrash Chemical Reactions Lab Lecture 9/5/2011 Q: Before we start discussing this week s lab, can we talk about our lab notebooks? Sure. Q: What makes a lab notebook a good notebook?
Teaching Introductory Chemistry through Concept Development Studies. John S. Hutchinson Department of Chemistry Rice University
 Teaching Introductory Chemistry through Concept Development Studies John S. Hutchinson Department of Chemistry Rice University Goals in General Chemistry: What should students gain? Fundamental Chemical
Teaching Introductory Chemistry through Concept Development Studies John S. Hutchinson Department of Chemistry Rice University Goals in General Chemistry: What should students gain? Fundamental Chemical
Lesson 2 Changes in State
 Lesson 2 Changes in State Student Labs and Activities Page Launch Lab 25 Content Vocabulary 26 Lesson Outline 27 MiniLab 29 Content Practice A 30 Content Practice B 31 Language Arts Support 32 School to
Lesson 2 Changes in State Student Labs and Activities Page Launch Lab 25 Content Vocabulary 26 Lesson Outline 27 MiniLab 29 Content Practice A 30 Content Practice B 31 Language Arts Support 32 School to
Name Class Date. What are the four fundamental forces in nature? How can forces affect the motion of an object? Why is friction sometime necessary?
 CHAPTER 11 SECTION Motion 3 Motion and Force KEY IDEAS As you read this section, keep these questions in mind: What are the four fundamental forces in nature? How can forces affect the motion of an object?
CHAPTER 11 SECTION Motion 3 Motion and Force KEY IDEAS As you read this section, keep these questions in mind: What are the four fundamental forces in nature? How can forces affect the motion of an object?
Chapter 2 Basic Chemistry Outline
 Chapter 2 Basic Chemistry Outline 1.0 COMPOSITION OF MATTER 1.1 Atom 1.2 Elements 1.21 Isotopes 1.22 Radioisotopes 1.3 Compounds 1.31 Compounds Formed by Ionic Bonding 1.32 Compounds Formed by Covalent
Chapter 2 Basic Chemistry Outline 1.0 COMPOSITION OF MATTER 1.1 Atom 1.2 Elements 1.21 Isotopes 1.22 Radioisotopes 1.3 Compounds 1.31 Compounds Formed by Ionic Bonding 1.32 Compounds Formed by Covalent
CHM 105 & 106 MO1 UNIT FIVE, LECTURE SIX 1 IN OUR PREVIOUS LECTURE WE WERE LOOKING AT EQUILIBRIUM APPLICATIONS TO DISSOLVING
 CHM 105 & 106 MO1 UNIT FIVE, LECTURE SIX 1 CHM 105/106 Program 46: Unit 5 Lecture 6 IN OUR PREVIOUS LECTURE WE WERE LOOKING AT EQUILIBRIUM APPLICATIONS TO DISSOLVING AND PRECIPITATION PROCESSES WHICH WE
CHM 105 & 106 MO1 UNIT FIVE, LECTURE SIX 1 CHM 105/106 Program 46: Unit 5 Lecture 6 IN OUR PREVIOUS LECTURE WE WERE LOOKING AT EQUILIBRIUM APPLICATIONS TO DISSOLVING AND PRECIPITATION PROCESSES WHICH WE
Nanoscale thermal transport and the thermal conductance of interfaces
 Nanoscale thermal transport and the thermal conductance of interfaces David G. Cahill Scott Huxtable, Zhenbin Ge, Paul Bruan Materials Research Laboratory and Department of Materials Science Zhaohui Wang,
Nanoscale thermal transport and the thermal conductance of interfaces David G. Cahill Scott Huxtable, Zhenbin Ge, Paul Bruan Materials Research Laboratory and Department of Materials Science Zhaohui Wang,
STATES OF MATTER NOTES..
 STATES OF MATTER NOTES.. While you are reading, answer the following which will help you with the States of Matter Project. What is matter (definition): What are the states of matter and what are the characteristics/properties
STATES OF MATTER NOTES.. While you are reading, answer the following which will help you with the States of Matter Project. What is matter (definition): What are the states of matter and what are the characteristics/properties
Isaac Newton was a British scientist whose accomplishments
 E8 Newton s Laws of Motion R EA D I N G Isaac Newton was a British scientist whose accomplishments included important discoveries about light, motion, and gravity. You may have heard the legend about how
E8 Newton s Laws of Motion R EA D I N G Isaac Newton was a British scientist whose accomplishments included important discoveries about light, motion, and gravity. You may have heard the legend about how
Topic 2: Bonding and properties of water Chapter 2 6. Define a covalent bond, and compare polar covalent bonds vs. nonpolar 5 covalent bonds.
 Name PACKET #1 Block Date Unit 1: Chemistry of Life, Part I Objectives: Upon completion of this part of the unit, you should be able to: Topic 1: The nature of matter Chapter 2 Packet P Objective Book
Name PACKET #1 Block Date Unit 1: Chemistry of Life, Part I Objectives: Upon completion of this part of the unit, you should be able to: Topic 1: The nature of matter Chapter 2 Packet P Objective Book
Topic C6 Chemical Synthesis Homework booklet Graph paper required for homework two Name Key terms and spellings on back page
 Topic C6 Chemical Synthesis Homework booklet Graph paper required for homework two Name Key terms and spellings on back page Due Date Teacher Comment Homework 1 Homework 2 Homework 3 Homework 4 Homework
Topic C6 Chemical Synthesis Homework booklet Graph paper required for homework two Name Key terms and spellings on back page Due Date Teacher Comment Homework 1 Homework 2 Homework 3 Homework 4 Homework
Chemical reactions. C2- Topic 5
 Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
8 th Grade Science. Directed Reading Packet. Chemistry. Name: Teacher: Period:
 8 th Grade Science Directed Reading Packet Chemistry Name: Teacher: Period: Chapter 1, Section 1: Inside the Atom Introduction 1. Atoms are the particles of an element that still have the element s. 2.
8 th Grade Science Directed Reading Packet Chemistry Name: Teacher: Period: Chapter 1, Section 1: Inside the Atom Introduction 1. Atoms are the particles of an element that still have the element s. 2.
DJO AP Biology Summer Assignment
 DJO AP Biology Summer Assignment Welcome to AP Biology! Complete the summer assignment and use the key to check your answers. Also read the graphing appendix and complete the graphing assignment. Finally,
DJO AP Biology Summer Assignment Welcome to AP Biology! Complete the summer assignment and use the key to check your answers. Also read the graphing appendix and complete the graphing assignment. Finally,
Balancing Chemical Equations
 Lesson Created by: Lauryn Atwood Length of lesson: 1 week Description of the class: Heterogeneous Name of course: Chemistry Grade level: 10-12 Honors or regular: Regular Balancing Chemical Equations Source
Lesson Created by: Lauryn Atwood Length of lesson: 1 week Description of the class: Heterogeneous Name of course: Chemistry Grade level: 10-12 Honors or regular: Regular Balancing Chemical Equations Source
Paper Reference. London Examinations IGCSE. Foundation Tier. Tuesday 10 November 2009 Afternoon Time: 1 hour 30 minutes
 Centre No. Candidate No. Paper Reference(s) 4335/1F London Examinations IGCSE Chemistry Paper 1F Foundation Tier Tuesday 10 November 2009 Afternoon Time: 1 hour 30 minutes Materials required for examination
Centre No. Candidate No. Paper Reference(s) 4335/1F London Examinations IGCSE Chemistry Paper 1F Foundation Tier Tuesday 10 November 2009 Afternoon Time: 1 hour 30 minutes Materials required for examination
Hot and Cold Balloons
 Hot and Cold Balloons Moira filled a balloon with air. She tightly tied the balloon so no air could get in or out of the balloon. She kept the balloon in a warm room. An hour later she put the balloon
Hot and Cold Balloons Moira filled a balloon with air. She tightly tied the balloon so no air could get in or out of the balloon. She kept the balloon in a warm room. An hour later she put the balloon
Real life example 1 Let s look at this series of chloroalcohols, and how fast the chloride gets displaced by an external nucleophile.
 Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
Isaac Newton was a British scientist whose accomplishments
 E8 Newton s Laws of Motion R EA D I N G Isaac Newton was a British scientist whose accomplishments included important discoveries about light, motion, and gravity. You may have heard the legend about how
E8 Newton s Laws of Motion R EA D I N G Isaac Newton was a British scientist whose accomplishments included important discoveries about light, motion, and gravity. You may have heard the legend about how
ESA Study Guide Year 10 Science
 Then and now Questions from pages 26, 27 of ESA Study Guide Year 10 Science 1. Which early scientist thought atoms would combine to form new substances? 2. Which New Zealand scientist found that most of
Then and now Questions from pages 26, 27 of ESA Study Guide Year 10 Science 1. Which early scientist thought atoms would combine to form new substances? 2. Which New Zealand scientist found that most of
Chapter 20: Mechanical Waves
 Chapter 20: Mechanical Waves Section 20.1: Observations: Pulses and Wave Motion Oscillation Plus Propagation Oscillation (or vibration): Periodic motion (back-and-forth, upand-down) The motion repeats
Chapter 20: Mechanical Waves Section 20.1: Observations: Pulses and Wave Motion Oscillation Plus Propagation Oscillation (or vibration): Periodic motion (back-and-forth, upand-down) The motion repeats
CHAPTER 2 ATOMS, MOLECULES,
 CHAPTER 2 ATOMS, MOLECULES, AND LIFE LECTURE OUTLINE Case Study: Unstable Atoms Unleashed 2.1 What Are Atoms? A. Atoms Are the Basic Structural Units of Elements (Figures 2-1 and 2-2, and Table 2-1) 1.
CHAPTER 2 ATOMS, MOLECULES, AND LIFE LECTURE OUTLINE Case Study: Unstable Atoms Unleashed 2.1 What Are Atoms? A. Atoms Are the Basic Structural Units of Elements (Figures 2-1 and 2-2, and Table 2-1) 1.
Rehearse your talk at least 3 /mes
 Presenta(ons 1 Organiza(on End on (me Right amount of material Have a (me budget for each slide Work towards a take- home message UncluAered slides that enhance your words Rehearse your talk at least 3
Presenta(ons 1 Organiza(on End on (me Right amount of material Have a (me budget for each slide Work towards a take- home message UncluAered slides that enhance your words Rehearse your talk at least 3
1 The Nature of Science
 CHAPTER 1 Introduction to Science 1 The Nature of Science SECTION KEY IDEAS As you read this section, keep these questions in mind: What processes do scientists use to answer questions? How is a scientific
CHAPTER 1 Introduction to Science 1 The Nature of Science SECTION KEY IDEAS As you read this section, keep these questions in mind: What processes do scientists use to answer questions? How is a scientific
Thermal Radiation and Line Emission 7/7/09. Astronomy 101
 Thermal Radiation and Line Emission 7/7/09 Astronomy 101 Astronomy Picture of the Day Astronomy 101 Outline for Today Astronomy Picture of the Day Astro News Article Business Return Lab 3 Q&A session Thermal
Thermal Radiation and Line Emission 7/7/09 Astronomy 101 Astronomy Picture of the Day Astronomy 101 Outline for Today Astronomy Picture of the Day Astro News Article Business Return Lab 3 Q&A session Thermal
Drying induced acidity at the mineral-water. interface: ATR-FTIR Spectroscopy
 Drying induced acidity at the mineral-water interface: ATR-FTIR Spectroscopy Dr. Javier Aguilar Department of Earth Sciences Stellenbosch University (South Africa) Introduction Why mineral-water interface
Drying induced acidity at the mineral-water interface: ATR-FTIR Spectroscopy Dr. Javier Aguilar Department of Earth Sciences Stellenbosch University (South Africa) Introduction Why mineral-water interface
Light & Atoms. Electromagnetic [EM] Waves. Light and several other forms of radiation are called electromagnetic waves or electromagnetic radiation.
![Light & Atoms. Electromagnetic [EM] Waves. Light and several other forms of radiation are called electromagnetic waves or electromagnetic radiation. Light & Atoms. Electromagnetic [EM] Waves. Light and several other forms of radiation are called electromagnetic waves or electromagnetic radiation.](/thumbs/88/117441686.jpg) Light & Atoms Electromagnetic [EM] Waves Light and several other forms of radiation are called electromagnetic waves or electromagnetic radiation. These have both and electric part and a magnetic part
Light & Atoms Electromagnetic [EM] Waves Light and several other forms of radiation are called electromagnetic waves or electromagnetic radiation. These have both and electric part and a magnetic part
U N I T T E S T P R A C T I C E
 South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
Most substances can be in three states: solid, liquid, and gas.
 States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
London Examinations IGCSE
 Centre No. Candidate No. Paper Reference(s) 4437/5 London Examinations IGCSE Science (Double Award) Chemistry Paper 5 igher Tier Wednesday 17 June 2009 Morning Time: 1 hour 30 minutes Materials required
Centre No. Candidate No. Paper Reference(s) 4437/5 London Examinations IGCSE Science (Double Award) Chemistry Paper 5 igher Tier Wednesday 17 June 2009 Morning Time: 1 hour 30 minutes Materials required
Self-Check. change in pressure. 6. I can explain how the volume of a gas is changed by a change in temperature S. Coates
 Self-Check YES NO 1. I can describe how atoms move in a solid, liquid, and gas 2. I can describe the speed/energy of the atoms in a solid, liquid, and gas. 3. I can explain how the distance between atoms
Self-Check YES NO 1. I can describe how atoms move in a solid, liquid, and gas 2. I can describe the speed/energy of the atoms in a solid, liquid, and gas. 3. I can explain how the distance between atoms
News English.com Ready-to-use ESL / EFL Lessons
 www.breaking News English.com Ready-to-use ESL / EFL Lessons 1,000 IDEAS & ACTIVITIES FOR LANGUAGE TEACHERS The Breaking News English.com Resource Book http://www.breakingnewsenglish.com/book.html NASA
www.breaking News English.com Ready-to-use ESL / EFL Lessons 1,000 IDEAS & ACTIVITIES FOR LANGUAGE TEACHERS The Breaking News English.com Resource Book http://www.breakingnewsenglish.com/book.html NASA
The Main Point. How do light and matter interact? Lecture #7: Radiation and Spectra II. How is light absorbed and emitted?
 Lecture #7: Radiation and Spectra II How is light absorbed and emitted? Models of Atomic Structure. Formation of Spectral Lines. Doppler Shift. Applications in Solar System Studies Detecting gaseous phases
Lecture #7: Radiation and Spectra II How is light absorbed and emitted? Models of Atomic Structure. Formation of Spectral Lines. Doppler Shift. Applications in Solar System Studies Detecting gaseous phases
PHYSICS. Chapter 16 Lecture FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E RANDALL D. KNIGHT Pearson Education, Inc.
 PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 16 Lecture RANDALL D. KNIGHT 2017 Pearson Education, Inc. Chapter 16 Traveling Waves IN THIS CHAPTER, you will learn the basic properties
PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 16 Lecture RANDALL D. KNIGHT 2017 Pearson Education, Inc. Chapter 16 Traveling Waves IN THIS CHAPTER, you will learn the basic properties
Evaporation and Intermolecular Attractions
 Evaporation and Intermolecular Attractions BACKGROUND A substance absorbs energy from its surroundings as it changes from the liquid to the gas phase. The absorption of heat by the evaporating substance
Evaporation and Intermolecular Attractions BACKGROUND A substance absorbs energy from its surroundings as it changes from the liquid to the gas phase. The absorption of heat by the evaporating substance
EDUCATION PROGRAMS GUIDE
 EDUCATION PROGRAMS GUIDE Inner Space Center University of Rhode Island Graduate School of Oceanography innerspacecenter.org ABOUT THE ISC The Inner Space Center (ISC) is an international leader in ocean
EDUCATION PROGRAMS GUIDE Inner Space Center University of Rhode Island Graduate School of Oceanography innerspacecenter.org ABOUT THE ISC The Inner Space Center (ISC) is an international leader in ocean
A few elements, including copper, silver, and gold, have been known for thousands of years.
 CHEMISTRY & YOU Chapter The Periodic Organizing the Elements Classifying the Elements Periodic Trends How can you organize and classify elements? If you have ever played a card game, then you have probably
CHEMISTRY & YOU Chapter The Periodic Organizing the Elements Classifying the Elements Periodic Trends How can you organize and classify elements? If you have ever played a card game, then you have probably
IES LAURETUM SCIENCE NAME.
 IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
IES LAURETUM SCIENCE NAME. 1 CONTENTS 1. STATES 6F 0ATTER 2. STATES 6F 0ATTER AND THE5R *R6*ERT5ES 3. 25NET5C THE6RY 4. CHANGE 6F STATE 6F 0ATTER Break the code: Find the letter for each number * = 0 =
Foundations of Chemistry
 Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
IR Spectrography - Absorption. Raman Spectrography - Scattering. n 0 n M - Raman n 0 - Rayleigh
 RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
RAMAN SPECTROSCOPY Scattering Mid-IR and NIR require absorption of radiation from a ground level to an excited state, requires matching of radiation from source with difference in energy states. Raman
How not to give a poster: Some suggestions based on years of experience. D. Lund (credit to J. Granger)
 How not to give a poster: Some suggestions based on years of experience D. Lund (credit to J. Granger) Purpose of a Poster Summarize research concisely and attractively Help publicize it and generate discussion
How not to give a poster: Some suggestions based on years of experience D. Lund (credit to J. Granger) Purpose of a Poster Summarize research concisely and attractively Help publicize it and generate discussion
Chemistry A: States of Matter Packet Name: Hour: Page!1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Evaluation Module: Care for the Chronically ill Person Spring 2010 Responsible for the evaluation process: Project manager, Mette Bro Jansen
 2010 Evaluation Module: Care for the Chronically ill Person Spring 2010 Responsible for the evaluation process: Project manager, Mette Bro Jansen Table of contents Questions concerning the learning outcome
2010 Evaluation Module: Care for the Chronically ill Person Spring 2010 Responsible for the evaluation process: Project manager, Mette Bro Jansen Table of contents Questions concerning the learning outcome
AP Chemistry Audit Syllabus Aubrey High School Teacher: Timothy Dean Mooney
 AP Chemistry Audit Syllabus Aubrey High School Teacher: Timothy Dean Mooney Textbook: Chemistry: A Molecular Approach AP Edition, 3 rd Edition, by Nivaldo J. Tro ( 2014) [CR1] Course Description: The purpose
AP Chemistry Audit Syllabus Aubrey High School Teacher: Timothy Dean Mooney Textbook: Chemistry: A Molecular Approach AP Edition, 3 rd Edition, by Nivaldo J. Tro ( 2014) [CR1] Course Description: The purpose
STATES OF MATTER. The Four States of Ma/er. Four States. Solid Liquid Gas Plasma
 STATES OF MATTER The Four States of Ma/er Solid Liquid Gas Plasma Four States STATES OF MATTER Ø What makes a substance a par:cular state of ma
STATES OF MATTER The Four States of Ma/er Solid Liquid Gas Plasma Four States STATES OF MATTER Ø What makes a substance a par:cular state of ma
GRADE 8: Materials 1. UNIT 8M.1 7 hours. Atoms and molecules. Resources. About this unit. Previous learning. Expectations
 GRADE 8: Materials 1 Atoms and molecules UNIT 8M.1 7 hours About this unit This is the first of four units on materials for Grade 8. This unit builds on all the units in Grade 7, providing a theoretical
GRADE 8: Materials 1 Atoms and molecules UNIT 8M.1 7 hours About this unit This is the first of four units on materials for Grade 8. This unit builds on all the units in Grade 7, providing a theoretical
