How do you think Alexander Fleming tested his hypothesis?
|
|
|
- Wilfred Rice
- 5 years ago
- Views:
Transcription
1 Chapter 1 Introduction to Chemistry 1.1 The Scope of Chemistry 1.2 Chemistry and You 1.3 Thinking Like a Scientist 1.4 Problem Solving in Chemistry 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY & YOU How do you think Alexander Fleming tested his hypothesis? In 1928, Alexander Fleming, a Scottish scientist, noticed that the bacteria he was studying did not grow in the presence of a yellowgreen mold. 2 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 1
2 An Experimental Approach to Science An Experimental Approach to Science How did Lavoisier help to transform chemistry? 3 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. An Experimental Approach to Science The word chemistry comes from the word alchemy. Alchemists were concerned with searching for a way to change other metals, such as lead, into gold. Alchemists developed the tools and techniques for working with chemicals. They designed equipment that is still in use today, including beakers, flasks, tongs, funnels, and the mortar and pestle. 4 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 2
3 An Experimental Approach to Science In France, Antoine-Laurent Lavoisier did work in the late 1700s that would revolutionize the science of chemistry. 5 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. An Experimental Approach to Science In France, Antoine-Laurent Lavoisier did work in the late 1700s that would revolutionize the science of chemistry. Lavoisier helped to transform chemistry from a science of observation to the science of measurement that it is today. 6 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 3
4 An Experimental Approach to Science Lavoisier designed a balance that could measure mass to the nearest gram. He also settled a long-standing debate about how materials burn. He was able to show that oxygen is required for a material to burn. 7 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Who is credited with transforming chemistry from a science of observation to a science of measurement? A. Fleming B. Lavoisier C. de Mestral D. Carothers 8 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 4
5 Who is credited with transforming chemistry from a science of observation to a science of measurement? A. Fleming B. Lavoisier C. de Mestral D. Carothers 9 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. What are the steps in the scientific method? 10 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 5
6 The scientific method is a logical, systematic approach to the solution of a scientific problem. 11 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Steps in the scientific method include making observations, proposing and testing hypotheses, and developing theories. 12 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 6
7 Making Observations When you use your senses to obtain information, you make an observation. This scientist is making observations with a microscope. Observation is an essential step in the scientific method. 13 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Making Observations Suppose you try to turn on a flashlight and you notice that it does not light. 14 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 7
8 Testing Hypotheses If you guess that the batteries in a flashlight are dead, you are making a hypothesis. A hypothesis is a proposed explanation for an observation. 15 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Testing Hypotheses Replacing the batteries is an experiment, a procedure that is used to test a hypothesis. 16 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 8
9 Testing Hypotheses Replacing the batteries is an experiment, a procedure that is used to test a hypothesis. The variable that you change during an experiment is the independent variable, also called the manipulated variable. The variable that is observed during the experiment is the dependent variable, also called the responding variable. 17 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Testing Hypotheses For the results of an experiment to be accepted, the experiment must produce the same result no matter how many times it is repeated, or by whom. This is why scientists are expected to publish a description of their procedures along with their results. 18 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 9
10 Testing Hypotheses Sometimes the experiment a scientist must perform to test a hypothesis is difficult or impossible. For example, atoms and molecules, which are some of the smallest units of matter, cannot be easily seen. A model is a representation of an object or event. Chemists may use models to study chemical reactions and processes. 19 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Developing Theories Once a hypothesis meets the test of repeated experimentation, it may be raised to a higher level of ideas. It may become a theory. A theory is a well-tested explanation for a broad set of observations. 20 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 10
11 Developing Theories When scientists say that a theory can never be proved, they are not saying that a theory is unreliable. They are simply leaving open the possibility that a theory may need to be changed at some point in the future to explain new observations or experimental results. 21 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Scientific Laws The figure below shows how scientific experiments can lead to laws as well as theories. Observations Hypothesis A hypothesis may be revised based on experimental data. Experiments An experiment can lead to observations that support or disprove a hypothesis. Theory A theory is tested by more experiments and modified if necessary. Scientific Law A scientific law summarizes the results of many observations and experiments. 22 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 11
12 Scientific Laws A scientific law is a concise statement that summarizes the results of many observations and experiments. 23 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Scientific Laws A scientific law is a concise statement that summarizes the results of many observations and experiments. A law doesn t try to explain the relationship it describes. That explanation requires a theory. 24 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 12
13 CHEMISTRY & YOU What was Alexander Fleming s hypothesis? How could he test his hypothesis? 25 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY & YOU What was Alexander Fleming s hypothesis? How could he test his hypothesis? Other scientists had made the same observation, but Fleming was the first to recognize its importance. He assumed that the mold had released a chemical that prevented the growth of the bacteria. 26 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 13
14 What is a hypothesis? A. information obtained from an experiment B. a proposed explanation for observations C. a concise statement that summarizes the results of many experiments D. a thoroughly tested model 27 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. What is a hypothesis? A. information obtained from an experiment B. a proposed explanation for observations C. a concise statement that summarizes the results of many experiments D. a thoroughly tested model 28 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 14
15 Collaboration and Communication Collaboration and Communication What role do collaboration and communication play in science? 29 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Collaboration and Communication No matter how talented the players on a team may be, one player cannot ensure victory for the team. Individuals must collaborate, or work together, for the good of the team. 30 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 15
16 Collaboration and Communication When scientists collaborate and communicate with one another, they increase the likelihood of a successful outcome. 31 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Collaboration and Communication Collaboration Scientists choose to collaborate for different reasons. Some research problems are so complex that no one person could have all the knowledge, skills, and resources to solve the problem. 32 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 16
17 Collaboration and Communication Collaboration Collaboration isn t always a smooth process. Working in pairs or in a group can be challenging, but it can also be rewarding. 33 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Collaboration and Communication Communication The way scientists communicate with each other and with the public has changed over the centuries. Scientists working as a team can communicate face to face. They also can exchange ideas by , by phone, and at local and international conferences. They publish their results in scientific journals. 34 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 17
18 Collaboration and Communication Communication Scientific journals are the most reliable source of information about new discoveries. Articles are published only after being reviewed by experts in the author s field. Reviewers may find errors in experimental design or challenge the author s conclusions. This review process is good for science because work that is not well founded is usually not published. 35 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Why are articles in scientific journals the most reliable source of information about new scientific discoveries? A. The articles are reviewed by experts in the author s field. B. Any article that is submitted is published. C. Everyone has access to the information. D. The articles are short and easy to read. 36 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 18
19 Why are articles in scientific journals the most reliable source of information about new scientific discoveries? A. The articles are reviewed by experts in the author s field. B. Any article that is submitted is published. C. Everyone has access to the information. D. The articles are short and easy to read. 37 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Key Concepts Lavoisier helped to transform chemistry from a science of observation to the science of measurement that it is today. Steps in the scientific method include making observations, testing hypotheses, and developing theories. When scientists collaborate and communicate with one another, they increase the likelihood of a successful outcome. 38 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 19
20 Glossary Terms scientific method: a logical, systematic approach to the solution of a scientific problem; steps in the scientific method include making observations, testing hypotheses, and developing theories observation: information obtained through the senses; observation in science often involves a measurement hypothesis: a proposed explanation for an observation experiment: a repeatable procedure that is used to test a hypothesis 39 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Glossary Terms independent variable: the variable that is changed during an experiment; also called manipulated variable dependent variable: the variable that is observed during an experiment; also called responding variable model: a representation of an event or object theory: a well-tested explanation for a broad set of observations scientific law: a concise statement that summarizes the results of many observations and experiments 40 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 20
21 BIG IDEA Chemists use the scientific method to solve problems and develop theories about the natural world. 41 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. END OF Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. 21
1.3 Thinking Like a Scientist > Chapter 1 Introduction to Chemistry. 1.3 Thinking Like a Scientist. 1.1 The Scope of Chemistry 1.2 Chemistry and You
 Chapter 1 Introduction to Chemistry 1.1 The Scope of Chemistry 1.2 Chemistry and You 1.3 Thinking Like a Scientist 1.4 Problem Solving in Chemistry 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 1 Introduction to Chemistry 1.1 The Scope of Chemistry 1.2 Chemistry and You 1.3 Thinking Like a Scientist 1.4 Problem Solving in Chemistry 1 Copyright Pearson Education, Inc., or its affiliates.
Introduction to Chemistry. d6retp6pq
 Introduction to Chemistry https://www.youtube.com/watch?v=j7 d6retp6pq Why Study Chemistry Chemistry can explain the natural world Chemistry can prepare people for careers Chemistry can produce informed
Introduction to Chemistry https://www.youtube.com/watch?v=j7 d6retp6pq Why Study Chemistry Chemistry can explain the natural world Chemistry can prepare people for careers Chemistry can produce informed
Answer: the study of the composition of matter and changes that matter undergoes
 Vocabulary Vocabulary and Math - 200-200 Vocabulary Vocabulary and Math - 200-200 Define chemistry Answer: the study of the composition of matter and changes that matter undergoes Vocabulary Vocabulary
Vocabulary Vocabulary and Math - 200-200 Vocabulary Vocabulary and Math - 200-200 Define chemistry Answer: the study of the composition of matter and changes that matter undergoes Vocabulary Vocabulary
Name Date Class. biochemistry analytical chemistry physical chemistry
 1.1 CHEMISTRY Section Review Objectives Identify five traditional areas of study in chemistry Relate pure chemistry to applied chemistry Identify reasons to study chemistry Vocabulary matter chemistry
1.1 CHEMISTRY Section Review Objectives Identify five traditional areas of study in chemistry Relate pure chemistry to applied chemistry Identify reasons to study chemistry Vocabulary matter chemistry
Every physical or chemical change in matter involves a change in energy.
 Sec. 2.1 Energy Objectives: 1. Explain that physical and chemical changes in matter involve transfers of energy 2. Apply the law of conservation of energy to analyze changes in matter 3. Distinguish between
Sec. 2.1 Energy Objectives: 1. Explain that physical and chemical changes in matter involve transfers of energy 2. Apply the law of conservation of energy to analyze changes in matter 3. Distinguish between
Scientific Method. Section 1. Observation includes making measurements and collecting data. Main Idea
 Scientific Method Section 1 2B, 2C, 2D Key Terms scientific method system hypothesis model theory s Observation includes making measurements and collecting data. Sometimes progress in science comes about
Scientific Method Section 1 2B, 2C, 2D Key Terms scientific method system hypothesis model theory s Observation includes making measurements and collecting data. Sometimes progress in science comes about
MIDDLE SCHOOL BIOLOGY LABORATORY 1ST SEMESTER NAME: DATE: Activity: for each text we will highlight the most important information.
 NAME: DATE: TEACHER: Albert Hernandez. GRADE: 2 nd I. Read text carefully and answer the questions bellow. Activity: for each text we will highlight the most important information. The Goal of Science
NAME: DATE: TEACHER: Albert Hernandez. GRADE: 2 nd I. Read text carefully and answer the questions bellow. Activity: for each text we will highlight the most important information. The Goal of Science
Chapter 3 Scientific Measurement
 Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
3.1 Using and Expressing Measurements > 3.1 Using and Expressing Measurements >
 Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
4.1 Defining the Atom > Chapter 4 Atomic Structure. 4.1 Defining the Atom. 4.2 Structure of the Nuclear Atom. 4.3 Distinguishing Among Atoms
 Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom 4.3 Distinguishing Among Atoms 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom 4.3 Distinguishing Among Atoms 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chemical Reactions: The Law of Conservation of Mass
 Chemical Reactions: The Law of Conservation of Mass What happens in a Chemical Reaction? Chemical bonds in the reactants are broken, then atoms are rearranged to form new substances (products). Reactants
Chemical Reactions: The Law of Conservation of Mass What happens in a Chemical Reaction? Chemical bonds in the reactants are broken, then atoms are rearranged to form new substances (products). Reactants
BIOLOGY 111. CHAPTER 1: An Introduction to the Science of Life
 BIOLOGY 111 CHAPTER 1: An Introduction to the Science of Life An Introduction to the Science of Life: Chapter Learning Outcomes 1.1) Describe the properties of life common to all living things. (Module
BIOLOGY 111 CHAPTER 1: An Introduction to the Science of Life An Introduction to the Science of Life: Chapter Learning Outcomes 1.1) Describe the properties of life common to all living things. (Module
Chapter 1. Biology: Exploring Life. Lecture by Richard L. Myers
 Chapter 1 Biology: Exploring Life PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Richard
Chapter 1 Biology: Exploring Life PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Richard
Scientific Method. Chapter 1.3. Copyright Cmassengale
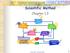 Scientific Method Chapter 1.3 1 Observation STEP 1 Employing your five senses to perceive objects or events 2 3 Asking a Question Based on observations; one or more questions are generated 4 Forming a
Scientific Method Chapter 1.3 1 Observation STEP 1 Employing your five senses to perceive objects or events 2 3 Asking a Question Based on observations; one or more questions are generated 4 Forming a
Chemistry: Introduction to Chemistry
 Chemistry: Introduction to Chemistry Name: Hr: Pure Science vs. Applied Science pure science ( science ) = In science, we often try to establish a cause-effect relationship What drives pure scientists?
Chemistry: Introduction to Chemistry Name: Hr: Pure Science vs. Applied Science pure science ( science ) = In science, we often try to establish a cause-effect relationship What drives pure scientists?
2.3 Elements and Compounds > Chapter 2 Matter and Change. 2.3 Elements and Compounds. 2.1 Properties of Matter 2.2 Mixtures. 2.4 Chemical Reactions
 Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 10 Chemical Quantities
 101 The Mole: A Measurement Chapter 10 Chemical Quantities 101 The Mole: A Measurement 102 Mole-Mass and Mole-Volume Relationships 103 Percent Composition and Chemical Formulas 1 Copyright Pearson Education,
101 The Mole: A Measurement Chapter 10 Chemical Quantities 101 The Mole: A Measurement 102 Mole-Mass and Mole-Volume Relationships 103 Percent Composition and Chemical Formulas 1 Copyright Pearson Education,
The Science of Biology
 Chapter 1 The Science of Biology Section 1 1 What Is Science? (pages 3 7) This section explains what the goal of science is and describes a scientific view of the world What Science Is and Is Not (page
Chapter 1 The Science of Biology Section 1 1 What Is Science? (pages 3 7) This section explains what the goal of science is and describes a scientific view of the world What Science Is and Is Not (page
How can there be different varieties of atoms?
 Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom 4.3 Distinguishing Among Atoms 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom 4.3 Distinguishing Among Atoms 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
1 September 19, 2006
 Title: What s Your Angle? (2005 and 2006 Exam) Authors: SALTers Grade: 8 Description: Students investigate the effect of the angle at which light strikes a block of wood on the surface temperature of that
Title: What s Your Angle? (2005 and 2006 Exam) Authors: SALTers Grade: 8 Description: Students investigate the effect of the angle at which light strikes a block of wood on the surface temperature of that
6/28/11. Avogadro s Number and the Mole. The Mole. The Mole. The Mole (mol)
 Avogadro s Number and the Mole Molecular weight: The sum of atomic weights of all atoms in a molecule. Formula weight: The sum of atomic weights of all atoms in one formula unit of any compound. Mole:
Avogadro s Number and the Mole Molecular weight: The sum of atomic weights of all atoms in a molecule. Formula weight: The sum of atomic weights of all atoms in one formula unit of any compound. Mole:
Section 4.1 Polynomial Functions and Models. Copyright 2013 Pearson Education, Inc. All rights reserved
 Section 4.1 Polynomial Functions and Models Copyright 2013 Pearson Education, Inc. All rights reserved 3 8 ( ) = + (a) f x 3x 4x x (b) ( ) g x 2 x + 3 = x 1 (a) f is a polynomial of degree 8. (b) g is
Section 4.1 Polynomial Functions and Models Copyright 2013 Pearson Education, Inc. All rights reserved 3 8 ( ) = + (a) f x 3x 4x x (b) ( ) g x 2 x + 3 = x 1 (a) f is a polynomial of degree 8. (b) g is
1.1 The Scope of Chemistry > Chapter 1 Introduction to Chemistry. 1.1 The Scope of Chemistry
 Chapter 1 Introduction to Chemistry 1.1 The Scope of Chemistry 1.2 Chemistry and You 1.3 Thinking Like a Scientist 1.4 Problem Solving in Chemistry 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 1 Introduction to Chemistry 1.1 The Scope of Chemistry 1.2 Chemistry and You 1.3 Thinking Like a Scientist 1.4 Problem Solving in Chemistry 1 Copyright Pearson Education, Inc., or its affiliates.
19.4 Neutralization Reactions > Chapter 19 Acids, Bases, and Salts Neutralization Reactions
 Chapter 19 Acids, Bases, and Salts 19.1 Acid-Base Theories 19.2 Hydrogen Ions and Acidity 19.3 Strengths of Acids and Bases 19.4 Neutralization Reactions 19.5 Salts in Solution 1 Copyright Pearson Education,
Chapter 19 Acids, Bases, and Salts 19.1 Acid-Base Theories 19.2 Hydrogen Ions and Acidity 19.3 Strengths of Acids and Bases 19.4 Neutralization Reactions 19.5 Salts in Solution 1 Copyright Pearson Education,
Scientific Method: a logical approach to understanding or solving problems that needs solved.
 Chapter 2 Section 1 Section 2-1 Objectives Describe the purpose of the scientific method. Distinguish between qualitative and quantitative observations. Describe the differences between hypotheses, theories,
Chapter 2 Section 1 Section 2-1 Objectives Describe the purpose of the scientific method. Distinguish between qualitative and quantitative observations. Describe the differences between hypotheses, theories,
Concept 1.3: Scientists use two main forms of inquiry in their study of nature
 Concept 1.3: Scientists use two main forms of inquiry in their study of nature The word Science is derived from Latin and means to know Inquiry is the search for information and explanation There are two
Concept 1.3: Scientists use two main forms of inquiry in their study of nature The word Science is derived from Latin and means to know Inquiry is the search for information and explanation There are two
1.1. KEY CONCEPT Biologists study life in all its forms. 4 Reinforcement Unit 1 Resource Book. Biology in the 21st Century CHAPTER 1
 1.1 THE STUDY OF LIFE KEY CONCEPT Biologists study life in all its forms. Biology is the scientific study of all forms of life. Living things are found almost everywhere on Earth, from very hot environments
1.1 THE STUDY OF LIFE KEY CONCEPT Biologists study life in all its forms. Biology is the scientific study of all forms of life. Living things are found almost everywhere on Earth, from very hot environments
EXPERIMENTAL DESIGN. u Science answers questions with experiments.
 EXPERIMENTAL DESIGN u Science answers questions with experiments. Define the Problem u Begin by asking a question about your topic u Represented by a problem statement u What is a good question for an
EXPERIMENTAL DESIGN u Science answers questions with experiments. Define the Problem u Begin by asking a question about your topic u Represented by a problem statement u What is a good question for an
6-12. Grades. Great extension activities for biology topics. Correlated to standards. Comprehensive biology vocabulary study
 Grades 6-12 CD-104643 Great extension activities for biology topics Correlated to standards Comprehensive biology vocabulary study Fascinating true-tolife illustrations 10 20 30 40 50 70 80 90 100 110
Grades 6-12 CD-104643 Great extension activities for biology topics Correlated to standards Comprehensive biology vocabulary study Fascinating true-tolife illustrations 10 20 30 40 50 70 80 90 100 110
SCIENTIFIC INQUIRY AND CONNECTIONS. Recognize questions and hypotheses that can be investigated according to the criteria and methods of science
 SUBAREA I. COMPETENCY 1.0 SCIENTIFIC INQUIRY AND CONNECTIONS UNDERSTAND THE PRINCIPLES AND PROCESSES OF SCIENTIFIC INQUIRY AND CONDUCTING SCIENTIFIC INVESTIGATIONS SKILL 1.1 Recognize questions and hypotheses
SUBAREA I. COMPETENCY 1.0 SCIENTIFIC INQUIRY AND CONNECTIONS UNDERSTAND THE PRINCIPLES AND PROCESSES OF SCIENTIFIC INQUIRY AND CONDUCTING SCIENTIFIC INVESTIGATIONS SKILL 1.1 Recognize questions and hypotheses
and just what is science? how about this biology stuff?
 Welcome to Life on Earth! Rob Lewis 512.775.6940 rlewis3@austincc.edu 1 The Science of Biology Themes and just what is science? how about this biology stuff? 2 1 The Process Of Science No absolute truths
Welcome to Life on Earth! Rob Lewis 512.775.6940 rlewis3@austincc.edu 1 The Science of Biology Themes and just what is science? how about this biology stuff? 2 1 The Process Of Science No absolute truths
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Law of conservation of mass If a piece of magnesium is burnt, will there be a gain or a loss in mass? Why?
 1 Atomic Theory Law of conservation of mass If a piece of magnesium is burnt, will there be a gain or a loss in mass? Why? Activity Measure the mass of 500 cm 3 of your favorite drink. Then compare your
1 Atomic Theory Law of conservation of mass If a piece of magnesium is burnt, will there be a gain or a loss in mass? Why? Activity Measure the mass of 500 cm 3 of your favorite drink. Then compare your
Science cause and effect Science as a global effort ethics matter!
 Scientific methods discussion Science cause and effect Science as a global effort ethics matter! Outline: the method linear? skepticism where are we in science? creativity ethics (Materials here from:
Scientific methods discussion Science cause and effect Science as a global effort ethics matter! Outline: the method linear? skepticism where are we in science? creativity ethics (Materials here from:
Section Objectives: Recognize some possible benefits from studying biology. Summarize the characteristics of living things.
 Section Objectives: Recognize some possible benefits from studying biology. Summarize the characteristics of living things. The Science of Biology The concepts, principles, and theories that allow people
Section Objectives: Recognize some possible benefits from studying biology. Summarize the characteristics of living things. The Science of Biology The concepts, principles, and theories that allow people
CONCEPT 4 Scientific Law. CONCEPT 3 Scientific Theory
 CONCEPT 1 Nature of Science is a way of learning about the natural world that is based on evidence and logic. The goal of science is to understand and things happen. Science advances as new evidence accumulates
CONCEPT 1 Nature of Science is a way of learning about the natural world that is based on evidence and logic. The goal of science is to understand and things happen. Science advances as new evidence accumulates
Chapter 1 Biology: Exploring Life
 Chapter 1 Biology: Exploring Life PowerPoint Lectures for Campbell Biology: Concepts & Connections, Seventh Edition Reece, Taylor, Simon, and Dickey Lecture by Edward J. Zalisko Figure 1.0_1 Chapter 1:
Chapter 1 Biology: Exploring Life PowerPoint Lectures for Campbell Biology: Concepts & Connections, Seventh Edition Reece, Taylor, Simon, and Dickey Lecture by Edward J. Zalisko Figure 1.0_1 Chapter 1:
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
9.1 The Arithmetic of Equations > Chapter 9 Stoichiometry. 9.1 The Arithmetic of Equations. 9.2 Chemical Calculations
 Chapter 9 Stoichiometry 9.1 The Arithmetic of Equations 9.2 Chemical Calculations 9.3 Limiting Reagent and Percent Yield 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 9 Stoichiometry 9.1 The Arithmetic of Equations 9.2 Chemical Calculations 9.3 Limiting Reagent and Percent Yield 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Study Guide Exam 1 BIO 301L Chinnery Spring 2013
 Study Guide Exam 1 BIO 301L Chinnery Spring 2013 Lecture 1: Biology and Life What are the big picture messages from this lecture? How can you associate the contents of this lecture with those of the other
Study Guide Exam 1 BIO 301L Chinnery Spring 2013 Lecture 1: Biology and Life What are the big picture messages from this lecture? How can you associate the contents of this lecture with those of the other
Effective January 2008 All indicators in Standard / 11
 Scientific Inquiry 8-1 The student will demonstrate an understanding of technological design and scientific inquiry, including process skills, mathematical thinking, controlled investigative design and
Scientific Inquiry 8-1 The student will demonstrate an understanding of technological design and scientific inquiry, including process skills, mathematical thinking, controlled investigative design and
Chapter 12 Stoichiometry
 12.2 Chemical Calculations > Chapter 12 Stoichiometry 12.1 The Arithmetic of Equations 12.22 Chemical Calculations 12.3 Limiting Reagent and Percent Yield 1 Copyright Pearson Education, Inc., or its affiliates.
12.2 Chemical Calculations > Chapter 12 Stoichiometry 12.1 The Arithmetic of Equations 12.22 Chemical Calculations 12.3 Limiting Reagent and Percent Yield 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
5.1/4.1 Scientific Investigation, Reasoning, and Logic Question/Answer Packet #1
 5.1/4.1 Scientific Investigation, Reasoning, and Logic Question/Answer Packet #1 The student will demonstrate an understanding of scientific reasoning, logic, and the nature of science by planning and
5.1/4.1 Scientific Investigation, Reasoning, and Logic Question/Answer Packet #1 The student will demonstrate an understanding of scientific reasoning, logic, and the nature of science by planning and
HISTORY OF CHEMISTRY BY SARA C. MIGUEL M. JESSICA S.
 HISTORY OF CHEMISTRY BY SARA C. MIGUEL M. JESSICA S. A Little Info! Chemistry is a branch of science Dates back to prehistoric times ( looong time ago ) Is separated into four different time periods Prehistoric
HISTORY OF CHEMISTRY BY SARA C. MIGUEL M. JESSICA S. A Little Info! Chemistry is a branch of science Dates back to prehistoric times ( looong time ago ) Is separated into four different time periods Prehistoric
Biology. Slide 1 of 21. End Show. Copyright Pearson Prentice Hall
 Biology 1 of 21 1-1 What Is Science? 2 of 21 1-1 What Is Science? What Science Is and Is Not What Science Is and Is Not What is the goal of science? 3 of 21 1-1 What Is Science? What Science Is and Is
Biology 1 of 21 1-1 What Is Science? 2 of 21 1-1 What Is Science? What Science Is and Is Not What Science Is and Is Not What is the goal of science? 3 of 21 1-1 What Is Science? What Science Is and Is
Understanding How Chemists View Molecules: The Laws of Definite and Multiple Proportions
 Understanding How Chemists View Molecules: The Laws of Definite and Multiple Proportions Name: Period: The period from 1780-1860 was critical for the development of chemistry. During that time period,
Understanding How Chemists View Molecules: The Laws of Definite and Multiple Proportions Name: Period: The period from 1780-1860 was critical for the development of chemistry. During that time period,
AP CHEMISTRY READING GUIDE
 Name: Due Date: AP CHEMISTRY READING GUIDE Chapters 1-3, Chemical Foundations & Stoichiometry Chapter 1 Chemical Foundations Define the following terms in your own words: Scanning Tunneling Microscope
Name: Due Date: AP CHEMISTRY READING GUIDE Chapters 1-3, Chemical Foundations & Stoichiometry Chapter 1 Chemical Foundations Define the following terms in your own words: Scanning Tunneling Microscope
7.1 Ions > Chapter 7 Ionic and Metallic Bonding. 7.1 Ions. 7.2 Ionic Bonds and Ionic Compounds 7.3 Bonding in Metals
 Chapter 7 Ionic and Metallic Bonding 7.1 Ions 7.2 Ionic Bonds and Ionic Compounds 7.3 Bonding in Metals 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY & YOU What
Chapter 7 Ionic and Metallic Bonding 7.1 Ions 7.2 Ionic Bonds and Ionic Compounds 7.3 Bonding in Metals 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY & YOU What
Chapter 1: Introduction: Themes in the Study of Life
 Name Period Begin your study of biology this year by reading Chapter 1. It will serve as a reminder about biological concepts that you may have learned in an earlier course and give you an overview of
Name Period Begin your study of biology this year by reading Chapter 1. It will serve as a reminder about biological concepts that you may have learned in an earlier course and give you an overview of
1. Why is it incorrect to say that the results of a measurement were accurate but not precise?
 Summer Assignment Name: Record your answers in a Google document and be prepared to share your work with me at the beginning of the school year. Cite examples where appropriate. Show your work. Be concise
Summer Assignment Name: Record your answers in a Google document and be prepared to share your work with me at the beginning of the school year. Cite examples where appropriate. Show your work. Be concise
Instant download and all chapter : Test Bank for Introductory Chemistry Essentials 4th Edition by Tro CLICK HERE
 Instant download and all chapter : Test Bank for Introductory Chemistry Essentials 4th Edition by Tro CLICK HERE Introductory Chemistry, 4e (Tro) Chapter 1 The Chemical World True/False Questions 1) Chemicals
Instant download and all chapter : Test Bank for Introductory Chemistry Essentials 4th Edition by Tro CLICK HERE Introductory Chemistry, 4e (Tro) Chapter 1 The Chemical World True/False Questions 1) Chemicals
Warm-up: Are accuracy and precision the same thing? (If so do you want to bet the house on it?)
 Obj: Students will: 1. Distinguish between accuracy and precision. 2. Examine various pieces of lab equipment for their accuracy. 3. Define and identify significant figures. Warm-up: Are accuracy and precision
Obj: Students will: 1. Distinguish between accuracy and precision. 2. Examine various pieces of lab equipment for their accuracy. 3. Define and identify significant figures. Warm-up: Are accuracy and precision
Diffusion and Osmosis
 Lab 3- Bio 160 Diffusion and Osmosis Name: OBJECTIVES: To gain a better understanding of diffusion and osmosis. To understand these terms: diffusion, osmosis, concentration gradient, Brownian motion, hypotonic,
Lab 3- Bio 160 Diffusion and Osmosis Name: OBJECTIVES: To gain a better understanding of diffusion and osmosis. To understand these terms: diffusion, osmosis, concentration gradient, Brownian motion, hypotonic,
3.3 Solving Conversion Problems > Chapter 3 Scientific Measurement. 3.3 Solving Conversion Problems. 3.1 Using and Expressing Measurements
 Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Chapter 1. A Physics Toolkit
 Chapter 1 A Physics Toolkit Chapter 1 A Physics Toolkit In this chapter you will: Use mathematical tools to measure and predict. Apply accuracy and precision when measuring. Display and evaluate data graphically.
Chapter 1 A Physics Toolkit Chapter 1 A Physics Toolkit In this chapter you will: Use mathematical tools to measure and predict. Apply accuracy and precision when measuring. Display and evaluate data graphically.
Law of Conservation of Mass
 .8: hemical Equations onding and hemical Reactions Law of onservation of Mass 1 2 hemical reactions are occurring both inside of us and all around us every second of every day. s a matter of fact, there
.8: hemical Equations onding and hemical Reactions Law of onservation of Mass 1 2 hemical reactions are occurring both inside of us and all around us every second of every day. s a matter of fact, there
INTRODUCTION TO SCIENCE CHAPTER 1
 INTRODUCTION TO SCIENCE CHAPTER 1 1 Science is the study of Everything!! A way of learning about the natural world. Scientist: a person who studies, or has expert WHAT IS SCIENCE? knowledge of a natural
INTRODUCTION TO SCIENCE CHAPTER 1 1 Science is the study of Everything!! A way of learning about the natural world. Scientist: a person who studies, or has expert WHAT IS SCIENCE? knowledge of a natural
8/11/2015 PHYSICAL SCIENCE 1.1 WHAT IS SCIENCE? BIG IDEAS OF PHYSICAL SCIENCE BRANCHES OF SCIENCE WHAT IS SCIENCE?
 PHYSICAL SCIENCE Chapter 1 Science Skills GOAL: Students will be able to distinguish what characterizes science and its methods. Standard: SC.912.N.1.2, SC.912.N.1.3, SC.912.N.1.4, SC.912.N.1.5, SC.912.N.1.6,
PHYSICAL SCIENCE Chapter 1 Science Skills GOAL: Students will be able to distinguish what characterizes science and its methods. Standard: SC.912.N.1.2, SC.912.N.1.3, SC.912.N.1.4, SC.912.N.1.5, SC.912.N.1.6,
5 Major Areas of Chemistry
 Chapter 1 What is Chemistry? Chemistry is the study of the composition of matter (matter is anything with mass and occupies space), its composition, properties, and the changes it undergoes. Has a definite
Chapter 1 What is Chemistry? Chemistry is the study of the composition of matter (matter is anything with mass and occupies space), its composition, properties, and the changes it undergoes. Has a definite
EARTH/SPACE SCIENCE. Earth Materials and Changes
 EARTH/SPACE SCIENCE This Earth/Space science course is designed to continue student investigations of the earth sciences that began in grades K-8 while providing students the experiences and necessary
EARTH/SPACE SCIENCE This Earth/Space science course is designed to continue student investigations of the earth sciences that began in grades K-8 while providing students the experiences and necessary
Chapter 6. September 17, Please pick up a calculator and take out paper and something to write with. Association and Correlation.
 Please pick up a calculator and take out paper and something to write with. Sep 17 8:08 AM Chapter 6 Scatterplots, Association and Correlation Copyright 2015, 2010, 2007 Pearson Education, Inc. Chapter
Please pick up a calculator and take out paper and something to write with. Sep 17 8:08 AM Chapter 6 Scatterplots, Association and Correlation Copyright 2015, 2010, 2007 Pearson Education, Inc. Chapter
BACK TO SCHO O L W ITH
 Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore BACK TO SCHO O L W ITH GEO - L ITERACY Essays on Geo-Literacy For
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore BACK TO SCHO O L W ITH GEO - L ITERACY Essays on Geo-Literacy For
14.3 Ideal Gases > Chapter 14 The Behavior of Gases Ideal Gases Properties of Gases The Gas Laws Gases: Mixtures and Movements
 Chapter 14 The Behavior of Gases 14.1 Properties of Gases 14.2 The Gas Laws 14.3 Ideal Gases 14.4 Gases: Mixtures and Movements 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Chapter 14 The Behavior of Gases 14.1 Properties of Gases 14.2 The Gas Laws 14.3 Ideal Gases 14.4 Gases: Mixtures and Movements 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
EVOLUTION: BIOLOGY S UNIFYING THEME
 EVOLUTION: BIOLOGY S UNIFYING THEME The history of life is a saga of a constantly changing Earth billions of years old. Fossils document this history. CONCEPT 3-7 2013 Pearson Education, Inc. Figure 1.9
EVOLUTION: BIOLOGY S UNIFYING THEME The history of life is a saga of a constantly changing Earth billions of years old. Fossils document this history. CONCEPT 3-7 2013 Pearson Education, Inc. Figure 1.9
Describe the main branches of natural science and relate them to each other. Describe the relationship between science and technology.
 Section 1 The Nature of Science Objectives Describe the main branches of natural science and relate them to each other. Describe the relationship between science and technology. Distinguish between scientific
Section 1 The Nature of Science Objectives Describe the main branches of natural science and relate them to each other. Describe the relationship between science and technology. Distinguish between scientific
Standards Alignment... 5 Safe Science... 9 Scienti c Inquiry Assembling Rubber Band Books...15
 Standards Alignment... 5 Safe Science... 9 Scienti c Inquiry... 11 Assembling Rubber Band Books...15 Earth in Space and Time The Scoop on Stars...17 Telescopes...19 Magnify the Sky...21 Star Samples...27
Standards Alignment... 5 Safe Science... 9 Scienti c Inquiry... 11 Assembling Rubber Band Books...15 Earth in Space and Time The Scoop on Stars...17 Telescopes...19 Magnify the Sky...21 Star Samples...27
8.1 Molecular Compounds > Chapter 8 Covalent Bonding. 8.1 Molecular Compounds
 Chapter 8 Covalent Bonding 8.1 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 8 Covalent Bonding 8.1 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 1. Chemical Foundations
 Chapter 1 Chemical Foundations Chapter 1 Table of Contents (1.1) (1.2) (1.3) (1.4) (1.5) (1.6) (1.7) Chemistry: An atoms-first approach The scientific method The early history of chemistry Fundamental
Chapter 1 Chemical Foundations Chapter 1 Table of Contents (1.1) (1.2) (1.3) (1.4) (1.5) (1.6) (1.7) Chemistry: An atoms-first approach The scientific method The early history of chemistry Fundamental
2 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
 CHEMISTRY & YOU Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom How did scientists determine the structures that are inside an atom? X-rays are used to see structures
CHEMISTRY & YOU Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom How did scientists determine the structures that are inside an atom? X-rays are used to see structures
Reading the Periodic Table
 62 Classifying the Elements > 62 Classifying the Elements > CHEMISTRY & YOU Chapter 6 The Periodic 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends What can you learn about each
62 Classifying the Elements > 62 Classifying the Elements > CHEMISTRY & YOU Chapter 6 The Periodic 61 Organizing the Elements 62 Classifying the Elements 63 Periodic Trends What can you learn about each
Scientific Method - the universal approach to solving scientific problems. 1. Problem Statement - Define the problem - ask question
 Biology: 7 Character of Life: 1. Organization of Cells 2. Response to Stimuli 3. Homeostasis 4. Metabolism 5. Growth & Development 6. Reproduction 7. Change Through Time Levels of Organization Atoms molecules
Biology: 7 Character of Life: 1. Organization of Cells 2. Response to Stimuli 3. Homeostasis 4. Metabolism 5. Growth & Development 6. Reproduction 7. Change Through Time Levels of Organization Atoms molecules
Introduction to Science. Section 1: The Nature of Science Section 2: The Way Science Works Section 3: Organizing Data
 Introduction to Science Section 1: The Nature of Science Section 2: The Way Science Works Section 3: Organizing Data Section 1: The Nature of Science Key Terms Science Technology Scientific Law Scientific
Introduction to Science Section 1: The Nature of Science Section 2: The Way Science Works Section 3: Organizing Data Section 1: The Nature of Science Key Terms Science Technology Scientific Law Scientific
Everything is a chemical!!!
 Why Take Chemistry? 1.Guidance 2. Career 3. How does the world work? Ice skating Sweat Water bugs Atomic bombs Fossil fuels Fireworks Why doesn t a gas tank explode? Everything is a chemical!!! 1 Chemistry
Why Take Chemistry? 1.Guidance 2. Career 3. How does the world work? Ice skating Sweat Water bugs Atomic bombs Fossil fuels Fireworks Why doesn t a gas tank explode? Everything is a chemical!!! 1 Chemistry
BIL 151 Laboratory Enzymes: Planning Your Project
 BIL 151 Laboratory Enzymes: Planning Your Project Science is a process we use to answer questions about the natural world in a logical, rigorous fashion that helps us better understand Life, the Universe,
BIL 151 Laboratory Enzymes: Planning Your Project Science is a process we use to answer questions about the natural world in a logical, rigorous fashion that helps us better understand Life, the Universe,
Introduction. Chapter 1. The Study of Chemistry. The scientific method is a systematic approach to research
 1 Introduction Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 2 Macroscopic The Study of Chemistry Microscopic 2 3 The scientific method is a systematic
1 Introduction Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 2 Macroscopic The Study of Chemistry Microscopic 2 3 The scientific method is a systematic
Welcome to Biology 160&
 Welcome to Biology 160& Course goals Know what ALL living things are made of Cells and cellular machinery. How do they work? Understand the genetic basis of evolutionary change Why so many organisms? How
Welcome to Biology 160& Course goals Know what ALL living things are made of Cells and cellular machinery. How do they work? Understand the genetic basis of evolutionary change Why so many organisms? How
Le Châtelier s Principle. 19 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. Equilibrium: Le Châtelier s Principle
 Factors Affecting : Le Châtelier s Principle Pressure Factors Affecting : Le Châtelier s Principle Pressure When volume decreases, the pressure increases. systems in which some reactants and products are
Factors Affecting : Le Châtelier s Principle Pressure Factors Affecting : Le Châtelier s Principle Pressure When volume decreases, the pressure increases. systems in which some reactants and products are
3.2 Units of Measurement > Chapter 3 Scientific Measurement. 3.2 Units of Measurement. 3.1 Using and Expressing Measurements
 Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Guided Reading Activities
 Name Period Chapter 1: Biology: Exploring Life Guided Reading Activities Big idea: Themes in the study of biology Answer the following questions as you read modules 1.1 1.4: 1. A cell phone is not alive.
Name Period Chapter 1: Biology: Exploring Life Guided Reading Activities Big idea: Themes in the study of biology Answer the following questions as you read modules 1.1 1.4: 1. A cell phone is not alive.
UNIT 1: NATURE OF SCIENCE
 Nature of Science UNIT 1: NATURE OF SCIENCE Chapter 1.1-1.3, pages 6-26 Honors Physical Science Pure science aims to come to a common understanding of the universe Scientists suspend judgment until they
Nature of Science UNIT 1: NATURE OF SCIENCE Chapter 1.1-1.3, pages 6-26 Honors Physical Science Pure science aims to come to a common understanding of the universe Scientists suspend judgment until they
2.1 Properties of Matter > Chapter 2 Matter and Change. 2.1 Properties of Matter. 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions
 21 Properties of Matter > 1 Copyright Pearson Education, Inc, or its affiliates All Rights Reserved Chapter 2 Matter and Change 21 Properties of Matter 22 Mixtures 23 Elements and Compounds 24 Chemical
21 Properties of Matter > 1 Copyright Pearson Education, Inc, or its affiliates All Rights Reserved Chapter 2 Matter and Change 21 Properties of Matter 22 Mixtures 23 Elements and Compounds 24 Chemical
Lecture Presentation. Chapter 1. Matter, Measurement, and Problem Solving. Christian Madu, Ph.D. Collin College Pearson Education, Inc.
 Lecture Presentation Chapter 1 Matter, Measurement, and Problem Solving Christian Madu, Ph.D. Collin College What Do You Think? What do you think is the most important idea in all of human knowledge? If
Lecture Presentation Chapter 1 Matter, Measurement, and Problem Solving Christian Madu, Ph.D. Collin College What Do You Think? What do you think is the most important idea in all of human knowledge? If
Aquarium Systems PCA Scenario Map, Results, and Scoring Rubrics
 Title: Aquarium Systems (2005 and 2006 Op Exam) Authors: SALTers Grade: High School Description: Students investigate how increasing the temperature of an aquarium affects the breathing rate of goldfish
Title: Aquarium Systems (2005 and 2006 Op Exam) Authors: SALTers Grade: High School Description: Students investigate how increasing the temperature of an aquarium affects the breathing rate of goldfish
1.02 Scientific Method
 1.02 Scientific Method Dr. Fred O. Garces Chemistry 100 Miramar College 1 Science and the meaning of life Ever since humans had the ability to think and reason, they have been trying to answer some basic
1.02 Scientific Method Dr. Fred O. Garces Chemistry 100 Miramar College 1 Science and the meaning of life Ever since humans had the ability to think and reason, they have been trying to answer some basic
Unit 1: Introduction to Chemistry
 Unit 1: Introduction to Chemistry I. Observations vs. Inferences Observation: information you gather using your five senses ***You will NEVER use taste in class! o Describes facts Examples You see the
Unit 1: Introduction to Chemistry I. Observations vs. Inferences Observation: information you gather using your five senses ***You will NEVER use taste in class! o Describes facts Examples You see the
Biologists Study the Interactions of Life
 What is Biology? Biologists Study the Interactions of Life Living things do not live in isolation. They interact with their environment and depend on other living/non-living things for survival. Biologists
What is Biology? Biologists Study the Interactions of Life Living things do not live in isolation. They interact with their environment and depend on other living/non-living things for survival. Biologists
Chapter 2. Secton 1 - Energy
 Chapter 2 Secton 1 - Energy Energy and Change Energy is the capacity to do work Any change in matter involves energy Physical or chemical Evaporation liquid gas Requires energy Hydrogen and oxygen react
Chapter 2 Secton 1 - Energy Energy and Change Energy is the capacity to do work Any change in matter involves energy Physical or chemical Evaporation liquid gas Requires energy Hydrogen and oxygen react
2 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
 Chapter 8 Covalent Bonding 8.1 What information does a molecular formula provide? 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc.,
Chapter 8 Covalent Bonding 8.1 What information does a molecular formula provide? 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc.,
Pick up a composition notebook Choose a seat Cut out the calendar and contents pages
 1. 2. 3. Pick up a composition notebook Choose a seat Cut out the calendar and contents pages p o t p a l a t Ge the om. o m r o fr age r o t s Login to a laptop Review the syllabus Join Remind NOW!!!
1. 2. 3. Pick up a composition notebook Choose a seat Cut out the calendar and contents pages p o t p a l a t Ge the om. o m r o fr age r o t s Login to a laptop Review the syllabus Join Remind NOW!!!
BIOLOGY NOTES - CHAPTER 1
 BIOLOGY NOTES - CHAPTER 1 SECTION 1 and 2 Biology is the study of life Bio = life Logy = study of The scientific study of all forms of life, or all types of organisms Science means to know Science is a
BIOLOGY NOTES - CHAPTER 1 SECTION 1 and 2 Biology is the study of life Bio = life Logy = study of The scientific study of all forms of life, or all types of organisms Science means to know Science is a
Parameter Estimation, Sampling Distributions & Hypothesis Testing
 Parameter Estimation, Sampling Distributions & Hypothesis Testing Parameter Estimation & Hypothesis Testing In doing research, we are usually interested in some feature of a population distribution (which
Parameter Estimation, Sampling Distributions & Hypothesis Testing Parameter Estimation & Hypothesis Testing In doing research, we are usually interested in some feature of a population distribution (which
Unit 2 Lesson 3 Physical and Chemical Changes. Copyright Houghton Mifflin Harcourt Publishing Company
 Florida Benchmarks SC.8.N.1.1 Define a problem from the eighth grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of
Florida Benchmarks SC.8.N.1.1 Define a problem from the eighth grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of
Chapter 1. Introduction: Biology Today. Lectures by Chris C. Romero, updated by Edward J. Zalisko
 Chapter 1 Introduction: Biology Today Lectures by Chris C. Romero, updated by Edward J. Zalisko PowerPoint Lectures for Campbell Essential Biology, Fourth Edition Eric Simon, Jane Reece, and Jean Dickey
Chapter 1 Introduction: Biology Today Lectures by Chris C. Romero, updated by Edward J. Zalisko PowerPoint Lectures for Campbell Essential Biology, Fourth Edition Eric Simon, Jane Reece, and Jean Dickey
Chemistry Chapter 3. Atoms: The Building Blocks of Matter
 Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Warm Up: Vocabulary. Unit 1 Introduction to Biology. Defining Key Terms. Defining Key Terms. Defining Key Terms (continued) Think About It
 Unit 1 Introduction to Biology Warm Up: Vocabulary Work in groups at your table to come up with basic scientific method vocabulary. Discuss and define these words in your groups. Defining Key Terms Science-
Unit 1 Introduction to Biology Warm Up: Vocabulary Work in groups at your table to come up with basic scientific method vocabulary. Discuss and define these words in your groups. Defining Key Terms Science-
5.2 Electron Arrangement in Atoms > Happy Thursday!
 Happy Thursday! Please take out your homework problems for me to check for a grade. Keep them out since we will be going over them. Also, take out your notes packet! 1 Chapter 5 Electrons In Atoms 5.1
Happy Thursday! Please take out your homework problems for me to check for a grade. Keep them out since we will be going over them. Also, take out your notes packet! 1 Chapter 5 Electrons In Atoms 5.1
Understanding the Atom
 Understanding the Atom CHAPTER 9 LESSON 1 Discovering Parts of an Atom What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column
Understanding the Atom CHAPTER 9 LESSON 1 Discovering Parts of an Atom What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column
1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2
 1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2 2.) A chemist has discovered what she thinks is a new molecule. In order for it to be a molecule,
1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2 2.) A chemist has discovered what she thinks is a new molecule. In order for it to be a molecule,
The Meaning Of Chemistry
 The Meaning Of Chemistry Experiences and observations alone never lead to finalities. Theory, however, creates reliable roads over which we may pursue our journeys through the world of observation. Anton
The Meaning Of Chemistry Experiences and observations alone never lead to finalities. Theory, however, creates reliable roads over which we may pursue our journeys through the world of observation. Anton
